Abstract
Objective
Objective Interspinous process spacers are used in the treatment of lumbar spinal stenosis by preventing extension at the implanted level and reducing claudication, which is a common symptom of lumbar spinal stenosis. This review assessed the current safety and performance of lumbar spinal stenosis treatments and the biomechanical effects of spinal position, range of motion, and the use of interspinous process spacers.
Method
Method EMBASE and PubMed were searched to find studies reporting on the safety and performance of nonsurgical treatment, including physical therapy and pharmacological treatment, and surgical treatment, including direct and indirect lumbar decompression treatment. Results were supplemented with manual searches to include studies reporting on the use of interspinous process spacers and the review of biomechanical testing performed on the Superion device.
Results
Results The effects of spinal position in extension and flexion have been shown to have an impact in the variation in dimensions of the spinal canal and foramina areas. Overall studies have shown that spinal positions from flexion to extension reduce the spinal canal and foramina dimensions and increase ligamentum flavum thickness. Biomechanical test data have shown that the Superion device resists extension and reduces angular movement at the implantation level and provides significant segmental stability.
Conclusions
Conclusions Superion interspinous lumbar decompression is a minimally invasive, low-risk procedure for the treatment of lumbar spinal stenosis, which has been shown to have a low safety profile by maintaining sagittal alignment, limiting the potential for device dislodgment or migration, and to preserve mobility and structural elements.
Keywords: Lumbar Spinal Stenosis, Biomechanics, Superion, X-Stop, Indirect Decompression, Interspinous Process Devices
Introduction
Lumbar spine stenosis (LSS) specifically refers to a narrowing of the neural foramina and/or the spinal canal, which compresses the nerve(s) and surrounding structures [1]. Symptoms of LSS result from direct compression or ischemia of neural elements [2]. Patients may present with symptoms of neurogenic claudication (pain in the buttocks or legs on walking or standing that resolves with sitting down or lumbar flexion), radicular pain, axial pain, or a combination of any of the manifestations [2,3].
Spinal stenosis is commonly classified by descriptive elements, such as etiology (i.e., degenerative or congenital), location (i.e., central, lateral, recess, foraminal, extraforaminal), and severity of the narrowing on advanced imaging (i.e., mild, moderate or severe) [4]. Degenerative lumbar stenosis results from changes in the spine that occur with aging, including facet joint hypertrophy, loss of intervertebral disc height, disc bulging, osteophyte formation, and hypertrophy of the ligamentum flavum [5]; alternatively, stenosis can be caused by congenital narrowing of the spinal canal [6]. Most cases of LSS are degenerative, resulting from changes in the spine with aging [7].
There are several treatment options for patients suffering from LSS, including surgical and nonsurgical treatment. Patients who exhibit mild or moderate pain effects or symptoms of LSS undergo conservative or nonsurgical treatment, such as physical therapy, medication or pharmacological treatment using nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids, and/or epidural steroid injections [8]. Adogwa et al. [9] conducted a retrospective cohort study that investigated the utilization and cost of long-term maximal nonoperative therapy before spinal fusion surgery in patients with symptomatic lumbar stenosis or spondylolisthesis. Of the total patients, 66.7% used NSAIDs, 84.4% used opioids, 58.6% used muscle relaxants, 65.5% received lumbar epidural steroid injections, and 24.9% received chiropractor treatment. Nunley et al. [10] investigated the prevalence of opioid medication use through five years of follow-up after interspinous process decompression (IPD) with the Superion device. Results showed that at baseline 49.5% of all study subjects (N = 190) were using opioid medication, and at the week 6 follow-up, opioid use had increased to 64.1% (116 of 181). At follow-up intervals of 12 months, 24 months, and 60 months, the opioid medication prevalence had significantly decreased to 25.2% (41 of 163), 13.3% (20 of 150), and 7.5% (8 of 107), respectively. Similarly, a reduction was observed in opioid medication prevalence for study subjects with an opioid history from 67.3% (66 of 98) at the time of enrollment to 9.1% (5 of 55) at the 60-month interval.
In those patients who exhibit moderate to severe symptoms, decompression surgery has been shown to be more effective [11]. A lack of a standardized approach for conservative treatment and nonsurgical treatment protocols hinders proper analysis of treatment [7, 8]; however, studies have shown that nonoperative treatment with the use of epidural injections significantly improves pain and quality of life over the short term [12].
Surgical treatments include direct decompression and stabilization and indirect decompression, which includes the use of interspinous process devices (IPDs) or spacers. Surgically, decompression of neural elements is the primary purpose of the operation in LSS, relieving symptoms and improving functions [11, 13]. Direct decompression is achieved by the resection of impinging bone, ligaments, and disc material, which directly compress neural elements [14]. Direct decompression, however, may result in posterior column instability, necessitating additional stabilization [15]. Spinal fusion is sometimes added to the decompression procedure to avoid or treat instability [5]. Various approaches to achieving decompression include traditional laminectomy, bilateral laminotomies, bilateral decompression through unilateral laminotomy, and different forms of laminoplasty [7]. Complications associated with direct decompression include spinal cord injury, nerve root injury, epidural hematoma, epidural fibrosis, and iatrogenic instability [14].
