Abstract
Objective:
PhytoSERM is a selective estrogen receptor beta (ERβ) modulator comprised of three phytoestrogens: genistein, daidzein, and S-equol. The PhytoSERM formulation promotes estrogenic action in the brain while largely inactive or inhibitory in reproductive tissue. A Phase Ib/IIa clinical trial (ClinicalTrial.gov ID: NCT01723917) of PhytoSERM demonstrated safety and pharmacokinetics profile of PhytoSERM1, 2. While this study was not powered for efficacy analysis, we conducted a pilot, retrospective analyses to identify potential responders to PhytoSERM treatment, and to determine the optimal populations to pursue in a Phase II clinical trial of efficacy of the PhytoSERM formulation.
Methods:
In this retrospective analysis involving 46 participants (N=16 placebo, N=18 50mg/day PhytoSERM, and N=12 100mg/day PhytoSERM), therapeutic effect of PhytoSERM was stratified by two genetic risk modulators for Alzheimer’s disease: mitochondrial haplogroup and APOE genotype.
Results:
Our retrospective responder analysis indicated that participants on 50mg of daily PhytoSERM (PS50) for 12 weeks significantly reduced hot flash frequency compared to their baseline (−1.61, [−2.79, −0.42] (mean, [95% CI]), p=0.007). Participants on 50mg of PhytoSERM also had significantly greater reduction in hot flash frequency at 12 weeks compared to the placebo group (−1.38, −0.17, (median PS50, median placebo), p=0.04). Fifty milligrams of daily PhytoSERM also preserved cognitive function in certain aspects of verbal learning and executive function. Our analysis further suggest that mitochondrial haplogroup and APOE genotype can modify PhytoSERM response.
Conclusion:
Our data support a precision medicine approach for further development of PhytoSERM as a safe and effective alternative to hormone therapy for menopause associated hot flash and cognitive decline. While definitive determination of PhytoSERM efficacy is limited by the small sample size, these data provide a reasonable rationale to extend analyses to a larger study set powered to address statistical significance.
Keywords: PhytoSERM, hot flash, cognitive function, Mitochondrial Haplogroup, APOE
Introduction
Loss of ovarian hormones during the menopausal transition is associated with vasomotor symptoms (hot flashes and night sweats), brain glucose hypometabolism, and cognitive decline3–8. Given the shared metabolic and cognitive phenotypes between menopausal transition and late onset Alzheimer’s disease (LOAD)9–14, this natural endocrinological aging transition is also considered a contributor to the two-fold higher life-time risk of LOAD in females compared to males9, 15, 16
Hormone therapy is a proven effective treatment to reduce hot flash frequency, improve brain glucose metabolism, and preserve cognitive function in selected cognitive domains17–26. However, unopposed estrogen or combined hormone therapy can elevate risks for stroke, heart attack, and breast cancer27–32, which has deterred estrogen therapy by menopausal females. For these reasons, safe and effective alternatives that selectively activate estradiol action in the brain but not in the reproductive system are of high priority for women’s health.
Prior studies indicate that physiologically-relevant levels of soy isoflavones promote neurogenesis in vitro33, 34, and provide benefits in memory and cognitive functions in some clinical studies35–40. Although some studies using plant-derived phytoestrogen in post-menopausal women reported positive impact on hot flashes, bone mineral density, risks of cardiovascular diseases, and cognitive function, results were generally mixed and inconclusive (reviewed by40). The conflicting results may be explained by the complex signaling pathways downstream of estrogen and different compositions of phytoestrogens, which could activate both agonistic and antagonistic signaling pathways. Furthermore, while activation of either ERα or ERβ can promote neuroprotection against various neurodegenerative insults, co-administration of ERα-selective agonist and ERβ-selective agonists was less efficacious41, 42. Because ERβ promotes estrogen-mediated neuronal plasticity and memory function, a phytoestrogen formula that selectively targets estrogen receptor beta (ERβ) may be a novel and plausible solution for menopause-related vasomotor symptoms and cognitive impairment43–45.
To address the need for a safe and efficacious intervention for menopausal symptoms and the concern regarding potential elevated health risks related to estrogen therapy, a formulation of three estrogen receptor beta selective isoflavones —genistein, daidzein, and S-equol, in equal parts was developed46. Preclinical translational studies demonstrated efficacy of PhytoSERM in reducing thermodysregulation while promoting cognitive function, mitochondrial respiration and overall health in a peri-menopausal rat model without adverse effects on the reproductive system34, 46–49. A recent Phase Ib/IIa clinical trial on PhytoSERM for management of menopause-associated vasomotor symptoms and cognitive decline (NCT01723917) showed favorable safety, feasibility, and pharmacokinetic profiles but did not show efficacy on a range of clinical measures1, 2. As the trial was not powered for an efficacy analysis, we conducted retrospective analyses to identify potential responders to PhytoSERM treatment, and to determine the optimal populations to pursue in a Phase II clinical trial of efficacy of the PhytoSERM formulation.
Mechanistic, preclinical studies revealed that PhytoSERM can potentiate mitochondrial function and bioenergetics49. Furthermore, two genetic risk modifiers for late onset Alzheimer’s disease – mitochondrial haplogroup and APOE genotype (reviewed by50) – are associated with mitochondrial bioenergetics and respiratory efficiency. It is therefore of interest to explore if these two genetic factors may modulate therapeutic effects of PhytoSERM on hot flash frequency and cognitive function. We report herein outcomes of this retrospective analysis, based on mitochondrial haplogroups and APOE genotypes.
Materials and Methods
Study design
This study is based on the randomized, double-blinded, placebo-controlled, parallel group, Phase Ib/IIa clinical trial for the safety and feasibility of estrogen receptor-β targeted PhytoSERM formulation for management of menopausal symptoms (NCT01723917) in peri- to postmenopausal females1, 2. The study design and participant characteristics have been previously described in detail1, 2. Eligible participants were generally healthy women between 45 and 60 years of age, with intact uteri and ovaries, who had at least one cognitive complaint and one vasomotor-related symptom (one hot flash or night sweat event per day). Study participants were randomized to receive either one 50mg tablet of PhytoSERM (PS50, n=23), one 100mg tablet of PhytoSERM (PS100, n=24), or matching placebo tablet (n=24) per day for 12 weeks. Six participants did not complete the study and were excluded from analysis (n=2 PS50, n=3 PS100, and n=1 placebo participants excluded)1. Within the PS100 and placebo groups, 6 participants on each arm were entered into a cross-over study, and were also excluded from this analysis.
