Abstract
The innate immune response is important in paraquat-induced acute lung injury, but the exact pathways involved are not elucidated. The objectives of this study were to determine the specific role of the NLRP3 inflammasome in the process. Acute lung injury was induced by administering paraquat (PQ) intraperitoneally. NLRP3 inflammasome including NLRP3, ASC, and caspase-1 mRNA and protein expression in lung tissue and IL-1β and IL-18 levels in BALF were detected at 4, 8, 24, and 72 h after PQ administration in rats. Moreover, rats were pretreated with 10, 30, and 50 mg/kg NLRP3 inflammasome blocker glybenclamide, respectively, 1 h before PQ exposure. At 72 h after PQ administration, lung histopathology changes, NLRP3, ASC, and caspase-1 protein expression, as well as secretion of cytokines including IL-1β and IL-18 in BALF were investigated. The NLRP3 inflammasome including NLRP3, ASC, caspase-1 expression, and cytokines IL-1β and IL-18 levels in PQ poisoning rats were significantly higher than that in the control group. NLRP3 inflammasome blocker glybenclamide pretreatment attenuated lung edema, inhibited the NLRP3, ASC, and caspase-1 activation, and reduced IL-1β and IL-18 levels in BALF. In the in vitro experiments, IL-1β and IL-18 secreted from RAW264.7 mouse macrophages treated with paraquat were attenuated by glybenclamide. In conclusion, paraquat can induce IL-1β/IL-18 secretion via NLRP3-ASC-caspase-1 pathway, and the NLRP3 inflammasome is essential for paraquat-induced acute lung injury.
KEY WORDS: paraquat, acute lung injury, NLRP3 inflammasome, toxicity
INTRODUCTION
Paraquat (1, 1’-dimethyl-4, 4’-bipyridinium dichloride (PQ)), as a highly effective, fast-acting, and nonselective herbicide, is widely used in the world, especially in agricultural countries. It is highly toxic to humans and animals, accumulating in the lungs, liver, and kidneys. An estimated of 250,000 to 370,000 people die globally from pesticide poisoning each year, and more than 90 % of the patients with acute poisoning have attempted suicide by ingesting PQ [1]. The very high case fatality of PQ is due both to its inherent toxicity and the lack of any effective treatment. The most common cause of death from PQ poisoning is ARDS, the most severe form of ALI, due to an oxidative redox cycling and subsequently inflammation. It is pathologically observed that pulmonary edema, bronchial and alveolar destruction, and ultimately fibrosis in the lungs after PQ poisoning.
It is well known that the oxidative damage is one of the initial factors to the toxic effects of PQ. PQ undergoes the redox cycling process and generates a great amount of reactive oxygen species (ROS) [2]. Production of ROS is crucial to the regulation of innate immune responses. ROS regulates activation of redox-regulated transcription factors (nuclear factor–κB (NF–κB) and AP-1) and cytokines production [3, 4], finally causes inflammation and destruction of lung tissue structure. Therefore, inflammation is one of the core contributors to acute lung injury induced by PQ.
“Inflammasomes” are intracellular macromolecular complexes that serve as platforms for the activation of the proinflammatory caspase-1, which in turn cleaves IL-1β and IL-18 from their respective proforms [5]. These inflammasome-activated cytokines belong to the IL-1 cytokine family [6] and play central roles in the propagation of the acute inflammatory response. The NLRP3 inflammasome, one of the inflammasome complexes, has a basic structure consisting of nucleotide-binding-domain, leucine-rich repeat domain containing protein (NLRP), and the adaptor protein ASC (apoptosis-associated speck-like protein containing caspase-1 activator domain), which recruit and activate procaspase-1 [7]. The NLRP3 inflammasome is expressed in many inflammatory cells, including macrophages and neutrophils, and also activated by many factors including environmental irritants, endogenous danger signals, pathogens, and distinct pathogen-associated molecular patterns (PAMPs) [8, 9]. Moreover, it is proposed that a lot of the cellular signals are responsible for NLRP3 activation, including potassium (K+) efflux, pore formation in cell membranes, lyposomes damage, the elevation of ROS, and damage in the mitochondria [9–11].
In previous studies [2, 12, 13], ROS and cytokines including interleukin-1β (IL-1β) were involved in the pathogenesis of PQ-induced lung damage. However, there are no reports that whether the NLRP3 inflammasome is involved in the development of PQ poisoning-induced acute lung injury. In this current study, our objective was to test the hypothesis that NLRP3 inflammasome signaling was required for acute lung injury induced by PQ.
MATERIALS AND METHODS
Animals
Adult male Sprague–Dawley rats weighing 200–250 g were purchased from the Experimental Animal Center of China Medical University. Rats were housed in cages in a temperature- (20–25 °C) and humidity-controlled (40 %–70 %) environment with a daily light–dark cycle. The rats had access to food and water ad libitum. All experimental procedures were conducted in accordance with the Institutional Animal Ethics Committee and Animal Care Guidelines of China Medical University governing the use of experimental animals.
