Abstract
Young infants with measles requiring respiratory support have a significant risk for death and long-term complications. Even in developed countries, the occurrence of spontaneous air-leaks and acute respiratory distress syndrome (ARDS) still represent the most severe clinical presentation in early childhood, with a high fatality rate. A clinical series review from a tertiary university paediatric intensive care unit (PICU) was undertaken. During the 2006–2007 outbreak in Rome, Italy, a young infant presented with ARDS/spontaneous air-leak and needed aggressive ventilatory management and haemodynamic support. Both nebulised iloprost and intravenous pentoxifylline were administered during the acute hypoxaemic phase; the role of this pharmacologic approach in critically ill patients is still under debate. We observed four further cases of respiratory impairment requiring a non-invasive approach. Clinical-radiological findings ranged from interstitial pneumonia to bronchiolitis-like pictures. All patients were imported cases, representing an important epidemiological factor and future medical issue, though they were not malnourished nor affected by chronic diseases. We conclude that early respiratory assessment and timely PICU referral is of mainstem importance in the youngest infants with measles-induced respiratory failure. The protean nature of clinical presentation and the possibility of rapid respiratory deterioration should be highlighted, and infants from immigrant families may represent a susceptible high-risk group.
Keywords: Continuous Positive Airway Pressure, Measle, Acute Respiratory Distress Syndrome, Paediatric Intensive Care Unit, Pentoxifylline
Introduction
Measles continues to be a menace to millions of children worldwide. Recently, the World Health Organization (WHO) has set 2010 as the target for the elimination in the European region; notwithstanding, a measles resurgence has been reported by several countries [1] with some localised outbreaks: overcrowding and viral susceptibility appear to play a crucial role. In younger infants, measles remains a serious threat, occasionally presenting with secondary infections and/or respiratory, neurologic and cardiovascular disturbances [1, 2]. Pneumonia accounts for most lethal cases [1, 2]: it is reported in about 50% of hospitalised cases, potentially leading to acute respiratory distress syndrome (ARDS) [2, 3]. Spontaneous air-leak has been reported in 0.5 to 2.4% of hospitalised children in the 1980s [2]. We present an unusual case of an infant with severe respiratory failure due to measles and four other infants with pulmonary and extra-pulmonary manifestations.
Illustrative cases
CR #1
A 5-month-old Rom-Sinti male was admitted to our paediatric intensive care unit (PICU) because of respiratory distress. He had been well until 3 days before admission, when he presented with cough, fever, poor appetite and dyspnoea. He was born at term from an uncontrolled pregnancy, with a family history of tubercular pulmonary disease (mother), and a 3-year-old sister was previously admitted for a mycobacterium tuberculosis (MTB) meningitis. Clinical assessment revealed dry cough and coryza, hyperpnoea and dyspnoea; rales and rhonchi were audible on both hemithoraces. Meningeal signs were negative. No typical rash with conjunctivitis nor Koplik’s spots were present. His body temperature was 39.5°C, heart rate (HR) 185/min, respiratory rate (RR) 65/min and blood pressure (BP) 79/38 mmHg. A chest radiograph on the first day revealed multiple opacities and