All participants kept daily diaries of their hot flash (frequency and severity) throughout the 12-week trial period. Diaries were collected at each visit at 4-week intervals. For this retrospective analysis, participants who had missing entries for more than 7 consecutive and those who had overall more than 25% missing entries were excluded to ensure participants compliance and data consistency (n=3 PS50, n=3 PS100, and n=1 placebo participants excluded). Due to inconsistency in pre-randomization hot flash diary entries, to capture comparable outcomes, we used daily average hot flash frequency at week 1 post-randomization and treatment as baseline for each participant.
Neuropsychological tests were administered at baseline, weeks 4, 8, and 12. The following tests were included in this analysis: the Verbal Fluency (FAS) test for verbal fluency51; the Rey Auditory Verbal Learning Test (RAVLT) as an assessment of multiple cognitive parameters associated with verbal learning and memory (immediate recall, delayed recall, recognition, and learning over trials)52; the Trail Making Test Parts A as a measurement of visual and motor search speed and Part B as an index of executive function or task-switching53; the Logical Memory Test I and II (immediate and delayed paragraph recall; from the Wechsler Memory Scale-Revised (WMS‐R)) as measures of immediate and episodic memory54; and Mini–Mental State Examination (MMSE) score as a measurement of global cognitive function. The Institutional Review Board at the University of Southern California approved the study (ClinicalTrial.gov Identifier NCT01723917), and all participants provided written informed consent.
Mitochondrial DNA Haplotyping
Total DNA was extracted from whole blood samples using QIAGEN QIAamp DNA Mini Kit following manufacturer’s instructions (Qiagen, Hilden, Germany). Isolated DNA was quantified by PicoGreen dsDNA quantitation assay (ThermoFisher, Waltham, MA). Mitochondrial DNA sequencing was done by University of Arizona Genomics Core. Briefly, DNA samples were first enriched for mitochondrial DNA by PCR reaction (see Supplement table 1 for primers and locations). Amplified segments were sequenced by dye-terminator sequencing on a 96-capillary 3730xl DNA Analyzer (see Supplement table 2 for primers and locations). Sequencing results were assembled and aligned to revised Cambridge Reference Sequence (rCRS, GenBank number NC_012920) using the CLC Main WorkBench software. Mitochondrial haplogroup for each sample was classified using mthap55, 56.
APOE genotyping
APOE genotyping was done as previously described with some modifications57. Briefly, the following primer sequences were used to amplify the DNA: FWD_TAAGCTTGGCACGGCTGTCCAAGGA and REV_ACAGAATTCGCCCCGGCCTGGRACACTGCC. Amplification was performed in a final volume of 25 μL containing 25ng/μL of DNA solution, 400nM of each primer, and 1x RT2 SYBR Green qPCR Mastermix. Reactions were done using Bio-Rad MyCycler Thermal cycler using the following conditions: initial denaturation at 95°C for 10 minutes, followed by 40 cycles of amplification (94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 1 minute), and a final extension step at 72°C for 7 minutes. Amplification products were digested with HhaI restriction endonuclease. APOE genotype for each sample was identified based on agarose gel electrophoresis results.
Statistical Analysis
Post-hoc analysis of changes in hot flash frequency and cognitive function within each treatment group were analyzed using Wilcoxon matched-pairs signed rank test between week 1 and week 12, and changes among treatment groups were analyzed using Kruskal-Wallis test followed by Dunn’s multiple comparison test corrected for multiple comparisons to compare PS50 or PS100 to the placebo group. Analyses were then stratified based on the APOE genotype and mitochondrial haplogroup of the participants to identify responder groups. Mann-Whitney test was used with an alpha value of 0.05. Alpha value was not adjusted for multiple comparison. Outliers defined as more than 2 times standard deviations away from average were excluded from statistical analysis. Analysis was performed by GraphPad Prism v7.
Results
Participant demographics and genotypes
A total of 46 participants with complete hot flash diaries were included in the responder identification analysis. Participant age ranged from 47 to 60 years old, with an average (SD) of 54.2 (3.3) years old. Participants had on average (SD) 17.3 (3.2) years of education. Nine (19.6%) participants were Hispanic or Latino, and 37 (80.4%) were non-Hispanic or Latino. Four participants (8.7%) were Asian, 2 (4.3%) were African-American, 35 (76.1%) were White, and 5 (10.9%) were of “unknown” race1, 2. See Table 1 for participants’ demographic information by treatment.
Table 1.
Participants demographic and baseline characteristics by treatment groups. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day.
| Placebo (n=16) |
PS50 (n=18) |
PS100 (n=12) |
p value | |
|---|---|---|---|---|
| Age, yrs, mean (SD) | 53.9 (3.14) | 53.8 (3.78) | 55.3 (2.83) | 0.43 |
| Education, yrs, mean (SD) | 18.2 (2.59) | 16.6 (2.79) | 17.0 (4.29) | 0.25 |
| Hispanic or Latino, n (%) | 2 (12.5%) | 4 (22.2%) | 3 (25.0%) | 0.67 |
| Race | 0.34 | |||
| Asian | 0 | 2 (11.1%) | 2 (16.67%) | |
| African-American | 1 (6.25%) | 1 (5.56%) | 0 | |
| White | 15 (93.75%) | 12 (66.67%) | 8 (66.67%) | |
| unknown | 0 | 3 (16.67%) | 2 (16.67%) | |
| Baseline Daily Hot Flash Frequency, mean (SD) | 2.4 (2.2) | 3.2 (2.5) | 4.4 (3.6) | 0.23 |
| Baseline RAVLT LOT, mean (SD) | 15.3 (5.4) | 12.5 (6.4) | 17.5 (2.8) | 0.09 |
| Baseline Trail B Time,s, mean (SD) | 50.3 (15.3) | 55.9 (15.7) | 52.5 (12.9) | 0.35 |
DNA sequencing results from 6 participants did not have sufficient mitochondrial genome coverage to generate confident haplogroup assignments, and these participants were excluded from mitochondrial haplogroup-based analysis. Of the 40 participants for which mitochondrial haplotype was determined, Haplogroup H had the greatest representation (n=11, 27.5%) in this cohort (Table 2). Due to the limited number of participants from other haplogroups, data from females not of haplogroup H were combined into a non-H category (n=29, 72.5%).