Experimental Design
Two sets of experiments were performed in vivo. In the first set of experiments, 48 rats were randomly distributed to two groups: control (1 mL normal saline solution, i.p. n = 24) and PQ poisoning group (n = 24). PQ (Sigma-Aldrich, MO, USA) was intraperitoneally administered at a dose of 20 mg/kg according to our previous research [13]. At 4, 8, 24, and 72 h after PQ administration, six rats were taken from each group at each endpoint for testing IL-1β and IL-18 levels in rats BALF, lung pathology, and NLRP3 inflammasome mRNA and protein expression. Glybenclamide has previously been used to block the NLRP3 inflammasome [14]. Therefore, in the second set of experiments, to study the NLRP3 inflammasome effect, the NLRP3 inflammasome blocker glybenclamide (Sigma-Aldrich, MO, USA) (i.p) at the dose of 10 mg/kg (Glyben-L, n = 6/group), 30 mg/kg (Glyben-M, n = 6/group) and 50 mg/kg (Glyben-H, n = 6/group) were pretreated respectively 1 h before PQ administration in rats. At 72 h after PQ administration, rats were anesthetized, then BALF was collected from the left lung for cytokines detection, and the right lung was excised for oxidase detection, histopathology, and Western blot analysis.
BALF Collection
After rats were euthanized, BALF was harvested from the left lung with 4 mL phosphate-balanced saline solution in 2.5 mL aliquots after cannulation of the left trachea. The collected BALF was centrifuged at 1,000 g for 10 min; the supernatant was collected and stored at −80 °C, for later cytokine measurements.
IL-1β and IL-18 Elisa Assays
The levels of IL-1β and IL-18 in the rats BALF were measured via Elisa assays using commercially available kits (R&D Systems, USA) according to the manufacturer’s recommended instructions. The levels of IL-1β and IL-18 in the samples were calculated based on a standard curve. The detection ranges of the IL-1β and IL-18 Elisa assays were 7.8–500 and 5–160 pg/ml, respectively. Samples that had a concentration that exceeded the limit of the standard curve were measured after dilution.
MDA Levels and MPO Activity Assays
MDA levels and Myeloperoxidase (MPO) activity in the lung tissue were spectrophotometrically assayed using assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). At 4, 8, 24, and 72 h after PQ treatment, rats were killed, and the left lungs were excised. One hundred milligrams of lung tissue was homogenized and fluidized in extraction buffer to obtain 5 % of the homogenate. The sample including 0.9 ml homogenate and 0.1 ml of reaction buffer was heated to 37 °C in a water bath for 15 min, and then the enzymatic activity was determined by measuring the changes in absorbance at 460 nm using a 96-well plate reader and expressed as units per gram weight. Both detective procedures were performed according to the manufacturer’s recommended instructions.
NLRP3, ASC, and Caspase-1 mRNA Expression Analysis
Complementary DNA synthesis from rats lungs RNA was performed by a reverse transcription reaction using oligo dT (Invitrogen, Grand Island, NY) and Moloney rat leukemia virus reverse transcriptase (Invitrogen). Quantitative polymerase chain reactions were performed using light Cycler SYBR green I master mix (Roche, Mijdrecht, The Netherlands) and measured in a Light Cycler 480 (Roche) apparatus using the following conditions—5 min 95 °C hot-start, followed by 40 cycles of amplification (95 °C for 10 s, 60 °C for 5 s, 72 °C for 15 s). For quantification, standard curves were constructed by polymerase chain reactions on serial dilutions of a concentrated complementary DNA sample, and data were analyzed using Light Cycler software. Gene expression was presented as a ratio of the expression to the housekeeping gene GAPDH for rat analysis. The following rat primer sequences were used: NLRP3 forward primer 5′-GCTAAGAAGGACCAGCCAGA-3′ and reverse primer 5′-CCAGCAAACCTATCCACTCC-3′; ASC forward primer 5′-TTGCTGGATGCTCTGTATGG-3′ and reverse primer 5′-CCAAGTAGGGCTGTGTTTGC-3′; Caspase-1 forward primer 5′-AGATGCCAACCACTGAAAGG-3′ and reverse primer 5′-GCATGATTCCCAACACAGGT-3′; GAPDH forward primer 5′-GAACATCATCCCTGCATCCA-3′ and reverse primer 5′-CCAGTGAGCTTCCCGTTCA-3′.
HE and Immunohistochemistry
Rat lung tissue samples were fixed in 4 % paraformaldehyde for 48 h at 4 °C and processed for paraffin embedding. Paraffin-embedded blocks were cut into 4 μm thick sections and mounted onto slides. Some sections were stained with hematoxylin and eosin and analyzed by a pathologist who was blinded for group identity. The lung injury was scored according to a histology scoring system [15]. Four pathologic parameters were scored on a scale of 0–4: (1) alveolar congestion, (2) hemorrhage, (3) leukocyte infiltration, and (4) thickness of alveolar wall/hyaline membranes. A score of 0 represents normal lungs; 1, mild, <25 % lung involvement; 2, moderate, 25–50 % lung involvement; 3, severe, 50–75 % lung involvement and 4, very severe, >75 % lung involvement. The total histology score was expressed as the sum of the score for all parameters.
The rest sections preparing for immunohistochemistry were pretreated at 60 °C for 1 h, then dewaxed in xylene, hydrated, and washed in 0.01 mol/L of citrate buffer. After inhibiting endogenous peroxidase using 3 % H2O2 in methanol, the sections were incubated with anti-caspase-1 polyclonal antibody (Santa Cruz, CA, USA) overnight at 4 °C. The sections were then thoroughly washed with a phosphate-buffered saline solution, after which point the corresponding secondary antibodies were applied and incubated at room temperature for 30 min. Reaction products were visualized following incubation with diaminobenzidine and then counterstained with hematoxylin. Negative controls were generated by omitting the primary antibodies.
