Abstract
Background
Therapeutic ultrasound may be offered to people experiencing mild to moderate symptoms of carpal tunnel syndrome (CTS). The effectiveness and duration of benefit of this non‐surgical intervention remain unclear.
Objectives
To review the effects of therapeutic ultrasound compared with no treatment, placebo or another non‐surgical intervention in people with CTS.
Search methods
On 27 November 2012, we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL (2012, Issue 11 in The Cochrane Library), MEDLINE (January 1966 to November 2012), EMBASE (January 1980 to November 2012), CINAHL Plus (January 1937 to November 2012), and AMED (January 1985 to November 2012).
Selection criteria
Randomised controlled trials (RCTs) comparing any regimen of therapeutic ultrasound with no treatment, a placebo or another non‐surgical intervention in people with CTS.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted data and assessed the risk of bias in the included studies. We calculated risk ratio (RR) and mean difference (MD) with 95% confidence intervals (CIs) for primary and secondary outcomes. We pooled results of clinically homogenous trials in a meta‐analysis using a random‐effects model, where possible, to provide estimates of the effect.
Main results
We included 11 studies including 414 participants in the review. Two trials compared therapeutic ultrasound with placebo, two compared one ultrasound regimen with another, two compared ultrasound with another non‐surgical intervention, and six compared ultrasound as part of a multi‐component intervention with another non‐surgical intervention (for example, exercises and splint). The risk of bias was low in some studies and unclear or high in other studies, with only two reporting that the allocation sequence was concealed and six reporting that participants were blinded. Overall, there is insufficient evidence that one therapeutic ultrasound regimen is more efficacious than another. Only two studies reported the primary outcome of interest, short‐term overall improvement (any measure in which patients indicate the intensity of their complaints compared with baseline, for example, global rating of improvement, satisfaction with treatment, within three months post‐treatment). One low quality trial with 68 participants found that when compared with placebo, therapeutic ultrasound may increase the chance of experiencing short‐term overall improvement at the end of seven weeks treatment (RR 2.36; 95% CI 1.40 to 3.98), although losses to follow‐up and failure to adjust for the correlation between wrists in participants with bilateral CTS in this study suggest that this data should be interpreted with caution. Another low quality trial with 60 participants found that at three months post‐treatment therapeutic ultrasound plus splint increased the chance of short‐term overall improvement (patient satisfaction) when compared with splint alone (RR 3.02; 95% CI 1.36 to 6.72), but decreased the chance of short‐term overall improvement when compared with low‐level laser therapy plus splint (RR 0.87; 95% CI 0.57 to 1.33), though participants were not blinded to treatment, it was unclear if the random allocation sequence was adequately concealed, and there was a potential unit of analysis error. Differences between groups receiving different frequencies and intensities of ultrasound, and between ultrasound as part of a multi‐component intervention versus other non‐surgical interventions, were generally small and not statistically significant for symptoms, function, and neurophysiologic parameters. No studies reported any adverse effects of therapeutic ultrasound, but this outcome was only measured in three studies. More adverse effects data are required before any firm conclusions on the safety of therapeutic ultrasound can be made.
Authors' conclusions
There is only poor quality evidence from very limited data to suggest that therapeutic ultrasound may be more effective than placebo for either short‐ or long‐term symptom improvement in people with CTS. There is insufficient evidence to support the greater benefit of one type of therapeutic ultrasound regimen over another or to support the use of therapeutic ultrasound as a treatment with greater efficacy compared to other non‐surgical interventions for CTS, such as splinting, exercises, and oral drugs. More methodologically rigorous studies are needed to determine the effectiveness and safety of therapeutic ultrasound for CTS.
Keywords: Humans, Carpal Tunnel Syndrome, Carpal Tunnel Syndrome/therapy, Combined Modality Therapy, Combined Modality Therapy/methods, Randomized Controlled Trials as Topic, Randomized Controlled Trials as Topic/standards, Time Factors, Treatment Outcome, Ultrasonic Therapy, Ultrasonic Therapy/methods
Plain language summary
Therapeutic ultrasound for carpal tunnel syndrome
Carpal tunnel syndrome is a condition where one of two main nerves in the wrist is compressed, which can lead to pain in the hand, wrist and sometimes forearm, and numbness and tingling in the thumb, index and long finger. In advanced cases some of the muscles of the hand can become weak. Carpal tunnel syndrome is more common in women and older age groups. Many people undergo surgery to treat this condition, though sometimes other treatments, such as therapeutic ultrasound, are offered. Therapeutic ultrasound involves applying a round‐headed instrument to the skin of the painful area, to deliver sound waves that are absorbed by the underlying tissues, to help relieve pain and lessen disability. We searched for study reports and found 11 randomised controlled trials including 443 participants overall that assessed the safety and benefit of therapeutic ultrasound for people with carpal tunnel syndrome. The risk of bias of studies was low in some studies and unclear or high in others. There is only poor quality evidence from very limited data to suggest that therapeutic ultrasound may be more effective than placebo for either short‐ or long‐term symptom improvement in people with carpal tunnel syndrome. There is insufficient evidence to support the greater benefit of one type of therapeutic ultrasound regimen over another or to support the use of therapeutic ultrasound as a treatment with greater efficacy compared with other non‐surgical interventions for carpal tunnel syndrome, such as splinting, exercises, and oral drugs. Few studies measured adverse effects to therapeutic ultrasound. More research is needed to find out how effective and safe therapeutic ultrasound is for people with carpal tunnel syndrome, particularly in the long term.
Summary of findings
Summary of findings for the main comparison. Therapeutic ultrasound compared with placebo for carpal tunnel syndrome (CTS).
| Therapeutic ultrasound compared with placebo for carpal tunnel syndrome | ||||||
| Patient or population: patients with CTS Settings: outpatient clinic of university department of physical medicine and rehabilitation, Vienna, Austria Intervention: therapeutic ultrasound Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Therapeutic ultrasound | |||||
| Short‐term overall improvement (three months or less) | Study population | RR 2.36 (1.4 to 3.98) | 68 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| 324 per 10001 | 765 per 1000 (454 to 1000) | |||||
| Adverse effects | See comment | See comment | Not estimable | 68 (1 study) | ⊕⊕⊝⊝ low2,3 | No adverse effects were reported in the intervention or control groups |
|
Short‐term improvement in pain and/or paraesthesia (after seven weeks of treatment) Scale from: zero to 10 |
The mean improvement in pain and/or paraesthesia (after seven weeks of treatment) in the control groups was 2.68 | The mean improvement in pain and/or paraesthesia (after seven weeks of treatment) in the intervention groups was 0.99 lower (1.77 to 0.21 lower) | 68 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| Short‐term improvement in hand grip strength (change from baseline to seven weeks) | The mean improvement in hand grip strength (change from baseline to seven weeks) in the control groups was ‐0.09 kilograms | The mean improvement in hand grip strength (change from baseline to seven weeks) in the intervention groups was 3.96 higher (1.31 to 6.61 higher) | 90 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| Long‐term improvement in CTS symptoms (number of participants with complete remission of subjective symptoms) (six months follow‐up) | Study population | RR 3.67 (1.74 to 7.74) | 60 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| 200 per 10001 | 734 per 1000 (348 to 1000) | |||||
|
Long‐term improvement in pain and/or paraesthesia (six months follow‐up) Scale from: zero to 10 |
The mean improvement in pain and/or paraesthesia (six months follow‐up) in the control group was 2.92 | The mean improvement in pain and/or paraesthesia (six months follow‐up) in the intervention groups was 1.86 lower (2.67 to 1.05 lower) | 60 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| Long‐term improvement in hand grip strength (six months follow‐up) | The mean improvement in hand grip strength (six months follow‐up) in the control groups was 18.1 kilograms | The mean improvement in hand grip strength (six months follow‐up) in the intervention groups was 4.16 higher (0.88 lower to 9.2 higher) | 60 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Assumed risk is based on the risk in the control group in the one study comparing therapeutic ultrasound to placebo (Ebenbichler 1998).
2 Unit of analysis error committed. 3 Reasons for loss‐to‐follow‐up not reported; not clear if participants were inappropriately excluded from the analyses.
Background
Description of the condition
Carpal tunnel syndrome (CTS) is a neuromuscular condition in which the median nerve at the level of the wrist undergoes irritation which is often attributed to increased pressure within the carpal tunnel (Keith 2009; Kerwin 1996). The most commonly reported symptoms of CTS include pain in the wrist and hand which can radiate to the arm (Rempel 1998) and paraesthesiae (numbness) in the thumb, index, middle and radial half of the ring finger (Szabo 1994). In advanced stages of the condition, thenar muscle weakness can occur (Szabo 1994).
Results of a Swedish study suggest that the prevalence of CTS in the general population is 3.8% for clinically diagnosed cases and 2.7% for electrophysiologically confirmed cases (Atroshi 1999). Recent evidence indicates that between 1981 to1985 the adjusted annual incidence of CTS was 258 per 100,000 person‐years, compared with 424 per 100,000 person‐years between 2000 to 2005 in Minnesota, USA, though it is not clear whether this apparent increase in incidence is due to increased diagnostic practice and awareness of CTS (Gelfman 2009). Age and gender are associated with the incidence of CTS. People aged less than 25 years accounted for only 2.4% of patients presenting to Australian general practices between 2000 and 2009 with the condition, compared with people aged 45 to 64 years who accounted for 45.5% of these cases (Charles 2009). As for gender, 67% of CTS encounters at Australian general practices were attributable to females (Charles 2009). Females in their fourth and fifth decades have been found to suffer CTS four times more commonly than males (Atroshi 1999). An association between obesity and an increased incidence of CTS has also been identified (Atroshi 1999; Bland 2005; Stallings 1997; Werner 1994).
Description of the intervention
CTS can be treated using surgery or non‐surgical interventions, or a combination of both (for example carpal tunnel release followed by rehabilitation exercises). Surgical treatment is usually offered to individuals who have persistent CTS symptoms, severe sensory disturbance or thenar motor weakness. By contrast, non‐surgical treatments are offered to those who experience intermittent symptoms of mild to moderate CTS, and sometimes temporarily to those awaiting carpal tunnel release. Surgical treatment options for patients with CTS have been examined in other Cochrane reviews: surgical treatment options for CTS (Scholten 2007), and the effect of surgical versus non‐surgical treatment (Verdugo 2008).
Many different non‐surgical options for the treatment of CTS exist, such as therapeutic ultrasound, splinting, exercises or mobilisation, ergonomic modification (equipment or positioning), oral medication, vitamins and complementary therapies. Therapeutic ultrasound is a physical therapy which involves application of a round‐headed instrument to the skin of the painful area to deliver sound waves that are absorbed by the underlying connective tissue, such as ligaments and tendons (Watson 2008) The intervention can vary in its intensity and frequency of sound waves, and the duration of treatment can range from a few days to months. Ultrasound can be administered by a range of trained health professionals (for example physiotherapists and chiropractors). Therapeutic ultrasound is also used to treat a number of musculoskeletal conditions, such as osteoarthritis (Rutjes 2010) and acute ankle sprain (Van den Bekerom 2011)
How the intervention might work
Early experimental studies suggest that therapeutic ultrasound can have an anti‐inflammatory and tissue stimulating effect, by enhancing blood flow, increasing membrane permeability, and altering connective tissue extensibility and nerve conduction, due to its thermal effect (Binder 1985; Hong 1988; Lehmann 1974). However, Yildiz 2011 highlights other research which suggests that ultrasound does not have an anti‐inflammatory effect but rather accelerates the process of formation and resolution of pressure in the carpal tunnel canal (Young 2002). Despite these alternative theories, therapeutic ultrasound has not always been associated with a beneficial effect in clinical settings, so the underlying mechanism of action remains unclear.
Why it is important to do this review
Following the publication of the original version of this review (Page 2012a), which was based on searches conducted up to February 2011, the evidence base for all non‐surgical interventions for CTS has grown. Given the personal and financial impact of CTS, there is a need to synthesise the most up‐to‐date evidence on the efficacy of therapeutic ultrasound for the treatment of CTS.
Objectives
The objective of this review was to compare the efficacy of therapeutic ultrasound for carpal tunnel syndrome (CTS) with no treatment, placebo or another non‐surgical treatment for improving clinical outcome.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished studies using or attempting to use a randomised methodology were eligible for inclusion. We included studies comparing therapeutic ultrasound with no treatment, placebo, or other non‐surgical treatments . We excluded studies comparing therapeutic ultrasound to surgical treatment, as these studies are the focus of another Cochrane systematic review (Verdugo 2008). There were no language restrictions for the inclusion of studies.
Types of participants
All participants with a diagnosis of CTS, as defined by the authors of each study. Participants who had previous surgery for CTS were excluded.
Types of interventions
All therapeutic ultrasound interventions (that is of any frequency, intensity, and duration). Comparison interventions included no treatment, placebo, or other non‐surgical interventions; surgical interventions were excluded as comparisons. Trials where therapeutic ultrasound was used as an adjunct to another treatment were included only if the comparison provided information on the additional effect of the therapeutic ultrasound intervention.
Types of outcome measures
We modified the outcomes reported in this review from the original review (O'Connor 2003) to be consistent with other Cochrane reviews on carpal tunnel syndrome (Marshall 2007; Scholten 2007; Verdugo 2008).
Primary outcomes
Short‐term overall improvement (any measure in which patients indicate the intensity of their complaints compared with baseline, for example global rating of improvement, satisfaction with treatment) (dichotomous outcome; three months or less)
Secondary outcomes
Adverse effects
Short‐term improvement in CTS symptoms (for example, pain, paraesthesia, nocturnal paraesthesia) (three months or less).
Short‐term improvement in functional ability or health‐related quality of life (three months or less).
Short‐term improvement in neurophysiologic parameters (three months or less).
Long‐term improvement in CTS symptoms (greater than three months).
Long‐term improvement in functional ability or health‐related quality of life (greater than three months).
Search methods for identification of studies
Electronic searches
On 27 November, we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL (2012 , Issue 11 in The Cochrane Library), MEDLINE (January 1966 to November 2012), EMBASE (January 1980 to November 2012), CINAHL Plus (January 1937 to November 2012), and AMED (January 1985 to November 2012).
The detailed search strategies are in the appendices: CENTRAL, Appendix 1, MEDLINE Appendix 2, EMBASE Appendix 3, CINAHL Plus Appendix 4 and AMED Appendix 5.
Searching other resources
We searched protocols of trials on the clinical trials register that is maintained by the US National Institute of Health at http://clinicaltrials.gov, and searched protocols of trials published after July 1st 2005 using the Clinical Trial Register at the International Clinical Trials Registry Platform of the World Health Organisation (http://www.who.int/ictrp/en/). We also reviewed the reference lists of randomised or quasi‐randomised trials identified from the electronic searches.
Data collection and analysis
The review authors followed the recommended strategies for data collection and analysis as documented in Chapter 7 and 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
At least two review authors independently selected trials for possible inclusion against a predetermined checklist of inclusion criteria (see Criteria for considering studies for this review). We screened titles and abstracts and initially categorised studies into the following groups.
Possibly relevant ‐ studies that met the inclusion criteria and studies from which it was not possible to determine whether they met the criteria either from their title or abstract.
Excluded ‐ those clearly not meeting the inclusion criteria.
If a title, or abstract, appeared to meet the eligibility criteria for inclusion of the review, or we could not tell, we obtained a full text version of the article and two review authors independently assessed it in order to determine whether it met the inclusion criteria. The review authors resolved discrepancies through discussion.
Data extraction and management
Two authors independently extracted data using a standard data extraction form developed for this review. The authors resolved any discrepancies through discussion until consensus was reached. We pilot tested the data extraction form and modified it accordingly before use. In addition to items for assessing risk of bias and study results, we also recorded the following study characteristics:
participant details, including demographic data and inclusion/exclusion criteria;
types of interventions used in the intervention and comparison groups;
outcomes reported, including the tools and timing for outcome measures.
One author compiled all comparisons and entered outcome data into Review Manager 5.1. At least one author cross‐checked data. For trials where the required data were not reported, one author requested further information. When unsuccessful, we included the study in the review and fully described it, but did not include it in any meta‐analysis. An entry of this process was made in the notes section of the Characteristics of included studies tables.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in included studies using The Cochrane Collaboration's tool for assessing risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). The following items were assessed for risk of bias based on information extracted from reports of the included studies:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data (defined separately for data measured at 12 weeks or less, and after 12 weeks);
selective reporting;
other sources of bias (for example inappropriate unit of analysis).
Each item was rated as being at 'Low risk', 'Unclear risk' or 'High risk' of bias. We resolved any discrepancies through discussion.
Measures of treatment effect
We used the Cochrane statistical software Review Manager 5.1 to perform data analysis. We expressed results as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes and (unstandardised) mean differences (MD) with 95% CI for continuous outcomes if the same measurement tool was used to measure the same outcome across separate studies. Alternatively, we summarised continuous outcomes using the standardised mean difference (SMD) when studies measured the same outcome but employed different measurement tools.
Unit of analysis issues
We sought information about the unit of randomisation used (that is, wrists or participants) where participants with bilateral CTS receive the same intervention for both wrists). In studies that randomised wrists, we sought information about whether wrists of each participant were allocated to different treatments, or whether there was no constraint that the two wrists be allocated to different treatments. Given that results for different wrists in participants with bilateral CTS are unlikely to be independent, we assessed how the investigators of studies which included participants with bilateral CTS took account of this dependence in their analyses (for example, use of paired or matched analyses, generalised estimating equations). If this information was not reported, we contacted trialists for clarification. We also requested individual wrist outcome data from trialists to re‐analyse the data. If we were unable to obtain individual wrist outcome data, we had planned to estimate parameters (such as an intra‐class correlation coefficient) from studies that reported sufficient information to calculate this, and to use these estimates to adjust the results in other studies, following the advice provided in sections 16.3 and 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). If unable to adjust the outcome data, we included the data as reported by the trialists, and commented on the validity of such analyses.
Dealing with missing data
We sought relevant missing information from the authors of included studies about study design, outcome data, or attrition rates such as drop‐outs, losses to follow‐up and withdrawn study participants, where possible.
Assessment of heterogeneity
We assessed clinical heterogeneity by determining whether the characteristics of participants, interventions, outcome measures and timing of outcome measurement were similar across studies. We assessed statistical heterogeneity using the Chi2 statistic and the I2 test (Higgins 2002). We interpreted the I2 statistic using the following as an approximate guide:
0% to 40% might not be important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% may represent considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
To assess publication bias, we would have generated funnel plots if at least 10 studies examining the same treatment comparison were included in the review (Sterne 2011). To assess outcome reporting bias, we planned to compare the outcomes specified in trial protocols with the outcomes reported in the corresponding study publications; if trial protocols were unavailable, we compared the outcomes reported in the methods and results sections of the study publications (Dwan 2011).
Data synthesis
We pooled results of studies with similar characteristics (participants, interventions, outcome measures and timing of outcome measurement) to provide estimates of the efficacy of therapeutic ultrasound for CTS. Where we could not combine data, we presented a narrative synthesis of results. We meta‐analysed pooled results using either a fixed‐effect or random‐effects model (depending on the level of clinical and methodological heterogeneity). We set statistical significance at P < 0.05 for primary and secondary outcome measures.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses according to severity of CTS symptoms and sex, since these factors may cause variations in outcomes. We defined subgroups as follows:
severity of CTS symptoms: early (E), intermediate (I) and advanced (A) symptoms (Szabo 1992);
sex: male, female.
Sensitivity analysis
We conducted sensitivity analyses for each item in the 'Risk of bias' table by excluding studies judged as 'High risk of bias'. We also conducted sensitivity analyses using the following filter.
Quality of diagnostic criteria: high (A), moderate (B) and low (C) quality (Rempel 1998).
Results
Description of studies
Results of the search
The search conducted up until 27 November 2012 identified a total of 221 records. Table 1 reports the number of hits retrieved by each search strategy. The number of records after removal of duplicates was 128. From these, we retrieved 26 full text papers for further examination. After screening the full text of the selected papers for eligibility, 11 studies (Bakhtiary 2004; Baysal 2006; Bilgici 2010; Dincer 2009; Duymaz 2012; Ebenbichler 1998; Ekim 2008; Koyuncu 1995; Oztas 1998; Piravej 2004; Yildiz 2011) fulfilled the inclusion criteria. The only new study included in this review update was Duymaz 2012. A flow diagram of the study selection process is presented in Figure 1. Searches of clinical trials registries resulted in the identification of one ongoing placebo‐controlled RCT of therapeutic ultrasound (NCT01590745).
1.

