Key Points
Question
Could administration of convalescent plasma transfusion be beneficial in the treatment of critically ill patients with coronavirus disease 2019 (COVID-19)?
Findings
In this uncontrolled case series of 5 critically ill patients with COVID-19 and acute respiratory distress syndrome (ARDS), administration of convalescent plasma containing neutralizing antibody was followed by an improvement in clinical status.
Meaning
These preliminary findings raise the possibility that convalescent plasma transfusion may be helpful in the treatment of critically ill patients with COVID-19 and ARDS, but this approach requires evaluation in randomized clinical trials.
Abstract
Importance
Coronavirus disease 2019 (COVID-19) is a pandemic with no specific therapeutic agents and substantial mortality. It is critical to find new treatments.
Objective
To determine whether convalescent plasma transfusion may be beneficial in the treatment of critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Design, Setting, and Participants
Case series of 5 critically ill patients with laboratory-confirmed COVID-19 and acute respiratory distress syndrome (ARDS) who met the following criteria: severe pneumonia with rapid progression and continuously high viral load despite antiviral treatment; Pao2/Fio2 <300; and mechanical ventilation. All 5 were treated with convalescent plasma transfusion. The study was conducted at the infectious disease department, Shenzhen Third People's Hospital in Shenzhen, China, from January 20, 2020, to March 25, 2020; final date of follow-up was March 25, 2020. Clinical outcomes were compared before and after convalescent plasma transfusion.
Exposures
Patients received transfusion with convalescent plasma with a SARS-CoV-2–specific antibody (IgG) binding titer greater than 1:1000 (end point dilution titer, by enzyme-linked immunosorbent assay [ELISA]) and a neutralization titer greater than 40 (end point dilution titer) that had been obtained from 5 patients who recovered from COVID-19. Convalescent plasma was administered between 10 and 22 days after admission.
Main Outcomes and Measures
Changes of body temperature, Sequential Organ Failure Assessment (SOFA) score (range 0-24, with higher scores indicating more severe illness), Pao2/Fio2, viral load, serum antibody titer, routine blood biochemical index, ARDS, and ventilatory and extracorporeal membrane oxygenation (ECMO) supports before and after convalescent plasma transfusion.
Results
All 5 patients (age range, 36-65 years; 2 women) were receiving mechanical ventilation at the time of treatment and all had received antiviral agents and methylprednisolone. Following plasma transfusion, body temperature normalized within 3 days in 4 of 5 patients, the SOFA score decreased, and Pao2/Fio2 increased within 12 days (range, 172-276 before and 284-366 after). Viral loads also decreased and became negative within 12 days after the transfusion, and SARS-CoV-2–specific ELISA and neutralizing antibody titers increased following the transfusion (range, 40-60 before and 80-320 on day 7). ARDS resolved in 4 patients at 12 days after transfusion, and 3 patients were weaned from mechanical ventilation within 2 weeks of treatment. Of the 5 patients, 3 have been discharged from the hospital (length of stay: 53, 51, and 55 days), and 2 are in stable condition at 37 days after transfusion.
Conclusions and Relevance
In this preliminary uncontrolled case series of 5 critically ill patients with COVID-19 and ARDS, administration of convalescent plasma containing neutralizing antibody was followed by improvement in their clinical status. The limited sample size and study design preclude a definitive statement about the potential effectiveness of this treatment, and these observations require evaluation in clinical trials.
This case series describes clinical outcomes in 5 Chinese patients with laboratory-confirmed COVID-19, ARDS, and high viral loads despite antiviral treatment who were given human plasma with SARS-CoV-2 antibodies obtained from previously infected and recovered patients.
