Abstract
Background
Post-tooth extraction socket preservation is necessary due to alveolar bone resorptive patterns through regenerative dentistry approaches that involve the use of stem cells, scaffold and growth factor. Stem cells derived from human exfoliated deciduous teeth (SHED) are known to potentially possess the osteogenic ability. Meanwhile, carbonate apatite scaffold (CAS) can act as a biocompatible scaffold capable of supporting mesenchymal stem cells (MSCs) to proliferate and differentiate optimally. The aim of this study is to investigate the expression of bone morphogenic protein-2 and 7 (BMP2, BMP7) and Matrix Metalloproteinase-8 (MMP-8) after the transplantation of SHED-incorporated CAS during in vivo bone remodeling.
Material and Methods
A total of 14 healthy, male, Wistar rats, whose mandible anterior teeth were extracted by means of sterile needle holder clamps, constituted the subjects of this study of alveolar bone defects. Two research groups were created: a control group (CAS) as group I and an experimental group (CAS + SHED) as group II. SHED with a density of 106 cells were incorporated into CAS before being transplanted into the experimental group. After 7 days, all the animals were sacrificed and their mandible anterior region extracted. The BMP2, BMP7 and MMP-8 expression were subsequently analyzed by means of immunostaining. An unpaired t-test was conducted to analyze the treatment and control group (p<0.01) data.
Results
The expression of BMP-2 and BMP-7 was higher in group II compared to group I. Meanwhile, the level of MMP-8 was lower in group II than group I. There was greater significant increased expression of BMP-2 and BMP-7 expression in Group II compared to Group I. There was significant decreased expression of MMP-8 between group II than group I (p<0.01).
Conclusion
SHED-incorporated CAS can enhance BMP-2 and BMP-7 expression while attenuating MMP-8 expression during in vivo alveolar bone remodeling.
Keywords: alveolar bone defect, bone regeneration, carbonate apatite scaffold, osteogenic ability, stem cell from human exfoliated deciduous teeth
Introduction
Indonesia is the largest archipelagic country in the world with a population of 271,786,538 in 2019.1 Its expansive territory and dense population induce numerous medical conditions such as oral health problems.2 The prevalence of oral disease within the country, including dental caries and periodontitis, is relatively high. According to the findings issued in 2018 by the Basic Health Research Department (Riset Kesehatan Dasar or RISKESDAS) of the Ministry of Health, 57.6% of Indonesia’s population experienced oral health problems that can affect the quality of life. Meanwhile, the prevalence of caries among that section of the populace aged between 12 and more than 65 years old ranged from 65.5% to 95%.3 Oral health problems such as dental caries must be treated immediately and appropriately in order to prevent caries of such severity that the affected teeth must be extracted. However, in Indonesia, dental caries are frequently neglected and remain untreated, with only 10.2% of oral health problems receiving attention.3 The most common etiology of tooth loss is dental caries. Moreover, in Indonesia, tooth extraction has been the most frequent form of treatment applied to severe dental caries that potentially lead to bone defects.4 The percentage of procedures involving tooth extraction in Indonesia stands at approximately 79.6%.4,5 Unnecessary tooth extraction can precipitate certain complications such as alveolar bone fractures (31.82%), prolong bleeding (4.54%), and inflammation (2.27%).4,6
The normal sequence of post-tooth extraction bone healing is resorptive, usually involving both hard and soft tissue defects in the alveolus area, but without socket preservation.7 The loss of periodontal tissue structure in the afflicted area is problematic and can affect both the aesthetic and functional outcomes of dental prosthetics.8 Post-extraction socket preservation, involving the use of bone graft as biomaterial scaffold, is necessary due to the alveolar bone resorptive pattern.9
