Highlights
-
•
The role of birds in cross-species transmission and emergence of novel viruses such as avian influenza A viruses are discussed.
-
•
The novel avian viruses identified between 2012 and 2014 are summarized.
-
•
The concept of ‘pathogen augmentation’ is introduced.
Abstract
Birds, the only living member of the Dinosauria clade, are flying warm-blooded vertebrates displaying high species biodiversity, roosting and migratory behavior, and a unique adaptive immune system. Birds provide the natural reservoir for numerous viral species and therefore gene source for evolution, emergence and dissemination of novel viruses. The intrusions of human into natural habitats of wild birds, the domestication of wild birds as pets or racing birds, and the increasing poultry consumption by human have facilitated avian viruses to cross species barriers to cause zoonosis. Recently, a novel adenovirus was exclusively found in birds causing an outbreak of Chlamydophila psittaci infection among birds and humans. Instead of being the primary cause of an outbreak by jumping directly from bird to human, a novel avian virus can be an augmenter of another zoonotic agent causing the outbreak. A comprehensive avian virome will improve our understanding of birds’ evolutionary dynamics.
Current Opinion in Virology 2015, 10:63–69
This review comes from a themed issue on Emerging viruses: interspecies transmission
Edited by Antoine Gessain and Fernando Garcia-Arenal
For a complete overview see the Issue and the Editorial
Available online 31st January 2015
http://dx.doi.org/10.1016/j.coviro.2015.01.006
1879-6257/© 2015 Elsevier B.V. All rights reserved.
Introduction
Birds are flying warm-blooded vertebrates of the class Aves. They are the most highly biodiversified tetrapods with about 10,000 species. The evolution of birds from bipedal carnivorous dinosaurs through sustained miniaturization and anatomical innovation over approximately 160 million years is one of the most compelling examples of macroevolution [1••]. Modern-day birds are believed to be the only living members of the Dinosauria clade. Their unparalleled biodiversity and length of evolution among animals have provided abundant opportunities for virus acquisition from various sources. Their unique adaptive immune system allows asymptomatic infection and virus co-evolution to occur [2•]. Their global distribution, annual long distance migrations, and habitual roosting in environments shared by other animals further enhance the mixing and dissemination of viruses to and from other species.
These biological, immunological, and ecological characteristics have given birds distinctive roles in the emergence of novel viruses and their cross-species transmission [2•]. Birds may act as a vehicle for vector dissemination, an amplifying host in bird–vector–bird cycles, or the gene source of emerging viruses in cross-species virus transmission (Figure 1 ). Transmission of tick-borne viruses such as Crimean-Congo hemorrhagic fever virus, tick-borne encephalitis virus, and louping ill virus is facilitated by migratory birds which carry infected ticks especially of the genera Ixodes, Hyalomma, and Haemaphysalis [3]. Mesostigmatic mites such as Dermanyssus gallinae which are found in birds are implicated in the transmission of various human and avian pathogens including equine encephalitis viruses, Fowlpox virus, Newcastle disease virus, and hantaviruses [4]. Flaviviruses such as West Nile virus, Japanese encephalitis virus, and St. Louis encephalitis virus are amplified by birds in bird–mosquito–bird cycles and then transmitted to dead-end hosts including human. Avian influenza viruses are classical examples of viruses emerging from birds through the reassortment of different gene segments [5•]. In this article, we use the novel avian influenza viruses as examples to illustrate the role of birds in the cross-species transmission and emergence of zoonotic avian viruses, and discuss on the types and significance of other non-zoonotic avian viruses identified in the past two years.
Figure 1.
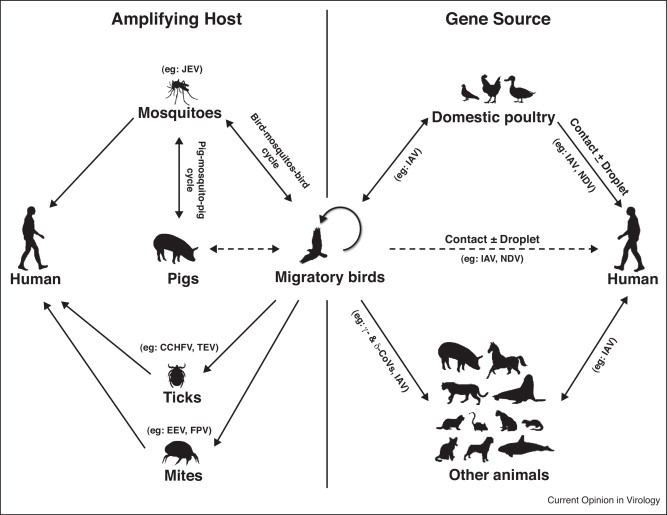
Birds as the gene source and amplifying host in cross-species transmission and emergence of novel viruses. Abbreviations: CCHFV, Crimean-Congo hemorrhagic fever virus; CoV, coronavirus; EEV, equine encephalitis virus; FPV, Fowlpox virus; IAV, influenza A virus; JEV, Japanese encephalitis virus; NDV, Newcastle disease virus; TEV, tick-borne encephalitis virus.
