Abstract
Objective
Lumbar spinal stenosis (LSS) can lead to compression of the neural and vascular elements and is becoming more common due to degenerative changes that occur because of aging processes. Symptoms may manifest as pain and discomfort that radiates to the lower leg, thigh, and/or buttocks. The traditional treatment algorithm for LSS consists of conservative management (physical therapy, medication, education, exercise), often followed by epidural steroid injections (ESIs), and when nonsurgical treatment has failed, open decompression surgery with or without fusion is considered. In this review, the variables that should be considered during the management of patients with LSS are discussed, and the role of each treatment option to provide optimal care is evaluated.
Results
This review leads to the creation of an evidence-based practical algorithm to aid clinicians in the management of patients with LSS. Special emphasis is directed at minimally invasive surgery, which should be taken into consideration when conservative management and ESI have failed.
Keywords: Lumbar Spinal Stenosis, Nonsurgical Treatment, Epidural Steroid Injection, Interspinous Lumbar Decompression, Minimally Invasive, Algorithmic Approach
Introduction
Lumbar spinal stenosis (LSS) is described as a condition in which there is diminished space available for the neural and vascular elements in the lumbar spine [1]. LSS may occur at three sites: central canal, lateral recess, and neuroforamen. Most cases of LSS are degenerative, resulting from changes in the spine with aging. Due to this normal aging process, changes occur in the discs, ligamentum flavum, and facet joints that cause narrowing of the spaces around the neurovascular structures of the spine. These changes may lead to symptomatic pain in the legs and back, as well as to impaired ambulation and other disabilities [1–3]. LSS affects more than 200,000 people in the United States and is considered the most common reason for spinal surgery in patients aged >65 years [3]. In one study, a prevalence of absolute LSS of 47.2% for patients aged 60–69 years was reported, with this number increasing with age [4]. However, not all patients with LSS develop significant or debilitating symptoms, and the natural history of mild to moderate LSS may be favorable for 33–50% of patients [1].
Patients with LSS have a history of symptoms that are notable for pain and discomfort that radiate to the lower leg, thigh, and/or buttocks (neurogenic claudication). Patients with more pronounced LSS may develop lower extremity weakness, muscle cramping, numbness, and imbalance in gait. Patients typically report symptoms of neurogenic claudication that abate with sitting down or leaning forward, referred to as the “shopping cart sign.” When spinal stenosis predominantly affects the neuroforamen or lateral recess, patients may report radicular pain following a specific dermatomal distribution. As LSS is a slowly progressing condition, rapid onset of these symptoms may refer to a different pathology.
To confirm clinical suspicion of LSS, magnetic resonance imaging (MRI) or computed tomography (CT) myelogram studies are required. MRI remains the most common imaging modality to assess LSS [5], as physical examination and x-ray imaging do not have a high sensitivity or specificity. There is great variability in the description and assessment of LSS among radiologists, and lack of standardization may contribute to increased heterogeneity of the patient population undergoing surgery for LSS. However, radiographs are low cost and are readily available. Radiological AP and lateral images may show nonspecific degenerative findings such as disc height loss, disc space narrowing, and osteophyte formation, whereas flexion/extension radiographs may show segmental instability and subtle degenerative spondylolisthesis. Causes that are unrelated to back pain, such as sacroiliac joint pathology, renal stones, or calcified aneurysmal dilatation of the aorta, may also be identified. However, soft tissue evaluation using is limited. MRI has high sensitivity in diagnosing stenosis, as it has high soft tissue contrast, and it best depicts cord, nerve roots, and bone marrow abnormalities. Nevertheless, imaging should be correlated with clinical presentation, as many LSS imaging studies may not have a direct correlation with clinical presentation, and treatment options may vary [6]. The physical findings that are most strongly linked to LSS include wide-based gait, normal Romberg test, thigh pain after 30 seconds of lumbar extension, and neuromuscular deficits [1]. An expert consensus was obtained, which demonstrated an 80% certainty of LSS diagnosis based on seven history items, which include “leg/buttock pain while walking,” “flex forward to relieve pain,” “relief when using a shopping cart or bicycle,” “motor/sensory,” “disturbance while walking,” “normal/symmetric foot pulses,” “lower extremity weakness,” and “low back pain” [7]. Based on physical examination, it is usually sufficient to diagnose for LSS, and MRI/CT diagnosis is often reserved for patients who are being considered for surgery after conservative management has failed.
Treatment Options
Conservative Management
For decades, the mainstay of LSS included physical therapy, exercise, and stretching. Other nonsurgical treatment has included bracing, analgesic medications, epidural injections, and lifestyle interventions.
