Abstract
Influenza viruses are a major threat to human health globally. In addition to further improving vaccine prophylaxis, disease management through antiviral therapeutics constitutes an important component of the current intervention strategy to prevent advance to complicated disease and reduce case-fatality rates. Standard-of-care is treatment with neuraminidase inhibitors that prevent viral dissemination. In 2018, the first mechanistically new influenza drug class for the treatment of uncomplicated seasonal influenza in 2 decades was approved for human use. Targeting the PA endonuclease subunit of the viral polymerase complex, this class suppresses viral replication. However, the genetic barrier against viral resistance to both drug classes is low, pre-existing resistance is observed in circulating strains, and resistant viruses are pathogenic and transmit efficiently. Addressing the resistance problem has emerged as an important objective for the development of next-generation influenza virus therapeutics. This review will discuss the status of influenza therapeutics including the endonuclease inhibitor baloxavir marboxil after its first year of clinical use and evaluate a subset of direct-acting antiviral candidates in different stages of preclinical and clinical development.
Abbreviations: RBC, red blood cells; PK, pharmacokinetics; CDC, centers for disease control and prevention; IAV, influenza A virus; IBV, influenza B virus; RdRP, RNA-dependent RNA polymerase
INTRODUCTION
Influenza virus affects approximately 10% of the population during every season. In most healthy individuals, these infections predominantly result in relatively mild, self-limiting disease that remains restricted to the upper respiratory tract and does not require therapeutic intervention. Reflecting the overall high disease prevalence, however, the World Health Organization estimates that 3–5 million infections lead to severe disease that advances to the lower respiratory tract and viral pneumonia, resulting in up to 650,000 deaths annually.1 High-risk groups for severe influenza infection include older adults, the immunocompromized, pregnant women, people with underlying pulmonary conditions, and, to a lesser degree, the very young. Yearly vaccination is recommended for everyone older than 6 months of age, but vaccine efficacy varies substantially based on how well circulating viruses and vaccine strains are matched, patient age and patient influenza history. In the 2017/18 influenza season, for instance, vaccine efficacy against the predominant H3N2 strain was only 25%, leading to the highest mortality rate since the 2009 H1N1 pandemic.2 Although disease burden was particularly high in that season, vaccination efficacy was on average below 50% also in the preceding years also.3 , 4 Moreover, effectiveness of the influenza vaccine is particularly low in older adults, leaving one of the primary at-risk groups poorly protected (reviewed in5). Due to these limitations to vaccine prophylaxis combined with continued high disease burden caused by seasonal influenza viruses, the threat of spill-over of highly pathogenic avian influenza viruses into the human population and a low barrier to viral escape of standard-of-care therapeutics (discussed in detail below), effective novel antiviral therapeutics are urgently needed for improved disease management especially in high risk patients and for heightened preparedness against the risk of future global pandemics.
THERAPEUTIC WINDOW FOR TARGETING OF INFLUENZA VIRUS REPLICATION
Whereas influenza virus infection causes direct cell damage in the airway epithelium, severe tissue damage during complicated disease is largely a consequence of immunopathogenesis and peaks after the acute infection has been cleared. Influenza virus load in the upper respiratory tract is highest approximately 2–3 days after infection, which coincides with peak fever and most pronounced respiratory clinical signs. After the third day of infection, virus replication is increasingly immune controlled and virus load drops rapidly.6 Rapid disease progression and, in the case of uncomplicated disease, immune control of virus replication outline a narrow therapeutic window for influenza drugs. Ideally, treatment should be initiated within 24–36 hours of infection. In fact, clinical studies assessing the impact of neuraminidase inhibitors (NAIs) have revealed a benefit for the patient when treatment was initiated within 48 hours of the onset of influenza symptoms,7 , 8 and therapeutic impact was greatest when antiviral drugs were administered within 24 hours of disease manifestation.9, 10, 11 Accordingly, public disease awareness, proactive patient behavior, and access to rapid diagnostics are paramount for therapeutic success. Several diagnostic methods are currently used in the clinic that shorten the time to treatment, recently comparatively reviewed in.12
In an attempt to pre-empt the challenges arising from a narrow therapeutic window, chemoprophylaxis has been explored. Whereas several studies support that prophylactic administration of NAIs lowered the risk of developing disease,8 , 13 the CDC recommends reserving chemoprophylaxis for people in high risk groups and for outbreak control among high risk individuals in institutional settings.14 In contrast, general chemoprophylaxis is not recommended due to the unclear risk-benefit for otherwise healthy patients and concerns of promoting the development of viral resistance.
INFLUENZA VIRUS RESISTANCE TO ANTIVIRALS
All currently approved influenza drugs interfere with viral protein function and therefore belong to the group of direct-acting antivirals (DAAs). In comparison with indirectly acting host-directed experimental antivirals, drugs of the DAA group have a lower tendency for undesirable side effects. However, rapid development of viral resistance has emerged as the predominant liability of DAAs, especially when directed against RNA viruses with error prone polymerases such as respiratory syncytial virus (RSV)15 , 16 or the influenza viruses.17
Exemplifying the scope of the problem, the adamantanes, amantadine and, subsequently, rimantadine, were the first drugs approved for the treatment of influenza A virus (IAV) infections. These inhibitors target the viral M2 ion channel, preventing dissociation of the viral ribonucleoprotein (RNP) genome from the matrix protein by blocking M2-mediated diffusion of protons into virions located in maturing endosomes.18 , 19 For some IAV strains, they can also affect virion assembly by disturbing M2-mediated pH-equilibration of the late Golgi.18 , 20 However, the administration of the adamantes has been discouraged by the CDC for more than a decade due to widespread pre-existing viral resistance.21, 22, 23 In the 2019 influenza season, for instance, >99% of circulating H3N2 and H1N1 strains carried resistance mutations to amantadine.14 This high prevalence of resistance to the adamantanes is thought to directly reflect their extensive use in the poultry industry,24 which also increased the risk of prolonged exposure of human viruses to the drug, since the adamantanes were detectable in agricultural animals for up to 13 days after treatment.25 In addition to seasonal influenza virus strains, signature amantadine resistance mutations were detected in highly pathogenic avian H5N1 strains.26 , 27 To lower the risk of triggering the emergence of resistance against other influenza drug classes in circulating viruses, many countries including the United States and China have banned their use in agriculture.
