Abstract
Dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin-related protein (DC-SIGNR) is a type II transmembrane protein which has been reported to bind a variety of pathogens as well as participate in immunoregulation. But the association between the level of DC-SIGNR and lung cancer is unknown. To investigate the clinical diagnostic significance of DC-SIGNR in lung cancer, we investigated serum DC-SIGNR levels in 173 lung cancer patients and 134 healthy individuals using enzyme-linked immunosorbent assay (ELISA). Results showed that serum DC-SIGNR levels in lung cancer patients were lower than that in healthy controls (P = 0.0003). A cut-off value of 3.8998 ng/L for DC-SIGNR predicted the presence of lung cancer with 78.03 % sensitivity and 49.25 % specificity (area under the curve = 0.6212, P = 0.0003). Strikingly, serum DC-SIGNR levels were significantly higher in lung cancer patients with brain metastasis compared to those without metastasis (P = 0.0283). Moreover, the serum concentrations of DC-SIGNR in lung cancer patients also correlated significantly with serum natural killer cells percentage (P = 0.0017). In addition, immunohistochemistry assay demonstrated that the expression of DC-SIGNR in lung tissues of 31 lung cancer patients and 13 tuberculosis patients was significantly lower than that in 18 normal lung tissues (P = 0.0418, 0.0289), and there is no significant difference between tuberculosis tissues and lung cancer tissues (P = 0.2696). These results suggest that DC-SIGNR maybe a promising biological molecule that has the potential for clinical research of lung cancer, whereas its underlying roles are needed to be investigated in further studies.
Electronic supplementary material
The online version of this article (doi:10.1007/s11010-015-2465-4) contains supplementary material, which is available to authorized users.
Keywords: DC-SIGNR, Lung cancer, Expression, Brain metastasis, Natural killer cells
Introduction
Lung cancer is the leading cause of cancer death worldwide among men and the second leading cancer site in women [1]. The high mortality rate of this disease is primarily due to the difficulty of early diagnosis, the high metastatic potential, and the poor responses to chemical therapy and radiotherapy [2]. Unfortunately, the unclear etiology of lung cancer presents a huge challenge for the treatment of lung cancer. Many groups’ results showed that smoking is the most important modifiable risk factor for lung cancer [3–5]. Otherwise, lung cancer susceptibility is also determined by host genetic factors [6, 7]. Infection may play a role in lung cancer, including human papilloma virus (PPV), Epstein-Barr virus, and human immunodeficiency virus (HIV) [8–10]. In addition, there are some biological molecules correlated with the risk of lung cancer such as P-selectin [11] and chondrolectin (CHODL) [12]. Other known risk factors for lung cancer include exposure to several occupational and environmental carcinogens such as asbestos, arsenic, and polycyclic aromatic hydrocarbons [13].
Recent studies from several groups suggest that lectins may play an important role in a variety of physiological and pathological processes, typically with tumor progression and metastasis. For example, Läubli and Borsig [14] reported that selectins were upregulated the pulmonary metastatic microenvironment is facilitated by P or L-selectin mediated interactions between tumor cells and blood components, which can significantly reduced metastasis. Other groups results also showed that high mannose-binding lectin (MBL) levels were associated with poorer lung cancer survival [15], and low MBL levels increased gastric cancer risk [16]. Additionally, liver sinusoidal endothelial cell lectin (LSECtin) can adhere to colorectal cells and plays an important role in colorectal carcinoma liver metastasis [17]. Recently, our laboratory has been reported that DC-SIGN and DC-SIGNR are low expressions in patients with non-Hodgkin’s lymphoma (NHL) and could be potentially useful in NHL clinical settings [18, 19]. DC-SIGN and DC-SIGNR are novel blood-based molecular markers which can potentially be used for the diagnosis of early stage of colon cancer patients [20]. Therefore, we are interested in the expression of DC-SIGNR in lung cancer patients.
DC-SIGNR, a closest homologues to the C-type lectin superfamily members DC-SIGN, LSECtin, and CD23, which consists of an N-terminal cytoplasmic tail, a transmembrane domain, an extracellular C-terminal neck region, and a C-type carbohydrate recognition domain (CRD) [21, 22]. The CRD domain of DC-SIGNR can interact with high mannose carbohydrates, and the neck domains that support the CRD may influence the ligand-binding properties of these lectins, and the intra-cellular domain located in the cytoplasmic tail containing recycling and internalization motifs [23]. DC-SIGNR is highly expressed on liver sinusoidal endothelial cells, lymph node endothelial cells, and a significant proportion in terms of placenta capillary endothelial cells but was not expressed in peripheral blood-derived DCs [24]. Furthermore, DC-SIGNR is also expressed in the lung in both cytokeratin positive alveolar epithelia, as well as a subset of cells that co-expressed angiotensin converting enzyme-2 (ACE2) but were negative for cytokeratin [25]. Like DC-SIGN, DC-SIGNR can recognize and bind several pathogens, including viruses, bacteria, fungi, and parasites [26]. DC-SIGNR also recognizes endogenous ligands, such as ICAM molecules [27], although their glycan epitopes have not been fully characterized. However, the role of DC-SIGNR in lung cancer was not been elucidated.
