Abstract
With the rapid increase in the use of nanotechnology and immunotherapy for cancer management in the recent past, there are great implications for using nanotechnology in immuno-oncology. However, to deliver clinical success, the scientific and clinical rationale must be critically evaluated when applying nanotechnology for immuno-oncology challenges. This opinion article distinguishes designing nanotherapeutics for immunotherapy and the past focus on the placement of chemotherapy agents in nanoparticles. We believe the integration of nanotechnology with cancer immunotherapy for ‘nano-immunotherapeutics’ provides unique opportunities for both fields, paving the way for entirely new therapeutic paradigms. As a particular focus in our article, we envision the necessities and challenges of nanotechnology in the development of in situ cancer vaccines, immune checkpoint inhibitors, adoptive cell transfer, and bispecific antibody therapy.
Keywords: cancer, nanotechnology, immunotherapy
Nanotherapeutics for Oncology: The beginning of the end or the end of the beginning?
Exploring nanotechnology for cancer therapy grew exponentially in the past forty years. By physical entrapment or chemical conjugation of various therapeutic or imaging agents into nanocarriers, nanotherapeutics enabled enhanced solubility, targeted delivery, reduced systemic toxicity, and augmented therapeutic efficacy in cancer therapy [1–4]. The benefits obtained from using nanoparticles for cancer therapy can be attributed to the unique nanoscale properties of carriers, flexible adjustment of the carrier size, morphology, as well as surface properties including charge and targeting moieties. Because of the enhanced permeability and retention (EPR) effect (see Glossary), nanoparticles preferentially accumulate within tumors owing to their leaky vasculature and poor lymphatic drainage [5, 6]. The EPR effect was also observed in patients with locally advanced cancers [7], although this effect varies depending on a patient’s pathological and physiological characteristics and clinical condition [8, 9]. For specific therapeutic modalities (e.g., gene therapy), nano delivery is indispensable to realize in vivo therapeutic application [10]. Nanocarriers can also be designed as ‘smart’ formulations for controlled drug release in response to the different stimuli in the tumor microenvironment, which is expected to further improve the therapeutic efficacy of nanoformulations (Figure 1) [11–13].
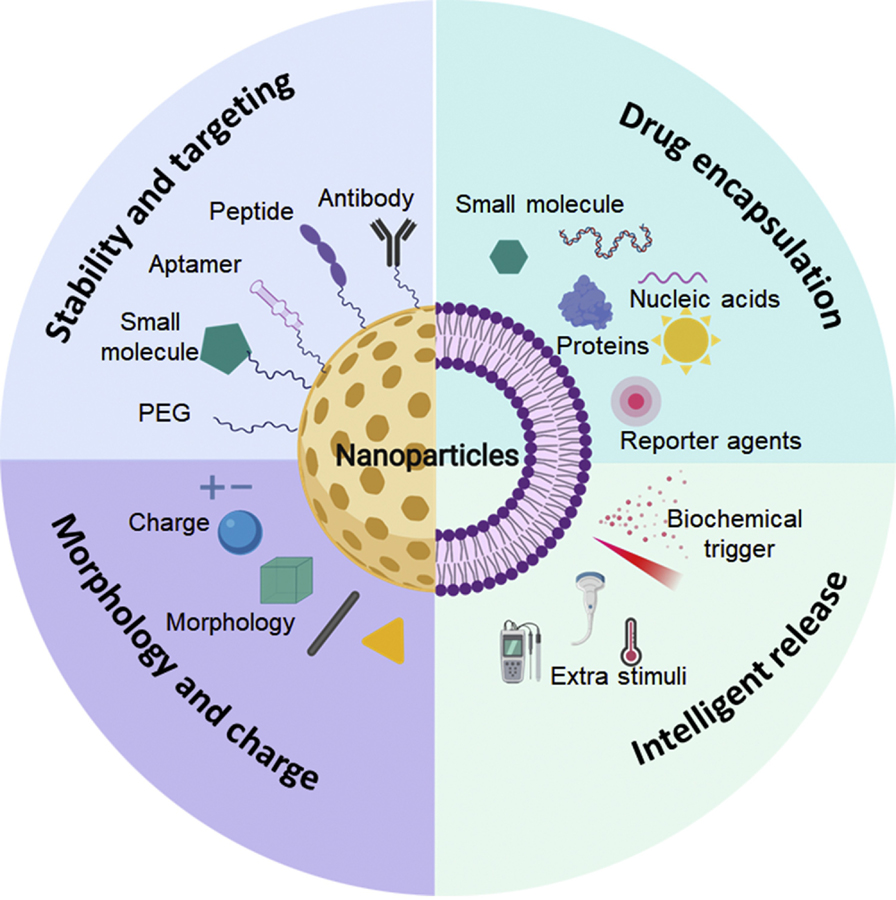
Figure 1. Nanoparticles with optimized properties for drug delivery.
Stability and targeting ability of nanoparticles can be optimized by surface modification with PEG or various targeting ligands (e.g., small molecules, aptamers, peptides and antibodies). Various small-molecule drugs, nucleic acids including DNA and RNA, proteins and reporter agents can be encapsulated into nanoparticles for increasing solubility/stability and reducing blood exposure. The nanoscale effect can be further optimized by tuning the morphology and surface charge of the nanoparticles. After reaching the target site, the loaded cargos can be released, actuated by biochemical triggers as well as extra stimuli. Figures created with BioRender.com. Abbreviations: PEG, poly(ethylene glycol).
With the growing body of academic research in this field, several nanomedicine drugs like Doxil®, Abraxane®, Genexol®, Onivyde®, and more recently Onpattro® (the first RNA interference drug) have been successfully brought to market. Nevertheless, there is pressure from diverse stakeholders ranging from funding agencies to clinicians in the field of cancer nanomedicine for more clinical translation. Recently, the U.S. National Cancer Institute (NCI) has announced that it will stop funding its Centers of Cancer Nanotechnology Excellence (CCNEs) because of “nanotechnology’s ‘natural transition’ from an emerging field requiring dedicated support to a more mature enterprise able to compete head to head with other types of cancer research” [14]. This action may imply a shift of the cancer research community’s attitude on the discipline of nanomedicine, marking “the beginning of the end of the nanomedicine hype” [15]. We, however, believe that this is the end of the beginning. Nanotechnology is merely a tool in anticancer drug development. There should not be an expectation that more than a hundred types of cancers can be cured by merely placing anticancer agents into a nanoparticle. Nanomedicine should be used in the right context for relevant patient populations, as we did in developing other types of drugs [16]. Looking forward, translatability should be prioritized when designing a new nanoformulation since translation is the ultimate goal of using nanotechnology in the biomedical research field. From another aspect, the cargos loaded inside nanoformulations matter, since nanotechnology only provides a platform for delivery, and the key to therapy is still the loaded cargos. We believe nanotechnology needs to tackle some grand challenges in its next phase, and one discipline to focus on is immuno-oncology.