Introduction
The epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) originating in Wuhan, China, has rapidly spread worldwide.1 As of March 24, 2020, China had reported 81 767 cases with 3281 deaths, and the World Health Organization declared coronavirus disease 2019 (COVID-19) a pandemic. As of March 18, 2020, cases were reported in approximately 195 countries.2
No specific therapeutic agents or vaccines for COVID-19 are available.3 Several therapies, such as remdesivir and favipiravir, are under investigation,3,4 but the antiviral efficacy of these drugs is not yet known. The use of convalescent plasma was recommended as an empirical treatment during outbreaks of Ebola virus in 2014, and a protocol for treatment of Middle East respiratory syndrome coronavirus with convalescent plasma was established in 2015.5 This approach with other viral infections such as SARS-CoV, H5N1 avian influenza, and H1N1 influenza also suggested that transfusion of convalescent plasma was effective.6,7,8,9,10 In previous reports, most of the patients received the convalescent plasma by single transfusion.9,10,11 In a study involving patients with pandemic influenza A(H1N1) 2009 virus infection, treatment of severe infection with convalescent plasma (n = 20 patients) was associated with reduced respiratory tract viral load, serum cytokine response, and mortality.10 In another study involving 80 patients with SARS, administration of convalescent plasma was associated with a higher rate of hospital discharge at day 22 from symptom onset compared with patients who did not receive convalescent plasma.12 Accordingly, these findings raise the hypothesis that use of convalescent plasma transfusion could be beneficial in patients infected with SARS-CoV-2.
The purpose of this study was to describe the initial clinical experience with convalescent plasma transfusion administered to critically ill patients with COVID-19.
Methods
This study was conducted at the infectious disease department, Shenzhen Third People's Hospital, Shenzhen, China, from January 20, 2020, to March 25, 2020, and the final date of follow-up was March 25, 2020. The study was approved by the ethics committees from Shenzhen Third People’s Hospital, and each patient gave written informed consent.
Patients
Patients with laboratory confirmed COVID-19, diagnosed using quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) (GeneoDX Co, Ltd)13 were eligible to receive convalescent plasma treatment if they fulfilled the following criteria: (1) had severe pneumonia with rapid progression and continuously high viral load despite antiviral treatment; (2) Pao2/Fio2 of <300 (Pao2 measured in mm Hg and Fio2 measured as fraction of inspired oxygen)14; and (3) were currently or had been supported with mechanical ventilation. The serum of each recipient was obtained and enzyme-linked immunosorbent assay (ELISA) and neutralizing antibody titers were tested one day prior to the convalescent plasma transfusion. The ABO blood types of the patients were determined for potential compatibility with the convalescent plasma donor, and each received 2 consecutive transfusions of 200 to 250 mL of ABO-compatible convalescent plasma (400 mL of convalescent plasma in total) on the same day it was obtained from the donor. The patients received antiviral agents continuously until the SARS-CoV-2 viral loads became negative.
Disease Severity Classification
Patients with laboratory-confirmed COVID-19 infection who had any of the following were considered in critical condition: (1) respiratory failure requiring mechanical ventilation, (2) shock, identified by the use of vasopressor therapy and elevated lactate levels (>2 mmol/L) despite adequate fluid resuscitation, or (3) failure of other organs requiring admission to the intensive care unit (ICU).
Donors
The 5 donors of convalescent plasma were between the ages of 18 and 60 years. The donors had recovered from SARS-CoV-2 infection and were invited to donate their convalescent plasma after written informed consent was obtained. All donors had been previously diagnosed with laboratory-confirmed COVID-19 and subsequently tested negative for SARS-CoV-2 and other respiratory viruses, as well as for hepatitis B virus, hepatitis C virus, HIV, and syphilis at the time of blood donation. The donors had been well (asymptomatic) for at least 10 days, with a serum SARS-CoV-2–specific ELISA antibody titer higher than 1:1000 and a neutralizing antibody titer greater than 40. Following donation, 400 mL of convalescent plasma was obtained from each donor by apheresis, and the plasma was immediately transfused to the recipients on the same day it was obtained.
Clinical Information
Clinical information for the 5 patients before and after convalescent plasma transfusion was obtained from a review of the hospital computer medical system and included the following: demographic data, days of admission from symptom onset, and presenting symptoms; data about various treatments, including mechanical ventilation, antiviral therapies, and steroids; clinical data, including body temperature, Pao2/Fio2, and Sequential Organ Failure Assessment (SOFA) score (range 0-24, with higher scores indicating more severe illness); laboratory data, including white blood cell count, lymphocyte count, chemistry panels assessing liver and kidney function, cycle threshold value (Ct), inflammatory factors C-reactive protein (CRP), procalcitonin, and IL-6, and serum antibody titer (IgG, IgM, and neutralizing antibodies); data from chest imaging studies; and information on complications, such as acute respiratory distress syndrome (ARDS), bacterial pneumonia, and multiple organ dysfunction syndrome.
