Abstract
Cholinergic basal forebrain (cBF) neurons are defined by their expression of the p75 neurotrophin receptor (p75NTR) and tropomyosin-related kinase (Trk) neurotrophin receptors in addition to cholinergic markers. It is known that the neurotrophins, particularly nerve growth factor (NGF), mediate cholinergic neuronal development and maintenance. However, the role of neurotrophin signalling in regulating adult cBF function is less clear, although in dementia, trophic signalling is reduced and p75NTR mediates neurodegeneration of cBF neurons. Here we review the current understanding of how cBF neurons are regulated by neurotrophins which activate p75NTR and TrkA, B or C to influence the critical role that these neurons play in normal cortical function, particularly higher order cognition. Specifically, we describe the current evidence that neurotrophins regulate the development of basal forebrain neurons and their role in maintaining and modifying mature basal forebrain synaptic and cortical microcircuit connectivity. Understanding the role neurotrophin signalling plays in regulating the precision of cholinergic connectivity will contribute to the understanding of normal cognitive processes and will likely provide additional ideas for designing improved therapies for the treatment of neurological disease in which cholinergic dysfunction has been demonstrated.
Keywords: axon innervation, axon guidance, basal forebrain, cholinergic, neurotrophins, synaptic plasticity
Introduction
Neurotrophin receptors in cholinergic basal forebrain neurons
One of the defining features of the cholinergic basal forebrain (cBF) neurons is their expression of the neurotrophin receptors, p75 neurotrophin receptor (p75NTR) and tropomyosin-related kinase A (TrkA), TrkB and TrkC, the receptors for nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophins 3 and 4 (NT3/4).
By binding to their cognate Trk and pan p75NTR receptors, neurotrophins mediate an array of morphological effects in mature neurons, which have been extensively reviewed elsewhere [1,2–4]. In the peripheral nervous system and hippocampus, where they have been most studied, neurotrophins can modulate neuronal development including neurogenesis and differentiation [5], circuit specification [6,7], and neuronal life and death decisions including programmed cell death [4,8]. Classically, neurotrophins are secreted both constitutively and in response to neuronal activity [1], acting as target-derived factors for post-mitotic neurons, with a need for the activated ligand–Trk receptor complexes to be retrogradely transported to mediate cell survival [9–11]. However, they can also act in a cell-autonomous manner [10,12], and within defined cellular compartments. In particular, neurotrophins directly modify synaptic activity (such as long-term potentiation and depression, LTP and LTD respectively), as well as regulating the morphology of synapses and spines [13,14], and the growth and pruning of axons and dendrites [15,16]. Although it is widely assumed that the effects of neurotrophins on basal forebrain neurons mimic these effects, this is far from resolved. Nevertheless, these growth factors are strong candidates for controlling and maintaining cBF connectivity and therefore cognitive and behavioural outcomes mediated by these neurons.
There are numerous unresolved questions pertaining to neurotrophins in cBF neurons. Do the neurotrophins play roles in all of the aforementioned aspects of cBF development and function? What is the major adult role of the neurotrophin receptors in this cell population? What is the evolutionary advantage for mammals to co-express all of the neurotrophin receptors in most cells of each of the nuclei that make up the basal forebrain, given their apparent overlapping functions? We propose that the complexity of cholinergic neurotransmission requires precise mechanisms for organising the heterogeneous population of cBF axonal projections to different classes of downstream neuronal targets, and this should be considered when developing therapies for conditions in which cBF neurons degenerate or are dysfunctional. This review will present the current knowledge regarding the roles of the neurotrophin receptors in cBF neurons during development, adulthood and dementia and consequently their influence on cognition.
Origin and development of basal forebrain neurons
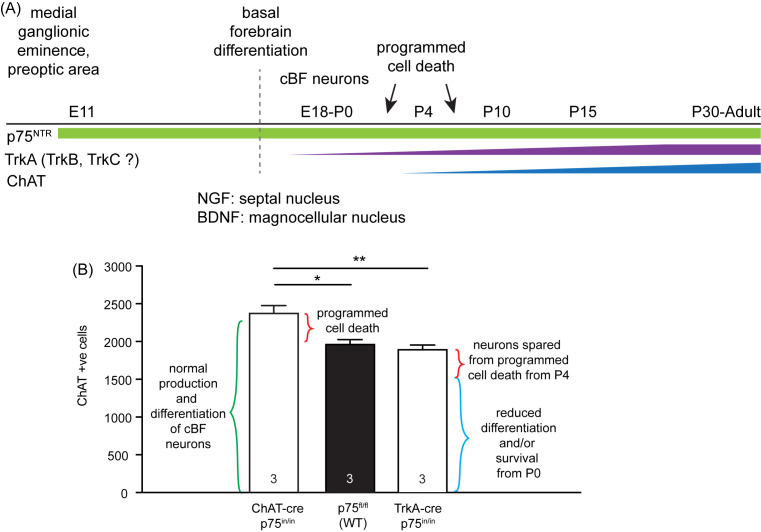
An excellent resource regarding the development of the basal forebrain in different species is provided by Semba [17]. In the mouse, p75NTR is expressed from embryonic day (E) 11 in the neuronal precursors of presumptive basal forebrain neurons (Figure 1). Expression of TrkA and markers of the cholinergic phenotype are found in the differentiating cBF neurons after birth, following their migration from the subventricular and marginal zones of the ventral telencephalon. TrkA is expressed from E18 to E20 [18] whereas ChAT and acetylcholine esterase expression are robustly detectable at approximately postnatal day (P) 4 in the medial septum (MS), vertical diagonal band of Broca (VDB) and preoptic area [17,19]. Their expression then significantly increases in the axons of cBF neurons, peaking at approximately P30 in mice.
Figure 1. p75NTR plays multiple roles in development.
(A) Timeline of mouse development indicating the expression of p75NTR, TrkA and ChAT. NGF and BDNF appear to play complementary roles in inducing the differentiation of septal and magnocellular cBF neurons, respectively. TrkA and TrkB also regulate axonal growth and ChAT expression but not cBF neuron survival. The age at which TrkB and TrkC expression is induced in cBF neurons is unclear. (B) Comparison of the number of cBF neurons in ‘wild-type’ (WT; p75fl/fl; black), and mice lacking p75NTR from either E18 (via TrkA-cre; MGI:4360700; >95% recombination) or P4 (via ChAT-cre). ChAT-positive cells were counted in every third section (as described in [125]) in the MS, VDB and HDB of P30 animals. As both knockout strains lack p75NTR expression during the postnatal period, which reduces programmed cell death (red bracket), the reduced cBF neuronal number in TrkA-cre p75NTR-deficient mice compared with ChAT-cre p75NTR-deficient animals indicates a role for p75NTR in cBF neuronal survival or differentiation between E18 and P4 (blue bracket compared with green bracket). n=3 mice per genotype. *P<0.05, **P<0.01, one-way ANOVA.
Cortical interneurons and nearly all cholinergic neurons of the ventral forebrain originate from progenitor zones in the ventral telencephalon that are positive for the homoeobox transcription factor Nkx2.1. In the mouse telencephalon, Nkx2.1 expression starts at approximately E10.5 and is restricted to the medial ganglionic eminences (MGE) and preoptic area, zones mainly known as the origin of parvalbumin- and somatostatin-expressing interneurons that migrate tangentially into the cortex (reviewed in [20]). However, lineage tracing in Nkx2.1-positive progenitors has revealed that these cells also give rise to neurons in subcortical structures including the globus pallidus and the septal nucleus of the basal forebrain [21]. Interestingly, the expression of Nkx2.1 in the basal forebrain is retained into adulthood, in contrast with cortical interneurons, in which Nkx2.1 is rapidly down-regulated during their tangential migration [21,22]. Consistent with this, deletion of Nkx2.1 during embryonic development leads to almost complete ablation of both cholinergic and parvalbumin-positive neurons in the basal forebrain. Postnatal deletion of Nkx2.1 in cBF neurons decreases the expression of p75NTR and results in a decline in cBF neuron number, indicating that Nkx2.1 regulates factors involved in the maturation and survival of these cells [22,23], such as Lhx8 and Islet-1 [24,25].
Although the ventral telencephalon (subpallium) is the birthplace of septal basal forebrain neurons (comprising the MS, the VDB and the horizontal diagonal band of Broca (HDB)), some reports suggest that a different progenitor population forms the magnocellular nucleus (that consists of the nucleus basalis of Meynert (NBM) and the substantia innominata (SI)), and that this population may originate from progenitor zones in the ventral pallium [17,26]. This is consistent with the observation that Nkx2.1-lineage derived neurons which co-stain for ChAT are found mainly in the MS and VDB, although not in the HDB [21,26]. Rather, cells that are negative for Nkx2.1 but positive for reelin expression (a marker not expressed by septal cBF neurons) migrate from the ventral pallium to the subpallium and differentiate into cholinergic neurons of the magnocellular nucleus [26]. Nonetheless, both populations express p75NTR and all Trk receptors, indicating important functional roles for these receptors in both septal and magnocellular cBF neurons.
Role of neurotrophins in basal forebrain development
Exactly how the expression of Trk and p75NTR receptors is regulated during embryogenesis is unclear. Although it is not unusual for TrkB and TrkC receptors to be co-expressed by neurons, the additional co-expression of TrkA, as occurs in the basal forebrain, is unusual in the brain [27]. Furthermore, TrkA expression in cBF neurons is an evolutionarily recent development, being found in mammals but not birds [28], which potentially indicates a new functional specialisation of cBF neurons. Several reports have demonstrated that bone morphogenetic protein (BMP) 9 is transiently expressed in the septum during basal forebrain development and up-regulates the expression of p75NTR, ChAT and acetylcholine synthesis both in vitro and in vivo [29–31]. Furthermore, BMP9 up-regulates NGF, ciliary neurotrophic factor and serpin F1, creating a trophic environment for cBF neurons. Non-cholinergic, TrkA-negative septal basal forebrain neurons also express low levels of NGF, permitting both autocrine and paracrine roles for NGF in the development and/or maintenance of cBF neurons [18,30]. Indeed, many earlier reports concluded that endogenous NGF regulates the differentiation of cBF neurons [32,33].
In the magnocellular nuclei, ChAT expression is induced by P14, via a BDNF-induced, p75NTR- and Trk-dependent mechanism [29]. Interestingly, NGF cannot act as a substitute for BDNF, perhaps reflecting the different embryonic origins of magnocellular and septal cBF neurons [27]. In P14 rat cultures cBF neuronal survival was mediated by NGF, BDNF and NT-4 whereas NT-3 had no effect at this time point but induced cholinergic neuron differentiation in cultures from E18 rats [34]. Co-treatment with NGF and BDNF resulted in a small but consistent increase in the number of surviving ChAT-positive neurons, whereas sequential treatments demonstrated that most cholinergic cells respond to both these factors, indicating that these cells co-express TrkA and TrkB.
As p75NTR and Trk receptors are expressed during the differentiation and/or specification of the cholinergic phenotype of developing cBF neurons, one might expect that mice in which individual neurotrophin receptor genes have been deleted would produce robust and obvious cBF phenotypes (see sections below). However, cBF neurons are unusual in co-expressing TrkA, B and/or C and have redundant mechanisms of development. Multiple trophic factors may act in concert to regulate the survival and growth of cBF neurons during the early postnatal critical period of development, with NGF being a particularly potent survival-promoting factor, and other cortically derived factors being more important for the growth, branching, and navigation of axons in the target regions [35]. Another possibility is that the methods used have not been sophisticated enough to detect the specialised functional changes each receptor modulates at particular time points.
Adult cBF anatomy
At completion of development, the basal forebrain consists of a heterogeneous cell population, including cholinergic, γ-aminobutyric acid-ergic (GABAergic) and glutamatergic neurons, which form complex networks that provide important modulation of cortical function [36,37]. As described above, the basal forebrain is subdivided into the septal nucleus and the basal magnocellular nucleus (Figure 2). In primates, the cholinergic nuclear groups are more commonly referred to as Ch1–Ch4 [38]. Ch1 (the MS) consists of ∼10% cholinergic neurons; Ch2 (VDB) has at least 70% cholinergic neurons, whereas only a minority (∼1%) of neurons in Ch3 (HDB) have been classified as cholinergic. Ch4, the basal magnocellular complex, is the least heterogeneous nucleus, with at least 90% of its neurons being cholinergic. However, some cholinergic neurons from the NBM can co-release GABA alongside acetylcholine, suggesting they perhaps have a different developmental origin [39].
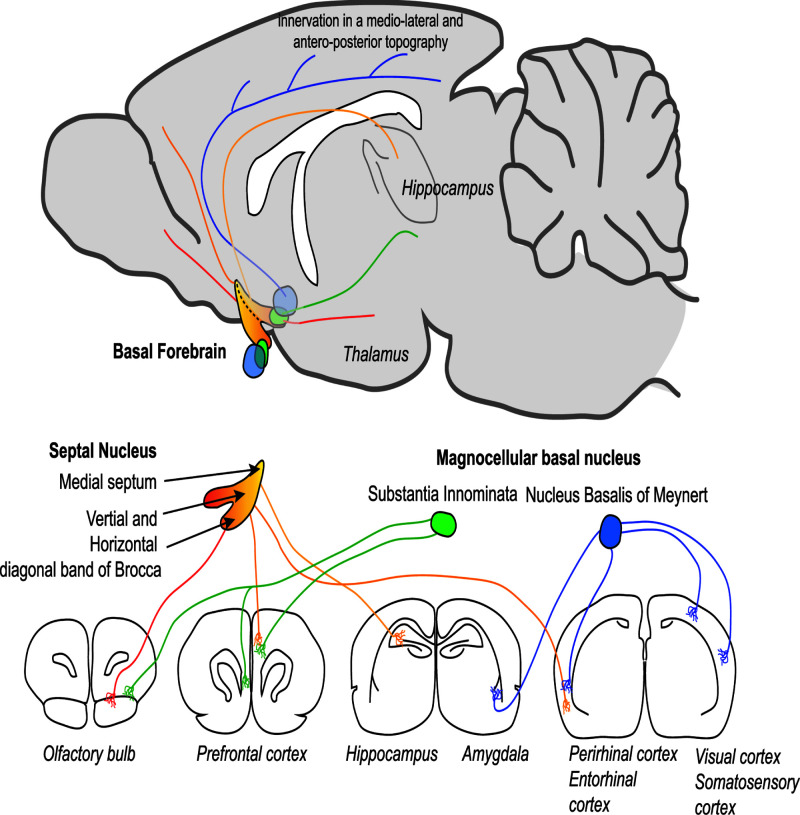
Figure 2. Anatomy and innervation of the basal forebrain nuclei.
Depiction of a midline sagittal section of the adult mouse brain illustrating the position of the basal forebrain nuclei (in ‘3D’) and their projections. Lower panels depict more precisely axonal innervation from specific basal forebrain nuclei to various brain areas portrayed in rostral to caudal coronal brain sections.
MS/VDB neurons project to the hippocampus via the septohippocampal pathway (as visualised by magnetic resonance tractography imaging; [40]). These neurons simultaneously innervate parts of the prefrontal cortex [41], whereas HDB neurons project mainly to the olfactory bulb, but also to the thalamus. The NBM and SI send projections to the amygdala and innervate the entire neocortex, particularly the somatosensory barrel and visual cortices [38,42]. More specific features of the basal forebrain circuitry of the mouse have recently been described in detail [43,44]. However an emerging principle is that a single cholinergic neuron can project to multiple brain areas of associated function across cortical and subcortical areas [44].