diffuse reticulonodular infiltrates of bilateral lungs (Fig. 1a). Room-air arterial blood gas (ABG) showed partial pressure of oxygen (PaO2) 41 mmHg, partial pressure of carbon dioxide (PaCO2) 39 mmHg, pH 7.345 and BE −6. Laboratory studies revealed haemoglobin (Hb) 7.7 g/dL and white blood cells (WBC) count 6,900/μL (80% neutrophils, 14% lymphocytes, 6% monocytes). Both the platelet count (PLT) and coagulation test were within normal limits; C-reactive protein (CRP) peaked at 46.5 mg/L (n.v. <3 mcg/L) at 48 h. His blood chemistry was unremarkable except for alanine aminotransferase (ALT) 68 UI/L, aspartate aminotransferase (AST) 25 UI/L and lactate dehydrogenase (LDH) 2,417 UI/L. Intravenous ceftriaxone and clarithromycin were given. Hypotension and hypoxaemia rapidly developed, requiring fluid support and increased oxygen administration. On the subsequent day, he developed a subcutaneous emphysaema, the chest film, thereby, also revealed pneumopericardium, pneumomediastinum and massive lung consolidation (Fig. 1b). He underwent tracheal intubation and needed further inotropic support; severe ARDS rapidly developed, while his oxygen need rapidly rose to 100% (Fig. 1c–d). On 2D-echocardiographic assessment, a good left ventricle (LV) function was documented, pulmonary artery pressure was slightly increased (45–50 mmHg) without right ventricle (RV) enlargement and inferior vena cava (IVC) dynamics was maintained. Recruitment manoeuvres and high-level positive end-expiratory pressure (PEEP) (up to 17 cmH2O) effectively improved oxygenation, achieving a FiO2 of 0.60. During the acute hypoxaemic phase, iloprost (1–1.5 mcg q3 h) was nebulised through an ultrasonic built-in device, while a pentoxifylline continuous infusion was set (2 mg/kg/h). Both the oxygenation index (OI = mean airway pressure × FiO2%/PaO2) and PaO2/FiO2 progressively improved; dynamic compliance increased from 0.35 to 0.48 mL/kg/cmH2O within 48 h (Fig. 2). A pleural drain was needed during the recovery phase, when spontaneous breathing was permitted (Fig. 1e–f). He was discharged after 20 days without further complications.
Fig. 1.
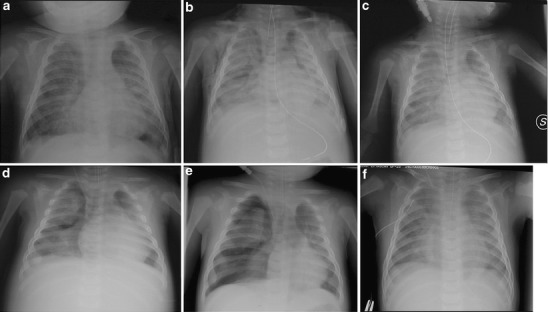
Radiological evolution of the acute respiratory distress syndrome (ARDS) and air-leak picture described in CR #1
Fig. 2.
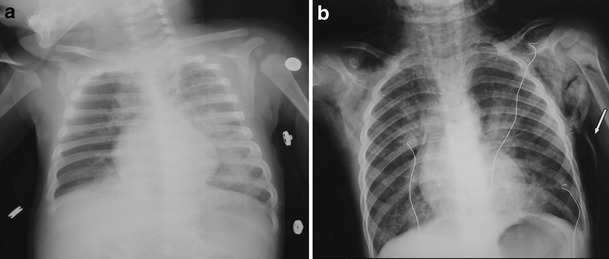
Chest X-rays of patients described in CR #2 (a) and CR #5 (b). The arrow indicates the subcutaneous air collection
CR #2
An 11-month old male (Rom-Sinti) was admitted to the PICU suffering from worsening respiratory distress. Parents reported feeding difficulties, decreased activity and cyanotic episodes from 2 days. On arrival, he presented with a comatose state, fever (39°C), dyspnoea, tachypnoea (RR 50/min), hypoxaemia (PaO2 50 mmHg) and hypotension (60/35 mmHg). His CRP was 120 mg/L. No cutaneous rash was appreciable. He underwent helmet continuous positive airway pressure (CPAP) and haemodynamic support; i.v. acyclovir and cefotaxime were introduced. Chest radiogram showed an interstitial pneumonia with left diffuse opacities (Fig. 3a); cerebral computed tomography (CT) scan was unremarkable, whereas bilateral slowing was detected by electroencephalography (EEG) tracing. Due to high-grade mucosal involvement, measles serology was performed (IgM-positive; IgG-negative). Blood, cerebro-spinal fluid (CSF) and urine cultures tested negative, as were CSF viral cultures and measles-specific antibodies. The child needed respiratory and haemodynamic support until day 5. Full respiratory and neurological recovery was obtained.