Quantitative RT-PCR
The qRT-PCR for SARS-CoV-2 was assessed as described previously.13 Nasopharyngeal specimens collected during hospitalization were sent to the laboratory in a viral transport case. Total nucleic acid extraction from the samples was performed using the QIAamp RNA Viral Kit (Qiagen), and qRT-PCR was performed using a commercial kit specific for 2019-nCoV detection (GeneoDX Co) approved by the China Food and Drug Administration. Each RT-PCR assay provided a Ct value, which is the number of cycles required for the fluorescent signal to cross the threshold for a positive test: a higher Ct value is correlated with a lower viral load. The specimens were considered positive if the Ct value was 37.0 or lower and negative if the results were undetermined. Specimens with a Ct value higher than 37 were repeated. The specimen was considered positive if the repeated results were the same as the initial result and between 37 and 40. If the repeated Ct was undetectable, the specimen was considered negative. All procedures involving clinical specimens and SARS-CoV-2 were performed in a biosafety level 3 laboratory. The Ct values of the 5 recipients were obtained on day −1, day 1, day 3, day 7, and day 12 after the transfusion.
ELISA
Microtiter plates (Sangon Biotech) were coated overnight at 4 °C with 4 μg/mL recombinant SARS-CoV-2 RBD (receptor binding domain) proteins (50 μL per well) expressed by our laboratory through 293-T cells. The plates were washed 3 times with phosphate-buffered saline (PBS) containing 0.1% vol/vol Tween-20 (PBST) and blocked with blocking solution (PBS containing 2% wt/vol nonfat dry milk) for 2 hours at 37 °C. The plates were then washed with PBST. The serum samples were diluted to 200-fold into PBS as initial concentration, and serial 3-fold dilutions of serum was added to the wells and incubated at 37 °C for 60 minutes. After 3 washes, 100 μL of horseradish peroxidase–conjugated goat anti–human IgG (for IgG antibody titer detection) and IgM (for IgM antibody titer detection) antibodies solution (Sangon Biotech) were added to each plate, respectively, and incubated at 37 °C for 60 minutes. After 5 washes, 100 μL of tetramethylbenzidine substrate (Sangon Biotech) was added at room temperature in the dark. After 15 minutes, the reaction was stopped with a 2 M H2SO4 solution (sulfuric acid). The absorbance was measured at 450 nm. All samples were run in triplicate. The ELISA titers were determined by end point dilution.
Serum Neutralization Assay
Vero cells (104) were seeded 24 hours before the infection in a 96-well plate (Costar). On the day of infection, the cells were washed twice. Serum samples from patients were incubated at 56 °C for 30 minutes and then diluted 2-fold in cell culture medium (modified eagle medium). Aliquots (40 μL) of diluted serum samples (from 2-fold to 2056-fold) were added to 50 μL of cell culture medium containing 50 times the tissue culture infective dose (TCID50) of the BetaCoV/Shenzhen/SZTH-003/2020 strain virus (isolated from this hospital, GISAID access number: EPI_ISL_406594)15 on a 96-well plate and incubated at 37 °C for 2 hours in CO2 5% vol/vol. Virus antibody mix was then added to cells in 96-well plates and plates were incubated at 37 °C with microscopic examination for cytopathic effect after a 5-day incubation. The highest dilution of serum that showed inhibition activity of SARS-CoV-2 was recorded as the neutralizing antibody titer. Assays were performed in triplicate with negative control samples from healthy volunteers.
Results
Five patients (age range, 36-73 years; 2 women) were treated with convalescent serum. None were smokers, and 4 of 5 had no preexisting medical conditions. All 5 had received various antiviral agents and steroids (Table 1). Convalescent plasma was administered between 10 and 22 days after admission.