Table 2.
Participants by treatment groups and mitochondrial haplogroups. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day.
| Placebo (n=14) |
PS50 (n=16) |
PS100 (n=10) |
Total (n=40) |
|
|---|---|---|---|---|
| A | 1 | 3 | 0 | 4 |
| B | 0 | 1 | 1 | 2 |
| C | 0 | 0 | 1 | 1 |
| D | 0 | 1 | 0 | 1 |
| H | 5 | 5 | 1 | 11 |
| K | 2 | 1 | 1 | 4 |
| L | 0 | 1 | 0 | 1 |
| M | 0 | 1 | 1 | 2 |
| T | 3 | 0 | 3 | 6 |
| U | 1 | 2 | 2 | 5 |
| V | 1 | 1 | 0 | 2 |
| W | 1 | 0 | 0 | 1 |
Thirty-two participants were APOE 3/3 carriers (67%) and 14 were APOE 3/4 carriers (33%), which is consistent with prevalence in the general population. There were no APOE 4/4 or APOE2 carriers in this analysis (Table 3).
Table 3.
Participants by treatment and APOE genotype. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day.
| Placebo | PS50 | PS100 | Total | |
|---|---|---|---|---|
| APOE 3/3 | 12 | 14 | 6 | 32 |
| APOE 3/4 | 4 | 4 | 6 | 14 |
Intention to Treat Analysis
We previously published the intention to treat analysis for this clinical trial1, including all participants who were randomized, dispensed study treatment, and had baseline and at least one postbaseline assessment. We reported no significant effect of PhytoSERM on 12-week change of either vasomotor composite score (hot flash frequency and Greene climacteric flushing items 19 and 20) or neuropsychological composite score (sum of standardized scores for multiple cognitive test, including verbal fluency, Rey Auditory Verbal Learning Test, Continuous Performance Test, Trial Making Test parts A and B, and Logical Memory Test) in comparison to the placebo group1. However, not all measurements included the composite scores have the same sensitivity to PhytoSERM treatment. Further, within the placebo and the PS100 group, 6 participants on each arm were involved in a nested crossover design, where they received either placebo or 100mg of daily PhytoSERM for the first 4 weeks, then crossed over to the other treatment for the remaining 8 weeks, and were assigned to the group of their second treatment. Thus, the intention to treat analysis could not accurately capture the treatment effect of PhytoSERM, and a retrospective analysis is necessary to dissect out potential PhytoSERM treatment effect, and identify measures sensitive to PhytoSERM treatment that should be included in future efficacy studies.
Effect of PhytoSERM on hot flash frequency
Daily average hot flash frequency at week 1 after randomization and initiation of double-blinded treatment was used as baseline for each participant because many participants had missing baseline diary entries and we wanted to capture more comparable entries. Change in hot flash frequency was calculated as the difference between week 12 and baseline hot flash frequency. No difference in baseline hot flash frequency was observed among the three treatment groups (Table 1), nor did baseline hot flash frequency significantly differ by different mitochondrial haplogroups or APOE genotypes.
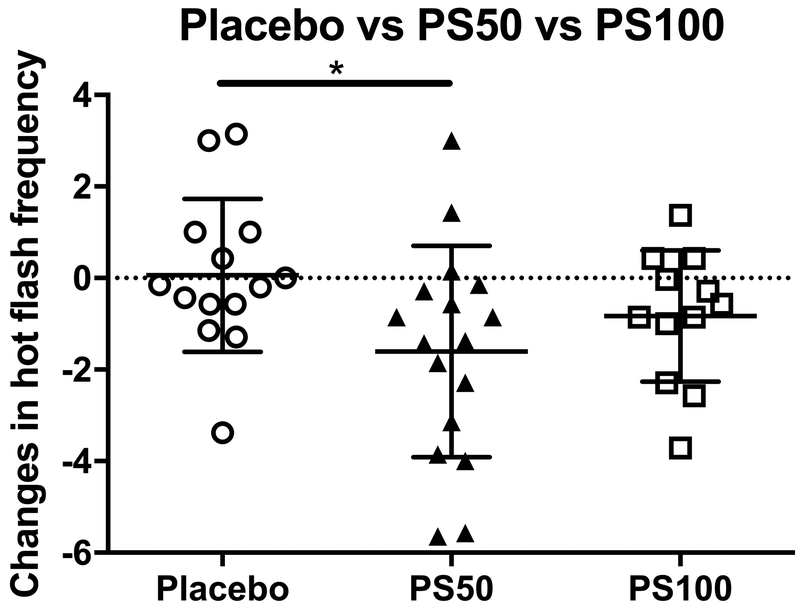
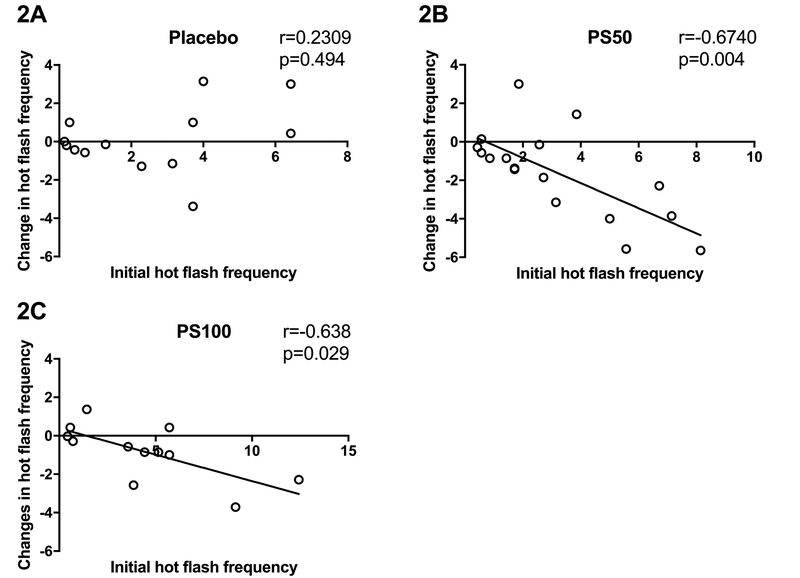
Compared to baseline, 12 weeks of PhytoSERM treatment significantly decreased hot flash frequency in the PS50 group, which was not observed in placebo or the PS100 group (Table 4). Among treatment groups, PS50 group had significantly greater 12-week reduction in hot flash frequency (−1.38, −0.17, (median PS50, median placebo), p=0.04, Figure 1) compared to the placebo group which was not observed in the PS100 group in comparison to the placebo (−0.71, −0.17, (median PS100, median placebo), p=0.49, Figure 1). Thus, 50mg of daily PhytoSERM appears to be the optimal dosage which is consistent with earlier preclinical analyses46–48. Further, baseline hot flash frequency was significantly positively correlated with PhytoSERM induced reduction in hot flash frequency in the PS50 group (r=−0.67, p=0.0038, Figure 2B) and the PS100 group (r=−0.64, p=0.03, Figure 2C), while no correlation was observed in the placebo group (r=−0.23, p=0.4, Figure 2A).
