Highlights
-
•
The replication and transfer of rolling circle-replicating plasmids is reviewed.
-
•
Comparisons of replication and transfer cassettes are presented.
-
•
The current understanding of the pMV158 DNA transfer mechanism is reviewed.
Abbreviations: AbR, antibiotic resistance(s); G−, Gram-negative; G+, Gram-positive; ICEs, integrative conjugative elements; LIC, Leading-strand initiation and control of replication; pRNA, primer RNA; RC, rolling circle; RCR-plasmids, rolling circle-replicating plasmids; RNAP, RNA polymerase; ss, single-stranded; ds, double-stranded; sso, single strand origin; T-DNA, transferred DNA strand; T4SSs, type IV secretion systems; T4CP, T4SS-coupling protein; VGT/HGT, vertical/horizontal gene transfer
Keywords: Conjugative transfer, Relaxases, Rolling-circle replicating plasmids, Origins of transfer, Firmicutes
Abstract
Rolling circle-replicating plasmids constitute a vast family that is particularly abundant in, but not exclusive of, Gram-positive bacteria. These plasmids are constructed as cassettes that harbor genes involved in replication and its control, mobilization, resistance determinants and one or two origins of lagging strand synthesis. Any given plasmid may contain all, some, or just only the replication cassette. We discuss here the family of the promiscuous streptococcal plasmid pMV158, with emphasis on its mobilization functions: the product of the mobM gene, prototype of the MOBV relaxase family, and its cognate origin of transfer, oriT. Amongst the subfamily of MOBV1 plasmids, three groups of oriT sequences, represented by plasmids pMV158, pT181, and p1414 were identified. In the same subfamily, we found four types of single-strand origins, namely ssoA, ssoU, ssoW, and ssoT. We found that plasmids of the rolling-circle Rep_2 family (to which pMV158 belongs) are more frequently found in Lactobacillales than in any other bacterial order, whereas Rep_1 initiators seemed to prefer hosts included in the Bacillales order. In parallel, MOBV1 relaxases associated with Rep_2 initiators tended to cluster separately from those linked to Rep_1 plasmids. The updated inventory of MOBV1 plasmids still contains exclusively mobilizable elements, since no genes associated with conjugative transfer (other than the relaxase) were detected. These plasmids proved to have a great plasticity at using a wide variety of conjugative apparatuses. The promiscuous recognition of non-cognate oriT sequences and the role of replication origins for lagging-strand origin in the host range of these plasmids are also discussed.
1. Introduction
The concept developed by Marshall McLuhanin the early 1960s on considering planet Earth as becoming a Global Village (http://projects.chass.utoronto.ca/mcluhan-studies/v1_iss2/1_2art2.htm) has proved to be true fifty years afterwards. Humankind has become global, indeed, not only virtually but physically as well: in addition to the World Wide Web, economical, commercial, and touristic activities have led to unquestionable benefits for the exchange of cultures, but it has also imposed a heavy burden on the rest of the biosphere. A huge part of it is formed by the microbial world, so that going global has increased health risks in the way of outbreaks of epidemics, and the appearance of new transmissible diseases: the swine and avian flu, and the Severe Acute Respiratory Syndrome, to name the most known (see, for instance, the reports by the US-Center for Disease Prevention and Control: http://www.bt.cdc.gov/publications, and its European counterpart: http://www.ecdc.europa.eu/en/Pages/home.aspx). Within this global scenario, not only viruses but also bacterial plasmids have increased their relevance as vehicles to disseminate genetic information among different species: the human activities and human contacts have resulted to be brutal selection processes that have accelerated the horizontal transfer of genetic information among microorganisms (Baquero, 2004, Wellington et al., 2013). Selection of novel bacterial traits derives not only from the need of better starters for fermentation/food production but also from the abuse and misuse of broad-spectrum antibiotics. These last activities have led to the selection of bacteria harboring genetic elements with multiple antibiotic-resistances (AbR), which are rapidly spread (even as epidemic outbursts in hospitals) by horizontal gene transfer, HGT (Anderson and Seifert, 2011, Baquero, 2009). Genes responsible for AbR frequently cluster in the bacterial mobile elements (the mobilome) within the Integrative and Conjugative Elements (ICEs) and transmissible plasmids that, in turn, are platforms to recruit various smaller mobile elements, such as insertion sequences, transposons and integrons that can also encode AbR genes (Frost et al., 2005). Thus, HGT mediated by self-replicating plasmids or by transfer of the islands, plays an essential role in the bacterial, and consequently in the global biodiversity.
In the Plasmid Biology field, the processes pertaining to the dissemination of genetic information stored in plasmids are of special relevance. Those processes involve the two main ways of inheritance, namely vertical (VGT, from mother to daughter cells) and HGT (cell-to-cell) gene transfer (Thomas, 2000). Whereas the former includes DNA replication and partition, this latter being coupled to the cell division, HGT is usually achieved by conjugation between cells of the same or different species (del Solar et al., 1998, Grohmann et al., 2003, Lanka and Wilkins, 1995). Replication and conjugation are, in addition to genetic recombination, the most important sources of genetic variability among plasmids and their hosts. The entire genetic content of the bacterial mobilome amounts up to 25% of the total DNA circulating among bacteria, thus being a shared strong task force for the evolution of the bacterial populations (Ochman et al., 2000, Thomas and Nielsen, 2005). A substantial part of the mobilome is constituted by plasmids, the so-called plasmidome (Walker, 2012).
Plasmids are much more than cloning vectors or tools to over-produce proteins. In addition to their role in the spread of genetic information, bacterial plasmids are excellent models to study a number of biological processes, such as transactions involving macromolecular interactions: protein-DNA, protein–protein, and DNA–RNA (Espinosa, 2013). They also constitute a wealth of information on control of gene expression (del Solar and Espinosa, 2000, del Solar et al., 2002), intracellular distribution of DNA molecules (Reyes-Lamothe et al., 2014), and can be considered as useful models for system biology (Paulson and Ehrenberg, 1998). Excellent books dealing with various aspects of the biology of plasmids have been published (Funnell and Phillips, 2004, Thomas, 2000) and a new one is in press (Alonso and Tomalsky, 2014). In the present review we will concentrate on plasmids replicating by the rolling circle mechanism (RCR-plasmids) because they may encode up to two proteins with endonuclease/topoisomerase-like activity that cleave supercoiled DNA at two different regions on the same molecule, making their study thought-provoking.
2. Replication, conjugation, and mobilization: common themes
Replication and transfer generally require that plasmid-encoded protein(s) interact with their cognate DNA sites to initiate the process. The key initiator players in these two processes are: (i) the generically called Rep proteins involved in vegetative replication and that interact with their cognate origin of replication, oriV, and (ii) the relaxases, usually termed Tra or Mob proteins, involved in transfer, and that recognize their cognate origin of transfer, the oriT. Thus, interplays between Rep-oriV and Tra/Mob-oriT will define the VGT and the HGT processes, respectively. The early discovery of conjugation (Lederberg and Tatum, 1946, Lederberg and Tatum, 1953) and the existence of plasmid-encoded proteins that relax the donor DNA as the first stage in the transfer process, led to a historically broad interest in plasmid transfer among Gram-negative (G−) bacteria. Conjugation in Gram-positive (G+) bacteria was studied relatively later and it is still a matter of active research (Goessweiner-Mohr et al., 2013, Grohmann et al., 2003). Replication involves a number of different strategies devoted to the melting of the DNA strands at the origin of replication (namely theta, strand-displacement and rolling-circle mechanisms), and is usually mediated by the Rep initiator alone or with the help of plasmid- or host-encoded proteins (del Solar et al., 1998). Although DNA melting to initiate the transfer is also required, conjugation, however, involves a single strategy devoted to the unidirectional transfer of mobile elements (plasmids or ICEs) from a donor bacterial cell to a recipient one through physical cell-to-cell contact (de la Cruz et al., 2010, Zechner et al., 2000). Based on their transfer machinery we can distinguish between: (i) self-transmissible (conjugative) plasmids and integrative conjugative elements (ICEs), which codify all the functions required for their HGT, and (ii) mobilizable plasmids and integrative and mobilizable elements (IMEs), which ‘travel light’ because they encode only the relaxase and its cognate oriT. These latter plasmids and IMEs make use of functions provided by either the host chromosome or by other auxiliary (also termed ‘helper’) plasmid for their transference.
The assembly of plasmid- and host-encoded proteins in a specific DNA region initiates replication and transfer, thus initiation of both processes requires the generation of multiprotein-DNA-complexes, the replisome or the relaxosome, respectively (del Solar et al., 1998, Lanka and Wilkins, 1995, Pansegrau and Lanka, 1996a). In the case of RCR-plasmids (del Solar et al., 1987, Khan et al., 1981, Novick, 1998, Puyet et al., 1988, te Riele et al., 1986a, te Riele et al., 1986b), initiation of replication and mobilization are mechanistically similar processes. Both, Rep and Tra/Mob proteins have endonuclease/topoisomerase-like activities on their DNA targets, the double-strand origin (dso) or the oriT. These proteins cleave their cognate DNA at the phosphodiester bond of a specific di-nucleotide (the nic site) generating a stable amino acyl-DNA adduct (Chandler et al., 2013, de la Campa et al., 1990, Guasch et al., 2003, Khan, 2003, Koepsel et al., 1986, Moscoso et al., 1997, Pansegrau and Lanka, 1996a). The nick introduced by Rep or Tra/Mob proteins generates a free 3′-OH end, which acts as a primer for leading-strand synthesis in both cases, VGT and HGT. In the RCR-plasmids, proteins from the host replicative machinery, at least DNA-polymerases I and III, single-strand (ss) DNA-binding protein and PcrA helicase, participate in the elongation from the 3′-OH end generated by the plasmid-encoded Rep initiator (Anand et al., 2005, Anand and Khan, 2004, Díaz et al., 1994, Khan, 2003, Khan, 2005, Machón et al., 2010, Ruiz-Masó et al., 2006, Soultanas et al., 1999, Thomas et al., 2013). To process their DNA substrates, initiators of replication and transfer require the nic site being exposed in a single-stranded configuration, which can be achieved by DNA melting and generation of hairpin structures. Hairpin formation would be mediated either by the binding of auxiliary associated proteins, and/or by binding of the initiator or the relaxase. In both cases, the di-nucleotide to be cleaved will be exposed in an unpaired form (Jin and Novick, 2001, Lorenzo-Díaz et al., 2011, Lucas et al., 2010, Noirot et al., 1990, Ruiz-Masó et al., 2007).
Besides the initiator (relaxase) and its binding site (oriT), conjugation requires an additional machinery that RCR does not, namely the protein complex that completes the conjugation apparatus. This is a highly specialized protein machinery encoded by the donor plasmid DNA (or the host chromosome in some cases) that includes the coupling protein (T4CP) and the Type IV Secretion System (T4SS) which translocate the relaxase-DNA complex to the recipient cell (Draper et al., 2005, Garcillán-Barcia et al., 2007). T4SSs recruit their substrate by mechanisms still not fully understood for conjugation, and they also participate in other processes involving DNA trafficking between prokaryotic donors and recipient (prokaryotic or eukaryotic) cells, like in the case of Ti and related plasmids (Baron et al., 2002, Bhatty et al., 2013, Cascales et al., 2013, Chen et al., 2005, de Paz et al., 2005, Goessweiner-Mohr et al., 2013, Gomis-Ruth et al., 2004, Hamilton et al., 2000, Llosa et al., 2009, Zhang et al., 2012). The T4CP and the T4SS proteins participate in pumping the transferred DNA (T-DNA)-relaxase complex into the recipient cell (Gomis-Rüth and Coll, 2006, Guasch et al., 2003, Llosa et al., 2002, Matilla et al., 2010). In RCR as well as in transfer, the parental DNA strand will be displaced until either the dso or the oriT are reconstituted. This last stage involves DNA strand transfer reactions that will terminate either leading strand replication (in the donor cell) or plasmid transfer (in the recipient cell). Intermediates of both processes will be ssDNA molecules that correspond to the parental plus strand (del Solar et al., 1998, Grohmann et al., 2003, Pansegrau and Lanka, 1996a, Pansegrau and Lanka, 1996b, Wilkins and Lanka, 1993). Synthesis of the lagging strand initiates from the so-called single-strand origins (sso), as described below.
3. Modular construction of RCR-plasmids
A relevant part of the mobilizable plasmids replicate by the RC mechanism (del Solar et al., 1987, Khan et al., 1981, Novick, 1998, Puyet et al., 1988, te Riele et al., 1986a, te Riele et al., 1986b), although not all of them are mobilizable. In the case of small plasmids, isolated primarily from G+ bacteria, two pioneer discoveries were made: (i) the identification of specific single-stranded DNA molecules (ssDNA) as intermediates of plasmid replication (Puyet et al., 1988, te Riele et al., 1986a, te Riele et al., 1986b), and (ii) the finding that their Rep initiator proteins exhibited a sequence-specific relaxing activity on supercoiled DNA (Koepsel et al., 1985). These results led to the discovery of RCR-plasmids, which constituted a new class of plasmids that replicate by the asymmetric rolling circle mechanism that share similarities with the replication of ssDNA coliphages (Novick, 1998). Furthermore, the presence of RCR-plasmids in G− bacteria (Yasukawa et al., 1991) helped to cast off the idea of a genetic barrier between the two types of bacteria (del Solar et al., 1993). Thus, the conclusion that plasmid RCR is mechanistically similar to conjugative transfer was soon achieved (Waters and Guiney, 1993). It was interesting to learn that some of the RCR-plasmids encoded, in addition to the Rep topoisomerase-like initiator, another protein with the ability to relax DNA (Caryl et al., 2004, Grohmann et al., 2003, Guzmán and Espinosa, 1997, Smith and Thomas, 2004). These proteins were thought to be involved in inter-plasmidic recombination (plasmid recombination enzymes, Pre; (Projan and Novick, 1988)), although it was later shown that Pre proteins were required for plasmid mobilization (Priebe and Lacks, 1989). Further, the relaxase activity of the pMV158_Pre protein (renamed MobM) on supercoiled DNA, and its nic site were demonstrated in vitro and in vivo (Grohmann et al., 1997, Guzmán and Espinosa, 1997). However, even up today, these proteins are grouped into a family termed Mob-Pre at the database of protein families Pfam (PF01076), and no further investigations on their participation in plasmid recombination have been performed.