Fig. 3.
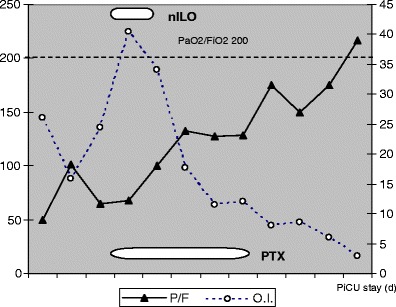
Trends of PaO2/FiO2 and oxygenation index (OI) values during the paediatric intensive care unit (PICU) stay of the infant described in CR #1 (PaO2/FiO2 ratio on the left axis, OI on the right axis; nebulised iloprost [nebILO] introduction and pentoxifylline [PTX] i.v. drip time course have been illustrated)
CR #3
A 4-month-old Filipino male presented with a history of cough and fever for 3 days, dyspnoea and poor feeding from 24 h. Physical examination showed adequate growth, rhinorrhoea, tachypnoea, chest retractions, wheezing and bibasilar rales. His temperature was 39.5°C, HR 160/min and RR 60/min. Oxygen saturation on room-air was 85%, PaO2 55 mmHg, PaCO2 42 mmHg, pH of 7.332 and BE −4. Bronchiolitis was supposed and fluids/oxygen supplementation was administered. His WBC count was 47,870/µL (32% neutrophils, 51% lymphocytes) and CRP 36 mg/L. Chest radiogram showed hyperinflation and peribronchial thickening. After 24 h, the respiratory distress increased, requiring 50% mask oxygen support. Nasopharyngeal aspirate tested negative for adenovirus, respiratory syncytial virus (RSV) and influenza–parainfluenza viruses. Two days after the admission, he developed conjunctivitis and a macular rash. Measles serology revealed IgM positivity; IgG titre subsequently increased. He was discharged after 10 days.
CR #4
A 15-month-old previously healthy Rom-Sinti female was admitted to the emergency department because of fever and respiratory distress. A 2-day history of non-purulent conjunctivitis, coryza, fever, rhinitis and sialorrhoea was reported. She had fever (38.5°C) and increasing dyspnoea (HR 160/min, RR 55/min, BP 90/42 mmHg). Room-air ABG revealed PaO2 31 mmHg, PaCO2 38 mmHg, pH 7.39 and BE −3. A chest radiograph showed interstitial and peribronchial flogosis, perihilar linear opacities and right upper lobe consolidation. Laboratory studies were: Hb 10.9 g/dL, WBC 4,130/μL (62% neutrophils, 31.7% lymphocytes, 6.1% monocytes), PLT 269,000/µL; coagulation test and CRP were within normal limits. Fluid support and 40% oxygen supplementation were started, together with i.v. amoxicillin and clarithromycin. Two days after the admission, she presented with maculopapular rash with cranial/caudal progression. Measles IgM serology tested positive. Early respiratory therapy achieved a significant clinical improvement within 1 week.
CR #5
A 3-year old boy from Nigeria was admitted to the PICU suffering from hyperpyrexia and severe wheezing. On admission, he was markedly hyperpneic (RR 50/min), tachycardic (150 bpm) and diaphoretic; crepitant rales were diffusely audible on chest auscultation. His physical assessment revealed conjunctival and pharyngeal hyperaemia and diarrhoea. Oxygen saturation on room-air was 82%: he was given mask CPAP (+5 cmH2O) using a starting FiO2 of 0.5 for 6 h from admission (subsequently, mask oxygen supplementation), whereas albuterol/ipratropium bromide were aerosolised every 2–3 h. An i.v. continuous aminophylline infusion was started at 0.8 mg/kg/hr after a 4 mg/kg load. The subsequent day, the dyspnoea was still present, with increased use of accessory respiratory muscles: a rapidly progressing mediastinal and subcutaneous emphysaema appeared (Fig. 2b, arrow). Arterial gas analysis showed an increased oxygen need (min. P/F ratio 180) and PaCO2 rose progressively to 68–70 mmHg. Tracheal intubation and invasive ventilation were avoided due to the significant air-leak: on the third day, a continuous i.v. albuterol infusion was added to control the diffuse wheezing and lung inflation. Despite a further heart rate increase (HR 170–175 bpm, even in normothermia), the hypercapnoea decreased and the respiratory status steadily improved. Serological test showed a positive measles IgM. Chest X-rays revealed the persistence of interstitial process, with a progressive reabsorption of the subcutaneous air: he could be discharged from the PICU on day 9. Immune-deficit tests and human immunodeficiency virus (HIV), Epstein–Barr virus (EBV) and cytomegalovirus (CMV) serology were negative.