Indirect decompression procedures enable the decompression of neural tissue without resecting the compressing tissue [14]. The rationale for using IPDs is that it has been demonstrated that symptoms of lumbar spinal stenosis are often relieved on flexion and exacerbated on extension. IPDs limit extension of the spine, which may help relieve pain or neurogenic claudication [16].
Methods
A literature search was performed to find studies reporting on the safety and performance of nonsurgical treatment, including physical therapy and pharmacological treatment, and surgical treatment, including direct and indirect lumbar decompression treatment. A literature search through December 2018 was performed using the following electronic databases: PubMed and EMBASE. The search was restricted to articles published in the English language. For the search strategy, titles, key words, and abstracts were searched for the following words: “mild lumbar stenosis,” “spinal stenosis,” “lumbar decompression,” “biomechanics,” and “treatment” [17]. Manual searches were also performed to include studies reporting on the use of interspinous process spacers. The following terms were searched for: “interspinous spacer,” “interspinous device,” and specific device trademark names such as X-STOP and Coflex.
Biomechanics
Position of Spine
Variations in the Dimensions of the Spinal Canal and Foramina, Specifically in Extension
The lumbar spinal canal is lengthened during flexion and shortened during extension [17]. In flexion, the length of the canal is measured along its center line and is increased in comparison with that of the neutral position because of the location of the instantaneous axis of rotation of the vertebrae [17]. In extension, the canal is shortened, as measured by the decrease in its anterior body, center line, and posterior border lengths [17].
Biomechanical studies have evaluated the variation in dimensions of the lumbar spinal canal under conditions of flexion and extension movements. Schönström et al. [18], when evaluating the dynamic changes in the dimension of the lumbar spine canal between flexion and extension, showed a reduction of 40 mm2 of the cross-sectional area of the spinal canal and 2 mm in the midsagittal diameter when moving from flexion to extension. This demonstrates that the dimensions of the lumbar spine canal increased when the spine was flexed from an extended position. A study performed by Inufusa et al. [19] showed a significant increase in cross-sectional areas, midsagittal diameter, and subarticular diameter of the canal in flexion (mean change = 10.9%) and a decrease in extension (mean change = 11.2%).
Assessing normal intervertebral rotation and translation is important in evaluating lumbar stability. The neutral zone is the part of the range of physiological motion, measured in the neutral position, within which the spinal motion is produced with minimal internal resistance, offered by the passive spinal column [20]. Literature reviewed suggests that the neutral zone for each level of the lumbar spine is at 5°, whereas rotation of less than 3° is well below the normal flexion extension neutral zone of the lumbar [21]. Sagittal plane translation of 4.5 mm is considered to be a criterion of instability [22]. A combination of intervertebral rotation and translation might provide a variable for assessing clinical instability [21].
Effects of Ligamentum Flavum Thickness
The ligamentum flavum extends from the anterior inferior border of the laminae above the posterior superior border of the laminae [17]. Calculations show that, with full extension of the spine from the neutral position, there is a decrease in the length of the ligamentum flavum of 10%, and because the ligamentum flavum has 15% pretension, it does not buckle into the spinal canal with extension [17]. Full flexion of the spine from the neutral position results in a 35% increase in length [17].
Recent studies [23] evaluating the changes in cross-sectional measurements of the spinal canal and intervertebral foramina as a function of body position have shown that at disc level the cross-sectional area of the spinal canal varies between body positions, from a mean of 268 mm2 in fully flexed position to a mean of 224 m2 in the fully extended position (P < 0.0001). The maximum thickness of the ligamentum flavum changes from a mean of 1.8 mm in the fully extended position to a mean of 4.3 mm in the fully extended position. Cross-sectional areas of the neural foramina increased significantly in flexion (vs neutral) and decreased in extension, with a change of as much as 44%. Therefore, the thickening of ligamentum flavum, especially in extension, contributes significantly to reduction in cross-sectional areas of the spinal canal.
Impact of Indirect Lumbar Decompression
Interspinous Spacers on Spinal Canal and Foramina Dimensions
Neurogenic claudication is the most common clinical feature attributed to lumbar spinal stenosis [10, 24]. The symptoms of neurogenic claudication are exacerbated upon extension and are relieved on resting or flexion of the lumbar spine [25]. The use of interspinous process spacers between the spinous processes allows flexion, axial rotation, and lateral bending but prevents extension of the stenotic levels of the spine [26].