Western Blot Analysis
Rat lung tissue samples were harvested and frozen in liquid nitrogen immediately until homogenization. Proteins were extracted from the lungs using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Biotechnology, China) according to the manufacturer’s protocol. To extract the total protein from lung tissue, protein concentrations were determined by BCA protein assay kit and equal amounts of protein were loaded per well on a 10 % sodium dodecyl sulfate polyacrylamide gel. Subsequently, proteins were transferred onto polyvinylidene difluoride membrane. The membranes were washed in Tris-buffered saline with Tween 20 and incubated in 5 % skim milk (Sigma) at room temperature for 2 h on a rotary shaker and followed by TBS-T washing. Incubations with rabbit polyclonal antibodies specific for NLRP3, ASC, and caspase-1 in diluent buffer (5 % skim milk in TBS-T) were performed overnight at 4 °C. Then the membrane was washed with TBS-T followed by incubation with the peroxidase-conjugated secondary antibody at room temperature for 1 h. Immunoreactive bands were visualized with an enhanced chemiluminescence Western blot kit in accordance with the manufacturer’s instructions. The β-actin Western blot was performed as the internal control of protein loading. The signals were detected with an enhanced chemiluminescence kit (Pierce) and exposed on X-ray film. After the film was scanned with a GS-700 imaging densitometer (Bio-Rad, Hercules, CA), a quantitative analysis was performed using Multi-Analyst software (Bio-Rad).
Cell Culture
RAW264.7 mouse macrophage was obtained from Cell bank, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, Shanghai, China. Cells were cultured in 10 cm plates and maintained in high-glucose Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) supplemented with 10 % fetal bovine serum (Gibco), 2 mM glutamine, and 1 % streptomycin/penicillin in a 37 °C humidified incubator with 5 % CO2. The medium was changed every 2 days.
MTT Assay for Cell Viability
The cytotoxic effects of paraquat to RAW264.7 mouse macrophages were assessed using the 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. The RAW264.7 mouse macrophages were seeded into 96-well plates (5 × 104 cells per well in 100 μL of medium). The cells were exposed to different concentrations (0, 10, 50, 100, 500, and 1,000 μM) of paraquat and incubated for 24 and 48 h after the cells were adhered. At the end of the incubation period, 10 μL of 0.5 mg/mL MTT was added to each well. After incubation for an additional 4 h in a 37 °C humidified incubator, the supernatant was discarded, and 150 μL of DMSO was added. After the formazan product was dissolved, the absorbance was measured at 570 nm, using a Bio-Tek MQX 680 (Bio-Tek Instruments Inc., Winooski, VT, USA).
Quantitative Analysis of IL-1β and IL-18 Production in Macrophage Supernatants after NLRP3 Inflammasome Inhibition
RAW264.7 mouse macrophages (1 × 105 cells/ml) in 96-well plates were treated with 100 μM PQ, and NLRP3 inflammasome blocker glybenclamide was individually preincubated with macrophages at different concentrations (0, 10, 50, and 200 μM) 30 min before PQ treatment to verify the inhibition. At 24 h later, the levels of IL-1β and IL-18 in macrophage supernatants were measured via Elisa assays prescribed above in vivo.
Statistical Analyses
The data were expressed as the mean ± SD. Statistical analyses were carried out using SPSS 16.0. Independent sample t test was used to compare means between the PQ poisoning group and control group. One-way ANOVA followed by the Student–Newman–Keuls test was used to compare the results that were obtained in glybenclamide pretreatment groups with different doses. P < 0.05 was considered to be statistically significant.
RESULTS
PQ Increased the Levels of IL-1β and IL-18 in Rats BALF
ELISA was used to analyze the production of IL-1β and IL-18 in the rats BALF at 4, 8, 24, and 72 h after PQ administration. As shown in Fig. 1a and b, the levels of IL-1β and IL-18 in the PQ poisoning rats BALF increased obviously compared with the control (*P < 0.05).
Fig. 1.
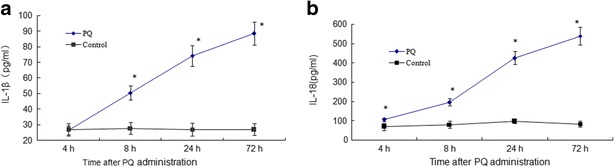
Levels of IL-1β and IL-18 in the rats BALF at 4, 8, 24, and 72 h after PQ administration. Both of them in the PQ poisoning rats BALF increased obviously compared with the control. The values presented are the mean ± SD (n = 6/group). *P < 0.05 versus control group.
PQ Increased MDA Levels and MPO Activity in Rats Lungs
MDA levels and MPO activity in rats lungs were significantly higher in the PQ group than those in the control group (*P < 0.05), as shown in Fig. 2a and b. Both of them were obviously elevated time-dependently.
Fig. 2.
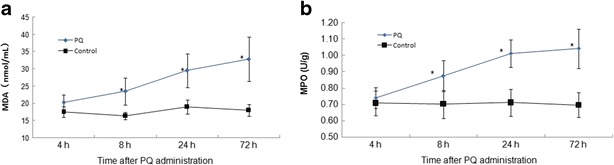
MDA levels and MPO activity in rat lungs at 4, 8, 24, and 72 h after PQ administration. Both of them in the PQ poisoning rats lungs increased significantly compared with the control. The values presented are the mean ± SD (n = 6/group). *P < 0.05 versus control group.
PQ Resulted in Relative mRNA Expression and Protein Levels of NLRP3, ASC, and Caspase-1 in Rats Lungs
As shown in Fig. 3a, b, and c, the relative ASC, NLRP3, and caspase-1 mRNA expression levels were increased time-dependently and were 2.7-, 3.8-, and 2.7-fold, respectively, compared with the control at 72 h. As shown in Fig. 3d and e, NLRP3 protein levels increased time-dependently in PQ poisoning rats lungs compared with the control (*P < 0.05). Protein levels of ASC and caspase-1 did not significantly change at 4 h, but, after 8 h, both of them increased compared with the control (*P < 0.05).
