Abstract
Severe burns are often treated by means of autologous skin grafts, preferably following early excision of the burnt tissue. In the case of, for example, a large surface trauma, autologous skin cells can be expanded in vitro prior to transplantation to facilitate the treatment when insufficient uninjured skin is a limitation. In this study we have analyzed the impact of the enzyme (trypsin or accutase) used for cell dissociation and the incubation time on cell viability and expansion potential, as well as expression of cell surface markers indicative of stemness. Skin was collected from five individuals undergoing abdominal reduction surgery and the epidermal compartment was digested in either trypsin or accutase. Trypsin generally generated more cells than accutase and with higher viability; however, after 7 days of subsequent culture, accutase-digested samples tended to have a higher cell count than trypsin, although the differences were not significant. No significant difference was found between the enzymes in median fluorescence intensity of the analyzed stem cell markers; however, accutase digestion generated significantly higher levels of CD117- and CD49f-positive cells, but only in the 5 h digestion group. In conclusion, digestion time appeared to affect the isolated cells more than the choice of enzyme.
Keywords: keratinocytes, epithelial cells, cell culture, stem cells, autografts
Introduction
In the care of a severely burnt patient, the damaged epithelial tissue needs to be repaired to maintain a barrier to microbial infections and to reduce loss of fluids. In a partial thickness burn the epidermis will be recovered through healing, largely by stem cells residing in the hair follicles1,2. In the event of a full-thickness burn, however, per definition the hair follicles are no longer capable of repairing the epithelium, which necessitates a surgical intervention both in terms of debridement and also to replace the epidermis by autologous skin grafts3. The cultured epithelial autografts (CEA) approach is sometimes used when widely meshed autologous grafts, or similar solutions, are not sufficient to cover the wound4,5. The use of CEA is, however, controversial, since no conclusive evidence has been presented that proves the efficacy and longevity of the procedure6-8. The use of in vitro expanded cells, either as such or in combination with a scaffold, has become an integral part of modern engineered tissue substitutes9. Within this field of research, there has been very little focus on optimizing the isolation procedure and exploring how different types of tissue-dissociation solutions affect the cell populations.
It has long been suspected that the most common procedure for isolation and expansion of the cells has a negative effect on the cells that ultimately leads to de novo ulceration9–14. Recent reports are also indicating that serial cultivation of skin cells enriches rapid dividing cells, possibly increasing the risk for skin cancers in the patients following autologous transplantation15.
Here we show that the isolation procedure using two common procedures, involving trypsin and accutase (STEMPRO® ACCUTASE), indeed have implications on the performance of the isolated cells, affecting both crude cell counts and viability but not stem cell markers. Cells isolated with the frequently used enzyme trypsin failed to recover the seeded cell number even after 7 days of subsequent culturing. The loss of cell surface proteins due to enzymatic degradation could affect the fate of stem cells in general and basal keratinocytes in particular16. Since basal keratinocytes are dependent on niche extracellular matrix (ECM) for their continued stemness, it would not be far-fetched to believe that trypsin therefore could affect the fate of skin keratinocytes17. Our findings indicate that the incubation time rather than choice of enzyme has the largest impact on how well the isolated cells will proliferate and what they express on their cell surface.
Materials and Methods
The study included 10 skin biopsies for each tested enzyme, taken from five volunteers undergoing abdominal reduction surgery in the Hand and Plastic surgery department at Linköping University Hospital. The research was carried out in accordance with the declaration of Helsinki on the ethical principles for medical research involving human subjects after obtaining permission from the regional ethics board in Linköping (2015/177-31). Verbal informed consent was obtained from the patients for their anonymized information to be published in a scientific article. Biopsies were used to study the effect of trypsin and accutase on keratinocyte crude cell counts, viability, and expression of stem cell markers. Two biopsies were taken from each individual and a mean value was calculated to reduce the inter-individual variance. A total of four skin biopsies from four volunteers were used to study the effect of trypsin and accutase on keratinocyte stem cell marker expression. All underlying research material is stored at Linköping University and can be accessed upon request.