Nanotherapeutics for Immuno-Oncology: Why over what?
It’s an unavoidable fact that cancer immunotherapy has been and will continue to be an essential player in cancer therapy [17, 18]. The most crucial attribute for cancer immunotherapy is utilizing the host’s immune system for cancer therapy. Using immunotherapy for cancer management has the potential for long term tumor inhibition or even cure since the response to immunotherapy is systemic and, immune induction can lead to a long-term memory response. Further, immuno-oncology drugs possess inherent advantages for late-stage and metastatic tumor therapy, which are significant hurdles for current chemotherapy or molecular targeted therapy. However, similar to chemotherapy, immunotherapeutic agents also face problems like instability, inefficient delivery, immune-related adverse effects (irAEs), and lack of efficacy in the majority of solid tumors. Therefore, there is plenty of room to leverage the accumulated experiences in cancer nanotechnology for the era of immunotherapy. Importantly, the characteristics of immunotherapy should be accounted for, in designing ‘nano-immunotherapeutics’ for more clinical success.
New players as delivery cargos
When we look back over the age of nanomedicine based on chemotherapeutic agents, the basic design principle was the development of new formulations that improved the solubility and druggability of the clinically used agents, reduced blood exposure and related adverse effects, and increased tumor site accumulation. For immunotherapeutic agents, although reducing the adverse effects of small molecular drugs is still an important aspect, protein and gene therapies, and even cell therapies are becoming more and more prevalent. Integration of nanotechnology with these new biotechnology participants to leverage the best of these technologies is the need of the hour.
Shifts in delivery targets
For chemotherapeutic drug delivery, since interaction with cancer cells is necessary for chemo agents to kill tumor cells, drug delivery to each tumor cell was a critical aspect for the drug delivery system design. However, for immunotherapy, since cytotoxic T cells and natural killer cells will travel systemically, drug delivery to each target cell is not necessarily required. Local immunotherapy may induce systemic immune responses and result in control over the distant tumors. In addition, many immunomodulating targets are stomal cells including fibroblasts and immune cells, and these cells are always more easily available than tumor cells for nanoparticles.
Why over what
To improve the translational potential of nanomedicine in the immunotherapy age, we still need to learn from the past. Leveraging nanotechnology for cancer immunotherapy is not simply transplanting the past technologies, but instead focusing on the specific medical questions in immunotherapy. Uniqueness and necessity are more critical than abundances for clinical translation. Also, it is necessary to establish evaluation criteria and methods for nanotechnologies in cancer immunotherapy to maximize clinical success.
In this opinion article, we will describe the opportunities of nanotechnology in four central themes of immuno-oncology in the present day: in situ cancer vaccine, immune checkpoint inhibitors, adoptive cell transfer (ACT), and bispecific antibody (BsAb) therapy. We will not cover the entire spectrum of cancer immunotherapy but primarily focus on the possible integrations of nanotechnology and immunotherapy to meet the unmet needs in immuno-oncology while maximizing the advantage and success rate of cancer nanotechnology.
Nanotechnology for in situ Cancer Vaccine
A cancer vaccine is a robust strategy to elicit an immune response against cancer. In contrast to traditional vaccines, cancer vaccines are expected to be therapeutic after subcutaneous injection of tumor-specific antigen and adjuvant. However, the development of cancer vaccines towards this goal is not entirely successful. Besides anti-human papillomavirus (HPV) vaccine approved by the United States Food and Drug Administration (FDA) for the prevention of specific subtypes of cervical cancer and precancerous lesions, traditional cancer vaccines with a combination of antigen with adjuvant have not received wide clinical success [19].
A number of researchers believe that subcutaneous administration of peptide-based antigen and adjuvant alone is not sufficient to elicit a strong immune response [20]. As a result, various nanoparticles were developed as a new adjuvant modality for promoting antigen uptake and presentation to antigen-presenting cells (APCs). Reports showed that simultaneous delivery of antigen and adjuvant to APCs would greatly improve the antigen-specific immune activation effect, and nanoparticle-based cancer vaccines had shown greater antitumor efficacy over naked therapeutic agents in animal models [21–23]. From another angle, a single type of antigen is not enough to elicit comprehensive anti-tumor immune responses due to the heterogeneity of a tumor [24]. Therefore, identification of neo-antigens by whole genome sequencing and a combination of a cocktail of neo-epitopes as a cancer vaccine is an emerging focus [19]. Personalized neo-epitope peptide and RNA vaccines unique for each patient has proven successful in small scale investigator-initiated trials [25, 26]. In addition, dendritic cell (DC)-based cancer vaccines have also been introduced as a new therapeutic strategy for personalized cancer therapy. In this approach, DCs are isolated, loaded with tumor antigens in vitro, and then re-infused back to patients [27]. The FDA approved the personalized DC-vaccine (Provenge®) in 2010 for prostate cancer therapy [28]. Clinical trials of personalized DC-vaccines using autologous whole-tumor cell lysates as antigen sources are also ongoing in metastatic ovarian cancer, and glioblastoma multiforme patients [29].
