Summary
Bacterial and viral infections occur early and recurrently in life and thereby impose a substantial disease burden. Besides causing clinical symptoms, a potential role of infection in the development of the asthma syndrome later in life has also been suggested. However, whether bacterial and viral infections unmask host factors in children at risk of asthma or whether they directly cause asthma remains unclear; both viewpoints could be justified, but the underlying mechanisms are complex and poorly understood. Recently, the role of the bacterial microbiome has been emphasised. But data are still sparse and future studies are needed for definitive conclusions to be made. In this Review, we discuss present knowledge of viruses and bacteria that infect and colonise the respiratory tract and mucosal surfaces, including their timepoint of action, host factors related to infection, and their effect on childhood asthma. Childhood asthma could be the result of a combination of altered host susceptibility and infectious agents.
Introduction
We all live, suffer, and prosper in a world of bacteria and viruses. The damage caused by bacteria as infectious agents—ie, invaders of host tissues and disease-causing organisms—is well accepted. Bacteria can trigger various diseases, many of which affect the respiratory tract. During the prenatal period and early childhood, bacterial infections can affect intrauterine lung development during important developmental time windows.1 Bacteria not only cause infections that elicit adaptive immune responses, but also populate habitats in our body in large numbers, outnumbering human cells about ten-fold. Besides metabolic functions, the microbiome—ie, the collective genomes of microbes living inside and on the human body—has a major effect on the development of immune responses early in life. Mice raised under germ-free conditions show several deficiencies in immune responses and are prone to the development of experimental asthma.2 Reconstitution of the gut microbiome of germ-free mice with microbiota from mice raised in specific pathogen-free conditions corrects these deficits, but only when administered early in life.2 The role of the lung microbiome in turn is less well known. Several studies3, 4, 5, 6 have reported specific colonisation with proteobacteria in bronchoalveolar lavage samples and sputum of patients with asthma. But whether this type of colonisation precedes the onset of asthma or occurs secondary to airway inflammation remains to be elucidated. Viruses are generally known as causes of disease and have been associated with upper respiratory tract infections (URTIs) and lower respiratory tract infections (LRTIs).7, 8 Numerous studies7, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 have linked early viral LRTIs to the development of childhood wheeze and asthma. The nature of this association is, however, not completely clear. Viruses might predispose to the onset of wheeze and asthma, but it is equally conceivable that viral infections merely unmask host factors underlying disease susceptibility. Alternatively, both notions might be justified: viruses might trigger wheeze and asthma and thereby aggravate airway inflammation.15, 18 The role of the bacterial microbiome in shaping immune responses to virus infections is currently unknown.
In this Review, we first discuss the controversial role of viruses in the development of childhood asthma. We then review the role of the bacterial microbiome and bacterial infections. Finally, we discuss the potential effect of viral and bacterial infections on the treatment and prevention of childhood asthma.
Viruses
The most commonly identified viruses causing respiratory tract infections in children are human rhinoviruses (HRVs), respiratory syncytial virus (RSV), influenza and parainfluenza viruses, coronavirus, adenovirus, human metapneumovirus, and bocavirus.7, 8, 22 HRVs (family Picornaviridae, genus Enterovirus) are single-stranded, non-enveloped, positive-sense RNA viruses with more than 100 different serotypes. Serotypes are subdivided by their receptor (major group: small intercellular adhesion molecule, minor group: low density lipoprotein receptor) and according to sequence variations—ie, HRV-A or HRV-B and lately also the potentially more virulent HRV-C.7, 25 HRVs were initially thought to be limited to URTIs.24 However, HRVs are able to spread to and replicate in the lower airways,26, 27, 28 and thus might lead to LRTIs.7, 24, 29, 30 RSV (family Paramyxoviridae, genus Pneumovirus) is a single-stranded, enveloped, negative-sense RNA virus that is classified into two subtypes, RSV-A and RSV-B. In most patients, RSV produces only mild symptoms of URTI, but in infants RSV can lead to bronchiolitis with season of birth as an important predictor of severe disease, especially in immunocompromised and premature infants.31, 32, 33, 34, 35
Key messages.
-
•
Viral and bacterial infections are important factors in asthma pathogenesis
-
•
Patients with asthma might be more susceptible to viral and bacterial infections because of impaired mucosal and systemic immune responses and atopy
-
•
Bacterial colonisation of the airway and gut mucosal surfaces might play an important part
-
•
Both host factors and harmful effects of infections probably contribute to the development and progression of asthma
By use of PCR-based viral diagnostics, viruses and multiple coexisting viral strains have been detected in biological samples not only from patients with respiratory tract infections, but also from asymptomatic individuals. Several HRV strains have been shown to circulate during one infectious season36 and HRV RNA was identified in nasal mucus as a remnant of previous infection long after individuals had become symptom-free.7, 8, 31, 37
Viruses predispose to asthma
Abundant epidemiological data have reported an association between respiratory viral infections and the development of childhood wheeze and asthma. Several prospective studies and a few studies of population-based cohorts have investigated children admitted to hospital for symptomatic LRTIs (table ).10, 17, 29, 30, 38, 39, 40, 41, 42, 43, 44, 45 Several of these studies30, 38, 39, 40, 41, 42, 43, 44, 45 undertook analyses of both HRVs and RSV. In the longitudinal Tucson Children's Respiratory Study,10 viral LRTI during the first 3 years of life was an independent determinant of wheeze at age 6 years (table). This risk decreased until age 11–13 years and was accompanied by a reduction in forced expiratory volume during 1 s and increased responsiveness to salbutamol, implying a lasting change in the regulation of airway smooth muscle tone.10 Finnish studies38, 41 replicated this finding and showed that the risk of asthma conferred by virus-induced LRTI might decrease over time (table). Another important population-based birth-cohort, the Tennessee Asthma Bronchiolitis Study (TABS),33 investigated children that were admitted to hospital or visited outpatient and emergency departments with symptoms of bronchiolitis secondary to viral LRTI. Although the risk of asthma was generally increased after viral LRTI during infancy, this risk was also dependent on the age of the child at the peak of the virus season with a maximum risk for those infants aged around 121 days.33 Furthermore, the risk differed between winter months, probably dominated by RSV infections, and non-winter months, probably dominated by HRV infections (table).46, 47 In support of these findings, a further study48 extended the population of the TABS cohort and added a further Californian population; investigators reported that about 13% of childhood asthma cases were attributable to infant LRTIs during the RSV season.
Table.
