Summary
Tropical countries are experiencing a substantial rise in type 2 diabetes, which is often undiagnosed or poorly controlled. Since diabetes is a risk factor for many infectious diseases, this increase probably adds to the large infectious disease burden in tropical countries. We reviewed the literature to investigate the interface between diabetes and infections in tropical countries, including the WHO-defined neglected tropical diseases. Although solid data are sparse, patients with diabetes living in tropical countries most likely face increased risks of common and health-care-associated infections, as well as infected foot ulcers, which often lead to amputation. There is strong evidence that diabetes increases the severity of some endemic infections such as tuberculosis, melioidosis, and dengue virus infection. Some HIV and antiparasitic drugs might induce diabetes, whereas helminth infections appear to afford some protection against future diabetes. But there are no or very scarce data for most tropical infections and for possible biological mechanisms underlying associations with diabetes. The rise in diabetes and other non-communicable diseases puts a heavy toll on health systems in tropical countries. On the other hand, complications common to both diabetes and some tropical infections might provide an opportunity for shared services—for example, for eye health (trachoma and onchocerciasis), ulcer care (leprosy), or renal support (schistosomiasis). More research about the interaction of diabetes and infections in tropical countries is needed, and the infectious disease burden in these countries is another reason to step up global efforts to improve prevention and care for diabetes.
Introduction
Diabetes mellitus seems to increase the incidence and severity of many common infections, especially those caused by bacteria and fungi, and for some infections there is evidence that hyperglycaemia and poor glycaemic control correlate with infection risk and outcome.1, 2 However, these findings are almost exclusively from studies performed in the resource-rich industrialised world, even though the global epidemic of type 2 diabetes is particularly thriving in low-income and middle-income countries. In this Review, we use “diabetes” when referring to type 2 diabetes. More than 80% of patients with diabetes live in low-income and middle-income countries, and 88% of deaths related to diabetes occur in these countries.3 According to the World Bank, more than 80 countries are classified as low income and lower-middle income, but in this Review we will use the term “tropical countries” to refer to a bigger number of countries (149 countries according to WHO) with tropical and subtropical conditions, many of which would be classified as upper-middle income (eg, Botswana, China, Colombia, Malaysia, Peru, and Thailand) or even high income (eg, Argentina and Venezuela). The number of patients with diabetes is projected to increase by 55% globally in the next 20 years, but this value disguises how the brunt of the increase will be borne by tropical countries, with predicted increases of 109% in Africa, 96% in the Middle East and north Africa, and 71% in southeast Asia.3 Studies suggest that these estimates could even be too conservative. For instance, in China, there are now an estimated 114 million people with diabetes, with a national prevalence of 11·6% in 2010 compared with 5·5% in 2001 and 1% in 1980.4 The proportion of diabetes that is undiagnosed or poorly controlled is also much higher in tropical countries than in better-resourced industrialised countries.3
In this Review, we first briefly discuss the risk from diabetes for infectious diseases in general, referring to potential underlying mechanisms. We then discuss what factors could contribute to a higher burden of diabetes-associated infections in tropical countries, specifically referring to diabetic foot and tropical diabetic hand syndrome. We briefly review what is known about diabetes and tuberculosis (reviewed previously)1, 5, 6 and HIV, and discuss what is known about diabetes with respect to malaria, emerging viral infections, and the so-called neglected tropical diseases (NTDs).7, 8, 9 Finally, we explore the implications of the increasing importance of diabetes-associated infections for health systems in tropical countries. In the absence of a good evidence base for many of the key questions, we end with some of the priorities for future research and action.
Diabetes mellitus and infectious diseases
Infections are a common cause of morbidity and mortality in patients with diabetes, with important health and socioeconomic implications. Some relatively rare infections—such as rhinocerebral mucormycosis,10 Fournier's gangrene, and Klebsiella pneumoniae liver abscess11—occur almost exclusively in patients with diabetes. Mucocutaneous and invasive fungal infections, mostly caused by Candida species, are also more common in individuals with diabetes; as are common infections including bacterial pneumonia, urinary tract infections, bloodstream infections, skin infections, soft-tissue infections, and eye infections.12, 13, 14 Infections are not only more common in people with diabetes but can also be more severe than in those without diabetes. For instance, observational studies have shown diabetes to be associated with both increased rate of hospital admission and increased time spent in hospital because of infections; increased rates of admission to intensive care units; increased mortality for pneumonia, bloodstream infections, and influenza;13, 14, 15, 16 increased rates of amputation and death for necrotising limb infections;17 and greatly increased rates of endogenous endophthalmitis among patients with fungaemia or bacteraemia.18 Diabetes might also affect antibiotic treatment; it has been associated with increased antimicrobial drug resistance—eg, among Enterobacteriaceae19, 20, 21—whereas impaired kidney function and liver steatosis associated with diabetes increase the risk of antimicrobial drug toxicity. Finally, preventive vaccination could be less effective in people with diabetes, as has been shown for influenza vaccination.16, 22
Several mechanisms contribute to the increased frequency and severity of infections in people with diabetes. Firstly, diabetes is associated with decreased host immunity to infections.23, 24, 25, 26 Diabetic neuropathy, macroangiopathy, and microangiopathy contribute to increased rates of skin lesions and poor wound healing, and patients with diabetes have been shown to have decreased functioning of immune cells, including neutrophils, macrophages, T cells, and antibody-producing B cells.24, 26 Other factors that could impair immune responses in diabetes include obesity, vitamin D deficiency, oxidative stress,25 and possibly diabetes drugs.27, 28 People living with diabetes have an increased rate of Staphylococcus aureus carriage, and glycosuria predisposes individuals to outgrowth of uropathogens. Finally, patients with diabetes have an increased rate of health-care-associated infections because of more frequent attendance at health-care facilities, hospitalisation, and instrumentation during medical procedures.29 As reviewed in The Lancet Diabetes and Endocrinology, epidemiological studies have linked more profound hyperglycaemia with increased infection rates and mortality, increased risk of hospitalisation from pneumonia, some bloodstream infections, and urinary tract infections.30
Diabetes and infections in tropical countries
For most infections associated with diabetes there is a striking paucity of comparative population-level data from tropical countries as compared with high-income countries, in terms of diabetes prevalence, infection rates, and risk estimates for infections in patients with diabetes. A major factor contributing to this knowledge gap is uncertainty about the reliability of case ascertainment because of weak diagnostic services (table 1 ). Also, reduced access to health services means that hospital-derived data miss an important proportion of cases, and might over-represent severe diseases. Although infections represent a larger proportion of hospitalisations in tropical countries than in high-income countries, this difference reflects casemix and is not a useful surrogate of comparative incidence. Similarly missing are data on the proportion of these common infections that are attributed to diabetes in tropical countries, or on differences in severity due to diabetes.
Table 1.
Diabetes in relation to infectious diseases in general, and in tropical countries in particular
| Comments | Relevance for resource-limited countries in the tropics | |
|---|---|---|
| Incidence risk | Many common infections (pneumonia, urinary tract infections, sepsis, skin tissue, soft tissue, etc) have increased frequency in diabetes | Increased numbers of patients with unknown, untreated, or poorly controlled diabetes |
| Severity | Infections in diabetes lead to increased and prolonged hospitalisation, increased intensive care unit admissions, and increased complications | Increased numbers of patients with unknown, untreated, or poorly controlled diabetes |
| Health-care-associated infections | Health-care-associated infections disproportionally affect patients with diabetes | Increased frequency in tropical countries |
| Antimicrobial drug resistance | Increased frequency in reported proportions of antimicrobial drug resistance in patients with diabetes—eg, Enterobacteriaceae in urinary tract infections | High rates of antimicrobial drug resistance in some tropical countries |
| Importance of diabetes care | Proper diabetes management (especially foot care) reduces infection risk and complications | Foot and other diabetes management more difficult; increased rates of amputations |
| Glycaemic control | Good glycaemic control reduces incidence and severity of many infections | Reduced number of patients achieve good glycaemic control |
| Health service access and retention | Reduced access and decreased quality of diabetes care is associated with decreased socioeconomic class in many settings | Large gaps at every step of cascade—reduced access to diagnosis thus reduced awareness of diabetes status, and much reduced linkage of diabetes diagnosis to care; access to diagnostic and therapeutic services for infections also likely reduced |
| Drug toxicity | Patients with diabetes experience increased toxicity and adverse events when using antibiotics | Monitoring of toxicity often more difficult than in resource-rich settings |
| Diabetes prevalence | All issues in the list above are more important in high diabetes prevalence settings than in lower diabetes prevalence settings | Most rapid increase and some of the highest diabetes prevalence rates occur in tropical countries |
Even though reliable data for most infections are unavailable, it is highly likely that patients with diabetes in tropical countries are at increased risk for common bacterial and other infections for several reasons. Firstly, diabetes is more likely to be undiagnosed (and therefore unmanaged) in resource-constrained tropical settings than in wealthier countries, and even if the condition is diagnosed, it is more likely to be associated with poor glycaemic control.31 Unavailability, insecure supply, or high costs of diabetes medication, and issues related to storage and use of insulin, all compromise glycaemic control in many resource-constrained settings.32, 33 Similarly, healthy diet and lifestyle are relatively low priorities for people living in poverty or under difficult conditions in tropical countries, and health education and self-management of diabetes are challenging. Additionally, the number of health providers available to properly educate, treat, and monitor diabetes is low in such settings. In sub-Saharan Africa, for instance, there were an estimated 16 physicians per 100 000 people in 2011 (17 times less than in the USA and UK) while for most African countries, large numbers of physicians continue to emigrate.34
Another contributing factor is reduced access to diagnostic and therapeutic services, or to good-quality care for infections (table 1). This will further lead to failing treatment, recurrent infections, and development of antimicrobial drug resistance. Hospitals in low-resource settings are often a source of infection, with risks of health-care-associated infection substantially higher than reported in industrialised countries.29 Although evidence is inadequate, it seems plausible that diabetes is over-represented among hospitalised patients in such settings. For instance, diabetic foot complications are the main cause of prolonged hospital stays for patients with diabetes in tropical countries, which could lead to high rates of drug resistance, especially in the case of treatment for diabetic foot ulcers. For example, in a hospital-based study of diabetes patients in Algeria, 86% of Staphylococcus aureus were meticillin resistant, and 60–80% of Escherichia coli and Klebsiella isolates from diabetic foot infections were multidrug resistant.35 Also, transmission of viral hepatitis and HIV in low-resource settings has been attributed to the shared use of glucose meters in health institutes.36 It should be noted that diabetes is not well captured by considering it as a single disease entity, and the risks faced by people with diabetes and the care provided is hugely variable between different countries, between rural and urban settings, and between primary health clinics and hospitals. However, good epidemiological data exploring the importance of these and other such factors are extremely rare (table 1).
