Abstract
Background
It has been unclear whether repeat dose(s) of prenatal corticosteroids are beneficial.
Objectives
To assess the effectiveness and safety of repeat dose(s) of prenatal corticosteroids.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (20 January 2015), searched reference lists of retrieved studies and contacted authors for further data.
Selection criteria
Randomised controlled trials of women who had already received a single course of corticosteroids seven or more days previously and considered still at risk of preterm birth.
Data collection and analysis
We assessed trial quality and extracted data independently.
Main results
We included 10 trials (a total of 4733 women and 5700 babies) with low to moderate risk of bias. Treatment of women who remain at risk of preterm birth seven or more days after an initial course of prenatal corticosteroids with repeat dose(s), compared with no repeat corticosteroid treatment, reduced the risk of their infants experiencing the primary outcomes respiratory distress syndrome (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.75 to 0.91, eight trials, 3206 infants, number needed to treat to benefit (NNTB) 17, 95% CI 11 to 32) and serious infant outcome (RR 0.84, 95% CI 0.75 to 0.94, seven trials, 5094 infants, NNTB 30, 95% CI 19 to 79).
Treatment with repeat dose(s) of corticosteroid was associated with a reduction in mean birthweight (mean difference (MD) ‐75.79 g, 95% CI ‐117.63 to ‐33.96, nine trials, 5626 infants). However, outcomes that adjusted birthweight for gestational age (birthweight Z scores, birthweight multiples of the median and small‐for‐gestational age) did not differ between treatment groups.
At early childhood follow‐up, no statistically significant differences were seen for infants exposed to repeat prenatal corticosteroids compared with unexposed infants for the primary outcomes (total deaths; survival free of any disability or major disability; disability; or serious outcome) or in the secondary outcome growth assessments. In women, for the two primary outcomes, there was no increase in infectious morbidity of chorioamnionitis or puerperal sepsis, and the likelihood of a caesarean birth was unchanged.
Authors' conclusions
The short‐term benefits for babies of less respiratory distress and fewer serious health problems in the first few weeks after birth support the use of repeat dose(s) of prenatal corticosteroids for women still at risk of preterm birth seven days or more after an initial course. These benefits were associated with a small reduction in size at birth. The current available evidence reassuringly shows no significant harm in early childhood, although no benefit.
Further research is needed on the long‐term benefits and risks for the woman and baby. Individual patient data meta‐analysis may clarify how to maximise benefit and minimise harm.
Plain language summary
Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease
This review shows that a repeat dose of prenatal corticosteroids given to women who remain at risk of an early birth after an initial course of prenatal corticosteroids helps the baby's lungs and reduces serious health problems in the first few weeks of life.
Infants born preterm (before 37 weeks' gestation) are at risk of difficulty breathing and lung disease because their lungs are not fully developed. Women at risk of preterm birth include those with ruptured membranes, antepartum haemorrhage, preterm labour, cervical incompetence, pre‐eclampsia or multiple pregnancy. Preterm babies who survive the early weeks of life are also at risk of long‐term neurological disabilities such as epilepsy and cerebral palsy. A single course of corticosteroids, given to women who may give birth early, helps develop the baby's lungs. This benefit does not last beyond seven days. This review of 10 randomised controlled trials, involving 4733 women who remained at risk of early birth more than seven days after an initial course of corticosteroids and 5700 babies between 23 and 34 weeks' gestation at trial enrolment showed that repeat dose(s) of prenatal corticosteroids reduced the risk of the baby having breathing difficulties and serious health problems in the first few weeks of life. Some of the trials showed that the baby may be smaller at birth, but not if adjusted for gestational age nor by the time of hospital discharge. In four trials that followed up the babies to early childhood, no long‐term benefits or harms were seen at 18 months to two years' corrected age. Further research is needed on the long‐term benefits and risks for the woman and baby, which should include later child health, growth and development.
Repeat prenatal corticosteroid treatment could increase the risk of infection and suppress pituitary‐adrenal function for the mother and her baby. For the women, there was no increase in infectious morbidity of chorioamnionitis or puerperal sepsis, and the likelihood of a caesarean birth was unchanged.
Betamethasone was the only corticosteroid evaluated. It is uncertain whether the effects seen for betamethasone would be the same for dexamethasone.
Background
Description of the condition
Infants born preterm (before 37 weeks' gestation) are at high risk of neonatal lung disease and its sequelae. The more preterm the baby the greater are the risks, especially when birth occurs before 32 weeks' gestation. In Australia, in 2003, 1.6% of all births were before 32 weeks' gestation (Laws 2005). Respiratory distress syndrome (RDS), as a consequence of immature lung development, is the principal cause of early neonatal mortality and morbidity and contributes significantly to the high costs of neonatal intensive care. Preterm babies who survive the early weeks of life are at risk of long‐term neurological disability (Johnson 1993). Parents are understandably worried and distressed when their baby is born preterm. Strategies to reduce the risk of neonatal respiratory disease for infants who are born preterm have received considerable attention (Roberts 2006; Rojas‐Reyes 2012).
A single course of prenatal corticosteroids reduces the risk of RDS from 26% to 17% (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.59 to 0.73, 21 trials, 4038 infants) (Roberts 2006). Other beneficial effects include a reduced risk of neonatal death, intraventricular haemorrhage, necrotising enterocolitis and early sepsis (Roberts 2006). Prenatal corticosteroids enhance the benefits of postnatal surfactant therapy (Jobe 1994) and reduce the need for blood pressure support (Moise 1995). Overall, there is a reduction in the cost and duration of neonatal care. The cost benefit of a single course of antenatal corticosteroids is estimated as USD 3000 (NIH 1995). Long‐term follow‐up into adulthood of babies in the New Zealand trial (Liggins 1972), exposed to prenatal corticosteroids have shown no adverse clinical outcomes (Dalziel 2005a; Dalziel 2005b). However, even though prenatal corticosteroids remain the most effective known strategy for reducing the adverse consequences of preterm birth, and despite postnatal intensive care and exogenous surfactant, there is still significant neonatal morbidity (Rojas‐Reyes 2012).
Description of the intervention
Prenatal corticosteroid treatment compared with no prenatal corticosteroid treatment has not been shown to be effective in babies who are born more than seven days after an initial treatment (Roberts 2006). Specifically no reduction in the incidence of RDS or neonatal mortality has been demonstrated (McLaughlin 2003; Roberts 2006), and birthweight is significantly reduced (Roberts 2006). There may be benefit in repeating the dose of prenatal corticosteroids to women who remain at risk of preterm birth more than seven days after the initial course. This was suggested by Liggins and Howie in the first reported controlled trial of antenatal glucocorticoid treatment for the prevention of RDS in premature infants (Liggins 1972). Indeed, in some clinical centres this has been standard practice. However, until recently, there has been little formal assessment of such a policy, and the effect of this practice on the women and infants has been unclear (NIH 2000).
How the intervention might work
Animal studies have suggested that repeat treatment with prenatal corticosteroids may be more effective than a single course in reducing the risk of RDS. In sheep fetuses; there is a dose‐dependent improvement in lung function with repeat doses of betamethasone (Ikegami 1997). In human infants, improved cardiovascular responses to preterm birth have been observed (Padbury 1996).
Why it is important to do this review
The potential benefits of repeat prenatal corticosteroid treatment on neonatal lung function and cardiovascular health may be balanced by increased maternal risks such as infection and suppression of hypothalamic‐pituitary‐adrenal function (Ashwood 2006; McKenna 2000). In addition, experimental reports raise concerns about the use of repeat doses of prenatal corticosteroids because of potential adverse effects for the offspring.
It is well known that corticosteroids inhibit cell growth and DNA replication. Studies in both small and large animals demonstrate that exogenous steroids inhibit fetal growth and increase fetal blood pressure (Fowden 1996; Jensen 2002). In sheep there is a dose‐dependent reduction in birthweight in lambs exposed to up to four doses of betamethasone administered to the ewe (Ikegami 1997), although exogenous steroids administered directly to the fetus do not inhibit fetal growth (Newnham 1999).
Other animal experimental studies have shown that repeat doses of corticosteroids may have harmful effects on neuronal myelination (Dunlop 1997), the development of the alveolar septa leaving 'emphysematous' ‐like alveoli (Tschanz 1995) and hypothalamic‐pituitary‐adrenal (HPA) function (Ikegami 1997). Effects on the HPA axis can persist into adulthood.
In humans, similar concerns have been raised from non‐randomised cohort studies, with adverse effects after repeat doses of corticosteroids on measures of growth at birth (French 1999), risk of neonatal infection, fetal pituitary‐adrenal axis function, neonatal blood pressure (Mildenhall 2006), childhood behaviour (French 1998), and high levels of stress in parents (French 1998). Long‐term developmental follow‐up studies of infants exposed to repeat doses of prenatal corticosteroids are limited to date and have produced conflicting results. Some non‐randomised studies suggest delayed development (Esplin 2000) and adverse effects on childhood behaviour (French 1998), whilst other non‐randomised studies have shown no difference between exposed and non‐exposed children (French 1999; Hasbargen 2001; Thorp 2002), or possible reduced cerebral palsy (French 2004). Another long‐term potential adverse outcome that requires further investigation is the possibility that single or repeat doses of antenatal corticosteroids could program cardiovascular settings in the fetus and lead to adult hypertension (Benediktsson 1993), and insulin resistance leading to diabetes mellitus (Dalziel 2005a). Increased exposure of the fetus to glucocorticoids has been proposed as a possible mechanism underlying the epidemiological association between small size at birth and adult cardiovascular and metabolic disease (Seckl 2004).
There remains uncertainty therefore about whether there is benefit in repeating the dose of prenatal corticosteroids for women who remain at risk of preterm birth after an initial course. This review will assess the benefits and harms of repeat doses of prenatal corticosteroids for women at risk of preterm birth seven or more days after an initial course.
Objectives
To assess the effectiveness and safety, using the best available evidence, of a repeat dose(s) of prenatal corticosteroids, given to women who remain at risk of preterm birth seven or more days after an initial course of prenatal corticosteroids with the primary aim of reducing fetal, infant and childhood morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised trials with reported data that compared outcomes for women at risk of preterm birth randomised to receive a repeat dose(s) of prenatal corticosteroids with outcomes in controls given a single course of prenatal corticosteroids, with or without additional placebo administration. The trials used some form of random allocation and reported data on one or more of the prestated outcomes. We excluded quasi‐randomised trials. Cross‐over trials were not eligible for inclusion. Cluster trials were eligible for inclusion.
Types of participants
Women considered to be at risk of preterm birth who have already received a single course of prenatal corticosteroid seven or more days previously.
Predefined subgroups were planned to examine separately the outcomes for women and infants based on the reasons the woman was considered to be at risk for preterm birth (e.g. presence or absence of ruptured membranes, antepartum haemorrhage, preterm labour, cervical incompetence, pre‐eclampsia, growth restriction), and the number of infants in utero (singleton, twin or higher order multiple pregnancy).
Types of interventions
Corticosteroid administered to the women intravenously, intramuscularly or orally, compared with either placebo or no placebo. We excluded trials in which the fetus received corticosteroids directly as these are included in another Cochrane review, 'Transplacental versus direct fetal corticosteroid treatment for accelerating fetal lung maturation where there is a risk of preterm birth’ (Utama 2010).
We planned predefined subgroups to examine separately the primary outcomes for women and infants based on the type of corticosteroid given, the planned interval between corticosteroid treatments, the number of repeat courses planned, the number of repeat courses actually given, the planned dose of corticosteroid given per repeat treatment, the planned dose of repeat corticosteroid drug exposure per week, the method of administration, and the gestational age at which the treatment was given.
Types of outcome measures
We prespecified clinically relevant outcomes after discussion amongst the authors.
Primary outcomes
We chose primary outcomes to be most representative of the clinically important measures of effectiveness and safety, including serious outcomes, for the women and their infants, the infant as a child and the infant as an adult.
For the infant
RDS;
severe lung disease (however defined by the authors);
composite serious outcome (however defined by authors);
birthweight;
fetal, neonatal or later death;
chronic lung disease (however defined by authors);
intraventricular haemorrhage.
For the child
Total deaths;
survival free of any disability (however defined by authors);
survival free of major disability (however defined by authors);
disability at childhood follow‐up (developmental delay or intellectual impairment, blindness, deafness, or cerebral palsy after 18 months of age) (however defined by authors);
composite serious outcome (however defined by authors).
For the child as an adult
Total deaths;
survival free of any disability (however defined by authors);
survival free of major disability (however defined by authors);
disability at adult follow‐up (developmental delay or intellectual impairment, blindness, deafness, or cerebral palsy) (however defined by authors);
major sensorineural disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay or intellectual impairment (defined as developmental quotient or intelligence quotient less than two standard deviations below mean));
composite serious outcome (however defined by authors).
For the women
Chorioamnionitis (however defined by authors);
puerperal sepsis (however defined by authors).
Secondary outcomes
These include other measures of effectiveness, complications, satisfaction with care and health service use.
For the infant
Gestational age at birth (preterm birth less than 37 weeks, very preterm birth less than 34 weeks, extremely preterm birth less than 28 weeks);
interval between trial entry and birth;
small‐for‐gestational age;
head circumference at birth;
length at birth;
skin‐fold thickness at birth;
placental weight;
Apgar score less than seven at five minutes;
use of respiratory support (mechanical ventilation or continuous positive airways pressure (CPAP)), or both;
use of mechanical ventilation;
use of CPAP;
duration of respiratory support;
use of oxygen supplementation;
duration of oxygen supplementation;
use of surfactant;
use of inotropic support;
use of nitric oxide for respiratory support;
intraventricular haemorrhage grade 3/4;
periventricular leukomalacia;
early systemic neonatal infection (however defined by authors);
proven infection while in the neonatal intensive care unit;
admission to neonatal intensive care unit;
air leak syndrome;
necrotising enterocolitis (however defined by authors);
patent ductus arteriosus (however defined by authors);
retinopathy of prematurity;
use of postnatal corticosteroids;
neonatal blood pressure (systolic, diastolic and mean arterial blood pressure);
cardiac hypertrophy;
growth assessments at primary hospital discharge (weight, head circumference, length, skin‐fold thickness);
growth assessments at infant follow‐up (weight, head circumference, length, skin‐fold thickness);
infant temperament;
infant behaviour;
developmental delay at infant follow‐up (however defined by the authors);
hypothalamo/pituitary/adrenal (HPA) axis suppression (however defined by the authors).
For the child
Growth assessments at childhood follow‐up (weight, head circumference, length, skin‐fold thickness, body mass index (BMI));
developmental delay (however defined by the authors);
intellectual impairment;
motor impairment;
blindness/visual impairment (however defined by authors);
deafness (however defined by authors);
cerebral palsy;
child behaviour (however defined by authors);
child temperament;
learning difficulties;
respiratory disease (however defined by authors);
insulin sensitivity;
dyslipidaemia;
blood pressure;
HPA axis function;
lung function;
bone density.
For the child as an adult
Age at puberty;
growth assessments in later life (weight, head circumference, length, skin‐fold thickness, BMI);
major sensorineural disability (defined as any of legal blindness, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy, or developmental delay or intellectual impairment (defined as developmental quotient or intelligence quotient less than two standard deviations below mean));
developmental delay (however defined by the authors);
intellectual impairment;
motor impairment;
blindness;
deafness;
cerebral palsy;
educational achievements;
learning difficulties;
insulin sensitivity;
dyslipidaemia;
blood pressure;
HPA axis function;
lung function;
bone density.
For the woman
Death;
pulmonary oedema;
admission to intensive care unit;
prelabour rupture of the membranes after trial entry;
hypertension (variously defined by the authors);
mode of birth;
length of labour;
pyrexia after trial entry requiring the use of antibiotics;
intrapartum fever requiring the use of antibiotics;
postpartum haemorrhage;
postnatal pyrexia (however defined by authors);
breastfeeding after hospital discharge;
postnatal depression;
side effects of therapy (including nausea, vomiting, hypertension, glucose intolerance, osteoporosis, adrenal insufficiency, insomnia, pain at the injection site, bruising at the injection site, haematoma at injection site);
discontinuation of therapy because of maternal side effects;
adverse drug reaction;
satisfaction with the therapy;
quality of life;
parenting stress.
Use of health services
Length of antenatal hospitalisation for the women;
length of postnatal hospitalisation for the women;
maternal admission to intensive care unit;
admission to and length of stay in neonatal intensive care unit;
length of neonatal hospitalisation;
costs of maternal care;
cost of neonatal care;
hospital re‐admission at childhood follow‐up.
While we sought all the above outcomes from the included trials, only those with data appear in the analysis tables. We included outcomes in the analysis if reasonable measures were taken to minimise observer bias and data were available for analysis according to original allocation.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (20 January 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched reference lists of trials and other review articles. We contacted authors on two studies listed under 'ongoing studies' in the previous version of the review for further information for this update.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous version of this review, see 'Crowther 2011'.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
We independently evaluated trials under consideration for inclusion without consideration of their results. We resolved any differences of opinion by discussion. There was no blinding of authorship.
Data extraction and management
Two review authors independently extracted study data, using a pre‐designed data form. Review author Philippa Middleton independently extracted data for the ACTORDS trial (Crowther 2006). We resolved discrepancies through discussion. When information was unclear, we attempted to contact authors of the original reports to provide further details. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI). We calculated number needed to treat to benefit (NNTB) from the summary risk differences and their 95% confidence limits (Higgins 2011).
Continuous data
For continuous data, we used the mean difference (MD) with 95% CI.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials were eligible for inclusion but we did not identify any. Should we include cluster‐randomised trials subsequently, we will include them in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in Handbook [Section 16.3.4 or 16.3.6]) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a separate meta‐analysis.
Cross‐over trials
Cross‐over trials were not eligible for inclusion.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analyses. If missing data were such that they might significantly affect the results, we excluded these data from the analysis. This decision rested with the review authors. If missing data become available subsequently, we will include them in the analyses.
For all outcomes we carried out analyses as far as possible on an intention‐to‐treat basis. We attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was taken as the number randomised minus any participants whose outcomes were known to be missing, ('available case' analysis).
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if the Tau² was greater than zero and either the I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. When we identified high levels of heterogeneity among the trials, we explored this by pre‐specified subgroup analysis.
Assessment of reporting biases
Where we suspected reporting bias (see selective reporting bias above), and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Where there were 10 or more studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots, although we identified none. In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Clinical subgroups
We planned secondary analyses to explore clinical diversity by examining separately the outcomes for women exposed to repeat dose(s) of prenatal corticosteroids compared with women receiving no repeat prenatal corticosteroids/placebo based on:
the reasons the woman was considered to be at risk of preterm birth (e.g. presence or absence of ruptured membranes, antepartum haemorrhage, preterm labour, cervical incompetence, pre‐eclampsia and growth restriction);
the number of babies in utero (singleton, twins or higher order multiples);
the type of corticosteroid given (betamethasone, dexamethasone);
the planned interval between corticosteroid treatments (minimum interval of seven days or less, between eight and less than 14 days, 14 days or more);
the planned number of repeat courses of corticosteroids to be given (one, two, three, four or more repeat courses);
the number of repeat courses of corticosteroids actually given post‐randomisation (one, two, three, four or more repeat courses);
the planned dosage of corticosteroid given per treatment (12 mg or less, more than 12 mg to 24 mg, more than 24 mg);
the planned dose of repeat dose of corticosteroid drug exposure/week (12 mg or less/week, more than 12 mg/week to 24 mg/week, more than 24 mg/week);
the method of treatment administration (intramuscular, intravenous, intra‐amniotic); and
the gestational age at which the first repeat treatment was given (less than 28, 28 to less than 32, 32 to 34, more than 34 completed weeks).
We used primary outcomes for the infant, women and child most representative of the clinically important measures of effectiveness and safety for the women and their infants.
In future updates, we may carry out formal subgroup analysis in order to investigate heterogeneity.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality on important outcomes in the review. Where there was risk of bias associated with a particular aspect of study quality (e.g. inadequate allocation concealment), we explored this by sensitivity analysis.
Results
Description of studies
Results of the search
Studies considered and included
We identified 14 trials for potential inclusion (Aghajafari 2002; Bontis 2011; Crowther 2006; Garite 2009; Guinn 2002; Mazumder 2008; McEvoy 2002; McEvoy 2010; Mercer 2001; Murphy 2008; Peltoniemi 2007; Romejko‐Wolniewicz 2013; Thorp 2000; Wapner 2006). There were 11 trials of repeat dose(s) of prenatal corticosteroids given to women who remain at risk of preterm birth seven or more days after an initial course of prenatal corticosteroids, of which 10 trials met our inclusion criteria (Aghajafari 2002; Crowther 2006; Garite 2009; Guinn 2002; Mazumder 2008; McEvoy 2002; McEvoy 2010; Murphy 2008; Peltoniemi 2007; Wapner 2006). We excluded four trials (Bontis 2011; Mercer 2001; Romejko‐Wolniewicz 2013; Thorp 2000).
Included studies
A total of 4733 women (and 5700 babies) were recruited into the 10 trials that met the prespecified criteria for inclusion in this review (12 women: 16 babies in Aghajafari 2002; 982 women: 1147 babies in Crowther 2006; 437 women: 577 babies in Garite 2009; 502 women: 496 babies in Guinn 2002; 76 women: 76 babies in Mazumder 2008; 37 women: 37 babies in McEvoy 2002, 85 women: 113 babies in McEvoy 2010; 1858 women: 2318 babies in Murphy 2008; 249 women: 326 babies in Peltoniemi 2007; and 495 women: 594 babies in Wapner 2006).
Five of the trials were conducted in the United States of America (Garite 2009; Guinn 2002; McEvoy 2002; McEvoy 2010; Wapner 2006); one each in Canada (Aghajafari 2002), India (Mazumder 2008), and Finland (Peltoniemi 2007); one in Australia and New Zealand (Crowther 2006); and one involved 20 countries (Murphy 2008).
