Abstract
Background
The chikungunya virus (CHIKV) is a re-emerging alphavirus that can cause chronic and potentially incapacitating rheumatic musculoskeletal disorders known as chronic chikungunya arthritis (CCA). We conducted a prospective cohort study of CHIKV-infected subjects during the 2013 chikungunya outbreak in Martinique. The aim of this study was to assess the prevalence of CCA at 12 months and to search for acute phase factors significantly associated with chronicity.
Methodology/Principal findings
A total of 193 patients who tested positive for CHIKV RNA via qRT-PCR underwent clinical investigations in the acute phase (<21 days), and then 3, 6, and 12 months after inclusion. The Asian lineage was identified as the circulating genotype. A total of 167 participants were classified as either with or without CCA, and were analyzed using logistic regression models. The overall prevalence of CCA at 12 months was 52.1% (95%CI: 44.5–59.7). In univariate analysis, age (RD 9.62, 95% CI, 4.87;14.38, p<0.0001), female sex (RD 15.5, 95% CI, 1.03;30.0, p = 0.04), headache (RD 15.42, 95% CI, 0.65;30.18 p = 0.04), vertigo (RD 15.33, 95% CI, 1.47;29.19, p = 0.03), vomiting (RD 12.89, 95% CI, 1.54;24.24, p = 0.03), dyspnea (RD 13.53, 95% CI, 0.73;26.33, p = 0.04), intravenous rehydration (RD -16.12, 95% CI, -31.58; -0.66 p = 0.04) and urea (RD 0.66, 95% CI, 0.12;1.20, p = 0.02) were significantly associated with the development of CCA. For the subpopulation with data on joint involvement in the acute phase, the risk factors significantly associated with CCA were at least one 1 enthesitis (RD 16.7, 95%CI, 2.8; 30.7, p = 0.02) and at least one tenosynovitis (RD 16.8, 95% CI, 1.4–32.2, p = 0.04).
Conclusions
This cohort study conducted in Martinique confirms that CCA is a common complication of acute chikungunya disease. Our analysis emphasized the importance of age and female sex for CCA occurrence, and highlighted the aggravating role of dehydration during the acute phase. Early and adequate hydration were found to reduce the risk chronic chikungunya disorders.
Trial registration
clinicaltrials.gov (NCT01099852).
Author summary
Chikungunya is a tropical, mosquito-borne virus that has been re-emerging in the last decade. It has caused major epidemics in recent years, including in Reunion Island and in Southeast Asia. Nearly 2.5 billion people around the world are at risk of contracting the virus. In the acute phase of the illness, patients experience a flu-like syndrome with fever, headache, myalgia, rash, and severe arthralgia. These symptoms can persist for several months in some patients, and can lead to significant functional disability. During the 2013 epidemic in Martinique, we followed nearly 200 patients who had contracted chikungunya. More than half of the patients had a chronic form of the disease—mainly women over 50 years of age. Our statistical analyses indicate that poor hydration in the acute phase is a risk factor for developing chronic rheumatism. This suggests that, in the context of a chikungunya epidemic, patients should drink plenty of fluids as soon as the first symptoms appear.
Introduction
Chikungunya virus (CHIKV) is a re-emerging alphavirus transmitted to humans by Aedes mosquitoes that has caused massive epidemics in Africa [1–3]; in the Indian Ocean islands [4–6]; in Southeast Asia [7,8]; in the Americas [9,10]; and in Italy, where the first European outbreak occurred in 2007 [11,12]. CHIKV is known to target human epithelial and endothelial cells, fibroblasts and macrophages [13–15], as well as human muscle satellite cells [16]. The virus is also suspected of neurotropism [17,18], which can lead to neurological complications [19–22].
Chikungunya, a Makonde term meaning “that which bends up” [23], is an acute illness similar to dengue that is characterized by the abrupt onset of high-grade fever, followed by constitutional symptoms such as polyarthritis, musculoskeletal pain, headache, and skin involvement [24], and in rare cases by severe manifestations such as encephalopathy [21,24], acute hepatitis [25,26], myocarditis [27–29], and multi-organ failure. The acute phase (viremic period) lasts from 5 to 10 days on average, and is resolved within a few weeks. In May 2015, the World Health Organization [30] defined a person with chronic chikungunya as a “person with previous clinical diagnosis of chikungunya after 12 weeks of the onset of the symptoms presenting with at least 1 of the following articular manifestations: pain, rigidity, or edema, continuously or recurrently.” Chronic arthralgia (i.e., “suspected chronic chikungunya arthritis” or “CCA”) is a common complication post CHIKV-infection. Some studies [31–37] have reported that 40 to 60% of patients with acute chikungunya experience significantly impaired quality of life in the long term. Other studies have found that patients affected by CCA were significantly older [31,33,38,39], were more likely to be female [6,31,39,40], had severe initial rheumatic symptoms [39,41,42], and had high CHIK-specific IgG titers [38,41]. Martinique is a French overseas department with a population of nearly 400,000 and located in the French West Indies. The first autochthonous cases of chikungunya were described in the region in November 2013 (in December 2013 in Martinique) [43]. The Asian lineage was identified as the circulating genotype [44]. At the end of the outbreak (in approximately January 2015), the number of affected people was estimated at 145,000 (36% of the population) [45]. The aim of the present study was: first, to determine the prevalence of CCA at 12 months of follow-up, and second, to search for acute phase factors significantly associated with chronicity.
Methods
Ethics statement
Ethical clearance was obtained from the French National Agency for the Safety of Medicines and Health Products (ANSM) (n°IDRCB 2010-A00282-37) and from the French Committee for the Protection of Individuals. Written and signed informed consent was obtained from all participants.
CARBO presentation
In June 2010, the Department of Infectious Diseases at the Hospital of Fort de France initiated a descriptive and prognostic study of dengue in the French West Indies and French Guiana known as the “DAG” Study. This study was based on a hospital cohort of children and adults with suspected dengue and had as its main objective to identify the predictive factors of severe dengue. Following the introduction of CHIKV in Martinique, the inclusion criteria of the DAG study were extended to chikungunya cases, and the protocol was adjusted accordingly (namely by including a broad description of rheumatic disorders specific to chikungunya disease). In May 2014, the “DAG” study became the “DAG-2” study, and included a cohort composed of 2 groups: the dengue group and the chikungunya group. This study is registered on clinicaltrials.gov (NCT01099852). The DAG-2 study is still ongoing (the duration of follow-up is 36 months), and the present analysis was conducted at 12 months of follow-up.
At the beginning of 2016, the DAG-2 cohort was extended to other arboviruses (including the Zika virus), and was renamed “the CARBO cohort.”
This study was conducted at Pierre Zobda-Quitman Hospital in Fort de France, capital of Martinique, in the departments most likely to receive patients infected with CHIKV (the emergency department and the department of infectious diseases). As a result, the cohort of patients was mainly ambulatory and reflected the health care-seeking population affected by the epidemic in Martinique.
Study population
Patients were included in the cohort between December 19, 2013, and December 4, 2014. Inclusion criteria were: age ≥ 16 years of age, ability to sign informed consent, blood sample positive for CHIKV RNA via qRT-PCR, and onset of symptoms (headache, arthralgia, myalgia, fever, rash, fatigue) ≤7 days.
Data collection and follow-up
The DAG questionnaire was adapted to the objectives of the study. Data were collected at the initial visit (in the acute phase), and if possible on day 3, between days 5 and 7, and between days 8 and 10 after the onset of symptoms. The following data were recorded: sociodemographic data, comorbidities and clinical characteristics at baseline (headache, arthralgia, myalgia, fever, rash, fatigue, etc.), impact on quality of life as measured by the EuroQol 5 Dimensions (EQ5D) scale (S1 Fig), and treatments used. At the beginning of the epidemic, the medical team of the infectious disease department was trained by a group of rheumatologists, one of whom was a member of the CHIKV patient care team. Patients with CCA were managed in collaboration with the rheumatology department. For patients enrolled from July 2014 onwards (i.e., after adjustment of the protocol for the DAG-2 cohort), an accurate description of affected joints (location, swelling, stiffness, arthritis, enthesitis, joint pain, etc.), and an assessment of peripheral neuropathy with the douleur neuropathique 4 (DN4, neuropathic pain 4) scale (S2 Fig) were also performed.
At 3 months (M3), patients were examined by a team physician to identify CCA cases. At 6 months (M6) and 12 months (M12), patients were interviewed by phone using the DAG questionnaire (which is made up of closed questions) to monitor persistent symptoms (in particular joint pain), the impact on quality of life (as measured by the “EQ5D” scale), and treatments received. Patients were definitively classified as having CCA if they answered NO at any time of follow-up from M3 onwards to the following question: “Do you feel completely recovered from CHIKV-infection, in other words, are you now free of joint pain, rigidity, or edema related to this infection?” A clinical examination to describe and manage remaining disorders was proposed to these patients. Only patients who felt completely recovered from chikungunya at all visits from M3 onwards were categorized as NO CCA (without chronic chikungunya disease). The global clinical assessment of CCA subjects was conducted using the “Multi-Dimensional Health Assessment Questionnaire” (MDHAQ). Patients with chikungunya are known to experience a fluctuation of their symptoms (continuously or recurrently) over time. Patients with relapsing CCA were defined as having “disorders present at 1 and/or 2 time points without recovery” and patients with lingering CCA were defined as having “disorders present at all 3 time points: M3, M6, and M12.”
The 12-month period of follow-up allowed for the comprehensive description of chronic chikungunya disorders and for patients to characterize the time point with the heaviest burden (M3, M6, or M12).
Laboratory tests
Only CHIKV-viremic patients were recruited in the study. Blood cell counts and biochemical tests (C-reactive protein (CRP), ionogram, urea, creatinine and hepatic transaminases) were performed upon a blood sample testing positive for CHIKV RNA via qRT-PCR (RealStar Chikungunya RT-PCR kit 1.0—Altona Diagnostics, Hamburg, Germany). A biological bank was created but never used, and the viral load of patients was not collected.