In our research, we compared the serum levels of DC-SIGNR between lung cancer patients and healthy volunteers using an ELISA. We found that lung cancer patients showed lower levels of DC-SIGNR compared with healthy controls and that there were correlations between serum levels of DC-SIGNR and clinical parameters of lung cancer patients. In immunohistochemistry (IHC) study, the DC-SIGNR is lower expression in lung tissues from lung cancer patients compared to those in normal lung tissues.
Materials and methods
Clinical samples
Serum samples of 173 lung cancer patients were obtained from the First and Second Affiliated Hospital of Dalian Medical University during March 2014–June 2014 and were stored at −80 °C until they were analyzed. The mean age of the 173 patients was 61 years (range, 32–83 years), 102 were male and 71 were female. According to the World Health Organization (WHO) classification, the lung cancer cases consisted of small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), the NSCLC is further divided into three major pathologic subtypes, including adenocarcinoma (ADC), squamous cell carcinoma (SCC), and large cell carcinoma [28]. Clinical staging of lung cancer patients was performed according to the IASLC Lung Cancer Staging [29]. Clinical data including gender, age, cancer stage, histological subtype, concentrations of tumor markers, and the percentage of T cell subsets were acquired from hospitalization records. The basic characteristics of these subjects were shown in Table 1 and Supplementary Table 1. The control group was composed of 134 healthy blood donor volunteers. Among the healthy subjects, the mean age was 56 years (range, 24–73 years). Each healthy control performed routine physical examinations, and the results were within the normal range. Lung cancer patients and healthy individuals with various pathogen infections and poor performance status were excluded in present study.
Table 1.
Clinical data of the lung cancer patients in ELISA study
| Clinical date | No. | Proportion | DC-SIGNR (ng/L) median (range) | P value |
|---|---|---|---|---|
| Gender (n = 173) | ||||
| Female | 71 | 41 | 3.689 (0.089–14.79) | 0.5723 |
| Male | 102 | 59 | 3.317 (0.124–14.40) | |
| Age (n = 173) | ||||
| ≤60 | 77 | 45 | 3.021 (0.124–10.64) | 0.1953 |
| >60 | 96 | 55 | 3.829 (0.089–14.79) | |
| Cancer stage (n = 160)* | ||||
| I–II | 41 | 26 | 2.661 (0.239–9.274) | 0.9299 |
| III–IV | 119 | 74 | 3.537 (0.089–14.79) | |
| Histologic subtype (n = 164)* | ||||
| SCLC | 31 | 19 | 3.740 (0.674–14.40) | 0.5016 |
| ADC | 106 | 65 | 3.235 (0.089–14.79) | |
| SCC | 27 | 16 | 3.124 (0.282–14.11) | |
SCLC small cell lung cancer, ADC adenocarcinoma, SCC squamous cell carcinoma
* The n shown reflects the number of patients for whom data are available
In addition, formalin-fixed and paraffin-embedded tissues of 31 lung cancer patients, 13 lung tissues from tuberculosis patients, 18 normal lung tissues, and 9 lymph nodes from 2009 to 2015 were obtained from The Second Affiliated Hospital of Dalian Medical University. Lung tissues from tuberculosis patients and normal lung tissues were used as control groups, and normal lymph nodes were used as either positive or negative controls. The clinical data for these subjects are summarized in Table 2 and Supplementary Table 2.
Table 2.