Studies investigating association between HRV and RSV infection or RSV infection and initiation of recurrent wheeze and asthma
| Study name, country | Type of study | Number of participants | Age at enrolment | Age at assessment of recurrent wheeze or asthma, or both | Assessment of recurrent wheeze or asthma, or both | Atopy | Association between virus-induced wheeze and subsequent recurrent wheeze and asthma | |
|---|---|---|---|---|---|---|---|---|
| HRV and RSV | ||||||||
| Hyvärinen et al38 Kotaniemi-Syrjänen et al41 |
NA, Finland | Prospective observational study of infants admitted to hospital for RTI-associated wheeze | Entry: 100 children Follow-up at 5·6–8·8 years: 82 children Follow-up at 11–13 years: 81 children |
1–23 months | 5·6–8·8 years and 11–13 years | Parental questionnaire, exercise-challenge test; asthma-specific medication | Entry: specific IgE Follow-up: skin prick tests, parental questionnaire on allergic diseases |
HRV: follow-up at 5·6–8·8 years; aOR for outcome asthma.*41 Follow-up at 11–13 years; aOR for outcome asthma.‡38 RSV: follow-up at 11–13 years; aOR for outcome asthma¶38 |
| Jackson et al39, 40 Lemanske et al44 |
Childhood origins of asthma study (COAST) Wisconsin, USA | High-risk birth cohort (at least one parent with allergic disease or asthma, or both) | Entry: 289 children Follow-up at 1 year: 285 children Follow-up at 3 years: 275 children Follow-up at 6 years: 259 children |
Birth | 3 years and 6 years | Parental questionnaire Follow-up at 3 years: wheeze Follow-up at 6 years: doctor-diagnosis of asthma, asthma-specific medication |
Follow-up at 1 year: specific IgE Follow-up at 5 years: skin prick test |
HRV: follow-up at 3 years; OR for outcome wheeze.*44 Follow-up at 6 years; OR for outcome asthma.*40 Markov model: allergic sensitisation precedes HRV-induced wheeze, HR 2·8 (95% CI 1·5–5·1).39 RSV: follow-up at 3 years; OR for outcome wheeze.†44 Follow-up at 6 years: OR for outcome asthma.†40 Markov model: allergic sensitisation does not precede RSV-induced wheeze, HR 0·71 (95% CI 0·25–2·0)39 |
| Kusel et al30, 42, 43 | Childhood asthma study (CAS) Perth, Australia | High-risk birth cohort (at least one parent with allergic disease or asthma, or both) | Entry: 263 children, Follow-up at 1 year: 236 children; Follow-up at 5 years: 198 children; Follow-up at 10 years: 147 children |
Birth | 12 months, 5 years, and 10 years | Follow-up at 12 months: telephone interviews every 2 weeks until resolution of symptoms of acute RTI Follow-up at 5 years and 10 years: past doctor-diagnosis of asthma and current doctor-diagnosis of asthma or current wheeze and wheeze; wheeze phenotypes |
Skin prick tests at 6 months, 2 years, 5 years, and 10 years | HRV: follow-up at 12 months; RR for outcome wheeze.*30 Follow-up at 5 years; ORs for outcomes current asthma†, persistent wheeze†, late-onset wheeze¶, current wheeze† (pronounced if atopic by age 2 years).42 Follow-up at 10 years; ORs for outcomes current asthma¶, persistent wheeze‡, current asthma¶ (if atopic by age 2 years†), persistent wheeze¶ (if atopic by age 2 years†).43 Findings for current asthma and persistent wheeze after any febrile LRTI pronounced if atopic before age 2 years.43 RSV: follow-up at 12 months; RR for outcome wheeze.†30 Follow-up at 5 years; OR for outcomes current asthma¶, persistent wheeze¶, late-onset wheeze¶, current wheeze§ (pronounced if atopic by age 2 years).42 Follow-up at 10 years; ORs for outcomes current asthma¶ (if febrile†), persistent wheeze¶ (if febrile†)43 |
| Valkonen et al45 | NA Turku, Finland |
Retrospective observational study of children admitted to hospital for virus-induced wheeze, <2 years | 416 children | First admission to hospital <2 years | 1 years, 2 years, and 3 years after hospital admission | Doctor-diagnosis of recurrent wheeze, asthma-specific medication | Not determined | ORs for wheeze induced by viruses other than RSV (including HRV) compared with RSV for first year outcomes wheeze*, for second year†, for third year†45 |
| RSV | ||||||||
| Blanken et al57 | MAKI trial One university and 15 regional hospitals in the Netherlands |
Prospective, double-blind, randomised, placebo-controlled multicentre study (intention-to-treat analysis) | 429 healthy, late preterm infants | Birth, GA 33–35 weeks | Up to 1 year | Number of wheezing days, confirmed RSV infections, admissions to hospital for confirmed RSV infections, wheezing episodes and prevalence of recurrent wheeze (≥3 episodes) | Family history of atopy | RRs for number of wheezing days**, number of confirmed RSV infections**, number of admissions to hospital for confirmed RSV infections**, any wheezing‖, recurrent wheezing‖57 |
| Sigurs et al35, 50, 51 | NA Sweden |
Prospective observational study | 140 children | Mean age of 116 days | Up to 13 years | Doctor-diagnosis of asthma and parent-reported recurrent wheeze | Skin prick tests, specific IgE in serum, allergic rhinoconjunctivitis and atopic dermatitis | RRs for outcomes; current asthma*, recurrent wheeze†35, 50, 51 |
| Simões et al54, 55 | Palivizumab Long-Term Respiratory Outcomes Study27 clinical centres in 6 countries | Prospective, unblinded, multicentre, matched, double cohort study | 421 premature children | Mean age of 19 months | 2–5 years | Doctor-diagnosis of recurrent wheeze and wheeze in general defined as one or more consecutive days of wheeze followed by at least 1 symptom-free week | Family history of atopy or food allergies, family history of asthma | Follow-up at age 3–4 years: palivizumab-treated versus palivizumab-untreated children, incidence of wheeze and of doctor-diagnosed recurrent wheeze significantly lower in palivizumab-treated children55 Follow-up at age 2–5 years: palivizumab-treated versus palivizumab-untreated children; ORs for outcomes; doctor-diagnosed recurrent wheeze** in children with no family history of asthma, pronounced if no family history of atopy or food allergies54 |
| Stein et al10 | Tucson Children's Respiratory Study (TCRS) Arizona, USA |
Prospective birth cohort of children to patients of Group Health Medical Associates (health maintenance organisation) | Subset of 1246 children originally included | Birth | 6 years, 8 years, 11 years, and 13 years | Parental questionnaire on child's history of wheeze at 6, 8, 11, and 13 years; lung function tests at 11 years | Skin prick tests at 6 and 11 years, specific IgE in serum at 9 months and at 6 and 11 years | Follow-up at 6 years: ORs for outcomes infrequent wheeze (≤3 episodes during previous year)†, frequent wheeze (>3 episodes during previous year)* Follow-up at 13 years: ORs for outcomes; infrequent wheeze¶, frequent wheeze¶ No association between RSV-induced LRTI and later atopic sensitisation10 |
| Wu et al33 Carrol et al46, 47 James et al48 |
Tennessee Asthma Bronchiolitis Study (TABS), Tennessee, USA | Population-based cohort study of children taking part in the Tennessee Medicaid programme and with clinically significant LRTI (requiring admission to hospital emergency department visit, or outpatient visit for viral LRTI) | 95 310 children | Birth | 3·5 years and 5·5 years | Doctor-diagnosis of asthma and asthma-specific medication use | Not determined | Follow-up at 3·5 and 5·5 years; OR for asthma after LRTI requiring admission to hospital.‡33 Follow-up at 5·5 years; aRR for asthma after LRTI requiring admission to hospital during winter months†, ‡during non-winter months46 |
| Thomsen et al49 | NA Denmark |
Population-based twin registry | 16 580 children (8290 twin pairs) | Birth | 3–9 years | Doctor-diagnosis of asthma, parent-reported asthma by questionnaire | Parent reported hay fever and atopic dermatitis as proxies for IgE-mediated disease | Model of asthma underlying RSV-bronchiolitis fitted data better than model of RSV-bronchiolitis underlying asthma49 |
>4.
between >2 and ≤4.
between >1 and ≤2.
p=values between >0·05 and <0·1.
not significantly increased.
between ≥0·5 and <1.
between ≥0·1 and <0.5. HRV=human rhinoviruses. RSV=respiratory syncytial virus. NA=not applicable. aOR=adjusted odds ratio. OR=odds ratio. HR=hazard ratio. RR=relative risk. GA=gestational age. aRR=adjusted relative risk. LRTI=lower respiratory tract infection.
RSV infection early in life might not only be associated with long-term effects on lung function and changed airway tone, but might also relate to allergic disease.34, 35, 49, 50, 51 Sigurs and coworkers35, 50, 51 undertook observational studies of infants admitted to hospital in Sweden for severe RSV-induced LRTI and reported that RSV-induced LRTI was linked to atopic sensitisation and allergic disease at 7 years of age. This increased risk of atopic sensitisation and allergic disease persisted up to age 13 years, when RSV-induced LRTI during infancy was also associated with allergic asthma.35 However, these findings are restricted to individuals with severe disease requiring admission to hospital. Further reviews34, 52, 53 have discussed the potential role of RSV bronchiolitis in the initiation of asthma.