Avian influenza viruses: the role of birds in cross-species transmission and emergence of novel viruses
Birds have had a close relationship with human throughout history. The intrusions of human into the natural habitats of wild birds, the popularity of rearing pet birds in developed countries, pigeon racing and the increasing consumption of poultry in developing countries may have facilitated the emergence and dissemination of novel avian influenza viruses that cause zoonosis [5•, 6]. Avian influenza viruses have been known to cause human diseases since 1959 [7]. In 1997, A(H5N1) virus caused the first large human outbreak in Hong Kong [8••]. Other avian influenza viruses causing human disease between 1998 and 2012 include A(H7N2), A(H7N3), A(H7N7), A(H9N2) and A(H10N7) viruses [7]. In the past two years, four novel avian influenza viruses have emerged to cause human infections confirmed by genome sequencing of the virus isolates (Table 1 ) [9, 10•, 11, 12]. Among these four viruses, A(H7N9) virus poses the most severe threat to humans, causing over 450 human cases with a case-fatality rate of about 30% [5•, 13].
Table 1.
Novel avian influenza viruses associated with human infection reported between 2012 and 2014 (as of 21 October 2014)
| Virus | Place (date) of first human case | No. of human cases (case-fatality rate) | Clinical significance in birds | References |
|---|---|---|---|---|
| A(H7N9) | Shanghai, China (February 2013) | 455 (∼30%) | Lowly pathogenic | [10•, 39] |
| A(H6N1) | Taiwan (May 2013) | 1 (0%) | Lowly pathogenic | [9] |
| A(H10N8) | Nanchang City, China (November 2013) | 3 (67%) | Variable | [11] |
| A(H5N6) | Sichuan, China (May 2014) | 1 (100%) | Highly pathogenic | [12, 40] |
Sixteen of 18 hemagglutinin (HA) subtypes and nine of 11 neuraminidase (NA) subtypes of influenza viruses can be found in birds, especially waterfowl and shorebirds [2•]. Out of a possible 144 HA and NA combinations, 112 were identified in wild birds, including 49 that were also found in domestic birds [14]. Since most of these avian influenza viruses are of low pathogenicity in birds, infected migratory birds can carry different influenza virus subtypes for long distances. Mixed infection is also commonly identified [15]. Therefore, waterfowls remain the most important source of genetic diversity for influenza viruses.
Although wild birds harbor the most diverse subtypes of influenza viruses, reassortment and amplification events of avian influenza viruses affecting humans most likely occur in live poultry markets (LPM) where there are high densities of poultries [16, 17••]. Surveillance studies showed that avian influenza viruses were found more commonly in poultry samples collected in LPM than in wild bird samples or backyard poultry samples [18]. Next-generation sequencing showed that mixed infection of different subtypes is common among poultries in LPM [19]. Phylogenetic studies suggested that A(H7N9) and A(H10N8) viruses are reassortants with internal genes originating from A(H9N2) viruses that are circulating in poultries [16, 17••, 20]. Unlike A(H5N1) virus which is highly pathogenic for poultries, A(H7N9) and A(H10N8) viruses tend to cause asymptomatic avian infections, and therefore widespread circulation of A(H7N9) and A(H10N8) viruses among poultries were not recognized until human cases appeared.
Only few avian influenza viruses can be transmitted directly from birds to humans. These avian influenza viruses are usually amplified and mixed in the poultry population before transmission from poultries to humans. For example, both A(H7N9) and A(H10N8) viruses affecting humans carry internal genes originating from A(H9N2) isolated from poultries [20]. Direct contact between humans and infected poultries therefore poses a high risk of transmission. Epidemiological studies suggested that humans without direct contact with infected poultries can also be infected via contacting the contaminated environment in live poultries markets [21]. Older age and presence of underlying diseases are risk factors for A(H7N9) virus infection, but most patients with A(H5N1) virus infection do not have these risk factors [22]. Beside epidemiological and host factors, many studies have tried to elucidate the virological characteristics allowing a particular virus subtype to cause human disease. Traditionally, the sialic acid receptor preference of the viral surface glycoprotein HA has been the focus of attention [5•, 23]. Avian influenza viruses and human seasonal influenza viruses preferentially bind to α2,3-linked sialic acid receptor (α2,3 SA) and α2,6-linked sialic acid receptor (α2,6 SA), respectively. Some human A(H5N1) virus isolates had increased affinity to α2,6 SA [24]. The distribution of receptors also differs between humans and birds. In humans, the nasal mucosa, paranasal sinuses, pharynx, trachea and bronchi are mainly lined by α2,6 SA, while the non-ciliated cuboidal bronchiolar cells and type II pneumocytes in the alveoli express α2,3 SA [25]. In chickens and quails, α2,3 SA and α2,6 SA are found on the epithelial cells of both the intestine and respiratory tract. In ducks, only α2,3 SA can be found in the intestine, while α2,3 SA are much more abundant than α2,6 SA in the trachea [26]. Although receptor binding is certainly an important attribute to the adaptation of avian influenza viruses to humans, the discrepant receptor binding results from different studies on wild-type avian influenza viruses isolated from patients, even using the same virus strain, make it difficult to generalize the importance of receptor binding in the natural setting [27]. Furthermore, some avian influenza virus isolates that do not cause human disease also have high preference for α2,6 SA. For example, non-human avian H10 viruses can bind to α2,6 SA, but these H10 viruses have not caused human disease [28]. Therefore, other genetic attributes are important for viral adaptation to humans.