Clinical data of lung cancer, tuberculosis patients and normal lung tissues included in IHC study
| Clinical date | Lung cancer patients (n = 31) | Tuberculosis patients (n = 13) | Normal lung tissues (n = 18) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Gender | ||||||
| Male | 19 | 61 | 7 | 54 | 8 | 44 |
| Female | 12 | 39 | 6 | 46 | 10 | 56 |
| Age | ||||||
| ≤60 | 17 | 55 | 12 | 92 | 10 | 56 |
| >60 | 14 | 45 | 1 | 8 | 8 | 44 |
| Mean destiny | ||||||
| <0.0535004 | 19 | 61 | 9 | 69 | 8 | 44 |
| >0.0535004 | 12 | 39 | 4 | 31 | 10 | 56 |
Enzyme-linked immunosorbent assay (ELISA)
We used standard sandwich ELISA to detect the levels of DC-SIGNR in human serum. 96-well microplates were coated with 100 µl anti-DC-SIGNR goat polyclonal antibody (pAb, Santa Cruz Biotechnology, Inc., USA, Catalog Number: sc-17261) at a final concentration of 0.27 μg/ml and incubated overnight at 4 °C. Washed the plates 3 times with phosphate buffered saline (PBS) containing 0.05 % Tween-20 (PBST) (pH 7.4), and the wells were blocked with blocking buffer (5 % bovine serum albumin) at 37 °C for 1 h. The plates were washed three times with PBST. And then, 100 μl serum from lung cancer patients and healthy controls was added to the wells. As a negative control, 100 µl PBS was used. Each plate was incubated at 37 °C for 2 h. Subsequently, PBST was used to wash the plates 3 times, and 100 μl anti-DC-SIGNR mouse monoclonal antibody (mAb, R&D Systems, Inc., USA, Catalog Number: MAB16211) diluted to a concentration of 0.5 μg/ml was added to each wells. The plates were incubated at 37 °C for 1.5 h. After washing, 100 μl peroxidase-conjugated goat anti-mouse (ZSGB-BIO) was added and the plates were incubated at 37 °C for 1 h. Finally, the plates were washed 3 times and incubated with 3,3′,5,5′-tetramethylbenzidine (TMB, TIANGEN BIOTECH CO, LTD.) at 37 °C for 0.5 h, and then 2 mol/L H2SO4 was added to stop the reaction. The optical density (OD) was measured at the 450 nm wavelength on a microplate reader. The quantitative DC-SIGNR concentrations were determined by comparing the optical density values with the standard curve.
The S100β levels in lung cancer patients with brain metastasis were detected by ELISA kit (WESTANG BIO-TECH, LTD). The patients who have other diseases which can affect S100β levels were excluded in this study. The concentrations of S100β were shown in SupplementaryTable 3.
Immunohistochemistry
Before being deparaffinized in xylene and rehydrated in a graded ethanol series, sections from paraffin-embedded blocks were incubated at 60 °C for 30 min. Sections were washed with PBS, and then endogenous peroxidase activity was blocked with 3 % hydrogen peroxide diluted in double-distilled water for 10 min at room temperature. Antigen retrieval was performed by heating slides in citrate buffer (0.01 mol/L citric acid, pH 6.0) for 20 min in a microwave oven. After being washed with PBS, the sections were incubated with goat serum for the purpose of blocking. Sections were incubated with anti-DC-SIGNR rabbit pAb (Abcam, Inc.) overnight at 4 °C (1:250). The next day, after being washed with PBS, the sections were incubated with horseradish peroxidase-labeled anti-rabbit immunoglobulin (1:1000) (ZSGB-BIO) at 37 °C for 1 h and were then washed again. Finally, all the sections were developed with 3,3′-diaminobenzidine tetrahydrochloride (DAB,ZSGB-BIO) within the cells and then counterstained with hematoxylin. Photographs were recorded on an Olympus BX51 microscope.
The mean density of DC-SIGNR expression was assessed using the Image-Pro Plus version 6.0 (Media Cybernetics, Inc. Silver Spring, MD USA). Integrated optical density (IOD) sum represents the protein content of DC-SIGNR in the area of interest (AOI), while Area equals the area of AOI. (IOD sum)/Area stands for “mean density”. Briefly, images were captured at 200× magnification from 3 AOIs/case, which were selected based on areas with maximal DC-SIGNR staining. The mean density values are shown in Supplementary Table 2. This quantitation was positively correlated with DC-SIGNR expression in tissue.
Statistical analysis
Non-parametric Mann–Whitney U test was used to determine the statistical significance among two groups. The statistical significance among more than two groups was determined with the Kruskal–Wallis non-parametric test. Correlation of DC-SIGNR values with clinical parameters was tested by the non-parametric Spearman’s rank correlation coefficient test. In all tests, 2-sided P values below 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism5 (GraphPad Software, Inc., San Diego, CA, USA).
Results
The levels and diagnostic values of DC-SIGNR in lung cancer patients
We detected serum DC-SIGNR levels in 307 participants, composing of 173 with lung cancer and 134 healthy controls by ELISA. The DC-SIGNR level in the serum of patients with lung cancer (14.9434 ± 0.3152 mg/ml) was significantly lower than that in healthy controls (3.4696 ± 0.2471 mg/ml) (P = 0.0003; Fig. 1a). A ROC curve is often used to assess the power of a novel serum marker for tumor prediction. In the present study, a cut-off value of 3.8998 ng/L for DC-SIGNR predicted the presence of lung cancer with 78.03 % sensitivity and 49.25 % specificity, and the area under curve (AUC) was 0.6212 (P = 0.0003) (Fig. 1b). Therefore, serum DC-SIGNR levels demonstrated low accuracy for the detection of lung cancer.
Fig. 1.