The main afferent inputs into the basal forebrain are glutamatergic projections from the ventral tegmental area [45], GABAergic axons from the nucleus accumbens [46] and cholinergic projections from the pons [47]. There are also afferents from cortical, amygdaloid, striatal neurons. It remains unclear exactly which afferents from these regions contact cholinergic neurons in the basal forebrain, although noradrenergic and dopaminergic axons contact cholinergic neurons in extensive portions of the basal forebrain [48]. In addition, there is a largely unexplored network of intrinsic connections within the basal forebrain. It has been reported that a small population of peptidergic neurons [49] and parvalbumin-expressing GABAergic neurons [50] form synapses with cholinergic neurons within this region, and that the subpopulations of basal forebrain glutamatergic cells innervate local parvalbumin-expressing cells [51].
It is important to note that cBF axons can synapse on to specific neuronal targets (point to point neurotransmission) or evoke downstream responses via volume transmission, whereby the release of acetylcholine affects many neurons or synapses in the vicinity of the cBF terminal (see reference [43]). The presence of extensive axonal arbors and generic involvement of cholinergic control of theta oscillations across large brain areas has resulted in a misconception that there is little specificity in cBF axonal innervation [52,53]. More recent studies are challenging this idea, providing evidence that the connectivity of cholinergic projection neurons to their cortical targets must have sufficient neuronal and/or synaptic specificity to provide selective functionality.
A recent series of studies revealed several organising principles of cBF neurons [44,54,55]. Firstly, cholinergic projections to the neocortex are not diffuse, but instead are organised into segregated or overlapping pools of projection neurons along a rostral caudal axis. The extent of overlap between cBF neuronal populations projecting to the cortex depends on the degree of connectivity between the cortical targets of these populations (e.g. olfactory bulb, piriform and entorhinal cortices; Figure 2). Secondly, axons from very distinct cBF areas can innervate immediately adjacent cortical regions, and immediately adjacent cBF neurons can innervate disparate brain areas. These findings suggest that the connectivity of cholinergic projection neurons to their cortical targets has sufficient specificity to provide functionally and spatially selective signalling.
Consistent with the anatomically derived connectivity maps [43,47], recent functional MRI (fMRI) of humans has revealed that the network connectivity between the basal forebrain and other brain areas falls into three functionally distinct connectivity subdivisions. Each functional group is linked to a subdomain of the basal forebrain and has specific target regions, including within the hippocampus, insula, thalamus, and cingulate gyrus. However there are also regions of functional overlap, such as the salience network which is associated with all three cBF functional clusters [56]. Thus there is mounting evidence that the wiring of cBF neurons, although extensive, is guided by unknown underlying mechanisms which may turn out to be a key component of functional modulation of cognitive processes by these neurons.
The function of adult cBF neurons
cBF neuronal effects on behaviour
cBF neurons, through their release of acetylcholine, have been implicated in a wide range of cognitive processes and behaviours, including attention, fear-related processes and reward-mediated behaviour, addiction, memory updating, extinction and renewal [57–61]. Much of the early research that revealed the role of these neurons in the behavioural outcomes used lesioning methods, in which a saporin–conjugated p75NTR-specific antibody was used to create a lesion that was specific to cBF neurons [62]. Depending on whether the injection was made into the ventricle or into a specific nucleus, either entire basal forebrain complex lesions or MS/VDB- and NBM-specific lesions could be induced in a range of animals from rodents to primates. Rat cBF neurons were shown to be required for various cognitive tasks, including motor learning [63] and fear consolidation [59]. Lesions in mice resulted in deficits in working spatial memory in the T-maze [64] and poor utilisation of spatial cues in the Morris water maze [65–67] and Barnes maze [66], as well as impaired cue-independent (egocentric) navigation [68]. These lesion studies were complemented by pharmacological approaches in which cholinergic transmission was blocked with the muscarinic receptor antagonist scopolamine, which resulted in similar cognitive changes [69–73].
More recently, manipulating cBF neurotransmission in mice via optogenetics has further demonstrated cBF neuronal influences on cognitive processes. For example, selective activation of cBF neurons in different cBF nuclei has been reported to improve complex cortically dependent behaviours such as the encoding of natural scenes [74] and olfactory discrimination [75], maintaining wakefulness [76], and mediating cognitive functions through responses to external reinforcement feedback [77]. Not surprisingly, the cBF nuclei required for particular behaviours depend on the circuity involved in the task. MS/VDB cBF neurons are needed for hippocampal-dependent functions such as the detection of spatial cues, exploration and cue-dependent allocentric navigation [78], and prefrontal cortex-dependent fear extinction [79], whereas NBM cBF neuron-mediated neurotransmission is necessary for amygdala-dependent functions including fear conditioning (but not extinction), object recognition [59,80] and egocentric navigation (Hamlin, Boskovic and Coulson, unpublished).
Mechanisms of cholinergic action on neurons and networks
Not all effects of the basal forebrain on behaviours and associative tasks require intact cBF circuits [68]. Rather, complex cognitive processing that requires the integration of information between multiple cortical regions appears to be specifically dependent on the appropriately timed release of acetylcholine from cBF neurons. In particular, it has been proposed that the basal forebrain is particularly important for regulating the output of cortical layer 3 neurons, responsible for invariant perceptual representation, as well as layer 5 pyramidal neurons that control memory output [81]. Layer 3 isocortical neurons are activated on association and recall of associative memories and project contralaterally to activate the layer 5 pyramidal neurons responsible for behavioural responses, which output to the cerebellum, pons, basal ganglia (specifically the subthalamic nucleus and striatum) and motor cortex.
Early work revealed that cBF-mediated neurotransmission also controls electrical oscillations in the hippocampus, as lesioning cBF neurons led to a decrease in low-frequency (6–12 Hz) theta oscillations [82]. These findings were further extended to show that denervation of the hippocampus via cBF axotomy affects the ability of animals to encode novel information i.e. form new memories within the hippocampus [83]. More recently, stimulation of cBF neurons in anaesthetized mice revealed that hippocampal theta synchronisation is mediated by an indirect pathway in which cholinergic neurons recruit and stimulate non-cholinergic neurons within the basal forebrain [84]. However, a direct septo-hippocampal cholinergic projection can alternatively increase the firing of hippocampal inhibitory interneurons with concomitant decreased firing of principal cells [84]. Activation of both cholinergic and non-cholinergic afferents causes a reduction in hippocampal pyramidal neuron firing and a more precise coupling to the theta oscillatory phase. These two anatomically and functionally distinct pathways appear to mediate information encoding, a requirement for memory formation. Furthermore, encoding of novel memories within the entorhinal cortex requires acetylcholine-mediated suppression of signalling at the interface of the CA3 and CA1 regions of the hippocampus [85,86]. Such changes in synaptic activity in the hippocampus are timing dependent [87] and require co-ordinated cholinergic modulation at both pre- and post-synaptic sites within this region [88].
Real‐time monitoring of acetylcholine release in the cortex of rats performing attentional tasks has revealed that precisely timed (transmission in the order of seconds; ‘transients’) cholinergic signals in the prefrontal cortex coincide with the detection of behaviourally significant cues [89], such as when animals are anticipating a meal [90], presented with sensory stimuli [91] or subjected to operant learning [92]. Cholinergic-mediated enhancement of post-synaptic activity, by optogenetic activation of cBF neurons, is not only associated with improved cue detection [93], but is also sufficient to condition cued-interval timing activity, thereby mimicking the reward timing activity that is observed after behavioural conditioning, and can improve visual discrimination [94]. Conversely, blocking cBF activity with optogenetics [92,93] or long temporal resolution cBF stimulation [95] synchronises activity across different cortical areas, thereby causing animals to ‘miss’ visual cues, and impairs visual responses.
cBF-mediated modulation of cortical activity also depends on the strength of concomitant inputs to the target neurons: cholinergic neurotransmission is inhibitory during quiescence, but excitatory when there is simultaneous post-synaptic activity [96]. By reducing the excitability of less active neurons and enhancing that of more active ones, cBF neurons increase the signal-to-noise ratio of incoming information. These results suggest that cBF neurons might facilitate memory formation by biasing the synaptic competition in favour of potentiated cells [97]. cBF neurons can respond to reward and punishment with high speed and precision [77]. Responses are scaled by the unexpectedness of reinforcement and are highly similar between the prefrontal and auditory cortices, which are innervated by the HDB and NBM nuclei, respectively [77]. This suggests that cBF neurons broadcast a rapid and precisely timed signal to enhance information integration [57,98,99].
Electrophysiological evidence points to the ability of the cBF-mediated cholinergic mechanisms to result in both (i) an enhanced probability of propagating cortical output in response to previously associated information, and alternatively, (ii) regulating new learning in response to salient or novel stimuli by releasing dendritic inhibition while suppressing low threshold pyramidal neuronal transmission [96]. Evidence of the ability of acetylcholine to enhance synaptic potentiation at single neuron resolution is provided by experiments involving stimulation of acetylcholine release, either by direct basal forebrain stimulation or via optogenetic activation of axons, leading to an enhanced response of glutamatergic neurons in the cortex, an effect which can last for more than 5 min [100–102]. However, this appears to be due to the ability of the cBF to drive the activity of a feed-forward inhibition/disinhibition microcircuit of inhibitory and excitatory neuronal subtypes within innervated regions, a mechanism that is conserved across the cortex [103,104]. Nonetheless, it has recently been reported that muscarinic activation of calcium channels within layer 5 pyramidal dendrites causes the generation of dendritic plateau potentials, powerfully transforming the pattern of dendritic integration of simultaneous input, leading to repetitive action potential output [102].
cBF neurons have axonal collaterals within all layers of a cortical column, but the modulation is layer specific, and can also vary according to cortical area and species. Examples are provided below and in Table 1 (for recent more comprehensive reviews, see references [105,106]). There is emerging evidence that cBF neurotransmission is particularly important for layer 1 interneurons which express nicotinic and muscarinic acetylcholine receptors (nAchRs and mAchRs, respectively [107,108]). Tonic (brief/resting) nAchR activation of layer 1 neurogliaform cells results in direct inhibition of layer 2/3 and 5 pyramidal dendrites [109,110]. However, phasic activation (high-quantity burst firing) causes silencing of the neurogliaform cells through M1 mAchRs, causing disinhibition of layer 2/3 apical dendrites [110]. The majority of layer 2/3 interneurons, including parvalbumin-positive basket and chandelier cells, do not express AchRs, with only ∼20% of layer 2/3 interneurons having depolarising nicotinic responses and ∼3% of interneurons becoming hyperpolarised in response to acetylcholine [107]. However, parvalbumin-positive interneurons can be inhibited by the activation of cholinergic-responsive layer 1 interneurons, which in turn results in somatic disinhibition of pyramidal neurons. Finally, despite sparse expression of nAChRs in layer 5/6 pyramidal neurons, robust mAChR expression is maintained predominantly at the soma [107,108], where the receptors act to suppress single synaptic responses, but facilitate synaptic responses evoked during stimulation trains [111,112]. However M1 mAChRs are also distributed on the extrasynaptic membrane of pyramidal cell dendrites and spines opposed to cholinergic varicosities indicative of volume release sites [113]. Activation of dendritic M1 receptors modulates of dendritic excitability of layer 5 pyramidal neocortical neurons and powerfully controls dendritic integrative operations and neuronal associative computations [102]. Similarly, the effect of acetylcholine through nicotinic and M1 or M2 mAChRs modifies the ratio between excitation and inhibition, switching layer 5 pyramidal neurons from tonic to burst firing, and enabling a potent and sustained enhancement of the neuronal synaptic response [114].
Table 1. Examples of cholinergic receptor activation in the cortex that modulate neuronal excitability and synaptic plasticity.
| Neuron affected | Cholinergic response | Direct effect | Downstream effects |
|---|---|---|---|
| Layer 1 inhibitory neuron (NGF expressing) | Tonic cholinergic transmission: activation of nChR-expressing cells | Direct inhibition of layer 2/3 and 5 pyramidal dendrites | Inhibition of PV-positive interneurons causing axo-somatic disinhibition of pyramidal neurons |
| Phasic cholinergic transmission: silencing of recipient cells through M1 mAchRs | Disinhibition of layer 2/3 apical dendrites | ||
| Pyramidal neurons (layers 2/3 and 5/6): (BDNF expressing) | Axo-somatic activation of M1 mAChRs at location | Suppression of single synaptic responses, but facilitation of synaptic responses evoked during stimulation trains | |
| Dendritic activation of M1 mAChRs | Compartment-dependent modulation of dendritic excitability | Direct enhancement of dendritic integration and computations | |
| Thalamic axons (layer 4) (NT3 expression?) | Activation of presynaptic nAChRs | Amplification of thalamic neurotransmission | Increased strength of incoming sensory information |
Taken together, the above data indicate that cBF neuron-mediated neurotransmission requires temporally precise modulation of post-synaptic targets by these cells. There is also a requirement for a high degree of anatomical organisation of (i) cBF axonal innervation to dendrites, neurons and microcircuits within cortical columns, (ii) cBF neurons within the same basal forebrain nuclei innervating distinct cortical regions, and (iii) coincident transmission to downstream targets by cBF neurons located in different nuclei. It is as yet unclear how cBF neurons are able to establish and maintain such a dynamic range of connections. This also raises the question of whether directed modulation of post-synaptic targets by cBF neurons is achieved through a high degree of plasticity of their connections in the adult brain. We propose that neurotrophins provide a key mechanism that could provide a cBF-cortical neuron organisational structure within target areas. However, how the cBF neurons receive incoming information from cortical or subcortical relays in an organised way, and innervate the cortex in a rostral caudal manner are topics outside the scope of this review.
The role of neurotrophin receptors in cBF function
The neurotrophin receptors are co-expressed to differing extents in developing cBF neurons [18,47,115] and their expression persists throughout adulthood (Figure 1A). Over 90% of cBF neurons express p75NTR in both rodents and primates [116,117]. In the adult human posterior NBM cBF nucleus, 54% of cBF neurons express TrkA, 75% express TrkB and 58% express TrkC [116]. The proportions are similar in the adult rat (at least 45% for TrkA and 77% for TrkB; [117]). The state of knowledge of the role of neurotrophin receptor in cBF neurons is explained below.
The role of p75NTR in cBF neurons
Effects of loss of p75NTR expression on cBF morphology
The majority of studies report that genetic knockout of p75NTR leads to an increase in the number of cBF neurons, a finding that is proposed to be due to a lack of developmentally regulated apoptosis [118–120]. This is consistent with the reported role of p75NTR developmental programmed cell death in a range of neuronal types, particularly peripheral neurons [121,122]. This phenotype was once controversial, due to conflicting reports from the use of germline knockout animals on a variety of different and mixed background strains, some of which express a remnant transcript [119,123,124]. However, we recently confirmed a role for p75NTR in mediating cBF neuronal death during the early postnatal period (but not in age-related decline in cBF cell number) based on a conditional knockout of p75NTR from P4 in the mouse cBF, using ChAT-cre-mediated deletion [25]. Interestingly, conditional knockout of p75NTR in cBF neurons at birth using a TrkA-cre reduced the number of cBF neurons (Figure 1B), revealing a role for p75NTR in facilitating cholinergic differentiation or survival between E18 and P4, prior to its cell death role. The programmed cell death period of septal cBF neurons occurs from P1 to P15, with a peak at P1 and a second peak at P5, following the induction of ChAT expression [126]. The apparent multiple roles of p75NTR in cBF neurons (Figure 1A,B) mirror the effects of p75NTR in mouse dorsal root ganglia, where p75NTR together with TrkA is trophic at E15–E18, but mediates programmed cell death at P2 [127]. Thus, p75NTR may facilitate Trk-mediated trophic effects during axonal targeting and prior to, or even concurrent with, its role in pruning ‘excess’ cBF neurons.