In the past 2 decades, major research efforts have been directed towards the discovery of host-directed antiviral drugs, lured by the prospect of a high barrier of these therapeutics against the development of resistance. Mechanistically unique is the host-directed viral entry inhibitor DAS181, a recombinant sialidase fusion protein, which targets cellular sialic acid residues required for influenza virus infection. In contrast to this extracellular host target, the majority of experimental host-directed antivirals aim to modulate host immune responses or have been developed against intracellular processes that are critical for successful virus replication (reviewed in28). The challenge, however, is the identification of a druggable intracellular target that has a major impact on virus replication but is dispensable for host function. Reflecting the conundrum that antiviral efficacy and undesirable adverse effects of host-directed antivirals follow in most cases the same structure-activity relationship, no intracellularly acting host-directed candidate has been approved yet for clinical use against influenza viruses29 , 30 or other respiratory RNA viruses of the myxo- and pneumovirus families.
CLINICALLY USED DRUGS AND SELECTED DAA CANDIDATES IN ADVANCED STAGES OF DEVELOPMENT
Table 1 shows influenza drugs currently approved and recommended for clinical use.
Table 1.
Influenza therapeutics currently approved for clinical use
| Drug | Target protein | Route of administration | Patient age group | Target virus |
|---|---|---|---|---|
| Baloxavir marboxil | PA | oral (PO) | ≥ 12 years | IAV and IBV |
| Oseltamivir | NA | oral (PO) | ≥ 1 year | IAV and IBV |
| Peramivir | NA | intravenous | ≥ 2 years | IAV and IBV |
| Zanamivir | NA | inhalation | ≥ 5 years | IAV and IBV |
| Amantadine* | M2 | oral (PO) | ≥ 1 year | IAV |
| Rimantadine* | M2 | oral (PO) | ≥ 1 year | IAV |
| Favipiravir† | RdRP | oral (PO) | adults | IAV and IBV |
Abbreviations: IAV, influenza A virus; IBV, influenza B virus; RdRP, RNA-dependent RNA polymerase.
not recommended for antiviral treatment against currently circulating influenza virus strains due to high level of preexisting resistance
conditionally approved in Japan against emerging influenza viruses resistant to other antivirals
Neuraminidase inhibitors
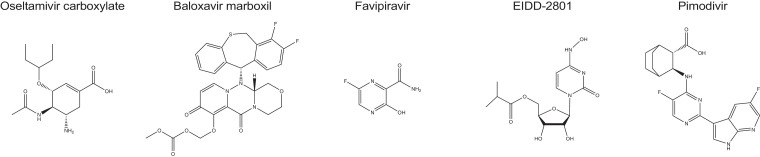
Of the NAIs in clinical use, oseltamivir (Fig 1 ), peramivir, and zanamivir, oseltamivir carboxylate is the most frequently prescribed and considered standard-of-care for influenza management since essentially all circulating influenza viruses have acquired resistance to the adamantanes. Mechanistically, NAIs competitively inhibits the enzymatic activity of IAV and influenza B virus (IBV) neuraminidases, preventing cleavage of the terminal sialic acid residues from glycoproteins and carbohydrates displayed on the surface of mammalian cells and influenza virus particles. Binding of virions to uncleaved sialic acid then impairs virion release and dissemination.31
Fig 1.
Chemical structures of oseltamivir carboxylate, baloxavir marboxil, favipiravir, EIDD-2801, and pimodivir.
Oseltamivir is orally bioavailable and well-tolerated. Reflecting this excellent safety profile, the drug has been approved for use also in pregnant women, pediatric patients, and neonates.29 , 32 As discussed above, clinical trials have shown that NAIs provide benefit when started early after infection.9 , 33 However, meta-analyses of clinical data revealed little effect on reducing influenza-associated complications or hospitalization,34, 35, 36, 37 and some data suggested that late treatment with NAIs could have enhanced the risk of progression to severe disease in the 2009/2010 pandemic.38
Resistance to oseltamivir can develop rapidly in both experimental settings and the clinic, and typically originates from substitutions at signature resistance sites in the viral NA protein such as H274Y and I223R (predominant in H1N1 and H5N1 viruses), and E119V, R292K, or N294S (predominant in H3N2 viruses).39 , 40 Major resistance mutations to zanamivir are NA substitutions Q136K and I223R (H1N1 viruses) and Q136K (H3N2 viruses), respectively.41 Whereas resistance to the NAIs is relatively rare in currently endemic influenza virus strains,42 , 43 the prevalence of escape mutations increased rapidly in the 2007/2008 influenza season44 and approximately 90% of strains circulating during the 2008/2009 season were resistant to NAIs,45, 46, 47 generating concern that this drug class could also be lost to resistance in the future.48
Neutralizing antibodies
Although no biologics have been approved for influenza therapy so far, neutralizing antibodies (nAbs) in particular have been extensively tested. Substantiated by the precedent established, for instance, by nAbs used clinically against RSV49 and Ebola virus infection,50 antibody-based therapeutics are typically well-tolerated and show favorable pharmacokinetic profiles. The influenza virus HA head domain containing the receptor binding sites (RBCs) is highly immunogenic and thus a primary target for nAbs. However, these head-directed anti-influenza nAbs typically show a very narrow indication spectrum, hampering their clinical development. Although some nAbs directed against the more conserved RBC have been identified,51, 52, 53 alternative targeting of the less variable stalk domain of the HA trimer has attracted major attention in recent years, due to cross-reactivity with multiple HA subtypes.54, 55, 56, 57, 58, 59
Three influenza virus HA stalk-targeting broadly neutralizing Abs (bnAbs), MHAA4548A, MEDI8852, and VIS410, have advanced to phase 2 clinical trials and demonstrated some antiviral efficacy when dosed therapeutically, accelerating symptom resolution and reducing virus replication.60, 61, 62 A half-life of approximately 3 weeks in humans61 makes therapeutic antibodies compatible with attractive single-dose administration. Whereas no resistant viruses emerged in these clinical trials, 2 viral escape pathways from HA stalk-directed antibodies have been identified experimentally63: after viral adaptation in cell culture, resistant variants either carried a point mutation in the stalk epitope preventing antibody binding, or harbored substitutions that enhanced HA fusion activity and therefore allowed viral entry in the presence of antibody. These experimental resistance mutations affected viral fitness, however, reducing viral replication in cell culture.