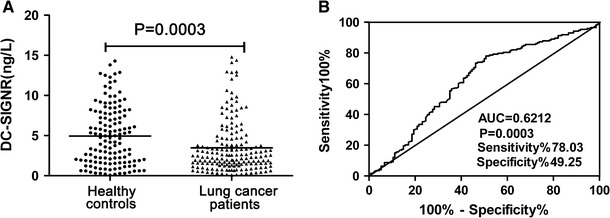
Low serum levels of DC-SIGNR in lung cancer patients. The scatter plot displays DC-SIGNR levels in serum samples from 173 lung cancer patients and 134 healthy controls were tested by enzyme-linked immunosorbent assay (ELISA). Each point represents the serum DC-SIGNR level of one sample. The horizontal lines indicate the median levels. Serum DC-SIGNR was significantly lower in lung cancer patients compared with healthy controls (P = 0.0003) (a). Receiver operating characteristic (ROC) curve was used for serum DC-SIGNR in lung cancer prediction. A cut-off value of 3.8998 ng/L for DC-SIGNR to predict the presence of lung cancer with 78.03 % sensitivity and 49.25 % specificity (Area under curve (AUC) = 0.6212, P = 0.0003) (b)
Serum concentrations of DC-SIGNR correlated significantly with lung cancer patients who have brain metastasis
We assessed the correlations between serum levels of DC-SIGNR and clinical data, including gender, age, cancer stage, histologic subtype, and metastasis to different organs. Strikingly, serum concentrations of DC-SIGNR of lung cancer patients with brain metastasis were higher than that without metastasis (P = 0.0283; Fig. 2b). However, the levels of DC-SIGNR in patients with lung cancer showed no significant difference between metastasis and non-metastasis (P = 0.3887; Fig. 2a). Furthermore, the serum DC-SIGNR levels were not significantly different between bone metastasis and non-metastasis (P = 0.3841; Fig. 2b), lung metastasis and non-metastasis (P = 0.6744; Fig. 2b), lymph node metastasis and non-metastasis (P = 0.9880; Fig. 2b).
Fig. 2.
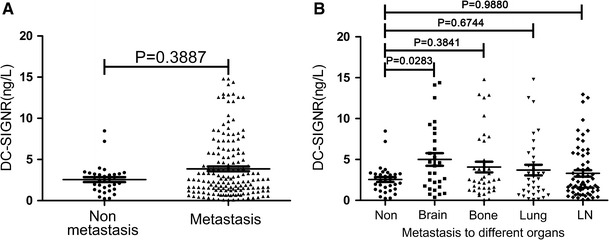
Serum DC-SIGNR levels were significantly correlated with brain metastasis. The serum levels of DC-SIGNR were not significantly different between metastasis and non-metastasis (P = 0.3887) (a). Serum concentrations of DC-SIGNR were higher in brain metastasis than in non-metastasis (P = 0.0283) (b). However, the serum DC-SIGNR levels were not significantly different between bone metastasis and non-metastasis (P = 0.3841), lung metastasis and non-metastasis (P = 0.6744), lymph node metastasis and non-metastasis (P = 0.9880) (b)
Serum concentrations of DC-SIGNR correlated significantly with serum natural killer (NK) cells percentage
Otherwise, we found that the serum levels of DC-SIGNR correlated significantly with serum NK cells percentage with the Spearman’s correlation coefficient of −0.3221 (P = 0.0017; Fig. 3a). However, there was no significant correlation between serum DC-SIGNR and CD3, with a Spearman’s correlation coefficient of 0.1583 (P = 0.1713; Fig. 3b). Moreover, serum levels of DC-SIGNR showed no association with CD4 with a Spearman’s correlation coefficient of 0.0513 (P = 0.6270; Fig. 3c). In addition, serum levels of DC-SIGNR displayed no correlation with CD8 with a Spearman’s correlation coefficient of 0.0723 (P = 0.4934; Fig. 3d).
Fig. 3.
