Abstract
Background
Miscarriage is a prevalent public health issue and many events occur before women are aware of their pregnancy, complicating research design. Thus, risk factors for miscarriage are critically understudied. Our goal was to identify environmental chemicals with a high number of interactions with miscarriage genes, based on known toxicogenomic responses.
Methods
We used miscarriage (MeSH: D000022) and chemical gene lists from the Comparative Toxicogenomics Database in human, mouse, and rat. We assessed enrichment for gene ontology biological processes among the miscarriage genes. We prioritized chemicals (n=25) found at Superfund sites or in the blood or urine pregnant women. For chemical-disease gene sets of sufficient size (n=13 chemicals, n=20 comparisons), chi-squared enrichment tests and proportional reporting ratios (PRR) were calculated. We cross-validated enrichment results.
Results
Miscarriage was annotated with 121 genes and overrepresented in inflammatory response (q=0.001), collagen metabolic process (q=1×10−13), cell death (q=0.02), and vasculature development (q=0.005) pathways. The number of unique genes annotated to a chemical ranged from 2 (bromacil) to 5,607 (atrazine). In humans, all chemicals tested were highly enriched for miscarriage gene overlap (all p<0.001; parathion PRR=7, cadmium PRR=6.5, lead PRR=3.9, arsenic PRR=3.5, atrazine PRR=2.8). In mice, highest enrichment (p<0.001) was observed for naphthalene (PRR=16.1), cadmium (PRR=12.8), arsenic (PRR=11.6), and carbon tetrachloride (PRR=7.7). In rats, we observed highest enrichment (p<0.001) for cadmium (PRR=8.7), carbon tetrachloride (PRR=8.3), and dieldrin (PRR=5.3). Our findings were robust to 1,000 permutations each of variable gene set sizes.
Conclusion
We observed chemical gene sets (parathion, cadmium, naphthalene, carbon tetrachloride, arsenic, lead, dieldrin, and atrazine) were highly enriched for miscarriage genes. Exposures to chemicals linked to miscarriage, and thus linked to decreased probability of live birth, may limit the inclusion of fetuses susceptible to adverse birth outcomes in epidemiology studies. Our findings have critical public health implications for successful pregnancies and the interpretation of adverse impacts of environmental chemical exposures on pregnancy.
Keywords: Miscarriage, spontaneous abortion, computational toxicology, toxicogenomics, environmental chemicals, prenatal
1. Introduction
Early pregnancy loss through miscarriage, also known as spontaneous abortion, is clinically defined as spontaneous pregnancy loss prior to 20 weeks of gestation (McNair and Altman 2011). Early pregnancy loss is the most common complication of pregnancy. Estimates of the rate of pregnancies that end in miscarriage typically range from 15 to 20% (Agenor and Bhattacharya 2015; Krieg et al. 2016); however, these values do not include miscarriages or implantation failures that occur before the mother is aware of pregnancy. When these early losses are considered, estimates for early pregnancy loss grow to as high as 50–70% of all pregnancies (Jeve and Davies 2014; Macklon et al. 2002; Salker et al. 2010) and can be an important cause of subfertility or infertility.
Successful pregnancy requires a complex interplay between immunological, hormonal, and genetic processes (Weselak et al. 2008). A large proportion of miscarriages are explained by chromosomal abnormalities of the offspring, particularly aneuploidy: chromosomal abnormalities affect 50% of first trimester and 20% of second trimester miscarried fetuses (Choi et al. 2014; Larsen et al. 2013; Tsuiko et al. 2018). Maternal conditions also influence miscarriage risk. Strong causal associations for miscarriage have been identified for acquired maternal thrombophilia and thyroid autoimmunity, as well as other immune or inflammatory related processes (Larsen et al. 2013). Maternal use of substances such as tobacco, cocaine and alcohol during pregnancy have shown significant associations with miscarriage (Ness et al. 1999; Weselak et al. 2008). Environmental toxicant exposures are also associated with miscarriage, including exposure to the pesticide DDT and the heavy metal arsenic (Krieg et al. 2016). Moreover, early pregnancy loss and adverse pregnancy outcomes occur at higher rates in and around contaminated waste sites such as landfills and in areas with drinking water contamination or high air pollution (Grippo et al. 2018; Vrijheid 2000). Relatively little is known, however, about the ~80,000 chemicals currently in commerce, and whether they contribute to the risk of miscarriage (Xia et al. 2018). Importantly, exposures are modifiable risk factors and represent an opportunity for miscarriage prevention.
Adverse pregnancy outcomes and reproductive toxicity endpoints are particularly challenging to evaluate in any single in vitro or in vivo model because they result from multiple complex and interrelated biological processes. Interpreting data from animal models can be challenging due to anatomical differences in the female reproductive tract, number of fetuses per pregnancy and endocrinology of pregnancy and parturition across species (Grigsby 2016; Malassiné et al. 2003; Napso et al. 2018). In particular, there are key differences between rodents and humans in physiological events involved in pregnancy and parturition, such as differences in the production of cytokines and prostaglandins and in placental structure and function (Clark 2014; Faas et al. 2005; Moffett and Loke 2006; Schmidt et al. 2015). While humans are the most relevant species for miscarriage investigation, studies in humans are limited due to ethical and other considerations. Animal studies have the advantage of controlled, multidose experimental designs, which can provide better insight into mechanisms of toxicity. No single animal model is ideal for studying miscarriage in humans, nonetheless, animal models such as mouse and rat can provide much needed insight into molecular mechanisms and/or signaling pathways underpinning chemical-miscarriage associations. For example, exposure to the trihalomethane compound bromodichloromethane is associated with miscarriage in epidemiology studies (Savitz et al. 2006; Waller et al. 1998). Bromodichloromethane also causes pregnancy loss in rat, likely by disrupting luteinizing hormone signaling (Bielmeier et al. 2001). Luteinizing hormone signaling is also highly relevant in human pregnancy and disruptions initially identified in another species can potentially be translated to humans.
Women seeking to become pregnant are exposed to mixtures of chemicals (Woodruff et al. 2011), providing another layer of complexity in hazard identification. Moreover, because women may experience pregnancy loss before they are aware of their pregnancy status, failure to account for a toxicant’s impact on miscarriage can bias potential associations between that toxicant and a birth outcome (Liew et al. 2015). For example, when testing an association between air pollution exposure during pregnancy and autism spectrum disorder diagnosis in children, restricting analyses to live births created a situation with two potential selection-bias processes at play: 1) the chance of live birth was influenced by both air pollution exposure as well as another risk factor for autism; and 2) the depletion of fetuses susceptible to autism from the high exposure group (Raz et al. 2018). Thus, various factors create challenges for evaluating chemical risks for adverse pregnancy outcomes. The current analysis is a proof-of-concept study designed to leverage toxicology results in multiple species to prioritize miscarriage-associated chemicals for follow up research.
Rigorous interrogation of chemicals and potential pathways involved in miscarriage requires integration of findings from multiple model systems as well as human data. The Comparative Toxicogenomics Database (CTD), developed by North Carolina State University and the National Institute for Environmental Health Sciences, provides a useful tool for such an analysis. The CTD features millions of curated gene-chemical, gene-disease and chemical-disease relationships as well as various other functions (Davis et al. 2017; Davis et al. 2018). To focus our analysis on a set of environmental chemicals relevant to a specific geographical location, we selected 25 chemicals found in maternal blood/urine samples, groundwater, tap water, or at Superfund sites in Puerto Rico, a region with elevated adverse pregnancy outcomes (Martin et al. 2018). Using a combination of CTD data and our own statistical approach, which included stratifying analysis by species and cross-validation, we evaluated the genes associated with each chemical and tested them for enrichment with genes annotated to the CTD disease term “Spontaneous Abortion”. To elucidate potential molecular or cellular mechanisms by which these chemicals could increase risk for miscarriage, we identified Gene Ontology defined Biological Processes associated with specific chemical-gene interactions for a subset of chemicals. The goals of this study were to: 1) identify chemicals associated with increased risk of miscarriage, based on toxicogenomic responses across multiple species (human, mouse and rat) using the CTD; 2) identify chemical impacts on specific molecular targets and cellular pathways involved in miscarriage; and 3) identify targets/pathways commonly impacted by multiple chemicals, suggesting potential toxicant mixtures of concern for this endpoint.
2. Methods
2.1. Datasets
Background gene lists for human, mouse and rat species
We stratified our analyses by species to compare chemical-gene-disease associations across several species while maintaining biological coherence across the diverse array of studies included in the CTD. The symbols for all genes annotated to each species (Homo sapiens, Mus musculus and Rattus norvegicus) were downloaded from the Comparative Toxicogenomics Database (CTD) (date of download 7/30/18). We then verified that the annotated genes were listed as “active” for each species in the PubMed gene database (queried 8/2/18) (Lu 2011). This returned a total of 42,830 human, 34,321 mouse and 25,150 rat genes.