Diabetic foot infections and tropical diabetic hand syndrome
In tropical countries, diabetic foot problems are a common complication of diabetes and an important cause of morbidity and mortality. For instance, data from a study in Ethiopia showed that diabetic foot ulcers accounted for 39% of admissions of patients with diabetes.37 Reports from Africa and Asia suggest that neuropathy rather than peripheral vascular disease underlies diabetic foot problems, although this situation might be changing because of an increase in cardiovascular disease and its risk factors.38, 39 Inadequate footwear and walking barefoot can contribute to neuropathic foot ulcers in tropical countries. Infection is usually a consequence rather than a cause of foot ulcers, but concomitant infections cause delays in wound healing, and in the absence of proper management, infected diabetic ulcers could progress to systemic infection, gangrene, limb loss, and death.40 As a result, diabetic foot infections are cited as a major cause of prolonged hospital stays, cause of amputation, and mortality in resource-constrained settings.38 As one Indian doctor wrote, “the severely infected foot is the hallmark of the diabetic foot commonly seen in India”,39 and this observation is probably true for many tropical countries.
Tropical diabetic hand syndrome is a more-aggressive, upper limb version of the diabetic foot and has been reported in Africa and Asia.41, 42, 43 So far, only small case series have been published, but the syndrome seems to range from localised cellulitis and swelling to ulceration of the hands, sepsis, and gangrene. It usually follows minor or unrecognised trauma and can lead to amputation. The prevalence of diabetic hand syndrome in tropical countries is due to a combination of factors, including rapid spread of infection in the hand, delayed access to health care, and poor glycaemic control.
Amputation is a marker not only of disease severity, but also of disease management and quality of health services. Although reliable epidemiological data are scarce, it appears that amputation rates for diabetic foot are much higher in tropical countries compared with industrialised countries.44 For instance, amputation was performed in 15% of patients in hospital with foot ulcers in a study in Pakistan,45 and in 33% of patients with foot ulcers in Tanzania.46 In India, although precise data are absent, it is estimated that 45 000 legs are amputated yearly, with approximately 75% resulting from infected neuropathic feet.39 Scarcity of knowledge, patients' delay in seeking treatment, paucity of foot care, and unavailability of suitable facilities and trained personnel challenge the implementation of multidisciplinary programmes that are used in industrialised settings.38 The success of specific projects, such as the “Step by Step” Diabetic Foot Project in Tanzania, suggests that there might be opportunities for reducing morbidity and mortality even when resources are limited.47
Mucormycosis
Mucormycosis is a fungal infection that is noteworthy for being particularly aggressive in patients with diabetes. It is caused by zygomycetes and is characterised by rapidly progressive tissue necrosis. It usually presents as rhino-orbital-cerebral disease, especially in patients with diabetes.48 However, it can also present as pulmonary, cutaneous, gastrointestinal, or disseminated disease. In developed countries incidence of mucormycosis is increasing. The infection is usually seen in immunocompromised patients, especially because widely used azole antifungal drugs are inactive against mucormycosis.49 However, in developing countries, especially India, the number of zygomycosis cases seems to be increasing because of increasing numbers of cases in patients with uncontrolled or unrecognised diabetes.50, 51, 52 In a single tertiary care centre in India, the number of cases witnessed in the past 25 years increased approximately six times;53 and a case series showed that 131 (74%) of 178 patients had mucormycosis associated with diabetes53 (compared with 86 [16%] of 531 patients in France49) and a quarter of patients did not know that they had diabetes. Mortality is high, especially in low-resource settings where use of amphotericin B, appropriate surgery with debridement of necrotic tissue, and correction of uncontrolled diabetes might be more difficult. As a result of diabetes, other invasive mould infections, especially those caused by Aspergillus flavus, might also be on the rise in tropical countries.54
Effect of diabetes mellitus on tuberculosis, HIV, and malaria
In addition to the universal presence of common bacterial and other infections, tropical countries are disproportionally affected by the so-called big three: HIV, tuberculosis, and malaria. The interaction between diabetes and tuberculosis has been reviewed extensively.1, 5, 6 People with diabetes are at three times increased risk of developing active tuberculosis compared with people who do not have diabetes,55 especially those with poor glycaemic control who have an even higher risk.56 Diabetes is also associated with increased rates of death, treatment failure, and recurrent disease.57 As such, the rise in diabetes prevalence in tuberculosis-endemic countries is seen as a major threat to further control of tuberculosis.
With regard to HIV, of the few studies done in tropical countries, a study in Tanzania found that the rate of glucose metabolism disorders (as measured by oral glucose tolerance test) among HIV patients on antiretroviral therapy was six times higher than that in HIV-negative patients matched for age and obesity.58 Studies in Ethiopia and South Africa have found that long duration of HIV treatment is associated with increased incidence of diabetes.59, 60 This effect might be explained by mitochondrial toxicity associated with older anti-HIV drugs (zidovudine, stavudine, didanosine, and saquinavir)61, 62 that are still prescribed in some tropical countries, as well as newer drugs like efavirenz, as shown in a very large cohort in South Africa.63 Additionally, in part due to the successful roll-out of HIV treatment in Africa, there are increasing numbers of older people at increased risk of developing diabetes.64 Concurrent hepatitis C virus infection has also been linked with diabetes in HIV-infected patients, although data are conflicting,61, 62 and dialysis for end-stage renal disease in diabetes constitutes a risk for transmission of blood-borne viruses, including HIV, in tropical countries.65, 66
Much less is known about the association between diabetes and malaria, which affects approximately 200 million individuals worldwide each year.67 We identified only one study examining a possible association. In a large case-control study in Ghana, asymptomatic malaria parasitaemia was more common in people with diabetes compared with people without diabetes (17·4% vs 11·3%), and was higher in people with poor glycaemic control than in those with adequate glycaemic control.68 Malaria is more common in pregnancy than in age-matched non-pregnant women, and is associated with anaemia, increased maternal mortality, abortion, preterm delivery, and neonatal deaths.69 If diabetes increases malaria parasitaemia, as the study from Ghana suggests, gestational diabetes might increase the incidence, morbidity, and mortality of pregnancy-associated malaria. Such an association would be relevant, especially in light of the rising global incidence of gestational diabetes.70 However, to our knowledge, this association has not been studied to date.
Diabetes and neglected tropical diseases
People living in tropical countries are affected by a range of specific tropical diseases, such as helminth, parasitic, mycobacterial, and viral infections. WHO has compiled a list of 18 important NTDs (table 2 )—but many other important conditions have not been included, at least eight of which were included in the Global Burden of Disease Study in 2010.7 NTDs have a lower mortality than HIV, tuberculosis, and malaria, but collectively NTD morbidity adds up to an estimated 48 million disability-adjusted life-years, similar to that for tuberculosis.7 Here we review what is known about NTDs in relation to diabetes, although data are scarce. Diabetes could increase the risk of some NTDs, whereas others appear to afford some protection against diabetes. Additionally, treatment of some NTDs could contribute to the development of diabetes or interfere with glycaemic control.
Table 2.