Table 1. Clinical Characteristics of SARS-CoV-2-Infected Patients Who Received Convalescent Plasma.
| Patient | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sex | Male | Male | Female | Female | Male |
| Age, y | 70s | 60s | 50s | 30s | 60s |
| Weight, kg | 55 | 85 | 60 | 41.5 | 87 |
| Smoking | No | No | No | No | No |
| Blood type | B | B | B | A | B |
| Coexisting chronic diseases | None | Hypertension; mitral insufficiency | None | None | None |
| Disease presentation and course | |||||
| Estimated incubation period, da | 1 | 7 | 3 | 7 | 15 |
| Interval between symptom onset and admission, d | 2 | 4 | 2 | 2 | 3 |
| Interval between admission and plasma transfusion, d | 22 | 10 | 20 | 19 | 20 |
| Complications prior to plasma transfusion | Bacterial pneumonia; severe ARDS; MODS | Bacterial pneumonia; fungal pneumonia; severe ARDS; myocardial damage | Severe ARDS | Severe ARDS | Severe ARDS |
| Most severe disease classification | Critical | Critical | Critical | Critical | Critical |
| Treatments | |||||
| Steroids | Methylprednisolone | Methylprednisolone | Methylprednisolone | Methylprednisolone | Methylprednisolone |
| Antivirals | Lopinavir/ritonavir; interferon alfa-1b; favipiravir | Lopinavir/ritonavir; arbidol; darunavir | Lopinavir/ritonavir; interferon alfa-1b; | Interferon alfa-1b; favipiravir | Lopinavir/ritonavir; interferon alfa-1b |
Abbreviations: ARDS, acute respiratory distress syndrome; MODS, multiple organ dysfunction syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Estimated incubation period defined as interval between estimated exposure to SARS-CoV-2 and symptom onset.
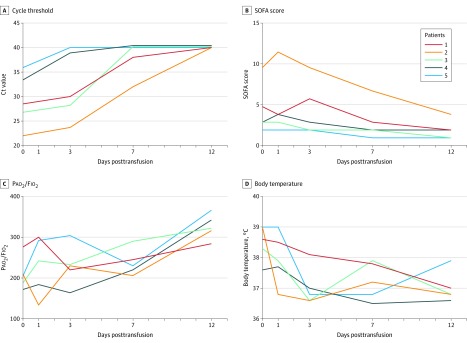
The Ct value at the time of admission ranged from 18.9 to 38.0, and on the day of plasma transfusion from 22.0 to 35.9 (Table 2 and Figure 1A). It increased (improved) within 1 day after transfusion. The Ct value of patient 5 became negative on posttransfusion day 1, patient 3 and patient 4 became negative on day 3, and patient 1 and patient 2 became negative on day 12 after the transfusion (Table 2).
Table 2. Comparison of Viral Load, Clinical Indexes, and Laboratory Results Before and After Convalescent Plasma Transfusion.
| Patient | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Clinical characteristics | |||||
| Body temperature, °C | |||||
| Just before transfusion | 38.6 | 39.0 | 37.6 | 38.3 | 39.0 |
| Day 1 posttransfusion | 38.5 | 36.8 | 37.7 | 37.9 | 39.0 |
| Day 3 posttransfusion | 38.1 | 36.6 | 37.0 | 36.6 | 36.8 |
| Day 7 posttransfusion | 37.8 | 37.2 | 36.5 | 37.9 | 36.8 |
| Day 12 posttransfusion | 37.0 | 36.8 | 36.6 | 36.8 | 37.9 |
| SOFA scorea | |||||
| Just before transfusion | 5 | 10 | 3 | 3 | 2 |
| Day 1 posttransfusion | 4 | 12 | 4 | 3 | 2 |
| Day 3 posttransfusion | 6 | 10 | 3 | 2 | 2 |
| Day 5 posttransfusion | 5 | 11 | 2 | 2 | 2 |
| Day 7 posttransfusion | 3 | 7 | 2 | 2 | 1 |
| Day 12 posttransfusion | 2 | 4 | 2 | 1 | 1 |
| Pao2/Fio2b | |||||
| Just before transfusion | 276 | 209 | 172 | 188 | 205 |
| Day 1 posttransfusion | 300 | 134 | 184 | 242 | 292 |
| Day 3 posttransfusion | 220 | 230 | 164 | 233 | 304 |
| Day 7 posttransfusion | 245 | 206 | 220 | 290 | 230 |
| Day 12 posttransfusion | 284 | 316 | 342 | 322 | 366 |
| Ct valuec (viral load proxy) | |||||
