Abstract
Background:
Identification of a peripheral biomarker is a major roadblock in the diagnosis of Parkinson’s disease (PD). Immunohistological identification of p-serine 129 (pS129) α-synuclein (αSyn) in the submandibular gland (SMG) tissues of PD patients has been recently reported.
Objective:
We report on a proof-of-principle study for using an ultra-sensitive and specific, real-time quaking-induced conversion (RT-QuIC) assay to detect the pathological α-synuclein (paS) in the SMG tissues of PD patients.
Methods:
The αSyn RT-QuIC assay was used to detect and quantify paS levels in PD, incidental Lewy body disease (ILBD), and control SMG tissues as well as in formalin-fixed paraffin-embedded (FFPE) sections.
Results:
We determined the quantitative seeding kinetics of paS present in SMG tissues from autopsied subjects using the αSyn RT-QuIC assay. A total of 32 cases comprising 13 PD, 3 ILBD, and 16 controls showed 100% sensitivity and 94% specificity. Interestingly, both PD and ILBD tissues showed 100% concordance for elevated levels of paS seeding activity compared to control tissues. End-point dilution kinetic analyses revealed that SMG had a wide dynamic range of paS seeding activity.
Conclusions:
Our results are the first to demonstrate the utility of using the RT-QuIC assay on peripherally accessible SMG tissues and FFPE tissue sections to detect PD-related pathological changes with high sensitivity and specificity. Additionally, the detection of seeding activity from ILBD cases containing immunohistochemically undetected paS demonstrates the αSyn RT-QuIC assay’s potential utility for identifying prodromal PD in SMG tissues.
Keywords: formalin-fixed paraffin-embedded (FFPE), pathological α-synuclein (paS), Parkinson’s disease (PD), real-time quaking-induced conversion (RT-QuIC), submandibular gland (SMG)
Introduction
Parkinson’s disease (PD) is the most common movement disorder, affecting predominantly the elderly population over the age of 60 years. Genetically linked forms of PD are known to have an early onset. The prevalence, mortality, and disability-adjusted life-years of PD have doubled in the past decade and will continue to increase in the near future.1 The current state-of-the-art diagnosis of PD essentially relies on the clinical signs judged by a clinician2 and may be further augmented by functional dopaminergic imaging of the basal ganglia.3 However, by the time of onset of clinically diagnostic symptoms, about 75% of striatal dopaminergic terminals and 50% of cell bodies in the substantia nigra have been lost.4 The lack of reliable diagnostic markers for diagnosis of PD has been a challenge in clinical neurology. Retrospective studies have revealed no improvement in the accuracy of clinical PD diagnosis, especially in its early stages, over the past two decades.5 The accumulation of misfolded pathological α-synuclein (paS) in brainstem, limbic, and cortical structures remains the major histopathological hallmark for confirming the diagnosis of PD.6 The discovery of peripheral biomarkers that aid in the detection of PD-related pathological processes would substantially improve clinical diagnostic accuracy, subject selection for clinical trials, assessment of target engagement, and the monitoring of disease progression and/or response to therapy.7–9 Considerable effort has been made to develop biomarkers, such as αSyn protein, for patient samples that can be used as diagnostic tools. Recently, our group10–14 and others15–17 established an ultrasensitive protein seeding assay called real-time quaking-induced conversion (RT-QuIC) to quantify multiple disease-specific misfolded proteins, including αSyn and prions, in clinical samples. The αSyn RT-QuIC assay has detected paS in CSF and brain homogenate samples from PD and dementia with Lewy body (DLB) subjects with high sensitivity and specificity. Further advantages of this method include the identification of different α-synucleinopathy strains and diagnostic applications from other tissues in prion disease.10, 17 With these advancements in improved detection of paS in biofluids, it may now be plausibly applied to peripherally biopsied tissues.18–20 In the current study, we evaluated the diagnostic potential of paS present in the readily accessible submandibular gland (SMG) using this ultra-sensitive αSyn RT-QuIC assay. Beach et al.21–25 recently reported the presence of paS in the SMG by immunohistochemical staining for pS129 αSyn on slide-mounted, formalin-fixed paraffin-embedded (FFPE) SMG sections from both human autopsies and needle biopsies in live patients. Therefore, we hypothesized that human SMG is a suitable test material to detect paS using the αSyn RT-QuIC assay. To achieve this goal, we optimized the RT-QuIC assay to analyze autopsied SMG tissues and, FFPE SMG tissue sections, and were able to differentiate PD, ILBD, and control subjects. Overall, we provide the first evidence for the potential of SMG αSyn RT-QuIC as a sensitive, robust and quantitative assay for a PD-related pathological process.
Methods
Sample details
Frozen SMG tissues and slide-mounted FFPE SMG tissue sections were provided by the Banner Sun Health Research Institute (BSHRI; Phoenix, AZ). All subjects had been recruited and clinically followed to autopsy and neuropathological examination as part of the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program (www.brainandbodydonationprogram.org).26, 27 All cases were neuropathologically examined using specific diagnostic criteria for PD and based on previous published criteria.26, 28, 29 Cases with Lewy bodies but not meeting the diagnostic criteria for PD were considered as ILBD. Controls consisted of normal elderly subjects or subjects with AD-related changes but insufficient for AD diagnosis. Our study utilized SMG tissues from 13 PD, 3 ILBD, and 12 control subjects and SMG FFPE sections from matching subjects and an additional 4 controls. The details and pathological diagnostic summary of samples are presented in Tables S1 and S2, respectively. Since all human tissues were obtained from the Arizona Study of Aging and Neurodegenerative Disorders/Brain and Body Donation Program, a separate Institutional Review Board (IRB) approval from Iowa State University was not required.
Processing of SMG tissues and FFPE sections
Frozen tissue was collected fresh, kept frozen at −80 °C, then shipped on dry ice and stored again at −80 °C until tested in the αSyn RT-QuIC assay. A small amount of SMG tissue consisting of 30-100 mg was homogenized (10% w/v) in sterile phosphate-buffered saline (PBS) using a Bullet Blender (Next Advance) with 0.5-mm zirconium oxide beads for 2 min at maximum speed and 4 °C. The 10% homogenates were then used to make various dilutions ranging from 10−2 to 10−8 with sterile PBS. For FFPE sections, paraffin-embedded SMG sections (6-μm thick) from the same autopsy cases were mounted onto electrostatically charged glass slides and shipped to Iowa State University. To recover slide-mounted FFPE SMG sections, we washed the slides twice in 100% xylene for 10 min each. After the xylene washes, slides were submersed for 10 minutes each in a descending series of alcohol dilutions (100%, 95%, and 70%) to rehydrate the tissues. Next, the slides were washed with sterile PBS as a final step. After washing, SMG tissue sections were scraped off from slides and then weighed. From this point, they were used as a regular tissue homogenate in the αSyn RT-QuIC assay.