Study flow diagram.
Table 1
| Database | Period searched | Date searched | Number of hits |
| Cochrane Neuromuscular Disease Group Specialized Register | to 27 November 2012 | 27 November 2012 | 20 |
| CENTRAL | to Issue 11, 2012 | 27 November 2012 | 23 |
| MEDLINE | January 1966 to November 2012 | 27 November 2012 | 55 |
| EMBASE | January 1980 to November 2012 | 27 November 2012 | 70 |
| CINAHL Plus | January 1937 to November 2012 | 27 November 2012 | 40 |
| AMED | January 1985 to November 2012 | 27 November 2012 | 13 |
Included studies
Eleven studies allocated adults with CTS to a therapeutic ultrasound regimen (delivered alone or with another non‐surgical intervention) or to placebo ("sham" ultrasound) or another non‐surgical intervention. A total of 414 participants with 664 CTS‐affected wrists were included. The sex of participants was unclear in the studies by Bakhtiary 2004 and Ebenbichler 1998; in the remaining studies there were 287 females and 29 males included. The ultrasound interventions varied in intensity and frequency, and duration of treatment across the studies.
Bakhtiary 2004 compared the effects of 15 sessions performed once a day, five times a week for three weeks of pulsed ultrasound treatment administered for 15 minutes per session to the area over the carpal tunnel at a frequency of 1 MHz and an intensity of 1.0 W/cm2, compared with low‐level laser therapy, on the outcomes pain, pinch strength, hand grip strength and neurophysiologic parameters in 50 participants with 90 CTS‐affected wrists.
Baysal 2006 compared three different treatment groups in 36 participants with 72 affected wrists. One group received therapeutic ultrasound plus a neutral volar wrist splint worn at day and night. The second group received therapeutic ultrasound plus splint plus nerve and tendon gliding exercises. The third group received splint plus nerve and tendon gliding exercises. Treatment duration was for three weeks, and outcome measurements included symptoms, pain, Tinel and Phalen sign, two‐point discrimination, hand function, grip strength, pinch strength, neurophysiologic parameters, and patient satisfaction. The ultrasound component was delivered pulsed at a frequency of 1 MHz and an intensity of 1.0 W/cm2, for 15 minutes once a day, five times a week, for three weeks.
In the study conducted by Bilgici 2010, 34 participants with 49 CTS‐affected wrists were randomly allocated to receive either therapeutic ultrasound at a frequency of 3 MHz and intensity of 1.5 W/cm2 for five minutes, five times a week for four weeks, or to local corticosteroid injection plus neutral‐positioned wrist splint worn as much as possible during the day and night for four weeks. Outcomes were symptoms, pain, hand function, grip strength, neurophysiologic parameters, and adverse effects.
In the study conducted by Dincer 2009, 60 female participants with bilateral CTS were randomly allocated to wearing a splint worn at night and during aggravating daytime activities for three months, or wearing a splint for three months and receiving continuous ultrasound at a frequency of 3 MHz and intensity of 1.0 W/cm2 for three minutes per session, with 10 sessions performed once a day, five times a week for two weeks, or wearing a splint for three months and receiving low‐level laser therapy administered at 10 sessions performed once a day, five times a week for two weeks. Outcomes assessed were pain, symptoms, function, neurophysiologic parameters, and patient satisfaction.
Duymaz 2012 compared therapeutic ultrasound (for five minutes per session, once a day five times a week for three weeks; intensity was 0.8 W/cm2 and frequency was 1 MHz) to dexamethasone iontophoresis and to placebo iontophoresis in 58 participants with 58 CTS‐affected wrists. All groups also received nerve and tendon gliding exercises plus a neutral wrist splint worn every night plus activity modification training. Outcomes assessed were symptoms, pain, Tinel's test, Phalen's test, Reverse Phalen's test, hand function, grip strength, pinch strength, and neurophysiologic parameters.
In the study conducted by Ebenbichler 1998, pulsed ultrasound therapy at 1.0 W/cm2 intensity and 1 MHz frequency was compared with placebo ("sham") ultrasound for seven weeks duration in 45 participants with 90 CTS‐affected wrists. Outcomes assessed were CTS symptoms, sensation, grip strength, pinch strength, neurophysiologic parameters, medication use, adverse effects and return to work.
Ekim 2008 randomly allocated 28 participants with 28 CTS‐affected wrists to either continuous ultrasound at 1.5 W/cm2 intensity and 3 MHz frequency plus splint worn at night, or placebo ultrasound at 0.0 W/cm2 intensity plus splint worn at night. Both the active and placebo ultrasound regimens were delivered for five minutes, five days a week for two weeks. Outcomes assessed were symptoms, pain, Tinel's test, Phalen's test, hand function, grip strength, and neurophysiologic parameters.
Koyuncu 1995 compared the delivery of circular ultrasound at two different frequencies (1 and 3 MHz), both at 1.0 W/cm2 intensity and delivered for eight minutes per session, five days per week, for four weeks in 16 participants with 21 CTS‐affected wrists. Outcomes assessed were pain, paraesthesiae, superficial touch sensation, large and small object grasping, sensory and motor nerve transmission delay and Tinel and Phalen sign.
In the study conducted by Oztas 1998, the use of continuous ultrasound at different intensities (1.5, 0.8 and 0.0W/cm2), all at 3 MHz frequency for five minutes a day, five days a week for two weeks were compared in 18 females with 30 CTS‐affected wrists. Outcomes assessed were pain, CTS symptoms, nocturnal wakening and neurophysiologic parameters.
In the study conducted by Piravej 2004, 18 participants with 30 CTS‐affected wrists were randomly allocated to either continuous ultrasound therapy performed at an intensity of 0.5 W/cm2 and frequency of 1 MHz for 10 minutes per session, five days a week for four weeks, plus placebo drug taken each day, or to "sham" ultrasound plus diclofenac 75 mg/day (a nonsteroidal anti‐inflammatory drug) taken in a divided dose each day for four weeks. Outcomes assessed were pain (measured using a visual analogue scale (VAS)), presence of nocturnal and/or diurnal pain and/or paraesthesia , frequency of awakening at night, and neurophysiologic parameters.
Yildiz 2011 investigated the effects of "sham" ultrasound for two weeks compared with ultrasound delivered at a frequency of 1 MHz and intensity of 1 W/cm2 for 15‐minute sessions, once a day, five times a week for two weeks, or to ultrasound with 2.5% ketoprofen gel (a nonsteroidal anti‐inflammatory drug) delivered at a frequency of 1 MHz and intensity of 1 W/cm2 for 15‐minute sessions, once a day, five times a week for two weeks. The 51 participants (76 CTS‐affected wrists) in all groups wore a splint at night and during the day for eight weeks, and the outcomes were pain, symptoms, function, adverse effects (complications) and neurophysiologic parameters.
The primary outcome, short‐term overall improvement using any measure where patients indicate the intensity of their complaints compared with baseline (over three months or less) was measured in only two of the 11 studies (Dincer 2009; Ebenbichler 1998). Adverse effects were only measured in three studies (Bilgici 2010; Ebenbichler 1998; Yildiz 2011).
In nine studies (Bakhtiary 2004; Baysal 2006; Bilgici 2010; Dincer 2009; Ebenbichler 1998; Koyuncu 1995; Oztas 1998; Piravej 2004; Yildiz 2011) some or all participants had bilateral CTS, where both wrists contributed to the analysis. In three of these nine studies (Baysal 2006; Dincer 2009; Piravej 2004), randomisation occurred at the level of participants, where the same intervention was delivered to both wrists in participants with bilateral CTS. In two studies (Bakhtiary 2004; Ebenbichler 1998), randomisation of wrists occurred, where all participants with bilateral CTS received a different intervention to each wrist. In two studies (Oztas 1998; Yildiz 2011), randomisation of wrists occurred, where there was no constraint that participants' wrists be allocated to the same or different treatments. It was unclear in Bilgici 2010 or Koyuncu 1995 whether participants with bilateral CTS received the same or different interventions to each wrist. All outcomes of interest to the review were analysed at the wrist level in these nine studies. In seven of these studies (Baysal 2006; Bilgici 2010; Dincer 2009; Koyuncu 1995; Oztas 1998; Piravej 2004; Yildiz 2011), the trialists did not report how the analysis accounted for correlation between wrists in bilateral CTS and attempts to obtain this information from the trialists were unsuccessful (so it is not clear whether a unit of analysis error occurred in these studies). However, personal communication with Bakhtiary 2004 and Ebenbichler 1998 confirmed that the correlation between wrists was not accounted for in the analysis (therefore a unit of analysis error occurred in these studies).
Excluded studies
We excluded a total of 15 studies after review of the full text publication. Reasons for exclusion of studies are provided in the Characteristics of excluded studies table. The main reasons for exclusion were that a non‐randomised study design had been employed and that therapeutic ultrasound plus another intervention was compared to a different intervention for CTS (so the additional effect of ultrasound could not be determined).
Risk of bias in included studies
Full details of our assessment of risk of bias in included studies are available in the 'Risk of bias' tables, and a summary is presented in Figure 2. In cases where risk of bias was rated as 'Unclear risk of bias', attempts to contact the trial authors for further information were made, and unless otherwise specified, these were unsuccessful.
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Generation of the randomisation sequence was judged to have been adequate and at 'Low risk of bias' in six studies (Bakhtiary 2004; Baysal 2006; Duymaz 2012; Ebenbichler 1998; Piravej 2004; Yildiz 2011), as all used computer‐generated randomisation sequences; in the remaining studies (Bilgici 2010; Dincer 2009; Ekim 2008; Koyuncu 1995; Oztas 1998), the method used to generate this sequence was unclear. Only two studies were judged to be at low risk of bias for the domain allocation concealment (Bilgici 2010; Ebenbichler 1998), as these studies reported using sealed, opaque, sequentially numbered envelopes to conceal the allocation sequence. The remaining nine studies were rated as being at 'Unclear risk of bias' on this domain, as they either did not report any method for concealing the allocation sequence (Ekim 2008; Koyuncu 1995; Oztas 1998; Piravej 2004), or reported only some components of an effective method (for example they reported that sealed, sequentially numbered envelopes were used, but did not report whether these were opaque) (Bakhtiary 2004; Baysal 2006; Dincer 2009; Duymaz 2012; Yildiz 2011).
Blinding
Participants in six studies were reported as being blinded to the intervention they received (Ebenbichler 1998; Ekim 2008; Koyuncu 1995; Oztas 1998; Piravej 2004; Yildiz 2011). Blinding in these studies was possible because they involved either a "sham" ultrasound regimen (Ebenbichler 1998; Ekim 2008; Oztas 1998; Piravej 2004; Yildiz 2011) or two different types of ultrasound that could not be differentiated by the participants (Koyuncu 1995; Oztas 1998). These studies were therefore rated at 'Low risk' of performance bias. Because of the nature of the interventions delivered in the studies conducted by Baysal 2006, Bilgici 2010, Dincer 2009, and Duymaz 2012, patients were not blinded in these studies. The study conducted by Bakhtiary 2004 was rated as being at 'Unclear risk of bias' for this performance bias domain. Three studies (Duymaz 2012; Ekim 2008; Oztas 1998) reported that blinding of assessors of all subjective and some objective outcomes was not done, while in Bilgici 2010 and Koyuncu 1995 it was unclear whether outcome assessors were blinded; all remaining studies were rated at low risk of detection bias.
Incomplete outcome data
Nine studies were judged as being at low risk of bias for completeness of outcome data at three months or less (Bakhtiary 2004; Baysal 2006; Bilgici 2010; Dincer 2009; Duymaz 2012; Ekim 2008; Koyuncu 1995; Piravej 2004; Yildiz 2011). Two studies were rated as 'Unclear risk of bias' for this domain (Ebenbichler 1998; Oztas 1998). Only two studies could be assessed for completeness of outcome data collected three months or more after treatment ended, with one study rated as 'High risk of bias' (Baysal 2006) and the other rated as 'Unclear risk of bias' (Ebenbichler 1998).
Selective reporting
Five studies were judged as being at 'Low risk of bias' for selective outcome reporting (Bakhtiary 2004; Baysal 2006; Bilgici 2010; Ekim 2008; Yildiz 2011). Judgements were based on comparing outcomes specified in the methods section with those reported in the results section of the publication, and the finding that the majority of effect estimates for the reported outcomes in these studies were not statistically significant. Two studies were rated as being at 'Unclear risk of bias' (Oztas 1998; Piravej 2004) because they did not report on function as an outcome (whereas all other included studies did), and a protocol for these studies was not available to confirm that this outcome was not measured. Four studies were rated as being at 'High risk' of reporting bias (Dincer 2009; Duymaz 2012; Ebenbichler 1998; Koyuncu 1995). Dincer 2009 introduced a new outcome in the results section which was not pre‐specified in the methods section of the publication. The authors reported the "number of completely normal hands based on electroneuromyography at three months", but did describe this outcome or define "completely normal" in the methods section. Duymaz 2012 fully reported means and standard deviations for some outcomes, but partially reported (that is, only specified whether differences between groups were statistically significant or not) or did not report any data at all for other outcomes. In the study conducted by Ebenbichler 1998, it was reported that nerve conduction studies assessing median motor nerve conduction and sensory nerve action potentials were conducted but results were not reported. The same study reported that three participants were off work; however, work status was not stated as an outcome measure in the methods section of the publication. The study conducted by Koyuncu 1995 was rated as being at a 'High risk' of reporting bias because outcomes were assessed every week throughout the four‐week treatment period but only results at baseline and at the end of treatment were reported. Further, results for motor nerve distal transmission delay and sensory nerve transmission delay were only partially reported as median endpoint values and mean change scores (without measures of variation). No protocols or trial registry entries for any of the included studies were identified, which limits our assessment of selective reporting.
Other potential sources of bias
All studies were judged to be at low risk of other potential sources of bias.
Effects of interventions
See: Table 1
Therapeutic ultrasound versus placebo
Two trials compared a regimen of therapeutic ultrasound with placebo ("sham" ultrasound) (Ebenbichler 1998 and Oztas 1998). Ebenbichler 1998 compared pulsed ultrasound therapy (1.0 W/cm2 intensity and 1 MHz frequency) with placebo ultrasound (0.0 W/cm2 intensity) for a duration of seven weeks, while Oztas 1998 compared different intensities of continuous ultrasound: 1.5 W/cm2 versus 0.8 W/cm2 versus 0.0 W/cm2 (placebo), all at 3 MHz frequency, for a duration of two weeks. In Ebenbichler 1998, the correlation between wrists in participants with bilateral CTS was not accounted for in the analysis. Whether this correlation was accounted for in the analysis in Oztas 1998 is unclear. Therefore, all outcome data reported in these two studies may be invalid due to a unit of analysis error. Attempts to retrieve individual wrist outcome data from the trialists were unsuccessful. Without access to the individual wrist data, and without being able to estimate parameters such as the intraclass correlation coefficient from other studies included in the review, we did not attempt to adjust the results of these two studies. We have included the outcome data as reported by the trialists, but emphasise that results of these studies should be interpreted with caution, as the lack of adjustment may have produced overly narrow 95% CIs with artificially smaller P values (Higgins 2011c). Both Ebenbichler 1998 and Oztas 1998 assessed some of the same outcomes, but owing to the potential unit of analysis errors, we did not pooldata.
Primary outcomes
1) Short‐term overall improvement (three months or less)
Reported as an outcome in Ebenbichler 1998 but not Oztas 1998.
In the study conducted by Ebenbichler 1998, participants were dichotomised into those who rated improvement in their wrists as 'good to excellent overall improvement' and those who did not. By the end of seven weeks of treatment, the chance of rating 'good to excellent overall improvement' was 136% higher for wrists receiving ultrasound compared with wrists receiving placebo (RR 2.36; 95% CI 1.40 to 3.98). However this outcome should be interpreted with caution, as 11 participants were not included in the analysis of outcomes measured at seven weeks because of non‐compliance in keeping appointments (eight participants) and excessive pain requiring additional therapeutic measures (three participants). While these losses were balanced evenly across groups, it is unclear whether it was still possible to assess and include some, or all, of the outcome data for these individuals or whether they had been inappropriately excluded from the analysis; therefore, the direction of potential bias is unclear.
Secondary outcomes
1) Adverse effects
Reported as an outcome in Ebenbichler 1998 but not Oztas 1998.
No side effects due to ultrasound treatment were reported by participants (Ebenbichler 1998).
2) Short‐term improvement in CTS symptoms (three months or less)
Reported as an outcome in Ebenbichler 1998 and Oztas 1998.
Ebenbichler 1998 found that wrists receiving ultrasound had pain or paraesthesia that was 0.33 points lower on an 11‐point scale after two weeks (MD ‐0.33; 95% CI ‐1.31 to 0.65) and 0.99 points lower at the end of seven weeks of treatment (endpoint MD ‐0.99; 95% CI ‐1.77 to ‐0.21) than wrists receiving placebo. Further, using a five‐point scale measuring subjective pain or paraesthesia, the authors dichotomised wrists into those that experienced 'satisfactory improvement or complete remission of symptoms' and those that did not, and found that therapeutic ultrasound increased the likelihood of reporting complete remission of symptoms by 77% (RR 1.77; 95% CI 1.09 to 2.88). Ebenbichler 1998 also found the mean change from baseline to two weeks in sensory loss was larger for the therapeutic ultrasound group compared with placebo (MD ‐1.24; 95% CI ‐2.36 to ‐0.12). The difference between groups in mean change from baseline to seven weeks for this outcome was also larger for the therapeutic ultrasound group, but the low precision of the effect estimate suggests a positive effect of placebo is also possible (MD ‐1.07; 95% CI ‐2.23 to 0.09).
After two weeks and five days of treatment, Oztas 1998 found that the VAS pain score (scale 0 to 10, with lower scores denoting less pain) was 1.10 points lower in wrists receiving ultrasound 1.5 W/cm2 intensity compared with placebo (MD ‐1.10; 95% CI ‐2.92 to 0.72), and was 0.40 points lower in wrists receiving ultrasound 0.8 W/cm2 intensity compared with placebo (MD ‐0.40; 95% CI ‐2.30 to 1.50). VAS pain or paraesthesia was no different between wrists receiving ultrasound 1.5 W/cm2 intensity compared with placebo (MD 0.00; 95% CI ‐0.68 to 0.68) and 0.30 points higher in wrists receiving ultrasound 0.8 W/cm2 intensity compared with placebo (MD 0.30; 95% CI ‐0.49 to 1.09). Further, there was no difference in frequency of nocturnal awakening at this time point between wrists receiving ultrasound 1.5 W/cm2 intensity and those receiving placebo (MD 0.00; 95% CI ‐0.92 to 0.92), and a small difference on this outcome between wrists receiving ultrasound 0.8 W/cm2 intensity and those receiving placebo (MD ‐0.40; 95% CI ‐1.36 to 0.56). None of the 95% CIs of these effect estimates rule out the possibility of negative effects of ultrasound on these outcomes.
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Reported as an outcome in Ebenbichler 1998 but not Oztas 1998.
Ebenbichler 1998 measured grip strength (kg) and pinch strength (kg) at two weeks and seven weeks post‐treatment, and reported mean change from baseline. The authors reported improvement from baseline to two weeks for hand grip strength was 1.32 kg higher for wrists receiving therapeutic ultrasound (MD 1.32; 95% CI ‐1.10 to 3.74); however, the 95% CI incorporates both positive and negative changes in hand grip strength compared with placebo. In contrast, the difference between wrists in improvement from baseline to seven weeks was greater, and favoured those receiving ultrasound (MD 3.96; 95% CI 1.31 to 6.61). The difference between groups in pinch strength at two weeks was 0.19 points higher for the ultrasound group (MD 0.19; 95% CI 0.05 to 0.33), and while the effect estimate at seven weeks indicates pinch strength was 0.27 points higher for the ultrasound group (MD 0.27; 95% CI ‐0.09 to 0.63), the 95% CI incorporates both increases and decreases in pinch strength compared with placebo.
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Reported as an outcome in Ebenbichler 1998 and Oztas 1998.
Ebenbichler 1998 found that therapeutic ultrasound resulted in a greater improvement in both motor distal latency at two weeks (MD ‐0.27; 95% CI ‐0.45 to ‐0.09) and at the end of seven weeks of treatment (MD ‐0.61; 95% CI ‐0.83 to ‐0.39), and in sensory nerve conduction velocity at two weeks (MD 5.34; 95% CI 5.06 to 5.62) and at the end of seven weeks of treatment (MD 8.24; 95% CI 7.96 to 8.52) compared with placebo.
After two weeks and five days of treatment, Oztas 1998 found median sensory distal latency was 0.15 ms slower in wrists receiving ultrasound with 1.5 W/cm2 intensity compared with wrists receiving placebo (MD 0.15; 95% CI ‐0.93 to 1.23) but 0.13 ms faster in wrists receiving ultrasound with 0.8 W/cm2 intensity when compared with wrists receiving placebo (MD ‐0.13; 95% CI ‐0.95 to 0.69); and motor distal latency was 0.64 ms slower in wrists receiving ultrasound with 1.5 W/cm2 intensity compared with wrists receiving placebo (MD 0.64; 95% CI ‐0.88 to 2.16) and 0.74 ms faster in wrists receiving ultrasound with 0.8 W/cm2 intensity when compared with wrists receiving placebo (MD 0.74; 95% CI ‐0.55 to 2.03). Further, antidromic sensory nerve conduction velocity was 2.10 m/s better for wrists receiving placebo when compared with wrists receiving ultrasound at 1.5 W/cm2 intensity (MD ‐2.10; 95% CI ‐11.87 to 7.67) and 6.4 m/s better for wrists receiving ultrasound with 0.8 W/cm2 intensity when compared with wrists receiving placebo (MD 6.40; 95% CI ‐4.05 to 16.85); and median motor forearm conduction velocity was 0.20 m/s better for wrists receiving placebo when compared with wrists receiving ultrasound at 1.5 W/cm2 intensity (MD ‐0.20; 95% CI ‐6.13 to 5.73), and 0.20 m/s better for wrists receiving ultrasound with 0.8 W/cm2 intensity when compared with wrists receiving placebo (MD 0.20; 95% CI ‐4.57 to 4.97). However, the 95%CIs for these effect estimates all incorporate effects that are positive or negative for ultrasound compared with placebo.
5) Long‐term improvement in CTS symptoms (more than three months)
Reported as an outcome in Ebenbichler 1998 but not Oztas 1998.
Ebenbichler 1998 dichotomised wrists into those rated by participants as experiencing an 'overall unsatisfactory outcome' or not. Six months after the seven‐week treatment period ended, ultrasound increased the likelihood of not experiencing an overall unsatisfactory outcome by 91% compared with placebo (RR 1.91; 95% CI 1.13 to 3.23). Also, wrists receiving ultrasound were reported as having pain or paraesthesia 1.86 points lower on an 11‐point scale (MD ‐1.86; 95% CI ‐2.67 to ‐1.05) and sensory loss 1.18 points lower on an 11‐point scale (MD ‐1.18; 95% CI ‐2.02 to ‐0.34) at this time point. Using a five‐point scale on subjective pain and/or paraesthesia, wrists were dichotomised into those who experienced 'satisfactory improvement or complete remission of symptoms' or not, and more wrists in the therapeutic ultrasound group (73% compared with 20%) were reported as having experienced complete remission of symptoms at six months follow‐up (RR 3.67; 95% CI 1.74 to 7.74).
6) Long‐term improvement in functional ability or health‐related quality of life (more than three months)
Reported as an outcome in Ebenbichler 1998 but not Oztas 1998.
Ebenbichler 1998 measured functional outcomes using grip strength (kg) and pinch strength (kg) at six months follow‐up. Wrists receiving ultrasound had 4.16 kg better grip strength than wrists receiving placebo (MD 4.16; 95% CI ‐0.88 to 9.20) and 0.74 kg better pinch strength than the wrists receiving placebo (MD 0.74; 95% CI ‐0.17 to 1.65); however, both effect estimates have 95% CI that do not exclude the possibility of no difference between groups, or a negative effect of ultrasound.
Therapeutic ultrasound: different frequencies
One trial compared the efficacy of therapeutic ultrasound delivered at different frequencies (Koyuncu 1995). In this study, circular ultrasound delivered at frequency 1 MHz was compared with ultrasound delivered at frequency 3 MHz, over a duration of four weeks. It was unclear whether the correlation between wrists in participants with bilateral CTS was accounted for in the analysis. Therefore, all outcome data reported in this study may be invalid due to a unit of analysis error. Attempts to retrieve individual wrist outcome data from the trialists were unsuccessful. Without access to the individual wrist data, and without being able to estimate parameters such as the intraclass correlation coefficient from other studies included in the review, we did not attempt to adjust the results of this study. We have included the outcome data as reported by the trialists, but emphasise that results should be interpreted with caution, as the possible lack of adjustment may have produced overly narrow 95% CIs with artificially smaller P values (Higgins 2011c).
Primary outcomes
1) Short‐term overall improvement (three months or less)
Not reported as an outcome.
Secondary outcomes
1) Adverse effects
Not reported as an outcome.
2) Short‐term improvement in CTS symptoms (three months or less)
From baseline to the end of four weeks of treatment, Koyuncu 1995 found that when compared with ultrasound at 1 MHz frequency, ultrasound at 3 MHz frequency reduced the risk of pain by 37% (RR 0.63; 95% CI 0.26 to 1.52), paraesthesia by 63% (RR 0.37; 95% CI 0.09 to 1.42), superficial sensation by 45% (RR 0.55; 95% CI 0.06 to 5.18), and positive Tinel sign by 44% (RR 0.66; 95% CI 0.21 to 2.08). In contrast, ultrasound at 1 MHz frequency increased the risk of positive Phalen sign by 10% when compared with ultrasound at 3 MHz frequency (RR 1.10; 95% CI 0.37 to 3.27). For all these outcomes, the low precision of the 95% CIs means that positive and negative effects of both treatment regimens are possible.
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
By the end of four weeks of treatment, the authors found that ultrasound at 1 MHz frequency increased the chances of having improvement in the grasping of large, and small, objects, both by 227% when compared with ultrasound at 3 MHz frequency (RR 3.27; 95% CI 0.15 to 72.23). However, the 95% CIs are very wide, making it difficult to make any firm conclusions about these outcomes.
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Koyuncu 1995 assesses motor nerve distal transmission delay and sensory nerve transmission delay, but only median values for these neurophysiologic endpoints were reported. Attempts to obtain summary data for inclusion in a meta‐analysis (for example, means and SDs) from the authors were unsuccessful.
5) Long‐term improvement in CTS symptoms (more than three months)
Not reported as an outcome.
6) Long‐term improvement in functional ability or health‐related quality of life (more than three months)
Not reported as an outcome.
Therapeutic ultrasound: different intensity
One trial compared regimens of therapeutic ultrasound delivered at different intensities (Oztas 1998). This study examined any differences between continuous ultrasound delivered at intensity 1.5 W/cm2, compared with intensity 0.8 W/cm2. It was unclear whether the correlation between wrists in participants with bilateral CTS was accounted for in the analysis. Therefore, all outcome data reported in this study may be invalid due to a unit of analysis error. Attempts to retrieve individual wrist outcome data from the trialists were unsuccessful. Without access to the individual wrist data, and without being able to estimate parameters such as the intraclass correlation coefficient from other studies included in the review, we did not attempt to adjust the results of this study. We have included the outcome data as reported by the trialists, but emphasise that results should be interpreted with caution, as the possible lack of adjustment may have produced overly narrow 95% CIs with artificially smaller P values (Higgins 2011c).
Primary outcomes
1) Short‐term overall improvement (three months or less)
Not reported as an outcome.
Secondary outcomes
1) Adverse effects
Not reported as an outcome.
2) Short‐term improvement in CTS symptoms (three months or less)
At the end of two weeks and five days of treatment, Oztas 1998 reported that pain intensity for wrists receiving ultrasound at 1.5 W/cm2 intensity was 0.70 points lower on an 11‐point scale (MD ‐0.70; 95% CI ‐2.28 with 0.88), and night pain/paraesthesia was 0.30 points lower on an 11‐point scale (MD ‐0.30; 95% CI ‐0.90 to 0.30) compared with wrists receiving ultrasound at 0.8 W/cm2 intensity. The group receiving ultrasound at 0.8 W/cm2 intensity awoke on average 0.40 fewer times at night per week than the group receiving ultrasound at 1.5 W/cm2 intensity (MD 0.40; 95% CI ‐0.41 to 1.21). However, none of the 95% CIs of these effect estimates exclude the possibility of effects in either direction for these two ultrasound intensities.
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Not reported as an outcome.
4) Short‐term improvement in neurophysiologic parameters (three months or less)