In general, RCR-plasmids appear to be constructed as gene cassettes (del Solar et al., 1993, Khan, 1997, Khan, 2005) that may have up to four independent modules involved in: (i) Leading-strand initiation and control of replication (LIC); (ii) AbR determinant (DET); (iii) Mobilization (MOB), and (iv) One or two origins for lagging strand replication, sso (Fig. 1 ). This latter kind of origins varies among the different RCR described, but they have been categorized in four types: ssoA, ssoU, ssoW, and ssoT depending on their DNA sequence (Khan, 2000, Khan, 2005, Kramer et al., 1998a, Kramer et al., 1997, Meijer et al., 1995b, van der Lelie et al., 1989), or in their role in plasmid promiscuity (Kramer et al., 1995, Lorenzo-Díaz and Espinosa, 2009). Their structure and roles will be described below (see Table 1 ). RCR-plasmids may harbor all the cassettes, like the streptococcal plasmids pMV158 and pRW35 (Priebe and Lacks, 1989, van der Lelie et al., 1989, Woodbury et al., 2008), or just the LIC cassette, as in the mycoplasma plasmids pADB201 (Bergemann et al., 1989) and pKMK1 (King and Dybvig, 1992), which are considered to be the smallest RCR-plasmids, unless we consider the hybrid phage-plasmid phasyl (Seufert et al., 1988) as one of them.
Fig. 1.
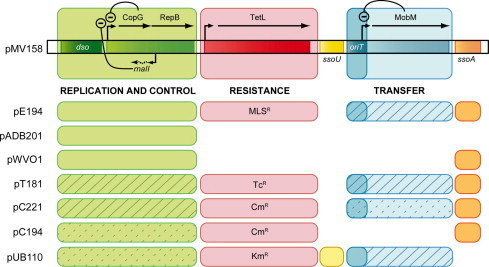
Genetic organization of representatives of RCR-plasmids. The modular organization of pMV158 from S. agalactiae is depicted in the upper part. Genes and the identified promoters are indicated as arrows (arrowheads pointing to the transcription direction). Negative (−) regulatory elements within the replication and control (green) and the transfer (blue) modules are indicated. Below pMV158, other RCR-plasmids are depicted: pADB201 from Mycoplasma mycoides, pWV01 from Lactococcus lactis and pE194, pT181, pC221, pC194, and pUB110 from S. aureus. The corresponding antibiotic resistance (red module) is indicated with the following abbreviations: MLSR, macrolide/lincosamide/streptogramin B; TcR, tetracycline; CmR, chloramphenicol; KmR, kanamycin. Plasmids sharing similar genetic modules are presented in the same color and filling. See Supplementary Tables S1 and S2 for detailed information on the plasmids.
Table 1.
MOBV1 plasmids.
| Plasmid name a | Nucleotide GenBank Acc. No. | Plasmid size (kb) | Relaxase GenBank Acc. No. b | Relaxase tree cluster c | Host | Replication initiator family d | sso type (position related to mob gene) e | Virulence or antibiotic/metal resistance f |
|---|---|---|---|---|---|---|---|---|
| pMV158 | NC_010096.1 | 5.541 | YP_001586274 | pMV158 | Streptococcus agalactiae | Rep_2 | ssoA (3′) + ssoU (5′) | Tc |
| pER13 | NC_002776.1 | 4.139 | NP_115336.1 | pMV158 | Streptococcus thermophilus | Rep_2 | ssoA (3′) | |
| pSMQ172 | NC_004958.1 | 4.230 | NP_862547.1 | pMV158 | Streptococcus thermophilus | Rep_2 | ssoA (3′) | |
| pGA2000 | NC_019252.1 | 4.967 | YP_006961085.1 | pMV158 | Streptococcus pyogenes | Rep_2 | ssoA (3′) | MLS(B) |
| pGB2002 | NC_015971.1 | 6.825 | YP_004831084.1 | pMV158 | Streptococcus agalactiae | Rep_2 | ssoA (3′) | MLS(B) |
| pRW35 | NC_010423.2 | 4.968 | YP_001716200.1 | pMV158 | Streptococcus pyogenes | Rep_2 | ssoA (3′) + ssoU (5′) | MLS(B) |
| pDRPIS7493 | NC_015876.1 | 4.727 | YP_004769541.1 | pMV158 | Streptococcus pseudopneumoniae | Rep_2 | ND | |
| pSSU1 | NC_002140.1 | 4.975 | NP_053061.1 | pMV158 | Streptococcus suis | Rep_2 | ssoA (3′) | |
| pYSI8 | NC_010936.1 | 4.973 | YP_001967741.1 | pYSI8 | Lactobacillus sakei | Rep_2 | ssoT (5′) | Lin |
| pK214 | NC_009751.1 | 29.871 | YP_001429536.1 | pYSI8 | Lactococcus lactis | Rep_trans and Rep_3 + L_lactis_RepB_C | ND | MEP, Strp, Chlr, Tc |
| YP_001429523.1 (MOBQ) | ||||||||
| pUR2941 | HF583290.1 | 20.876 | CCQ43999.1 | pYSI8 | Staphylococcus aureus | RepA_N + DnaB_2 and unknown and truncated | ssoA (5′) | Kan/Neo, Tc, MLS(B), Cd, Cu |
| pCPS49 | NC_019142.1 | 5.292 | YP_006958108.1 | pYSI8 | Staphylococcus aureus | Rep_2 | ssoA (3′) | PLS(A) |
| pSTE1 | NC_020237.1 | 11.951 | YP_007419104.1 | pSYI8 | Staphylococcus hyicus | Rep_trans and HTH_Hin_like and truncated | ND | Strp, MLS(B), Tc |
| YP_007419109.1 | pKKS825 | |||||||
| pKKS825 | NC_013034.2 | 14.363 | YP_003084337.1 | pKKS825 | Staphylococcus aureus | Rep_1 and HTH_Hin_like and Rep_3 | ND | Kan/Neo, Tc, Trim, PLS(A) |
| YP_003084330.1 | pKKS825 | |||||||
| YP_004679012.1 (MOBQ, truncated and fused to Rep_1) | ||||||||
| pDB2011 | NC_021513.1 | 7.641 | YP_008119849.1 | pKKS825 | Listeria innocua | Rep_1 and HTH_Hin_like | ND | MLS(B), Spec, Trim |
| pS130a | AUPT01000023.1 | 8.882 | EPZ04218.1 | pKKS825 | Staphylococcus aureus | HTH_11 | ND | Ery, Tc, Kan, Ble |
| pSCFS1 | NC_005076.1 | 17.108 | NP_899176.1 | pKKS825 | Staphylococcus sciuri | HTH_Hin_like and Rep_3 | ND | Flr/Chlr, MLS(B), Spec |
| NP_899168.1 | pNM11 | |||||||
| pLB4 | M33531.1 | Incomplete | AAA25252.1 | pLB4 | Lactobacillus plantarum | Rep_2 | ssoT (5′) | |
| pMRI_5.2 | NC_019900.1 | 5.206 | YP_007215174.1 | pLB4 | Lactobacillus plantarum | Rep_1 and Rep_2 | ssoT (3′) | |
| pLAC1 | NC_014164.1 | 3.478 | YP_003650630.1 | pLB4 | Lactobacillus acidipiscis | Rep_1 | ND | |
| pPLA4 | AF304384.2 | 8.135 | ABG23031.1 | pLB4 | Lactobacillus plantarum | Rep_3 | ND | Bacteriocin |
| pPB1 | NC_006399.1 | 2.899 | YP_138221.1 | pLB4 | Lactobacillus plantarum | Rep_2 | ssoT (5′) | |
| LkipL48 | NC_014135.1 | 3.196 | YP_003620509.1 | pLB4 | Leuconostoc kimchii | Rep_2 | ND | |
| pMBLR00 | NC_019353.1 | 3.370 | YP_006964795.1 | pLB4 | Leuconostoc mesenteroides | Rep_2 | ND | |
| pLAB1000 | M55222.1 | Incomplete | P35856.1 | pLB4 | Lactobacillus hilgardii | Rep_1 | ND | |
| pSMA23 | NC_010242.1 | 3.497 | YP_001649176.1 | pSMA23 | Lactobacillus casei | Rep_1 | ND | |
| pLC88 | U31333.1 | Incomplete | AAA74581.1 | pSMA23 | Lactococcus casei | Rep_1 | ND | |
| p141 | AB517606.1 | Incomplete | BAH97325.1 | pSMA23 | Lactobacillus plantarum | Rep_1 | ND | |
| pCD034-1 | NC_016035.1 | 3.424 | YP_004869658.1 | pSMA23 | Lactobacillus buchneri | Rep_1 | ssoT (3′) | |
| pM4 | NC_009666.2 | 3.320 | YP_001621756.1 | pSMA23 | Lactobacillus plantarum | Rep_1 | sso-new | |
| pF8801 | NC_007593 | 5.558 | YP_398641.1 | pSMA23 | Pediococcus damnosus | Rep_1 | ND | |
| pCD034-2 | NC_016034.1 | 2.707 | YP_004869655.1 | pSMA23 | Lactobacillus buchneri | Rep_2 | ssoT (3′) | |
| pG6301 | NC_019372.1 | 3.516 | YP_006965557.1 | pSMA23 | Lactobacillus plantarum | Rep_1 | ND | |
| pLB925A02 | NC_012549.1 | 3.524 | YP_002790952.1 | pSMA23 | Lactobacillus brevis | Rep_1 | ND | |
| pSD11 | NC_014919.1 | 3.225 | YP_004134615.1 | pSMA23 | Lactobacillus brevis | Rep_1 | ND | |
| pGL2 | NC_016981.1 | 4.572 | YP_005352352.1 | pGL2 | Lactococcus garvieae | Rep_2 | ssoW (3′) | Bacteriocin |
| pAMalpha1 | NC_005013.1 | 9.759 | NP_863358.1 | pAMalpha1 | Enterococcus faecalis | Rep_1 + Rep_1 and Rep_3 | ssoU (5′) | Tc |
| NP_863352.1 | pUB110 | |||||||
| Unnamed | GG670384.1 | Incomplete | EEU18290.1 | pAMalpha1 | Enterococcus faecalis | Rep_3 | ND | |
| Unnamed | GG692894.1 | Incomplete | EEU66435.1 | pAMalpha1 | Enterococcus faecalis | Rep_1 + Rep_1 and Rep_3 | ND | Tc |
| EEU66441.1 | pUB110 | |||||||
| EF62pA | NC_017314.1 | 5.143 | YP_005706998.1 | pAMalpha1 | Enterococcus faecalis | Rep_3 | ||
| pBMO2 | NC_004930.1 | 3.854 | NP_862027.1 | pBM02 | Lactococcus lactis | Rep_2 | ssoW (3′) | |
| pI4 | AF300457.1 | Incomplete | AAG28767.1 | pI4 | Bacillus coagulans | – | ssoT (5′) | Coagulin |
| pTXW | NC_013952.1 | 3.178 | YP_003517730.1 | pTXW | Lactobacillus paracasei | Rep_2 | ssoW (5′) | |
| pWCZ | NC_019669.1 | 3.078 | YP_007027014.1 | pTXW | Lactobacillus paracasei | Rep_2 | ND | |
| pLA106 | NC_004985.1 | 2.862 | NP_862697.1 | pTXW | Lactobacillus acidophilus | Rep_2 | ND | |
| pRCEID2.9 | NC_017466.1 | 2.952 | YP_005849229.1 | pTXW | Lactobacillus casei | Rep_2 | ND | |
| pT181 | NC_001393.1 | 4.439 | NP_040472.1 | pT181 | Staphylococcus aureus | Rep_trans | ssoA (3′) | |
| pSEQU3 | AVBD01000026.1 | 4.846 | ERH33926.1 | pT181 | Staphylococcus equorum | Rep_trans | ND | Tc |
| pKH17 | NC_010284.1 | 4.441 | YP_001654074.1 | pT181 | Staphylococcus aureus | Rep_trans | ND | Tc |
| pSE-12228-01 | NC_005008.1 | 4.439 | NP_863257.1 | pT181 | Staphylococcus epidermidis | Rep_trans | ND | Tc |
| pKH6 | NC_001767.1 | 4.439 | NP_053796.1 | pT181 | Staphylococcus aureus | Rep_trans | ND | Tc |
| pS0385-1 | NC_017334.1 | 5.246 | YP_005735514.1 | pT181 | Staphylococcus aureus | Rep_trans | ND | Tc |
| SAP095B | NC_013312.1 | 4.439 | YP_006937497.1 | pT181 | Staphylococcus aureus | Rep_trans | ND | Tc |
| pSBK203 | U35036.1 | Incomplete | AAA79055.1 | pT181 | Staphylococcus aureus | Rep_trans | ND | Chlr |
| pKH7 | NC_002096.1 | 4.118 | NP_052168.1 | pT181 | Staphylococcus aureus | Rep_trans | ND | Chlr |
| pS1c | AUPS01000031.1 | 3.899 | EQM91159.1 | pT181 | Staphylococcus aureus | Rep_trans | ND | |
| SAP047A | NC_013331.1 | 28.974 | YP_006938074.1 | pT181 | Staphylococcus aureus | Rep_1 and Rep_3 and RepA_N | ND | Cd, β-lac, enterotoxin G |
| pPCZ1 | NC_013539.1 | 4.738 | YP_003329162.1 | pPCZ1 | Planococcus sp. | Rep_3 | ssoA (3′) | |
| pGI1 | NC_004335.1 | 8.254 | NP_705753.1 | pGI1 | Bacillus thuringiensis | Rep_1 | ssoT (5′) | |
| pCT8513 | NC_017207.1 | 8.513 | YP_005569975.1 | pGI1 | Bacillus thuringiensis | Rep_1 | ND | |
| pB52y | AVEZ01000046.1 | 6.283 | EQM25212.1 | pGI1 | Bacillus licheniformis | Unknown | ND | |
| pBMB9741 | NC_001272.2 | 6.578 | YP_724461.1 | pGI1 | Bacillus thuringiensis | Rep_1 | ND | |
| pIS56-8 | NC_020377.1 | 8.251 | YP_007482091.1 | pGI1 | Bacillus thuringiensis | Rep_1 | ND | |
| BTB_7p | NC_018882.1 | 7.635 | YP_006931124.1 | pGI1 | Bacillus thuringiensis | Rep_1 | ND | |
| pHT7 | NC_020243.1 | 7.635 | YP_007425204.1 | pGI1 | Bacillus thuringiensis | Rep_1 | ND | |
| BTB_9p | NC_018886.1 | 8.513 | YP_006931150.1 | pGI1 | Bacillus thuringiensis | Rep_1 | ND | |
| pE33L5 | NC_007104.1 | 5.108 | YP_245942.1 | pE33L5 | Bacillus cereus | HTH_36 | ssoT (5′) | |
| pW_3 | ABCZ02000102.1 | Incomplete | EDX54234.1 | pE33L5 | Bacillus cereus | Truncated | ND | |
| pH308197_11 | NC_011340.1 | 11.567 | YP_002267516.1 | pE33L5 | Bacillus cereus | HTH_CRP | ND | |
| p1414 | NC_002075.1 | 7.949 | NP_049443.1 | p1414 | Bacillus subtilis | Rep_1 | ssoT (3′) | |