Brain homogenate preparation
Post-mortem human brain samples used for the end-point dilution analysis were provided by the University of Miami Brain Endowment Bank of the NIH NeuroBioBank. Substantia nigra tissues from pathologically confirmed PD cases were used to make 10% w/v homogenates in sterile PBS using a Bullet Blender with 0.5-mm Zirconium oxide beads for 2 min at maximum speed. The 10% homogenates were used to make dilutions ranging from 10−2 to 10−7 using PBS and tested in the αSyn RT-QuIC assay.
Purification of recombinant human αSyn protein by fast protein liquid chromatography (FPLC)
Purification of recombinant human WT αSyn was performed as described in our recent publication.10 In brief, E. coli Rosetta DE2 cells were transformed with a human WT αSyn-expressing plasmid and plated on LB agar containing kanamycin (50 μg/mL). Single colonies from this culture were individually inoculated into tubes containing 5 mL LB media supplemented with kanamycin. The mini-cultures were grown overnight in an incubator at 225 rpm and 37 °C and expanded to 1 L cultures. When the bacterial cultures reached an OD600 of 0.7, 1 mM IPTG was added to induce αSyn expression, and after 4.5 h, cultures were harvested by pelleting at 4200 x g for 20 min at 4 °C. Later, bacterial pellets were lysed by adding 10 mL of 50 mM Tris and 500 mM NaCl at pH 7.4 using an Omni tissue homogenizer and then heat-precipitated at 85 °C for 15 min. The tubes were cooled on ice and all the subsequent steps were carried out at 4 °C. The precipitates were removed by centrifugation at 15,000 x g for 20 min at 4 °C and DNA was precipitated out of the supernatants by incubating with streptomycin (10 mg/mL) for 30 min. The resulting solutions were centrifuged at 23,000 x g for 30 min, and the supernatants were diluted 6-fold and dialyzed using a 3-kDa molecular weight cut-off (MWCO) snakeskin dialysis tubing in a 20 mM Tris HCl buffer at pH 8 overnight at 4 °C. On the following morning, the dialyzed supernatants were concentrated and 0.2-μm filtered before loading onto a Sephacryl 200 column (GE Healthcare Life Sciences) for size-exclusion FPLC. The column was pre-equilibrated in 20 mM Tris-HCl buffer of pH 8 at 4 °C. Fractions having recombinant αSyn were determined based on Coomassie staining of the peak fractions. The fractions positive for recombinant αSyn were combined, concentrated, and 0.2-μm filtered before loading onto a HiPrep Q FF 16/10 anion-exchange column. After injecting into FPLC, a wash step was performed using 50 mM NaCl for 5 column volumes followed with a linear gradient in 10 column volumes up to 1 M NaCl in 20 mM Tris, pH 8, and αSyn protein was recovered between 300 and 350 mM NaCl. The peak fractions were collected and analyzed through polyacrylamide gel electrophoresis followed by Coomassie staining to visualize the protein fractions. Fractions having protein were pooled and dialyzed overnight in 20 mM Tris, pH 8, at 4 °C. On the next day, the buffer was replaced with a new buffer for one hour and the protein was 0.2-μm filtered. Protein concentrations were estimated using a NanoDrop spectrophotometer with an extinction coefficient of 0.5960 mg·mL−1·cm−1. The protein was stored in aliquots with a final concentration of ~1 mg at −80 °C until use.
Polyacrylamide gel electrophoresis
Protein fractions from size-exclusion or anion-exchange chromatography were mixed with an equal volume of 2X sample-loading buffer (2X Laemmli sample buffer, Bio-Rad) with 2-mercaptoethanol, vortexed, and then boiled for 5 min. Proteins were separated by polyacrylamide gel electrophoresis using 4-20% gradient gels (Bio-Rad) followed by staining with Coomassie blue and destaining.
Determining the purity and activity of recombinant αSyn protein as a suitable RT-QuIC substrate
The purity of monomeric recombinant αSyn was confirmed by Coomassie stain. Additionally, we compared the thioflavin T (ThT) fluorescence intensity between monomeric recombinant αSyn versus aggregated αSyn (αSynagg) as described in our recent publication.10 Briefly, 95 μL of 25 μM ThT was added to a 384-well plate with 2.5 μL of monomeric recombinant αSyn or αSynagg and incubated at room temperature for 2 min. Fluorescence readings were taken at excitation and emission wavelengths of 450 and 480 nm, respectively. We noticed an enhanced ThT fluorescence >80-fold with αSynagg compared to monomeric recombinant αSyn.
αSyn RT-QuIC assay
The αSyn RT-QuIC assay was performed as described previously10–14, 30 using a 96-well clear-bottom plate (Nalgene Nunc International). For all the αSyn RT-QuIC assays, the reaction mixture consisted of final concentrations of 40 mM phosphate buffer (pH 8.0), 170 mM NaCl, 10 μM ThT, 0.00125% sodium dodecyl sulfate (SDS) and 0.1 mg/mL of recombinant αSyn. Prior to each reaction setup, the recombinant αSyn was filtered through a 100-kDa MWCO filter to retain monomer filtrate. After optimizing the RT-QuIC assay test conditions for the SMG tissue homogenates, we tested the remaining samples using these conditions. Next, as a test validation, we performed blind testing of all the same samples in the αSyn RT-QuIC assay. To seed the RT-QuIC reactions, a 10−4 % w/v dilution of SMG tissue homogenates or a 10−2 % w/v dilution for FFPE tissue sections was used. Samples were homogenized to 10% w/v and diluted 10-fold with sterile PBS as described previously.10–14 Each reaction consisted of 5 μL of test sample as seed and 95 μL of αSyn RT-QuIC reaction mixture per well of a 96-well plate preloaded with six 0.8-mm silica beads (OPS Diagnostics) per well. Next, the plates were sealed with a plate sealer (Nalgene Nunc International) and reactions were initiated in a CLARIOstar (BMG) plate reader with alternating 1-min shake and rest cycles (double orbital, 400 rpm) at 42 °C. ThT fluorescence readings were recorded at excitation and emission wavelengths of 450 and 480 nm, respectively, every 30 min over a period of either 24 h for frozen samples or 60 h for FFPE tissue sections. Samples were run in quadruplicates and considered positive when at least two wells crossed the threshold fluorescence, defined as the average fluorescence of the first 10 cycles for all samples plus 10 standard deviations. The protein aggregation rate (PAR) for each sample was calculated by taking the inverse of the time required to cross the threshold fluorescence.