The possible causal role of viral infections can only be established by intervention trials that attempt to prevent viral infection and thereby prevent subsequent wheeze and asthma. Passive immunisation with antibodies against RSV has been investigated. Simões and coworkers54, 55 undertook prospective, multicentre, matched double cohort studies in children born prematurely, and showed that the administration of palivizumab—a humanised antibody against the RSV fusion protein56—was associated with a significant decrease in relative risk of recurrent wheeze as diagnosed by a doctor at 2–5 years of age, but only in children without a family history of asthma or atopy.54 The keenly anticipated results of the MAKI trial,57 a more robust multicentre, placebo-controlled, double-blind, randomised clinical trial of palivizumab in 429 healthy preterm babies, showed that the number of cumulative wheezing days during the first year of life was significantly (p<0·001) lower after palivizumab prophylaxis. Moreover, significant reductions in the number of RSV infections and hospital admissions for RSV were reported in the palivizumab treatment group compared with the placebo group (table).57
Viral infections unmask a predisposition for asthma
Host and environmental factors
Rather than causing asthma, viral LRTI might merely unveil the underlying risk. In other words, infection might trigger symptoms in individuals with pre-existing host factors who would have become symptomatic anyway. These host factors include altered airway function or mechanics, genetic background, atopic sensitisation, and impaired mucosal and systemic immune responses. Environmental exposures might additionally affect the probability that a patient with asthma exacerbates with viral infections.
Altered airway function or mechanics
Wheeze is a symptom of expiratory flow limitation as a function of airway calibre and airway mechanics,58 which results in fluttering of the airway walls and a whistling sound. After oedema, bronchospasm, or other changes in airway mechanics due to LRTI-induced inflammation, any narrowing of the airway can result in wheeze, especially in infants. Decreased airway function and bronchial hyper-responsiveness is not only present with acute wheeze, but might also precede its onset.59, 60 Impaired lung growth due to insults during rapid development in early time windows might adversely affect the anatomy or wall mechanics of the airway, which might then be unmasked by infection with respiratory viruses leading to the first manifestation of wheeze.58, 61
Genetic background
A prominent host factor is the genetic background of the child, which can directly affect lung growth and development or induce changes in immune responses.62 Historically, linkage studies, analyses by positional cloning, and candidate gene studies have identified many genetic variants associated with asthma.62, 63 Since high-throughput techniques have become less expensive, 33 genome-wide association studies (GWASs) of asthma have been reported until August 16, 2013. The first GWAS of asthma described the GSDMB-ORMDL3 locus on chromosome 17q21, which has been replicated in many populations and has the greatest effect on childhood-onset asthma.64, 65, 66 Notably, Smit and coworkers67 did an association analysis in children from the Epidemiological Study on the Genetics and Environment of Asthma (EGEA) and showed that neither the effect of 17q21 risk alleles nor of early LRTI on asthma are independent of each other. Odds ratios (ORs) for early-onset asthma after early LRTI were higher for carriers of risk alleles (3·42–6·36) than for non-carriers (1·84–2·44, pinteraction 0·008–0·05).67 These findings suggest that early RTIs uncover carriers of risk and enhance detrimental viral effects. Çalişkan and coworkers68 confirmed this notion by investigation of children from the Childhood Origins of Asthma (COAST) study, and replicated the findings in individuals from the Copenhagen Studies on Asthma in Childhood (COPSAC), both high-risk cohorts. The investigators showed that 17q21 variants increased the risk of asthma and that HRV-induced LRTI modified this risk in both cohorts. ORs for asthma in homozygous risk allele carriers of rs7216389 were 26·1 in the COAST study (3·9 in COPSAC) if the children had at least one HRV-induced LRTI compared with 0·8 (0·7 in COPSAC) if there was no previous HRV-LRTI (pinteraction ≤0·01 vs 0·08 in COPSAC).68 Thomsen and coworkers49 did a retrospective analysis of data from the large Danish twin registry and used advanced genetic variance components models and direction of causation models to show that RSV infection was an indicator of genetic predisposition to asthma, but does not seem to cause asthma (table).
Atopic sensitisation
Another important host factor is atopic sensitisation. Atopy and allergic disease are independently associated with subsequent asthma.61 The association between early and persistent sensitisation and asthma lasts through early childhood and into adulthood.22, 61 Sensitisation to inhalant allergens during childhood is associated with decreased lung growth, which is an independent risk factor for asthma later in life.22, 61 Studies of two Australian birth cohorts, the population-based Raine Study and the high-risk Childhood Asthma Study (CAS),30, 42, 43, 69, 70 investigated the potential role of atopic sensitisation in the complex interplay with viral infections. In the Raine Study,69 sensitisation at age 6 years was associated with an increased risk of current asthma, but this risk was higher if study participants had LRTI during the first year of life. In the CAS, Kusel and coworkers42 reported that the risk of both current asthma at 5 years (diagnosed by a doctor) and persistent wheeze (which started before age 3 years and persisted until age 5 years) was increased after HRV-induced LRTI compared to outcome without exposure, whereas after RSV-induced LRTI, the risk was only increased for wheeze (table). This increased risk was, however, restricted to children sensitised by age 2 years.42 Results from follow-up at age 10 years showed similar trends.43 Children with virus-induced, particularly febrile, LRTIs were at increased risk of asthma and wheeze, which was pronounced if the children were sensitised by age 2 years (table).43 In the COAST study (table), the risk of wheeze at age 3 years and 6 years was increased after either HRV-induced or RSV-induced LRTI during the first 3 years of life.40, 44 Early sensitisation was a predisposing factor in the time course of events preceding the development of wheeze.39
Impaired mucosal and systemic immune responses
Hansel and coworkers71 have reviewed the role of mucosal immune responses to microorganisms in the development of asthma. Thus, we will only focus on aspects of impaired immune responses to viral infections that predispose to asthma. During infancy, the immune system, including both the innate and adaptive immune responses, is immature compared with the immunity attained as an adult. Some evidence suggests that infants who develop atopy and asthma have a particularly delayed maturation of immune responses, which might predispose to development of viral LRTIs and result in asthma.15, 21, 22, 23, 24, 70, 72
During infancy, dendritic cells are less capable of presenting antigen compared with later in childhood.23 T cells, as part of the adaptive defence alliance, are less able to produce cytokines and form memory cells after birth, and instead might even undergo apoptosis upon antigen contact.72 Moreover, adaptive immune responses of neonates show an inherent T-helper cell type 2 (Th2) bias, with maturation of the T-helper cell type 1 (Th1) responses lagging;72, 73 this bias is more pronounced in individuals with an atopic predisposition.72 This results in a less ordered Th1 response and decreased interferon-γ concentration on contact with pathogens.74 The combination of inefficacious immune response towards viral pathogens and a delay in the development of more focused and effective responses is related to an increased number of RTIs early in life, possibly because of increased viral spread to the lower airways.21, 22, 23, 73, 75, 76
The airway epithelium is regarded as the first line of defence against respiratory viruses; its role is complex77 and it plays a central part in mucosal immunity.71 Although viruses have been shown to disrupt the integrity of the respiratory mucosa, such disruptions are also a component of the asthmatic phenotype. Epithelial integrity in children with asthma has been related to genetic risk as conferred by polymorphisms in SPINK5, a gene encoding a serine proteinase inhibitor known to be involved in the retention of epithelial barrier function.78
Environmental exposures
The most robust and consistent finding conferring pre-existent risk of LRTIs and wheeze is related to environmental tobacco smoke (ETS) exposure either prenatally or postnatally. ETS has been shown to affect early lung function by disrupting growth and development of the lungs during important time windows of rapid growth.79 Moreover, a link between early ETS exposure, virus-induced LRTI, and later wheeze and asthma has been shown.38, 40, 41, 80, 81 This association could be attributable to the effect of ETS on the maturing innate immune response, which could account for increased susceptibility to infections in infants exposed to ETS.82, 83 Studies in mice showed that ETS alters antiviral immune responses thereby mitigating viral infections.84 The genetic background of a child might furthermore modify the association between an adverse environmental exposure and premorbid risk of asthma. In Smit and coworkers'67 analysis of children from the EGEA study, ETS exposure further enhanced the effects of early viral LRTIs on 17q21 risk allele carriers. As a result, children with a history of ETS exposure and a specific genetic background could be especially susceptible to viral infection.