Data analyses and statistical tests
Results were expressed as mean and standard deviation or frequency (percentage), as appropriate. Comparisons between the no CCA group and the CCA group were performed using chi-square test, Fisher’s exact test, or Wilcoxon test, as appropriate. Results were reported as risk difference with 95% confidence interval.
A predictive set of factors associated with CCA were selected using the least absolute shrinkage and selection operator (LASSO) method, after multiple imputations with chained equations to address missing data for variable selection. The penalty term required by the LASSO method was selected using 5-fold cross-validation. Factors with less than 10% of missing data were considered for the analysis. Factors selected by LASSO have non-null coefficient. A final non-regularized logistic regression was conducted on factors with non-null coefficient, in which the effect of each factor was reported as an adjusted odds-ratio with 95% confidence interval.
All tests were two-sided, and P values of less than 0.05 were considered significant. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
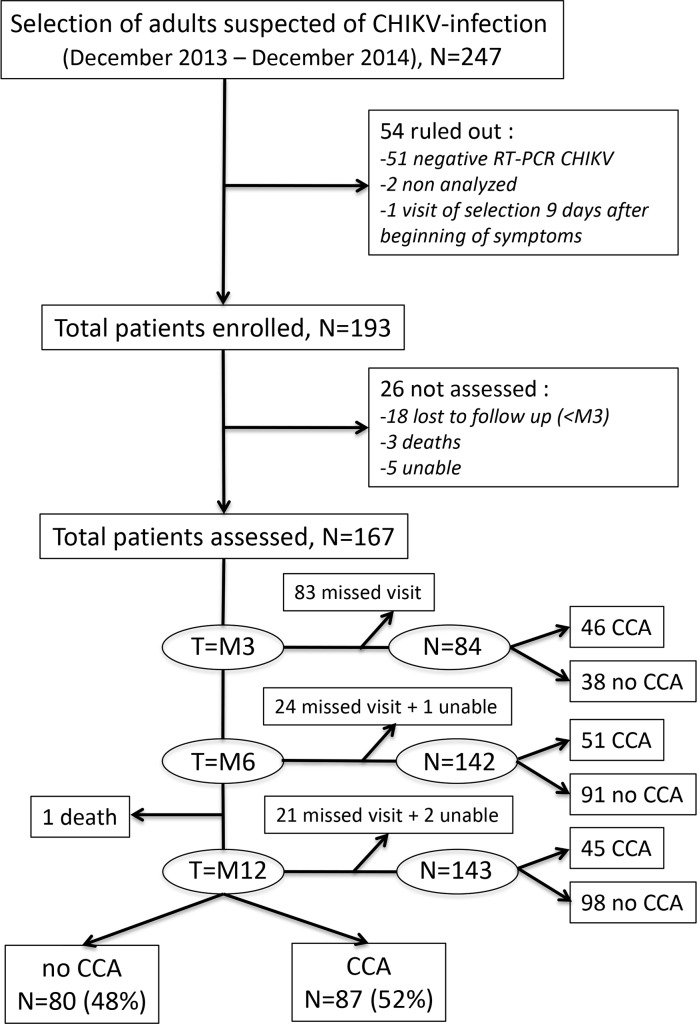
A total of 247 patients were eligible and consented to participate, 193 of whom were enrolled in the study (54 patients were excluded: 51 tested negative for CHIKV RNA via qRT-PCR, 2 were not tested for CHIKV RNA due to a temporary technical problem in the laboratory, and 1 was first evaluated more than 7 days after the onset of symptoms). The flowchart of the study is displayed in Fig 1.
Fig 1. Diagram of the study population.
“Unable”: patients unable to answer the question “Do you feel completely recovered from CHIKV-infection, in other words, are you now free of joint pain, rigidity, or edema related to this infection?”; “missed visit”: not present at a specific time point; “lost to follow-up”: patients who missed all follow-up visits from M3 onwards; “death”: dead patients; “CCA”: patients with chronic chikungunya arthritis; “no CCA”: patients without chronic chikungunya arthritis.
Overall prevalence of CCA at 12 months
Of the 193 patients, 21 were lost to follow-up (i.e., they did not attend any of the follow-up visits from M3 onwards), 3 of whom had died before M3 (causes of death were: adenocarcinoma of the prostate, multiple organ failure in the acute phase of CHIKV-infection, and unknown cause). Between M6 and M12, 1 patient died of myeloma; however, this patient was not excluded from the study because he had been reported as CCA at M3 (i.e., prior to his death). Five subjects had an undetermined CHIKC status at all follow-up visits, and were therefore excluded from the analysis.
A total of 167 patients were classified as either CCA or no CCA. Close to half the patients missed the M3 visit, as only 46 CCA patients and 38 no CCA patients were counted at the time. However, a greater number of patients were evaluated at M6 and M12, respectively 142 (85%; 51 CCA and 91 no CCA) and 143 (86%; 45 CCA and 98 no CCA) patients. Of the 167 participants, 87 had experienced CCA by M12, giving an overall prevalence of 52.10% (CI 95% 44.5–59.7). Ten CCA patients attended only 1 visit, and so their CCA profile (“relapsing” or “lingering”) could not be assessed. Of the 77 CCA patients, 64 (83.12%) had relapsing CCA and 13 (16.88%) had lingering CCA.
Clinical data in the acute phase
The ratio of women to men was 1.80 (59 males and 106 females). Of the 167 included patients (age range from 20 to 91 years), 53.9% were aged over 50 years, with a mean (±SD) age of 51.35 (±16.10) years. Native American origins (n = 65, 38.92%) and Sub-Saharan African origins (n = 50, 29.94%) were the most represented. Cardiovascular and rheumatologic diseases were the most common pre-existing comorbidities: 36 (21.56%) participants had high blood pressure, 28 (16.77%) had healed fractures, 14 (8.38%) had osteoarthritis, 13 (7.78%) had diabetes, 13 (7.78%) had dyslipidemia, 11 (6.59%) had rheumatologic inflammatory diseases, and 9 (5.39%) had cancer.
The most frequently recorded clinical signs in the acute phase were fever (95.21%), intensity of pain > 4/10 on the visual analog scale (83.83%), headache (59.28%), myalgia (56.29%), and vertigo (31.74%); digestive symptoms (diarrhea, vomiting, and abdominal pain) were less common.
In the subpopulation with data on joint involvement in the acute phase (n = 73, including 37 no CCA and 36 CCA), the majority had articular disorders (69 patients, 94.52%) with joint pain (90.41%), swelling (42.46%), stiffness (27.40%), arthritis (20.55%), tenosynovitis (13.70%), and enthesitis (10.96%). The most affected joints were the ankles (68.49%), the wrists (58.90%), the distal interphalangeal joints of the hands (36.97%), the knees (50.68%), and the proximal interphalangeal joints of the hands (45.20%).
The clinical and biological characteristics of patients at the onset of disease are displayed in Table 1.
Table 1. Clinical characteristics and results of biological tests in CCA and no CCA adults at disease onset.
DAG-2 study 2014–2016. Martinique (N = 167).