| pBamNAU-B3a | NC_022531.1 | 8.438 | YP_008628645.1 | p1414 | Bacillus amyloliquefaciens | – | ND | |
| pBA45-1 | NC_020273.1 | 8.009 | YP_007447244.1 | p1414 | Bacillus amyloliquefaciens | Rep_1 | ND | |
| pPL1 | NC_013537.1 | 6.704 | YP_003329154.1 | p1414 | Bacillus subtilis | Rep_1 and unknown | ND | |
| pTA1015 | NC_001765.1 | 5.807 | NP_053784.1 | p1414 | Bacillus subtilis | Rep_1 | ssoT (3′) | |
| pBS608 | NC_006825.1 | 6.611 | YP_195753.1 | p1414 | Bacillus subtilis | Rep_1 | ND | |
| pTA1060 | NC_001766.1 | 8.737 | NP_053788.1 | p1414 | Bacillus subtilis | Rep_1 and unknown | ssoT (3′) | |
| pBSG3 | NC_014104.1 | 8.439 | YP_003600423.1 | p1414 | Bacillus amyloliquefaciens | Rep_1 | ssoT (3′) | |
| pSD853_7.9 | NC_015392.1 | 7.860 | YP_004376195.1 | p1414 | Salmonella enterica | Rep_1 | ND | |
| pTRACA20 | NC_013279.1 | 3.780 | YP_003208332.1 | pTRACA20 | Uncultured bacterium | DNA_primase_S | ssoW (3′) | |
| pUB110 | NC_001384.1 | 4.548 | NP_040431.1 | pUB110 | Staphylococcus aureus | Rep_1 + Rep_1 | ssoU (5′) | Neo, Ble |
| pSES22 | NC_007621.1 | 4.040 | YP_415518.1 | pUB110 | Staphylococcus saprophyticus | Rep_1 | ND | MLS(B) |
| pERGB | JN970906.1 | Incomplete | AEW23141.1 | pUB110 | Staphylococcus aureus | Rep_1 and Rep_1 | ND | PLS(A), Tb, Tc, Trim |
| pTB19 | M63891.1 | Incomplete | AAA98305.1 | pUB110 | Geobacillus stearothermophilus | Rep_1 | ssoU (5′) | Tc, Ble |
| AAA98307.1 | pUB110 | |||||||
| pV7037 | HF586889.1 | Incomplete | CCQ71694.1 | pUB110 | Staphylococcus aureus | RepA_N and truncated | ND | Tc, Cd |
| pBC16 | NC_001705.1 | 4.630 | NP_043522.1 | pUB110 | Bacillus cereus | Rep_1 + Rep_1 | ssoU (5′) | Tc |
| pSWS47 | NC_022618.1 | 28.743 | YP_008719890.1 | pUB110 | Staphylococcus epidermidis | Rep_3 and truncated and truncated and RepA_N | ND | PLS(A), Kan/Neo, Tc, Trim |
| YP_008719902.1 (MOBP) | ||||||||
| pTB53 | D14852.1 | Incomplete | BAA03580.1 | pUB110 | Bacillus sp. | – | ND | |
| pIP1714 | AF015628.1 | Incomplete | AAC61672.1 | pUB110 | Staphylococcus cohnii | Rep_1 + Rep_1 | ND | PLS(A), MLS(B) |
| pNM11 | NC_019558.1 | 11.383 | YP_007016413.1 | pNM11 | Planococcus citreus | Rep_3 | ND | |
| pBS-03 | JQ394981.1 | Incomplete | AFJ49144.1 | pNM11 | Bacillus sp. | Rep_1 | ND | Flr/Chlr, Strp |
| AFJ49142.1 (MOBV, truncated) | ||||||||
| pSS-03 | NC_016054.1 | 7.122 | YP_004888092.1 | pNM11 | Staphylococcus arlettae | Rep_1 | ND | Flr/Chlr, MLS(B) |
| YP_004888090.1 (MOBV, truncated) | ||||||||
| pJ612 | NC_019186.1 | 5.048 | YP_006959664.1 | pJ612 | Haemophilus influenzae | Rep_3 | ND | β-lac |
| pA1606 | NC_019180.1 | 5.646 | YP_006959644.1 | pJ612 | Haemophilus influenzae | Rep_3 | ND | β-lac |
Plasmids whose relaxases were retrieved by a PSI-BLAST using MobM_pMV158 (300-N terminal residues) as a query are listed.
Underlined accession numbers denote those relaxase genes that are probably misannotated in the GenBank database (i.e. extended N-terminal sequence respect to that of MobM_pMV158).
It locates the corresponding plasmid in one of the cartooned clusters of Fig. 4, for which a prototype was selected.
Replication initiation protein family. When more than one, their names are separated by “and”. “+” is used for initiators that contain more than one pfam domain. Further details on replication initiation protein families can be found at http://pfam.sanger.ac.uk/.
Only the previously identified ssos are annotated. Most of the sso sequences span 200–300 bp and are located in close proximity to the transfer module, with the exception of plasmid pUR2941 (ssoA was mapped 7 kb upstream mob). pM4 plasmid has a new type of sso as described in (Yin et al., 2009). ND, not determined.
Antibiotic or metal resistance to: Tc, tetracycline; MLS(B), macrolide/lincosamide/streptogramin B; Lin, lincosamide; MEP, macrolide efflux protein; Strp, Streptomycin; Chlr, chloramphenicol; Kan, kanamycin; Neo, neomycin; Cu, copper; Cd, cadmium; PLS(A), pleuromutilins/lincosamide/streptogramin A; Trim, trimethoprim; Spec, spectinomycin; Ery: erythromycin; Ble, bleomycin; Flr, florfenicol; β-lac, beta-lactam; Tb, tobramycin.
Many of these plasmids have left their ‘print’ on the chromosome of the hosts they might have colonized and, in fact, a bioinformatics survey detected RCR plasmids-related MOBV-relaxase genes in 63 out of 1207 chromosomes analyzed (Guglielmini et al., 2011). The AbR trace could be considered as a thought-provoking approach to follow the RCR-plasmid ‘fate’ along evolutionary history. Although we have not performed any further search, a homolog of the pMV158-tetL determinant was found in the chromosome of Bacillus subtilis (Lacks et al., 1986), whereas a cat gene, homologous to the one harbored by plasmids pC194 and pC221 has been described to be present in the chromosome of G+ bacteria, like Bacillus pumilus (Harwood et al., 1983) or Streptococcus pneumoniae (Pepper et al., 1988), and even in the chromosome of Clostridium perfringens (Bannam and Rood, 1991). These findings argue in favor of the role of these RCR-plasmids in the integration and dispersion of resistance genes in the chromosome of hosts that they have colonized.
4. The family: pMV158 and relatives
In general, plasmids have been grouped according to their replicon, since this is the hallmark of the plasmid (Chang et al., 2000, del Solar et al., 1998, Khan, 1996, Nordström et al., 1984, Novick, 1989). As mentioned above, RCR-plasmids were grouped in several families, although the most studied are the Rep_1 family (PF01446), whose prototypes are the staphylococcal plasmids pC194, and pUB110, the Rep_2 family (PF01719) of which the streptococcal plasmid pMV158 and the staphylococcal plasmid pE194 are the representatives, and the Rep_trans family (PF02486) represented by the staphylococcal plasmids pT181 and pC221 (del Solar et al., 1998, del Solar et al., 1993, Gruss and Ehrlich, 1989, Khan, 1997, Khan, 2000, Khan, 2005, Novick, 1989). These three families harbor all the cassettes with the exceptions of pC194 that does not contain a MOB module and the lagging-strand origin ssoU, which is reported to be present only in pUB110 and pMV158 (Fig. 1). Curiously, these two latter plasmids also share identical oriT sequences. In vitro, the MobM-protein from pMV158 was able to relax supercoiled DNA from both plasmids (Fernández-López et al., 2013a). Out of the representatives of the different families, pC194 has been reported to be one of the most promiscuous, since it was shown to replicate not only in staphylococci, streptococci and bacilli (Ballester et al., 1990, Horinouchi and Weisblum, 1982), but also in Escherichia coli and in the yeast Saccharomyces as well (Goursot et al., 1982). In the case of pMV158, it has been shown to exhibit an extraordinary host range. The plasmid was primarily isolated from Streptococcus agalactiae (Burdett, 1980) and, along the years, it has been transferred in the laboratory (either by mobilization or by transformation) to more than 20 bacterial species, from Firmicutes to α-Proteobacteria and in all of them it replicates stably (del Solar et al., 1998, Espinosa, 2013, Lacks et al., 1986).
After a BlastP search using prototypes of the three RCR-plasmid families, we found the following distribution: (i) in Rep_1 (prototype RepA from pC194), 334 non-redundant homologs in Bacillales, and 157 in Lactobacillales; (ii) in Rep_2 (prototype taken, RepE initiator from pE194), 44 non-redundant homologs in Bacillales and 242 in Lactobacillales; (iii) in Rep_trans (RepC from pT181 as prototype), 176 non-redundant homologs in Bacillales and 241 in Lactobacillales. These figures suggest that whereas the Rep_1 family of RCR-plasmids would prefer to colonize Bacillales and the Rep_2 family of pMV158 would be better fitted to Lactobacillales, the Rep_trans family of RCR-plasmids would be distributed more evenly. In general, we found a good correlation between the G + C content of RCR-plasmids and their respective hosts (Espinosa et al., 1995), with the exception of the staphylococcal plasmid pUB110 which has a G + C content close to 45%, making it a plasmid which has been considered more like a bacilli than a staphylococci replicon (Alonso et al., 1988, McKenzie et al., 1986).
Traditionally, RCR-plasmids have been classified according their LIC module (del Solar et al., 1998, del Solar et al., 1993). According to this criterion, we performed a PSI-BLAST search for plasmids of the pMV158 family using the initiator RepBpMV158 as the query. The results are compiled in Supplementary Table S1. The 78 plasmids retrieved belong to the Rep_2 family. Out of them, 55 did not encode any relaxase whereas the remaining 23 contained relaxase genes belonging to the MOBV family (see below).
Many of the retrieved plasmids lack any distinguishable marker, although resistance to a variety of antibiotics, and even one instance of resistance to arsenate was found. A phylogenetic tree of the 78 plasmids retrieved was constructed (Supplementary Fig. S1), using the protein GpA initiator from phage ΦX174 as the out-group representative. There will be a detailed in-depth analysis on the LIC module of plasmids of the pMV158 family to be published elsewhere (G. del Solar and J.A. Ruiz-Masó, personal communication).
Furthermore, most of the retrieved plasmids were primarily isolated from Lactobacillales, but as shown in Supplementary Fig. S1 they are also widely distributed in other taxonomic orders of Firmicutes, Tenericutes and Proteobacteria, with notable examples of plasmids from Mycoplasma (eight plasmids). Although not retrieved in this search, there is an RCR-plasmid from Mycoplasma yeatsii, pMyBK1, which deserves a mention here because of two features (Kent et al., 2012, Breton et al., 2012). Firstly, it is unique at encoding an initiator of RCR different from the ones described here. Secondly, pMyBK1 is the only example of mobilizable plasmid in genus Mycoplasma, and it is precisely a MOBV plasmid. Inspecting the sequence of the mob gene of pMyBK1, we found that it harbors 39 UGA codons (out of a total of 520 codons). Since in Mycoplasma UGA specifies “tryptophan” instead of “stop” (Halbedel and Stülke, 2007, Inamine et al., 1990, Yamao et al., 1985), we can conclude that the transfer of pMyBK1 would be limited to Mycoplasma species.