Identification of neo-antigens is time-consuming and labor intensive, and ex vivo engineering of personalized DC-vaccines and reinfusion back to patients is expensive. As a result, the development of a universal and inexpensive vaccine will be impactful for cancer therapy. An in situ vaccine, which utilizes the tumor itself as the antigen source, with injection of various immune agonists into tumors to stimulate tumor antigen-specific immune responses is a new research highlight [30]. For example, Saguv-Barfi et al. reported that the combination of locally injected unmethylated CG-enriched oligodeoxynucleotide (CpG) – a Toll-like receptor 9 (TLR9) ligand – and anti-OX40 (CD134) antibody eradicated spontaneous malignancy in both injected and untreated distant tumors. The injected CpG induced the expression of OX40 on CD4+ T cells in the tumor, and anti-OX40 antibody then triggered tumor specific T cell responses. This combination of a TLR9 ligand and an anti-OX40 antibody was effective in multiple types of murine cancers and is now under clinical trials for treating patients with low-grade B-cell non-Hodgkin lymphomas [31]. In another study, Hammerich et al. reported an in situ vaccine that combined Fms-related tyrosine kinase 3 ligand (Flt3L), radiotherapy, and a Toll-like receptor 3 (TLR3) agonist. The in situ vaccine treatment induced anti-tumor CD8+ T cell responses and distant tumor regression in patients with advanced stage indolent non-Hodgkin’s lymphoma and is currently being investigated in a clinical trial [32].
We believe there are great opportunities in applying nanotechnologies for in situ vaccine development (Figure 2a). First, the immune stimulators used for an in situ vaccine generally have severe side effects, and quickly diffuse to systemic compartments even after local injection. Nanotechnology can help to maintain these adjuvants locally after intratumoral injection as well as increasing the immune stimulation efficiency [33–36]. Second, current in situ vaccine therapy was mainly carried out by direct injection of various agents into the superficial tumor model, while for deep tumors, delivery technology will be necessary. Deep tumors may also have different immune microenvironments compared to superficial tumors, so the drugs used in the same vaccine can have different responses [37]. Third, nanoparticles can protect biologic immune activator drugs from degradation, and facilitate selective accumulation inside the tumor by the EPR effect [38, 39]. Because of the intrinsic pathogen-like properties (e.g., the dimensions), nanoparticles are readily taken up by APCs, greatly promoting the immune stimulation efficiency [40, 41].
Figure 2. Integration of nanotechnology and immuno-oncology for cancer therapy.
(A) For in situ cancer vaccine: Intravenously or peritumorally injected nanoparticles induce tumor cell necrosis and actuate antigen release, then the antigens are captured by nanoparticles and delivered to the tumor-draining lymph nodes, where antigens are presented, and antigen-presenting cells mature and prime T cells. Then the activated T cells infiltrate into the tumor and kill tumor cells. Activated T cells can also systematically distribute and eliminate distal tumors or metastases. (B) For checkpoint inhibitor therapy: Nanoparticles loaded with genes encoding protein therapeutics can be delivered for local production of cytokines, immune checkpoint antibodies or other protein therapeutics including BiTE. (C) For ACT therapy: Nanotechnology can be integrated with CAR-T cells and facilitate construction of ‘armed’ CAR-T cells by multiplexing with necessary cytokines and immunomodulators. This process can be realized either ex vivo or in vivo to minimize ex vivo engineering workflow. (D) For BsAb therapy: Nanotechnology can be integrated with BiTE for construction of mfBiNE, leveraging the unique properties like flexible surface decoration and cargo loading ability of nanoparticles. mfBiNE holds the advantages of long circulation, multivalent or multi-type decoration, as well as loading with supporting drugs to augment the capability of killer cells against solid tumors, which is so far unachievable. Figures created with BioRender.com. Abbreviations: ACT, adoptive cell transfer; CAR, chimeric antigen receptor; BiTE, bi-specific T cell engager; mfBiNE, multifunctional bispecific nano-engager.
We need to emphasize that current nanotechnology does not realize truly tumor-specific drug delivery. Liver and spleen accumulation happens inevitably for systemically injected nanoparticles due to clearance by the reticuloendothelial system (RES). Non-specific stimulation of the immune cells in RES sites can result in a cytokine storm and related side effects [42]. Therefore, realizing tumor-specific immune stimulation still requires additional investigations. In a recent study, Hewitt et al. reported that intratumoral injection of lipid nanoparticles (LNPs) loaded with messenger RNAs (mRNAs) encoding cytokines including interleukin-23, interleukin-36γ and OX40L produced robust antitumor responses in a broad range of tumors [43]. All mRNAs were included with a 3’UTR (untranslated region) microRNA-122 (miR122) binding site, to attenuate potential protein expression post translation of the injected mRNAs in hepatocytes. Since miR122 expression is high in hepatocytes, this strategy successfully bypassed liver expression [43]. There are also many other tumor microenvironment specific factors (e.g., low pH, hypoxia, high reactive oxygen species), that can be harnessed to specifically target the immune activators to the tumor. The concept of a tumor-activated “pro-drug” has been widely applied in chemotherapeutic applications [44–46] and can be transplanted to immuno-oncology as well.
Nanotechnology for immune checkpoint therapy
Immune checkpoint inhibitors are the primary driving force for the current excitement behind cancer immunotherapy. By eliminating the negative immune regulation between T cells and tumor cells (programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) axis), as well as the negative feedback between T cells and APCs mediated by the interaction between cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and CD80 and CD86, checkpoint inhibitors gained considerable traction in melanoma, metastatic lung cancer, mismatch-repair deficient cancers, and numerous other cancer types [47–49]. Checkpoint molecules are expressed on cell surfaces, with some of them serving as co-stimulatory receptors or ligands, while others serve as co-inhibitory molecules [50]. As we are identifying newer checkpoint mechanisms, more immune checkpoint monoclonal antibodies are being developed for cancer immunotherapy [51, 52].
Immune checkpoints are natural regulators of the immune system aimed at maintaining immune homeostasis. Although negative or positive immune regulation in the tumor could effectively modulate the immune balance inside the tumor and influence tumor growth, activating a systemic immune response results in severe irAEs in healthy tissues [53]. As greater numbers of checkpoint targeting agents are entering clinical stages, irAEs are becoming a critical concern for the stakeholders in immuno-oncology drug development [54, 55]. For example, the general irAEs of anti-PD-1 in non-small cell lung cancer patients occur within a few weeks to three months after treatment initiation, and nearly all significant organs can be affected. In some cases, the onset of irAEs like pulmonary and hepatic toxicity is delayed up to a year after treatment initiation [56]. In addition, some immune checkpoint co-stimulators like anti-OX40 and anti-CD40 antibodies have also been reported with treatment-related adverse events such as cytokine release syndrome and hepatotoxicity in the clinic and can only be applied with intratumoral injection in clinical trials [57, 58].