| Variables | N | no CCA (%) (N1 = 80) | CCA (%) (N2 = 87) | Risk difference [95%CI] |
p-value |
|---|---|---|---|---|---|
| DEMOGRAPHIC DATA | |||||
| Mean Age | 164 | 46.37 (15.83)# | 55.99 (15.01)# | 9.62 [4.87;14.38] | <0.0001° |
| Age | <0.0001* | ||||
| <35 years | 22 (27.85) | 4 (4.71) | |||
| 35–50 years | 26 (32.91) | 22 (25.88) | |||
| 51–65 years | 22 (27.85) | 42 (49.41) | |||
| >65 years | 9 (11.39) | 17 (20.00) | |||
| Female | 165 | 45 (56.25) | 61 (71.76) | 15.5 [1.03;30.0] | 0.04* |
| BMI (kg/m2) | 161 | 26.63 (6.52)# | 26.61 (5.91)# | -0.013 [-1.95;1.92] | 1* |
| Employment | 160 | 59 (75.64) | 60 (73.17) | -2.47 [-15.99;11.05] | 0.7* |
| CLINICAL SIGNS AT DISEASE ONSET | |||||
| Arthralgia | 167 | 77 (96.25) | 84 (96.55) | 0.3 [-5.36;5.96] | 1* |
| Fever | 167 | 77 (96.25) | 82 (94.25) | -2.0 [-8.42; 4.43] | 0.7* |
| Visual analog scale (VAS) > 4 | 167 | 67 (83.75) | 73 (83.91) | 0.16 [-11.02;11.34] | 1* |
| Headache | 167 | 41 (51.25) | 58 (66.67) | 15.42 [0.65;30.18] | 0.04* |
| Myalgia | 167 | 46 (57.50) | 48 (55.17) | -2.33[-17.38;12.72] | 0.8* |
| Abdominal pain | 167 | 28 (35.00) | 27 (31.03) | -3.97 [-18.24;10.31] | 0.6* |
| Rash | 167 | 19 (23.75) | 16 (18.39) | -5.36 [-17.74;7.02] | 0.4* |
| Number of signs | 167 | 3.27 (1.12) | 3.40 (1.13) | 0.13 [-0.22;0.47] | 0.5° |
| OTHERS SIGNS IN THE ACUTE PHASE | |||||
| Dorsalgia | 167 | 56 (70.0) | 60 (68.97) | -1.03 [-15.01;12.94] | 0.9* |
| Adenopathy | 167 | 33 (41.25) | 43 (49.43) | 8.18 [-6.88;23.23] | 0.3* |
| Asthenia | 73 | 33 (89.19) | 32 (88.89) | -0.3 [-14.64;14.03] | 1* |
| Confinement to bed | 94 | 24 (55.81) | 36 (70.59) | 14.77 [-4.63;34.18] | 0.1* |
| Non-palmoplantar rash | 94 | 26 (60.47) | 29 (56.86) | -3.6 [-23.56;16.36] | 0.7* |
| Vertigo | 167 | 19 (23.75) | 34 (39.08) | 15.33 [1.47;29.19] | 0.03* |
| Anorexia | 73 | 23 (62.16) | 24 (66.67) | 4.50 [-17.43;26.44] | 0.7* |
| Diarrhea | 167 | 18 (22.50) | 25 (28.74) | 6.24 [-6.96;19.43] | 0.4* |
| Vomiting | 167 | 9 (11.25) | 21 (24.14) | 12.89 [1.54;24.24] | 0.03* |
| Malaise | 167 | 15 (18.75) | 15 (17.24) | -1.51 [-13.18;10.16] | 0.8* |
| Cough | 167 | 16 (20.00) | 12 (13.79) | -6.21 [-17.58;5.17] | 0.3* |
| Nausea | 167 | 7 (8.75) | 16 (18.39) | 9.64 [-0.59;19.87] | 0.07* |
| Dyspnea | 167 | 14 (17.50) | 27 (31.03) | 13.53 [0.73;26.33] | 0.04* |
| Retro-orbital pain | 73 | 6 (16.22) | 10 (27.78) | 11.56 [-7.28;30.41] | 0.2** |
| Peripheral neuropathy | 46 | 6 (23.08) | 3 (15.00) | -8.08 [-30.60;14.44] | 0.5** |
| Confusional syndrome | 167 | 4 (5.00) | 1 (1.15) | -3.85 [-9.13;1.42] | 0.2** |
| Meningeal irritation | 94 | 0 | 0 | ||
| Convulsions | 94 | 0 | 0 | ||
| Coma | 94 | 0 | 0 | ||
| Melena | 95 | 0 | 1 (1.92) | 1.92 [-1.81;5.66] | 1** |
| Menorrhagia | 167 | 1 (1.25) | 0 | -1.25 [-3.68;1.18] | 0.5** |
| Petechial purpura | 167 | 0 | 1 (1.15) | 1.15 [-1.09;3.39] | 1** |
| Pleural effusion | 167 | 0 | 1 (1.15) | 1.15 [-1.09;3.39] | 1** |
| Shock | 167 | 0 | 1 (1.15) | 1.15 [-1.09;3.39] | 1** |
| Cerebral failure | 167 | 0 | 1 (1.15) | 1.15 [-1.09;3.39] | 1** |
| Low blood pressure (SBP < 90mmhg and DBP < 60mmhg) | 155 | 4 (5.33) | 7 (8.75) | 3.42 [-4.60;11.43] | 0.4** |
| High Blood pressure (SBP> 140mmhg and DBP > 90mmhg) | 155 | 5 (6.67) | 3 (3.66) | -2.92 [-9.93;4.10] | 0.4** |
| Recoloration time >3 sec | 147 | 9 (12.86) | 4 (5.19) | -7.66 [-16.94;1.61] | 0.1** |
| Pulse (beats per minute) | 157 | 80.99 (19.09)# | 76.96 (19.13)# | -4.02 [-10.05;2.00] | 0.2° |
| Respiration frequency (breathing cycles) | 131 | 18.42 (6.67) | 19.48 (9.59) | 1.07 [-1.77;3.91] | 0.5° |
| Scale scores | |||||
| EQ5D scale score in the acute phase (total score out of 100) | 144 | 60.38 (23.10) | 56.05 (20.67) | -4.32 [-11.53;2.89] | 0.2° |
| DN4 scale score (total score out of 10) | 46 | 2.31 (1.89) | 1.85 (1.56) | -0.46 [-1.51;0.60] | 0.4° |
| MEDICAL HISTORY | |||||
| High Blood Pressure (HBP) | 167 | 15 (18.75) | 21 (24.14) | 5.39 [-7.02;17.80] | 0.4* |
| Healed fractures | 167 | 15 (18.75) | 13 (14.94) | -3.81 [-15.18;7.56] | 0.5* |
| Allergy | 167 | 6 (7.50) | 9 (10.34) | 2.84 [-5.77;11.46] | 0.5** |
| Osteoarthritis | 167 | 5 (6.25) | 9 (10.34) | 4.09 [-4.22;12.41] | 0.3** |
| HIV | 167 | 6 (7.50) | 8 (9.20) | 1.70 [-6.68;10.07] | 0.7** |
| Diabetes | 167 | 5 (6.25) | 8 (9.20) | 2.95 [-5.12;11.01] | 0.5** |
| Dyslipidemia | 167 | 5 (6.25) | 8 (9.20) | 2.95 [-5.12;11.01] | 0.5** |
| Rheumatologic and inflammatory diseases | 167 | 5 (6.25) | 6 (6.90) | 0.65 [-6.87;8.16] | 0.9** |
| Cancer | 167 | 4 (5.00) | 5 (5.75) | 0.75 [-6.09;7.58] | 0.8** |
| Other immunosuppression (immunosuppressive therapy, chemotherapy) | 167 | 2 (2.50) | 5 (5.75) | 3.25 [-2.72;9.22] | 0.3** |
| Thrombopathy or chronic thrombocytopenia | 167 | 1 (1.25) | 6 (6.90) | 5.65 [-0.21;11.50] | 0.07** |
| Asthma | 167 | 2 (2.50) | 5 (5.75) | 3.25 [-2.72;9.22] | 0.4** |
| Dengue | 167 | 4 (5.00) | 2 (2.30) | -2.70[-8.42;3.02] | 0.4** |
| Hemoglobinopathy | 167 | 1 (1.25) | 5 (5.75) | 4.50 [-0.97;9.96] | 0.2** |
| Rheumatologic and degenerative diseases | 167 | 1 (1.25) | 2 (2.30) | 1.05 [-2.93;5.03] | 1** |
| Other rheumatologic diseases | 167 | 2 (2.50) | 1 (1.15) | -1.35 [-5.44;2.74] | 0.6** |
| Spondyloarthropathy | 167 | 0 | 2 (2.30) | 2.30 [-0.85;5.45] | 0.5** |
| Lupus | 167 | 1 (1.25) | 1 (1.15) | -0.10 [-3.41;3.21] | 1** |
| APS | 167 | 1 (1.25) | 0 | -1.25 [-3.68;1.18] | 0.5** |
| HBV | 167 | 1 (1.25) | 0 | -1.25 [-3.68;1.18] | 0.5** |
| HCV | 167 | 0 | 0 | ||
| Rheumatoid polyarthritis | 167 | 0 | 0 | ||
| Arthropathic psoriasis | 167 | 0 | 0 | ||
| Psoriasis | 167 | 0 | 0 | ||
| TREATMENTS | |||||
| Analgesic level 1 | 167 | 74 (92.50) | 76 (87.36) | -5.14 [-14.20;3.92] | 0.3* |
| Paracetamol | 165 | 67 (83.75) | 71 (83.53) | -0.22 [-11.51;11.07] | 1* |
| Analgesic level 2 | 167 | 24 (30.0) | 31 (35.63) | 5.63 [-8.58;19.85] | 0.4* |
| Antihistaminic | 167 | 20 (25.0) | 29 (33.33) | 8.33 [-5.38;22.05] | 0.2* |
| Non-steroidal anti-inflammatory drug (NSAID) | 167 | 17 (21.25) | 28 (32.18) | 10.93 [-2.36;24.23] | 0.1* |
| Phytotherapy (herbal medicine) | 71 | 14 (37.84) | 19 (55.88) | 18.04 [-4.82;40.91] | 0.1* |