Fig. 3.
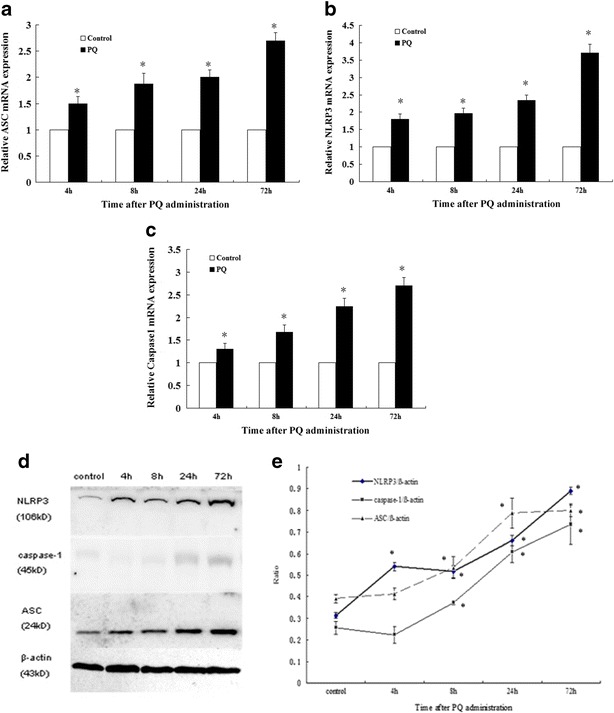
The mRNA expression (a, b, and c) and protein (d and e) levels of NLRP3, ASC, and caspase-1 in rats lungs at 4, 8, 24, and 72 h after PQ administration. The values presented are the mean ± SD (n = 6/group). *P < 0.05 versus control group.
ALI Induced by PQ Is Partly Attenuated by NLRP3 Inflammasome Blocker Glybenclamide in Rats
As shown in Fig. 4a and b, the lungs of rats that were exposed to PQ for 72 h displayed significant inflammatory alterations that were characterized by lung edema, alveolar hemorrhage, inflammatory cell infiltration, and destruction of epithelial and endothelial cell structure. Pretreatment with glybenclamide relieved markedly many histopathological changes of PQ-induced acute lung injury that were outlined above.
Fig. 4.
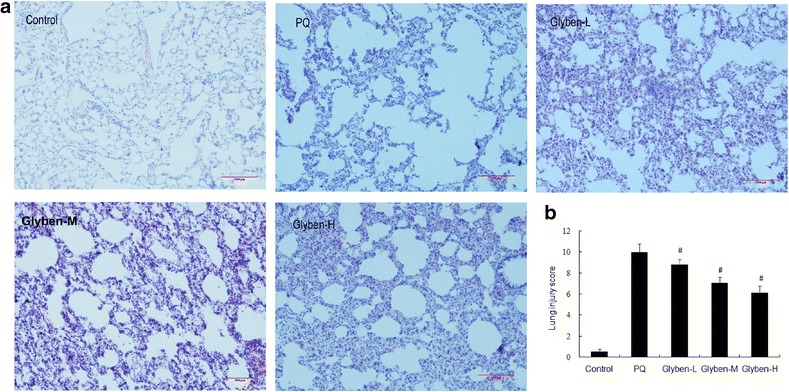
Histopathological changes (a and b) in lung tissues of paraquat-induced acute lung injury rats after glybenclamide pretreatment. Glybenclamide was intraperitoneally administered 1 h before intraperitoneal administration of PQ. Lung tissue was collected and lung histological evaluation was determined after PQ administration for 72 h. Glyben-L: glybenclamide at the low dose of 10 mg/kg, Glyben-M: glybenclamide at the middle dose of 30 mg/kg, Glyben-H: glybenclamide at the high dose of 50 mg/kg. The values presented are the mean ± SD (n = 6/group). #P < 0.05 versus PQ group.
MPO, But Not MDA in Rats Lungs Was Attenuated by Glybenclamide
As shown in Fig. 5a and b, the MPO in rats lungs was decreased when pretreated with middle and high dose of glybenclamide (#P < 0.05). Although there was a downward trend in MDA levels, it was not statistically significant compared with the PQ group (P > 0.05).
Fig. 5.
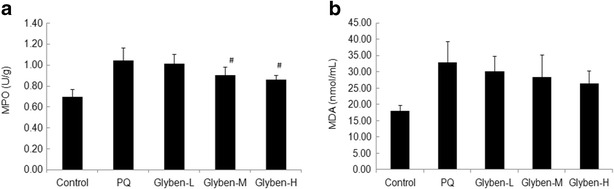
MPO activity and MDA levels (a and b) in lung tissues of paraquat-induced acute lung injury rats after glybenclamide pretreatment. Glybenclamide was intraperitoneally administered 1 h before intraperitoneal administration of PQ. Lung tissue was collected and lung histological evaluation was determined after PQ administration for 72 h. Glyben-L: glybenclamide at the low dose of 10 mg/kg, Glyben-M: glybenclamide at the middle dose of 30 mg/kg, Glyben-H: glybenclamide at the high dose of 50 mg/kg. The values presented are the mean ± SD (n = 6/group). #P < 0.05 versus PQ group.