The absence of p75NTR also affects the size of cBF neurons, which is thought to reflect an increase in the metabolic need of an enlarged axonal arbor [35]. Indeed, an increased density of axonal innervation of the hippocampus, somatosensory and visual cortices and amygdala has been reported for p75NTR-deficient cBF neurons [118,120,124,125,128–130]. However, the increased cBF axonal density could also be due to the increased cBF neuronal number, rather than, or as well as, an increase in the size of individual neuronal arbors. Although this is difficult to accurately delineate, labelling of individual axonal arbors has revealed that the arbor size of MS/VDB cBF neurons innervating the hippocampus and prefrontal cortex is not substantially affected by the lack of p75NTR cBF neuronal expression [79], suggesting that the previously reported increased innervation was due to the overlapping arbors of the additional surviving cBF neurons. Despite this, the authors reported a ten-fold increase in synaptic connectivity between cBF neurons and target cells in the prefrontal cortex, an outcome that could be reversed by re-expression of p75NTR in adult cBF neurons [79], thereby confirming a role for p75NTR in pruning adult cBF connections. By contrast, synaptic connections to the dorsal hippocampus were relatively rare, and septo-hippocampal synaptogenesis was unaffected by manipulation of p75NTR expression [79]. These observations indicate that p75NTR controls presynaptic mechanisms of cBF synapse formation. They also highlight the possibility that different mechanisms control axonal and synaptic growth, and/or control cBF innervation of the hippocampus and prefrontal cortex. One possible mechanism could be mediated by the differential expression of neurotrophic ligands in the cBF target cells, acting through cognate Trk receptors expressed by cBF neurons, as discussed in the next section.
Effects of loss of p75NTR expression on behaviour and synaptic function
One of the most consistent observations of p75NTR mutants lacking exon III (p75exonIII−/−) and cholinergic-specific p75NTR knockout mice are changes in behaviour in cognitively complex tasks, with little change in baseline behaviour. In comparison with littermate controls, mice lacking p75NTR have generally been reported to have improved spatial learning and/or memory performance in an egocentric passive place avoidance task, as well as in cued, hippocampal-dependent Barnes and Morris water mazes. The mice also display changes in the amount of time spent in light–dark compartments, and reduced cued fear conditioning and fear extinction consolidation [79,124,125,128,131]. These behavioural phenotypes are consistent with altered cBF neuron function, being often the opposite phenotype to that of mice with cBF lesions. Indeed, most of the aforementioned studies concluded that increased cell number and/or cholinergic innervation is responsible for the behavioural change. In only one study was re-expression of p75NTR in adult animals attempted in order to reverse the observed phenotype. The study demonstrated that acute p75NTR signalling, rather than the increased number of neurons (a developmental phenotype), is important for cBF neuron synaptic innervation and fear extinction consolidation [79]. However, it remains unclear which cortical cell types have ectopic basal forebrain synaptic connectivity, and what effect (excitatory or inhibitory) the enhanced cholinergic innervation causes.
Juvenile (2–3 weeks old) p75exon III−/− and p75exon IV−/− mice have been shown to have reduced hippocampal LTD, without an accompanying change in LTP [132,133]. Interestingly, 3–6 months old p75exon III−/− mice have been reported to have enhanced LTP in the hippocampus and amygdala [130,131]. In each case, the absence of p75NTR promotes the persistence of transmission between neurons, albeit by different synaptic mechanisms. Increased complexity of the dendritic spines of CA1 hippocampal pyramidal neurons, changes to glutamatergic receptors, and increased cholinergic transferase activity in p75exonIII−/− mice have also been demonstrated [124,131,133,134]. In addition, it has been reported that, in adult mice lacking p75NTR, the excitability and persistent firing of pyramidal neurons in layer 5 of the entorhinal cortex is unregulated due to a lack of inhibition [135]. As the expression of p75NTR in the entorhinal cortex occurs in the innervating cBF axons, the role of p75NTR could be to regulate cBF neuron structure or function thereby indirectly mediating the inhibition or activation of post-synaptic pyramidal cell activity.
Although these phenotypes are consistent with the increased cBF arborisation of cBF neurons in p75NTR-deficient mice, whether they are due to p75NTR signalling in cBF neurons during development or in the adult is difficult to determine. Experiments carried out using brain slices of developing animals have provided evidence of post-synaptic p75NTR expression (e.g [133]), but cannot rule out effects of systemic changes to hippocampal physiology due to altered cBF innervation or other p75NTR-dependent developmental/neurogenic ramifications [124,136–138], which may also be mediated by changes in cholinergic function [139]. Therefore, the physiological results should be interpreted with caution. Nonetheless, altered cBF innervation to cortical neurons in p75NTR−/− mice is expected to result in altered cortical synaptic plasticity, with associated cognitive changes. However demonstration of this will be best interrogated using adult conditional p75NTR knockout strains.
It remains unclear what ligands drive p75NTR signalling in cBF neurons, and whether p75NTR acts to facilitate Trk receptor signalling as well as acting independently to facilitate synaptic pruning, driven by the developmental stage or expression levels of co-receptors. The current dogma would suggest that proneurotrophins, acting together with the sortilin/SorCS family of co-receptors, is likely to be responsible for the pruning of synapses and axons via p75NTR (as has been recently reported for a developmentally transient, p75NTR-positive, prefrontal cortex-projecting hippocampal neuronal population [140]). Although mice in which sortilin receptor members are conditionally knocked out in cBF neurons have not been analysed, sequestration of proNGF with specific antibodies causes mild impairment in cognitively taxing, distractor attention tasks without affecting baseline attention [141], suggestive of changes to cholinergic neurotransmission.
The role of Trk receptors in the cBF
Neurotrophin-mediated Trk receptor signalling appears to regulate both the development and adult function of cBF neurons. However, as with the analysis of p75NTR-deficient mice, it has often been difficult to differentiate the adult role from the developmental functions. Despite current dogma, target-derived neurotrophins are not essential for the survival of cBF neurons [142], but rather regulate their morphology and the extent of their cholinergic phenotype.
TrkA-NGF mediated regulation of cBF neurons
The first study of TrkA-deficient mice revealed dramatically reduced acetylcholine esterase-positive fibres in the hippocampus and somatosensory cortex of those animals which survived to adulthood [143]. A personal communication cited in the report (L.F. Kroner, unpublished) indicated that these mice displayed no change in cBF neuron number. More recently, analysis of conditional NGF- and TrkA-deficient animals in which the gene was removed from E10.5 in nestin-positive progenitors revealed a 30% reduction in the number of cBF neurons at P7 that was still evident at 20 months of age when compared with cre-negative controls [144]. This was concomitant with a decrease in cholinergic axonal and terminal innervation to the somatosensory cortex and hippocampus, as measured by ChAT activity. The authors concluded that the decrease in ChAT- (and p75NTR-) positive cell number was due to the increased developmental death of cholinergic cells observed at P7. Although it is unclear whether the innervation phenotype represents loss of axons/cells or merely down-regulation of ChAT/p75NTR expression, the authors reported that the expression of ChAT in the remaining neurons and the density of the remaining cholinergic varicosities were unchanged, suggesting that there was no difference in the connectivity of the surviving neurons. Therefore it is possible that a subpopulation of neurons was affected by the loss of NGF-TrkA signalling. Surprisingly, these TrkA-deficient animals displayed normal function across a large number of assays of cognitive domains, which included attention performance, anxiety, exploration and working and spatial memory. The authors concluded that deficits in TrkA signalling are not sufficient to induce cognitive impairments in adult animals, despite the mice displaying a significant cellular phenotype.
However, contrasting findings were reported in a study published the same year which used a different conditional TrkA knockout mouse line, in which the gene was removed from E9.5 in dlx5/6-expressing telencephalon cells [145]. These TrkA-deficient mice displayed mild cognitive deficits, with impaired associative and working memory, as manifested through decreased memory in a Pavlovian fear-conditioning task, and decreased object discrimination in a novel object recognition task (but no change in spatial memory in the Morris water maze task) compared with control animals. The number and size of cBF neurons in P30 mice was unchanged, although a significant decrease in cholinergic innervation to the hippocampus and frontal cortex was observed, as measured by p75NTR immunostaining in the postnatal period, as well as a decrease in total ChAT expression in the basal forebrain across all ages investigated from P14 to P180. It is again unclear from these experiments whether this result represents a loss of the axons themselves or only down-regulation of ChAT and p75NTR expression. However the authors suggested that loss of NGF-TrkA signalling (demonstrated by reduced Akt phosphorylation) specifically decreased the level of ChAT (and p75NTR) synthesis within cells, and that this in turn was sufficient to affect cholinergic neurotransmission.
It is likely that the explanation of these contrasting findings is, at least partially, due to methodological issues. In adult rats, down-regulation of TrkA in the magnocellular nuclei had no effect on cBF neuron survival but decreased the density of cortical cholinergic axons and reduced their capacity to release acetylcholine, impairing attention of aged animals [146]. Similarly, TrkA down-regulation in mice impaired performance in attention tasks, an effect that could be rescued by simultaneously sequestering proNGF [141]. Therefore, a common phenotype, evident from neonatal development onwards, is represented by reduced axonal expression of ChAT, and/or reduced innervation of cBF axons to the cortex. The most parsimonious overall conclusion is that NGF and TrkA signalling controls the expression of ChAT within cBF neurons and their axons, and has only mild effects on neuronal specification and/or survival. The conclusion that the major influence of TrkA is on acetylcholine production and release is consistent with the function of NGF, as reported for mice with reduced NGF expression, and is the opposite of that displayed by p75NTR-deficient mice.
The role of NGF in cBF function
The first study of complete NGF knockout mice indicated that the production and survival of cBF neurons was unaffected (when compared with the obvious effects on the peripheral nervous system; [147]). However, reanalysis of the published images suggests a decrease in adult cBF neuron number. Indeed, heterozygous NGF−/+ mice show a reduced number and size of adult cBF neurons, as well as deficits in memory updating in a Morris water maze reversal learning task [148], a phenotype that is highly reminiscent of MS cBF-lesioned [149] and p75exonIII−/− mice [136], but is not seen in TrkA-deficient mice (see above). Nestin-cre driven conditional NGF−/− mice, have a 30% loss of cBF neurons, reduced ChAT expression in axons (although not as severe as that in TrkA−/− mice), and a reduced number of nerve terminals in the hippocampus and frontal cortex [144]. Similarly, mice with impaired NGF signalling through the expression of NGF antibodies from early postnatal development display a progressive loss of ChAT-positive cells in the adult cBF, cell shrinkage and reduced ChAT expression, as well as poor learning and retention in an 8-arm radial maze [150]. Experiments in which anti-NGF or anti-TrkA antibodies were applied to the rat basal forebrain at P2 have also been shown to cause a decrease of ∼75% in cBF cell number at P9–P22 and a reduction in the size of the remaining cBF neurons [151,152]. However, the effects of NGF antibody infusion in the latter experiment were reversible, indicating that NGF-TrkA signalling is not an essential survival factor during neonatal cBF neuronal development and the programmed cell death period [151].
One explanation for this is that NGF signalling plays a different role in regulating synaptic activity in immature and mature cBF neurons. Cultured cBF neurons from E16/17 rats are dependent on NGF for survival between the second and fourth week in vitro. However, at earlier or later time points, they are largely resistant to NGF deprivation. The addition of BDNF, NT3, NT4/5 or glial-derived neurotrophic factor to cBF neurons, or co-culture with cortical neurons that enable synaptic innervation, confers resistance to NGF deprivation during this critical period [35]. During the same period, when sequestration of endogenous NGF caused no effect on LTP or LTD in P16–P18 neonatal rat visual cortex slices, application of exogenous NGF blocked high frequency-stimulated LTP. In contrast, at P30–P35, high-frequency stimulation did not evoke LTP unless NGF was sequestered or muscarine was applied to the slices, indicating that the NGF effect was mediated by cBF neurons [153]. Therefore, NGF appears to act first to promote differentiation (via p75NTR?) and survival during maturation, then primarily as a mediator of postnatal axonal growth, and subsequently to fine-tune cortical synaptic function.
Several studies have directly tested the role of NGF signalling in adult cBF neurons, revealing that NGF can control synaptic plasticity, circuit development and rewiring. Intraventricular infusion of NGF into adult NGF−/+ mice was not only able to reverse the learning deficit induced by chronic NGF reduction, but also to cause an increase in cBF cell size and ‘number’ in the adult, indicating that NGF-mediated signalling controls the adult cBF neuronal phenotype [148]. Similarly, lesion of septo-hippocampal projections via the fornix of adult cBF neurons resulted in cell shrinkage and loss of ChAT expression, which could be restored by application of NGF [154]. NGF expression can also modulate adult cBF connectivity, and has been reported to augment plasticity and behavioural learning through cholinergic-dependent mechanisms [155–158]. Stimulation of NGF release through learning or transgenic expression has also been shown to attract and rewire cBF axonal collaterals from multiple cBF cell bodies, facilitating the recall of learned memories [159,160]. Moreover, it has been reported that topical application of NGF to the barrel cortex of an adult rat augmented the size of the functional representation of a whisker within minutes, replicating the effect of nicotinic stimulation [156]. NGF application also results in an increase in the amplitude of the activity-dependent intrinsic signal in the barrel of a stimulated whisker [155]. The timescale of this result indicates a role for NGF-TrkA signalling in regulating neurotransmission and/or synaptic plasticity rather than (or in addition to) structural change of the axon.
NGF expression is limited to interneurons
A particularly important detail is the target-derived source of NGF (secreted as proNGF; [161]) that cBF neurons encounter, with inhibitory interneurons emerging as the primary source of neuronal NGF. In the cortex, >90% of NGF-labelled cells are interneurons, with less than 3% of pyramidal neurons expressing NGF mRNA [162]. In the hippocampus, ∼70% of NGF mRNA-positive neurons are parvalbumin-positive, although ∼20% of parvalbumin-expressing interneurons do not express NGF. In contrast, only a subset of calbindin- and calretinin-positive interneurons (24 and 23%, respectively) contain NGF mRNA [163]. Although two-thirds of NGF-positive cells co-express parvalbumin, many parvalbumin-positive cells do not co-express NGF, and only a minority of NGF-positive cells are calbindin-positive (29.1 ± 3.9%) [164].
It has been demonstrated that cholinergic-responsive layer 1 vasoactive intestinal peptide (VIP)- and/or somatostatin-expressing interneurons can disinhibit the layer 2/3 parvalbumin-positive interneurons, thereby regulating pyramidal neuron firing [165]. However, in an apparent paradox to the NGF expression in parvalbumin-positive interneurons, those in layer 2/3 do not express receptors for acetylcholine, with only ∼20% of layer 2/3 interneurons having depolarising nicotinic responses and ∼3% becoming hyperpolarised in response to acetylcholine [107]. Furthermore, virtually every parvalbumin-positive neuron innervated by TrkA-negative GABAergic basal forebrain neurons expresses NGF mRNA [162]. One explanation is that NGF released by parvalbumin-positive neurons regulates en passant cholinergic neurotransmission to that local area. Indeed there appears to be a link between cholinergic and non-cholinergic transmission in cortical microcircuits regulated by the basal forebrain. For example, we recently found that a specific change of p75NTR expression in cBF neurons has a feedforward effect on the cortical synaptic connectivity of non-cBF neurons as well as cBF neurons [79]. Whether this dual reorganisation is typical of the cBF neuronal response to target-derived influences deserves further attention. Nonetheless, it is clear that NGF is an important mediator of cholinergic synaptic function.