Besides a change of receptor binding affinity for human receptors, it is believed that the ability of the virus to replicate efficiently in human cells is also essential for cross-species transmission of avian influenza viruses. While influenza virus utilizes viral polymerase to replicate viral genome, it relies on host machinery to transcribe viral mRNA and translate viral proteins of new virions. Trafficking of viral genome between nucleus, where virus genome is synthesized, and the cytoplasm, where viral proteins are translated and virion are packaged, could be another critical barrier of host restriction. Adaptations that affect viral polymerase function have been extensively studied and several markers which enhanced virus replication in mammals have been identified in the basic polymerase 2 (PB2), basic polymerase 1 (PB1), acid polymerase (PA), nucleoprotein (NP) and nuclear export protein (NEP) [29]. One of the most important substitution is the PB2 E627K, which has been associated with enhanced viral replication at the temperature of the human upper respiratory tract and were identified in many human isolates of A(H7N9) and A(H10N8) viruses [10•, 27, 30]. PB2 K562R substitution, which is present in some A(H7N9) strains and 80% of A(H5N1) strains, also increase viral polymerase activity [31]. Other important mutations of PB2 include D701N, Q591K, and T271A [29]. Recent findings appear to favor a hypothesis that compatibility between subunits of ribonucleoprotein (RNP) and NEP which facilitate viral genome trafficking from nucleus to cytoplasm for virus packaging at late stage of infection determine the fitness of viral genome for host adaptation [32].
Several groups have investigated mammalian adaptation of avian influenza viruses by generating mutant avian influenza viruses capable of non-contact transmission between ferrets. Studies on A(H5N1) viruses generated by serial passage in ferrets showed that mutations affecting receptor binding, fusion of viral and host cell membrane, and polymerase activity are important for transmission via the non-contact route [33, 34]. A study using influenza viruses generated with gene segments originating from circulating avian influenza viruses and the 1918 pandemic A(H1N1) virus showed that substitutions in the HA, PB2 and PA are important for virulence and efficient transmission in ferrets [35].
In addition to the avian influenza viruses subtypes H5, H7, H9 and H10 that are transmitted directly from birds or the contaminated environment to humans, several pandemic influenza viruses also carry gene segments originating from birds. These gene segments from avian viruses reassort with those from circulating human or swine influenza viruses. The PB1, HA and NA of the 1957 A(H2N2) and the PB1 and HA of the 1968 A(H3N2) originated from avian influenza viruses [23]. For the 1918 pandemic A(H1N1) virus, the timing of the introduction of the genes from avian influenza viruses is controversial. However, a recent phylogenomic study showed that the internal genes of the 1918 pandemic A(H1N1) virus originated from the western hemisphere avian influenza virus lineage [36]. Therefore, avian influenza viruses remain to be an important gene source for future pandemic influenza viruses, and continued surveillance among wild birds and poultries is necessary for better understanding of the virus origin in future pandemics.
Non-zoonotic avian virus discoveries and their significance: the novel concept of ‘pathogen augmentation’
Besides avian influenza viruses, advances in molecular sequencing technologies in recent years have led to the identification of many other novel viruses in avian species. Between January 2012 and September 2014, at least 73 viruses belonging to more than 17 families were identified in birds and published in NCBI-indexed journals. The clinical significance of many of these novel viruses remains unknown as only molecular detection of viral sequences without virus isolation or histological evidence of infection was reported. Among the 40 novel avian DNA viruses, Adenoviridae, Herpesviridae, and Poxviridae had the most clearly documented veterinary significance (see also Supplementary data, Table A). Most of them are associated with clinical symptoms and histological evidence of infection in addition to the molecular detection of viral sequences in clinical specimens. Among the 33 novel RNA viruses, those in the Coronaviridae were the most numerous (see also Supplementary data, Table B). This wealth of virus genetic data reformed the International Committee on Taxonomy of Viruses (ICTV) classification of Coronaviridae. In 2011, Deltacoronavirus, a new genus that mainly consists of avian coronaviruses, was added to the existing Alphacoronavirus, Betacoronavirus, and Gammacoronavirus under the subfamily Coronavirinae in the family Coronaviridae. Phylogenetic analysis showed that most members of Deltacoronavirus and Gammacoronavirus have likely originated from birds and may subsequently cross species barriers to infect other animals as exemplified by the possible emergence of porcine coronavirus HKU15 in pigs originating from sparrows [37].