Enzymatic Digestion
Skin was obtained from abdominoplasty surgeries after informed consent and with ethical permission from the regional ethics board in Linköping (2015/177-31), and cut into 1 cm2 pieces in the operation room. The skin pieces were placed in a sterile container with Dulbeccos Modified Eagles Medium (Life Technologies, Carlsbad, CA USA) supplemented with 10% Fetal Calf Serum (Life Technologies) and 1% Penicillin and Streptomycin (Life Technologies). Two 1 cm2 pieces of skin were used for each sample and placed dermal side down in 0.5% Dispase (Life Technologies) for 15–17 h at 37°C, 95% humidity, and 5% CO2. The epidermis was subsequently peeled off of the dermis and was used for further digestion in either 0.05% trypsin (Life Technologies) or 1X (STEMPRO®) Accutase (Life Technologies) for 15 min, 1 h, 5 h or 24 h at 37°C, 95% humidity, and 5% CO2. The isolated cells were cultured in Keratinocyte Serum Free Medium (Life Technologies) supplemented with epidermal growth factor (EGF, Life Technologies) and bovine pituitary extract (Life Technologies) according to the manufacturer’s instructions at 37°C, 95% humidity, and 5% CO2 for 7 days.
Cell Counts and Viability
The resulting cell suspensions were analyzed immediately after isolation and after 7 days of culture with respect to crude cell counts and viability (viable percentage of total count). Cell counts and viability measurements were performed with a Luna automatic cell counter (Logos Biosystems Inc, Annandale, VA, USA), based on trypan blue staining per the manufacturer’s instruction.
Flow Cytometry
Forward scatter (size) and side scatter (granularity) as well as antibody-labeled cell surface and intracellular markers were analyzed immediately after isolation using fluorescence assisted cell sorting (FACS). Analysis was performed on a BD FACS Aria II flow cytometer (Becton Dickinson, San Jose, CA, USA) using the following protocol: 100,000 cells were transferred to individual flow cytometry tubes and were fixed and permeabilized in TF Fix/Perm Buffer for 40–50 min on ice followed by further permeabilization in TF Perm/Wash Buffer according to the manufacturer’s instructions (Transcription factor Buffer Set, BD Pharmingen, San Jose, CA, USA). Unconjugated mouse IgG1 anti-human cytokeratin 19 (Thermo Fisher Scientific, Waltham, MA, USA) was then added to each tube followed by incubation for 45 min and washing in FACS staining buffer (0.5% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) in phosphate buffered saline (PBS, Life Technologies). Secondary antibody, V450-conjugated rat anti-mouse IgG1 (BD Biosciences, San Jose, CA, USA), was then added to each tube followed by incubation for 1 h at 4°C and washing in FACS staining buffer. Finally, APC-conjugated mouse anti-human CD29 (BD Biosciences), PE-CF594-conjugated rat IgG2a anti-human CD49f (BD Biosciences) and PerCP-Cy5.5-conjugated mouse IgG1 anti-human CD117 (BD Biosciences) were added followed by incubation for 20 min and washing in FACS staining buffer. Unspecific binding of directly conjugated antibodies was evaluated using corresponding isotype control antibodies (BD Biosciences). Unspecific binding of secondary antibodies was evaluated by omission of primary antibodies. Unstained cells were used as control. Optimal antibody concentration was determined by titration (data not shown). Prior to analysis, cells were re-suspended in FACS staining buffer and analyzed within 24 h. Analysis of flow cytometry data was performed in Kaluza software v1.3 (Beckman Coulter, Brea, CA, USA). Both the percentage of positive cells as well as the median fluorescence intensity (MFI) was analyzed.
Statistical Analysis
Data are presented as median and 10th to 90th centiles or median and range. The significances of differences between enzymes at each incubation time were assessed using the Mann–Whitney U-test. Differences depending on incubation time were analyzed by Kruskal–Wallis ANOVA with Dunn’s test as post hoc. Differences over time were assessed by the Wilcoxon matched pairs signed-rank test. GraphPad Prism v.6 (GraphPad Software Inc. La Jolla, CA, USA) was used for statistical analysis. Probabilities of less than 0.05 were considered statistically significant.
Results
Cell Counts and Cell Viability
Skin was obtained from abdominoplasty surgeries after informed consent. The epidermis was isolated and subjected to proteolytic digestion using either trypsin or accutase in order to isolate the cells. The number of cells was determined both immediately after isolation and after 7 days of culture. The highest cell counts obtained directly after isolation of keratinocytes were obtained using trypsin treatment for 15 min. Longer incubation times with trypsin did not improve the cell count. Accutase only generated more cells than trypsin after an incubation period of 24 h (Fig. 1). Regardless of the incubation times, trypsin generated cells with higher viability than accutase, albeit the difference was not significant (Fig. 2).