Using nanotechnology to deliver checkpoint inhibitor proteins, leveraging decades of insights in the usage of nanoparticles in protein delivery, is a possible way to solve the above problems in immune checkpoint therapy. Wang et al. designed a targeted delivery platform of immune checkpoint inhibitors for adjuvant immunotherapy by conjugating anti-PD-L1 monoclonal antibodies (mAbs) onto platelets; this strategy takes advantage of the natural homing ability of platelets to the resection cavity [59]. In another study, Mi et al. constructed a nanoparticle system for precise spatiotemporal codelivery of anti-PD-1 and anti-OX40 mAb – T cell activation was improved when both immunomodulatory agents were simultaneously engaged with T cells [60]. It should be noted that because platelets or many other blood cells have both Fc receptors and other immune receptors on their surface, some immune checkpoint mAbs (e.g., anti-CD40L) will cause embolic thrombosis in patients [61]. Removing Fc fragments from the antibodies can reduce the risk of thrombotic complications, while also impairing the blood circulation time of the proteins [62]. Using nanoparticles for constructing multivalent antibody-conjugated nanoparticles is a potentially robust approach to bypass this pharmacokinetic challenge while retaining therapeutic efficacy.
Constructing a gene delivery system for local expression of therapeutic proteins provides an alternative strategy for checkpoint-based therapy (Figure 2b). In 2017, Huang and his team members reported a nanoparticle-loaded plasmid, targeting tumor-associated fibroblasts (TAFs) for expression of secreted cytotoxic proteins, as an anticancer strategy. The uptake of the chemotherapeutic drug-loaded nanoparticles by fibroblasts was previously considered as an “off-target” event, and this novel idea turned the TAFs into in situ “factory” for producing therapeutic proteins and holds great promise for developing unique cancer therapy strategies [63]. Following up on this idea, genes encoding antibody-mimicking trap proteins against C-X-C motif chemokine 12 (CXCL12), PD-L1, interleukin-10, Wnt Family Member 5A, lipopolysaccharide, C-C type chemokine receptor type 7, etc. were developed and delivered using LNPs, enabling tumor selective immunotherapeutic protein expression and reduction of autoimmune syndromes [37, 64–69]. Oncolytic virus (OV) as a gene delivery carrier has gained much attention recently in cancer immunotherapy. OVs can also be functionalized with gene vectors for long term expression [70, 71]. Both OVs and nanoparticles as gene delivery carriers have advantages and disadvantages: OVs allow high transfection efficiency, while nanoparticles enable low immunogenicity and have limited safety concerns. The delivery vehicle is a critical component in gene therapy. Therefore, there is immense scope for improving nanomaterial-based gene carriers in immune-oncology.
Nanotechnology for Adoptive Cell Transfer Therapy
ACT has become a prominent player in cancer immunotherapy today. There are several types of ACT: endogenous T-cell therapy, tumor-infiltrating lymphocyte (TIL) therapy, engineered T-cell receptor (TCR) therapy, and chimeric antigen receptor (CAR) T-cell therapy[72]. In CAR T-cell therapy, killer immune cells isolated from a donor are engineered with synthetic antigen receptors and costimulatory molecules, expanded ex vivo, followed by re-infusion back to patients. These therapies had demonstrated a robust therapeutic effect in leukemia and lymphomas [73]. However, there are still two significant limitations for current ACT. First, ACT therapy has only succeeded in hematological malignancies to date, performing poorly against solid tumors. The immunosuppressive tumor microenvironment in solid tumors limits T cell infiltration and function [74]. Second, ACT technology demands a time-consuming and expensive workflow, and there are risks of infection during the ex vivo manufactoring process [75, 76]. Therefore, improvements on current cell-based cancer immunotherapy will be clinically significant.
The combination of nanotechnology and bioengineering provides new opportunities to bypass the hurdles mentioned above of immunosuppressive solid tumors and labor-intensive manufacturing process of cell therapies and may lead to a new generation of biotechnology products (Figure 2c). For example, conjugation of nanoparticles loaded with agents to relieve the immunosuppressive microenvironment to the surface of adoptively transferred cells may lead to persistent autocrine-like signaling among these cells. Consistently released agents can help these cells to overcome the immunosuppressive factors adjacent. In one study, Tang et al. described a strategy to “backpack” large quantities of interleukin-15 superagonists on T cells by using protein nanogels that selectively released these cargos in response to the T cell receptor activation after antigen recognition [77]. Compared to systemically administered adjuvant therapies with therapy promoting cytokines or tumor microenvironment modulators, the hitchhiking interleukin-15 nanogel delivery enhanced T cell infiltration in tumors by sixteen-fold, and allowed over eight-fold higher doses of cytokine administration without toxicity [77].
Nanoparticles can be absorbed onto the adoptive cells via nonspecific adherence, chemical conjugation, and antibody binding [78]. Maleimide functionalized nanoparticles can be attached to T cells by chemical conjugation with free surface thiols [79]. Nanoparticles can also be functionalized with monoclonal antibodies targeting various cluster of differentiation (CD) molecules on T cells. It is noteworthy that these receptors on T cells showed substantial internalization, and CD45 targeting exhibited prolonged cell surface retention than other CD molecules [80]. The strategy for decorating adoptive T cells with nanocarriers enables weaponizing these killer cells to perturb immunosuppression, and provides an opportunity to develop more potent ‘killing machines.’ Compared to genetic engineering approaches for modifying T cells, decorating T cells with nanoparticles is easier and enables on-demand modular designs. Also, decorating immune cells with nanoparticles enables targeted delivery of chemotherapeutic drugs by the tissue-homing effect of lymphocytes [81].