Richards et al. [26] performed a study to quantify the effects of interspinous process spacer implants (X-STOP) on the dimensions of the spinal canal and neural foraminal during flexion and extension. Canal and foramina dimensions were compared with intact and implanted specimens positioned at 15° flexion and 15° extension. Although there was no significant difference in canal and foramina areas between intact and implanted spines, the studies concluded that in extension the implant showed significant increases in the canal area (18%), the subarticular diameter (50%), the canal diameter (10%), the foraminal area (25%), and the foraminal width (41%). In turn, the interspinous process spacer prevented narrowing of the spinal canal and foramina at the treated levels during extension.
Range of Motion
Studies have been performed to evaluate the impact on range of motion once interspinous spacers have been implanted. Interspinous spacers were developed to treat intermittent neurogenic claudication symptoms by restricting extension and placing the motion segments in flexion [27].
Siddiqui et al. [27] conducted an in vivo study to understand the sagittal kinematics of the lumbar spine and changes in disc height, segmental range of motion, and total lumbar range of motion before and after X-STOP implantation. All patients had pre- and postoperative positional magnetic resonance imagining in standing, supine, and sitting positions, in both flexion and extension. The results showed no changes in overall lumbar range of motion and segmental range of motion for the single-level procedure; however, there was a significant reduction in range of movement in flexion extension after double implantation at the caudad level.
Lindsey et al. [25] conducted an in vitro study to understand the kinematics of the instrument and adjacent levels after the implantation of the interspinous implant (X-STOP). The results show that the implant significantly affected the instrumented level during flexion extension; the range of motion of the implanted level from flexion to neutral and from flexion to extension decreased significantly after implantation.
Biomechanical Effects of Interspinous Process Spacers
Biomechanical studies were performed to evaluate the effects of interspinous process spacers upon facet loading and intradiscal pressure and to understand the importance of spinous process strength. Interspinous process spacers have been demonstrated to significantly reduce facet loading at the implanted level, and they did not alter facet loading at the adjacent levels [3]; after implantation, they show no significant change in intradiscal pressure in extension or flexion [28].
Spinous process fractures are a reported complication of interspinous process spacer implantation [7] due to interspinous spacers converting the spinous process from naturally tension-bearing structures to compression-loading structures [16]. Due to this complication, it is important to understand the impact of spinous process failure load limits compared with load limits required to insert IPS implants [29]. Recent studies have shown that the mean lateral insertion load of the X-STOP (65.6 ± 46.2N) was significantly lower than the mean spinous process failure load (316.9 ± 196.5N) [29].
Biomechanical Testing—Superion
Biomechanical testing on human cadavers was performed to evaluate the impact of the Superion device on the biomechanics of the lumbar spine and as a verification of surgical techniques used in simulated surgery.
Range of Motion—Kinematic and Kinetic Behavior
The range of motion of intact spines was compared with those having a Superion ISS located at a single level and at two levels in a kinematic biomechanical study in human cadavers. An evaluation of the impact of the Superion device on intradiscal pressure was also performed. A study was conducted on six lumbar spine specimens <70 years old and consisting of contiguous levels L1 to S1. Specimens were first radiographed and DEXA scanned to ensure the adequacy of the anatomical structure and bone mineral density, and specimens exhibiting significant degeneration, ostephyte bridging, narrowing disc space, signs of metastatic disease, or evidence of prior spine surgery were excluded.
Preconditioning was performed on each spine for three cycles in each loading plan (flexion, extension, lateral bending, and rotation) to a maximum bending moment of 7.5 Nm. The motion of the L1, L2, L3, L4, and L5 vertebrae relative to the fixed sacrum was measured. Intradiscal pressures were measured at the “pathological” level (the level at which the Superion device was implanted) and at the level immediately above that level. For each spine, implanted Superion devices included the nominal size (as determined by the gauge provided with the System instrument set), one size smaller than nominal, and one size larger than nominal.
Testing was conducted in the following sequence for each of the six spines: 1) intact spine; 2) spine with undersized (one less than nominal) Superion device implanted at L4-L5; 3) spine with nominal size Superion device implanted at L4-L5; 4) spine with oversized (one size larger than nominal) Superion device implanted at L4-L5; 5) spine with two nominal size Superion spacers, with one each implanted at the L3-L4 and L4-L5 levels.
In each condition, quasi-static loads were applied to the L1 vertebral body, leading to pure moments of 1.5, 3.0, 4.5, 6.0, 7.5, and 10.0 Nm. Moments were applied to generate the following six loading modes: flexion, extension, right and left lateral bending, and right and left axial rotation. A follower-type preload of 400 N was applied to the spines throughout the testing. To overcome each spine’s viscoelastic effect, specimens were ranged maximally in all directions at least three times before data collection. The results of testing indicate the following.
