Case report
A 62-year-old woman with a 32 pack-year history of tobacco use and a distant history of early-stage breast cancer treated with breast conserving therapy and tamoxifen was diagnosed with metastatic lung adenocarcinoma involving the right lung, thoracic lymph nodes, and vertebral spine. The PD-L1 tumor proportion score was 90% and next generation sequencing of >400 cancer related genes at our institution1 demonstrated the presence of a BRAF V600E mutation; the sample was negative for oncogenic alterations in KRAS, EGFR, ALK, ROS1, MET, and RET. Plasma genotyping also identified a BRAF V600E mutation and no other significant oncogenic driver mutations.
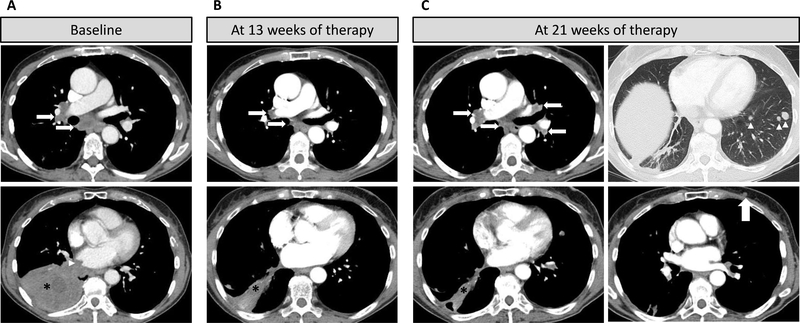
The patient’s cancer progressed after three cycles of first-line carboplatin and pemetrexed and again after three cycles of second-line pembrolizumab (Figure 1A). She was then started on dabrafenib and trametinib and experienced marked response to therapy with a decrease in the sizes of the dominant lung mass, satellite lung nodules and mediastinal adenopathy, reaching the nadir of tumor burden at 13 weeks (Figure 1B). However, at 21 weeks of therapy, while the dominant right lower lobe mass remained small, she developed progressive disease with the development of multiple new lung nodules and a new chest wall nodule (Figure 1C). An excisional biopsy of a chest wall nodule confirmed metastatic lung adenocarcinoma. Repeat sequencing of both the tumor and plasma revealed the acquisition of an NRAS Q61K mutation in addition to the original BRAF V600E mutation (Figure 2). No other clear genomic mechanisms of resistance to dabrafenib and trametinib were identified.
Figure 1:
Computed tomography imaging shows initial response to combination dabrafenib and trametinib and subsequent progression
A. CT scan of the chest at baseline prior to dabrafenib plus trametinib therapy demonstrated a dominant consolidated mass (asterisk) in the right lower lobe with heterogenous CT density and invasion into the right atrium (arrowhead), and right hilar and subcarinal lymphadenopathy (arrows).
B. CT scan at 13 weeks of therapy demonstrated a marked response to therapy with significant decrease of the dominant mass (asterisk) and lymph nodes (arrows).
C. CT scan at 21 weeks of therapy demonstrated continued response in the dominant right lower lobe lesion (asterisk) but progressive disease with increased bilateral hilar and subcarinal lymphadenopathy (arrows), multiple new lung nodules (arrowheads) and a new left chest wall nodule (large arrow).
Figure 2:
Tumor and cell-free DNA sequencing at diagnosis and after progression on combination dabrafenib and trametinib shows acquisition of an NRAS Q61K mutation
A. Aligned sequencing reads from a pre-treatment tumor biopsy (top) detected a BRAF c. 1799T>A point mutation (p.V600E); sequencing of a repeat biopsy after progression on combination dabrafenib and trametinib (bottom) demonstrated persistence of the BRAF 1799T>A mutation and the acquisition of a NRAS c.181C>A point mutation (p.Q61K).
B. Commercial plasma genotyping also detected BRAF V600E in both pre-treatment and post-treatment samples, but NRAS Q61K was only detected after progression on combination dabrafenib and trametinib.s
Due to a rapidly declining performance status, the patient transitioned to hospice care and she died one month later.