Regenerative dentistry involving the application of tissue engineering techniques is emerging and becoming increasingly popular. Triad tissue engineering combines biocompatible scaffold seeds, growth factor, and stem cells which are then transplanted into affected areas to enhance the remodeling or regeneration of defective tissue.10 Bone regeneration can be optimally achieved by the application of mesenchymal stem cells (MSCs).11 The oral cavity is a unique site with potential resources for MSCs such as gingival mesenchymal stem cells (GMSCs),12–15 dental pulp mesenchymal stem cells (DPSCs)16,17 and stem cells from human exfoliated deciduous teeth (SHED).18 MSCs demonstrate the potential ability to differentiate into various cell types within the mesenchymal lineage through osteogenic, chondrogenic and adipogenic differentiation.19 In addition, MSCs also possess the ability to accelerate wound healing by means of their paracrine and autocrine effects which enhance cell migration to injured tissue.20 One promising source of MSCs in the orofacial region is SHED which demonstrates potential osteogenic differentiation ability supportive of bone defect regeneration.21 Nevertheless, biocompatible scaffold is necessary to support and optimally facilitate MSCs proliferation.10,11 Carbonate apatite scaffold (CAS) biomaterial is relatively popular and frequently employed in Indonesia to stimulate bone regeneration as a means of overcoming excessive bone resorption. GAMA-CHA®, a product used in this study, is a scaffold fabricated and formulated in Indonesia which consists of carbonate apatite and gelatin.22 SHED can attach and proliferate optimally due to CAS (GAMA-CHA®) rather than hydroxyapatite.18 Meanwhile, at present, no study has been conducted that analyzes the ability of the SHED incorporated in CAS (GAMA-CHA®) to ameliorate alveolar bone defect in animal models. The hypothesis of this study is that the transplantation of SHED-incorporated CAS enhances bone remodeling through bone morphogenetic 2 and 7 expressions (BMP-2 and BMP-7) and attenuates matrix metalloproteinase-8 (MMP-8) expression in animal models. Therefore, the aim of this study is to investigate the expression of BMP-2, BMP-7 and MMP-8 following the transplantation of SHED-incorporated CAS to animal models.
Materials and Methods
Study Design and Animal Study Preparation
This study constituted a true experimental study with post-test-only control group design. The sample was selected by means of simple randomized blind sampling. The study was approved by the Institutional Review Board (IRB) of the Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia (No. 042/HRECC. FODM/IV/2017).
A total of 14 healthy, male, 12 to 16-week-old, Wistar rats approximately 200–250 grams in weight supplied by the Animal Breeding Laboratory of the Faculty of Veterinary Medicine, Airlangga University constituted the animal subjects. Animal ethics and welfare guidelines formed the study protocol were carried out in accordance with the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines to ameliorate any suffering of the animals.
During a one-week adaptation period, the subjects were provided with food and water ad libitum, with husk replacement occurringevery second day. Two groups participated in this study, namely; a control group and an experimental group (CAS + SHED). CAS (GAMA-CHA, Jogjakarta, Indonesia). The subjects were administered a rodent anesthetic intramuscularly (Sigma Aldrich, St. Louis, US) (xylazine and ketamine 0.1 mL/10 g body weight). After the subjects had been anesthetized, tooth extraction was completed.
Alveolar Bone Defect Animal Model
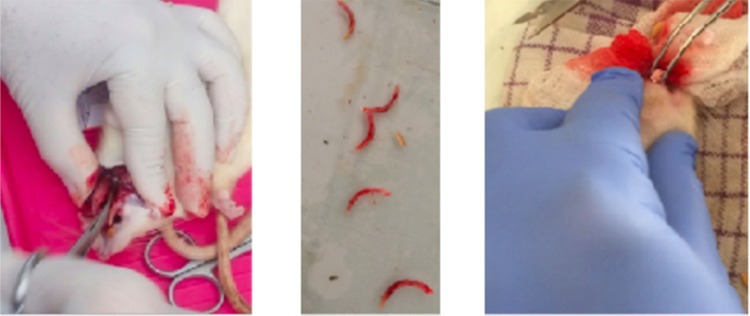
An alveolar bone defect was effected which facilitated the extraction of the mandible anterior teeth. The incisors and surrounding areas were cleaned with iodine. The extraction was performed slowly and gently on the left lower incisors using a PT2 (Hu-Friedy) periotome, using special modifications and forceps. Tooth extraction sockets were filled with CAS only or SHED incorporated CAS to be studied (Figure 1). The extracted mandibular incisors area was then closed using a 5.0 suture monofilament thread following the previous method by Khoswanto.23
Figure 1.
An alveolar bone defect was effected which facilitated the extraction of the mandible anterior teeth animal model.