To shorten the ex vivo engineering workflow, an alternative approach for decoration of ACT cells is in vivo targeting. For example, Zheng et al. reported in vivo stimulation of ACT cells using F(ab’)2 fragments against a unique cell surface antigen (Thy1.1) expressed on the surface of the injected T cells; the study demonstrated that more than 95% of the ACT cells could be conjugated with nanoparticles following a single injection [82]. In another study, Smith et al. designed DNA-carrying nanoparticles, which could efficiently introduce leukemia-targeting CAR genes into circulating T cells, and avoided the intricacies of ex vivo CAR-T cell manufacturing [83]. The nanoparticles were loaded with plasmid DNA encoding the leukemia-specific 194–1BBz CAR and furnished with anti-CD3e F(ab’)2 for targeting T cells. The nanoparticle-programmed CAR lymphocytes enabled tumor regression with efficacies similar to adoptive T-cell therapy, while simplified the storage conditions and reduced costs in CAR-T therapy [83].
Nanotechnology for Bispecific Antibody Therapy
Using BsAb for redirecting killer cells to target cells in vivo can be a powerful approach for cancer immunotherapy. BsAb was designed to bind simultaneously with two distinct antigens. A typical example involves one arm binding to CD3 on T cells while the other arm binds to receptors overexpressed on target cells. By bridging the interaction between T cells and tumor cells, BsAb induced targeted tumor cell killing in a T-cell receptor-independent manner [84, 85]. However, clinical translation of this idea has not been very promising to date. Catumaxomab is a BsAb consisting of one half of an anti-epithelial cell adhesion molecule (EpCAM) antibody and one half of an anti-CD3 antibody. The European Medicines Agency (EMA) approved this drug in 2009 to treat malignant ascites; however, due to severe toxicity, this drug was withdrawn from the market in 2014 [86]. The BsAb design with an Fcγ portion can contribute to non-conditional T-cell activation as T cells bridge with Fcγ receptor-expressing blood cells, resulting in low systemic tolerability and cytokine release syndrome. Bi-specific T-cell engager (BiTE) was developed by linking the single-chain Fv fragment (scFv) directly with a flexible linker, eliminating the Fcγ portion, to mitigate this challenge [87]. Blinatumomab, a BiTE combining CD3 and CD19 was approved by FDA in 2014 as a second-line treatment for Philadelphia chromosome-negative relapsed or refractory acute lymphoblastic leukemia.
However, BiTE technology is still far from satisfactory. Since lacking the Fc portion, the blood circulation time of BiTE is quite short, and constant administration is required for effective therapy [88]. Similar to CAR-T treatment, BiTE is ineffective against solid tumors [89]. Besides, BiTE does not direct T cells for tumor killing. The binding affinity between the monomeric chain of BiTE and T cells is rather low, and a firm binding only appeared when a multivalent transient matrix of CD3-binding sites formed on the surface of the target cells [90].
There are opportunities for harnessing nanotechnology for constructing multifunctional bi-specific nano-engager (mfBiNE) for re-directing immune cells in cancer therapy [91] (Figure 2d). Firstly, nanoparticles are characterized by long circulation ability, and linking small fragments of proteins to nanoparticle surface can prolong the circulation time of protein therapeutics and reduce drug administration frequency. Secondly, nanoparticles have flexible surface modification capabilities, thus providing a platform for surface conjugation with multivalent antibodies, or multi-type antibodies engaging different targets with unique antibodies. Multivalent interactions can greatly increase the binding affinity and stimulation efficacy while using protein engineering for constructing a multivalent antibody is highly complicated and expensive [92]. Thirdly, nanoparticles loaded with supportive drugs can augment the capability of killer cells allowing robust efficacy against solid tumors, which is so far unachievable.
Chiu et al. showed that antibody potency was increased up to twenty five-fold when the antibodies were presented in a multivalent liposome formulation with trastuzumab and rituximab grafted onto the liposome membranes [93]. Yuan et al. reported a multivalent bi-specific nanobioconjugate engager by simultaneously targeting the human epidermal growth factor receptor 2 (HER2) expressed by cancer cells, and pro-phagocytosis signaling mediated by calreticulin. This platform allowed selective, immune-mediated eradication of cancer cells, and even induced systemic, durable antitumor immunity [91]. To solve the problem of fast clearance of BiTEs from the blood, Cheng et al. explored endogenously derived exosomes as carriers for the BiTE targeting both CD3 on T cells and epidermal growth factor receptor (EGFR) on tumor cells. The so-called synthetic multivalent antibodies retargeted exosomes (SMART-Exos) were constructed by a genetic display of BiTEs on the exosomal surface, and the resulted SMART-Exos demonstrated enhanced antitumor immunity both in vitro and in vivo [94].
We must acknowledge that there are possible challenges of designing mfBiNEs which deserve more investigation. For example, antibody conjugation with nanoparticles does not always guarantee long circulation. Although rituximab and tratuzumab both enhanced in vitro cell viability when conjugated to LNPs, rituximab-LNPs did not improve in vivo efficacy because of reduced blood circulation time, while tratuzumab-LNPs both increased the pharmacokinetics and efficacy in breast tumor xenograft models [95]. The reason for these distinct in vivo behaviors of rituximab-LNPs and tratuzumab-LNPs is still unclear, and it is apparent that the nature of the antibody influences the in vivo performance of the multivalent antibodies. Secondly, the strong binding affinity of multivalent antibody on T cells or NK cells may result in non-specific activation and cytokine release. Current understanding regarding the balance of the binding affinity is still not enough, but several recent studies showed that the multivalent antibodies activate T cells or NK cells only in the presence of target cells. For example, the multivalent αCD3/αEGFR SMART-Exos resulted in dose-dependent activation of T cells only in the presence of EGFR-positive cells, while no cytokine release was detected in the absence of target cells or the presence of EGFR-negative cells [94]. The multivalent rituximab-LNPs (each LNPs consisted of 38–47 rituximab with about 90 valencies capable of binding) elicited superior complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC) over rituximab, and this effect was observed only in the presence of target cells [95]. Since the valency on the nanoparticle surface is tunable, further investigation on the integration of nanotechnology and multi-type antibodies for cancer therapy is necessary.
Concluding Remarks
In this opinion article, we discussed the opportunities and challenges of nanotechnology in the age of cancer immunotherapy, with emphasis on the integration of nanotechnology with in situ cancer vaccines, immune checkpoint inhibitors, ACT, and bispecific antibody therapies. We also discussed the numerous gaps and concerns for expanding nanotechnology to the above modalities (see Outstanding Questions). Importantly, it should be noted that in the era of immunotherapy, nanomaterials go beyond an ‘adjuvant’ or ‘formulation,’ and should be integrated into new-age biotechnology solutions. Encouragingly, an increasingly high volume of publications on the topic of “nano” and “immunotherapy” is appearing and there were over 70 publications in this interdisciplinary domain in 2019 alone. Based on these efforts, a new sub-discipline called “nano-immunotherapeutics” may soon emerge. We anticipate more disease-focused designs in this framework for the benefit of patients, in the days to come.