Major concerns for the use of broadly reactive Abs against uncomplicated seasonal influenza are the target restriction to IAVs, the lack of oral bioavailability, a necessity for high-dose injections as a consequence of poor distribution from blood to the upper airways, and the high manufacturing costs of biologics. Since both IAVs and IBVs are human pathogens and clinically relevant,64 efficacy against pathogens of both genera should constitute a developmental objective of next-generation drugs. This target breadths cannot be achieved with traditional bnAbs. However, engineering of multidomain antibodies has been explored experimentally and proof-of-concept efficacy against both IAVs and IBVs has been demonstrated in mice,65 potentially illuminating a path to overcome this limitation. Furthermore, better compliance from the large patient group of nonhospitalized adults suffering from uncomplicated disease is expected when the drug is orally bioavailable and amenable to cost-effective manufacture. bnAbs appear therefore best suited for use in high risk groups or hospitalized patients with complicated disease.
Inhibitors of the viral polymerase complex
In the last decade, the viral RNA-dependent RNA polymerase (RdRP) complex has emerged as an important target for novel small-molecule therapeutics that have progressed to advanced development and/or clinical testing. Influenza virus RdRP is comprised of 3 subunits, the PB1, PB2, and PA proteins, that are responsible for both replication and transcription of the 8 genome segments. The segments do not exist as naked RNA but are encapsidated by viral NP proteins, forming RNP complexes. Whereas replication requires the generation of complementary positive polarity RNA intermediates (cRNA) that are then copied into progeny negative polarity segments (vRNPs), viral message is directly synthesized from vRNPs. Since the influenza virus RdRP lacks enzymatic activity to form 5′ mRNA cap structures, endonuclease activity of the PA subunit is instrumental for the generation of bioactive viral mRNAs through transfer of 5′-capped RNA primers derived from host mRNAs in a cap-snatching mechanism. PB2 is involved in binding of the capped primers, whereas the PB1 subunit harbors enzymatic activity for phosphodiester bond formation.66, 67, 68, 69 The overall replication strategy and RdRP organization is highly conserved across all IAV subtypes and among IAVs and IBVs, increasing the likelihood that therapeutic targeting of the polymerase complex will result in the development of broad-spectrum influenza virus inhibitors.
Baloxavir marboxil – founding member of the PA endonuclease inhibitor class
Licensed in Japan and the United States since 2018, baloxavir marboxil (Fig 1) is the first new influenza drug class approved in 2 decades for the treatment of uncomplicated influenza infection in patients older than 12 years of age.70 Taken orally, baloxavir marboxil is a prodrug that prevents cap-snatching by blocking PA endonuclease activity when hydrolyzed to free baloxavir acid. A single dose is typically sufficient for clinical benefit, and efficacy is similar to, or better than, current standard-of-care.71 , 72 In clinical trials, participants showed significantly lower viral burden 1 day after initiation of treatment compared to placebo groups, and the time to alleviation of clinical signs was decreased on average by approximately 26 hours, which resembled the improvements that can be achieved with NAIs.72 Quite remarkably, twice daily experimental treatment of mice infected with a lethal dose of a highly pathogenic avian IAV resulted in complete survival of the animals and reduction of viral load in the respiratory tract. Similarly profound efficacy was observed in immunocompromized mice.73, 74, 75 In addition to activity against IAVs, IBVs and influenza viruses of the C and D genera are susceptible to baloxavir inhibition, making the drug a truly broad-spectrum influenza virus inhibitor.71 , 76 , 77
Baloxavir marboxil was well tolerated in clinical trials with only mild and transient adverse events that resolved without further intervention. Side effects included headaches and increased eosinophil and white blood cell counts. PK properties in pediatric recipients were similar to those seen in adults, and a trial involving 107 pediatric patients receiving 1 weight-adjusted dose of baloxavir marboxil established equivalent efficacy in adults and children against uncomplicated influenza.78 , 79 Although efficacy of baloxavir marboxil and NAIs were comparable, baloxavir marboxil provides full benefit after a single oral dose, giving the drug an advantage over current standard-of-care. Consistent with distinct protein targets of baloxavir marboxil and the NAIs, viruses harboring signature NAI resistance mutations were shown to remain susceptible to inhibition by baloxavir marboxil.71 , 80
Despite the exciting safety and efficacy performance of baloxavir marboxil, resistant viruses emerged in up to 9.7% of adult clinical trial participants and 23.4% of children.72 , 79 In several cases, the emergence of resistance coincided with a rebounds in shed virus titers and prolonged manifestation of clinical signs. Resembling resistance profiles generated for different PA endonuclease inhibitor chemotypes in cell culture,81 the signature hotspot for escape from baloxavir marboxil is PA residue 38, for which several substitutions (PA I38T/M/F) have been described. The initial characterization of PA I38 mutant viruses in cell culture suggested that resistance may carry a severe fitness penalty.71 , 82 However, subsequent follow-up analyses of the fitness of H1N1 and H3N2 strains containing PA I38 mutants in the ferret model revealed that pathogenesis and transmission success of resistant and the corresponding sensitive viruses were equivalent.83 , 84 In mice and ferrets, both loss of animal body weight and lung viral loads were identical after infection with resistant or sensitive viruses, and substitutions at PA I38 remained stable over 4 passages through mice.85 , 86 A small percentage of influenza viruses circulating in the United States in the 2016/2017 and 2017/2018 seasons, and thus prior to approval of baloxavir marboxil, contained mutations at PA residue 38.80 , 87 , 88 Moreover, a resistant H3N2 virus was detected in a pediatric patient without prior exposure to baloxavir marboxil, supporting that this mutant strain spread between humans.89 Even though baloxavir marboxil has not yet been used extensively in the clinic and long-term field data are scarce, the available evidence indicates that the genetic barrier against resistance to the drug is low and that escape comes with little penalty to viral fitness, pathogenesis, or transmission success. Accordingly, there is considerable risk that pre-exiting resistance to baloxavir marboxil could become widespread with increasing duration of clinical use.