The mean resultant moments necessary to replicate the same motion as the intact (unimplanted) situations for 10 Nm of moment were measured, and it was observed that flexion and extension required the greatest motions, under all test conditions, to achieve the 10 Nm of moment, as compared with lateral bending and rotation. All of the implanted situations (undersized, oversized, one nominal, and two nominal spacers) demonstrated significantly greater moments in extension than the intact situation. A single spacer implanted at L4-L5 has a resultant moment greater than the intact situation in extension, and when two spacers were implanted at L4-L5 and L3-L4, the greatest moment was observed in extension. There were minimal changes in moments between the implanted and intact spines, which were observed in flexion, lateral bending, and axial rotation. Spacers exhibited effective stabilizing effects in extension, when compared with the intact (unimplanted) spine.
When measuring the mean discrete angular displacement for the L4-L5 and L5-S1 interspaced, it was observed that there was a reduction in angular motion for both flexion and extension at the implanted L4-L5 level, although, consistent with the design intent, the greatest reduction was seen in extension. The greatest reduction in angular displacement was observed in extension when two nominal size spacers were implanted at L3-L4 and L4-L5. At L5-S1, the inferior level adjacent to the implanted spacer, greater angular displacement was observed in extension in all the interspinous spacer configurations. Overall, greater angular displacement was seen at the L5-S1 level in extension with the single or double implanted nominally sized spacer, although the undersized and oversized implants also elicited additional angular displacement in extension, and few, if any, differences in motion were observed at any level in lateral bending and axial rotation.
When evaluating overall motion of the entire spinal segment for an applied bending moment of 7.5 Nm, it was observed that all interspinous spacer configurations reduced angular displacement in extension, with the single and double nominally sized implants eliciting the greatest reduction in overall spinal motion in extension. In flexion, the double spacer implant produced reduction in overall motion, and few, if any, differences in motion were observed in the spine in either lateral bending or axial rotation, among the implanted specimens.
When evaluating the intradiscal pressures at the adjacent (L3-L4) level during testing, it was observed that the nominally sized spacers produced reductions in anterior intradiscal pressure at the adjacent segment in both flexion and extension. In flexion, the oversized implant produced the greatest reduction in anterior disc pressure at the adjacent level, whereas in extension, the nominal size spacer performed optimally in reducing adjacent level disc pressure. For posterior disc pressure, the nominal size implant produced the greatest reduction in disc pressure in flexion. All three (oversized, undersized, and nominal) produced an increase in posterior disc pressure in extension, although this may be attributed to the greater bending moments necessary to achieve the same motion as that of the intact (unimplanted) specimen.
Overall, the test data support the design intent of the Superion device, in that implantation of the spacer 1) resists extension at the implanted level while having little or no significant effect at other levels, 2) reduces angular moment at the implanted level, and 3) has little or no significant effect on lateral bending or axial rotation.
Supraspinous Ligament in Biomechanical Stability
The Superion surgical technique requires that the supraspinous ligament (SLL) be progressively dilated (rather than transected) to enable surgical placement of the device using minimally invasive techniques. To determine if unintended disruption of the SLL has a biomechanical consequence to segmental stability, a cadaveric study was conducted to assess the biomechanical contribution of the human lumber supraspinous ligament (SLL) with regard to the overall stability of the spinal motion segment after implantation of the Superion Spacer. The biomechanical stability of the motion segment with and without the SLL was assessed using a complete series of loading paradigms that mimic activities of daily living.
A study was conducted on three human cadaveric lumbar spine specimens (L1-Sacrum, <70 years of age). Each specimen was radiographed to determine anatomical integrity. Any specimen exhibiting significant degeneration, osteophytic bridging, narrowing disc space, or signs of metastatic disease, or evidence of prior spine surgery that was either instrumented or noninstrumented, was excluded.
Testing was conducting in the following sequence on six motion segments (three L2-L3 and three L4-L5) for six stages: 1) intact, 2) dilation of the SLL with interspinous spacer (DS + ISS), 3) 50% transection of the SSL with interspinous spacer (50%S + ISS), 4) 100% transection of the SSL with interspinous spacer (100%S + ISS), and 5) spacer removed (injured).
For implantation, the dilation sequence was performed in the midline of the SSL using an initial longitudinal division of the SSL. The dilation sequence involved a series of three dilators to gain access to the interspinous space. Following access, the space was gauged and the implant delivered.
Using the hybrid biomechanical testing principle (unconstrained pure moments) combined with follower load principle (addition of a compressive follower load to simulate torso weight and musculature), each specimen was tested intact, initially for zero Newtons, followed by adding a compressive follower load to define the baseline kinematic behavior and stiffness for each level (400 N).