Fig 1.
Cell counts obtained by study enzymes at different time points. Kruskal–Wallis ANOVA showed a significant difference in immediate crude cell count for trypsin over different treatment times (p = 0.047). Post hoc test using Dunn’s test revealed that the significant differences were found between 15 min–24 h and 1h–24 h (p = 0.016). No significant difference was found for accutase (p = 0.52). Furthermore, a significant difference was observed for the 7 days group within the enzyme group (p = 0.007) but not for accutase (p = 0.066). Dunn’s test localized the differences to 15min–24 h, 1h–24 h, 5h–24 h (p = 0.03). No significant differences were found between trypsin and accutase at any of the time points. No significant difference was found between immediate crude cell count and crude cell count after 7 days of culture.
Fig 2.
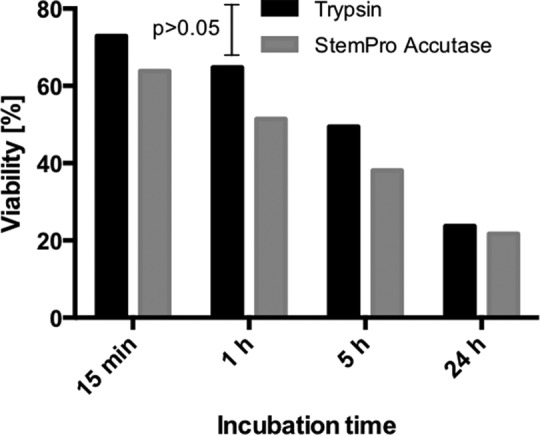
The effect of incubation time on cell viability for trypsin and accutase. Kruskal–Wallis ANOVA p-value was p < 0.016 for trypsin and p < 0.0094 for accutase. Kruskal–Wallis ANOVA also did not reveal any significant difference between the groups. In the case of trypsin, the significant difference was according to Dunn’s test found between 15 min/24 h and 1 h/24 h (p < 0.01). In the case of accutase, the significant difference was according to Dunn’s test found between 15 min/5 h (p = 0.032), 15 min/24 h (p > 0.01) and 1 h/24 h (p = 0.032).
Interestingly, however, whereas cells isolated by trypsin failed to recover the number of cells obtained directly after isolation after 7 days of culture, accutase-treated cells showed slightly higher cell counts after 7 days of culture than directly after isolation (Fig. 1). This was, however, only seen when using the shorter accutase treatment times (15 min and 1 h). After 7 days of culture, cells isolated using accutase for 1 h generated the highest number of cells but not significantly different from trypsin for any of the four incubation times (Fig. 1). In addition, when considering the viability of the isolated cells no significant differences were observed between any of the groups (Fig. 3).
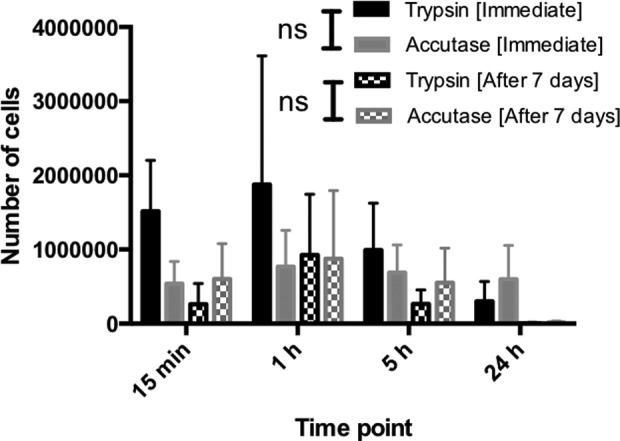
Fig 3.