Immunoblotting
Conformation of the αSyn RT-QuIC end products was analyzed by dot blot using a Bio-Dot Microfiltration System as described previously. 10, 12 The RT-QuIC plate was collected and stored in −20 °C until analysis. Then, 2.5 μL of the end products from the RT-QuIC assay plate were diluted in 100 μL of PBS and blotted on to a nitrocellulose membrane for 1 h. Next, membranes incubated in 1X LI-COR blocking buffer (LBB) for 30 min after washing with 1X Tris-buffered saline (TBS) using a gentle vacuum. Later, membranes were incubated for one hour with a rabbit monoclonal αSyn filament conformation-specific antibody (MJFR-14-6-4-2; 1:2,000) and a mouse monoclonal total αSyn antibody (BD Biosciences, #610787; 1:2,000) and triple-washed with 1X TBS for 10 min each. The membranes were later incubated with secondary antibodies conjugated with IR dye (LI-COR) made in LBB (1:15,000) for 30 min followed by 3 washes with 1X TBS. Densitometric quantification of dots was done using ImageJ software.
Statistical analysis
GraphPad 7.0 was used for statistical analysis. Raw data were analyzed using Student’s two-sample t-test for comparing two groups and one-way ANOVA with Tukey’s post hoc test for comparing more than two groups. Asterisks were assigned as follows: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001. The number of biological replicates is expressed as “n” unless otherwise mentioned. JMP Pro 14 statistical software was used for multivariate comparisons. For quantification of seeding activity using endpoint dilution analysis, Spearman-Kärber analysis was performed as described previously.31
Results
Determination of optimal test conditions for the SMG αSyn RT-QuIC assay
We10–14 and others32 have previously shown that an optimal concentration of SDS in the reaction mixture is critical for the sensitivity of the RT-QuIC assay. Therefore, we first optimized the SDS concentration to detect paS seeding activity in the SMG RT-QuIC assay. Initially, we ran the αSyn RT-QuIC assay with and without SDS on SMG tissue homogenates prepared from PD and control (n=6 each) cases obtained from BSHRI. We observed an increase in ThT fluorescence and protein aggregation rate (PAR) as determined based on the inverse of time to cross the threshold fluorescence, with 0.00125% SDS (Fig 1A & 1B) compared to the reactions seeded without SDS in the reaction mixture (Fig 1C & 1D). Well-scan pattern displayed a greater deposition of paS amyloids in a PD sample compared to control SMG sample (Fig. 1E). Reactions without SDS exhibited delayed RT-QuIC amplification with substantially increased lag periods. Thus, the 0.00125% SDS was determined to be a rate-limiting factor for efficient amplification of paS in the SMG αSyn RT-QuIC assay. Next, using the optimized reaction conditions, we assessed the reproducibility of RT-QuIC by repeating the experiment three times. The repeated assay results were identical, suggesting a high reproducibility for αSyn RT-QuIC to determine the seeding activity of SMG paS.
Figure 1.
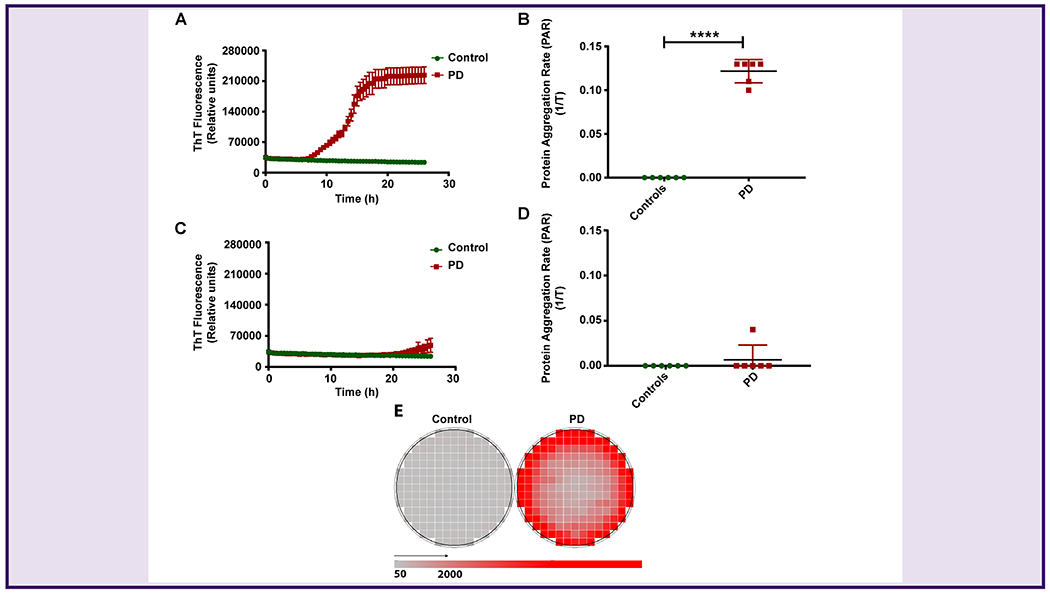
Optimization of the αSyn RT-QuIC assay for SMG homogenates.
(A, B) Testing of SMG samples in the αSyn RT-QuIC assay using 0.00125% SDS in the RT-QuIC reaction mixture. PD samples showed enhanced ThT fluorescence (A) and higher PAR (B) compared to control samples. (C, D) Testing of SMG samples in the αSyn RT-QuIC assay without SDS in the RT-QuIC reaction mixture. The lag period for enhanced ThT fluorescence increased in PD cases compared to controls (C), and no significant differences in PAR were observed between controls and PD (D). (E) Well-scan image of control and PD SMG samples showing deposition of protein aggregates in a PD sample in response to shake and rest cycles (red color) of the plate reader compared to a control (grey color). Each symbol represents the average of 4 technical replicates. A total of 6 PD and 6 controls were tested in the αSyn RT-QuIC assay. Student’s two-sample t-test was used to compare the two groups data expressed here as the mean and standard error of 4 technical replicates. ****p ≤ 0.0001.
Rapid detection of paS seeding activity in SMG tissues
After establishing the optimal SDS concentration in the SMG RT-QuIC assay, we tested the paS seeding activity of frozen SMG tissues collected from 13 PD, 3 ILBD, and 12 age-matched control subjects. The SMG homogenates from PD cases rapidly seeded the reactions, crossing the threshold fluorescence within 7 h, whereas paS seeding activity in ILBD tissue homogenates needed 20 h. In contrast, paS seeding activity in SMG tissue homogenates from controls did not occur during the 24-h period (Fig 2A). Similarly, SMG tissue homogenates from PD and ILBD samples exhibited significantly higher PARs when compared with the age-matched control samples (Fig 2B). When we blindly tested all the SMG homogenates, results are highly correlated, thereby further enhancing the rigor of the αSyn RT-QuIC assay. These results demonstrate that the RT-QuIC determination of paS seeding activity and PAR may be used to effectively discriminate PD, ILBD, and control samples. To validate the successful amplification of misfolded αSyn in the RT-QuIC assay, we subjected the RT-QuIC end products present in the 96-well plate to a dot blot analysis with an αSyn filament conformation-specific antibody. The immunoblots also were stained for total αSyn to reveal the presence of total αSyn levels among these groups. Consistent with the RT-QuIC ThT fluorescence data, the RT-QuIC end products of PD cases contained the highest levels of misfolded αSyn compared to ILBD and control cases, whereas total αSyn levels were higher in controls compared to ILBD and PD (Fig 2C & 2D).