Each specimen was preconditioned for three cycles in each loading plane (flexion, extension, lateral bending, and rotation) to condition the spinal tissue. Each specimen was tested nondestructively to a maximum being moment of 10 Nm in flexion, extension, lateral bending, and axial rotation for the intact treatment. The ration of motion and bending moments was the measure for all iterations. The loading was repeated each time after intact testing, dilation of the SSL, and 50% or 100% transaction of the SSL with device implantation.
For the various cases of SSL transection, the measured bending moments required to achieve the same range of motion as the intact spine were the highest in extension when compared with other loading modes. There was a rapid reduction in the bending moment required for the injured segment (transected supraspinous and no interspinous spacer) to reach the same range of motion as that of the intact.
The bending moments in extension for the implanted Superion stages of each specimen were 100% to 135% greater than the intact moments. In flexion, the Superion spacer contributed less support, yet an increase in bending moments of 60% to 75% was measured in flexion after the interspinous spacer implantation. This may be attributed to the added posterior support from the implanted interspinous spacer. The interspinous spacer acts as a hyperextension-limiting device, which in turn contributes to added support in flexion. Once the spacer was removed, there was a significant reduction (33%) in flexion stability when compared with the intact state (P > 0.05). There was no statistical difference observed in bending moments between the transected (50% and 100%) and dilated groups with the interspinous spacer. This suggests that the supraspinous ligament plays little role in the segmental stability of the motion segment with an implanted interspinous spacer.
The study results suggest that the Superion implantation provided more stability across the degenerative segment than the intact ligamentous tissue. The lack of significant difference between the biomechanical stability of the implant groups with and without a transected supraspinous ligament (i.e., dilated vs transected) further suggests that the supraspinous ligament played a minor role in biomechanical stability, as the Superion device was mainly responsible for the load bearing during motion.
In regards to the discrete range of motion measured across the implanted spinal segment, there was less motion in extension for all of the treatment groups with the implanted Superion, with the exception of the injured state. Overall, the results implied that the motion at the implanted Superion level has decreased in extension, irrespective of the supraspinous ligament integrity.
From the results, it can be established that the supraspinous ligament played an insignificant role in segmental stability based on the lack of difference between dilated and transected groups. Furthermore, the Superion device provided significant stability through increased bending moments and decreased range of motion that greatly exceeded the intact spine. Therefore, it is safe to conclude that transection of the supraspinous ligament during implantation of the Superion spacer will not affect the biomechanical stabilizing effect of the Superion device in a degenerative spinal segment. The stability of the Superion implant greatly exceeds the stability of the posterior ligamentous tissues, in particular the supraspinous ligament, and will absorb the majority of the posterior load, resulting in less loading of the posterior ligaments. In turn, the supraspinous ligament will have little bearing on segmental stability with the Superion spacer in place.
Radiographic Studies of Superion
A radiographic study was conducted on seven human cadaver lumbar spine segments. The aim of the study was to evaluate and quantify the impact of implantation of the Superion ISS device on the dimensions of the spinal canal and the neural foramina in flexion, extension, and neutral positions.
Dimension evaluation was performed using computed tomography (CT) imaging. Each specimen was first radiographed and DEXA scanned to ensure anatomic integrity and bone mineral density. Specimens exhibiting significant degeneration, osteophytic bridging, narrowed disc space, and signs of metastatic disease were excluded from the study. This yielded a total of seven each of the L2-L3 and L4-L5 motion segments, although a total sample size of seven was used. L4-L5 segments was the preferred level, with the L2-L3 level being used in the event that abnormalities in the former were observed.
The dimensions of the spinal canal and neural foramina were quantified during flexion, extension, and in the neutral position using CT imaging. Two series of scans were performed, first without the Superior ISS implant and, second, once the Superior ISS had been placed in accordance with the recommended implantation technique. Specimens were scanned in each of three positions: neutral, 10° flexion, and 5° extension.
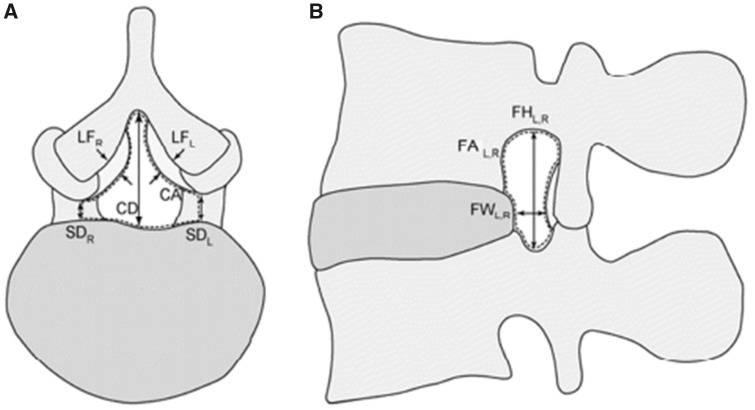
Noncontiguous axial slices, taken in 4-mm increments and parallel to each intervertebral disc, were used to measure the canal area (CA), both left and right subarticular diameter (SDL, SDR), canal diameter (CD), and ligamentum flavum thickness at the L2/L3, L3/L4, and L4/L5 levels (LFL, LRF). The locations of each measurement can be seen in Figure 1A. Noncontiguous slices, taken in 3-mm increments and parallel to the left and right pedicular plane, were used to measure the left and right foramen area (FAL, FAR), foramen height (FHL, FHR), and foramen width at the L2/L3, L3/L4, and L4/L5 levels (FWL, FWR). The locations of each measurement can be seen in Figure 1B.