Caspase-1 Activation Was Inhibited by Glybenclamide in ALI, Including NLRP3 and ASC
Caspase-1 localization in the lung tissues was illustrated in Fig. 6a. In the control group, caspase-1-positive signals were weakly detected in the lung tissues. At 72 h after PQ administration, caspase-1 positive signals were observed to be obviously increased in the lung tissues. Notably, caspase-1-positive signals in the lung tissues were significantly reduced by pretreatment with glybenclamide. The results of the Western blot analyses were illustrated in Fig. 6b. The PQ-poisoning group displayed increased caspase-1 expression compared with the control group. Glybenclamide pretreatment reduced caspase-1 protein expression in the lung tissues significantly (#P < 0.05). Meanwhile, the expression of NLRP3 and ASC protein was reduced when pretreated with glybenclamide at the dose of 30 and 50 mg/kg (#P < 0.05).
Fig. 6.
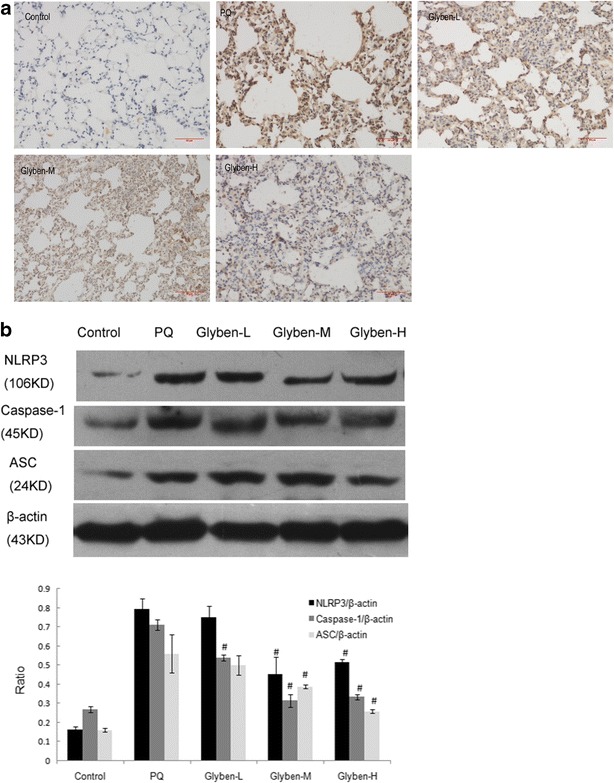
Effects of glybenclamide on NLRP3, ASC, and caspase-1 protein expression in rats lung induced by PQ. Glybenclamide was intraperitoneally administered 1 h before intraperitoneal administration of PQ. At 72 h after PQ administration, lung tissue was collected to determine the caspase-1 positive signal by immunohistochemistry (a) and NLRP3, ASC, caspase-1 protein expression of Western blotting (b). Glyben-L: glybenclamide at the low dose of 10 mg/kg, Glyben-M: glybenclamide at the middle dose of 30 mg/kg, Glyben-H: glybenclamide at the high dose of 50 mg/kg. The values presented are the mean ± SD (n = 6/group). #P < 0.05 versus PQ group.
Secretion of IL-1β and IL-18 Was Inhibited by Glybenclamide in ALI
The increased IL-1β and IL-18 protein levels were attenuated significantly by glybenclamide at 72 h, especially at the dose of 30 and 50 mg/kg, as shown in Fig. 7a and b (#P < 0.05).
Fig. 7.
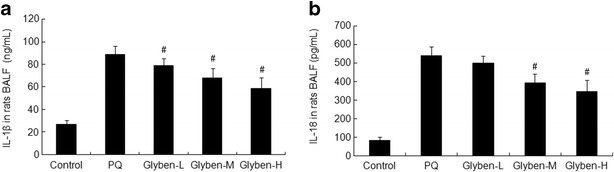
Effects of glybenclamide on cytokines secretion in BALF induced by PQ. Glybenclamide was intraperitoneally administered 1 h before intraperitoneal administration of PQ. At 72 h after PQ administration, BALF was collected to determine the expression of IL-1β and IL-18 by ELISA (a and b). Glyben-L: glybenclamide at the low dose of 10 mg/kg, Glyben-M: glybenclamide at the middle dose of 30 mg/kg, Glyben-H: glybenclamide at the high dose of 50 mg/kg. The values presented are the mean ± SD (n = 6/group). #P < 0.05 versus PQ group.
Paraquat Decreased RAW264.7 Mouse Macrophages Viability
To analyze the effect of paraquat on the viability of RAW264.7 mouse macrophages, the cells were exposed to different concentrations of paraquat for 24 and 48 h. The results from the MTT assay showed that paraquat significantly decreased the viability of RAW264.7 mouse macrophages in a dose-dependent manner (Fig. 8). After a 24 h incubation with 100 μM paraquat, the viability of the cells was reduced by about 14 %. The optimum concentration (100 μM) of paraquat was chosen for next experiments.
Fig. 8.
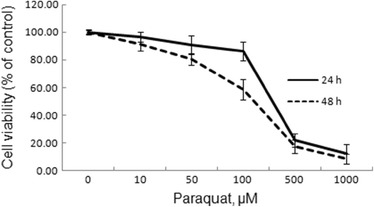
Effects of paraquat (PQ) on RAW264.7 mouse macrophage viability. RAW264.7 mouse macrophages were exposed with or without PQ (0, 10, 50, 100, 500, and 1,000 μM) for 24 and 48 h. The cell viability was assessed by MTT assay. The values presented are the mean ± SD (n = 3).