Discussion
Measles remains a common infectious disease worldwide, despite the availability of effective vaccines [1]. Measles-induced respiratory failure is infrequently seen in Italian PICUs, with some recent increase due to the presence of measles-susceptible infants in the general population. We report our 2-year experience, illustrating the persisting role of measles virus as an agent of severe—and possibly lethal—respiratory illness. We describe both a severe ARDS picture and air-leak syndromes that required a global therapeutic and intensive care approach. Approximately 5% of children with typical measles clinically manifest pneumonia and ARDS is the most severe complication [2, 4], as confirmed by reports in the last two decades.
In Italy, measles vaccine was first introduced in 1979 and combined measles–mumps–rubella (MMR) vaccines became available in the early 1990s. Measles hospitalisations in Italy in 2002–2003 mainly occurred in the central and southern regions, reflecting the different regional vaccination strategies. Epidemiological evidence shows that a higher proportion of children susceptible to measles can be found in regions with coverage rates below 70% [5–7]. Recently, coverage rates in children by 2 years of age have been improving across Italy, and, in 2005, reached 87%; for this reason, measles hospitalisation became more frequent among immigrants or Rom people [8], according to our observations. For this reason, without any intent to differentiate patients by ethnicity, the patients’ origins have been investigated in the case illustrations.
In a 1993 review, infants with measles pneumonia and hypoxaemic acute respiratory failure (ARF) had a 56% mortality. Interestingly, an oxygenation index >40 separated survivors from non-survivors [9]. Also, in the 1990s, infants with measles and severe ARF yet presented a mortality rate exceeding 50% [1, 2, 9]. In the Dublin measles outbreak in 2000, 11.7% of the 111 hospitalised children required ICU admission: seven of them underwent mechanical ventilation, with three fatalities [10]. Air-leak syndromes have been associated to measles-induced lung involvement both in the acute phase and during recovery. It appears that the coexistence of severe ARDS and major air-leaks denotes a poor prognosis. In a 1995 series, all children with both air-leak and ARDS died, despite airway pressure limitation policy and modern ICU care [2].
Traditional therapeutic approach to measles pneumonia consisted of supportive measures, such as supplementary oxygen and parenteral fluids. Systemic steroids and vitamin A supplementation have been suggested for severe cases [4], but their value is uncertain. At our institution, antiviral therapy (e.g. ribavirin) is not routinely adopted, except for immunosuppressed or haemato-oncologic patients. Antimicrobial therapy has been prophylactically administered in high-risk patients [1]. Severe ARDS often requires an aggressive ventilatory setting and full intensive care support. Iatrogenic lung injury can be minimised by a “lung-protective” strategy (including pressure limitation and permissive hypercapnoea), aiming at early FiO2 limitation to decrease lung inflammatory and oxidative phenomena [11]. Pharmacological adjunctive therapies (such as iloprost nebulisation and pentoxifylline infusion) [11, 12] or inhaled nitric oxide [11, 13] may have a role in the acute hypoxaemic phase to prevent further clinical deterioration. As for iNO, the effect of nebILO relates to its selective vasodilating ability, i.e. vasodilation of ventilated vs. non-ventilated lung areas, partly correcting the ventilation/perfusion mismatch (induced by both diffuse atelectasis and exudative phenomena of ARDS) and reducing intrapulmonary shunt blood flow [14–17]. Similarly, there is a considerable body of evidence on the clinical synergistic effect of PTX and related compounds such as anti-inflammatory and anti-oedema agents [18–20].