Spontaneous abortion gene list
We downloaded CTD genes linked to the clinical term for miscarriage, “Spontaneous Abortion” (Medical Subject Heading (MeSH) Identifier: D000022, date of download: 7/12/18). This list contains 121 curated human genes. Of these, 111 have known mouse homologs and 112 have known rat homologs. All statistical tests were conducted using human, mouse or rat species data as separate, independent analyses. Because CTD disease terms are curated using a hierarchic annotation system (Davis et al. 2012), we also identified and quantified genes belonging to additional “child” disease terms contained below the “parent” term of “Spontaneous Abortion” (see Supplementary Table 1).
Chemical gene lists
We selected an optimized number of 25 chemicals for analysis to allow for: 1) analysis of multiple, diverse chemical classes (e.g. metals, organophosphate pesticides, volatile organic compounds, etc.); and 2) feasibility of downstream follow up analysis of chemicals enriched with miscarriage genes. We focused our analysis on chemicals relevant to exposures in the population of greatest concern for the miscarriage endpoint (i.e., pregnant women) within a specific geographical location (Puerto Rico), a region with high rates of adverse pregnancy outcomes (Martin et al. 2018) and extensive contamination with Superfund chemicals (USEPA, 2018). Therefore, we selected chemicals based on their presence in maternal blood/urine samples, groundwater, tap water, Superfund sites or reported usage in Puerto Rico. The total list of 25 chemicals included heavy metals [arsenic, cadmium, lead and chromium (Padilla et al. 2011)]; phthalate esters [dibutyl phthalate, diethyl phthalate and diethylhexyl phthalate (Cantonwine et al. 2014; Padilla et al. 2011)], volatile organic compounds [perchloroethylene, trichloroethylene, carbon tetrachloride, chloroform and methylene chloride (Padilla et al. 2011)], pesticides [aldrin, atrazine, bromacil, dieldrin, glyphosate, methyl parathion and parathion (Irizarry et al. 1989; Marnio et al. 2016; Padilla et al. 2011)], phenols [triclosan and benzophenone-3 (Meeker et al. 2013)], polycyclic aromatic hydrocarbons [naphthalene, phenanthrene and pyrene (Cathey et al. 2018)] and a personal care product [N,N-Diethyl-meta-toluamide (Lewis et al. 2014)]. We compared our a priori selected chemicals to the full list of chemicals associated with the “Spontaneous Abortion” term in the CTD online portal and quantified overlap with our list. Gene lists for these chemicals were downloaded for human, mouse and rat from the CTD website on 9/29/18. Bromacil was dropped for further analysis because it had an exceptionally small gene list (n=2 genes).
2.2. Statistical tests
Enrichment testing of chemical gene lists and miscarriage genes
All analyses were conducted in R statistical software. R markdown code used to conduct analyses and to generate figures and tables is publicly available (https://github.com/bakulskilab), facilitating future applications of the workflow to other exposures and outcomes. We conducted univariate descriptive statistics on each species-specific chemical gene list. Within species, we generated 2×2 descriptive tables for genes associated with each chemical by genes associated with miscarriage. A minimum number of chemical-gene annotations were required to meet the assumptions to conduct statistical tests for enrichment with miscarriage genes (required expected frequency of genes per cell required to be greater than one). Therefore, based on the gene distributions for each chemical and each species, we selected appropriate enrichment test statistics as follows. When expected frequencies per cell were greater than or equal to five, enrichment testing was conducted using standard chi-square test for independence. When the expected frequency of genes per cell was less than five and greater than one, we used the ‘N-1’ chi-squared test (Campbell 2007). Chemicals with expected frequency less than one in any cell of the 2×2 table were dropped from further analysis for that species. Out of the 72 possible comparisons (24 remaining chemicals x three species), we proceeded with 20 enrichment tests that met the chi-square or ‘N-1’ chi-square assumptions. See Supplementary Figure 1 for a flow chart illustrating chemical inclusions/exclusions by species. We considered p<0.05 to be suggestively enriched and a Bonferroni correction was used to account for multiple comparisons (n=20 tests; p<0.0025).
Sensitivity analyses
We used Fisher’s exact tests to confirm chemical-disease gene enrichment associations observed in the primary analyses. Because the gene sample size was always greater than 1,000 in this study, we prioritized the chi-squared test and used Fisher exact test as a comparison. For all chemicals showing significant gene-disease/gene-chemical associations, the proportional reporting ratio (PRR) was calculated to identify the direction and magnitude of enrichment (i.e. more overlapping genes than expected by chance) or depletion (i.e. fewer overlapping genes than expected by chance). A PRR>1 indicates enrichment while a PRR<1 indicates depletion (PRR=1 indicates neither).
Cross-validation of enrichment results
To assess the susceptibility of the miscarriage gene list to false-positive results, we performed cross-validation for enrichment testing with miscarriage genes. We randomly permuted 1000 pseudo-chemical gene lists each of specific sizes (100, 500, 1000, 2500, 5000 and 10000 genes) to reflect the range of genes associated with chemicals in our dataset. Genes in these random lists were then tested for enrichment with the exact miscarriage genes. We calculated the number of permuted tests meeting our significance criteria by chance. We visualized the patterns in results with a density plot of observed association p-values.
Gene Ontology enrichment analysis of miscarriage gene list
To gain insight into specific biological functions represented in the 121 genes contained in the CTD term “Spontaneous Abortion” we identified enriched Gene Ontology terms represented by the 121 miscarriage genes. For statistical transparency, assumption testing, and scripting reproducibility, we tested gene ontology enrichment with a commonly used tool (DAVID) (Huang et al. 2009a, b). In addition, we used the REVIGO web based platform (Supek et al. 2011) to exclude redundant Gene Ontology terms. The 121 miscarriage gene symbols were used as input for enrichment testing in DAVID and “Homo sapiens” was used as the background or reference gene list. We then removed redundant Gene Ontology terms from DAVID results using REVIGO (Supek et al. 2011). As further confirmation of our results, we also analyzed the miscarriage genes using the gene set enrichment (“Set Analyzer”) tool in the CTD online portal.
Identification of chemical-gene associations for enriched miscarriage Gene Ontology terms and Gene Ontology enrichment analysis of chemical gene lists
Based on enrichment testing results, we prioritized a subset of five chemicals that were enriched from the human chemical data (arsenic, cadmium, lead, atrazine and parathion). We visualized overlap in miscarriage genes interacting with these chemicals using a heatmap. Gene membership in each of four selected miscarriage Gene Ontology terms was highlighted. Although we prioritized chemicals that were enriched in human, we also conducted this analysis for chemicals enriched in mouse and rat data. Finally, we performed gene ontology analysis using DAVID and the genes annotated to our prioritized chemicals in our order to identify biological pathways likely to be affected by these chemicals.
3. Results
3.1. Gene-chemical associations
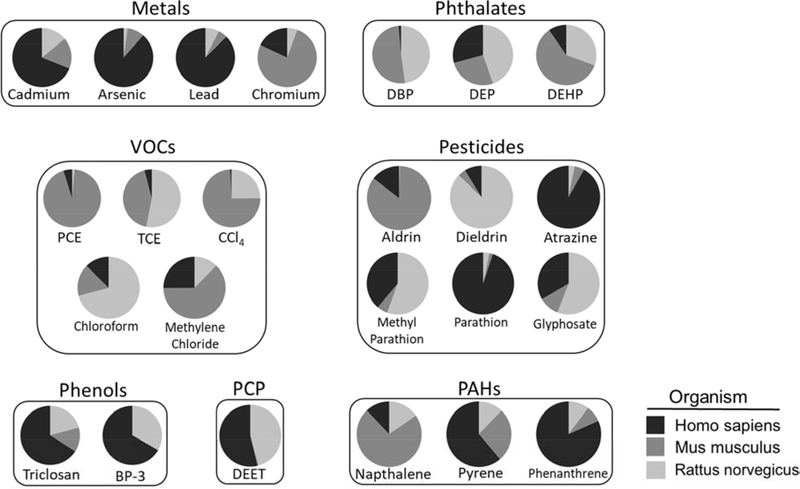
We surveyed 25 chemicals for their associated genes in the CTD and noted that the number of unique genes per chemical ranged from two (bromacil) to 5,607 (atrazine). We observed a mean of 1,441 genes per chemical and a median of 746 genes per chemical (Supplementary Table 1). Figure 1 shows the proportion of genes associated with each chemical varied by species (human, rat, mouse). Parathion had the highest proportion of genes (95%) obtained from human studies and carbon tetrachloride had the lowest proportion (0.7%). Twenty-three out of the 25 chemicals analyzed included annotated genomic data from all three species. The exceptions were benzophenone-3 with genes annotated to human and rat and bromacil with genes annotated in humans only.