Figure 1.
Dimensional locations.
The impact of the superior space on canal and foraminal dimension is shown in Table 1, following the average value for each measurement.
Table 1.
Canal and foraminal dimension after implantation of the Superion interspinous process decompression
| Canal area | 5% increase in neutral position |
| No change in flexion | |
| 13% increase in extension | |
| Subarticular diameter | 11% increase (right side) and 5% increase (left side) in neutral |
| 6% decrease (right side) and 3% decrease (left side) in flexion | |
| 5% increase (right side) and 1% decrease (left side) in extension | |
| Canal diameter | 1% decrease in neutral |
| No change in flexion | |
| 6% increase in extension | |
| Ligamentum flavum thickness | 13% decrease (right side) and 15% decrease (left side) in neutral |
| 17% decrease (right side) and 6% increase (left side) in flexion | |
| 4% decrease (right side) and 9% decrease (left side) in extension | |
| Foraminal area | 3% increase (right side) and 1% increase (left side) in neutral |
| 3% decrease (right side) and 6% increase (left side) in flexion | |
| 7% increase (right side) and 15% increase (left side) in extension | |
| Foraminal height | 2% increase (right side) and 4% increase (left side) in neutral |
| 1% decrease (right side) and no change (left side) in flexion | |
| 2% increase (right side) and 10% increase (right side) in extension | |
| Foraminal width | 12% increase (right side) and 27% increase (left side) in neutral |
| 1% decrease (right side) and 4% increase (left side) in flexion | |
| 29% increase (right side) and 25% increase (left side) in extension |
From the data generated, the following general observation can be made. Overall, canal area increased in extension, to a lesser extent in neutral, with little or no impact in flexion. The effects of the spacer on canal diameter and subarticular diameter are largely consistent with these measurements in extension, albeit to a lesser extent, although subarticular diameter tended to decrease in flexion. The thickness of the ligamentum flavum decreased in neutral, flexion, and extension, although differences in impact were seen between left- and right-side flexion. Foraminal dimensions generally increased markedly in extension, and to a lesser extent in neutral. As was observed in the ligamentum flavum diameter, differences in the impact of the spacer on foraminal dimensions were seen between the left and right side in flexion, with the right side tending to decrease slightly in area, height, and width, whereas the left side increased slightly.
In addition, following approximately 60,000 cycles of coupled motions of 15° flexion extension and ±3 axial rotation, implant sites were assessed by comparing precycling locations of the Superion ISS implants with postcycling locations, as determined by CT imaging. Following the dissection of the cycled specimens, spinous processes were also examined for evidence, in the form of scrapes or other markings, of device motion or migration. The study demonstrated that there was no radiographic evidence of device/migration/mislocation or motion during cyclic testing, and there were no fractures of the spinous process.
Interspinous Process Devices
The Superion Indirect Decompression System (IDS) is indicated to treat skeletally mature patients suffering from pain, numbness, and/or cramping in the legs (neurogenic intermittent claudication) secondary to a diagnosis of moderate lumbar spinal stenosis, with or without Grade 1 spondyloisthesis, evidence of thickened ligamentum flavum, narrowing lateral recess, and/or central canal or foraminal narrowing. The Superion IDS is indicated for those patients with impaired physical function who have experienced relief in flexion from symptoms of leg/buttock/groin pain with or without back pain.
Other interspinous process devices on the market (X-STOP, Coflex) state similar indication for use claims. The X-STOP implant fits between the spinous process of the lumbar spine. It consists of two components: a spacer assembly and a wing assembly. The spacer assembly is comprised of a tissue expander, an oval spacer, and a fixed wing. The wing assembly component is comprised of an adjustable wing and locking screw. The Coflex device is an interlaminar stabilization device used to treat moderate to severe spinal stenosis. Unlike the Superion and X-STOP IPD, the Coflex is implanted after direct decompression surgery of stenosis at the affect level(s) has been performed.
The Superion implant is a stand-alone device with a straightforward minimally invasive delivery. The implant is placed between the spinous process of the symptomatic levels and deployed. The device is designed to limit extension at the symptomatic level(s) while concurrently maintaining sagittal alignment, preserving mobility and structural elements. The superior and inferior projections of the Superion implant capture the spinous process, which limits potential for implant migration.