IL-1β and IL-18 Secreted from RAW264.7 Mouse Macrophages Treated with Paraquat Were Attenuated by Glybenclamide
To confirm that that glybenclamide was acting directly on macrophages secretion of IL-1β and IL-18, macrophages exposed with PQ were pretreated with varying concentrations of glybenclamide. As depicted in Fig. 9, glybenclamide with high concentrations (50 and 200 μM) significantly decreased cytokine levels in the supernatant of macrophages. In correspondence with our in vivo data, we found that glybenclamide reduced IL-1β and IL-18 secretion from macrophage in a dose-dependent fashion.
Fig. 9.
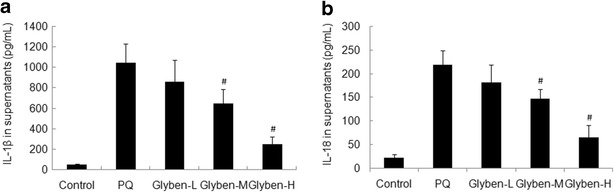
Glybenclamide inhibited PQ-induced IL-1β and IL-18 secretion from mouse macrophages. RAW264.7 mouse macrophages were exposed with 100 μM PQ for 24 h. Glybenclamide (10, 50, 200 μM) was individually preincubated with macrophages 30 min before PQ exposure. Supernatants were analysed by ELISA for IL -1β and IL-18. Glyben-L: glybenclamide at the concentration of 10 μM, Glyben-M: glybenclamide at the concentration of 50 μM, Glyben-H: glybenclamide at the concentration of 200 μM. The values presented are the mean ± SD (n = 3). #P < 0.05 versus PQ group.
DISCUSSION
Paraquat is a very toxic substance for humans and animals. Intoxication with PQ causes severe clinical situations including multiple organ damage. PQ is also a strong pneumotoxicant, especially due to its accumulation in the lung through a polyamine uptake system and to its capacity to induce redox cycling, leading to oxidative stress-related damage. Although the mechanism underlying the development of lung injury by PQ is not clear, previous studies showed that oxidative and inflammatory mediators induced by PQ could result in tissue injury. PQ undergoes cyclic single electron reduction/oxidation through its quaternary ammonium nitrogen atoms and bipyridyl ring, producing ROS and elevating oxidase levels. As an oxidative stress marker, MDA levels in rats lungs induced by PQ were significantly increased in our study. Another marker for neutrophil infiltration, MPO, was also increased in the PQ-induced lungs. On other hand, oxidative stress stimulates inflammation by promoting production and secretion of inflammatory mediators from immune cells. Several cytokines have been identified to be involved in the molecular pathogenesis of PQ poisoning, including IL-1β, TNF-α, IL-6, etc. [16, 17]. PQ-induced ROS can activate intracellular transcription factors such as NF–κB [13], thereby enhancing IL-1β expression. IL-1β can upregulate the expression of endothelial intercellular adhesion molecules essential for the recruit of inflammatory cells to an area of inflammation [18]. In an in vivo experiment, we found that the production of IL-1β and IL-18 in the PQ-poisoning group of rats, BALF increased obviously in a time-dependent manner compared with the control. Meanwhile, these cytokines were mainly secreted by monocytes/macrophages [19], implying that macrophage recruitment may be indispensable for the development of PQ-induced lung damage. It was reported that BALF cells consisted of more than 99 % of macrophages [20]. Therefore, RAW264.7 mouse macrophages were selected for the target in vitro study. PQ decreased RAW264.7 mouse macrophages viability in a dose-dependent fashion and resulted in a significantly increase of IL-1β and IL-18 in supernatant of macrophages.
The NLRP3 inflammasome was described to be involved in host responses to a wide variety of pathogens. Many factors including the efflux of intracellular potassium ions [21], nickel (Ni2+) [22], the lysosomal rupture [23], and bacterial RNA [24] could result in NLRP3 activation. NLRP3 was also activated upon mitochondrial damage and release of ROS [25]. This activity was dependent on the mitochondrial voltage-dependent ion channels which facilitate the exchange of ions between the intermembrane space and the cell cytosol. Complex I inhibition with rotenone led to increased mitochondrial ROS production [26]. Similarly, inhibitors of ROS production or scavengers of ROS, including NAD (P) H oxidase inhibitors can suppress NLRP3 activation. In addition, ROS may activate NF–κB signaling pathway, then NF–κB may induce NLRP3 expression and NLRP3 inflammasome activity [19]. NF–κB was transferred from cytoplasm to nucleus, combined with the promoter regions of target gene transcription and induced the release of inflammatory cytokines including TNF-α, pro-IL-lβ, and pro-IL-l8 [27]. These potent pro-inflammatory cytokines were controlled by two checkpoints: transcription as well as maturation and release [28]. Caspase-1-mediated activation and mature of members of the IL-1β cytokine family led to the recruitment and the activation of other immune cells, such as neutrophils, at the site of infection and/or tissue damage [29]. Interestingly, the increased production of IL-1β and IL-18 was specific to and dependent on NLRP3 inflammasome activation. As previously described [30], PQ-induced redox cycling could generate a great amount of ROS including superoxide anions, NO, and other free radicals, leading to oxidative damage. Meanwhile, PQ can cause intense macrophage activation and leukocyte infiltration in lung, and increase inflammatory cytokines and chemokines. Interestingly, granulocytes, monocytes, and lymphocytes all express NLRP3, suggesting an important role for NLRP3 in primary defense mechanisms of the body [31]. In our study, NLRP3 inflammasome including NLRP3, ASC, and caspase-1 expression was significantly increased in rats lungs induced by PQ poisoning compared with the control. Meanwhile, IL-1β and IL-18 cytokines production in PQ group were also raised significantly. We considered that PQ generated a great amount of ROS, induced inflammatory cells infiltration, and resulted in NLRP3 inflammasome activation and cytokines including IL-1β and IL-18 secretion.