| Intravenous rehydration | 99 | 13 (27.66) | 6 (11.54) | -16.12 [-31.58;-0.66] | 0.04** |
| Analgesic level 3 | 167 | 0 | 1 (1.15) | 1.15 [-1.09;3.39] | 1** |
| Cumulative dose of paracetamol since the beginning of symptoms (milligrams) | 83 | 5.57 (4.48)# | 8.21 (7.48)# | 2.63 [-0.15;5.41] | 0.06° |
| BIOLOGY | |||||
| PT (Prothrombin Time) (%) | 139 | 94.67 (27.03)# | 94.23 (24.78)# | -0.44 [-9.13;8.82] | 0.9° |
| ACT patient (seconds) | 139 | 35.45 (3.99)# | 35.49 (4.49)# | 0.036 [-1.39;1.46] | 0.9° |
| ACT control (seconds) | 139 | 32.76 (0.59)# | 32.68 (0.65)# | -0.07 [-0.28;0.13] | 0.5° |
| Fibrinogen (G/L) | 27 | 4.08 (1.20)# | 3.83 (1.30)# | -0.25 [-1.24;0.74] | 0.6° |
| Leukocyte (G/L) | 160 | 4.98 (2.03)# | 4.95 (2.41)# | -0.03 [-0.73;0.67] | 0.9° |
| Red blood cells (G/L) | 160 | 4.59 (0.59)# | 4.46 (0.56)# | -0.14 [-0.32;0.04] | 0.1° |
| Hemoglobin (G/dl) | 160 | 13.29 (1.84)# | 12.88 (1.47)# | -0.41 [-0.92;0.11] | 0.1° |
| MCV (μm3) | 160 | 87.68 (5.94)# | 85.88 (13.61)# | -1.80 [-5.15;1.56] | 0.3° |
| Neutrophils (G/L) | 157 | 3.38 (1.75)# | 3.39 (2.14)# | 0.009 [-0.61;0.63] | 1° |
| Eosinophils (G/L) | 157 | 0.07 (0.09)# | 0.05 (0.08)# | -0.02 [-0.04;0.01] | 0.1° |
| Basophils (G/L) | 157 | 0.02 (0.02)# | 0.02 (0.02)# | 0.002 [-0.005;0.008] | 0.7° |
| Lymphocyte (G/L) | 156 | 0.94 (0.47)# | 0.97 (0.52)# | 0.03 [-0.13;0.19] | 0.7° |
| Monocyte (G/L) | 157 | 0.44 (0.21)# | 0.46 (0.22)# | 0.02 [-0.04;0.09] | 0.5° |
| Platelets (G/L) | 159 | 204.60 (65.81)# | 205.90 (69.56)# | 1.3 [-19.97;22.64] | 0.9° |
| Sodium (mmol/L) | 151 | 137.60 (2.96)# | 137.50 (2.65)# | -0.12 [-1.02;0.78] | 0.8° |
| Potassium (mmol/L) | 151 | 3.90 (0.44)# | 3.91 (0.36)# | 0.008 [-0.12;0.14] | 0.9° |
| Chlorine (mmol/L) | 151 | 87.94 (28.72)# | 85.94 (30.81)# | -2.00 [-11.63;7.62] | 0.7° |
| Urea (mmol/L) | 148 | 3.96 (1.22)# | 4.62 (1.95)# | 0.66 [0.12;1.20] | 0.02° |
| Creatinine (μmol/L) | 156 | 83.39 (18.86)# | 79.72 (23.08)# | -3.67 [-10.38;3.04] | 0.3° |
| Protein (g/L) | 31 | 75.14 (5.19)# | 72.06 (6.35)# | -3.08 [-7.41;1.24] | 0.2° |
| Calcium (mmol/L) | 61 | 2.29 (0.10)# | 2.29 (0.10)# | -0.004 [-0.06;0.05] | 0.9° |
| Albumin (g/L) | 11 | 39.90 (2.95)# | 39.20 (3.47)# | -0.70 [-5.07;3.67] | 0.7° |
| Total bilirubin (μmol/L) | 157 | 9.07 (9.22)# | 8.30 (6.67)# | -0.77 [-3.29;1.75] | 0.5° |
| Conjugated bilirubin (μmol/L) | 155 | 3.09 (2.32)# | 2.84 (1.49)# | -0.26 [-0.87;0.36] | 0.4° |
| AST (UI/L) | 158 | 36.17 (48.42)# | 31.18 (16.59)# | -5.00 [-16.10;6.11] | 0.4° |
| ALT (UI/L) | 158 | 27.19 (18.96)# | 28.71 (18.79)# | 1.53 [-4.42;7.47] | 0.6° |
| GGT (UI/L) | 156 | 38.88 (28.58)# | 38.71 (42.99)# | -0.17 [-11.88;11.55] | 1° |
| CK (UI/L) | 133 | 206.90 (228.10)# | 197.80 (218.10)# | -9.11 [-85.66;67.43] | 0.8° |
| Lipase (UI/L) | 14 | 26.00 (11.93)# | 21.43 (1.00)# | -4.57 [-17.43;8.29] | 0.5° |
| ALP (UI/L) | 156 | 69.68 (43.11)# | 69.04 (24.80)# | -0.65 [-11.61;10.31] | 0.9° |
| Troponin (ng/mL) | 34 | 5.87 (3.75)# | 8.83 (9.00)# | 2.96 [-1.68;7.59] | 0.2° |
| CRP (mg/L) | 153 | 25.57 (70.12)# | 22.35 (30.89)# | -3.21 [-20.27;13.84] | 0.7° |
| Lactates (mmol/L) | 3 | 251.0 | 121.0 (169.7)# | -130.0 [-2770.7;2510.7] | 0.6° |
| Hematocrite (%) | 160 | 40.25 (5.15)# | 38.98 (3.93) # | 1.28 [-0.15;2.70] | 0.08° |
*Chi-square test
**Fisher’s exact test
°Wilcoxon test
#Standard deviation; bold: significant variable; 1no CCA: 1 Sub-Saharan Africa + Western Europe, 1 Sub-Saharan Africa + Western Europe + Native America, 1 Sub-Saharan Africa + India, 1 Sub-Saharan Africa + Western Europe + India / CCA: 5 Sub-Saharan Africa + Western Europe, 2 Sub-Saharan Africa + India, 2 Sub-Saharan Africa + Western Europe + India; HIV: human immunodeficiency virus; HBV: hepatitis B virus; HCV: hepatitis C virus; DN4: douleur neuropathique 4/neuropathic pain 4; EQ-5D: EuroQol-5 dimension; APS: antiphospholipid syndrome; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ACT: activated cephalin time; MCV: mean corpuscular volume; ALT: alanine aminotransferase test; AST: aspartate aminotransferase test; GGT: gamma-glutamyltransferase; CK: creatine kinase; ALP: alkaline phosphatase; CRP: C reactive protein.
Identification of risk factors for chronic outcome (CCA)
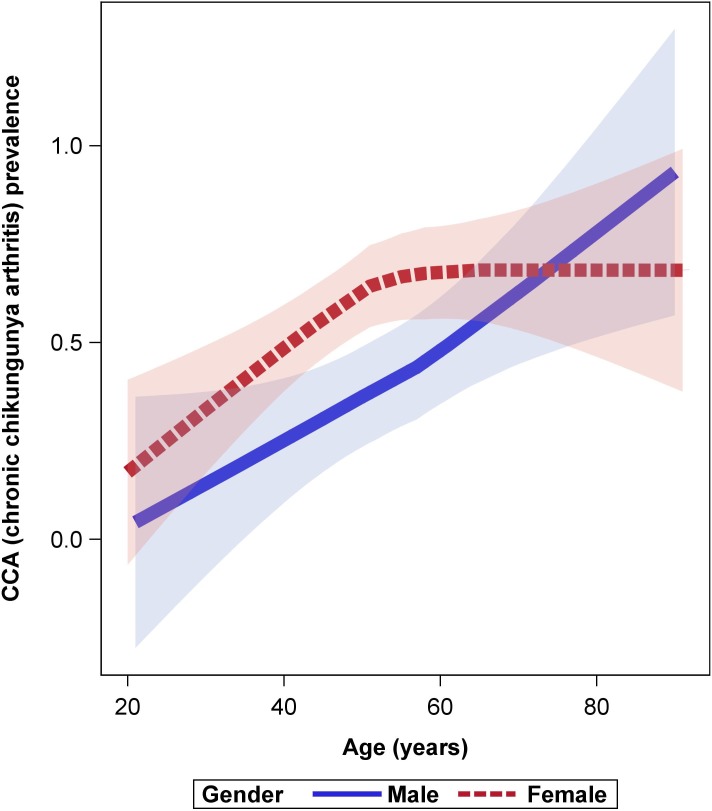
Characteristics at disease onset in the 2 groups (no CCA and CCA) were compared to identify initial predictive factors for CCA. These characteristics are detailed in S1 and S2 Tables. Significant results are listed in Table 1. CCA patients were mainly women aged over 50 years (Fig 2). Overall, 58% (61/106) of the women developed CCA, versus 41% (24/59) of the men (sex data available for 165 patients). In univariate analysis (Table 1), the probability of being CCA increased with age (RD 9.62, 95% CI, 4.87;14.38, p<0.0001); female sex (RD 15.5, 95% CI, 1.03;30.0, p = 0.04); and some clinical signs at disease onset, namely headache (RD 15.42, 95% CI, 0.65;30.18 p = 0.04), vertigo (RD 15.33, 95% CI, 1.47;29.19, p = 0.03), vomiting (RD 12.89, 95% CI, 1.54;24.24, p = 0.03), dyspnea (RD 13.53, 95% CI, 0.73;26.33, p = 0.04), and intravenous rehydration (RD -16.12, 95% CI, -31.58; -0.66 p = 0.04). Biologically, the increase of urea (RD 0.66, 95% CI, 0.12; 1.20, p = 0.02) was significantly associated with the development of CCA. For the subpopulation with data on joint involvement in the acute phase, the risk factors significantly associated with CCA in univariate analysis were at least one enthesitis (RD 16.7, 95%CI, 2.8;30.7, p = 0.02) and at least one tenosynovitis (RD 16.8, 95% CI, 1.4–32.2, p = 0.04) (Table 2). Comorbidities (cardiovascular or rheumatologic diseases) were not found to be a risk factor for CCA (Table 1). Impact on quality of life, as measured by the EQ5D scale in the acute phase, was not significantly different between CCA and no CCA patients (Table 1).
Fig 2. Loess curve between age and prevalence of chronic chikungunya according to sex.
Pink corresponds to female sex and blue to male sex. DAG-2 study 2014–2016, Martinique.
Table 2. Joint involvement in the acute phase in a subpopulation of the DAG-2 study cohort 2014–2016, Martinique (N = 73): comparison between CCA and no CCA adults.