Outstanding questions:
For the era of immunotherapy, how to improve the translational rate of nanomedicine?
Are there any criteria in designing nanotherapeutics for immuno-oncology drugs that can broaden the proportion of patients with an effective response, and expand the benefit to patients with solid tumor malignancies?
How to realize truly tumor-specific immune modulation or gene expression in leveraging nanotechnologies for cancer immunotherapy?
How to optimize the benefits of multivalent interaction and safety concerns from multicomponent assemblies of nanoparticle-based bi-specific antibodies?
Highlights.
Nanotechnology plays unique roles in enhancing the efficacy, enabling translational potential or even developing novel therapeutic paradigms based on current cancer immunotherapy.
For in situ cancer vaccines, nanotechnology can reduce the adverse effects of immune agonists, and enable exploiting deep tumors as an antigen source.
For immune checkpoint inhibitors, nano delivery for protein therapeutics or genes may aid in the translation of newly identified checkpoint molecules – into tightly controlled clinically relevant drugs.
For adoptive cell transfer therapy, backpacking of nanoparticles onto the adoptive cells will amplify the potency of ‘super’ killer cells.
For bispecific antibody therapy, the nanoparticle-based formulation will enable more flexible construction of multivalent or multi-armed structures, and expand the application in solid tumors.
Acknowledgments:
This work is supported by the National Natural Science Foundation of China (51673185, 51973215, 51673189, 51833010, 51520105004), the Jilin Province Science and Technology Development Plan (20170101100JC, 20190103112JH), the National Key Research and Development Program of China (2016YFC1100701), and National Institutes of Health (NIH) grant CA198999.
Glossary:
- Adoptive cell transfer (ACT)
a kind of immunotherapy extracting immune cells from the patient, which are then genetically modified and cultured ex vivo and returned to the same patient.
- Bispecific antibody (BsAb)
an artificial protein which can simultaneously bind to two different types of antigen.
- Bi-specific T-cell engager (BiTE)
a class of artificial bispecific monoclonal antibodies consisting of two single chain variable fragments (scFv) connected in tandem by a flexible linker.
- Chimeric antigen receptor (CAR) T-cell therapy
a kind of adoptive cell therapy where isolated T cells are genetically engineered to produce chimeric T-cell receptors combining both an antigen-binding and T-cell activating function.
- Enhanced permeability and retention (EPR) effect
a phenomenon where macromolecular drugs or nanoparticles with certain sizes (generally considered between 20–200 nm) tend to permeate and accumulate in tumor tissues more than they do in normal tissues, due to impaired blood vessel architecture and less effective lymphatic drainage in fast growing solid tumors.
- Engineered T-cell receptor (TCR) therapy
a kind of adoptive cell therapy whereby T cells are isolated from a patient, equipped with a new T cell receptor, and then re-infused into the patient.
- Immune checkpoint inhibitors
a kind of immunotherapy whereby inhibitory checkpoints are blocked thus, restoring immune system function.
- Immuno-oncology
study and development of treatments that take advantage of the body’s immune system to fight cancer.
- In situ cancer vaccine
an approach to stimulate tumor-specific immune responses in the body by injecting some immune stimulators directly into the tumor.
- Immune-related adverse effects (irAEs)
side effects observed in immunotherapy because of nonspecific activation of the immune system.
- Neo-antigens
molecules newly minted by mutations that occur in cancer cells which may serve as substances for provoking an immune response.
- Tumor-infiltrating lymphocyte (TIL) therapy
a kind of adoptive cell therapy where tumor infiltrating lymphocytes are isolated from a patient’s tumor sample, activated and expanded ex vivo, and re-infused back to the patient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors declare no conflict of interests.
References:
- 1.Shi JJ et al. (2017) Cancer nanomedicine: progress, challenges and opportunities. Nature Reviews Cancer 17 (1), 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peer D et al. (2007) Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology 2, 751. [DOI] [PubMed] [Google Scholar]
- 3.Min Y et al. (2015) Clinical Translation of Nanomedicine. Chemical reviews 115 (19), 11147–11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitragotri S et al. (2015) Accelerating the Translation of Nanomaterials in Biomedicine. ACS nano 9 (7), 6644–6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda H et al. (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65 (1–2), 271–84. [DOI] [PubMed] [Google Scholar]
- 6.Maeda H et al. (2016) A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy--Problems, Solutions, and Prospects. Microcirculation 23 (3), 173–82. [DOI] [PubMed] [Google Scholar]
- 7.Harrington KJ et al. (2001) Effective Targeting of Solid Tumors in Patients With Locally Advanced Cancers by Radiolabeled Pegylated Liposomes. Clinical Cancer Research 7 (2), 243–254. [PubMed] [Google Scholar]
- 8.Maeda H (2015) Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev 91, 3–6. [DOI] [PubMed] [Google Scholar]
- 9.Wang AZ (2015) EPR or no EPR? The billion-dollar question. Science Translational Medicine 7 (294), 294ec112. [Google Scholar]
- 10.Morrison C (2018) Alnylam prepares to land first RNAi drug approval. Nature Reviews Drug Discovery 17, 156. [DOI] [PubMed] [Google Scholar]
- 11.Sun QH et al. (2017) Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Advanced Materials 29 (14), 1606628. [DOI] [PubMed] [Google Scholar]
- 12.Du JZ et al. (2018) Tumor-Acidity-Cleavable Maleic Acid Amide (TACMAA): A Powerful Tool for Designing Smart Nanoparticles To Overcome Delivery Barriers in Cancer Nanomedicine. Accounts of Chemical Research 51 (11), 2848–2856. [DOI] [PubMed] [Google Scholar]
- 13.Chen J et al. (2017) Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors. Adv Mater 29 (32), 201701170. [DOI] [PubMed] [Google Scholar]
- 14.Service, R.F. (2019) U.S. cancer institute cancels nanotech research centers. https://www.sciencemag.org/news/2019/05/us-cancer-institute-cancels-nanotech-research-centers.