Key observations on diabetes and neglected tropical diseases from published literature
| Articles identified | Link with diabetes | Common end-organ morbidity? | Shared health system needed? | Comments | |
|---|---|---|---|---|---|
| Buruli ulcer | 0 | No data | No (aggressive non-neuropathic ulceration) | Tissue viability services* | Mycobacterial disease |
| Chagas disease | 14 | Hyperglycaemia more common in Chagas cardiomyopathy than in controls71 | Cardiomyopathy and gastrointestinal motility | Cardiology | Parasite control diminished and mortality increased in diabetic mice71 |
| Dengue | 11 | Diabetes increases risk of severe dengue and exacerbates thrombocytopenia72, 73, 74, 75, 76, 77, 78 | No | No | .. |
| Chikungunya | 1 | No data | No | No | .. |
| Dracunculiasis | 3 | No data | No | No | .. |
| Echinococcosis | 1 | No data | No | No | .. |
| Yaws | 23† | No data | No | No | .. |
| Food-borne trematodiases | 2‡ | No data | No | No | .. |
| Human African trypanosomiasis | 21 | No data | No | No | Only murine studies |
| Leishmaniasis | 40 | Only sporadic case reports of modified phenotype | No | No | Pentamidine is diabetogenic79, 80, 81 |
| Leprosy | 99 | Diabetes prevalence higher in lepromatous than tuberculoid82 | Neuropathic ulcers and blindness | Tissue viability services and eye health services | Steroid-induced diabetes in therapy of leprosy reactions83 |
| Lymphatic filariasis | 2 | Lymphatic filariasis prevalence reduced in diabetes patients84 | No | Tissue viability services | Reduced proinflammatory cytokines in lymphatic filariasis85 |
| Onchocerciasis | 4 | No data | Blindness | Eye health services | .. |
| Rabies | 3 | No data | No | No | .. |
| Schistosomiasis | 31 | Diabetes inversely associated with previous schistosomiasis in China;86 attenuated schistosomiasis phenotype in diabetes (decreased egg counts and reduced granulomas) | Renal impairment | No | Murine data suggestive that egg exposure reduces subsequent IDDM87, 88, 89, 90 |
| Soil-transmitted helminthiases | 57 | Soil-transmitted helminthiases inversely associated with diabetes (Indonesia);91 previous strongyloides inversely associated with diabetes (Australia)92 | No | No | Murine data suggestive that Fasciola antigen exposure reduces subsequent IDDM93 |
| Taeniasis or cysticercosis | 2 | .. | No | No | Murine taeniasis attenuates diabetes94 |
| Trachoma | 15 | .. | Blindness | Eye health services | .. |
IDDM=insulin-dependent diabetes.
Tissue viability services encompasses foot care services.
23 articles were identified using MeSH terms “diabetes mellitus” AND (“yaws” OR “treponem*”) and almost all results related to peridontal disease.
MeSH terms used were “diabetes mellitus” AND (“clonorchis” OR “opisthorchis” OR “fasciola” OR “paragonimus”).
Bacterial infections
Regarding bacterial infections, diabetes might affect the incidence and phenotype of leprosy. In India, diabetes was identified in 14·2% of patients with leprosy in Uttar Pradesh, compared with only 2% in people without leprosy.82 Diabetes prevalence was strikingly different when disaggregated by disease phenotype: 19·3% in lepromatous leprosy and 6·4% in tuberculoid leprosy,82 a finding that was replicated in a smaller study in Kuwait in 2012.95 Diabetes might also interact with leprosy treatment. In India, ulcer management was more complex and consequently treatment in hospital was longer in patients with diabetes than in those without diabetes,96 in addition prolonged use of steroids for leprosy reversal reactions might induce diabetes.97
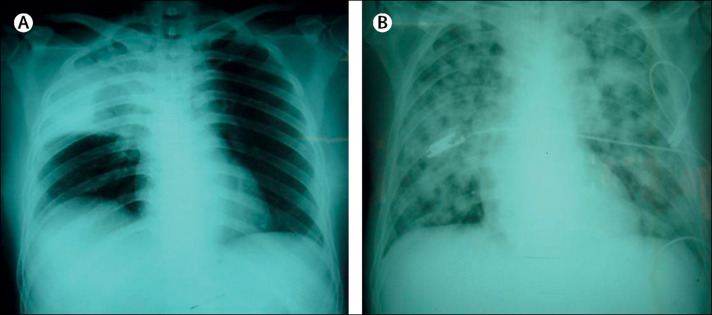
Melioidosis, although not included in the WHO list of NTDs, is a tropical disease that has long been recognised for its association with diabetes. It is a severe infection that can manifest as sepsis, pneumonia, or abscesses (figure ). It is caused by the Gram-negative bacterium, Burkholderia pseudomallei, and is particularly well recognised in southeast Asia and northern Australia. Precise risk estimates are scarce, but 40–60% of patients with melioidosis in different studies also have diabetes.99, 100, 101 These epidemiological data are supported by results from numerous immunological studies showing decreased B pseudomallei phagocytosis and killing, and altered cytokine responses by neutrophils and monocytes from people with diabetes.23, 102, 103 Diabetes medication might also affect susceptibility and presentation of melioidosis. The use of sulfonylurea derivatives was strongly associated with decreased proinflammatory cytokine responses and increased severity of melioidosis in a study in Singapore,28 although the opposite was found in a large cohort in Thailand.104
Figure.
Chest radiographs of two patients with melioidosis
(A) Right upper lobe consolidation in patient with bacteraemia, pneumonia, pyelonephritis, and subcutaneous abscesses. (B) Widespread bilateral pulmonary shadowing in patient who also had bacteraemia and multiple abscesses in liver and spleen. Reproduced from Peacock,98 by permission of Elsevier.
There are no published data on an interaction with diabetes for other bacterial infections such as Buruli ulcer, a destructive skin and subcutaneous tissue disease caused by Mycobacterium ulcerans, despite the well-established association of diabetes with disease due to Mycobacterium tuberculosis. The same is true for yaws, the most common of the endemic treponemal infections, which presents as skin and bone disease and is the subject of a renewed WHO-led initiative aimed at elimination, as well as for trachoma, an eye infection caused by Chlamydia trachomatis.
Viral infections
Viral infections constitute a growing global health problem. They include infections with dengue virus, which is included in the list of NTDs; other arboviruses transmitted by mosquitoes; respiratory viral infections, including severe acute respiratory syndrome, Middle East respiratory syndrome, and infection with certain influenza strains; and haemorrhagic fever viral infections, such as Ebola virus disease. Regarding a possible relation with diabetes, most is known about dengue. Dengue is a mosquito-borne viral infection that has had an enormous geographical expansion, with an estimated 390 million cases in 2012.105 Epidemiological data have shown that diabetes is associated with increased severity of dengue-induced thrombocytopenia,72 a two-to-three times increased risk of severe dengue (previously known as dengue shock syndrome and dengue haemorrhagic fever),73, 74, 75, 76 an independent five times increased risk of acute kidney injury,77 and increased admission to the intensive care unit.78 For infection with West Nile virus—an arbovirus that has spread from Africa to the western hemisphere, southern Europe, and elsewhere in the last two decades—diabetes is strongly associated with neuroinvasive disease.106 We could not find data for other arboviral infections endemic in tropical and subtropical countries, such as chikungunya and Zika, two emerging viral infections that share vectors and some clinical features with dengue, or for other arboviral infections such as yellow fever and Japanese encephalitis.
There are also good data to support a relationship between diabetes and emerging respiratory viral infections. For severe acute respiratory syndrome, which affected thousands of people in 2003, diabetes and poor glycaemic control were strongly associated with mortality (odds ratio 3·0; 95% CI 1·4–6·3).107 In recent outbreaks of Middle East respiratory syndrome in Saudi Arabia, also caused by a novel coronavirus, diabetes was detected in 68% of cases and 66% of deaths.108 This association could be due to increased susceptibility to infection or to increased likelihood of severe disease leading to health-care attendance. There is no indication that diabetes is associated with the 2014 Ebola virus disease outbreak; a diabetes prevalence of 2% was found in one case series in Guinea.109 Rabies is an almost universally fatal viral disease usually acquired through the bite of a dog or bat. One case of fatal rabies despite post-exposure vaccination in a patient with diabetes was reported from India,110 but attribution of vaccine failure to diabetes cannot be inferred from this single report.
Protozoan infections
There is some uncertainty regarding a possible effect of diabetes on leishmaniasis, a disease caused by a sandfly-borne kinetoplastid parasite. Leishmaniasis is phylogeographically determined, with South American species mostly causing cutaneous disease and species in Africa and Eurasia causing cutaneous as well as visceral disease. Isolated case reports suggest that diabetes can affect the phenotype of cutaneous disease, with unusually ulcerovegetative Leishmania major lesions occurring in patients with diabetes in Morocco,111 and more numerous lesions reported in diabetes patients in France and Italy from Leishmania infantum compared with those without diabetes.112 Subclinical (or perhaps prepatent) leishmanial infection is increasingly recognised, but whether diabetes is a risk factor for progression to clinical disease has not been reported. In a small series in Saudi Arabia, six of 13 patients with presumed recrudescent disease were noted to have diabetes.113 Finally, the diabetogenic side-effect of pentamidine, a key drug for leishmaniasis, is well recognised, often necessitating the use of insulin.79, 80, 81
Chagas disease, or American trypanosomiasis, which is caused by the parasite Trypanosoma cruzi, leads to end-organ damage to the heart and gastrointestinal tract in around a third of infected patients. In a single study, diabetes and hyperglycaemia were more common in patients with Chagas cardiomyopathy than in patients with Chagas but without cardiomyopathy and healthy people.71 This study supports the hypothesis that the reduced parasympathetic activity caused by T cruzi leads to relative sympathetic hyperactivity, and is consistent with a small study by the same group implicating T cruzi infection in subtle intrapancreatic denervation changes.114 Whether these data really implicate T cruzi in the development of impaired glucose tolerance or whether hyperglycaemia predisposes patients to more symptomatic infection remains unclear. In a mouse model of diabetes, hyperglycaemia was associated with markedly reduced control of experimental T cruzi parasitaemia and increased mortality.115 For African trypanosomiasis—a parasitic disease spread by tsetse flies—no link with diabetes has been published, although murine infection with Trypanosoma brucei rhodesiense (which causes East African trypanosomiasis) appeared to offer some protection against inducible diabetes.116
Helminth infections
Some helminth NTDs are associated with reduced diabetes prevalence. In a cross-sectional study in Indonesia, infection with the soil-transmitted helminths ascaris, trichuris, hookworm, or strongyloides was inversely correlated with insulin resistance, and this association was not explained by differences in BMI.91 Similarly, serological evidence of previous strongyloides infection was independently associated with a 60% lower prevalence of type 2 diabetes in an Indigenous Australian population with high rates of both,92 although the opposite was found in a much smaller study in Brazil.117 Strongyloides hyperinfection, a disseminated manifestation of the disease with a high parasite burden that mainly occurs in immunocompromised individuals, has been reported in patients with diabetes,118, 119, 120 although these reports provide insufficient evidence of increased susceptibility.