Table 4.
Within-group daily hot flash frequency 12-week change from baseline. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day.
| Placebo | PS50 | PS100 | |
|---|---|---|---|
| Mean | 0.06 | −1.61 | −0.83 |
| 95% CI | [−0.90, 1.03] | [−2.79, −0.42] | [−1.74, 0.08] |
| p value | 0.83 | 0.007 | 0.07 |
Figure 1.
Impact of different doses of PhytoSERM on hot flash frequencies. Participants in the PS50 group experienced significantly greater 12-week reduction in hot flash frequency in comparison to those in the placebo group. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day. * p<0.05.
Figure 2.
Initial hot flash frequency predicts therapeutic outcomes of PhytoSERM on hot flash frequency reduction in the PS50 group only. 2A, no correlation was observed in the placebo group; 2B and 2C, participants with higher initial hot flash frequency showed greater reduction in hot flash frequency in PS50 and PS100 respectively. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day.
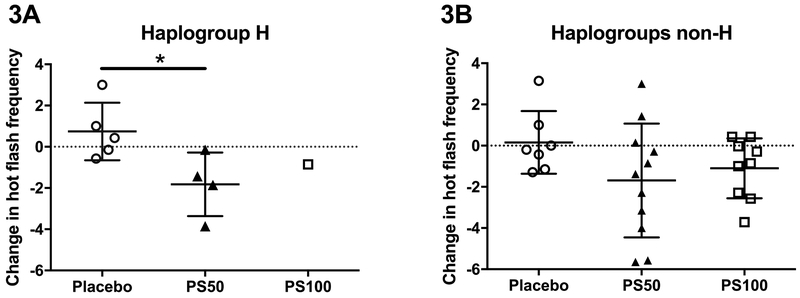
When stratified by mitochondrial haplogroup, those belonging to mitochondrial haplogroup H had significantly decreased hot flash frequency when treated with 50mg of PhytoSERM per day compared to the placebo group (−1.64, 0.43, (median PS50, median placebo), p=0.04, Figure 3A). Because only one haplogroup H participant was assigned to the PS100 group, no statistical analysis was conducted. Non-H participants on PS50 demonstrated comparable average reduction in hot flash frequency compared to haplogroup H participants, however effect was not statistically significant due to variation within the group (−1.38, −0.2, (median PS50, median placebo), p=0.15, Figure 3B).
Figure 3.
Change in hot flash frequency from week 1 to week 12 in participants when stratified by mitochondrial haplogroup. 3A, haplogroup H in the PS50 group had significantly greater reduction in hot flash frequency compared to those on placebo. 3B, no statistical significant therapeutic effect was observed in non-H participants. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day. * p<0.05.
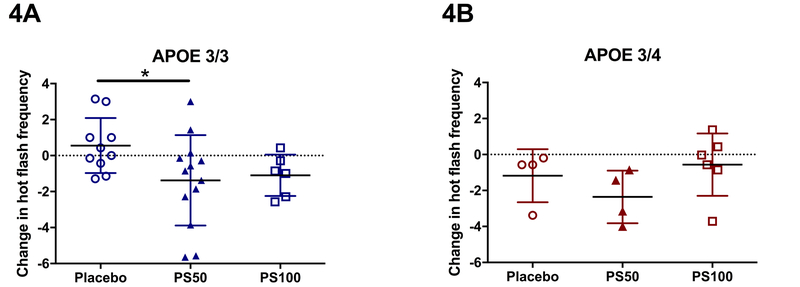
When stratified by APOE genotype, APOE4 non-carriers in the PS50 group had significantly greater reduction in hot flash frequency compared to those in the placebo group (−0.86, 0.21, (median PS50, median placebo), p=0.04, Figure 4A). A non-significant trend towards reduced hot flash frequency was observed in APOE4 carriers, likely due to limited sample size (−2.29, −0.57, (median PS50, median placebo), p=0.17, Figure 4B). Participants in PS100 did not experience significant improvement regardless of APOE genotype.
Figure 4.
Change in hot flash frequency stratified by APOE genotype. 4A, APOE3/3 participants on PS50 showed significant reduction in hot flash frequency. 4B, APOE3/4 participants on PS50 showed non-significant decline in hot flash frequency relative to the placebo group. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day. * p<0.05.