Historically, the significance of newly identified avian viruses has mainly been determined by the viral pathogenicity in causing disease in humans or birds directly. Recently, the novel concept of ‘pathogen augmentation’ was introduced and brought insights into the significance of avian viruses in cross-species transmission of pathogens. A novel adenovirus was detected in Mealy Parrots during an outbreak of avian chlamydiosis and human psittacosis amongst epidemiologically linked workers in an animal detention center in Hong Kong [38••]. This novel psittacine adenovirus was detected only in the lung, kidney, liver, spleen and cloacal swabs of birds with Chlamydophila psittaci but not in other healthy birds. Moreover, the bacterial load of C. psittaci was higher in adenovirus-infected birds with higher viral load. The adenovirus likely caused immunosuppression of the infected birds which allowed C. psittaci to multiply to high levels with subsequent transmission to humans. These findings demonstrated that infection of birds by an emerging novel virus could lead to an outbreak of re-emerging infections such as C. psittaci infection among birds and epidemiologically linked humans. Instead of being the primary cause of the outbreak by jumping directly from bird to human, the novel avian virus acted as an augmenter of another zoonotic agent causing the outbreak. This concept of ‘pathogen augmentation’ in birds is analogous to the enhanced epidemiological dissemination of Mycobacterium tuberculosis in patients with human immunodeficiency virus infection. It is different from human infection by flaviviruses which are amplified in birds in bird–mosquito–bird cycles. Thus, an unusual outbreak of zoonotic diseases in human may warrant further investigations of the animal source to identify the possible underlying viral infection in epidemiologically linked birds.
Conclusions
In the recent two years, we have witnessed an explosion in avian virus discovery through the application of state-of-the art molecular diagnostic tools. The emerging avian influenza A(H7N9) virus reminds the global health community of the pandemic potential of avian viruses that are capable of crossing species barriers. Continuous, systematic, and global bird surveillance programs are important for the identification of other avian viruses with zoonotic potential. Molecular studies are important in identifying the appropriate adaptive mutations for cross-species transmission or virulent mutants of veterinary significance. The novel concept of ‘pathogen augmentation’ demonstrated in a recent outbreak of C. psittasi infection associated with a new avian adenovirus highlights the importance of investigating for an underlying viral infection in epidemiologically linked avian source associated with outbreaks of re-emerging zoonoses in human.
Conflict of interest
The authors declare no conflict of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by the Providence Foundation Limited in memory of the late Lui Hac Minh, the commissioned research grant from the Health and Medical Research Fund of the Food and Health Bureau, and Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease for the Department of Health, Hong Kong Special Administrative Region. The funding sources had no role in the study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the article for publication.
Footnotes
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.coviro.2015.01.006.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1••.Lee M.S., Cau A., Naish D., Dyke G.J. Dinosaur evolution. Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds. Science. 2014;345:562–566. doi: 10.1126/science.1252243. [DOI] [PubMed] [Google Scholar]; Bayesian approaches were used to identify the drivers underlying the dinosaur-bird transition as an example of macroevolution.
- 2•.Chan J.F., To K.K., Tse H., Jin D.Y., Yuen K.Y. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21:544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review of how the unique avian adaptive immune system facilitates interspecies transmission and emergence of novel viruses.
- 3.Bente D.A., Forrester N.L., Watts D.M., McAuley A.J., Whitehouse C.A., Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100:159–189. doi: 10.1016/j.antiviral.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Valiente Moro C., Chauve C., Zenner L. Vectorial role of some dermanyssoid mites (Acari, Mesostigmata, Dermanyssoidea) Parasite. 2005;12:99–109. doi: 10.1051/parasite/2005122099. [DOI] [PubMed] [Google Scholar]
- 5•.To K.K., Chan J.F., Chen H., Li L., Yuen K.Y. The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: a tale of two cities. Lancet Infect Dis. 2013;13:809–821. doi: 10.1016/S1473-3099(13)70167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comparative analysis of the similarities and differences between the outbreaks caused by avian influenza A(H5N1) virus in Hong Kong in 1997 and avian influenza A/H7N9 virus in mainland China in 2013.
- 6.Anonymous: U.S. Pet Ownership Statistics. Available at https://www.avma.org/KB/Resources/Statistics/Pages/Market-research-statistics-US-pet-ownership.aspx (accessed on 21.10.14).
- 7.To K.K., Tsang A.K., Chan J.F., Cheng V.C., Chen H., Yuen K.Y. Emergence in China of human disease due to avian influenza A(H10N8) — cause for concern? J Infect. 2014;68:205–215. doi: 10.1016/j.jinf.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 8••.Yuen K.Y., Chan P.K., Peiris M., Tsang D.N., Que T.L., Shortridge K.F., Cheung P.T., To W.K., Ho E.T., Sung R. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]; The first report of severe human disease caused by any avian influenza A virus in history.