Figure 1. Proportion of genes obtained for each chemical by species.
Lists of genes interacting with 24 different chemicals in any of three different species (Homo sapiens, Mus musculus and Rattus norvegicus) were downloaded from the Comparative Toxicogenomics Database (date of download: 7/30/18). The proportion of genes associated with each species varied across compounds. For example, among genes annotated to Homo sapiens, parathion had the highest (95%) and carbon tetrachloride the lowest (0.7%) proportion of genes. VOCs: volatile organic carbons, PCE: perchloroethylene, TCE: trichloroethylene, CCl4: carbon tetrachloride, BP-3: benzophenone-3, DEET: N,N-Diethyl-meta-toluamide, PCP: personal care product, PAHs: polycyclic aromatic hydrocarbons (To be printed in black and white.)
Some chemicals could not be tested for enrichment in miscarriage genes in each of the three target species due to an insufficient number of annotated genes. These included bromacil, which was removed from consideration for all species. In addition, we excluded chemicals with insufficient annotated genes for 19 human, 15 mouse, and 18 rat chemical analyses. This left 13 total chemicals tested for enrichment with miscarriage genes in at least one species. These chemicals were: cadmium, arsenic, lead, chromium, DBP, DEHP, PCE, TCE, CCl4, dieldrin, atrazine, parathion and naphthalene.
3.2. Enrichment testing for chemical associations with miscarriage
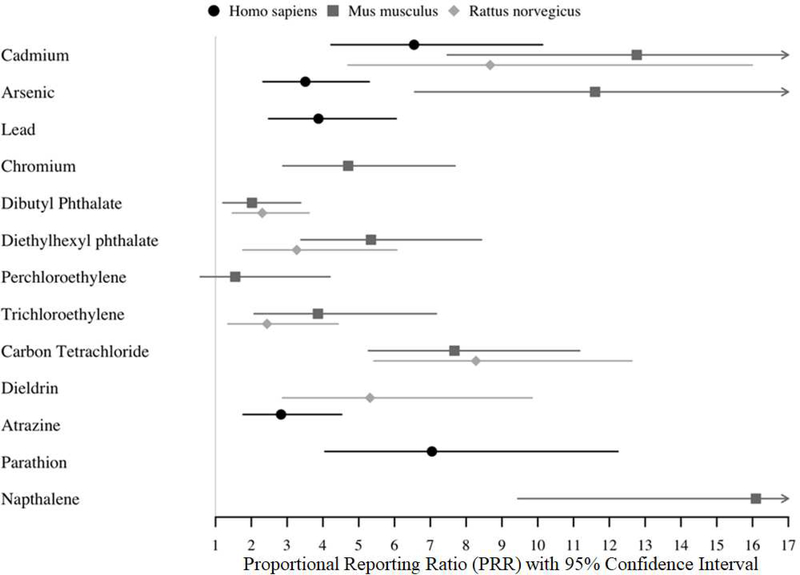
Twelve of the 13 chemicals tested were enriched with miscarriage genes (corrected chi-squared p<0.05) in one or more species. Perchloroethylene and DBP were tested in the mouse but not significantly enriched (Figure 2 and Supplementary Table 3). Cadmium was the only chemical tested in all three species and it was enriched in miscarriage genes in all three species. Of the chemicals tested, five were enriched with miscarriage genes for human, seven for mouse and six for rat. Metals had the highest percentage of chemicals with 4/4 (100%) enriched for miscarriage genes in at least one species. This was followed by phthalates with 2/3 (66.7%) enriched compounds, then pesticides (3/6, 50%), VOCs (2/5, 40%), PAHs (1/3, 33%), phenols (0/2, 0%) and the personal pesticide product DEET (0/1, 0%). The PPRs>1 for these associations reflect higher risk of miscarriage due to more overlap with miscarriage genes (as opposed to depletion or lower risk) (Figure 2). The highest PRRs were observed for arsenic (11.6), cadmium (12.8) and naphthalene (16.1). The confidence intervals for some chemicals were relatively large (e.g. cadmium, dieldrin) (Figure 2 and Supplementary Table 3) suggest a higher degree uncertainty around the magnitude of enrichment for these compounds. No chemical associations were observed with PPR<1, which would have indicated a protective or null association between that chemical and miscarriage.
Figure 2. Proportional reporting ratios (PRRs) and confidence intervals for chemical associations with miscarriage genes.
Proportional reporting ratios were calculated for chemicals significantly enriched with miscarriage genes to identify the direction and magnitude of enrichment or depletion. Errors show 95% confidence intervals. (To be printed in black and white.)
As a sensitivity environment-wide analysis in the CTD online portal, we observed 5,337 chemicals were linked to the term “Spontaneous Abortion”, either through curated association or through an inferred association via a gene. Among our 25 chemicals selected a priori, 4 (16%) were among the CTD curated associations and 18 (72%) were among the CTD inferred associations with this term. The chemicals with curated associations were arsenic, DEHP, DEP and triclosan.
3.3. Cross-validation
We randomly permuted human gene sets (n=100, 500, 100, 2500, or 5000) and tested for enrichment in the miscarriage genes. We observed 0.37% of tests achieved chi-square corrected p-value<0.05 across all gene set sizes and 0.34% achieved Fisher’s test corrected p-value<0.05. For randomly permuted mouse gene data, 0.39% of tests achieved chi-square corrected p-value<0.05 and 0.40% achieved Fisher’s test corrected p-value<0.05. For randomly permuted rat gene data, 0.40% of tests achieved chi-square corrected p<0.05 and 0.35% of tests achieved Fisher’s test corrected p-value<0.05. Cross-validation results across all p-value thresholds for human data are shown in Supplementary Figure 2.
3.4. Functional analysis of Spontaneous Abortion gene list
Among the 121 “Spontaneous Abortion” genes, 7 (6%) were annotated to the term “Abortion, Habitual”, 3 (2%) were annotated to “Uterine Cervical Incompetence”, 73 (60%) were annotated to “Abortion, Veterinary”, 117 (97%) were annotated to “Embryo Loss”, 2 (1.7%) were annotated to “Preimplantation Embryonic Lethality”, and 3 (2%) were annotated to “Pregnancy Loss, Susceptibility To”. Supplementary Table 1 shows all 121 Spontaneous Abortion genes and parent-child term hierarchical relationships in the CTD. Among the 121 miscarriage genes, 68 (56%) were linked to at least one of the five prioritized chemicals. There were 2 (1.7%) genes linked to all five prioritized chemicals and 33 (27%) genes linked to only one of the five prioritized chemicals. The 68 genes impacted by at least one of the five chemicals included genes coding for: cytokines (IL11, IL16, IL1β, IL20RA, IL24, IL25RA, IL6, IL9, TGF-β1, TNSF10 and TNSF13), matrix metalloproteinases/tissue inhibitors of metalloproteinases (MMPs 11, 15, 19 and TIMPs 2 and 3), insulin like growth factors (IGF1, IGF2, IGFBP3 and IGFBP6), collagen (COL1A1, COL 5A1, COL6A1 and COL6A3) and receptors (AHR, PRLR).
Pathway enrichment analysis of the 121 miscarriage genes identified 143 enriched Gene Ontology terms (FDR<0.05). After removal of redundant terms using REVIGO, 60 enriched terms remained. Consistent with the current understanding of the etiology of miscarriage, enriched terms included processes associated with inflammation and immune processes (“inflammatory response”, GO:0006954, q=0.001; “immune response”, GO:0006955, q=0.0001), collagen metabolism (“collagen metabolic process”, GO:0032963, q=1×10−13), cell death (“cell death”, GO:0008219, q=0.02) and tissue development (“vasculature development”, GO:0001944, q=0.0005; “skeletal system development”, GO:0001501, q=0.02). Based on these results, we identified the following enriched Gene Ontology terms for functional analysis: “collagen metabolic process” (GO:0032963), “inflammatory response” (GO:0006954), “cell death” (GO:0008219) and “vasculature development” (GO:0001944). These terms were also identified as significantly enriched among the miscarriage genes when we conducted analysis using the CTD online portal “set analyzer” function (data not shown) and are phenotypes associated with spontaneous abortion in the CTD (see Supplementary Table 5, date of download 1/13/20).