5. The MOBV family
Classification of plasmids according to the relaxases and origins of transfer they carry has supposed a novel definition of plasmid families (Francia et al., 2004, Garcillán-Barcia et al., 2009). This classification provided a more global view of HGT by conjugation, whereas classification by replicons would point more to the vertical transfer. The HGT-based classification has showed that there are many plasmids that do not encode transfer functions (Garcillán-Barcia et al., 2011, Smillie et al., 2010). RCR-plasmids of Firmicutes fall mostly in the so-called MOBV family, being the MobM relaxase from the streptococcal plasmid pMV158 the representative of the family (Garcillán-Barcia et al., 2009). It was believed that plasmids are DNA molecules that result from shuffling of various gene cassettes, evolving independently one of each other (Osborn et al., 2000). As we will discuss below, analysis of evolutionary roots of these cassettes motivates to think that there could be crosstalk between them, as some module combinations prevail among others.
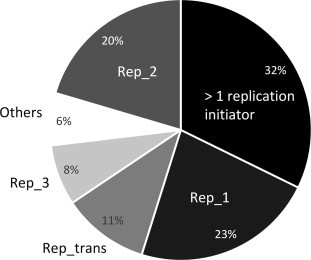
To update the inventory of elements composing the MOBV1 subfamily we have performed a PSI-BLAST search using MobMpMV158 as the query. The retrieved MOBV1 plasmids ranged from 2.7 to 30 kb (median = 5.1 kb; Fig. 2 ). Roughly half of them coded for antibiotic resistance genes (mainly to a wide variety of protein synthesis inhibitors, such as macrolides, lincosamides, aminoglycosides, streptogramins, amphenicols and tetracyclines) or other virulence traits like resistance to heavy metals and production of bacteriocins (Table 1). The organization of the mobilization region was found to be similar in all members: the oriT located upstream, close to the mob gene. Furthermore, the nic site was placed on the same strand as the mob gene, as expected.
Fig. 2.
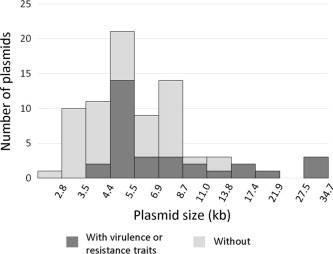
Distribution of MOBV1 plasmids according to their size. The X axis was built by using the log10 of plasmid size values. Each bar represents the abundance of plasmids for a given size range, which is indicated at each side of the bar. Plasmids encoding genes for metal or antibiotic resistance and/or bacteriocin production are indicated in dark gray. The rest are indicated in light gray. Data was obtained from Table 1.
5.1. The origin of transfer (oriT)
Plasmid conjugation initiates through the assembly of the relaxosome on the oriT, a region that contains inverted (IR) and/or direct (DR) repeats, A + T-rich tracts and, most importantly, the nic site. The relaxase requires that its target is presented as ssDNA to cleave it and generate the relaxase-DNA adduct, which will be pumped through a T4SS. Once in the recipient, the oriT is reconstituted by a transesterification reaction mediated by the relaxase to close the incoming molecule (reviewed in (Chandler et al., 2013).
The oriT of pMV158 spans 41 bp upstream of the mobM gene, exhibits a high A + T content (75.6%) and has three IRs (IR1 to IR3; Lorenzo-Díaz et al., 2011). Furthermore, a 6-bp sequence (5′-ACTTTA-3′) is repeated in the IR1/IR3 left-arm and the IR2 right-arm (Fig. 3 ). The nic site was firstly mapped in vitro between coordinates 3595–3596 (dinucleotide 5′-GpT-3′) in the pMV158 sequence (GenBank Acc. No. NC_010096; (Guzmán and Espinosa, 1997)). Then, the nic sites for the pMV158 and pE194 oriTs were determined in their respective hosts in vivo (Grohmann et al., 1997), mapping exactly in the same position as already suggested by the previous in vitro results. In silico analysis revealed that the IRs could generate three alternative stem-loop structures in which the position of the nic site would be placed in different positions: (i) located 8-bp upstream to IR1, (ii) in the IR2 inter-arm region, or (iii) at the 3′-end of the IR3 (Lorenzo-Díaz et al., 2011). IR3 includes the IR1 sequence and, since IR1/3 and IR2 partially overlap (see Fig. 3), the generation of cruciform secondary structures by one of them would hinder the formation of the other, indicating that the target DNA accessibility by the relaxase could depend on the plasmid DNA superhelicity (Fernández-López et al., 2014). Our in vitro analysis demonstrated that MobM binds specifically to ssDNA encompassing IR1/3 with high affinity (Lorenzo-Díaz et al., 2011), which allowed the MobM protein to repress its own synthesis (Lorenzo-Díaz et al., 2012). Functional relevance of the IRs in the different steps of the conjugative process is currently under exploration. We hypothesize that IR1/3 may be involved in the Mob-recognition of the oriT at the initiation of the relaxosome formation (in the donor cell) and IR2 at the termination reaction to close the T-strand (in the recipient cell).
Fig. 3.
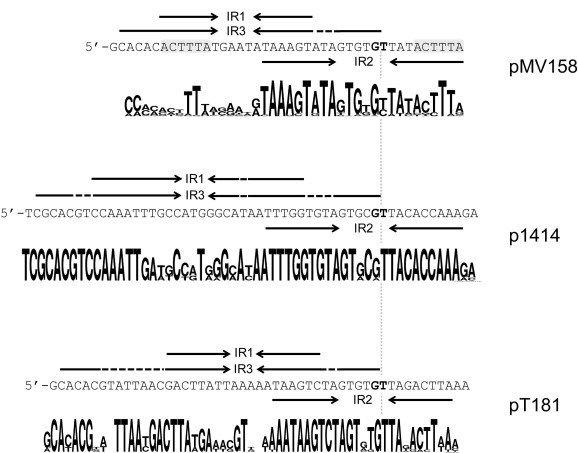
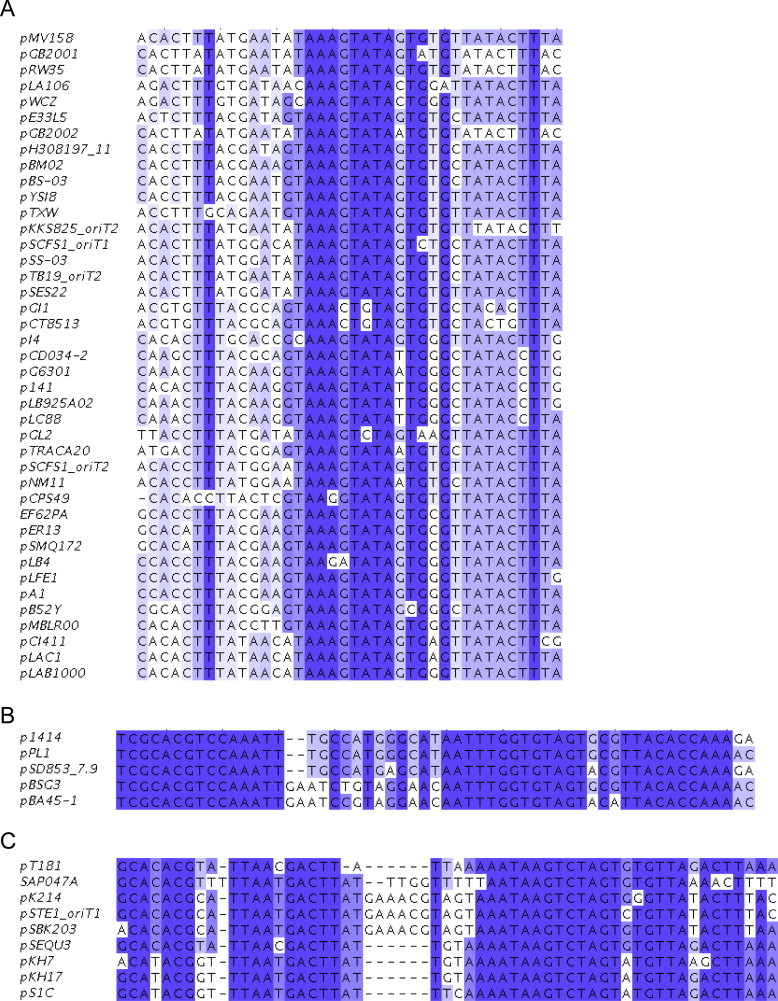
The origin of transfer (oriT) in MOBV1 representative plasmids. Comparison of the oriT sequences located in the pMV158, p1414 and pT181 plasmids, lined up by the position of the nic site (boldface letters). The three overlapping inverted repeats (IR1, IR2 and IR3) are depicted by arrows, and dashed lines indicate the position of those unpaired bases in the predicted secondary structures they could form. Gray background in the pMV158-oriT sequence denotes the position of a conserved repeated region. Consensus of the oriT sequences aligned in Supplementary Fig. S2 were prepared using WebLogo (version 2.8.2; Crooks et al., 2004).
Based on the sequence and structure of oriT pMV158, we inspected the oriT regions of the 93 MOBV-plasmids listed in Table 1. A total of 97 sequences were manually identified upstream to their respective relaxase encoded genes (five of them exhibiting two different MOBV-related oriTs: pSTE1, pKKS285, pSCFS1, unnamed (GenBank Acc. No GG692894.1), and pTB19. Analysis of the region showed a high degree of sequence conservation in the majority of the oriTs, being composed by three IRs and exhibiting the consensus sequence ‘GTGBG↓T’ for the nic site (B denoting a G, C or T following IUPAC code; ‘↓’ being the nic site). Two other minor plasmid groups, represented by pT181 and p1414, grouped in different clusters given their differences respect to the oriT sequence of pMV158 (Fig. 3 and Supplementary Fig. S2). However, these two clusters maintain the number and distribution of the IRs. Unfortunately, the experimental evidences for mapping the specific nic site in these plasmids are scarce and only a few oriT predictions, such as for plasmids like pSMQ172 and pPB1, have been published (de las Rivas et al., 2004, Turgeon and Moineau, 2001).
Given that the oriT pMV158 is the only element required in cis for pMV158 to be transferred (Farías and Espinosa, 2000), it is plausible to assume that any RCR-plasmid containing an oriT-like sequence would be potentially mobilizable. Such is the case of pXY3 (Zhou et al., 2010) and pCI411 (Coffey et al., 1994) plasmids (not included in Table 1), which only seem to contain an orphan and non-canonical oriT. In fact, the prototype RCR-plasmid pC194, which lacks both a mob gene and a canonical oriT, has been mobilized by using the conjugative transposon Tn916 as a helper (Naglich and Andrews, 1988, Showsh and Andrews, 1999). It is assumed that the relaxase of the helper should be able to recognize a suitable oriT in the Δmob plasmid. It is more intriguing the finding that pC194 and ΔoriT-Δmob-derivatives of plasmids pUB110 and pTA1060 were efficiently mobilized by the mating apparatus of ICEBs1, but without the intervention of the ICEBs1 NicK relaxase (Lee et al., 2012). Thus, in this case, the plasmids were not transferred by cross-recognition of an oriT by the relaxase of the helper. They were neither transferred by forming cointegrates with ICEBs1. Unexpectedly, the RCR initiation proteins of the mobilized plasmids were crucial for transfer (Lee et al., 2012). Thus, the strategy we have followed to search for plasmids containing MOBV1 relaxases might underestimate the real population of mobile elements.
5.2. The MOBV1 relaxases
The second element that belongs to the transfer module of the pMV158 RCR-family is the mobM gene, which codifies the MobM relaxase (Guzmán and Espinosa, 1997). Thus, in addition to using the homology of the Rep proteins to define the pMV158 RCR-plasmid family (Supplementary Fig. S1 and Table S1), we decided to find out whether any relationship between the Rep- and the Mob-proteins of the plasmid family existed. The classification system for mobilizable plasmids based on the amino acid sequence of the relaxases allowed defining MobM from pMV158 as the prototype of the MOBV superfamily (Francia et al., 2004, Garcillán-Barcia et al., 2011, Garcillán-Barcia et al., 2009). This superfamily is composed of more than 200 relaxases, of which about 140 were located in plasmids and the rest in bacterial chromosomes (Guglielmini et al., 2011).
Five subfamilies were described, and MobM from pMV158 was taken as the prototype of the MOBV1 subfamily (Garcillán-Barcia et al., 2009). Members of this subfamily showed three conserved motifs: (i) Motif I (HxxR), of yet unknown function; (ii) Motif II (NY(D/E)L), which is the proposed catalytic domain, and (iii) Motif III (HxDE…PHxH), which corresponds to the metal-coordination motif, also known as the 3H motif (Chandler et al., 2013). The MOBV2 family, represented by the Mob protein of the theta-replicating plasmid pBBR1 (Szpirer et al., 2001), exhibited Motifs I and III but lacked Motif II. The three motifs are located in the N-terminal moiety of the MOBV relaxases. This moiety harbors the DNA binding and nicking activities, since a truncated version of MobM, which only contains the first N-terminal 199 residues, retained the relaxase activity on supercoiled DNA (Fernández-López et al., 2013a, Fernández-López et al., 2013b, Lorenzo-Díaz et al., 2011). The C-terminal moiety of MobM could be involved in, at least, two functions: (i) protein–protein interactions (dimerization and interactions with the auxiliary plasmid-encoded coupling protein) and (ii) association with the cell membrane, through a proposed coiled-coil region located between residues 400 and the C-terminal end of MobM. Disruption of the alpha helical-rich region by mutations (changes to Pro residues) resulted in failure of MobM-association with membranes; further, the pMV158-derivative harboring these mutations lost its ability to be transferred (de Antonio et al., 2004). There was no indication of a helicase activity in the C-terminal moiety of MobM (our unpublished observations).