- 9.Wei S.H., Yang J.R., Wu H.S., Chang M.C., Lin J.S., Lin C.Y., Liu Y.L., Lo Y.C., Yang C.H., Chuang J.H. Human infection with avian influenza A H6N1 virus: an epidemiological analysis. Lancet Respir Med. 2013;1:771–778. doi: 10.1016/S2213-2600(13)70221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W., Chen J., Jie Z., Qiu H., Xu K. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]; The first three cases of severe human disease caused by avian influenza A/H7N9 virus infection.
- 11.Chen H., Yuan H., Gao R., Zhang J., Wang D., Xiong Y., Fan G., Yang F., Li X., Zhou J. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383:714–721. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- 12.Centre for Health Protection: Detection of avian influenza A(H5N6) virus from a patient in Sichuan closely monitored by DH. Available at http://www.chp.gov.hk/en/content/116/34591.html (accessed on 21.10.14).
- 13.Centre for Health Protection . 2014. Avian influenza report. Available at http://www.chp.gov.hk/en/guideline1_year/29/134/441/332.html (accessed 21.10.14) [Google Scholar]
- 14.Olson S.H., Parmley J., Soos C., Gilbert M., Latorre-Margalef N., Hall J.S., Hansbro P.M., Leighton F., Munster V., Joly D. Sampling strategies and biodiversity of influenza a subtypes in wild birds. PLoS One. 2014;9:e90826. doi: 10.1371/journal.pone.0090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G., Zhang T., Li X., Jiang Z., Jiang Q., Chen Q., Tu X., Chen Z., Chang J., Li L. Serological evidence of H7, H5 and H9 avian influenza virus co-infection among herons in a city park in Jiangxi, China. Sci Rep. 2014;4:6345. doi: 10.1038/srep06345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui L., Liu D., Shi W., Pan J., Qi X., Li X., Guo X., Zhou M., Li W., Li J. Dynamic reassortments and genetic heterogeneity of the human-infecting influenza A (H7N9) virus. Nat Commun. 2014;5:3142. doi: 10.1038/ncomms4142. [DOI] [PubMed] [Google Scholar]
- 17••.Chen Y., Liang W., Yang S., Wu N., Gao H., Sheng J., Yao H., Wo J., Fang Q., Cui D. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381:1916–1925. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study to demonstrate the epidemiological and phylogenetic link between human cases of avian influenza A/H7N9 and infected wet market poultry.
- 18.Ni X., He F., Hu M., Zhou X., Wang B., Feng C., Wu Y., Li Y., Tu J., Li H. Investigation of avian influenza virus in poultry and wild birds due to novel avian-origin influenza A(H10N8) in Nanchang City, China. Microbes Infect. 2014;17:48–53. doi: 10.1016/j.micinf.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Yu X., Jin T., Cui Y., Pu X., Li J., Xu J., Liu G., Jia H., Liu D., Song S. Influenza H7N9 and H9N2 viruses: coexistence in poultry linked to human H7N9 infection and genome characteristics. J Virol. 2014;88:3423–3431. doi: 10.1128/JVI.02059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D., Shi W., Gao G.F. Poultry carrying H9N2 act as incubators for novel human avian influenza viruses. Lancet. 2014;383:869. doi: 10.1016/S0140-6736(14)60386-X. [DOI] [PubMed] [Google Scholar]
- 21.Li Q., Zhou L., Zhou M., Chen Z., Li F., Wu H., Xiang N., Chen E., Tang F., Wang D. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014;370:520–532. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowling B.J., Jin L., Lau E.H., Liao Q., Wu P., Jiang H., Tsang T.K., Zheng J., Fang V.J., Chang Z. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. 2013;382:129–137. doi: 10.1016/S0140-6736(13)61171-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horimoto T., Kawaoka Y. Pandemic threat posed by avian influenza A viruses. Clin Microbiol Rev. 2001;14:129–149. doi: 10.1128/CMR.14.1.129-149.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe Y., Ibrahim M.S., Ellakany H.F., Kawashita N., Mizuike R., Hiramatsu H., Sriwilaijaroen N., Takagi T., Suzuki Y., Ikuta K. Acquisition of human-type receptor binding specificity by new H5N1 influenza virus sublineages during their emergence in birds in Egypt. PLoS Pathog. 2011;7:e1002068. doi: 10.1371/journal.ppat.1002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinya K., Ebina M., Yamada S., Ono M., Kasai N., Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 26.Ge S., Wang Z. An overview of influenza A virus receptors. Crit Rev Microbiol. 2011;37:157–165. doi: 10.3109/1040841X.2010.536523. [DOI] [PubMed] [Google Scholar]