Physical Therapy
Physical therapy is commonly described as the initial treatment method for LSS. Patients typically require four to six weeks of physical therapy, attending two to three times a week [8]. In a systematic review by Slater et al. [9], the authors demonstrated that exercise is effective to reduce pain, disability, and pain medication intake and that it provides physiological stability by decreasing anger, depression, and mood disturbance. Furthermore, patients who eventually will go on to have spine surgery and who have performed physical therapy show faster recovery [10]. The use of lumbosacral braces may further provide pain relief and may increase walking distance when compared with patients who do not wear braces [11]. There is, however, limited evidence supporting the use of physical therapy for the long-term treatment of LSS, and many patients fail to commit to physical therapy protocols. Bracing also does not cure LSS.
Medications
The use of analgesic medication for treatment of LSS usually begins as firstline treatment and is often combined with physical therapy. However, there is limited evidence to determine the effectiveness of medication as it is often combined with other treatment modalities. Further, any pain medication may interact with other medications a patient is taking. Although nonsteroidal anti-inflammatory drugs provide short-term pain relief, long-term use has been associated with gastric ulcers or other gastrointestinal issues and, depending on a patient’s pain level, and may not provide enough pain relief [12]. Other analgesics such as acetaminophen may not reduce pain adequately and generally cannot be used long term. Other medications, such as gabapentin and pregabalin, have been shown to be effective to reduce neuropathic pain. When used in patients with LSS, gabapentin was shown to increase walking distance and improve pain scores. It also allowed for recovery of sensory deficits [12]; however, the study had a short follow-up period, and no long-term benefits have been proven. When there is still inadequate pain control, opioid therapy may be cautiously considered. However, opioid therapy can cause constipation, dependence, and drowsiness, and there is limited evidence of the efficacy of opioids in the treatment of LSS [8].
Other Conservative Treatment
Other treatment modalities include manipulation and lifestyle changes. Manipulation therapies are aimed at reducing stenosis of the ligament around the spine, decreasing intradiscal pressure, and expanding the intervertebral foramen, which may aid in the recovery of damaged spinal nerves and functional recovery of the surrounding structures. In a study by Murphy et al., distraction manipulation and neural mobilization were associated with improved clinical outcomes and a decrease in pain up to 16 months [13]. When compared with conservative management (physical therapy), flexion-distraction manipulation therapy showed a greater decrease in pain and greater reduction of disability, as shown by the Oswestry Disability Index (ODI) [14]. However, there is limited evidence on the use of manipulation, and the long-term benefits of manipulation are currently unknown.
Lifestyle changes may be part of conservative management, as patients with LSS are at a risk for diseases such as obesity. A pilot study by Tomkins-Lane et al. [15] suggested that a spinal stenosis pedometer and nutrition lifestyle intervention was feasible, attractive to participants, and led to a decrease in fat mass and symptom severity and an increase in mental health. Although not many clinical trials have investigated the effectiveness of lifestyle changes on LSS, they should be considered as part of the treatment program to improve general health and decrease comorbidities in patients.
Spinal Injections
ESI
Interlaminar and transforaminal epidural steroid injections (ESIs) with or without local anesthetic are the most commonly performed nonsurgical spinal procedure, with 65.5% of patients with lumbar spinal stenosis undergoing at least one ESI. In a study by Adogwa et al. [8], the authors reported 18,494 ESIs that were performed between 2007 and 2016 in a subgroup of adults with degenerative spinal diagnosis that underwent an index 1–, 2–, or 3–level decompression and fusion. In a systematic review by Manchikanti et al., it was demonstrated that caudal epidural injections and lumbar interlaminar epidural injections of local anesthetic with or without steroid provided effective and significant improvement in pain and function in central spinal stenosis [16]. In contrast, in a meta-analysis by Liu et al., it was shown that epidural steroid injections provide limited improvement in short-term and long-term benefits in LSS patients [17]. There are limited data demonstrating the long-term efficacy of ESI. The Spine Outcomes Research Trial (SPORT) results further did not support the use of ESI [18, 19]. In general, ESI is suggested to provide short-term pain relief (two weeks to six months) in patients with neurogenic claudication or radiculopathy. There is, however, conflicting evidence concerning long-term (21.5–24 months) efficacy [1, 20].
Nerve (Medial Branch) Block and RFA
Lumbar RFA is considered a treatment for lumbar spondylosis or facet joint arthropaty [21, 22]; however, because of its degenerative etiology, many patients with LSS also have degenerative spondylosis and associated symptoms.
In a comparative study by Park et al. [23], it was shown that nerve (medial branch) block and radiofrequency neurotomy were effective in the treatment of LSS in elderly patients with back pain, radiculopathy, and neurogenic claudication. Excellent and good results were shown in 64% of the patients with nerve block, compared with 71% of the patients with radiofrequency neurotomy. Poor results were shown in 8% of the patients after nerve block, compared with 3% of the patients after radiofrequency neurotomy. The results, however, were limited to patients who had mild to moderate stenosis, and long-term benefits were not assessed for all treatments.