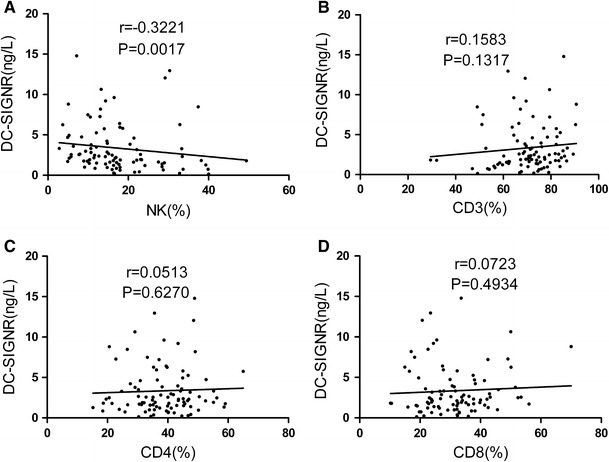
Correlations of serum levels of DC-SIGNR with NK, CD3, CD4, and CD8 levels. Serum DC-SIGNR levels correlated significantly with percent of NK cells in lung cancer patients (P = 0.0017) (a). Serum levels of DC-SIGNR showed no significant correlation with CD3 (b), CD4 (c), and CD8 (d)
No significant correlations were observed between serum levels of DC-SIGNR and clinical parameters, such as age, gender, cancer stage, and histologic subtype
Contrary to above results, serum levels of DC-SIGNR in patients with lung cancer showed no significant difference between male and female (P = 0.5723; Fig. 4a). Serum levels of DC-SIGNR also showed no significant difference between patients of ≤60 years old and patients >60 years old (P = 0.1213; Fig. 4b). Serum levels of DC-SIGNR expressed no association with age with a Spearman’s correlation coefficient of 0.0706 (P = 0.3559; Fig. 4c). Furthermore, the serum DC-SIGNR levels were not significantly different among the stage I–II, and stage III–IV (P = 0.9299; Fig. 4d). Additionally, for different histologic subtype, no significant difference was observed between lung cancer patients with SCLC and those with NSCLC (P = 0.6000; Fig. 4e). Similarly, the difference of serum DC-SIGNR levels was not apparent between SCLC, ADC and SCC (P = 0.5016; Fig. 4f).
Fig. 4.
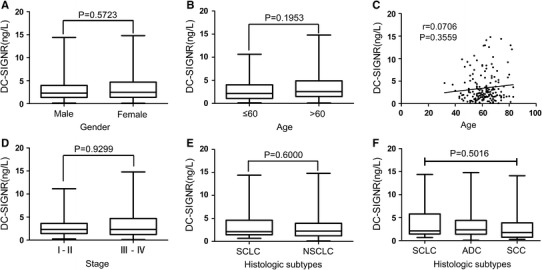
Correlations of serum levels of DC-SIGNR with clinical data. Serum levels of DC-SIGNR showed no significant correlation with gender (a), age (b, c), cancer stage (d), and histologic subtype (e, f)
Serum DC-SIGNR levels showed no significant correlation with S100β, carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and CYFRA21-1
In our study, the levels of DC-SIGNR in lung cancer patients with brain metastasis is higher than that without metastasis. S100β as a marker of brain metastasis, so we compare the correlation between the two molecular. However, there is no significant correlation between DC-SIGNR and S100β, with a Spearman’s correlation coefficient of 0.003847 (P = 0.9854; Fig. 5a). In present study, serum levels of DC-SIGNR expressed no association with CEA, with a Spearman’s correlation coefficient of 0.1982 (P = 0.1007; Fig. 5b). Similarly, serum levels of DC-SIGNR were not significantly correlated with NSE, with a Spearman’s correlation coefficient of −0.0709 (P = 0.3742; Fig. 5c). Furthermore, the data also showed no correlation between serum DC-SIGNR and CYFRA21-1 levels, with a Spearman’s correlation coefficient of 0.0168 (P = 0.8346; Fig. 5d).
Fig. 5.

Correlations of serum levels of DC-SIGNR with tumor markers. Serum levels of DC-SIGNR displayed no significant correlation with S100β (a) CEA (b), NSE (c), and CYFRA21-1 (d)
The expression of DC-SIGNR in lung cancer patients and tuberculosis patients was significantly lower than that in normal lung tissues
We determined the expression level of DC-SIGNR in serum. Moreover, it has been reported that DC-SIGNR is expressed in the lung in both cytokeratin positive alveolar epithelia, as well as a subset of cells that co-expressed ACE2 [25]. Therefore we want to know whether the DC-SIGNR expression in lung cancer tissues. We performed IHC analysis, using anti-DC-SIGNR pAb in 31 lung cancer tissues (1 SCLC, 16 ADC, and 14 SCC) and 13 lung tissues of tuberculosis patients, and 18 normal lung tissues were used as controls. Strikingly, the mean density of DC-SIGNR expression in the lung cancer patients and tuberculosis patients was significantly lower than that in normal lung tissues (P = 0.0418, 0.0289). But, there is no significant difference between tuberculosis tissues and lung cancer tissues (P = 0.2696) (Figs. 6, 7). The normal lymph nodes of tumor patients were used as positive controls and negative controls.
Fig. 6.

IHC for DC-SIGNR expression in tissues of lung cancer tissues, tuberculosis patients, and normal tissues. IHC analysis was used to determine DC-SIGNR expression and arrows indicate positive staining. Areas in the black boxes of A, C, E, G, and I were enlarged below. DC-SIGNR expression was detected in lung tissues from tuberculosis patients (a–b). DC-SIGNR expression was detected in the lung tissues from lung cancer patients (c–d). DC-SIGNR expression in alveolar epithelia cells of normal lung tissues (e–f). DC-SIGNR expression in lymphatic endothelial cells exhibited strong positive (g–h). Normal lymph node tissues were used as a negative control (i–j)
Fig. 7.