Figure 2.
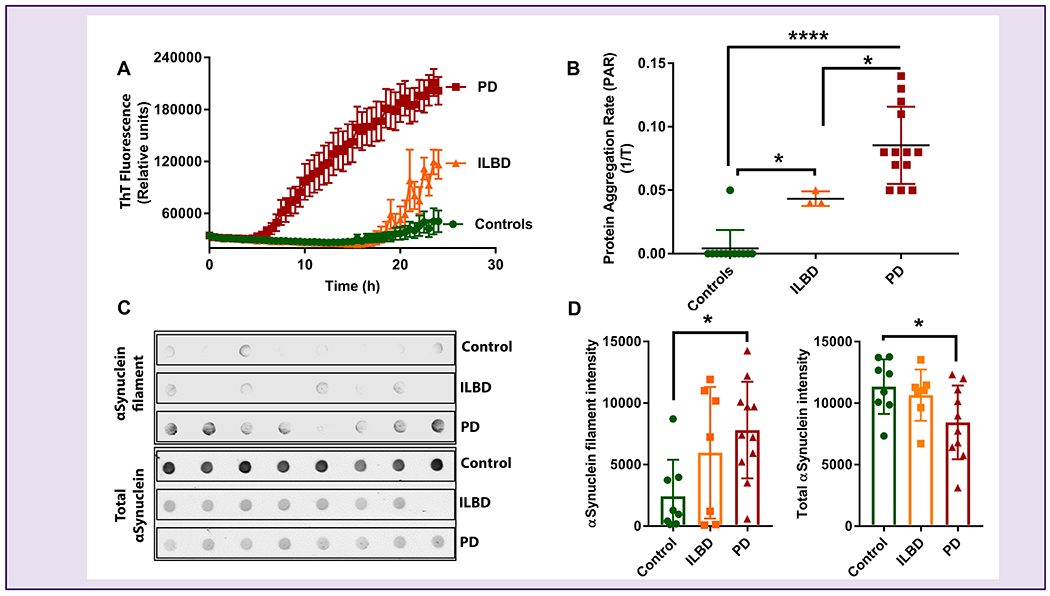
Reproducible detection of paS seeding activity in SMG homogenates.
(A) ThT fluorescence in PD, ILBD, and control SMG homogenates, showing more paS in PD and ILBD samples. (B) Comparison of PAR between the PD, ILBD, and control SMG homogenate samples showing higher paS load in PD and ILBD. We tested 13 PD (red), 3 ILBD (orange), and 12 control (green) samples in the αSyn RT-QuIC assay. One-way ANOVA was used to compare the three groups and all samples were tested in quadruplicates and expressed as the mean and standard error of 4 technical replicates. (C) A representative Dot blot image consisting of PD, ILBD, and control SMG RT-QuIC end products with αSyn filament conformation-specific (top panel) and total αSyn (bottom panel) antibodies. (D) Densitometric analyses of αSyn filament and total αSyn levels, showing that the RT-QuIC end products of PD wells showed the highest levels of misfolded αSyn compared to ILBD and control wells, while total αSyn levels were higher in controls compared to other groups. ****p ≤ 0.0001, *p ≤ 0.05.
Detection of paS seeding activity in FFPE sections of SMG tissues
Next, we sought to determine whether the αSyn RT-QuIC assay can be adopted for detecting paS in slide-mounted FFPE autopsied SMG tissue sections (6-μm thick) obtained from the same population of immunohistologically confirmed PD (n=13), ILBD (n=3), and control (n=16) subjects. The FFPE sections of autopsied SMG tissues were processed for RT-QuIC as described in the Methods. Remarkably, our RT-QuIC results showed that the SMG FFPE section-derived homogenates from PD cases rapidly seeded the reactions within 10 h, as determined by the time to cross the threshold fluorescence (Fig 3A). While the 3 ILBD FFPE sections required 30-40 h to cross the threshold fluorescence, control FFPE sections did not cross the threshold fluorescence up to 40 h (Fig. 3A). As such, SMG FFPE sections from PD samples exhibited a significantly higher PAR than both ILBD and age-matched control samples (Fig 3B). Overall, these results clearly demonstrate the potential diagnostic utility of the αSyn RT-QuIC assay on peripherally accessible SMG biopsy tissue specimens from histological slides.
Figure 3.
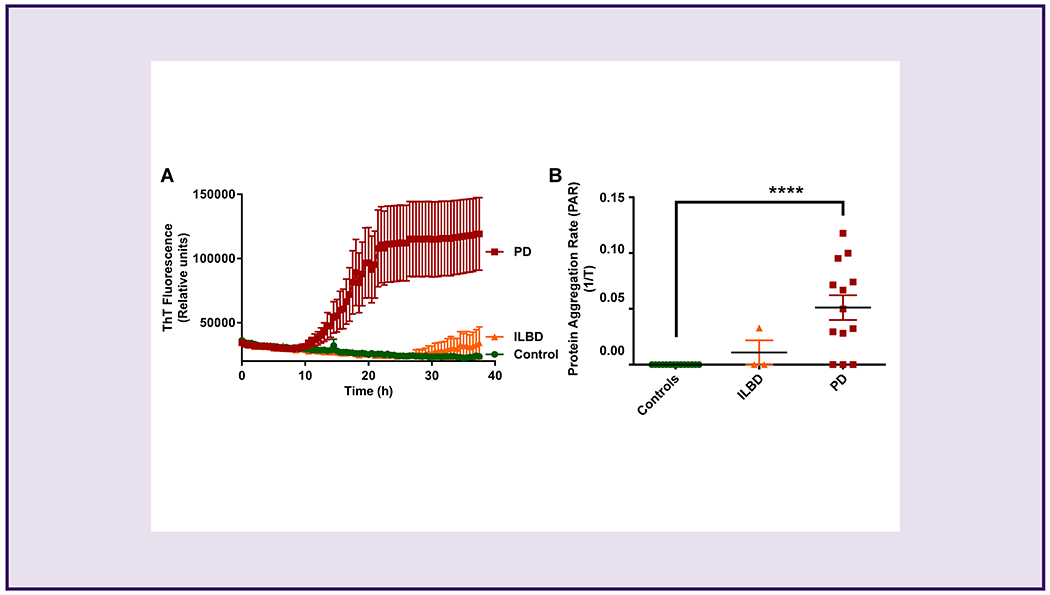
Detection of paS seeding activity in SMG FFPE sections.