TrkB-BDNF/NT4-mediated regulation of cBF neurons
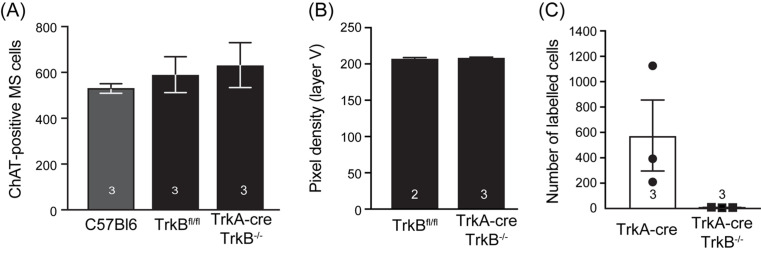
Like NGF-deficient mice, complete BDNF heterozygous mice have fewer and smaller cBF neurons at P15, a phenotype that is maintained into adulthood. Although BDNF−/+ mice display decreased ChAT activity [166], there is also evidence that the reduced cell number is due to cell death, with increased DNA fragmentation in the MS at P6 being reported in these animals [167]. In contrast, we have preliminary evidence that conditional knockout of TrkB in cBF neurons from birth (using a TrkA-cre) does not influence adult septal cBF neuron number or the density of somatosensory cortex innervation to any layer (Figure 3A,B). This suggests that the reduction in BDNF expression throughout the brain during embryogenesis indirectly affects the postnatal survival of cBF neurons, rather than this being cell autonomous. If so, neither NGF nor BDNF is required for cBF neuronal survival during the postnatal programmed cell death period, although each may compensate for the other. Nonetheless, TrkB appears to be crucial for cBF synaptogenesis, as the number of synaptic connections made by TrkB−/− cBF neurons to prefrontal cortical cells (labelled using an anterograde synaptic tracer; [79]) is significantly reduced so as to be almost negligible (Figure 3C), the opposite of the increase in synaptic connectivity observed in cBF-specific p75NTR-deficient animals [79].
Figure 3. Postnatal loss of TrkB from cBF neurons affects synaptic connectivity.
(A) Quantification of the number of cBF neurons in the MS (defined as per [68]) of C57Bl6 mice, TrkB floxed (TrkBfl/fl) mice and animals lacking p75NTR from E18 (via TrkA-cre). (B) Quantification of the cBF axonal innervation to the somatosensory cortex of TrkB floxed mice and TrkA-cre TrkB−/− mice. (C) Quantification of the number of cells labelled by synaptic transfer of a herpes simplex virus expressing TdTomato from TrkA-cre expressing cBF neurons (methods are as described in reference [79]). No difference in cBF neuron number or innervation density was observed. However the number of prefrontal cortex cells labelled by cBF synaptic innervation in TrkA-cre TrkB−/− mice was less than five per animal, compared with several hundred labelled cells in control TrkA-cre mice, the latter being equivalent to that reported for ChAT-cre animals [79]. The number of mice of each genotype is indicated in each graph.
In contrast with NGF, BDNF is expressed by pyramidal neurons and not interneurons and is therefore the best candidate for establishing pyramidal neuron cholinergic transmission via synaptic connections. Interestingly, topical application of BDNF has the opposite effect on the functional representation in the barrel cortex of a stimulated whisker to that of NGF application. BDNF results in a rapid and long-lasting decrease in whisker representation size and decreases the amplitude of the activity-dependent intrinsic signals [155]. This finding appears consistent with the actions of BDNF-expressing excitatory compared with NGF-expressing inhibitory neurons, although the explanation is unlikely to be that simple.
The effect of NGF, which mimics whisker training that causes the expansion of the receptive field for a specific whisker [155], may be due to a larger cholinergic neurotransmission release area within the cortical column (the area exposed to NGF) than is typically recruited by stimulation of whisker movement. In turn, the area which responds to cholinergic neurotransmission upon whisker stimulation (causing disinhibition/excitation) is larger. Surprisingly, this is not the effect of BDNF application, even though both NGF and BDNF are expected to enhance cBF axonal innervation, albeit to inhibitory and excitatory neurons, respectively. Rather, the effect of BDNF appears equivalent to denervation of a whisker, or alternatively, resulting in a more precise exclusive whisker representation field following whisker training [168]. That is, the effect of BDNF appears to be increased inhibition to (or reduced activation of) the application area. It is possible that this is because BDNF, unlike NGF, affects local cortical neurons (both inhibitory and excitatory) as well as cBF axons.
In summary, additional investigations need to be undertaken to reveal the mechanism by which BDNF-TrkB signalling modulates cBF neurons and thus cortical neuronal activity. BDNF appears to be required for the specification of magnocellular cBF neurons during embryonic development, and subsequently regulates the developmental and/or adult cBF synaptic innervation of the cortex, likely mirroring the role of NGF, but in relation to a different cellular target.
TrkC-NT3 mediated regulation of cBF neurons
Finally, almost nothing has been reported regarding the role of NT3-TrkC in cBF neurons. It is known that NT3 plays a role in thalamic innervation of the cortex [169], where it is expressed in the rat from birth, but with expression in cortical layers varying during postnatal development [170]. NT3 may perform this role by modulating the extent of cBF innervation of the cortex, acting as a mediator of cholinergic control over afferent thalamic neurotransmission within the cortex. It could also control cBF innervation to specific cortical layers in order to establish the cholinergic regulation of thalamic or other afferent neurotransmission. In the adult, the expression of NT3 is much more limited than that of BDNF, which is expressed by all pyramidal neurons. Hippocampal cells expressing NT-3 mRNA include those in the CA2 region, in the most medial portion of the CA1 region, and in the dentate gyrus [171]. These cells are primarily small subsets of parvalbumin- and calretinin-positive interneurons and form a distinct subset from the GABAergic cells that express NGF [162]. Surprisingly then, intraventricular infusion of NT3 is equally as potent as infusion of NGF in promoting cholinergic function and spatial maze learning of adult mice with lesioned cBF neurons [172]. This could be due to redundancy or overlapping functions, but given the expression of TrkC by cBF neurons, it is likely that the more restricted expression of NT3 controls aspects of cBF innervation to particular neurons or regions, which are not mediated sufficiently by TrkA and TrkB.
Cholinergic degeneration in dementia
Almost 40 years old, the first major evidence-based hypothesis regarding the aetiology of Alzheimer’s disease (AD) was the cholinergic hypothesis (reviewed in reference [173]). It proposed that the depletion of brain acetylcholine in the cerebral cortex [174,175] and loss of cBF neurons, particularly in the NBM [176], was a primary cause of the cognitive decline which occurs in advanced age and AD. The loss is not specific for AD however, with cholinergic dysfunction and degeneration being associated with a range of other neurodegenerative conditions involving cognitive impairment, including Down’s syndrome, dementia with Lewy bodies and Parkinson’s disease with dementia [177–179].
In AD (the most prevalent and best studied dementia), it is now clear that atrophy of the basal forebrain (as measured by a reduced volume in MRI images) precedes and predicts clinical decline in cohorts with both mild cognitive impairment and idiopathic AD, as well as in individuals who have not yet been diagnosed with cognitive impairment [180–183]. As in animals with lesioned cBF neurons, only higher order cognitive functions such as spatial awareness and navigation are impaired in early dementia [184].
Furthermore, atrophy of the posterior NBM (Ch4p nuclei) has shown similar or superior diagnostic accuracy to hippocampal atrophy in identifying AD subjects [185,186], and recent work by others suggests that cBF degeneration precedes and, together with coincident amyloid peptide (Aβ) accumulation into plaques (the classic hallmark of AD), drives entorhinal cortical degeneration [187]. These findings are significant because adult loss of cBF neurons alone is sufficient to drive cognitive impairment and subsequent Aβ, but not tau, pathology in a range of transgenic mouse models of familial AD [149,188–192]. Conversely, we have recently demonstrated that deletion of p75NTR from cBF neurons (resulting in more cBF neurons and more cholinergic synapses [79,125]) improves cognitive performance and reduces Aβ accumulation in the cortex of APP/PS1 familial AD mice [193]. These data strongly suggest that cholinergic degeneration is a major contributor to AD pathogenesis, whereas the maintenance of cholinergic function should be protective.
Neurotrophins and AD
Neurotrophin dysfunction has been strongly linked to cholinergic degeneration in AD, particularly decreased NGF/TrkA trophic signalling [194,195]. This underpinned the rationale for neurotrophin-based treatments of this condition [196,197], including ongoing clinical trials delivering NGF to the NBM by implant or virus [198,199]. The specific neurotrophin-related changes which occur in AD include down-regulation of BDNF signalling, the accumulation of proNGF in the cortex, and reduced TrkA and TrkB neurotrophic signalling in cBF neurons in association with increased cholinergic dysfunction and cognitive decline (comprehensively reviewed in reference [161]).
p75NTR small molecule modulators are also being tested for the treatment of AD [200]. Unlike in the healthy brain, there is evidence that p75NTR expression is induced in the cortex and hippocampus of animal AD models and human patients [201–203]. Furthermore, neuronal dystrophy induced by Aβ in both cultured cells and mouse cBF neurons can be mediated by p75NTR [204–206]. It has been shown that the extracellular domain of p75NTR can bind to and sequester and/or endocytose Aβ [207–210]. Conversely, deletion of p75NTR prevents the degeneration of cBF neurons in animal models of familial AD [204,206]. This evidence suggests that p75NTR may be a key player in mediating the ongoing selective degeneration of cBF neurons, which in turn could promote not only cognitive decline but further Aβ accumulation and cortical degeneration.
The major question regarding neurotrophin-based therapeutic options is how best to prevent or restore the loss of cBF connectivity in order to preserve cognitive function. Is it sufficient to merely keep cBF neurons alive? The provision of NGF to the NBM appears to promote neuronal survival [199,211], but it is unclear whether the cBF axons remain connected to or reinnervate the cortex. The neurotrophic hypothesis suggests that the neurotrophin should be provided at the target. Whether an even distribution of axonal innervation across the cortex would suffice is unknown, although the evidence presented above regarding the precision of cholinergic innervation linked to function would suggest not. However, acetylcholineesterase inhibitors such as Aricept show mild to moderate efficacy even when the specificity of timing and/or neuronal response cannot be maintained by this class of drug [212,213]. Therefore, it remains to be demonstrated whether specific timing of and/or a precise location of axonal innervation and acetylcholine release in the cortex is required for efficacious neurotransmission, and whether neurotrophins or mimetics can be used to enhance cholinergic-based treatment avenues for dementia.
Summary, future perspectives and predictions
cBF neurons are regulated by neurotrophins through their receptors from as early as cell specification and differentiation, mediating the extent of their cholinergic phenotype, and controlling axonal outgrowth and target innervation. In addition it is clear that the adult expression of neurotrophin receptors in cBF neurons regulates aspects of cell morphology, both growth and degeneration, which in turn can affect cortical function and thus behaviour. However, the precise role that each Trk receptor plays in these neurons is less clear, with redundancy being an unlikely explanation. Rather we propose that the neurotrophin receptors control specific aspects of cBF axonal innervation, and that the neurons remain in a developmental-like or plastic state throughout life, constantly rewiring in response to salient external stimuli to assist in encoding experiences required for future behaviourally relevant responses.
The specificity of innervation using different Trk receptors is illustrated by the sensory neurons of the dorsal root ganglia and neurons within the spinal cord [7]. These neurons also express p75NTR and each Trk receptor (albeit in restricted subpopulations) during development, and depend on these receptors for their appropriate innervation of different targets based on the expression of target-derived neurotrophins. Furthermore, it has been proposed that neurons with overlapping axonal fields predominantly express TrkA receptors, whereas those neurons with restricted axonal fields predominantly express TrkB and/or TrkC receptors [27]. Extrapolating, this could suggest that NGF is responsible for volume transmission and BDNF for synaptic innervation. However, this model is too simplistic. No particular interneuron subpopulation or cortical layer consistently expresses NGF, indicating that only some cells or circuits have the ability to up-regulate cholinergic neurotransmission via NGF signalling, and there is no reported reason why all neurotrophins could not equally induce varicosities and en passant release sites. More akin to the peripheral nervous system, co-expression of multiple Trk receptors by a single neuron may ensure that all relevant cells receive innervation by cBF axons, to guarantee widespread coverage of the cortex. However, this too seems simplistic, given the apparent importance of the location and timing of cholinergic transmission for cortical function.
It has been proposed that the activity-dependent release of NGF could serve as a feedback signal between highly plastic cortical inhibitory microcircuits and cholinergic neurotransmission, whereby NGF acts not only to increase cholinergic activity but also to reorganise the circuits for cortical function [164]. In other words, NGF, acting through TrkA, promotes the cBF axonal innervation of the NGF-producing cortical targets, after which TrkA-mediated signalling tunes the degree of cholinergic neurotransmission by regulating the amount of acetylcholine that is produced and/or released. Alternatively, the up-regulation of ChAT by NGF may reflect the number or extent of axonal arbors or neurotransmitter release sites of individual neurons, which expand in response to an increase in the number of cortical cells producing NGF. In both examples, the expression of NGF is regulated by recently, robustly or repeatedly activated interneurons within particular microcircuits of the cortex.
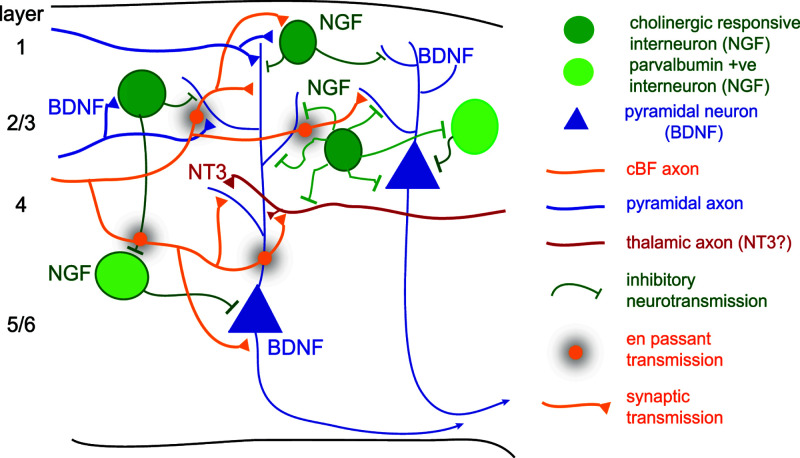
An extension of this hypothesis would be that input requiring the encoding of new memories, which involves the rewiring of circuits, alters the NGF expression level or the neuronal cells that express NGF, to drive circuit or neurotransmission change, whereas recalling learned memory does not require NGF expression, or its expression remains unchanged. Furthermore, the release of proNGF by inactive interneurons, could stimulate pruning or LTD of local cBF connections via p75NTR. However, in this model (Figure 4) it remains unclear whether NGF only mediates cBF synaptic innervation of inhibitory neurons, or also regulates pyramidal neuronal activity through regulation of volume transmission within local cortical microcircuits.