The SHED used in this study was obtained according to specific inclusion criteria such as the presence of vital conditions, the absence of caries, and the root resorption of deciduous teeth.
The method of producing SHED culture adhered to that employed in previous studies. Normal exfoliated human deciduous were collected from 7- to 10-year-old children due to orthodontic treatment under approved guidelines set by the National Institutes of Health Office of Human Subjects Research. SHED culture was produced at the Tissue Bank of the Diagnostic Center, Dr. Soetomo Surabaya General Hospital, using Dulbeccos Modified Eagle Medium (DMEM®, Life Technologies, Gibco BRLTM, USA) with the addition of 20% fetal bovine serum (FBS, Biochrom AG®, Germany), 5 mm L-glutamine (Gibco Invitrogen®, 25, USA), 100 U/mL penicillin-G, 100 ug/mL streptomycin, and 100 ug/mL kanamycin (Gibco Invitrogen®, 25, USA). The SHED culture medium was changed once every 3 days in order to eliminate those cells which were not attached to the culture plate and transfer the SHED to a new culture medium. The pulp was separated from a remnant crown and then digested in a solution of 3 mg/mL collagenase type I (Worthington Biochem, Freehold, NJ) and 4 mg/mL dispase (Roche Molecular Biochemicals) for 1 h at 37°C. Single-cell suspensions were cultured in a regular medium as reported.18,20,21 SHED within passage three with a density of 106 cells in single cell was incorporated into CAS before being transplanted directly after the tooth extraction.24
All the animals were observed for 7 days at which point all were sacrificed by means of rodent anaesthesia (60 mg/b w of ketamine and xylazine 3 mg/bw). The affected area of the periodontal tissue in each sample was then extracted. Each tissue sample was immersed in 10% formalin (OneMed, Indonesia) for 3 days to achieve fixation, after which the sample was decalcified with 5% EDTA (OneMed®, Indonesia) for a period of 1 month. In addition, tissue processing, including clearing and impregnation, was performed on all samples. The paraffin blocks were subsequently serially sectioned using a microtome.25
Immunohistochemistry Staining
Immunohistochemistry staining was performed by means of a 3.3ʹ-diaminobenzidine stain kit (DAB) (Sigma Aldrich, St. Louis, US). Monoclonal antibodies (AbMo) of BMP-2 (sc-137087), BMP-7 (sc-53917), and MMP-8 (sc-514803) were used in this study (Santa Cruz Biotechnology™, US). The observation and examination of the expression number of BMP-2, BMP-7, and MMP-8 in the alveolar bone were conducted in five different visual fields by two observers using a Nikon H600L light microscope (Japan) at 1000x magnification (Nikon, Japan).26
Statistical Analysis
Statistical analysis was performed using a Statistical Package for Social Science (SPSS) 20.0 version (IBM corporation, Illinois, Chicago, United State). An unpaired t-test was employed to analyze the treatment and control group data. The expression of BMP-2, BMP-7, and MMP-8 in each group (p<0.01) was then compared.