| Variables | N | no CCA (%) (N = 37) | CCA (%) (N = 36) | Risk difference [IC95%] |
P-value |
|---|---|---|---|---|---|
| Sites | |||||
| Ankles | 73 | 26 (70.3) | 24 (66.7) | -3.6 [-24.9;17.7] | 0.7 |
| Wrists | 73 | 22 (59.5) | 21 (58.3) | -1.1 [-23.7;21.5] | 0.9 |
| Distal interphalangeal joints (hands) | 73 | 10 (27.0) | 17 (47.22) | 20.2 [-1.5;41.9] | 0.07 |
| Knees | 73 | 18 (48.7) | 19 (52.8) | 4.1 [-18.8;27.1] | 0.7 |
| Shoulders | 73 | 17 (46.0) | 17 (47.2) | 1.3 [-21.6;24.2] | 0.9 |
| Proximal interphalangeal joints (hands) | 73 | 16 (43.2) | 17 (47.2) | 4.0 [-18.8;26.8] | 0.7 |
| Metacarpophalangeal joints | 73 | 11 (29.7) | 14 (38.9) | 9.2 [-12.5;30.9] | 0.4 |
| Elbows | 73 | 10 (27.0) | 10 (27.8) | 0.8 [-19.7;21.2] | 0.9 |
| Metatarsophalangeal joints | 73 | 9 (24.3) | 9 (25.0) | 0.7 [-19.1;20.5] | 0.9 |
| Feet | 73 | 9 (24.3) | 8 (22.2) | -2.1 [-21.5;17.3] | 0.8 |
| Hips | 73 | 7 (18.9) | 8 (22.2) | 3.3 [-15.2;21.8] | 0.7 |
| Hands | 73 | 5 (13.5) | 6 (16.7) | 3.2 [-13.3;19.6] | 0.7 |
| Ribs | 73 | 0 | 1 (2.8) | 2.8 [-2.6;8.2] | 0.5 |
| Back | 73 | 5 (13.5) | 7 (19.4) | 5.9 [-11.1;22.9] | 0.5 |
| Neck | 73 | 10 (27.0) | 13 (36.1) | 9.1 [-12.2;30.3] | 0.4 |
| Rachis | 73 | 16 (43.2) | 10 (27.8) | -15.5 [-37.1;6.2] | 0.2 |
| Sternum | 73 | 2 (5.4) | 5 (13.9) | 8.5 [-5.0;21.9] | 0.2 |
| Proximal interphalangeal joints (feet) | 73 | 5 (13.5) | 5 (13.9) | 0.4 [-15.4;16.2] | 1.0 |
| Distal interphalangeal joints (feet) | 73 | 4 (10.8) | 5 (13.9) | 3.1 [-12.0;18.2] | 0.7 |
| Articular disorders | |||||
| Joint pain | 73 | 33 (89.2) | 33 (91.7) | 2.5 [-11.0;16.0] | 0.7 |
| Joint swelling without arthritis | 73 | 17 (46.0) | 14 (38.9) | -7.1 [-29.7;15.6] | 0.5 |
| Joint stiffness | 73 | 7 (18.9) | 13 (36.1) | 17.2 [-2.9;37.3] | 0.1 |
| Arthritis | 73 | 7 (18.9) | 8 (22.2) | 3.3 [-15.2;21.8] | 0.7 |
| Asymmetric joint involvement | 73 | 7 (18.9) | 5 (13.9) | -5.0 [-22.0;11.9] | 0.6 |
| Tenosynovitis | 73 | 2 (5.4) | 8 (22.2) | 16.8 [1.4;32.2] | 0.04 |
| Enthesitis | 73 | 1 (2.7) | 7 (19.4) | 16.7 [2.8;30.7] | 0.02 |
| Arthritis with synovitis | 73 | 1 (2.7) | 0 | -2.7 [-7.9;2.5] | 0.3 |
| Periostitis | 73 | 0 | 0 | - |
*test; bold: significant variable
Of the 77 chronic patients who answered at least 2 of the 3 questionnaires, 64 (83%) had relapsing joint symptoms and 13 (17%) had lingering joint symptoms. Moreover, 54 out of 87 CCA patients were clinically evaluated in the chronic phase (after adjustment of the protocol for the DAG-2 cohort), with joint pain (87%), stiffness (33.3%), and swelling (22%) being the most common chronic symptoms (Table 3). Enthesitis (13%), arthritis (7.4%), and tenosynovitis (5.6%) were less frequently observed. Ankles (31.5%) and knees (29.6%) were the most affected joints, while the proximal interphalangeal joints of the hands (24.1%), the wrists (22.2%), and the shoulders (22.2%) were less frequently involved. A quarter of patients presented peripheral neuropathy (27.7%), and 10 out of 30 patients had a mean MDHAQ score of 10.7. The mean EQ5D score was 51.56, indicating a significant decrease in quality of life relative to before CHIKV-infection (defined as 100/100).
Table 3. Clinical profile of CCA patients in the chronic phase between M3 and M12 (N = 54).
| Variable | N | Value n (%) | min-max |
|---|---|---|---|
| Age: mean in years | 48 | 49.86 | 21–91 |
| Sex ratio Women/Men | 53 | 2.78 | |
| Number of joint assessments | 54 | 7 | 0–32 |
| Joint pain | 54 | 47 (87.04) | |
| Joint swelling without arthritis | 54 | 12 (22.00) | |
| Joint stiffness | 54 | 18 (33.33) | |
| Arthritis | 54 | 4 (7.41) | |
| Tenosynovitis | 54 | 3 (5.56) | |
| Enthesitis | 54 | 7 (12.96) | |
| Arthritis with synovitis | 54 | 1 (1.85) | |
| Periostitis | 54 | 1 (1.85) | |
| Peripheral neuropathy | 47 | 13 (27.66) | |
| DN4 score mean value in the chronic phase | 47 | 2.2 | 0–7 |
| EQ5D score mean value in the chronic phase: mean points | 49 | 51.56 | 1–100 |
| MDHAQ score mean value in the chronic phase: mean points | 43 | 10.7 | 0–22.7 |
| Sites of assessment | |||
| Ankles | 54 | 17 (31.48) | |
| Knees | 54 | 16 (29.63) | |
| Proximal interphalangeal joint (hand) | 54 | 13 (24.07) | |
| Wrists | 54 | 12 (22.22) | |
| Shoulder | 54 | 12 (22.22) | |
| Metacarpophalangeal joint | 54 | 10 (18.52) | |
| Metatarsophalangeal joint | 54 | 9 (16.67) | |
| Elbow | 54 | 7 (12.96) | |
| Distal interphalangeal joint (hand) | 54 | 7 (12.96) | |
| Hand | 54 | 3 (5.56) | |
| Foot | 54 | 3 (5.56) | |
| Hip | 54 | 3 (5.56) | |
| Distal interphalangeal joint (foot) | 54 | 2 (3.70) | |
| Proximal interphalangeal joint (foot) | 54 | 1 (1.85) |
DN4: douleur neuropathique 4/neuropathic pain 4; EQ-5D: EuroQol-5 dimension; MDHAQ: multidimensional health assessment questionnaire.
A multivariable analysis performed using the LASSO method is presented in S1 Table. Age (aOR 1.08 95%CI, 1.05–1.12, p<0.0001), female sex (aOR 2.44 95%CI, 1.03–5.88, p = 0.046), vomiting (aOR 3.37 95%CI, 1.06–11.8, p = 0.045), and Low blood pressure (aOR 6.67 95%CI, 1.47–36.6, p = 0.02) were identified as the mains risk factors for CCA.
Discussion
This was the first prospective cohort study of CHIKV-infected cases to be conducted in Martinique during the 2013-outbreak. The overall prevalence of chronic chikungunya in our cohort was 52.10% (CI 95% 44.50–59.70), which confirms that it is a common sequela after acute infection. Indeed, previous studies have reported prevalences ranging between 53.70% and 87.20% globally [6,31–33]—except in India, where prevalences have been shown to vary between 4.10% and 7.00% [46,47] (possibly due to viral or population specificities).
During previous outbreaks, age over 50 and female sex were shown to be the main risk factors for chronic chikungunya disease [6,32,34,40,41,48,49]. Some studies focusing on gender have suggested that the menstrual cycle [50] and ovulation influence the production of pro‐inflammatory cytokines by monocytes [50,51] and that estradiol plays a role in the increase of antibody production [52,53]. However, these hypotheses have not been fully tested.
In our study, increased urea (p = 0.02) and reduced frequency of intravenous rehydration (p = 0.04) were associated with the development of CCA in univariate analysis. This finding suggests that initial dehydration is one of the potential factors leading to the development of CCA, just as it can cause complications in other arbovirus infections [54,55]. Our results also indicate that vertigo (p = 0.03) and headache (p = 0.04) are symptoms of dehydration, and that vomiting (p = 0.03) is an aggravating factor for dehydratation. Note that vomiting was a significant factor in the multivariable analysis, along with age and sex. The difficulty in diagnosing dehydration and the lack of human and material resources explain why rehydration was not systematically performed in our cohort of patients with CHIKV.
Because cartilage is largely composed of water [56], dehydration may result in its degradation by causing cracks and increasing fragility [57,58]. A study by Sahni et al. [59] on the impact of prolonged dehydration on osseous tissue and calcium phosphate metabolism in mice concluded that prolonged water deficiency leads to decreased bone mass, with an increased rate of bone resorption and mineralization defect. It is also established that calcium metabolism disorders stimulate parathyroid hormone (PTH) secretion and activation of bone resorption by osteoclast. In our study, no difference in calcemia was observed between CCA and no CCA patients in the acute phase. However, the rate of calcium was available for only 61 patients (as our study sample was small), and this one-off measure does not reflect metabolic disorders over the medium to long term. In fact, hydration may help dilute pro-inflammatory cells and cytokines recruited in joints and may enhance anti-inflammatory response. While articular cartilage is a mechanically highly challenged material with very limited regenerative ability, recent data by Boettcher et al. [60] have shown that a broad range of mechanical and structural properties of cartilage can be restored after rehydration with physiological salt solution.
CHIKV has been shown to damage cartilage [61] and synovial tissue [62]. In view of this, we make the hypothesis that patients suffering from dehydration at the onset of the disease are at higher risk of subsequently developing damaged cartilage, which may lead to more severe joint injury, slower recovery, and chronic injury. While it has been suggested that the intensity of symptoms at presentation is predictive of CCA [41], our results do not allow us to formally respond to the hypothesis we have formulated: dehydration during the acute phase of CHIKV infection is a cause of CCA. Indeed, we did not report on the occurrence of dehydration per se. Future studies should explicitly test this hypothesis (i) by accurately quantifying the volume of liquid ingested by patients during the acute phase in prospective studies; (ii) by conducting a randomized clinical trial comparing high intravenous and oral rehydration versus rehydration advice with subsequent quantification. Lastly, dehydration may contribute to the increase in viremia through serum concentration, as some studies have reported higher CHIKV plasma viral loads in CCA patients [62,63]. However, we did not investigate this parameter in our study due to a lack of funding.