Figure 4. Model of cBF connectivity of the cortex driven by different neurotrophins.
cBF neuronal axons (orange) can release acetylcholine by both volume and synaptic neurotransmission. Some interneurons (green) express NGF and mediate inhibitory transmission, thereby regulating disinhibition. Other undefined interneuron subtypes do not express NGF whereas others do not respond to acetylcholine (e.g. parvalbumin-positive). Pyramidal neurons (blue) express BDNF and regulate cBF synaptic innervation of dendrites, to directly modulate dendritic input and scaling, as well as axo-somatic sites. The role of NT3 is as yet unclear but could modulate cBF regulation of thalamic axonal input (red), and/or control region specific innervation within the hippocampus (not shown).
As BDNF is only expressed by pyramidal neurons, and given our preliminary data from TrkB−/− cBF neurons (Figure 3), it is reasonable to conclude that BDNF-TrkB acts to regulate the cBF synaptic connectivity of pyramidal neurons (Figure 4). The formation of cholinergic synapses onto pyramidal neuronal dendrites and their axo-somatic regions would help to co-ordinate dendritic integration and cortical synaptic output. However, BDNF can be released from axonal terminals as well as dendritic spines [214], and the release of BDNF from innervating glutamatergic axons could promote en passant cholinergic discharge and therefore modulate presynaptic nAChRs located at extra-synaptic sites on glutamatergic afferents [215], and thereby the strength of the incoming information as well as the integration within and the output of the information from cortical neurons.
NT3-TrkC is proposed to regulate other specialised functions of cBF neurons. Speculatively, this could include modulation of specific afferent neurotransmission, gatekeeping of information from brain areas (e.g. CA2/3 output to CA1), or control of innervation that needs to be coordinated between brain areas such as the hippocampus and cortex. However, NT3 may simply regulate a third layer of innervation such as a specific interneuronal class.
Finally, p75NTR acts to limit cBF neurotransmission, which could be crucial when the information that cBF connections encode is no longer salient or requires modification. It appears that p75NTR might act in a continuum, from inhibiting LTP and enhancing LTD at cholinergic synapses, to pruning cholinergic synapses and/or non-synaptic acetylcholine release sites, to pruning axons. It is possible that p75NTR also acts to temper cholinergic transmission presynaptically, being able to regulate G-protein coupled inwardly rectifying channels that reduce the membrane potential, acting to prevent excitotoxicity in these highly metabolically active neurons [216,217]. Whether p75NTR is activated by proneurotropins accumulating in the absence of sufficient circuit activity and cleavage to mature forms, or by other cell types that act to remove inactive synapses (e.g microglia), and/or in the absence of sufficient Trk receptor signalling, will require sophisticated in vivo experimentation to reveal. It is clear nonetheless that expression of p75NTR makes cBF neurons susceptible to degeneration in conditions such as AD, including in response to Aβ.
Given the importance of acetylcholine on cortical disinhibition, particularly dendritic gating, further elucidating the mechanisms which control the cBF axonal innervation that results in synaptic and volume neurotransmission, as well as deciphering which neurons within cortical columns are so innervated, and under what conditions, will significantly increase our understanding of the cortical computations underpinning associative learning. Moreover determining the time-frame of cBF axonal and synaptic change mediated by neurotrophin receptors in the adult is of key importance for further understanding the mechanisms by which this influential group of neurons affects higher order cortical function and experience-dependent learning. This knowledge is also crucial for designing efficacious therapies to prevent or restore cBF dysfunction and thus cognitive impairment in the context of neurological and degenerative conditions including AD.
Acknowledgements
We thank other members of our laboratories and Professor Stephen R. Williams and Rowan Tweedale for critical reading and editing of the manuscript.
Abbreviations
- Abeta
amyloid beta
- AD
Alzheimer’s disease
- BDNF
brain-derived neurotrophic factor
- BMP
bone morphogenetic protein
- cBF
cholinergic basal forebrain
- GABAergic
γ-aminobutyric acid-ergic
- HDB
horizontal diagonal band of Broca
- LTD
long-term depression
- LTP
long-term potentiation
- mAchR
muscarinic acetylcholine receptor
- MS
medial septum
- nAchR
nicotinic acetylcholine receptor
- NBM
nucleus basalis of Meynert
- NGF
nerve growth factor
- NT3/4
neurotrophin 3 and 4
- p75NTR
p75 neurotrophin receptor
- SI
substantia innominata
- Trk
tropomyosin-related kinase
- VDB
vertical diagonal band of Broca
Funding
This work was supported by the Australian National Health and Medical Research Council of Australia [grant number 1144731 (to E.J.C.)]; and the Chinese Council Scholarship (to Y.W.).
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Bothwell M. (2016) Recent advances in understanding neurotrophin signaling. F1000Res. 5, 10.12688/f1000research.8434.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meeker R. and Williams K. (2014) Dynamic nature of the p75 neurotrophin receptor in response to injury and disease. J. Neuroimmune Pharmacol. 9, 615–628 10.1007/s11481-014-9566-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitre M., Mariga A. and Chao M.V. (2017) Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin. Sci. (Lond.) 131, 13–23 10.1042/CS20160044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reichardt L.F. (2006) Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1545–1564 10.1098/rstb.2006.1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomellini E., Lagadec C., Polakowska R. and Le Bourhis X. (2014) Role of p75 neurotrophin receptor in stem cell biology: more than just a marker. Cell. Mol. Life Sci. 71, 2467–2481 10.1007/s00018-014-1564-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva S. and Wang F. (2011) Retrograde neural circuit specification by target-derived neurotrophins and growth factors. Curr. Opin. Neurobiol. 21, 61–67 10.1016/j.conb.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keefe K.M., Sheikh I.S. and Smith G.M. (2017) Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int. J. Mol. Sci. 10.3390/ijms18030548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murase S. (2014) A new model for developmental neuronal death and excitatory/inhibitory balance in hippocampus. Mol. Neurobiol. 49, 316–325 10.1007/s12035-013-8521-8 [DOI] [PubMed] [Google Scholar]
- 9.Bronfman F.C., Lazo O.M., Flores C. and Escudero C.A. (2014) Spatiotemporal intracellular dynamics of neurotrophin and its receptors. Implications for neurotrophin signaling and neuronal function. Handb. Exp. Pharmacol. 220, 33–65 10.1007/978-3-642-45106-5_3 [DOI] [PubMed] [Google Scholar]
- 10.Zweifel L.S., Kuruvilla R. and Ginty D.D. (2005) Functions and mechanisms of retrograde neurotrophin signalling. Nat. Rev. Neurosci. 6, 615–625 10.1038/nrn1727 [DOI] [PubMed] [Google Scholar]
- 11.Matusica D. and Coulson E.J. (2014) Local versus long-range neurotrophin receptor signalling: endosomes are not just carriers for axonal transport. Semin. Cell Dev. Biol. 31, 57–63 10.1016/j.semcdb.2014.03.032 [DOI] [PubMed] [Google Scholar]
- 12.Herrmann K.A. and Broihier H.T. (2018) What neurons tell themselves: autocrine signals play essential roles in neuronal development and function. Curr. Opin. Neurobiol. 51, 70–79 10.1016/j.conb.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Palacio-Schjetnan A. and Escobar M.L. (2013) Neurotrophins and synaptic plasticity. Curr. Topics Behav. Neurosci. 15, 117–136 10.1007/7854_2012_231 [DOI] [PubMed] [Google Scholar]
- 14.Leal G., Afonso P.M., Salazar I.L. and Duarte C.B. (2015) Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 1621, 82–101 10.1016/j.brainres.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 15.Guo W., Nagappan G. and Lu B. (2018) Differential effects of transient and sustained activation of BDNF-TrkB signaling. Dev. Neurobiol. 78, 647–659 10.1002/dneu.22592 [DOI] [PubMed] [Google Scholar]
- 16.Lu B., Pang P.T. and Woo N.H. (2005) The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 6, 603–614 10.1038/nrn1726 [DOI] [PubMed] [Google Scholar]
- 17.Semba K. (2004) Phylogenetic and ontogenetic aspects of the basal forebrain cholinergic neurons and their innervation of the cerebral cortex. Prog. Brain Res. 145, 3–43 [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Holtzman D.M., Kromer L.F., Kaplan D.R., Chua-Couzens J., Clary D.O. et al. (1995) Regulation of TrkA and ChAT expression in developing rat basal forebrain: evidence that both exogenous and endogenous NGF regulate differentiation of cholinergic neurons. J. Neurosci. 15, 2888–2905 10.1523/JNEUROSCI.15-04-02888.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thal L.J., Gilbertson E., Armstrong D.M. and Gage F.H. (1992) Development of the basal forebrain cholinergic system: phenotype expression prior to target innervation. Neurobiol. Aging 13, 67–72 10.1016/0197-4580(92)90011-L [DOI] [PubMed] [Google Scholar]
- 20.Marin O. and Rubenstein J.L. (2001) A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2, 780–790 10.1038/35097509 [DOI] [PubMed] [Google Scholar]
- 21.Xu Q., Tam M. and Anderson S.A. (2008) Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J. Comp. Neurol. 506, 16–29 10.1002/cne.21529 [DOI] [PubMed] [Google Scholar]
- 22.Magno L., Kretz O., Bert B., Ersozlu S., Vogt J., Fink H. et al. (2011) The integrity of cholinergic basal forebrain neurons depends on expression of Nkx2-1. Eur. J. Neurosci. 34, 1767–1782 10.1111/j.1460-9568.2011.07890.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magno L., Barry C., Schmidt-Hieber C., Theodotou P., Hausser M. and Kessaris N. (2017) NKX2-1 Is Required in the embryonic septum for cholinergic system development, learning, and memory. Cell Rep. 20, 1572–1584 10.1016/j.celrep.2017.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fragkouli A., van Wijk N.V., Lopes R., Kessaris N. and Pachnis V. (2009) LIM homeodomain transcription factor-dependent specification of bipotential MGE progenitors into cholinergic and GABAergic striatal interneurons. Development 136, 3841–3851 10.1242/dev.038083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liodis P., Denaxa M., Grigoriou M., Akufo-Addo C., Yanagawa Y. and Pachnis V. (2007) Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J. Neurosci. 27, 3078–3089 10.1523/JNEUROSCI.3055-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pombero A., Bueno C., Saglietti L., Rodenas M., Guimera J., Bulfone A. et al. (2011) Pallial origin of basal forebrain cholinergic neurons in the nucleus basalis of Meynert and horizontal limb of the diagonal band nucleus. Development 138, 4315–4326 10.1242/dev.069534 [DOI] [PubMed] [Google Scholar]
- 27.von Bartheld C.S. and Fritzsch B. (2006) Comparative analysis of neurotrophin receptors and ligands in vertebrate neurons: tools for evolutionary stability or changes in neural circuits? Brain Behav. Evol. 68, 157–172 10.1159/000094085 [DOI] [PubMed] [Google Scholar]
- 28.von Bartheld C.S., Schober A., Kinoshita Y., Williams R., Ebendal T. and Bothwell M. (1995) Noradrenergic neurons in the locus coeruleus of birds express TrkA, transport NGF, and respond to NGF. J. Neurosci. 15, 2225–2239 10.1523/JNEUROSCI.15-03-02225.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Formaggio E., Dalfini A.C., Fazzini F., Fumagalli G. and Chiamulera C. (2011) GABAergic neurons expressing p75 in rat substantia innominata and nucleus basalis. Mol. Cell. Neurosci. 46, 625–632 10.1016/j.mcn.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 30.Schnitzler A.C., Mellott T.J., Lopez-Coviella I., Tallini Y.N., Kotlikoff M.I., Follettie M.T. et al. (2010) BMP9 (bone morphogenetic protein 9) induces NGF as an autocrine/paracrine cholinergic trophic factor in developing basal forebrain neurons. J. Neurosci. 30, 8221–8228 10.1523/JNEUROSCI.5611-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Coviella I., Follettie M.T., Mellott T.J., Kovacheva V.P., Slack B.E., Diesl V. et al. (2005) Bone morphogenetic protein 9 induces the transcriptome of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. U.S.A. 102, 6984–6989 10.1073/pnas.0502097102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Large T.H., Bodary S.C., Clegg D.O., Weskamp G., Otten U. and Reichardt L.F. (1986) Nerve growth factor gene expression in the developing rat brain. Science 234, 352–355 10.1126/science.3764415 [DOI] [PubMed] [Google Scholar]
- 33.Vantini G., Schiavo N., Di Martino A., Polato P., Triban C., Callegaro L. et al. (1989) Evidence for a physiological role of nerve growth factor in the central nervous system of neonatal rats. Neuron 3, 267–273 10.1016/0896-6273(89)90251-1 [DOI] [PubMed] [Google Scholar]
- 34.Nonomura T., Nishio C., Lindsay R.M. and Hatanaka H. (1995) Cultured basal forebrain cholinergic neurons from postnatal rats show both overlapping and non-overlapping responses to the neurotrophins. Brain Res. 683, 129–139 10.1016/0006-8993(95)00357-V [DOI] [PubMed] [Google Scholar]
- 35.Ha D.H., Robertson R.T., Ribak C.E. and Weiss J.H. (1996) Cultured basal forebrain cholinergic neurons in contact with cortical cells display synapses, enhanced morphological features, and decreased dependence on nerve growth factor. J. Comp. Neurol. 373, 451–465 [DOI] [PubMed] [Google Scholar]
- 36.Gritti I., Mainville L., Mancia M. and Jones B.E. (1997) GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J. Comp. Neurol. 383, 163–177 [DOI] [PubMed] [Google Scholar]
- 37.Tingley D., Alexander A.S., Quinn L.K., Chiba A.A. and Nitz D.A. (2015) Cell assemblies of the basal forebrain. J. Neurosci. 35, 2992–3000 10.1523/JNEUROSCI.4432-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mesulam M.M., Mufson E.J., Wainer B.H. and Levey A.I. (1983) Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10, 1185–1201 10.1016/0306-4522(83)90108-2 [DOI] [PubMed] [Google Scholar]
- 39.Saunders A., Granger A.J. and Sabatini B.L. (2015) Corelease of acetylcholine and GABA from cholinergic forebrain neurons. Elife 4, 10.7554/eLife.06412, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerbler G.M., Hamlin A.S., Pannek K., Kurniawan N.D., Keller M.D., Rose S.E. et al. (2013) Diffusion-weighted magnetic resonance imaging detection of basal forebrain cholinergic degeneration in a mouse model. Neuroimage 66, 133–141 10.1016/j.neuroimage.2012.10.075 [DOI] [PubMed] [Google Scholar]