- 15.story C (2019) The beginning of the end of the nanomedicine hype. Journal of Controlled Release S0168–3659 (19), 30308–6. [DOI] [PubMed] [Google Scholar]
- 16.Das M and Huang L (2019) Liposomal Nanostructures for Drug Delivery in Gastrointestinal Cancers. J Pharmacol Exp Ther 370 (3), 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellman I et al. (2011) Cancer immunotherapy comes of age. Nature 480 (7378), 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanmamed MF and Chen L (2018) A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 175 (2), 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Burg SH et al. (2016) Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nature Reviews Cancer 16 (4), 219–233. [DOI] [PubMed] [Google Scholar]
- 20.Hollingsworth RE and Jansen K (2019) Turning the corner on therapeutic cancer vaccines. npj Vaccines 4 (1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irvine DJ et al. (2015) Synthetic Nanoparticles for Vaccines and Immunotherapy. Chemical Reviews 115 (19), 11109–11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song W et al. (2017) Nanomaterials for cancer immunotherapy. Biomaterials 148, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuai R et al. (2017) Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature Materials 16 (4), 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furness AJ et al. (2016) Neoantigen heterogeneity: a key driver of immune response and sensitivity to immune checkpoint blockade? Immunotherapy 8 (7), 763–766. [DOI] [PubMed] [Google Scholar]
- 25.Ott PA et al. (2017) An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547 (7662), 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahin U et al. (2017) Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547 (7662), 222–226. [DOI] [PubMed] [Google Scholar]
- 27.Gilboa E (2007) DC-based cancer vaccines. The Journal of clinical investigation 117 (5), 1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner TA et al. (2012) Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother 8 (4), 534–9. [DOI] [PubMed] [Google Scholar]
- 29.Tanyi JL et al. (2018) Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Science Translational Medicine 10 (436), eaao5931. [DOI] [PubMed] [Google Scholar]
- 30.Marabelle A et al. (2017) Intratumoral immunotherapy: using the tumor as the remedy. Annals of Oncology 28, 33–43. [DOI] [PubMed] [Google Scholar]
- 31.Sagiv-Barfi I et al. (2018) Eradication of spontaneous malignancy by local immunotherapy. Science Translational Medicine 10 (426), eaan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammerich L et al. (2019) Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nature Medicine 25 (5), 814–824. [DOI] [PubMed] [Google Scholar]
- 33.Lynn GM et al. (2015) In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nature Biotechnology 33 (11), 1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuhn L et al. (2018) Nanoparticle-Conjugate TLR7/8 Agonist Localized Immunotherapy Provokes Safe Antitumoral Responses. Advanced Materials 30 (45), e1803397. [DOI] [PubMed] [Google Scholar]
- 35.Mottas I et al. (2019) Amphiphilic nanoparticle delivery enhances the anticancer efficacy of a TLR7 ligand via local immune activation. Biomaterials 190–191, 111–120. [DOI] [PubMed] [Google Scholar]
- 36.Kwong B et al. (2011) Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy. Biomaterials 32 (22), 5134–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song WT et al. (2018) Trapping of Lipopolysaccharide to Promote Immunotherapy against Colorectal Cancer and Attenuate Liver Metastasis. Advanced Materials 30 (52), e1805007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das M et al. (2019) Nanoparticle Delivery of RIG-I Agonist Enables Effective and Safe Adjuvant Therapy in Pancreatic Cancer. Molecular Therapy 27 (3), 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng N et al. (2018) A nanoparticle-incorporated STING activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. Jci Insight 3 (22), 120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song W et al. (2019) Leveraging biomaterials for cancer immunotherapy: targeting pattern recognition receptors. Materials Today Nano 5, 100029. [Google Scholar]
- 41.Thomas SN et al. (2014) Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials 35 (2), 814–824. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y et al. (2018) Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nature Communications 9 (1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hewitt SL et al. (2019) Durable anticancer immunity from intratumoral administration of IL-23, IL-36 gamma, and OX40L mRNAs. Science Translational Medicine 11 (477), eaat9143. [DOI] [PubMed] [Google Scholar]
- 44.Yang SC et al. (2019) Selectively Potentiating Hypoxia Levels by Combretastatin A4 Nanomedicine: Toward Highly Enhanced Hypoxia-Activated Prodrug Tirapazamine Therapy for Metastatic Tumors. Advanced Materials 31 (11), 1805955. [DOI] [PubMed] [Google Scholar]
- 45.Ueki N et al. (2013) Selective cancer targeting with prodrugs activated by histone deacetylases and a tumour-associated protease. Nature Communications 4, 2735. [DOI] [PubMed] [Google Scholar]
- 46.Trouet A et al. (2001) Extracellularly Tumor-activated Prodrugs for the Selective Chemotherapy of Cancer. Cancer Research 61 (7), 2843–2846. [PubMed] [Google Scholar]
- 47.Le DT et al. (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New England Journal of Medicine 372 (26), 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zou WP et al. (2016) PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science Translational Medicine 8 (328), 328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma P and Allison JP (2015) Immune Checkpoint Targeting in Cancer Therapy: Toward Combination Strategies with Curative Potential. Cell 161 (2), 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer 12 (4), 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J et al. (2019) Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nature Medicine 25 (4), 656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J et al. (2019) Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 176 (1–2), 334–347.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martins F et al. (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nature Reviews Clinical Oncology 16 (9), 563–580. [DOI] [PubMed] [Google Scholar]
- 54.Weber JS et al. (2012) Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. Journal of Clinical Oncology 30 (21), 2691–2697. [DOI] [PubMed] [Google Scholar]
- 55.Postow MA et al. (2018) Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. New England Journal of Medicine 378 (2), 158–168. [DOI] [PubMed] [Google Scholar]
- 56.Remon J et al. (2018) Immune-related adverse events with immune checkpoint inhibitors in thoracic malignancies: focusing on non-small cell lung cancer patients. Journal of Thoracic Disease 10, S1516–S1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vonderheide RH et al. (2007) Clinical Activity and Immune Modulation in Cancer Patients Treated With CP-870,893, a Novel CD40 Agonist Monoclonal Antibody. Journal of Clinical Oncology 25 (7), 876–883. [DOI] [PubMed] [Google Scholar]
- 58.ClinicalTrials.gov (2019) SD-101 and BMS-986178 in Treating Patients With Advanced or Metastatic Solid Malignancies. Identifier: NCT03831295.