In addition to ETS, exposure to outdoor air pollution could play an important part not only as a trigger of asthma exacerbations, but also as an inducer of alterations in airway function or mechanics, and in immune response, leading to asthma. Studies85 have investigated the density of car traffic, in particular truck traffic on roads in close proximity to the child's residence, and identified that such exposure could have direct effects on lung growth,86 and might also lead to changes in immune function, because exposure to air pollution is directly associated with an increased risk of respiratory infections during infancy.87
Another important environmental exposure relates to maternal and infant nutrition. Malnutrition might directly affect the respiratory system by affecting growth and development. Children of mothers exposed to the Dutch famine during early and mid-gestation were shown to be at risk of obstructive airway disease, suggesting direct nutritional effects on lung development and postnatal airway mechanics.88 Maternal dietary vitamin D and vitamin E concentrations during pregnancy were inversely related to wheeze in children.89 Whereas vitamin E has been shown to interfere with airway development via epigenetic changes,90 low serum vitamin D concentrations have been related to respiratory infections in infants.91 Although the exact role of vitamin D in the context of immune modulation is not yet clear, it could be important for regulation of both innate and adaptive immune response.92
Bacteria
The bacterial microbiome
The human body harbours tens of trillions of bacteria, far more than the number of human cells. Individuals have great variation in the bacterial species at different body sites and mucosal surfaces.93 Interindividual diversity is much greater than the variation in composition of these microbiota in the individual over time.94 The gut microbiome has mainly been studied in mice and humans. The Human Microbiome Project95 has extensively characterised faecal samples from healthy individuals. Unfortunately, the airway microbiome has not been included in this ground-breaking project and therefore characterisation of airway samples in healthy populations is scarce. Unlike the gastrointestinal tract, from which faecal samples can easily be obtained, accessibility of the airways, particularly in children, restricts the feasibility of such studies. Technical issues such as the comparability of microbiome analyses from the upper and lower respiratory tract and the potential contamination of bronchoscopic samples have not been resolved.
Nevertheless, some conclusions can be made. Children and adults with asthma seem to be primarily colonised with gammaproteobacteria—ie, Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. 3–6,96 However, in individuals with asthma, whether this pattern of colonisation occurs secondary to airway epithelium damage, impaired barrier function, and inflammatory changes in the airways21, 23, 97 or is a primary cause of the disease remains to be elucidated. One birth cohort study3 suggests that colonisation of the hypopharynx with H influenzae, M catarrhalis, and S pneumoniae very early in life precedes the onset of asthma. Notably, this risk associated with colonisation at 1 month of age was independent of colonisation at later timepoints, suggesting a potential window of opportunity for disease development.3 However, because these findings were from children of mothers who had asthma, generalisation of the results is limited. Moreover, the investigators used conventional culture methods;3 studies that use DNA-based techniques are needed because they might identify an increased variety of microbiota. Furthermore, whether these findings truly reflect colonisation of the lower airway mucosa remains unclear because these studies used either bronchoalveolar lavage samples, sputum samples, or samples from the hypopharynx. Although bacteria might colonise mucosal surfaces of the upper respiratory tract and fluid and sputum in the lower airways, true colonisation of mucosal surfaces in the lower respiratory tract remains a matter of debate.
The role of the abundant gut microbiome in respiratory diseases furthermore awaits elucidation. In experimental studies,2 reconstitution of a normal faecal microbiome early in life in gnotobiotic mice abolishes the risk of asthma associated with germ-free upbringing. Whether the gut–lung axis is of equal importance in humans, particularly in those prone to develop asthma, has been indirectly investigated in a few studies.98, 99 The microbiome of the gut of an infant could be affected by the mode of birth delivery, because children born by caesarean section are colonised differently than children who were vaginally delivered.98 Vaginal flora could affect the gut colonisation of an infant and affect the infant's risk of asthma. Benn and coworkers99 did a population-based cohort study in Denmark, and showed that the composition of the maternal vaginal microflora affected wheeze of their offspring during the first 3 years of life, and affected risk of asthma in children aged 4–5 years. Whereas Ureaplasma urealyticum was associated with infant wheeze only (adjusted odds ratio [OR] 2·0), Staphylococcus aureus (adjusted OR 2·2) and maternal antibiotic use during pregnancy (adjusted OR 1·7) were associated with asthma in the child at 5 years of age as defined by use of asthma-specific medication.99 However, the data for U urealyticum were unaffected by adjustment for antibiotic treatment. Moreover, U urealyticum has been shown to invade the amniotic sac, where it can reproduce.99 Keski-Nisula and coworkers100 reported that intrauterine growth of potential pathogenic bacteria such as Bacteroides spp, Clostridium spp, and Streptococcus spp were associated with doctor-diagnosed asthma at age 15–17 years.
Distortion of the microbiome could occur after administration of antibiotics. Maternal antibiotic use could therefore affect a child's risk of asthma by changing the vaginal and gut microbiome of the mother.101 Moreover, most antibiotics cross the placenta and might therefore affect the fetal and postnatal microbiota of the child. In a cross-sectional case-control study102 of 338 children with asthma and 467 controls, aged 6–7 years, Calvani and coworkers identified that episodes of maternal fever as a proxy measure of infection were associated with childhood asthma independently of maternal asthma. After adjustment for maternal antibiotic use, the results were not significant suggesting a primary role for antibiotics. Other studies103, 104, 105 have supported these findings. The role of administration of antibiotics to the child compared with the mother is prone to bias in epidemiological studies. Many children are given antibiotics because of respiratory trouble; confounding by indication severely limits the validity of these studies. In fact, when only considering children receiving antibiotics for other non-respiratory indications, the positive associations disappear.106, 107
In environments rich in microbials, such as traditional European farms, exposure is associated with protection from atopic sensitisation, hay fever, and childhood wheeze and asthma.108, 109, 110, 111 The abundant and diverse microbial burden within these environments has an important protective role in childhood asthma,109 in which the timepoint of exposure can be crucial.111 However, bacterial exposure in urban environments has also been shown to be inversely related to atopy and asthma.112 Whether these environmental exposures affect the human airway and gut microbiome is still unknown. In mice, the biogeography of their habitat was shown to shape the diversity of their intestinal microbiota.113
Additionally, environmental microbial exposure might directly affect immune responses.21, 23 In the cord blood of infants born to mothers exposed to farming activity and farm dairy products during pregnancy, interferon-γ and TNF-α concentrations were higher compared with infants born to mothers who did not have any farm contact,114 suggesting a strengthening of antiviral responses early in life. Moreover, innate immune receptors such as CD14, TLR2, and TLR4 were persistently upregulated in children who grew up on farms.111, 115 Adaptive immunity was also affected; CD4+CD25hiTreg cells were both more abundant and more capable of suppressing T-cell proliferation in the cord blood of newborn babies with farming mothers than babies of non-farming mothers.116 Furthermore, allergen-induced Th2-associated production of cytokines, such as interleukin 4 and interleukin 13, and immunoglobulins and their isotypes, such as IgG1, IgG4, and IgE inhibition of Ig-class switching, seems to be reduced in farm environments.111
Bacterial infections
Bacterial infections are regarded as a distorted equilibrium of microbial communities, which gives rise to the overgrowth of one species. The association between maternal infections (urinary tract infections and chorioamnionitis) during pregnancy and subsequent wheeze and asthma in children has been assessed through data from questionnaires and medical records and registers.117, 118, 119, 120, 121 Kumar and coworkers120 further investigated chorioamnionitis by assessment of objective histological changes. Results from these studies suggest a potential role of prenatal bacterial infections in the initiation of wheeze and asthma in children.