- 41.Bloem B., Schoppink L., Rotaru D.C., Faiz A., Hendriks P., Mansvelder H.D. et al. (2014) Topographic mapping between basal forebrain cholinergic neurons and the medial prefrontal cortex in mice. J. Neurosci. 34, 16234–16246 10.1523/JNEUROSCI.3011-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gozzi A., Jain A., Giovannelli A., Bertollini C., Crestan V., Schwarz A.J. et al. (2010) A neural switch for active and passive fear. Neuron 67, 656–666 10.1016/j.neuron.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 43.Ballinger E.C., Ananth M., Talmage D.A. and Role L.W. (2016) Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 91, 1199–1218 10.1016/j.neuron.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Yu B., Sun Q., Zhang Y., Ren M., Zhang X. et al. (2018) Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. U.S.A. 115, 415–420 10.1073/pnas.1703601115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Souza Silva M.A., Dolga A., Pieri I., Marchetti L., Eisel U.L., Huston J.P. et al. (2006) Cholinergic cells in the nucleus basalis of mice express the N-methyl-D-aspartate-receptor subunit NR2C and its replacement by the NR2B subunit enhances frontal and amygdaloid acetylcholine levels. Genes Brain Behav. 5, 552–560 10.1111/j.1601-183X.2006.00206.x [DOI] [PubMed] [Google Scholar]
- 46.Zaborszky L. and Cullinan W.E. (1992) Projections from the nucleus accumbens to cholinergic neurons of the ventral pallidum: a correlated light and electron microscopic double-immunolabeling study in rat. Brain Res. 570, 92–101 10.1016/0006-8993(92)90568-T [DOI] [PubMed] [Google Scholar]
- 47.Zaborszky L., van den Pol A. and Gyengesi E. (2012) The basal forebrain cholinergic projection system in mice. In The Mouse Nervous System, Watson C., Paxinos G. and Puelles L. eds) pp. 684–718, Academic Press Elsevier, Cambridge, Massachusetts [Google Scholar]
- 48.Zaborszky L., Duque A., Gielow M., Gombkoto P., Nadasdy Z. and Somogyi J. (2015) Chapter 19 - Organization of the basal forebrain cholinergic projection system: specific or diffuse. In The Rat Nervous System, 4th edn, (Paxinos G., ed.), pp. 491–507, Academic Press, San Diego [Google Scholar]
- 49.Duque A., Tepper J.M., Detari L., Ascoli G.A. and Zaborszky L. (2007) Morphological characterization of electrophysiologically and immunohistochemically identified basal forebrain cholinergic and neuropeptide Y-containing neurons. Brain Struct. Funct. 212, 55–73 10.1007/s00429-007-0143-3 [DOI] [PubMed] [Google Scholar]
- 50.Zaborszky L. and Duque A. (2000) Local synaptic connections of basal forebrain neurons. Behav. Brain Res. 115, 143–158 10.1016/S0166-4328(00)00255-2 [DOI] [PubMed] [Google Scholar]
- 51.Hajszan T., Alreja M. and Leranth C. (2004) Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: novel glutamatergic local circuit cells. Hippocampus 14, 499–509 10.1002/hipo.10195 [DOI] [PubMed] [Google Scholar]
- 52.Saper C.B. (1984) Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J. Comp. Neurol. 222, 313–342 10.1002/cne.902220302 [DOI] [PubMed] [Google Scholar]
- 53.Woolf N.J. (1991) Cholinergic systems in mammalian brain and spinal cord. Prog. Neurobiol. 37, 475–524 10.1016/0301-0082(91)90006-M [DOI] [PubMed] [Google Scholar]
- 54.Kondo H. and Zaborszky L. (2016) Topographic organization of the basal forebrain projections to the perirhinal, postrhinal, and entorhinal cortex in rats. J. Comp. Neurol. 524, 2503–2515 10.1002/cne.23967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaborszky L., Csordas A., Mosca K., Kim J., Gielow M.R., Vadasz C. et al. (2015) Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb. Cortex 25, 118–137 10.1093/cercor/bht210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan R., Biswal B.B. and Zaborszky L. (2018) Functional subdivisions of magnocellular cell groups in human basal forebrain: test-retest resting-state study at ultra-high field, and meta-analysis. Cereb. Cortex 10.1093/cercor/bhy150, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasselmo M.E. and Sarter M. (2011) Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 36, 52–73 10.1038/npp.2010.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lustig C. and Sarter M. (2016) Attention and the cholinergic system: relevance to schizophrenia. Curr. Top. Behav. Neurosci. 28, 327–362 10.1007/7854_2015_5009 [DOI] [PubMed] [Google Scholar]
- 59.Knox D. (2016) The role of basal forebrain cholinergic neurons in fear and extinction memory. Neurobiol. Learn. Mem. 133, 39–52 10.1016/j.nlm.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson M.A. and Fadel J.R. (2017) Cholinergic regulation of fear learning and extinction. J. Neurosci. Res. 95, 836–852 10.1002/jnr.23840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitchers K.K., Phillips K.B., Jones J.L., Robinson T.E. and Sarter M. (2017) Diverse roads to relapse: a discriminative cue signaling cocaine availability is more effective in renewing cocaine seeking in goal trackers than sign trackers and depends on basal forebrain cholinergic activity. J. Neurosci. 37, 7198–7208 10.1523/JNEUROSCI.0990-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wiley R.G., Oeltmann T.N. and Lappi D.A. (1991) Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 562, 149–153 10.1016/0006-8993(91)91199-B [DOI] [PubMed] [Google Scholar]
- 63.Conner J.M., Culberson A., Packowski C., Chiba A.A. and Tuszynski M.H. (2003) Lesions of the Basal forebrain cholinergic system impair task acquisition and abolish cortical plasticity associated with motor skill learning. Neuron 38, 819–829 10.1016/S0896-6273(03)00288-5 [DOI] [PubMed] [Google Scholar]
- 64.Johnson D.A., Zambon N.J. and Gibbs R.B. (2002) Selective lesion of cholinergic neurons in the medial septum by 192 IgG-saporin impairs learning in a delayed matching to position T-maze paradigm. Brain Res. 943, 132–141 10.1016/S0006-8993(02)02623-9 [DOI] [PubMed] [Google Scholar]
- 65.Berger-Sweeney J., Stearns N.A., Murg S.L., Floerke-Nashner L.R., Lappi D.A. and Baxter M.G. (2001) Selective immunolesions of cholinergic neurons in mice: effects on neuroanatomy, neurochemistry, and behavior. J. Neurosci. 21, 8164–8173 10.1523/JNEUROSCI.21-20-08164.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moreau P.H., Cosquer B., Jeltsch H., Cassel J.C. and Mathis C. (2008) Neuroanatomical and behavioral effects of a novel version of the cholinergic immunotoxin mu p75-saporin in mice. Hippocampus 18, 610–622 10.1002/hipo.20422 [DOI] [PubMed] [Google Scholar]
- 67.Gil-Bea F.J., Solas M., Mateos L., Winblad B., Ramirez M.J. and Cedazo-Minguez A. (2011) Cholinergic hypofunction impairs memory acquisition possibly through hippocampal Arc and BDNF downregulation. Hippocampus 21, 999–1009 [DOI] [PubMed] [Google Scholar]
- 68.Hamlin A.S., Windels F., Boskovic Z., Sah P. and Coulson E.J. (2013) Lesions of the basal forebrain cholinergic system in mice disrupt idiothetic navigation. PLoS ONE 8, e53472 10.1371/journal.pone.0053472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prado-Alcala R.A., Haiek M., Rivas S., Roldan-Roldan G. and Quirarte G.L. (1994) Reversal of extinction by scopolamine. Physiol. Behav. 56, 27–30 10.1016/0031-9384(94)90257-7 [DOI] [PubMed] [Google Scholar]
- 70.Roldan G., Cobos-Zapiain G., Quirarte G.L. and Prado-Alcala R.A. (2001) Dose- and time-dependent scopolamine-induced recovery of an inhibitory avoidance response after its extinction in rats. Behav. Brain Res. 121, 173–179 10.1016/S0166-4328(01)00157-7 [DOI] [PubMed] [Google Scholar]
- 71.Santini E., Sepulveda-Orengo M. and Porter J.T. (2012) Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology 37, 2047–2056 10.1038/npp.2012.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yousefi B., Nasehi M., Khakpai F. and Zarrindast M.R. (2012) Possible interaction of cholinergic and GABAergic systems between MS and CA1 upon memory acquisition in rats. Behav. Brain Res. 235, 231–243 10.1016/j.bbr.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 73.Blake M.G., Boccia M.M., Krawczyk M.C., Delorenzi A. and Baratti C.M. (2012) Choline reverses scopolamine-induced memory impairment by improving memory reconsolidation. Neurobiol. Learn. Mem. 98, 112–121 10.1016/j.nlm.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 74.Goard M. and Dan Y. (2009) Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci. 12, 1444–1449 10.1038/nn.2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma M. and Luo M. (2012) Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J. Neurosci. 32, 10105–10116 10.1523/JNEUROSCI.0058-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anaclet C., Pedersen N.P., Ferrari L.L., Venner A., Bass C.E., Arrigoni E. et al. (2015) Basal forebrain control of wakefulness and cortical rhythms. Nat. Commun. 6, 8744 10.1038/ncomms9744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hangya B., Ranade S.P., Lorenc M. and Kepecs A. (2015) Central cholinergic neurons are rapidly recruited by reinforcement feedback. Cell 162, 1155–1168 10.1016/j.cell.2015.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okada K., Nishizawa K., Kobayashi T., Sakata S. and Kobayashi K. (2015) Distinct roles of basal forebrain cholinergic neurons in spatial and object recognition memory. Sci. Rep. 5, 13158 10.1038/srep13158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boskovic Z., Milne M.R., Qian L., Clifton H.D., McGovern A.E., Turnbull M.T. et al. (2018) Cholinergic basal forebrain neurons regulate fear extinction consolidation through p75 neurotrophin receptor signaling. Transl. Psychiatry 8, 199 10.1038/s41398-018-0248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Staib J.M., Della Valle R. and Knox D.K. (2018) Disruption of medial septum and diagonal bands of Broca cholinergic projections to the ventral hippocampus disrupt auditory fear memory. Neurobiol. Learn. Mem. 152, 71–79 10.1016/j.nlm.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solari S.V. and Stoner R. (2011) Cognitive consilience: primate non-primary neuroanatomical circuits underlying cognition. Front. Neuroanat. 5, 65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee M.G., Chrobak J.J., Sik A., Wiley R.G. and Buzsaki G. (1994) Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience 62, 1033–1047 10.1016/0306-4522(94)90341-7 [DOI] [PubMed] [Google Scholar]
- 83.McGaughy J., Koene R.A., Eichenbaum H. and Hasselmo M.E. (2005) Cholinergic deafferentation of the entorhinal cortex in rats impairs encoding of novel but not familiar stimuli in a delayed nonmatch-to-sample task. J. Neurosci. 25, 10273–10281 10.1523/JNEUROSCI.2386-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dannenberg H., Pabst M., Braganza O., Schoch S., Niediek J., Bayraktar M. et al. (2015) Synergy of direct and indirect cholinergic septo-hippocampal pathways coordinates firing in hippocampal networks. J. Neurosci. 35, 8394–8410 10.1523/JNEUROSCI.4460-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hasselmo M.E. and Schnell E. (1994) Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J. Neurosci. 14, 3898–3914 10.1523/JNEUROSCI.14-06-03898.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kremin T. and Hasselmo M.E. (2007) Cholinergic suppression of glutamatergic synaptic transmission in hippocampal region CA3 exhibits laminar selectivity: implication for hippocampal network dynamics. Neuroscience 149, 760–767 10.1016/j.neuroscience.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parikh V. and Sarter M. (2008) Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann. N.Y. Acad. Sci. 1129, 225–235 10.1196/annals.1417.021 [DOI] [PubMed] [Google Scholar]
- 88.Gu Z., Lamb P.W. and Yakel J.L. (2012) Cholinergic coordination of presynaptic and postsynaptic activity induces timing-dependent hippocampal synaptic plasticity. J. Neurosci. 32, 12337–12348 10.1523/JNEUROSCI.2129-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarter M., Lustig C., Berry A.S., Gritton H., Howe W.M. and Parikh V. (2016) What do phasic cholinergic signals do? Neurobiol. Learn. Mem. 130, 135–141 10.1016/j.nlm.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Inglis F.M., Day J.C. and Fibiger H.C. (1994) Enhanced acetylcholine release in hippocampus and cortex during the anticipation and consumption of a palatable meal. Neuroscience 62, 1049–1056 10.1016/0306-4522(94)90342-5 [DOI] [PubMed] [Google Scholar]
- 91.Inglis F.M. and Fibiger H.C. (1995) Increases in hippocampal and frontal cortical acetylcholine release associated with presentation of sensory stimuli. Neuroscience 66, 81–86 10.1016/0306-4522(94)00578-S [DOI] [PubMed] [Google Scholar]
- 92.Orsetti M., Casamenti F. and Pepeu G. (1996) Enhanced acetylcholine release in the hippocampus and cortex during acquisition of an operant behavior. Brain Res. 724, 89–96 10.1016/0006-8993(96)00292-2 [DOI] [PubMed] [Google Scholar]
- 93.Gritton H.J., Howe W.M., Mallory C.S., Hetrick V.L., Berke J.D. and Sarter M. (2016) Cortical cholinergic signaling controls the detection of cues. Proc. Natl. Acad. Sci. U.S.A. 113, E1089–97 10.1073/pnas.1516134113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu J., Dosher B.A. and Lu Z.L. (2015) Augmented Hebbian reweighting accounts for accuracy and induced bias in perceptual learning with reverse feedback. J. Vision 15, 10 10.1167/15.10.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pinto L., Goard M.J., Estandian D., Xu M., Kwan A.C., Lee S.H. et al. (2013) Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci. 16, 1857–1863 10.1038/nn.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eggermann E., Kremer Y., Crochet S. and Petersen C.C.H. (2014) Cholinergic signals in mouse barrel cortex during active whisker sensing. Cell Rep. 9, 1654–1660 10.1016/j.celrep.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 97.Unal C.T., Pare D. and Zaborszky L. (2015) Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J. Neurosci. 35, 853–863 10.1523/JNEUROSCI.2706-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu A.J. and Dayan P. (2002) Acetylcholine in cortical inference. Neural Netw. 15, 719–730 10.1016/S0893-6080(02)00058-8 [DOI] [PubMed] [Google Scholar]
- 99.Froemke R.C., Merzenich M.M. and Schreiner C.E. (2007) A synaptic memory trace for cortical receptive field plasticity. Nature 450, 425–429 10.1038/nature06289 [DOI] [PubMed] [Google Scholar]
- 100.Tremblay N., Warren R.A. and Dykes R.W. (1990) Electrophysiological studies of acetylcholine and the role of the basal forebrain in the somatosensory cortex of the cat. I. Cortical neurons excited by glutamate. J. Neurophysiol. 64, 1199–1211 10.1152/jn.1990.64.4.1199 [DOI] [PubMed] [Google Scholar]
- 101.Tremblay N., Warren R.A. and Dykes R.W. (1990) Electrophysiological studies of acetylcholine and the role of the basal forebrain in the somatosensory cortex of the cat. II. Cortical neurons excited by somatic stimuli. J. Neurophysiol. 64, 1212–1222 10.1152/jn.1990.64.4.1212 [DOI] [PubMed] [Google Scholar]