Effect of PhytoSERM on estrogen-dependent cognitive function
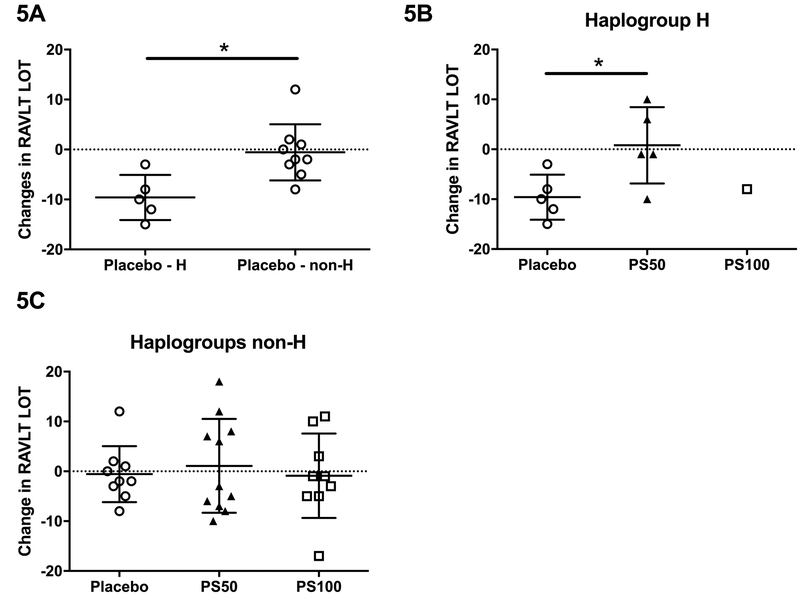
The following aspects of the audio verbal learning performance (RAVLT) were examined: immediate recall (total from trial 1), delayed recall (total after long delay), recognition, and learning over trials (LOT) (total from trials 1 through 5 minus 5 times of total from trial 1, as a measure of verbal learning ability), as previously described58. We observed that treatment with neither 50mg nor 100mg of PhytoSERM per day improved immediate recall, delayed recall, or recognition, nor did genetic variation (by mitochondrial haplotype H vs non-H, and by APOE4 genotype) modulate any of these outcomes (data not shown). Intriguingly, however, haplogroup H on placebo had significantly decreased LOT during the clinical study compared to non-H haplogroups (−10, −2, (median H, median non-H), p=0.007, Figure 5A), whereas 50mg of PhytoSERM per day successfully prevented the decline (−1, −10, (median PS50, median placebo), p=0.048, Figure 5B). No such preventative effect was observed in non-H haplogroups (−2, −3, −1, (median placebo, median PS50, median PS100), Figure 5C). Overall, PhytoSERM treatment did not result in significant improvement in index of executive function compared to the placebo, as measured by Trails making B. However, the PS50 group participants exhibited significantly enhanced Trails B performance compared to their own baseline, while no significant reduction in Trails B time was observed in either placebo or the PS100 group (Table 5). We also observed that Trails B, Index of executive function, was not modulated by mitochondrial genetic variances or APOE genotype (data not shown).
Figure 5.
Change in participants’ RAVLT Learning Over Trial score (verbal learning ability) stratified by mitochondrial haplogroup. 5A, haplogroup H participants on placebo displayed significantly more decline in verbal learning ability compared to their non-haplogroup H counterparts. 5B, in haplogroup H participants, treatment with 50mg of daily PhytoSERM preserved verbal learning ability in comparison to those on placebo. 5C, no difference was observed among three treatment groups in non-haplogroup H participants. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day. * P<0.05.
Table 5.
Within-group trial B time 12-week change from baseline. PS50, PhytoSERM 50gm/day; PS100, PhytoSERM 100mg/day.
| Placebo | PS50 | PS100 | |
|---|---|---|---|
| Mean (s) | −2.4 | −7.71 | −5.91 |
| 95% CI (s) | [−6.10, 1.3] | [−12.79, −2.63] | [−16.55, 4.73] |
| p value | 0.16 | 0.006 | 0.12 |
No effect of PhytoSERM was observed on verbal fluency, episodic memory or global cognition throughout the trial, with or without stratification by genotype groups.
Discussion
This retrospective analysis is based on a 12-week, Phase I clinical trial of the safety of PhytoSERM, which included measurement of menopause-associated vasomotor symptoms and cognitive function1, 2. Although the clinical trial was not powered for efficacy evaluation, we sought to identify indicators of efficacy to advance in a Phase II clinical trial of PhytoSERM. The goal of the current study was to determine the specificity of PhytoSERM action in reducing menopause-associated hot flash frequency while promoting estrogen-dependent cognitive function, and to identify potential responders to PhytoSERM treatment. To address this issue, we stratified participants based on two genetic factors: mitochondrial haplogroup and APOE genotype. These two factors were selected because both are demonstrated risk modifiers for late onset AD, with known effect on brain glucose metabolism and mitochondrial bioenergetics (reviewed by50). Further, estrogen promotes both mitochondrial function and bioenergetic respiratory capacity in the brain45, 59–61, whereas estrogen dysregulation can lead to decline in both glucose metabolism and mitochondrial respiration62, 63.
Outcomes of these exploratory analyses indicated that PhytoSERM reduced hot flash frequency in menopausal females, which was in agreement with our preclinical study using the rat surgical menopausal model47, and in accordance with literature reporting benefit of estrogen or phytoestrogens in reducing menopause-associated vasomotor symptoms64–67. Consistent with our preclinical translational analysis, 50mg, but not 100mg, of daily PhytoSERM was optimal for reducing hot flash frequency46–48. Correlational analysis suggested that efficacy of PhytoSERM is the most apparent in females with greater hot flash frequency. This outcome suggested that females experiencing higher hot flash frequencies are more responsive to estrogenic intervention and may serve as an indicator of the therapeutic window. One limitation of this analysis is the use of week 1 post-treatment as the baseline due to the lack of consistent pre-randomization hot flash frequency data among participants. Because we did not observe significant differences in week 1 hot flash frequency between placebo and treatment groups, albeit less ideal, it still constitutes a valid baseline for our 12-week change analysis.
When stratified by mitochondrial haplogroups, the therapeutic effect of 50mg of PhytoSERM on change in hot flash frequency was statistically significant in haplogroup H, the most common haplogroup among European descendants. While the therapeutic effect was not significant in non-H participants, the average 12-week reduction in hot flash frequency was comparable to haplogroup H. Given the limited sample size and variance observed in non-H participants, we cannot eliminate the possibility that there are other responding haplogroups, and that the effect of PhytoSERM may occur in other haplogroups that were under-represented in this study. The Phase II clinical trial of PhytoSERM could be designed to include sufficient sample size and hence greater mitochondrial haplogroup diversities to confirm the hypothesis.