Postnatal childhood infection with atypical bacteria, such as chlamydia and mycoplasma, have also been related to increased risk of development of asthma.96 However, similar to virus infections, several host factors, including family history of allergy,96 pre-existing atopy,122, 123, 124 and impaired clearance of bacteria after acute infections,18 also play a part in bacterial infections. Furthermore, children with early asthma and atopy might have reduced serum concentrations of bacteria-specific IgG, supporting a change in the underlying host immune response.15
Implications for prevention and treatment
Potential targets for the prevention and treatment of childhood asthma relate to either viruses or bacteria or to host factors that convey risk of asthma development. The most important route of viral transmission seems to be virus particles via self-inoculation of the conjunctivae and accessible respiratory mucosa after touching contaminated surfaces, hence purposeful hand hygiene is important.125 Although first results of anti-RSV antibodies (palivizumab54, 55, 57) for prevention of viral induced wheeze are very promising in premature children, their preventive potential for children at risk of asthma has not been investigated. However, the prohibitive costs discourage clinical application without robust scientific evidence.
Antibiotic treatment, particularly early in life, might do more harm than good as a preventive measure. Nevertheless, antibiotics are indispensable in pneumonia or other overt bacterial infections. Macrolide antibiotics might have a role in the prevention of asthma exacerbations in adults with severe asthma,126 but data for children are scarce. Whether manipulation of the microbiota could in turn succeed is still open to debate. Because of conflicting results and safety issues in infants and immunocompromised individuals, at present no evidence supports prebiotic (containing nutrients such as oligosaccharides fostering growth of beneficial bacteria in the colon), probiotic (beneficial bacteria), or synbiotic (containing both nutrients and beneficial bacteria) manipulation of the gastrointestinal microbiome to effectively prevent asthma.127 Furthermore, evidence for use of the bacteria mix Broncho-Vaxom (OM-85 BV) consisting of H influenzae, Diplococcus pneumoniae, Klebsiella pneumoniae, Klebsiella ozaenae, S aureus, Streptococcus pyogenes, Streptococcus viridans, and Neisseria catarrhalis is insufficient.125 Although the evidence of any benefit of microbial products is weak, future developments in their application for the prevention and treatment of asthma are in progress. In view of the strong epidemiological evidence for protection from microbial exposures, these approaches could be promising as long as the safety of administered products is guaranteed.
Alternatively, host factors that increase susceptibility to viral and bacterial infections could be targeted. External interferon β application, which aims to reduce virus shedding, especially to the lower airways after primary infection, is effective in adults with asthma,29 and prophylactic intranasal treatment has effectively reduced HRV infection in adults.125 However, the adverse systemic reactions, ethical constraints for testing in children, and the related cost prohibit broad use of interferon β in paediatric populations.
Secondary prevention of atopy with subcutaneous or sublingual immunotherapy is possible.128 Immunotherapy started early in life could have potential preventive action. However, subcutaneous injections are not acceptable for children younger than 5 years and sublingual administration has not been tested in young children. Administration of anti-IgE-antibodies (omalizumab) has decreased rates of (probably virus-induced) asthma exacerbations in inner-city schoolchildren,129 but results have not yet been replicated.
Conclusions
In view of the outlined evidence, we propose a comprehensive theory for the role of viruses and bacteria in the development of childhood asthma. Four primary components (host factors) could be attributable to the development of asthma: (i) altered airway function and mechanics; (ii) impaired mucosal immune responses; (iii) impaired systemic immune responses; and (iv) atopic sensitisation (figure ). These components could be formed prenatally depending on the genetic background of the mother and offspring and the maternal environmental exposures during pregnancy, such as tobacco smoke, antibiotic use, infections, and microbial environments.
Figure.
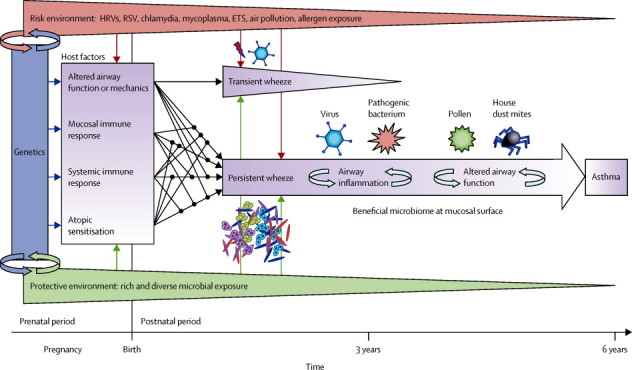
Contributions of viruses and bacteria to the development of asthma
Four essential host factors (purple) are formed both prenatally and postnatally depending on genetic background and environmental exposures. Although viruses and bacteria primarily interact with the mucosa, there is also interaction with the systemic immune response, local changes of which will be further boosted by atopic sensitisation, allergen exposure, and continuous infection. Transient wheeze is triggered by virus infections (red bolt) on the basis of altered airway function, but will be outgrown by children. Development of asthma might be determined by one or a combination of the four primary host factors and prenatal and postnatal environmental exposures, which might contribute to (red) or protect from (green) the development of asthma. HRVs=human rhinoviruses. RSV=respiratory syncytial virus. ETS=environmental tobacco smoke.
Viruses and bacteria mostly interact with the second component, but in patients with asthma the interface between the airways and the environment is most likely defective and disordered. This change in epithelial defence might be attributable to one or a combination of factors such as impaired repair mechanisms of damaged epithelia,77 dysfunction of the epithelial barrier,78 or deficient innate immune responses at the mucosal surface,15 which could result in airway inflammation and particularly suitable conditions for dysbiosis and overgrowth of some bacteria—these bacteria might in turn reinforce airway inflammation. Likewise, a disordered airway mucosa and weakened antiviral defence might hamper virus clearance and increase damage to the epithelial surface with each viral infection.77, 130 Viral and bacterial communities might actively interact; overgrowth of specific bacteria can follow viral infections in the lower respiratory tract, suggesting bidirectional synergism (ie, viral predisposition to bacterial colonisation and bacterial predisposition to viral colonisation).130 Although viral infection might also affect immune responses against pathogenic bacteria,130 whether colonisation with specific bacteria such as those identified in the airways of patients with asthma affect antiviral responses is unknown.
The third component, impaired systemic immune responses—such as a Th2 bias in infancy and delayed immune maturation—could be involved in viral and bacterial infections or colonisation and might interact with local immune responses at the airway mucosa. The development of atopic sensitisation could in turn be affected by impaired systemic immune responses and innate immunity at mucosal surfaces. Thus, a widely diversified network of interactions between the four primary components could have already been established at birth.
The fourth component, atopy sensitisation, could be important because allergen exposure might induce a constant low-level inflammatory state in the airways, facilitating subsequent allergen uptake by airway mucosal dendritic cells and mediation of Th2 responses.15 Viral infection triggers both local and systemic changes to this steady state of constant basal inflammation.15, 21 Local Th2-polarisation is directly induced via binding of HRVs and house dust mite allergens to toll-like receptors,131, 132 potentially enhancing each other's effect.