(A) ThT fluorescence in PD, ILBD, and control SMG homogenates, showing more paS in PD and ILBD samples. (B) Comparison of PAR between the PD, ILBD, and control samples showing higher paS load in PD than in ILBD and control SMG homogenates. No statistical significance was observed between the ILBD and control SMG homogenates. We tested 13 PD (red), 3 ILBD (orange), and 16 control (green) samples in the αSyn RT-QuIC assay. One-way ANOVA with Tukey’s post hoc test was used to compare the three groups, and all samples were tested in quadruplicates and expressed here as the mean and standard error of 4 technical replicates. ****p ≤ 0.0001.
Quantitative assessment of paS seeding activity in SMG and brain tissues from PD cases
We further compared SMG and brain tissue homogenates from confirmed PD cases in an end-point dilution analysis to determine the relative seeding activity of paS from the two tissues. Spearman-Kärber analyses were performed to determine the seeding-competent dilutions based on the percentage of the RT-QuIC reactions that turn positive in each dilution tested. This analysis provides an estimation of the median seeding dose (SD50) representing the seeding concentration at which 50% of the replicates turn positive in the RT-QuIC assay. For brain tissues, we tested log-fold dilutions from 10−3 to 10−7 in quadruplicates (Fig 4A). Positive reactions were observed in all four replicates of 10−3 and 10−4, in two for 10−5, and one replicate amplified at 10−6. None of the replicates amplified in the 10−7 dilution. For SMG tissues, we tested two samples with log-fold dilutions from 10−2 to 10−8 in quadruplicates in the αSyn RT-QuIC assay. All four replicates (100%) of the two SMG cases exhibited positive reactions in dilutions ranging from 10−2 to 10−5, while 75% and 25% of the replicates showed positive reactions in 10−6 and 10−7, respectively (Fig 4B). None of the replicates amplified in the 10−8 dilution. Brain homogenate and SMG tissues showed SD50 titers of 8.8x108 and 2x106 per mg of tissue, respectively. These results support SMG tissue as an efficient seeding material whose high dynamic range on the αSyn RT-QuIC assay approaches that of brain homogenates, albeit with differences in the RT-QuIC reaction mixture composition in both types of samples. Collectively, these results further reveal that SMG biopsy tissues of PD patients harbor misfolded αSyn with high seeding activity.
Figure 4.
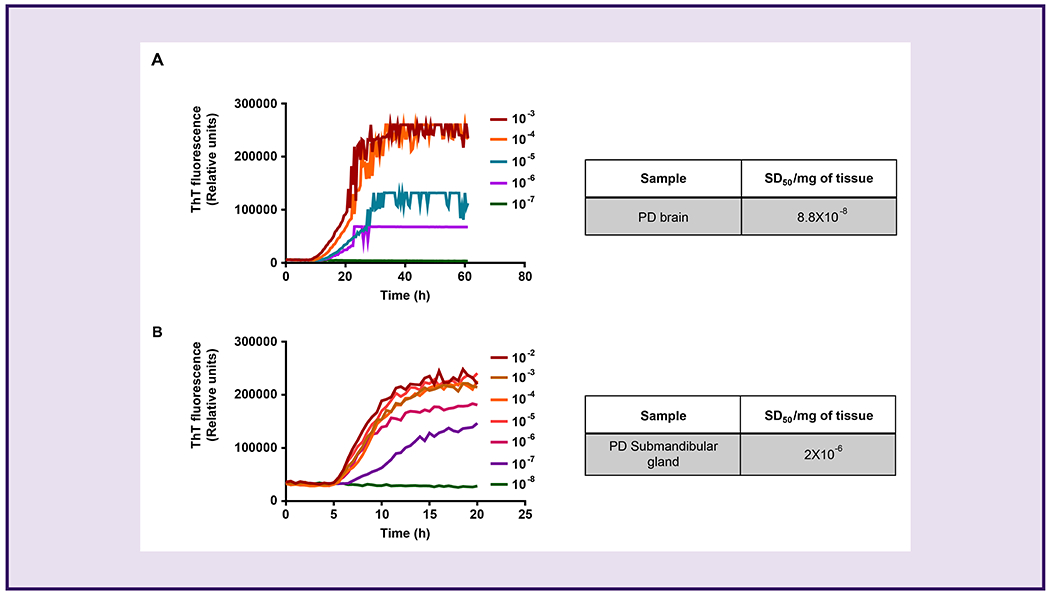
Determination of median seeding dose (SD50) of paS in PD samples using the αSyn RT-QuIC assay.
End-point dilutions of PD brain homogenates (A) and SMG tissue samples (B) by αSyn RT-QuIC. Each trace represents the average of 4 replicates. Tables to the right of each graph indicate the concentration of SD50 units calculated by Spearman-Kärber analysis representing one brain homogenate and two SMG samples.
Discussion
Despite the lack of clinically reliable biomarkers for PD-related pathology, their development has been a major goal of several biomarker studies. The protein αSyn is implicated not only in the etiopathology of PD, but also in the development of a biomarker for PD.33 Extensive efforts have been made to identify the αSyn present in the cerebrospinal fluid (CSF) of PD cases and to assess its use as a diagnostic biomarker.34 Mollenhauer et al, measured the levels of αSyn present in the CSF of PD and healthy cases using an enzyme-linked immunosorbent assay (ELISA) and found lower levels of total αSyn in PD patients relative to healthy controls. Other forms of αSyn, such as its oligomeric or phosphorylated forms, were increased in the CSF of PD patients compared to controls.35 Overall, a trend of decreased total αSyn levels and increased oligomeric or phosphorylated αSyn levels in the CSF samples of PD compared to controls were reported.36–38 In a similar manner, total, oligomeric, and phosphorylated forms of αSyn 39–41 have also been reported in the plasma, demonstrating the potential biomarker role for plasma αSyn. However, these tests are difficult to employ as clinically supportive diagnostic assays due to inconsistences in the detection of paS in the biological samples, lack of reproducibility and complexity in antibody-based approaches, and low sensitivities and specificities.42–45
Recent developments in protein misfolding assays, such as RT-QuIC and protein misfolding cyclic amplification (PMCA), have made the rapid and quantitative detection of misfolded proteins in biological samples possible thus allowing to overcome some challenges posed by antibody based methods discussed.11, 12, 46–48 RT-QuIC was first established as a diagnostic tool for prion diseases due to its ultra-sensitive detection of prions from a variety of samples.10–12, 14, 49, 50 Since αSyn shares an aggregation mechanism similar to prion propagation, misfolding techniques, such as PMCA and RT-QuIC, have been used to detect paS in brain and CSF from patients affected by synucleinopathies.10, 15, 51–53 However, both approaches have limitations. CSF sampling is more invasive and is often associated with health risks including infection, back pain, and headaches, and brain tissue is only available after autopsy.54 Therefore, utilizing more peripherally biopsied tissues may be a preferable strategy. In this regard, Beach and colleagues25, 55 examined tissue sections from autopsied PD patients and demonstrated that the SMG, which embeds components of the peripheral nervous system, exhibited significantly higher pS129 αSyn immunoreactivity compared to age-matched control sections. Also, it was previously reported that the SMG possesses the highest burden of phosphorylated αSyn compared to other biopsy sites.5 Therefore, immunohistochemical detection of pS129 αSyn in autopsied and biopsied SMGs has since been shown to be a highly specific biomarker of PD and dementia with Lewy bodies.21, 24,22, 23 However, current immunohistochemical methods for specific detection of paS require pathology expertise to accurately interpret the slides and may have inherently challenges associated with antibody based assays and lower sensitivity as compared to RT-QuIC assays.