3.5. Overlap of genes targeted by enriched chemicals and functional analysis
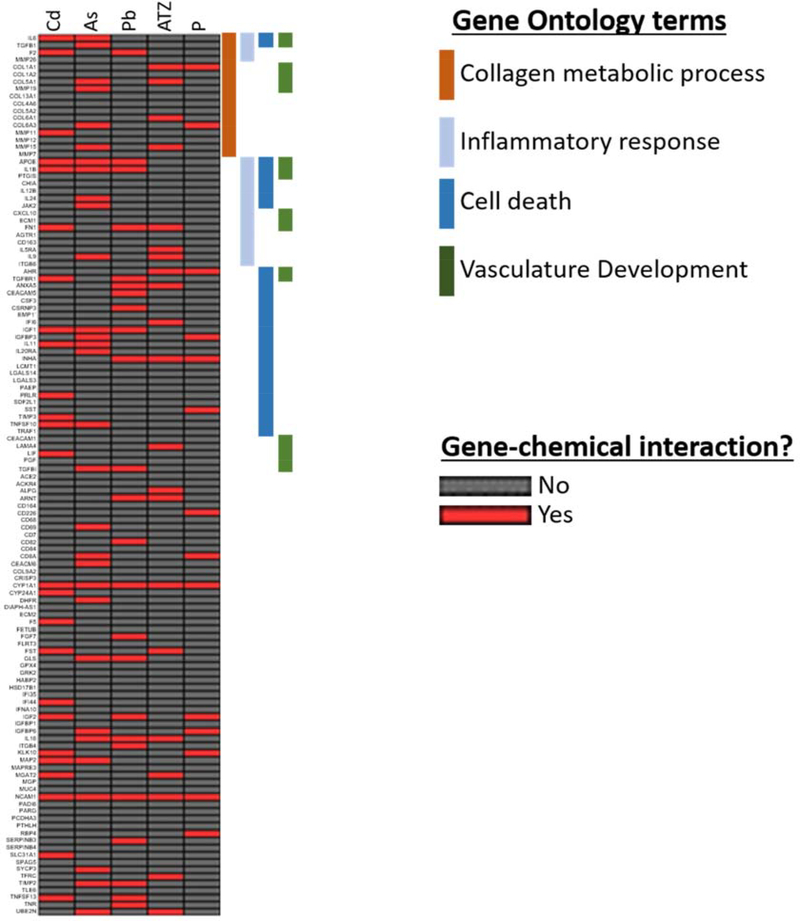
We prioritized the five chemicals enriched in the human data for further study: arsenic, cadmium, lead, atrazine and parathion. Each of the five chemicals are shown to impact multiple genes in the miscarriage gene list (Figure 3 and Supplementary Table 4). Some genes are impacted by multiple chemicals, such as CYP1A1 and NCAM1 that were impacted by all five chemicals. Other genes are unique to specific chemicals, such as IL24 and JAK2 (specific to arsenic) or COL6A1 and LAMA4 (specific to atrazine). We observed chemical-gene interactions across all four of the Gene Ontology terms investigated. Genes did not appear to cluster by any particular Gene Ontology process or chemical. CYP1A1 and NCAM1 were not involved in the Gene Ontology processes investigated. We observed similar results in the mouse and rat data (Supplementary Figures 3 and 4), with most chemicals impacting genes in at >1 gene in all four of the relevant biological processes. In contrast to the human data, we observed no genes that were impacted by all of the chemicals enriched in the mouse or rat.
Figure 3. Chemical-gene interactions for 121 miscarriage genes and associations with selected Gene Ontology terms.
Chemical interactions for 121 miscarriage genes were plotted for 5 chemicals (Cd: cadmium, As: arsenic, Pb: lead, ATZ: atrazine and P: parathion). Associations with select Gene Ontology terms relevant to the etiology of miscarriage (collagen metabolic process, inflammatory response, cell death and vasculature development) were plotted in parallel in order to identify potential toxicological mechanisms linking chemical exposure to miscarriage. (To be printed in color.)
3.6. Functional analysis of chemical gene lists
We conducted pathway analysis for chemical-gene interactions for the five prioritized chemicals using DAVID and identified significantly enriched biological processes among all five chemical gene lists. Arsenic was associated with 2,381 significantly enriched processes (p<0.05), cadmium with 2,372, lead with 1,525, atrazine with 1,074 and parathion with 835. Two of the relevant processes enriched for miscarriage genes (“cell death” and “vasculature development”) were also enriched for lead, atrazine and parathion while four (“collagen metabolism”, “inflammatory response”, “cell death” and “vasculature development”) were enriched for arsenic and cadmium (see Table 1).
Table 1.
Relevant Gene Ontology defined Biological Processes enriched in chemical gene lists and among Spontaneous Abortion genes. Values reported are fold enrichment (p-value). n/a: process not enriched.
| Chemicals | Collagen metabolic process | Inflammatory response | Cell death | Vasculature Development |
|---|---|---|---|---|
| Arsenic | 1.6 (0.002) | 1.6 (1.4*10−13) | 1.5 (1.9*10−30) | 1.7 (2.9*10−17) |
| Cadmium | 2.3 (3.9*10−4) | 2.7 (5.3*10−29) | 1.9 (9.1*10−35) | 2.2 (6.7*10−17) |
| Lead | n/a | n/a | 1.3 (7*10−8) | 1.3 (0.001) |
| Atrazine | n/a | n/a | 1.3 (1*10−8) | 1.3 (8*10−4) |
| Parathion | n/a | n/a | 1.3 (0.003) | 1.5 (0.01) |
| Spontaneous Abortion genes | 22.0 (5.5*10−17) | 4.2 (5.4*10−7) | 2.3 (1.1*10−5) | 4.4 (2.9*10−7) |
| Fold enrichment (p-value) | ||||
4. Discussion
Miscarriage is a prevalent public health issue and risk factors for miscarriage are critically understudied. The present study evaluated a set of chemicals detected in Superfund sites and in pregnant women for associations with molecular markers of miscarriage using rigorous analyses of publicly available toxicogenomic data. Chemicals from multiple classes, including metals, pesticides, volatile organic carbons and polycyclic aromatic hydrocarbons, affected more miscarriage genes than would be expected by chance in at least one of the species studied (human, mouse or rat). These findings were robust to permutation testing. Given the diversity in chemical structures that were enriched with miscarriage genes and the diversity of chemicals known or suspected to cause miscarriage (Krieg et al. 2016), it seems unlikely that a generalizable set of characteristics will identify chemicals that present a hazard for this endpoint.
Miscarriage is an important consideration for reproductive epidemiology studies. When investigating postnatal health effects, increased rates of miscarriage may bias results. Specifically in environmental epidemiology, chemical exposures causing miscarriage may “deplete susceptible” fetuses, resulting in apparent null or even protective associations between chemical exposures and postnatal health effects (Weisskopf et al. 2015). That is, individuals included in pregnancy cohorts with successful births may be a select group of individuals resistant to the toxicant induced health effects whereas those more sensitive are traditionally excluded (Weisskopf et al. 2015). Indeed, chemically sensitive pregnancies may be lost before subject recruitment into epidemiology studies. Quantitative bias analysis may be needed to assess the potential influence of live birth bias in birth outcome or developmental studies (Lash et al. 2014; Weuve et al. 2018). Our results suggest that several chemicals could generate such biases.
Our results are consistent with epidemiological studies showing associations between heavy metal exposure and miscarriage. Specifically, our study shows that arsenic, cadmium, chromium and lead were highly enriched with miscarriage genes. Consistent with these results, meta-analyses of epidemiological literature concluded that high levels of arsenic exposure (>50 ppb) are associated with miscarriage and that plausible mechanisms exist for arsenic causing miscarriage due to its endocrine disrupting properties (Milton et al. 2017; Rahman et al. 2016). Lead levels in the hair of pregnant woman were associated with risk of missed abortion (loss of pregnancy with retention of products of conception) (Zhao et al. 2017). Similarly, increased incidence of miscarriage has also been reported in women with exposure to chromium (Remy et al. 2017). Animal studies found that cadmium decreases incidence of pregnancy through decreased implantation and fetal resorptions (Jacobo-Estrada et al. 2017). A biological basis for impacts on pregnancy outcomes is suggested by cadmium’s ability to decrease levels of hormones necessary to maintain pregnancy, such as progesterone (Jacobo-Estrada et al. 2017; Nampoothiri and Gupta 2008; Piasek and Laskey 1994). However, Buck-Louis et al. reported no association with pregnancy loss at environmentally relevant levels of exposure to cadmium in humans (Buck Louis et al. 2017). The results of our study contribute to the body of evidence that exposure to arsenic, cadmium, chromium and lead constitute a risk factor for miscarriage, although the threshold of exposure for increased risk is not well characterized.