Lymphatic filariasis, a filarial nematode disease caused by Wuchereria bancrofti or Brugia malayi, might also protect against diabetes. The disease accounts for substantial disability due to chronic—sometimes gross—lower limb lymphoedema, particularly in Africa and Asia. In Chennai, India, prevalence of lymphatic filariasis was inversely correlated with type 1 diabetes,84 with a reduction in proinflammatory cytokines proposed as a possible explanation for a protective effect.85
Schistosomiasis or bilharzia, a helminth infection transmitted by freshwater snails, might also protect against diabetes. In mice, exposure to schistosomal egg antigen reduces the risk of insulin-dependent diabetes, possibly through induction of adipose tissue M2 macrophages and expansion of regulatory T cells,87, 88, 89, 90 both of which might provide a protective effect against diabetes. In a large cross-sectional study in China (n=9359), previous schistosomal infection was 50% less common among people with diabetes than in people without diabetes.86
No published data were found on a possible relation between other helminth infections and diabetes. For instance, no clinical data have been published for cysticercosis, a major cause of epilepsy caused by the larval stage of the pork tapeworm Taenia solium, although in mice pre-existing Taenia crassiceps infection attenuated the diabetogenic effect of streptozotocin.94 No human data have been published for food-borne helminths such as Fasciola, Clonorchis, Opisthorchis, and Paragonimus, but the onset of diabetes in non-obese diabetic mice can be prevented through early exposure to secreted or excreted antigens of Fasciola hepatica. 93 There are also no data for echinococcosis, also known as hydatid disease, a parasitic disease caused by a dog tapeworm, aside from a single case report of hydatid disease of the pancreas resulting in diabetes.121 The same is true for onchocerciasis (river blindness) and for dracunculiasis (guinea worm disease), the latter of which is now mainly limited to Sudan, and is acquired through ingestion of water fleas infected with worm larvae. Overall, however, data seem to suggest that some helminth infections might protect against diabetes. As such, more research is needed to see whether decreasing rates of helminth infections or deliberate global deworming through mass drug administration programmes is subsequently accompanied by an increase in the prevalence of diabetes.122
Implications for health services
Health services in tropical countries are affected by a double burden of disease: infectious and non-communicable diseases.123 There is an estimated shortage of more than 4 million health workers globally,124 and this number is increasing alongside the rise of non-communicable diseases and failing government health systems in many countries.125 Health-care workers in impoverished settings often struggle to address even the most urgent and critical health problems, including those caused by infectious diseases. As a result, there is a severe inadequacy in awareness and prevention of non-communicable diseases in tropical countries. Meanwhile, these same countries face a rapid increase in relevant risk factors for diabetes, such as obesity and unhealthy diets.126 This increase is caused by an epidemiological transition and urbanisation in tropical countries,127 combined with a genetic and epigenetic mismatch of their inhabitants with new (and more affluent) environments.128 Therefore, it is essential for international organisations to take action and to partner with fragile services in tropical countries to deal with both infectious diseases and non-communicable diseases. Such actions include integration of surveillance, prevention, and care for different diseases; innovations to lessen the burden for health services; task shifting and self-management of diseases; and more funding to strengthen current health systems (table 3 ).
Table 3.
Suggested approaches to strengthen services in tropical countries to deal with communicable and non-communicable diseases
| Integrated approach to disease prediction and control | |
|---|---|
| Integration of care | |
| Prevention | Innovative model for incentives to prevent infectious and non-communicable diseases129 |
| Screening | Screening HIV patients for cardiovascular risk factors130 |
| Care | Integration of care for diabetes and tuberculosis6 |
| Technical solutions | |
| Simplified diagnostics | Smartphone applications to replace laboratory immunoassays131 |
| Disease monitoring | Use of new collective methods and digital images132 |
| Electronic health | Text messages to increase adherence to antiretroviral therapies133 |
| Depot medication | Simplify chronic treatment using depot medication134 |
| Health service delivery | |
| Task-shifting | Diabetes management shifted from senior health workers towards nurses135, 136 |
| Peer support | Peer support for diabetes and hypertension patients137, 138 |
| Work-based support | Health prevention and treatment in work environments139, 140 |
| Funding | |
| Government | Commitment to UN declaration to mobilise funds for medication141 |
| Research | Scholarships for studies in global health with preference for combining communicable and non-communicable diseases |
Primary prevention
At the level of primary prevention, well-distributed health education for infectious diseases, such as handwashing and hygiene in schools and other public spaces, could be extended to messages related to prevention of non-communicable diseases such as diabetes. Counselling and education on behavioural change for prevention of chronic diseases tend not to have strong effects in western settings,142 but these interventions could have more effect in tropical countries where awareness is low and motivation often becomes high once people are well informed.143 These health education programmes might even be linked with health surveillance in factories and other work environments where good hygiene and health among employees is increasingly supported for economic reasons.139, 144 Besides healthy behaviour and awareness, there should also be increased emphasis on coordination of combined screening and diagnosis of infectious and non-communicable diseases, both in health-care settings and community and work spaces.145
Strengthening chronic care
Growing awareness and screening of patients at high risk for developing diabetes would lead to an increase of patients attending health-care services, where treatment and monitoring should be strengthened and better integrated. This approach has shown to be effective, for instance, in a programme addressing diabetes and HIV in African countries.146 Treatment adherence—which is often low in tropical countries, for infectious and non-communicable diseases—should be emphasised in the pathway of care. New technologies such as electronic health and mobile health have shown to be effective in this respect, for instance, in maintaining adherence to antiretroviral therapy,133 but also in maintaining a healthy diet147 and diabetes prevention,148 as shown in randomised clinical trials in Latin America and India, respectively. New technologies might also alleviate the burden on health services by simplifying diabetes diagnosis, these developments are underway for infectious diseases for example, through smartphone applications that replace laboratory-quality immunoassays,131 through innovative mosquito traps and digital images that can replace skilled entomologists in monitoring the spread of malaria-carrying mosquitoes,132 or with depot medication that simplifies chronic treatment, such as is being developed for HIV treatment.134 As far as we are aware, there are no nascent technologies for diabetes diagnosis or monitoring.
Finally, practical solutions should be implemented to diminish the increasing burden of diabetes on health services. Solutions include shifting care of diabetes from senior health-care workers towards nurses,135, 136 involvement of community health-care workers,149 and introduction of peer support for patients with diabetes in low-resource settings,137, 138 all of which have been shown to be effective in the management of diabetes or cardiovascular risk. It should be noted that integration of care for NTDs with other services is likely to be more challenging than, for instance, for HIV. NTDs include a whole range of infectious diseases, some rare, some lacking awareness, and many with a scarcity of good diagnostic tools or effective treatment options.
Overlapping morbidity
The morbidity arising from some NTDs overlaps with that of diabetes. The two most important NTDs that cause blindness are trachoma and onchocerciasis. Trachoma results from chronic inflammation mediated by C trachomatis. Although there appear to be no biological associations, the key interaction with diabetes is the relative demand for eye health services.150 In 2010, 1·4% of global blindness (total 32·4 million people) was attributable to trachoma, and 2·4% to diabetic retinopathy. Cataract, which is frequently a complication of diabetes, was the cause of a third of all blindness. Onchocerciasis results from ocular inflammation caused by the microfilariae of the nematode Onchocerca volvulus. No interaction with diabetes has been reported, but as with trachoma, onchocerciasis represents a competing demand on eye health services in endemic regions. A more optimistic view would be that it represents an opportunity for added value from service provision. The global map of trachoma is currently being populated by data; it is hoped that efforts at control through the SAFE strategy will start to shrink areas of hyperendemicity. Onchocerciasis prevalence is falling as intermittent mass drug therapy with ivermectin is rolled out in sub-Saharan Africa, and the last remaining areas in Latin America are close to achieving elimination.