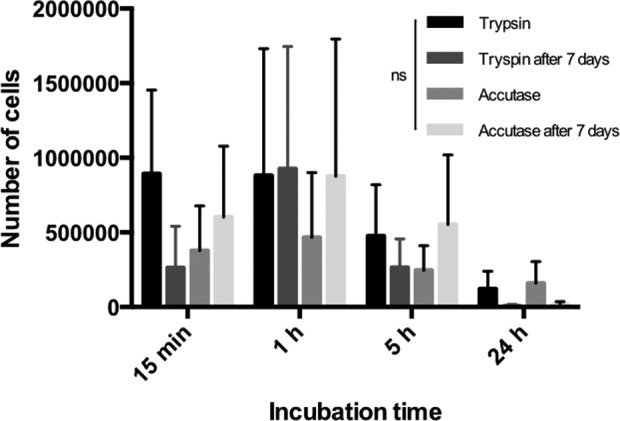
Number of viable cells generated by each tissue-dissociation solution at different incubation time directly after cell isolation and after 7 days of culture. Wilcoxon’s matched pairs signed-rank test was used to analyze significant difference over time. No significant differences were observed between any of the groups.
Effect on Cell Surface Markers
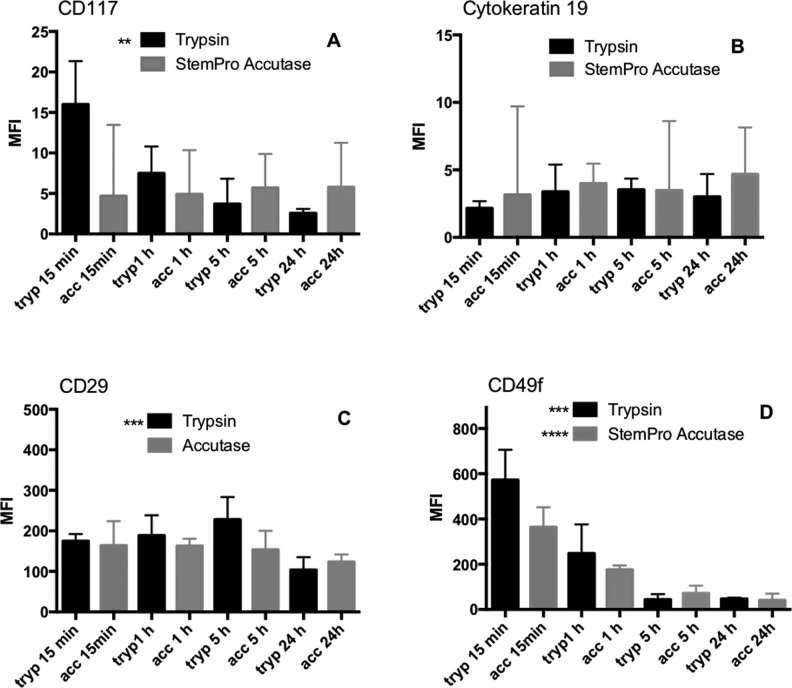
The detachment and dissociation of the cells using trypsin or accutase treatment will result in hydrolysis and loss of extracellular membrane proteins, which can consequently affect cell interactions with ECM proteins and be detrimental for stem cell homeostasis, which is dependent on the correct niche being maintained17. Flow cytometric analysis was employed to elucidate the relative difference in loss of keratinocyte stem cell markers of cells isolated using trypsin or accutase. The difference in MFI and the difference in percentage of positive cells were measured (Figs 4 and 5, respectively).
Fig 4.
Effect of the two different enzymes and incubation time on the median fluorescence intensity. No significant difference was observed between the enzyme groups at any time point in any of the analyzed markers (A–D). A significantly lower MFI was, however, observed over incubation time in the case of CD117 (A) and CD29 (C) in the trypsin group and both trypsin and accutase in the CD49f (D) group.
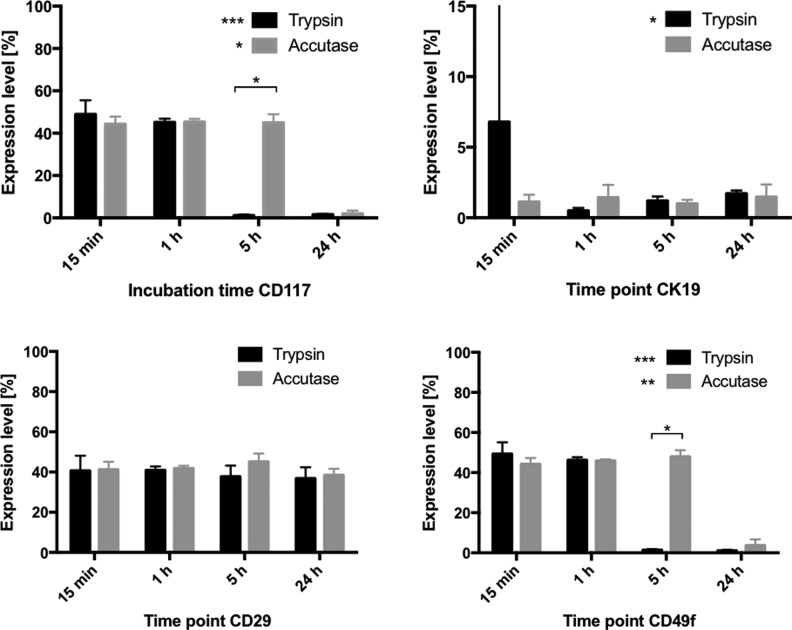
Fig 5.