- 102.Williams S.R. and Fletcher L.N. (2019) A dendritic substrate for the cholinergic control of neocortical output neurons. Neuron, 10.1016/j.neuron.2018.11.035, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 103.Higley M.J., Soler-Llavina G.J. and Sabatini B.L. (2009) Cholinergic modulation of multivesicular release regulates striatal synaptic potency and integration. Nat. Neurosci. 12, 1121–1128 10.1038/nn.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen N., Sugihara H. and Sur M. (2015) An acetylcholine-activated microcircuit drives temporal dynamics of cortical activity. Nat. Neurosci. 18, 892–902 10.1038/nn.4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Obermayer J., Verhoog M.B., Luchicchi A. and Mansvelder H.D. (2017) Cholinergic modulation of cortical microcircuits is layer-specific: evidence from rodent, monkey and human brain. Front. Neural Circuits 11, 100 10.3389/fncir.2017.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Coppola J.J. and Disney A.A. (2018) Is there a canonical cortical circuit for the cholinergic system? anatomical differences across common model systems Front. Neural Circuits 12, 8 10.3389/fncir.2018.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Disney A.A., Domakonda K.V. and Aoki C. (2006) Differential expression of muscarinic acetylcholine receptors across excitatory and inhibitory cells in visual cortical areas V1 and V2 of the macaque monkey. J. Comp. Neurol. 499, 49–63 10.1002/cne.21096 [DOI] [PubMed] [Google Scholar]
- 108.Gulledge A.T., Bucci D.J., Zhang S.S., Matsui M. and Yeh H.H. (2009) M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J. Neurosci. 29, 9888–9902 10.1523/JNEUROSCI.1366-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Letzkus J.J., Wolff S.B., Meyer E.M., Tovote P., Courtin J., Herry C. et al. (2011) A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335 10.1038/nature10674 [DOI] [PubMed] [Google Scholar]
- 110.Brombas A., Fletcher L.N. and Williams S.R. (2014) Activity-dependent modulation of layer 1 inhibitory neocortical circuits by acetylcholine. J. Neurosci. 34, 1932–1941 10.1523/JNEUROSCI.4470-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Radzicki D., Pollema-Mays S.L., Sanz-Clemente A. and Martina M. (2017) Loss of M1 receptor dependent cholinergic excitation contributes to mPFC deactivation in neuropathic pain. J. Neurosci. 37, 2292–2304 10.1523/JNEUROSCI.1553-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sparks D.W. and Chapman C.A. (2014) Contribution of Ih to the relative facilitation of synaptic responses induced by carbachol in the entorhinal cortex during repetitive stimulation of the parasubiculum. Neuroscience 278, 81–92 10.1016/j.neuroscience.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 113.Yamasaki M., Matsui M. and Watanabe M. (2010) Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J. Neurosci. 30, 4408–4418 10.1523/JNEUROSCI.5719-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nunez A., Dominguez S., Buno W. and Fernandez de Sevilla D. (2012) Cholinergic-mediated response enhancement in barrel cortex layer V pyramidal neurons. J. Neurophysiol. 108, 1656–1668 10.1152/jn.00156.2012 [DOI] [PubMed] [Google Scholar]
- 115.Mufson E.J., Ginsberg S.D., Ikonomovic M.D. and DeKosky S.T. (2003) Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J. Chem. Neuroanat. 26, 233–242 10.1016/S0891-0618(03)00068-1 [DOI] [PubMed] [Google Scholar]
- 116.Salehi A., Verhaagen J., Dijkhuizen P.A. and Swaab D.F. (1996) Co-localization of high-affinity neurotrophin receptors in nucleus basalis of Meynert neurons and their differential reduction in Alzheimer’s disease. Neuroscience 75, 373–387 10.1016/0306-4522(96)00273-4 [DOI] [PubMed] [Google Scholar]
- 117.Milne M.R., Haug C.A., Abraham I.M. and Kwakowsky A. (2015) Estradiol modulation of neurotrophin receptor expression in female mouse basal forebrain cholinergic neurons in vivo. Endocrinology 156, 613–626 10.1210/en.2014-1669 [DOI] [PubMed] [Google Scholar]
- 118.Yeo T.T., Chua-Couzens J., Butcher L.L., Bredesen D.E., Cooper J.D., Valletta J.S. et al. (1997) Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. J. Neurosci. 17, 7594–7605 10.1523/JNEUROSCI.17-20-07594.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Naumann T., Casademunt E., Hollerbach E., Hofmann J., Dechant G., Frotscher M. et al. (2002) Complete deletion of the neurotrophin receptor p75NTR leads to long-lasting increases in the number of basal forebrain cholinergic neurons. J. Neurosci. 22, 2409–2418 10.1523/JNEUROSCI.22-07-02409.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Greferath U., Trieu J. and Barrett G.L. (2012) The p75 neurotrophin receptor has nonapoptotic antineurotrophic actions in the basal forebrain. J. Neurosci. Res. 90, 278–287 10.1002/jnr.22735 [DOI] [PubMed] [Google Scholar]
- 121.Ward N.L. and Hagg T. (1999) p75NGFR and cholinergic neurons in the developing forebrain: a re-examination. Brain Res. Dev. Brain Res. 118, 79–91 10.1016/S0165-3806(99)00133-9 [DOI] [PubMed] [Google Scholar]
- 122.Underwood C.K. and Coulson E.J. (2008) The p75 neurotrophin receptor. Int. J. Biochem. Cell Biol. 40, 1664–1668 10.1016/j.biocel.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 123.Peterson D.A., Dickinson-Anson H.A., Leppert J.T., Lee K.F. and Gage F.H. (1999) Central neuronal loss and behavioral impairment in mice lacking neurotrophin receptor p75. J. Comp. Neurol. 404, 1–20 [DOI] [PubMed] [Google Scholar]
- 124.Dokter M., Busch R., Poser R., Vogt M.A., von Bohlen Und Halbach V., Gass P. et al. (2015) Implications of p75NTR for dentate gyrus morphology and hippocampus-related behavior revisited. Brain Struct. Funct. 220, 1449–1462 10.1007/s00429-014-0737-5 [DOI] [PubMed] [Google Scholar]
- 125.Boskovic Z., Alfonsi F., Rumballe B.A., Fonseka S., Windels F. and Coulson E.J. (2014) The role of p75NTR in cholinergic basal forebrain structure and function. J. Neurosci. 34, 13033–13038 10.1523/JNEUROSCI.2364-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sophou S., Dori I., Antonopoulos J., Parnavelas J.G. and Dinopoulos A. (2006) Apoptosis in the rat basal forebrain during development and following lesions of connections. Eur. J. Neurosci. 24, 573–585 10.1111/j.1460-9568.2006.04929.x [DOI] [PubMed] [Google Scholar]
- 127.Barrett G.L. and Bartlett P.F. (1994) The p75 nerve growth factor receptor mediates survival or death depending on the stage of sensory neuron development. Proc. Natl. Acad. Sci. U.S.A. 91, 6501–6505 10.1073/pnas.91.14.6501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Greferath U., Bennie A., Kourakis A., Bartlett P.F., Murphy M. and Barrett G.L. (2000) Enlarged cholinergic forebrain neurons and improved spatial learning in p75 knockout mice. Eur. J. Neurosci. 12, 885–893 10.1046/j.1460-9568.2000.00976.x [DOI] [PubMed] [Google Scholar]
- 129.Vonbuh V. and Vonbuh O. (2016) Deletion of p75NTR enhances the cholinergic innervation pattern of the visual cortex. Vis. Neurosci. 33, E012 10.1017/S0952523816000080 [DOI] [PubMed] [Google Scholar]
- 130.Busch R., Baldus M., Vogt M.A., Berger S.M., Bartsch D., Gass P. et al. (2017) Effects of p75NTR deficiency on cholinergic innervation of the amygdala and anxiety-like behavior. J. Neurochem. 141, 461–471 10.1111/jnc.14006 [DOI] [PubMed] [Google Scholar]
- 131.Barrett G.L., Reid C.A., Tsafoulis C., Zhu W., Williams D.A., Paolini A.G. et al. (2010) Enhanced spatial memory and hippocampal long-term potentiation in p75 neurotrophin receptor knockout mice. Hippocampus 20, 145–152 [DOI] [PubMed] [Google Scholar]
- 132.Rosch H., Schweigreiter R., Bonhoeffer T., Barde Y.A. and Korte M. (2005) The neurotrophin receptor p75NTR modulates long-term depression and regulates the expression of AMPA receptor subunits in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 102, 7362–7367 10.1073/pnas.0502460102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Woo N.H., Teng H.K., Siao C.J., Chiaruttini C., Pang P.T., Milner T.A. et al. (2005) Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 8, 1069–1077 10.1038/nn1510 [DOI] [PubMed] [Google Scholar]
- 134.Zagrebelsky M., Holz A., Dechant G., Barde Y.A., Bonhoeffer T. and Korte M. (2005) The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J. Neurosci. 25, 9989–9999 10.1523/JNEUROSCI.2492-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gibon J., Buckley S.M., Unsain N., Kaartinen V., Seguela P. and Barker P.A. (2015) proBDNF and p75NTR control excitability and persistent firing of cortical pyramidal neurons. J. Neurosci. 35, 9741–9753 10.1523/JNEUROSCI.4655-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Catts V.S., Al-Menhali N., Burne T.H., Colditz M.J. and Coulson E.J. (2008) The p75 neurotrophin receptor regulates hippocampal neurogenesis and related behaviours. Eur. J. Neurosci. 28, 883–892 10.1111/j.1460-9568.2008.06390.x [DOI] [PubMed] [Google Scholar]
- 137.Bernabeu R.O. and Longo F.M. (2010) The p75 neurotrophin receptor is expressed by adult mouse dentate progenitor cells and regulates neuronal and non-neuronal cell genesis. BMC Neurosci. 11, 136 10.1186/1471-2202-11-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zanin J.P., Abercrombie E. and Friedman W.J. (2016) Proneurotrophin-3 promotes cell cycle withdrawal of developing cerebellar granule cell progenitors via the p75 neurotrophin receptor. Elife 5, 10.7554/eLife.16654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kaneko N., Okano H. and Sawamoto K. (2006) Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells 11, 1145–1159 10.1111/j.1365-2443.2006.01010.x [DOI] [PubMed] [Google Scholar]
- 140.Giza J.I., Kim J., Meyer H.C., Anastasia A., Dincheva I., Zheng C.I. et al. (2018) The BDNF Val66Met prodomain disassembles dendritic spines altering fear extinction circuitry and behavior. Neuron 99, 163.e6–178.e6 10.1016/j.neuron.2018.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yegla B. and Parikh V. (2014) Effects of sustained proNGF blockade on attentional capacities in aged rats with compromised cholinergic system. Neuroscience 261, 118–132 10.1016/j.neuroscience.2013.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sofroniew M.V., Cooper J.D., Svendsen C.N., Crossman P., Ip N.Y., Lindsay R.M. et al. (1993) Atrophy but not death of adult septal cholinergic neurons after ablation of target capacity to produce mRNAs for NGF, BDNF, and NT3. J. Neurosci. 13, 5263–5276 10.1523/JNEUROSCI.13-12-05263.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Smeyne R.J., Klein R., Schnapp A., Long L.K., Bryant S., Lewin A. et al. (1994) Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368, 246–249 10.1038/368246a0 [DOI] [PubMed] [Google Scholar]
- 144.Muller M., Triaca V., Besusso D., Costanzi M., Horn J.M., Koudelka J. et al. (2012) Loss of NGF-TrkA signaling from the CNS is not sufficient to induce cognitive impairments in young adult or intermediate-aged mice. J. Neurosci. 32, 14885–14898 10.1523/JNEUROSCI.2849-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanchez-Ortiz E., Yui D., Song D., Li Y., Rubenstein J.L., Reichardt L.F. et al. (2012) TrkA gene ablation in basal forebrain results in dysfunction of the cholinergic circuitry. J. Neurosci. 32, 4065–4079 10.1523/JNEUROSCI.6314-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Parikh V., Howe W.M., Welchko R.M., Naughton S.X., D’Amore D.E., Han D.H. et al. (2013) Diminished trkA receptor signaling reveals cholinergic-attentional vulnerability of aging. Eur. J. Neurosci. 37, 278–293 10.1111/ejn.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Crowley C., Spencer S.D., Nishimura M.C., Chen K.S., Pitts-Meek S., Armanini M.P. et al. (1994) Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76, 1001–1011 10.1016/0092-8674(94)90378-6 [DOI] [PubMed] [Google Scholar]
- 148.Chen K.S., Nishimura M.C., Armanini M.P., Crowley C., Spencer S.D. and Phillips H.S. (1997) Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits. J. Neurosci. 17, 7288–7296 10.1523/JNEUROSCI.17-19-07288.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Turnbull M.T., Boskovic Z. and Coulson E.J. (2018) Acute down-regulation of BDNF signaling does not replicate exacerbated amyloid-β levels and cognitive impairment induced by cholinergic basal forebrain lesion. Front. Mol. Neurosci. 11, 51 10.3389/fnmol.2018.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ruberti F., Capsoni S., Comparini A., Di Daniel E., Franzot J., Gonfloni S. et al. (2000) Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J. Neurosci. 20, 2589–2601 10.1523/JNEUROSCI.20-07-02589.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cattaneo A., Capsoni S., Margotti E., Righi M., Kontsekova E., Pavlik P. et al. (1999) Functional blockade of tyrosine kinase A in the rat basal forebrain by a novel antagonistic anti-receptor monoclonal antibody. J. Neurosci. 19, 9687–9697 10.1523/JNEUROSCI.19-22-09687.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Molnar M., Tongiorgi E., Avignone E., Gonfloni S., Ruberti F., Domenici L. et al. (1998) The effects of anti-nerve growth factor monoclonal antibodies on developing basal forebrain neurons are transient and reversible. Eur. J. Neurosci. 10, 3127–3140 10.1046/j.1460-9568.1998.00314.x [DOI] [PubMed] [Google Scholar]
- 153.Pesavento E., Capsoni S., Domenici L. and Cattaneo A. (2002) Acute cholinergic rescue of synaptic plasticity in the neurodegenerating cortex of anti-nerve-growth-factor mice. Eur. J. Neurosci. 15, 1030–1036 10.1046/j.1460-9568.2002.01937.x [DOI] [PubMed] [Google Scholar]
- 154.Hefti F. (1986) Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J. Neurosci. 6, 2155–2162 10.1523/JNEUROSCI.06-08-02155.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Prakash N., Cohen-Cory S. and Frostig R.D. (1996) RAPID and opposite effects of BDNF and NGF on the functional organization of the adult cortex in vivo. Nature 381, 702–706 10.1038/381702a0 [DOI] [PubMed] [Google Scholar]
- 156.Prakash N., Cohen-Cory S., Penschuck S. and Frostig R.D. (2004) Basal forebrain cholinergic system is involved in rapid nerve growth factor (NGF)-induced plasticity in the barrel cortex of adult rats. J. Neurophysiol. 91, 424–437 10.1152/jn.00489.2003 [DOI] [PubMed] [Google Scholar]
- 157.Gutierrez H., Miranda M.I. and Bermudez-Rattoni F. (1997) Learning impairment and cholinergic deafferentation after cortical nerve growth factor deprivation. J. Neurosci. 17, 3796–3803 10.1523/JNEUROSCI.17-10-03796.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Conner J.M., Franks K.M., Titterness A.K., Russell K., Merrill D.A., Christie B.R. et al. (2009) NGF is essential for hippocampal plasticity and learning. J. Neurosci. 29, 10883–10889 10.1523/JNEUROSCI.2594-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brooks A.I., Cory-Slechta D.A. and Federoff H.J. (2000) Gene-experience interaction alters the cholinergic septohippocampal pathway of mice. Proc. Natl. Acad. Sci. U.S.A. 97, 13378–13383 10.1073/pnas.230169397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brooks A.I., Cory-Slechta D.A., Bowers W.J., Murg S.L. and Federoff H.J. (2000) Enhanced learning in mice parallels vector-mediated nerve growth factor expression in hippocampus. Hum. Gene Ther. 11, 2341–2352 10.1089/104303400750038453 [DOI] [PubMed] [Google Scholar]