- 27.To K.K., Chan J.F., Yuen K.Y. Viral lung infections: epidemiology, virology, clinical features, and management of avian influenza A(H7N9) Curr Opin Pulm Med. 2014;20:225–232. doi: 10.1097/MCP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 28.Vachieri S.G., Xiong X., Collins P.J., Walker P.A., Martin S.R., Haire L.F., Zhang Y., McCauley J.W., Gamblin S.J., Skehel J.J. Receptor binding by H10 influenza viruses. Nature. 2014;511:475–477. doi: 10.1038/nature13443. [DOI] [PubMed] [Google Scholar]
- 29.Manz B., Schwemmle M., Brunotte L. Adaptation of avian influenza A virus polymerase in mammals to overcome the host species barrier. J Virol. 2013;87:7200–7209. doi: 10.1128/JVI.00980-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.To K.K., Song W., Lau S.Y., Que T.L., Lung D.C., Hung I.F., Chen H., Yuen K.Y. Unique reassortant of influenza A(H7N9) virus associated with severe disease emerging in Hong Kong. J Infect. 2014;69:60–68. doi: 10.1016/j.jinf.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song W., Wang P., Mok B.W., Lau S.Y., Huang X., Wu W.L., Zheng M., Wen X., Yang S., Chen Y. The K526R substitution in viral protein PB2 enhances the effects of E627K on influenza A virus replication. Nat Commun. 2014;5:5509. doi: 10.1038/ncomms6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paterson D., Fodor E. Emerging roles for the influenza A virus nuclear export protein (NEP) PLoS Pathog. 2012;8:e1003019. doi: 10.1371/journal.ppat.1003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linster M., van Boheemen S., de Graaf M., Schrauwen E.J., Lexmond P., Manz B., Bestebroer T.M., Baumann J., van Riel D., Rimmelzwaan G.F. Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell. 2014;157:329–339. doi: 10.1016/j.cell.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong X., Coombs P.J., Martin S.R., Liu J., Xiao H., McCauley J.W., Locher K., Walker P.A., Collins P.J., Kawaoka Y. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature. 2013;497:392–396. doi: 10.1038/nature12144. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T., Zhong G., Russell C.A., Nakajima N., Hatta M., Hanson A., McBride R., Burke D.F., Takahashi K., Fukuyama S. Circulating avian influenza viruses closely related to the 1918 virus have pandemic potential. Cell Host Microbe. 2014;15:692–705. doi: 10.1016/j.chom.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worobey M., Han G.Z., Rambaut A. A synchronized global sweep of the internal genes of modern avian influenza virus. Nature. 2014;508:254–257. doi: 10.1038/nature13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.To K.K., Tse H., Chan W.M., Choi G.K., Zhang A.J., Sridhar S., Wong S.C., Chan J.F., Chan A.S., Woo P.C. A novel psittacine adenovirus identified during an outbreak of avian chlamydiosis and human psittacosis: zoonosis associated with virus-bacterium coinfection in birds. PLoS Negl Trop Dis. 2014;8:e3318. doi: 10.1371/journal.pntd.0003318. [DOI] [PMC free article] [PubMed] [Google Scholar]; The novel concept of ‘pathogen augmentation’ in birds is illustrated in an outbreak of avian chalmydiosis and human psittacosis where the Mealy Parrots were co-infected by a new avian adenovirus.
- 39.Kalthoff D., Bogs J., Grund C., Tauscher K., Teifke J.P., Starick E., Harder T., Beer M. Avian influenza H7N9/13 and H7N7/13: a comparative virulence study in chickens, pigeons, and ferrets. J Virol. 2014;88:9153–9165. doi: 10.1128/JVI.01241-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The World Organisation for Animal Health: Update on highly pathogenic avian influenza in animals (type H5 and H7). Available at http://www.oie.int/wahis_2/public%5C.%5Ctemp%5Creports/en_fup_0000015698_20140731_162951.pdf (accessed 22.10.14).