Results
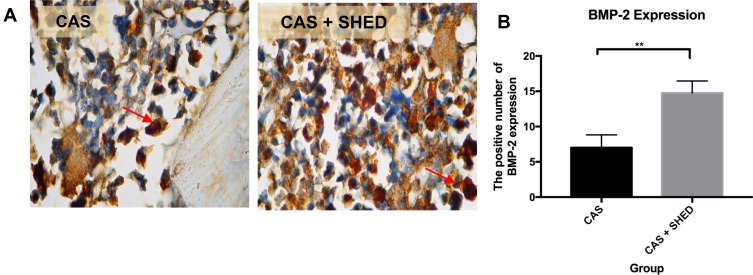
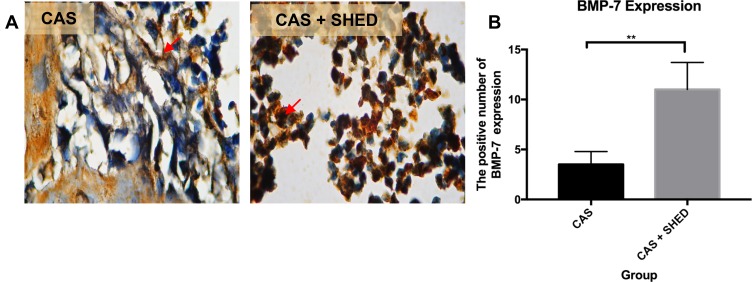
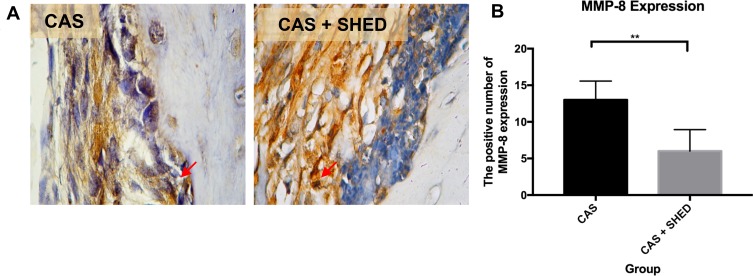
The BMP-2 and BMP-7 expressions were higher in group II than in group 1 (Figures 2 and 3). MMP-8 levels were lower in group II than in group I (Figure 4). There were more significantly increased expressions of BMP-2 and BMP-7 in group II compared to group I (p<0.01). Meanwhile, there was a significantly decreased expression of MMP-8 in group II compared to group I (p<0.01) (Table 1).
Figure 2.
The periodontal tissue histological section of an afflicted subject. (A) AbMo and DAB were used to perform immunohistochemical analysis of BMP-2 expression. Positive cells appeared brown in color (red arrow) through a light microscope at 1000x magnification. (B) The number of positive expressions of BMP-2 is shown. The statistical significance of differences between groups was examined by means of an unpaired t-test (n = 7; **Information: significant at p < 0.01).
Figure 3.
The periodontal tissue histological section of an afflicted subject. (A) AbMo and DAB were used to perform immunohistochemical analysis of BMP-7 expression. Positive cells appeared brown in color (red arrow) through a light microscope at 1000x magnification. (B) The number of positive expressions of BMP-7 is shown. The statistical significance of differences between groups was examined by means of an unpaired t-test (n = 7; **Information: significant at p < 0.01).
Figure 4.
The periodontal tissue histological section of an afflicted subject. (A) AbMo and DAB were used to perform immunohistochemical analysis of MMP-8 expression. Positive cells appeared brown in color (red arrow) through a light microscope at 1000x magnification. (B) The number of positive expressions of MMP-8 is shown. The statistical significance of differences between groups was examined by means of an unpaired t-test (n = 7; **Information: significant at p < 0.01).
Table 1.
The Description of Each Group Mean ± Standart Deviation (SD), Result of t-Test of Each Marker Between Groups (n=7)
| Group | Molecular Marker | ||
|---|---|---|---|
| Mean ± SD | |||
| BMP-2 | BMP-7 | MMP-8 | |
| CAS | 7 ± 0.91 | 3.5 ± 0.65 | 13 ± 1.29 |
| CAS+SHED | 14.75 ± 0.85 | 11 ± 1.35 | 6 ± 1.47 |
| *Sig | 0.0010 | 0.0001 | 0.0001 |
Note: *Significant at p value < 0.01.
Discussion
In this study, it was found that the post-transplantation incorporation of SHED into CAS could increase the BMP-2 and BMP-7 expression higher than the group transplanted only with CAS and with a significant difference. Enhancement of BMPs' expression constitutes the marker that bone remodeling had occurred. At present, 20 types of BMP exist and several studies have asserted that BMP-2 and BMP-7 can accelerate the regeneration of lost or damaged bone tissue such as occurs in critical sized bone defects and skeletal fractures.27–29 Osteoblast is differentiated and proliferated by stimulation of BMPs.30 MSCs differentiation, bone formation or remodeling, and skeletal development are controlled by BMPs through several forms of signaling and transduction.31 For example, BMP-2 and BMP-7 can enhance osteogenic bone regeneration.32 Previous studies have asserted that the BMP2 knockdown (KO) model results in the absence of bone marrow cavity formation with the result that the cortical bone or trabecular bone is defective. Osterix expression was also reduced in the BMP2 KO model.33 MSCs can induce BMP-2 and BMP-7 expression to stimulate bone regeneration. The CAS (GAMACHA) employed in this study served as bioscaffold material that supports the optimal differentiation and proliferation of SHED into osteogenic lineage.18 A previous study conducted by Wu and team stated that post-transplantation of MSCs in a rabbit model of avascular necrosis of femoral head can enhance the BMP-2 expression level that stimulates in vivo revascularization and bone remodeling of the afflicted area.34 Meanwhile, a study conducted by Liu and team mentions the significantly increased expression of BMP-7 in BMSCs in vitro culture indicating that the enhancement of BMP-7 expression can be used as BMSC-based cell therapy for osteoarthritis.35 The results of those studies were in line with and support the findings of the authors that showed post-transplantation of SHED can stimulate the enhancement of BMP-2 and BMP-7 in the alveolar bone defect area.