We are aware of three other trials of repeat prenatal corticosteroids for women at risk of preterm birth. One trial from the UK stopped recruitment and publication is awaited (TEAMS 1999); one trial in women with preterm prelabour rupture of the membranes has only been published in abstract form with no usable data as yet (Sobhrabvand 2001); and one trial from Iran is awaiting classification to establish its eligibility for inclusion. We are awaiting a response from the authors (Atarod 2014).
The gestational age at trial entry varied between the trials: 24 to 30 weeks in Aghajafari 2002; 25 to 32 weeks in Murphy 2008; 25 to less than 33 weeks in Guinn 2002 and Garite 2009; 26 to 33 weeks in Mazumder 2008 and McEvoy 2010; 25 to 33 weeks in McEvoy 2002; 23 to less than 32 weeks in Wapner 2006; less than 32 weeks in Crowther 2006; and less than 34 weeks in Peltoniemi 2007. All women were at increased risk of preterm birth (seeCharacteristics of included studies table) and had received a single course of antenatal corticosteroids one week or more before trial entry. The type, amount and timing regimen for administration of the corticosteroid given for the pre‐trial course of antenatal corticosteroids varied between the trials.
In six trials women were eligible for inclusion seven or more days after a pre‐trial course (Aghajafari 2002; Crowther 2006; Guinn 2002; Mazumder 2008; McEvoy 2002; Peltoniemi 2007); in one trial between seven and 10 days after a pre‐trial course (Wapner 2006); in two trials 14 or more days after a pre‐trial course (Garite 2009: McEvoy 2010); and in one trial between 14 and 21 days after a pre‐trial course (Murphy 2008).
Exclusion criteria for recruitment to the trials included in this review are listed below
Aghajafari 2002; if women required chronic doses of corticosteroids secondary to medical conditions, had a contra‐indication to corticosteroids, had clinical evidence of chorioamnionitis, or if their fetus(es) had a known lethal congenital anomaly.
Crowther 2006; in second stage of labour, chorioamnionitis needing urgent delivery, or if further corticosteroid therapy was judged to be essential.
Garite 2009; major fetal anomaly, cervical dilatation 5 cm or more, higher order multiples, ruptured membranes, documented lung maturity, receiving corticosteroids for other indications, human immunodeficiency virus infection or active tuberculosis.
Guinn 2002; required immediate delivery, fetal anomalies incompatible with life, documented fetal lung maturity, and maternal active tuberculosis or human immunodeficiency virus infection.
Mazumder 2008; unreliable gestational age, frank chorioamnionitis and major fetal malformation, not available for follow‐up.
McEvoy 2002; insulin‐dependent diabetics, drug‐addiction, or known lethal congenital anomaly, multiple pregnancy.
McEvoy 2010; insulin‐dependent diabetics, major documented fetal or chromosomal abnormality; multiple pregnancy greater than twins; clinical chorioamnionitis; first course of antenatal corticosteroids given before 24 weeks' gestation; chronic steroid use during pregnancy for clinical care.
Murphy 2008; contraindication to corticosteroid use, needed chronic doses of corticosteroid drugs, had evidence of chorioamnionitis, known lethal congenital abnormality, had an initial course of corticosteroids before 23 weeks' gestation, previously participated in MACS, women with a multiple pregnancy with fetal death after 13 weeks' gestation.
Peltoniemi 2007; long‐term maternal corticosteroid use, clinical chorioamnionitis, or lethal disease of the fetus.
Wapner 2006; preterm premature rupture of the membranes prior to randomisation, confirmed fetal lung maturity, chorioamnionitis, a major fetal anomaly, non‐reassuring fetal status, systemic corticosteroid use during the current pregnancy, or insulin‐dependent diabetes.
Interventions
The type of corticosteroid planned to be given as treatment was betamethasone for all the trials, although the gestational age at which treatment could begin or was continued until varied slightly between the trials.
In five trials a planned treatment course was two doses of 12 mg/dose betamethasone, intramuscularly, at weekly intervals (Aghajafari 2002; Guinn 2002; Mazumder 2008; McEvoy 2002; Wapner 2006). For Aghajafari 2002, a weekly course of betamethasone was given (two doses of 12 mg/dose betamethasone (Celestone Soluspan; Schering Canada Inc.) intramuscularly, 24 hours apart) until 33 weeks or birth if the woman remained at increased risk of preterm birth. Guinn 2002 used a weekly course of betamethasone (two doses of 12 mg/dose betamethasone, intramuscularly 24 hours apart) until 34 weeks or birth, whichever came first. Mazumder 2008 used betamethasone 12 mg intramuscularly, two doses, 24 hours apart until the end of the 33rd week of gestation. McEvoy 2002 used a weekly course of betamethasone (two doses of 12 mg/dose betamethasone (Celestone Soluspan; Schering Corporation, Kenilworth, New Jersey), intramuscularly, until 34 weeks or birth. Wapner 2006 used a weekly course of betamethasone (two doses of 12 mg betamethasone as 6 mg betamethasone sodium phosphate and 6 mg betamethasone acetate, intramuscularly in 24 hours) until birth or 33 weeks and six days, limited to four repeat courses after the first 67 women.
One trial, Crowther 2006, used a single intramuscular injection of 11.4 mg Celestone Chronodose (Schering‐Plough, Sydney, Australia) containing 7.8 mg betamethasone sodium phosphate and 6 mg betamethasone acetate, repeated weekly if the woman remained undelivered and less than 32 weeks' gestation and the responsible clinician regarded her as at continued risk of preterm birth.
One trial, Murphy 2008, used a course of betamethasone (two doses of 12 mg/dose betamethasone (as 6 mg betamethasone sodium phosphate and 6 mg betamethasone acetate: Celestone Schering‐Plough Corporation, Madison, New Jersey, USA), intramuscularly, 24 hours apart, every 14 days (if the woman remained at risk of preterm birth after their first course of study treatment) until 33 weeks' gestation or birth. For women with preterm premature rupture of membranes, it was recommended that treatment stop at 32 weeks' gestation.
Three trials planned only a single repeat course of treatment; Garite 2009 used a single course consisting of two doses of 12 mg betamethasone, intramuscularly, 24 hours apart (preparation not specified); McEvoy 2010 used a single course of two doses of 12 mg/dose betamethasone (Celestone Soluspan; Schering Corporation, Kenilworth, New Jersey), intramuscularly, 24 hours apart; and Peltoniemi 2007 used a single intramuscular injection of 12 mg intramuscular of betamethasone, preparation not specified. For Garite 2009, due to unavailability of betamethasone, dexamethasone (6 mg, intramuscularly every 12 hours up to four doses or similar placebo regimen) was used for 61 (14%) of women.
Primary outcomes
The primary outcomes for Aghajafari 2002 were the rate of recruitment over a 12‐month period, risk of complications requiring discontinuation of study treatment, concentrations of plasma cortisol and adrenocorticotropic hormone in cord blood and in maternal blood immediately following birth, perinatal or neonatal mortality or significant neonatal morbidity.
For Crowther 2006, the primary outcomes were occurrence of neonatal RDS, severity of any respiratory disease present, use and duration of oxygen therapy, use and duration of mechanical ventilation, and weight, length and head circumference at birth and at discharge from hospital.
In Garite 2009 the primary outcome was a composite of neonatal mortality/morbidity in babies born before 34 weeks' gestation. The composite outcome was defined as one or more of: perinatal death (defined as stillbirth or death before neonatal discharge); RDS (oxygen requirement, clinical diagnosis and consistent chest radiograph); bronchopulmonary dysplasia (defined as a requirement for oxygen at 30 days of age); severe intraventricular haemorrhage (grades 3 or 4); periventricular leukomalacia; blood culture‐proven sepsis; or necrotising enterocolitis (not defined in the publication).
The Guinn 2002 trial had a composite neonatal morbidity primary outcome of any of the following: severe RDS, bronchopulmonary dysplasia, severe intraventricular haemorrhage, periventricular leukomalacia, necrotising enterocolitis, proven sepsis or death between randomisation and nursery discharge.
The primary outcome in Mazumder 2008 was severe RDS, defined as respiratory distress within six hours of birth in a preterm infant with either a negative gastric shake test or a typical chest radiograph. Severe RDS was defined as requiring mechanical ventilation for at least 12 hours. Mechanical ventilation was started in infants with hypoxaemia (Pa02 < 50 mmHg) or hypercapnic acidosis (PaC02 > 50 mmHg with pH < 7.25) or worsening acidosis or clinically worsening respiratory fatigue/apnoea/work of breathing despite continuous positive airway pressure (maximum 8 cm water pressure).
The primary outcomes for McEvoy 2002 and McEvoy 2010 were functional residual capacity and respiratory compliance.
Murphy 2008 used a composite primary outcome consisting of one of the following: stillbirth or neonatal death (defined as death during the first 28 days of life or before hospital discharge); severe RDS (defined as needing assisted ventilation via endotracheal tube and supplemental oxygen within the first 24 hours of life and for 24 hours or more, and either a radiographic scan compatible with RDS or surfactant given between the two and 24 hours after birth); bronchopulmonary dysplasia (defined as needing oxygen at a postmenstrual age of 36 completed weeks and radiographic scan compatible with bronchopulmonary dysplasia); intraventricular haemorrhage grade 3 or 4; cystic periventricular leukomalacia, necrotising enterocolitis.
For Peltoniemi 2007 the primary outcome was survival without RDS or severe intraventricular haemorrhage during the first hospitalisation.
For Wapner 2006 the primary outcome was one of the following: severe RDS (defined as clinical features of RDS with the need for oxygen and respiratory support from six to 24 hours or more of age, an abnormal chest x‐ray, and either administration of a full course of surfactant or a fraction of inspired oxygen (FiO2 of at least 60%); grade 3 or 4 intraventricular haemorrhage; periventricular leukomalacia; chronic lung disease (defined as the need for supplemental oxygen at 36 weeks' corrected age in infants born before 34 weeks' gestation); or stillbirth or neonatal death.
All the trials had a range of secondary outcomes of clinical relevance.
At early childhood follow‐up, primary outcomes varied by trial. For Crowther 2006, the pre‐specified primary outcomes were survival at two years' corrected age free of major neurodisability (defined as survival free of moderate or severe disability); and body size (weight, height and head circumference). For Murphy 2008, the primary outcome was death or the presence of neurologic impairment at 18 to 24 months of age, corrected for gestational age at birth. Neurologic impairment was defined as the presence of cerebral palsy or cognitive delay. For Peltoniemi 2007, the power analysis at follow‐up was based on survival without neurodevelopmental impairment. For Wapner 2006, the power analysis was based on the prespecified developmental outcome of the Bayley Mental Developmental Index score. Other prespecified outcomes for Wapner were the Bayley Psychomotor Developmental Index score: measurements of weight, height and head circumference and the occurrence of cerebral palsy.
Excluded studies
We excluded four trials (Bontis 2011; Mercer 2001; Romejko‐Wolniewicz 2013; Thorp 2000). Bontis 2011 was not a 'randomized' trial; in Mercer 2001 women recruited to the trial did not have corticosteroids before entry (the objective of this trial was to evaluate the need for and benefits of weekly antenatal corticosteroids in women at risk of preterm birth; in Romejko‐Wolniewicz 2013, this is a head‐to‐head trial of 2 different regimens and is eligible for the Cochrane review entitled 'Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth' Brownfoot 2013; and in Thorp 2000 the treatment to which women were randomised was not repeat dose(s) of prenatal corticosteroids.
Risk of bias in included studies
SeeIncluded studies, 'Risk of bias' tables and 'Risk of bias' summary figures: Figure 1; Figure 2. Overall, the included trials were assessed as having a low to moderate risk of bias.
1.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Adequate sequence generation was clearly reported for eight trials. For Aghajafari 2002, randomisation was computer‐generated and was centrally controlled by one pharmacist at each hospital who kept the randomisation code with stratification by gestational age (24 to 27 weeks versus 28 to 30 weeks), and by hospital using block sizes of two. Crowther 2006 used a central telephone randomisation service for study number and then treatment pack allocation. The randomisation numbers were generated by a computer with variable block sizes, stratified by centre, gestational age (two groups: less than 28 weeks and 28 weeks or more) and number of fetuses (three groups: singleton, twin and triplet). In Garite 2009, a blocked randomisation sequence was used prepared centrally, with the random sequence generated by computer. Guinn 2002 used computer‐generated randomisation logs prepared centrally, stratified by centre, and distributed to the research pharmacist at each clinical site. Mazumder 2008 used a website‐generated random number list. In McEvoy 2002, assignment was via the pharmacy using a random‐number table. No stratification was reported. McEvoy 2010 used a random number table, with stratification of 28 weeks or less versus 28 or more weeks' gestation and multiple gestation (twins versus singletons). For Wapner 2006, randomisation sequences for the treatment kits were generated by the independent data co‐ordinating centre with stratification by centre, type of qualifying corticosteroid course and whether an in‐patient or out‐patient using the urn design.
The method of sequence generation was unclear from the published reports for Peltoniemi 2007 and Murphy 2008. Murphy 2008 used a 24‐hour telephone service for randomisation. Information on the generation of the allocation sequence was not provided in the published paper but was probably adequate. For Peltoniemi 2007, randomisation was performed centrally and was stratified according to centre by using four sets of sequentially‐labelled, opaque, sealed envelopes (for the four strata of gestational age (28 weeks or less or between 28 and 34 weeks or less) and multiple gestation (singleton or multiple pregnancies)), which were sent to each centre. How the randomisation sequence was generated was not described in the paper but was probably adequate.
Allocation concealment
Adequate allocation concealment was reported in all 10 trials. For Aghajafari 2002, randomisation was controlled by one pharmacist at each hospital who kept the randomisation code. Crowther 2006 used a central telephone randomisation service for study number and then treatment pack allocation. In Garite 2009, the research pharmacist at each site prepared the medication based on the blocked randomisation sequence. In Guinn 2002, the research pharmacist at each clinical site allocated the treatment from the randomisation log. In Mazumder 2008, women were randomised by opening the next serially numbered opaque sealed envelope prepared using the random number list. For McEvoy 2002 and McEvoy 2010, assignment was via the pharmacy where the medication was prepared, and was stratified according to centre by using four sets of sequentially‐labelled, opaque, sealed envelopes (for the four strata of gestational age (28 weeks or less or between 28 and 34 weeks or less) and multiple gestation (singleton or multiple pregnancies)), which were sent to each centre. Murphy 2008 used a 24‐hour telephone service for randomisation. Peltoniemi 2007 used centrally‐labelled, opaque, sealed envelopes sent to each centre, with the sealed envelope opened after informed consent was obtained. For Wapner 2006, the woman was assigned the next sequentially‐numbered treatment kit by a centralised research pharmacy.
Blinding
A placebo was used in all the trials, except Mazumder 2008, to blind participants and caregivers to treatment allocation. Normal saline was used as placebo in Aghajafari 2002, Crowther 2006, Garite 2009 and Peltoniemi 2007. McEvoy 2002 and McEvoy 2010 used 25 mg cortisone acetate, an inactive steroid. Murphy 2008 used a similarly appearing intramuscular injection of dilute concentration of aluminium monostearate (an inert substance used as a filler in pharmaceutical preparations). The type of placebo preparation used was not stated for Guinn 2002 or Wapner 2006.
In Aghajafari 2002, the pharmacist prepared the study treatments in a syringe covered with yellow tape and the injection of the study treatment was given by a designated research nurse in each hospital, who was not caring for the woman. For Crowther 2006, the treatment packs looked identical and contained an opaque study‐labelled syringe. In Garite 2009 the study syringes were completely covered by a label to conceal the contents. For Guinn 2002, the placebo syringes were indistinguishable from the syringes containing betamethasone. For McEvoy 2002 and McEvoy 2010, the placebo was identical in appearance to betamethasone. In Murphy 2008, the study treatments were similar in appearance. In Peltoniemi 2007 the study medication and placebo were prepared in identical syringes which were masked with opaque tape. In Wapner 2006, the placebo was identical in appearance to betamethasone.
There are no details for blinding of outcome assessors reported by Aghajafari 2002, Garite 2009, Guinn 2002, Mazumder 2008, McEvoy 2002, McEvoy 2010.
Of the four trials reporting early childhood follow‐up data in full (Crowther 2006; Murphy 2008; Peltoniemi 2007; Wapner 2006), three stated blinding of outcome assessors. For Crowther 2006 staff making the assessments and the families "were unaware of group assignment"; in Peltoniemi 2007 "the examiners and families were unaware of treatment‐group assignment" and in Wapner 2006 assessments were performed by "centrally trained and certified study personnel who were unaware of the treatment assignment". Murphy 2008 does not stipulate whether outcome assessors were blinded to treatment group.
Incomplete outcome data
All 10 trials provided data on women and children up to the time of primary hospital discharge after birth. No losses to follow‐up to this time were reported for Aghajafari 2002, Crowther 2006, McEvoy 2002 or Peltoniemi 2007. Garite 2009 reported 19 (3.3%) babies (13 in the repeat corticosteroid group and six in the placebo group) lost to follow‐up. In the Guinn 2002 trial, 16 (3.2%) women and one (0.2%) neonate were lost to follow‐up. Partial data were available for women who were lost to follow‐up for the birth date, weight, and health status for the neonate. The denominators presented in the trial report vary slightly from one variable to another because of missing data (Guinn 2002). For Mazumder 2008 one (1.3%) women was lost to follow‐up before birth in the repeat corticosteroid group. In McEvoy 2010, one (0.9%) neonate was lost to follow‐up in the placebo group. For Murphy 2008, five (0.3%) babies alive at randomisation were lost to follow‐up, two in the repeat corticosteroid group and three in the placebo group. For Wapner 2006, three (0.6%) women were lost to follow‐up.
At early childhood follow‐up, five trials have reported some data (Crowther 2006; Mazumder 2008; Murphy 2008; Peltoniemi 2007; Wapner 2006), with varied losses to follow‐up reported. In Crowther 2006, for 86 (7.5%) children alive at randomisation, no data were available to include in the primary outcome of survival free of major neurosensory disability at two years' corrected age follow‐up. In Murphy 2008, for 205 (8.9%) children alive at randomisation no data were available to include in the primary outcome of death or the presence of neurologic impairment at 18 to 24 months corrected age. For Peltoniemi 2007, 69 (21.2%) children from trial entry were not available to include in the primary outcome of survival without severe neurological, cognitive or sensory impairment at follow‐up of the children between two to three years of age. For Wapner 2006, at the time of two‐year corrected age assessment of the 582 known survivors, 96 (16.5%) were not able to be seen, 46 in the repeat corticosteroid group and 50 in the placebo group; 117 (20.1%) did not have a Bayley assessment performed, 59 in the repeat corticosteroid group and 58 in the placebo group. Only interim data have been reported by Mazumder 2008 on 44 (58%) infants at 18 months of age and final follow‐up at 18 months of age on the children remaining is awaited.
Selective reporting
Overall, there was no obvious risk of selective reporting for nine of the trials. There was insufficient detail in the publication to permit judgement for Mazumder 2008. Only outcomes relating to body size were reported by number of repeat corticosteroid courses in Wapner 2006.
Other potential sources of bias
Guinn 2002 made the decision to stop recruitment at 500 women following the first interim analysis when 308 women had been randomised. The reason was because of "safety concerns" and the finding of only a marginal difference in short‐term outcomes between treatment groups.
In the Peltoniemi 2007 trial, recruitment was terminated early, after 249 women had been enrolled, primarily because of safety concerns due a decrease in intact survival in the repeat corticosteroid group. There were additional concerns about the long‐term adverse effects of glucocorticoid. There was a potential imbalance at trial entry for multiple pregnancy and gestational age that was not addressed by the trial authors.
In the Wapner 2006 trial, recruitment was stopped early based on safety concerns after the recruitment of 495 women. "At the second interim analysis the Data Safety Monitoring Committee recommended that enrolment be halted because of a tendency towards decreased birthweight in the repeat steroid group without evident reduction in the primary morbidity outcome and also because of difficulties with recruitment."
Effects of interventions
We included 10 trials involving more than 4730 women and 5650 babies.
(1) Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment (all included trials)
Primary outcomes for the infant
Data were available for all the primary outcomes for the infant.
Significantly fewer infants exposed to repeat dose(s) of corticosteroids had respiratory distress syndrome (RDS) compared with infants exposed to placebo or no treatment (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.75 to 0.91, eight trials, 3206 infants, number needed to treat to benefit (NNTB) 17, 95% CI 11 to 32 ‐ Analysis 1.1).