We have updated the inventory of elements that cluster with pMV158 into the MOBV1 subfamily. 97 non-redundant plasmid MOBV1 relaxases were retrieved from a PSI-BLAST search using the N-terminal 300 amino acids of MobM from pMV158 as a query (Table 1). All of them shared the three Motifs described above (Fig. 4 A). The inferred phylogeny of MOBV1 relaxases, rooted by a MOBV2 relaxase, showed well-supported external clusters of highly related sequences and poorly-supported internal nodes (Fig. 4B). It is a fact that reflects the low overall similarity of the taxa, mainly circumscribed to the three relaxase motifs described (Francia et al., 2004, Garcillán-Barcia et al., 2009). The plasmids coding these relaxases are primarily distributed in several genera of Lactobacillales and Bacillales, with a few members out of the phylum Firmicutes. Curiously, the plasmids encoding relaxases comprised in a monophyletic clade in the phylogenetic tree shown in Fig. 4B are generally hosted in a single taxonomic order (either Lactobacillales or Bacillales), suggesting less inter-order transfer than expected.
Fig. 4.
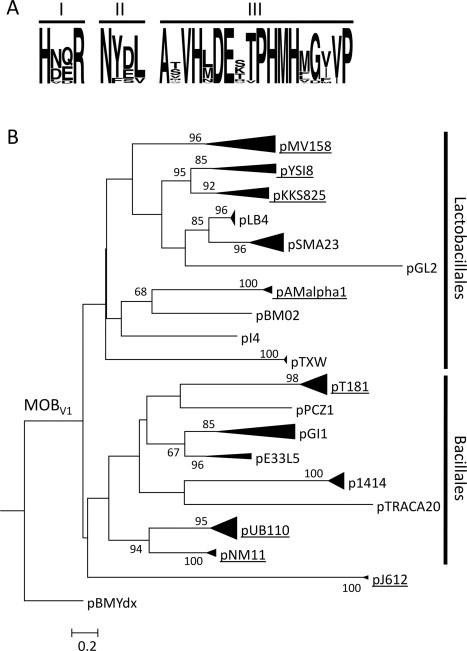
Phylogeny of MobMpMV158 homologs. (A) Logos of the MOBV1 relaxase motifs. MOBV1 relaxases were analyzed using WebLogo (version 2.8.2) (Crooks et al., 2004). (B) The 300 N-terminal residues of the MobM relaxase of plasmid pMV158 were used as query in a PSI-BLAST search (Altschul et al., 1997) (e-value: 1 × E−6 and limited to 100 non-redundant plasmid hits). The search converged in the sixth iteration. The 300 N-terminal residues of the homologs were aligned using MUSCLE (Edgar, 2004). The phylogenetic reconstruction was carried out by maximum likelihood (ML), using RAxML version 7.2.7 (Stamatakis, 2006). 100 ML trees were executed using the JTTGAMMA model. 1000 bootstrap trees were then inferred to obtain the confidence values for each node of the best ML tree. Only bootstrap values >50% are indicated. The MOBV2 relaxase of plasmid pBMYdx (GenBank Acc. No. NP_981974.1) was used as outgroup. Highly related clusters are compressed and a prototype member is indicated. The names and features of all members included in the tree are recorded in Table 1. Clusters grouping plasmids that encode antibiotic resistance traits are underlined. Vertical bars delimit clades for which most of their members are hosted either in Lactobacillales or in Bacillales.
No genes associated with conjugative transfer others than the relaxase ones were encoded by these plasmids. Thus, they are classified as mobilizable, requiring the conjugative machinery of other plasmids to be transferred (namely, a coupling protein and a mating-pair formation apparatus composed by a T4SS and a conjugative pilus). Eight mating-pair formation (MPF) types have been phylogenetically described (Guglielmini et al., 2011); three of them, MPFFATA, MPFFA and MPFT were able to mobilize MOBV1 plasmids. Specifically, pMV158 was mobilized between G+ bacteria by functions supplied by helper MPFFATA plasmids of the Inc18 family, like pIP501 and pAMβ1 (Grohmann et al., 1999, Priebe and Lacks, 1989, van der Lelie et al., 1990), and even to the G− bacterium E. coli by MPFT plasmids RP4 and R388 but not by MPFF plasmid F (Farías and Espinosa, 2000). Other elements, such as pC194 and pUB110, were mobilized by auxiliary MPFFA elements such as Tn916 (Lee et al., 2012, Naglich and Andrews, 1988), and MPFFATA plasmids, like pLS20, mobilized pUB110 and pBC16 (Koehler and Thorne, 1987, Selinger et al., 1990).
Most of the MOBV1 plasmids replicate by the rolling circle mechanism (Fig. 5 and Table 1). MOBV1 relaxases are predominantly linked to RCR initiators of the three different subgroups: Rep_1 (PF01446), Rep_2 (PF01719), and Rep_trans (PF02486). A small fraction of MOBV1 relaxases is linked to a wide variety of theta replication families, of which Rep_3 is the most abundant. Congruently, with the abovementioned taxonomic bias in the abundance of RCR initiators and the MOBV1 relaxase distribution, most relaxases encoded in Rep_2 RCR plasmids grouped separately from those encoded in Rep_1 RCR plasmids (clade Lactobacillales vs. Bacillales in Fig. 6 and Supplementary Table S2). An example of this bias is found in Enterococci (Lactobacillales), where Rep_1 initiators are commonly found in multireplicon plasmids, and may not be functional, as it is the case for plasmid pAMα1 (Clewell et al., 2014). Nevertheless, a few functional Rep_1 initiators can be also found in the Lactobacillales clade, such as those grouped in the cartooned pSMA23 cluster of Fig. 4B. Curiously, within the pSMA23 group some exceptions also exist: plasmid pCD034-2 encodes a Rep_2 instead of a Rep_1 initiator, an indication of recent recombination events that led to new backbone combinations. Besides, more ancient recombination events could give rise to arrangements present in plasmids of the Bacillales MOBV1 clade, since the highly divergent relaxases included in that group are linked to initiators of the three RCR subgroups (Fig. 6A).
Fig. 5.
Distribution of replication initiation protein families in MOBV1 plasmids. The percentage of replication initiator families included in Table 1 is presented. Plasmids with more than one initiator are included in “>1 replication initiator”. Plasmids with a single initiator are grouped in “Rep_1”, “Rep_2” and “Rep_trans” families (when RCR), or in “Rep_3” and “Others” (non-RCR).
Fig. 6.
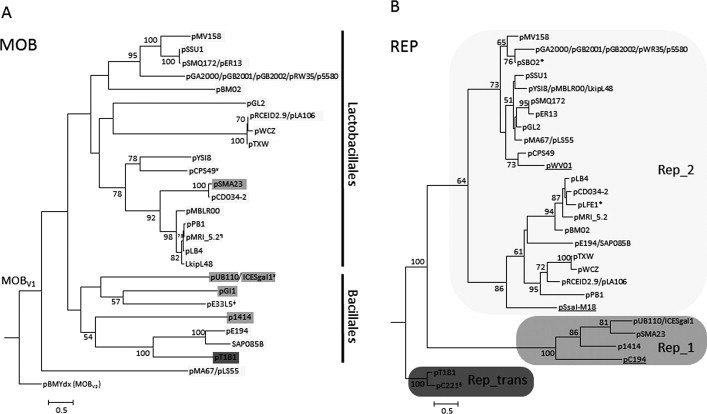
Phylogeny of the Rep and Mob (MOBV1) proteins of relevant RCR-plasmids. Representative RCR plasmids and MOBV1 elements included in Supplementary Table S2 were used to trace the evolutionary relationships of their relaxase and replication initiation proteins. The homologs were aligned using MUSCLE (Edgar, 2004). The phylogenetic reconstruction was carried out by maximum likelihood (ML), using RAxML version 7.2.7 (Stamatakis, 2006). 100 ML trees were executed using the JTTGAMMA model. 1000 bootstrap trees were then inferred to obtain the confidence values for each node of the best ML tree. Only bootstrap values >50% are indicated. (A) Phylogenetic tree of the N-terminal 300 residues of MOBV1 relaxases. Each plasmid is shadowed in gray according to the RCR initiator subgroup as indicated in the legend of panel B. ≠Indicates an exceptional MOBV1 plasmid, pE33L5, which does not encode an RCR initiator but a HTH_36 (PF13730) replication initiation protein. Vertical bars delimit clades for which most of their members are hosted either in Lactobacillales or in Bacillales. ¥Indicates an element not hosted in the taxonomic order indicated by the bars. ¶Indicates that plasmid pMRI_5.2 also encodes a Rep_1 RCR initiator. (B) Phylogenetic tree of the RCR initiators. Plasmids pT181 and pC221 were used as outgroups. A gray color palette was used to indicate clades containing different RCR initiators families: Rep_1 (PF01446) and Rep_2 (PF01719), as well as the Rep_trans (PF02486) used to root the tree. ∗According to their GenBank annotated sequences, plasmids pSBO2 and pLFE1 encode truncated MOBV relaxases and thus were not included in the MOB phylogeny, neither were the underlined plasmids (pWV01, pSsal-M18 and pC194) since they do not encode relaxases. §pC221 is a mobilizable RCR plasmid, but it encodes a MOBP7 instead of a MOBV relaxase.
Fig. 6 also provides an interesting example of the switch of plasmids from and to integrative elements. ICESgal1 is an integrative element highly similar to plasmid pUB110 (both in relaxase and initiator) but located in the chromosome of Streptococcus gallolyticus UCN34 (Lactobacillales order). It is tempting to speculate that this element integrated in the chromosome once transferred from Bacillales to the Lactobacillales background (maybe helped by a Tn916-like element located close to ICESgal1), where it was not able to replicate and became an integrative and mobilizable element (IME).
A significant proportion (one third) of MOBV1 plasmids recorded in Table 1 encoded more than one replication initiation protein (Fig. 5). Besides, 10 out of the 93 plasmids coded more than one relaxase gene. Both facts suggest the frequent arising of plasmid cointegrates. Precisely, it is known the ability of RCR-plasmid encoded relaxases to promote site-specific DNA recombination at oriT rendering plasmid cointegrates during conjugative mobilization, where the host rec system may function to stimulate the recombination process (Gennaro et al., 1987, Novick et al., 1984, Projan and Novick, 1988). Further, it is worth recalling that the staphylococcal plasmid pE194 was able to integrate into the B. subtilis chromosome by a RecA-independent recombination mechanism, and using as little as 6–14 bp homologies (Dempsey and Dubnau, 1989). This, in conjunction with the finding of RCR-plasmids integrated into other bigger plasmids (Oskam et al., 1991), raises the question of whether RCR-plasmids that are integrated on IMEs would participate in their replication and mobilization; in fact, we have found a putative initiator of replication of the Rep_1 family within an streptococcal island which also encode a MOBV1 protein (our unpublished observations). Besides, the cross-recognition of heterologous oriTs by MOBV relaxases (Fernández-López et al., 2013a) could be favored in the multi-relaxase cointegrates, potentiating their spreading.
Recombination seems to be the most likely cause of the different topologies exhibited in the dendrograms of initiators and relaxases (compare trees in panels A and B of Fig. 6). Despite the absence of a strong coevolution between initiators and relaxases, there is a clear tendency to find stable backbones Rep_2-MOBV1 and Rep_1-MOBV1 differentially adapted to different taxonomic orders.
6. The single-strand origins (sso)
Rolling circle replication in ssDNA coliphages and in plasmids requires the existence of origins that are involved in lagging strand synthesis (Kornberg and Baker, 1992, Novick, 1998). When RCR-plasmids were discovered (te Riele et al., 1986a, te Riele et al., 1986b), it was apparent that the ssDNA intermediates should harbor signals for the conversion of the ssDNA to plasmid dsDNA. This was first reported for plasmid pT181 (Gruss et al., 1987) and soon afterwards for the pMV158-derivative plasmid pLS1 (del Solar et al., 1987). In these two plasmids, it was shown that their ssoAs were located in non-coding 200–300 base-pair-long regions that have the potential to generate one or several secondary structures on ssDNA. These signals were orientation-dependent (this means that they were functional only when placed in the displaced strand). Deletion of the region encompassing the ssoA led to accumulation of ssDNA intermediates and to plasmid instability, but the plasmids were still able to replicate (del Solar et al., 1987, Dempsey et al., 1995, Gruss et al., 1987, Kramer et al., 1995, Murray et al., 1989, Seegers et al., 1995). This finding led to the hypothesis that alternative, albeit less efficient, ssos could replace the genuine conversion signal (del Solar et al., 1987, Kramer et al., 1998a, Meijer et al., 1995a, Meijer et al., 1995b). Although accumulation of ssDNA intermediates and plasmid unstable inheritance by VGT were thought to be related phenomena (Meijer et al., 1995a, Meijer et al., 1995b), cloning of signals that allowed an efficient ssDNA → dsDNA conversion, at least in plasmid pLS1, did not lead to stable plasmid inheritance (Hernández-Arriaga et al., 2000). Apart from ssoA, other three sso types have been described based on sequence similarity and structure analysis (Khan, 1996, Khan, 2000, Kramer et al., 1998a): ssoU (pUB110, pMV158), ssoT (pTA1060, pBAA1), and ssoW (pWV01). A fifth type was described in plasmid pM4 from Lactobacillus plantarum, which exhibited no significant sequence or structural similarity with any of the four classical sso (Yin et al., 2009). Plasmids pMV158 and pRW35 have the unusual feature of harboring two sso, ssoU and ssoA. Out of the two origins, the former has a more complex structure than the latter (Fig. 7 ).
Fig. 7.