On the other hand, it was found that MMP-8 expression had significantly decreased in the group with CAS and SHED compared to the group with CAS only. Matrix metalloproteinases (MMPs) are groups of proteolytic enzymes that play a major role in tissue destruction, remodeling, and immune responses.36 MMP-8 at higher concentrations was found in individuals with periodontitis compared to those in good health.37 Abnormalities or overexpression of MMPs in ECM can induce bone resorption or osteolysis.38 MMP-8 was the pro-active collagenase that can be found in the Western blot results of peri-implantitis patients.39 MMP-8 can be used as a predictor marker for alveolar bone loss in peri-implantitis during its active period.39
Differentiation of MSCs into several lineages is controlled by MMPs. Exogenous MMPs at their surface interact with MSCs. Specific MMPs regulate the differentiation, angiogenesis, proliferation and migration of MSCs. A study conducted by Mayney and Volloch found increased expression of tissue inhibitor of metalloproteinases (TIMP) such as TIMP-2 and TIMP-4, while levels of MMP-1 and MMP-8 were both reduced during osteogenic differentiation. This indicated the downregulated or upregulated expression of MMPs and TIMPs in differentiation-specific manners.40,41 These results were similar to the ones produced by this study showed that the SHED incorporated CAS group had lower MMP-8 expression compared to the control group. CAS has abilities such as osteoinductive and osteoconductive which induce the microenvironment suitable for SHED differentiation into osteogenic lineage.18,22 SHED also proliferate optimally within CAS that can secrete various beneficial growth factors and cytokines that support bone regeneration. Compared with hDPSCs or hBMSCs, SHED has highly proliferative and superior osteogenic differentiation ability. Unlike BMSCs, SHED can also be obtained through minimal invasive surgical procedures. SHED has been suggested as a potentially promising MSCs source from the orofacial region for bone regeneration treatment.42 Nevertheless, some pathological conditions and some therapies influence the behavior of the bone tissue that we could not ignore. This study results only limited for animal study. Thus, further study is still required with a better sample size and design for future investigation and application in human being. This is an important step that needs more variables before we can reproduce and generalize the results.
Conclusion
Based on the research results above, it can be concluded that SHED-incorporated CAS can enhance BMP-2 and BMP-7 expression while attenuating MMP-8 expression during alveolar bone remodeling in wistar rats (R. norvegicus). In the future, SHED-incorporated CAS may become a promising treatment for orofacial tissue region regeneration in human being.
Acknowledgment
The authors would like to thank the Faculty of Dental Medicine at Universitas Airlangga (UNAIR), and Dr. Soetomo Surabaya General Hospital. This research was funded by Airlangga University (Hibah Mandat Research Grants, 2016).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division. World population prospects 2019: highlights (ST/ESA/SER.A/423); 2019. Available from: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf. Accessed November26, 2019.