Publications for further reading and for Supplementary tables
- 41.Bodewes R., van de Bildt M.W., Schapendonk C.M., van Leeuwen M., van Boheemen S., de Jong A.A., Osterhaus A.D., Smits S.L., Kuiken T. Identification and characterization of a novel adenovirus in the cloacal bursa of gulls. Virology. 2013;440:84–88. doi: 10.1016/j.virol.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Marek A., Ballmann M.Z., Kosiol C., Harrach B., Schlotterer C., Hess M. Whole-genome sequences of two turkey adenovirus types reveal the existence of two unknown lineages that merit the establishment of novel species within the genus Aviadenovirus. J Gen Virol. 2014;95:156–170. doi: 10.1099/vir.0.057711-0. [DOI] [PubMed] [Google Scholar]
- 43.Park Y.M., Kim J.H., Gu S.H., Lee S.Y., Lee M.G., Kang Y.K., Kang S.H., Kim H.J., Song J.W. Full genome analysis of a novel adenovirus from the South Polar skua (Catharacta maccormicki) in Antarctica. Virology. 2012;422:144–150. doi: 10.1016/j.virol.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S.Y., Kim J.H., Park Y.M., Shin O.S., Kim H., Choi H.G., Song J.W. A novel adenovirus in Chinstrap penguins (Pygoscelis antarctica) in Antarctica. Viruses. 2014;6:2052–2061. doi: 10.3390/v6052052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph H.M., Ballmann M.Z., Garner M.M., Hanley C.S., Berlinski R., Erdelyi K., Childress A.L., Fish S.S., Harrach B., Wellehan J.F., Jr. A novel siadenovirus detected in the kidneys and liver of Gouldian finches (Erythura gouldiae) Vet Microbiol. 2014;172:35–43. doi: 10.1016/j.vetmic.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Chu D.K., Poon L.L., Chiu S.S., Chan K.H., Ng E.M., Bauer I., Cheung T.K., Ng I.H., Guan Y., Wang D. Characterization of a novel gyrovirus in human stool and chicken meat. J Clin Virol. 2012;55:209–213. doi: 10.1016/j.jcv.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seimon T.A., McAloose D., Raphael B., Honkavuori K.S., Chang T., Hirschberg D.L., Lipkin W.I. A novel herpesvirus in 3 species of pheasants: mountain peacock pheasant (Polyplectron inopinatum), Malayan peacock pheasant (Polyplectron malacense), and Congo peafowl (Afropavo congensis) Vet Pathol. 2012;49:482–491. doi: 10.1177/0300985811424733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shivaprasad H.L., Phalen D.N. A novel herpesvirus associated with respiratory disease in Bourke's parrots (Neopsephotus bourkii) Avian Pathol. 2012;41:531–539. doi: 10.1080/03079457.2012.732692. [DOI] [PubMed] [Google Scholar]
- 49.Varsani A., Kraberger S., Jennings S., Porzig E.L., Julian L., Massaro M., Pollard A., Ballard G., Ainley D.G. A novel papillomavirus in Adelie penguin (Pygoscelis adeliae) faeces sampled at the Cape Crozier colony, Antarctica. J Gen Virol. 2014;95:1352–1365. doi: 10.1099/vir.0.064436-0. [DOI] [PubMed] [Google Scholar]
- 50.Phan T.G., Vo N.P., Boros A., Pankovics P., Reuter G., Li O.T., Wang C., Deng X., Poon L.L., Delwart E. The viruses of wild pigeon droppings. PLoS One. 2013;8:e72787. doi: 10.1371/journal.pone.0072787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baron H.R., Howe L., Varsani A., Doneley R.J. Disease screening of three breeding populations of adult exhibition budgerigars (Melopsittacus undulatus) in New Zealand reveals a high prevalence of a novel polyomavirus and avian malaria infection. Avian Dis. 2014;58:111–117. doi: 10.1637/10604-063013-REG.1. [DOI] [PubMed] [Google Scholar]
- 52.Offerman K., Carulei O., Gous T.A., Douglass N., Williamson A.L. Phylogenetic and histological variation in avipoxviruses isolated in South Africa. J Gen Virol. 2013;94:2338–2351. doi: 10.1099/vir.0.054049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niemeyer C., Favero C.M., Kolesnikovas C.K., Bhering R.C., Brandao P., Catao-Dias J.L. Two different avipoxviruses associated with pox disease in Magellanic penguins (Spheniscus magellanicus) along the Brazilian coast. Avian Pathol. 2013;42:546–551. doi: 10.1080/03079457.2013.849794. [DOI] [PubMed] [Google Scholar]
- 54.Sikorski A., Kearvell J., Elkington S., Dayaram A., Arguello-Astorga G.R., Varsani A. Novel ssDNA viruses discovered in yellow-crowned parakeet (Cyanoramphus auriceps) nesting material. Arch Virol. 2013;158:1603–1607. doi: 10.1007/s00705-013-1642-6. [DOI] [PubMed] [Google Scholar]
- 55.Chu D.K., Leung C.Y., Perera H.K., Ng E.M., Gilbert M., Joyner P.H., Grioni A., Ades G., Guan Y., Peiris J.S. A novel group of avian astroviruses in wild aquatic birds. J Virol. 2012;86:13772–13778. doi: 10.1128/JVI.02105-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Honkavuori K.S., Briese T., Krauss S., Sanchez M.D., Jain K., Hutchison S.K., Webster R.G., Lipkin W.I. Novel coronavirus and astrovirus in Delaware Bay shorebirds. PLoS One. 2014;9:e93395. doi: 10.1371/journal.pone.0093395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolf S., Reetz J., Hoffmann K., Grundel A., Schwarz B.A., Hanel I., Otto P.H. Discovery and genetic characterization of novel caliciviruses in German and Dutch poultry. Arch Virol. 2012;157:1499–1507. doi: 10.1007/s00705-012-1326-7. [DOI] [PubMed] [Google Scholar]