To address the potential gaps in biomarker-based diagnostic approaches, we adopted an αSyn RT-QuIC assay to detect the seeding activity associated with paS from the SMG tissues of autopsied PD patients. In this study, we demonstrate paS seeding activity from both frozen and FFPE SMG sections of PD and ILBD patients. In this study, SMG RT-QuIC was able to discriminate PD and ILBD subjects from age-matched controls with 100% sensitivity and 94% specificity in frozen tissues and 76% sensitivity and 100% specificity in FFPE sections. These findings have important implications for developing an RT-QuIC assay on SMG biopsy tissues from live patients.22 When frozen tissues were tested, we achieved 100% sensitivity with SMG RT-QuIC, but the relatively low specificity (94%) might be due to both the age and AD-related pathology of the control subjects.56 The FFPE slides we tested demonstrated a 100% specificity but with a low sensitivity, revealing important implications of RT-QuIC when testing archived samples. The perfect specificity may be due to the tissue processing steps that would remove factors or contaminants capable of triggering non-specific amplification in the RT-QuIC assay. However, the low sensitivity (76%) may be due to insufficient quantity of seeds obtained from the 6-μm thick slides. Despite successfully detecting paS seeding activity from SMG tissues, one limitation associated with the collection of core needle biopsies is an insufficient amount of glandular tissue captured in the needle bore, which can be overcome by pooling repeated biopsies or by using larger core needles.22, 23 However, this may not be a major limitation given the RT-QuIC’s ability to detect pathological seeding from minuscule protein concentrations. Another limitation is that the SMG biopsy procedure is invasive and although rare, adverse events (AEs) such as hematoma, infection, and facial nerve damage have been documented.57 Recently, other groups58 also reported the occurrence of AEs associated with the collection of SMG biopsies as due to an increasing number of penetrations into the gland with the core needle biopsy tool, hence considerations should be given in future studies to minimize such events. Furthermore, to confirm the specific RT-QuIC amplification associated with the misfolded αSyn levels, we tested the RT-QuIC end products using an αSyn filament conformation-specific antibody and found the highest levels of misfolded αSyn in PD patients compared to ILBD and controls. Overall, these results demonstrate that RT-QuIC-based seeding activity may now be extended to testing larger cohort sets of both frozen tissues and FFPE sections of SMG. A schematic representation of the scope of this assay as a potential biomarker for PD and the key steps involved in sample collection and testing are depicted in Fig 5. Moreover, the associations of RT-QuIC PAR values with age, sex, MMSE scores, post-mortem interval, Braak stages, and the amount of pathological α-synuclein were assessed using multivariate regression analysis. In control, ILBD, and PD cases, no effect of sex (p=0.5681), age (p=0.2809), MMSE scores (p=0.2142), UPDRS scores (p=0.5633), Braak staging (p=0.5668), or post-mortem time (p=0.4876) was observed on RT-QuIC assay results. Only pathological discrimination of PD from control and the amount of paS based on unified LB staging differed significantly (p<0.0001) with RT-QuIC PAR values (Table S3). Although our RT-QuIC method is robust at distinguishing PD from control samples, due to the lack of association of PAR with disease severity (e.g. UPDRS), this method in its current form is unlikely to serve as a biomarker of disease progression or response to therapy. However, inclusion of additional cases is expected to yield much stronger correlations between PAR values and MMSE scores, UPDRS scores, and Braak staging. Thus, the full utility of the RT-QuIC assay in biomarker discovery can only be properly realized in a longitudinal study of biopsied patients stretching from the early prodromal stages all the way to autopsy. Such a study would allow the systematic comparison of paired RT-QuIC and IHC analyses of repeated biopsies collected over the prolonged course of the disease. Altogether, our results demonstrate that the ultrasensitive RT-QuIC assay can be readily employed for detection of paS in SMG biopsy samples, enabling an easy, relatively less-invasive approach for molecular diagnosis of PD and potentially other related synucleinopathies. Although the αSyn RT-QuIC assay has previously been shown to differentiate the strains in synucleinopathy cases using brain tissue17, the use of SMG αSyn RT-QuIC for the differential diagnosis of other Parkinsonism-related disorders such as DLB, MSA, and PDD as well as other αSyn strain typing remains to be explored. Prospective studies are warranted to test the performance of the assay in clinical settings.
Figure 5.
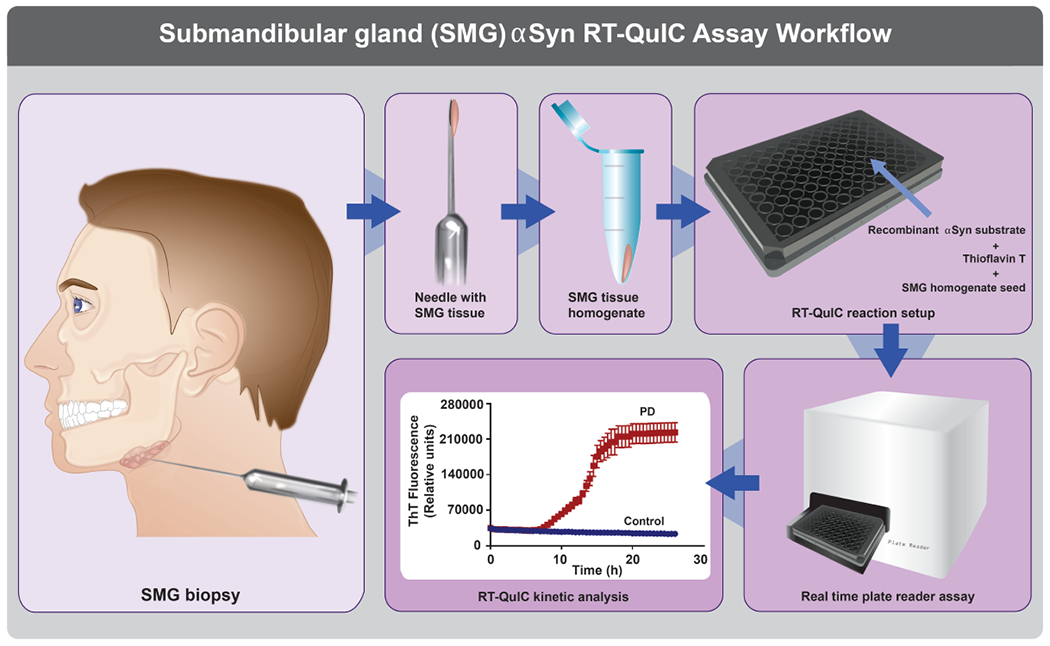
Schematic representation of the αSyn RT-QuIC assay as a tool for monitoring PD using SMG biopsy.