| On admission to hospital | 23.0 | 19.7 | 18.9 | 38.0 | 28.0 |
| Lowest value during hospitalizationd (highest viral load) | 19.2 | 19.7 | 18.9 | 26.6 | 26.5 |
| Just before plasma transfusion | 28.5 | 22.0 | 33.0 | 26.6 | 35.9 |
| Day 1 posttransfusion | 30.0 | 23.7 | 38.5 | 28.0 | Negative |
| Day 3 posttransfusion | 34.4 | 25.0 | Negative | Negative | Negative |
| Day 7 posttransfusion | 38.0 | 32.0 | Negative | Negative | Negative |
| Day 12 posttransfusion | Negative | Negative | Negative | Negative | Negative |
| Mechanical ventilation | |||||
| Onset, days before transfusion | 11 | 2 | 12 | 9 | 2 |
| Extubated, days posttransfusion | Intubated | Intubated | 2 | 9 | 9 |
| ECMO | |||||
| Onset, days before transfusion | Not received | 1 | Not received | Not received | Not received |
| Removal, days posttransfusion | NA | 5 | NA | NA | NA |
| Laboratory findings | |||||
| C-reactive protein, mg/L (normal range, <8) | |||||
| Before transfusion | 163.4 | 242.8 | 65. | 156.0 | 173.1 |
| Day 1 posttransfusion | 146.2 | 223.0 | 108.3 | NT | 186.8 |
| Day 3 posttransfusion | 115.1 | 75.2 | 78.7 | 160.8 | 233.7 |
| Day 5 posttransfusion | 31.3 | 10.4 | 74.7 | NT | 260.4 |
| Day 7 posttransfusion | 31.2 | 13.9 | 6.2 | 9.6 | 5.5 |
| Day 12 posttransfusion | 5.3 | 33.1 | NT | 5.8 | 3.2 |
| Procalcitonin, ng/mL (normal range, <0.1) | |||||
| Before transfusion | 1.2 | 7.3 | 0.1 | 0.2 | 0.2 |
| Day 1 posttransfusion | 1.3 | 19.7 | 0.1 | 0.08 | 0.4 |
| Day 3 posttransfusion | 1.6 | 13.9 | 0.09 | 0.07 | 1.5 |
| Day 5 posttransfusion | 0.9 | 1.8 | 0.08 | NT | 0.9 |
| Day 7 posttransfusion | 1.1 | 0.1 | 0.04 | 0.04 | 0.09 |
| Day 12 posttransfusion | 0.4 | 0.2 | NT | 0.04 | 0.07 |
| IL-6, pg/mL (normal range, 0-7) | |||||
| Before transfusion | 70.5 | 438.2 | 63.9 | 79.1 | 87.8 |
| Day 1 posttransfusion | 74.9 | NT | 118.5 | 39.3 | NT |
| Day 3 posttransfusion | 34.5 | 1045.0 | 67.0 | 25.8 | 797.9 |
| Day 5 posttransfusion | 24.1 | 334.1 | 590.5 | NT | NT |
| Day 7 posttransfusion | 30.8 | 29.8 | 174.3 | 34.0 | 69.9 |
| Day 12 posttransfusion | 6.1 | 31.8 | NT | 2.7 | 54.9 |
| Length of hospital stay, d | Remains hospitalized | Remains hospitalized | 53 | 51 | 55 |
| Current status as of March 25, 2020 | Stable, still receiving mechanical ventilation | Stable, still receiving mechanical ventilation | Discharged home | Discharged home | Discharged home |
Abbreviations: Ct, cycle threshold; ECMO, extracorporeal membrane oxygenation; NT, not tested.
The SOFA score is calculated using 6 systems: respiratory, coagulation, hepatic, cardiovascular, central nervous system, and kidney. A score of 0 is given for normal function through to 4 for most abnormal for each system. The worst values on each day are recorded, and the final SOFA score is the sum of the scores of each system.
Pao2/Fio2 ratio was defined as the ratio of the partial pressure of arterial oxygen to the percentage of inspired oxygen.
Cycle threshold is the number of polymerase chain reaction cycles required for gene amplification. A higher Ct value is correlated with a lower viral load.
Lowest value (highest viral load) between hospital admission and plasma transfusion.
Figure 1. Temporal Changes of Cycle Threshold Value, Pao2/Fio2, SOFA Score, and Body Temperature in Patients Receiving Convalescent Plasma Transfusion .