- 59.Wang C et al. (2017) In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nature Biomedical Engineering 1 (2), 0011. [Google Scholar]
- 60.Mi Y et al. (2018) A Dual Immunotherapy Nanoparticle Improves T-Cell Activation and Cancer Immunotherapy. Advanced Materials 30 (25), 1706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robles-Carrillo L et al. (2010) Anti-CD40L Immune Complexes Potently Activate Platelets In Vitro and Cause Thrombosis in FCGR2A Transgenic Mice. Journal of Immunology 185 (3), 1577–1583. [DOI] [PubMed] [Google Scholar]
- 62.Shock A et al. (2015) CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: an in vivo study. Arthritis Research & Therapy 17, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miao L et al. (2017) Targeting Tumor-Associated Fibroblasts for Therapeutic Delivery in Desmoplastic Tumors. Cancer Research 77 (3), 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodwin TJ et al. (2016) Local and transient gene expression primes the liver to resist cancer metastasis. Science Translational Medicine 8 (364), 364ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miao L et al. (2017) Transient and Local Expression of Chemokine and Immune Checkpoint Traps To Treat Pancreatic Cancer. Acs Nano 11 (9), 8690–8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen LM et al. (2018) Local Blockade of Interleukin 10 and C-X-C Motif Chemokine Ligand 12 with Nano-Delivery Promotes Antitumor Response in Murine Cancers. Acs Nano 12 (10), 9830–9841. [DOI] [PubMed] [Google Scholar]
- 67.Liu Q et al. (2018) Nanoparticle-Mediated Trapping of Wnt Family Member 5A in Tumor Microenvironments Enhances Immunotherapy for B-Raf Proto-Oncogene Mutant Melanoma. Acs Nano 12 (2), 1250–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.An S et al. (2019) Locally Trapping the C-C Chemokine Receptor Type 7 by Gene Delivery Nanoparticle Inhibits Lymphatic Metastasis Prior to Tumor Resection. Small 15 (9), 1805182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song WT et al. (2018) Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nature Communications 9 (1), 2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Twumasi-Boateng K et al. (2018) Oncolytic viruses as engineering platforms for combination immunotherapy. Nature Reviews Cancer 18 (7), 419–432. [DOI] [PubMed] [Google Scholar]
- 71.Freedman JD et al. (2018) An Oncolytic Virus Expressing a T-cell Engager Simultaneously Targets Cancer and Immunosuppressive Stromal Cells. Cancer Research 78 (24), 6852–6865. [DOI] [PubMed] [Google Scholar]
- 72.Rosenberg SA and Restifo NP (2015) Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348 (6230), 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.June CH et al. (2018) CAR T cell immunotherapy for human cancer. Science 359 (6382), 1361–1365. [DOI] [PubMed] [Google Scholar]
- 74.D’Aloia MM et al. (2018) CAR-T cells: the long and winding road to solid tumors. Cell Death & Disease 9 (3), 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaiser AD et al. (2015) Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer gene therapy 22 (2), 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernanda Masri ECSA (2019) Viral vector manufacturing: how to address current and future demands? Cell & Gene Therapy Insights 5 (S5), 949–970. [Google Scholar]
- 77.Tang L et al. (2018) Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nature Biotechnology 36 (8), 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cole C et al. (2005) Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nature Medicine 11 (10), 1073–1081. [DOI] [PubMed] [Google Scholar]
- 79.Stephan MT et al. (2010) Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nature Medicine 16 (9), 1035–U135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng Y et al. (2017) Enhancing Adoptive Cell Therapy of Cancer through Targeted Delivery of Small-Molecule Immunomodulators to Internalizing or Noninternalizing Receptors. Acs Nano 11 (3), 3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang BN et al. (2015) Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Science Translational Medicine 7 (291), 291ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng Y et al. (2013) In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. Journal of Controlled Release 172 (2), 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith TT et al. (2017) In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nature Nanotechnology 12 (8), 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krishnamurthy A and Jimeno A (2018) Bispecific antibodies for cancer therapy: A review. Pharmacology & Therapeutics 185, 122–134. [DOI] [PubMed] [Google Scholar]
- 85.Chames P and Baty D (2009) Bispecific antibodies for cancer therapy: the light at the end of the tunnel? mAbs 1 (6), 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duell J et al. (2019) Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin Pharmacol Ther 106 (4), 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baeuerle PA and Reinhardt C (2009) Bispecific T-Cell Engaging Antibodies for Cancer Therapy. Cancer Research 69 (12), 4941–4944. [DOI] [PubMed] [Google Scholar]
- 88.Dreier T et al. (2003) T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3-bispecific single-chain antibody construct. Journal of Immunology 170 (8), 4397–4402. [DOI] [PubMed] [Google Scholar]
- 89.Yu S et al. (2017) Recent advances of bispecific antibodies in solid tumors. Journal of Hematology & Oncology 10 (1), 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brischwein K et al. (2007) Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. Journal of Immunotherapy 30 (8), 798–807. [DOI] [PubMed] [Google Scholar]
- 91.Yuan HF et al. (2017) Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nature Nanotechnology 12 (8), 763–769. [DOI] [PubMed] [Google Scholar]
- 92.Nunez-Prado N et al. (2015) The coming of age of engineered multivalent antibodies. Drug Discovery Today 20 (5), 588–594. [DOI] [PubMed] [Google Scholar]
- 93.Chiu GNC et al. (2007) Modulation of cancer cell survival pathways using multivalent liposomal therapeutic antibody constructs. Molecular Cancer Therapeutics 6 (3), 844–855. [DOI] [PubMed] [Google Scholar]
- 94.Cheng Q et al. (2018) Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. Journal of the American Chemical Society 140 (48), 16413–16417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Popov J et al. (2011) Multivalent rituximab lipid nanoparticles as improved lymphoma therapies: indirect mechanisms of action and in vivo activity. Nanomedicine 6 (9), 1575–1591. [DOI] [PubMed] [Google Scholar]