Discussion
Activating mutations in BRAF have been identified in a diverse array of malignancies, including approximately 1–2 percent of patients with non-small cell lung cancer (NSCLC)2–4. In June 2017, the U.S. Food and Drug Administration approved the combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib for patients with metastatic BRAF V600E mutant NSCLC, with an overall response rate of 64% and a disease control rate of 75% in the first line setting and an overall response rate of 63% and a disease control rate of 79% as subsequent line therapy5,6. Unfortunately, the duration of response is limited, with a median progression free survival of 10.9 months or 9.7 months as first line or subsequent line therapy respectively5,6.
Resistance to targeted therapies can be acquired through multiple mechanisms including genomic alterations of the target (including mutations and amplification) and/or bypass track activation. While multiple mechanisms of resistance to combined RAF and MEK inhibition have been described in melanoma that serve to reactivate MAPK signaling include BRAF amplification, resistance-associated BRAF splice variants, and gain-of-function RAS and/or MEK mutations7–9, but mechanisms of resistance to these drugs in NSCLC have not been elucidated.
In this patient, acquired resistance to combined BRAF and MEK inhibition in NSCLC was coincident with the appearance of a gain-of-function NRAS mutation on both tumor and cell-free DNA sequencing suggesting a dominant/clonal resistance mechanism. Interestingly, while NRAS mutations are only rarely identified as potential driver mutations in NSCLC10, they have also been implicated in acquired resistance to targeted therapies in NSCLC including EGFR11 and RET12 inhibition.
Our finding is also in line with previous observations implicating NRAS Q61K in resistance to both single agent BRAF inhibition13 as well as combination BRAF/MEK inhibition8,9 in melanoma. In the case of combination BRAF and MEK inhibition in NSCLC, it is not clear if this mutation leads to resistance by overcoming MAPK pathway inhibition, for example by activating compensatory signaling though other RAF family members14, or by activating the PI3K pathway or by a combination of these two mechanisms. As such, the optimal approach to overcome this resistance is unclear at this time, though emerging approaches to directly targeting RAS could prove useful in either case.
Footnotes
Conflict of interest statement:
Dr. Abravanel has nothing to disclose.
Dr. Nishino reports personal fees from WorldCare Clinical, Toshiba Medical Systems, and Daiichi Sankyo; grants from Merck, Toshiba Medical Systems, AstraZeneca and the NIH; and, personal fees from Bayer and Roche, all outside the submitted work.
Dr. Sholl has nothing to disclose.
Dr. Ambrogio has nothing to disclose.
Dr. Awad has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1(19). doi: 10.1172/JCI.INSIGHT.87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 3.Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455(7216):1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19(16):4532–4540. doi: 10.1158/1078-0432.CCR-13-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17(7):984–993. doi: 10.1016/S1470-2045(16)30146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18(10):1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 7.Wagle N, Van Allen EM, Treacy DJ, et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discov. 2014;4(1):61–68. doi: 10.1158/2159-8290.CD-13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long G V, Fung C, Menzies AM, et al. Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat Commun. 2014;5:1–9. doi: 10.1038/ncomms6694. [DOI] [PubMed] [Google Scholar]
- 9.Moriceau G, Hugo W, Hong A, et al. Tunable-Combinatorial Mechanisms of Acquired Resistance Limit the Efficacy of BRAF/MEK Cotargeting but Result in Melanoma Drug Addiction. Cancer Cell. 2015;27(2):240–256. doi: 10.1016/j.ccell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohashi K, Sequist L V, Arcila ME, et al. Characteristics of lung cancers harboring NRAS mutations. Clin Cancer Res. 2013;19(9):2584–2591. doi: 10.1158/1078-0432.CCR-12-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ninomiya K, Ohashi K, Makimoto G, et al. MET or NRAS amplification is an acquired resistance mechanism to the third-generation EGFR inhibitor naquotinib. Sci Rep. 2018;8(1):1955. doi: 10.1038/s41598-018-20326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson-Taylor SK, Le AT, Yoo M, et al. Resistance to RET-Inhibition in RET-Rearranged NSCLC Is Mediated By Reactivation of RAS/MAPK Signaling. Mol Cancer Ther. 2017;16(8):1623–1633. doi: 10.1158/1535-7163.MCT-17-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorard C, Estrada C, Barbotin C, et al. RAF proteins exert both specific and compensatory functions during tumour progression of NRAS-driven melanoma. Nat Commun. 2017;8:15262. doi: 10.1038/ncomms15262. [DOI] [PMC free article] [PubMed] [Google Scholar]