Contrary to other authors, we found no association between CCA and pre-existing diseases—whether rheumatic disorders [48] like osteoarthritis [64] and rheumatoid arthritis [65–69] or cardiovascular diseases like hypertension [70] and diabetes [34].
Some studies have described the inflammatory and autoimmune processes in the acute and chronic phases of chikungunya disease [62,71]. Several of them have identified a positive relationship at disease onset between CRP level [34,65], pro-inflammatory cytokines [62,71], and severity at presentation [41,42,64]. However, no autoimmune markers for CHIKV have been highlighted in observational studies [34]. In other studies, biomarkers associated with CCA were IL-6 secretion [63] and GM-CSF at disease onset [62,63]. In a study conducted in Reunion Island, the proper role of specific IgG titers in predicting CCA was observed and positively correlated with age, female sex, and severity of initial rheumatic symptoms [41]. Yet, this study found no association between CRP level at disease onset and CCA.
The main clinical characteristics of chronic patients in our study were consistent with previous descriptions [8,24,38]: polyarthralgia, articular stiffness, swollen joints, and arthritis. Moreover, as was the case in previous studies [38,72–74], enthesitis and tenosynovitis were observed in our cohort. One study reported relapsing arthralgia for the majority of examined patients [40]. In our study, the development of CCA was associated with at least 1 tenosynovitis or 1 enthesitis in the acute phase. These 2 conditions, which are highly specific to symptomatic CHIKV infection, have not yet been identified as risk factors for CCA [75–77].
Some studies have highlighted the impact of CCA on daily activities and quality of life [35–38,41]. Patients suffering from CCA have been shown to require a multidisciplinary medical care program [78,79]. One study recommends long-term follow up by a general practitioner, often in cooperation with a rheumatologist or a rehabilitation physician [80]. Chikungunya disease and especially CCA with relapsing sequela have been shown to induce anxiety, stress, and even a nervous breakdown [81,82]. Medical treatments include analgesics, anti-inflammatory drugs, corticosteroids, and in severe cases anticonvulsant and immunosuppressive therapy [79,83,84]. Moreover, treatment may be combined with physiotherapy, physical therapy, and psychiatric and psychological evaluation [81,82–85]. Long-term disabilities caused by chikungunya are costly at the individual and collective scales. The economic impact of the 2005–2006 chikungunya outbreak in Reunion Island was estimated at 51.63 million US dollars [86].
One of the strengths of our study was the early inclusion and prospective follow-up of patients located at the epicenter of the epidemic. Another strength was that patients were examined physically by a clinician investigator at disease onset and at M3 to monitor clinical manifestations. This objective assessment of symptoms probably prevented an overestimation of symptoms on the part of patients, though some data (e.g., those collected via joint pain assessment) remain subjective.
Our study also has limitations that must be acknowledged. First, the phone interviews conducted at M6 and M12 may have led to a declarative bias. The rate of lost to follow-up was high, though phone calls helped to reduce it. Second, the study cohort did not fully reflect the population affected by the epidemic because subjects were not sampled from the general population; thus, data for elders may be missing as their symptoms are atypical and easily misdiagnosed [87,88]. Third, the plasma viral load of CCA and no CCA patients could not be measured due to a lack of funding; however, future studies could exploit our biological bank to assess this variable and to conduct other immunological and virological investigations.
To conclude, this first prospective study conducted during the chikungunya outbreak in Martinique has described the course of the disease in the local population and has estimated the burden at 1 year. Our findings confirm that more than half of adult symptomatic infections become chronic, with age and female sex as major risk factors for incident chronic chikungunya. The predictors of chronicity identified in the acute phase lead us to suggest that dehydration is a compounding factor in the occurrence of persisting chikungunya-associated rheumatic disorders. This hypothesis opens up new avenues for prevention and research in CHIKV pathways.
Supporting information
(DOCX)
(DOCX)
Multivariable analysis using least absolute shrinkage and selection operator. DAG-2 study 2014–2016, Martinique (N = 167).
(DOCX)
(DOC)
Acknowledgments
We would like to thank both Elsevier and our copy editor Arianne Dorval for editorial assistance as well as the Chronic Chikungunya working group at the University Medical Center of Martinique (Bally Jacques, Brouste Yannick, Brunier Lauren, Godaert-Simon Lidvine, Hochedez Patrick, Jean-Marie Janick, Komla-Souhka Isabelle, Molkkar Sabine, Pelonde-Erimée Véronique, Perreau Caroline, René-Corail Patrick, Signate Aissatou, Trois-Gros Odile, Ursulet Gilbert, Valentino Ruddy). The protocol was prepared with the help of the INSERM Research and Action Targeting Emerging Infectious Disease (REACTing) network.
Data Availability
At the promoter's demand, data are only available on request : Department of Research and Innovation, Martinique University Hospital 97261 Fort-de-France, Martinique, France. Contact: Cedric CONTARET; email: cedric.contaret@chu-fortdefrance.fr.
Funding Statement
Funding was obtained by the CHU of Martinique from the French Ministry of Research and Higher Education (PHRC 2019 29-01). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, et al. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne Zoonotic Dis Larchmt N. 2010; 10(3): 259–66. [DOI] [PubMed] [Google Scholar]
- 2.Pastorino B, Muyembe-Tamfum JJ, Bessaud M, Tock F, Tolou H, Durand JP, et al. Epidemic resurgence of Chikungunya virus in Democratic Republic of the Congo: identification of a new central African strain. J Med Virol. 2004; 74(2): 277–82. 10.1002/jmv.20168 [DOI] [PubMed] [Google Scholar]
- 3.Diagne CT, Faye O, Guerbois M, Knight R, Diallo D, Faye O, et al. Vector Competence of Aedes aegypti and Aedes vittatus (Diptera: Culicidae) from Senegal and Cape Verde Archipelago for West African Lineages of Chikungunya Virus. Am J Trop Med Hyg. 2014; 91(3): 635–41. 10.4269/ajtmh.13-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, Fontenille D. [Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control]. Parasite Paris Fr. 2008; 15(1): 3–13. [DOI] [PubMed] [Google Scholar]
- 5.Vazeille M, Moutailler S, Coudrier D, Rousseaux C, Khun H, Huerre M, et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PloS One. 2007; 2(11): e1168 10.1371/journal.pone.0001168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Essackjee K, Goorah S, Ramchurn SK, Cheeneebash J, Walker-Bone K. Prevalence of and risk factors for chronic arthralgia and rheumatoid-like polyarthritis more than 2 years after infection with chikungunya virus. Postgrad Med J. 2013; 89(1054): 440–7. 10.1136/postgradmedj-2012-131477 [DOI] [PubMed] [Google Scholar]
- 7.Ngwe Tun MM, Inoue S, Thant KZ, Talemaitoga N, Aryati A, Dimaano EM, et al. Retrospective seroepidemiological study of chikungunya infection in South Asia, Southeast Asia and the Pacific region. Epidemiol Infect. 2016; 144(11): 2268–75. 10.1017/S095026881600056X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijayakumar KP, Nair Anish TS, George B, Lawrence T, Muthukkutty SC, Ramachandran R. Clinical Profile of Chikungunya Patients during the Epidemic of 2007 in Kerala, India. J Glob Infect Dis. 2011; 3(3): 221–6. 10.4103/0974-777X.83526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villabona-Arenas CJ, de Oliveira JL, Capra Cde S, Balarini K, Loureiro M, Fonseca CR, et al. Detection of four dengue serotypes suggests rise in hyperendemicity in urban centers of Brazil. PLoS Negl Trop Dis. 2014; 8: e2620 10.1371/journal.pntd.0002620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camacho D, Reyes J, Negredo A, Hernández L, Sánchez-Seco M, Comach G. Asian genotype of Chikungunya virus circulating in Venezuela during 2014. Acta Trop. 2017; 174: 88–90. 10.1016/j.actatropica.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 11.Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli A, Panning M, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. The Lancet. 2007; 370(9602): 1840–6. [DOI] [PubMed] [Google Scholar]
- 12.Horcada ML, Díaz-Calderón C, Garrido L. Chikungunya fever. Rheumatic manifestations of an emerging disease in Europe. Reumatol Clin. 2015; 11(3): 161–4. 10.1016/j.reuma.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 13.Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, et al. Characterization of reemerging chikungunya virus. PLoS Pathog. 2007; 3(6): e89 10.1371/journal.ppat.0030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgs S, Ziegler SA. A nonhuman primate model of chikungunya disease. J Clin Invest. 2010; 120(3): 657–60. 10.1172/JCI42392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffar-Bandjee MC, Gasque P. [Physiopathology of chronic arthritis following chikungunya infection in man]. Médecine Trop Rev Corps Santé Colon. 2012; 72 Spec No: 86–7. [PubMed] [Google Scholar]