A, Change in cycle threshold (Ct) value in nasopharyngeal swabs of infected patients at day 0, day 3, day 7, and day 12 after the plasma transfusion. A Ct value of 40 was defined as undetectable. B, Change in Sequential Organ Failure Assessment (SOFA) score of the patients with convalescent plasma treatment (range 0-24, with higher scores indicating more severe illness; see footnote to Table 2 for more complete definition). C, Change in Pao2/Fio2 ratio of the treated patients from day 0 to day 12 after treatment. D, Change in body temperature of the 5 patients following plasma transfusion.
The SOFA score ranged from 2 to 10 prior to plasma transfusion, and decreased to a range of 1 to 4 at 12 days following transfusion (Table 2 and Figure 1B). The Pao2/Fio2 ranged from 172 to 276 prior to transfusion, and increased (improved) for 4 of 5 patients within 7 days after transfusion (overall range, 206-290), and increased substantially (range, 284-366) on the 12th day after the plasma treatment (Table 2 and Figure 1C). Body temperature ranged from 37.6 to 39.0 °C before plasma transfusion and declined to the normal range on the third day after the transfusion (Table 2 and Figure 1D).
After the treatment, the values of the inflammatory biomarkers CRP, procalcitonin, and IL-6 of patients 1, 2, 4, and 5 decreased; the values of CRP and procalcitonin of patient 3 decreased (Table 2).
The computed tomography scans of the lungs of these patients all demonstrated severe pneumonia prior to plasma transfusion and showed improvement of the pulmonary lesion of patient 1 on the third day after the plasma transfusion (eFigure 1 in the Supplement) and gradual resolution of pulmonary lesions of other patients at 3 days after the plasma treatment (eFigures 2, 3, 4, and 5 in the Supplement).
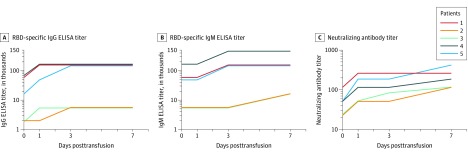
One day prior to convalescent plasma administration, the RBD-specific IgG and IgM ELISA titers of the donors ranged between 1800 and 16 200 (ELISA end point dilution titers) (Table 3). The neutralization titers against SARS-CoV-2 ranged between 80 and 480 (neutralizing end point dilution titers). The RBD-specific IgG ELISA titers of 5 recipients ranged between 1800 and 48 600 and the IgM titers between 5400 and 145 800 a day prior to the convalescent transfusion (eTable in the Supplement). After the transfusion of convalescent plasma, the titers of IgG and IgM in the sera of these patients increased in a time-dependent manner. The IgG titers of the treated patients increased to 145 800, 5400, 5400, 145 800 and 145 800, and the IgM titers increased to 145 800, 5400, 5400, 437 400 and 145 800, respectively, at 3 days after transfusion. These IgG and IgM titers maintained a high level at 7 days after transfusion (Figure 2A and 2B; eTable in the Supplement). The neutralizing antibody titers of the 5 recipients ranged between 40 and 160 before transfusion; one day after transfusion, the titers increased to 320, 80, 80, 160, and 240; on day 7, they were 320, 160, 160, 240, and 480, respectively (Figure 2C; eTable in the Supplement).
Table 3. Characteristics and Antibody Titer of Convalescent Plasma Donors.
| Donorsa | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Blood type | B | B | B | A | B |
| Donated plasma volume, mL | 400 | 400 | 400 | 400 | 400 |
| Interval between symptom onset and discharge, d | 11 | 11 | 13 | 13 | 11 |
| Interval between discharge and plasma donation, d | 11 | 11 | 13 | 11 | 12 |
| RBD-specific IgG ELISA titerb | 16 200 | 1800 | 1800 | 5400 | 16 200 |
| RBD-specific IgM ELISA titerc | 16 200 | 1800 | 5400 | 5400 | 5400 |
| Neutralizing antibody titerd | 240 | 80 | 120 | 240 | 480 |
Abbreviation: RBD, receptor binding domain.
Donors-patients were matched by number (donor 1 gave plasma to patient 1, etc).
ELISA end point dilution titers (IgG antibody). The expected titer of negative control from a healthy person is ≤200.
ELISA end point dilution titers (IgM antibody). The expected titer of negative control from a healthy person is ≤200.
Neutralization end point dilution titers. The expected titer of negative control from a healthy person is ≤10.