Effect of the two different enzymes and incubation time on expression of the four stem cell markers. Significantly more cells digested with accutase expressed the stem cell markers CD117 and CD49f after 5 h incubation as compared with cells incubated with trypsin. A significant difference over incubation time was observed in both enzyme groups in the case of CD117 and CD49f and in the trypsin group only in the case of cytokeratin 19.
Median Fluorescence Intensity
CD117
No significant differences in MFI were observed between the enzymes in terms of which enzyme was used. A significant difference in MFI was found within the trypsin group p = 0.0027. Dunn’s test revealed a difference between 15 min incubation and 24 h incubation. No significant difference was found in MFI between the incubation times in the accutase group (Fig. 4).
CK19
No significant differences in MFI were observed between the enzymes, either in terms of which enzyme was used or between the incubation times within each enzyme group (Fig 4).
CD29
No significant differences in MFI were observed between the enzymes in terms of which enzyme was used. A significant difference was observed between the incubation times within the trypsin group (p = 0.0009). Dunn’s test revealed that the difference was found between 15 min and 5 h, 15 min and 24 h, and 1 h and 24 h, as well as between 5 h and 24 h (p = 0.0286) (Fig. 4).
CD49f
No significant differences in MFI were observed between the enzymes in terms of which enzyme was used. A significant difference was, however, found between the different incubation times within each enzyme group (p = 0.0004 for trypsin and p < 0.0001 for accutase). In the case of trypsin, the differences were found to be between 15 min and 5 h, 15 min and 24 h, 1 h and 5 h, and 1 h and 24 h (p = 0.0286). In the case of accutase, the differences were found between 15 min and 1 h, 15 min and 5 h, 15 min and 24 h, 1 h and 5 h, and 1 h and 24 h (p = 0.0286) (Fig. 4).
Percentage of Positive Cells
CD117
Kruskal–Wallis analysis revealed a significant difference in percent CD117-positive cells over time within each enzyme group (trypsin p = 0.0003, accutase p = 0.0163). Dunn’s test identified the differences to be between 15 min and 5 h, 15 min and 24 h, 1 h and 5 h, and 1 h and 24 h in the trypsin group (p = 0.0286), and between 24 h and all other incubation times for the accutase group (p = 0.0286). Pairwise comparison between the enzyme groups using Mann–Whitney test revealed a significant difference at the 5 h time point (Fig. 5).
Cytokeratin 19
Kruskal–Wallis analysis revealed a significant difference between the incubation times within the trypsin group (p = 0.0292), but not the accutase group (p = 0.8991). Post hoc analysis localized the difference to 1 h and 5 h, and 1 h and 24 h (p = 0.0286). No significant difference was found between the enzyme groups (Fig. 5).
CD29
No significant difference was found either between the different incubation times in any of the enzyme groups or between the enzyme groups (Fig. 5).
CD49f
Kruskal–Wallis analysis revealed a significant difference within each enzyme group over time (trypsin p = 0.0005, accutase p = 0.0053). Dunn’s test localized the difference to 15 min and 5 h, 15 min and 24 h, 1 h and 5 h, and 1 h and 24 h for the trypsin group (p = 0.0286), and 15 min and 24 h, 1 h and 24 h, and 5 h and 24 h in the accutase group (p = 0.0286). Pairwise comparison between the enzyme groups using Mann–Whitney revealed a significant difference between trypsin and accutase at 5 h incubation (Fig. 5).