Favipiravir (T-705) – broad-spectrum ribonucleoside analog inhibitor of RNA viruses
T-705 is a broad-spectrum pyrazinecarboxamide (Fig 1) that is converted intracellularly to a ribonucleoside analog through phosphoribosylation, thus competitively targeting the viral RdRP complex. Anabolism efficiency can vary between cells of different tissue origin, but has been shown to be low in disease-relevant primary human airway epithelium cells.90 The drug has broad-spectrum antiviral activity in cell culture, inhibiting RNA viruses of the arenavirus, bunyavirus, flavivirus, alphavirus, norovirus, picornavirus, paramyxovirus, and rhabdovirus families, in addition to influenza viruses.91, 92, 93, 94
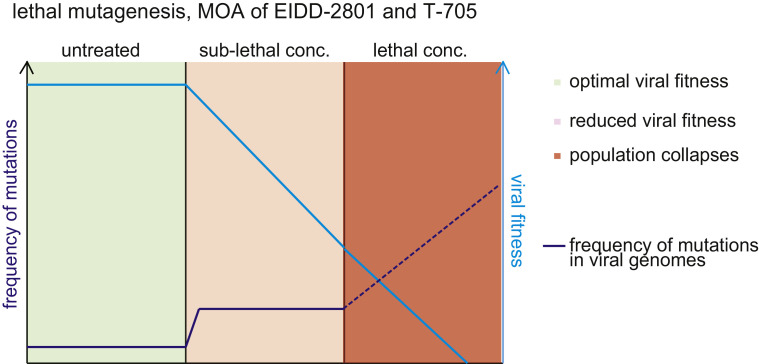
Mechanistically, T-705 is incorporated into newly synthesized RNA by the viral polymerase in place of purines but not pyrimidines,95 resulting in increased frequencies of C-to-U and G-to-A transition mutations as demonstrated for influenza viruses and hepatitis C virus both in vitro and in vivo.96, 97, 98 Collapse of virus replication is considered to be a consequence of error catastrophe or lethal mutagenesis, resulting from the accumulation of random low-frequency mutations in the viral genome (Fig 2 ). The genetic barrier of T-705 against resistance is favorably high,99 although a recent study has identified a pair of point mutations in the PB1 (K229R) and PA (P659L) polymerase subunits that reduce viral sensitivity to the compound by approximately 30-fold in cell culture.100 , 101
Fig 2.
Principle of influenza virus inhibition by lethal mutagenesis induced by ribonucleoside analogs EIDD-2801 and T-705. In the absence of drug, random mutation frequency in the virus population is low (green). Sublethal concentrations of the inhibitor increase the frequency of random transition mutations, reducing overall fitness of the virus population (salmon). At sterilizing concentrations, the drug increases the random mutation frequency beyond a tolerable threshold, resulting in collapse of the virus population (red); MOA, mechanism of action. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Safety testing revealed that T-705 is teratogenic and embryotoxic at concentrations close to the approved human dose.99 , 102 Although conditionally licensed in Japan for the treatment of pathogenic influenza viruses resistant to other therapeutics, these safety concerns prevent drug administration to some high-risk groups such as children or pregnant women.103 Activity assessment against other potential viral targets in animal models furthermore revealed that in vivo efficacy is quite poor and dependent on extremely high dose concentrations.104 , 105 These results lower the clinical value of T-705 and undermine its use against viruses predominantly associated with pediatric diseases, such as pathogens of the pneumovirus and paramyxovirus families.
EIDD-2801 – next-generation influenza virus inhibitor candidate in early development
A search for inhibitors with broad antirespiratory RNA virus activity yielded the ribonucleoside analog N 4-hydroxycytidine (NHC), which showed oral efficacy against RSV and both highly-pathogenic avian and seasonal influenza viruses in mouse models.90 Mechanistic characterization revealed that the compound is efficiently anabolized to the bioactive tri-phosphate form in ex vivo primary human airway epithelium cultures and in animal tissues, followed by incorporation as cytidine into viral RNA by influenza virus polymerases.90 , 106 Tautomeric interconversions then cause base-pairing of the incorporated analog either as cytosine or uracil, resulting in high-frequency random C-to-U and G-to-A transition mutations in viral RNA replication or transcription products and thus inhibition through error catastrophe.106 Additional studies have expanded the antiviral target spectrum of NHC to viruses of the flavivirus, alphavirus, coronavirus, and togavirus families,107, 108, 109, 110, 111 demonstrating broad anti-RNA virus activity.
Whereas rodent PK properties of NHC were good, oral bioavailability of the drug in non-human primates such as cynomolgus macaques was limited. This liability was addressed through the development of the prodrug EIDD-2801, a 5′-isopropylester (Fig 1) of NHC, which demonstrated good oral bioavailability in all species tested including nonhuman primates. Upon intestinal absorption, EIDD-2801 was efficiently hydrolyzed to free NHC.106 Oral daily doses of 14 mg/kg EIDD-2801 were reportedly efficacious against H1N1 and H3N2 IAV strains in the ferret model, causing significant reduction of viral load in both the upper and lower respiratory tract and alleviation of clinical signs. The compound showed potent antiviral activity in disease-relevant well-differentiated human airway epithelium model cultures, inhibiting both IAV and IBV strains with active concentrations around 0.08 μM and selectivity indices >1700.
EIDD-2801/NHC appears to establish an unusually high barrier against viral resistance, since several studies have unsuccessfully tried to induce viral escape through dose-escalation and sublethal fixed-dose adaptation strategies. Next-generation sequencing of the resulting treatment-experienced virus populations revealed an elevated number of low-frequency mutations, but none of these became allele-dominant or mediated robust resistance to the drug.106 , 108 , 109 Although these results are encouraging, adaptation through serial passaging in vivo will likely be necessary to better predict whether resistance to EIDD-2801/NHC could ultimately emerge.
Toxicity concerns are a major potential liability of ribonucleoside analogs as underscored, for instance, by the compromised safety profile of T-705.99 , 102 A primary source for adverse effects is incorporation of the ribonucleoside analogs into host RNAs by nuclear and mitochondrial RNA polymerases.112 , 113 The integrity of mitochondrial transcripts is particularly at risk due to the proofreading inability of mitochondrial RNA polymerases in contrast to nuclear RNA Pol II.114 Not surprisingly, NHC was incorporated by human mitochondrial polymerases in in vitro studies115 and an elevated frequency of NHC-characteristic transition events was noted after prolonged exposure of primary human airway epithelium cells to the drug. However, the transition mutation frequency in ferret lung tissue was unchanged from that in controls after extended treatment of animals with 200 mg/kg daily dose and thus over 14-times the efficacious dose for influenza therapy.106 Potential adverse effects of EIDD-2801 on embryo development are untested at present, and formal 2-species multi-dose toxicity studies will be required to establish initial human doses examined in phase 1 clinical trials.
Pimodivir (VX-787) – PB2 inhibitor in clinical development
Pimodivir is a cyclohexyl carboxylic acid analogue (Fig 1) that inhibits influenza virus replication by targeting the polymerase PB2 subunit. The drug outperformed current standard-of-care in animal infection models of H3N2 and H1N1 viruses, although potency was highest against H1N1 strains.116 Also pimodivir remained active against viruses harboring signature resistance mutations to the NAIs,117 and is presumably at low risk of cross-resistance with baloxavir marboxil as well. The compound was well-tolerated in a phase 2 clinical trial, reduced viral load, and resulted in slightly faster resolve of clinical signs.118
However, specific resistance mutations to pimodivir emerged in the PB2 protein of 6.4% of trial participants, indicating that the genetic barrier to viral escape from the drug is low. Compared to standard-of-care, baloxavir marboxil and ribonucleoside analogs in development, another substantial limitation of pimodivir is a narrowed antiviral indication spectrum. Exclusively IAVs are susceptible to the drug,119 restricting its use to only 1 of the 2 clinically-significant influenza virus genera.