When stratified by APOE genotype, APOE 3/3 participants on 50mg of daily PhytoSERM had significantly greater reduction in hot flash frequency compared to placebo. APOE 3/4 participants on 50mg of daily PhytoSERM displayed a trend towards decline in hot flash frequency, likely due to individual differences and small number of APOE 3/4 participants. However, the magnitude of change was similar between APOE4 carriers and non-carriers, and neither group showed reduction of hot flash frequency with 100mg of daily PhytoSERM.
PhytoSERM treatment was also associated with improved executive function (Trails B) and preserved verbal learning (RAVLT LOT). Participants on the PS50 group had significantly reduced Trails B time compared to their own baseline, although the 12-week change was not significant compared to that of the placebo group. This effect was independent of mitochondrial haplogroup and APOE genotype. More intriguing is the effect of PhytoSERM on verbal learning ability, as measured by the “Learning Over Trial” (LOT) parameter of the RAVLT. Haplogroup H participants on placebo had significantly lower LOT score compared to non-H counterparts, whereas treatment with 50mg of PhytoSERM for 12 weeks effectively prevented a decline in LOT (Figure 5). One concern over the statistical significance of these observations is limited sample size within each haplogroup and genotype. However, our observation was consistent with population studies showing haplogroup H has a higher risk for late onset AD50, 68–80. These observations were consistent with our previous study demonstrating that 9-months of PhytoSERM treatment promoted spatial working memory in both ovariectomized wildtype mice and an ovariectomized Alzheimer’s disease mouse model47, 48. The selective protective effect of PhytoSERM on specific cognitive functions is consistent with estrogen preferentially effected cognitive tasks of greater complexity, temporal demand and associative challenge45. While definite interpretation of PhytoSERM efficacy is limited by the small sample size, these data provide a reasonable rationale to extend analyses to a larger study set powered to assess statistical significance.
Again, this study is a retrospective analysis based on a Phase I safety and feasibility study. We are aware that the statistical power of this analysis was limited by the small sample size and multiple comparison. Future prospective, adequately statistically powered clinical studies are necessary to validate the observations made in this pilot study. While our analysis suggested that haplogroup H can particularly benefit from PhytoSERM treatment, given the limited sample size and within-group-variance in other genetic groups, we could not rule out the possibility of additional responder groups based on these genetic markers. Future studies powered to detect impact of mitochondrial haplogroup and APOE genotype are required to confirm this hypothesis. As such, we do not intend for this exploratory analysis to be a definitive pharmacogenetic study. Rather, outcomes on PhytoSERM treatment effect as well as genetic modification effect are intended to provide insights and reference dosage for a Phase II clinical study.
Conclusion
The purpose of the retrospective PhytoSERM responder identifying analysis was to determine parameters of efficacy on which to design a Phase II clinical trial. Results from this analysis demonstrated potential beneficial effect of PhytoSERM at a daily dosage of 50mg to reduce hot flash frequency and to preserve cognitive function, particularly verbal learning and cognitive flexibility. While the observations made in this retrospective analysis await confirmation in a prospective, larger Phase II clinical study, the data support further development of PhytoSERM as a therapy to ameliorate menopause-associated hot flash and sustain cognitive function. Furthermore, our data support a precision medicine approach for further development of PhytoSERM as a safe and effective alternative to hormone therapy for menopause associated symptoms.
Supplementary Material
Supplement table 1.
Supplement table 2.
Sources of funding:
This work was supported by NIA R01AG033288 and P50AG05142 to LSS, and P01AG026572 to RDB.
Footnotes
Conflicts of Interest / Financial disclosures: None reported.
References
- 1.Lon S Schneider, Liqin, Franke Adrian A., Chen Yu-Ling, Pawluczyk Sonia, Mack Wendy J., Brinton Roberta D.. Safety and feasibility of estrogen receptor-b targeted phytoSERM formulation for menopausal symptoms: phase 1b/2a randomized clinical trial. Menopause (New York, NY). 2019;26(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez G, Zhao L, Franke AA, et al. Pharmacokinetics and safety profile of single-dose administration of an estrogen receptor beta-selective phytoestrogenic (phytoSERM) formulation in perimenopausal and postmenopausal women. Menopause (New York, NY). 2018;25(2):191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erlik Y, Meldrum DR, Judd HL. Estrogen levels in postmenopausal women with hot flashes. Obstetrics and gynecology. 1982;59(4):403–7. [PubMed] [Google Scholar]
- 4.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278(16):1349–56. [PubMed] [Google Scholar]
- 5.Kim SA, Jung H. Prevention of Cognitive Impairment in the Midlife Women. Journal of Menopausal Medicine. 2015;21(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. American journal of epidemiology. 2000;152(5):463–73. [DOI] [PubMed] [Google Scholar]
- 7.Greendale GA, Derby CA, Maki PM. Perimenopause and Cognition. Obstetrics and gynecology clinics of North America. 2011;38(3):519–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosconi L Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. 2017;12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Santi S, de Leon MJ, Rusinek H, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiology of aging. 2001;22(4):529–39. [DOI] [PubMed] [Google Scholar]
- 10.Ishii K, Sasaki M, Kitagaki H, et al. Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1997;38(6):925–8. [PubMed] [Google Scholar]
- 11.Mosconi L, De Santi S, Li J, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiology of aging. 2008;29(5):676–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):284–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60(8):1374–7. [DOI] [PubMed] [Google Scholar]