End-stage kidney disease provides another example of potential for sharing of health services. In tropical countries, poorly managed and recurrent common urinary tract infections, and obstructive uropathy due to Schistosoma haematobium contribute, like diabetes, to the burden of end-stage renal failure that is poorly served in resource-constrained countries.151, 152
Leprosy and diabetes share several morbidity sequelae; both diseases feature in the differential diagnosis of peripheral neuropathy and mononeuritis multiplex, and are among the causes of Charcot joint (neuropathic arthropathy). As such, there is potential for overlap of use of clinical services related to peripheral neuropathy and neuropathic foot ulcers, although the additional vascular compromise seen in the diabetic foot is not a feature of leprosy neuropathic ulcers. Surveillance data for eye, kidney, and neuropathic complications of NTDs and diabetes in tropical countries are scarce. As a result, it is impossible to estimate the number of diagnosed and undiagnosed patients who would benefit from integrated services. What seems obvious, though, is that the projected global growth of diabetes will greatly increase the need and costs of preventive and therapeutic services. Brazil for instance, witnessed a 20% growth in diabetes between 2006 and 2010, with an estimated annual direct cost of diabetes of almost US$4 billion.153 Diabetes-associated infections are likely to be a major factor in these costs.
Conclusions
Several conclusions can be made with respect to the relation between diabetes and infectious diseases in tropical countries. First, the global diabetes epidemic disproportionally affects tropical countries, where chronic care, glycaemic control, and wound and foot care can be very challenging. Diabetes increases incidence and severity, and affects treatment and outcome of a large number of infectious diseases, including many—such as tuberculosis and dengue—that have the highest burden in tropical countries. This incidence puts a heavy burden on health services that are often already overstretched. Second, health-care-associated infections and antimicrobial drug resistance are a major problem for patients with diabetes in tropical countries. Third, while some helminth infections might afford protection against diabetes, diabetes might increase the risk or severity of other helminth infections, although data are scarce. Fourth, there is a general scarcity of epidemiological, clinical, and health systems research addressing the interaction of diabetes and infectious diseases in tropical countries except for tuberculosis25, 154 and melioidosis,28, 102, 103 and there are very few data on underlying mechanisms or the effect of diabetes medication. As such, there is an urgent need for more research to address all these issues, and for efforts to better link or integrate care for communicable and non-communicable diseases. And of course, in light of the dramatic rise in diabetes prevalence in tropical countries, the interaction between diabetes and infectious diseases is yet another reason to improve prevention and management of diabetes in high-burden tropical countries.
Search strategy and selection criteria
We searched MEDLINE between its inception and Dec 1, 2015, for articles in English using the search terms (alone or in combination) “diabetes”, “hyperglycaemia/-mic” in combination with “infection” and MeSH (medical subject heading) terms for each of the neglected tropical diseases and their causative agents, and “HIV”, “malaria”, “melioidosis”, “Burkholderia pseudomallei”, “HCV”, “dengue”, “chikungunya”, “MERS”, “SARS”, “Ebola”, or “Zika”. For the relation between diabetes and tuberculosis we used a recent Review in The Lancet Diabetes and Endocrinology.
We manually searched the references of individual articles for additional publications that were not previously identified. Additional searches were performed for gestational diabetes, certain antidiabetic drugs, epidemiology of antimicrobial drug resistance, nosocomial infections in tropical countries, and public health implications. We included articles that we felt were most relevant and of highest available quality. We used no geographical criteria and had no preference for papers published from a particular country or continent. We also used the WHO website on NTDs as a reference.
Acknowledgments
Acknowledgments
RvC and DAJM are supported by the TANDEM project on tuberculosis and diabetes, which is funded by the European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement number 305279.
Contributors
RvC designed the manuscript, performed search strategies, and wrote the first draft. SvdV was responsible for the section “Implications for health services”. DAJM was responsible for additional literature searches and the section on NTDs. All authors contributed to the final draft.
Declaration of interests
We declare no competing interests.
References
- 1.Riza AL, Pearson F, Ugarte-Gil C. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol. 2014;2:740–753. doi: 10.1016/S2213-8587(14)70110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg AY, Weerarathna T, McCarthy JS, Davis TME. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2006;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation . IDF diabetes. 7 edn. International Diabetes Federation; Brussels, Belgium: 2015. http://www.diabetesatlas.org (accessed July 21, 2016). [Google Scholar]
- 4.Ma RC, Lin X, Jia W. Causes of type 2 diabetes in China. Lancet Diabetes Endocrinol. 2014;2:980–991. doi: 10.1016/S2213-8587(14)70145-7. [DOI] [PubMed] [Google Scholar]
- 5.Lonnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2014;2:730–739. doi: 10.1016/S2213-8587(14)70109-3. [DOI] [PubMed] [Google Scholar]
- 6.Odone A, Houben RM, White RG, Lönnroth K. The effect of diabetes and undernutrition trends on reaching 2035 global tuberculosis targets. Lancet Diabetes Endocrinol. 2014;2:754–764. doi: 10.1016/S2213-8587(14)70164-0. [DOI] [PubMed] [Google Scholar]
- 7.Hotez PJ, Alvarado M, Basáñez M-G. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e2865. doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh V, Sharma B, Sen R, Agrawal S, Bhagol A, Bali R. Rhinocerebral mucormycosis: a diagnostic challenge and therapeutic dilemma in immunocompetent host. J Oral Maxillofac Surg. 2012;70:1369–1375. doi: 10.1016/j.joms.2011.06.209. [DOI] [PubMed] [Google Scholar]
- 11.Siu LK, Yeh K-M, Lin J-C, Fung C-P, Chang F-Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 12.Hirji I, Guo Z, Andersson SW, Hammar N, Gomez-Caminero A. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD) J Diabetes Complications. 2012;26:513–516. doi: 10.1016/j.jdiacomp.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Kornum JB, Thomsen RW, Riis A, Lervang H-H, Schønheyder HC, Sørensen HT. Type 2 diabetes and pneumonia outcomes: a population-based cohort study. Diabetes Care. 2007;30:2251–2257. doi: 10.2337/dc06-2417. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen RW, Riis AH, Kjeldsen S, Schønheyder HC. Impact of diabetes and poor glycaemic control on risk of bacteraemia with haemolytic streptococci groups A, B, and G. J Infect. 2011;63:8–16. doi: 10.1016/j.jinf.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Thomsen RW, Hundborg HH, Lervang H-H, Johnsen SP, Sørensen HT, Schønheyder HC. Diabetes and outcome of community-acquired pneumococcal bacteremia: a 10-year population-based cohort study. Diabetes Care. 2004;27:70–76. doi: 10.2337/diacare.27.1.70. [DOI] [PubMed] [Google Scholar]
- 16.Grijalva CG, Zhu Y, Williams DJ. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA. 2015;314:1488–1497. doi: 10.1001/jama.2015.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uehara K, Yasunaga H, Morizaki Y, Horiguchi H, Fushimi K, Tanaka S. Necrotising soft-tissue infections of the upper limb: risk factors for amputation and death. Bone Joint J. 2014;96-B:1530–1534. doi: 10.1302/0301-620X.96B11.34888. [DOI] [PubMed] [Google Scholar]
- 18.Vaziri K, Pershing S, Albini TA, Moshfeghi DM, Moshfeghi AA. Risk factors predictive of endogenous endophthalmitis among hospitalized patients with hematogenous infections in the United States. Am J Ophthalmol. 2015;159:498–504. doi: 10.1016/j.ajo.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Søraas A, Sundsfjord A, Sandven I, Brunborg C, Jenum PA. Risk factors for community-acquired urinary tract infections caused by ESBL-producing enterobacteriaceae—a case-control study in a low prevalence country. PLoS One. 2013;8:e69581. doi: 10.1371/journal.pone.0069581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y-H, Chen P-L, Hung Y-P, Ko W-C. Risk factors and clinical impact of levofloxacin or cefazolin nonsusceptibility or ESBL production among uropathogens in adults with community-onset urinary tract infections. J Microbiol Immunol Infect. 2014;47:197–203. doi: 10.1016/j.jmii.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Guh AY, Bulens SN, Mu Y. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US Communities, 2012–2013. JAMA. 2015;314:1479–1487. doi: 10.1001/jama.2015.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castilla J, Godoy P, Domínguez A. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis. 2013;57:167–175. doi: 10.1093/cid/cit194. [DOI] [PubMed] [Google Scholar]