Postnatally, two major developments can be delineated by their timecourse (figure). Transient wheeze can be prompted by viral infections, but not by other triggers such as allergens.133 We propose that individuals with transient wheeze have normal mucosal and systemic immune responses to viral and bacterial exposures. In turn, changes in airway function or mechanics might play an essential part in the manifestation of symptoms early in life,58 which disappear around age 2–3 years.
Persistent wheeze or later development of asthma symptoms could occur, and might be determined by one or a combination of the four primary components at birth and postnatal environmental exposures. Non-atopic wheeze could mainly involve impaired and delayed mucosal and systemic immune responses, which might mature until school age when remission of symptoms occurs.134 In individuals with atopic sensitisation early in life, viral and bacterial infections might enhance airway inflammation, thereby further changing airway function and eventually resulting in chronic asthma. Environments rich in microbial exposures will in turn protect from a chronic course because they can counterbalance the impaired mucosal and systemic immune responses to strengthen antiviral, Th1, and innate immune responses. These exposures might also prevent harmful bacterial colonisation of the airway mucosa. Although evidence for mutualism between viruses and their host exists in nature,135 whether there is also a role for “good” viruses in human beings, particularly in the context of airway disease, is unknown.
Although this concept is oversimplified and contains many facets in each component and pathway, the proposed conceptual framework could help to relate the various effects of viral and bacterial infection to the development of asthma.
Search strategy and selection criteria
We searched PubMed (MeSH) with the terms “wheeze” or “asthma” in combination with “prenatal”, “childhood”, and “infections”. We largely selected publications from Nov 1, 2012, and Mar 31, 2013, but did not exclude commonly referenced older publications. In total, more than 468 abstracts were screened. We also searched the reference lists of identified articles and selected articles we judged relevant. Review articles are cited to provide readers with more detail and further references.
Acknowledgments
Acknowledgments
OF and EvM are members of the German Centre for Lung Research (DZL) as part of the Comprehensive Pneumology Centre in Munich (CPC-M).
Contributors
OF and EvM did the literature search, designed the figure, contributed to the Review, and approved its final version.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Kunzmann S, Collins JJ, Kuypers E, Kramer BW. Thrown off balance: the effect of antenatal inflammation on the developing lung and immune system. Am J Obstet Gynecol. 2013;208:429–437. doi: 10.1016/j.ajog.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Olszak T, An D, Zeissig S. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bisgaard H, Hermansen MN, Buchvald F. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas PA, Cooper PJ, Cox MJ. Upper airways microbiota in antibiotic-naive wheezing and healthy infants from the tropics of rural Ecuador. PLoS One. 2012;7:e46803. doi: 10.1371/journal.pone.0046803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilty M, Burke C, Pedro H. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131:346–352. doi: 10.1016/j.jaci.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kieninger E, Fuchs O, Latzin P, Frey U, Regamey N. Rhinovirus infections in infancy and early childhood. Eur Respir J. 2013;41:443–452. doi: 10.1183/09031936.00203511. [DOI] [PubMed] [Google Scholar]
- 8.Xepapadaki P, Skevaki CL, Papadopoulos NG. The role of viral and bacterial infections on the development and exacerbations of asthma. Eur Resp Mono. 2012;56:115–127. [Google Scholar]
- 9.Gern JE, Busse WW. Association of rhinovirus infections with asthma. Clin Microbiol Rev. 1999;12:9–18. doi: 10.1128/cmr.12.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein RT, Sherrill D, Morgan WJ. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 11.Gavala ML, Bertics PJ, Gern JE. Rhinoviruses, allergic inflammation, and asthma. Immunol Rev. 2011;242:69–90. doi: 10.1111/j.1600-065X.2011.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gern JE. Rhinovirus and the initiation of asthma. Curr Opin Allergy Clin Immunol. 2009;9:73–78. doi: 10.1097/ACI.0b013e32831f8f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gern JE. The ABCs of rhinoviruses, wheezing, and asthma. J Virol. 2010;84:7418–7426. doi: 10.1128/JVI.02290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holgate ST. Rhinoviruses in the pathogenesis of asthma: the bronchial epithelium as a major disease target. J Allergy Clin Immunol. 2006;118:587–590. doi: 10.1016/j.jaci.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med. 2012;18:726–735. doi: 10.1038/nm.2768. [DOI] [PubMed] [Google Scholar]
- 16.Holtzman MJ. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J Clin Invest. 2012;122:2741–2748. doi: 10.1172/JCI60325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson DJ. The role of rhinovirus infections in the development of early childhood asthma. Curr Opin Allergy Clin Immunol. 2010;10:133–138. doi: 10.1097/ACI.0b013e3283352f7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James KM, Peebles RS, Jr, Hartert TV. Response to infections in patients with asthma and atopic disease: an epiphenomenon or reflection of host susceptibility? J Allergy Clin Immunol. 2012;130:343–351. doi: 10.1016/j.jaci.2012.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jartti T, Gern JE. Rhinovirus-associated wheeze during infancy and asthma development. Curr Respir Med Rev. 2011;7:160–166. doi: 10.2174/157339811795589423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jartti T, Korppi M. Rhinovirus-induced bronchiolitis and asthma development. Pediatr Allergy Immunol. 2011;22:350–355. doi: 10.1111/j.1399-3038.2011.01170.x. [DOI] [PubMed] [Google Scholar]
- 21.Sly PD. The early origins of asthma: who is really at risk? Curr Opin Allergy Clin Immunol. 2011;11:24–28. doi: 10.1097/ACI.0b013e328342309d. [DOI] [PubMed] [Google Scholar]
- 22.Sly PD, Boner AL, Bjorksten B. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sly PD, Holt PG. Role of innate immunity in the development of allergy and asthma. Curr Opin Allergy Clin Immunol. 2011;11:127–131. doi: 10.1097/ACI.0b013e32834487c6. [DOI] [PubMed] [Google Scholar]
- 24.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125:1202–1205. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Palmenberg AC, Spiro D, Kuzmickas R. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science. 2009;324:55–59. doi: 10.1126/science.1165557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally induced infection. Am J Respir Crit Care Med. 1997;155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 27.Mosser AG, Vrtis R, Burchell L. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 28.Papadopoulos NG, Bates PJ, Bardin PG. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 29.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165–1174. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal LA, Avila PC, Heymann PW. Viral respiratory tract infections and asthma: the course ahead. J Allergy Clin Immunol. 2010;125:1212–1217. doi: 10.1016/j.jaci.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heymann PW, Carper HT, Murphy DD. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu P, Dupont WD, Griffin MR. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh AM, Moore PE, Gern JE, Lemanske RF, Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175:108–119. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 35.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 36.Olenec JP, Kim WK, Lee WM. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jartti T, Lehtinen P, Vuorinen T, Koskenvuo M, Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 38.Hyvärinen MK, Kotaniemi-Syrjanen A, Reijonen TM, Korhonen K, Korppi MO. Teenage asthma after severe early childhood wheezing: an 11-year prospective follow-up. Pediatr Pulmonol. 2005;40:316–323. doi: 10.1002/ppul.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson DJ, Evans MD, Gangnon RE. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am J Respir Crit Care Med. 2012;185:281–285. doi: 10.1164/rccm.201104-0660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson DJ, Gangnon RE, Evans MD. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotaniemi-Syrjänen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy—the first sign of childhood asthma? J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kusel MM, de Klerk NH, Kebadze T. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kusel MM, Kebadze T, Johnston SL, Holt PG, Sly PD. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J. 2012;39:876–882. doi: 10.1183/09031936.00193310. [DOI] [PubMed] [Google Scholar]