Two of the phthalates tested were enriched for miscarriage genes, with DBP enriched for rat and DEHP enriched for mouse. These results are consistent with several reports in human observational studies. Both DBP and DEHP levels in maternal hair samples were associated with missed abortion (Zhao et al. 2017). In addition, urinary levels of the main DEHP metabolite (monoethylhexyl phthalate) were higher in women with missed abortion (Yi et al. 2016) and in women with pregnancy loss undergoing medically assisted reproduction (Messerlian et al. 2016; Toft et al. 2012; Zhao et al. 2017). In another study, a DBP metabolite (monobutyl phthalate) was not associated with recurrent miscarriage. However, a structurally similar phthalate (mono-isobutyl phthalate) did show a significant association (Peng et al. 2016). Phthalates are endocrine disruptors (Qureshi et al. 2016), and several phthalates stimulate oxidative stress (Tetz et al. 2013; Wang et al. 2010), inflammation (Wang et al. 2010) and epigenetic changes (Grindler et al. 2018; Strakovsky and Schantz 2018) in gestational tissues and cells in culture, suggesting potential mechanisms explaining associations with miscarriage. These studies are consistent with our observation that DBP was enriched for miscarriage genes in the rat data. However, we did not observe an association between DBP and miscarriage genes in the mouse. Species differences in phthalate reproductive effects are known to vary between mouse and rat (Johnson et al. 2012). We were not able to conduct a statistical test using human DBP data due to a small available gene list, reflecting a lack of available human gene data.
There is epidemiological evidence that exposure to solvents, including carbon tetrachloride and trichloroethylene, is associated with low birth weight and birth defects (Bove et al. 1995; Forand et al. 2012; Ruckart et al. 2014). Carbon tetrachloride induced abortion in a rat model (Narotsky et al. 1997), consistent with our finding that carbon tetrachloride was enriched with miscarriage genes. Although epidemiological and experimental data link the pesticides atrazine, dieldrin and parathion (enriched for miscarriage in our study) to some adverse pregnancy outcomes, evidence for miscarriage is mixed. For example, the spouses of workers who used higher levels of atrazine had an increased risk of miscarriage (Petrelli et al. 2003), though a recent systematic review of the literature found a cumulative lack of evidence linking atrazine to adverse birth outcomes, including miscarriage (Goodman et al. 2014). Dieldrin was associated with adverse outcomes in pregnancy, such as gestational hypertension (Savitz et al. 2014) and altered thyroid hormone levels in cord blood (Luo et al. 2017). However, another study observed maternal serum dieldrin levels were not associated with between missed abortion (Bercovici et al. 1983). Parathion did not affect the number of live fetuses per litter in rats (Keith et al. 2017), but one small study in pregnant women (n=20) showed altered placental morphology in an exposed group, an impact that could have implications for miscarriage (Levario-Carrillo et al. 2001). It is possible that the solvent and pesticide enrichment for genomic markers of miscarriage identified in our study reflects responses in gestational tissues not linked to miscarriage specifically but that may have implications for other pregnancy outcomes.
Miscarriage genes impacted by one or more of the five prioritized chemicals represent a diverse array of signaling/functional pathways including cytokines, matrix metalloproteinases and insulin like growth factors (IGFs). These observations provide a potential mechanistic link between chemical exposures and miscarriage. Miscarriage is linked with altered levels of circulating matrix metalloproteinases (Castruita-De la Rosa et al. 2019; Nissi et al. 2013) as well as altered cytokine levels in the gestational compartment (Calleja-Agius et al. 2012; Giannubilo et al. 2012). Less is known about IGFs and miscarriage, but one study showed that women with polycystic ovarian syndrome who miscarried had increased levels of circulating and decidual IGFs (Luo et al. 2016).
Miscarriage genes were enriched in the GO processes “collagen metabolic process”, “inflammatory response”, “cell death”, and “vasculature development”. These four processes are listed as phenotypes associated with spontaneous abortion in the CTD and are consistent with known etiologies of miscarriage (date of download 1/13/20). Some or all of these processes were also enriched for all five of our prioritized chemicals, supporting the biological plausibility of these chemicals causing miscarriage via alteration of these pathways. For example, we observed miscarriage genes were enriched for “collagen metabolic process”, consistent with research showing that women with a history of miscarriages had decreased levels of collagen types IV and V in the decidua (Iwahashi et al. 1996; Iwahashi and Nakano 1998). Notably, several of the enriched metals have been shown to disrupt collagen metabolism in animal models. Specifically, arsenic exposure (50 ppb in drinking water) decreased collagen gene expression and disrupted collagen deposition in the lungs and heart of mice (Hays et al. 2008), cadmium disrupted collagen metabolism in the bones of rats (Galicka et al. 2004) and lead disrupts collagen metabolism in the brain capillaries of calves (Ahrens 1993). Less is known about the effects of the pesticides atrazine and parathion on collagen metabolism, but a study of male reproductive effects of atrazine exposure in rats noted reduced testicular collagen fiber (Kniewald et al. 2000). Exposure to these the chemicals may increase risk for miscarriage by disrupting collagen metabolism in the decidua.
Miscarriage genes were also enriched for the inflammatory response pathway. Although, the role of inflammation in implantation, pregnancy, and parturition is complex, an overall shift from Th2 (anti-inflammatory) to Th1 (pro-inflammatory) cytokines in the decidua is a proposed miscarriage cause (Berger 2000; Romero et al. 2004). Furthermore, cytokines like interferon-γ (IFNγ), IL-2 and TNFα are increased in women with recurrent miscarriage (Ng et al. 2002; Pandey et al. 2005). In humans, exposure to arsenic (Ahmed et al. 2011; Dutta et al. 2015; Prasad and Sinha 2017), cadmium (Everson et al. 2018; Lin et al. 2009; Messner and Bernhard 2010), and lead (Machon-Grecka et al. 2018; Sirivarasai et al. 2013; Wang et al. 2009) are associated with increased markers of inflammation, including in placental gestational tissues. For example, cadmium increases blood levels of complement component 5 fragment (C5a), which is associated with miscarriage (Jacobo-Estrada et al. 2017; Zhang et al. 2016). Parathion exposure was linked to asthma (characterized by airway inflammation) in farmworkers (Hoppin et al. 2009). Atrazine caused inflammation in the prostate of rats (Scialli et al. 2014). We have not yet noted evidence of parathion or atrazine causing inflammation in gestational tissues.
Cell death was another pathway enriched among miscarriage genes. Apoptosis and cell death are consistently associated with miscarriage. Placentas from women with recurrent miscarriage have increased levels of apoptosis compared to women with normal pregnancies (Atia 2017). Toxic chemicals such as polycyclic aromatic hydrocarbons (PAHs) induced apoptotic pathways, leading to embryo loss in mice, a proposed mechanism explaining higher miscarriage rates in women who smoke (Detmar et al. 2006). Arsenic and atrazine also cause apoptosis in mouse embryos (Liu et al. 2003; Scialli et al. 2014), and lead and cadmium induce apoptosis in rat placenta (Erboga and Kanter 2016; Wang et al. 2014), consistent with our results.
Finally, miscarriage genes and genes annotated to all five chemicals in the human data were enriched for vasculature development. Vasculature development at the fetal-maternal interface is critical for pregnancy. Women undergoing miscarriage have decreased vascularization in placental chorionic villi (Meegdes et al. 1988). Arsenic exposure disrupts placental vascularization in mouse models, leading to miscarriage (He et al. 2007). In non-gestational tissues, cadmium disrupts vasculature development in zebrafish (Cheng et al. 2001) while lead disrupts the structure of the blood brain barrier in developing rats, resulting in capillary leakage into the brain parenchyma (Wang et al. 2007). Atrazine or parathion both interact with multiple genes in the vasculature development pathway, but there is currently little reported evidence for their effects on vasculature development in gestational tissues.
All of the chemicals enriched with miscarriage genes interacted with genes across in one or more of the Gene Ontology terms we studied. This trend was observed across all three species we investigated. Our results highlight that diverse chemicals can affect multiple molecular targets within biological pathways relevant to miscarriage, and that publicly available databases can be used to “scan” a wide range of chemicals for interactions with these targets. This approach could prove useful for analyzing complex mixtures of chemicals found at Superfund or hazardous waste sites. Strengths of this study and our statistical approach include the ability to screen a large number of chemicals for potential risks to a complex toxicological endpoint in a relatively short amount of time. Moreover, by analyzing the pathways enriched among miscarriage genes we were able to gain insight into specific mechanisms by which chemicals could contribute to this disorder, providing more biological coherence to our interpretations than would be achieved by simply testing chemicals for associations with disease terms. In addition, before conducting our enrichment tests, we performed cross-validation in order to verify that the miscarriage gene list was resistant to false-positives. Our results showed overlap with findings from the online CTD portal, which presented an important positive control. Our methods improved statistical transparency of the calculations and involve species specific evaluation (while the CTD portal results were species agnostic). Finally, by conducting enrichment testing in each of the three species (human, mouse and rat), we were able to focus our downstream analysis on chemicals that were most relevant to currently available human data (i.e., cadmium, arsenic, lead, atrazine and parathion) and follow up with chemicals enriched in other species in subsequent analysis. This approach allowed us to identify cadmium as being enriched for miscarriage across human, mouse and rat, illustrating a potentially conserved response across several mammalian species.