Figure 2. Changes of Receptor Binding Domain–Specific IgG and IgM ELISA and Neutralizing Antibody Titers Before and After Convalescent Plasma Transfusion in Patients.
Higher titer values indicate greater protection. A, Variation of RBD-specific IgG ELISA titer. B, Variation of RBD-specific IgM ELISA titer. C, Variation of neutralizing antibody titer against SARS-CoV-2 in recipients in day 0, day 1, day 3, and day 7 following transfusion. The identical line segments were adjusted slightly to avoid superimposition. RBD indicates receptor binding domain.
All 5 patients were receiving mechanical ventilation at the time of transfusion, and 3 patients (patients 3, 4, and 5) were weaned from mechanical ventilation (Table 2). Patient 2 was receiving ECMO at the time of plasma treatment but did not require ECMO on day 5 after transfusion (Table 2). Patients 3, 4, and 5 were discharged from the hospital (length of stay: 53, 51, and 55 days, respectively). As of March 25, 2020, patients 1 and 2 remained hospitalized, with lengths of stay of 37 days each.
Discussion
In this case series, 5 patients who were critically ill with COVID-19 were treated with convalescent plasma. As assessed by Ct, viral load declined within days of treatment with convalescent plasma, and the clinical conditions of these patients improved, as indicated by body temperature reduction, improved Pao2/Fio2, and chest imaging. Four patients who had been receiving mechanical ventilation and ECMO no longer required respiratory support by 9 days after plasma transfusion.
Previous studies have reported the use of convalescent plasma transfusion in the treatment of various infections.6,10,16 For example, patients (n = 50) with SARS had a significantly higher discharge rate by day 22 following onset of illness (73.4% vs 19.0%; P<.001) and lower case-fatality rate (0% vs 23.8%; P = .049) in the convalescent plasma treatment group (n = 19 patients) when compared with steroid treatment group (n = 21).17 In another study of 93 patients with influenza A(H1N1), patients who received convalescent plasma treatment (n = 20) compared with those in the control group (n = 73) had significantly fewer deaths (20% vs 54.8%; P = .01) and a lower median lymphocyte count on ICU admission.10
In this study, collection and transfusion of the plasma were done as previously reported.10 In addition, plasma was obtained from the donors and transfused in the recipients on the same day, which helps preserve the natural activity of the plasma.
Studies have shown that viral loads are highly correlated with disease severity and progression.18 Fatal outcome of human influenza A(H5N1) has been associated with high viral load and hypercytokinemia.19 Apart from antiviral treatment, virus-specific neutralizing antibody, which could accelerate virus clearance and prevent entry into target cells, serves as the main mechanism for the restriction and clearance of the viruses by the host.20,21,22 In the current study, SARS-CoV-2 was still detectable in all 5 patents even though antiviral treatment had been given for at least 10 days, although viral load decreased and became undetectable soon after convalescent plasma treatment. As determined by ELISA, all plasma from the donors had high virus-specific IgG and IgM ELISA titers. Moreover, the neutralizing antibody titers, vital for the restriction of viral infection of the 5 recipients, significantly increased after plasma transfusion. The results highlight the possibility that antibodies from convalescent plasma may have contributed to the clearance of the virus and also the improvement of symptoms. In addition to viral neutralizing antibodies, acceleration of infected cell clearance by antibodies has also been found in an in vivo study of HIV-1 virus.23 In the current study, all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, which also may have contributed to the viral clearance observed.
Limitations
This study has several limitations. First, this was a small case series that included no controls. Second, it is unclear if these patients would have improved without transfusion of convalescent plasma, although the change in Ct and Pao2/Fio2 represent encouraging findings. Third, all patients were treated with multiple other agents (including antiviral medications), and it is not possible to determine whether the improvement observed could have been related to therapies other than convalescent plasma. Fourth, plasma transfusion was administered 10 to 22 days after admission; whether a different timing of administration would have been associated with different outcomes cannot be determined. Fifth, whether this approach would reduce case-fatality rates is unknown.