- 44.Lemanske RF, Jr, Jackson DJ, Gangnon RE. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 45.Valkonen H, Waris M, Ruohola A, Ruuskanen O, Heikkinen T. Recurrent wheezing after respiratory syncytial virus or non-respiratory syncytial virus bronchiolitis in infancy: a 3-year follow-up. Allergy. 2009;64:1359–1365. doi: 10.1111/j.1398-9995.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- 46.Carroll KN, Wu P, Gebretsadik T. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;123:964–966. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll KN, Wu P, Gebretsadik T. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–1061. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James KM, Gebretsadik T, Escobar GJ. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J Allergy Clin Immunol. 2013;132:227–229. doi: 10.1016/j.jaci.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomsen SF, van der Sluis S, Stensballe LG. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179:1091–1097. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 50.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 51.Sigurs N, Gustafsson PM, Bjarnason R. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 52.Drysdale SB, Milner AD, Greenough A. Respiratory syncytial virus infection and chronic respiratory morbidity–is there a functional or genetic predisposition? Acta Paediatr. 2012;101:1114–1120. doi: 10.1111/j.1651-2227.2012.02825.x. [DOI] [PubMed] [Google Scholar]
- 53.Mailaparambil B, Grychtol R, Heinzmann A. Respiratory syncytial virus bronchiolitis and asthma–insights from recent studies and implications for therapy. Inflamm Allergy Drug Targets. 2009;8:202–207. doi: 10.2174/187152809788681056. [DOI] [PubMed] [Google Scholar]
- 54.Simões EA, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick L, Groothuis JR. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126:256–262. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simões EA, Groothuis JR, Carbonell-Estrany X. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 56.Wang D, Bayliss S, Meads C. Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses. Health Technol Assess. 2011;15:1–124. doi: 10.3310/hta15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blanken MO, Rovers MM, Molenaar JM. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–1799. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 58.Frey U. Why are infants prone to wheeze? Physiological aspects of wheezing disorders in infants. Swiss Med Wkly. 2001;131:400–406. doi: 10.4414/smw.2001.06137. [DOI] [PubMed] [Google Scholar]
- 59.Haland G, Carlsen KC, Sandvik L. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 60.Turner SW, Young S, Goldblatt J, Landau LI, Le Souef PN. Childhood asthma and increased airway responsiveness: a relationship that begins in infancy. Am J Respir Crit Care Med. 2009;179:98–104. doi: 10.1164/rccm.200805-804OC. [DOI] [PubMed] [Google Scholar]
- 61.Lodrup-Carlsen KC, Custovic A. Lung development and the role of asthma and allergy. Eur Resp Mono. 2012;56:82–96. [Google Scholar]
- 62.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sleiman PM, Hakonarson H. Recent advances in the genetics and genomics of asthma and related traits. Curr Opin Pediatr. 2010;22:307–312. doi: 10.1097/MOP.0b013e328339553d. [DOI] [PubMed] [Google Scholar]
- 64.Moffatt MF, Gut IG, Demenais F. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moffatt MF, Kabesch M, Liang L. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 66.Granell R, Henderson AJ, Timpson N. Examination of the relationship between variation at 17q21 and childhood wheeze phenotypes. J Allergy Clin Immunol. 2013;131:685–694. doi: 10.1016/j.jaci.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smit LA, Bouzigon E, Pin I. 17q21 variants modify the association between early respiratory infections and asthma. Eur Respir J. 2010;36:57–64. doi: 10.1183/09031936.00154509. [DOI] [PubMed] [Google Scholar]
- 68.Çalişkan M, Bochkov YA, Kreiner-Moller E. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oddy WH, de Klerk NH, Sly PD, Holt PG. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur Respir J. 2002;19:899–905. doi: 10.1183/09031936.02.00103602. [DOI] [PubMed] [Google Scholar]
- 70.Holt PG, Rowe J, Kusel M. Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. J Allergy Clin Immunol. 2010;125:653–659. doi: 10.1016/j.jaci.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 71.Hansel TT, Johnston SL, Openshaw PJ. Microbes and mucosal immune responses in asthma. Lancet. 2013;381:861–873. doi: 10.1016/S0140-6736(12)62202-8. [DOI] [PubMed] [Google Scholar]
- 72.Holt PG, Sly PD. Interaction between adaptive and innate immune pathways in the pathogenesis of atopic asthma: operation of a lung/bone marrow axis. Chest. 2011;139:1165–1171. doi: 10.1378/chest.10-2397. [DOI] [PubMed] [Google Scholar]
- 73.Holt PG, Upham JW, Sly PD. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. J Allergy Clin Immunol. 2005;116:16–24. doi: 10.1016/j.jaci.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 74.Tang ML, Kemp AS, Thorburn J, Hill DJ. Reduced interferon-gamma secretion in neonates and subsequent atopy. Lancet. 1994;344:983–985. doi: 10.1016/s0140-6736(94)91641-1. [DOI] [PubMed] [Google Scholar]
- 75.Gern JE, Brooks GD, Meyer P. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–78. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–841. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 77.Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. 2011;24:210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kabesch M, Carr D, Weiland SK, von Mutius E. Association between polymorphisms in serine protease inhibitor, kazal type 5 and asthma phenotypes in a large German population sample. Clin Exp Allergy. 2004;34:340–345. doi: 10.1111/j.1365-2222.2004.01860.x. [DOI] [PubMed] [Google Scholar]
- 79.Cook DG, Strachan DP, Carey IM. Health effects of passive smoking. 9. Parental smoking and spirometric indices in children. Thorax. 1998;53:884–893. doi: 10.1136/thx.53.10.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strachan DP, Cook DG. Health effects of passive smoking. 4. Parental smoking, middle ear disease and adenotonsillectomy in children. Thorax. 1998;53:50–56. doi: 10.1136/thx.53.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113(suppl 4):1007–1015. [PubMed] [Google Scholar]
- 82.Noakes PS, Hale J, Thomas R, Lane C, Devadason SG, Prescott SL. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur Respir J. 2006;28:721–729. doi: 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- 83.Prescott SL. Effects of early cigarette smoke exposure on early immune development and respiratory disease. Paediatr Respir Rev. 2008;9:3–9. doi: 10.1016/j.prrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 85.Salam MT, Islam T, Gilliland FD. Recent evidence for adverse effects of residential proximity to traffic sources on asthma. Curr Opin Pulm Med. 2008;14:3–8. doi: 10.1097/MCP.0b013e3282f1987a. [DOI] [PubMed] [Google Scholar]
- 86.Latzin P, Roosli M, Huss A, Kuehni CE, Frey U. Air pollution during pregnancy and lung function in newborns: a birth cohort study. Eur Respir J. 2009;33:594–603. doi: 10.1183/09031936.00084008. [DOI] [PubMed] [Google Scholar]
- 87.Stern G, Latzin P, Roosli M. A prospective study of the impact of air pollution on respiratory symptoms and infections in infants. Am J Respir Crit Care Med. 2013;187:1341–1348. doi: 10.1164/rccm.201211-2008OC. [DOI] [PubMed] [Google Scholar]