Radiofrequency Thermal Ablation
In a study by Jacobson et al. [24], the authors investigated the use of radiofrequency thermal ablation (RFA) in patients with soft tissue stenosis. Results up to six months showed 58% relief of clinical symptoms, back pain, and claudication with increased spinal movement and required no further intervention. Ten (22%) of the patients who did not have relief of clinical symptoms or who did not maintain favorable results went on for surgical treatment. No detrimental effects of the RFA were observed in patients who required surgery.
In general, in patients who have mild to moderate LSS and who initially received medical or interventional treatment and were followed for two to 10 years, approximately 20–40% will ultimately require surgical intervention. Of the patients who do not require surgical intervention, eventually 50–70% will have improvement in their pain [1].
Surgical Interventions
When conservative treatment fails, surgical treatment options may be considered. Predictive factors for surgery are, for example, patients with cauda equine symptoms, degenerative scoliosis or spondylolisthesis, and a long disease duration [12]. The purpose of surgery in patients with LSS is to decompress the spinal canal while maintaining spinal stability and to prevent or slow further structural deterioration. Surgical options range from minimally invasive decompression surgery for indirect lateral and central stenosis using interspinous spacers to more conventional invasive decompression surgery, either with or without fusion.
Interspinous Spacers
Stand-alone interspinous spacers are designed for the treatment of symptoms of intermittent neurogenic claudication secondary to moderate lumbar spinal stenosis and are implanted by minimally invasive methods through a cannula. Interspinous spacers aim to provide indirect decompression of the lateral and central spinal canal without violating the spinal canal and to support the segments. Range of motion, flexion, and bending are preserved. Interspinous spacers originated with the X-STOP implant using a novel procedure to indirectly decompress the lumbar spine by placing an implant between the spinous processes. The first studies showed promising results [25–27], with 45% of the patients experiencing an improvement at two years compared with 7% in the control group [27]. An overall complication of 3.3% was reported in some series. However, more recently higher complication rates have been reported [28, 29], and Bowers showed a complication rate of 38%, with 85% requiring additional surgery [29, 30]. Other interspinous spacers include the Superion and Coflex implants, which are both approved by the Food and Drug Administration (FDA) for mild to moderate LSS. Both implants have dynamic stability without rigidity of pedicle screw instrumentation. Lauryssen et al. [31] compared Superion vs decompressive laminectomy. Both treatments provided effective and durable symptom relief of neurogenic claudication symptoms. Superion patients revealed improvement in back and leg pain severity after 12 and 24 months compared with laminectomy patients (65 vs 52% and 70% vs 62%, respectively). Patients with Superion implants showed comparable disability, physical function, and symptoms outcomes and had a slightly higher improvement by outcome measurement compared with laminectomy. In another randomized study, these results were confirmed, with Superion showing significantly greater individual patient success based on the Zurich Claudication Questionnaire (ZCQ), no re-operations at the index level, no procedure-related complications, and no clinically significant confounding treatments when compared with the X-STOP (52.5% vs 38.0%) up to three years after the procedure [32]. Four- and five-year follow-up results of the same study showed the same trend, with decreased pain and improved ODI scores compared with baseline [33, 34]. Re-operation rates for interspinous spacers decreased over time, with 14.2% re-operations at one-year follow-up and 3.2% at five-year follow-up, suggesting that early clinical improvement is indicative of long-term sustained clinical benefit. This is in contrast to laminectomy procedures that tend to show increased re-operations rates with time. In general, Superion is minimally invasive with a shorter procedure time and involves significantly less blood loss and significantly fewer complications [32, 34, 35] when compared with other interspinous spacers and laminectomy; it has shown sustained clinical benefit up to five years [34]. However, not all patients are suitable for treatment with an interspinous spacer. Patients with osteoporosis (risk of spinous process fracture) and spondylolisthesis with dynamic instability (risk of posterior migration of implant) are not appropriate candidates for interspinous spacers.
Minimally Invasive Lumbar Decompression
Minimal invasive lumbar decompression (MILD) has been described for direct decompression for central stenosis. This procedure involves inserting a cannula through a six-gauge portal and using tissue and bone sculptors to perform a minimal laminotomy and resect the hypertrophied ligamentum flavum in order to decompress the affected dural sac or nerve roots. This procedure is performed using fluoroscopic guidance to maintain safety. In a prospective clinical trial, 78 patients were treated with the MILD procedure, and early follow-up at six weeks showed significant improvements in clinical outcomes on the VAS, ODI, ZCQ, and quality of life with no major device- or procedure-related events [36]. Similar results were obtained in a retrospective review of MILD patients who underwent two levels of bilateral decompression [37]. In a systematic review by Kreiner et al. [38], the authors revealed one RCT and 12 studies that provided information on the use of MILD in patients with degenerative LSS. All studies showed significant improvements in pain and functional outcomes when compared with pretreatment values or compared with a control group. Although these data seem promising, there is a lack of high-quality studies, and the long-term benefits of the procedure are currently unknown.