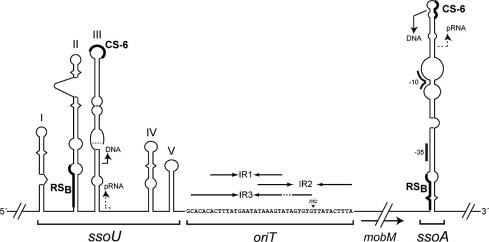
Predicted secondary structures of the lagging-strand origins of replication ssoA and ssoU. The oriT and the mobM gene of plasmid pMV158 are flanked by two lagging-strand origins of replication (ssoA and ssoU). oriTpMV158 sequence (coordinates 3564–3605 from pMV158; GenBank Acc. No. NC_010096) is shown at the center of the image. Its three inverted repeats are represented by arrows and the nic site by a vertical arrowhead. Both ssos can generate long hairpin-loop structures that function as ‘ssDNA promoters’ (Kramer et al., 1999, Kramer et al., 1997, Masai and Arai, 1997). The RNAP-binding site (RSB), located in the base of the hairpin is recognized by the RNAP to synthesize a short pRNA. A consensus sequence (CS-6), located in the loop of the hairpin, acts as the termination point for the pRNA synthesis. The pRNA is then used by DNA polymerase I for limited extension synthesis, followed by replication of the lagging strand by DNA Pol III. The figure was modified with permission from the American Society for Microbiology from (Fernández-López et al., 2014). No further reproduction or distribution is permitted without the prior written permission of American Society for Microbiology.
Two regions were mapped in the ssoA: the recombination site B (RSB) and a 6-nucleotide consensus sequence (CS-6) (del Solar et al., 1987, Gruss et al., 1987). These two regions acted as efficient signals only in ssDNA configuration (replicative intermediates). In these molecules, the ssoA would adopt a long stem-loop structure where the RSB would be located at the stem and the CS-6 in the loop of the hairpin (Fig. 7). Biochemical and genetic analyses demonstrated that RSB was recognized as the binding site of the host RNA polymerase (RNAP), thus acting as ssDNA promoter (Kramer et al., 1997). These promoters were shown to generate within paired secondary structures on ssDNA molecules and harbor sequences that are recognized by the host RNAP to initiate lagging strand synthesis (Glucksmann-Kuis et al., 1992, Masai and Arai, 1997). From the ssoA pMV158 promoter, the RNAP synthesized a short 20 nt-long primer RNA (pRNA) that stopped at the CS-6 sequence, which acted as a transcription terminator (Kramer et al., 1997). The pRNA was processed by DNA polymerase I and was proposed to be elongated by the host DNA polymerase III to finish the lagging DNA strand synthesis (Kramer et al., 1998b).
Efficient ssDNA → dsDNA conversion is also needed in the conjugative process. Within the recipient cell the transferred ssDNA should be converted into dsDNA either prior or after the circularization of the T-DNA by a strand-transfer reaction. During transfer, a fast conversion of ssDNA intermediates would be critical to finish the process, since no proteins essential for vegetative replication and control would be synthesized in the recipient cell until the first dsDNA plasmid copy is generated (Lorenzo-Díaz and Espinosa, 2009). Despite its importance, the synthesis of the lagging strand in the recipient cell remains an unresolved issue. For plasmids F and ColIb-P9 single-stranded promoters were identified in the leading strand region, which is the one that enters first into the recipient cell, and that includes genes that promote the establishment of the incoming plasmid (Bates et al., 1999, Masai and Arai, 1997, Nasim et al., 2004).
We have explored the sso diversity and distribution among MOBV1 plasmids. Based on the ssos previously identified by homology or characterized by in vivo and/or in vitro approaches, we annotated 35 sso elements in 33 out of 93 plasmids in Table 1. This limited number is due to the little overall homology among ssos, even those of the same type. All the reported sso types were found: ssoT (13 plasmids), ssoA (11 plasmids), ssoU (6 plasmids) and ssoW (4 plasmids). Two plasmids (pMV158 and pRW35) contained two ssos (ssoA and ssoU). No new members arose in our search of the new type of sso reported for plasmid pM4 (Zhai et al., 2009). Curiously enough, we have observed that while ssoA and ssoW are generally found downstream and close to the mob gene, the ssoU is always upstream with respect to the oriT sequence. In the case of the ssoT element we have found that it can be located upstream (n = 5) or downstream (n = 8) of the MOB module.
It has been demonstrated that the sso type is one of the key elements in determining the host range of a plasmid: whereas ssoU and ssoT support a wide-host range, ssoA and ssoW seem to evolve in the other direction, working efficiently only in their natural hosts in vivo (Khan, 2005). From the analysis abovementioned, it seems apparent that highly related plasmids do not always contain the same type of sso, whereas distantly related do. These facts complicate even more the picture of the putative host range a MOBV1 plasmid can reach. Whether the location of the sso affects the efficiency of a given plasmid for replication and/or conjugative transfer and, consequently, its host range has not been explored yet.
7. Conclusions and perspectives
RCR-plasmids represent a pool of genetic information that is shared by many bacteria. We have found them in Lactobacillales, Bacillales, Mollicutes, etc. Among the bacteria that host them, we have found several of the most relevant G+ pathogens, namely Staphylococcus aureus, S. pneumoniae, and Enterococcus faecalis. These three bacterial species are important for human health because they: (i) exhibit very high rates of human morbidity and mortality; (ii) are the cause of many health-care associated infections; (iii) have acquired elevated resistance to antibiotics; (iv) play an important role as reservoir of AbR and virulence genes, and (v) carry conjugative or mobilizable broad host-range plasmids, thus contributing to the spread of resistances. Infections caused by the above G+ bacteria represent, in addition to a high economic impact, a threat to hospitals, children, elder population and immuno-compromised people (Jones, 2001). Furthermore, plasmid-encoded genes related not only to AbR but also to replication and/or transfer, are also found within the ICEs. However, and in spite of the relevance of these bacteria, very little is known on the genetics and biochemistry of the transfer functions of the RCR-plasmids studied here with the exception of the streptococcal plasmid pMV158 (reviewed in (Espinosa, 2013, Fernández-López et al., 2014) and the staphylococcal plasmid pC221 (Caryl et al., 2004, Caryl and Thomas, 2006, Smith and Thomas, 2004). And even in these two plasmids there is little, if any, information on the interactions of the relaxases encoded by pMV158 or pC221 with the machinery provided by auxiliary plasmids (Arends et al., 2013, Goessweiner-Mohr et al., 2013, Grohmann et al., 2003).
In addition to the above, it would be interesting to explore how the conjugative transfer is regulated in plasmids that contain more than one MOBV cassette as well as the level of cross-recognition between the MobM-like relaxases and their non-cognate oriTs. We have shown that MobM is able to relax supercoiled DNAs from plasmids with oriTs that share total (plasmid pUB110) or partial (plasmid pDL287) homology with the oriT of pMV158 (Fernández-López et al., 2013a), a phenomenon that could play an important role in the plasmid spreading between bacteria in natural environments. Furthermore, the fact that RCR initiators relax DNA in a way highly similar to conjugative relaxases and the recent finding of RCR initiators involvement in plasmid mobilization (Lee et al., 2012) open a new research field in the conjugative transmission of RCR plasmids.
Acknowledgments
Thanks are due to members of our labs for information and fruitful discussions. We regret that we have been unable to include all the contributions of people working in our field due to space limitations, and apologize for missing references. Research was funded by the Spanish Ministry of Economy and Competitiveness (grants CSD-2008-00013-INTERMODS to M.E., Sara Borrell CD13/00304 to F.L.-D., and BFU2011-26608 to M.P.G.-B.), and by the 7th Framework Programme (FP7-REGPOT-2012-CT2012-31637-IMBRAIN to F.L.-D. and by 282004/FP7-HEALTH-2011-2.3.1-2 to M.P.G.-B.).
Communicated by Saleem Khan
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.plasmid.2014.05.004.
Appendix A. Supplementary data
Supplementary Fig. S1.
Supplementary Fig. S2.
References
- Alonso J.C. Functional analysis of the leading strand replication origin of plasmid pUB110 in Bacillus subtilis. Nucleic Acids Res. 1988;16:9127–9145. doi: 10.1093/nar/16.19.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, J.C., Tomalsky, M., 2014. Plasmids: biology and impact in biotechnology and discovery. In: A. Press, (Ed.).
- Altschul S.F. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S.P. The PcrA3 mutant binds DNA and interacts with the RepC initiator protein of plasmid pT181 but is defective in its DNA helicase and unwinding activities. Plasmid. 2005;54:104–113. doi: 10.1016/j.plasmid.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Anand S.P., Khan S.A. Structure-specific DNA binding and bipolar helicase activities of PcrA. Nucleic Acids Res. 2004;32:3190–3197. doi: 10.1093/nar/gkh641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.T., Seifert H.S. Opportunity and means: horizontal gene transfer from the human host to a bacterial pathogen. MBio. 2011;2:e00005–e00011. doi: 10.1128/mBio.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends K. TraG encoded by the pIP501 type IV secretion system is a two-domain peptidoglycan-degrading enzyme essential for conjugative transfer. J. Bacteriol. 2013;195:4436–4444. doi: 10.1128/JB.02263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester S. Comparative expression of the pC194-cat gene in Streptococcus pneumoniae, Bacillus subtilis and Escherichia coli. Gene. 1990;86:71–79. doi: 10.1016/0378-1119(90)90115-8. [DOI] [PubMed] [Google Scholar]
- Bannam T.L., Rood J.I. Relationship between the Clostridium perfringens catQ gene product and chloramphenicol acetyltransferases from other bacteria. Antimicrob. Agents Chemother. 1991;35:471–476. doi: 10.1128/aac.35.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquero F. From pieces to patterns: evolutionary engineering in bacterial pathogens. Nat. Rev. Microbiol. 2004;2:510–518. doi: 10.1038/nrmicro909. [DOI] [PubMed] [Google Scholar]
- Baquero F. Environmental stress and evolvability in microbial systems. Clin. Microbiol. Infect. 2009;15:5–10. doi: 10.1111/j.1469-0691.2008.02677.x. [DOI] [PubMed] [Google Scholar]
- Baron C. Bacterial secrets of secretion: EuroConference on the biology of type IV secretion processes. Mol. Microbiol. 2002;43:1359–1365. doi: 10.1046/j.1365-2958.2002.02816.x. [DOI] [PubMed] [Google Scholar]
- Bates S. Expression of leading region genes on IncI 1 plasmid ColIb-P9: genetic evidence for single-stranded DNA transcription. Microbiology. 1999;145:2665–2672. doi: 10.1099/00221287-145-10-2655. [DOI] [PubMed] [Google Scholar]
- Bergemann A.D. Homology of mycoplasma plasmid pADB201 and staphylococcal plasmid pE194. J. Bacteriol. 1989;171:593–595. doi: 10.1128/jb.171.1.593-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatty M. The expanding bacterial type IV secretion lexicon. Res. Microbiol. 2013;164:620–639. doi: 10.1016/j.resmic.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton M. Distribution and diversity of mycoplasma plasmids: lessons from cryptic genetic elements. BMC Microbiol. 2012;12:257. doi: 10.1186/1471-2180-12-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B) Antimicrob. Agents Chemother. 1980;18:753–760. doi: 10.1128/aac.18.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caryl J.A. Reconstitution of a staphylococcal plasmid–protein relaxation complex in vitro. J. Bacteriol. 2004;186:3374–3383. doi: 10.1128/JB.186.11.3374-3383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caryl J.A., Thomas C.D. Investigating the basis of substrate recognition in the pC221 relaxosome. Mol. Microbiol. 2006;60:1302–1318. doi: 10.1111/j.1365-2958.2006.05188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E. DNA substrate-induced activation of the Agrobacterium VirB/VirD4 type IV secretion system. J. Bacteriol. 2013;195:2691–2704. doi: 10.1128/JB.00114-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D.B. Extrachromosomal and mobile elements in enterococci: transmission, maintenance, and epidemiology. In: Gilmore M.S., Clewell D.B., Ike Y, editors. Massachusetts Eye and Ear Infirmary; Boston: 2014. (Enterococci: From Commensals to Leading Causes of Drug Resistant Infection). [PubMed] [Google Scholar]
- Coffey A. Nucleotide sequence and structural organization of the small broad-host-range plasmid pCI411 from Leuconostoc lactis. Microbiology. 1994;140:2263–2269. doi: 10.1099/13500872-140-9-2263. [DOI] [PubMed] [Google Scholar]
- Crooks G.E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M. Breaking and joining single-stranded DNA: the HUH endonuclease superfamily. Nat. Rev. Microbiol. 2013;11:625–638. doi: 10.1038/nrmicro3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.-L. Role of individual monomers of a dimeric initiator protein in the initiation and termination of plasmid rolling circle replication. J. Biol. Chem. 2000;275:13529–13534. doi: 10.1074/jbc.275.18.13529. [DOI] [PubMed] [Google Scholar]
- Chen I. The ins and outs of DNA transfer in bacteria. Science. 2005;310:1456–1460. doi: 10.1126/science.1114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Antonio C. Features of the plasmid pMV158-encoded MobM, a protein involved in its mobilization. J. Mol. Biol. 2004;335:733–743. doi: 10.1016/j.jmb.2003.11.017. [DOI] [PubMed] [Google Scholar]
- de la Campa A.G. Initiation of replication of plasmid pLS1. The initiator protein RepB acts on two distant DNA regions. J. Mol. Biol. 1990;213:247–262. doi: 10.1016/S0022-2836(05)80188-3. [DOI] [PubMed] [Google Scholar]
- de la Cruz F. Conjugative DNA metabolism in gram-negative bacteria. FEMS Microbiol. Rev. 2010;34:18–40. doi: 10.1111/j.1574-6976.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- de las Rivas B. Complete nucleotide sequence and structural organization of pPB1, a small Lactobacillus plantarum cryptic plasmid that originated by modular exchange. Plasmid. 2004;52:203–211. doi: 10.1016/j.plasmid.2004.09.001. [DOI] [PubMed] [Google Scholar]
- de Paz H.D. Functional interactions between type IV secretion systems involved in DNA transfer and virulence. Microbiology. 2005;151:3505–3516. doi: 10.1099/mic.0.28410-0. [DOI] [PubMed] [Google Scholar]
- del Solar G., Espinosa M. Plasmid copy number control: an ever-growing story. Mol. Microbiol. 2000;37:492–500. doi: 10.1046/j.1365-2958.2000.02005.x. [DOI] [PubMed] [Google Scholar]
- del Solar G. Replication and control of circular bacterial plasmids. Microbiol. Mol. Biol. Rev. 1998;62:434–464. doi: 10.1128/mmbr.62.2.434-464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Solar G. A genetically economical family of plasmid-encoded transcriptional repressors in control of plasmid copy number. J. Bacteriol. 2002;184:4943–4951. doi: 10.1128/JB.184.18.4943-4951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Solar G. Rolling circle-replicating plasmids from gram-positive and gram-negative bacteria: a wall falls. Mol. Microbiol. 1993;8:789–796. doi: 10.1111/j.1365-2958.1993.tb01625.x. [DOI] [PubMed] [Google Scholar]
- del Solar G. Initiation signals for the conversion of single stranded to double stranded DNA forms in the streptococcal plasmid pLS1. Nucleic Acids Res. 1987;15:5561–5580. doi: 10.1093/nar/15.14.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey L.A., Dubnau D.A. Identification of plasmid and Bacillus subtilis chromosomal recombination sites used for pE194 integration. J. Bacteriol. 1989;171:2856–2865. doi: 10.1128/jb.171.5.2856-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey L.A. Localization of the start sites of lagging-strand replication of rolling circle plasmids from gram-positive bacteria. Mol. Microbiol. 1995;15:679–687. doi: 10.1111/j.1365-2958.1995.tb02377.x. [DOI] [PubMed] [Google Scholar]
- Díaz A. Multiple roles for DNA polymerase I in establishment and replication of the promiscuous plasmid pLS1. Mol. Microbiol. 1994;14:773–783. doi: 10.1111/j.1365-2958.1994.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Draper O. Site-specific recombinase and integrase activities of a conjugative relaxase in recipient cells. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16385–16390. doi: 10.1073/pnas.0506081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa M. Plasmids as models to study macromolecular interactions: the pMV158 paradigm. Res. Microbiol. 2013;164:199–204. doi: 10.1016/j.resmic.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Espinosa M. Plasmid rolling circle replication and its control. FEMS Microbiol. Lett. 1995;130:111–120. doi: 10.1111/j.1574-6968.1995.tb07707.x. [DOI] [PubMed] [Google Scholar]
- Farías M.E., Espinosa M. Conjugal transfer of plasmid pMV158: uncoupling of the pMV158 origin of transfer from the mobilization gene mobM, and modulation of pMV158 transfer in Escherichia coli mediated by IncP plasmids. Microbiology. 2000;146:2259–2265. doi: 10.1099/00221287-146-9-2259. [DOI] [PubMed] [Google Scholar]
- Fernández-López, C. et al., 2014. Mobilizable rolling-circle replicating plasmids from Gram-positive bacteria: a low-cost conjugative transfer. In: Alonso, J.C., Tomalsky, M. (Eds.). vol. ASM Press, in press.