- 16.Ozden S, Huerre M, Riviere J-P, Coffey LL, Afonso PV, Mouly V, et al. Human muscle satellite cells as targets of Chikungunya virus infection. PloS One. 2007; 2(6): e527 10.1371/journal.pone.0000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashyap RS, Morey S, Bhullar S, Baheti N, Chandak N, Purohit H, et al. Determination of Toll-Like Receptor-Induced Cytokine Profiles in the Blood and Cerebrospinal Fluid of Chikungunya Patients. Neuroimmunomodulation. 2014; 21(6): 338–46. 10.1159/000358240 [DOI] [PubMed] [Google Scholar]
- 18.Arpino C, Curatolo P, Rezza G. Chikungunya and the nervous system: what we do and do not know. Rev Med Virol. 2009; 19(3): 121–9. 10.1002/rmv.606 [DOI] [PubMed] [Google Scholar]
- 19.Chandak NH, Kashyap RS, Kabra D, Karandikar P, Saha SS, Morey SH, et al. Neurological complications of Chikungunya virus infection. Neurol India. 2009; 57(2): 177–80. 10.4103/0028-3886.51289 [DOI] [PubMed] [Google Scholar]
- 20.Bank AM, Batra A, Colorado RA, Lyons JL. Myeloradiculopathy associated with chikungunya virus infection. J Neurovirol. 2016; 22(1): 125–8. 10.1007/s13365-015-0372-9 [DOI] [PubMed] [Google Scholar]
- 21.Gérardin P, Couderc T, Bintner M, Tournebize P, Renouil M, Lémant J, et al. Chikungunya virus-associated encephalitis: A cohort study on La Réunion Island, 2005–2009. Neurology. 2016; 86(1): 94–102. 10.1212/WNL.0000000000002234 [DOI] [PubMed] [Google Scholar]
- 22.Mazaud R, Salaün JJ, Montabone H, Goube P, Bazillio R. [Acute neurologic and sensorial disorders in dengue and Chikungunya fever]. Bull Société Pathol Exot Ses Fil. 1971; 64(1): 22–30. [PubMed] [Google Scholar]
- 23.Mason PJ, Haddow AJ. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53; an additional note on chikungunya virus isolations and serum antibodies. Trans R Soc Trop Med Hyg. 1957; 51(3): 238–40. 10.1016/0035-9203(57)90022-6 [DOI] [PubMed] [Google Scholar]
- 24.Palacios-Martínez D, Díaz-Alonso RA, Arce-Segura LJ, Díaz-Vera E. Chikungunya, una enfermedad vírica emergente. Propuesta de un algoritmo de manejo clínico. Semergen-Med Fam. 2015; 41(4): 221–225. [DOI] [PubMed] [Google Scholar]
- 25.Casolari S, Briganti E, Zanotti M, Zauli T, Nicoletti L, Magurano F, et al. A fatal case of encephalitis associated with Chikungunya virus infection. Scand J Infect Dis. 2008; 40(11–12): 995–6. 10.1080/00365540802419055 [DOI] [PubMed] [Google Scholar]
- 26.Lemant J, Boisson V, Winer A, Thibault L, André H, Tixier F, et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit Care Med. 2008; 36(9): 2536–41. [DOI] [PubMed] [Google Scholar]
- 27.Hrnjaković Cvjetković IB, Cvjetković D, Patić A, Nikolić N, Stefan Mikić S, Milošević V. Chikungunya—a serious threat for public health. Med Pregl. 2015; 68(3–4): 122–5. 10.2298/mpns1504122h [DOI] [PubMed] [Google Scholar]
- 28.Simon F, Paule P, Oliver M. Chikungunya virus-induced myopericarditis: toward an increase of dilated cardiomyopathy in countries with epidemics? Am J Trop Med Hyg. 2008; 78(2): 212–3. [PubMed] [Google Scholar]
- 29.Maiti CR, Mukherjee AK, Bose B, Saha GL. Myopericarditis following chikungunya virus infection. J Indian Med Assoc. 1978; 70(11): 256–8. [PubMed] [Google Scholar]
- 30.Lemant J, Boisson V, Winer A, Thibault L, André H, Tixier F, et al. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit Care Med. 2008; 36(9): 2536–41. [DOI] [PubMed] [Google Scholar]
- 31.Chikungunya: case definitions for acute, atypical and chronic cases. Conclusions of an expert consultation, Managua, Nicaragua, 20–21 May 2015. Wkly Epidemiol Rec Health Sect Secr Leag Nations. 2015;90(33): 410–4. [PubMed] [Google Scholar]
- 32.Rodriguez-Morales AJ, Gil-Restrepo AF, Ramírez-Jaramillo V, Montoya-Arias CP, Acevedo-Mendoza WF, Bedoya-Arias JE, et al. Post-chikungunya chronic inflammatory rheumatism: results from a retrospective follow-up study of 283 adult and child cases in La Virginia, Risaralda, Colombia. F1000Research. 2016;5: 360 10.12688/f1000research.8235.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Morales AJ, Cardona-Ospina JA, Villamil-Gómez W, Paniz-Mondolfi AE. How many patients with post-chikungunya chronic inflammatory rheumatism can we expect in the new endemic areas of Latin America? Rheumatol Int. 2015; 35(12): 2091–4. 10.1007/s00296-015-3302-5 [DOI] [PubMed] [Google Scholar]
- 34.Schilte C, Staikowsky F, Staikovsky F, Couderc T, Madec Y, Carpentier F, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013; 7(3): e2137 10.1371/journal.pntd.0002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Couturier E, Guillemin F, Mura M, Léon L, Virion J-M, Letort M-J, et al. Impaired quality of life after chikungunya virus infection: a 2-year follow-up study. Rheumatol Oxf Engl. 2012; 51(7): 1315–22. [DOI] [PubMed] [Google Scholar]
- 36.Ramachandran V, Malaisamy M, Ponnaiah M, Kaliaperuaml K, Vadivoo S, Gupte MD. Impact of Chikungunya on health related quality of life Chennai, South India. PloS One. 2012; 7(12): e51519 10.1371/journal.pone.0051519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Andrade DC, Jean S, Clavelou P, Dallel R, Bouhassira D. Chronic pain associated with the Chikungunya Fever: long lasting burden of an acute illness. BMC Infect Dis. 2010; 10:31 10.1186/1471-2334-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marimoutou C, Vivier E, Oliver M, Boutin J-P, Simon F. Morbidity and impaired quality of life 30 months after chikungunya infection: comparative cohort of infected and uninfected French military policemen in Reunion Island. Medicine (Baltimore). 2012; 91(4): 212–9. 10.1097/MD.0b013e318260b604 [DOI] [PubMed] [Google Scholar]
- 39.Moro ML, Grilli E, Corvetta A, Silvi G, Angelini R, Mascella F, et al. Long-term chikungunya infection clinical manifestations after an outbreak in Italy: a prognostic cohort study. J Infect. 2012; 65(2): 165–72. 10.1016/j.jinf.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 40.Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, et al. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis. 2009; 3(3): e389 10.1371/journal.pntd.0000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gérardin P, Fianu A, Michault A, Mussard C, Boussaïd K, Rollot O, et al. Predictors of Chikungunya rheumatism: a prognostic survey ancillary to the TELECHIK cohort study. Arthritis Res Ther. 2013; 15(1): R9 10.1186/ar4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yaseen HM, Simon F, Deparis X, Marimoutou C. Identification of initial severity determinants to predict arthritis after chikungunya infection in a cohort of French gendarmes. BMC Musculoskelet Disord. 2014; 15:249 10.1186/1471-2474-15-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ledrans M, Najioullah F, Cesaire R, Locatelli Jouans C, Vincent J, Daudens E, et al. Émergence du chikungunya dans les départements français d’Amérique: organisation et résultats de la surveillance épidémiologique, avril 2014. Bull Epidemiol Hebd. 2014; (21–22): 368–79. [Google Scholar]
- 44.Gallian P, de Lamballerie X, Salez N, Piorkowski G, Richard P, Paturel L, et al. Prospective detection of chikungunya virus in blood donors, Caribbean 2014. Blood. 2014; 123(23): 3679–81. 10.1182/blood-2014-03-564880 [DOI] [PubMed] [Google Scholar]
- 45.Point épidémiologique du 12 janvier au 1er mars 2015 (S2015-03 à S2015-09) [cited 2016 May 17]. [Internet]. Available from: http://www.ars.martinique.sante.fr/fileadmin/MARTINIQUE/Actualites/Autres_actu/2015/fin_epidemie_CHIK/PE_Antilles_2015-2_Chik.pdf
- 46.Chopra A, Anuradha V, Ghorpade R, Saluja M. Acute Chikungunya and persistent musculoskeletal pain following the 2006 Indian epidemic: a 2-year prospective rural community study. Epidemiol Infect. 2012; 140(5): 842–50. 10.1017/S0950268811001300 [DOI] [PubMed] [Google Scholar]
- 47.Ramachandran V, Kaur P, Kanagasabai K, Vadivoo S, Murhekar M. Persistent arthralgia among Chikungunya patients and associated risk factors in Chennai, South India. J Postgrad Med. 2014; 60(1): 3 10.4103/0022-3859.128795 [DOI] [PubMed] [Google Scholar]
- 48.Rahim AA, Thekkekara RJ, Bina T, Paul BJ. Disability with Persistent Pain Following an Epidemic of Chikungunya in Rural South India. J Rheumatol. 2016; 43(2): 440–4. 10.3899/jrheum.141609 [DOI] [PubMed] [Google Scholar]
- 49.Heath CJ, Lowther J, Noël TP, Mark-George I, Boothroyd DB, Mitchell G, et al. The Identification of Risk Factors for Chronic Chikungunya Arthralgia in Grenada, WestIndies: A Cross-Sectional Cohort Study. Open Forum Infect Dis. 2018; 5(1): ofx234 10.1093/ofid/ofx234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willis C, Morris JM, Danis V, Gallery EDM. Cytokine production by peripheral blood monocytes during the normal human ovulatory menstrual cycle. Hum Reprod. 2003; 18(6): 1173–8. 10.1093/humrep/deg231 [DOI] [PubMed] [Google Scholar]
- 51.Her Z, Malleret B, Chan M, Ong EKS, Wong S-C, Kwek DJC, et al. Active Infection of Human Blood Monocytes by Chikungunya Virus Triggers an Innate Immune Response. J Immunol. 2010;184(10): 5903–13. 10.4049/jimmunol.0904181 [DOI] [PubMed] [Google Scholar]
- 52.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11(4): 411–23. 10.1093/humupd/dmi008 [DOI] [PubMed] [Google Scholar]