Seven trials reported a composite for serious infant outcome. Treatment with repeat dose(s) of corticosteroid was associated with a reduction in serious infant outcome (RR 0.84, 95% CI 0.75 to 0.94, seven trials, 5094 infants, NNTB 30, 95% CI 19 to 79 ‐ Analysis 1.3). The composite outcome was variously defined by the trialists: Aghajafari 2002: one or more of the following: stillborn or neonatal death during the first 28 days of life or before hospital discharge, whichever was sooner; RDS; bronchopulmonary dysplasia (requiring oxygen at 36 corrected postnatal gestational age); grade 3 or 4 intraventricular haemorrhage and necrotising enterocolitis; Crowther 2006: one or more of the following: air leak syndrome, patent ductus arteriosus, need for oxygen at 36 weeks' postmenstrual age, severe intraventricular haemorrhage (grade 3 or 4), periventricular leukomalacia, proven necrotising enterocolitis or retinopathy of prematurity; Garite 2009: one or more of: perinatal death in babies born before 34 weeks' gestation, perinatal death (defined as stillbirth or death before neonatal discharge); RDS (oxygen requirement, clinical diagnosis and consistent chest radiograph); bronchopulmonary dysplasia (defined as a requirement for oxygen at 30 days of age); severe intraventricular haemorrhage (grades 3 or 4); periventricular leukomalacia; blood culture‐proven sepsis; or necrotising enterocolitis (not defined); Guinn 2002: the presence of any of the following: severe RDS, bronchopulmonary dysplasia, severe intraventricular haemorrhage, periventricular leukomalacia, necrotising enterocolitis, proven sepsis or death between randomisation and nursery discharge; Murphy 2008: one of the following: stillbirth or neonatal death (defined as death during the first 28 days of life or before hospital discharge); severe RDS (defined as needing assisted ventilation via endotracheal tube and supplemental oxygen within the first 24 hours of life and for 24 hours or more, and either a radiographic scan compatible with RDS or surfactant given between the first two to 24 hours of life); bronchopulmonary dysplasia (defined as needing oxygen at a postmenstrual age of 36 completed weeks and radiographic scan compatible with bronchopulmonary dysplasia); intraventricular haemorrhage grade 3 or 4; cystic periventricular leukomalacia, necrotising enterocolitis; Peltoniemi 2007: death or RDS or severe intraventricular haemorrhage (expressed as survival without RDS or severe intraventricular haemorrhage during the first hospitalisation); Wapner 2006: one of the following: severe RDS, grade 3 or 4 intraventricular haemorrhage; periventricular leukomalacia, chronic lung disease (defined as the need for supplemental oxygen at 36 weeks' corrected age in infants born before 34 weeks' gestation), or stillbirth or neonatal death.
1.1. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 1 Respiratory distress syndrome.
1.3. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 3 Composite serious outcome (variously defined).
Treatment with repeat dose(s) of corticosteroid was associated with a reduction in unadjusted mean birthweight. However, birthweight outcomes that adjusted for gestational age (birthweight Z scores, birthweight multiples of the median and small‐for‐gestational age) did not differ statistically between treatment groups.
Mean birthweight (mean difference (MD) ‐75.79 g, 95% CI ‐117.63 to ‐33.96, nine trials, 5626 infants ‐ Analysis 1.4);
birthweight Z scores (MD ‐0.11, 95% CI ‐0.23 to 0.00, two trials, 1256 infants ‐ Analysis 1.5);
birthweight multiples of the median (MD 0.00, 95% CI ‐0.03 to 0.03, one trial, 590 infants ‐ Analysis 1.6).
1.4. Analysis.
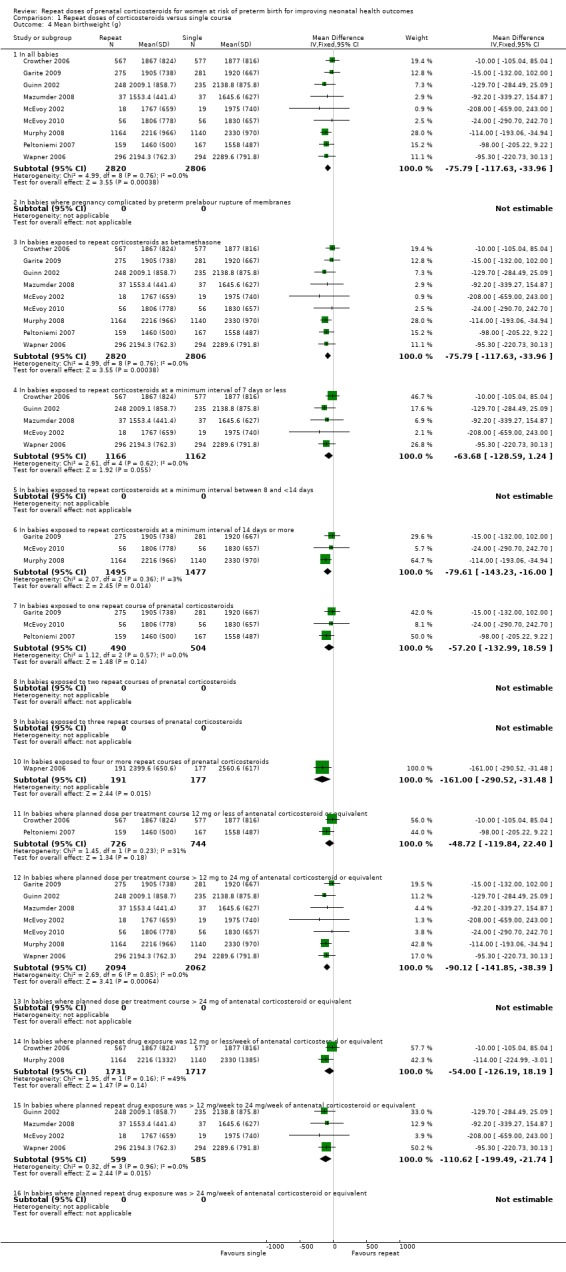
Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 4 Mean birthweight (g).
1.5. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 5 Birthweight Z scores.
1.6. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 6 Birthweight multiples of the median.
No statistically significant differences were seen in infants in the repeat corticosteroid group compared with infants in the placebo/no treatment group for any of the other primary clinical outcomes:
severe lung disease (average RR 0.80, 95% CI 0.56 to 1.14, I² = 76%, Tau² = 0.12; random‐effects, six trials, 4826 infants ‐ Analysis 1.2);
fetal and neonatal mortality (RR 0.94, 95% CI 0.71 to 1.23, nine trials, 5554 infants ‐ Analysis 1.8);
chronic lung disease (RR 1.06, 95% CI 0.87 to 1.30, eight trials, 5393 infants ‐ Analysis 1.11);
intraventricular haemorrhage (RR 0.94, 95% CI 0.75 to 1.18, six trials, 3065 infants ‐ Analysis 1.12).
1.2. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 2 Severe lung disease.
1.8. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 8 Fetal and neonatal mortality.
1.11. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 11 Chronic lung disease.
1.12. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 12 Intraventricular haemorrhage.
Primary outcomes for the child
Only four trials have reported data in full from early childhood follow‐up (Crowther 2006, Murphy 2008; Peltoniemi 2007, Wapner 2006) and we have included these in the meta‐analysis. We have not included the interim data reported by Mazumder 2008 on 44 (58%) infants at 18 months of age, due to the high proportion of unavailable data (42%).
Data were available for all the primary outcomes for the child, but not from all the trials. No statistically significant differences were seen for infants in the repeat corticosteroids group compared with infants in the placebo or no treatment for any of the primary outcomes for the child:
total deaths up to early childhood follow‐up (RR 1.06, 95% CI 0.80 to 1.41, four trials, 4370 infants ‐ Analysis 1.65);
survival free of any disability (RR 1.01, 95% CI 0.97 to 1.05, two trials, 3155 infants ‐ Analysis 1.66);
survival free of any major disability (average RR 1.01, 95% CI 0.92 to 1.11; I² = 88%, Tau² = 0.00, random‐effects, two trials, 1317 infants ‐ Analysis 1.67);
any disability at childhood follow‐up (RR 0.98, 95% CI 0.83 to 1.16, one trial, 999 infants ‐ Analysis 1.69);
major disability at childhood follow‐up (RR 1.08, 95% CI 0.31 to 3.76, two trials, 1256 infants ‐ Analysis 1.68);
composite serious outcome at childhood follow‐up (RR 0.99, 95% CI 0.87 to 1.12, two trials, 3164 infants ‐ Analysis 1.70), defined in Crowther 2006 as death or any neurosensory disability; and defined in Murphy 2008 as death or any neurologic impairment.
1.65. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 65 Total mortality up to early childhood follow up.
1.66. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 66 Survival free of any disability to early childhood follow up.
1.67. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 67 Survival free of major neurosensory disability to early childhood follow up.
1.69. Analysis.
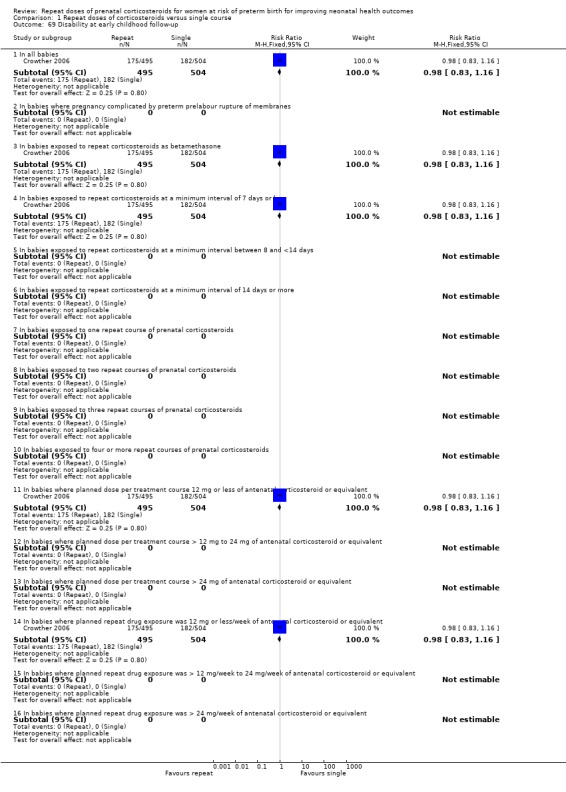
Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 69 Disability at early childhood follow‐up.
1.68. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 68 Major neurosensory disablity at early childhood follow‐up.
1.70. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 70 Composite serious outcome at early childhood follow‐up (however defined by authors).
Primary outcomes for the child as an adult
No data available for inclusion.
Primary outcomes for the women
No statistically significant differences were seen for women treated with repeat dose(s) of prenatal corticosteroids compared with women given placebo or no treatment for the two primary outcomes for the women of maternal infectious morbidity:
chorioamnionitis (RR 1.16, 95% CI 0.92 to 1.46, six trials, 4261 women ‐ Analysis 1.15);
puerperal sepsis (RR 1.15, 95% CI 0.83 to 1.60, five trials, 3091 women ‐ Analysis 1.16).
1.15. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 15 Chorioamnionitis.
1.16. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 16 Puerperal sepsis.
Secondary outcomes for the infant
In keeping with the reduction in RDS seen, treatment with repeat dose(s) of corticosteroid compared with placebo or no treatment was associated with a reduction in the use of:
mechanical ventilation (average RR 0.84, 95% CI 0.71 to 0.99; I² = 61%, Tau² = 0.02, random‐effects, six trials, 4918 infants ‐ Analysis 1.27);
oxygen supplementation (RR 0.92, 95% CI 0.85 to 0.99, two trials, 3448 infants ‐ Analysis 1.29);
surfactant (average RR 0.78, 95% CI 0.65 to 0.95; I² = 66%, Tau² = 0.05, random‐effects, nine trials, 5525 infants ‐ Analysis 1.31);
inotropic support (RR 0.80, 95% CI 0.66 to 0.97, two trials, 1470 infants ‐ Analysis 1.32).
1.27. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 27 Use of mechanical ventilation.
1.29. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 29 Use of oxygen supplementation.
1.31. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 31 Use of surfactant.
1.32. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 32 Use of inotropic support.
Fewer infants exposed to repeat corticosteroids compared with infants exposed to placebo or no treatment had a patent ductus arteriosus (RR 0.80, 95% CI 0.64 to 0.98, six trials, 4356 infants ‐ Analysis 1.39).
1.39. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 39 Patent ductus arteriosus.
At birth, treatment with repeat dose(s) of corticosteroid was associated with a reduction in mean head circumference (MD ‐0.32 cm, 95% CI ‐0.49 to ‐0.15, nine trials, 5626 infants ‐ Analysis 1.21), head circumference Z scores (MD ‐0.14, 95% CI ‐0.27 to ‐0.00, two trials, 1256 infants ‐ Analysis 1.22), mean length (average MD ‐0.56 cm, 95% CI ‐0.89 to ‐0.23, six trials, 4550 infants ‐ Analysis 1.23) and in one trial, significantly lower length multiples of the mean at birth (MD ‐0.01, 95% CI ‐0.02 to 0.00, one trial, 590 infants ‐ Analysis 1.25). In contrast, length Z scores were not significantly reduced by treatment with repeat dose(s) of corticosteroid in two trials (MD ‐0.05, 95% CI ‐0.19 to 0.09, two trials, 1256 infants ‐ Analysis 1.24). There was no statistically significant difference associated with repeat antenatal corticosteroids for small‐for‐gestational age (RR 1.18, 95% CI 0.97 to 1.43, seven trials, 3975 infants ‐ Analysis 1.7).
1.21. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 21 Mean head circumference at birth (cm).
1.22. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 22 Head circumference Z scores at birth.
1.23. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 23 Mean length at birth (cm).
1.25. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 25 Length multiples of the median at birth.
1.24. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 24 Length Z scores at birth.
1.7. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 7 Small‐for‐gestational age at birth.
At primary hospital discharge, two trials (1195 infants) reported infant growth assessments. No significant differences were seen between infants exposed to repeat corticosteroids or placebo/no treatment for mean weight, head circumference or length, or in Z scores for weight, head circumference or length.
The mean gestational age at birth for the infants (MD ‐0.09 weeks, 95% CI ‐0.33 to 0.15, eight trials, 3179 infants ‐ Analysis 1.17) was not significantly different between treatment groups, nor was the proportion of infants born before 37 weeks', 34 weeks' or 28 weeks' gestation. However, data are not available to include in these analyses from Murphy 2008, the largest trial, where there was a significant difference in the mean gestational age women gave birth reported (34.5 weeks (standard deviation (SD) 3.6 for the repeat corticosteroid group and 34.9 weeks (SD 3.6) for the placebo group, MD ‐0.40 weeks, 95% CI ‐0.73 to ‐0.07).
1.17. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 17 Mean gestational age at birth (weeks).
No statistically significant differences were seen in infants exposed to repeat dose(s) of corticosteroids compared with infants exposed to placebo or no treatment for the other reported secondary respiratory outcomes (including duration of respiratory support, duration of oxygen supplementation, use of postnatal corticosteroids), infectious morbidity outcomes (early systemic neonatal infection, proven infection while in the neonatal intensive care unit), and other neonatal morbidity (including air leak syndrome, intraventricular haemorrhage grade 3 or 4, periventricular leukomalacia, necrotising enterocolitis, and retinopathy of prematurity).
In the one trial (Crowther 2006) that reported blood pressure and cardiac outcomes in a subgroup of infants (recruited at two of the collaborating hospitals), no significant differences were seen between treatment groups for mean blood pressure on the first day of life or at six weeks postnatally, or in the risk of neonatal cardiac hypertrophy. In the one trial that reported on hypothalamo/pituitary/adrenal axis suppression in a subgroup of infants (recruited at one of the collaborating hospitals), infants exposed to repeat dose(s) of corticosteroids had significantly lower mean cortisol concentrations at birth (MD ‐44.90 nmol/L, 95% CI ‐78.41 to ‐11.39, one trial, 67 infants ‐ Analysis 1.46).
1.46. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 46 Mean basal cortisol concentrations (nmol/L) at birth.
Secondary outcomes for the child
From the limited data available from four trials (Crowther 2006; Murphy 2008; Peltoniemi 2007; Wapner 2006), no statistically significant differences were seen for infants in the repeat corticosteroids group compared with infants in the placebo or no treatment groups for any of the reported secondary developmental outcomes for the child. These included developmental delay, blindness, deafness, and cerebral palsy, mental development index (MDI), and psychomotor developmental index (PDI):
developmental delay (RR 0.97, 95% CI 0.84 to 1.13, three trials, 3202 infants ‐ Analysis 1.80);
blindness (RR 1.17, 95% CI 0.65 to 2.10, two trials, 3151 infants ‐ Analysis 1.83);
deafness (RR 0.85, 95% CI 0.29 to 2.52, three trials, 3405 infants ‐ Analysis 1.84);
cerebral palsy (RR 1.03, 95% CI 0.71 to 1.50, four trials, 3800 infants ‐ Analysis 1.85) (More than a 10‐fold variation in the rate of cerebral palsy was seen between the trials);
mental developmental index (MDI) (MD 1.23, ‐0.65 to 3.11, two trials, 1162 infants ‐ Analysis 1.81);
psychomotor developmental index (PDI) (MD 0.40, ‐1.75 to 2.55, one trial, 958 infants ‐ Analysis 1.82).
1.80. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 80 Developmental delay at early childhood follow‐up.
1.83. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 83 Bindness at early childhood follow‐up.
1.84. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 84 Deafness at early childhood follow‐up.
1.85. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 85 Cerebral palsy at early childhood follow‐up.
1.81. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 81 Mental Developmental Index at early childhood follow‐up.
1.82. Analysis.
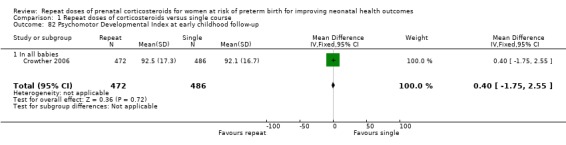
Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 82 Psychomotor Developmental Index at early childhood follow‐up.
Data were not able to be included in the meta‐analysis for Murphy 2008 for MDI or PDI scores expressed as means, as standard deviations were not available in the published report. In this trial, the mean MDI scores were 93.91 and 94.74 and the PDI scores were 97.86 and 98.98 for the prenatal corticosteroid therapy and placebo groups, respectively.
At early childhood follow‐up, all four trials reported some growth assessments for the children. Unlike assessments of body size at birth, at childhood follow‐up no significant differences were seen for children exposed to repeat corticosteroids compared with children in the control group for a variety of measures of body size, includingmean weight, head circumference and length, Z scores for weight, head circumference and height, and assessments of small for age for weight, head circumference and or height:
mean weight (MD ‐0.03 kg, 95% CI ‐0.21 to 0.15, three trials, 1776 infants ‐ Analysis 1.74);
mean head circumference (MD ‐0.05 cm, 95% CI ‐0.22 to 0.11, three trials, 1776 infants ‐ Analysis 1.75);
mean height (MD ‐0.13 cm, 95% CI ‐0.55 to 0.30, three trials, 1776 infants ‐ Analysis 1.76);
weight Z scores (MD ‐0.03, 95% CI ‐0.19 to 0.13, one trial, 1047 infants ‐ Analysis 1.77);
head circumference Z scores (MD 0.04, 95% CI ‐0.09 to 0.18, two trials, 1290 infants ‐ Analysis 1.78);
height Z scores (MD ‐0.04, 95% CI ‐0.17 to 0.09, two trials, 1290 infants ‐ Analysis 1.79);
weight small for age (RR 0.92, 95% CI 0.71 to 1.19, two trials, 1448 infants ‐ Analysis 1.71);
head circumference small for age (average RR 1.10, 95% CI 0.77 to 1.56, two trials; I² = 57%, Tau² = 0.04, random‐effects, 1442 infants ‐ Analysis 1.72);
height small for age (average RR 1.12, 95% CI 0.63 to 2.02, two trials; I² = 65%, Tau² = 0.12, random‐effects, 1441 infants ‐ Analysis 1.73).
1.74. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 74 Mean weight at early childhood follow‐up.
1.75. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 75 Mean head circumference at early childhood follow‐up.
1.76. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 76 Mean height at early childhood follow‐up.
1.77. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 77 Weight Z score at early childhood follow‐up.
1.78. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 78 Head circumference Z score at early childhood follow‐up.
1.79. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 79 Height Z score at early childhood follow‐up.
1.71. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 71 Weight small for age at early childhood follow‐up.
1.72. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 72 Head circumference small for age at early childhood follow‐up.
1.73. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 73 Height small for age at early childhood follow‐up.
Data were not able to be included in the meta‐analysis for Murphy 2008 for anthropometric assessments expressed as means, as standard deviations were not available in the published report. In this trial, mean weight, head circumference and height for children exposed to repeated courses of prenatal corticosteroids were not significantly different from those for children who were exposed to a single course.
Using a random‐effects model because of heterogeneity, we found no impact on asthma or wheezing at early childhood follow‐up on average between treatment groups (average RR 0.89, 95% CI 0.63 to 1.27; I² = 62%, Tau² = 0.06, random‐effects, three trials, 1720 children ‐ Analysis 1.87). Two trials reported on blood pressure at early childhood follow‐up. One trial (Wapner 2006) reported a significantly lower mean systolic blood pressure for children who had been exposed to repeat corticosteroids compared with children exposed to a single course (MD ‐2.90 mmHg, 95% CI ‐5.40 to ‐0.40, one trial, 486 children ‐ Analysis 1.89), although no differences were seen for mean diastolic blood pressure, or, in another trial (Crowther 2006), in the proportion of children who were hypertensive.
1.87. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 87 Asthma/Wheezing at early childhood follow‐up.
1.89. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 89 Mean systolic blood pressure at early childhood follow‐up.
Two trials (Crowther 2006; Murphy 2008) reported on behaviour.Crowther 2006 used the Child Behavior Checklist, a rating scale that screens for behavioural and emotional problems. The trial showed no significant differences between the groups in the total or individual mean scores for internalising, externalising, emotional reactivity, anxiety or depression, somatic complaints, withdrawal, sleep problems, attention problems, or aggressive behaviour between treatment groups. No differences were seen in the proportion of children with a behaviour score within the clinical range (RR 1.09, 95% CI 0.79 to 1.51, one trial, 1045 children ‐ Analysis 1.86). Murphy 2008 used the Behavior Rating Scale of the BSID‐II and found scores were non‐optimal or questionable for 285 (31.6%) of 902 children in the prenatal corticosteroid therapy group and 239 (27.3%) of 874 children in the placebo group.
1.86. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 86 Behaviour score at early childhood follow‐up within clinical range.
Secondary outcomes for the child as an adult
No information is currently available for outcomes for the child when an adult.
Secondary outcomes for the women
There was one maternal death reported for a women allocated to the placebo group and withdrawn from the trial before study treatment was given (Garite 2009). No statistically significant differences were seen between treatment groups, where data were available, for the following outcomes: risk of prelabour rupture of the membranes after trial entry, hypertension, mode of birth, postpartum haemorrhage orpostnatal pyrexia.