Endoscopic Decompression (for Foraminal Stenosis)
Minimally invasive discectomy can achieve decompression through nucleotomy and indirectly relieves pressure on the exiting nerve root, whereas minimally invasive transforaminal endoscopic decompression procedures can achieve spinal decompression through either a direct or an indirect approach. These techniques may vary according to the type of stenosis, including interlaminar, transforaminal percutaneous endoscopic decompression or endoscopic lumbar foraminotomy [39]. Sclafani et al. performed a post hoc analysis of an intracanal endoscopic decompression technique evaluating 86 patients with disc herniation or foraminal stenosis [40]. The authors showed that there was a significant improvement in pain and functional outcome scores up to one year after the procedure. Patients with foraminal stenosis showed improvement in back and leg pain at one year but did not show an overall improvement in ODI scores, with a one-year re-operation rate of 28% compared with 2% in patients with disc herniation. In another study [41], patients who underwent percutaneous endoscopic transforaminal lumbar spinal canal decompression for LSS were evaluated and showed significant improvement in terms of pain relief and ODI scores at final follow-up when compared with baseline values. Two (3%) of the patients had a relapse and had subsequent open decompression surgery. Similar pain relief and functional improvement was found for lateral recess stenosis in geriatric patients using the same percutaneous endoscopic decompression via transforaminal approach [42].
In general, minimally invasive techniques are associated with minimal blood loss, minimal disruption to the musculature and tissue surrounding the decompression site, and are mostly done under intravenous sedation at an outpatient surgery center. However, minimally invasive techniques are also associated with a higher learning curve [43, 44] and may require greater radiation exposure, longer procedure time, and no visualization of the surgical technique [45].
Invasive Open Decompression Surgery
Laminectomy has been the standard surgical treatment for LSS, demonstrating significant improvement in symptoms and functioning. Laminectomy can be either with or without fusion, depending on the disease characteristics and surgeon preference. Different studies have found conflicting results, with some reporting a more favorable outcome of decompression surgery alone and others reporting the opposite [46]. In general, treatment with decompression alone without fusion was shown to be effective in 80% of patients with severe symptoms of LSS [1]. The primary goal of spinal fusion would be to improve regional back pain and improve stability. Another distinction is made between limited single-level (less than two to three levels) decompression and multilevel decompression. Several studies have aimed to determine the best surgical approach, thereby comparing single-level fusion vs multilevel fusion, or decompression surgery with or without fusion vs traditional medical/interventional treatment in patients with LSS.
Limited vs Multilevel Decompression Surgery
Limited open decompression may be performed when one to three affected segments are involved. However, there remains controversy concerning how many levels need to be operated on in case of multilevel LSS for the best clinical outcome. In a randomized controlled trial by Park et al., nonoperative LSS surgery was compared with single-level decompression and fusion and multiple-level decompression and fusion [47]. Patients with multiple stenosis were older and more likely to be male, whereas single-level patients were more often smokers and had more depression. No significant differences were obtained in terms of ODI and pain when comparing one, two, three, or more levels; only patient satisfaction at two years was higher in patients that had single-level decompression and fusion. For patients with LSS and associated degenerative spondylolisthesis, SF-36 bodily pain, mental health, and ODI showed better results after single-level fusion when compared with multilevel fusion. Blood loss increased with more levels, and an overall trend toward more complications was reported for multilevel surgery [47, 48]. In the SPORT study, Weinstein reported on nonoperative treatment vs single-level decompression with or without fusion with significant crossover between the trials, with one observational and one randomized cohort. Better improvement in clinical symptoms and other functional outcomes was obtained after surgery and was maintained up to four years [19]. A retrospective study by Adilay and Guclu demonstrated better functional outcomes scores and less pain in single-level decompressive laminectomy vs multilevel laminectomy, although no significant differences in complications were reported between the two procedures. Spinal instability was found to be higher in multilevel decompressive laminectomy, and four patients had postoperative spondylolisthesis that required posterior fusion [49]. In another study, patients with multisegmental LSS who underwent multilevel decompression without fusion had significantly less favorable spinal stenosis (SSM) scores as compared with single-level decompression. Other functional outcomes were similar between the two procedures. No differences in intra- or postoperative complications were reported [50].