- 23.Gan Y-H. Host susceptibility factors to bacterial infections in type 2 diabetes. PLoS Pathog. 2013;9:e1003794. doi: 10.1371/journal.ppat.1003794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodgson K, Morris J, Bridson T, Govan B, Rush C, Ketheesan N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology. 2015;144:171–185. doi: 10.1111/imm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronacher K, Joosten SA, van Crevel R, Dockrell HM, Walzl G, Ottenhoff THM. Acquired immunodeficiencies and tuberculosis: focus on HIV/AIDS and diabetes mellitus. Immunol Rev. 2015;264:121–137. doi: 10.1111/imr.12257. [DOI] [PubMed] [Google Scholar]
- 26.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16(suppl 1):S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singhal A, Jie L, Kumar P. Metformin as adjunct antituberculosis therapy. Sci Transl Med. 2014;6:263ra159. doi: 10.1126/scitranslmed.3009885. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Foo G, Lim WP. Sulphonylurea usage in melioidosis is associated with severe disease and suppressed immune response. PLoS Negl Trop Dis. 2014;8:e2795. doi: 10.1371/journal.pntd.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allegranzi B, Bagheri Nejad S, Combescure C. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 30.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4:148–158. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 31.Manne-Goehler J, Atun R, Stokes A, et al. Unmet need for diabetes care in sub-Saharan Africa: individual pooled analysis in 12 countries. Lancet Diabetes Endocrinol (in press). [DOI] [PubMed]
- 32.Beran D, Ewen M, Laing R. Constraints and challenges in access to insulin: a global perspective. Lancet Diabetes Endocrinol. 2016;4:275–285. doi: 10.1016/S2213-8587(15)00521-5. [DOI] [PubMed] [Google Scholar]
- 33.Grant P. Management of diabetes in resource-poor settings. Clin Med. 2013;13:27–31. doi: 10.7861/clinmedicine.13-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tankwanchi ABS, Ozden C, Vermund SH. Physician emigration from sub-Saharan Africa to the United States: analysis of the 2011 AMA physician masterfile. PLoS Med. 2013;10:e1001513. doi: 10.1371/journal.pmed.1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Djahmi N, Messad N, Nedjai S. Molecular epidemiology of Staphylococcus aureus strains isolated from inpatients with infected diabetic foot ulcers in an Algerian University Hospital. Clin Microbiol Infect. 2013;19:E398–E404. doi: 10.1111/1469-0691.12199. [DOI] [PubMed] [Google Scholar]
- 36.Thompson ND, Perz JF. Eliminating the blood: ongoing outbreaks of hepatitis B virus infection and the need for innovative glucose monitoring technologies. J Diabetes Sci Technol. 2009;3:283–288. doi: 10.1177/193229680900300208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gizaw M, Harries AD, Ade S. Diabetes mellitus in Addis Ababa, Ethiopia: admissions, complications and outcomes in a large referral hospital. Public Health Action. 2015;5:74–78. doi: 10.5588/pha.14.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbas ZG, Archibald LK. Challenges for management of the diabetic foot in Africa: doing more with less. Int Wound J. 2007;4:305–313. doi: 10.1111/j.1742-481X.2007.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pendsey S. Clinical profile of diabetic foot in India. Int J Low Extrem Wounds. 2010;9:180–184. doi: 10.1177/1534734610380025. [DOI] [PubMed] [Google Scholar]
- 40.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361:1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Lv L, Wen X. A clinical analysis of diabetic patients with hand ulcer in a diabetic foot centre. Diabet Med. 2010;27:848–851. doi: 10.1111/j.1464-5491.2010.03012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raimi TH, Alese OO. Tropical diabetes hand syndrome with autoamputation of the digits: case report and review of literature. Pan Afr Med J. 2014;18:199. doi: 10.11604/pamj.2014.18.199.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbas ZG, Archibald LK. Tropical diabetic hand syndrome. Epidemiology, pathogenesis, and management. Am J Clin Dermatol. 2005;6:21–28. doi: 10.2165/00128071-200506010-00003. [DOI] [PubMed] [Google Scholar]
- 44.Boulton AJM, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 45.Mehmood K, Akhtar ST, Talib A. Clinical profile and management outcome of diabetic foot ulcers in a tertiary care hospital. J Coll Physicians Surg Pak. 2008;18:408–412. [PubMed] [Google Scholar]
- 46.Gulam-Abbas Z, Lutale JK, Morbach S, Archibald LK. Clinical outcome of diabetes patients hospitalized with foot ulcers, Dar es Salaam, Tanzania. Diabet Med. 2002;19:575–579. doi: 10.1046/j.1464-5491.2002.00740.x. [DOI] [PubMed] [Google Scholar]
- 47.Abbas ZG, Lutale JK, Bakker K, Baker N, Archibald LK. The ‘Step by Step’ Diabetic Foot Project in Tanzania: a model for improving patient outcomes in less-developed countries. Int Wound J. 2011;8:169–175. doi: 10.1111/j.1742-481X.2010.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun HY, Singh N. Mucormycosis: its contemporary face and management strategies. Lancet Infect Dis. 2011;11:301–311. doi: 10.1016/S1473-3099(10)70316-9. [DOI] [PubMed] [Google Scholar]
- 49.Bitar D, van Cauteren D, Lanternier F. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15:1395–1401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhansali A, Bhadada S, Sharma A. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670–674. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakshi SS. Rhino-orbital mucormycosis in a patient with diabetes. Lancet Diabetes Endocrinol. 2016 doi: 10.1016/S2213-8587(15)00522-7. published online April 29. [DOI] [PubMed] [Google Scholar]
- 52.Meis JF, Chakrabarti A. Changing epidemiology of an emerging infection: zygomycosis. Clin Microbiol Infect. 2009;15(suppl 5):10–14. doi: 10.1111/j.1469-0691.2009.02973.x. [DOI] [PubMed] [Google Scholar]
- 53.Chakrabarti A, Chatterjee SS, Das A. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009;85:573–581. doi: 10.1136/pgmj.2008.076463. [DOI] [PubMed] [Google Scholar]
- 54.Chakrabarti A, Singh R. The emerging epidemiology of mould infections in developing countries. Curr Opin Infect Dis. 2011;24:521–526. doi: 10.1097/QCO.0b013e32834ab21e. [DOI] [PubMed] [Google Scholar]
- 55.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabarsi P, Baghaei P, Marjani M, Vollmer WM, Masjedi M-R, Harries AD. Changes in glycosylated haemoglobin and treatment outcomes in patients with tuberculosis in Iran: a cohort study. J Diabetes Metab Disord. 2014;13:123. doi: 10.1186/s40200-014-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker MA, Harries AD, Jeon CY. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maganga E, Smart LR, Kalluvya S. Glucose metabolism disorders, HIV and antiretroviral therapy among Tanzanian adults. PLoS One. 2015;10:e0134410. doi: 10.1371/journal.pone.0134410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohammed AE, Shenkute TY, Gebisa WC. Diabetes mellitus and risk factors in human immunodeficiency virus-infected individuals at Jimma University Specialized Hospital, Southwest Ethiopia. Diabetes Metab Syndr Obes. 2015;8:197–206. doi: 10.2147/DMSO.S80084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abrahams Z, Dave JA, Maartens G, Levitt NS. Changes in blood pressure, glucose levels, insulin secretion and anthropometry after long term exposure to antiretroviral therapy in South African women. AIDS Res Ther. 2015;12:24. doi: 10.1186/s12981-015-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capeau J, Bouteloup V, Katlama C. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26:303–314. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 62.Rasmussen LD, Mathiesen ER, Kronborg G, Pedersen C, Gerstoft J, Obel N. Risk of diabetes mellitus in persons with and without HIV: a Danish nationwide population-based cohort study. PLoS One. 2012;7:e44575. doi: 10.1371/journal.pone.0044575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karamchand S, Leisegang R, Schomaker M. Risk factors for incident diabetes in a cohort taking first-line nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Medicine (Baltimore) 2016;95:e2844. doi: 10.1097/MD.0000000000002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Werfalli M, Engel ME, Musekiwa A, Kengne AP, Levitt NS. The prevalence of type 2 diabetes among older people in Africa: a systematic review. Lancet Diabetes Endocrinol. 2016;4:72–84. doi: 10.1016/S2213-8587(15)00363-0. [DOI] [PubMed] [Google Scholar]
- 65.El Sayed NM, Gomatos PJ, Beck-Sagué CM. Epidemic transmission of human immunodeficiency virus in renal dialysis centers in Egypt. J Infect Dis. 1999;181:91–97. doi: 10.1086/315167. [DOI] [PubMed] [Google Scholar]
- 66.Fabrizi F, Messa P. Transmission of hepatitis C virus in dialysis units: a systematic review of reports on outbreaks. Int J Artif Organs. 2015;38:471–480. doi: 10.5301/ijao.5000437. [DOI] [PubMed] [Google Scholar]
- 67.Murray CJ, Ortblad KF, Guinovart C. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danquah I, Bedu-Addo G, Mockenhaupt FP. Type 2 diabetes mellitus and increased risk for malaria infection. Emerg Infect Dis. 2010;16:1601–1604. doi: 10.3201/eid1610.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desai M, ter Kuile FO, Nosten F. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 70.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103:176–185. doi: 10.1016/j.diabres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Santos dos VM, da Cunha SF, de P Teixeira V. Frequency of diabetes mellitus and hyperglycemia in chagasic and non-chagasic women. Rev Soc Bras Med Trop. 1999;32:489–496. doi: 10.1590/s0037-86821999000500004. (in Portuguese). [DOI] [PubMed] [Google Scholar]