There are several limitations to the current study that should be noted. The results presented demonstrate statistical associations between genes impacted by chemicals and those involved in miscarriage. This analysis was not able to address matters of dose-response or directionality of gene expression changes (up- or down-regulation). The lack of tissue specificity is another limitation that should be noted, as the chemical-gene lists were obtained from a variety of studies using various tissues which may not reflect the chemical-gene interactions occurring in reproductive tissues relevant to the physiological processes of miscarriage. It should also be noted that chemicals that were dropped from analysis due to an insufficient number of gene-chemical annotations are not necessarily irrelevant to miscarriage, but rather may be understudied compared to chemicals with a larger number of curated gene interactions. Future studies will address these limitations, through use of cell models or additional databases.
Taken together, our results contribute to the body of evidence that exposure to several diverse chemicals, including metals and pesticides, may constitute a risk factor for miscarriage. Our results show that publicly available databases, such as the CTD, can be used to screen a large number of chemicals for effects on molecular responses related to this complex reproductive endpoint. This approach identified multiple compounds that affect more miscarriage genes than would be expected by chance and provided insight into which cellular pathways are targeted by these toxicants. Moreover, our findings provided insight into disease-chemical relationships and common modes of action across chemicals. This approach could be extended to identify potential mixture effects (synergistic, additive, etc.) for risk for miscarriage by identifying toxicants that target similar cellular pathways. Finally, our findings have critical implications for the design and interpretation of pregnancy cohort analyses with environmental exposures because chemicals contributing to miscarriage could lead to sensitive pregnancies being lost before recruitment into epidemiology studies.
Supplementary Material
Highlights.
Miscarriage is a prevalent public health challenge that is difficult to study
Pregnant women are commonly exposed to multiple chemical types
Miscarriage influences inflammation, collagen, vasculature, and cell death pathways
Genes and pathways influenced by chemicals overlap with those influenced by miscarriage
Chemicals may influence risk of miscarriage through multiple pathways
Funding acknowledgements
This research was supported by the Michigan Center on Lifestage Environmental Exposures and Disease (P30 ES017885). Drs. Harris, Meeker, Padilla, and Loch-Caruso were supported by a research grant from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (P42 ES017198). Dr. Bakulski was supported by research grants from the NIEHS (R01 ES025531; R01 ES025574), National Institute of Aging, NIH (R01 AG055406) and National Institute on Minority Health and Health Disparities, NIH (R01 MD013299). Additional funding for Dr. Harris was provided by the National Center for Advancing Translational Sciences (UL1TR002240). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, NIH or the University of Michigan.
Abbreviations
- PRR
proportional reporting ratio
- CTDm
Comparative Toxicogenomics Database
- GO
Gene Ontology
- As
arsenic
- Cd
cadmium
- Pb
lead
- Cr
chromium
- DBP
dibutyl phthalate
- DEP
diethyl phthalate
- DEHP
diethylhexyl phthalate
- PCE
perchloroethylene
- TCE
trichloroethylene
- CCl4
carbon tetrachloride
- ATZ
atrazine
- P
parathion
- BP-3
benzophenone-3
- DEET
N,N-Diethyl-meta-toluamide
Footnotes
Competing Financial Interests:
The authors declare they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Agenor A, Bhattacharya S. 2015. Infertility and miscarriage: Common pathways in manifestation and management. Womens Health (Lond) 11:527–541. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Mahabbat-e Khoda S, Rekha RS, Gardner RM, Ameer SS, Moore S, et al. 2011. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environmental health perspectives 119:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens FA. 1993. Effects of lead on glucose metabolism, ion flux, and collagen synthesis in cerebral capillaries of calves. Am J Vet Res 54:808–812. [PubMed] [Google Scholar]
- Atia TA. 2017. Placental apoptosis in recurrent miscarriage. Kaohsiung J Med Sci 33:449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovici B, Wassermann M, Cucos S, Ron M, Wassermann D, Pines A. 1983. Serum levels of polychlorinated biphenyls and some organochlorine insecticides in women with recent and former missed abortions. Environ Res 30:169–174. [DOI] [PubMed] [Google Scholar]
- Berger A 2000. Th1 and th2 responses: What are they? BMJ 321:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielmeier SR, Best DS, Guidici DL, Narotsky MG. 2001. Pregnancy loss in the rat caused by bromodichloromethane. Toxicological sciences : an official journal of the Society of Toxicology 59:309–315. [DOI] [PubMed] [Google Scholar]
- Bove FJ, Fulcomer MC, Klotz JB, Esmart J, Dufficy EM, Savrin JE. 1995. Public drinking water contamination and birth outcomes. Am J Epidemiol 141:850–862. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Smarr MM, Sundaram R, Steuerwald AJ, Sapra KJ, Lu Z, et al. 2017. Low-level environmental metals and metalloids and incident pregnancy loss. Reproductive toxicology 69:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja-Agius J, Jauniaux E, Muttukrishna S. 2012. Inflammatory cytokines in maternal circulation and placenta of chromosomally abnormal first trimester miscarriages. Clin Dev Immunol 2012:175041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I 2007. Chi-squared and fisher-irwin tests of two-by-two tables with small sample recommendations. Stat Med 26:3661–3675. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, et al. 2014. Urinary phthalate metabolite concentrations among pregnant women in northern puerto rico: Distribution, temporal variability, and predictors. Environ Int 62:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castruita-De la Rosa C, Garza-Veloz I, Delgado-Enciso I, Olivas-Chavez JC, Cardenas-Vargas E, Rodriguez-Sanchez IP, et al. 2019. Spontaneous abortion is preceded by an altered serum concentration of matrix metalloproteinases. J Matern Fetal Neonatal Med:1–9. [DOI] [PubMed] [Google Scholar]
- Cathey A, Ferguson KK, McElrath TF, Cantonwine DE, Pace G, Alshawabkeh A, et al. 2018. Distribution and predictors of urinary polycyclic aromatic hydrocarbon metabolites in two pregnancy cohort studies. Environ Pollut 232:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SH, Chan PK, Wu RS. 2001. The use of microangiography in detecting aberrant vasculature in zebrafish embryos exposed to cadmium. Aquat Toxicol 52:61–71. [DOI] [PubMed] [Google Scholar]
- Choi TY, Lee HM, Park WK, Jeong SY, Moon HS. 2014. Spontaneous abortion and recurrent miscarriage: A comparison of cytogenetic diagnosis in 250 cases. Obstet Gynecol Sci 57:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA. 2014. The use and misuse of animal analog models of human pregnancy disorders. Journal of reproductive immunology 103:1–8. [DOI] [PubMed] [Google Scholar]
- Davis AP, Wiegers TC, Rosenstein MC, Mattingly CJ. 2012. Medic: A practical disease vocabulary used at the comparative toxicogenomics database. Database (Oxford) 2012:bar065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AP, Grondin CJ, Johnson RJ, Sciaky D, King BL, McMorran R, et al. 2017. The comparative toxicogenomics database: Update 2017. Nucleic acids research 45:D972–D978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AP, Grondin CJ, Johnson RJ, Sciaky D, McMorran R, Wiegers J, et al. 2018. The comparative toxicogenomics database: Update 2019. Nucleic acids research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmar J, Rabaglino T, Taniuchi Y, Oh J, Acton BM, Benito A, et al. 2006. Embryonic loss due to exposure to polycyclic aromatic hydrocarbons is mediated by bax. Apoptosis 11:1413–1425. [DOI] [PubMed] [Google Scholar]
- Dutta K, Prasad P, Sinha D. 2015. Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. Int J Hyg Environ Health 218:564–574. [DOI] [PubMed] [Google Scholar]
- Erboga M, Kanter M. 2016. Effect of cadmium on trophoblast cell proliferation and apoptosis in different gestation periods of rat placenta. Biol Trace Elem Res 169:285–293. [DOI] [PubMed] [Google Scholar]
- Everson TM, Punshon T, Jackson BP, Hao K, Lambertini L, Chen J, et al. 2018. Cadmium-associated differential methylation throughout the placental genome: Epigenome-wide association study of two u.S. Birth cohorts. Environmental health perspectives 126:017010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas MM, Bouman A, Veenstra van Nieuwenhoven AL, van der Schaaf G, Moes H, Heineman MJ, et al. 2005. Species differences in the effect of pregnancy on lymphocyte cytokine production between human and rat. J Leukoc Biol 78:946–953. [DOI] [PubMed] [Google Scholar]