Two surgical approaches can be used to deliver the Superion Implant: The fluoroscopically guided technique and the mini-open approach offering direct visualization of the supraspinous ligament. These minimally invasive surgical approaches and posterior placement of the Superion Implant allow delivery of the implant while reducing the trauma to surrounding tissue and anatomical structures and reducing the chance of device dislodgment.
A clinical study was performed to determine a reasonable assurance of the safety and effectiveness of the Superion IDS for the treatment of moderate degenerative lumbar spine stenosis in the United States. The study was a prospective, multicenter, single-blinded, randomized controlled clinical trial comparing the Superion IDS with a control group consisting of the X-STOP IPD.
Overall, the data presented demonstrate a reasonable assurance of the safety of the Superion IDS device compared with the X-STOP IPD, and the safety profile of the Superion IDS device was determined to be similar to that of the X-STOP IPD device when considering adverse event incidence. However, radiographic data reported that the X-STOP IPD device subjects had device dislodgment or migration (11.9%), whereas none of the Superion IDS subjects exhibited dislodgment or migration, and once in place, the Superion IDS appeared to retain its postoperative position between the spinous process. These data are in line with results observed in the aforementioned radiographic study in this review paper. Furthermore, the radiographic data reported that there was a higher rate of spinous process fractures in Superion IDS (16.3%) compared with X-STOP IPD (8.5%). However, characteristics of the spinous process fractures showed that a majority of Superion IDS fractures (80.6%) present were coincident or in contact with the device, whereas a majority of the X-STOP IPD fractures (70.6%) were present anterior to the location of the device.
Nunley et al. [24] conducted a randomized controlled, multicenter, US Food and Drug Administration (FDA) noninferiority trial. The study evaluated the five-year clinical outcomes for stand-alone interspinous spacer decompression in the treatment of subjects aged 45 years or older with moderate symptoms of intermittent neurogenic claudication, secondary to a diagnosis of moderate degenerative lumbar spinal stenosis at one or two contiguous levels from L1-L5. All clinical outcomes showed successful results. Outcome methods and results are as follows: Zurich Claudication Questionnaire (ZCQ) symptoms severity (ss) was 75% (66 of 88), physical function (pf) was 81% (71 of 88), and patient satisfaction (ps) was 90% (79 of 88); leg and back pain visual analog scale was 80% (68 of 85) and 65% (55 of 85), respectively; and Oswestry Disability Index had a rate of 65% (57 of 88). Of the 190 patients randomized to receive treatment, 142 (75%) were free from reoperation, revision, or supplemental fixation at their index level at five years. Therefore, it was concluded that after five years of postoperative follow-up, IPD with a stand-alone spacer provides sustained clinical benefits.
Conclusions
Surgical decompression has shown successful clinical outcomes in the treatment of patients with intermittent neurogenic claudication associated with moderate lumbar spinal stenosis.
Interspinous process decompression (IPD) and the use of interspinous process spacers, such as Superion, are components of this minimally invasive device, which has been shown to have a low safety profile by maintaining sagittal alignment, to limit the potential for device dislodgment or migration, to preserve mobility and structural elements, and to reduce the risk of further surgery or intervention.
References
- 1. Jackson RP, McManus AC, Moore J.. Lumbar spinal stenosis: Treatment options for an aging population. Mo Med 2012;1096:466–9. [PMC free article] [PubMed] [Google Scholar]
- 2. Patel J, Osburn I, Wanaselja A, et al. Optimal treatment for lumbar spinal stenosis: An update. Curr Opin Anaesthesiol 2017;305:598–603. [DOI] [PubMed] [Google Scholar]
- 3. Wiseman CM, Lindsey DP, Fredrick AD, et al. The effect of an interspinous process implant on facet loading during extension. Spine (Phila Pa 1976) 2005;308:903–7. [DOI] [PubMed] [Google Scholar]
- 4. Schroeder GD, Kurd MF, Vaccaro AR.. Lumbar spinal stenosis: How is it classified? J Am Acad Orthop Surg 2016;2412:843–52. [DOI] [PubMed] [Google Scholar]
- 5. Zaina F, et al. Surgical versus non-surgical treatment for lumbar spinal stenosis. Cochrane Database Syst Rev 2016;1:CD010264.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siebert E, Prüss H, Klingebiel R, Failli V, Einhäupl KM, Schwab JM. Lumbar spinal stenosis: Syndrome, diagnostics and treatment. Nat Rev Neurol 2009;57:392–403. [DOI] [PubMed] [Google Scholar]