- 72.Chen C-Y, Lee M-Y, Lin K-D. Diabetes mellitus increases severity of thrombocytopenia in dengue-infected patients. Int J Mol Sci. 2015;16:3820–3830. doi: 10.3390/ijms16023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mallhi TH, Khan AH, Adnan AS, Sarriff A, Khan YH, Jummaat F. Clinico-laboratory spectrum of dengue viral infection and risk factors associated with dengue hemorrhagic fever: a retrospective study. BMC Infect Dis. 2015;15:399. doi: 10.1186/s12879-015-1141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pang J, Salim A, Lee VJ. Diabetes with hypertension as risk factors for adult dengue hemorrhagic fever in a predominantly dengue serotype 2 epidemic: a case control study. PLoS Negl Trop Dis. 2012;6:e1641. doi: 10.1371/journal.pntd.0001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Htun NSN, Odermatt P, Eze IC, Boillat-Blanco N, D'Acremont V, Probst-Hensch N. Is diabetes a risk factor for a severe clinical presentation of dengue? Review and meta-analysis. PLoS Negl Trop Dis. 2015;9:e0003741. doi: 10.1371/journal.pntd.0003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Figueiredo MAA, Rodrigues LC, Barreto ML. Allergies and diabetes as risk factors for dengue hemorrhagic fever: results of a case control study. PLoS Negl Trop Dis. 2010;4:e699. doi: 10.1371/journal.pntd.0000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mallhi TH, Khan AH, Adnan AS, Sarriff A, Khan YH, Jummaat F. Incidence, characteristics and risk factors of acute kidney injury among dengue patients: a retrospective analysis. PLoS One. 2015;10:e0138465. doi: 10.1371/journal.pone.0138465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pang J, Thein T-L, Leo YS, Lye DC. Early clinical and laboratory risk factors of intensive care unit requirement during 2004–2008 dengue epidemics in Singapore: a matched case-control study. BMC Infect Dis. 2014;14:649. doi: 10.1186/s12879-014-0649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jha TK, Sharma VK. Pentamidine-induced diabetes mellitus. Trans R Soc Trop Med Hyg. 1984;78:252–253. doi: 10.1016/0035-9203(84)90289-x. [DOI] [PubMed] [Google Scholar]
- 80.Assan R, Perronne C, Assan D. Pentamidine-induced derangements of glucose homeostasis. Determinant roles of renal failure and drug accumulation. A study of 128 patients. Diabetes Care. 1995;18:47–55. doi: 10.2337/diacare.18.1.47. [DOI] [PubMed] [Google Scholar]
- 81.Belehu A, Naafs B. Diabetes mellitus associated with pentamidine mesylate. Lancet. 1982;1:1463–1464. doi: 10.1016/s0140-6736(82)92468-0. [DOI] [PubMed] [Google Scholar]
- 82.Nigam P, Dayal SG, Srivastava P. Diabetic status in leprosy. Hansenol Int. 1979;4:7–14. [PubMed] [Google Scholar]
- 83.Saini C, Ramesh V, Nath I. CD4+ Th17 cells discriminate clinical types and constitute a third subset of non-Th1, non-Th2 T cells in human leprosy. PLoS Negl Trop Dis. 2013;7:e2338. doi: 10.1371/journal.pntd.0002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aravindhan V, Mohan V, Surendar J. Decreased prevalence of lymphatic filariasis among subjects with type-1 diabetes. Am J Trop Med Hyg. 2010;83:1336–1339. doi: 10.4269/ajtmh.2010.10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aravindhan V, Mohan V, Surendar J. Decreased prevalence of lymphatic filariasis among diabetic subjects associated with a diminished pro-inflammatory cytokine response (CURES 83) PLoS Negl Trop Dis. 2010;4:e707. doi: 10.1371/journal.pntd.0000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Lu J, Huang Y. Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J Clin Endocrinol Metab. 2013;98:E283–E287. doi: 10.1210/jc.2012-2517. [DOI] [PubMed] [Google Scholar]
- 87.Cooke A, Tonks P, Jones FM. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 88.Zaccone P, Burton OT, Gibbs S. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J Biomed Biotechnol. 2010;2010:795210. doi: 10.1155/2010/795210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hulstijn M, Barros L de A, Neves RH, de Moura EG, Machado-Silva JR. Parasitological and morphological study of Schistosoma mansoni and diabetes mellitus in mice. Exp Parasitol. 2011;129:42–47. doi: 10.1016/j.exppara.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 90.Hussaarts L, García-Tardón N, van Beek L. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015;29:3027–3039. doi: 10.1096/fj.14-266239. [DOI] [PubMed] [Google Scholar]
- 91.Wiria AE, Hamid F, Wammes LJ, Prasetyani MA. Infection with soil-transmitted helminths is associated with increased insulin sensitivity. PLoS One. 2015;10:e0127746. doi: 10.1371/journal.pone.0127746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hays R, Esterman A, Giacomin P, Loukas A, McDermott R. Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? Evidence from Australian Aboriginal adults. Diabetes Res Clin Pract. 2015;107:355–361. doi: 10.1016/j.diabres.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 93.Lund ME, O'Brien BA, Hutchinson AT. Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS One. 2014;9:e86289. doi: 10.1371/journal.pone.0086289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Espinoza-Jiménez A, Rivera-Montoya I, Cárdenas-Arreola R, Morán L, Terrazas LI. Taenia crassiceps infection attenuates multiple low-dose streptozotocin-induced diabetes. J Biomed Biotechnol. 2010;2010:850541. doi: 10.1155/2010/850541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saraya MA, Al-Fadhli MA, Qasem JA. Diabetic status of patients with leprosy in Kuwait. J Infect Public Health. 2012;5:360–365. doi: 10.1016/j.jiph.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Smith WC. Screening for diabetes mellitus in leprosy patients with complicated ulcers. Lepr India. 1979;51:236–238. [PubMed] [Google Scholar]
- 97.Papang R, John AS, Abraham S, Rao PSSS. A study of steroid-induced diabetes mellitus in leprosy. Indian J Lepr. 2009;81:125–129. [PubMed] [Google Scholar]
- 98.Peacock SJ. Melioidosis. In: Cohen J, Opal SM, Powderly WG, editors. Infectious diseases. 3rd edn. Elsevier; London: 2010. pp. 1213–1217. [Google Scholar]
- 99.Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36:111–125. doi: 10.1055/s-0034-1398389. [DOI] [PubMed] [Google Scholar]
- 100.Meumann EM, Cheng AC, Ward L, Currie BJ. Clinical features and epidemiology of melioidosis pneumonia: results from a 21-year study and review of the literature. Clin Infect Dis. 2012;54:362–369. doi: 10.1093/cid/cir808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hassan MRA, Pani SP, Peng NP. Incidence, risk factors and clinical epidemiology of melioidosis: a complex socio-ecological emerging infectious disease in the Alor Setar region of Kedah, Malaysia. BMC Infect Dis. 2010;10:302. doi: 10.1186/1471-2334-10-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morris J, Williams N, Rush C. Burkholderia pseudomallei triggers altered inflammatory profiles in a whole-blood model of type 2 diabetes-melioidosis comorbidity. Infect Immun. 2012;80:2089–2099. doi: 10.1128/IAI.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan KS, Lee KO, Low KC. Glutathione deficiency in type 2 diabetes impairs cytokine responses and control of intracellular bacteria. J Clin Invest. 2012;122:2289–2300. doi: 10.1172/JCI57817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koh GC, Maude RR, Schreiber MF. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis. 2011;52:717–725. doi: 10.1093/cid/ciq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhatt S, Gething PW, Brady OJ. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jean CM, Honarmand S, Louie JK, Glaser CA. Risk factors for West Nile virus neuroinvasive disease, California, 2005. Emerg Infect Dis. 2007;13:1918–1920. doi: 10.3201/eid1312.061265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang JK, Feng Y, Yuan MY. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 108.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barry M, Traoré FA, Sako FB. Ebola outbreak in Conakry, Guinea: epidemiological, clinical, and outcome features. Med Mal Infect. 2014;44:491–494. doi: 10.1016/j.medmal.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Deshmukh RA, Yemul VL. Fatal rabies encephalitis despite post-exposure vaccination in a diabetic patient: a need for use of rabies immune globulin in all post-exposure cases. J Assoc Physicians India. 1999;47:546–547. [PubMed] [Google Scholar]
- 111.Chiheb S, Oudrhiri L, Zouhair K, Soussi Abdallaoui M, Riyad M, Benchikhi H. Unusual clinical presentation of cutaneous leishmaniasis in three diabetic patients. Ann Dermatol Venereol. 2012;139:542–545. doi: 10.1016/j.annder.2012.05.013. (in French). [DOI] [PubMed] [Google Scholar]
- 112.Ceyhan AM, Yildirim M, Basak PY, Akkaya VB. Unusual multifocal cutaneous leishmaniasis in a diabetic patient. Eur J Dermatol. 2009;19:514–515. doi: 10.1684/ejd.2009.0732. [DOI] [PubMed] [Google Scholar]
- 113.Sharquie KE, Najim RA, Hussein AK. Reinfestation in cutaneous leishmaniasis: a new look at predisposing conditions. Saudi Med J. 2000;21:464–467. [PubMed] [Google Scholar]