Milder forms of hypoxaemic respiratory distress can be non-invasively managed by helmet CPAP, even in young infants who frequently do not tolerate mask- or nasal-CPAP. This technique can help to clear diffuse atelectatic areas and to prevent worsening hypoxia and respiratory muscle fatigue [21, 22].
As illustrated by our small series, measles respiratory involvement can present under several different pictures and the clinical outcome is often unpredictable. Although severe pneumonia/ARF represents a rare complication, it still largely accounts for the mortality from this preventable disease [1, 4, 10]. We recommend that infants with increasing oxygen need should be monitored in a intensive–subintensive setting due to the risk of rapid deterioration.
This report suggests the importance of informing parents and physicians of the life-threatening complications of measles, enhancing efforts to protect children in those settings where immunisation rates have fallen so strikingly.
References
- 1.Asaria P, MacMahon E. Measles in the United Kingdom: can we eradicate it by 2010? BMJ. 2006;333:890–895. doi: 10.1136/bmj.38989.445845.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abramson O, Dagan R, Tal A, Sofer S. Severe complications of measles requiring intensive care in infants and young children. Arch Pediatr Adolesc Med. 1995;149:1237–1240. doi: 10.1001/archpedi.1995.02170240055008. [DOI] [PubMed] [Google Scholar]
- 3.Freebeck PC, Clark S, Fahey PJ. Hypoxemic respiratory failure complicating nosocomial measles in a healthy host. Chest. 1992;102:625–626. doi: 10.1378/chest.102.2.625. [DOI] [PubMed] [Google Scholar]
- 4.Rupp ME, Schwartz ML, Bechard DE. Measles pneumonia. Treatment of a near-fatal case with corticosteroids and vitamin A. Chest. 1993;103:1625–1626. doi: 10.1378/chest.103.5.1625. [DOI] [PubMed] [Google Scholar]
- 5.Filia A, Brenna A, Panà A, Cavallaro GM, Massari M, Ciofi degli Atti ML. Health burden and economic impact of measles-related hospitalizations in Italy in 2002–2003. BMC Public Health. 2007;7:169. doi: 10.1186/1471-2458-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciofi degli Atti ML, D’Argenio P, di Giorgio G, Filonzi A, Grandori L. Measles in Italy 2002: studies show correlation between vaccine coverage and incidence. EuroSurveill Weekly. 2002;6:021205. [Google Scholar]
- 7.Salmaso S, Rota MC, Ciofi degli Atti ML, Tozzi AE, Kreidl P, ICONA Study Group Infant immunization coverage in Italy: estimates by simultaneous EPI cluster surveys of regions. Bull World Health Organ. 1999;77(10):843–851. [PMC free article] [PubMed] [Google Scholar]
- 8.Filia A, Curtale F, Kreidl P, Morosetti G, Nicoletti L, Perrelli F, Mantovani J, Campus D, Rossi G, Sanna MC, Zanetti A, Magurano F, Fortuna C, Iannazzo S, Pompa MG, Ciofi degli Atti M. Cluster of measles cases in the Roma/Sinti population, Italy, June–September 2006. EuroSurveill Weekly. 2006;11(10):E061012.2. doi: 10.2807/esw.11.41.03062-en. [DOI] [PubMed] [Google Scholar]
- 9.Swift JD, Barruga MC, Perkin RM, van Stralen D. Respiratory failure complicating rubeola. Chest. 1993;104:1786–1787. doi: 10.1378/chest.104.6.1786. [DOI] [PubMed] [Google Scholar]