CONCLUSIONS
Influenza viruses continue to present a major threat to human health globally. In the past year, the first new drug class in over 2 decades was approved for influenza therapy, providing a much-needed expansion of the available antiviral arsenal. However, low genetic barriers of both neuraminidase and PA endonuclease inhibitors against viral escape create an urgent need for next-generation therapeutics. Of the different developmental candidates currently explored, bnAbs are attractive for the protection of high-risk groups if treatment costs can be managed. Competitive ribonucleoside analog inhibitors with high resistance barrier are promising for use against seasonal and pandemic viruses, provided the risk of adverse effects is acceptably low or can be appropriately mitigated.
Acknowledgments
The study conforms to the relevant ethical guidelines for human and animal research. We thank AL Hammond for critical reading of the manuscript. No further editorial support was used. Both authors have read the journal's policy on disclosure of potential conflicts of interest. Both authors declare no competing interests. Both authors have read the journal's authorship agreement. The manuscript has been reviewed by and approved by both named authors. This work was supported, in part, by public health service grant AI141222 (to RKP) from the NIH/NIAID. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Reeves J.D., Gallo S.A., Ahmad N. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci U S A. 2002;99:16249–16254. doi: 10.1073/pnas.252469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umino Y., Kohama T., Sato T.A., Sugiura A., Klenk H.D., Rott R. Monoclonal antibodies to three structural proteins of Newcastle disease virus: biological characterization with particular reference to the conformational change of envelope glycoproteins associated with proteolytic cleavage. J Gen Virol. 1990;71(Pt 5):1189–1197. doi: 10.1099/0022-1317-71-5-1189. [DOI] [PubMed] [Google Scholar]
- 3.Garten R., Blanton L., Elal A.I.A., Alabi N., Barnes J., Biggerstaff M. Update: influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67:634–642. doi: 10.15585/mmwr.mm6722a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle J.D., Chung J.R., Kim S.S. Interim estimates of 2018-19 seasonal influenza vaccine effectiveness—United States, February 2019. MMWR Morb Mortal Wkly Rep. 2019;68:135–139. doi: 10.15585/mmwr.mm6806a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dugan H.L., Henry C., Wilson P.C. Aging and influenza vaccine-induced immunity. Cell Immunol. 2019 doi: 10.1016/j.cellimm.2019.103998. [DOI] [PubMed] [Google Scholar]
- 6.Baccam P., Beauchemin C., Macken C.A., Hayden F.G., Perelson A.S. Kinetics of influenza A virus infection in humans. J Virol. 2006;80:7590–7599. doi: 10.1128/JVI.01623-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson J., Whitley R.J., Pocock S., Monto A.S. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729–1737. doi: 10.1016/S0140-6736(14)62449-1. [DOI] [PubMed] [Google Scholar]
- 8.Jefferson T., Jones M.A., Doshi P. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;348 doi: 10.1002/14651858.CD001265.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki F.Y., Macleod M.D., Paggiaro P. Early administration of oral oseltamivir increases the benefits of influenza treatment. J Antimicrob Chemother. 2003;51:123–129. doi: 10.1093/jac/dkg007. [DOI] [PubMed] [Google Scholar]
- 10.Treanor J.J., Hayden F.G., Vrooman P.S. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson K.G., Aoki F.Y., Osterhaus A.D. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 12.Vemula S.V., Zhao J., Liu J., Wang X., Biswas S., Hewlett I. Current approaches for diagnosis of influenza virus infections in humans. Viruses. 2016;8:96. doi: 10.3390/v8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson R.J., Cooper K.L., Tappenden P. Oseltamivir, zanamivir and amantadine in the prevention of influenza: a systematic review. J Infect. 2011;62:14–25. doi: 10.1016/j.jinf.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clincians; 2019. Available at: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm#Treatment. (Accessed February 27, 2020).
- 15.DeVincenzo J.P., Whitley R.J., Mackman R.L. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 16.Chemaly R.F., Dadwal S.S., Bergeron A. A phase 2, randomized, double-blind, placebo-controlled trial of presatovir for the treatment of respiratory syncytial virus upper respiratory tract infection in hematopoietic-cell transplant recipients. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz1166. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain M., Galvin H.D., Haw T.Y., Nutsford A.N., Husain M. Drug resistance in influenza A virus: the epidemiology and management. Infect Drug Resist. 2017;10:121–134. doi: 10.2147/IDR.S105473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing X., Ma C., Ohigashi Y. Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel. Proc Natl Acad Sci U S A. 2008;105:10967–10972. doi: 10.1073/pnas.0804958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stouffer A.L., Acharya R., Salom D. Structural basis for the function and inhibition of an influenza virus proton channel. Nature. 2008;451:596–599. doi: 10.1038/nature06528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Qiu J.X., Soto C., DeGrado W.F. Structural and dynamic mechanisms for the function and inhibition of the M2 proton channel from influenza A virus. Curr Opin Struct Biol. 2011;21:68–80. doi: 10.1016/j.sbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiraishi K., Mitamura K., Sakai-Tagawa Y., Goto H., Sugaya N., Kawaoka Y. High frequency of resistant viruses harboring different mutations in amantadine-treated children with influenza. J Infect Dis. 2003;188:57–61. doi: 10.1086/375799. [DOI] [PubMed] [Google Scholar]
- 22.Abed Y., Goyette N., Boivin G. Generation and characterization of recombinant influenza A (H1N1) viruses harboring amantadine resistance mutations. Antimicrob Agents Chemother. 2005;49:556–559. doi: 10.1128/AAC.49.2.556-559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krumbholz A., Schmidtke M., Bergmann S. High prevalence of amantadine resistance among circulating European porcine influenza A viruses. J Gen Virol. 2009;90:900–908. doi: 10.1099/vir.2008.007260-0. [DOI] [PubMed] [Google Scholar]
- 24.Parry J. Use of antiviral drug in poultry is blamed for drug resistant strains of avian flu. BMJ. 2005;331:10. doi: 10.1136/bmj.331.7507.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You X., Yang S., Zhao J. Study on the abuse of amantadine in tissues of broiler chickens by HPLC-MS/MS. J Vet Pharmacol Ther. 2017;40:539–544. doi: 10.1111/jvp.12388. [DOI] [PubMed] [Google Scholar]