Decompression Surgery with or Without Fusion
In a randomized study, Malmivaari compared decompressive surgery with medical/interventional treatment in patients with moderate LSS. Both groups showed improvement in clinical symptoms at two-year follow-up. Greater improvement was obtained after surgery in disability and leg and back pain [51]. In another case–control study, patients were treated with either medical intervention or decompression alone, depending on the severity of their symptoms, and one group was randomly assigned to either surgery or medical intervention [6]. Improved short-term outcomes at six months were obtained for both treatment groups, with higher improvement in the surgery group (70%, 79%). Of the patients who were randomly assigned to the surgical group, 92% reported improvement at six months. Long-term results from this study showed that 70% maintained improvement after medical/surgical intervention, although quite a number of patients had crossed over to surgery. Other studies showed that there is less blood loss and shorter hospitalization after decompression alone compared with decompression with fusion [52]. In a meta-analysis by Ahmed et al. [46], the authors compared decompression surgery with vs without fusion. Fusion with decompression surgery was found to have better functional outcomes and reduced back and leg pain when compared with decompression alone. Higher rates of satisfaction and decreased leg pain scores were observed for patients with lumbar degenerative spondylolisthesis who underwent decompression and fusion rather than decompression alone. Decompression with fusion was found to be 2.55 times better as compared with no fusion in terms of ODI scores. In another meta-analysis [53], however, the authors did not show a significant difference between decompression alone and fusion in terms of functional and satisfaction outcome parameters. However, operation time, blood loss, and length of hospital stay were remarkably higher in the fusion group, though no difference in re-operation rate was reported between both groups [46, 53]. In a meta-analysis by Phan et al., minimally invasive unilateral laminectomy was compared with open decompression surgery [54]. Higher satisfaction rates were found in the minimally invasive group, with pain rates being significantly lower compared with the open laminectomy group. The operative duration was longer in the minimally invasive group, but less blood loss, a shorter hospital stay, and fewer re-operations were reported.
In general, although open decompression surgery allows for direct visualization of the decompression site and has been shown to be safe and effective in the majority of patients, the procedure is also associated with higher morbidity, secondary spinal instability, longer recovery time, and more risks and might be less tolerated in patients with advanced age. The decision of whether to perform decompression surgery alone or to combine with fusion is largely based on the clinical judgment of the surgeon. Generally, multilevel spinal stenosis involving foraminal and lateral stenosis with significant central canal stenosis, compounded with multilevel spondylosis with significant segmental dynamic instability, will require extensive multilevel decompression including medial facetectomy and multilevel spinal fusion.
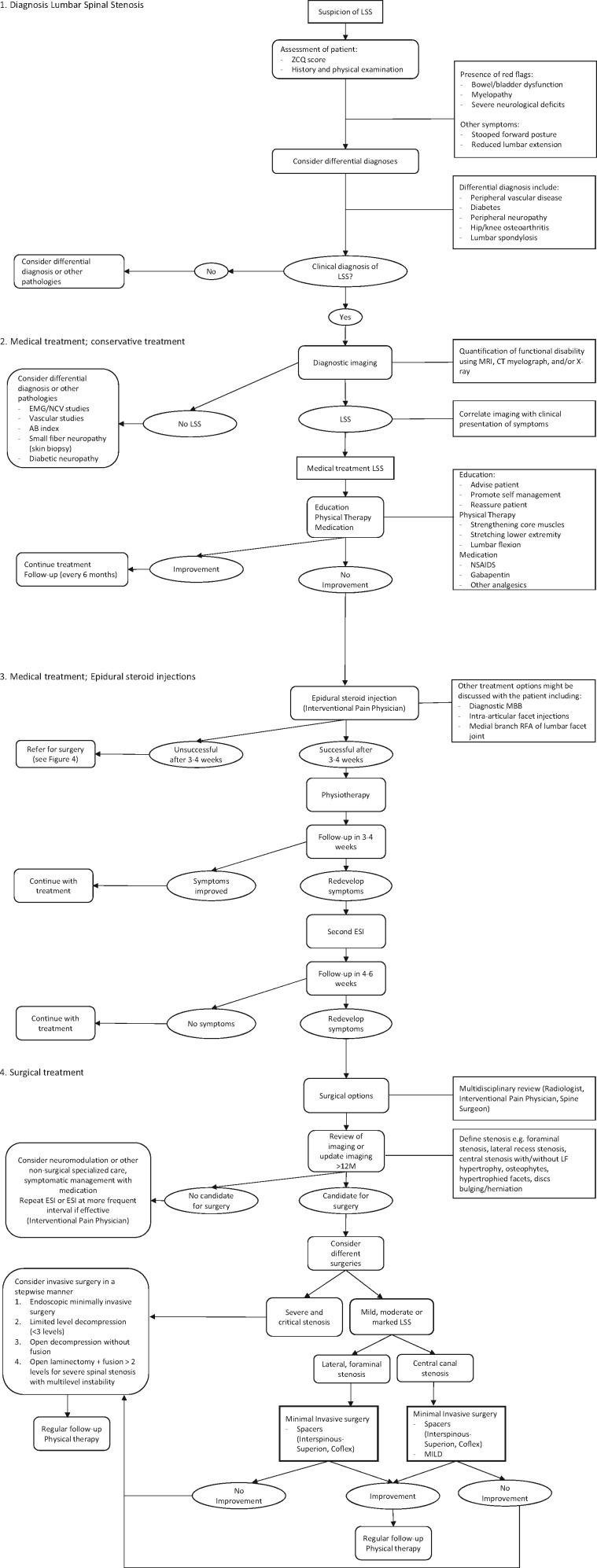
Algorithm for Spinal Stenosis Treatment
The traditional treatment algorithm for LSS consists of conservative management (physical therapy, medication, education, exercise), followed by ESI, and finally open surgical decompression [55]. As shown in this paper, recently several minimally invasive procedures have been developed, thereby expanding the treatment options. The MIST guideline provides guidance on the treatment choice of different minimally invasive procedures depending on the patient’s disease characteristics [20]. The algorithm proposed here (Figure 1) serves as a guide to help clinicians improve treatment selection for patients diagnosed with LSS or who have a suspicion of LSS. The following points are considered of special interest:
Figure 1.