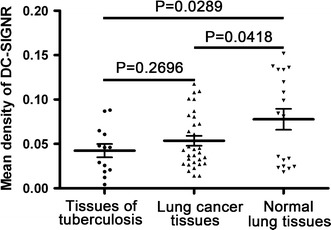
Semi-quantitative image analysis of DC-SIGNR expression in tissues. The graph displays a scatter plot of the levels of DC-SIGNR expression in lung tissues from lung cancer patients, tuberculosis patients, and normal controls. There was a statistical significance in IHC for DC-SIGNR expression between lung cancer tissues and normal lung tissues (P = 0.0418), tuberculosis tissues and normal controls (P = 0.0289). There was no significantly difference between lung cancer patients and tuberculosis patients (P = 0.2696). DC-SIGNR expression in lung cancer patients was lower than in normal lung tissues
Discussion
The expression of DC-SIGNR mainly occurs in infective diseases, such as acquired immune deficiency syndrome (AIDS) with HIV infection, hepatitis C infection disease, and Ebola virus infection disease [26]. Recently, our laboratory reported that DC-SIGNR expression is lower in patients with NHL than in healthy individuals and higher expression in colon cancer patient serum [18, 20]. To extend the knowledge of the possible clinical applications of DC-SIGNR, we studied the serum levels of DC-SIGNR in patients with lung cancer, and we also studied the expression and location levels of DC-SIGNR in lung cancer tissues. Our study demonstrated that serum levels of DC-SIGNR in lung cancer patients were significantly lower than those in healthy individuals. Furthermore, we found that the serum DC-SIGNR levels correlated significantly with brain metastasis and serum NK cells percentage in lung cancer patients.
Brain metastasis as one of the most dreaded complications of lung cancer has a poor prognosis [30]. Up to 50 % of lung cancer patients develop brain metastasis during their course of disease [31]. The occurrence of brain metastasis is associated with poor prognosis and high morbidity in patients with advanced lung cancer, even after intensive multimodal therapy [32]. Furthermore, little is known about the pathobiology and the molecular mechanisms involved in the brain metastatic cascade which limits the possibilities for focused drug development and clinical trial [33]. S100β and ProApolipoprotein A1 as a serum marker of brain metastasis in lung cancer have been investigated in a recent study [34–36]. In our study, the DC-SIGNR levels in lung cancer patients with brain metastasis are significantly higher than those without metastasis. So, we compared the correlation between serum DC-SIGNR and S100β levels. However, there is no significant correlation between them. S100β which is a protein found in astrocytes and only released into serum when the blood–brain barrier is breached [37]. The expression of DC-SIGNR in brain tissues is still unknown. Thus, we guessed that the two molecular may have a difference mechanism to affect the brain metastasis of lung cancer and it can be investigated in further study. We also compared the serum levels of DC-SIGNR in bone, lung, and lymph nodes metastasis with non-metastasis, respectively. But we found no significant difference between them.
Natural killer (NK) cells, as a major component of the innate immune system, can limit the growth and dissemination of several types of tumors [38]. Unlike T cells and B cells, NK cells can directly exert cellular cytotoxicity on tumor cells without prior sensitization and secrete immunostimulatory cytokines, which control both local tumor growth and metastasis [39]. An epidemiologic survey showed that low NK cell activity is associated with increased cancer risk [40]. Numerous studies have demonstrated that the infiltration of NK cells appears to have prognostic value in gastric carcinoma [41], colorectal carcinoma [42], and lung carcinomas [43, 44], as a relatively higher level of NK cell infiltrate correlates with a better prognosis, thus suggesting relevant protective roles for NK cell infiltrate. In the present study, we detected a significantly negative correlation between serum levels of DC-SIGNR and serum percentage of NK cells in lung cancer patients. The molecular mechanism for this negative correlation is not yet known. On the contrary, we found no significant correlations between serum levels of DC-SIGNR and serum percentage of CD3, CD4, and CD8 in the present study.
In order to know the DC-SIGNR whether it has the ability to distinguish the benign and malignant diseases of the lung, we choose tuberculosis (TB) patients, which results in mycobacterium tuberculosis infections as a benign disease group. Mycobacterium tuberculosis can targets DC-SIGN to inhibit the immuno-stimulatory function of DC through the interaction of the mycobacterial mannosylated lipoarabinomannan (ManLAM) to DC-SIGN [45]. Like DC-SIGN, DC-SIGNR also can capture ManLAM and rapidly internalizes it to lysosomes, and may be involved in the clearance of mycobacteria [45]. Tailleux L et al. [46] showed DC-SIGN present in the alveolar CD11b+ MΦs in the lungs of patients with TB. In present study, we found the DC-SIGNR expression in lungs of patients with TB. However, there was no significantly difference between the DC-SIGNR expression in lung cancer tissue and tuberculosis. Strikingly, both of them are lower than those in normal lung tissues.