According to the recent reports, the NLRP3 inflammasome played an important role in many disorders such as atherosclerosis, cancer, diabetes, acute gout, and several chronic pulmonary diseases [32], except host protection against microbial infection. The NLRP3 inflammasome was required for the development of acute lung injury caused by LPS/mechanical ventilation [33], hyperoxia [34], hemorrhagic shock [35], etc. NLRP3 deletion decreased lung epithelial cell death and caspase-3 levels; attenuated the recruitment of inflammatory cells and elevation of IL-1β, TNF-α, macrophage inflammatory protein-2, and monocyte chemoattractant protein-1; suppressed NF–κB levels; and exerted a protective effect against acute lung injury [34]. To verify the role of NLRP3 inflammasome in ALI induced by PQ, its blocker glybenclamide was used in our study. Glybenclamide, a type 2 diabetes drug, is an ATP-sensitive potassium channel inhibitor and can exert anti-inflammatory effects by inhibiting the activation of NLRP3 inflammasome induced by PAMP and DAMP, as well as crystalline substances [14, 36]. Although the mechanism remains to be fully elucidated, evidence suggests that this inhibitor acts downstream of the P2X7 receptor but upstream of NLRP3. It also blocks maturation of caspase-1 and pro-IL-1β. Glybenclamide has been shown to prevent neutrophil extravasation and accumulation in animal models [37]. Glybenclamide treatment was associated with a survival benefit during lipopolysaccharide induced lethality in mice [14] and also in patients with gram-negative sepsis [38]. In ventilator-induced lung injury model, glybenclamide reduced lung edema, neutrophil influx, and IL-6 levels in BALF. Our results suggested that glybenclamide pretreatment also attenuated lung edema and inflammatory cell infiltration, decreased IL-1β and IL-18 levels in BALF, reduced the MPO levels in lungs, and inhibited the NLRP3 inflammasome activation. In addition, although there was a downward trend in MDA levels, it was not statistically significant compared with the PQ group (P > 0.05). We considered that the NLRP3 inflammasome blocker glybenclamide only inhibited inflammation by reducing the secretion of IL-1β and IL-18 but did not exert anti-oxidant effects. We also found that the secretion of IL-1β and IL-18 from RAW264.7 mouse macrophages treated with paraquat was reduced in vitro after the NLRP3 inflammasome was blocked by glybenclamide. NLRP3/IL-1β and IL-18 pathways may play important roles in ALI, but the potential mechanism needs to be investigated further.
CONCLUSIONS
In previous studies, it was found that mature IL-1β and IL-18 secretion was largely dependent on the NLRP3 inflammasome activation. NLRP3 inflammasome activation contributed to accelerating caspase-1 maturation and finally resulting in IL-1β and IL-18 over-production. In this study, the NLRP3 inflammasome activation and cytokines (IL-1β and IL-18) secretion were induced by PQ in acute lung injury, and both of them were investigated to be involved in the inflammation process. Additionally, the NLRP3 inflammasome inhibition by using glybenclamide can ameliorate the proinflammatory activity and protect the lung against inflammatory injury induced by PQ in rats. To reconfirm the effect, IL-1β and IL-18 secreted from RAW264.7 mouse macrophages treated with PQ were also attenuated by glybenclamide in vitro experiments. In conclusion, PQ can generate ROS and induce IL-1β/IL-18 secretion via NLRP3-ASC-caspase-1 pathway. So, the intracellular danger sensor NLRP3 inflammasome is essential for PQ-induced acute lung injury.
ACKNOWLEDGMENTS
This study is supported by the National Natural Science Foundation of China (Grant No. 81171793 /H1503) and the Science Research Plan of Liaoning Province Education Administration (Grant No. L2014300).
Conflict of Interest
The authors declare that they have no conflict of interest.