The two trials that reported on the outcome any side effects of therapy showed significant heterogeneity. Crowther 2006 reported a significant increase in side effects with treatment with repeat dose(s) of corticosteroid compared with saline placebo (RR 1.97, 95% CI 1.23 to 3.18, one trial, 982 women), whilst Wapner 2006 reported a significant reduction in side effects with repeat dose(s) corticosteroid treatment compared with an unnamed placebo (RR 0.49, 95% CI 0.39 to 0.61, one trial, 492 women). Using a random‐effects model, no impact was seen for side effects of therapy on average between treatment groups (average RR 0.97, 95% CI 0.24 to 3.90, random‐effects, two trials, 1474 women ‐ Analysis 1.60). Similar heterogeneity was seen for pain at the site of the injection; Crowther 2006 reported a non‐significant increase in side effects with treatment with repeat dose(s) of corticosteroid compared with placebo (RR 2.02, 95% CI 0.95 to 4.26, one trial, 982 women), whilst Wapner 2006 a significant reduction (RR 0.28, 95% CI 0.19 to 0.41, one trial, 492 women). Overall, no average difference was seen using a random‐effects model (average RR 0.73, 95% CI 0.11 to 5.05, random‐effects, two trials, 1474 women ‐ Analysis 1.63). Wapner 2006 reported a significant reduction in bruising at the site of the injection with treatment with repeat dose(s) of corticosteroid compared with an unnamed placebo (RR 0.38, 95% CI 0.21 to 0.71, one trial, 492 women ‐ Analysis 1.64). Three trials reported on insomnia (Aghajafari 2002; Crowther 2006; Wapner 2006). More women receiving repeat corticosteroids compared with no repeat treatment reported insomnia (RR 2.63, 95% CI 1.10 to 6.30, three trials, 1486 women ‐ Analysis 1.62). Wapner 2006 found no significant differences for maternal glucose intolerance between treatment groups.
1.60. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 60 Any maternal side effects of therapy.
1.63. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 63 Pain at injection site.
1.64. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 64 Bruising at injection site.
1.62. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 62 Insomnia.
No data from any of the trials were reported on other secondary systematic review outcomes for the women including postnatal depression, parenting stress, breastfeeding after discharge from hospital, satisfaction with the therapy, andquality of life.
Secondary outcomes on use of health services
Few data were reported that related to health services use. No differences were seen between treatment groups for the two trials (Crowther 2006; Murphy 2008) that reported on need for admission to the neonatal intensive care unit. Similarly, no differences were seen between treatment groups for length of postnatal hospitalisation for the women in the one trial with data (Guinn 2002).
All four trials reported on readmission to hospital by early childhood follow‐up and no significant differences were seen on readmission to hospital between treatment groups (RR 1.02, 95% CI 0.93 to 1.11, four trials, 3824 children ‐ Analysis 1.91).
1.91. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 91 Hospital re‐admission by early childhood follow‐up.
(2) Sensitivity analyses based on trial quality
All 10 trials were rated as being adequate for allocation concealment, so sensitivity analyses were not performed based on trial quality.
(3) Planned population subgroup analyses
(3.1) Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by reason for being at risk of preterm birth (e.g. presence or absence of ruptured membranes, antepartum haemorrhage, preterm labour, cervical incompetence, pre‐eclampsia and growth restriction)
Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by the presence or absence of ruptured membranes at trial entry or after trial entry
Only one trial (Guinn 2002), reported data for a subgroup of 161 women with preterm prelabour rupture of membranes (120 with ruptured membranes at trial entry and 41 women who ruptured membranes after trial entry). For the infants, no statistically significant differences were seen for any of the primary outcomes where data were available, namely RDS; fetal, neonatal, infant death; andchronic lung disease. In this small subgroup of women with ruptured membranes, treatment with repeat dose(s) of corticosteroid compared with placebo was associated with an increased risk of chorioamnionitis (RR 1.56, 95% 1.05 to 2.31, one trial, 160 women ‐ Analysis 1.15), although no differences were seen for puerperal sepsis between treatment groups (RR 0.65, 95% CI 0.19 to 2.22, one trial, 160 women ‐ Analysis 1.16).
Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by the presence or absence at trial entry of antepartum haemorrhage, preterm labour, cervical incompetence, pre‐eclampsia and growth restriction
No data have been reported on these clinical subgroups to date.
(3.2) Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by number of babies in utero (singleton, twins or higher order multiples)
No data have been reported on these clinical subgroups to date.
(3.3) Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by type of corticosteroid given (e.g. betamethasone, dexamethasone, hydrocortisone)
All 10 trials planned to use betamethasone, so we were not able to perform subgroup analyses as to the type of repeat corticosteroid treatment given.
(3.4) Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by the planned interval between corticosteroid treatments (at a minimum interval of seven days or less, at a minimum interval between eight and less than 14 days, at a minimum interval of 14 days or greater)
Planned interval between corticosteroid treatments of a minimum interval of seven days or less
Seven trials used a minimum interval of seven days or less between corticosteroid treatments. (Aghajafari 2002; Crowther 2006; Guinn 2002; Mazumder 2008; McEvoy 2002; Peltoniemi 2007; Wapner 2006).
Women were eligible for inclusion seven or more days after a pre‐trial course in six trials (Aghajafari 2002; Crowther 2006; Guinn 2002; Mazumder 2008; McEvoy 2002; Peltoniemi 2007), and in Wapner 2006 between seven and 10 days after a pre‐trial course. The upper gestational age at trial entry varied between the trials: being up to 30 weeks in Aghajafari 2002, less than 32 weeks in Crowther 2006 and Wapner 2006, less than 33 weeks in Guinn 2002, Mazumder 2008 and McEvoy 2002, and up to 34 weeks in Peltoniemi 2007.
In five trials a planned treatment course was two doses of 12 mg/dose betamethasone (Aghajafari 2002; Guinn 2002; Mazumder 2008; McEvoy 2002; Wapner 2006), repeated at weekly intervals up to 33 weeks in Aghajafari 2002, until less than 34 weeks in Wapner 2006 and until 34 weeks in Guinn 2002, Mazumder 2008, and McEvoy 2002. In two trials (Crowther 2006; Peltoniemi 2007), a planned treatment course was a single injection of 12 mg betamethasone, given only once in Peltoniemi 2007 and repeated weekly if still considered at risk of preterm birth up to less than 32 weeks' gestation in Crowther 2006.
Primary outcomes for the infant
Treatment with repeat dose(s) of corticosteroid treatments at a minimum interval of seven days or less compared with no repeat treatment was associated with a reduction in:
respiratory distress syndrome (RR 0.86, 95% CI 0.77 to 0.96, six trials, 2538 infants ‐ Analysis 1.1);
composite serious outcome (RR 0.78, 95% CI 0.66 to 0.91, five trials, 2232 infants ‐ Analysis 1.3).
No significant differences were seen between treatment groups for the other primary clinical outcomes:
severe lung disease (average RR 0.72, 95% CI 0.47 to 1.12, I² = 78%, Tau² = 0.15 random‐effects, five trials, 2522 infants ‐ Analysis 1.2);
fetal and neonatal mortality (RR 0.96, 95% CI 0.67 to 1.37, six trials, 2871 infants ‐ Analysis 1.8);
chronic lung disease (RR 0.97, 95% CI 0.78 to 1.21, six trials, 2538 infants ‐ Analysis 1.11);
intraventricular haemorrhage (RR 0.98, 95% CI 0.77 to 1.26, five trials, 2519 infants ‐ Analysis 1.12).
Most of the outcomes related to birthweight were significantly less for infants exposed to repeat corticosteroid treatment at intervals of seven days, or less compared with infants exposed to placebo. Infants exposed to repeat corticosteroids had lower mean birthweights, lower birthweight Z scores and were more likely to be small‐for‐gestational age, although there was no difference in birthweight multiples of the median:
birthweight (MD ‐63.68 g, 95% CI ‐128.59 to 1.24, five trials, 2328 infants ‐ Analysis 1.4);
birthweight Z score (MD ‐0.13, 95% CI ‐0.26 to 0.00, one trial, 1144 infants ‐ Analysis 1.5);
birthweight multiples of the median (MD 0.00, 95% CI ‐0.03 to 0.03, one trial, 590 infants ‐ Analysis 1.6);
small‐for‐gestational age (RR 1.38, 95% CI 1.04 to 1.82, four trials, 1006 infants ‐ Analysis 1.7).
Primary outcomes for the child
At early childhood follow‐up, no significant differences were seen when treatment was given at a minimum interval of seven days or less between treatment groups for any of the primary outcomes:
total deaths up to early childhood follow‐up (RR 1.11, 95% CI 0.74 to 1.67, three trials, 2066 infants ‐ Analysis 1.65);
survival free of any disability (RR 1.02, 95% CI 0.92 to 1.12, one trial, 1060 infants ‐ Analysis 1.66);
survival free of any major disability (average RR 1.01, 95% CI 0.92 to 1.11, random‐effects, two trials, 1317 infants ‐ Analysis 1.67);
any disability at childhood follow‐up (RR 0.98, 95% CI 0.83 to 1.16, one trial, 999 infants ‐ Analysis 1.69);
major disability at childhood follow‐up (RR 1.08, 95% CI 0.31 to 3.76, two trials, 1256 infants ‐ Analysis 1.68);
composite serious outcome at childhood follow‐up (RR 0.98, 95% CI 0.84 to 1.13, one trial, 1060 infants ‐ Analysis 1.70), defined in this trial as death or any neurosensory disability.
Primary outcomes for the women
No statistically significant differences for the two primary outcomes of maternal infectious morbidity were seen for women treated with repeat dose(s) of prenatal corticosteroids at a minimum interval of seven days or less compared with women given placebo/no treatment:
chorioamnionitis (RR 1.23, 95% CI 0.95 to 1.59, four trials, 1971 women ‐ Analysis 1.15);
puerperal sepsis (average RR 1.01, 95% CI 0.58 to 1.74, I² = 32%, Tau² = 0.08 random‐effects not shown in forest plot, four trials, 1238 women ‐ Analysis 1.16.4).
Planned interval between corticosteroid treatments of a minimum interval of eight and less than 14 days
No trial used a minimum interval between eight and less than 14 days so no data have been reported on these subgroups to date.
Planned interval between corticosteroid treatments of a minimum interval of 14 days or more
Three trials used a minimum interval of 14 days or more between corticosteroid treatment(s) (Garite 2009; McEvoy 2010; Murphy 2008), with Garite 2009 and McEvoy 2010 only planning a single repeat course of treatment.
The gestational age at trial entry was similar between the trials: 25 to 32 weeks in Murphy 2008, 25 to less than 33 weeks in Garite 2009, and 26 to 33 weeks in McEvoy 2010. Women were eligible for inclusion 14 or more days after a pre‐trial course in Garite 2009 and McEvoy 2010 and between 14 and 21 days after a pre‐trial course in Murphy 2008.
In all three trials the planned treatment course was two doses of 12 mg/dose betamethasone (Garite 2009; McEvoy 2010; Murphy 2008) given singly for Garite 2009 and McEvoy 2010, and for Murphy 2008, given every 14 days if the woman remained at risk of preterm birth, until 33 weeks' gestation or birth.
Primary outcomes for the infant
Treatment with repeat dose(s) of corticosteroid treatments at a minimum interval of 14 days or more between treatments compared with placebo was associated with a reduction in RDS (RR 0.72, 95% CI 0.58 to 0.89, two trials, 668 infants ‐ Analysis 1.1).
No significant differences were seen between treatment groups for the other primary clinical outcomes:
severe lung disease (average RR 1.11, 95% CI 0.82 to 1.49, random‐effects, one trial, 2304 infants ‐ Analysis 1.2);
composite serious outcome (average RR 0.88, 95% CI 0.64 to 1.20, I² = 76%, Tau² = 0.04 random‐effects not shown in forest plot, two trials, 2862 infants ‐ Analysis 1.3.6);
fetal and neonatal mortality (RR 1.02, 95% CI 0.69 to 1.51, three trials, 2993 infants ‐ Analysis 1.8);
chronic lung disease (RR 1.49, 95% CI 0.96 to 2.32, two trials, 2855 infants ‐ Analysis 1.11);
intraventricular haemorrhage (RR 0.77, 95% CI 0.43 to 1.36, one trial, 546 infants ‐ Analysis 1.12).
Birthweight and birthweight Z scores were significantly reduced for infants exposed to repeat corticosteroid treatment at intervals of 14 days or more compared with infants exposed to placebo, although not the proportion of infants small‐ for‐ gestational age:
birthweight (MD ‐79.61 g, 95% CI ‐143.23 to ‐16.00, three trials, 2972 infants ‐ Analysis 1.4);
birthweight Z score (MD 0.0, 95% CI ‐0.34 to 0.34, one trial, 112 infants ‐ Analysis 1.5);
small‐for‐gestational age (RR 1.03, 95% CI 0.79 to 1.35, three trials, 2969 infants ‐ Analysis 1.7).
Primary outcomes for the child
Data from early childhood follow‐up were available for inclusion from one trial (Murphy 2008).
Limited data were available for the primary outcomes for the child for this subgroup. No statistically significant differences were seen for infants exposed to repeat dose(s) of corticosteroid treatments at a minimum interval of 14 days or more between treatments compared with placebo for any of the primary outcomes for the child reported:
total deaths up to early childhood follow‐up (RR 1.02, 95% CI 0.69 to 1.51, one trial, 2304 infants ‐ Analysis 1.65);
survival free of any disability to early childhood follow‐up (RR 1.00, 95% CI 0.97 to 1.03, one trial, 2104 infants ‐ Analysis 1.66);
composite serious outcome at childhood follow‐up (RR 1.01, 95% CI 0.81 to 1.25, one trial, 2104 infants ‐ Analysis 1.70), defined in Murphy 2008 as death or any neurologic impairment.
Primary outcomes for the women
No statistically significant differences were seen for the two outcomes of maternal infectious morbidity chorioamnionitis and puerperal sepsis for women treated with repeat dose(s) of prenatal corticosteroids at a minimum interval of 14 days or more between treatments compared with women given placebo.
(3.5) Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by the number of repeat courses actually given post‐randomisation (one, two, three, four or more repeat courses of prenatal corticosteroids)
One repeat course of corticosteroids planned or actually given
One single repeat course (variously defined) was planned for three trials (Garite 2009; McEvoy 2010; Peltoniemi 2007). Two trials used a single course consisting of two doses of 12 mg betamethasone (Garite 2009; McEvoy 2010) and for one trial the course of repeat corticosteroids was a single dose of 12 mg betamethasone (Peltoniemi 2007). Women were eligible for inclusion seven or more days after a pre‐trial course in Peltoniemi 2007, and 14 or more days after a pre‐trial course in Garite 2009 and McEvoy 2010. The eligible gestational age at trial entry was similar between these three trials: being 25 to less than 33 weeks in Garite 2009, 26 to 33 weeks McEvoy 2010, and up to 34 weeks in Peltoniemi 2007.
Primary outcomes for the infant
Treatment with one planned repeat course of corticosteroid treatment compared with placebo was associated with a reduction in a composite serious outcome (RR 0.75, 95% CI 0.60 to 0.93, one trial, 558 infants ‐ Analysis 1.3);
No significant differences were seen between treatment groups for the other primary clinical outcomes. There was significant heterogeneity for some of the outcomes (respiratory distress syndrome, and intraventricular haemorrhage):
RDS (average RR 0.83, 95% CI 0.61 to 1.15, I² = 72%, Tau² = 0.05 random‐effects not shown in forest plot, three trials, 994 infants ‐ Analysis 1.1.7);
severe lung disease (RR 1.23, 95% CI 0.94 to 1.60, one trial, 326 infants ‐ Analysis 1.2);
fetal and neonatal mortality (RR 1.41, 95% CI 0.64 to 3.08, three trials, 1015 infants ‐ Analysis 1.8);
chronic lung disease (RR 1.27, 95% CI 0.83 to 1.96, two trials, 877 infants ‐ Analysis 1.11);
intraventricular haemorrhage (average RR 0.99, 95% CI 0.64 to 1.54, I² = 31%, Tau² = 0.03 random‐effects not shown in forest plot, two trials, 872 infants ‐ Analysis 1.12).
No significant effects were seen on birthweight, birthweight Z scores or the proportion of infants small‐for‐gestational age for infants exposed to one planned repeat course of corticosteroid treatment compared with infants exposed to placebo:
birthweight (MD ‐57.20 g, 95% CI ‐132.99 to 18.59, three trials, 994 infants ‐ Analysis 1.4);
birthweight Z score (MD 0.00, 95% CI ‐0.34 to 0.34, one trial, 112 infants ‐ Analysis 1.5);
small‐for‐gestational age (RR 1.16, 95% CI 0.87 to 1.54, three trials, 991 infants ‐ Analysis 1.7).
Primary outcomes for the child
Data from early childhood follow‐up were available for inclusion from one trial (Peltoniemi 2007).
Limited data were available for all the primary outcomes for the child for this subgroup. No statistically significant differences were seen for infants exposed to one planned repeat course of corticosteroid compared with infants exposed to placebo for any of the primary outcomes for the child:
total deaths up to early childhood follow‐up (RR 2.31, 95% CI 0.82 to 6.50, one trial, 326 infants ‐ Analysis 1.65);
survival free of any major disability (RR 0.98, 95% CI 0.95 to 1.01, one trial, 257 infants ‐ Analysis 1.67);
major disability at childhood follow‐up (RR 3.53, 95% CI 0.37 to 33.52, one trial, 257 infants ‐ Analysis 1.68).
Primary outcomes for the women
No statistically significant differences were seen for women treated with one planned repeat course of corticosteroid compared with placebo for the two primary outcomes for the women of maternal infectious morbidity:
chorioamnionitis (RR 0.64, 95% CI 0.23 to 1.77, one trial, 437 women ‐ Analysis 1.15);
puerperal sepsis (RR 1.57, 95% CI 0.80 to 3.10, one trial, 249 women ‐ Analysis 1.16).
Four or more courses of repeat corticosteroids planned or actually given.
One trial reported anthropometric outcomes but not clinical outcomes for infants when four or more courses of repeat corticosteroids actually had been given (Wapner 2006). Women were eligible for inclusion in the trial from 23 to less than 32 weeks' gestation and between seven and 10 days after a pre‐trial course of corticosteroids. This trial used a weekly course of two doses of 12 mg betamethasone until birth or 33 weeks and six days whichever was earlier, limited to four study courses after the first 67 women.
Primary outcomes for the infant
Infants exposed to four or more repeat courses of betamethasone compared with infants not exposed to repeat corticosteroids had a reduction in mean birthweight (MD ‐161.00 g, 95% CI ‐290.52 to ‐31.48, one trial, 368 infants ‐ Analysis 1.4), lower birthweight multiples of the median (MD ‐0.04, 95% CI ‐0.07 to ‐0.01, one trial, 368 infants ‐ Analysis 1.6), and were more likely to be small‐for‐gestational age (RR 2.00, 95% CI 1.07 to 3.73, one trial, 368 infants ‐ Analysis 1.7).
No data were reported for the other primary outcomes.
Primary outcomes for the child
No data reported for this subgroup.
Primary outcomes for the women
No data reported for this subgroup.
(3.6) Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by the planned dose of betamethasone or equivalent given per treatment (12 mg or less of betamethasone or equivalent, greater than 12 mg to 24 mg or less of betamethasone or equivalent, greater than 24 mg or more of betamethasone or equivalent)
Planned treatment with repeat dose(s) of corticosteroid of 12 mg or less of betamethasone or equivalent compared with placebo
The planned dose of corticosteroid given per treatment was 12 mg or less of betamethasone for two trials (Crowther 2006; Peltoniemi 2007). Women were eligible for inclusion seven or more days after a pre‐trial course in both trials. The eligible gestational age at trial entry was less than 32 weeks in Crowther 2006 and up to 34 weeks in Peltoniemi 2007. A course of study treatment consisted of one 12 mg injection of betamethasone in both trials, given only once in Peltoniemi 2007 and in Crowther 2006 repeated weekly if the woman remained at risk of preterm birth up to 32 weeks' gestation.
Primary outcomes for the infant
Treatment with repeat dose(s) of corticosteroid of 12 mg or less of betamethasone compared with placebo was associated with a reduction in composite serious neonatal outcome in the one trial that reported data (RR 0.77, 95% CI 0.62 to 0.96, one trial, 1144 infants ‐ Analysis 1.3).
There was significant heterogeneity when comparing the effects of treatment on mean birthweight for these two trials. Using a random‐effects model there was no significant reduction in mean birthweight on average (average MD ‐50.36 g, 95% CI ‐136.30 to 35.58, random‐effects, two trials, 1470 infants ‐ Analysis 1.4). No difference between treatment groups was reported in one trial for the risk of being small‐for‐gestational age (RR 1.33, 95% CI 0.92 to 1.94, one trial, 326 infants ‐ Analysis 1.7). One trial reported a reduction in birthweight Z scores (MD ‐0.13, 95% CI ‐0.26 to ‐0.00, one trial, 1144 infants ‐ Analysis 1.5).
No significant differences were seen between treatment groups for the other primary clinical outcomes. There was significant heterogeneity for several of the outcomes (respiratory distress syndrome, severe lung disease, and fetal and neonatal mortality), where a random‐effects model was used:
RDS (average RR 0.91, 95% CI 0.68 to 1.24, I² = 81%, Tau² = 0.04 random‐effects not shown in forest plot, two trials, 1470 infants ‐ Analysis 1.1);
severe lung disease (average RR 0.84, 95% CI 0.40 to 1.78, I² = 93%, Tau² = 0.27 random‐effects, two trials, 1470 infants ‐ Analysis 1.2);
fetal and neonatal mortality (average RR 1.37, 95% CI 0.50 to 3.75, random‐effects not shown in forest plot, two trials, 1472 infants ‐ Analysis 1.8);
chronic lung disease (RR 0.97, 95% CI 0.74 to 1.27, two trials, 1470 infants ‐ Analysis 1.11);
intraventricular haemorrhage (RR 1.02, 95% CI 0.74 to 1.40, two trials, 1470 infants ‐ Analysis 1.12).