After two weeks and five days of treatment, median motor distal latency was 0.10 ms faster for wrists receiving ultrasound at 1.5 W/cm2 intensity compared with wrists receiving ultrasound at 0.8 W/cm2 intensity (MD ‐0.10; 95% CI ‐1.61 to 1.41). In contrast, wrists receiving ultrasound at 0.8 W/cm2 intensity had 0.28 ms faster median sensory distal latency (MD 0.28; 95% CI ‐0.72 to 1.28), 0.40 m/s better median motor forearm conduction velocity (MD ‐0.40; 95% CI ‐5.90 to 5.10), and 8.50 m/s better sensory nerve conduction velocity (MD ‐8.50; 95% CI ‐18.91 to 1.91) compared with wrists receiving ultrasound at 1.5 W/cm2 intensity. It must be cautioned that the precision of these effect estimates is low and the 95% CIs incorporate changes in either direction for both of the ultrasound intensities.
5) Long‐term improvement in CTS symptoms (more than three months)
Not reported as an outcome.
6) Long‐term improvement in functional ability or health‐related quality of life (more than three months)
Not reported as an outcome.
Therapeutic ultrasound (single intervention) versus other non‐surgical intervention
Two trials compared therapeutic ultrasound delivered as a single intervention versus another non‐surgical intervention. One trial compared therapeutic ultrasound with low‐level laser therapy delivered for three weeks (Bakhtiary 2004), while Bilgici 2010 compared therapeutic ultrasound with local corticosteroid injection plus splint for four weeks. In Bakhtiary 2004, the correlation between wrists in participants with bilateral CTS was not accounted for in the analysis. Whether this correlation was accounted for in the analysis in Bilgici 2010 is unclear. Therefore, all outcome data reported in these two studies may be invalid due to a unit of analysis error. Attempts to retrieve individual wrist outcome data from the trialists were unsuccessful. Without access to the individual wrist data, and without being able to estimate parameters such as the intraclass correlation coefficient from other studies included in the review, we did not attempt to adjust the results of these two studies. We have included the outcome data as reported by the trialists, but emphasise that results of these studies should be interpreted with caution, as the lack of adjustment may have produced overly narrow 95% CIs with artificially smaller P values (Higgins 2011c).
Primary outcomes
1) Short‐term overall improvement (three months or less)
Not reported as an outcome in Bakhtiary 2004 or Bilgici 2010.
Secondary outcomes
1) Adverse effects
Reported as an outcome in Bilgici 2010 but not Bakhtiary 2004.
Bilgici 2010 found no side effects due to ultrasound treatment were reported by participants, whereas some participants receiving local corticosteroid injection plus splint reported transient local injection pain (however the number of participants reporting this were not reported).
2) Short‐term improvement in CTS symptoms (three months or less)
Reported as an outcome in Bakhtiary 2004 and Bilgici 2010.
Bakhtiary 2004 assessed pain using a 0 to 10 VAS, and found a greater improvement in pain in the ultrasound group compared with the low‐level laser therapy group in terms of mean change from baseline with the end of three weeks of treatment (MD ‐3.20; 95% CI ‐3.76 to ‐2.64) and mean change from baseline to seven weeks follow‐up (MD ‐4.30; 95% CI ‐4.90 to ‐3.70). Given that it is not clear whether patients were blinded, this outcome should be interpreted with caution, as it is possible that participants' expectations of ultrasound or low‐level laser therapy may have biased their self‐reported assessment for pain.
Bilgici 2010 reported that wrists receiving ultrasound had a symptom severity score (measured using a Turkish‐validated version of the Levine questionnaire (Levine 1993)) that was 0.66 points lower at the end of four weeks of treatment (MD ‐0.66; 95% CI ‐1.89 to 0.57), but 0.18 points higher at four weeks post‐treatment (MD 0.18; 95% CI ‐0.45 to 0.81), and pain (measured using a visual analogue scale; scale units not reported) that was 0.55 points lower at the end of four weeks of treatment (MD ‐0.55; 95% CI ‐2.17 to 1.07) and 0.12 points lower at four weeks post‐treatment (MD ‐0.12; 95% CI ‐1.39 to 1.15), compared with wrists receiving local corticosteroid injection plus splint. The precision of each of these effect estimates was low, and opposite effects of treatment are possible.
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Reported as an outcome in Bakhtiary 2004 and Bilgici 2010.
Bakhtiary 2004 found a greater improvement in hand grip strength in wrists receiving ultrasound compared with wrists receiving low‐level laser therapy in terms of mean change from baseline to the end of three weeks of treatment (MD 17.20; 95% CI 10.05 to 24.35) and mean change from baseline to seven weeks follow‐up (MD 18.10; 95% CI 9.83 to 26.37). Further, a difference in pinch strength favoured the ultrasound group at the end of three weeks of treatment (MD 6.50; 95% CI 5.27 to 7.73) and at seven weeks follow‐up (MD 7.00; 95% CI 5.33 to 8.67).
Bilgici 2010 reported that wrists receiving ultrasound had a functional status score (measured using a Turkish‐validated version of the Levine questionnaire (Levine 1993)) that was 0.81 points lower at the end of four weeks of treatment (MD ‐0.81; 95% CI ‐1.70 to 0.08) and 0.24 points lower at four weeks post‐treatment (MD ‐0.24; 95% CI ‐1.01 to 0.53), and grip strength that was 2.80 mmHg better at the end of four weeks of treatment (MD 2.80; 95% CI 1.01 to 4.59) and 3.43 mmHg better at four weeks post‐treatment (MD 3.43; 95% CI 1.71 to 5.15) compared with wrists receiving local corticosteroid injection plus splint. Of all these effect estimates, only the grip strength results had 95% CIs that ruled out a null or alternative effect of treatment.
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Reported as an outcome in Bakhtiary 2004 and Bilgici 2010.
In Bakhtiary 2004, wrists receiving ultrasound had a greater change from baseline than wrists receiving low level laser therapy in: motor distal latency after three weeks of treatment (MD ‐0.70; 95% CI ‐0.90 to ‐0.50) and at seven weeks follow‐up (MD ‐0.90; 95% CI ‐1.06 to ‐0.74); in compound muscle action potential (CMAP) amplitude after three weeks of treatment (MD 2.00; 95% CI 1.03 to 2.97) and at seven weeks follow‐up (MD 2.50; 95% CI 1.55 to 3.45); in thumb sensory latency after three weeks of treatment (MD ‐0.50; 95% CI ‐0.75 to ‐0.25) and at seven weeks follow‐up (MD ‐0.50; 95% CI ‐0.73 to ‐0.27); in thumb sensory action potential (SAP) amplitude after three weeks of treatment (MD 5.00; 95% CI 1.92 to 8.08) and at seven weeks follow‐up (MD 5.70; 95% CI 2.74 to 8.66); in index sensory latency after three weeks of treatment (MD ‐0.90; 95% CI ‐1.36 to ‐0.44) and at seven weeks follow‐up (MD ‐0.90; 95% CI ‐1.33 to ‐0.47); and in index sensory action potential (SAP) amplitude after three weeks of treatment (MD 9.10; 95% CI 2.76 to 15.44) and at seven weeks follow‐up (MD 10.30; 95% CI 4.66 to 15.94).
Bilgici 2010 reported that wrists receiving ultrasound had a median nerve motor distal latency that was 0.05 msec faster at the end of four weeks of treatment (MD ‐0.05; 95% CI ‐0.55 to 0.45) and 0.11 msec slower at four weeks post‐treatment (MD 0.11; 95% CI ‐0.66 to 0.88), and a sensory nerve conduction velocity that was 3.71 m/sec higher at the end of four weeks of treatment (MD 3.71; 95% CI ‐0.45 to 7.87) and 2.32 m/sec higher at four weeks post‐treatment (MD 2.32; 95% CI ‐1.89 to 6.53), compared with wrists receiving local corticosteroid injection plus splint. The 95% CIs of all these effect estimate were wide and incorporate both null and opposite effects of treatment.
5) Long‐term improvement in CTS symptoms (more than three months)
Not reported as an outcome in Bakhtiary 2004 or Bilgici 2010.
6) Long‐term improvement in functional ability or health‐related quality of life (more than three months)
Not reported as an outcome in Bakhtiary 2004 or Bilgici 2010.
Therapeutic ultrasound (as part of multiple interventions) versus other non‐surgical interventions
Six trials compared therapeutic ultrasound delivered as part of a multi‐component intervention with another non‐surgical intervention (Baysal 2006; Dincer 2009; Duymaz 2012; Ekim 2008; Piravej 2004; Yildiz 2011). In the study conducted by Baysal 2006, therapeutic ultrasound plus splint was compared with therapeutic ultrasound plus nerve and tendon gliding exercises plus splint and with nerve and tendon gliding exercises plus splint. While there are three possible comparisons in the study by Baysal 2006, only the two comparisons where therapeutic ultrasound was delivered to one of the groups were compared (that is we did not include data on the comparison between therapeutic ultrasound plus nerve and tendon gliding exercises plus splint versus therapeutic ultrasound plus splint). Dincer 2009 compared splint worn at night and during aggravating daytime activities, with splint and continuous ultrasound, or splint and low‐level laser therapy. Duymaz 2012 compared therapeutic ultrasound with dexamethasone iontophoresis and with placebo iontophoresis (all groups also received nerve and tendon gliding exercises plus night splint plus activity modification). Ekim 2008 compared ultrasound plus splint with placebo ultrasound plus splint. Piravej 2004 compared continuous ultrasound therapy plus placebo drug, with "sham" ultrasound plus diclofenac 75 mg/day. Yildiz 2011 compared "sham ultrasound" plus splint with either ultrasound plus splint or to ultrasound with 2.5% ketoprofen gel plus splint. In Baysal 2006, Dincer 2009, Piravej 2004, and Yildiz 2011 it was unclear whether the correlation between wrists in participants with bilateral CTS was accounted for in the analysis. Therefore, all outcome data reported in these four studies may be invalid due to a unit of analysis error. Attempts to retrieve individual wrist outcome data from the trialists were unsuccessful. Without access to the individual wrist data, and without being able to estimate parameters such as the intraclass correlation coefficient from other studies included in the review, we did not attempt to adjust the results of these four studies. We have included the outcome data as reported by the trialists, but emphasise that results should be interpreted with caution, as the possible lack of adjustment may have produced overly narrow 95% CIs with artificially smaller P values (Higgins 2011c). Only two of these studies were deemed to be relatively similar (Ekim 2008 and Yildiz 2011), but were not combined because of heterogeneity of intensity, frequency, and duration of ultrasound treatment. We have provided a narrative synthesis of the results.
Primary outcomes
1) Short‐term overall improvement (three months or less)
Reported as an outcome in Dincer 2009 but not Baysal 2006, Duymaz 2012, Ekim 2008, Piravej 2004 or Yildiz 2011.
Dincer 2009 found that ultrasound and splint increased the chance of being satisfied with treatment (RR 3.02; 95% CI 1.36 to 6.72), and of having completely normal hands based on electroneuromyography (RR 3.17; 95% CI 1.30 to 7.77) compared with splint alone, at three months after treatment ended. Compared with low‐level laser therapy plus splint, the ultrasound plus splint group had slightly fewer participants who were satisfied with treatment (RR 0.87; 95% CI 0.57 to 1.33), and fewer completely normal hands based on electroneuromyography (RR 0.88; 95% CI 0.54 to 1.45), though the precision of these effect estimates was low. The results regarding the number of people with completely normal hands based on electroneuromyography should be interpreted with caution as they are associated with a high risk of selective reporting bias, as the authors did not pre‐specify this outcome or define "completely normal hands" in the Methods section of the publication.
Secondary outcomes
1) Adverse effects
Reported as an outcome in Yildiz 2011 but not Baysal 2006, Dincer 2009, Duymaz 2012, Ekim 2008 or Piravej 2004.
None of the participants in the study by Yildiz 2011 reported complications or side effects of treatment during the study period.
2) Short‐term improvement in CTS symptoms (three months or less)
Reported as an outcome in Baysal 2006, Dincer 2009, Duymaz 2012, Ekim 2008, Piravej 2004 and Yildiz 2011.
In the study conducted by Baysal 2006, wrists receiving ultrasound and splint had lower mean VAS pain scores (on a zero to 10 scale) at the end of three weeks of treatment (MD ‐1.10; 95% CI ‐2.59 to 0.39) and eight weeks post‐treatment (MD ‐0.10; 95% CI ‐1.87 to 1.67), and lower mean symptom severity scores (assessed using the Levine questionnaire (Levine 1993)) at the end of three weeks of treatment (MD ‐2.60; 95% CI ‐7.81 to 2.61) and eight weeks post‐treatment (MD ‐1.10; 95% CI ‐7.31 to 5.11), than wrists receiving exercise and splint. The low precision of these effect estimates does not rule out beneficial effects of exercise and splint only. Wrists receiving ultrasound and exercises and splint also had a lower mean VAS pain scores at the end of three weeks treatment (MD ‐2.00; 95% CI ‐3.46 to ‐0.54) and at eight weeks post‐treatment (MD ‐1.80; 95% CI ‐3.00 to ‐0.60), and lower mean symptom severity scores at the end of treatment (MD ‐3.60; 95% CI ‐7.80 to 0.60) and at eight weeks post‐treatment (MD ‐4.60; 95% CI ‐9.36 to 0.16) compared with exercises and splint only. However, the 95% CIs do not exclude the possibility of a small beneficial effect of exercise and splint alone, and the risk of bias associated with non‐blinding of patients for these self‐reported outcomes is high. Ultrasound and splint reduced the risk of having a positive Phalen's sign by 18% at the end of three weeks of treatment (RR 0.82; 95% CI 0.38 to 1.76) and by 32% at eight weeks post‐treatment (RR 0.68; 95% CI 0.29 to 1.59), compared with exercises and splint only. Ultrasound and splint also reduced the risk of having a positive Tinel's sign by 37% at the end of three weeks of treatment (RR 0.63; 95% CI 0.27 to 1.43), but increased the risk by 7% at eight weeks post‐treatment (RR 1.07; 95% CI 0.41 to 2.79), compared with exercises and splint only. In comparison to the exercises and splint group, receiving ultrasound and exercises and splint reduced the risk of having a positive Phalen's sign at the end of three weeks of treatment by 5% (RR 0.95; 95% CI 0.47 to 1.93) and by 32% at eight weeks post‐treatment (RR 0.68; 95% CI 0.29 to 1.59), and reduced the risk of having a positive Tinel's sign by 37% at the end of three weeks of treatment (RR 0.63; 95% CI 0.27 to 1.43) and by 79% at eight weeks post‐treatment (RR 0.21; 95% CI 0.03 to 1.58). None of the 95% CIs of these effect estimates rule out a negative effect of the interventions comprising ultrasound.
In the study by Dincer 2009, wrists receiving ultrasound and splint had less symptom severity at one month (MD ‐0.34; 95% CI ‐0.53 to ‐0.15) and three months after treatment ended (MD ‐0.70; 95% CI ‐1.06 to ‐0.34), and less pain (VAS) at one month (MD ‐2.60; 95% CI ‐3.46 to ‐1.74) and three months after treatment ended (MD ‐2.53; 95% CI ‐3.52 to ‐1.54), compared with wrists receiving splint alone. In contrast, wrists receiving low‐level laser therapy and splint had less symptom severity (as assessed using the Levine questionnaire (Levine 1993)) at one month (MD 0.45; 95% CI 0.15 to 0.75) and three months after treatment ended (MD 0.71; 95% CI 0.29 to 1.13), and less pain (VAS) at one month (MD 0.61; 95% CI ‐0.30 to 1.52) and three months after treatment ended (MD 1.25; 95% CI 0.22 to 2.28), compared with wrists receiving ultrasound and splint (however, the low precision of the VAS pain effect estimate at one month follow‐up means an opposite effect of treatment is possible). The possible lack of allocation concealment and lack of patient blinding may have biased these results in favour of low‐level laser therapy and should therefore be interpreted with caution.
Duymaz 2012 reported that wrists receiving ultrasound plus exercises, night splint and activity modification had a symptom severity score (as assessed using the Levine questionnaire (Levine 1993)) that was 4.25 points higher (worse) at the end of treatment (MD 4.25; 95% CI ‐1.12 to 9.62) and 5.2 points higher at three months follow‐up (MD 5.20; 95% CI 0.27 to 10.13) compared with wrists receiving dexamethasone iontophoresis plus exercises, night splint and activity modification, and that was 0.45 points higher at the end of treatment (MD 0.45; 95% CI ‐5.88 to 6.78) and 1.10 points lower (better) at three months follow‐up (MD ‐1.10; 95% CI ‐7.11 to 4.91) compared with wrists receiving placebo iontophoresis plus exercises, night splint and activity modification. Compared with the ultrasound group, the change from baseline to the end of treatment in: (i) VAS pain on movement was 1.45 points larger (better) in the dexamethasone iontophoresis group (MD ‐1.45; 95% CI ‐2.55 to ‐0.35) and 0.64 points smaller (worse) in the placebo iontophoresis group (MD 0.64; 95% CI ‐0.32 to 1.60); (ii) VAS pain at rest was 1.35 points larger in the dexamethasone iontophoresis group (MD ‐1.35; 95% CI ‐2.43 to ‐0.27) and 0.70 points smaller in the placebo iontophoresis group (MD 0.70; 95% CI ‐0.14 to 1.54); and (iii) VAS pain at night was 0.10 points larger in the dexamethasone iontophoresis group (MD ‐0.10; 95% CI ‐1.49 to 1.29) and 0.64 points smaller in the placebo iontophoresis group (MD 0.64; 95% CI ‐0.67 to 1.95). For all these self‐reported outcomes, results should be interpreted with caution due to the lack of participant blinding. Trialists also reported measuring Phalen's test, Reverse Phalen's test, Tinel's test and carpal compression test, but only reported whether differences between groups on these outcomes were statistically significant (therefore no useable data for these outcomes have been included in the review).
Ekim 2008 found that wrists receiving therapeutic ultrasound plus splint worn at night reduced the risk of having a positive Tinel's test by 13% (RR 0.87; 95% CI 0.46 to 1.64), reduced the risk of having a positive Phalen's test by 26% (RR 0.74; 95% CI 0.33 to 1.65), and reduced symptom severity score (as assessed using Turkish translated version of the Levine questionnaire (Levine 1993)) by 6.4 points (MD ‐6.40; 95% CI ‐8.40 to ‐4.40) at the end of two weeks of treatment when compared with wrists receiving placebo ultrasound plus splint worn at night (only the result for symptom severity score was statistically significant). Ekim 2008 also measured pain using a 100 mm VAS but reported medians and interquartile ranges (IQR) only as the data were skewed, and therefore could not be entered into RevMan. The ultrasound plus splint group had a median VAS pain of 30 (IQR 25 to 39.25) and the placebo ultrasound plus splint group had a higher median VAS pain of 50 (IQR 40 to 65) at the end of two weeks of treatment. All these results should be interpreted with caution because it was not clear whether the random allocation sequence was adequately concealed.
When comparing wrists receiving ultrasound plus placebo to wrists receiving "sham" ultrasound plus NSAID, Piravej 2004 found small differences in the following outcomes at the end of four weeks of treatment: VAS pain score (MD ‐0.20; 95% CI ‐1.53 to 1.13), pain/paraesthesia (MD ‐0.07; 95% CI ‐0.52 to 0.38) and frequency of awakening at night (MD 0.07; 95% CI ‐0.42 to 0.56). The low precision of effect estimates means the results cannot be interpreted as one intervention being of greater benefit than the other. The above effect estimates are based on endpoint scores; results based on change from baseline scores were also reported in the publication, and were similar in terms of direction, magnitude and statistical significance of effect for all outcomes except for VAS pain score and frequency of awakening, where the direction of effect changed.
Yildiz 2011 found small differences between wrists receiving ultrasound and splint and wrists receiving "sham" ultrasound and splint on the following outcomes: VAS pain score at the end of two weeks of treatment (MD ‐0.31; 95% CI ‐1.55 to 0.93) and six weeks after treatment ended (MD ‐0.51; 95% CI ‐2.01 to 0.99), and symptom severity score (as assessed using the Levine questionnaire (Levine 1993)) at the end of two weeks of treatment (MD 0.10; 95% CI ‐0.22 to 0.42) and six weeks after treatment ended (MD ‐0.11; 95% CI ‐0.52 to 0.30). The precision of all these effect estimates was low, and opposite effects of interventions cannot be ruled out. Small, nonsignificant differences between wrists receiving ultrasound with 2.5% ketoprofen gel plus splint and wrists receiving ultrasound plus splint were also shown for VAS pain score at the end of two weeks of treatment (MD ‐0.62; 95% CI ‐1.83 to 0.59), and symptom severity score at the end of two weeks of treatment (MD 0.26; 95% CI ‐0.12 to 0.64) and six weeks after treatment ended (MD 0.34; 95% CI ‐0.04 to 0.72). However, at six weeks after treatment ended, the VAS pain score was lower in the wrists receiving ultrasound with 2.5% ketoprofen gel and splint, (MD 1.79; 95% CI 0.55 to 3.03). All the above effect estimates are based on an intention‐to‐treat (ITT) analysis; results based on a per‐protocol analysis were also reported in the publication, and were similar in terms of direction, magnitude, and statistical significance of effect.
3) Short‐term improvement in functional ability or health‐related quality of life (three months or less)
Reported as an outcome in Baysal 2006, Dincer 2009, Duymaz 2012, Ekim 2008, and Yildiz 2011, but not Piravej 2004.
Baysal 2006 found that wrists receiving ultrasound and splint had a mean self‐reported functional status that was 1.30 points lower on a 40‐point scale at the end of three weeks of treatment (MD 1.30; 95% CI ‐3.83 to 6.43) and 1.20 points lower at eight weeks post‐treatment (MD 1.20; 95% CI ‐3.81 to 6.21) compared with wrists receiving exercises and splint. The ultrasound and splint group wrists had hand grip strength that was 0.70 kg better at the end of three weeks of treatment (MD 0.70; 95% CI ‐4.82 to 6.22) and 0.80 kg better at eight weeks post‐treatment (MD 0.80; 95% CI ‐2.42 to 4.02) when compared with the exercises and splint wrists, but had pinch strength which was 0.60 kg worse at the end of three weeks of treatment (MD ‐0.60; 95% CI ‐1.98 to 0.78) and at eight weeks post‐treatment (MD ‐0.60; 95% CI ‐1.92 to 0.72) compared with exercises and splint group wrists. Further, wrists receiving ultrasound and exercises and splint had a mean self‐reported functional status that was 3.10 points lower (better) on a 40‐point scale at the end of three weeks of treatment (MD ‐3.10; 95% CI ‐6.58 to 0.38) and 2.30 points lower at eight weeks post‐treatment (MD ‐2.30; 95% CI ‐5.42 to 0.82) compared with wrists receiving exercises and splint, and hand grip strength was 0.60 kg better at the end of treatment (MD 0.60; 95% CI ‐3.09 to 4.29), but 0.40 kg worse at eight weeks post‐treatment (MD ‐0.40; 95% CI ‐4.27 to 3.47). Pinch strength was 0.70 kg better at the end treatment (MD 0.70; 95% CI ‐0.56 to 1.96) and at eight weeks post‐treatment (MD 0.70; 95% CI ‐0.57 to 1.97) compared with wrists receiving exercises and splint only. All of these effect estimates have 95% CIs that do no exclude the possibility of no difference between groups or effects that favour either treatment group.
Wrists in the study by Dincer 2009 that received ultrasound and splint had better self‐reported functional ability than wrists receiving splint only at one month (MD ‐0.13; 95% CI ‐0.28 to 0.02), and three months (MD ‐0.65; 95% CI ‐0.82 to ‐0.48) after treatment ended. Alternatively, wrists receiving low‐level laser therapy and splint had better self‐reported functional ability than wrists receiving ultrasound and splint at one month (MD 0.32; 95% CI 0.07 to 0.57), and three months after treatment ended (MD 0.18; 95% CI ‐0.10 to 0.46). However, the lack of patient blinding means this self‐reported outcome could be biased based on participant expectations of the benefits offered by low‐level laser therapy as being greater than ultrasound and either of these interventions being greater than splint alone.
Duymaz 2012 reported that wrists receiving ultrasound plus exercises, night splint and activity modification had a functional status score (as assessed using the Levine questionnaire (Levine 1993)) that was 3.20 points higher (worse) at the end of treatment (MD 3.20; 95% CI ‐0.76 to 7.16) and 3.5 points higher at three months follow‐up (MD 3.50; 95% CI ‐0.53 to 7.53) compared with wrists receiving dexamethasone iontophoresis plus exercises, night splint and activity modification, and that was 2.94 points higher at the end of treatment (MD 2.94; 95% CI ‐1.73 to 7.61) and 1.85 points higher at three months follow‐up (MD 1.85; 95% CI ‐2.74 to 6.44) compared to wrists receiving placebo iontophoresis plus exercises, night splint and activity modification. Compared to the ultrasound group, Health Assessment Questionnaire scores were 0.24 points lower (better) at the end of treatment (MD 0.24; 95% CI ‐0.12 to 0.60) and 0.07 points lower at three months follow‐up (MD 0.07; 95% CI ‐0.26 to 0.40) in the dexamethasone iontophoresis group, and 0.07 points lower at the end of treatment (MD 0.07; 95% CI ‐0.31 to 0.45) and 0.02 points higher (worse) at three months follow‐up (MD ‐0.02; 95% CI ‐0.36 to 0.32) in the placebo iontophoresis group. For all these outcomes, results should be interpreted with caution due to the lack of participant and outcome assessor blinding. Trialists also reported measuring grip and pinch strength, but only reported whether differences between groups on these outcomes were statistically significant (therefore no data for these outcomes have been included in the review).
Ekim 2008 found that wrists receiving therapeutic ultrasound plus splint worn at night had a functional status score (as assessed using Turkish translated version of the Levine questionnaire (Levine 1993)) that was 1.00 points lower (better) (MD ‐1.00; 95% CI ‐4.45 to 2.45), and grip strength that was better (MD 0.04; 95% CI ‐0.02 to 0.10) (units of measurement not specified) at the end of two weeks of treatment when compared with wrists receiving placebo ultrasound plus splint worn at night. However, the wide 95% CIs incorporate effects in either direction.
Yildiz 2011 found small differences favouring wrists that received ultrasound plus splint over wrists receiving "sham" ultrasound plus splint on self‐reported functional status at the end of two weeks of treatment (MD ‐0.15; 95% CI ‐0.52 to 0.22) and six weeks after treatment ended (MD ‐0.21; 95% CI ‐0.67 to 0.25), though the precision of effect estimates was low. Differences in self‐reported functional status between wrists receiving ultrasound with 2.5% ketoprofen gel plus splint compared with wrists receiving ultrasound plus splint were also small and imprecise at the end of two weeks of treatment (MD ‐0.23; 95% CI ‐0.61 to 0.15) and six weeks after treatment ended (MD 0.19; 95% CI ‐0.24 to 0.62). All the above effect estimates are based on an ITT analysis; results based on a per‐protocol analysis were also reported in the publication, and were similar in terms of direction, magnitude, and statistical significance of effect.
4) Short‐term improvement in neurophysiologic parameters (three months or less)
Reported as an outcome in Baysal 2006, Dincer 2009, Duymaz 2012, Ekim 2008, Piravej 2004 and Yildiz 2011.
In the study by Baysal 2006, wrists receiving ultrasound and splint had a mean motor distal latency that was 0.20 ms faster at the end of treatment (MD ‐0.20; 95% CI ‐0.95 to 0.55) and 0.30 ms faster at eight weeks post‐treatment (MD ‐0.30; 95% CI ‐0.91 to 0.31), and a mean sensory distal latency that was 0.10 ms slower at the end of treatment (MD 0.10; 95% CI ‐0.28 to 0.48) and no different at eight weeks post‐treatment (MD 0.00; 95% CI ‐0.36 to 0.36), compared with wrists receiving exercises and splint only. Comparisons between wrists receiving ultrasound plus exercises plus splint and wrists receiving exercises plus splint alone indicate that mean motor distal latency was 0.20 ms faster at the end of treatment (MD ‐0.20; 95% CI ‐1.37 to 0.97) and 0.20 ms faster at eight weeks post‐treatment (MD ‐0.20; 95% CI ‐1.46 to 1.06), and mean sensory distal latency was 0.20 ms slower at the end of treatment (MD 0.20; 95% CI ‐0.13 to 0.53) and 0.20 ms slower at eight post‐treatment (MD 0.20; 95% CI ‐0.12 to 0.52) when compared with wrists receiving exercises and splint only. None of these effect estimates had high precision though as indicated by the 95% CI that incorporates effects of the intervention in either direction.
Dincer 2009 found that wrists receiving ultrasound and splint had better median nerve motor distal latency at one month (MD ‐0.15; 95% CI ‐0.26 to ‐0.04) and three months (MD ‐0.29; 95% CI ‐0.46 to ‐0.12) after treatment ended, and better second digit‐wrist median nerve sensory velocity at one month (MD 3.09; 95% CI 1.42 to 4.76) and three months (MD 3.29; 95% CI ‐0.35 to 6.93) after treatment ended, compared with wrists receiving splint only. Further, wrists receiving low‐level laser therapy had slightly better median nerve motor distal latency at one month (MD 0.05; 95% CI ‐0.07 to 0.17) and three months (MD 0.07; 95% CI ‐0.10 to 0.24) after treatment ended, and better second digit‐wrist median nerve sensory velocity at one month (MD ‐2.69; 95% CI ‐4.80 to ‐0.58) and three months (MD ‐2.67; 95% CI ‐6.38 to 1.04) after treatment ended, compared with wrists receiving ultrasound and splint. However, the precision of the effect estimates was low and in a number of cases, opposite effects cannot be ruled out.
Duymaz 2012 reported means and SDs for sensory nerve distal latency, sensory nerve amplitude, sensory nerve conduction velocity, motor nerve distal latency, motor nerve amplitude, motor nerve conduction velocity, compound muscle action potential (CMAP) of the abductor pollicis brevis muscle, but it is not clear whether the data are endpoint values at end of treatment, endpoint values at three months follow‐up, change from baseline to end of treatment values, or change from baseline to three months follow‐up values. For this reason, we did not extract the mean (SD) outcome data reported for any these six neurophysiologic parameters.
Ekim 2008 found that at the end of two weeks of treatment, wrists receiving ultrasound plus splint worn at night had motor distal latency that was 0.10 msec slower (MD 0.10; 95% CI ‐0.46 to 0.66), motor nerve conduction velocity that was 2.70 m/sec higher (MD 2.70; 95% CI ‐1.08 to 6.48), sensory distal latency that was 0.10 msec faster (MD ‐0.10; 95% CI ‐0.32 to 0.12), and palm‐wrist conduction velocity that was 0.90 m/sec lower (MD ‐0.90; 95% CI ‐4.31 to 2.51) compared with wrists receiving placebo ultrasound plus splint worn at night. The low precision of these effect estimates suggests that null or alternative effects are possible though.