- Fernández-López C. Nicking activity of the pMV158 MobM relaxase on cognate and heterologous origins of transfer. Plasmid. 2013:120–130. doi: 10.1016/j.plasmid.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Fernández-López C. Functional properties and structural requirements of the plasmid pMV158-encoded MobM relaxase domain. J. Bacteriol. 2013;195:3000–3008. doi: 10.1128/JB.02264-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia M.V. A classification scheme for mobilization regions of bacterial plasmids. FEMS Microbiol. Rev. 2004;28:79–100. doi: 10.1016/j.femsre.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Frost L. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 2005;3:722–732. doi: 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- Funnell B.E., Phillips G.J., editors. Plasmid Biology. ASM Press; Washington, DC: 2004. [Google Scholar]
- Garcillán-Barcia M.P. Identification of bacterial plasmids based on mobility and plasmid population biology. FEMS Microbiol. Rev. 2011;35:936–956. doi: 10.1111/j.1574-6976.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- Garcillán-Barcia M.P. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol. Rev. 2009;33:657–687. doi: 10.1111/j.1574-6976.2009.00168.x. [DOI] [PubMed] [Google Scholar]
- Garcillán-Barcia M.P. Conjugative transfer can be inhibited by blocking relaxase activity within recipient cells with intrabodies. Mol. Microbiol. 2007;63:404–416. doi: 10.1111/j.1365-2958.2006.05523.x. [DOI] [PubMed] [Google Scholar]
- Gennaro M.L. A site-specific recombination function in Staphylococcus aureus plasmids. J. Bacteriol. 1987;169:2601–2610. doi: 10.1128/jb.169.6.2601-2610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glucksmann-Kuis M.A. Specific sequences and a hairpin structure in the template strand are required for N4 virion RNA polymerase promoter recognition. Cell. 1992;70:491–500. doi: 10.1016/0092-8674(92)90173-a. [DOI] [PubMed] [Google Scholar]
- Goessweiner-Mohr N. Conjugative type IV secretion systems in gram-positive bacteria. Plasmid. 2013;70:289–302. doi: 10.1016/j.plasmid.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Rüth F.X., Coll M. Cut and move: protein machinery for DNA processing in bacterial conjugation. Curr. Opin. Struct. Biol. 2006;16:744–752. doi: 10.1016/j.sbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Gomis-Ruth F.X. Coupling factors in macromolecular type-IV secretion machineries. Curr. Pharm. Des. 2004;10:1551–1565. doi: 10.2174/1381612043384817. [DOI] [PubMed] [Google Scholar]
- Goursot R. Plasmids from Staphylococcus aureus replicate in yeast Saccharomyces cerevisiae. Nature. 1982;298:488–490. doi: 10.1038/298488a0. [DOI] [PubMed] [Google Scholar]
- Grohmann E. Mobilisation of the streptococcal plasmid pMV158: interactions of MobM protein with its cognate oriT DNA region. Mol. Gen. Genet. 1999;261:707–715. doi: 10.1007/s004380050014. [DOI] [PubMed] [Google Scholar]
- Grohmann E. Conjugative plasmid transfer in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 2003;67:277–301. doi: 10.1128/MMBR.67.2.277-301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann E. Determination of specific DNA strand discontinuities with nucleotide resolution in exponentially growing bacteria harbouring rolling circle-replicating plasmids. FEMS Microbiol. Lett. 1997;152:363–369. doi: 10.1111/j.1574-6968.1997.tb10453.x. [DOI] [PubMed] [Google Scholar]
- Gruss A.D., Ehrlich S.D. The family of highly interrelated single-stranded deoxyribonucleic avid plasmids. Microbiol. Rev. 1989;53:231–241. doi: 10.1128/mr.53.2.231-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A.D. Functional analysis of a palindromic sequence required for normal replication of several staphylococcal plasmids. Proc. Natl. Acad. Sci. U.S.A. 1987;84:2165–2169. doi: 10.1073/pnas.84.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch A. Recognition and processing of the origin of transfer DNA by conjugative relaxase TrwC. Nat. Struct. Biol. 2003;10:1002–1010. doi: 10.1038/nsb1017. [DOI] [PubMed] [Google Scholar]
- Guglielmini J. The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. PLoS Genet. 2011;7:e1002222. doi: 10.1371/journal.pgen.1002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán L., Espinosa M. The mobilization protein, MobM, of the streptococcal plasmid pMV158 specifically cleaves supercoiled DNA at the plasmid oriT. J. Mol. Biol. 1997;266:688–702. doi: 10.1006/jmbi.1996.0824. [DOI] [PubMed] [Google Scholar]
- Halbedel S., Stülke J. Tools for the genetic analysis of Mycoplasma. Int. J. Med. Microbiol. 2007;297:37–44. doi: 10.1016/j.ijmm.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Hamilton C.M. TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58. J. Bacteriol. 2000;182:1541–1574. doi: 10.1128/jb.182.6.1541-1548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C.R. Nucleotide sequence of a Bacillus pumilus gene specifying chloramphenicol acetyltransferase. Gene. 1983;24:163–169. doi: 10.1016/0378-1119(83)90076-8. [DOI] [PubMed] [Google Scholar]
- Hernández-Arriaga A.M. A functional lagging strand origin does not stabilize plasmid pMV158 inheritance in Escherichia coli. Plasmid. 2000;43:49–58. doi: 10.1006/plas.1999.1430. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J. Bacteriol. 1982:815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine J.M. Evidence that UGA is read as a tryptophan codon rather than as a stop codon by Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum. J. Bacteriol. 1990;172:504–506. doi: 10.1128/jb.172.1.504-506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R., Novick R.P. Role of the double-strand origin cruciform in pT181 replication. Plasmid. 2001;46:95–105. doi: 10.1006/plas.2001.1535. [DOI] [PubMed] [Google Scholar]
- Jones R.N. Resistance patterns among nosocomial pathogens: trends over the past few years. Chest. 2001;119(2 Suppl.):397S–404S. doi: 10.1378/chest.119.2_suppl.397s. [DOI] [PubMed] [Google Scholar]
- Kent B.N. Development of a novel plasmid as a shuttle vector for heterologous gene expression in Mycoplasma yeatsii. J. Microbiol. Methods. 2012;91:121–127. doi: 10.1016/j.mimet.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Khan S.A. Mechanism of replication and copy number control of plasmids in gram-positive bacteria. In: Setlow J.K., editor. vol. 18. Plenum Press; New York: 1996. (Genetic Engineering). [DOI] [PubMed] [Google Scholar]
- Khan S.A. Rolling-circle replication of bacterial plasmids. Microbiol. Mol. Biol. Rev. 1997;61:442–455. doi: 10.1128/mmbr.61.4.442-455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.A. Plasmid rolling-circle replication: recent developments. Mol. Microbiol. 2000;37:477–484. doi: 10.1046/j.1365-2958.2000.02001.x. [DOI] [PubMed] [Google Scholar]
- Khan S.A. DNA-protein interactions during the initiation and termination of plasmid pT181 rolling-circle replication. Prog. Nucleic Acid Res. Mol. Biol. 2003;75:113–137. doi: 10.1016/s0079-6603(03)75004-1. [DOI] [PubMed] [Google Scholar]
- Khan S.A. Plasmid rolling-circle replication: highlights of two decades of research. Plasmid. 2005;53:126–136. doi: 10.1016/j.plasmid.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Khan S.A. Replication of plasmid pT181 in vitro: requirement for a plasmid-encoded product. Proc. Natl. Acad. Sci. U.S.A. 1981;78:4902–4906. doi: 10.1073/pnas.78.8.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King K.W., Dybvig K. Nucleotide sequence of Mycoplasma mycoides subspecies mycoides plasmid pKMK1. Plasmid. 1992:86–91. doi: 10.1016/0147-619x(92)90039-d. [DOI] [PubMed] [Google Scholar]
- Koehler T.M., Thorne C.B. Bacillus subtilis (natto) plasmid pLS20 mediates interspecies plasmid transfer. J. Bacteriol. 1987;169:5271–5278. doi: 10.1128/jb.169.11.5271-5278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsel R.R. Sequence-specific interaction between the replication initiator protein of plasmid pT181 and its origin of replication. Proc. Natl. Acad. Sci. U.S.A. 1986;83:5484–5488. doi: 10.1073/pnas.83.15.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsel R.R. The replication initiator protein of plasmid pT181 has sequence-specific endonuclease and topoisomerase-like activities. Proc. Natl. Acad. Sci. U.S.A. 1985;82:6845–6849. doi: 10.1073/pnas.82.20.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A., Baker T. Freeman and Co.; New York: 1992. DNA Replication. [Google Scholar]
- Kramer M.G. Lagging-strand origins of the promiscuous plasmid pMV158: physical and functional characterization. Microbiology. 1995;141:655–662. doi: 10.1099/13500872-141-3-655. [DOI] [PubMed] [Google Scholar]
- Kramer M.G. Lagging strand replication of rolling-circle plasmids: specific recognition of the ssoA-type origins in different gram-positive bacteria. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10505–10510. doi: 10.1073/pnas.95.18.10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M.G. Characterization of a single-strand origin, ssoU, required for broad host range replication of rolling-circle plasmids. Mol. Microbiol. 1999;33:466–475. doi: 10.1046/j.1365-2958.1999.01471.x. [DOI] [PubMed] [Google Scholar]
- Kramer M.G. Plasmid rolling circle replication: identification of the RNA polymerase-directed primer RNA and requirement of DNA polymerase I for lagging strand initiation. EMBO J. 1997;16:5784–5795. doi: 10.1093/emboj/16.18.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M.G. Lagging-strand replication from the ssoA origin of plasmid pMV158 in Streptococcus pneumoniae: in vivo and in vitro influences of mutations in two conserved ssoA regions. J. Bacteriol. 1998;180:83–89. doi: 10.1128/jb.180.1.83-89.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S.A. Identification and analysis of genes for tetracycline resistance and replication functions in the broad-host-range plasmid pLS1. J. Mol. Biol. 1986;192:753–765. doi: 10.1016/0022-2836(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Lanka E., Wilkins B.M. DNA processing reactions in bacterial conjugation. Annu. Rev. Biochem. 1995;64:141–169. doi: 10.1146/annurev.bi.64.070195.001041. [DOI] [PubMed] [Google Scholar]
- Lederberg J., Tatum E. Gene recombination in E. coli. Nature. 1946;158:558. doi: 10.1038/158558a0. [DOI] [PubMed] [Google Scholar]
- Lederberg J., Tatum E. Sex in bacteria; genetic studies, 1945–1952. Science. 1953;118:169–175. doi: 10.1126/science.118.3059.169. [DOI] [PubMed] [Google Scholar]
- Lee C.A. The Bacillus subtilis conjugative transposon ICEBs1 mobilizes plasmids lacking dedicated mobilization functions. J. Bacteriol. 2012;194:3165–3172. doi: 10.1128/JB.00301-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Díaz F. The MobM-relaxase domain of plasmid pMV158: thermal stability and activity upon Mn2+ and DNA specific-binding. Nucleic Acids Res. 2011;39:4315–4329. doi: 10.1093/nar/gkr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Díaz F., Espinosa M. Lagging strand DNA replication origins are required for conjugal transfer of the promiscuous plasmid pMV158. J. Bacteriol. 2009;191:720–727. doi: 10.1128/JB.01257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-Díaz F. Autoregulation of the synthesis of the MobM relaxase encoded by the promiscuous plasmid pMV158. J. Bacteriol. 2012;194:1789–1799. doi: 10.1128/JB.06827-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M. Relaxase DNA binding and cleavage are two distinguishable steps in conjugative DNA processing that involve different sequence elements of the nic site. J. Biol. Chem. 2010;285:8918–8926. doi: 10.1074/jbc.M109.057539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa M. Bacterial conjugation: a two-step mechanism for DNA transport. Mol. Microbiol. 2002;45:1–8. doi: 10.1046/j.1365-2958.2002.03014.x. [DOI] [PubMed] [Google Scholar]