Abbreviations
- PQ
Paraquat
- NLRP3
Nucleotide-binding domain and leucine-rich repeat containing proteins
- ASC
Apoptosis-associated speck-like protein containing caspase-1 activator domain
- BALF
Bronchoalveolar lavage fluid
- TNF-α
Tumor necrosis factor-α
- IL-1β
Interleukin-1β
- IL-18
Interleukin-18
- MPO
Myeloperoxidase
- MDA
Malonyldialdehyde
- ROS
Reactive oxygen species
- ALI
Acute lung injury
- ARDS
Acute respiratory distress syndrome
- NF–κB
Nuclear factor–κB
- AP-1
Activator protein-1
REFERENCES
- 1.Gawarammana IB, Dawson AH. Peripheral burning sensation: a novel clinical marker of poor prognosis and higher plasma-paraquat concentrations in paraquat poisoning. Clinical Toxicology (Philadelphia) 2010;48:347–349. doi: 10.3109/15563651003641794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Critical Reviews in Toxicology. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 3.Ogier-Denis E, Mkaddem SB, Vandewalle A. NOX enzymes and Toll-like receptor signaling. Seminars in Immunopathology. 2008;30:291–300. doi: 10.1007/s00281-008-0120-9. [DOI] [PubMed] [Google Scholar]
- 4.Iriti M, Faoro F. Review of innate and specific immunity in plants and animals. Mycopathologia. 2007;164:57–64. doi: 10.1007/s11046-007-9026-7. [DOI] [PubMed] [Google Scholar]
- 5.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 6.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. The Journal of Experimental Medicine. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, Reed JC. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Molecular Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. The Journal of Immunology. 2010;187:597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- 9.Chen S, Sun B. Negative regulation of NLRP3 inflammasome signaling. Protein & Cell. 2013;4:251–258. doi: 10.1007/s13238-013-2128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin interacting protein links oxidative stress to inflammasome activation. Nature Immunology. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 11.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunology. 2010;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erroi A, Bianchi M, Ghezzi P. The pneumotoxicant paraquat potentiates IL-1 and TNF production by human mononuclear cells. Agents and Actions. 1992;36:66–69. doi: 10.1007/BF01991230. [DOI] [PubMed] [Google Scholar]
- 13.Liu ZN, Wang Y, Zhao HY, Zheng Q, Xiao L, Zhao M. CB2 receptor activation ameliorates the proinflammatory activity in acute lung injury induced by paraquat. Biomed Research International. 2014;2014:971750. doi: 10.1155/2014/971750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. The Journal of Cell Biology. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belperio JA, Keane MP, Burdick MD, et al. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. The Journal of Clinical Investigation. 2002;110:1703–1716. doi: 10.1172/JCI0215849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida Y, Takayasu T, Kimura A, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Gene expression of cytokines and growth factors in the lungs after paraquat administration in mice. Legal Medicine. 2006;8:102–109. doi: 10.1016/j.legalmed.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Agelaki S, Tsatsanis C, Gravanis A, Margioris AN. Corticotropin-releasing hormone augments proinflammatory cytokine production from macrophages in vitro and in lipopolysaccharide-induced endotoxin shock in mice. Infection and Immunity. 2002;70:6068–6074. doi: 10.1128/IAI.70.11.6068-6074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leff JA, Baer JW, Bodman ME, Kirkman JM, Shanley PF, Patton LM, Beehler CJ, McCord JM, Repine JE. Interleukin-1-induced lung neutrophil accumulation and oxygen metabolite-mediated lung leak in rats. American Journal of Physiology. 1994;266:L2–L8. doi: 10.1152/ajplung.1994.266.1.L2. [DOI] [PubMed] [Google Scholar]
- 19.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF–kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. The Journal of Immunology. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi G, Wolthuis EK, Bresser P, Levi M, van der Poll T, Dzoljic M, Vroom MB, Schultz MJ. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology. 2006;105:689–695. doi: 10.1097/00000542-200610000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Franchi L, Kanneganti TD, Dubyak GR, Núñez G. Differential requirement of P2X7 receptor and intracellular K + for caspase-1 activation induced by intracellular and extracellular bacteria. The Journal of Biological Chemistry. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Zhong F. Nickel induces interleukin-1β secretion via the NLRP3-ASC-caspase-1 pathway. Inflammation. 2014;37:457–466. doi: 10.1007/s10753-013-9759-z. [DOI] [PubMed] [Google Scholar]
- 23.Davis BK, Wen H, Ting JPY. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual Review of Immunology. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, Ryffel B, Swanson JA, Müller M, Blander JM. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474:385–389. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 26.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. The Journal of Biological Chemistry. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 27.Jian XD, Li M, Zhang YJ, Ruan YJ, Guo G, Sui H, Zhang YC. Role of growth factors in acute lung injury induced by paraquat in a rat model. Human & Experimental Toxicology. 2011;30:460–469. doi: 10.1177/0960327110372648. [DOI] [PubMed] [Google Scholar]
- 28.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nature Reviews Immunology. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nature Immunology. 2012;13:325–3322. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2012;180:65–77. doi: 10.1016/S0300-483X(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 31.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. Journal of Histochemistry & Cytochemistry. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 32.Lawlor KE, Vince JE. Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? Biochimica et Biophysica Acta. 2014;1840:1433–1440. doi: 10.1016/j.bbagen.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Jones HD, Crother TR, Gonzalez-Villalobos RA, Jupelli M, Chen S, Dagvadorj J, Arditi M, Shimada K. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. American Journal of Respiratory Cell and Molecular Biology. 2014;50:270–280. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukumoto J, Fukumoto I, Parthasarathy PT, Cox R, Huynh B, Ramanathan GK, Venugopal RB, Allen-Gipson DS, Lockey RF, Kolliputi N. NLRP3 deletion protects from hyperoxia-induced acute lung injury. American Journal of Physiology-Cell Physiology. 2013;305:C182–C189. doi: 10.1152/ajpcell.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiang M, Shi X, Li Y, Xu J, Yin L, Xiao G, Scott MJ, Billiar TR, Wilson MA, Fan J. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. The Journal of Immunology. 2011;187:4809–4817. doi: 10.4049/jimmunol.1102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, Corradin G, Tschopp J. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4:e6510. doi: 10.1371/journal.pone.0006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pompermayer K, Amaral FA, Fagundes CT, Vieira AT, Cunha FQ, Teixeira MM, Souza DG. Effects of the treatment with glibenclamide, an ATP-sensitive potassium channel blocker, on intestinal ischemia and reperfusion injury. European Journal of Pharmacology. 2007;556:215–222. doi: 10.1016/j.ejphar.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 38.Koh GC, Maude RR, Schreiber MF, Limmathurotsakul D, Wiersinga WJ, Wuthiekanun V, Lee SJ, Mahavanakul W, Chaowagul W, Chierakul W, White NJ, van der Poll T, Day NP, Dougan G, Peacock SJ. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clinical Infectious Diseases. 2011;52:717–725. doi: 10.1093/cid/ciq192. [DOI] [PMC free article] [PubMed] [Google Scholar]