Primary outcomes for the child
At early childhood follow‐up no significant differences were seen for any of the primary outcomes when treatment with repeat dose(s) of corticosteroid of 12 mg or less of betamethasone was compared with placebo, although there was significant heterogeneity for several of the outcomes:
total deaths up to early childhood follow‐up (average RR 1.30, 95% CI 0.54 to 3.10, I² = 60%, Tau² = 0.25 random‐effects not shown in forest plot, two trials, 1472 infants ‐ Analysis 1.65);
survival free of any disability (RR 1.02, 95% CI 0.92 to 1.12, one trial, 1060 infants ‐ Analysis 1.66);
survival free of any major disability (average RR 1.01, 95% CI 0.92 to 1.11, I² = 88%, Tau² = 0.00 random‐effects, two trials, 1317 infants ‐ Analysis 1.67);
any disability at childhood follow‐up (RR 0.98, 95% CI 0.83 to 1.16, one trial, 999 infants ‐ Analysis 1.69);
major neurosensory disability at childhood follow‐up (average RR 1.08, 95% CI 0.31 to 3.76, I² = 42%, Tau² = 0.49 random‐effects, two trials, 1256 infants ‐ Analysis 1.68);
composite serious outcome at childhood follow‐up (RR 0.98, 95% CI 0.84 to 1.13, one trial, 1060 infants ‐ Analysis 1.70), defined in this trial as death or any neurosensory disability.
Primary outcomes for the women
No statistically significant differences were seen for women treated with repeat dose(s) of prenatal corticosteroids of 12 mg or less of betamethasone compared with women given placebo for the two primary outcomes of maternal infectious morbidity;
chorioamnionitis (RR 1.08, 95% CI 0.72 to 1.62, P = 0.70, one trial, 982 women ‐ Analysis 1.15);
puerperal sepsis ( RR 1.57, 95% CI 0.80 to 3.10, P = 0.19, one trial, 249 women ‐ Analysis 1.16.
Planned treatment with repeat dose(s) of corticosteroid of greater than 12 mg to 24 mg or less of betamethasone compared with placebo or no treatment
The planned dose of corticosteroid given per treatment was greater than 12 mg to 24 mg or less of betamethasone for eight trials (Aghajafari 2002; Garite 2009; Guinn 2002; Mazumder 2008; McEvoy 2002; McEvoy 2010; Murphy 2008; Wapner 2006).
Women were eligible for inclusion seven or more days after a pre‐trial course in four trials (Aghajafari 2002; Guinn 2002; Mazumder 2008; McEvoy 2002); between seven and 10 days after a pre‐trial course in Wapner 2006; between 14 to 21 days after a pre‐trial course in Murphy 2008; and 14 or more days after a pre‐trial course in Garite 2009 and McEvoy 2010.
The upper gestational age at trial entry varied between the trials: being up to 30 weeks in Aghajafari 2002, less than 32 weeks in Wapner 2006, to 32 weeks in Murphy 2008; less than 33 weeks in Garite 2009 ,Guinn 2002, Mazumder 2008 and McEvoy 2002 and McEvoy 2010.
In all eight trials a planned treatment course was two doses of 12 mg/dose betamethasone given only once for Garite 2009 and McEvoy 2010; repeated at weekly intervals up to 33 weeks in Aghajafari 2002, until before 34 weeks in Wapner 2006 and until 34 weeks in Guinn 2002, Mazumder 2008 and McEvoy 2002; and repeated every 14 days up to 33 weeks' gestation in Murphy 2008.
Primary outcomes for the infant
Treatment with repeat dose(s) of corticosteroid treatments of greater than 12 mg to 24 mg or less of betamethasone compared with no repeat treatment was associated with a reduction in:
RDS (RR 0.78, 95% CI 0.67 to 0.92, six trials, 1736 infants ‐ Analysis 1.1);
composite serious outcome (RR 0.87, 95% CI 0.76 to 0.99, six trials, 3950 infants ‐ Analysis 1.3).
These benefits were associated with a significantly lower mean birthweight (MD ‐90.12 g, 95% CI ‐141.85 to ‐38.39, seven trials, 4156 infants ‐ Analysis 1.4), although no differences between treatment groups were seen when adjustment was made for gestational age using birthweight Z scores (MD 0.00, 95% CI ‐0.34 to 0.34, one trial, 112 infants ‐ Analysis 1.5) or birthweight multiples of the median (MD 0.00, 95% CI ‐0.03 to 0.03, one trial, 590 infants ‐ Analysis 1.6) or in the number of infants small‐for‐gestational age (RR1.13, 95% CI 0.90 to 1.42, six trials, 3649 infants ‐ Analysis 1.7).
No significant differences were seen between treatment groups for the other primary outcomes:
severe lung disease (average RR 0.78, 95% CI 0.50 to 1.20, I² = 56%, Tau² = 0.09 random‐effects, four trials, 3356 infants ‐ Analysis 1.2);
fetal and neonatal mortality (RR 0.85, 95% CI 0.61 to 1.19, seven trials, 4082 infants ‐ Analysis 1.8);
chronic lung disease (RR 1.18, 95% CI 0.88 to 1.59 six trials, 3923 infants ‐ Analysis 1.11);
intraventricular haemorrhage (RR 0.88, 95% CI 0.64 to 1.21, four trials, 1595 infants ‐ Analysis 1.12).
Primary outcomes for the child
Data from early childhood follow‐up were available for inclusion from two trials (Murphy 2008; Wapner 2006).
No significant differences between treatment groups when treatment was given of greater than 12 mg to 24 mg or less of betamethasone for total deaths (RR 1.04, 95% CI 0.72 to 1.50, two trials, 2898 infants ‐ Analysis 1.65); survival free of any disability to early childhood follow‐up (RR 1.00, 95% CI 0.97 to 1.03, one trial, 2104 infants ‐ Analysis 1.66); and composite serious outcome at childhood follow‐up (RR 1.01, 95% CI 0.81 to 1.25, one trial, 2104 infants ‐ Analysis 1.70), defined in Murphy 2008 as death or any neurologic impairment.
Primary outcomes for the women
No statistically significant differences were seen for women treated with repeat dose(s) of prenatal corticosteroids of greater than 12 mg to 24 mg or less of betamethasone compared with women not given repeat corticosteroids for the two outcomes of maternal infectious morbidity of chorioamnionitis and puerperal sepsis.
Planned treatment with repeat dose(s) of corticosteroid greater than 24 mg or more of betamethasone or equivalent compared with placebo/no treatment
No data have been reported on this subgroup.
(3.7). Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by the planned repeat drug exposure per week (12 mg/week or less of betamethasone or equivalent, greater than 12 mg/week to 24 mg/week of betamethasone or equivalent, greater than 24 mg/week or more of betamethasone or equivalent). Trials only included if able to give multiple trial treatments.
Planned treatment with repeat dose(s) of corticosteroid of 12 mg/week or less of betamethasone or equivalent compared with placebo/no treatment
The planned repeat drug exposure per week was 12 mg/week or less of betamethasone or equivalent for two trials (Crowther 2006; Murphy 2008). Women were eligible for inclusion seven or more days after a pre‐trial course in Crowther 2006 and between 14 to 21 days after a pre‐trial course in Murphy 2008. The eligible gestational age at trial entry was similar being less than 32 weeks in Crowther 2006 and 25 to 32 weeks in Murphy 2008. A course of study treatment consisted of one 12 mg injection of betamethasone repeated weekly if still at risk up to less than 32 weeks' gestation in Crowther 2006 and two 12 mg betamethasone injections repeated every 14 days up to 33 weeks' gestation in Murphy 2008.
Primary outcomes for the infant
Treatment with repeat dose(s) of corticosteroid where the planned repeat drug exposure per week was 12 mg/week or less of betamethasone compared with placebo was associated with a reduction the risk of RDS, in the one trial that reported data:
RDS (RR 0.79, 95% CI 0.68 to 0.92, one trial, 1144 infants ‐ Analysis 1.1).
Significant heterogeneity was seen for all the other primary outcomes except fetal and neonatal mortality where data were reported from both trials. No significant differences were seen between treatment groups for the other primary outcomes:
severe lung disease (average RR 0.80, 95% CI 0.42 to 1.51, I² = 90%, Tau² = 0.19 random‐effects, two trials, 3448 infants ‐ Analysis 1.2);
composite serious outcome (average RR 0.89, 95% CI 0.67 to 1.18, I² = 70%, Tau² = 0.03 random‐effects not shown in forest plot two trials, 3448 infants ‐ Analysis 1.3);
fetal and neonatal mortality (RR 1.01, 95% CI 0.73 to 1.40, two trials, 3450 infants ‐ Analysis 1.8);
chronic lung disease (average RR 1.14, 95% CI 0.67 to 1.95, I² = 52%, Tau² = 0.09 random‐effects not shown in forest plot, two trials, 3448 infants ‐ Analysis 1.11);
intraventricular haemorrhage (RR 0.89, 95% CI 0.57 to 1.38, one trial, 1144 infants ‐ Analysis 1.12);
birthweight (average MD ‐57.89 g, 95% CI ‐159.49 to 43.71, I² = 49%, Tau² = 2628.81 random‐effects not shown in forest plot, two trials, 3448 infants ‐ Analysis 1.4);
birthweight Z score (MD ‐0.13, 95% CI ‐0.26 to 0.00, one trial, 1144 infants ‐ Analysis 1.5);
small‐for‐gestational age at birth (RR 1.06,95% CI 0.75 to 1.50, one trial, 2304 infants ‐ Analysis 1.7).
Primary outcomes for the child
At early childhood follow‐up two trials reported data (Crowther 2006; Murphy 2008). No significant differences were seen between treatment groups for any of the primary outcomes when the planned repeat drug exposure per week was 12 mg/week or less of betamethasone compared with placebo:
total deaths up to early childhood follow‐up (RR 0.98, 95% CI 0.72 to 1.33, two trials, 3450 ‐ Analysis 1.65);
survival free of any disability (RR 1.00, 95% CI 0.97 to 1.04, two trials, 3164 infants ‐ Analysis 1.66);
survival free of any major disability (average RR 1.04, 95% CI 0.99 to 1.10, one trial, 1060 infants ‐ Analysis 1.67);
any disability at childhood follow‐up (RR 0.98, 95% CI 0.83 to 1.16, one trial, 999 infants ‐ Analysis 1.69);
major disability at childhood follow‐up (RR 0.77, 95% CI 0.55 to 1.08, one trial, 999 infants ‐ Analysis 1.68);
composite serious outcome at childhood follow‐up (RR 0.99, 95% CI 0.87 to 1.12, two trials, 3164 infants ‐ Analysis 1.70), defined in Crowther 2006 as death or any neurosensory disability; and defined in Murphy 2008 as death or any neurologic impairment.
Primary outcomes for the women
No statistically significant differences were seen for women treated with repeat dose(s) of prenatal corticosteroids for the two outcomes of maternal infectious morbidity when the planned repeat drug exposure per week was 12 mg/week or less of betamethasone compared with placebo:
chorioamnionitis (RR 1.08, 95% CI 0.77 to 1.51, two trials, 2835 women ‐ Analysis 1.15);
puerperal sepsis (RR 1.34, 95% CI 0.80 to 2.22, one trial, 1853 women ‐ Analysis 1.16).
Planned treatment with repeat dose(s) of corticosteroid of greater than 12 mg/week to 24 mg/week or less of betamethasone or equivalent compared with placebo/no treatment
The planned repeat drug exposure per week was greater than 12 mg/week to 24 mg/week or less of betamethasone or equivalent for five trials (Aghajafari 2002; Guinn 2002; Mazumder 2008; McEvoy 2002; Wapner 2006).
Women were eligible for inclusion seven or more days after a pre‐trial course in four trials (Aghajafari 2002; Guinn 2002; Mazumder 2008; McEvoy 2002) and between seven and 10 days after a pre‐trial course in Wapner 2006.
The upper gestational age at trial entry varied between the trials: being up to 30 weeks in Aghajafari 2002, less than 32 weeks in Wapner 2006, and less than 33 weeks in Guinn 2002, Mazumder 2008 and McEvoy 2002.
In all five trials a planned treatment course was two doses of 12 mg/dose betamethasone repeated at weekly intervals up to 33 weeks in Aghajafari 2002, until less than 34 weeks in Wapner 2006 and until 34 weeks in Guinn 2002, Mazumder 2008 and McEvoy 2002.
Primary outcomes for the infant
Treatment with repeat dose(s) of corticosteroid, where the planned repeat drug exposure per week was greater than 12 mg/week to 24 mg/week or less of betamethasone, compared with placebo was associated with a reduction in the risk of severe lung disease, composite serious outcome and fetal and neonatal mortality:
severe lung disease (average RR 0.62, 95% CI 0.44 to 0.87, random‐effects, three trials, 1052 infants ‐ Analysis 1.2);
composite serious outcome (average RR 0.77, 95% CI 0.60 to 0.99, random‐effects, four trials, 1088 infants ‐ Analysis 1.3);
fetal and neonatal mortality (RR 0.51, 95% CI 0.26 to 1.00, four trials, 1089 infants ‐ Analysis 1.8).
No significant heterogeneity was seen for the other primary outcomes reported. No significant differences were seen between treatment groups for the other primary clinical outcomes. Although mean birthweight was lower in exposed infants compared with unexposed infants there was no difference in the risk of being small‐for‐gestational age or birthweight multiples of the median:
RDS (RR 0.86, 95% CI 0.68 to 1.10, four trials, 1068 infants ‐ Analysis 1.1);
chronic lung disease (RR 0.97, 95% CI 0.65 to 1.44, four trials, 1068 infants ‐ Analysis 1.11);
intraventricular haemorrhage (RR 0.95, 95% CI 0.64 to 1.40, three trials, 1017 infants ‐ Analysis 1.12);
birthweight (MD ‐110.62 g, 95% CI ‐199.49 to ‐21.74, four trials, 1184 infants ‐ Analysis 1.4);
small‐for‐gestational age at birth (RR 1.29,95% CI 0.88 to 1.90, four trials, 792 infants ‐ Analysis 1.7);
birthweight multiples of the median (MD 0.00, 95% CI ‐0.03 to 0.03, one trial, 590 infants ‐ Analysis 1.6).
Primary outcomes for the child
At early childhood follow‐up one trial reported data (Wapner 2006). No significant differences were seen between treatment groups for the only primary outcome with data when the planned repeat drug exposure per week was greater than 12 mg/week to 24 mg/week or less of betamethasone compared with placebo:
total deaths up to early childhood follow‐up (RR 1.15, 95% CI 0.39 to 3.38, one trial, 594 infants ‐ Analysis 1.65).
Primary outcomes for the women
No statistically significant differences were seen for women treated with repeat dose(s) of prenatal corticosteroids for the two outcomes of maternal infectious morbidity when the planned repeat drug exposure per week was greater than 12 mg/week to 24 mg/week or less of betamethasone compared with placebo:
chorioamnionitis (RR 1.35, 95% CI 0.96 to 1.88, three trials, 989 women ‐ Analysis 1.15);
puerperal sepsis (RR 0.76, 95% CI 0.42 to 1.36, three trials, 989 women ‐ Analysis 1.16).
Planned treatment with repeat dose(s) of corticosteroid greater than 24 mg/week or more of betamethasone or equivalent compared with placebo or no treatment
No data have been reported on this subgroup.
(3.8) Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by the method of administration (intramuscular, intravenous, oral)
No data have been reported on these subgroups to date. All the included trials used intramuscular injection as the method of administration of the treatment.
(3.9) Repeat dose(s) of prenatal corticosteroids versus placebo/no treatment by the gestational age at entry to the trial
No data have been reported on these subgroups to date.
Discussion
Summary of main results
A total of 4733 women (5700 babies) were randomised into trials comparing women given repeat dose(s) of prenatal corticosteroids seven or more days after an initial course with women not receiving repeat corticosteroids after an initial course of prenatal corticosteroids seven or more days earlier. The use of repeat dose(s) of corticosteroid for women at risk of preterm birth reduced the risk of neonatal respiratory distress syndrome (RDS) by 17% (number needed to treat to benefit (NNTB) 17 (95% CI 11 to 32) women; 60 fewer babies with RDS per 1000 women treated) with a similar reduction, 16%, in the risk of serious infant outcome (NNTB 30 (95% CI 19 to 79) women; 33 fewer babies with a serious health outcome per 1000 women treated). These are clinically important neonatal benefits and there is clear evidence that repeat doses leads to a reduction in the use of mechanical ventilation, less need for oxygen supplementation, less inotropic support treatment, less use of surfactant and a reduced risk of a patent ductus arteriosus. No substantive effect was seen on fetal or neonatal mortality or for total mortality at early childhood follow‐up.
The reduction in risk of RDS and/or composite serious neonatal outcomes was consistent across most of the planned subgroups providing data (timing of repeat corticosteroid treatment seven days or less, or 14 days or more; planned number of doses only one; dose per treatment 12 mg or less, or more than 12 mg to 24 mg or less betamethasone; or dose per week).
In contrast to these clinical benefits, at birth, infants exposed to repeat corticosteroids compared with infants not exposed to repeat corticosteroids had a reduction in some measures of growth (mean weight, mean length and mean head circumference, head circumference Z scores, length multiples of the mean), although not in Z scores for weight or length, or multiples of the median for weight, or in the risk of being small‐for‐gestational age. By the time of discharge from hospital after birth and at later childhood follow‐up, no differences were seen between children exposed to repeat prenatal corticosteroids and those not exposed for weight, head circumference and height. Measures of body size are dependant on gestational age at birth and without adjustment for gestational age, as in Z scores or multiples of the mean/median, it is not possible to fully assess the effects repeat prenatal corticosteroid treatment may have on fetal growth.
In the update of this review in 2011, data from early childhood follow‐up were added from four trials. These data show no substantive differences in children exposed to repeat prenatal corticosteroids compared with unexposed children for survival or neurosensory disability at 18 months to two years' corrected age, childhood behaviour, or general health. Further information from one existing trial on outcome in early childhood is awaited (Mazumder 2008). The evidence available provides some reassurance about the health of the infants into early childhood when repeat prenatal corticosteroids are used for women at risk of preterm birth. However, there are as yet no information on the health, neurodevelopmental, growth, cardiovascular and metabolic status in later childhood, adulthood to assess the long‐term significance of the observed neonatal clinical benefits or the effects on anthropometric assessments observed at birth. Such information will be needed to assess overall benefits and risks of using repeat prenatal corticosteroids for women at risk of preterm birth. Several trials have conducted later assessment of their children (Crowther 2006 ‐ early school‐age follow‐up at six to eight years' corrected age; and Murphy 2008 ‐ childhood follow‐up at five years of age) and publications arising will be included in the next update of this review.
For the women, there was no increase in infectious morbidity of chorioamnionitis or puerperal sepsis, and the likelihood of a caesarean birth was unchanged providing some reassurance about the safety of repeat prenatal corticosteroid treatment for the mothers. There was significant heterogeneity for the trials reporting on side effects with no differences overall for women reporting any side effects. Although few women reported insomnia (2.3% in the repeat prenatal corticosteroid group, 0.8% in the non repeat corticosteroid group), this was more likely for women treated with repeat prenatal corticosteroids. Reduction in bruising at the injection site was reported in one trial for women given repeat corticosteroid injections compared with placebo.
Overall completeness and applicability of evidence
Women in these trials have been recruited in a range of countries and settings. The trials were all conducted at either tertiary or provincial hospitals, but apart from one trial, they were conducted in high‐income countries. The results are considered applicable to hospital settings worldwide. Betamethasone has been the only corticosteroid evaluated in randomised trials of repeat prenatal corticosteroids prior to preterm birth. Dexamethasone is often used because it is less expensive and often more easily available, particularly in low‐ to middle‐income countries. It is uncertain whether the effects seen for betamethasone would be the same for dexamethasone. Appropriate trials are needed.
There has been no cost‐effectiveness analysis of the use of repeat prenatal corticosteroids for women at risk of preterm birth seven or more days after initial corticosteroid treatment.
Quality of the evidence
The quality of the studies included in this review ranged from moderate to excellent. Of the 10 included trials, all are recent publications of moderate to high methodological quality. All had adequate allocation concealment, all except one used a placebo, and losses to follow‐up to the time of primary hospital discharge after birth were nil or minimal. At early childhood follow‐up losses to follow‐up in the three trials that have reported data ranged between 7.5% and 21.2%.
Potential biases in the review process
The evidence for this review is derived from studies identified from a detailed, systematic search process without language restriction. It is possible (but unlikely) that additional trials comparing the use of repeat corticosteroids prior to preterm birth versus placebo or no treatment have been published but not identified. It is also possible that there are other studies, additional to those of which we are aware, that have been conducted but are not yet published. Should any such studies be identified, we will include them in future updates of this review.
Two of the review authors (CAC, JEH) were investigators for the ACTORDS trial (Crowther 2006). Data extraction for this trial was performed by the other review authors (PM and CM).
Agreements and disagreements with other studies or reviews
Since the update of this review in 2011, further follow‐up data from the Crowther 2006 (two publications), McEvoy 2010 (one publication) and Murphy 2008 (two publications) have been published adding to the evidence base assessing whether repeat dose(s) of prenatal corticosteroids improve fetal lung maturation, and thereby reduce infant morbidity and mortality. This systematic review presents strong evidence that repeat dose(s) of prenatal corticosteroid for women at risk of preterm birth provides significant clinical benefits in the short term for the infant and is not associated with any evidence of any effect on benefit or harm for either the woman or the child at early childhood follow‐up.