SMG tissue sampled using needle biopsies is processed for testing in the RT-QuIC assay to assist in clinical practice. The RT-QuIC workflow runs in a high-throughput format using a 96-well plate. Each well consists of 5 μL of SMG homogenate as a seed and 95 μL of αSyn RT-QuIC reaction mixture containing recombinant human αSyn protein as a substrate. During the reaction process, the samples with paS show higher ThT fluorescence compared to controls, and the reactions can be monitored in real-time with final data processing being completed in a 24-48 h window for visualization and reporting. The SMG αSyn RT-QuIC assay may find clinical applications in PD diagnosis, progression, and therapy monitoring.
Supplementary Material
Acknowledgments
The Lloyd and Armbrust endowments to AGK and Salisbury endowment to AK, and Iowa State University Big Data Brain Initiative are also acknowledged. We thank Dr. Thomas G. Beach for his critical review of the manuscript and providing the submandibular gland tissues. We would like to acknowledge the Brain and Body Donation Program at Banner Sun Health Research Institute for maintaining the tissues. We thank Dr. Julien Roche, Iowa State University, for providing us with human WT αSyn-expressing plasmid. We also thank Gary Zenitsky for proofreading the manuscript and blinding the samples and Maddlyn Haller for technical assistance. We would like to thank Devlen Dailey- Gempis, Kayla Guthals and Mica Post for assistance with the figures.
Funding sources: National Institutes of Health grants ES026892 and NS100090 to AGK, NS088206 to AK, and NS112008 to AGK and XH. This work was also supported in part by The U.S. Army Medical Research Materiel Command endorsed by the US Army, through the Parkinson’s Research Program (PRP), Investigator-Initiated Research Award (IIRA), Program Announcement Funding Opportunity Announcement Number W81XWH-17-PRP-IIRA, under Award, No. W81XWH1810106.
Footnotes
Potential Conflicts of Interest
AGK and VA have an equity interest in PK Biosciences Corporation located in Ames, IA. The terms of this arrangement have been reviewed and approved by Iowa State University in accordance with its conflict of interest policies. All other authors declare no potential conflicts of interest.
References
- 1.Dorsey ER, Elbaz A, Nichols E, et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology 2018;17(11):939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 3.Langston JW, Wiley JC, Tagliati M. Optimizing Parkinson’s disease diagnosis: the role of a dual nuclear imaging algorithm. npj Parkinson’s Disease 2018;4(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain : a journal of neurology 1991;114 ( Pt 5):2283–2301. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease. A systematic review and meta-analysis 2016;86(6):566–576. [DOI] [PubMed] [Google Scholar]
- 6.Irwin DJ, Lee VMY, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nature reviews Neuroscience 2013;14(9):626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delenclos M, Jones DR, McLean PJ, Uitti RJ. Biomarkers in Parkinson’s disease: Advances and strategies. Parkinsonism & related disorders 2016;22 Suppl 1:S106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology 2014;83(5):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beach TG, Adler CH. Importance of low diagnostic Accuracy for early Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 2018;33(10):1551–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manne S, Kondru N, Hepker M, et al. Ultrasensitive Detection of Aggregated α-Synuclein in Glial Cells, Human Cerebrospinal Fluid, and Brain Tissue Using the RT-QuIC Assay: New High-Throughput Neuroimmune Biomarker Assay for Parkinsonian Disorders. Journal of Neuroimmune Pharmacology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manne S, Kondru N, Nichols T, et al. Ante-mortem detection of chronic wasting disease in recto-anal mucosa-associated lymphoid tissues from elk (Cervus elaphus nelsoni) using real-time quaking-induced conversion (RT-QuIC) assay: A blinded collaborative study. Prion 2017;11(6):415–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondru N, Manne S, Greenlee J, et al. Integrated Organotypic Slice Cultures and RT-QuIC (OSCAR) Assay: Implications for Translational Discovery in Protein Misfolding Diseases. Sci Rep 2017;7:43155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore SJ, West Greenlee MH, Kondru N, et al. Experimental Transmission of the Chronic Wasting Disease Agent to Swine after Oral or Intracranial Inoculation. J Virol 2017;91(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West Greenlee MH, Lind M, Kokemuller R, et al. Temporal Resolution of Misfolded Prion Protein Transport, Accumulation, Glial Activation, and Neuronal Death in the Retinas of Mice Inoculated with Scrapie. Am J Pathol 2016;186(9):2302–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groveman BR, Orru CD, Hughson AG, et al. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta neuropathologica communications 2018;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairfoul G, McGuire LI, Pal S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Annals of clinical and translational neurology 2016;3(10):812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candelise N, Schmitz M, Llorens F, et al. Seeding variability of different alpha synuclein strains in synucleinopathies. Annals of neurology 2019;85(5):691–703. [DOI] [PubMed] [Google Scholar]
- 18.Munoz DG, Lee JM, Freeman R, et al. The Search for a Peripheral Biopsy Indicator of α-Synuclein Pathology for Parkinson Disease. Journal of Neuropathology & Experimental Neurology 2017;76(1):2–15. [DOI] [PubMed] [Google Scholar]
- 19.Beach TG, Serrano GE, Kremer T, et al. Immunohistochemical Method and Histopathology Judging for the Systemic Synuclein Sampling Study (S4). Journal of neuropathology and experimental neurology 2018;77(9):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visanji NP, Mollenhauer B, Beach TG, et al. The Systemic Synuclein Sampling Study: toward a biomarker for Parkinson’s disease. Biomarkers in medicine 2017;11(4):359–368. [DOI] [PubMed] [Google Scholar]
- 21.Beach TG, Adler CH, Dugger BN, et al. Submandibular gland biopsy for the diagnosis of Parkinson disease. Journal of neuropathology and experimental neurology 2013;72(2):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler CH, Dugger BN, Hinni ML, et al. Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology 2014;82(10):858–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler CH, Dugger BN, Hentz JG, et al. Peripheral synucleinopathy in early Parkinson’s disease: Submandibular gland needle biopsy findings. Movement disorders : official journal of the Movement Disorder Society 2017;32(5):722–723. [DOI] [PubMed] [Google Scholar]