- 26.Li K.S., Guan Y., Wang J. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- 27.Guan Y., Poon L.L., Cheung C.Y. H5N1 influenza: a protean pandemic threat. Proc Natl Acad Sci U S A. 2004;101:8156–8161. doi: 10.1073/pnas.0402443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson S. Treating influenza infection, from now and into the future. Front Immunol. 2018;9:1946. doi: 10.3389/fimmu.2018.01946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Principi N., Camilloni B., Alunno A., Polinori I., Argentiero A., Esposito S. Drugs for influenza treatment: is there significant news? Front Med-Lausanne. 2019;6:109. doi: 10.3389/fmed.2019.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw M.L. The next wave of influenza drugs. ACS Infect Dis. 2017;3:691–694. doi: 10.1021/acsinfecdis.7b00142. [DOI] [PubMed] [Google Scholar]
- 31.Laborda P., Wang S.Y., Voglmeir J. Influenza neuraminidase inhibitors: synthetic approaches, Derivatives and Biological Activity. Molecules. 2016;21:1513. doi: 10.3390/molecules21111513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKimm-Breschkin J.L. Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Resp Viruses. 2013;7:25–36. doi: 10.1111/irv.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oboho I.K., Reed C., Gargiullo P. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis. 2016;214:507–515. doi: 10.1093/infdis/jiw033. [DOI] [PubMed] [Google Scholar]
- 34.Muthuri S.G., Myles P.R., Venkatesan S., Leonardi-Bee J., Nguyen-Van-Tam J.S. Impact of neuraminidase inhibitor treatment on outcomes of public health importance during the 2009-2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis. 2013;207:553–563. doi: 10.1093/infdis/jis726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bassetti M., Castaldo N., Carnelutti A. Neuraminidase inhibitors as a strategy for influenza treatment: pros, cons and future perspectives. Expert Opin Pharmaco. 2019;20:1711–1718. doi: 10.1080/14656566.2019.1626824. [DOI] [PubMed] [Google Scholar]
- 36.Ebell M.H. Oseltamivir and zanamivir have limited effect on symptoms and do not reduce hospitalisation or serious complications of influenza. Evid Based Med. 2014;19:211. doi: 10.1136/ebmed-2014-110033. [DOI] [PubMed] [Google Scholar]
- 37.Jefferson T., Jones M., Doshi P., Spencer E.A., Onakpoya I., Heneghan C.J. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2545. doi: 10.1136/bmj.g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michiels B., Van Puyenbroeck K., Verhoeven V., Vermeire E., Coenen S. The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews. PLoS One. 2013;8:e60348. doi: 10.1371/journal.pone.0060348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tai C.Y., Escarpe P.A., Sidwell R.W. Characterization of human influenza virus variants selected in vitro in the presence of the neuraminidase inhibitor GS 4071. Antimicrob Agents Chemother. 1998;42:3234–3241. doi: 10.1128/aac.42.12.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gubareva L.V. Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res. 2004;103:199–203. doi: 10.1016/j.virusres.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 41.Han J., Perez J., Schafer A. Influenza virus: small molecule therapeutics and mechanisms of antiviral resistance. Curr Med Chem. 2018;25:5115–5127. doi: 10.2174/0929867324666170920165926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee N., Hurt A.C. Neuraminidase inhibitor resistance in influenza: a clinical perspective. Curr Opin Infect Dis. 2018;31:520–526. doi: 10.1097/QCO.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 43.Nitsch-Osuch A., Brydak L.B. Influenza viruses resistant to neuraminidase inhibitors. Acta Biochim Pol. 2014;61:505–508. [PubMed] [Google Scholar]
- 44.Hauge S.H., Dudman S., Borgen K., Lackenby A., Hungnes O. Oseltamivir-resistant influenza viruses A (H1N1), Norway, 2007-08. Emerg Infect Dis. 2009;15:155–162. doi: 10.3201/eid1502.081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dharan N.J., Gubareva L.V., Meyer J.J. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA J Am Med Assoc. 2009;301:1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- 46.Hurt A.C., Ernest J., Deng Y.M. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antivir Res. 2009;83:90–93. doi: 10.1016/j.antiviral.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Esposito S., Molteni C.G., Colombo C. Oseltamivir-induced resistant pandemic A/H1N1 influenza virus in a child with cystic fibrosis and Pseudomonas aeruginosa infection. J Clin Virol. 2010;48:62–65. doi: 10.1016/j.jcv.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 48.van der Vries E., Schutten M., Fraaij P., Boucher C., Osterhaus A. Influenza virus resistance to antiviral therapy. Adv Pharmacol. 2013;67:217–246. doi: 10.1016/B978-0-12-405880-4.00006-8. [DOI] [PubMed] [Google Scholar]
- 49.McLaurin K.K., Farr A.M., Wade S.W., Diakun D.R., Stewart D.L. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. 2016;36:990–996. doi: 10.1038/jp.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laursen N.S., Wilson I.A. Broadly neutralizing antibodies against influenza viruses. Antiviral Res. 2013;98:476–483. doi: 10.1016/j.antiviral.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ekiert D.C., Kashyap A.K., Steel J. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature. 2012;489:526–532. doi: 10.1038/nature11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsibane T., Ekiert D.C., Krause J.C. Influenza human monoclonal antibody 1F1 interacts with three major antigenic sites and residues mediating human receptor specificity in H1N1 viruses. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baranovich T., Jones J.C., Russier M. The hemagglutinin stem-binding monoclonal antibody VIS410 controls influenza virus-induced acute respiratory distress syndrome. Antimicrob Agents Chemother. 2016;60:2118–2131. doi: 10.1128/AAC.02457-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corti D., Voss J., Gamblin S.J. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 56.Dreyfus C., Laursen N.S., Kwaks T. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337:1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekiert D.C., Friesen R.H., Bhabha G. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333:843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kallewaard N.L., Corti D., Collins P.J. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell. 2016;166:596–608. doi: 10.1016/j.cell.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tharakaraman K., Subramanian V., Cain D., Sasisekharan V., Sasisekharan R. Broadly neutralizing influenza hemagglutinin stem-specific antibody CR8020 targets residues that are prone to escape due to host selection pressure. Cell Host Microbe. 2014;15:644–651. doi: 10.1016/j.chom.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hershberger E., Sloan S., Narayan K. Safety and efficacy of monoclonal antibody VIS410 in adults with uncomplicated influenza A infection: Results from a randomized, double-blind, phase-2, placebo-controlled study. EBioMedicine. 2019;40:574–582. doi: 10.1016/j.ebiom.2018.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McBride J.M., Lim J.J., Burgess T. Phase 2 randomized trial of the safety and efficacy of MHAA4549A, a broadly neutralizing monoclonal antibody, in a human influenza A virus challenge model. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali S.O., Takas T., Nyborg A.C. A phase 2a study to evaluate the safety of MEDI8852 in outpatient adults with acute, uncomplicated influenza A. Open Forum Infect Dis. 2017;4:S519. [Google Scholar]