- 15.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. American journal of epidemiology. 1994;140(3):256–61. [DOI] [PubMed] [Google Scholar]
- 16.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends in neurosciences. 2008;31(10):529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. The Journal of clinical endocrinology and metabolism. 2010;95(7 Suppl 1):s1–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of alzheimer disease in older women: The cache county study. JAMA. 2002;288(17):2123–9. [DOI] [PubMed] [Google Scholar]
- 19.Tang M-X, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. The Lancet. 1996;348(9025):429–32. [DOI] [PubMed] [Google Scholar]
- 20.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48(6):1517–21. [DOI] [PubMed] [Google Scholar]
- 21.Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Archives of internal medicine. 1996;156(19):2213–7. [PubMed] [Google Scholar]
- 22.Waring SC, Rocca WA, Petersen RC, O’Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology. 1999;52(5):965–70. [DOI] [PubMed] [Google Scholar]
- 23.Maki PM, Zonderman AB, Resnick SM. Enhanced Verbal Memory in Nondemented Elderly Women Receiving Hormone-Replacement Therapy. American Journal of Psychiatry. 2001;158(2):227–33. [DOI] [PubMed] [Google Scholar]
- 24.Shaywitz SE, Naftolin F, Zelterman D, et al. Better oral reading and short-term memory in midlife, postmenopausal women taking estrogen. Menopause (New York, NY). 2003;10(5):420–6. [DOI] [PubMed] [Google Scholar]
- 25.Maki PM. Hormone therapy and cognitive function: Is there a critical period for benefit? Neuroscience. 2006;138(3):1027–30. [DOI] [PubMed] [Google Scholar]
- 26.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of Estrogen Replacement Therapy on PET Cerebral Blood Flow and Neuropsychological Performance. Hormones and Behavior. 1998;34(2):171–82. [DOI] [PubMed] [Google Scholar]
- 27.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297(13):1465–77. [DOI] [PubMed] [Google Scholar]
- 28.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288(3):321–33. [DOI] [PubMed] [Google Scholar]
- 29.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. Jama. 2011;305(13):1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chlebowski RT, Kuller LH, Prentice RL, et al. Breast cancer after use of estrogen plus progestin in postmenopausal women. N Engl J Med. 2009;360(6):573–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chlebowski RT, Anderson G, Manson JE, et al. Estrogen alone in postmenopausal women and breast cancer detection by means of mammography and breast biopsy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(16):2690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. Jama. 2010;304(15):1684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Martin M, Salazar V, Castillo C, et al. Estradiol and soy extract increase the production of new cells in the dentate gyrus of old rats. Experimental gerontology. 2005;40(5):450–3. [DOI] [PubMed] [Google Scholar]
- 34.Zhao L, Chen Q, Diaz Brinton R. Neuroprotective and neurotrophic efficacy of phytoestrogens in cultured hippocampal neurons. Experimental biology and medicine (Maywood, NJ). 2002;227(7):509–19. [DOI] [PubMed] [Google Scholar]
- 35.File SE, Jarrett N, Fluck E, Duffy R, Casey K, Wiseman H. Eating soya improves human memory. Psychopharmacology. 2001;157(4):430–6. [DOI] [PubMed] [Google Scholar]
- 36.File SE, Hartley DE, Elsabagh S, Duffy R, Wiseman H. Cognitive improvement after 6 weeks of soy supplements in postmenopausal women is limited to frontal lobe function. Menopause (New York, NY). 2005;12(2):193–201. [DOI] [PubMed] [Google Scholar]
- 37.Duffy R, Wiseman H, File SE. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacology, biochemistry, and behavior. 2003;75(3):721–9. [DOI] [PubMed] [Google Scholar]
- 38.Kritz-Silverstein D, Von Muhlen D, Barrett-Connor E, Bressel MA. Isoflavones and cognitive function in older women: the SOy and Postmenopausal Health In Aging (SOPHIA) Study. Menopause (New York, NY). 2003;10(3):196–202. [DOI] [PubMed] [Google Scholar]
- 39.Casini ML, Marelli G, Papaleo E, Ferrari A, D’Ambrosio F, Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: a randomized, double-blind, crossover, placebo-controlled study. Fertility and sterility. 2006;85(4):972–8. [DOI] [PubMed] [Google Scholar]
- 40.Zhao L, Brinton RD. WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert review of neurotherapeutics. 2007;7(11):1549–64. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010(1–2):22–34. [DOI] [PubMed] [Google Scholar]
- 42.Zhao L, Brinton RD. Estrogen receptor alpha and beta differentially regulate intracellular Ca(2+) dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. [DOI] [PubMed] [Google Scholar]
- 43.Zhao L, Brinton RD. Estrogen receptor β as a therapeutic target for promoting neurogenesis and preventing neurodegeneration. Drug Development Research. 2005;66(2):103–17. [Google Scholar]
- 44.Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):3996–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends in pharmacological sciences. 2009;30(4):212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. 2009;150(2):770–83. [DOI] [PubMed] [Google Scholar]
- 47.Zhao L, Mao Z, Schneider LS, Brinton RD. Estrogen receptor beta-selective phytoestrogenic formulation prevents physical and neurological changes in a preclinical model of human menopause. Menopause (New York, NY). 2011;18(10):1131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao L, Mao Z, Chen S, Schneider LS, Brinton RD. Early intervention with an estrogen receptor beta-selective phytoestrogenic formulation prolongs survival, improves spatial recognition memory, and slows progression of amyloid pathology in a female mouse model of Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2013;37(2):403–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao J, Zhao L, Mao Z, et al. Potentiation of brain mitochondrial function by S-equol and R/S-equol estrogen receptor beta-selective phytoSERM treatments. Brain Res. 2013;1514:128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Brinton RD. Triad of Risk for Late Onset Alzheimer’s: Mitochondrial Haplotype, APOE Genotype and Chromosomal Sex . Frontiers in aging neuroscience. 2016;8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harold Goodglass EK. The Assessment of Aphasia and Related Disorders. Philadelphia: Le & Febiger; 1972. [Google Scholar]