At the end of four weeks of treatment, Piravej 2004 found small differences between wrists receiving ultrasound plus placebo and wrists receiving "sham" ultrasound plus NSAID for median nerve sensory distal latency (endpoint scores: MD ‐0.35; 95% CI ‐0.74 to 0.04), sensory nerve action potential (SNAP) (endpoint scores: MD 13.11; 95% CI ‐7.12 to 33.34), median nerve motor distal latency (change from baseline scores: MD ‐0.32; 95% CI ‐0.73 to 0.09), and CMAP (endpoint scores: MD 1.37; 95% CI ‐0.87 to 3.61). However, the low precision of effect estimates means that results cannot be interpreted as one intervention having clear benefit over another. The change from baseline score for the outcome, median nerve motor distal latency, is reported here because the standard deviation for the endpoint score was reported incorrectly in the trial publication. While the direction, magnitude and statistical significance of the outcomes, median nerve sensory distal latency and CMAP were similar when comparing endpoint to change from baseline scores, the endpoint score for sensory nerve action potential revealed no statistically significant difference between groups, whereas the change from baseline score for this outcome revealed a statistically significant difference favouring the ultrasound plus placebo group (MD ‐19.27; 95% CI ‐34.36 to ‐4.18). Twelve participants had bilateral CTS and six had unilateral CTS and the authors did not report controlling for inter‐correlation between the outcomes of both hands per participant with bilateral CTS. It is possible that unit of analysis error may have occurred, which may have artificially narrowed the 95% CIs, so these results should be interpreted with caution.
When comparing wrists receiving ultrasound plus splint to wrists receiving "sham" ultrasound plus splint, Yildiz 2011 found small differences in the outcomes, median nerve motor distal latency at the end of two weeks of treatment (MD 0.15; 95% CI ‐0.19 to 0.49) and six weeks after treatment ended (MD 0.11; 95% CI ‐0.21 to 0.43), and median nerve sensory distal latency at the end of two weeks of treatment (MD ‐0.04; 95% CI ‐0.24 to 0.16) and six weeks after treatment ended (MD ‐0.07; 95% CI ‐0.29 to 0.15). Further, when comparing wrists that received ultrasound with 2.5% ketoprofen gel and wrists that received ultrasound plus splint, small differences were found in the outcomes, median nerve motor distal latency at the end of two weeks of treatment (MD 0.20; 95% CI ‐0.07 to 0.47) and six weeks after treatment ended (MD 0.28; 95% CI 0.03 to 0.53), and median nerve sensory distal latency at the end of two weeks of treatment (MD 0.08; 95% CI ‐0.10 to 0.26) and six weeks after treatment ended (MD 0.08; 95% CI ‐0.09 to 0.25). The low precision of all these effect estimates makes it is impossible to conclude that one intervention is more beneficial than the other. All the above effect estimates are based on an ITT analysis; results based on a per‐protocol analysis were also reported in the publication, and were similar in terms of direction, magnitude, and statistical significance of effect for all outcomes, except for the outcome median nerve motor distal latency at six weeks after treatment ended when comparing ultrasound plus splint to "sham" ultrasound plus splint, which found a non‐significant effect (MD 0.28; 95% CI ‐0.00 to 0.56).
5) Long‐term improvement in CTS symptoms (more than three months)
Reported as an outcome in Baysal 2006 but not Dincer 2009, Duymaz 2012, Ekim 2008, Piravej 2004 and Yildiz 2011.
Baysal 2006 asked participants to report their satisfaction with treatment for each wrist at an average of 11 ± 4.5 months after the end of treatment. The authors reported the number of participants rated as 'excellent/good' (asymptomatic or rarely symptomatic), 'fair' (symptomatic only during compelling activity), and 'poor' (continuing symptoms without relief following treatment). In the therapeutic ultrasound and splint group, 25% of participants had 'excellent/good' satisfaction compared with 0% of participants receiving exercises and splint only (RR 9.69; 95% CI 0.55 to 171.98). Further, 61% of participants receiving ultrasound plus exercises plus splint had 'excellent/good' satisfaction compared with 0% of participants receiving exercises and splint only (RR 21.86; 95% CI 1.38 to 347.18). However, the precision of both effect estimates was very low, and the lack of patient blinding and unclear reasons for incomplete data for these outcomes suggests these results should be interpreted with caution.
6) Long‐term improvement in functional ability or health‐related quality of life (more than three months)
Not reported as an outcome in Baysal 2006, Dincer 2009, Duymaz 2012, Ekim 2008, Piravej 2004 or Yildiz 2011.
Subgroup and sensitivity analyses
We could not perform the planned subgroup and sensitivity analyses given the small number of studies that could be pooled. This may be possible in future updates of the review.
Assessment of reporting bias
The recommended number of studies required to generate a funnel plot (Sterne 2011) is 10, and in the absence of meta‐analyses, we could not assess publication bias. We did not locate protocols or trial registry entries for any of the studies included in the review, so our assessment of selective reporting was limited to comparing outcomes reported in the methods and results sections of publications.
Discussion
Summary of main results
We set out to determine the effectiveness of therapeutic ultrasound compared with no treatment, a placebo, or other non‐surgical treatments for improving clinical outcome in people with CTS. Eleven studies randomising 414 participants were included; two studies compared ultrasound with placebo, two studies compared different frequencies or intensities of ultrasound, and eight studies compared ultrasound with other non‐surgical interventions. No studies comparing ultrasound with 'no treatment' were found.
Overall there is insufficient evidence to recommend one therapeutic ultrasound regimen over another, or to recommend therapeutic ultrasound over other non‐surgical interventions for CTS. There is low quality evidence that therapeutic ultrasound may result in greater short‐term overall improvement and greater improvement in pain or paraesthesia compared with placebo at the end of seven weeks of treatment, and at six months follow‐up (Ebenbichler 1998). These results must be interpreted with caution given the unclear reasons for loss to follow‐up and failure to adjust for the correlation between wrists in participants with bilateral CTS in Ebenbichler 1998. However, differences between ultrasound and placebo for symptoms of pain or paraesthesia and neurophysiologic parameters after two weeks treatment, when pooled in a meta‐analysis, were small and not statistically significant (Ebenbichler 1998; Oztas 1998). This suggests that any beneficial effects of ultrasound may take more than a couple of weeks to become apparent. Studies comparing different frequencies (Koyuncu 1995) and different intensities of ultrasound (Oztas 1998) both had small study samples and results indicate there is limited evidence to recommend one type of ultrasound frequency or intensity over others, particularly in relation to short‐term overall improvement, symptoms, function and neurophysiologic parameters. We also found evidence that therapeutic ultrasound may be more effective than low‐level laser therapy for short‐term symptoms, function (hand grip strength and pinch strength) and neurophysiologic parameters (Bakhtiary 2004), but the investigators of this trial did not adjust for the correlation between wrists in participants with bilateral CTS so these results must be interpreted with caution. There is no high quality evidence that therapeutic ultrasound, when delivered as part of a multi‐component intervention, is any more effective than other non‐surgical interventions for CTS in terms of short‐term overall improvement, CTS symptoms, function and neurophysiological parameters (Baysal 2006; Dincer 2009; Duymaz 2012; Ekim 2008; Piravej 2004; Yildiz 2011). No studies reported any adverse effects of therapeutic ultrasound, but this outcome was only measured in three studies (Bilgici 2010; Ebenbichler 1998; Yildiz 2011). More adverse effects data are required before any firm conclusions on the safety of therapeutic ultrasound can be made.
Overall completeness and applicability of evidence
The evidence included in this review is limited in its completeness and applicability. There were a number of important pieces of information about study conduct and data that were not provided by the authors of the included studies (either in the publication or when requested). For example, the authors of nine studies did not report sufficient information to determine whether an adequate method of allocation concealment was used (Bakhtiary 2004; Baysal 2006; Dincer 2009; Duymaz 2012; Ekim 2008; Koyuncu 1995; Oztas 1998, Piravej 2004; Yildiz 2011). This is an important component of study design, given the meta‐epidemiological evidence to suggest that inadequate allocation concealment can result in biased treatment effects (Savović 2012). The included studies were also limited in the timing of outcome assessment, in that only two studies assessed outcomes more than three months post‐treatment cessation (Baysal 2006; Ebenbichler 1998), and the majority only assessed outcomes at the end of treatment. As a result there is limited evidence about the long‐term effects of therapeutic ultrasound for people with CTS. Further, of the 414 participants recruited in total, only 29 were reported as being male (note that two studies did not report the sex distribution of patients (Bakhtiary 2004, Ebenbichler 1998)). While there is a higher prevalence of CTS in females in the general population (Atroshi 1999, Charles 2009), the few males included in these studies limits the extent to which the results of the studies can be applied to men. In addition, only two small studies compared different regimens of therapeutic ultrasound (Koyuncu 1995; Oztas 1998), which limits any conclusions about the most effective ultrasound regimen. Finally, no studies provided a head‐to‐head comparison of therapeutic ultrasound delivered over different durations (for example sessions delivered over two weeks versus 10 weeks). Therefore, there is insufficient evidence regarding the most efficacious duration of therapeutic ultrasound delivery.
Quality of the evidence
The methodological quality varied across the studies. All of the studies were small (the largest included 60 participants with 120 CTS‐affected wrists (Dincer 2009)). Two of the studies reported using a random allocation sequence that was adequately concealed (Bilgici 2010; Ebenbichler 1998), six reported blinding of participants (Ebenbichler 1998; Ekim 2008; Koyuncu 1995; Oztas 1998; Piravej 2004; Yildiz 2011), and all but five of the studies (Bilgici 2010; Duymaz 2012; Ekim 2008; Koyuncu 1995; Oztas 1998) reported blinded assessment of objective outcomes. The lack of participant blinding in four studies is of concern given that many outcomes were self‐reported, and empirical evidence indicates that trials with self‐reported outcomes show exaggerated treatment effects (Savović 2012). Further, unit of analysis errors clearly occurred in two studies (Bakhtiary 2004; Ebenbichler 1998) and possibly occurred in another seven studies (Baysal 2006; Bilgici 2010; Dincer 2009; Koyuncu 1995; Oztas 1998; Piravej 2004; Yildiz 2011). Some type of selective outcome reporting was present in four studies (Dincer 2009; Duymaz 2012; Ebenbichler 1998; Koyuncu 1995), and suspected (though unclear) in another two (Oztas 1998; Piravej 2004). The latter finding is concerning given the results of a recent study which suggests that selective outcome reporting of "positive" or statistically significant trial results can bias the results and conclusions of a systematic review (Kirkham 2010).
Potential biases in the review process
While our described methods attempted to minimise bias in the selection of studies, collection of published data, and analysis for the review, our searches were limited to electronic databases, and as a result we have only included published studies. In future updates of this review, we will attempt to identify grey literature, given that empirical evidence suggests that published studies tend to have exaggerated treatment effects compared with unpublished studies (Hopewell 2007). It was also difficult to obtain relevant unpublished data from the authors of included studies. Further, it was difficult to assess selective outcome reporting as no protocols or trial registry entries for the included studies were identified.
Agreements and disagreements with other studies or reviews
To our knowledge, no other systematic reviews specifically focusing on therapeutic ultrasound for CTS exist. However, the findings of this review are generally consistent with those of other systematic reviews of non‐surgical interventions for CTS, which conclude there is limited or insufficient evidence for the effectiveness of therapeutic ultrasound for CTS (Ashworth 2010; Gerritsen 2002; Goodyear‐Smith 2004; Huisstede 2010; Muller 2004; Ono 2010; Piazzini 2007; Robertson 2001). In comparison to this review, the most recent systematic review of all non‐surgical interventions for CTS by Huisstede 2010 also included the studies conducted by Bakhtiary 2004, Baysal 2006, Koyuncu 1995, Ebenbichler 1998 and Oztas 1998; however, it did not include the studies conducted by Bilgici 2010, Dincer 2009, Duymaz 2012, Ekim 2008, Piravej 2004 or Yildiz 2011. Based on the date we conducted our searches, to our knowledge the current review is the most comprehensive and up‐to‐date review of randomised trials assessing the efficacy of therapeutic ultrasound for CTS.
Authors' conclusions
Implications for practice.
There is only poor quality evidence from very limited data to suggest that therapeutic ultrasound may be more effective than placebo for either short‐ or long‐term symptom improvement in people with carpal tunnel syndrome. There is insufficient evidence to support the greater benefit of one type of therapeutic ultrasound regimen over another or to support the use of therapeutic ultrasound as a treatment with greater efficacy compared with other non‐surgical interventions for CTS, such as splinting, exercises, and oral drugs. The preferences of both clinicians and patients should be taken into consideration when deciding whether to offer therapeutic ultrasound to people with CTS.
Implications for research.
Large scale, methodologically rigorous randomised trials are needed to assess the safety and efficacy of different therapeutic ultrasound regimens as compared with other non‐surgical interventions for CTS. More randomised trials are needed to ascertain the most effective frequency and intensity of therapeutic ultrasound to use. Trials should blind participants, personnel and outcome assessors where possible, and test the success of blinding (for example by asking participants to indicate which intervention they believe they received). Trialists should consider collecting data on overall improvement, adverse effects CTS symptoms, function, and neurophysiologic parameters. Finally, the long‐term effects of CTS need to be determined (that is outcomes should be assessed at least three months post‐treatment cessation).
What's new
| Date | Event | Description |
|---|---|---|
| 5 February 2013 | New citation required but conclusions have not changed | Searches updated to November 2012 and results incorporated |
| 8 January 2013 | New search has been performed | One new RCT identified from updated searches and included. Davis 1998 was incorrectly included in the previous version of this review (Page 2012a) and has been excluded from the current version. Davis 1998 compared the effect of therapeutic ultrasound delivered along with manual thrusts, massage and wrist splints to ibuprofen and wrist splint, so the additional effect of therapeutic ultrasound cannot be determined in this study. Davis 1998 is currently included in the 'Exercise and mobilisation interventions for carpal tunnel syndrome' review (Page 2012b). The meta‐analyses reported in the previous version of this review (Page 2012a) under Comparison 1: Therapeutic ultrasound verus placebo have been removed. The reason for removal is that following publication of the review, we determined that the correlation between wrists in participants with bilateral carpal tunnel syndrome had not been accounted for in the analyses reported by Ebenbichler 1998 and it was unclear whether an appropriate analysis had been conducted by Oztas 1998. Based on the potentially inappropriate analyses reported in these trials, we decided it was inappropriate to pool results in a meta‐analysis and have instead presented study‐specific effect estimates per trial. |
Notes
This is one of six reviews that will update the currently published review 'Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome' (O'Connor 2003). Three, in addition to this title, have been published as new reviews (O'Connor 2012; Page 2012b; Page 2012c) and the scope of an existing review (Marshall 2007) is to be widened to include oral corticosteroids. When all six reviews are published we will withdraw the original review from publication. This review includes a new search, revised review question and selection criteria, updated methodology and an updated review team.
Acknowledgements
We thank Ruth Brassington, Louisa Dunn, Kate Jewitt, Carolyn Reid, Angela Gunn and Rachel Barton from the Cochrane Neuromuscular Disease Group for their assistance in devising the search strategy, helping to locate people to translate the non‐English trials and ongoing support for this review. We thank Shawn Marshall for his substantial contribution to the original version of this review. We thank Katherine Beringer from the Monash Institute of Health Services Research for her assistance in retrieving studies relevant to the review. We thank the trialist Gerold Ebenbichler who corresponded with the principal reviewer to clarify additional information and/or provided additional data for the review. We thank the translators, in particular Murat Zinnuroglu, for their assistance in translating abstracts and papers for the review. We thank the peer reviewers for their helpful comments.
We thank the following institutions for their support during the review:
School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia;
School of Occupational Therapy, University of South Australia, Adelaide, Australia.
Editorial support from the Cochrane Neuromuscular Disease Group is funded by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. CENTRAL search strategy
#1"carpal tunnel syndrome" #2(("nerve entrapment" or "nerve compression" or "entrapment neuropath*") and carpal) #3(#1 OR #2) #4ultrasound or ultrasonic* #5(#3 AND #4)
Appendix 2. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to November Week 3 2012> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (342057) 2 controlled clinical trial.pt. (85675) 3 randomized.ab. (244680) 4 placebo.ab. (136464) 5 drug therapy.fs. (1586933) 6 randomly.ab. (175076) 7 trial.ab. (253559) 8 groups.ab. (1144975) 9 or/1‐8 (2957994) 10 Carpal Tunnel Syndrome.tw. or Carpal Tunnel Syndrome/ (7533) 11 ((nerve entrapment or nerve compression or entrapment neuropath$) and carpal).mp. (1004) 12 10 or 11 (7637) 13 Ultrasonic Therapy/ (7786) 14 13 or (ultrasound or ultrasonic$).mp. (165487) 15 9 and 12 and 14 (55) 16 15 and 20110201:20121127.(ed). (14)
Appendix 3. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2012 Week 47> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure/ (35555) 2 double‐blind procedure/ (111920) 3 randomized controlled trial/ (332920) 4 single‐blind procedure/ (16668) 5 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or (doubl$ adj blind$) or (singl$ adj blind$) or assign$ or allocat$ or volunteer$).tw. (1177991) 6 or/1‐5 (1256891) 7 exp animals/ (17803412) 8 exp humans/ (13950314) 9 7 not (7 and 8) (3853098) 10 6 not 9 (1129193) 11 limit 10 to embase (875712) 12 carpal tunnel syndrome/ or carpal tunnel syndrome.tw. (10746) 13 ((nerve entrapment or nerve compression or entrapment neuropath$) and carpal).mp. (1717) 14 12 or 13 (10873) 15 ultrasound therapy/ (6848) 16 15 or (ultrasound or ultrasonic$).mp. (265491) 17 11 and 14 and 16 (70) 18 17 and 20110201:20121127.(dd). (20)
Appendix 4. CINAHL Plus (EBSCOhost) search strategy
Tuesday, November 27, 2012 12:24:44 PM S33 S31 AND S32 14 S32 EM 20110201‐ 702,548 S31 s18 and s24 and s30 40 S30 s28 or s29 18,959 S29 ultrasound or ultrasonic* 18,959 S28 MM "Ultrasonic Therapy" 1,008 S27 "ultrasound therapy" 147 S26 ultrasound therapy 342 S25 s19 or s20 or s21 or s22 or s23 1,995 S24 s19 or s20 or s21 or s22 or s23 1,995 S23 entrapment neuropath* and carpal 45 S22 nerve compression and carpal 156 S21 nerve entrapment and carpal 58 S20 carpal tunnel syndrome 1,987 S19 (MH "Carpal Tunnel Syndrome") 1,769 S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 574,889 S17 ABAB design* 78 S16 TI random* or AB random* 116,691 S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 239,937 S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 81,509 S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 24,425 S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 18,888 S11 PT ("clinical trial" or "systematic review") 106,933 S10 (MH "Factorial Design") 852 S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 192,209 S8 (MH "Meta Analysis") 15,224 S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 32 S6 (MH "Quasi‐Experimental Studies") 5,714 S5 (MH "Placebos") 7,897 S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 25,508 S3 (MH "Clinical Trials+") 151,619 S2 (MH "Crossover Design") 9,918 S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 59,145
Appendix 5. AMED (OvidSP) search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to November 2012> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Randomized controlled trials/ (1560) 2 Random allocation/ (304) 3 Double blind method/ (454) 4 Single‐Blind Method/ (33) 5 exp Clinical Trials/ (3227) 6 (clin$ adj25 trial$).tw. (5526) 7 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. (2273) 8 placebos/ (524) 9 placebo$.tw. (2532) 10 random$.tw. (13034) 11 research design/ (1687) 12 Prospective Studies/ (522) 13 meta analysis/ (112) 14 (meta?analys$ or systematic review$).tw. (1931) 15 control$.tw. (28043) 16 (multicenter or multicentre).tw. (743) 17 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. (9917) 18 or/1‐17 (43215) 19 carpal tunnel syndrome/ or carpal tunnel syndrome.tw. (455) 20 ((nerve entrapment or nerve compression or entrapment neuropath$) and carpal).mp. (55) 21 19 or 20 (456) 22 ultrasonic therapy/ (235) 23 22 or (ultrasound or ultrasonic$).mp. (1427) 24 18 and 21 and 23 (13) 25 24 and 20110201:20121127.(up). (1)
Data and analyses
Comparison 1. Therapeutic ultrasound versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term overall improvement (number of participants with good to excellent improvement) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 7 weeks (end of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (number of participants with complete remission of subjective symptoms) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 7 weeks (end of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (VAS pain score) (2 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 2 weeks of treatment (1.5 W/cm2 intensity) (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 After 2 weeks of treatment (0.8 W/cm2 intensity) (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in CTS symptoms (pain and/or paraesthesia) (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 After 2 weeks of treatment (endpoint values of Ebenbichler 1998) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 After 2 weeks of treatment (endpoint values of Oztas 1998 1.5 W/cm2 intensity) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 After 2 weeks of treatment (endpoint values of Oztas 1998 0.8 W/cm2 intensity) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 After 7 weeks of treatment (endpoint values in Ebenbichler 1998) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in CTS symptoms (sensory loss) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Change from baseline to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Change from baseline to seven weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in CTS symptoms (nocturnal waking) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 After 2 weeks of treatment (1.5 W/cm2 intensity) (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 After 2 weeks of treatment (0.8 W/cm2 intensity) (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Short‐term improvement in functional ability (hand grip strength) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Change from baseline to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Change from baseline to seven weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Short‐term improvement in functional ability (pinch strength) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Change from baseline to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Change from baseline to seven weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Short‐term improvement in motor distal latency (ms) (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 After 2 weeks treatment (change from baseline values in Ebenbichler 1998) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 After 2 weeks treatment (endpoint values in Oztas 1998 1.5 W/cm2 intensity) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 After 2 weeks treatment (endpoint values in Oztas 1998 0.8 W/cm2 intensity) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Change from baseline to 7 weeks in Ebenbichler 1998 | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Short‐term improvement in motor nerve conduction velocity (m/s) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 After 2 weeks 5 days treatment (1.5 W/cm2 intensity) (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 After 2 weeks 5 days treatment (0.8 W/cm2 intensity) (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Short‐term improvement in sensory distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 After 2 weeks 5 days treatment (1.5 W/cm2 intensity) (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 After 2 weeks 5 days treatment (0.8 W/cm2 intensity) (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Short‐term improvement in sensory nerve conduction velocity (3 months or less) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 After 2 weeks treatment (change from baseline values in Ebenbichler 1998) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 After 2 weeks treatment (endpoint values in Oztas 1998 1.5 W/cm2 intensity) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 After 2 weeks treatment (endpoint values in Oztas 1998 0.8 W/cm2 intensity) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 Change from baseline to 7 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Long‐term improvement in CTS symptoms (>3 months) (number of participants who did not have an overall unsatisfactory outcome) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 At 7 months and 3 weeks (endpoint values) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Long‐term improvement in CTS symptoms (number of participants with complete remission of subjective symptoms) (>3 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.1 At 7 months and 3 weeks (endpoint values) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Long‐term improvement in CTS symptoms (pain and/or paraesthesia) (>3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 At 7 months 3 weeks (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Long‐term improvement in CTS symptoms (sensory loss) (>3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 At 7 months 3 weeks (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Long‐term improvement in functional ability (grip and pinch strength) (>3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Grip strength (kg) at 7 months and 3 weeks (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Pinch strength (kg) at 7 months and 3 weeks (endpoint values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 1 Short‐term overall improvement (number of participants with good to excellent improvement) (3 months or less).
1.2. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 2 Short‐term improvement in CTS symptoms (number of participants with complete remission of subjective symptoms) (3 months or less).
1.3. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 3 Short‐term improvement in CTS symptoms (VAS pain score) (2 months or less).
1.4. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 4 Short‐term improvement in CTS symptoms (pain and/or paraesthesia) (3 months or less).
1.5. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 5 Short‐term improvement in CTS symptoms (sensory loss) (3 months or less).
1.6. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 6 Short‐term improvement in CTS symptoms (nocturnal waking) (3 months or less).
1.7. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 7 Short‐term improvement in functional ability (hand grip strength) (3 months or less).
1.8. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 8 Short‐term improvement in functional ability (pinch strength) (3 months or less).
1.9. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 9 Short‐term improvement in motor distal latency (ms) (3 months or less).
1.10. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 10 Short‐term improvement in motor nerve conduction velocity (m/s) (3 months or less).
1.11. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 11 Short‐term improvement in sensory distal latency (ms) (3 months or less).
1.12. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 12 Short‐term improvement in sensory nerve conduction velocity (3 months or less).
1.13. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 13 Long‐term improvement in CTS symptoms (>3 months) (number of participants who did not have an overall unsatisfactory outcome).
1.14. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 14 Long‐term improvement in CTS symptoms (number of participants with complete remission of subjective symptoms) (>3 months).
1.15. Analysis.