- 63.Chai N., Swem L.R., Reichelt M. Two escape mechanisms of influenza A virus to a broadly neutralizing stalk-binding antibody. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kotey E., Lukosaityte D., Quaye O., Ampofo W., Awandare G., Iqbal M. Current and novel approaches in influenza management. Vaccines (Basel) 2019;7:53. doi: 10.3390/vaccines7020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laursen N.S., Friesen R.H.E., Zhu X. Universal protection against influenza infection by a multidomain antibody to influenza hemagglutinin. Science. 2018;362:598–602. doi: 10.1126/science.aaq0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ortin J., Martin-Benito J. The RNA synthesis machinery of negative-stranded RNA viruses. Virology. 2015;479:532–544. doi: 10.1016/j.virol.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 67.Pflug A., Lukarska M., Resa-Infante P., Reich S., Cusack S. Structural insights into RNA synthesis by the influenza virus transcription-replication machine. Virus Res. 2017;234:103–117. doi: 10.1016/j.virusres.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 68.De Vlugt C., Sikora D., Pelchat M. Insight into Influenza: a Virus Cap-Snatching. Viruses-Basel. 2018;10:641. doi: 10.3390/v10110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevaert A., Naesens L. The influenza virus polymerase complex: an update on its structure, functions, and significance for antiviral drug design. Med Res Rev. 2016;36:1127–1173. doi: 10.1002/med.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aschenbrenner D.S. FDA approves new antiviral for influenza. Am J Nurs. 2019;119:21. doi: 10.1097/01.NAJ.0000553202.36589.e2. [DOI] [PubMed] [Google Scholar]
- 71.Noshi T., Kitano M., Taniguchi K. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antiviral Res. 2018;160:109–117. doi: 10.1016/j.antiviral.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Hayden F.G., Sugaya N., Hirotsu N. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379:913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 73.Kiso M., Yamayoshi S., Furusawa Y., Imai M., Kawaoka Y. Treatment of highly pathogenic H7N9 virus-infected mice with baloxavir marboxil. Viruses. 2019;11:1066. doi: 10.3390/v11111066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taniguchi K., Ando Y., Nobori H. Inhibition of avian-origin influenza A(H7N9) virus by the novel cap-dependent endonuclease inhibitor baloxavir marboxil. Sci Rep. 2019;9:3466. doi: 10.1038/s41598-019-39683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukao K., Ando Y., Noshi T. Baloxavir marboxil, a novel cap-dependent endonuclease inhibitor potently suppresses influenza virus replication and represents therapeutic effects in both immunocompetent and immunocompromised mouse models. Plos One. 2019;14:e0217307. doi: 10.1371/journal.pone.0217307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomassini J., Selnick H., Davies M.E. Inhibition of cap (m7GpppXm)-dependent endonuclease of influenza virus by 4-substituted 2,4-dioxobutanoic acid compounds. Antimicrob Agents Chemother. 1994;38:2827–2837. doi: 10.1128/aac.38.12.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mishin V.P., Patel M.C., Chesnokov A. Susceptibility of influenza A, B, C, and D viruses to baloxavir(1) Emerg Infect Dis. 2019;25:1969–1972. doi: 10.3201/eid2510.190607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koshimichi H., Ishibashi T., Wajima T. Population pharmacokinetics of baloxavir marboxil in Japanese pediatric influenza patients. J Pharm Sci. 2019;108:3112–3117. doi: 10.1016/j.xphs.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 79.Hirotsu N., Sakaguchi H., Sato C. Baloxavir marboxil in Japanese pediatric patients with influenza: safety and clinical and virologic outcomes. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz908. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takashita E., Morita H., Ogawa R. Susceptibility of influenza viruses to the novel cap-dependent endonuclease inhibitor baloxavir marboxil. Front Microbiol. 2018;9:3026. doi: 10.3389/fmicb.2018.03026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones J.C., Kumar G., Barman S. Identification of the I38T PA substitution as a resistance marker for next-generation influenza virus endonuclease inhibitors (vol 9, e00a30-18, 2018) Mbio. 2018;9:e00430-18. doi: 10.1128/mBio.00430-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omoto S., Speranzini V., Hashimoto T. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep. 2018;8:9633. doi: 10.1038/s41598-018-27890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Imai M., Yamashita M., Sakai-Tagawa Y. Influenza A variants with reduced susceptibility to baloxavir isolated from Japanese patients are fit and transmit through respiratory droplets. Nat Microbiol. 2019;5:27–33. doi: 10.1038/s41564-019-0609-0. [DOI] [PubMed] [Google Scholar]
- 84.Takashita E., Kawakami C., Morita H. Detection of influenza A(H3N2) viruses exhibiting reduced susceptibility to the novel cap-dependent endonuclease inhibitor baloxavir in Japan, December 2018. Euro Surveill. 2019;24:3026. doi: 10.2807/1560-7917.ES.2019.24.3.1800698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chesnokov A., Patel M.C., Mishin V.P. Replicative fitness of seasonal influenza A viruses with decreased susceptibility to baloxavir. J Infect Dis. 2020;221:367–371. doi: 10.1093/infdis/jiz472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Checkmahomed L., M'Hamdi Z., Carbonneau J. Impact of the baloxavir-resistant polymerase acid (PA) I38T substitution on the fitness of contemporary influenza A(H1N1)pdm09 and A(H3N2) strains. J Infect Dis. 2020;221:63–70. doi: 10.1093/infdis/jiz418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gubareva L.V., Mishin V.P., Patel M.C. Assessing baloxavir susceptibility of influenza viruses circulating in the United States during the 2016/17 and 2017/18 seasons. Euro Surveill. 2019;24:1800666. doi: 10.2807/1560-7917.ES.2019.24.3.1800666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koszalka P., Tilmanis D., Roe M., Vijaykrishna D., Hurt A.C. Baloxavir marboxil susceptibility of influenza viruses from the Asia-Pacific, 2012-2018. Antiviral Res. 2019;164:91–96. doi: 10.1016/j.antiviral.