- 2.Ministry of Health Republic of Indonesia. The Republic of Indonesia health system review. Health Syst Transit. 2017;7(1):1–291. [Google Scholar]
- 3.Ministry of Health Republic of Indonesia; Basic health research in oral health result; 2018. Available from: https://www.depkes.go.id/resources/download/info-terkini/hasil-riskesdas-2018.pdf. Accessed November26, 2019. [Google Scholar]
- 4.Nugraha AP, Narmada IB, Ernawati DS, et al. Osteogenic potential of gingival stromal progenitor cells cultured in platelet rich fibrin is predicted by core-binding factor subunit-α1/Sox9 expression ratio (in vitro). F1000Res. 2018;7:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health Republic of Indonesia: Health Profile in Indonesia 2014. Ministry of health Republic of Indonesia. Sekretaris Jenderal. Jakarta; 2015. [Google Scholar]
- 6.Lande R, Kepel BJ, Siagian KV. Profile of risk factor and complication of tooth extraction at RSGM PSPDG-FK unsrat. J E Gigi (Eg). 2015;3(2):1–6. [Google Scholar]
- 7.Agarwal G, Thomas R, Mehta D. Postextraction maintenance of the alveolar ridge: rationale and review. Compend Cont Educ Dent. 2012;33(5):320–324;quiz 327, 336. [PubMed] [Google Scholar]
- 8.Hansson S, Halldin S. Alveolar ridge resorption after tooth extraction: a consequence of a fundamental principle of bone physiology. J Dent Biomech. 2012;3:1758736012456543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313–323. [PubMed] [Google Scholar]
- 10.Sari DS, Maduratna E, Latief FDE, Nugraha AP, Sudiana K, Rantam FA. Osteogenic differentiation and biocompatibility of bovine teeth scaffold with rat adipose-derived mesenchymal stem cells. Eur J Dent. 2019;13(02):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nugraha AP, Narmada IB, Ernawati DS, et al. Bone alkaline phosphatase and osteocalcin expression of rat’s gingival mesenchymal stem cells cultured in platelet-rich fibrin for bone remodeling (in vitro). study). Eur J Dent. 2018;12(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nugraha AP, Rantam FA, Ernawati DS, et al. Gingival mesenchymal stem cells from Wistar rat’s gingiva (Rattus norvegicus) isolation and characterization (in vitro study). J Int Dent Med Res. 2018;11(2):694–699. [Google Scholar]
- 13.Nugraha AP, Narmada IB, Ernawati DS, et al. Somatic cells acceleration by platelet rich fibrin. Indian Vet J. 2019;96(04):30–34. [Google Scholar]
- 14.Nugraha AP, Narmada IB, Ernawati DS, et al. In vitro bone sialoprotein-I expression in combined gingival stromal cells and platelet rich fibrin during osteogenic differentiation. Trop J Pharm Res. 2018;17(12):2341–2345. [Google Scholar]
- 15.Nugraha AP, Narmada IB, Ernawati DS, et al. The Aggrecan expression post platelet rich fibrin administration in gingival medicinal signaling cells in Wistar rats (Rattus norvegicus) during the early osteogenic differentiation (in vitro) Kafkas. Univ Vet Fak Derg. 2019;25(3):421–425. [Google Scholar]
- 16.Narmada IB, Laksono V, Nugraha AP, et al. Regeneration of salivary gland defects of diabetic wistar rats post human dental pulp stem cells intraglandular transplantation on acinar cell vacuolization and interleukin-10. Pesqui Bras Odontopediatria Clin Integr. 2019;19(1):1–10. [Google Scholar]
- 17.Suciadi SP, Nugraha AP, Ernawati DS, et al. The efficacy of human dental pulp stem cells in regenerating submandibular gland defects in diabetic wistar rats (Rattus novergicus). Res J Pharm Technol. 2019;12(4):1573–1579. [Google Scholar]
- 18.Saskianti T, Ramadhani R, Budipramana ES, Pradopo S, Suardita K. Potential proliferation of stem cell from human exfoliated deciduous teeth (SHED) in carbonate apatite and hydroxyapatite scaffold. J Int Dent Med Res. 2017;10(2):350–353. [Google Scholar]
- 19.Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry – part I: stem cell sources. J Prosthodont Res. 2012;56:151‑65. [DOI] [PubMed] [Google Scholar]
- 20.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alhasyimi AA, Pudyani PP, Asmara W, Ana ID. Enhancement of post-orthodontic tooth stability by carbonated hydroxyapatite-incorporated advanced platelet-rich fibrin in rabbits. Orthod Craniofac Res. 2018;21(2):112–118. [DOI] [PubMed] [Google Scholar]