Comparison 1 Therapeutic ultrasound versus placebo, Outcome 15 Long‐term improvement in CTS symptoms (pain and/or paraesthesia) (>3 months).
1.16. Analysis.
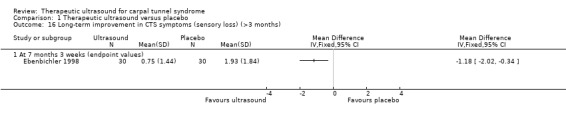
Comparison 1 Therapeutic ultrasound versus placebo, Outcome 16 Long‐term improvement in CTS symptoms (sensory loss) (>3 months).
1.17. Analysis.
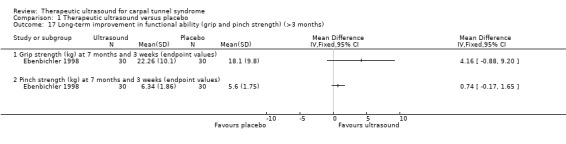
Comparison 1 Therapeutic ultrasound versus placebo, Outcome 17 Long‐term improvement in functional ability (grip and pinch strength) (>3 months).
Comparison 2. Therapeutic ultrasound (varying frequency).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (pain) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (paraesthesia) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (superficial sensation) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in CTS symptoms (Tinel's sign) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in CTS symptoms (Phalen's sign) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 After 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in functional ability (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Grasp of large objects after 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Grasp of small objects after 4 weeks of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.