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 89.Takashita E., Kawakami C., Ogawa R. Influenza A(H3N2) virus exhibiting reduced susceptibility to baloxavir due to a polymerase acidic subunit I38T substitution detected from a hospitalised child without prior baloxavir treatment, Japan, January 2019. Eurosurveillance. 2019;24:9–12. doi: 10.2807/1560-7917.ES.2019.24.12.1900170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoon J.J., Toots M., Lee S. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62:e00766-18. doi: 10.1128/AAC.00766-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rocha-Pereira J., Jochmans D., Dallmeier K., Leyssen P., Nascimento M.S.J., Neyts J. Favipiravir (T-705) inhibits in vitro norovirus replication. Biochem Bioph Res Co. 2012;424:777–780. doi: 10.1016/j.bbrc.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 92.Julander J.G., Smee D.F., Morrey J.D., Furuta Y. Effect of T-705 treatment on western equine encephalitis in a mouse model. Antiviral Res. 2009;82:169–171. doi: 10.1016/j.antiviral.2009.02.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morrey J.D., Taro B.S., Siddharthan V. Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antiviral Res. 2008;80:377–379. doi: 10.1016/j.antiviral.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gowen B.B., Wong M.H., Jung K.H. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007;51:3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Furuta Y., Takahashi K., Kuno-Maekawa M. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baranovich T., Wong S.S., Armstrong J. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol. 2013;87:3741–3751. doi: 10.1128/JVI.02346-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Avila A.I., Gallego I., Soria M.E. Lethal mutagenesis of hepatitis C virus induced by favipiravir. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arias A., Thorne L., Goodfellow I. Favipiravir elicits antiviral mutagenesis during virus replication in vivo. Elife. 2014;3:e03679. doi: 10.7554/eLife.03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldhill D.H., Langat P., Xie H.Y. Determining the mutation bias of favipiravir in influenza virus using next-generation sequencing. J Virol. 2019;93:e01217-18. doi: 10.1128/JVI.01217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goldhill D.H., Te Velthuis A.J.W., Fletcher R.A. The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci U S A. 2018;115:11613–11618. doi: 10.1073/pnas.1811345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagata T., Lefor A.K., Hasegawa M., Ishii M. Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med Public Health Prep. 2015;9:79–81. doi: 10.1017/dmp.2014.151. [DOI] [PubMed] [Google Scholar]
- 103.Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 104.Yamada K., Noguchi K., Kimitsuki K. Reevaluation of the efficacy of favipiravir against rabies virus using in vivo imaging analysis. Antiviral Res. 2019;172 doi: 10.1016/j.antiviral.2019.104641. [DOI] [PubMed] [Google Scholar]
- 105.Jochmans D., van Nieuwkoop S., Smits S.L., Neyts J., Fouchier R.A., van den Hoogen B.G. Antiviral activity of favipiravir (T-705) against a broad range of paramyxoviruses in vitro and against human metapneumovirus in hamsters. Antimicrob Agents Chemother. 2016;60:4620–4629. doi: 10.1128/AAC.00709-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Toots M., Yoon J.J., Cox R.M. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med. 2019;11:eaax5866. doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stuyver L.J., Whitaker T., McBrayer T.R. Ribonucleoside analogue that blocks replication of bovine viral diarrhea and hepatitis C viruses in culture. Antimicrob Agents Ch. 2003;47:244–254. doi: 10.1128/AAC.47.1.244-254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Urakova N., Kuznetsova V., Crossman D.K. Beta-D-N-4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J Virol. 2018;92:e01965-17. doi: 10.1128/JVI.01965-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Agostini M.L., Pruijssers A.J., Chappell J.D. Small molecule antiviral beta-D-N (4)-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol. 2019 doi: 10.1128/JVI.01348-19. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reynard O., Nguyen X.N., Alazard-Dany N., Barateau V., Cimarelli A., Volchkov V.E. Identification of a new ribonucleoside inhibitor of Ebola virus replication. Viruses. 2015;7:6233–6240. doi: 10.3390/v7122934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Painter G.R., Bowen R.A., Bluemling G.R. The prophylactic and therapeutic activity of a broadly active ribonucleoside analog in a murine model of intranasal venezuelan equine encephalitis virus infection. Antiviral Res. 2019;171 doi: 10.1016/j.antiviral.2019.104597. [DOI] [PubMed] [Google Scholar]
- 112.Lu G., Bluemling G.R., Mao S. Simple in vitro assay to evaluate the incorporation efficiency of ribonucleotide analog 5′-triphosphates into RNA by human mitochondrial DNA-dependent RNA polymerase. Antimicrob Agents Ch. 2018;62:e01830-17. doi: 10.1128/AAC.01830-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feng J.Y., Xu Y.L., Barauskas O. Role of mitochondrial RNA polymerase in the toxicity of nucleotide inhibitors of hepatitis C virus. Antimicrob Agents Ch. 2016;60:806–817. doi: 10.1128/AAC.01922-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sultana S., Solotchi M., Ramachandran A., Patel S.S. Transcriptional fidelities of human mitochondrial POLRMT, yeast mitochondrial Rpo41, and phage T7 single-subunit RNA polymerases. J Biol Chem. 2017;292:18145–18160. doi: 10.1074/jbc.M117.797480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sticher Z.M., Lu G., Mitchell D.G. Analysis of the potential for N(4)-hydroxycytidine to inhibit mitochondrial replication and function. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01719-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smee D.F., Barnard D.L., Jones S.M. Activities of JNJ63623872 and oseltamivir against influenza A H1N1pdm and H3N2 virus infections in mice. Antivir Res. 2016;136:45–50. doi: 10.1016/j.antiviral.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 117.Byrn R.A., Jones S.M., Bennett H.B. Preclinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit. Antimicrob Agents Ch. 2015;59:1574–1587. doi: 10.1128/AAC.04623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Finberg R.W., Lanno R., Anderson D. Phase 2b study of pimodivir (JNJ-63623872) as monotherapy or in combination with oseltamivir for treatment of acute uncomplicated seasonal influenza A: TOPAZ trial. J Infect Dis. 2019;219:1026–1034. doi: 10.1093/infdis/jiy547. [DOI] [PubMed] [Google Scholar]
- 119.Clark M.P., Ledeboer M.W., Davies I. Discovery of a novel, first-in-class, orally bioavailable azaindole inhibitor (VX-787) of influenza PB2. J Med Chem. 2014;57:6668–6678. doi: 10.1021/jm5007275. [DOI] [PubMed] [Google Scholar]