Lumbar Spinal Stenosis management algorithm.
Following suspicion of LSS, and based on physical examination and ZCQ score, conservative treatment may be initiated. The presence of so-called red flags should always lead to (additional) imaging studies and consultation with neurological specialists.
When physical therapy and medication have failed, other treatments such as diagnostic medial branch block (MBB), intra-articular facet injections, and medial branch RFA of the lumbar facet joint nerve may be considered.
Assessment of instability is of utmost importance to assess if minimally invasive surgical options are suitable for a patient. When instability is suspected, this may be confirmed by examination of flexion and extension films, as well as the presence of significant spondylolisthesis. Other factors that may point to instability are facet joint hypertrophy with fluid collection or facet cyst formation. If patients are deemed unstable with grade >2 spondylolisthesis, they are not suitable candidates for minimally invasive indirect decompression with an interspinous spacer.
Invasive decompression surgery should be considered in a stepwise manner: from limited open decompression with one to three segments to limited level fusion and finally extensive decompression and fusion.
References
- 1. Kreiner DS, Shaffer WO, Baisden JL, Gilbert TJ, Summers JT, Toton JF.. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update). Spine J 2013;137:734–43. [DOI] [PubMed] [Google Scholar]
- 2. Katz JN, Harris MB.. Clinical practice. Lumbar spinal stenosis. N Engl J Med 2008;3588:818–25. [DOI] [PubMed] [Google Scholar]
- 3. Lurie J, Tomkins-Lane C.. Management of lumbar spinal stenosis. BMJ 2016;352:h6234.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: The Framingham Study. Spine J 2009;97:545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Schepper EI, Overdevest GM, Suri P, et al. Diagnosis of lumbar spinal stenosis: An updated systematic review of the accuracy of diagnostic tests. Spine 2013;388:E469–81. [DOI] [PubMed] [Google Scholar]
- 6. Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleås F.. Lumbar spinal stenosis: Conservative or surgical management?: A prospective 10-year study. Spine 2000;2511:1424–35; discussion 35–6. [DOI] [PubMed] [Google Scholar]
- 7. Tomkins-Lane C, Melloh M, Lurie J, et al. ISSLS prize winner: Consensus on the clinical diagnosis of lumbar spinal stenosis: Results of an international Delphi study. Spine 2016;4115:1239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adogwa O, Davison MA, Vuong VD, et al. Long term costs of maximum non-operative treatments in patients with symptomatic lumbar stenosis or spondylolisthesis that ultimately required surgery: A five-year cost analysis. Spine. In press. [DOI] [PubMed]
- 9. Slater J, Kolber MJ, Schellhase KC, et al. The influence of exercise on perceived pain and disability in patients with lumbar spinal stenosis. A systematic review of randomized controlled trials. Am J Lifestyle Med 2016;102:136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindback Y, Tropp H, Enthoven P, Abbott A, Oberg B.. PREPARE: Presurgery physiotherapy for patients with degenerative lumbar spine disorder: A randomized controlled trial. Spine J 2018;188:1347–55. [DOI] [PubMed] [Google Scholar]
- 11. Prateepavanich P, Thanapipatsiri S, Santisatisakul P, Somshevita P, Charoensak T.. The effectiveness of lumbosacral corset in symptomatic degenerative lumbar spinal stenosis. J Med Assoc Thai 2001;844:572–6. [PubMed] [Google Scholar]
- 12. Matsudaira K, Hara N, Oka H, et al. Predictive factors for subjective improvement in lumbar spinal stenosis patients with nonsurgical treatment: A 3-year prospective cohort study. PLoS One 2016;112:e0148584.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murphy DR, Hurwitz EL, Gregory AA, Clary R.. A non-surgical approach to the management of lumbar spinal stenosis: A prospective observational cohort study. BMC Musculoskelet Disord 2006;716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi J, Lee S, Jeon C.. Effects of flexion-distraction manipulation therapy on pain and disability in patients with lumbar spinal stenosis. J Phys Ther Sci 2015;276:1937–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tomkins-Lane CC, Lafave LM, Parnell JA, et al. The Spinal Stenosis Pedometer And Nutrition Lifestyle Intervention (SSPANLI): Development and pilot. Spine J 2015;154:577–86. [DOI] [PubMed] [Google Scholar]