Comparison 2 Therapeutic ultrasound (varying frequency), Outcome 1 Short‐term improvement in CTS symptoms (pain) (3 months or less).
2.2. Analysis.

Comparison 2 Therapeutic ultrasound (varying frequency), Outcome 2 Short‐term improvement in CTS symptoms (paraesthesia) (3 months or less).
2.3. Analysis.

Comparison 2 Therapeutic ultrasound (varying frequency), Outcome 3 Short‐term improvement in CTS symptoms (superficial sensation) (3 months or less).
2.4. Analysis.

Comparison 2 Therapeutic ultrasound (varying frequency), Outcome 4 Short‐term improvement in CTS symptoms (Tinel's sign) (3 months or less).
2.5. Analysis.
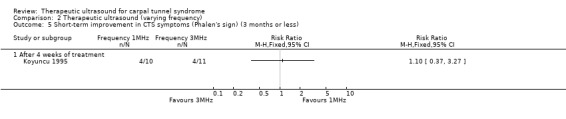
Comparison 2 Therapeutic ultrasound (varying frequency), Outcome 5 Short‐term improvement in CTS symptoms (Phalen's sign) (3 months or less).
2.6. Analysis.
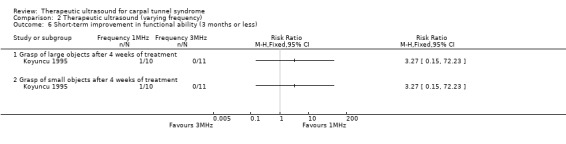
Comparison 2 Therapeutic ultrasound (varying frequency), Outcome 6 Short‐term improvement in functional ability (3 months or less).
Comparison 3. Therapeutic ultrasound (single intervention) versus low‐level laser therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Change from baseline to end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Change from baseline to 7 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in functional ability (hand grip strength, N) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Change from baseline to end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Change from baseline to 7 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in functional ability (pinch strength, N) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Change from baseline to end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change from baseline to 7 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in motor distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Change from baseline to end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Change from baseline to 7 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in compound muscle action potential (CMAP) amplitude (mV) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Change from baseline to end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Change from baseline to 7 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in thumb sensory latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Change from baseline to end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Change from baseline to 7 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Short‐term improvement in thumb sensory action potential (SAP) amplitude (µV) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Change from baseline to end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Change from baseline to 7 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Short‐term improvement in index sensory latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Change from baseline to end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Change from baseline to 7 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Short‐term improvement in index sensory action potential (SAP) amplitude (µV) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Change from baseline to end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Change from baseline to 7 weeks follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 Therapeutic ultrasound (single intervention) versus low‐level laser therapy, Outcome 1 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
3.2. Analysis.

Comparison 3 Therapeutic ultrasound (single intervention) versus low‐level laser therapy, Outcome 2 Short‐term improvement in functional ability (hand grip strength, N) (3 months or less).
3.3. Analysis.

Comparison 3 Therapeutic ultrasound (single intervention) versus low‐level laser therapy, Outcome 3 Short‐term improvement in functional ability (pinch strength, N) (3 months or less).
3.4. Analysis.
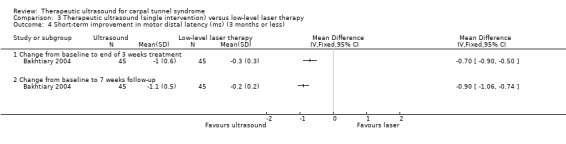
Comparison 3 Therapeutic ultrasound (single intervention) versus low‐level laser therapy, Outcome 4 Short‐term improvement in motor distal latency (ms) (3 months or less).
3.5. Analysis.

Comparison 3 Therapeutic ultrasound (single intervention) versus low‐level laser therapy, Outcome 5 Short‐term improvement in compound muscle action potential (CMAP) amplitude (mV) (3 months or less).
3.6. Analysis.

Comparison 3 Therapeutic ultrasound (single intervention) versus low‐level laser therapy, Outcome 6 Short‐term improvement in thumb sensory latency (ms) (3 months or less).
3.7. Analysis.

Comparison 3 Therapeutic ultrasound (single intervention) versus low‐level laser therapy, Outcome 7 Short‐term improvement in thumb sensory action potential (SAP) amplitude (µV) (3 months or less).
3.8. Analysis.

Comparison 3 Therapeutic ultrasound (single intervention) versus low‐level laser therapy, Outcome 8 Short‐term improvement in index sensory latency (ms) (3 months or less).
3.9. Analysis.

Comparison 3 Therapeutic ultrasound (single intervention) versus low‐level laser therapy, Outcome 9 Short‐term improvement in index sensory action potential (SAP) amplitude (µV) (3 months or less).
Comparison 4. Therapeutic ultrasound (varying intensity).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After 2 weeks 5 days of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (night pain / paraesthesia) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After 2 weeks 5 days of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (nocturnal awakening) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 After 2 weeks 5 days of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in motor distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 After 2 weeks 5 days treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in motor nerve conduction velocity (m/s) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 After 2 weeks 5 days treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in sensory distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 After 2 weeks 5 days treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Short‐term improvement in sensory nerve conduction velocity (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 After 2 weeks 5 days treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.

Comparison 4 Therapeutic ultrasound (varying intensity), Outcome 1 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
4.2. Analysis.

Comparison 4 Therapeutic ultrasound (varying intensity), Outcome 2 Short‐term improvement in CTS symptoms (night pain / paraesthesia) (3 months or less).
4.3. Analysis.

Comparison 4 Therapeutic ultrasound (varying intensity), Outcome 3 Short‐term improvement in CTS symptoms (nocturnal awakening) (3 months or less).
4.4. Analysis.

Comparison 4 Therapeutic ultrasound (varying intensity), Outcome 4 Short‐term improvement in motor distal latency (ms) (3 months or less).
4.5. Analysis.

Comparison 4 Therapeutic ultrasound (varying intensity), Outcome 5 Short‐term improvement in motor nerve conduction velocity (m/s) (3 months or less).
4.6. Analysis.

Comparison 4 Therapeutic ultrasound (varying intensity), Outcome 6 Short‐term improvement in sensory distal latency (ms) (3 months or less).
4.7. Analysis.

Comparison 4 Therapeutic ultrasound (varying intensity), Outcome 7 Short‐term improvement in sensory nerve conduction velocity (3 months or less).
Comparison 5. Therapeutic ultrasound (single intervention) versus local corticosteroid injection plus splint.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At the end of 4 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 4 weeks post‐treatment cessation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At the end of 4 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 4 weeks post‐treatment cessation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in functional ability (functional status score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At the end of 4 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 4 weeks post‐treatment cessation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in functional ability (grip strength) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At the end of 4 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 4 weeks post‐treatment cessation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in median nerve motor distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At the end of 4 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 4 weeks post‐treatment cessation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in median sensory nerve conduction velocity (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At the end of 4 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 4 weeks post‐treatment cessation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.

Comparison 5 Therapeutic ultrasound (single intervention) versus local corticosteroid injection plus splint, Outcome 1 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less).
5.2. Analysis.

Comparison 5 Therapeutic ultrasound (single intervention) versus local corticosteroid injection plus splint, Outcome 2 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
5.3. Analysis.

Comparison 5 Therapeutic ultrasound (single intervention) versus local corticosteroid injection plus splint, Outcome 3 Short‐term improvement in functional ability (functional status score) (3 months or less).
5.4. Analysis.

Comparison 5 Therapeutic ultrasound (single intervention) versus local corticosteroid injection plus splint, Outcome 4 Short‐term improvement in functional ability (grip strength) (3 months or less).
5.5. Analysis.

Comparison 5 Therapeutic ultrasound (single intervention) versus local corticosteroid injection plus splint, Outcome 5 Short‐term improvement in median nerve motor distal latency (3 months or less).
5.6. Analysis.

Comparison 5 Therapeutic ultrasound (single intervention) versus local corticosteroid injection plus splint, Outcome 6 Short‐term improvement in median sensory nerve conduction velocity (3 months or less).
Comparison 6. Therapeutic ultrasound plus splint versus exercises plus splint.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (Phalen sign) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 At end of treatment (3 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 11 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in CTS symptoms (Tinel sign) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 At end of treatment (3 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At 11 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in functional ability (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in functional ability (hand grip strength) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Short‐term improvement in functional ability (pinch strength) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Short‐term improvement in motor distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Short‐term improvement in sensory distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Long‐term improvement in CTS symptoms (>3 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 At 11 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 1 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
6.2. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less).
6.3. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 3 Short‐term improvement in CTS symptoms (Phalen sign) (3 months or less).
6.4. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 4 Short‐term improvement in CTS symptoms (Tinel sign) (3 months or less).
6.5. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 5 Short‐term improvement in functional ability (Levine) (3 months or less).
6.6. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 6 Short‐term improvement in functional ability (hand grip strength) (3 months or less).
6.7. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 7 Short‐term improvement in functional ability (pinch strength) (3 months or less).
6.8. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 8 Short‐term improvement in motor distal latency (ms) (3 months or less).
6.9. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 9 Short‐term improvement in sensory distal latency (ms) (3 months or less).
6.10. Analysis.

Comparison 6 Therapeutic ultrasound plus splint versus exercises plus splint, Outcome 10 Long‐term improvement in CTS symptoms (>3 months).
Comparison 7. Therapeutic ultrasound plus exercises plus splint versus exercises plus splint.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (Phalen sign) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 At end of treatment (3 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 11 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in CTS symptoms (Tinel sign) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 At end of treatment (3 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At 11 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in functional ability (Levine) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in functional ability (hand grip strength) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Short‐term improvement in functional ability (pinch strength) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Short‐term improvement in motor distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Short‐term improvement in sensory distal latency (ms) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 After end of 3 weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 At 11 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Long‐term improvement in CTS symptom (>3 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 At 11 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.

Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 1 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
7.2. Analysis.
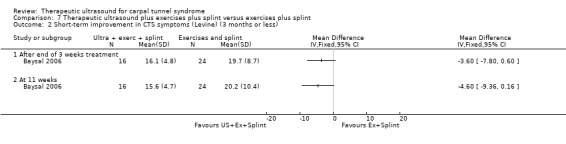
Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 2 Short‐term improvement in CTS symptoms (Levine) (3 months or less).
7.3. Analysis.

Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 3 Short‐term improvement in CTS symptoms (Phalen sign) (3 months or less).
7.4. Analysis.

Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 4 Short‐term improvement in CTS symptoms (Tinel sign) (3 months or less).
7.5. Analysis.

Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 5 Short‐term improvement in functional ability (Levine) (3 months or less).
7.6. Analysis.

Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 6 Short‐term improvement in functional ability (hand grip strength) (3 months or less).
7.7. Analysis.

Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 7 Short‐term improvement in functional ability (pinch strength) (3 months or less).
7.8. Analysis.

Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 8 Short‐term improvement in motor distal latency (ms) (3 months or less).
7.9. Analysis.

Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 9 Short‐term improvement in sensory distal latency (ms) (3 months or less).
7.10. Analysis.

Comparison 7 Therapeutic ultrasound plus exercises plus splint versus exercises plus splint, Outcome 10 Long‐term improvement in CTS symptom (>3 months).
Comparison 8. Therapeutic ultrasound plus splint versus splint.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term overall improvement (completely normal hands based on electroneuromyography) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Short‐term overall improvement (patient satisfaction) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in functional ability (functional status score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in median nerve motor distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Short‐term improvement in second digit‐wrist median nerve sensory velocity (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.

Comparison 8 Therapeutic ultrasound plus splint versus splint, Outcome 1 Short‐term overall improvement (completely normal hands based on electroneuromyography) (3 months or less).
8.2. Analysis.

Comparison 8 Therapeutic ultrasound plus splint versus splint, Outcome 2 Short‐term overall improvement (patient satisfaction) (3 months or less).
8.3. Analysis.

Comparison 8 Therapeutic ultrasound plus splint versus splint, Outcome 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less).
8.4. Analysis.

Comparison 8 Therapeutic ultrasound plus splint versus splint, Outcome 4 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
8.5. Analysis.

Comparison 8 Therapeutic ultrasound plus splint versus splint, Outcome 5 Short‐term improvement in functional ability (functional status score) (3 months or less).
8.6. Analysis.

Comparison 8 Therapeutic ultrasound plus splint versus splint, Outcome 6 Short‐term improvement in median nerve motor distal latency (3 months or less).
8.7. Analysis.

Comparison 8 Therapeutic ultrasound plus splint versus splint, Outcome 7 Short‐term improvement in second digit‐wrist median nerve sensory velocity (3 months or less).
Comparison 9. Therapeutic ultrasound plus splint versus low‐level laser therapy plus splint.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term overall improvement (completely normal hands based on electroneuromyography) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Short‐term overall improvement (patient satisfaction) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in functional ability (functional status score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in median nerve motor distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Short‐term improvement in second digit‐wrist median nerve sensory velocity (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At 1 month after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 At 3 months after treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.

Comparison 9 Therapeutic ultrasound plus splint versus low‐level laser therapy plus splint, Outcome 1 Short‐term overall improvement (completely normal hands based on electroneuromyography) (3 months or less).
9.2. Analysis.

Comparison 9 Therapeutic ultrasound plus splint versus low‐level laser therapy plus splint, Outcome 2 Short‐term overall improvement (patient satisfaction) (3 months or less).
9.3. Analysis.

Comparison 9 Therapeutic ultrasound plus splint versus low‐level laser therapy plus splint, Outcome 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less).
9.4. Analysis.

Comparison 9 Therapeutic ultrasound plus splint versus low‐level laser therapy plus splint, Outcome 4 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
9.5. Analysis.

Comparison 9 Therapeutic ultrasound plus splint versus low‐level laser therapy plus splint, Outcome 5 Short‐term improvement in functional ability (functional status score) (3 months or less).
9.6. Analysis.

Comparison 9 Therapeutic ultrasound plus splint versus low‐level laser therapy plus splint, Outcome 6 Short‐term improvement in median nerve motor distal latency (3 months or less).
9.7. Analysis.

Comparison 9 Therapeutic ultrasound plus splint versus low‐level laser therapy plus splint, Outcome 7 Short‐term improvement in second digit‐wrist median nerve sensory velocity (3 months or less).
Comparison 10. Therapeutic ultrasound plus nerve and tendon gliding exercises plus night splint plus activity modification versus dexamethasone iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (BCTQ symptom severity score) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 End of treatment (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Three months post‐treatment cessation (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (VAS pain on movement) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Change from baseline to end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (VAS pain at rest) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Change from baseline to end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in CTS symptoms (VAS pain at night) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Change from baseline to end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in functional ability (BCTQ functional status score) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 End of treatment (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Three months post‐treatment cessation (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in functional ability (Health Assessment Questionnaire) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 End of treatment (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Three months post‐treatment cessation (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.

Comparison 10 Therapeutic ultrasound plus nerve and tendon gliding exercises plus night splint plus activity modification versus dexamethasone iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 1 Short‐term improvement in CTS symptoms (BCTQ symptom severity score) (3 months or less).
10.2. Analysis.

Comparison 10 Therapeutic ultrasound plus nerve and tendon gliding exercises plus night splint plus activity modification versus dexamethasone iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 2 Short‐term improvement in CTS symptoms (VAS pain on movement) (3 months or less).
10.3. Analysis.

Comparison 10 Therapeutic ultrasound plus nerve and tendon gliding exercises plus night splint plus activity modification versus dexamethasone iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 3 Short‐term improvement in CTS symptoms (VAS pain at rest) (3 months or less).
10.4. Analysis.

Comparison 10 Therapeutic ultrasound plus nerve and tendon gliding exercises plus night splint plus activity modification versus dexamethasone iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 4 Short‐term improvement in CTS symptoms (VAS pain at night) (3 months or less).
10.5. Analysis.

Comparison 10 Therapeutic ultrasound plus nerve and tendon gliding exercises plus night splint plus activity modification versus dexamethasone iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 5 Short‐term improvement in functional ability (BCTQ functional status score) (3 months or less).
10.6. Analysis.

Comparison 10 Therapeutic ultrasound plus nerve and tendon gliding exercises plus night splint plus activity modification versus dexamethasone iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 6 Short‐term improvement in functional ability (Health Assessment Questionnaire) (3 months or less).
Comparison 11. Therapeutic ultrasound plus nerve and tendon gliding exercise plus night splint plus activity modification versus placebo iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (BCTQ symptom severity score) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 End of treatment (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Three months post‐treatment cessation (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (VAS pain on movement) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Change from baseline to end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (VAS pain at rest) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Change from baseline to end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in CTS symptoms (VAS pain at night) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Change from baseline to end of treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in functional ability (BCTQ functional status score) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 End of treatment (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Three months post‐treatment cessation (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in functional ability (Health Assessment Questionnaire) (3 months or less) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 End of treatment (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Three months post‐treatment cessation (endpoint values) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
11.1. Analysis.

Comparison 11 Therapeutic ultrasound plus nerve and tendon gliding exercise plus night splint plus activity modification versus placebo iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 1 Short‐term improvement in CTS symptoms (BCTQ symptom severity score) (3 months or less).
11.2. Analysis.

Comparison 11 Therapeutic ultrasound plus nerve and tendon gliding exercise plus night splint plus activity modification versus placebo iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 2 Short‐term improvement in CTS symptoms (VAS pain on movement) (3 months or less).
11.3. Analysis.

Comparison 11 Therapeutic ultrasound plus nerve and tendon gliding exercise plus night splint plus activity modification versus placebo iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 3 Short‐term improvement in CTS symptoms (VAS pain at rest) (3 months or less).
11.4. Analysis.
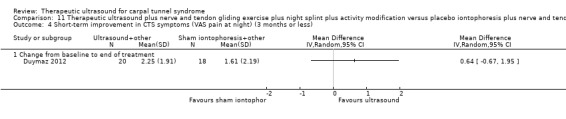
Comparison 11 Therapeutic ultrasound plus nerve and tendon gliding exercise plus night splint plus activity modification versus placebo iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 4 Short‐term improvement in CTS symptoms (VAS pain at night) (3 months or less).
11.5. Analysis.

Comparison 11 Therapeutic ultrasound plus nerve and tendon gliding exercise plus night splint plus activity modification versus placebo iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 5 Short‐term improvement in functional ability (BCTQ functional status score) (3 months or less).
11.6. Analysis.

Comparison 11 Therapeutic ultrasound plus nerve and tendon gliding exercise plus night splint plus activity modification versus placebo iontophoresis plus nerve and tendon gliding exercises plus night splint plus activity modification, Outcome 6 Short‐term improvement in functional ability (Health Assessment Questionnaire) (3 months or less).
Comparison 12. Therapeutic ultrasound plus splint versus placebo ultrasound plus splint.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (Tinel's sign) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At the end of two weeks treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (Phalen's sign) (3 months or less) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At the end of two weeks treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At the end of two weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in functional ability (functional status score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At the end of two weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in functional ability (grip strength) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At the end of two weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in motor distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At the end of two weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Short‐term improvement in motor nerve conduction velocity (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 At the end of two weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Short‐term improvement in sensory distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At the end of two weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Short‐term improvement in palm‐wrist conduction velocity (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 At the end of two weeks treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
12.1. Analysis.

Comparison 12 Therapeutic ultrasound plus splint versus placebo ultrasound plus splint, Outcome 1 Short‐term improvement in CTS symptoms (Tinel's sign) (3 months or less).
12.2. Analysis.

Comparison 12 Therapeutic ultrasound plus splint versus placebo ultrasound plus splint, Outcome 2 Short‐term improvement in CTS symptoms (Phalen's sign) (3 months or less).
12.3. Analysis.

Comparison 12 Therapeutic ultrasound plus splint versus placebo ultrasound plus splint, Outcome 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less).
12.4. Analysis.

Comparison 12 Therapeutic ultrasound plus splint versus placebo ultrasound plus splint, Outcome 4 Short‐term improvement in functional ability (functional status score) (3 months or less).
12.5. Analysis.

Comparison 12 Therapeutic ultrasound plus splint versus placebo ultrasound plus splint, Outcome 5 Short‐term improvement in functional ability (grip strength) (3 months or less).
12.6. Analysis.

Comparison 12 Therapeutic ultrasound plus splint versus placebo ultrasound plus splint, Outcome 6 Short‐term improvement in motor distal latency (3 months or less).
12.7. Analysis.

Comparison 12 Therapeutic ultrasound plus splint versus placebo ultrasound plus splint, Outcome 7 Short‐term improvement in motor nerve conduction velocity (3 months or less).
12.8. Analysis.
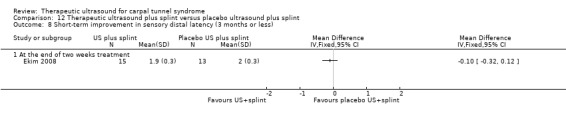
Comparison 12 Therapeutic ultrasound plus splint versus placebo ultrasound plus splint, Outcome 8 Short‐term improvement in sensory distal latency (3 months or less).
12.9. Analysis.

Comparison 12 Therapeutic ultrasound plus splint versus placebo ultrasound plus splint, Outcome 9 Short‐term improvement in palm‐wrist conduction velocity (3 months or less).
Comparison 13. Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term improvement in CTS symptoms (pain and/or paraesthesia) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Endpoint scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Change from baseline scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Short‐term improvement in CTS symptoms (frequency of awakening) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Endpoint scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Change from baseline scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in median nerve sensory distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Endpoint scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Change from baseline scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in sensory nerve action potential (SNAP) (endpoint) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Endpoint scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in sensory nerve action potential (SNAP) (change from baseline) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Change from baseline scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in median nerve motor distal latency (change from baseline) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Change from baseline scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Short‐term improvement in compound muscle action potential (CMAP) (endpoint) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Endpoint scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Short‐term improvement in compound muscle action potential (CMAP) (change from baseline) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Change from baseline scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Endpoint scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Change from baseline scores | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.1. Analysis.

Comparison 13 Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID, Outcome 1 Short‐term improvement in CTS symptoms (pain and/or paraesthesia) (3 months or less).
13.2. Analysis.

Comparison 13 Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID, Outcome 2 Short‐term improvement in CTS symptoms (frequency of awakening) (3 months or less).
13.3. Analysis.

Comparison 13 Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID, Outcome 3 Short‐term improvement in median nerve sensory distal latency (3 months or less).
13.4. Analysis.

Comparison 13 Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID, Outcome 4 Short‐term improvement in sensory nerve action potential (SNAP) (endpoint) (3 months or less).
13.5. Analysis.

Comparison 13 Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID, Outcome 5 Short‐term improvement in sensory nerve action potential (SNAP) (change from baseline) (3 months or less).
13.6. Analysis.

Comparison 13 Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID, Outcome 6 Short‐term improvement in median nerve motor distal latency (change from baseline) (3 months or less).
13.7. Analysis.

Comparison 13 Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID, Outcome 7 Short‐term improvement in compound muscle action potential (CMAP) (endpoint) (3 months or less).
13.8. Analysis.

Comparison 13 Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID, Outcome 8 Short‐term improvement in compound muscle action potential (CMAP) (change from baseline) (3 months or less).
13.9. Analysis.
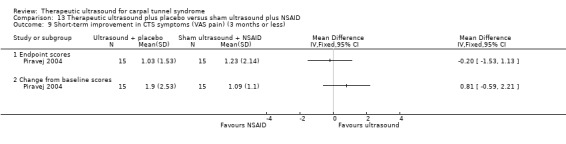
Comparison 13 Therapeutic ultrasound plus placebo versus sham ultrasound plus NSAID, Outcome 9 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
Comparison 14. Therapeutic ultrasound plus splint versus sham ultrasound plus splint.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 ITT analysis | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Per protocol analysis | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At end of two weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At end of two weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in functional ability (functional status score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At end of two weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in median nerve motor distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At end of two weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in median nerve sensory distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At end of two weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.

Comparison 14 Therapeutic ultrasound plus splint versus sham ultrasound plus splint, Outcome 1 Adverse events.
14.2. Analysis.

Comparison 14 Therapeutic ultrasound plus splint versus sham ultrasound plus splint, Outcome 2 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
14.3. Analysis.

Comparison 14 Therapeutic ultrasound plus splint versus sham ultrasound plus splint, Outcome 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less).
14.4. Analysis.

Comparison 14 Therapeutic ultrasound plus splint versus sham ultrasound plus splint, Outcome 4 Short‐term improvement in functional ability (functional status score) (3 months or less).
14.5. Analysis.

Comparison 14 Therapeutic ultrasound plus splint versus sham ultrasound plus splint, Outcome 5 Short‐term improvement in median nerve motor distal latency (3 months or less).
14.6. Analysis.

Comparison 14 Therapeutic ultrasound plus splint versus sham ultrasound plus splint, Outcome 6 Short‐term improvement in median nerve sensory distal latency (3 months or less).
Comparison 15. Therapeutic ultrasound plus splint versus therapeutic ultrasound plus splint plus ketoprofen phonophoresis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 ITT analysis | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Per protocol analysis | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At end of 2 weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At end of 2 weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Short‐term improvement in functional ability (functional status score) (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 At end of 2 weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Short‐term improvement in median nerve motor distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 At end of 2 weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Short‐term improvement in median nerve sensory distal latency (3 months or less) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At end of 2 weeks treatment (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At end of 2 weeks treatment (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 6 weeks after treatment ended (ITT analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 6 weeks after treatment ended (per protocol analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
15.1. Analysis.
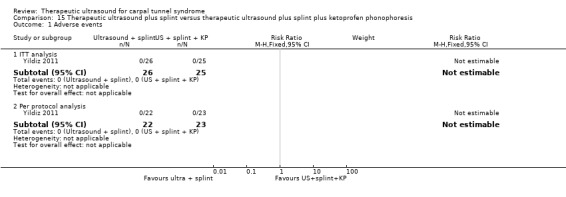
Comparison 15 Therapeutic ultrasound plus splint versus therapeutic ultrasound plus splint plus ketoprofen phonophoresis, Outcome 1 Adverse events.
15.2. Analysis.