Conclusions
In this preliminary uncontrolled case series of 5 critically ill patients with COVID-19 and ARDS, administration of convalescent plasma containing neutralizing antibody was followed by improvement in the patients’ clinical status. The limited sample size and study design preclude a definitive statement about the potential effectiveness of this treatment, and these observations require evaluation in clinical trials.
eTable. Variation of IgM, IgG ELISA titers and NAT before and after convalescent plasma transfusion in recipients.
eFigure 1. Computed tomography (CT) scan of the patient 1 (A) before and (B) 3 days-post convalescent plasma transfusion.
eFigure 2. Computed tomography (CT) scan of the patient 2 (A) before and (B) 3 days-post convalescent plasma transfusion.
eFigure 3. Computed tomography (CT) scan of the patient 3 (A) before and (B) 3 days-post convalescent plasma transfusion.
eFigure 4. Computed tomography (CT) scan of the patient 4 (A) before and (B) 3 days-post convalescent plasma transfusion.
eFigure 5. Computed tomography (CT) scan of the patient 5 (A) before and (B) 3 days-post convalescent plasma transfusion.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Novel coronavirus (COVID-19) situation. Updated March 24, 2020. https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. Published online February 24, 2020. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020;14(1):69-71. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraft CS, Hewlett AL, Koepsell S, et al. ; Nebraska Biocontainment Unit and the Emory Serious Communicable Diseases Unit . The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis. 2015;61(4):496-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Griensven J, Edwards T, de Lamballerie X, et al. ; Ebola-Tx Consortium . Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florescu DF, Kalil AC, Hewlett AL, et al. Administration of brincidofovir and convalescent plasma in a patient with Ebola virus disease. Clin Infect Dis. 2015;61(6):969-973. [DOI] [PubMed] [Google Scholar]
- 9.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450-1451. [DOI] [PubMed] [Google Scholar]
- 10.Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnouf T, Radosevich M. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9(4):309. [PubMed] [Google Scholar]
- 12.Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Yang M, Shen C, et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. Preprint. medRxiv Preprint posted online February 17, 2020. doi: 10.1101/2020.02.11.20021493 [DOI]
- 14.Villar J, Blanco J, del Campo R, et al. ; Spanish Initiative for Epidemiology, Stratification & Therapies for ARDS (SIESTA) Network . Assessment of PaO₂/FiO₂ for stratification of patients with moderate and severe acute respiratory distress syndrome. BMJ Open. 2015;5(3):e006812. doi: 10.1136/bmjopen-2014-006812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Yang Y, Gao Y, et al. Viral architecture of SARS-CoV-2 with post-fusion spike revealed by Cryo-EM. bioRxiv Preprint posted online March 5, 2020. doi: 10.1101/2020.03.02.972927 [DOI]
- 16.Yeh KM, Chiueh TS, Siu LK, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56(5):919-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. ; Convalescent Plasma Study Group . The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng KT, Oong XY, Lim SH, et al. Viral load and sequence analysis reveal the symptom severity, diversity, and transmission clusters of rhinovirus infections. Clin Infect Dis. 2018;67(2):261-268. [DOI] [PubMed] [Google Scholar]
- 19.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen C, Chen J, Li R, et al. A multimechanistic antibody targeting the receptor binding site potently cross-protects against influenza B viruses. Sci Transl Med. 2017;9(412):eaam5752. [DOI] [PubMed] [Google Scholar]
- 21.Shen C, Zhang M, Chen Y, et al. An IgM antibody targeting the receptor binding site of influenza B blocks viral infection with great breadth and potency. Theranostics. 2019;9(1):210-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. bioRxiv Preprint posted online March 12, 2020. doi: 10.1101/2020.03.11.987958 [DOI]
- 23.Lu CL, Murakowski DK, Bournazos S, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352(6288):1001-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Variation of IgM, IgG ELISA titers and NAT before and after convalescent plasma transfusion in recipients.
eFigure 1. Computed tomography (CT) scan of the patient 1 (A) before and (B) 3 days-post convalescent plasma transfusion.
eFigure 2. Computed tomography (CT) scan of the patient 2 (A) before and (B) 3 days-post convalescent plasma transfusion.
eFigure 3. Computed tomography (CT) scan of the patient 3 (A) before and (B) 3 days-post convalescent plasma transfusion.
eFigure 4. Computed tomography (CT) scan of the patient 4 (A) before and (B) 3 days-post convalescent plasma transfusion.
eFigure 5. Computed tomography (CT) scan of the patient 5 (A) before and (B) 3 days-post convalescent plasma transfusion.