- 88.Lopuhaa CE, Roseboom TJ, Osmond C. Atopy, lung function, and obstructive airways disease after prenatal exposure to famine. Thorax. 2000;55:555–561. doi: 10.1136/thorax.55.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127:724–733. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 90.Turner SW, Campbell D, Smith N. Associations between fetal size, maternal {alpha}-tocopherol and childhood asthma. Thorax. 2010;65:391–397. doi: 10.1136/thx.2008.111385. [DOI] [PubMed] [Google Scholar]
- 91.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63:473–477. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 92.Morales E, Romieu I, Guerra S. Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology. 2012;23:64–71. doi: 10.1097/EDE.0b013e31823a44d3. [DOI] [PubMed] [Google Scholar]
- 93.Ursell LK, Clemente JC, Rideout JR, Gevers D, Caporaso JG, Knight R. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J Allergy Clin Immunol. 2012;129:1204–1208. doi: 10.1016/j.jaci.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Biscardi S, Lorrot M, Marc E. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–1346. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 97.Hollams EM, Hales BJ, Bachert C. Th2-associated immunity to bacteria in teenagers and susceptibility to asthma. Eur Respir J. 2010;36:509–516. doi: 10.1183/09031936.00184109. [DOI] [PubMed] [Google Scholar]
- 98.Dominguez-Bello MG, Costello EK, Contreras M. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benn CS, Thorsen P, Jensen JS. Maternal vaginal microflora during pregnancy and the risk of asthma hospitalization and use of antiasthma medication in early childhood. J Allergy Clin Immunol. 2002;110:72–77. doi: 10.1067/mai.2002.125833. [DOI] [PubMed] [Google Scholar]
- 100.Keski-Nisula L, Katila ML, Remes S, Heinonen S, Pekkanen J. Intrauterine bacterial growth at birth and risk of asthma and allergic sensitization among offspring at the age of 15 to 17 years. J Allergy Clin Immunol. 2009;123:1305–1311. doi: 10.1016/j.jaci.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 101.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 102.Calvani M, Alessandri C, Sopo SM. Infectious and uterus related complications during pregnancy and development of atopic and nonatopic asthma in children. Allergy. 2004;59:99–106. doi: 10.1046/j.1398-9995.2003.00338.x. [DOI] [PubMed] [Google Scholar]
- 103.McKeever TM, Lewis SA, Smith C, Hubbard R. The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands General Practice Database. Am J Respir Crit Care Med. 2002;166:827–832. doi: 10.1164/rccm.200202-158OC. [DOI] [PubMed] [Google Scholar]
- 104.Rusconi F, Galassi C, Forastiere F. Maternal complications and procedures in pregnancy and at birth and wheezing phenotypes in children. Am J Respir Crit Care Med. 2007;175:16–21. doi: 10.1164/rccm.200512-1978OC. [DOI] [PubMed] [Google Scholar]
- 105.Stensballe LG, Simonsen J, Jensen SM, Bonnelykke K, Bisgaard H. Use of antibiotics during pregnancy increases the risk of asthma in early childhood. J Pediatr. 2013;162:832–838. doi: 10.1016/j.jpeds.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 106.Celedon JC, Fuhlbrigge A, Rifas-Shiman S, Weiss ST, Finkelstein JA. Antibiotic use in the first year of life and asthma in early childhood. Clin Exp Allergy. 2004;34:1011–1016. doi: 10.1111/j.1365-2222.2004.01994.x. [DOI] [PubMed] [Google Scholar]
- 107.Heintze K, Petersen KU. The case of drug causation of childhood asthma: antibiotics and paracetamol. Eur J Clin Pharmacol. 2013;69:1197–1209. doi: 10.1007/s00228-012-1463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ege MJ, Frei R, Bieli C. Not all farming environments protect against the development of asthma and wheeze in children. J Allergy Clin Immunol. 2007;119:1140–1147. doi: 10.1016/j.jaci.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 109.Ege MJ, Mayer M, Normand AC. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 110.Fuchs O, Genuneit J, Latzin P. Farming environments and childhood atopy, wheeze, lung function, and exhaled nitric oxide. J Allergy Clin Immunol. 2012;130:382–388. doi: 10.1016/j.jaci.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 111.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 112.Heederik D, von Mutius E. Does diversity of environmental microbial exposure matter for the occurrence of allergy and asthma? J Allergy Clin Immunol. 2012;130:44–50. doi: 10.1016/j.jaci.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 113.Linnenbrink M, Wang J, Hardouin EA, Kunzel S, Metzler D, Baines JF. The role of biogeography in shaping diversity of the intestinal microbiota in house mice. Mol Ecol. 2013;22:1904–1916. doi: 10.1111/mec.12206. [DOI] [PubMed] [Google Scholar]
- 114.Pfefferle PI, Buchele G, Blumer N. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: the PASTURE Study. J Allergy Clin Immunol. 2010;125:108–115. doi: 10.1016/j.jaci.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 115.Ege MJ, Bieli C, Frei R. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. 2006;117:817–823. doi: 10.1016/j.jaci.2005.12.1307. [DOI] [PubMed] [Google Scholar]
- 116.Schaub B, Liu J, Hoppler S. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009;123:774–782. doi: 10.1016/j.jaci.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 117.Xu B, Pekkanen J, Jarvelin MR, Olsen P, Hartikainen AL. Maternal infections in pregnancy and the development of asthma among offspring. Int J Epidemiol. 1999;28:723–727. doi: 10.1093/ije/28.4.723. [DOI] [PubMed] [Google Scholar]
- 118.Algert CS, Bowen JR, Lain SL, Allen HD, Vivian-Taylor JM, Roberts CL. Pregnancy exposures and risk of childhood asthma admission in a population birth cohort. Pediatr Allergy Immunol. 2011;22:836–842. doi: 10.1111/j.1399-3038.2011.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Getahun D, Strickland D, Zeiger RS. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med. 2010;164:187–192. doi: 10.1001/archpediatrics.2009.238. [DOI] [PubMed] [Google Scholar]
- 120.Kumar R, Yu Y, Story RE. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. J Allergy Clin Immunol. 2008;121:878–884. doi: 10.1016/j.jaci.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Collier CH, Risnes K, Norwitz ER, Bracken MB, Illuzzi JL. Maternal infection in pregnancy and risk of asthma in offspring. Matern Child Health J. 2013 doi: 10.1007/s10995-013-1220-2. published online Jan 22. [DOI] [PubMed] [Google Scholar]
- 122.Chu HW, Honour JM, Rawlinson CA, Harbeck RJ, Martin RJ. Effects of respiratory Mycoplasma pneumoniae infection on allergen-induced bronchial hyperresponsiveness and lung inflammation in mice. Infect Immun. 2003;71:1520–1526. doi: 10.1128/IAI.71.3.1520-1526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hardy RD, Jafri HS, Olsen K. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect Immun. 2001;69:3869–3876. doi: 10.1128/IAI.69.6.3869-3876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin RJ, Chu HW, Honour JM, Harbeck RJ. Airway inflammation and bronchial hyperresponsiveness after Mycoplasma pneumoniae infection in a murine model. Am J Respir Cell Mol Biol. 2001;24:577–582. doi: 10.1165/ajrcmb.24.5.4315. [DOI] [PubMed] [Google Scholar]
- 125.Ahanchian H, Jones CM, Chen YS, Sly PD. Respiratory viral infections in children with asthma: do they matter and can we prevent them? BMC Pediatr. 2012;12:147. doi: 10.1186/1471-2431-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brusselle GG, Vanderstichele C, Jordens P. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322–329. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 127.Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr. 2010;22:626–634. doi: 10.1097/MOP.0b013e32833d9728. [DOI] [PubMed] [Google Scholar]
- 128.Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18:736–749. doi: 10.1038/nm.2754. [DOI] [PubMed] [Google Scholar]
- 129.Busse WW, Morgan WJ, Gergen PJ. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9:e1003057. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Q, Nagarkar DR, Bowman ER. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol. 2009;183:6989–6997. doi: 10.4049/jimmunol.0901386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brand PL, Baraldi E, Bisgaard H. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J. 2008;32:1096–1110. doi: 10.1183/09031936.00002108. [DOI] [PubMed] [Google Scholar]
- 134.Illi S, von Mutius E, Lau S. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 135.Roossinck MJ. The good viruses: viral mutualistic symbioses. Nat Rev Microbiol. 2011;9:99–108. doi: 10.1038/nrmicro2491. [DOI] [PubMed] [Google Scholar]