Comparison 15 Therapeutic ultrasound plus splint versus therapeutic ultrasound plus splint plus ketoprofen phonophoresis, Outcome 2 Short‐term improvement in CTS symptoms (VAS pain) (3 months or less).
15.3. Analysis.

Comparison 15 Therapeutic ultrasound plus splint versus therapeutic ultrasound plus splint plus ketoprofen phonophoresis, Outcome 3 Short‐term improvement in CTS symptoms (symptom severity score) (3 months or less).
15.4. Analysis.

Comparison 15 Therapeutic ultrasound plus splint versus therapeutic ultrasound plus splint plus ketoprofen phonophoresis, Outcome 4 Short‐term improvement in functional ability (functional status score) (3 months or less).
15.5. Analysis.

Comparison 15 Therapeutic ultrasound plus splint versus therapeutic ultrasound plus splint plus ketoprofen phonophoresis, Outcome 5 Short‐term improvement in median nerve motor distal latency (3 months or less).
15.6. Analysis.

Comparison 15 Therapeutic ultrasound plus splint versus therapeutic ultrasound plus splint plus ketoprofen phonophoresis, Outcome 6 Short‐term improvement in median nerve sensory distal latency (3 months or less).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bakhtiary 2004.
| Methods | Randomised controlled trial No blinding reported by authors Randomisation occurred at the level of wrists, where participants with bilateral CTS received a different intervention for each wrist |
|
| Participants | Total n = 50 (90 wrists) randomised Intervention group n = 45 wrists Control group n = 45 wrists Sex not reported Mean ± SD age: Intervention group: 45 ± 17.1 yrs Control group: 48 ± 13.4 yrs Mean ± SD duration of CTS symptoms* Intervention group: 7.1 ± 6.9 months Control group: 6.7 ± 6.5 months Inclusion criteria: 1. Numbness in the median nerve distribution and night waking lasting more than one month 2. Positive Phalen’s test. 3. Positive Tinel’s test. 4. Participants had to fulfil standard electrophysiological criteria including prolongation of nerve conduction velocity (i.e. motor latency > 4 ms or sensory latency > 3.5 ms). Exclusion criteria: 1. Secondary entrapment neuropathies 2. Electroneurographic and clinical signs of axonal degeneration of the median nerve 3. Treated with ultrasound or low level laser therapy for the syndrome 4. Required regular analgesic or anti‐inflammatory drugs 5. History of steroid injection into the carpal tunnel, thyroid disease, diabetes, or systemic peripheral neuropathy |
|
| Interventions | Intervention: Ultrasound treatment was administered for 15 minutes per session to the area over the carpal tunnel at a frequency of 1 MHz and an intensity of 1.0 W/cm2, with pulsed mode duty cycle of 1:4 and a transducer area of 5 cm2, using an Enraf Sonopuls 434 machine with aquasonic gel as the couplant. The apparatus was initially standard and the output was controlled regularly by a simple under‐water radiation balance. A total of 15 ultrasound treatments were performed once a day, five times a week for three weeks. Control: Low‐level laser therapy was administered by applying a low intensity (9 J), infrared laser diode (Enraf, Endolaser 830 nm) at five points (1.8 J/point) over the course of the median nerve at the wrist. The output of the laser beam was controlled each session by a simple infrared photocell. A total of 15 laser therapies were performed once a day, 5 times a week for 3 weeks. |
|
| Outcomes | Outcomes assessed at baseline, at the end of three weeks treatment, and 4 weeks after treatment ended (7 weeks from baseline): 1. Pain using a zero to 10 VAS** 2. Pinch strength (N) using with a standard dynamometer between the tips of the thumb and the little finger** 3. Hand grip strength (N) using a handheld dynamometer: average force of three consecutive trials was calculated** 4. Nerve conduction: median motor distal latency (msec), median sensory distal latency (msec), compound muscle action potential amplitude (mV), sensory action potential amplitude (uV)** |
|
| Notes | *Measured as 'duration of current main complaints (months)' **Data reported only as change from baseline scores (no endpoint data reported). Analysis was undertaken at the wrist level for all outcomes, though some participants in each group had bilateral CTS. Bilateral cases had a different intervention applied to each wrist. The trialists did not report in the publication how the correlation between both wrists was accounted for in the analysis, and when contacted, confirmed that no such method was used. Therefore, a unit of analysis error is likely to have occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "As there were two categories of carpal tunnel syndrome patients, patients with bilateral involvement (n = 40), and patients with unilateral involvement (n = 10), a computer generated randomisation list was drawn up by the statistician for each category...Thus patients with both wrists affected were assigned randomly to one of the two following treatment groups: Group A, who received ultrasound in the right hand and low level laser therapy in the left hand; or Group B, who received low level laser therapy in the right hand and ultrasound in the left hand. The patients with one wrist affected were also assigned randomly to the following treatment groups: Group C, who received ultrasound treatment; and Group D, who received low level laser therapy treatment." Comment: The allocation sequence was probably adequately generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "....a computer generated randomisation list was drawn up by the statistician for each category. It was given to the physiotherapy department in two sets of sealed numbered envelopes, one set for bilateral carpal tunnel syndrome patients and one set for unilateral carpal tunnel syndrome patients. When the patients qualified to enter the study and had signed informed consent, according to their bilateral or unilateral involvement the appropriate numbered envelope was opened at the reception; the card inside indicated the patient's allocation to a treatment group. This information was then given to the physiotherapist to administer appropriate intervention." Comment: It is not clear whether the sealed numbered envelopes were opaque and sequentially numbered, therefore it is not clear whether the allocation sequence was adequately concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | Unclear risk | Comment: While unclear, participants are unlikely to have been blinded to which treatment they received, as there was no report that, for example, they were blindfolded to which wrist was receiving which treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The staff who assessed the outcomes were different from the staff administering the treatments, and they were blinded to the type of treatment (low level laser therapy or ultrasound) each patient had received." Comment: Staff administering the treatments are unlikely to have been blinded, but outcome assessors of objectively‐measured outcomes were likely to have been blinded successfully. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "In order to reduce the number lost to follow‐up, we guaranteed to complete the treatment regimen with more effective treatments if there was no benefit from the applied treatment at the end of the study. Thus, all patients completed the study to the end of the four‐week follow up period." Comment: Outcome data are likely to be complete |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes stated in the methods section of the publication were reported in the results in their pre‐specified way. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Baysal 2006.
| Methods | Randomised single‐blind controlled trial Blinded outcome assessors Randomisation occurred at the level of participants, not wrists (i.e. participants with bilateral CTS received the same intervention for both wrists) |
|
| Participants | Total n = 36 (72 wrists) randomised Intervention group 1 n = 12 (24 wrists) randomised; 12 (24 wrists) completed Intervention group 2 n = 12 (24 wrists) randomised; 8 (16 wrists) completed Intervention group 3 n = 12 (24 wrists) randomised; 8 (16 wrists) completed 0 males, 36 females Mean ± SD age: Intervention group 1: 47.8 ± 5.5 yrs Intervention group 2: 50.1 ± 7.3 yrs Intervention group 3: 51.4 ± 5.2 yrs Mean± SD duration of CTS symptoms: Intervention group 1: 1.5 ± 1.6 yrs Intervention group 2: 1.4 ± 0.8 yrs Intervention group 3: 1.4 ± 0.8 yrs Inclusion criteria: 1. Subjectively reported history of paraesthesia or pain in the median nerve distribution, nocturnal pain, and dysaesthesia 2. Tinel’s test, Phalen’s test, pain measurement, two‐point discrimination test, and grip and pinch strength measurement (no information provided on which criteria for these physician‐assessed outcomes had to be fulfilled by participants) Exclusion criteria: 1. Secondary entrapment neuropathies 2. Treated with ultrasound for CTS 3. Required regular analgesic or anti‐inflammatory drugs. 4. Clinical sign for axonal degeneration of the median nerve (thenar atrophy) on electromyographic examination of the abductor pollicis brevis muscle 5. Evidence of denervation (abnormal spontaneous activity in the form of fibrillations and positive sharp waves) on electromyographic examination of the abductor pollicis brevis muscle 6. History of steroid injection into the carpal tunnel, thyroid disease, diabetes, systemic peripheral neuropathy, pregnancy, or splint use |
|
| Interventions | Intervention group 1: Splinting and exercise therapy Intervention group 2: Splinting and ultrasound therapy Intervention group 3: Splinting, exercise, and ultrasound therapy (No description of how these combinations of treatments were completed by participants over the three week treatment period) Splinting ‐ A custom‐made neutral volar splint was given to patients. The patients were instructed to wear the splints all night and during the day for 3 weeks. Ultrasound therapy ‐ Ultrasound treatment was administered 15 min per session to the palmar carpal tunnel area at a frequency of 1 MHz and intensity of 1.0 W/cm2, pulsed mode 1 : 4, with a transducer of 5 cm2 (Electronica Pagani FP‐942/S) and with aquasonic gel as the couplant. The apparatus was standardized initially, and the output was controlled regularly by a simple underwater radiation balance. A total of 15 ultrasound treatments were performed once a day, five times a week, for 3 weeks. Exercise therapy ‐ Participants were instructed to perform nerve‐and tendon gliding exercises developed by Totten and Hunter. Brochures describing exercises were also given to patients.The exercises were applied as five sessions daily. Each exercise was repeated 10 times at each session. Exercise treatment was continued for 3 weeks. |
|
| Outcomes | Outcomes assessed at the first treatment session, at the end of therapy, and 8 weeks after treatment ended (11 weeks from baseline):* 1. Pain using a visual scale (VAS), on which the patients could indicate their assessment along a distance of 10 cm, ranging from 0 (no pain at all) to 10 (the most intense pain that I can imagine)* 2. Tinel's sign (rated as positive or not) 3. Phalen's sign (rated as positive or not) 4. Two‐point discrimination: performed on the pulp of the three radial digits 5. Symptoms using carpal tunnel questionnaire (rates 11 items on ordinal scale 1: mildest, to 5: most severe)* (Levine 1993) 6. Hand function using carpal tunnel questionnaire (rates 8 items on ordinal scale 1: no difficulty with the activity, to 5: cannot perform the activity at all)* (Levine 1993) 7. Hand grip strength using a handheld dynamometer: average force of three consecutive trials calculated* 8. Pinch grip strength using a standard dynamometer between the tips of the thumb and the little finger: average force of three consecutive trials calculated* 9. Nerve conduction: Median motor distal latency (msec), and sensory distal latency (msec)* 10. Satisfaction using a question asked over the telephone: rated as excellent if a patient is asymptomatic, good: rarely symptomatic, fair: symptomatic only during compelling activity or poor: continuing symptoms (without relief following treatment) (only measured at the final follow‐up). Authors report that patients' satisfaction investigation was performed at an average of 11 ± 4.5 months |
|
| Notes | *Endpoint scores for all outcomes were reported in the trial publication. Change from baseline scores were reported for these outcomes only if the difference between time points was statistically significant (P < 0.05). As the risk of outcome reporting bias was high for these change from baseline scores, endpoint scores (which were completely reported) were entered in RevMan. Analysis was undertaken at the wrist level for all outcomes, though all participants in each group had bilateral CTS. Bilateral cases had the same intervention applied to each wrist. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomization list was created by a biostatistician." Comment: The allocation sequence was probably adequately generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Computer‐generated randomization list was created by a biostatistician. It was given to the physiotherapy department in sealed numbered envelopes. When the patients qualified to enter the study, appropriate numbered envelope was opened at the reception; the card inside indicated the patient's allocation to a treatment group." Comment: It is not clear whether the sealed numbered envelopes were opaque and sequentially numbered, therefore it is not clear whether the allocation sequence was adequately concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | High risk | Comment: Not reported, and given the nature of the intervention it is unlikely that participants were not aware of which group they were assigned to. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The staff who assessed the outcomes were different from the staff administering the treatments and were blinded to the type of treatment each patient had received." Comment: Outcome assessors of objectively measured outcomes were probably blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: Twenty‐eight patients (56 wrists) completed the study. The eight dropouts are described as follows: two patients (group II) underwent surgery, two patients (group II) were lost to follow‐up. In group III, two patients were lost to follow‐up, and another two patients (group III) refused electrophysiologic study due to improvement of symptoms." Comment: The eight randomised participants who were drop‐outs and losses to follow‐up were clearly described, |
| Incomplete outcome data (attrition bias) After 3 months | High risk | Comment: There is no explanation for why results of the patient satisfaction questionnaire is based on fewer than 28 participants with 56 wrists. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes were fully reported as endpoint scores at the end of treatment and at eight weeks follow‐up. The authors also reported change from baseline scores for some (not all) of the outcomes, but numerical data suitable for inclusion in a meta‐analysis were only reported if the effect estimate was statistically significant. For non‐significant effects, the authors only reported that the result was "NS". Given endpoint scores were available and no meta‐analysis was performed, this selective reporting of data is unlikely to affect the results. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Bilgici 2010.
| Methods | Study design: Randomised controlled trial It is unclear whether randomisation occurred at the level of participants or wrists, and whether all bilateral CTS participants received the same or different intervention for each wrist |
|
| Participants | Details of sampling frame: Total N randomised = 34 participants (49 wrists) randomised; 31 participants (45 wrists) completed Intervention group 1 N = 16 participants (24 wrists) randomised; 15 participants (23 wrists) completed Intervention group 2 N = 18 participants (25 wrists) randomised; 16 participants (22 wrists) completed Group 1: 5 males; 10 females Group 2: 4 males; 12 females Group‐specific sex only reported for participants who completed trial. Overall 24 women and 10 men were randomised Mean ± SD (range) age: Group 1: 47.33 ± 7.44 Group 2: 44.15 ± 9.30 Group‐specific age only reported for participants who completed trial Mean ± SD (range) duration of CTS symptoms: Group 1: 46.33 ± 34.04 months Group 2: 46.29 ± 61.36 months Group‐specific duration of symptoms only reported for participants who completed trial Inclusion criteria: 1. Had clinical symptoms and signs of CTS confirmed by standard electrodiagnosis, with no abnormalities in the radial or ulnar nerve. Exclusion criteria: 1. Had thenar atrophy or spontaneous activity (fibrillation potentials and positive sharp waves) on electromyographic examination of the abductor pollicis brevis muscle 2. Pregnant 3. Had previous wrist trauma 4. Had a history of steroid injection into the carpal tunnel 5. Had rheumatic diseases 6. Had cervical radiculopathy 7. Had diabetes or other pathologic conditions predisposing to peripheral neuropathies |
|
| Interventions | Group 1: Ultrasound treatment delivered under water at a frequency of 3MHz and with an intensity of 1.5W/cm2 for five minutes, five times per week for four weeks. Group 2: Local corticosteroid injection plus neutral‐positioned wrist splint worn as much as possible during the day and night for four weeks. Local corticosteroid injection was given using a 22‐gauge needle at the proximal part of the carpal tunnel to the wrist crease just medial to the tendons of the flexor radial muscle involving a single 4mg dexamethasone injection without lidocaine |
|
| Outcomes | Outcomes assessed at baseline, at the end of the four week treatment period, and at four weeks post‐treatment. 1. Symptoms using the Turkish‐translated Boston Carpal Tunnel Questionnaire, calculated as the mean of 11 items scored from 1 (mildest) to 5 (most severe) (Levine 1993) 2. Pain using a VAS 3. Function using the Turkish‐translated Boston Carpal Tunnel Questionnaire, calculated as the mean of eight items scored from 1 (no difficulty in the activity to 5 (cannot perform the activity at all) (Levine 1993) 4. Grip strength measured using a hand‐held dynamometer, where the participants positioning was standardised and the average force of three consecutive trials was calculated 5. Two‐point discrimination performed on the pulp of three radial digits and the mean recorded (not an outcome of interest to the review) 6. Nerve conduction: median nerve motor distal latency (msec), median sensory nerve conduction velocity (m/sec) 7. Adverse effects |
|
| Notes | Analysis was undertaken at the wrist level for all outcomes, though some participants in each group had bilateral CTS. It is not clear whether bilateral CTS participants received the same intervention for both wrists. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomization was performed by using sequentially numbered and sealed opaque envelopes. Following the baseline assessment, patients were randomised to either ultrasound treatment (group A) or local corticosteroid injection plus splinting (group B)." Comment: No information on how the random sequence was generated was provided. |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was performed by using sequentially numbered and sealed opaque envelopes. Following the baseline assessment, patients were randomised to either ultrasound treatment (group A) or local corticosteroid injection plus splinting (group B)." Comment: The allocation sequence was probably adequately generated. |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | High risk | Comment: Due to the nature of the interventions delivered (ultrasound versus splint plus corticosteroid injection), it is unlikely that participants and personnel were unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All patients were examined by the same physician". Comment: The authors did not report whether the outcome assessor of objective outcomes was blind to treatment allocation |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "A total of 49 hands of 34 patients (24 women and 10 men) were enrolled in this study. 16 patients were randomly assigned to the group A, and 18 patients were randomly assigned to the group B. Three patients did not complete the 8 week follow‐up. One patient in group B did not allow to be injected into her hand after randomization. Two patients (one in each group), could not be reached and were lost to follow‐up. They were excluded from the study and data analysis. Thus, 15 patients (23 hands) in the Group A, and 16 patients (22 hands) in the Group B completed the follow‐up at 8 weeks". Quote: "The per‐protocol analyses included 45 hands". Comment: The overall amount of attrition, and reasons for this is small and relatively similar across groups, and unlikely to have affected the results of outcomes. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes specified in the Methods section were reported in the Results section in sufficient detail to be included in a meta‐analysis. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Dincer 2009.
| Methods | Randomised controlled trial Blinded outcome assessors Randomisation occurred at the level of participants, not wrists (i.e. participants with bilateral CTS received the same intervention for both wrists) |
|
| Participants | Total n = 60 (120 wrists) randomised
Intervention group 1 n = 40 wrists randomised; 34 wrists completed
Intervention group 2 n = 40 wrists randomised; 30 wrists completed Intervention group 3 n = 40 wrists randomised; 36 wrists completed 0 males; 60 females Mean ± SD age*: Intervention group 1 51.8 ± 6.6 yrs Intervention group 2 49.7 ± 9.5 yrs Intervention group 3 52.2 ± 9.1 yrs Inclusion criteria: 1. Diagnosed with mild to moderate CTS according to the American Association of Electrodiagnostic Medicine guidelines Exclusion criteria: 1. Diagnosed with severe CTS according to the American Association of Electrodiagnostic Medicine guidelines 2. Underlying metabolic disorders such as diabetes mellitus, thyroid or kidney disease 3. Connective tissue disorders 4. Malignancy 5. Distal radial fracture 6. Cervical radiculopathy 7. Brachial plexopathy 8. Tenosynovitis 9. Fibromyalgia 10. Any other CTS treatment or surgical procedure during the past year 11. Pregnant |
|
| Interventions | Intervention group 1: Neutral standard light‐weight wrist splint worn at night and during aggravating daytime activities for three months Intervention group 2: Neutral standard light‐weight wrist splint worn at night and during aggravating activities for three months plus ultrasound therapy administered to each hand for 3 minutes per session, with 10 sessions performed once a day, five times a week for two weeks. Ultrasound was administered at a frequency of 3 MHz and an intensity of 1.0 W/cm2 in a continuous mode Intervention group 3: Neutral standard light‐weight wrist splint worn at night and during aggravating activities for three months plus low‐level laser therapy applied to three points over the course of the median nerve for 30 seconds at each point, with 10 sessions performed once a day, five times a week for two weeks. An infrared GaAs diode laser with a wavelength of 904 nm, frequency range of 5‐7000 Hz, pulse duration of 200 nsec, maximum power output of 27 W, average power of 2.4 mW, and spot size of 0.07cm2 was used. |
|
| Outcomes | Outcome assessed at baseline, one month and three months after end of treatment 1. Pain using zero to 10 VAS (0 = "no pain", 10 = "the most intense pain one can imagine") 2. Symptoms using Boston carpal tunnel questionnaire (rates 11 items on ordinal scale 1: no symptom, to 5: the most severe symptom) (Levine 1993) 3. Function using Boston carpal tunnel questionnaire (rates 8 items on ordinal scale 1: no symptom, to 5: the most severe symptom) (Levine 1993) 4. Patient satisfaction measured using a five point scale, including "completely satisfied", "almost satisfied", "moderately satisfied", "somewhat satisfied", and "dissatisfied"** 5. Nerve conduction***: median nerve motor distal latency, median nerve motor conduction velocity, compound muscle action potential at the wrist and elbows, second digit‐wrist median nerve sensory velocity |
|
| Notes | *The authors reported the age of randomised participants, not those who completed the study **The authors dichotomised this outcome into "satisfied" (based on rating either "almost satisfied" or "completely satisfied") or not ***The authors reported an outcome, "number of completely normal hands according to electroneuromyography at 3 months", but did not report what the specific criteria for "completely normal" Analysis was undertaken at the wrist level for all outcomes, though all participants in each group had bilateral CTS. Bilateral cases had the same intervention applied to each wrist. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed by the use of numbered envelopes". Comment: Not clear how, and whether or not, the randomisation sequence was adequately generated. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed by the use of numbered envelopes". Comment: Not clear whether the allocation sequence was adequately concealed (i.e. whether the numbered envelopes were sealed and opaque and sequentially numbered). |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | High risk | Quote: "Ultrasound therapy was administered to each hand for 3 min per session, on the area over the carpal tunnel…with aquasonic gel. Quote: "Laser therapy was applied to three points over the course of the median nerve at the wrist. The laser probe was applied directly and perpendicularly in contact with the skin for 30 sec at each point..At each treatment session, the patients and physiotherapist wore protective glasses." Comment: It is possible participants were aware of treatment allocation based on the different treatment modalities delivered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At the beginning, all of the patients were assessed by the same physiatrist (E.C.) and at the first and third month the assessments were performed by another physiatrist who was blinded to treatment modality (M.Z.K.). Electrodiagnostic evaluations were performed by another physiatrist (U.D.) Comment: Blinding of outcome assessors was probably done. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "A total of 60 females patients with bilateral mild to moderate CTS (120 hands) were included in this study and randomised into three groups (40 hands in each group). Four patients did not finish therapy and six patients did not come to follow‐up assessments, thus the study was completed with a total of 100 hands; 34 in the Sp group, 30 in the SpUS group, and 36 hands in the SpLLL group" Comment: The number of drop‐outs in each group was clearly reported, was small, and relatively similar across groups. |
| Selective reporting (reporting bias) | High risk | Comment: The authors reported in the Methods section that median nerve motor distal latency, median nerve motor conduction velocity, compound muscle action potential at the wrist and elbows, and median nerve sensory velocity were measured, however only reported the results in numerical format suitable for meta‐analysis for the outcomes median nerve motor distal latency and second digit‐wrist median nerve sensory velocity. Further, the authors reported a new outcome in the Results section, "number of completely normal hands based on electroneuromyography at 3 months", but did not report how "completely normal" was defined in the Methods section. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Duymaz 2012.
| Methods | Randomised double‐blind controlled trial Blinded participants (only for two of the three groups) and outcome assessors (only for neurophysiologic parameters) |
|
| Participants | Total n = 58 (58 wrists) randomised Intervention group 1 n = 20 (20 wrists) randomised and completed Intervention group 2 n = 20 (20 wrists) randomised and completed Intervention group 3 n = 18 (28 wrists) randomised and completed Intervention group 1: 1 male, 19 females Intervention group 2: 2 males, 18 females Intervention group 3: 0 males, 18 females Mean ± SD age: Intervention group 1: 51.25 ± 6.88 yrs Intervention group 2: 51.5 ± 8.08 yrs Intervention group 3: 53.7 ± 8.35 yrs Mean ± SD duration of CTS symptoms: Not reported Inclusion criteria: 1. Diagnosis of idiopathic CTS based on provocation tests and electromyography during examination and complaints of numbness, tingling, weakness and pain in the hands lasting at least three months Exclusion criteria: 1. Presence of symptoms for more than a year 2. Acute findings 3. History of steroid injections or physical therapy 4. Systemic disease 5. Two‐point discrimination distance of greater than 6 mm 6. Presence of thenar atrophy, more proximal complex neuropathy, cervical discopathy, cervical Da Costa’s syndrome, shoulder, elbow, wrist, or finger problems (frozen shoulder, epicondylitis, cubital tunnel syndrome, history of wrist fracture, trigger finger) 7. Presence of a pacemaker 8. Other etiological causes leading to CTS, e.g. rheumatoid arthritis, gout, pregnancy 9. Previous CTS surgery |
|
| Interventions | Intervention group 1: Therapeutic ultrasound (for 5 min per session, once a day 5 times a week for 3 weeks; intensity was 0.8 W/cm2, and frequency was 1 MHz) plus 3 sets of 10 nerve and tendon gliding exercises performed every day plus neutral wrist splint worn every night plus activity modification training. Intervention group 2: Dexamethasone iontophoresis (dexamethasone sodium diphosphonate 0.4% solution was poured in the activated carbon electrode pad of 25cm2 placed over the carpal tunnel, and administration was performed by applying a current of 2 mA for 20 minutes) plus three sets of 10 nerve and tendon gliding exercises performed every day plus neutral wrist splint worn every night plus activity modification training. Intervention group 3: Placebo iontophoresis (water was poured in the activated carbon electrode pad of 25cm2 placed over the carpal tunnel, and administration was performed by applying a current of 2 mA for 20 minutes) plus three sets of 10 nerve and tendon gliding exercises performed every day plus neutral wrist splint worn every night plus activity modification training. |
|
| Outcomes | Outcome assessed at the end of 3 weeks treatment and 3 months after the end of treatment 1. Pain on movement, pain at rest, and pain at night, using a VAS* 2. Symptoms using Boston carpal tunnel questionnaire (BCTQ) (rates 11 items on ordinal scale 1: no symptom, to 5: the most severe symptom) (Levine 1993) 3. Function using Boston carpal tunnel questionnaire (BCTQ) (rates 8 items on ordinal scale 1: no symptom, to 5: the most severe symptom) (Levine 1993) 4. Function using the Health Assessment Questionnaire 5. Wrist flexion/extension range of motion (not an outcome of interest to the review) 6. Muscle test for the muscles in the carpal tunnel region and the abductor pollicis brevis (using the five points scale) (not an outcome of interest to the review) 7. Grip strength** 8. Pinch strength** 9. Two‐point discrimination (not an outcome of interest to the review) 10. Sensation using the Semmes‐Weinstein monofilaments (not an outcome of interest to the review) 11. Phalen's test** 12. Reverse Phalen's test** 13. Tinel's test** 14. Carpal compression test** 15. Neurophysiological parameters (sensory nerve distal latency, sensory nerve amplitude, sensory nerve conduction velocity, motor nerve distal latency, motor nerve amplitude, motor nerve conduction velocity, compound muscle action potential of the abductor pollicis brevis muscle)**** |
|
| Notes | *Trialists reported outcome data for change from baseline to end of treatment and change from end of treatment to three months follow‐up. We only extracted the change from baseline to end of treatment values. **No outcome data sufficient for entry into RevMan was reported in the study publication. ***Trialists reported means and SDs for each of the six neurophysiologic parameters, but it is not clear whether the data are endpoint values at end of treatment, endpoint values at three months follow‐up, change from baseline to end of treatment values, or change from baseline to three months follow‐up values. For this reason, we did not extract the mean (SD) outcome data reported for any the six neurophysiologic parameters. All participants contributed only one CTS‐affected wrist to the study. Therefore, a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly divided into three groups using computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The patients were randomly divided into three groups using computer‐generated random numbers. After the examination of each patient, the physician sent the number in an envelope to the physiotherapist." Comment: Trialists did not report whether the envelopes were sequentially numbered, sealed, and opaque, so it is unclear whether all essential safeguards to conceal the allocation sequence were utilised. |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | High risk | Quote: "The persons’ who performed the statistical analysis and electrophysiological assessment was blind to the therapy as were the patients who received dexamethasone iontophoresis or sham iontophoresis." Comment: Participants and personnel delivering ultrasound were not blind to treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The persons’ who performed the statistical analysis and electrophysiological assessment was blind to the therapy as were the patients who received dexamethasone iontophoresis or sham iontophoresis." Comment: The only outcome assessed by a blinded assessor was neurophysiological parameters. Other objective outcomes (e.g. grip strength) and self‐reported outcomes were assessed by unblinded assessors. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: No drop‐outs, losses to follow‐up or exclusions were reported, |
| Selective reporting (reporting bias) | High risk | Comment: Outcome data was not fully reported for all outcomes. For some outcomes (e.g. Phalen's test, grip strength, pinch strength), trialists only reported whether the differences between groups were statistically significant or not. For other outcomes (e.g. wrist extension and flexion range of motion), no information about the results was reported. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Ebenbichler 1998.
| Methods | Randomised, triple‐blind, placebo‐controlled trial Blinded participants, personnel and outcome assessors Randomisation occurred at the level of wrists, where participants with bilateral CTS received a different intervention for each wrist |
|
| Participants | Total n = 45 (90 wrists) randomised
Intervention group n = 45 (45 wrists)
Control group n = 45 (45 wrists) Sex not reported Mean ± SD age: 51 ± 15 yrs Inclusion criteria: 1. Bilateral idiopathic CTS confirmed with electrodiagnostic testing 2. Mild to moderate pain lasting longer than 3 months 3. Informed written consent Exclusion criteria: 1. Secondary entrapment neuropathies 2. Systemic disease 3. Electroneurographic and clinical signs of median nerve axonal degeneration 4. Previous CTR 5. Previous ultrasound treatment 6. History of steroid injection into carpal tunnel 7. Regular analgesic or anti‐inflammatory drug requirements |
|
| Interventions | Intervention: Pulsed ultrasound therapy using 1.0 W/cm2 intensity and 1 MHz frequency, 15 minute session daily, 5 times a week for 2 weeks, followed by twice a week for 5 weeks Control: Placebo ultrasound therapy using 0.0 W/cm2 intensity, 15 minute session daily, 5 times a week for 2 weeks, followed by twice a week for 5 weeks |
|
| Outcomes | Outcome assessed at 2 weeks (after 10 sessions), 7 weeks (at end of treatment) and 6 months after end of treatment 1. Symptoms using zero to 10 VAS (0 = "no complaints at all", 10 = "the most intense complaints I can imagine") 2. General symptom improvement (ordinal scale 1 = free of symptoms, 5 = much worse) (at 7 weeks and 6 months only) 3. Sensation using sharp pin wheel and VAS. 4. Grip strength in kilograms using Preston dynamometer 5. Pinch strength in kilograms using Preston dynamometer 6. Nerve conduction: median distal motor latency, sensory nerve action potentials*, sensory nerve conduction velocity, median motor nerve conduction velocity* 7. Medication use 8. Adverse effects 9. Return to work (selectively reported) |
|
| Notes | *No data reported Sex of participants not reported Mean and SD endpoint values for symptoms, sensation, grip strength, pinch strength and nerve conduction outcomes were provided by authors to facilitate entry into RevMan, as data were reported in the publication as mean change from baseline (with 95% CIs of the change from baseline). Analysis was undertaken at the wrist level for all outcomes of interest to the review, though all participants in each group had bilateral CTS. Bilateral cases had a different intervention applied to each wrist. The trialists did not report in the publication how the correlation between both wrists was accounted for in the analysis, and when contacted, confirmed that no such method was used. Therefore, a unit of analysis error is likely to have occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation list was produced with a random number generator of a popular spreadsheet program (Lotus Symphony)". |
| Allocation concealment (selection bias) | Low risk | Quote: "An ultrasound therapist not involved in the treatment allocated the dominant wrist of each consecutive patient to ultrasound or sham treatment‐by means of sequentially numbered sealed opaque envelopes containing the group allocation..." |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | Low risk | Quote: "The patients, study physician, and the therapists who delivered the ultrasound treatment were all unaware of the treatment allocation". Quote: "An on/off key introduced into the transducer circuit allowed mock insonation to be given to a sham group without affecting the normal ultrasonic output when the key was turned to the "on" position". Quote: "Intensity of ultrasound treatment was below sensitivity threshold." Comment: Outcomes including subjective symptom assessment, sensation, and overall symptom improvement were self‐reported by participants who were blinded to group assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "(The ultrasound therapist not involved in the treatment...) was the only person aware of treatment allocation during the trial". Comment: Outcomes including grip strength, pinch strength and nerve conduction studies were probably measured and assessed by personnel blinded to group assignments. |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Comment: Eleven participants were not included in the analysis of outcomes measured at two weeks and seven weeks because of non‐compliance in keeping appointments (8) and excessive pain requiring additional therapeutic measures (3). It is unclear whether it was still possible to assess and include some or all of the outcome data for these individuals or whether they had been inappropriately excluded from the analysis. |
| Incomplete outcome data (attrition bias) After 3 months | Unclear risk | Comment: Four participants were not accounted for in outcomes assessed at six months follow‐up. Reasons were not provided in order to determine whether these were genuine losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | High risk | Comment: Nerve conduction studies assessing median motor nerve conduction and sensory nerve action potentials were conducted but results were not reported. Change scores were reported for all continuous outcomes probably due to slight group imbalances at baseline that were noted by the authors (mean end point scores and standard deviations have since been obtained for some time points through personal communication with the authors). It was reported that three participants were off work however, work status was not stated as an outcome measure in the methods section. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Ekim 2008.
| Methods | Randomised, single‐blind placebo‐controlled trial Blinded participants |
|
| Participants | Total n = 28 (28 wrists) randomised
Intervention group n = 15 wrists randomised; 15 wrists completed
Control group n = 13 wrists randomised; 13 wrists completed 8 males; 20 females Mean ± SD age*: Intervention group 50.73 ± 10.5 yrs Control group 46.23 ± 10.6 yrs Median (interquartile range) duration of symptoms Intervention group 2.5 (1.5, 3.75) years Control group 2 (1.875, 3) years Inclusion criteria: 1. Diagnosed with CTS, as based on at least one of the following: (i) abnormal sensory nerve conduction of the palm‐wrist segment; or (ii) prolonged motor distal latency. The median motor distal latency over 3.9 msec or reduced sensory nerve conduction velocity of the palm‐wrist segment below 35.2 m/sec was accepted as CTS Exclusion criteria: 1) Diabetes mellitus, hypothyroiditis, acromegaly, rheumatoid arthritis, cervical radiculopathy or severe polyneuropathy and conditions that occur secondarily to causes such as wrist trauma, 2) Patients who had physical therapy or steroid injection for CTS within the last 3 months, 3) The presence of muscle atrophy, anaesthesia or ongoing (unhealed or intractable) pain, 4) The presence of reinnervation or fibrillation potentials on EMG, 5) Any medical problem that does not allow the patients to be treated by US. |
|
| Interventions | Intervention group: Active continuous ultrasound of 1.5 W/cm2 intensity and 3 MHz frequency for five minutes, five days a week for two weeks, plus neutral splint worn at night for two weeks. Control group: Placebo ultrasound of 0.0 W/cm2 intensity for five minutes, five days a week for two weeks, plus neutral splint worn at night for two weeks. |
|
| Outcomes | Outcomes assessed at baseline and at the end of two weeks treatment: 1. Pain using a 100 mm VAS 2. Symptoms using the Turkish‐translated version of the Levine questionnaire (Levine 1993) 3. Function using the Turkish‐translated version of the Levine questionnaire (Levine 1993) 4. Grip strength using a dynamometer, with the average of three measurements recorded 5. Tinel's test 6. Phalen's test 7. Nerve conduction: motor distal latency, motor nerve conduction velocity, sensory distal latency, palm‐wrist conduction velocity |
|
| Notes | Article is published in Turkish, and was translated by a translator organised by the Neuromuscular Disease Review Group. All participants had unilateral CTS. Therefore, a unit of analysis error resulting from the correlation between two wrists in bilateral CTS participants could not have occurred. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (translated from Turkish to English): "28 patients with idiopathic CTS fulfilling the criteria for the study were randomly allocated to 2 groups." Comment: No information on how the random sequence was generated was reported. |
| Allocation concealment (selection bias) | Unclear risk | Quote (translated from Turkish to English): "28 patients with idiopathic CTS fulfilling the criteria for the study were randomly allocated to 2 groups." Comment: No information on how the random sequence was concealed from those responsible for recruitment was reported. |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | Low risk | Quote (translated from Turkish to English): "This study was planned as a single‐blinded, randomized and prospective study..."
Quote (translated from Turkish to English): "28 patients with idiopathic CTS fulfilling the criteria for the study were randomly allocated to 2 groups. Group 1 patients (n: 15) underwent active continuous US of 1,5 W/cm2 dose while the Group 2 patients had placebo US (0,0 W/cm2). The generator of US was the Enraf Nonius Sonoplus 434 with a frequency of 3 MHz and a head size of 0,5 cm2. Aquasonic gel was used for coupling. The same equipment was used for placebo application. The equipment was switched on however no US waves were applied to the treatment area." Comment: Participants were probably blinded to the treatment they were receiving. However, personnel delivering the treatment were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (translated from Turkish to English): "This study was planned as a single‐blinded, randomized and prospective study..."
Quote (translated from Turkish to English): "28 patients with idiopathic CTS fulfilling the criteria for the study were randomly allocated to 2 groups. Group 1 patients (n: 15) underwent active continuous US of 1,5 W/cm2 dose while the Group 2 patients had placebo US (0,0 W/cm2). The generator of US was the Enraf Nonius Sonoplus 434 with a frequency of 3 MHz and a head size of 0,5 cm2. Aquasonic gel was used for coupling. The same equipment was used for placebo application. The equipment was switched on however no US waves were applied to the treatment area." Quote (translated from Turkish to English): "The same person performed the treatments to the palmar carpal tunnel area in a circular pattern for 5 minutes every 5 days of 2 weeks for both groups...The same physician performed the clinical evaluations of all patients at onset and the end of the study" Comment: The individual responsible for delivering the treatment was the same individual or assessed outcomes, and was not blind to treatment. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote (translated from Turkish to English): "...patients were evaluated daily and there were no patients who discontinued the study." Comment: No drop‐outs or losses to follow‐up were reported. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcomes reported in the Methods section of the publication were reported in the Results section of the publication. Outcomes were all reported as means and SDs, except for the outcome, VAS pain, which was reported as medians and interquartile ranges because the data were skewed. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Koyuncu 1995.