Authors' conclusions
Implications for practice.
The short‐term benefits seen for babies support the use of repeat dose(s) of prenatal corticosteroids for women who have received an initial course of prenatal corticosteroids seven of more days previously and who remain at risk of preterm birth.
Repeat dose(s) of prenatal corticosteroids given to women at risk of preterm birth, compared to no repeat treatment, reduce the occurrence of respiratory distress syndrome (RDS) by 17% and the risk of serious health problems in the first few weeks of life for the infant by 16%. For one baby to benefit by not developing RDS, 17 women (95% CI 11 to 32) would need to be treated with repeat prenatal corticosteroids. The number needed to treat to benefit (NNTB) for serious neonatal outcome is 30 women (95% CI 19 to 79). At birth the neonatal benefits are associated with a reduction in some measures of mean body size (mean weight, head circumference and length), although not for anthropometric assessments that adjusted for gestational age. These differences in body size measurements are no longer seen by hospital discharge in the two trials that report data. The limited evidence available from early childhood reassuringly shows no significant harm, although no benefit. No differences are seen in mortality, neurodisability or childhood weight, head circumference or height. There is insufficient evidence on the longer‐term benefits and risks.
Corticosteroids should be available in all hospitals providing care for women at risk of preterm birth (Roberts 2006). Repeat prenatal corticosteroids should be considered for women who have received a course of prenatal corticosteroids seven or more days previously, and who remain at risk of preterm birth before 34 weeks' gestation. Women eligible for repeat prenatal corticosteroid treatment should be informed of the known benefits and risks and counselled about the available but limited information about childhood health outcomes. Until clearer information is available on optimal dose of treatment, and frequency of administration it would seem prudent to use a treatment regimen of one of the trials reporting neonatal benefit.
Implications for research.
Further information is awaited from one existing trial on outcome in early childhood (Mazumder 2008). There are still no data published for later childhood or adulthood on health, neurodevelopmental, growth, cardiovascular and metabolic status. Such information is needed to assess overall benefits and risks of using repeat prenatal corticosteroids for women at risk of preterm birth. Several trials have completed later assessment of their children (Crowther 2006 ‐ early school‐age follow‐up at six to eight years' corrected age; and Murphy 2008 ‐ childhood follow‐up at five years of age). Data from these later assessments will be included in the next planned update of this review.
We are aware of three other trials of repeat prenatal corticosteroids for women at risk of preterm birth. One trial from the UK stopped recruitment and publication is awaited (TEAMS 1999); one trial in women with preterm prelabour rupture of the membranes has only been published in abstract form with data not suitable for inclusion in the meta‐analysis (Sobhrabvand 2001); and one trial from Iran is awaiting classification to establish its eligibility for inclusion. We are awaiting a response from the authors (Atarod 2014).
Individual patient data meta‐analysis using the existing trials is ongoing and may clarify how to maximise benefit and minimise harm using factors relating to the women (reasons the woman was considered at risk of preterm birth), her pregnancy (number of babies in‐utero, gestational age repeat prenatal corticosteroid treatment was given, time prior to birth repeat prenatal corticosteroid treatment was given), and drug frequency and dosage (Crowther 2012).
Further trials are difficult to justify until the results of the individual patient data meta‐analysis are available. Any further trials of repeat dose(s) of prenatal corticosteroids for women who remain at risk of preterm birth after a course of corticosteroids should be of high quality; be large enough to assess mortality and serious morbidity; consider comparing different corticosteroid preparations (all trials to date have used betamethasone for repeat treatment); provide further evaluation of the times between repeat courses; evaluate the optimal amount of corticosteroid given at each course; provide data on all relevant maternal and infant outcomes; ensure assessment of neurodevelopmental status of the child at follow‐up and organise assessment of longer‐term outcomes including behaviour, educational achievement, cardiovascular status, bone density, hypothalamic‐pituitary‐adrenal axis function, glucose intolerance and lung function.
Cost‐effectiveness analysis of the use of repeat corticosteroids should be conducted.
Feedback
Murphy, 5 July 2012
Summary
As the authors of the Multiple courses of Antenatal Corticosteroids for preterm birth Study (MACS), which is one of the trials included in this review, we have a few comments that need to be addressed. 1. In MACS, we collected data on ‘severe respiratory distress syndrome (RDS)’ rather than ‘RDS’. In this review, data from MACS for ‘severe RDS’ are included in the meta‐analysis for ‘severe lung disease’ but not in the meta‐analysis for ‘RDS’. As a result, the data from MACS are currently not able to contribute to answering the question as to the effect of multiple courses of antenatal corticosteroids on RDS. Because RDS is defined differently in the various trials we believe it is reasonable that the outcome RDS should include both mild and severe disease. Subgroup analyses could then look at mild RDS and severe RDS separately. Given that MACS is the largest trial included in the review, it is particularly important that its results should contribute to answering the question about the effect of repeated doses of antenatal corticosteroids on RDS. 2. The review authors have chosen to include data for ‘severe RDS’ from the various trials in an outcome of ‘severe lung disease’. We find the labelling of severe RDS as ‘severe lung disease’ ambiguous. Severe lung disease could theoretically include meconium aspiration, pneumothorax, group B streptococcal pneumonia and other conditions that are not thought to benefit from antenatal corticosteroids. 3. In the Results text section ‘secondary outcomes for the child’, the review states that “Data were not able to be included in the meta‐analysis for Murphy 2008 for anthropometric assessments expressed as means, as standard deviations were not available in the published report.” In our report of the 18‐24 month follow‐up for MACS (Asztalos 20081, not Murphy 2008 as stated in the review) the data are expressed as means, mean differences and confidence intervals. The within‐group standard deviations are actually not needed for the meta‐analysis, as all that is required is the mean difference (which we reported) and its variance. The variance can be derived by squaring the width of the CI divided by 2*1.96. That is: Variance of Mean Difference = [(UL – LL)/(2*1.96)]2. In any case, an estimate of the within‐group standard deviations can be calculated from the confidence limits and the group sample sizes.
The table below shows the summary statistic for the mean difference in birth weight at early childhood follow‐up with and without the MACS data. When MACS data are included, the difference in birth weight between the two groups is statistically significant.. Table Summary statistic for the mean difference in birth weight at early childhood follow‐up.
| Mean Difference in Weight | Variance of Mean Difference | Lower Limit | Upper Limit | P value | |
| Summary without MACS | ‐0.0297 | 0.0086 | ‐0.2114 | 0.1520 | 0.7488 |
| Summary with MACS | ‐0.2003 | 0.0046 | ‐0.3327 | ‐0.0678 | 0.0030 |
The long term impact of weighing less in early childhood is not known. Nevertheless, that repeated doses of antenatal corticosteroids have adverse effects on growth is worrisome in that it signifies the potential for repeated doses to cause harm.
Follow‐up studies have thus far been reassuring, demonstrating no difference in death or neurologic impairment in early childhood. However, we should not be falsely reassured by these studies. The long‐term follow‐up studies have limited power to rule out the effect of repeated doses of antenatal corticosteroids on infrequent adverse events. Thus, finding no difference in long‐term outcome does not necessarily prove safety. We are reminded of the RCTs of postnatal corticosteroid treatment which initially demonstrated short term benefits and then over time, with the completion of long‐term studies, demonstrated the potential for harm.2
4. We believe that the findings of short term neonatal benefits from repeated courses of antenatal corticosteroids are important. However, given the adverse effect of antenatal corticosteroids on birth weight and weight at early childhood follow‐up, and the uncertain effect on long term outcomes, we believe that the conclusions and recommendations for clinical practice in this review should be more cautious than they currently are. We would suggest the review authors consider a statement such as “Although the short term neonatal benefits of repeated courses of antenatal corticosteroids support their use, long‐term benefits have not been demonstrated and long‐term adverse effects have not been ruled out. The adverse effect of repeated doses of antenatal corticosteroids on birth weight and weight at early childhood follow‐up is a concern. Caution should therefore be exercised to ensure that only those women who are at particularly high risk of very early preterm birth are offered treatment with repeated courses of antenatal corticosteroids.” References 1. Asztalos EV, Murphy KE, Hannah ME, Willan AR, Matthews SG, Ohlsson A, et al. Multiple courses of antenatal corticosteroids for preterm birth study: 2‐year outcomes. Pediatrics 2010;126:e1045‐e1055.
2. Halliday HL, Ehrenkranz RA, Doyle LW. Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochran Database of Sys Rev 2010, Issue 5.
Kellie E. Murphy, Andrew R Willan, Mary E. Hannah, Elizabeth Asztalos, Arne Ohlsson, Edmond N Kelly, Stephen G Matthews, Saroj Saigal, Susan Ross, Marie‐France Delisle, Kofi Amankwah, Patricia Guselle, Amiram Gafni, Shoo K Lee, B Anthony Armson, for the MACS Collaborative Group. July 2012
[Feedback received from Kellie E Murphy, 5 July 2012]
Reply
Many thanks to the authors of the Multiple courses of Antenatal Corticosteroids for preterm birth Study (MACS).
1. The MACS trial reported on the outcome of 'severe respiratory distress syndrome'. The authors query why the data from MACS were only included in the outcome for 'severe lung disease' rather than RDS (any).
We do not feel that it would be appropriate to combine data from trials reporting respiratory distress syndrome (mild or severe) with severe RDS. This is likely to result in significant heterogeneity and therefore we chose to report data for RDS and severe lung disease as separate outcomes. If the authors of the MACS trial can provide data for RDS (any) we would certainly be willing to add it to the appropriate analysis.
2. The authors of the MACS trial query the labelling of the outcome 'severe lung disease'.
This outcome was selected by the review authors and is defined in the review protocol.
3. The authors of the MACS trial query why their data for anthropometric assessments at 18 to 24 months were not included in the meta‐analysis.
Data were not included as SD's were not available in the trial report. We acknowledge that the author of this report was Asztalos 2008, however Cochrane methodology requires that it is the primary trial that is cited in the text. The reference to Asztalos can be found in the reference section of this review. In order to enter data in to the statistical programme we require mean values for each group with the associated standard deviation or standard error. If the authors of the MACS trial can provide us with the mean and standard deviations for the treatment and the control groups with the numbers of children in each group we will certainly be willing to add these data to the appropriate analyses.
4. The authors of the MACS trial query the conclusions of the review.
The review authors consider the conclusions and both the clinical and research recommendations to be appropriate summaries of the available evidence.
Contributors
Caroline Crowther
What's new
| Date | Event | Description |
|---|---|---|
| 5 January 2015 | New citation required but conclusions have not changed | There are no changes to the conclusions of this review. |
| 5 January 2015 | New search has been performed | Search updated and two new trials excluded (Bontis 2011; Romejko‐Wolniewicz 2013). |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 24 July 2012 | Feedback has been incorporated | Comments from Kellie Murphy added ‐ see Feedback 1. |
| 18 July 2011 | Amended | Corrected reference error ‐ Ashworth 2006 amended to read Ashwood 2006. |
| 18 April 2011 | New citation required and conclusions have changed | Conclusions are now stronger; early benefit now without evidence of longer term harm. Two additional authors joined the review team (C McKinlay and P Middleton). |
| 31 March 2011 | New search has been performed | Search updated in March 2011 and data from five new trials added (Garite 2009; Mazumder 2008; McEvoy 2010; Murphy 2008; Peltoniemi 2007). Published longer term follow‐up in early childhood now included for four trials (Crowther 2006; Murphy 2008; Peltoniemi 2007; Wapner 2006) and information on follow up for one further trial added (Mazumder 2008). Acknowledgement of unpublished information provided on caesarean section by Emeritus Professor Thomas Garite for the Garite 2009 trial. |
| 20 September 2008 | Amended | Converted to new review format. |
| 11 May 2007 | New search has been performed | Search updated in November 2006 and data from two trials now published added (Crowther 2006; Wapner 2006). We updated the search just before submission for publication and identified the published report for the previously listed Peltoniemi ongoing study. We have added it to the ' Studies awaiting assessment' section and will consider it for inclusion in the next update (Peltoniemi 2007). |
Acknowledgements
We acknowledge the support from the Cochrane Pregnancy and Childbirth Review editorial team in Liverpool, the Australia and New Zealand Satellite of Cochrane Pregnancy and Childbirth (funded by the NHMRC) and the Liggins Institute, University of Auckland, New Zealand.
We especially acknowledge the support of this update from Dr Julie Brown who was the Project Manager for the Antenatal corticosteroids given to women prior to birth to improve fetal, infant, child and adult health: New Zealand and Australian Clinical Practice Guideline 2015.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser (2011).
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Repeat doses of corticosteroids versus single course.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Respiratory distress syndrome | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 In all babies | 8 | 3206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.75, 0.91] |
| 1.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.24] |
| 1.3 In babies exposed to repeat corticosteroids as betamethasone | 8 | 3206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.75, 0.91] |
| 1.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 6 | 2538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.96] |
| 1.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 2 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.58, 0.89] |
| 1.7 In babies exposed to one repeat course of prenatal corticosteroids | 3 | 994 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.73, 0.99] |
| 1.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 2 | 1470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.76, 0.98] |
| 1.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 6 | 1736 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.67, 0.92] |
| 1.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 1 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.92] |
| 1.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 4 | 1068 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.10] |
| 1.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Severe lung disease | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 In all babies | 6 | 4826 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.56, 1.14] |
| 2.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 In babies exposed to repeat corticosteroids as betamethasone | 6 | 4826 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.56, 1.14] |
| 2.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 5 | 2522 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.47, 1.12] |
| 2.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 1 | 2304 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.82, 1.49] |
| 2.7 In babies exposed to one repeat course of prenatal corticosteroids | 1 | 326 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.94, 1.60] |
| 2.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 2 | 1470 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.40, 1.78] |
| 2.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 4 | 3356 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.50, 1.20] |
| 2.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 2 | 3448 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.42, 1.51] |
| 2.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 3 | 1052 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.44, 0.87] |
| 2.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Composite serious outcome (variously defined) | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 In all babies | 7 | 5094 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.75, 0.94] |
| 3.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 In babies exposed to repeat corticosteroids as betamethasone | 7 | 5094 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.75, 0.94] |
| 3.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 5 | 2232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.66, 0.91] |
| 3.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 2 | 2862 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 3.7 In babies exposed to one repeat course of prenatal corticosteroids | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.93] |
| 3.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 1 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.62, 0.96] |
| 3.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 6 | 3950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 0.99] |
| 3.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 2 | 3448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 3.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 4 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.60, 1.00] |
| 3.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mean birthweight (g) | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 In all babies | 9 | 5626 | Mean Difference (IV, Fixed, 95% CI) | ‐75.79 [‐117.63, ‐33.96] |
| 4.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 In babies exposed to repeat corticosteroids as betamethasone | 9 | 5626 | Mean Difference (IV, Fixed, 95% CI) | ‐75.79 [‐117.63, ‐33.96] |
| 4.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 5 | 2328 | Mean Difference (IV, Fixed, 95% CI) | ‐63.68 [‐128.59, 1.24] |
| 4.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 3 | 2972 | Mean Difference (IV, Fixed, 95% CI) | ‐79.61 [‐143.23, ‐14.00] |
| 4.7 In babies exposed to one repeat course of prenatal corticosteroids | 3 | 994 | Mean Difference (IV, Fixed, 95% CI) | ‐57.20 [‐132.99, 18.59] |
| 4.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐161.0 [‐290.52, ‐31.48] |
| 4.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 2 | 1470 | Mean Difference (IV, Fixed, 95% CI) | ‐48.72 [‐119.84, 22.40] |
| 4.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 7 | 4156 | Mean Difference (IV, Fixed, 95% CI) | ‐90.12 [‐141.85, ‐38.39] |
| 4.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 2 | 3448 | Mean Difference (IV, Fixed, 95% CI) | ‐52.00 [‐126.19, 18.19] |
| 4.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 4 | 1184 | Mean Difference (IV, Fixed, 95% CI) | ‐110.62 [‐199.49, ‐21.74] |
| 4.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Birthweight Z scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 In all babies | 2 | 1256 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.23, 0.00] |
| 5.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 In babies exposed to repeat corticosteroids as betamethasone | 2 | 1256 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.23, 0.00] |
| 5.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 1 | 1144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.26, ‐0.00] |
| 5.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.34, 0.34] |
| 5.7 In babies exposed to one repeat course of prenatal corticosteroids | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.34, 0.34] |
| 5.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 1 | 1144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.26, ‐0.00] |
| 5.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.34, 0.34] |
| 5.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 1 | 1144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.26, ‐0.00] |
| 5.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid equivalent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Birthweight multiples of the median | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 In all babies | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 6.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 In babies exposed to repeat corticosteroids as betamethasone | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 6.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 6.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.7 In babies exposed to one repeat course of prenatal corticosteroids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 6.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 6.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.03, 0.03] |
| 6.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Small‐for‐gestational age at birth | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 In all babies | 7 | 3975 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
| 7.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 In babies exposed to repeat corticosteroids as betamethasone | 7 | 3975 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
| 7.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 4 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.04, 1.82] |
| 7.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 3 | 2969 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 7.7 In babies exposed to one repeat course of prenatal corticosteroids | 3 | 991 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.54] |
| 7.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.07, 3.73] |
| 7.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.92, 1.94] |
| 7.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 6 | 3649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.90, 1.42] |
| 7.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.75, 1.50] |
| 7.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 4 | 792 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.88, 1.90] |
| 7.16 In babies where planned repeat drug exposure was >24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Fetal and neonatal mortality | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 In all babies | 9 | 5554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.23] |
| 8.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.13, 1.88] |
| 8.3 In babies exposed to repeat corticosteroids as betamethasone | 9 | 5554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.23] |
| 8.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 6 | 2871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.67, 1.37] |
| 8.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 3 | 2993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.51] |
| 8.7 In babies exposed to one repeat course of prenatal corticosteroids | 3 | 1015 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.64, 3.08] |
| 8.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 2 | 1472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.70, 1.79] |
| 8.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 7 | 4082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.19] |
| 8.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 2 | 3450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
| 8.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 4 | 1089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.26, 1.00] |
| 8.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Fetal death | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 In all babies | 7 | 2755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.24, 2.84] |
| 9.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 In babies exposed to repeat corticosteroids as betamethasone | 7 | 2755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.24, 2.84] |
| 9.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 4 | 1740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.57] |
| 9.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 2 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.86] |
| 9.7 In babies exposed to one repeat course of prenatal corticosteroids | 3 | 1015 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.14, 7.23] |
| 9.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 2 | 1472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.15, 7.31] |
| 9.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 5 | 1283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.55] |
| 9.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 1 | 1146 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.23] |
| 9.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 3 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.08, 4.42] |
| 9.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Neonatal death | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 In all babies | 7 | 2713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.34] |
| 10.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 In babies exposed to repeat corticosteroids as betamethasone | 7 | 2713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.34] |
| 10.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 5 | 2045 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.61, 1.38] |
| 10.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 2 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.28, 2.66] |
| 10.7 In babies exposed to one repeat course of prenatal corticosteroids | 3 | 994 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.65, 3.33] |
| 10.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 2 | 1470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.70, 1.80] |
| 10.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 5 | 1243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.32, 1.20] |
| 10.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 1 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.56, 1.59] |
| 10.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 3 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.23, 1.18] |
| 10.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Chronic lung disease | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 In all babies | 8 | 5393 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.30] |
| 11.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.41] |
| 11.3 In babies exposed to repeat corticosteroids as betamethasone | 8 | 5393 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.30] |
| 11.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 6 | 2538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 11.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 2 | 2855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.96, 2.32] |
| 11.7 In babies exposed to one repeat course of prenatal corticosteroids | 2 | 877 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.83, 1.96] |
| 11.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.11 In babies where planned dose per treatment course 12 mg or less of betamethasone or equivalent | 2 | 1470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 11.12 In babies where planned dose per treatment course > 12 mg to 24 mg of betamethasone or equivalent | 6 | 3923 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.88, 1.59] |
| 11.13 In babies where planned dose per treatment course > 24 mg of betamethasone or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.14 In babies where planned repeat drug exposure was 12 mg or less/week of betamethasone or equivalent | 2 | 3448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 11.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of betamethasone or equivalent | 4 | 1068 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.65, 1.44] |
| 11.16 In babies where planned repeat drug exposure was > 24 mg/week of betamethasone or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Intraventricular haemorrhage | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 In all babies | 6 | 3065 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.18] |
| 12.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 In babies exposed to repeat corticosteroids as betamethasone | 6 | 3065 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.18] |
| 12.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 5 | 2519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.26] |
| 12.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and 14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 1 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.36] |
| 12.7 In babies exposed to one repeat course of prenatal corticosteroids | 2 | 872 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.42] |
| 12.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.11 In babies where planned dose per treatment course 12 mg or less of betamethasone or equivalent | 2 | 1470 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.74, 1.40] |
| 12.12 In babies where planned dose per treatment course > 12 mg to 24 mg of betamethasone or equivalent | 4 | 1595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 12.13 In babies where planned dose per treatment course > 24 mg of betamethasone or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.14 In babies where planned repeat drug exposure was 12 mg or less/week of betamethasone or equivalent | 1 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.57, 1.38] |
| 12.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of betamethasone or equivalent | 3 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.40] |
| 12.16 In babies where planned repeat drug exposure was > 24 mg/week of betamethasone or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Intraventricular haemorrhage grade 3/4 | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 In all babies | 6 | 4819 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.69, 1.86] |
| 14 Periventricular leukomalacia | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 In all babies | 7 | 4888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.37] |
| 15 Chorioamnionitis | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 In all women | 6 | 4261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.92, 1.46] |
| 15.2 In women where pregnancy complicated by preterm prelabour rupture of membranes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.05, 2.31] |
| 15.3 In women given repeat corticosteroids as betamethasone | 6 | 4261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.92, 1.46] |
| 15.4 In women given repeat corticosteroids at a minimum interval of 7 days or less | 4 | 1971 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.95, 1.59] |
| 15.5 In women given repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.6 In women given repeat corticosteroids at a minimum interval of 14 days or more | 2 | 2290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.56, 1.57] |
| 15.7 In women given one repeat course of prenatal corticosteroids | 1 | 437 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.23, 1.77] |
| 15.8 In women given two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.9 In women given three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.10 In women given four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.11 In women where planned dose per treatment course 12 mg or less of betamethasone or equivalent | 1 | 982 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.72, 1.62] |