- 53.Matalka KZ. The effect of estradiol, but not progesterone, on the production of cytokines in stimulated whole blood, is concentration-dependent. Neuro Endocrinol Lett. 2003; 24(3–4): 185–91. [PubMed] [Google Scholar]
- 54.Harris E, Pérez L, Phares CR, Pérez Mde L, Idiaquez W, Rocha J, et al. Fluid intake and decreased risk for hospitalization for dengue fever, Nicaragua. Emerg Infect Dis. 2003; 9(8): 1003–6. 10.3201/eid0908.020456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nasir NH, Mohamad M, Lum LCS, Ng CJ (2017) Effectiveness of a fluid chart in outpatient management of suspected dengue fever: A pilot study. PLoS ONE. 2017; 12: e0183544 10.1371/journal.pone.0183544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fox AJS, Bedi A, Rodeo SA. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health Multidiscip Approach. 2009; 1(6): 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fick JM, Espino DM. Articular cartilage surface failure: an investigation of the rupture rate and morphology in relation to tissue health and hydration. Proc Inst Mech Eng [H]. 2012; 226(5): 389–96. [DOI] [PubMed] [Google Scholar]
- 58.Wang Q, Yang Y-Y, Niu H-J, Zhang W-J, Feng Q-J, Chen W-F. An ultrasound study of altered hydration behaviour of proteoglycan-degraded articular cartilage. BMC Musculoskelet Disord. 2013; 14: 289 10.1186/1471-2474-14-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahni M, Peignoux-Deville J, Lopez E, Lallier F, Martelly E, Vidal B. Effet d’une carence hydrique sur certains aspects du métabolisme phosphocalcique d’un rongeur semi-désertique (Meriones shawi) en croissance. Reprod Nutr Dév. 1987; 27(1A): 1–12. [PubMed] [Google Scholar]
- 60.Boettcher K, Kienle S, Nachtsheim J, Burgkart R, Hugel T, Lieleg O. The structure and mechanical properties of articular cartilage are highly resilient towards transient dehydration. Acta Biomater. 2016; 29:180–7. 10.1016/j.actbio.2015.09.034 [DOI] [PubMed] [Google Scholar]
- 61.Lokireddy S, Vemula S, Vadde R. Connective tissue metabolism in chikungunya patients. Virol J. 2008; 5: 31 10.1186/1743-422X-5-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoarau J-J, Bandjee M-CJ, Trotot PK, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent Chronic Inflammation and Infection by Chikungunya Arthritogenic Alphavirus in Spite of a Robust Host Immune Response. J Immunol. 2010; 184(10): 5914–27. 10.4049/jimmunol.0900255 [DOI] [PubMed] [Google Scholar]
- 63.Chow A, Her Z, Ong EKS, Chen J, Dimatatac F, Kwek DJC, et al. Persistent Arthralgia Induced by Chikungunya Virus Infection is Associated with Interleukin-6 and Granulocyte Macrophage Colony-Stimulating Factor. J Infect Dis. 2011; 203(2): 149–57. 10.1093/infdis/jiq042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, et al. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis. 2009; 3(3): e389 10.1371/journal.pntd.0000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouquillard É, Combe B. A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Joint Bone Spine. 2009; 76(6): 654–7. 10.1016/j.jbspin.2009.08.005 [DOI] [PubMed] [Google Scholar]
- 66.Miner JJ, Aw-Yeang H-X, Fox JM, Taffner S, Malkova ON, Oh ST, et al. Brief Report: Chikungunya viral arthritis in the United States: A mimic of seronegative rheumatoid arthritis. Arthritis Rheumatol Hoboken NJ. 2015; 67(5): 1214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foissac M, Javelle E, Ray S, Guérin B, Simon F. Post-Chikungunya Rheumatoid Arthritis, Saint Martin. Emerg Infect Dis. 2015; 21(3): 530–2. 10.3201/eid2103.141397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arroyo-Ávila M, Vilá LM. Rheumatic Manifestations in Patients with Chikungunya Infection. P R Health Sci J. 2015; 34(2): 71–7. [PubMed] [Google Scholar]
- 69.Foissac M, Javelle E, Ray S, Guérin B, Simon F. Post-Chikungunya Rheumatoid Arthritis, Saint Martin. Emerg Infect Dis. 2015; 21(3): 530–2. 10.3201/eid2103.141397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Economopoulou A, Dominguez M, Helynck B, Sissoko D, Wichmann O, Quenel P, et al. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Réunion. Epidemiol Infect. 2009; 137(4): 534–41. [DOI] [PubMed] [Google Scholar]
- 71.Her Z, Malleret B, Chan M, Ong EKS, Wong S-C, Kwek DJC, et al. Active Infection of Human Blood Monocytes by Chikungunya Virus Triggers an Innate Immune Response. J Immunol. 2010; 184(10): 5903–13. 10.4049/jimmunol.0904181 [DOI] [PubMed] [Google Scholar]
- 72.Chaaithanya IK, Muruganandam N, Raghuraj U, Sugunan AP, Rajesh R, Anwesh M, et al. Chronic inflammatory arthritis with persisting bony erosions in patients following chikungunya infection. Indian J Med Res. 2014; 140(1): 142–5. [PMC free article] [PubMed] [Google Scholar]
- 73.Javelle E, Ribera A, Degasne I, Gaüzère B-A, Marimoutou C, Simon F. Specific Management of Post-Chikungunya Rheumatic Disorders: A Retrospective Study of 159 Cases in Reunion Island from 2006–2012. PLoS Negl Trop Dis. 2015; 9(3): e0003603 10.1371/journal.pntd.0003603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathew AJ, Goyal V, George E, Thekkemuriyil DV, Jayakumar B, Chopra A, et al. Rheumatic-musculoskeletal pain and disorders in a naïve group of individuals 15 months following a Chikungunya viral epidemic in south India: a population based observational study. Int J Clin Pract. 2011; 65(12): 1306–12. 10.1111/j.1742-1241.2011.02792.x [DOI] [PubMed] [Google Scholar]
- 75.Seijo A. [Tenosynovitis associated to Chikungunya virus (CHKV) infection: a response]. Medicina (Mex). 2015; 75(2): 130. [PubMed] [Google Scholar]
- 76.Yağcı Çağlayık D, Uyar Y, Korukluoğlu G, Ertek M, Unal S. [An imported Chikungunya fever case from New Delhi, India to Ankara, Turkey: the first imported case of Turkey and review of the literature]. Mikrobiyoloji Bül. 2012; 46(1): 122–8. [PubMed] [Google Scholar]
- 77.Parola P, Simon F, Oliver M. Tenosynovitis and Vascular Disorders Associated with Chikungunya Virus–Related Rheumatism. Clin Infect Dis. 2007; 45(6): 801–2. [DOI] [PubMed] [Google Scholar]
- 78.Chikungunya. Point sur les connaissances et la conduite à tenir en Martinique. 2013 Dec [cited 2015 Mar 28] [Internet]. Available from: http://www.ars.martinique.sante.fr/fileadmin/MARTINIQUE/Votre_Sante/Veille_sanitaire/Les_champs_de_competences/Chikungunya/Telecharger/Chik_Fiche_info_medecin_-_V1_Dec_2013.pdf
- 79.Prise en charge du chikungunya subaigu et chronique. Fiche de synthèse. 2014 Oct 17 [cited 2015 Mar 28] [Internet]. Available from: http://www.ars.martinique.sante.fr/fileadmin/MARTINIQUE/Actualites/Autres_actu/2014
- 80.Vijayan V, Sukumaran S. Chikungunya Virus Disease: An Emerging Challenge for the Rheumatologist. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2016; 22(4): 203–11. [DOI] [PubMed] [Google Scholar]
- 81.Soumahoro M-K, Gérardin P, Boëlle P-Y, Perrau J, Fianu A, Pouchot J, et al. Impact of Chikungunya virus infection on health status and quality of life: a retrospective cohort study. PloS One. 2009; 4(11): e7800 10.1371/journal.pone.0007800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhatia MS, Gautam P, Jhanjee A. Psychiatric Morbidity in Patients with Chikungunya Fever: First Report from India. J Clin Diagn Res JCDR. 2015; 9(10):VC01–VC03. 10.7860/JCDR/2015/14569.6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wadhwani GG. Homeopathic drug therapy Homeopathy in Chikungunya Fever and Post-Chikungunya Chronic Arthritis: an observational study. Homeopathy. 2013; 102(3): 193–8. 10.1016/j.homp.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 84.Abdelnabi R, Neyts J, Delang L. Antiviral Strategies Against Chikungunya Virus. Methods Mol Biol Clifton NJ. 2016; 1426: 243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yaseen HM, Simon F, Deparis X, Marimoutou C. Identification of initial severity determinants to predict arthritis after chikungunya infection in a cohort of French gendarmes. BMC Musculoskelet Disord. 2014; 15: 249 10.1186/1471-2474-15-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soumahoro M-K, Boelle P-Y, Gaüzere B-A, Atsou K, Pelat C, Lambert B, et al. The Chikungunya epidemic on La Réunion Island in 2005–2006: a cost-of-illness study. PLoS Negl Trop Dis. 2011; 5(6): e1197 10.1371/journal.pntd.0001197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Godaert L, Najioullah F, Bousquet L, Malmontet T, Fournet B, Césaire R, Fanon JL, Dramé M. Do Two Screening Tools for Chikungunya Virus Infection that were Developed among Younger Population Work Equally as Well in Patients Aged over 65 Years? PLoS Negl Trop Dis. 2017; 11(1): e0005256 10.1371/journal.pntd.0005256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Godaert L, Bartholet S, Gazeuse Y, Brouste Y, Najioullah F, Kanagaratnam L, Césaire R, Fanon JL, Dramé M. Misdiagnosis of Chikungunya Virus Infection: Comparison of Old and Younger Adults. J Am Geriatr Soc. 2018; 66(9): 1768–1772. 10.1111/jgs.15492 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Multivariable analysis using least absolute shrinkage and selection operator. DAG-2 study 2014–2016, Martinique (N = 167).
(DOCX)
(DOC)
Data Availability Statement
At the promoter's demand, data are only available on request : Department of Research and Innovation, Martinique University Hospital 97261 Fort-de-France, Martinique, France. Contact: Cedric CONTARET; email: cedric.contaret@chu-fortdefrance.fr.