- Forand SP, Lewis-Michl EL, Gomez MI. 2012. Adverse birth outcomes and maternal exposure to trichloroethylene and tetrachloroethylene through soil vapor intrusion in new york state. Environmental health perspectives 120:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicka A, Brzoska MM, Sredzinska K, Gindzienski A. 2004. Effect of cadmium on collagen content and solubility in rat bone. Acta Biochim Pol 51:825–829. [PubMed] [Google Scholar]
- Giannubilo SR, Landi B, Pozzi V, Sartini D, Cecati M, Stortoni P, et al. 2012. The involvement of inflammatory cytokines in the pathogenesis of recurrent miscarriage. Cytokine 58:50–56. [DOI] [PubMed] [Google Scholar]
- Goodman M, Mandel JS, DeSesso JM, Scialli AR. 2014. Atrazine and pregnancy outcomes: A systematic review of epidemiologic evidence. Birth Defects Res B Dev Reprod Toxicol 101:215–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby PL. 2016. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin Reprod Med 34:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindler NM, Vanderlinden L, Karthikraj R, Kannan K, Teal S, Polotsky AJ, et al. 2018. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women. Sci Rep 8:6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo A, Zhang J, Chu L, Guo Y, Qiao L, Zhang J, et al. 2018. Air pollution exposure during pregnancy and spontaneous abortion and stillbirth. Rev Environ Health 33:247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays AM, Lantz RC, Rodgers LS, Sollome JJ, Vaillancourt RR, Andrew AS, et al. 2008. Arsenic-induced decreases in the vascular matrix. Toxicol Pathol 36:805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Greenwell RJ, Brooks DM, Calderon-Garciduenas L, Beall HD, Coffin JD. 2007. Arsenic exposure in pregnant mice disrupts placental vasculogenesis and causes spontaneous abortion. Toxicological sciences : an official journal of the Society of Toxicology 99:244–253. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Coble J, et al. 2009. Pesticide use and adult-onset asthma among male farmers in the agricultural health study. Eur Respir J 34:1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. 2009a. Systematic and integrative analysis of large gene lists using david bioinformatics resources. Nat Protoc 4:44–57. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. 2009b. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research 37:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry H, Edmundo R, Beauchamp de Caloni I, Guadalupe R. 1989. Performance of elite banana (musa acuminata, aaa) cultivars in four locations of puerto rico. The Journal of Agriculture of the University of Puerto Rico 73:209–221. [Google Scholar]
- Iwahashi M, Muragaki Y, Ooshima A, Nakano R. 1996. Decreased type iv collagen expression by human decidual tissues in spontaneous abortion. The Journal of clinical endocrinology and metabolism 81:2925–2929. [DOI] [PubMed] [Google Scholar]
- Iwahashi M, Nakano R. 1998. Decreased type v collagen expression in human decidual tissues of spontaneous abortion during early pregnancy. J Clin Pathol 51:44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo-Estrada T, Santoyo-Sanchez M, Thevenod F, Barbier O. 2017. Cadmium handling, toxicity and molecular targets involved during pregnancy: Lessons from experimental models. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeve YB, Davies W. 2014. Evidence-based management of recurrent miscarriages. J Hum Reprod Sci 7:159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KJ, Heger NE, Boekelheide K. 2012. Of mice and men (and rats): Phthalate-induced fetal testis endocrine disruption is species-dependent. Toxicological sciences : an official journal of the Society of Toxicology 129:235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith S, Williams M, Fay M, Wilson J, Llados F, Carlson-Lynch H, et al. 2017. Toxicological profile for parathion.U.S. Department of Health and Human Services. [Google Scholar]
- Kniewald J, Jakominic M, Tomljenovic A, Simic B, Romac P, Vranesic D, et al. 2000. Disorders of male rat reproductive tract under the influence of atrazine. J Appl Toxicol 20:61–68. [PubMed] [Google Scholar]
- Krieg SA, Shahine LK, Lathi RB. 2016. Environmental exposure to endocrine-disrupting chemicals and miscarriage. Fertil Steril 106:941–947. [DOI] [PubMed] [Google Scholar]
- Larsen EC, Christiansen OB, Kolte AM, Macklon N. 2013. New insights into mechanisms behind miscarriage. BMC Med 11:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. 2014. Good practices for quantitative bias analysis. Int J Epidemiol 43:1969–1985. [DOI] [PubMed] [Google Scholar]
- Levario-Carrillo M, Feria-Velasco A, De Celis R, Ramos-Martinez E, Cordova-Fierro L, Solis FJ. 2001. [DOI] [PubMed] [Google Scholar]
- Parathion, a cholinesterase-inhibiting plaguicide induces changes in tertiary villi of placenta of women exposed: A scanning electron microscopy study. Gynecol Obstet Invest 52:269–275. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Cantonwine DE, Anzalota Del Toro LV, Calafat AM, Valentin-Blasini L, Davis MD, et al. 2014. Urinary biomarkers of exposure to insecticides, herbicides, and one insect repellent among pregnant women in puerto rico. Environ Health 13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Olsen J, Cui X, Ritz B, Arah OA. 2015. Bias from conditioning on live birth in pregnancy cohorts: An illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol 44:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Rathod D, Ho WC, Caffrey JJ. 2009. Cadmium exposure is associated with elevated blood c-reactive protein and fibrinogen in the u. S. Population: The third national health and nutrition examination survey (nhanes iii, 1988–1994). Ann Epidemiol 19:592–596. [DOI] [PubMed] [Google Scholar]
- Liu L, Trimarchi JR, Navarro P, Blasco MA, Keefe DL. 2003. Oxidative stress contributes to arsenic-induced telomere attrition, chromosome instability, and apoptosis. J Biol Chem 278:31998–32004. [DOI] [PubMed] [Google Scholar]
- Lu Z 2011. Pubmed and beyond: A survey of web tools for searching biomedical literature. Database (Oxford) 2011:baq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Pu Y, Tian H, Wu W, Sun X, Zhou T, et al. 2017. Association of in utero exposure to organochlorine pesticides with thyroid hormone levels in cord blood of newborns. Environ Pollut 231:78–86. [DOI] [PubMed] [Google Scholar]
- Luo L, Wang Q, Chen M, Yuan G, Wang Z, Zhou C. 2016. Igf-1 and igfbp-1 in peripheral blood and decidua of early miscarriages with euploid embryos: Comparison between women with and without pcos. Gynecol Endocrinol 32:538–542. [DOI] [PubMed] [Google Scholar]
- Machon-Grecka A, Dobrakowski M, Kasperczyk A, Birkner E, Pryzwan T, Kasperczyk S. 2018. The effect of subacute lead exposure on selected blood inflammatory biomarkers and angiogenetic factors. J Occup Health 60:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon NS, Geraedts JP, Fauser BC. 2002. Conception to ongoing pregnancy: The ‘black box’ of early pregnancy loss. Hum Reprod Update 8:333–343. [DOI] [PubMed] [Google Scholar]
- Malassiné A, Frendo JL, Evain‐Brion D. 2003. A comparison of placental development and endocrine functions between the human and mouse model. Human Reproduction Update 9:531–539. [DOI] [PubMed] [Google Scholar]
- Marnio YA, Perez M, Gallardo F, Trifilio M, Cruz M, Bayman P. 2016. Sun vs. Shade affects infestation, total population and sex ratio of the coffee berry borer (hypothenemus hampei) in puerto rico. Agriculture, Ecosystems & Environment 222:258–266. [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. 2018. Births: Final data for 2016. Natl Vital Stat Rep 67:1–55. [PubMed] [Google Scholar]
- McNair T, Altman K. 2011. Miscarriage and recurrent pregnancy loss. In: The johns hopkins manual of gynecology and obstetrics, (Hurt KJ, Guile MW, Bienstock JL, Fox HE, Wallach EE, eds), 438–439. [Google Scholar]
- Meegdes BH, Ingenhoes R, Peeters LL, Exalto N. 1988. Early pregnancy wastage: Relationship between chorionic vascularization and embryonic development. Fertil Steril 49:216–220. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. 2013. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in puerto rico. Environ Sci Technol 47:3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Wylie BJ, Minguez-Alarcon L, Williams PL, Ford JB, Souter IC, et al. 2016. Urinary concentrations of phthalate metabolites and pregnancy loss among women conceiving with medically assisted reproduction. Epidemiology 27:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner B, Bernhard D. 2010. Cadmium and cardiovascular diseases: Cell biology, pathophysiology, and epidemiological relevance. Biometals 23:811–822. [DOI] [PubMed] [Google Scholar]
- Milton AH, Hussain S, Akter S, Rahman M, Mouly TA, Mitchell K. 2017. A review of the effects of chronic arsenic exposure on adverse pregnancy outcomes. Int J Environ Res Public Health 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett A, Loke C. 2006. Immunology of placentation in eutherian mammals. Nat Rev Immunol 6:584–594. [DOI] [PubMed] [Google Scholar]
- Nampoothiri LP, Gupta S. 2008. Biochemical effects of gestational coexposure to lead and cadmium on reproductive performance, placenta, and ovary. J Biochem Mol Toxicol 22:337–344. [DOI] [PubMed] [Google Scholar]
- Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. 2018. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Frontiers in Physiology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narotsky MG, Brownie CF, Kavlock RJ. 1997. Critical period of carbon tetrachloride-induced pregnancy loss in fischer-344 rats, with insights into the detection of resorption sites by ammonium sulfide staining. Teratology 56:252–261. [DOI] [PubMed] [Google Scholar]
- Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, et al. 1999. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med 340:333–339. [DOI] [PubMed] [Google Scholar]
- Ng SC, Gilman-Sachs A, Thaker P, Beaman KD, Beer AE, Kwak-Kim J. 2002. Expression of intracellular th1 and th2 cytokines in women with recurrent spontaneous abortion, implantation failures after ivf/et or normal pregnancy. Am J Reprod Immunol 48:77–86. [DOI] [PubMed] [Google Scholar]
- Nissi R, Talvensaari-Mattila A, Kotila V, Niinimaki M, Jarvela I, Turpeenniemi-Hujanen T. 2013. Circulating matrix metalloproteinase mmp-9 and mmp-2/timp-2 complex are associated with spontaneous early pregnancy failure. Reproductive biology and endocrinology : RB&E 11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla I, Irizarry C, Steele K. 2011. Historical contamination of groundwater resources in the north coast karst aquifers of puerto rico. Rev Dimens 3:7–12. [PMC free article] [PubMed] [Google Scholar]
- Pandey MK, Rani R, Agrawal S. 2005. An update in recurrent spontaneous abortion. Arch Gynecol Obstet 272:95–108. [DOI] [PubMed] [Google Scholar]
- Peng F, Ji W, Zhu F, Peng D, Yang M, Liu R, et al. 2016. A study on phthalate metabolites, bisphenol a and nonylphenol in the urine of chinese women with unexplained recurrent spontaneous abortion. Environ Res 150:622–628. [DOI] [PubMed] [Google Scholar]
- Petrelli G, Figa-Talamanca I, Lauria L, Mantovani A. 2003. Spontaneous abortion in spouses of greenhouse workers exposed to pesticides. Environ Health Prev Med 8:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasek M, Laskey JW. 1994. Acute cadmium exposure and ovarian steroidogenesis in cycling and pregnant rats. Reproductive toxicology 8:495–507. [DOI] [PubMed] [Google Scholar]
- Prasad P, Sinha D. 2017. Low-level arsenic causes chronic inflammation and suppresses expression of phagocytic receptors. Environ Sci Pollut Res Int 24:11708–11721. [DOI] [PubMed] [Google Scholar]
- Qureshi MS, Yusoff AR, Wirzal MD, Sirajuaddin, Barek J, Afridi HI, et al. 2016. Methods for the determination of endocrine-disrupting phthalate esters. Crit Rev Anal Chem 46:146–159. [DOI] [PubMed] [Google Scholar]
- Rahman A, Kumarathasan P, Gomes J. 2016. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Sci Total Environ 569–570:1022–1031. [DOI] [PubMed] [Google Scholar]
- Raz R, Kioumourtzoglou MA, Weisskopf MG. 2018. Live-birth bias and observed associations between air pollution and autism. Am J Epidemiol 187:2292–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy LL, Byers V, Clay T. 2017. Reproductive outcomes after non-occupational exposure to hexavalent chromium, willits california, 1983–2014. Environ Health 16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Mazor M. 2004. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril 82:799–804. [DOI] [PubMed] [Google Scholar]
- Ruckart PZ, Bove FJ, Maslia M. 2014. Evaluation of contaminated drinking water and preterm birth, small for gestational age, and birth weight at marine corps base camp lejeune, north carolina: A cross-sectional study. Environ Health 13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, et al. 2010. Natural selection of human embryos: Impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PloS one 5:e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Singer PC, Herring AH, Hartmann KE, Weinberg HS, Makarushka C. 2006. Exposure to drinking water disinfection by-products and pregnancy loss. Am J Epidemiol 164:1043–1051. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Klebanoff MA, Wellenius GA, Jensen ET, Longnecker MP. 2014. Persistent organochlorines and hypertensive disorders of pregnancy. Environ Res 132:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Morales-Prieto DM, Pastuschek J, Frohlich K, Markert UR. 2015. Only humans have human placentas: Molecular differences between mice and humans. Journal of reproductive immunology 108:65–71. [DOI] [PubMed] [Google Scholar]
- Scialli AR, DeSesso JM, Breckenridge CB. 2014. Developmental toxicity studies with atrazine and its major metabolites in rats and rabbits. Birth Defects Res B Dev Reprod Toxicol 101:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirivarasai J, Wananukul W, Kaojarern S, Chanprasertyothin S, Thongmung N, Ratanachaiwong W, et al. 2013. Association between inflammatory marker, environmental lead exposure, and glutathione s-transferase gene. Biomed Res Int 2013:474963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakovsky RS, Schantz SL. 2018. Impacts of bisphenol a (bpa) and phthalate exposures on epigenetic outcomes in the human placenta. Environ Epigenet 4:dvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek F, Bosnjak M, Skunca N, Smuc T. 2011. Revigo summarizes and visualizes long lists of gene ontology terms. PloS one 6:e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetz LM, Cheng AA, Korte CS, Giese RW, Wang P, Harris C, et al. 2013. Mono-2-ethylhexyl phthalate induces oxidative stress responses in human placental cells in vitro. Toxicology and applied pharmacology 268:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft G, Jonsson BA, Lindh CH, Jensen TK, Hjollund NH, Vested A, et al. 2012. Association between pregnancy loss and urinary phthalate levels around the time of conception. Environmental health perspectives 120:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuiko O, Jatsenko T, Parameswaran Grace LK, Kurg A, Vermeesch JR, Lanner F, et al. 2018. A speculative outlook on embryonic aneuploidy: Can molecular pathways be involved? Dev Biol. USEPA. National priorities list (npl) sites - by state. Available: https://www.epa.gov/superfund/national-priorities-list-npl-sites-state#PR [accessed July, 30, 2018 2018]. [DOI] [PubMed]
- Vrijheid M 2000. Health effects of residence near hazardous waste landfill sites: A review of epidemiologic literature. Environmental health perspectives 108 Suppl 1:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller K, Swan SH, DeLorenze G, Hopkins B. 1998. Trihalomethanes in drinking water and spontaneous abortion. Epidemiology 9:134–140. [PubMed] [Google Scholar]
- Wang Q, Luo W, Zheng W, Liu Y, Xu H, Zheng G, et al. 2007. Iron supplement prevents lead-induced disruption of the blood-brain barrier during rat development. Toxicology and applied pharmacology 219:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Shang L, Wang J, Wu N, Wang S. 2010. Effect of phthalate esters on the secretion of prostaglandins (f2alpha and e2) and oxytocin in cultured bovine ovarian and endometrial cells. Domest Anim Endocrinol 39:131–136. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hu H, Li H, Ma H, Xu F, Qu B. 2014. Effects of lead exposure on placental cellular apoptosis and endoplasmic reticulum stress in rats. Chin Med J (Engl) 127:1744–1748. [PubMed] [Google Scholar]
- Wang YY, Sui KX, Li H, Ma HY. 2009. The effects of lead exposure on placental nf-kappab expression and the consequences for gestation. Reproductive toxicology 27:190–195. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Sparrow D, Hu H, Power MC. 2015. Biased exposure-health effect estimates from selection in cohort studies: Are environmental studies at particular risk? Environmental health perspectives 123:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weselak M, Arbuckle TE, Walker MC, Krewski D. 2008. The influence of the environment and other exogenous agents on spontaneous abortion risk. Journal of toxicology and environmental health Part B, Critical reviews 11:221–241. [DOI] [PubMed] [Google Scholar]
- Weuve J, Sagiv SK, Fox MP. 2018. Quantitative bias analysis for collaborative science. Epidemiology 29:627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. 2011. Environmental chemicals in pregnant women in the united states: Nhanes 2003–2004. Environmental health perspectives 119:878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Huang R, Shi Q, Boyd WA, Zhao J, Sun N, et al. 2018. Comprehensive analyses and prioritization of tox21 10k chemicals affecting mitochondrial function by in-depth mechanistic studies. Environmental health perspectives 126:077010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H, Gu H, Zhou T, Chen Y, Wang G, Jin Y, et al. 2016. A pilot study on association between phthalate exposure and missed miscarriage. Eur Rev Med Pharmacol Sci 20:1894–1902. [PubMed] [Google Scholar]
- Zhang Q, Huang Y, Zhang K, Huang Y, Yan Y, Wang F, et al. 2016. Cadmium-induced immune abnormality is a key pathogenic event in human and rat models of preeclampsia. Environ Pollut 218:770–782. [DOI] [PubMed] [Google Scholar]
- Zhao R, Wu Y, Zhao F, Lv Y, Huang D, Wei J, et al. 2017. The risk of missed abortion associated with the levels of tobacco, heavy metals and phthalate in hair of pregnant woman: A case control study in chinese women. Medicine (Baltimore) 96:e9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.