| 15.12 In women where planned dose per treatment course > 12 mg to 24 mg of betamethasone or equivalent | 5 | 3279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.90, 1.58] |
| 15.13 In women where planned dose per treatment course > 24 mg of betamethasone or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.14 In women where planned repeat drug exposure was 12 mg or less/week of betamethasone or equivalent | 2 | 2835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.77, 1.51] |
| 15.15 In women where planned repeat drug exposure was > 12 mg/week to 24 mg/week of betamethasone or equivale | 3 | 989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.96, 1.88] |
| 15.16 In women where planned repeat drug exposure was > 24 mg/week of betamethasone or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Puerperal sepsis | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 In all women | 5 | 3091 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.83, 1.60] |
| 16.2 In women where pregnancy complicated by preterm prelabour rupture of membranes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.19, 2.22] |
| 16.3 In women given repeat corticosteroids as betamethasone | 5 | 3091 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.83, 1.60] |
| 16.4 In women given repeat corticosteroids at a minimum interval of 7 days or less | 4 | 1238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.66, 1.59] |
| 16.5 In women given repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.6 In women given repeat corticosteroids at a minimum interval of 14 days or more | 1 | 1853 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.80, 2.22] |
| 16.7 In women given one repeat course of prenatal corticosteroids | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.80, 3.10] |
| 16.8 In women given two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.9 In women given three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.10 In women given four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.11 In women where planned dose per treatment course 12 mg or less of betamethasone or equivalent | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.80, 3.10] |
| 16.12 In women where planned dose per treatment course > 12 mg to 24 mg of betamethasone or equivalen | 4 | 2842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.72, 1.54] |
| 16.13 In women where planned dose per treatment course > 24 mg of betamethasone or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.14 In women where planned repeat drug exposure was 12 mg or less/week of betamethasone or equivalent | 1 | 1853 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.80, 2.22] |
| 16.15 In women where planned repeat drug exposure was > 12 mg/week to 24 mg/week of betamethasone or equivale | 3 | 989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.42, 1.36] |
| 16.16 In women where planned repeat drug exposure was > 24 mg/week of betamethasone or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Mean gestational age at birth (weeks) | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 In all babies | 8 | 3179 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.33, 0.15] |
| 18 Preterm birth before 37 weeks | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 In all babies | 2 | 1181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.92, 1.02] |
| 19 Very preterm birth before 34 weeks | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 In all babies | 4 | 2140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 20 Extremely preterm birth before 28 weeks | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 In all babies | 2 | 1632 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.83, 1.38] |
| 21 Mean head circumference at birth (cm) | 9 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 In all babies | 9 | 5626 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.49, ‐0.15] |
| 22 Head circumference Z scores at birth | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 22.1 In all babies | 2 | 1256 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.27, ‐0.00] |
| 23 Mean length at birth (cm) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.1 In all babies | 6 | 4550 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐0.89, ‐0.23] |
| 24 Length Z scores at birth | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 In all babies | 2 | 1256 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.19, 0.09] |
| 25 Length multiples of the median at birth | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 25.1 In all babies | 1 | 590 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.02, ‐0.00] |
| 26 Apgar score less than 7 at 5 minutes | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 In all babies | 3 | 4004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.64, 1.10] |
| 27 Use of mechanical ventilation | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 In all babies | 6 | 4918 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 0.99] |
| 28 Duration of respiratory support in days | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 28.1 In all babies | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.90, 1.50] |
| 29 Use of oxygen supplementation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 In all babies | 2 | 3448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 0.99] |
| 30 Duration of oxygen supplementation in days | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 30.1 In all babies | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | 3.3 [‐2.31, 8.91] |
| 31 Use of surfactant | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 31.1 In all babies | 9 | 5525 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.95] |
| 32 Use of inotropic support | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 In all babies | 2 | 1470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.97] |
| 33 Use of nitric oxide for respiratory support | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 33.1 In all babies | 1 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.29, 1.17] |
| 34 Early systemic neonatal infection (variously defined) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 34.1 In all babies | 3 | 1544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.11] |
| 35 Proven infection while in the neonatal intensive care unit | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 In all babies | 6 | 5002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.83, 1.20] |
| 36 Admission to the neonatal intensive care unit | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 36.1 In all babies | 2 | 3448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.07] |
| 37 Air leak syndrome | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 37.1 In all babies | 3 | 2192 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.29, 1.97] |
| 38 Necrotising enterocolitis | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 38.1 In all babies | 8 | 5394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.51, 1.08] |
| 39 Patent ductus arteriosus | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 39.1 In all babies | 6 | 4356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 0.98] |
| 40 Retinopathy of prematurity | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 40.1 In all babies | 7 | 4883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.81, 1.28] |
| 41 Use of postnatal steroids | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 41.1 In all babies | 3 | 3931 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.99, 1.93] |
| 42 Mean neonatal blood pressure on first day after birth | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 42.1 In all babies | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐2.47, 3.87] |
| 43 Mean neonatal blood pressure 6 weeks after birth | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 43.1 In all babies | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐5.06, 2.26] |
| 44 Neonatal cardiac hypertrophy as measured by interventricular septal thickness (IVSd) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 44.1 In all babies | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.24, 0.26] |
| 45 Neonatal cardiac hypertrophy as measured by left ventricular wall thickness in diastole | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 45.1 In all babies | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.27, 0.19] |
| 46 Mean basal cortisol concentrations (nmol/L) at birth | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 46.1 In all babies | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐44.9 [‐78.41, ‐11.39] |
| 47 Mean weight (g) at primary hospital discharge | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 47.1 In all babies | 1 | 1090 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐77.15, 75.15] |
| 48 Weight Z scores at primary hospital discharge | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 In all babies | 2 | 1195 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.16, 0.06] |
| 49 Mean head circumference (cm) at primary hospital discharge | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 49.1 In all babies | 2 | 1195 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.10, 0.35] |
| 50 Head circumference Z scores at primary hospital discharge | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 50.1 In all babies | 2 | 1195 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.15, 0.10] |
| 51 Mean length (cm) at primary hospital discharge | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 51.1 In all babies | 2 | 1189 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.44, 0.47] |
| 52 Length Z score at primary hospital discharge | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 52.1 In all babies | 2 | 1189 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.23, 0.10] |
| 53 Prelabour rupture of membranes after trial entry | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 53.1 In all women | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.57] |
| 54 Maternal Hypertension (variously defined by the authors) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 54.1 In all women | 3 | 3327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.32] |
| 55 Vaginal birth | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 55.1 In all women | 7 | 4062 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 56 Caesarean section | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 56.1 In all women | 7 | 4062 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.99, 1.10] |
| 57 Postpartum haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 57.1 In all women | 1 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.33, 1.07] |
| 58 Postnatal pyrexia (variously defined by authors) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 58.1 In all women | 1 | 982 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.38] |
| 59 Length of postnatal hospitalisation (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 59.1 In all babies | 1 | 483 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.22, 0.22] |
| 60 Any maternal side effects of therapy | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 60.1 In all women | 2 | 1474 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.24, 3.90] |
| 61 Maternal hyperglycaemia (variously defined by authors) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 61.1 In all women | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.89, 1.93] |
| 62 Insomnia | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 62.1 In all women | 3 | 1486 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.10, 6.30] |
| 63 Pain at injection site | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 63.1 In all women | 2 | 1474 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.11, 5.05] |
| 64 Bruising at injection site | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 64.1 In all women | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.21, 0.71] |
| 65 Total mortality up to early childhood follow up | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 65.1 In all babies | 4 | 4370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.80, 1.41] |
| 65.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 65.3 In babies exposed to repeat corticosteroids as betamethasone | 4 | 4370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.80, 1.41] |
| 65.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 3 | 2066 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.74, 1.67] |
| 65.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 65.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 1 | 2304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.51] |
| 65.7 In babies exposed to one repeat course of prenatal corticosteroids | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.82, 6.50] |
| 65.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 65.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 65.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 65.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 2 | 1472 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.72, 1.71] |
| 65.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 2 | 2898 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.72, 1.50] |
| 65.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 65.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 2 | 3450 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.72, 1.33] |
| 65.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 1 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.39, 3.38] |
| 65.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 66 Survival free of any disability to early childhood follow up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 66.1 In all babies | 2 | 3155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.97, 1.05] |
| 66.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 66.3 In babies exposed to repeat corticosteroids as betamethasone | 2 | 3164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.04] |
| 66.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 1 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.12] |
| 66.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 66.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 1 | 2104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.03] |
| 66.7 In babies exposed to one repeat course of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 66.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 66.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 66.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 66.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 1 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.12] |
| 66.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 1 | 2104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.03] |
| 66.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 66.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 2 | 3164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.04] |
| 66.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 66.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 67 Survival free of major neurosensory disability to early childhood follow up | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 67.1 In all babies | 2 | 1317 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 67.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 67.3 In babies exposed to repeat corticosteroids as betamethasone | 2 | 1317 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 67.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 2 | 1317 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 67.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 67.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 67.7 In babies exposed to one repeat course of prenatal corticosteroids | 1 | 257 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 67.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 67.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 67.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 67.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 2 | 1317 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 67.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 67.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 67.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 1 | 1060 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.99, 1.10] |
| 67.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 67.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68 Major neurosensory disablity at early childhood follow‐up | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 68.1 In all babies | 2 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.31, 3.76] |
| 68.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68.3 In babies exposed to repeat corticosteroids as betamethasone | 2 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.31, 3.76] |
| 68.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 2 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.31, 3.76] |
| 68.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68.7 In babies exposed to one repeat course of prenatal corticosteroids | 1 | 257 | Risk Ratio (M‐H, Random, 95% CI) | 3.53 [0.37, 33.52] |
| 68.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 2 | 1256 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.31, 3.76] |
| 68.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 1 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.08] |
| 68.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 68.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 69 Disability at early childhood follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 69.1 In all babies | 1 | 999 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.16] |
| 69.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.3 In babies exposed to repeat corticosteroids as betamethasone | 1 | 999 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.16] |
| 69.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 1 | 999 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.16] |
| 69.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.7 In babies exposed to one repeat course of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 1 | 999 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.16] |
| 69.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 1 | 999 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.16] |
| 69.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 69.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70 Composite serious outcome at early childhood follow‐up (however defined by authors) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 70.1 In all babies | 2 | 3164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.12] |
| 70.2 In babies where pregnancy complicated by preterm prelabour rupture of membranes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70.3 In babies exposed to repeat corticosteroids as betamethasone | 2 | 3164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.12] |
| 70.4 In babies exposed to repeat corticosteroids at a minimum interval of 7 days or less | 1 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.84, 1.13] |
| 70.5 In babies exposed to repeat corticosteroids at a minimum interval between 8 and <14 days | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70.6 In babies exposed to repeat corticosteroids at a minimum interval of 14 days or more | 1 | 2104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.25] |
| 70.7 In babies exposed to one repeat course of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70.8 In babies exposed to two repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70.9 In babies exposed to three repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70.10 In babies exposed to four or more repeat courses of prenatal corticosteroids | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70.11 In babies where planned dose per treatment course 12 mg or less of antenatal corticosteroid or equivalent | 1 | 1060 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.84, 1.13] |
| 70.12 In babies where planned dose per treatment course > 12 mg to 24 mg of antenatal corticosteroid or equivalent | 1 | 2104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.25] |
| 70.13 In babies where planned dose per treatment course > 24 mg of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70.14 In babies where planned repeat drug exposure was 12 mg or less/week of antenatal corticosteroid or equivalent | 2 | 3164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.87, 1.12] |
| 70.15 In babies where planned repeat drug exposure was > 12 mg/week to 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 70.16 In babies where planned repeat drug exposure was > 24 mg/week of antenatal corticosteroid or equivalent | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 71 Weight small for age at early childhood follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 71.1 In all babies | 2 | 1448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.19] |
| 72 Head circumference small for age at early childhood follow‐up | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 72.1 In all babies | 2 | 1442 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.56] |
| 73 Height small for age at early childhood follow‐up | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 73.1 In all babies | 2 | 1441 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.63, 2.02] |
| 74 Mean weight at early childhood follow‐up | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 74.1 In all babies | 3 | 1776 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.21, 0.15] |
| 75 Mean head circumference at early childhood follow‐up | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 75.1 In all babies | 3 | 1776 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.22, 0.11] |
| 76 Mean height at early childhood follow‐up | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 76.1 In all babies | 3 | 1776 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.55, 0.30] |
| 77 Weight Z score at early childhood follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 77.1 In all babies | 1 | 1047 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.19, 0.13] |
| 78 Head circumference Z score at early childhood follow‐up | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 78.1 In all babies | 2 | 1290 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.09, 0.18] |
| 79 Height Z score at early childhood follow‐up | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 79.1 In all babies | 2 | 1290 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.17, 0.09] |
| 80 Developmental delay at early childhood follow‐up | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 80.1 In all babies | 3 | 3202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.13] |
| 81 Mental Developmental Index at early childhood follow‐up | 2 | 1162 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [‐0.65, 3.11] |
| 81.1 In all babies | 2 | 1162 | Mean Difference (IV, Fixed, 95% CI) | 1.23 [‐0.65, 3.11] |
| 82 Psychomotor Developmental Index at early childhood follow‐up | 1 | 958 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.75, 2.55] |
| 82.1 In all babies | 1 | 958 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.75, 2.55] |
| 83 Bindness at early childhood follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 83.1 In all babies | 2 | 3151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.65, 2.10] |
| 84 Deafness at early childhood follow‐up | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 84.1 In all babies | 3 | 3405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.29, 2.52] |
| 85 Cerebral palsy at early childhood follow‐up | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 85.1 In all babies | 4 | 3800 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.71, 1.50] |
| 86 Behaviour score at early childhood follow‐up within clinical range | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 86.1 In all babies | 1 | 1045 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
| 87 Asthma/Wheezing at early childhood follow‐up | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 87.1 In all babies | 3 | 1720 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.63, 1.27] |
| 88 Hypertensive at early childhood follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 88.1 In all babies | 1 | 628 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| 89 Mean systolic blood pressure at early childhood follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 89.1 In all babies | 1 | 486 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐5.40, ‐0.40] |
| 90 Mean diasystolic blood pressure at early childhood follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 90.1 In all babies | 1 | 486 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.86, 0.86] |
| 91 Hospital re‐admission by early childhood follow‐up | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 91.1 In all babies | 4 | 3824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
1.9. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 9 Fetal death.
1.10. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 10 Neonatal death.
1.13. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 13 Intraventricular haemorrhage grade 3/4.
1.14. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 14 Periventricular leukomalacia.
1.18. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 18 Preterm birth before 37 weeks.
1.19. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 19 Very preterm birth before 34 weeks.
1.20. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 20 Extremely preterm birth before 28 weeks.
1.26. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 26 Apgar score less than 7 at 5 minutes.
1.28. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 28 Duration of respiratory support in days.
1.30. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 30 Duration of oxygen supplementation in days.
1.33. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 33 Use of nitric oxide for respiratory support.
1.34. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 34 Early systemic neonatal infection (variously defined).
1.35. Analysis.
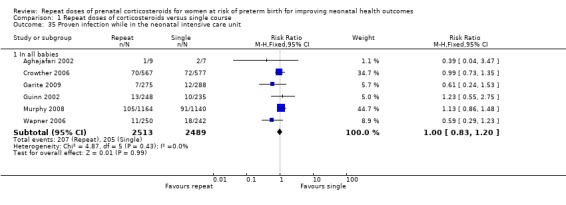
Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 35 Proven infection while in the neonatal intensive care unit.
1.36. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 36 Admission to the neonatal intensive care unit.
1.37. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 37 Air leak syndrome.
1.38. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 38 Necrotising enterocolitis.
1.40. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 40 Retinopathy of prematurity.
1.41. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 41 Use of postnatal steroids.
1.42. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 42 Mean neonatal blood pressure on first day after birth.
1.43. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 43 Mean neonatal blood pressure 6 weeks after birth.
1.44. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 44 Neonatal cardiac hypertrophy as measured by interventricular septal thickness (IVSd).
1.45. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 45 Neonatal cardiac hypertrophy as measured by left ventricular wall thickness in diastole.
1.47. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 47 Mean weight (g) at primary hospital discharge.
1.48. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 48 Weight Z scores at primary hospital discharge.
1.49. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 49 Mean head circumference (cm) at primary hospital discharge.
1.50. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 50 Head circumference Z scores at primary hospital discharge.
1.51. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 51 Mean length (cm) at primary hospital discharge.
1.52. Analysis.
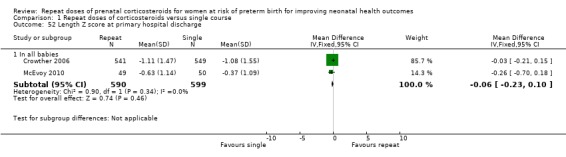
Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 52 Length Z score at primary hospital discharge.
1.53. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 53 Prelabour rupture of membranes after trial entry.
1.54. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 54 Maternal Hypertension (variously defined by the authors).
1.55. Analysis.
Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 55 Vaginal birth.
1.56. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 56 Caesarean section.
1.57. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 57 Postpartum haemorrhage.
1.58. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 58 Postnatal pyrexia (variously defined by authors).
1.59. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 59 Length of postnatal hospitalisation (days).
1.61. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 61 Maternal hyperglycaemia (variously defined by authors).
1.88. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 88 Hypertensive at early childhood follow‐up.
1.90. Analysis.

Comparison 1 Repeat doses of corticosteroids versus single course, Outcome 90 Mean diasystolic blood pressure at early childhood follow‐up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aghajafari 2002.
| Methods | Type of study: randomised trial. Small pilot study to determine the feasibility of a larger trial. | |
| Participants | Location: 2 hospitals in Toronto, Canada. Timeframe: September 1999‐August 2000. Eligibility criteria: women at 24‐30 weeks' gestation at continued increased risk of preterm birth who remained undelivered 7 or more days following a single course of antenatal corticosteroids (defined as 2 doses of 12 mg/dose intramuscular betamethasone, given at 12‐ or 24‐hour intervals; or 4 doses of 5‐6 mg/dose intramuscular dexamethasone, given at 12‐hour intervals. To be at increased risk of preterm birth, women had to have 1 or more of the following: regular uterine contractions; a shortened cervical length or cervical dilatation; preterm prelabour rupture of the membranes; antepartum bleeding secondary to placental separation or placenta praevia; history of preterm birth; maternal hypertension; other medical condition increasing the risk of preterm delivery or intrauterine growth restriction; or other fetal conditions increasing the risk of preterm delivery. Gestational age range: 24‐30 weeks. Exclusion criteria: women were excluded if they required chronic doses of corticosteroids secondary to medical conditions, had a contra‐indication to corticosteroids, had clinical evidence of chorioamnionitis, or of their fetus(es) had a known lethal congenital anomaly. Total recruited: 12 in total: 6 in the multiple course of antenatal corticosteroid group and 6 in the placebo group. | |
| Interventions | Multiple course of antenatal corticosteroid group: a weekly course of betamethasone (2 doses of 12 mg/dose betamethasone (Celestone Soluspan; Schering Canada Inc) intramuscularly, 24 hours apart) until 33 weeks or delivery if the woman remained at increased risk of preterm birth. In the placebo group: a weekly course of placebo consisting of 2 doses of normal saline, intramuscularly 24 hours apart, until 33 weeks or birth if the woman remained at increased risk of preterm birth. | |
| Outcomes | Outcomes: rate of recruitment over 12‐month period, risk of complications requiring discontinuation of study treatment, concentrations of plasma cortisol and ACTH in cord blood and in maternal blood immediately following birth. Perinatal or neonatal mortality or significant neonatal morbidity, defined as one or more of the following: stillborn or neonatal death during the first 28 days of life or prior to hospital discharge, whichever was sooner; respiratory distress syndrome; BPD (requiring oxygen at 36 corrected postnatal gestational age); IVH (grade 3 or 4; and necrotising enterocolitis. | |
| Funding Support | Funding: support from Canadian Institutes of Health Research Senior Scientist Award. | |
| Notes | Sample‐size calculation: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was computer‐generated and was centrally controlled by 1 pharmacist at each hospital who kept the randomisation code. Stratification: by gestational age (24‐27 weeks; 28‐30 weeks) and by hospital using block sizes of 2. |
| Allocation concealment (selection bias) | Low risk | Method of treatment allocation: 1 pharmacist at each hospital who kept the randomisation code. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The injection of the study treatment was given by a designated research nurse in each hospital who was not caring for the woman. Placebo of normal saline was used. The physical appearance of the study solutions had to be kept masked since the betamethasone is opaque, while saline is clear. To minimise unblinding, the pharmacist prepared the study treatments in a syringe covered with yellow tape. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported. Intention‐to‐treat analyses: yes. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Crowther 2006.