- 16. Manchikanti L, Kaye AD, Manchikanti K, Boswell M, Pampati V, Hirsch J.. Efficacy of epidural injections in the treatment of lumbar central spinal stenosis: A systematic review. Anesthesiol Pain Med 2015;51:e23139.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu K, Liu P, Liu R, Wu X, Cai M.. Steroid for epidural injection in spinal stenosis: A systematic review and meta-analysis. Drug Des Devel Ther 2015;9:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: Four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine 2008;3325:2789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009;916:1295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deer TR, Grider JS, Pope JE, et al. The MIST guidelines: The lumbar spinal stenosis consensus group guidelines for minimally invasive spine treatment. Pain Pract. In press. [DOI] [PubMed] [Google Scholar]
- 21. Abejon D, Ortego R, Solis R, Alaoui N, del Saz J, del Pozo C.. Trans-facet-joint approach to pulsed radiofrequency ablation of the L5 dorsal root ganglion in a patient with degenerative spondylosis and scoliosis. Pain Pract 2008;83:202–5. [DOI] [PubMed] [Google Scholar]
- 22. Klessinger S. Radiofrequency neurotomy for treatment of low back pain in patients with minor degenerative spondylolisthesis. Pain Phys 2012;151:E71–8. [PubMed] [Google Scholar]
- 23. Park CK, Kim SB, Kim MK, et al. Comparison of treatment methods in lumbar spinal stenosis for geriatric patient: Nerve block versus radiofrequency neurotomy versus spinal surgery. Korean J Spine 2014;113:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacobson RE, Granville M, Targeted HD.. Intraspinal radiofrequency ablation for lumbar spinal stenosis. Cureus 2017;93:e1090.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel VV, Whang PG, Haley TR, et al. Two-year clinical outcomes of a multicenter randomized controlled trial comparing two interspinous spacers for treatment of moderate lumbar spinal stenosis. BMC Musculoskelet Disord 2014;15:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller LE, Block JE.. Interspinous spacer implant in patients with lumbar spinal stenosis: Preliminary results of a multicenter, randomized, controlled trial. Pain Res Treat 2012;2012:823509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hartjen CA, Resnick DK, Hsu KY, Zucherman JF, Hsu EH, Skidmore GA.. Two-year evaluation of the X-STOP interspinous spacer in different primary patient populations with neurogenic intermittent claudication because of lumbar spinal stenosis. Clin Spine Surg 2016;297:305–11. [DOI] [PubMed] [Google Scholar]
- 28. Lonne G, Johnsen LG, Aas E, et al. Comparing cost-effectiveness of X-Stop with minimally invasive decompression in lumbar spinal stenosis: A randomized controlled trial. Spine 2015;408:514–20. [DOI] [PubMed] [Google Scholar]
- 29. Gazzeri R, Galarza M, Alfieri A.. Controversies about interspinous process devices in the treatment of degenerative lumbar spine diseases: Past, present, and future. Biomed Res Int 2014;2014:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poetscher AW, Gentil AF, Ferretti M, Lenza M.. Interspinous process devices for treatment of degenerative lumbar spine stenosis: A systematic review and meta-analysis. PLoS One 2018;137:e0199623.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lauryssen C, Jackson RJ, Baron JM, et al. Stand-alone interspinous spacer versus decompressive laminectomy for treatment of lumbar spinal stenosis. Expert Rev Med Devices 2015;126:763–9. [DOI] [PubMed] [Google Scholar]
- 32. Patel VV, Nunley PD, Whang PG, et al. Superion((R)) interspinous spacer for treatment of moderate degenerative lumbar spinal stenosis: Durable three-year results of a randomized controlled trial. J Pain Res 2015;8:657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nunley PD, Patel VV, Orndorff DG, Lavelle WF, Block JE, Geisler FH.. Superion interspinous spacer treatment of moderate spinal stenosis: 4-year results. World Neurosurg 2017;104:279–83. [DOI] [PubMed] [Google Scholar]