- 10.McBrien J, Murphy J, Gill D, Cronin M, O’Donovan C, Cafferkey MT. Measles outbreak in Dublin, 2000. Pediatr Infect Dis J. 2003;22:580–584. doi: 10.1097/00006454-200307000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Turner DA, Arnold JH. Insights in pediatric ventilation: timing of intubation, ventilatory strategies, and weaning. Curr Opin Crit Care. 2007;13:57–63. doi: 10.1097/MCC.0b013e32801297f9. [DOI] [PubMed] [Google Scholar]
- 12.Harris KW, O’Riordan TG, Smaldone GC. Aerosolized iloprost customized for the critically ill. Respir Care. 2007;52(11):1507–1509. [PubMed] [Google Scholar]
- 13.Kimura A, Kobayashi J, Fawaz M, Masutani S, Nagasaka H, Ohtake A, Sasaki N. Measles pneumonia: treatment of a near-fatal case with nitric oxide inhalation. Pediatr Int. 2002;44(4):451–452. doi: 10.1046/j.1442-200X.2002.01570.x. [DOI] [PubMed] [Google Scholar]
- 14.Wiedemann HP, Arroliga AC, Komara JJ., Jr Emerging systemic pharmacologic approaches in acute respiratory distress syndrome. Respir Care Clin N Am. 2003;9(4):419–435. doi: 10.1016/S1078-5337(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 15.Adhikari N, Burns KE, Meade MO (2004) Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev (4):CD004477 [DOI] [PMC free article] [PubMed]
- 16.Schuster KM, Alouidor R, Barquist ES. Nonventilatory interventions in the acute respiratory distress syndrome. J Intensive Care Med. 2008;23:19–32. doi: 10.1177/0885066607310166. [DOI] [PubMed] [Google Scholar]
- 17.Dahlem P, van Aalderen WMC, Bos AP. Pediatric acute lung injury. Paediatr Respir Rev. 2007;8:348–362. doi: 10.1016/j.prrv.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Krakauer T. Pentoxifylline inhibits ICAM-1 expression and chemokine production induced by proinflammatory cytokines in human pulmonary epithelial cells. Immunopharmacology. 2000;46(3):253–261. doi: 10.1016/S0162-3109(99)00186-1. [DOI] [PubMed] [Google Scholar]
- 19.Schermuly RT, Krupnik E, Tenor H, Schudt C, Weissmann N, Rose F, Grimminger F, Seeger W, Walmrath D, Ghofrani HA. Coaerosolization of phosphodiesterase inhibitors markedly enhances the pulmonary vasodilatory response to inhaled iloprost in experimental pulmonary hypertension. Maintenance of lung selectivity. Am J Respir Crit Care Med. 2001;164(9):1694–1700. doi: 10.1164/ajrccm.164.9.2105060. [DOI] [PubMed] [Google Scholar]
- 20.Schermuly RT, Leuchte H, Ghofrani HA, Weissmann N, Rose F, Kohstall M, Olschewski H, Schudt C, Grimminger F, Seeger W, Walmrath D. Zardaverine and aerosolised iloprost in a model of acute respiratory failure. Eur Resp J. 2003;22:342–347. doi: 10.1183/09031936.03.00093802. [DOI] [PubMed] [Google Scholar]
- 21.Thia LP, McKenzie SA, Blyth TP, Minasian CC, Kozlowska WJ, Carr SB. Randomised controlled trial of nasal continuous positive airways pressure (CPAP) in bronchiolitis. Arch Dis Child. 2008;93(1):45–47. doi: 10.1136/adc.2005.091231. [DOI] [PubMed] [Google Scholar]
- 22.Essouri S, Chevret L, Durand P, Haas V, Fauroux B, Devictor D. Noninvasive positive pressure ventilation: five years of experience in a pediatric intensive care unit. Pediatr Crit Care Med. 2006;7(4):329–334. doi: 10.1097/01.PCC.0000225089.21176.0B. [DOI] [PubMed] [Google Scholar]