In summary, we have shown that the serum level of DC-SIGNR is a promising marker to help distinguish lung cancer patients from healthy individuals. Additional studies are warranted to assess the potential value of DC-SIGNR in vivo. These studies will most likely make DC-SIGNR a useful biological molecule for clinical research on lung cancer mechanisms in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by grants from the Chinese National Natural Science Foundation Projects (81372669,31270867, 31470800) and Science and Technology Planning Project of Liaoning province, China (2012225020).
Compliance with Ethical Standards
The study was approved by the Research Ethics Committee of Dalian Medical University, and informed consent was obtained from all participants, in agreement with institutional guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Shuangyi Ren, Email: rsydl@aliyun.com.
Yunfei Zuo, Email: zyf04112002@dlmedu.edu.cn.
References
- 1.Jemal A, Bray F, Center MM. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Mitsuhashi A, Goto H, Kuramoto T, et al. Surfactant protein a suppresses lung cancer progression by regulating the polarization of tumor-associated macrophages. Am J Pathol. 2013;182:1843–1853. doi: 10.1016/j.ajpath.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warner KE, Mendez D. Tobacco control policy in developed countries: yesterday, today, and tomorrow. Nicot Tob Res. 2010;12:876–887. doi: 10.1093/ntr/ntq125. [DOI] [PubMed] [Google Scholar]
- 5.Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz AG, Prysak GM, Bock CH, et al. The molecular epidemiology of lung cancer. Carcinogenesis. 2007;28:507–518. doi: 10.1093/carcin/bgl253. [DOI] [PubMed] [Google Scholar]
- 8.Rezazadeh A, Laber DA, Ghim SJ, et al. The role of human papilloma virus in lung cancer: a review of the evidence. Am J Med Sci. 2009;338:64–67. doi: 10.1097/MAJ.0b013e3181a393ba. [DOI] [PubMed] [Google Scholar]
- 9.Castro CY, Ostrowski ML, Barrios R, et al. Relationship between Epstein-Barr virus and lymphoepithelioma-like carcinoma of the lung: a clinicopathologic study of 6 cases and review of the literature. Hum Pathol. 2001;32:863–872. doi: 10.1053/hupa.2001.26457. [DOI] [PubMed] [Google Scholar]
- 10.Kirk GD, Merlo C, O’Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45:103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roselli M, Mineo TC, Martini F, et al. Soluble selectin levels in patients with lung cancer. Int J Biol Mark. 2002;17:56–62. doi: 10.5301/jbm.2008.911. [DOI] [PubMed] [Google Scholar]
- 12.Masuda K, Takano A, Oshita H, et al. Chondrolectin is a novel diagnostic biomarker and a therapeutic target for lung cancer. Clin Cancer Res. 2011;17:7712–7722. doi: 10.1158/1078-0432.CCR-11-0619. [DOI] [PubMed] [Google Scholar]
- 13.Spitz M, Wu X, Wilkinson A, et al. Cancer of the Lung. Oxford: Oxford University Press; 2006. [Google Scholar]
- 14.Läubli H, Borsig L. Selectins as mediators of lung metastasis. Cancer Microenviron. 2010;3:97–105. doi: 10.1007/s12307-010-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pine SR, Mechanic LE, Ambs S, et al. Lung cancer survival and functional polymorphisms in MBL2, an innate-immunity gene. J Natl Cancer Inst. 2007;99:1401–1409. doi: 10.1093/jnci/djm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baccarelli A, Hou L, Chen J, et al. Mannose-binding lectin-2 genetic variation and stomach cancer risk. Int J Cancer. 2006;119:1970–1975. doi: 10.1002/ijc.22075. [DOI] [PubMed] [Google Scholar]
- 17.Zuo Y, Ren S, Wang M, et al. Novel roles of liver sinusoidal endothelial cell lectin in colon carcinoma cell adhesion, migration and in vivo metastasis to the liver. Gut. 2013;62:1169–1178. doi: 10.1136/gutjnl-2011-300593. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Chen K, Yan L, et al. Low expression of dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin-related protein in non-Hodgkin lymphoma and significant correlations with lactic acid dehydrogenase and b2-microglobulin. Biochem Cell Biol. 2013;91:214–220. doi: 10.1139/bcb-2012-0110. [DOI] [PubMed] [Google Scholar]
- 19.Ding D, Chen W, Zhang C, et al. Low expression of dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin in non-Hodgkin lymphoma and a significant correlation with b2-microglobulin. Med Oncol. 2014;31:202–214. doi: 10.1007/s12032-014-0202-6. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Zhang C, Chen K, et al. The clinical significance of DC-SIGN and DC-SIGNR, which are novel markers expressed in human colon cancer. PLoS One. 2014;9:e114748. doi: 10.1371/journal.pone.0114748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pohlmann S, Soilleux EJ, Baribaud F, et al. DC-SIGNR, a DC-SIGN homologue expressed in endothelial cells, binds to human and simian immunodeficiency viruses and activates infection in trans. Proc Natl Acad Sci USA. 2001;98:2670–2675. doi: 10.1073/pnas.051631398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinberg H, Mitchell DA, Drickamer K, et al. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–2166. doi: 10.1126/science.1066371. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR: subunit organization and binding to multivalent ligands. J Biol Chem. 2001;276:28939–28945. doi: 10.1074/jbc.M104565200. [DOI] [PubMed] [Google Scholar]