- 34. Nunley PD, Patel VV, Orndorff DG, Lavelle WF, Block JE, Geisler FH.. Five-year durability of stand-alone interspinous process decompression for lumbar spinal stenosis. Clin Interv Aging 2017;12:1409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nunley PD, Patel VV, Orndorff DG, Lavelle WF, Block JE, Geisler FH.. Interspinous process decompression improves quality of life in patients with lumbar spinal stenosis. Minim Invasive Surg 2018;2018:1035954.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deer TR, Kapural L.. New image-guided ultra-minimally invasive lumbar decompression method: The mild procedure. Pain Phys 2010;131:35–41. [PubMed] [Google Scholar]
- 37. Lingreen R, Grider JS.. Retrospective review of patient self-reported improvement and post-procedure findings for mild (minimally invasive lumbar decompression). Pain Phys 2010;136:555–60. [PubMed] [Google Scholar]
- 38. Kreiner DS, MacVicar J, Duszynski B, Nampiaparampil DE.. The mild(R) procedure: A systematic review of the current literature. Pain Med 2014;152:196–205. [DOI] [PubMed] [Google Scholar]
- 39. Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices 2014;116:605–16. [DOI] [PubMed] [Google Scholar]
- 40. Sclafani JA, Raiszadeh K, Laich D, et al. Outcome measures of an intracanal, endoscopic transforaminal decompression technique: Initial findings from the MIS prospective registry. Int J Spine Surg 2015;9:69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wen B, Zhang X, Zhang L, Huang P, Zheng G.. Percutaneous endoscopic transforaminal lumbar spinal canal decompression for lumbar spinal stenosis. Medicine (Baltimore) 2016;9550:e5186.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen X, Qin R, Hao J, et al. Percutaneous endoscopic decompression via transforaminal approach for lumbar lateral recess stenosis in geriatric patients. Int Orthop. In press. [DOI] [PubMed]
- 43. Sharif S, Afsar A.. Learning curve and minimally invasive spine surgery. World Neurosurg 2018;119:472–8. [DOI] [PubMed] [Google Scholar]
- 44. Epstein NE. Learning curves for minimally invasive spine surgeries: Are they worth it? Surg Neurol Int 2017;8:61.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park Y, Seok SO, Lee SB, Ha JW.. Minimally invasive lumbar spinal fusion is more effective than open fusion: A meta-analysis. Yonsei Med J 2018;594:524–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ahmed SI, Javed G, Bareeqa SB, et al. Comparison of decompression alone versus decompression with fusion for stenotic lumbar spine: A systematic review and meta-analysis. Cureus 2018;108:e3135.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park DK, An HS, Lurie JD, et al. Does multilevel lumbar stenosis lead to poorer outcomes?: A subanalysis of the Spine Patient Outcomes Research Trial (SPORT) lumbar stenosis study. Spine 2010;354:439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smorgick Y, Park DK, Baker KC, et al. Single- versus multilevel fusion for single-level degenerative spondylolisthesis and multilevel lumbar stenosis: Four-year results of the spine patient outcomes research trial. Spine 2013;3810:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adilay U, Guclu B.. Comparison of single-level and multilevel decompressive laminectomy for multilevel lumbar spinal stenosis. World Neurosurg 2018;111:e235–40. [DOI] [PubMed] [Google Scholar]
- 50. Ulrich NH, Burgstaller JM, Held U, et al. The influence of single-level versus multilevel decompression on the outcome in multisegmental lumbar spinal stenosis: Analysis of the Lumbar Spinal Outcome Study (LSOS) data. Clin Spine Surg 2017;3010:E1367–75. [DOI] [PubMed] [Google Scholar]
- 51. Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine 2007;321:1–8. [DOI] [PubMed] [Google Scholar]
- 52. Aihara T, Toyone T, Murata Y, Inage K, Urushibara M, Ouchi J.. Degenerative lumbar spondylolisthesis with spinal stenosis: A comparative study of 5-year outcomes following decompression with fusion and microendoscopic decompression. Asian Spine J 2018;121:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shen J, Xu S, Xu S, Ye S, Hao J.. Fusion or not for degenerative lumbar spinal stenosis: A meta-analysis and systematic review. Pain Phys 2018;211:1–8. [PubMed] [Google Scholar]
- 54. Phan K, Mobbs RJ.. Minimally invasive versus open laminectomy for lumbar stenosis: A systematic review and meta-analysis. Spine 2016;412:E91–100. [DOI] [PubMed] [Google Scholar]
- 55. Doorly TP, Lambing CL, Malanga GA, Maurer PM, Rashbaum RF.. Algorithmic approach to the management of the patient with lumbar spinal stenosis. J Fam Pract 2010;59(8 Suppl Algorithmic):S1–8. [PubMed] [Google Scholar]