- 52.Rey A L’examen clinique en psychologie. Paris: Presses universitaires de France; 1964. [Google Scholar]
- 53.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and motor skills. 1958;8(3):271–6. [Google Scholar]
- 54.Wechsler D Instruction Manual for the Wechsler Memory Scale Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- 55.Wang YK, Yao J, Han X, et al. Investigation of mtDNA control region sequences in a Tibetan population sample from China. Mitochondrial DNA Part A, DNA mapping, sequencing, and analysis. 2016;27(3):2215–20. [DOI] [PubMed] [Google Scholar]
- 56.Aissani B, Shrestha S, Wiener HW, Tang J, Kaslow RA, Wilson CM. Mitochondrial DNA variation and virologic and immunological HIV outcomes in African Americans. AIDS (London, England). 2014;28(13):1871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.William Rebeck G, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: Allelic variation and receptor interactions. Neuron. 1993;11(4):575–80. [DOI] [PubMed] [Google Scholar]
- 58.Teruya LC, Ortiz KZ, Minett TS. Performance of normal adults on Rey Auditory Learning Test: a pilot study. Arquivos de neuro-psiquiatria. 2009;67(2A):224–8. [DOI] [PubMed] [Google Scholar]
- 59.Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35(1):8–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao J, Brinton RD. Estrogen regulation of mitochondrial bioenergetics: implications for prevention of Alzheimer’s disease. Advances in pharmacology (San Diego, Calif). 2012;64:327–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao J, Chen S, Cadenas E, Brinton RD. Estrogen protection against mitochondrial toxin-induced cell death in hippocampal neurons: Antagonism by progesterone. Brain Research. [Article]. 2011;1379:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao J, Irwin R, Chen S, Hamilton R, Cadenas E, Brinton RD. Ovarian hormone loss induces bioenergetic deficits and mitochondrial beta-amyloid. Neurobiology of aging. 2012;33(8):1507–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bioenergetics and shift to ketogenic profile in brain during reproductive senescence. Biochimica et biophysica acta. 2010;1800(10):1121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burke GL, Legault C, Anthony M, et al. Soy protein and isoflavone effects on vasomotor symptoms in peri- and postmenopausal women: the Soy Estrogen Alternative Study. Menopause (New York, NY). 2003;10(2):147–53. [DOI] [PubMed] [Google Scholar]
- 65.Teekachunhatean S, Mattawanon N, Khunamornpong S. Short-Term Isoflavone Intervention in the Treatment of Severe Vasomotor Symptoms after Surgical Menopause: A Case Report and Literature Review. Case reports in obstetrics and gynecology. 2015;2015:962740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobs A, Wegewitz U, Sommerfeld C, Grossklaus R, Lampen A. Efficacy of isoflavones in relieving vasomotor menopausal symptoms - A systematic review. Molecular nutrition & food research. 2009;53(9):1084–97. [DOI] [PubMed] [Google Scholar]
- 67.Clarkson TB, Utian WH, Barnes S, et al. The role of soy isoflavones in menopausal health: report of The North American Menopause Society/Wulf H. Utian Translational Science Symposium in Chicago, IL (October 2010). Menopause (New York, NY). 2011;18(7):732–53. [DOI] [PubMed] [Google Scholar]
- 68.Chinnery PF, Taylor GA, Howell N, et al. Mitochondrial DNA haplogroups and susceptibility to AD and dementia with Lewy bodies. Neurology. 2000;55(2):302–4. [DOI] [PubMed] [Google Scholar]
- 69.Coto E, Gomez J, Alonso B, et al. Late-onset Alzheimer’s disease is associated with mitochondrial DNA 7028C/haplogroup H and D310 poly-C tract heteroplasmy. Neurogenetics. 2011;12(4):345–6. [DOI] [PubMed] [Google Scholar]
- 70.Edland SD, Tobe VO, Rieder MJ, et al. Mitochondrial genetic variants and Alzheimer disease: a case-control study of the T4336C and G5460A variants. Alzheimer disease and associated disorders. 2002;16(1):1–7. [DOI] [PubMed] [Google Scholar]
- 71.Elson J, Herrnstadt C, Preston G, et al. Does the mitochondrial genome play a role in the etiology of Alzheimer’s disease? Human genetics. 2006;119(3):241–54. [DOI] [PubMed] [Google Scholar]
- 72.Fachal L, Mosquera-Miguel A, Pastor P, et al. No evidence of association between common European mitochondrial DNA variants in Alzheimer, Parkinson, and migraine in the Spanish population. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2015;168B(1):54–65. [DOI] [PubMed] [Google Scholar]
- 73.Fesahat F, Houshmand M, Panahi MS, Gharagozli K, Mirzajani F. Do haplogroups H and U act to increase the penetrance of Alzheimer’s disease? Cellular and molecular neurobiology. [Comparative Study]. 2007;27(3):329–34. [DOI] [PubMed] [Google Scholar]
- 74.Mancuso M, Nardini M, Micheli D, et al. Lack of association between mtDNA haplogroups and Alzheimer’s disease in Tuscany. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2007;28(3):142–7. [DOI] [PubMed] [Google Scholar]
- 75.Maruszak A, Canter JA, Styczynska M, Zekanowski C, Barcikowska M. Mitochondrial haplogroup H and Alzheimer’s disease--is there a connection? Neurobiology of aging. 2009;30(11):1749–55. [DOI] [PubMed] [Google Scholar]
- 76.Maruszak A, Safranow K, Branicki W, et al. The impact of mitochondrial and nuclear DNA variants on late-onset Alzheimer’s disease risk. Journal of Alzheimer’s disease : JAD. [Research Support, Non-U.S. Gov’t]. 2011;27(1):197–210. [DOI] [PubMed] [Google Scholar]
- 77.Ridge PG, Maxwell TJ, Corcoran CD, et al. Mitochondrial Genomic Analysis of Late Onset Alzheimer’s Disease Reveals Protective Haplogroups H6A1A/H6A1B: The Cache County Study on Memory in Aging. PloS one. 2012;7(9):e45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santoro A, Balbi V, Balducci E, et al. Evidence for sub-haplogroup h5 of mitochondrial DNA as a risk factor for late onset Alzheimer’s disease. PloS one. 2010;5(8):e12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Walt JM, Dementieva YA, Martin ER, et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neuroscience letters. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. 2004;365(1):28–32. [DOI] [PubMed] [Google Scholar]
- 80.van der Walt JM, Scott WK, Slifer S, et al. Maternal lineages and Alzheimer disease risk in the Old Order Amish. Human genetics. 2005;118(1):115–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement table 1.
Supplement table 2.