- Llosa M. Bacterial type IV secretion systems in human disease. Mol. Microbiol. 2009;73:141–151. doi: 10.1111/j.1365-2958.2009.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machón C. RepD-mediated recruitment of PcrA helicase at the Staphylococcus aureus pC221 plasmid replication origin, oriD. Nucleic Acids Res. 2010;38:1874–1888. doi: 10.1093/nar/gkp1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Arai K. Frpo: a novel single-stranded DNA promoter for transcription and for primer RNA synthesis of DNA replication. Cell. 1997;89:897–907. doi: 10.1016/s0092-8674(00)80275-5. [DOI] [PubMed] [Google Scholar]
- Matilla I. The conjugative DNA translocase TrwB is a structure-specific DNA-binding protein. J. Biol. Chem. 2010;285:17537–17544. doi: 10.1074/jbc.M109.084137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie T. The nucleotide sequence of pUB110: some salient features in relation to replication and its regulation. Plasmid. 1986;15:93–103. doi: 10.1016/0147-619x(86)90046-6. [DOI] [PubMed] [Google Scholar]
- Meijer W.J.J. Effects of the generation of single-stranded DNA on the maintenance of plasmid pMV158 and derivatives in different Bacillus subtilis strains. Plasmid. 1995;33:79–89. doi: 10.1006/plas.1995.1010. [DOI] [PubMed] [Google Scholar]
- Meijer W.J.J. Effects of the generation of single-stranded DNA on the maintenance of plasmid pMV158 and derivatives in Lactococcus lactis. Plasmid. 1995;33:91–99. doi: 10.1006/plas.1995.1011. [DOI] [PubMed] [Google Scholar]
- Moscoso M. Initiation of replication of plasmid pMV158: mechanisms of DNA strand transfer reactions mediated by the initiator RepB protein. J. Mol. Biol. 1997;268:840–856. doi: 10.1006/jmbi.1997.1012. [DOI] [PubMed] [Google Scholar]
- Murray R.W., Koepsel R.R., Khan S.A. Synthesis of single-stranded plasmid pT181 DNA in vitro. J. Biol. Chem. 1989;264:1051–1057. [PubMed] [Google Scholar]
- Naglich J.G., Andrews R.E.J. Tn916-dependent conjugal transfer of pC194 and pUB110 from Bacillus subtilis into Bacillus thuringiensis subsp. israelensis. Plasmid. 1988;20:113–126. doi: 10.1016/0147-619x(88)90014-5. [DOI] [PubMed] [Google Scholar]
- Nasim M.T. The activity of a single-stranded promoter of plasmid ColIb-P9 depends on its secondary structure. Mol. Microbiol. 2004;53:405–417. doi: 10.1111/j.1365-2958.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- Noirot P. Initiation of rolling circle replication in pT181 plasmid: initiator protein enhances cruciform extrusion at the origin. Proc. Natl. Acad. Sci. U.S.A. 1990;87:8560–8564. doi: 10.1073/pnas.87.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K. Control of replication of bacterial plasmids: genetics, molecular biology, and physiology of the plasmid R1 system. Plasmid. 1984;12:71–90. doi: 10.1016/0147-619x(84)90054-4. [DOI] [PubMed] [Google Scholar]
- Novick R.P. Staphylococcal plasmids and their replication. Annu. Rev. Microbiol. 1989;43:537–565. doi: 10.1146/annurev.mi.43.100189.002541. [DOI] [PubMed] [Google Scholar]
- Novick R.P. Contrasting lifestyles of rolling-circle phages and plasmids. Trends Biochem. Sci. 1998;23:434–438. doi: 10.1016/s0968-0004(98)01302-4. [DOI] [PubMed] [Google Scholar]
- Novick R.P. Staphylococcal plasmid cointegrates are formed by host- and phage-mediated general rec systems that act on short regions of homology. Mol. Gen. Genet. 1984;195:374–377. doi: 10.1007/BF00332777. [DOI] [PubMed] [Google Scholar]
- Ochman H. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:229–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- Osborn M. The evolution of bacterial plasmids. In: Thomas C.M., editor. Harwood Academic Publishers; Amsterdam: 2000. (The Horizontal Gene Pool). [Google Scholar]
- Oskam L. The large Bacillus plasmid pTB19 contains two integrated rolling-circle plasmids carrying mobilization functions. Plasmid. 1991;26:30–39. doi: 10.1016/0147-619x(91)90034-t. [DOI] [PubMed] [Google Scholar]
- Pansegrau W., Lanka E. Enzymology of DNA strand transfer by conjugative mechanisms. Prog. Nucleic Acid Res. Mol. Biol. 1996;54:197–251. doi: 10.1016/s0079-6603(08)60364-5. [DOI] [PubMed] [Google Scholar]
- Pansegrau W., Lanka E. Mechanisms of initiation and termination reactions in conjugative DNA processing: independence of tight substrate binding and catalytic activity of relaxase (TraI) of IncPα plasmid RP4. J. Biol. Chem. 1996;271:13068–13076. doi: 10.1074/jbc.271.22.13068. [DOI] [PubMed] [Google Scholar]
- Paulson J., Ehrenberg M. Trade-off between segregational stability and metabolic burden: a mathematical model of plasmid ColE1 replication control. J. Mol. Biol. 1998;279:73–88. doi: 10.1006/jmbi.1998.1751. [DOI] [PubMed] [Google Scholar]
- Pepper K. Heterogeneity of chromosomal genes encoding chloramphenicol resistance in streptococci. Plasmid. 1988;19:71–74. doi: 10.1016/0147-619x(88)90065-0. [DOI] [PubMed] [Google Scholar]
- Priebe S.D., Lacks S.A. Region of the streptococcal plasmid pMV158 required for conjugative mobilization. J. Bacteriol. 1989;171:4778–4784. doi: 10.1128/jb.171.9.4778-4784.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan S.J., Novick R.P. Comparative analysis of five related staphylococcal plasmids. Plasmid. 1988;19:203–221. doi: 10.1016/0147-619x(88)90039-x. [DOI] [PubMed] [Google Scholar]
- Puyet A. Identification of the origin and direction of replication of the broad-host-range plasmid pLS1. Nucleic Acids Res. 1988;16:115–133. doi: 10.1093/nar/16.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Lamothe R. High-copy bacterial plasmids diffuse in the nucleoid-free space, replicate stochastically and are randomly partitioned at cell division. Nucleic Acids Res. 2014;42:1042–1051. doi: 10.1093/nar/gkt918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Masó J.A. Genetic and biochemical characterization of the Streptococcus pneumoniae PcrA helicase and its role in plasmid rolling circle replication. J. Bacteriol. 2006;188:7416–7425. doi: 10.1128/JB.01010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Masó J.A. Interactions between the RepB initiator protein of plasmid pMV158 and two distant DNA regions within the origin of replication. Nucleic Acids Res. 2007;35:1230–1244. doi: 10.1093/nar/gkl1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegers J.F.M.L. Structural and functional analysis of the single-strand origin of replication from the lactococcal plasmid pWV01. Mol. Gen. Genet. 1995;249:43–50. doi: 10.1007/BF00290234. [DOI] [PubMed] [Google Scholar]
- Selinger L.B. Mobilization of closely related plasmids pUB110 and pBC16 by Bacillus plasmid pXO503 requires trans-acting open reading frame beta. J. Bacteriol. 1990;172:3290–3297. doi: 10.1128/jb.172.6.3290-3297.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert W. A novel replicon occurring naturally in Escherichia coli is a phage-plasmid hybrid. EMBO J. 1988;7:4005–4010. doi: 10.1002/j.1460-2075.1988.tb03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showsh S.A., Andrews R.E., Jr. Analysis of the requirement for a pUB110 mob region during Tn916-dependent mobilization. Plasmid. 1999;41:179–186. doi: 10.1006/plas.1999.1398. [DOI] [PubMed] [Google Scholar]
- Smillie C. Mobility of plasmids. Microbiol. Mol. Biol. Rev. 2010;74:434–452. doi: 10.1128/MMBR.00020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.C.A., Thomas C.D. An accessory protein is required for relaxosome formation by small staphylococcal plasmids. J. Bacteriol. 2004;186:3363–3373. doi: 10.1128/JB.186.11.3363-3373.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soultanas P. Plasmid replication initiator protein RepD increases the processivity of PcrA DNA helicase. Nucleic Acids Res. 1999;27:1421–1428. doi: 10.1093/nar/27.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Szpirer C.Y. Mobilization function of the pBHR1 plasmid, a derivative of the broad-host-range plasmid pBBR1. J. Bacteriol. 2001;183:2101–2110. doi: 10.1128/JB.183.6.2101-2110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H. Are single-stranded circles intermediates in plasmid DNA replication? EMBO J. 1986;5:631–637. doi: 10.1002/j.1460-2075.1986.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H. Single-stranded plasmid DNA in Bacillus subtilis and Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 1986;83:2541–2545. doi: 10.1073/pnas.83.8.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C.M. Harwood Academic Publishers; Amsterdam: 2000. The Horizontal Gene Pool. p. 419. [Google Scholar]
- Thomas C.M., Nielsen K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- Thomas J. A conserved helicase processivity factor is needed for conjugation and replication of an integrative and conjugative element. PLoS Genet. 2013;9:e1003198. doi: 10.1371/journal.pgen.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon N., Moineau S. Isolation and characterization of a Streptococcus thermophilus plasmid closely related to the pMV158 family. Plasmid. 2001;45:171–183. doi: 10.1006/plas.2001.1517. [DOI] [PubMed] [Google Scholar]
- van der Lelie D. Similarity of minus origins of replication and flanking open reading frames of plasmids pUB110, pTB913 and pMV158. Nucleic Acids Res. 1989;17:7283–7294. doi: 10.1093/nar/17.18.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lelie D. Conjugal mobilization of streptococcal plasmid pMV158 between strains of Lactococcus lactis subsp. lactis. J. Bacteriol. 1990;172:47–52. doi: 10.1128/jb.172.1.47-52.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A. Welcome to the plasmidome. Nat. Rev. Microbiol. 2012;10:379. doi: 10.1038/nrmicro2804. [DOI] [PubMed] [Google Scholar]
- Waters V.L., Guiney D.G. Processes at the nick region link conjugation, T-DNA transfer and rolling-circle replication. Mol. Microbiol. 1993;9:1123–1130. doi: 10.1111/j.1365-2958.1993.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Wellington E.M. The role of the natural environment in the emergence of antibiotic resistance in gram-negative bacteria. Lancet Infect. Dis. 2013;13:155–165. doi: 10.1016/S1473-3099(12)70317-1. [DOI] [PubMed] [Google Scholar]
- Wilkins B., Lanka E. Plenum Press; New York: 1993. DNA Processing and Replication During Plasmid Transfer Between Gram-Negative Bacteria. [Google Scholar]
- Woodbury R.L. Plasmid-borne erm(T) from invasive, macrolide-resistant Streptococcus pyogenes strains. Antimicrob. Agents Chemother. 2008;52:1140–1143. doi: 10.1128/AAC.01352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao F. UGA is read as tryptophan in Mycoplasma capricolum. Proc. Natl. Acad. Sci. U.S.A. 1985;82:2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa H. Rolling-circle replication of the plasmid pKYM isolated from a gram-negative bacterium. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10282–10286. doi: 10.1073/pnas.88.22.10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S. Functional analysis of the plasmid pM4 replicon from Lactobacillus plantarum M4: determination of the minimal replicon and functionality identification of the putative sso. Plasmid. 2009;62:166–171. doi: 10.1016/j.plasmid.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Zechner E.L. Conjugative-DNA transfer processes. In: Thomas C.M., editor. The Horizontal Gene Pool. Harwood Academic publishers; Amsterdam: 2000. [Google Scholar]
- Zhai Z. Characterization of a novel rolling-circle replication plasmid pYSI8 from Lactobacillus sakei YSI8. Plasmid. 2009;62:30–34. doi: 10.1016/j.plasmid.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Zhang W. Type-IVC secretion system: a novel subclass of type IV secretion system (T4SS) common existing in gram-positive genus Streptococcus. PLoS ONE. 2012;7:e46390. doi: 10.1371/journal.pone.0046390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H. Characterization of a rolling-circle replication plasmid pXY3 from Lactobacillus plantarum XY3. Plasmid. 2010;64:36–40. doi: 10.1016/j.plasmid.2010.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