- 23.Khoswanto C. A new technique for research on wound healing through extraction of mandibular lower incisors in wistar rats. Euro Dent J. 2019;9:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prahasanti C, Subrata LH, Saskianti T, Suardita K, Ernawati DS. Combined hydroxyapatite scaffold and stem cell from human exfoliated deciduous teeth modulating alveolar bone regeneration via regulating receptor activator of nuclear factor-Κb and osteoprotegerin system. Iran J Med Sci. 2019;44(5):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narmada IB, Husodo KRD, Ardani IGAW, Rahmawati D, Nugraha AP, Iskandar RPD. Effect of vitamin D during orthodontic tooth movement on receptor activator of nuclear factor Kappa-B ligand expression and osteoclast number in pregnant wistar rat (Rattus novergicus). Krishna Inst Med Sci Univ. 2019;8(1):37–42. [Google Scholar]
- 26.Nareswari RAAR, Narmada IB, Djaharu’ddin I, Rahmawati D, Putranti NAR, Nugraha AP. Effect of vitamin D administration on vascular endothelial growth factor expression and angiogenesis number in orthodontic tooth movement of pregnant Wistar rats. J Postgrad Med Inst. 2019;33(3):182–188. [Google Scholar]
- 27.Sheikh Z, Javaid MA, Hamdan N, Hashmi R. Bone regeneration using bone morphogenetic proteins and various biomaterial carriers. Materials (Basel). 2015;8(4):1778–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Termaat MF, Den Boer FC, Bakker FC, Patka P, Haarman HJ. Bone morphogenetic proteins. Development and clinical efficacy in the treatment of fractures and bone defects. J Bone Joint Surg Am. 2005;87(6):1367–1378. [DOI] [PubMed] [Google Scholar]
- 29.Bibbo C, Nelson J, Ehrlich D, Rougeux B. Bone morphogenetic proteins: indications and uses. Clin Podiatr Med Surg. 2015;32(1):35–43. [DOI] [PubMed] [Google Scholar]
- 30.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77(8):626–631. [DOI] [PubMed] [Google Scholar]
- 31.Beederman M, Lamplot JD, Nan G, et al. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013;6(8A):32–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo KWH, Ulery BD, Ashe KM, Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev. 2012;64(12):1277–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2(12):e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Zhang C, Wu J, Han Y, Wu C. Angiogenesis and bone regeneration by mesenchymal stem cell transplantation with danshen in a rabbit model of avascular necrotic femoral head. Exp Ther Med. 2019;18(1):163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Li Z, Zhu Y, Luo C, Yang D. Bone morphogenetic protein-7 promoted the differentiation of bone marrow-derived mesenchymal stromal cells towards chondrocytes. Int J Clin Exp Med. 2018;11(3):1835–1844. [Google Scholar]
- 36.Savonius O, Roine I, Alassiri S, et al. The potential role of matrix metalloproteinases 8 and 9 and myeloperoxidase in predicting outcomes of bacterial meningitis of childhood. Mediators Inflamm. 2019;7436932:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Morais EF, Pinheiro JC, Leite RB, Santos PPA, Barboza CAG, Freitas RA. Matrix metalloproteinase-8 levels in periodontal disease patients: a systematic review. J Periodontal Res. 2018;53(2):156–163. [DOI] [PubMed] [Google Scholar]
- 38.Paiva KBS, Granjeiro JM. Matrix metalloproteinases in bone resorption, remodeling, and repair. Prog Mol Biol Transl Sci. 2017;148:203–303. [DOI] [PubMed] [Google Scholar]
- 39.Arakawa H, Uehara J, Hara ES, et al. Matrix metalloproteinase-8 is the major potential collagenase in active peri-implantitis. J Prosthodont Res. 2012;56(4):249–255. [DOI] [PubMed] [Google Scholar]
- 40.Mauney J, Volloch V. Adult human bone marrow stromal cells regulate expression of their MMPs and TIMPs in differentiation type-specific manner. Matrix Biol. 2010;29(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almalki SG, Agrawal DK. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res Ther. 2016;7(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunimatsu R, Nakajima K, Awada T, et al. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2018;501(1):193–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- United Nations, Department of Economic and Social Affairs, Population Division. World population prospects 2019: highlights (ST/ESA/SER.A/423); 2019. Available from: https://population.un.org/wpp/Publications/Files/WPP2019_Highlights.pdf. Accessed November26, 2019.