- 24.Beach TG, Adler CH, Serrano G, et al. Prevalence of Submandibular Gland Synucleinopathy in Parkinson’s Disease, Dementia with Lewy Bodies and other Lewy Body Disorders. J Parkinsons Dis 2016;6(1):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta neuropathologica 2010;119(6):689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beach TG, Adler CH, Sue LI, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology : official journal of the Japanese Society of Neuropathology 2015;35(4):354–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beach TG, Sue LI, Walker DG, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987-2007. Cell and tissue banking 2008;9(3):229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta neuropathologica 2009;117(6):613–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Archives of neurology 1999;56(1):33–39. [DOI] [PubMed] [Google Scholar]
- 30.Harischandra DS, Rokad D, Neal ML, et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein. Science Signaling 2019;12(572):eaau4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilham JM, Orru CD, Bessen RA, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog 2010;6(12):e1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orru CD, Hughson AG, Groveman BR, et al. Factors That Improve RT-QuIC Detection of Prion Seeding Activity. Viruses 2016;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science (New York, NY) 1997;276(5321):2045–2047. [DOI] [PubMed] [Google Scholar]
- 34.Mollenhauer B, Trautmann E, Taylor P, et al. Total CSF alpha-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neuroscience letters 2013;532:44–48. [DOI] [PubMed] [Google Scholar]
- 35.Hansson O, Hall S, Öhrfelt A, et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimer’s Research & Therapy 2014;6(3):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tokuda T, Salem SA, Allsop D, et al. Decreased α-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson’s disease. Biochemical and biophysical research communications 2006;349(1):162–166. [DOI] [PubMed] [Google Scholar]
- 37.Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. The Lancet Neurology 2011;10(3):230–240. [DOI] [PubMed] [Google Scholar]
- 38.Parnetti L, Chiasserini D, Bellomo G, et al. Cerebrospinal fluid Tau/α-synuclein ratio in Parkinson’s disease and degenerative dementias. Movement Disorders 2011;26(8):1428–1435. [DOI] [PubMed] [Google Scholar]
- 39.Tinsley RB, Kotschet K, Modesto D, et al. Sensitive and specific detection of alpha-synuclein in human plasma. Journal of neuroscience research 2010;88(12):2693–2700. [DOI] [PubMed] [Google Scholar]
- 40.El-Agnaf OMA, Salem SA, Paleologou KE, et al. Detection of oligomeric forms of α-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. The FASEB Journal 2006;20(3):419–425. [DOI] [PubMed] [Google Scholar]
- 41.Foulds PG, Mitchell JD, Parker A, et al. Phosphorylated alpha-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2011;25(12):4127–4137. [DOI] [PubMed] [Google Scholar]
- 42.Parnetti L, Cicognola C, Eusebi P, Chiasserini D. Value of cerebrospinal fluid α-synuclein species as biomarker in Parkinson’s diagnosis and prognosis. Biomarkers in medicine 2016;10(1):35–49. [DOI] [PubMed] [Google Scholar]
- 43.Mollenhauer B, El-Agnaf OM, Marcus K, Trenkwalder C, Schlossmacher MG. Quantification of alpha-synuclein in cerebrospinal fluid as a biomarker candidate: review of the literature and considerations for future studies. Biomarkers in medicine 2010;4(5):683–699. [DOI] [PubMed] [Google Scholar]
- 44.Eusebi P, Giannandrea D, Biscetti L, et al. Diagnostic utility of CSF alpha-synuclein species in Parkinson’s disease: protocol for a systematic review and meta-analysis. BMJ Open 2016;6(6):e011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cariulo C, Martufi P, Verani M, et al. Phospho-S129 Alpha-Synuclein Is Present in Human Plasma but Not in Cerebrospinal Fluid as Determined by an Ultrasensitive Immunoassay. Frontiers in Neuroscience 2019;13(889). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groveman BR, Orrù CD, Hughson AG, et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathologica Communications 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fairfoul G, McGuire LI, Pal S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Annals of Clinical and Translational Neurology 2016;3(10):812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Becker K, Wang X, Vander Stel K, Chu Y, Kordower J, Ma J. Detecting Alpha Synuclein Seeding Activity in Formaldehyde-Fixed MSA Patient Tissue by PMCA. Molecular neurobiology 2018;55(11):8728–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orrú CD, Bongianni M, Tonoli G, et al. A Test for Creutzfeldt–Jakob Disease Using Nasal Brushings. New England Journal of Medicine 2014;371(6):519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atarashi R, Satoh K, Sano K, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med 2011;17(2):175–178. [DOI] [PubMed] [Google Scholar]
- 51.Paciotti S, Bellomo G, Gatticchi L, Parnetti L. Are We Ready for Detecting α-Synuclein Prone to Aggregation in Patients? The Case of “Protein-Misfolding Cyclic Amplification” and “Real-Time Quaking-Induced Conversion” as Diagnostic Tools. Frontiers in Neurology 2018;9(415). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Rumund A, Green AJE, Fairfoul G, Esselink RAJ, Bloem BR, Verbeek MM. alpha-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Annals of neurology 2019;85(5):777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garrido A, Fairfoul G, Tolosa ES, Martí MJ, Green A, Group tBLS. α-synuclein RT-QuIC in cerebrospinal fluid of LRRK2-linked Parkinson’s disease . Annals of clinical and translational neurology 2019;6(6):1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruff RL, Dougherty JH Jr. Complications of lumbar puncture followed by anticoagulation. Stroke 1981;12(6):879–881. [DOI] [PubMed] [Google Scholar]
- 55.Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta neuropathologica 2010;119(6):703–713. [DOI] [PubMed] [Google Scholar]
- 56.Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK. Senile dementia of Lewy body type: A clinically and neuropathologically distinct form of Lewy body dementia in the elderly. Journal of the Neurological Sciences 1990;95(2):119–139. [DOI] [PubMed] [Google Scholar]
- 57.Song IH, Song JS, Sung CO, et al. Accuracy of Core Needle Biopsy Versus Fine Needle Aspiration Cytology for Diagnosing Salivary Gland Tumors. J Pathol Transl Med 2015;49(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chahine LM, Beach TG, Seedorff N, et al. Feasibility and Safety of Multicenter Tissue and Biofluid Sampling for alpha-Synuclein in Parkinson’s Disease: The Systemic Synuclein Sampling Study (S4). J Parkinsons Dis 2018;8(4):517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