- 114.dos Santos VM, de P Teixeira V, da Cunha DF. Pancreatic anatomopathologic changes in chronic chagasic women. Preliminary data. Arq Gastroenterol. 1999;36:127–132. (in Portuguese). [PubMed] [Google Scholar]
- 115.Tanowitz HB, Amole B, Hewlett D, Wittner M. Trypanosoma cruzi infection in diabetic mice. Trans R Soc Trop Med Hyg. 1988;82:90–93. [PubMed] [Google Scholar]
- 116.Gould CL, De Gee AL, Mansfield JM, Sonnenfeld G. Trypanosoma brucei rhodesiense infection in mice prevents virus-induced diabetes: possible role of interferon and immunological mechanisms. J Interferon Res. 1986;6:499–506. doi: 10.1089/jir.1986.6.499. [DOI] [PubMed] [Google Scholar]
- 117.Mendonça SC, Gonçalves-Pires Mdo R, Rodrigues RM, Ferreira A, Jr, Costa-Cruz JM. Is there an association between positive Strongyloides stercoralis serology and diabetes mellitus? Acta Trop. 2006;99:102–105. doi: 10.1016/j.actatropica.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 118.Murali A, Rajendiran G, Ranganathan K, Shanthakumari S. Disseminated infection with Strongyloides stercoralis in a diabetic patient. Indian J Med Microbiol. 2010;28:407–408. doi: 10.4103/0255-0857.71854. [DOI] [PubMed] [Google Scholar]
- 119.Debussche X, Toublanc M, Camillieri JP, Assan R. Overwhelming strongyloidiasis in a diabetic patient following ACTH treatment and keto-acidosis. Diabetes Metab. 1988;14:294–298. [PubMed] [Google Scholar]
- 120.Coovadia YM, Rajput MC, Bhana RH. Disseminated strongyloidiasis in a diabetic patient. Trop Geogr Med. 1993;45:179–180. [PubMed] [Google Scholar]
- 121.Arnaud A, Sarles JC, Sahel J, Sastre B. Distal pancreatic atrophy and diabetes associated with an intrapancreatic hydatid cyst. Pancreas. 1992;7:394–396. doi: 10.1097/00006676-199205000-00019. [DOI] [PubMed] [Google Scholar]
- 122.Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14:1150–1162. doi: 10.1016/S1473-3099(14)70771-6. [DOI] [PubMed] [Google Scholar]
- 123.Bygbjerg IC. Double burden of noncommunicable and infectious diseases in developing countries. Science. 2012;337:1499–1501. doi: 10.1126/science.1223466. [DOI] [PubMed] [Google Scholar]
- 124.Aluttis C, Bishaw T, Frank MW. The workforce for health in a globalized context—global shortages and international migration. Glob Health Action. 2014;7:23611. doi: 10.3402/gha.v7.23611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Samb B, Desai N, Nishtar S. Prevention and management of chronic disease: a litmus test for health-systems strengthening in low-income and middle-income countries. Lancet. 2010;376:1785–1797. doi: 10.1016/S0140-6736(10)61353-0. [DOI] [PubMed] [Google Scholar]
- 126.Lim SS, Vos T, Flaxman AD. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Santosa A, Wall S, Fottrell E, Högberg U, Byass P. The development and experience of epidemiological transition theory over four decades: a systematic review. Glob Health Action. 2014;7:23574. doi: 10.3402/gha.v7.23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koopman JJ, van Bodegom D, Ziem JB, Westendorp RG. An emerging epidemic of noncommunicable diseases in developing populations due to a triple evolutionary mismatch. Am J Trop Med Hyg. 2016;94:1189–1192. doi: 10.4269/ajtmh.15-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patel DN, Nossel C, Alexander E, Yach D. Innovative business approaches for incenting health promotion in sub-Saharan Africa: progress and persisting challenges. Prog Cardiovasc Dis. 2013;56:356–362. doi: 10.1016/j.pcad.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 130.Edwards JK, Bygrave H, van den Bergh R. HIV with non-communicable diseases in primary care in Kibera, Nairobi, Kenya: characteristics and outcomes 2010–2013. Trans R Soc Trop Med Hyg. 2015;109:440–446. doi: 10.1093/trstmh/trv038. [DOI] [PubMed] [Google Scholar]
- 131.Laksanasopin T, Guo TW, Nayak S. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci Transl Med. 2015;7:273re1. doi: 10.1126/scitranslmed.aaa0056. [DOI] [PubMed] [Google Scholar]
- 132.Pombi M, Guelbeogo WM, Calzetta M. Evaluation of a protocol for remote identification of mosquito vector species reveals BG-Sentinel trap as an efficient tool for Anopheles gambiae outdoor collection in Burkina Faso. Malar J. 2015;14:161. doi: 10.1186/s12936-015-0674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lester RT, Ritvo P, Mills EJ. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 134.Araínga M, Guo D, Wiederin J, Ciborowski P, McMillan J, Gendelman HE. Opposing regulation of endolysosomal pathways by long-acting nanoformulated antiretroviral therapy and HIV-1 in human macrophages. Retrovirology. 2015;12:5. doi: 10.1186/s12977-014-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Labhardt ND, Balo J-R, Ndam M, Grimm J-J, Manga E. Task shifting to non-physician clinicians for integrated management of hypertension and diabetes in rural Cameroon: a programme assessment at two years. BMC Health Serv Res. 2010;10:339. doi: 10.1186/1472-6963-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Some D, Edwards JK, Reid T. Task shifting the management of non-communicable diseases to nurses in Kibera, Kenya: does it work? PLoS One. 2016;11:e0145634. doi: 10.1371/journal.pone.0145634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fisher EB, Boothroyd RI, Coufal MM. Peer support for self-management of diabetes improved outcomes in international settings. Health Aff (Millwood) 2012;31:130–139. doi: 10.1377/hlthaff.2011.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Labhardt ND, Balo J-R, Ndam M, Manga E, Stoll B. Improved retention rates with low-cost interventions in hypertension and diabetes management in a rural African environment of nurse-led care: a cluster-randomised trial. Trop Med Int Health. 2011;16:1276–1284. doi: 10.1111/j.1365-3156.2011.02827.x. [DOI] [PubMed] [Google Scholar]
- 139.Chen J, Wu X, Gu D. Hypertension and cardiovascular diseases intervention in the capital steel and iron company and Beijing Fangshan community. Obes Rev. 2008;9(suppl 1):142–145. doi: 10.1111/j.1467-789X.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 140.Lara A, Yancey AK, Tapia-Conye R. Pausa para tu Salud: reduction of weight and waistlines by integrating exercise breaks into workplace organizational routine. Prev Chronic Dis. 2008;5:A12. [PMC free article] [PubMed] [Google Scholar]
- 141.Hogerzeil HV, Liberman J, Wirtz VJ. Promotion of access to essential medicines for non-communicable diseases: practical implications of the UN political declaration. Lancet. 2013;381:680–689. doi: 10.1016/S0140-6736(12)62128-X. [DOI] [PubMed] [Google Scholar]
- 142.de Waure C, Lauret G-J, Ricciardi W. Lifestyle interventions in patients with coronary heart disease: a systematic review. Am J Prev Med. 2013;45:207–216. doi: 10.1016/j.amepre.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 143.Uthman OA, Hartley L, Rees K, Taylor F, Ebrahim S, Clarke A. Multiple risk factor interventions for primary prevention of cardiovascular disease in low- and middle-income countries. Cochrane Database Syst Rev. 2015;8 doi: 10.1002/14651858.CD011163.pub2. CD011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Engelgau MM, Karan A, Mahal A. The economic impact of non-communicable diseases on households in India. Global Health. 2012;8:9. doi: 10.1186/1744-8603-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van de Vijver S, Oti S, Addo J, de Graft-Aikins A, Agyemang C. Review of community-based interventions for prevention of cardiovascular diseases in low- and middle-income countries. Ethn Health. 2012;17:651–676. doi: 10.1080/13557858.2012.754409. [DOI] [PubMed] [Google Scholar]
- 146.Rabkin M, Melaku Z, Bruce K. Strengthening health systems for chronic care: leveraging HIV programs to support diabetes services in Ethiopia and Swaziland. J Trop Med. 2012;2012:137460. doi: 10.1155/2012/137460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rubinstein A, Miranda JJ, Beratarrechea A. Effectiveness of an mHealth intervention to improve the cardiometabolic profile of people with prehypertension in low-resource urban settings in Latin America: a randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:52–63. doi: 10.1016/S2213-8587(15)00381-2. [DOI] [PubMed] [Google Scholar]
- 148.Ramachandran A, Snehalatha C, Ram J. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013;1:191–198. doi: 10.1016/S2213-8587(13)70067-6. [DOI] [PubMed] [Google Scholar]
- 149.Mishra SR, Neupane D, Briffa TG, Kallestrup P. mHealth plus community health worker interventions: the future research agenda. Lancet Diabetes Endocrinol. 2016;4:387–388. doi: 10.1016/S2213-8587(16)30001-8. [DOI] [PubMed] [Google Scholar]
- 150.Bourne RR, Stevens GA, White RA. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1:e339–e349. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 151.Shaheen FA, Al-Khader AA. Preventive strategies of renal failure in the Arab world. Kidney Int Suppl. 2005;98:S37–S40. doi: 10.1111/j.1523-1755.2005.09807.x. [DOI] [PubMed] [Google Scholar]
- 152.Essamie MA, Soliman A, Fayad TM, Barsoum S, Kjellstrand CM. Serious renal disease in Egypt. Int J Artif Organs. 1995;18:254–260. [PubMed] [Google Scholar]
- 153.Bertoldi AD, Kanavos P, França GVA. Epidemiology, management, complications and costs associated with type 2 diabetes in Brazil: a comprehensive literature review. Global Health. 2013;9:62. doi: 10.1186/1744-8603-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44:617–626. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]