- 58.Chen G.Q., Zhuang Q.Y., Wang K.C., Liu S., Shao J.Z., Jiang W.M., Hou G.Y., Li J.P., Yu J.M., Li Y.P. Identification and survey of a novel avian coronavirus in ducks. PLoS One. 2013;8:e72918. doi: 10.1371/journal.pone.0072918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liais E., Croville G., Mariette J., Delverdier M., Lucas M.N., Klopp C., Lluch J., Donnadieu C., Guy J.S., Corrand L. Novel avian coronavirus and fulminating disease in guinea fowl, France. Emerg Infect Dis. 2014;20:105–108. doi: 10.3201/eid2001.130774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yun T., Ye W., Ni Z., Zhang D., Zhang C. Identification and molecular characterization of a novel flavivirus isolated from Pekin ducklings in China. Vet Microbiol. 2012;157:311–319. doi: 10.1016/j.vetmic.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 61.Huang X., Han K., Zhao D., Liu Y., Zhang J., Niu H., Zhang K., Zhu J., Wu D., Gao L. Identification and molecular characterization of a novel flavivirus isolated from geese in China. Res Vet Sci. 2013;94:774–780. doi: 10.1016/j.rvsc.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 62.Kessell A., Hyatt A., Lehmann D., Shan S., Crameri S., Holmes C., Marsh G., Williams C., Tachedjian M., Yu M. Cygnet River virus, a novel orthomyxovirus from ducks, Australia. Emerg Infect Dis. 2012;18:2044–2046. doi: 10.3201/eid1812.120500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Briand F.X., Henry A., Massin P., Jestin V. Complete genome sequence of a novel avian paramyxovirus. J Virol. 2012;86:7710. doi: 10.1128/JVI.00946-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Day J.M., Zsak L. Molecular and phylogenetic analysis of a novel turkey-origin picobirnavirus. Avian Dis. 2014;58:137–142. doi: 10.1637/10593-061313-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 65.Wang X., Liu N., Wang F., Ning K., Li Y., Zhang D. Genetic characterization of a novel duck-origin picornavirus with six 2A proteins. J Gen Virol. 2014;95:1289–1296. doi: 10.1099/vir.0.063313-0. [DOI] [PubMed] [Google Scholar]
- 66.Boros A., Nemes C., Pankovics P., Kapusinszky B., Delwart E., Reuter G. Genetic characterization of a novel picornavirus in turkeys (Meleagris gallopavo) distinct from turkey galliviruses and megriviruses and distantly related to the members of the genus Avihepatovirus. J Gen Virol. 2013;94:1496–1509. doi: 10.1099/vir.0.051797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pankovics P., Boros A., Kiss T., Reuter G. Identification and complete genome analysis of kobuvirus in faecal samples of European roller (Coracias garrulus): for the first time in a bird. Arch Virol. 2014;160:345–351. doi: 10.1007/s00705-014-2228-7. [DOI] [PubMed] [Google Scholar]
- 68.Boros A., Kiss T., Kiss O., Pankovics P., Kapusinszky B., Delwart E., Reuter G. Genetic characterization of a novel picornavirus distantly related to the marine mammal-infecting aquamaviruses in a long-distance migrant bird species, European roller (Coracias garrulus) J Gen Virol. 2013;94:2029–2035. doi: 10.1099/vir.0.054676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farkas T., Fey B., Hargitt E., 3rd, Parcells M., Ladman B., Murgia M., Saif Y. Molecular detection of novel picornaviruses in chickens and turkeys. Virus Genes. 2012;44:262–272. doi: 10.1007/s11262-011-0695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boros A., Nemes C., Pankovics P., Kapusinszky B., Delwart E., Reuter G. Identification and complete genome characterization of a novel picornavirus in turkey (Meleagris gallopavo) J Gen Virol. 2012;93:2171–2182. doi: 10.1099/vir.0.043224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pankovics P., Boros A., Reuter G. Novel picornavirus in domesticated common quail (Coturnix coturnix) in Hungary. Arch Virol. 2012;157:525–530. doi: 10.1007/s00705-011-1192-8. [DOI] [PubMed] [Google Scholar]
- 72.Bullman S., Kearney K., O’Mahony M., Kelly L., Whyte P., Fanning S., Morgan J.G. Identification and genetic characterization of a novel picornavirus from chickens. J Gen Virol. 2014;95:1094–1103. doi: 10.1099/vir.0.061085-0. [DOI] [PubMed] [Google Scholar]
- 73.Kapoor A., Tesh R.B., Duraisamy R., Popov V.L., Travassos da Rosa A.P., Lipkin W.I. A novel mosquito-borne Orbivirus species found in South-east Asia. J Gen Virol. 2013;94:1051–1057. doi: 10.1099/vir.0.046748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Z., Zhu Y., Li C., Liu G. Outbreak-associated novel duck Reovirus, China, 2011. Emerg Infect Dis. 2012;18:1209–1211. doi: 10.3201/eid1807.120190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palacios G., Forrester N.L., Savji N., Travassos da Rosa A.P., Guzman H., Detoy K., Popov V.L., Walker P.J., Lipkin W.I., Vasilakis N. Characterization of Farmington virus, a novel virus from birds that is distantly related to members of the family Rhabdoviridae. Virol J. 2013;10:219. doi: 10.1186/1743-422X-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