- 161.Fahnestock M. and Shekari A. (2019) ProNGF and neurodegeneration in Alzheimer’s disease. Front. Neurosci. Neurodegener., [Accepted manuscript in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rocamora N., Pascual M., Acsady L., de Lecea L., Freund T.F. and Soriano E. (1996) Expression of NGF and NT3 mRNAs in hippocampal interneurons innervated by the GABAergic septohippocampal pathway. J. Neurosci. 16, 3991–4004 10.1523/JNEUROSCI.16-12-03991.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rocamora N., Welker E., Pascual M. and Soriano E. (1996) Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J. Neurosci. 16, 4411–4419 10.1523/JNEUROSCI.16-14-04411.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Biane J., Conner J.M. and Tuszynski M.H. (2014) Nerve growth factor is primarily produced by GABAergic neurons of the adult rat cortex. Front. Cell Neurosci. 8, 220 10.3389/fncel.2014.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Batista-Brito R., Zagha E., Ratliff J.M. and Vinck M. (2018) Modulation of cortical circuits by top-down processing and arousal state in health and disease. Curr. Opin. Neurobiol. 52, 172–181 10.1016/j.conb.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 166.Chourbaji S., Hellweg R., Brandis D., Zorner B., Zacher C., Lang U.E. et al. (2004) Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res. Mol. Brain Res. 121, 28–36 10.1016/j.molbrainres.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 167.Ward N.L. and Hagg T. (2000) BDNF is needed for postnatal maturation of basal forebrain and neostriatum cholinergic neurons in vivo. Exp. Neurol. 162, 297–310 10.1006/exnr.1999.7346 [DOI] [PubMed] [Google Scholar]
- 168.Guic E., Carrasco X., Rodriguez E., Robles I. and Merzenich M.M. (2008) Plasticity in primary somatosensory cortex resulting from environmentally enriched stimulation and sensory discrimination training. Biol. Res. 41, 425–437 10.4067/S0716-97602008000400008 [DOI] [PubMed] [Google Scholar]
- 169.Ma L., Harada T., Harada C., Romero M., Hebert J.M., McConnell S.K. et al. (2002) Neurotrophin-3 is required for appropriate establishment of thalamocortical connections. Neuron 36, 623–634 10.1016/S0896-6273(02)01021-8 [DOI] [PubMed] [Google Scholar]
- 170.Patz S. and Wahle P. (2006) Developmental changes of neurotrophin mRNA expression in the layers of rat visual cortex. Eur. J. Neurosci. 24, 2453–2460 10.1111/j.1460-9568.2006.05126.x [DOI] [PubMed] [Google Scholar]
- 171.Phillips H.S., Hains J.M., Laramee G.R., Rosenthal A. and Winslow J.W. (1990) Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science 250, 290–294 10.1126/science.1688328 [DOI] [PubMed] [Google Scholar]
- 172.Lee Y.S., Danandeh A., Baratta J., Lin C.Y., Yu J. and Robertson R.T. (2013) Neurotrophic factors rescue basal forebrain cholinergic neurons and improve performance on a spatial learning test. Exp. Neurol. 249, 178–186 10.1016/j.expneurol.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Douchamps V. and Mathis C. (2017) A second wind for the cholinergic system in Alzheimer’s therapy. Behav. Pharmacol. 28, 112–123 10.1097/FBP.0000000000000300 [DOI] [PubMed] [Google Scholar]
- 174.Bowen D.M., Smith C.B., White P. and Davison A.N. (1976) Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain 99, 459–496 10.1093/brain/99.3.459 [DOI] [PubMed] [Google Scholar]
- 175.Davies P. and Maloney A.J. (1976) Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2, 1403 10.1016/S0140-6736(76)91936-X [DOI] [PubMed] [Google Scholar]
- 176.Whitehouse P.J., Price D.L., Struble R.G., Clark A.W., Coyle J.T. and Delon M.R. (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239 10.1126/science.7058341 [DOI] [PubMed] [Google Scholar]
- 177.Pepeu G. and Grazia Giovannini M. (2017) The fate of the brain cholinergic neurons in neurodegenerative diseases. Brain Res. 1670, 173–184 10.1016/j.brainres.2017.06.023 [DOI] [PubMed] [Google Scholar]
- 178.Liu A.K., Chang R.C., Pearce R.K. and Gentleman S.M. (2015) Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 129, 527–540 10.1007/s00401-015-1392-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schliebs R. and Arendt T. (2011) The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 221, 555–563 10.1016/j.bbr.2010.11.058 [DOI] [PubMed] [Google Scholar]
- 180.Grothe M.J., Schuster C., Bauer F., Heinsen H., Prudlo J. and Teipel S.J. (2014) Atrophy of the cholinergic basal forebrain in dementia with Lewy bodies and Alzheimer’s disease dementia. J. Neurol. 261, 1939–1948 10.1007/s00415-014-7439-z [DOI] [PubMed] [Google Scholar]
- 181.Grothe M.J., Ewers M., Krause B., Heinsen H., Teipel S.J. and Alzheimer’s Disease Neuroimaging I (2014) Basal forebrain atrophy and cortical amyloid deposition in nondemented elderly subjects. Alzheimers Dement. 10, S344–S353 10.1016/j.jalz.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Grothe M., Heinsen H. and Teipel S. (2013) Longitudinal measures of cholinergic forebrain atrophy in the transition from healthy aging to Alzheimer’s disease. Neurobiol. Aging 34, 1210–1220 10.1016/j.neurobiolaging.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kerbler G.M., Fripp J., Rowe C.C., Villemagne V.L., Salvado O., Rose S. et al. (2015) Basal forebrain atrophy correlates with amyloid beta burden in Alzheimer’s disease. Neuroimage Clin. 7, 105–113 10.1016/j.nicl.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kerbler G.M., Nedelska Z., Fripp J., Laczo J., Vyhnalek M., Lisy J. et al. (2015) Basal Forebrain atrophy contributes to allocentric navigation impairment in Alzheimer’s disease patients. Front. Aging Neurosci. 7, 185 10.3389/fnagi.2015.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kilimann I., Grothe M., Heinsen H., Alho E.J., Grinberg L., Amaro E. Jr et al. (2014) Subregional basal forebrain atrophy in Alzheimer’s disease: a multicenter study. J. Alzheimers Dis. 40, 687–700 10.3233/JAD-132345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Teipel S., Heinsen H., Amaro E. Jr, Grinberg L.T., Krause B., Grothe M. et al. (2014) Cholinergic basal forebrain atrophy predicts amyloid burden in Alzheimer’s disease. Neurobiol. Aging 35, 482–491 10.1016/j.neurobiolaging.2013.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schmitz T.W., Nathan Spreng R. and Alzheimer’s Disease Neuroimaging I (2016) Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat. Commun. 7, 13249 10.1038/ncomms13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Laursen B., Mork A., Plath N., Kristiansen U. and Bastlund J.F. (2013) Cholinergic degeneration is associated with increased plaque deposition and cognitive impairment in APPswe/PS1dE9 mice. Behav. Brain Res. 240, 146–152 10.1016/j.bbr.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 189.Ramos-Rodriguez J.J., Pacheco-Herrero M., Thyssen D., Murillo-Carretero M.I., Berrocoso E., Spires-Jones T.L. et al. (2013) Rapid β-amyloid deposition and cognitive impairment after cholinergic denervation in APP/PS1 mice. J. Neuropathol. Exp. Neurol. 72, 272–285 10.1097/NEN.0b013e318288a8dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hartig W., Saul A., Kacza J., Grosche J., Goldhammer S., Michalski D. et al. (2014) Immunolesion-induced loss of cholinergic projection neurones promotes β-amyloidosis and tau hyperphosphorylation in the hippocampus of triple-transgenic mice. Neuropathol. Appl. Neurobiol. 40, 106–120 10.1111/nan.12050 [DOI] [PubMed] [Google Scholar]
- 191.Laursen B., Mork A., Plath N., Kristiansen U. and Bastlund J.F. (2014) Impaired hippocampal acetylcholine release parallels spatial memory deficits in Tg2576 mice subjected to basal forebrain cholinergic degeneration. Brain Res. 1543, 253–262 10.1016/j.brainres.2013.10.055 [DOI] [PubMed] [Google Scholar]
- 192.Turnbull M.T. and Coulson E.J. (2017) Cholinergic basal forebrain lesion decreases neurotrophin signaling without affecting tau hyperphosphorylation in genetically susceptible mice. J. Alzheimers Dis. 55, 1141–1154 10.3233/JAD-160805 [DOI] [PubMed] [Google Scholar]
- 193.Qian L., Milne M.R., Shepheard S., Rogers M.L., Medeiros R. and Coulson E.J. (2018) Removal of p75 neurotrophin receptor expression from cholinergic basal forebrain neurons reduces amyloid-β plaque deposition and cognitive impairment in aged APP/PS1 mice. Mol. Neurobiol. 10.1007/s12035-018-1404-2, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 194.Mufson E.J., Counts S.E., Fahnestock M. and Ginsberg S.D. (2007) Cholinotrophic molecular substrates of mild cognitive impairment in the elderly. Curr. Alzheimer Res. 4, 340–350 10.2174/156720507781788855 [DOI] [PubMed] [Google Scholar]
- 195.Counts S.E. and Mufson E.J. (2005) The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J. Neuropathol. Exp. Neurol. 64, 263–272 10.1093/jnen/64.4.263 [DOI] [PubMed] [Google Scholar]
- 196.Hefti F. and Schneider L.S. (1989) Rationale for the planned clinical trials with nerve growth factor in Alzheimer’s disease. Psychiatr. Dev. 7, 297–315 [PubMed] [Google Scholar]
- 197.Lu B., Nagappan G., Guan X., Nathan P.J. and Wren P. (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 405–416 10.1038/nrn3505 [DOI] [Google Scholar]
- 198.Rafii M.S., Tuszynski M.H., Thomas R.G. et al. (2018) Adeno-associated viral vector (serotype 2)–nerve growth factor for patients with alzheimer disease: a randomized clinical trial. JAMA Neurol. 75, 834–841 10.1001/jamaneurol.2018.0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Eyjolfsdottir H., Eriksdotter M., Linderoth B., Lind G., Juliusson B., Kusk P. et al. (2016) Targeted delivery of nerve growth factor to the cholinergic basal forebrain of Alzheimer’s disease patients: application of a second-generation encapsulated cell biodelivery device. Alzheimers Res. Ther. 8, 30 10.1186/s13195-016-0195-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Longo F.M. and Massa S.M. (2013) Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat. Rev. Drug Discov. 12, 507–525 10.1038/nrd4024 [DOI] [PubMed] [Google Scholar]
- 201.Chakravarthy B., Gaudet C., Menard M., Atkinson T., Brown L., Laferla F.M. et al. (2010) Amyloid-β peptides stimulate the expression of the p75NTR neurotrophin receptor in SHSY5Y human neuroblastoma cells and AD transgenic mice. J. Alzheimers Dis. 19, 915–925 10.3233/JAD-2010-1288 [DOI] [PubMed] [Google Scholar]
- 202.Mufson E.J. and Kordower J.H. (1992) Cortical neurons express nerve growth factor receptors in advanced age and Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 89, 569–573 10.1073/pnas.89.2.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Perez S.E., He B., Muhammad N., Oh K.J., Fahnestock M., Ikonomovic M.D. et al. (2011) Cholinotrophic basal forebrain system alterations in 3xTg-AD transgenic mice. Neurobiol. Dis. 41, 338–352 10.1016/j.nbd.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sotthibundhu A., Sykes A.M., Fox B., Underwood C.K., Thangnipon W. and Coulson E.J. (2008) β-amyloid1-42 induces neuronal death through the p75 neurotrophin receptor. J. Neurosci. 28, 3941–3946 10.1523/JNEUROSCI.0350-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yang T., Knowles J.K., Lu Q., Zhang H., Arancio O., Moore L.A. et al. (2008) Small molecule, non-peptide p75 ligands inhibit Aβ-induced neurodegeneration and synaptic impairment. PLoS ONE 3, e3604 10.1371/journal.pone.0003604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Knowles J.K., Rajadas J., Nguyen T.V., Yang T., LeMieux M.C., Vander Griend L. et al. (2009) The p75 neurotrophin receptor promotes amyloid-β1-42-induced neuritic dystrophy in vitro and in vivo. J. Neurosci. 29, 10627–10637 10.1523/JNEUROSCI.0620-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yaar M., Zhai S., Pilch P.F., Doyle S.M., Eisenhauer P.B., Fine R.E. et al. (1997) Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer’s disease. J. Clin. Invest. 100, 2333–2340 10.1172/JCI119772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Knowles J.K., Simmons D.A., Nguyen T.V., Vander Griend L., Xie Y., Zhang H. et al. (2013) A small molecule p75 ligand prevents cognitive deficits and neurite degeneration in an Alzheimer’s mouse model. Neurobiol. Aging 34, 2052–2063 10.1016/j.neurobiolaging.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ovsepian S.V., Antyborzec I., O’Leary V.B., Zaborszky L., Herms J. and Oliver Dolly J. (2014) Neurotrophin receptor p75 mediates the uptake of the amyloid beta (Aβ) peptide, guiding it to lysosomes for degradation in basal forebrain cholinergic neurons. Brain Struct. Funct. 219, 1527–1541 10.1007/s00429-013-0583-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yao X.Q., Jiao S.S., Saadipour K., Zeng F., Wang Q.H., Zhu C. et al. (2015) p75NTR ectodomain is a physiological neuroprotective molecule against amyloid-β toxicity in the brain of Alzheimer’s disease. Mol. Psychiatry 20, 1301–1310 10.1038/mp.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ferreira D., Westman E., Eyjolfsdottir H., Almqvist P., Lind G., Linderoth B. et al. (2015) Brain changes in Alzheimer’s disease patients with implanted encapsulated cells releasing nerve growth factor. J. Alzheimers Dis. 43, 1059–1072 10.3233/JAD-141068 [DOI] [PubMed] [Google Scholar]
- 212.Dou K.X., Tan M.S., Tan C.C., Cao X.P., Hou X.H., Guo Q.H. et al. (2018) Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer’s disease: a network meta-analysis of 41 randomized controlled trials. Alzheimers Res. Ther. 10, 126 10.1186/s13195-018-0457-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Knight R., Khondoker M., Magill N., Stewart R. and Landau S. (2018) A systematic review and meta-analysis of the effectiveness of acetylcholinesterase inhibitors and memantine in treating the cognitive symptoms of dementia. Dement. Geriatr. Cogn. Disord. 45, 131–151 10.1159/000486546 [DOI] [PubMed] [Google Scholar]
- 214.Harward S.C., Hedrick N.G., Hall C.E., Parra-Bueno P., Milner T.A., Pan E. et al. (2016) Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature 538, 99–103 10.1038/nature19766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jones I.W. and Wonnacott S. (2004) Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J. Neurosci. 24, 11244–11252 10.1523/JNEUROSCI.3009-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Coulson E.J., May L.M., Osborne S.L., Reid K., Underwood C.K., Meunier F.A. et al. (2008) p75 neurotrophin receptor mediates neuronal cell death by activating GIRK channels through phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 28, 315–324 10.1523/JNEUROSCI.2699-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.May L.M., Anggono V., Gooch H.M., Jang S.E., Matusica D., Kerbler G.M. et al. (2017) G-protein-coupled inwardly rectifying potassium (GIRK) channel activation by the p75 neurotrophin receptor is required for amyloid β toxicity. Front. Neurosci. 11, 455 10.3389/fnins.2017.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]