- 24.Kwon DS, Gregorio G, Bitton N, et al. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16:135–144. doi: 10.1016/S1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 25.Chan VS, Chan KY, Chen Y, et al. Homozygous L-SIGN (CLEC4 M) plays a protective role in SARS coronavirus infection. Nat Genet. 2006;38:38–46. doi: 10.1038/ng1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoo US, Chan KY, Chan VS, et al. DC-SIGN and L-SIGN: the SIGNs for infection. J Mol Med. 2008;86:861–874. doi: 10.1007/s00109-008-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder GA, Ford J, Torabi-Parizi P, et al. Characterization of DC-SIGN/R interaction with human immunodeficiency virus type 1 gp120 and ICAM molecules favors the receptor’s role as an antigen-capturing rather than an adhesion receptor. J Virol. 2005;79:4589–4598. doi: 10.1128/JVI.79.8.4589-4598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brambilla E, Travis WD, Colby TV, et al. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18:1059–1068. doi: 10.1183/09031936.01.00275301. [DOI] [PubMed] [Google Scholar]
- 29.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 30.Ampil F, Caldito G, Milligan S, et al. The elderly with synchronous non-small cell lung cancer and solitary brain metastasis: does palliative thoracic radiotherapy have a useful role. Lung Cancer. 2007;57:60–65. doi: 10.1016/j.lungcan.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Nolte SM, Venugopal C, McFarlane N, et al. A cancer stem cell model for studying brain metastases from primary lung cancer. J Natl Cancer Inst. 2013;105:551–562. doi: 10.1093/jnci/djt022. [DOI] [PubMed] [Google Scholar]
- 32.Hanibuchi M, Kim SJ, Fidler IJ, et al. The molecular biology of lung cancer brain metastasis: an overview of current comprehensions and future perspectives. J Med Invest. 2014;61:241–253. doi: 10.2152/jmi.61.241. [DOI] [PubMed] [Google Scholar]
- 33.Preusser M, Winkler F, Collette L, et al. Trial design on prophylaxis and treatment of brain metastases: lessons learned from the EORTC Brain Metastases Strategic Meeting 2012. Eur J Cancer. 2012;48:3439–3447. doi: 10.1016/j.ejca.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Pang X, Min J, Liu L, et al. S100B protein as a possible participant in the brain metastasis of NSCLC. Med Oncol. 2012;29:2626–2632. doi: 10.1007/s12032-012-0169-0. [DOI] [PubMed] [Google Scholar]
- 35.Jiang W, Jia Q, Liu L, et al. S100B promotes the proliferation, migration and invasion of specific brain metastatic lung adenocarcinoma cell line. Cell Biochem Funct. 2011;29:582–588. doi: 10.1002/cbf.1791. [DOI] [PubMed] [Google Scholar]
- 36.Marchi N, Mazzone P, Fazio V, et al. ProApolipoprotein A1: a serum marker of brain metastases in lung cancer patients. Cancer. 2008;112:1313–1324. doi: 10.1002/cncr.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapural M, Krizanac-Bengez L, Barnett G, et al. Serum S100β as a possible marker of blood-brain barrier disruption. Brain Res. 2002;940:102–104. doi: 10.1016/S0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]
- 38.Vivier E, Tomasello E, Baratin M, et al. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 39.Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011;2011:676198. doi: 10.1155/2011/676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Imai K, Matsuyama S, Miyake S, et al. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 41.Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. doi: 10.1002/(SICI)1097-0142(20000201)88:3<577::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 42.Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(SICI)1097-0142(19970615)79:12<2320::AID-CNCR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 43.Villegas FR, Coca S, Villarrubia VG, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/S0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 44.Takanami I, Takeuchi K, Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2001;121:1058–1063. doi: 10.1067/mtc.2001.113026. [DOI] [PubMed] [Google Scholar]
- 45.Koppel EA, Ludwig IS, Hernandez MS, et al. Identification of the mycobacterial carbohydrate structure that binds the C-type lectins DC-SIGN, L-SIGN and SIGNR1. Immunobiology. 2004;209:117–127. doi: 10.1016/j.imbio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, et al. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2005;2:e381. doi: 10.1371/journal.pmed.0020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