Discussion
The results show that there were no significant differences in cell viability or cell count for trypsin and accutase-treated tissue, either immediately after isolation or after 7 days of subsequent culture. However, in all cases, trypsin treatment resulted in a lower number of cells after 7 days of culture compared with the cell count immediately after isolation. In contrast, tissue treated with accutase for either 15 min or 1 h generated more cells after 7 days of culture than immediately after isolation. Accutase also generated more cells than trypsin after 7 days of culture irrespective of the incubation time, although this difference was not statistically significant. These findings clearly indicate that the isolation procedure of keratinocytes for CEA has an effect on the viability and performance of the cells, and that the treatments have a detrimental effect on the viability of the cells for both enzymes investigated in this study. In particular, our findings clearly indicate the necessity to reduce the dissociation time as much as possible. The choice of enzyme might also affect the outcome of the CEA expansion process regardless of how many cells were obtained immediately upon isolation, although this needs to be investigated more thoroughly. To further investigate the hypothesis that the choice of enzyme and treatment time will affect the presence of stem cells in the CEA population, flow cytometric analysis of the stem cell markers CD29, CD49f, CD117, Δp63, and CK19 was performed on the cells immediately after isolation. A significant difference was found between trypsin and accutase in CD29+/CD49f-positive cells and Δp63-positive cells, where trypsin generated a higher number of Δp63-positive cells while accutase generated a higher number of CD29+/CD49f-positive cells. The treatment time had a noticeable effect on the amount of stem cells, where longer incubation times significantly reduced the levels of CD29+CD49f+, CD117+, and Δp63+ cells, irrespective of the enzyme used. Albeit only a few significant differences between trypsin and accutase isolated keratinocytes could be confirmed due to the large deviations between samples, cells isolated by accutase digestion had a clear tendency to both generate a higher number of cells after 7 days of culture and also to contain a higher number of cells expressing the stem cell markers CD29 and CD49f (p = 0.03) and CK19 (p > 0.05) as compared with keratinocytes isolated by trypsin. Trypsin, on the other hand, tended to generate a higher number of cells immediately after isolation and with a higher percentage of cells expressing CD117 and Δp63.
Our work supports the finding by Jeschke and Herndon that the isolation process affects the quality of CEA, as the process affects cell viability and has a detrimental effect on the expression of stem cell markers10. It is widely accepted that binding of integrins to certain ECM proteins is a key trigger for keratinocyte stem cells to maintain their stem cell phenotype. Loss of integrins during isolation may thus reduce the efficacy of the CEA treatment12–14. Some authors further advocate avoiding expanding the cells, as this has a negative effect on the cells and decreases their ability to proliferate, and have instead focused on optimizing procedures to isolate minced skin pieces containing cells both epidermis and dermis, including skin appendices such as hair follicles, sweat glands, and sebaceous glands, with a high viability which they use directly on wounds of various etiology18–20. The findings presented here support this strategy.
Conclusions
In summary, few significant differences were observed between the cells isolated using the two different enzymatic tissue-dissociation solutions trypsin and accutase. However, longer treatment times clearly resulted in fewer cells, and the treatment time also reduced the concentration of stem cell markers, strongly indicating that the isolation process indeed can affect the quality of the CEAs. No significant differences were seen between the two tissue-dissociation solutions in terms of cell numbers or cell viability, and only a few significant differences were observed between the two enzymes in terms of level of expression of stem cell markers or percent of cells expressing stem cell markers (Figs 1 –5). This indicates that the choice of enzyme has less impact than treatment time on the quality of the isolated cells. However, it cannot be excluded that other factors can affect the outcome of the clinical use of CEAs. In particular, the conditions for expansion of the cells prior to transplantation and how the transplant is handled post transplantation are most likely of great importance, and are therefore currently being explored by our research team at the burn unit at Linköping University Hospital.
Acknowledgements
We thank Dr. Florence Sjögren for technical support with the flow cytometry analysis.
Footnotes
Ethical Approval: Ethical approval to report this case was obtained from the regional ethics board in Linköping, Sweden (2015/177-31).
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the regional ethics board in Linköping, Sweden (2015/177-31), approved protocols.
Statement of Informed Consent: Verbal informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Integrative Regenerative Medicine (IGEN) center at Linköping University.