| Methods | Randomised, double‐blind clinical trial Blinded participants and outcome assessors It is unclear whether randomisation occurred at the level of participants or wrists, and whether all bilateral CTS participants received the same or different intervention for each wrist |
|
| Participants | Total n = 16 participants (21 wrists) randomised
Group 1 n = 10 wrists randomised
Group 2 n = 11 wrists randomised 1 male; 15 females Median ± SD age: 49.4 ± 2.7 yrs Inclusion criteria: 1. Clinical diagnosis of CTS based on physical findings and confirmed with electrodiagnostic testing (detail not specified) Exclusion criteria: None stated |
|
| Interventions | Group 1: Circular ultrasound therapy over volar wrist surface using 1.0 W/cm2 intensity and 1MHz frequency, 8 minute session, 5 days per week, for 4 weeks (total of 20 sessions) Group 2: Circular ultrasound therapy over volar wrist surface using 1.0 W/cm2 intensity and 3MHz frequency, 8 minute session, 5 days per week, for 4 weeks (total of 20 sessions) |
|
| Outcomes | Outcome assessed weekly and at end of treatment (4 weeks) 1. Pain using ordinal scale 0‐3 (0 = no pain, 1 = mild, 2 = moderate, 3 = severe) 2. Paraesthesiae using ordinal scale 0‐3 (0 = none, 1 = mild, 2 = moderate, 3 = severe) 3. Superficial touch sensation using dichotomous scale (0 = normal, 1 = decreased) 4. Large object grasping using dichotomous scale (0 = normal, 1 = decreased) 5. Small object grasping using dichotomous scale (0 = normal, 1 = decreased) 6. Motor nerve distal transmission delay* 7. Sensory nerve transmission delay* 8. Tinel's sign 9. Phalen's sign |
|
| Notes | Article is published in Turkish, and was translated by a translator organised by the Neuromuscular Disease Review Group. Attempts to clarify allocation method with authors were unsuccessful *Note. Only median values for neurophysiological endpoints were published by authors. Attempts to obtain mean and SD data were unsuccessful. Analysis was undertaken at the wrist level for all outcomes, though some participants in each group had bilateral CTS. It is not clear whether bilateral CTS participants received the same intervention for both wrists. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly divided into two groups". Comment: Insufficient information to determine whether the method used to generate the allocation sequence was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to determine whether allocation was concealed. |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | Low risk | Comment: The study is described as double‐blinded however it does not specify who was blinded in the trial. It is likely that participants were blinded which would minimise bias in the assessment of self‐reported outcomes including pain, paraesthesia, and superficial touch sensation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: The study is described as double blind, however the authors do not specify who was blinded (i.e. those delivering the intervention or those assessing outcomes). |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: There are no apparent losses to follow‐up or exclusions in the results reported from this study. |
| Selective reporting (reporting bias) | High risk | Comment: Outcomes were assessed every week throughout the 4 week treatment period however, only results for before and after treatment are reported. Results for motor nerve distal transmission delay and sensory nerve transmission delay may have been selectively reported as only median values and mean change scores (without measures of variance) were reported. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Oztas 1998.
| Methods | Randomised, single‐blind, placebo‐controlled trial Blinded participants Randomisation occurred at the level of wrists, with no constraint that all participants' wrist be allocated to the same or different treatments |
|
| Participants | Total n = 18 (30 wrists) randomised
Intervention group 1 n = 7 (10 wrists) randomised
Intervention group 2 n = 9 (10 wrists) randomised
Control group n = 9 (10 wrists) randomised 0 males, 18 females Mean ± SD age: 52 ± 7 yrs Inclusion criteria: 1. Clinical diagnosis of CTS confirmed with electrodiagnostic studies 2. Symptom duration greater or equal to 6 months Exclusion criteria: 1. Diabetes mellitus 2. Rheumatic disease 3. Acute trauma 4. Pregnancy 5. Physical or medical therapy in previous month 6. Corticosteroid injection in previous 3 months 7. Serious medical problems interfering with electrodiagnostic studies 8. Medical problems contraindicating use of ultrasound 9. Muscle atrophy, anaesthesia or intractable pain due to CTS |
|
| Interventions | Intervention group 1: Continuous ultrasound therapy using 1.5 W/cm2 intensity and 3 MHz frequency, 5 minute session, 5 days per week, for 2 weeks Intervention group 2: Continuous ultrasound therapy using 0.8 W/cm2 intensity and 3 MHz frequency, 5 minute session, 5 days per week, for 2 weeks Control: Placebo treatment using 0.0 W/cm2 intensity without energy emission, 5 minute session, 5 days per week, for 2 weeks |
|
| Outcomes | Outcome assessed at 2 weeks 5 days 1. Pain severity (100mm horizontal VAS) 2. Symptoms* (nocturnal, day pain, paraesthesia on ordinal scale: 0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe) 3. Nocturnal waking* (ordinal scale: 0 = never wake, 1 = awaken 1‐2 times a week, 2 = awaken 3‐6 times per week, 3 = awaken 7 times or more) 4. Nerve conduction: median motor and sensory distal latencies, median motor forearm conduction velocity, sensory nerve conduction velocity |
|
| Notes | Attempts to clarify allocation method with authors were unsuccessful *Note. These outcomes used short ordinal scales which should be treated as binary data. Authors reported as continuous data. Attempts to obtain raw data from authors were unsuccessful. Analysis was undertaken at the wrist level for all outcomes, though some participants in each group had bilateral CTS. Some bilateral CTS participants received the same intervention for both wrists while others received different interventions for each wrist. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eighteen patients who were found to have a total of 30 idiopathic cases of CTS were randomly divided into three groups, each with 10 cases of CTS." Comment: Not enough information to determine the adequacy of the randomisation sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eighteen patients who were found to have a total of 30 idiopathic cases of CTS were randomly divided into three groups, each with 10 cases of CTS." Comment: Not enough information to determine whether the allocation sequence was adequately concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | Low risk | Quote: "The ultrasound therapy lasted 5 minutes per session, 5 days a week for 2 weeks, and patients were unaware of treatment groups." Comment: Participants were probably blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Design: Patient‐blinded, placebo‐controlled, before‐after treatment trial." Comment: It is likely that outcome assessors were not blinded to treatment allocation as it can be assumed that is this were the case, this would be stated in the above quote. |
| Incomplete outcome data (attrition bias) 3 months or less | Unclear risk | Comment: No withdrawals or drop‐outs were reported, but since the number of cases included in each analysis was not reported in any of the tables of results, it cannot be determined whether all outcomes were based on a complete dataset. |
| Selective reporting (reporting bias) | Unclear risk | Comment: All outcomes specified in the Methods section of the publication were reported in the pre‐specified way. However, unlike the majority of other included studies, this study did not measure the outcome, function or health‐related quality of life. As no protocol for the study could be obtained, it is not clear whether this outcome was measured and subsequently not reported. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Piravej 2004.
| Methods | Randomised double‐blind clinical trial Blinded participants and outcome assessors Randomisation occurred at the level of participants, not wrists (i.e. participants with bilateral CTS received the same intervention for both wrists) |
|
| Participants | Total n = 18 (30 wrists) randomised and completed
Intervention group 1 n = 10 (15 wrists) randomised and completed
Intervention group 2 n = 8 (15 wrists) randomised and completed 0 males and 18 females Mean ± SD age Intervention group 1: 49.07 ± 8.88 yrs Intervention group 2: 44.87 ± 7.55 yrs Inclusion criteria: 1. Clinical manifestation of CTS of less than 12 months 2. Musculoskeletal problems or specific predisposing factors, such as rheumatic diseases, diabetes mellitus, cervical spondylosis, acute trauma and pregnancy 3. No treatment for at least one month 4. No local corticosteroid injection during the last three months 5. No serious co‐existing medical condition that may prohibit electrophysiological test during the study 6. No allergy or contraindication for diclofenac and ultrasound therapy 7. No muscle atrophy, anaesthesia or intractable pain due to CTS 8. Electrophysiologic test showed the presence of median nerve sensory and motor responses with sensory distal latency longer than 2.8 msec but not more than 4.50 msec, sensory nerve action potential (SNAP) amplitude exceeding 10 uv, median‐ulnar mixed nerve latency difference longer than 0.5 msec, motor distal latency (MDL) longer than 4.2 msec but not more than 6.50 msec and compound muscle action potential (CMAP) amplitude not less than 5.0 mV 9. Electromyography of the abductor pollicis brevis (APB) muscle showed no spontaneous activity or markedly reduced firing frequency 10. The patient accepted the study and signed the consent form Exclusion criteria: Not reported (implied by inclusion criteria) |
|
| Interventions | Intervention group 1: Continuous ultrasound therapy in a circular fashion performed at intensity of 0.5 W/cm2 and frequency of 1 MHz for 10 minutes per session, 5 days a week for 4 weeks, plus placebo drug taken each day. Intervention group 2: "Sham" ultrasound therapy in a circular fashion performed at intensity of 0.0 W/cm2 and frequency of 1 MHz for 10 minutes per session, 5 days a week for 4 weeks, plus diclofenac 75 mg/day (a nonsteroidal anti‐inflammatory drug) taken in a divided dose each day. |
|
| Outcomes | Outcomes assessed at baseline and within five days after the end of four weeks treatment 1. Pain using zero to 100 VAS (0 = "no pain", 10 = "unbearable pain") 2. Presence of pain and/or paraesthesia symptoms at night and/or day, scored as follows: 0 = no symptoms, 1 = mild (nocturnal and/or diurnal paraesthesia ), 2 = moderate (nocturnal pain and paraesthesia ), and 3 = severe (nocturnal and diurnal pain and paraesthesia) 3. Frequency of awakening of symptoms at night per week scored as follows: 0 = never wake up; 1 = 1‐2 times a week; 2 = 3‐6 times a week; and 3 = 7 times or more 4. Nerve conduction: median nerve sensory distal latency, sensory nerve action potential amplitude (SNAP), median nerve motor distal latency* and compound muscle action potential (CMAP) |
|
| Notes | The authors reported the mean and SD for all outcomes as endpoint scores and change from baseline scores, however, the SD of the median nerve motor distal latency endpoint score appears to have been reported incorrectly in the study (SD before treatment is 0.67 whereas for after treatment it is reported as 25.67). Therefore, only the change from baseline scores for the outcome, median nerve motor distal latency, was entered into RevMan. Attempts to contact the authors for clarification of this were unsuccessful. Analysis was undertaken at the wrist level for all outcomes, though some participants in each group had bilateral CTS. Bilateral cases had the same intervention applied to each wrist. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A physician simple randomized the patients into 2 groups by drawing a paper which was labelled A or B of 15 cases without replacement." Comment: Randomisation sequence was probably adequately concealed. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A physician simple randomized the patients into 2 groups by drawing a paper which was labelled A or B of 15 cases without replacement." Comment: Not clear whether the allocation sequence was adequately concealed. |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | Low risk | Quote: "...patients were unaware of the treatment groups." Comment: Blinding of participants was probably achieved, given that placebo drug and sham ultrasound were used. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The electrophysiological tests were performed by the same physician, who did not involve with treatment assignment." Comment: Outcome assessor was probably blind to treatment allocation. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Comment: No drop‐outs were reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: All outcomes reported in the Methods section of the publication were reported fully in the Results section. However, unlike the majority of other included studies, this study did not measure the outcome, function or health‐related quality of life. As no protocol for the study could be obtained, it is not clear whether this outcome was measured and subsequently not reported. |
| Other bias | Low risk | Comment: No other sources of bias identified. |
Yildiz 2011.
| Methods | Randomised double‐blind clinical trial Blinded participants and outcome assessors Randomisation occurred at the level of wrists, with no constraint that all participants' wrists be allocated to the same or different treatments |
|
| Participants | Total n = 51 (76 wrists) randomised; 44 (68 wrists) completed
Intervention group 1 n = 17 (25 wrists) randomised; 16 (23 wrists) completed
Intervention group 2 n = 17 (26 wrists) randomised; 13 (22 wrists) completed
Intervention group 3 n = 17 (26 wrists) randomised; 15 (23 wrists) completed 8 males and 43 females randomised; 8 males; 36 females completed Mean ± SD age Intervention group 1 (randomised): 47.47 ± 8.07 yrs Intervention group 1 (completed): 47.5 ± 8.33 yrs Intervention group 2 (randomised): 48.41 ± 9.51 yrs Intervention group 2 (completed): 48.76 ± 10.94 yrs Intervention group 3 (randomised): 50.17 ± 10.47 yrs Intervention group 3 (completed): 50.26 ± 11.19 yrs Inclusion criteria: 1. Diagnosis of mild and moderate CTS according to American Association of Electrodiagnostic Medicine Guidelines 2. Symptom duration greater than one month Exclusion criteria: 1. Corticosteroid injection before the study 2. Physical or medical therapy in the previous three months 3. Muscle atrophy due to CTS 4. Evidence of obvious underlying causes of CTS such as hypothyroidism, diabetes mellitus, inflammatory rheumatic diseases, arthritis of wrist, acute trauma, pregnancy 5. Medical problems that would have been contraindicated for ultrasound therapy 6. Clinical or electrophysiologic evidence of accompanying conditions that could mimic CTS or interfere with its evaluation such as cervical radiculopathy, or significant polyneuropathy 7. Presence of either fibrillation potentials or reinnervation on needle electromyography in the abductor pollicis brevis muscle |
|
| Interventions | Intervention group 1: Neutral (0° to 5°) custom‐moulded thermoplastic volar wrist splint worn at night and during the day for eight weeks plus sham ultrasound (delivered with an acoustic gel without any medication via a Chattonooga Group, Model 27335 ultrasound system in off‐mode) for 15 minutes sessions, once a day, five times a week for two weeks Intervention group 2: Neutral (0° to 5°) custom‐moulded thermoplastic volar wrist splint worn at night and during the day for eight weeks plus pulsed mode (1:4) ultrasound with an acoustic gel without any medication at 1 MHz frequency and 1 W/cm2 intensity for 15 minute sessions, once a day, five times a week for two weeks. Intervention group 3: Neutral (0° to 5°) custom‐moulded thermoplastic volar wrist splint worn at night and during the day for eight weeks plus pulsed mode (1:4) ultrasound with 2.5% ketoprofen gel (a nonsteroidal anti‐inflammatory drug) at 1 MHz frequency and 1 W/cm2 intensity for 15 minute sessions, once a day, five times a week for two weeks. |
|
| Outcomes | Outcomes assessed at baseline, at the end of two week treatment and six weeks after treatment ended* 1. Pain using zero to 10 VAS (0 = "no pain", 10 = "worst possible pain") 2. Symptoms using Boston carpal tunnel questionnaire (rates 11 items on ordinal scale 1: mildest, to 5: most severe) (Levine 1993) 3. Function using Boston carpal tunnel questionnaire (rates 8 items on ordinal scale 1: no difficulty with the activity, to 5: can not perform the activity at all) (Levine 1993) 4. Complications or side effects (not defined) 5. Nerve conduction: median nerve motor distal latency, median nerve sensory distal latency |
|
| Notes | *Both an intention‐to‐treat and per‐protocol analysis was conducted for all outcomes. Analysis was undertaken at the wrist level for all outcomes, though some participants in each group had bilateral CTS. Some bilateral CTS participants received the same intervention for both wrists while others received different interventions for each wrist. The trialists did not report how the correlation between both wrists was accounted for in the analysis, and attempts to clarify this information from the trialists were unsuccessful. Therefore, it is not clear whether a unit of analysis error occurred. No attempt was made to adjust outcome data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation list was produced with a random number generator" Comment: Randomisation sequence was likely adequately generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After eligible patients had been enrolled, a physiatrist (GOG) who was not involved in the treatment allocated the involved wrist of each consecutive patient to PH, US or sham treatment group (same procedure was repeated for other involved wrist in patients with bilateral CTS) by means of sequentially numbered sealed envelopes containing the group allocation (sham, US or PH). This physiatrist was the only person aware of treatment allocation during the trial." Comment: It is not clear whether the sequentially numbered sealed envelopes were opaque, therefore it is not clear whether the allocation sequence was adequately concealed until interventions were assigned. |
| Blinding of participants and personnel (performance bias) Self‐reported outcomes | Low risk | Quote: "In addition to patients, NY, NSA and ES who delivered the treatment were all unaware of the treatment allocation. Only the physician (GOG) who was in charge of group allocation switched the ultrasonic generator to the respective modes before each treatment session. Both ketoprofen and acoustic gel tubes were covered. Sessions were arranged on a non‐overlapping timetable so that different groups of patients were not able to see each other. This procedure allowed blinding of both the patients and the physicians delivering the treatment. Intensity of US treatment was below sensitivity threshold." Comment: Patients were likely blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In addition to patients, NY, NSA and ES who delivered the treatment were all unaware of the treatment allocation. Only the physician (GOG) who was in charge of group allocation switched the ultrasonic generator to the respective modes before each treatment session. Both ketoprofen and acoustic gel tubes were covered. Sessions were arranged on a non‐overlapping timetable so that different groups of patients were not able to see each other. This procedure allowed blinding of both the patients and the physicians delivering the treatment. Intensity of US treatment was below sensitivity threshold." Quote: "Pre‐ and post‐treatment (2nd and 8th week) evaluations of the patients were made with the following clinical outcome parameters by a physiatrist (NY) and electrophysiological outcome parameters by the other physiatrist (NSA)". Comment: Outcome assessors were reported to be blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) 3 months or less | Low risk | Quote: "After completing two weeks of treatment, seven out of 51 randomised patients did not finish the study protocol (one in Group 1, four in Group 2 and two in Group 3) due to non‐compliance to splinting, illness and lost to follow‐up." Quote: "The ITT analysis included all randomised patients who received treatment at least once" Comment: The number of drop‐outs in each group, and the reasons why, was clearly reported in text and in a Figure. The number of drop‐outs was small and relatively similar across groups, suggesting low risk of bias. |
| Selective reporting (reporting bias) | Low risk | Comment: All outcome measures described in the methods of the published article were reported in the results although a protocol was not available to determine whether all intended outcomes were included in the publication. Standard outcome measures have been reported and selective outcome reporting is unlikely. Data for all outcomes were reported separately based on an intention‐to‐treat and per‐protocol analysis |
| Other bias | Low risk | Comment: No other sources of bias identified. |
CI: confidence interval CTS: carpal tunnel syndrome EMG: electroneuromyography SD: standard deviation US: ultrasound VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avci 2004 | Compares the effect of ultrasound plus paraffin bath versus other interventions for CTS (so we cannot determine the effect of ultrasound in isolation). |
| Bakhtiary 2011 | Investigates the effect of phonophoresis, which is the use of therapeutic ultrasound to enhance delivery of topically applied drugs, and so differs to the types of therapeutic ultrasound addressed in this review. |
| Coskun 2011 | Therapeutic ultrasound was provided to both groups (along with other non‐surgical interventions for CTS). |
| Dakowicz 2005 | Not a randomised controlled trial; all patients received ultrasound therapy |
| Davis 1998 | Compares the effect of therapeutic ultrasound delivered as one component of a chiropractic intervention which also comprised manual thrusts, massage, and wrist splints, compared with ibuprofen and wrist splint (so we cannot determine the effect of ultrasound in isolation). |
| Deliss 1998 | Not a randomised controlled trial. This is a clinical commentary on the Ebenbichler 1998 trial. |
| Gurcay 2012 | Investigates the effect of phonophoresis, which is the use of therapeutic ultrasound to enhance delivery of topically applied drugs, and so differs to the types of therapeutic ultrasound addressed in this review. |
| Hui 2004 | Long‐term follow‐up of patients in Wong 2011 RCT, where efficacy of steroid injection is compared with oral steroids. Awaiting assessment in Marshall 2007 Cochrane review. |
| Jarvik 2009 | RCT comparing ultrasound with other non‐surgical interventions to a surgical intervention for CTS; RCTs comparing surgical to non‐surgical interventions for CTS are the focus of another Cochrane review (Verdugo 2008) |
| Lucas 2002 | Not a randomised controlled trial. This is a critical appraisal of the Ebenbichler 1998 RCT. |
| Robertson 2001 | Review of therapeutic ultrasound for treating pain, musculoskeletal injuries and soft tissue lesions. |
| Sucher 1999 | Not a randomised clinical trial. This is a clinical commentary on the Oztas 1998 trial. |
| Taspinar 2007 | Compares the effect of ultrasound plus transcutaneous electrical nerve stimulation (TENS) versus other interventions for CTS (so we cannot determine the effect of ultrasound in isolation) |
| Toro 1997 | Therapeutic ultrasound was provided to both groups (along with other non‐surgical interventions for CTS). |
| Walling 1998 | Not a randomised controlled trial. This is a brief summary of the Ebenbichler 1998 RCT. |
CTS: carpal tunnel syndrome RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01590745.
| Trial name or title | Ultrasound therapy for carpal tunnel syndrome (CTS) |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | ClinicalTrials.gov ID NCT01590745 (http://clinicaltrials.gov/show/NCT01590745). |
Differences between protocol and review
This is a split review replacing the therapeutic ultrasound interventions included in the previous review titled 'Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome' (O'Connor 2003).
In the review by O'Connor et al. (O'Connor 2003), types of outcome measures included in the review were as follows:
Primary outcome:
The primary outcome measure was improvement in clinical symptoms, such as pain and paraesthesiae, at least three months after the end of treatment.
Secondary outcome measures included: 1. improvement in functional status and/or health‐related quality of life parameters at least three months after treatment; 2. improvement in objective physical examination measures, such as grip, pinch strength, and sensory perception at least three months after treatment; 3. improvement in neurophysiological parameters after three months after treatment; 4. clinical improvement at less than three months of follow‐up; 5. clinical improvement at one year after treatment; 6. need for surgical release of the flexor retinaculum during follow‐up.
The outcomes reported in this review have been modified from the original review (O'Connor 2003) to make them as consistent as possible with other Cochrane reviews on carpal tunnel syndrome (O'Connor 2012; Page 2012b; Page 2012c; Marshall 2007; Scholten 2007; Verdugo 2008).
Assessment for study risk of bias has been performed using The Cochrane Collaboration's 'Risk of bias' tool in this update of the review. We have included a 'Summary of findings' table.
The 'Types of interventions' criteria for considering studies for this review has been modified to make it clearer that trials where therapeutic ultrasound was used as an adjunct to another treatment were included only if the comparison provided information on the additional effect of the therapeutic ultrasound intervention. This modification resulted in the exclusion of Davis 1998 which was incorrectly included in the previous version of this review (Page 2012a). Davis 1998 compared the effect of therapeutic ultrasound delivered along with manual thrusts, massage, and wrist splints, to ibuprofen and wrist splint, so the additional effect of therapeutic ultrasound cannot be determined in this study. Davis 1998 is currently included in the 'Exercise and mobilisation interventions for carpal tunnel syndrome' review (Page 2012b).
Contributions of authors
MATTHEW PAGE (MP) was involved in the following stages of the review: design of the review (in collaboration with DOC); undertaking the search of studies; screening the search results (independently of, but in addition to DOC); organising retrieval of papers; screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to DOC); appraising the risk of bias of papers (independently of, but in addition to DOC and VP); extracting data from papers (independently of, but in addition to DOC, VP, and NMW); writing to study investigators for additional information; summarising the risk of bias of the studies (independently, but in addition to DOC and VP); compiling the summary of comparisons, tables of included, excluded, awaiting and ongoing studies; entering data into RevMan; performing analysis of data; interpreting the findings; writing of the review (in collaboration with DOC, VP and NMW); final approval of the version to be published.
DENISE O'CONNOR (DOC) was responsible for: design of the review (in collaboration with MP); developing the search strategy; screening the search results (independently of, but in addition to MP); screening retrieved papers against inclusion/exclusion criteria (independently of, but in addition to MP); appraising the risk of bias of papers (independently of, but in addition to MP and VP); extracting data from papers (independently of, but in addition to MP, VP and NMW); checking data entered into RevMan by MP (independently, but in addition to NMW) writing to study investigators for additional information; summarising the risk of bias of the studies (independently of, but in addition to MP and VP); writing the review (with contribution from MP, VP and NMW).
VERONICA PITT (VP) was involved in the following stages of the review: extracting data from papers (independently of, but in addition to MP, DOC and NMW); appraising the risk of bias of papers (independently of, but in addition to MP and DOC); summarising the risk of bias of papers (independently of, but in addition to MP and DOC) contributing to the writing of the review (in collaboration with MP, DOC and NMW).
NICOLA MASSY‐WESTROPP (NMW) was involved in the following stages of the review: extracting data from papers (independently of, but in addition to MP, DOC and VP); checking data entered into RevMan (independently, but in addition to DOC); contributing to the writing of the review (in collaboration with MP, DOC, and VP).
Sources of support
Internal sources
Australasian Cochrane Centre, Australia.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bakhtiary 2004 {published data only}
- Bakhtiary AH, Rashidy‐Pour A. Ultrasound and laser therapy in the treatment of carpal tunnel syndrome. Australian Journal of Physiotherapy 2004;50:147‐51. [PUBMED: 15482245] [DOI] [PubMed] [Google Scholar]
Baysal 2006 {published data only}
- Baysal O, Altay Z, Ozcan C, Ertem K, Yologlu S, Kayhan A. Comparison of three conservative treatment protocols in carpal tunnel syndrome. International Journal of Clinical Practice 2006;60(7):820‐8. [PUBMED: 16704676] [DOI] [PubMed] [Google Scholar]
Bilgici 2010 {published data only}
- Biligici A, Ulusoy H, Canturk F. The comparison of ultrasound treatment and local steroid injection plus splinting in the carpal tunnel syndrome: a randomized controlled trial. Bratislavske Lekarske Listy 2010;111(12):659‐65. [PUBMED: 21384736] [PubMed] [Google Scholar]
Dincer 2009 {published data only}
- Dincer U, Cakar E, Kiralp MZ, Kilac H, Dursun H. The effectiveness of conservative treatments of carpal tunnel syndrome: splinting, ultrasound, and low‐level laser therapies. Photomedicine and Laser Surgery 2009;27(1):119‐25. [PUBMED: 19196106] [DOI] [PubMed] [Google Scholar]
Duymaz 2012 {published data only}
- Duymaz T, Sindel D, Kesiktaş N, Müslümanoğlu L. Efficacy of some combined conservative methods in the treatment of carpal tunnel syndrome: a randomized controlled clinical and electrophysiological trial. Turkish Journal of Rheumatology 2012;27(1):38‐46. [Google Scholar]
Ebenbichler 1998 {published data only}
- Ebenbichler GR, Resch KL, Nicolakis P, Wiesinger GF, Uhl F, Ghanem A, et al. Ultrasound treatment for treating the carpal tunnel syndrome: randomised 'sham' controlled trial. BMJ 1998;316(7133):731‐5. [PUBMED: 9529407] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekim 2008 {published data only}
- Ekim A, Colak E. Ultrasound treatment in carpal tunnel syndrome: a placebo controlled study. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2008;54(3):96‐101. [EMBASE: 2008610958] [Google Scholar]
Koyuncu 1995 {published data only}
- Koyuncu H, Unver FN, Sahin U, Togay P. 1MHz ‐ 3MHz ultrasound applications in carpal tunnel syndrome [Karpal tunel sendromunda 1MHz ‐ 3MHz ultrason uygulamasi]. Fizik Tedavi ve Rehabilitasyon Dergisi 1995;19:141‐5. [EMBASE: 1995350540] [Google Scholar]
Oztas 1998 {published data only}
- Oztas O, Turan B, Bora I, Karakaya MK. Ultrasound therapy effect in carpal tunnel syndrome. Archives of Physical Medicine & Rehabilitation 1998;79(12):1540‐4. [PUBMED: 9862296] [DOI] [PubMed] [Google Scholar]
Piravej 2004 {published data only}
- Piravej K, Boonhong J. Effect of ultrasound thermotherapy in mild to moderate carpal tunnel syndrome. Journal of the Medical Association of Thailand 2004;87(Suppl 2):S100‐6. [PUBMED: 16083171] [PubMed] [Google Scholar]
Yildiz 2011 {published data only}
- Yildiz N, Atalay NS, Gungen GO, Sanal E, Akkaya N, Topuz O. Comparison of ultrasound and ketoprofen phonophoresis in the treatment of carpal tunnel syndrome. Journal of Back and Musculoskeletal Rehabilitation 2011;24(1):39‐47. [PUBMED: 21248399] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Avci 2004 {published data only}
- Avci S, Günaydin R, Öztura I. Comparison of effectiveness of splint and splint with physical therapy in carpal tunnel syndrome [Karpal tunel sendromunda atel ve atel ile birlikte fizik tedavinin etkinliginin karsilastirilmasi]. Ftr Turkiye Fiziksel Tip Ve Rehabilitasyon Dergisi 2004;50(2):22‐6. [Google Scholar]
Bakhtiary 2011 {published data only}
- Bakhtiary AH, Fatemi E, Emami M, Malek M. Comparing the effects of iontophoresis and phonophoresis of dexamethasone on the treatment of carpal tunnel syndrome. Koomesh 2011;13(1):83‐93. [Google Scholar]
Coskun 2011 {published data only}
- Coskun G, Kirdi N, Can F. Effects of wrist traction on pain and hand function in the treatment of carpal tunnel syndrome [Karpal tünel sendromunun tedavisinde bilek traksiyonunun ağrı ve elin fonksiyonelliği üzerine etkisi]. Fizyoterapi Rehabilitasyon 2011;22(1):3‐10. [Google Scholar]
Dakowicz 2005 {published data only}
- Dakowicz A, Latosiewicz R. The value of iontophoresis combined with ultrasound in patients with the carpal tunnel syndrome. Roczniki Akademii Medycznej w Bialymstoku 2005;50(Suppl 1):196‐8. [PUBMED: 16119664] [PubMed] [Google Scholar]
Davis 1998 {published data only}
- Davis PT, Hulbert JR, Kassak KM, Meyer JJ. Comparative efficacy of conservative medical and chiropractic treatments for carpal tunnel syndrome: a randomized clinical trial. Journal of Manipulative & Physiological Therapeutics 1998;21(5):317‐26. [PUBMED: 9627862] [PubMed] [Google Scholar]
Deliss 1998 {published data only}
- Deliss L. Ultrasound treatment for carpal tunnel syndrome: emphasis must be on return of sensation and function. BMJ 1998;317(7158):601. [PUBMED: 9721127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurcay 2012 {published data only}
- Gurcay E, Unlu E, Gurcay AG, Tuncay R, Cakci A. Assessment of phonophoresis and iontophoresis in the treatment of carpal tunnel syndrome: a randomized controlled trial. Rheumatology International 2012;32(3):717‐22. [DOI] [PubMed] [Google Scholar]
Hui 2004 {published data only}
- Hui ACF, Wong SM, Tang A, Mok V, Hung LK, Wong KS. Long‐term outcome of carpal tunnel syndrome after conservative treatment. International Journal of Clinical Practice 2004;58(4):337‐9. [PUBMED: 15161116] [DOI] [PubMed] [Google Scholar]
Jarvik 2009 {published data only}
- Jarvik JG, Comstock BA, Kliot M, Turner JA, Chan L, Heagerty PJ, et al. Surgery versus non‐surgical therapy for carpal tunnel syndrome: a randomised parallel‐group trial. Lancet 2009;374(9695):1074‐81. [PUBMED: 19782873] [DOI] [PubMed] [Google Scholar]
Lucas 2002 {published data only}
- Lucas N. A critical appraisal of an article examining the efficacy of ultrasound treatment for carpal tunnel syndrome. Journal of Osteopathic Medicine 2002;5(1):28‐30. [Google Scholar]
Robertson 2001 {published data only}
- Robertson VJ, Baker KG. A review of therapeutic ultrasound: effectiveness studies. Physical Therapy 2001;81(7):1339‐50. [PUBMED: 11444997] [PubMed] [Google Scholar]
Sucher 1999 {published data only}
- Sucher BM. Ultrasound therapy effect in carpal tunnel syndrome. Archives of Physical Medicine & Rehabilitation 1999;80(9):1117. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Taspinar 2007 {published data only}
- Taspinar S, Sahin F, Ercalik C, Kuran B, Barkut K, Celik M, et al. Comparison of the efficacy of corticosteroid injection, night splint and physiotherapy in diabetic corpal tunnel syndrome. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2007;53(2):54‐60. [Google Scholar]
Toro 1997 {published data only}
- Toro JR, Rodriguez IG, Espinel JP, Caballero MLG, Rodriquez FA. Chronic idiopathic carpal tunnel syndrome: effectiveness of iontophoresis‐corticoid treatment compared with iontophoresis‐placebo (galvanization). Rehabilitacion 1997;31(2):118‐26. [Google Scholar]
Walling 1998 {published data only}
- Walling AD. Effects of ultrasound treatment in carpal tunnel syndrome. American Family Physician 1998;58(4):961‐2. [Google Scholar]
References to ongoing studies
NCT01590745 {published data only}
- NCT01590745. Ultrasound Therapy for Carpal Tunnel Syndrome (CTS). http://clinicaltrials.gov/show/NCT01590745.
Additional references
Ashworth 2010
- Ashworth N. Carpal tunnel syndrome. Clinical Evidence 2010;03:1114. [PUBMED: 21718565] [PMC free article] [PubMed] [Google Scholar]
Atroshi 1999
- Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999;282(2):153‐8. [PUBMED: 10411196] [DOI] [PubMed] [Google Scholar]
Binder 1985
- Binder A, Hodge G, Greenwood AM, Hazleman BL, Page Thomas DP. Is therapeutic ultrasound effective in treating soft tissue lesions?. British Medical Journal 1985;290(6467):512‐4. [PUBMED: 3918652] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 2005
- Bland JD. The relationship of obesity, age, and carpal tunnel syndrome: more complex than was thought?. Muscle & Nerve 2005;32(4):527‐32. [DOI] [PubMed] [Google Scholar]
Charles 2009
- Charles J, Fahridin S, Britt H. Carpal tunnel syndrome. Australian Family Physician 2009;38(9):665. [PUBMED: 19893791] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Dwan 2011
- Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.MR000031.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelfman 2009
- Gelfman R, Melton LJ 3rd, Yawn BP, Wollan PC, Amadio PC, Stevens JC. Long‐term trends in carpal tunnel syndrome. Neurology 2009;72(1):33‐41. [PUBMED: 19122028] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerritsen 2002
- Gerritsen AAM, Krom MCTFM, Struijs MA, Scholten RJPM, Vet HCW, Bouter LM. Conservative treatment options for carpal tunnel syndrome: a systematic review of randomised controlled trials. Journal of Neurology 2002;249(3):272‐80. [PUBMED: 11993525] [DOI] [PubMed] [Google Scholar]
Goodyear‐Smith 2004
- Goodyear‐Smith F, Arroll B. What can family physicians offer patients with carpal tunnel syndrome other than surgery? A systematic review of nonsurgical management. Annals of Family Medicine 2004;2(3):267‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG. Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Hong 1988
- Hong CZ, Liu HH, Yu LJ. Ultrasound thermotherapy effect on the recovery of nerve conduction in experimental compression neuropathy. Archives of Physical Medicine and Rehabilitation 1988;69:410‐4. [PubMed] [Google Scholar]
Hopewell 2007
- Hopewell S, McDonald S, Clarke MJ, Egger M. Grey literature in meta‐analyses of randomised trials of health care interventions. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000010.pub3; PUBMED: 17443631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huisstede 2010
- Huisstede BM, Hoogvliet P, Randsdorp MS, Glerum S, Middelkoop M, Koes BW. Carpal tunnel syndrome. Part 1: Effectiveness of nonsurgical treatments ‐ a systematic review. Archives of Physical Medicine and Rehabilitation 2010;91(7):981‐1004. [PUBMED: 20599038] [DOI] [PubMed] [Google Scholar]
Keith 2009
- Keith MW, Masear V, Chung K, Maupin K, Andary M, Amadio PC, et al. Diagnosis of carpal tunnel syndrome. Journal of the American Academy of Orthopaedic Surgeons 2009;17(6):389‐96. [PUBMED: 19474448] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerwin 1996
- Kerwin G, Williams CS, Seiler JG 3rd. The pathophysiology of carpal tunnel syndrome. Hand Clinics 1996;12(2):243‐51. [PUBMED: 8724576] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Lehmann 1974
- Lehmann JF, Warren CG, Scham SM. Therapeutic heat and cold. Clinical Orthopaedics 1974;99:207‐45. [PUBMED: 4133064] [DOI] [PubMed] [Google Scholar]
Levine 1993
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self‐administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. Journal of Bone and Joint Surgery 1993;75(11):1585‐92. [PUBMED: 8245050] [DOI] [PubMed] [Google Scholar]
Marshall 2007
- Marshall SC, Tardif G, Ashworth NL. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD001554.pub2] [DOI] [PubMed] [Google Scholar]
Muller 2004
- Muller M, Tsui D, Schnurr R, Biddulph‐Deisroth L, Hard J, MacDermid JC. Effectiveness of hand therapy interventions in primary management of carpal tunnel syndrome: a systematic review. Journal of Hand Therapy 2004;17(2):210‐28. [PUBMED: 15162107] [DOI] [PubMed] [Google Scholar]
O'Connor 2012
- O'Connor D, Page MJ, Massy‐Westropp N. Ergonomic positioning or equipment for treating carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 1. [Art. No: CD009600; DOI: 10.1002/14651858.CD009600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ono 2010
- Ono S, Clapham PJ, Chung KC. Optimal management of carpal tunnel syndrome. International Journal of General Medicine 2010;3:255‐61. [PUBMED: 20830201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2012b
- Page MJ, O’Connor D, Pitt V, Massy‐Westropp N. Exercise and mobilisation interventions for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009899] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2012c
- Page MJ, Massy‐Westropp N, O'Connor D, Pitt V. Splinting for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD010003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piazzini 2007
- Piazzini DB, Aprile I, Ferrera PE, Bertolini C, Tonali P, Maggi L, et al. A systematic review of conservative treatment of carpal tunnel syndrome. Clinical Rehabilitation 2007;21(4):299‐314. [PUBMED: 17613571] [DOI] [PubMed] [Google Scholar]
Rempel 1998
- Rempel D, Evanoff B, Amadio PC, Krom M, Franklin G, Franzblau A, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. American Journal of Public Health 1998;88(10):1447‐51. [PUBMED: 9772842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2010
- Rutjes AWS, Nuesch E, Sterchi R, Juni P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003132.pub2; PUBMED: PMID:20091539] [DOI] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Scholten 2007
- Scholten RJPM, Mink van der Molen A, Uitdehaag BMJ, Bouter LM, Vet HCW. Surgical treatment options for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003905.pub3; PUBMED: 17943805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stallings 1997
- Stallings SP, Kasdan ML, Soergel TM, Corwin HM. A case‐control study of obesity as a risk factor for carpal tunnel syndrome in a population of 600 patients presenting for independent medical examination. The Journal of Hand Surgery 1997;22(2):211‐5. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Available from www.cochrane‐handbook.org. [Google Scholar]
Szabo 1992
- Szabo RM, Madison M. Carpal tunnel syndrome. Orthopedic Clinics of North America 1992;23(1):103‐9. [PUBMED: 1729659] [PubMed] [Google Scholar]
Szabo 1994
- Szabo RM, Steinberg DR. Nerve entrapment syndromes in the wrist. Journal of the American Academy of Orthopedic Surgeons 1994;2(2):115‐23. [PUBMED: 10708999] [DOI] [PubMed] [Google Scholar]
Van den Bekerom 2011
- Bekerom MP, Windt DA, ter Riet G, Heijden GJ, Bouter LM. Therapeutic ultrasound for acute ankle sprains. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD001250.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verdugo 2008
- Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non‐surgical treatment for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001552.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2008
- Watson T, Young, SR. Therapeutic ultrasound. In: Watson T editor(s). Electrotherapy: evidence‐based practice. 12. Edinburgh; New York: Churchill Livingstone, 2008. [Google Scholar]
Werner 1994
- Werner RA, Albers JW, Franzblau A, Armstrong TJ. The relationship between body mass index and the diagnosis of carpal tunnel syndrome. Muscle & Nerve 1994;17(6):632‐6. [DOI] [PubMed] [Google Scholar]
Wong 2011
- Wong SM, Hui ACF, Tang A, Ho PC, Hung LK, Wond KS, et al. Local vs systemic corticosteroids in the treatment of carpal tunnel syndrome. Neurology 2001;56(11):1565‐7. [DOI] [PubMed] [Google Scholar]
Young 2002
- Young S. Ultrasound therapy. In: Kitchen S editor(s). Electrotherapy: Evidence‐Based Practice. 11th Edition. Churchill Livingstone, 2002:221‐230. [Google Scholar]
References to other published versions of this review
O'Connor 2003
- O'Connor D, Marshall S, Massy‐Westropp N. Non‐surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2012a
- Page MJ, O'Connor D, Pitt V, Massy‐Westropp N. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009601] [DOI] [PubMed] [Google Scholar]