- 7. Lurie J, Tomkins-Lane C.. Management of lumbar spinal stenosis. BMJ 2016;352:h6234.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Backstrom KM, Whitman JM, Flynn TW.. Lumbar spinal stenosis-diagnosis and management of the aging spine. Man Ther 2011;164:308–17. [DOI] [PubMed] [Google Scholar]
- 9. Adogwa O, Davison MA, Vuong VD, et al. Long-term costs of maximum nonoperative treatments in patients with symptomatic lumbar stenosis or spondylolisthesis that ultimately required surgery: A 5-year cost analysis. Spine (Phila Pa 1976) 2019;446:424–30. [DOI] [PubMed] [Google Scholar]
- 10. Nunley PD, Deer TR, Benyamin RM, et al. Interspinous process decompression is associated with a reduction in opioid analgesia in patients with lumbar spinal stenosis. J Pain Res 2018;11:2943–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inoue G, Miyagi M, Takaso M.. Surgical and nonsurgical treatments for lumbar spinal stenosis. Eur J Orthop Surg Traumatol 2016;267:695–704. [DOI] [PubMed] [Google Scholar]
- 12. Beyer F, Geier F, Bredow J, et al. Non-operative treatment of lumbar spinal stenosis. Technol Health Care 2016;244:551–7. [DOI] [PubMed] [Google Scholar]
- 13. Malmivaara A, Slätis P, Heliövaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine (Phila Pa 1976) 2007;321:1–8. [DOI] [PubMed] [Google Scholar]
- 14. Yoshihara H. Indirect decompression in spinal surgery. J Clin Neurosci 2017;44:63–8. [DOI] [PubMed] [Google Scholar]
- 15. Malham GM, Parker RM, Goss B, et al. Indirect foraminal decompression is independent of metabolically active facet arthropathy in extreme lateral interbody fusion. Spine (Phila Pa 1976) 2014;3922:E1303–10. [DOI] [PubMed] [Google Scholar]
- 16. Phan K, Rao PJ, Ball JR, et al. Interspinous process spacers versus traditional decompression for lumbar spinal stenosis: Systematic review and meta-analysis. J Spine Surg 2016;21:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panjabi MM, White AA.. Basic biomechanics of the spine. Neurosurgery 1980;71:76–93. 3rd, [DOI] [PubMed] [Google Scholar]
- 18.Schönström N, Lindahl S, Willén J, Hansson T. Dynamic changes in the dimensions of the lumbar spinal canal: An experimental study in vitro. J Orthop Res 1989;71:115–21. [DOI] [PubMed] [Google Scholar]
- 19. Inufusa A, An HS, Lim T-H, et al. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine (Phila Pa 1976) 1996;2121:2412–20. [DOI] [PubMed] [Google Scholar]
- 20. Panjabi MM. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord 1992;54:390–6; discussion 397. [DOI] [PubMed] [Google Scholar]
- 21. Staub BN, Holman PJ, Reitman CA, et al. Sagittal plane lumbar intervertebral motion during seated flexion-extension radiographs of 658 asymptomatic nondegenerated levels. J Neurosurg Spine 2015;236:731–8. [DOI] [PubMed] [Google Scholar]
- 22. Balderston RA, Auerbach JD.. The definition of lumbar spinal instability and its clinical significance. Semin Spine Surg 2005;174:240–2. [Google Scholar]
- 23. Schmid MR, Stucki G, Duewell S, et al. Changes in cross-sectional measurements of the spinal canal and intervertebral foramina as a function of body position: In vivo studies on an open-configuration MR system. AJR Am J Roentgenol 1999;1724:1095–102. [DOI] [PubMed] [Google Scholar]
- 24. Nunley PD, Patel VV, Orndorff DG, et al. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging 2017;12:1409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindsey DP, Swanson KE, Fuchs P, et al. The effects of an interspinous implant on the kinematics of the instrumented and adjacent levels in the lumbar spine. Spine (Phila Pa 1976) 2003;2819:2192–7. [DOI] [PubMed] [Google Scholar]
- 26. Richards JC, Majumdar S, Lindsey DP, et al. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine (Phila Pa 1976) 2005;307:744–9. [DOI] [PubMed] [Google Scholar]
- 27. Siddiqui M, Karadimas E, Nicol M, et al. Effects of X-STOP device on sagittal lumbar spine kinematics in spinal stenosis. J Spinal Disord Tech 2006;195:328–33. [DOI] [PubMed] [Google Scholar]
- 28. Wilke H-J, Drumm J, Häussler K, et al. Biomechanical effect of different lumbar interspinous implants on flexibility and intradiscal pressure. Eur Spine J 2008;178:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Talwar V, Lindsey DP, Fredrick A, et al. Insertion loads of the X STOP interspinous process distraction system designed to treat neurogenic intermittent claudication. Eur Spine J 2006;156:908–12. [DOI] [PMC free article] [PubMed] [Google Scholar]