ORCID iD: M. Skog  https://orcid.org/0000-0002-1935-4891
https://orcid.org/0000-0002-1935-4891
Daniel Aili  https://orcid.org/0000-0002-7001-9415
https://orcid.org/0000-0002-7001-9415
References
- 1. Ito M, Cotsarelis G. Is the hair follicle necessary for normal wound healing? J Invest Dermatol. 2008;128(5):1059–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351–1354. [DOI] [PubMed] [Google Scholar]
- 3. Tompkins RG, Remensnyder JP, Burke JF, Tompkins DM, Hilton JF, Schoenfeld DA, Behringer GE, Bondoc CC, Briggs SE, Quinby WC., Jr Significant reductions in mortality for children with burn injuries through the use of prompt eschar excision. Ann Surg. 1988;208(5):577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cuono C, Langdon R, McGuire J. Use of cultured epidermal autografts and dermal allografts as skin replacement after burn injury. Lancet. 1986;1(8490):1123–1124. [DOI] [PubMed] [Google Scholar]
- 5. O’Connor NE, Mulliken JB, Banks-Schlegel S, Kehinde O, Green H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;1(8211):75–78. [PubMed] [Google Scholar]
- 6. Counter CM, Press W, Compton CC. Telomere shortening in cultured autografts of patients with burns. Lancet. 2003;361(9366):1345–1346. [DOI] [PubMed] [Google Scholar]
- 7. Gardien KL, Marck RE, Bloemen MC, Waaijman T, Gibbs S, Ulrich MM, Middelkoop E. Outcome of burns treated with autologous cultured proliferating epidermal cells: a prospective randomized multicenter intrapatient comparative trial. Cell Transplant. 2016;25(3):437–448. [DOI] [PubMed] [Google Scholar]
- 8. Williamson JS, Snelling CF, Clugston P, Macdonald IB, Germann E. Cultured epithelial autograft: five years of clinical experience with twenty-eight patients. J Trauma. 1995;39(2):309–319. [DOI] [PubMed] [Google Scholar]
- 9. Hirsch T, Rothoeft T, Teig N. et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551(7680):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeschke MG, Herndon DN. Burns in children: standard and new treatments. Lancet. 2014;383(9923):1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–343. [DOI] [PubMed] [Google Scholar]
- 12. Poumay Y, Leclercq-Smekens M, Grailly S, Degen A, Leloup R. Specific internalization of basal membrane domains containing the integrin alpha 6 beta 4 in dispase-detached cultured human keratinocytes. Eur J Cell Biol. 1993;60(1):12–20. [PubMed] [Google Scholar]
- 13. Poumay Y, Roland IH, Leclercq-Smekens M, Leloup R. Basal detachment of the epidermis using dispase: tissue spatial organization and fate of integrin alpha 6 beta 4 and hemidesmosomes. J Invest Dermatol. 1994;102(1):111–117. [DOI] [PubMed] [Google Scholar]
- 14. Stenn KS, Link R, Moellmann G, Madri J, Kuklinska E. Dispase, a neutral protease from Bacillus polymyxa, is a powerful fibronectinase and type IV collagenase. J Invest Dermatol. 1989;93(2):287–290. [DOI] [PubMed] [Google Scholar]
- 15. Singh M, Nuutlia K, Chauhan AS, Eriksson E. Invasive squamous cell carcinoma in full-thickness burn wounds after treatment with cultured epithelial autografts. Plast Reconstr Surg Glob Open. 2015;3(7):e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strobl H, Krump C, Borek I. Micro-environmental signals directing human epidermal Langerhans cell differentiation. Semin Cell Dev Biol. 2019;86:36–43. [DOI] [PubMed] [Google Scholar]
- 17. Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20(8):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Svensjo T, Pomahac B, Yao F, Slama J, Wasif N, Eriksson E. Autologous skin transplantation: comparison of minced skin to other techniques. J Surg Res. 2002;103(1):19–29. [DOI] [PubMed] [Google Scholar]
- 19. Hackl F, Bergmann J, Granter SR, Koyama T, Kiwanuka E, Zuhaili B, Pomahac B, Caterson EJ, Junker JP, Eriksson E. Epidermal regeneration by micrograft transplantation with immediate 100-fold expansion. Plast Reconstr Surg. 2012;129(3):443e–452e. [DOI] [PubMed] [Google Scholar]
- 20. Danks RR, Lairet K. Innovations in caring for a large burn in the Iraq war zone. J Burn Care Res. 2010;31(4):665–669. [DOI] [PubMed] [Google Scholar]