| Methods | Type of study: ACTORDS randomised controlled trial. | |
| Participants | Location: 23 hospitals in Australia and New Zealand. Timeframe: April 1998 to July 2004. Included: single, twin or triplet pregnancy at less than 32 weeks' gestation if women had received an initial treatment of corticosteroid 7 or more days previously and their responsible clinician regarded them to be at continued risk of preterm birth, and there was no contraindication to further corticosteroid therapy. Exclusion criteria: in second stage of labour, had chorioamnionitis needing urgent delivery, or if further corticosteroid therapy was judged to be essential. Gestational age recruited up to less than 32 weeks. Total recruited 982 women (1146 babies). 489 women in the repeat corticosteroid group and 493 women in the placebo group. | |
| Interventions | Repeat corticosteroids: 11.4 mg Celestone Chronodose (as 7.8 mg betamethasone sodium phosphate and 6 mg betamethasone acetate. Placebo: saline intramuscular injection. Every week, if the woman remained undelivered and less than 32 weeks' gestation, and the responsible clinician regarded her as at continued risk of preterm birth, a further treatment pack from the same treatment group was allocated by the telephone randomisation service. | |
| Outcomes | To time of primary hospital discharge: primary outcomes: frequency and severity of RDS (defined as clinical signs of respiratory distress and a ground‐glass appearance on chest radiograph); weight, length and head circumference at birth and primary discharge from hospital. Secondary outcomes included clinical chorioamnionitis (defined as requiring intrapartum antibiotics); maternal postpartum pyrexia (38.0 degrees Centrigrade or greater); any side effects of the injection for the mother and other measures of neonatal morbidity. Composite outcome was post hoc (defined as one of air leak syndrome, patent ductus arteriosus, need for oxygen at 36 weeks' post menstrual age, severe IVH (grade 3 or 4), periventricular haemorrhage, proven necrotising enterocolitis, or retinopathy of prematurity). For the early childhood follow‐up at 2 years' corrected age: primary outcomes: survival at 2 years' corrected age free of major neurosensory disability, defined as survival free of moderate to severe disability. Body size (weight, height and head circumference). Secondary outcomes were general health, including the use of health services since primary hospital discharge; respiratory morbidity; blood‐pressure; child behaviour; incidence of neurosensory impairments and disabilities; total number of deaths by 2 years of corrected age; and the combined adverse outcome of death or any neurosensory disability at 2 years' corrected age. |
|
| Funding Support | Funding: Australian National Health and Medical Research Council, The Channel 7 Research Foundation of South Australia, The Women's and Children's Hospital Research Foundation, Adelaide, and The Department of Obstetrics and Gynaecology, The University of Adelaide, South Australia. | |
| Notes | Sample‐size calculation: yes. 980 women needed to detect a 25% reduction in the risk of respiratory distress syndrome form 30% to 22.5% with 80% power and a 2‐sided significance level of 5%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number sequence generated by computer with variable block sizes and stratification by centre, gestational age and number of fetuses. |
| Allocation concealment (selection bias) | Low risk | Method of treatment allocation: central telephone randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Normal saline placebo was used. Study treatment packs and study syringes identical appearance (opaque study‐labelled syringe). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At the follow‐up at 2 years' corrected age: staff making the assessments and the families "were unaware of group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up to time of primary hospital discharge: none. Losses to follow‐up to time of 2 year corrected age assessment: 38 survivors (4%) had no paediatric assessment, 18 in the repeat corticosteroid group and 20 in the placebo group; 86 (8%) had no psychological tests, 44 in the repeat corticosteroid group and 42 in the placebo group. Intention‐to‐treat analyses: yes. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. No obvious risk of selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. Pre‐specified that analyses would be adjusted for gestational age at trial entry and for prognostic variables with imbalance (antepartum haemorrhage and preterm, prelabour rupture of the membranes). |
Garite 2009.
| Methods | Type of study: randomised placebo‐controlled trial. | |
| Participants | Location: 15 private and 3 university centres, USA. The participants were largely private/non‐goverment funded. Time frame: May 2003 to February 2008. Included: women with singleton or twin pregnancies from 25 weeks' to less than 33 weeks' gestation who had received a course of betamethasone 14 or more days previously and who were judged to have recurrent or continued risk of preterm birth. Exclusion criteria: major fetal anomaly, cervical dilatation 5 cm or more, higher order multiples, ruptured membranes, documented lung maturity, receiving corticosteroids for other indications, human immunodeficiency virus infection or active tuberculosis. Total recruited: 437 women (223 repeat corticosteroid, 214 placebo). 577 infants were enrolled (289 repeat corticosteroid, 288 placebo), although 1 fetal twin in the corticosteroid group died before randomisation. |
|
| Interventions | Repeat corticosteroid: a single course of intramuscular betamethasone given as 2 doses of 12 mg, 24 hours apart (preparation not specified). Placebo: a similarly administered saline intramuscular injection. In some centres betamethasone became unavailable and was replaced with dexamethasone 6 mg intramuscularly, 4 doses, every 12 hours. 31 women received dexamethasone and 30 women received an equivalent placebo. |
|
| Outcomes | Primary outcome: composite neonatal mortality/morbidity in babies born before 34 weeks' gestation. The composite outcome was defined as 1 or more of: perinatal death (defined as stillbirth or death before neonatal discharge); RDS (oxygen requirement, clinical diagnosis and consistent chest radiograph); BPD (defined as a requirement for oxygen at 30 days of age); severe IVH (grades 3 or 4); PVL; blood culture‐proven sepsis; or necrotising enterocolitis (not defined). Secondary outcomes: preterm birth before 34 weeks' gestation; RDS; gestational age at birth; small‐for‐gestational age (< 10th percentile); head circumference; birthweight; surfactant therapy; pneumothorax; maternal infectious morbidity. |
|
| Funding Support | Funding: Pediatrix Medical Group. | |
| Notes | Sample‐size calculation: yes, based on a 40% reduction in the primary outcome, which was estimated to be 28% in the control group. The planned sample size was 217 women in each arm (2 tailed alpha 0.05, beta 0.2). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A "blocked randomisation sequence" was used that was "prepared centrally". Although not described in the paper, the study protocol states that the random sequence was generated by computer. |
| Allocation concealment (selection bias) | Low risk | The research pharmacist (unblinded) at each site prepared the medication based on the blocked randomisation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Normal saline placebo was used. The study syringes (betamethasone or saline) were completely covered by a label to conceal the contents. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Neonatal data were unavailable on 13 babies in the repeat corticosteroid group and 6 babies in the placebo group. No reason was given for the missing data and no sensitivity analysis was performed. Data were missing for some of the secondary outcomes in a few participants (for example BPD missing data for 2 babies in the repeat corticosteroid group and 3 in the placebo group; and similarly for PVL, missing data for 6 versus 9 babies). Intention‐to‐treat analysis was performed on babies with known outcomes. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | Low risk | No other obvious risk of bias identified. |
Guinn 2002.
| Methods | Type of study: randomised controlled trial. | |
| Participants | Location: 13 academic centres in USA. Timeframe: February 1996‐April 2000. Eligibility criteria: 502 women (502 babies) at 24 weeks to < 33 weeks' gestation at high risk of preterm birth who remained undelivered 1 week following an initial course of antenatal corticosteroids (defined as 2 doses of 12 mg/dose intramuscular betamethasone, repeated at 24 hours; or 4 doses of 6 mg/dose intramuscular dexamethasone, given at 12 hour‐intervals. To be at high risk of preterm birth qualifying criteria were: preterm labour with intact membranes (either a history of regular uterine contractions associated with cervical dilatation of > 2 cm and effacement > 80% in a nulliparous participant or cervical dilatation of > 3 cm and > 80% effacement in a multiparous participant at the time of presentation: or regular uterine contractions with documented cervical change); preterm premature rupture of the membranes (rupture of the membranes occurring > 1 hour prior to the onset of preterm labour); maternal medical illness (pre‐eclampsia, hypertension, diabetes, renal disease, systemic lupus erythematosus, trauma); or suspected fetal jeopardy (intrauterine growth restriction < 10th percentile, oligohydramnios, abnormal antepartum testing, progression of a fetal anomaly compatible with like, twin‐twin transfusion syndrome). Gestational age range: 24 weeks to < 33 weeks' gestation. Exclusion criteria: women were excluded if they required immediate delivery, there were fetal anomalies incompatible with life, documented fetal lung maturity, and maternal active tuberculosis or human immunodeficiency virus infection. Total recruited: 502‐256 in the weekly‐course group and 246 in the single‐course group. | |
| Interventions | In the weekly‐course group: a weekly course of betamethasone (2 doses of 12 mg/dose betamethasone repeated after 24 hours, intramuscularly), until 34 weeks or birth whichever came first. In the single‐course group: a similarly administered placebo. | |
| Outcomes | Primary outcomes: composite neonatal morbidity defined as presence of any of the following: severe RDS, BPD, severe IVH, PVL, necrotising enterocolitis, proven sepsis or death between randomisation and nursery discharge. Secondary outcomes: frequency and severity of RDS; need for and duration of oxygen therapy; need for and duration of ventilatory support; BPD (defined as need for oxygen > 21% and usually ventilatory therapy for at least 28 days of life; in cases were no additional ventilatory support was needed but oxygen was required, chest radiographs consistent with BPD were used; in the case of neonatal death, BPD was diagnosed on autopsy findings); severe IVH was defined as intraventricular bleeding with dilatation of the cerebral ventricles (grade 3) or parenchymal haemorrhage (grade 4), as diagnosed with an imaging technique or autopsy, PVL was defined as the presence of more than 1 obvious hypoechoic cyst in the periventricular white matter; necrotising enterocolitis (defined as proven); proven sepsis; perinatal death defined as death of a fetus or neonate at any time between randomisation and nursery discharge. | |
| Funding Support | Funding: March of Dimes grant, the Berlex Foundation, the Wisconsin Perinatal Association, the Perinatal Clinical Research Center at the University of Colorado Health Sciences Center (grant from the General Clinical Research Centers Program, National Centers for Research Resources, National Institutes of Health), and the participating departments. | |
| Notes | Sample‐size calculation: yes. A sample of 1000 women was required to have a 90% power to detect a one‐third reduction in composite morbidity from 25.0% to 16.5% (2‐tailed alpha = 0.05). 2 interim analyses were planned for efficacy and safety. Recruitment was stopped early based on safety concerns. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation logs prepared centrally and distributed to the research pharmacist at each clinical site. Stratification: by centre. |
| Allocation concealment (selection bias) | Low risk | Method of treatment allocation: participants were assigned by the pharmacy to a treatment group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo syringes were indistinguishable from the syringes containing betamethasone. Patients and healthcare workers were blinded to study group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses: yes. Losses to follow‐up: 16 women and 1 neonate were lost to follow‐up. Partial data were available for participants who were lost to follow‐up. In some cases the investigators were able to ascertain the birth date, weight, and health status for the neonate. The denominators presented very slightly from 1 variable to another because of missing data. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | High risk | After the first interim analysis when 308 women have been randomised the decision was made to stop recruitment at 500 women, because of "safety concerns" and the finding of only a marginal difference in short‐term outcomes between treatment groups. |
Mazumder 2008.
| Methods | Type of study: open‐label, randomised trial. | |
| Participants | Location: tertiary hospital in northern India. Time frame: August 2004 onwards. Included: mothers from 26 to 33 weeks' gestation who were at risk of preterm birth and who had received a course of betamethasone 7 or more days previously. Mothers had to be available for follow‐up every week until birth. Exclusion criteria: unreliable gestational age, frank chorioamnionitis, and major fetal malformation. Total recruited: 76 mothers (38 repeat corticosteroid, 38 control). 83 babies were born to 75 mothers but only the first born of multiple pregnancies were assessed (37 repeat corticosteroid, 38 control). |
|
| Interventions | Repeat corticosteroid: betamethasone 12 mg intramuscularly, 2 doses, 24 hours apart. The course was repeated every 7 days until delivery or the end of the 33rd week of gestation. Control: no intervention. |
|
| Outcomes | Primary outcome: severe RDS. RDS was defined as respiratory distress within 6 hours of birth in a preterm infant with either a negative gastric shake test or a typical chest radiograph. Severe RDS was defined as requiring mechanical ventilation for at least 12 hours. Mechanical ventilation was started in infants with hypoxaemia (Pa02 < 50 mmHg) or hypercapnic acidosis (PaC02 > 50mmHg with pH < 7.25) or worsening acidosis or clinically worsening respiratory fatigue/apnoea/work of breathing despite continuous positive airway pressure (maximum 8 cm water pressure). Secondary outcomes: RDS; IVH; necrotising enterocolitis (not defined); patent ductus arteriosus (not defined); BPD (not defined), sepsis (not defined), retinopathy of prematurity, stillbirth and neonatal death, weight, length and occipital‐frontal circumference at birth and at 6 months' corrected age, and body size and development (evaluated by the Denver Development Screening Test II) at 6 months' corrected age. |
|
| Funding Support | None. | |
| Notes | Sample‐size calculation: yes, based on a reduction in severe RDS from an estimated 33% in the control group to 6% in the repeat corticosteroid group (2 tailed alpha 0.05, beta 0.2). The planned sample size was 70 (35 women in each arm). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Website‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Participants were "randomised" to receive by a process of simple randomisation. "Slips bearing the random allocation" (generated from the random number list) "were placed in opaque envelopes, sealed and numbered serially on the outside". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo not used so no blinding of participants or staff. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One mother in the repeat corticosteroid group was lost to follow‐up before birth. Outcomes were recorded only for the first‐born of multiple pregnancies. Intention‐to‐treat analysis was performed on babies with known outcomes. Neonatal outcomes reported on 75 (99%) infants, 37 in the repeat corticosteroid group and 38 in the control group. Infant outcomes reported to date for 44 (58%) infants, 21 in the repeat corticosteroid group and 23 in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient detail to permit judgement. Outcomes specified in the research methods reported. |
| Other bias | Low risk | No other obvious risk of bias identified. |
McEvoy 2002.
| Methods | Type of study: randomised trial. | |
| Participants | Location: single centre in USA (Sacred Heart Hospital, University of Florida, Pensacola, Florida). Timeframe: 3‐year period ending in December 1999. Eligibility criteria: women at 25‐33 weeks' gestation who remained undelivered 1 week after a single course of antenatal corticosteroids (defined as 2 doses of 12 mg/dose intramuscular betamethasone), given because of increased risk of preterm delivery. Gestational age range: 25‐33 weeks. Exclusion criteria: women were excluded if they were insulin‐dependent diabetics, had a drug‐addiction, or fetus had a known lethal congenital anomaly. Total recruited: 37 women (37 babies). 18 women in the repetitive courses of antenatal corticosteroid group and 19 women in the single course remote group. | |
| Interventions | In the repetitive courses of antenatal corticosteroid group: a weekly course of betamethasone (2 doses of 12 mg/dose betamethasone (Celestone Soluspan; Schering Corporation, Kenilworth, New Jersey), intramuscularly, until birth or 34 weeks' gestation. In the single‐course remote group: weekly courses of placebo intramuscularly, until 34 weeks or birth. | |
| Outcomes | Primary outcomes: functional residual capacity, respiratory compliance. Secondary outcomes: admission head circumference, surfactant administration, days on oxygen, and mechanical ventilation. | |
| Funding Support | American Lung Association. | |
| Notes | Sample‐size calculation: yes. Based on 37 women the average functional residual capacity in the single course remote group is not > 12% smaller than the functional residual capacity in the repetitive group (P = 0.05, power 80%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group assignment done through pharmacy using a randomisation table. Stratification: none stated. |
| Allocation concealment (selection bias) | Low risk | Method of treatment allocation: "group assignment done through pharmacy using a randomisation table." "The study medication was prepared by the pharmacy." Stratification: none stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Investigators and clinical care providers were unaware of the treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: none stated. Intention‐to‐treat analyses: yes. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | Low risk | No other obvious risk of bias detected. |
McEvoy 2010.
| Methods | Type of study: randomised trial. | |
| Participants | Location: 2 centres in USA (Sacred Heart Hospital, University of Florida, Pensacola, Florida recruited first 8 patients; Oregon Health and Science University).
Timeframe: From June 2001 to May 2007.
Eligibility criteria: women at 26 to < 34 weeks' gestation; at least 14 days after first course of antenatal corticosteroids (93% received betamethasone); at continued risk of preterm delivery as determined by their care provider; who provided informed consent. Gestational age range: 26 to < 34 weeks' gestation. Exclusion criteria: women were excluded if they were insulin‐dependent diabetics, had a major documented fetal or chromosomal abnormality; multiple pregnancy greater than twins; clinical chorioamnionitis; first course of antenatal corticosteroids given < 24 weeks' gestation; chronic steroid use during pregnancy for clinical care. Total recruited: 85 women randomised (113 babies alive at randomisation). 44 women (56 babies) in the rescue corticosteroids group and 41 women (57 babies) in the placebo group. |
|
| Interventions | In the rescue group: course of antenatal corticosteroids (2 doses of 12 mg/dose betamethasone (Celestone Soluspan; Schering Corporation, Kenilworth, New Jersey), intramuscularly, 24 hours apart. In the placebo group: 2 doses of placebo (identical in appearance to betamethasone: 25 mg cortisone acetate, an inactive steroid). | |
| Outcomes | Primary outcomes: respiratory compliance and functional residual capacity (measured within 72 hours of birth (before any surfactant). Secondary outcomes: growth measurements including weight, head circumference and length at birth and hospital discharge; surfactant administration; RDS (defined as clinical signs of respiratory distress with radiographic appearance and needing supplemental oxygen with FiO2 > 0.21); respiratory distress requiring ≥ 0.30 and ≥ 0.40 at 24 hours of age; days on mechanical ventilation and days on supplemental oxygen. |
|
| Funding Support | Oregon Health and Science University, GCRC/PHS Grant 5 MO1 RR000334; OCTRI UL1 RR02414001; and The American Lung Association. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group assignment performed using a randomisation table. A staff pharmacist performed randomisation and study drug preparation at each institution. Stratification: ≤ 28 versus > 28 weeks' gestation; multiple gestation (twins versus singletons). |
| Allocation concealment (selection bias) | Low risk | Method of treatment allocation: "group assignment was performed using a randomisation table. A staff pharmacist performed randomisation and study drug preparation". Stratification: ≤ 28 versus > 28 weeks' gestation; multiple gestation (twins versus singletons). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All patients, investigators and care providers were unaware of the treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: neonatal data not available for 1 baby in the placebo group. Intention‐to‐treat analyses: yes. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting. |
| Other bias | Low risk | No other obvious risk of bias detected. |
Murphy 2008.
| Methods | Type of study: MACS randomised controlled trial. | |
| Participants | Location: 80 centres, 20 countries. Timeframe: April 2001 to August 2006. Included: single, twin or triplet pregnancy between 25 and 32 weeks' gestation if women remained undelivered 14‐21 days after an initial course of antenatal corticosteroids (either betamethasone or dexamethasone) and continued to be at high risk of preterm birth. Exclusion criteria: contraindication to corticosteroid use, needed chronic doses of these drugs, had evidence of chorioamnionitis, carried a fetus with a known lethal congenital abnormality, had an initial course of corticosteroids before 23 weeks' gestation, previously participated in MACS, women with a multiple pregnancy with fetal death after 13 weeks' gestation. Gestational age recruited up to less than 32 weeks. Total recruited 1858 women (2304 babies). 935 women in the antenatal corticosteroid group and 918 women in the placebo group. | |
| Interventions | Repeat corticosteroid group: each course consisted of 2 intramuscular injections of betamethasone 12 mg (as 6 mg betamethasone sodium phosphate and 6 mg betamethasone acetate; Celestone Schering‐Plough Corporation, Madison, NJ, USA)) 24 hours apart. Placebo group: similarly appearing intramuscular injection of dilute concentration of aluminium monostearate (an inert substance used as a filler in pharmaceutical preparations). Women who remained at risk of preterm birth after their first course of study medication continued to receive 2 doses of 12 mg betamethasone or placebo, 24 hours apart, every 14 days until 33 weeks' gestation or birth, whichever happened first. For women with preterm rupture of the membranes the recommendation was to stop the study medication at 32 weeks' gestation. | |
| Outcomes | Primary outcome: composite of perinatal or neonatal mortality and neonatal morbidity; 1 of the following: stillbirth or neonatal death (defined as death during the first 28 days of life or before hospital discharge); severe RDS (defined as needing assisted ventilation via endotracheal tube and supplemental oxygen within the first 24 hours of life and for 24 hours or more, and either a radiographic scan compatible with RDS or surfactant given between the first 2‐24 hours of life); BPD (defined as needing oxygen at a postmenstrual age of 36 completed weeks and radiographic scan compatible with BPD); IVH grade III or IV; cystic PVL, necrotising enterocolitis. For the early childhood follow‐up at 2 years' corrected age: primary outcome was death or the presence of a neurologic impairment at 18 to 24 months of age, corrected for gestational age at birth. Neurologic impairment was defined as the presence of cerebral palsy or a cognitive delay. Cerebral palsy was diagnosed if the child had a non‐progressive motor impairment characterised by abnormal muscle tone and decreased range of movements. Cognitive delay were defined as Mental Developmental Index scores of 70 (2 SDs below the mean of 100) on the BSID‐II or equivalent scores on another standardised assessment. Secondary outcomes were anthropometric measurements (weight, height, and head circumference), general health, illnesses, and operations occurring after the primary hospitalisation was recorded, Psychomotor Developmental Index and the Behavior Rating Scale of the BSID‐II. |
|
| Funding Support | Funding: Canadian Institutes of Health Research (CIHR). | |
| Notes | ISRCTN 72654148 Sample‐size calculation: yes. A sample size of 1900 women (950 per group) was needed to have 80% probability of achieving a significant difference between the 2 groups (2‐tailed type 1 error 0.05, if multiple courses of antenatal corticosteroids reduced the risk of RDS from 12% to 8%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was done with a 24‐hour telephone service". Information on the generation of the allocation sequence not provided in the paper. Comment: probably done. "The study was stratified by gestational age and centre." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was done with a 24‐hour telephone service after patient eligibility and baseline information were recorded. A study number was assigned corresponding to a box of study medication at the participating centre." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were probably blinded as the injections were of similar appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Later publication reporting follow‐up data state that "the children’s families, clinicians, and researchers associated with the trial remained unaware of the random assignments ....” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analyses: yes.
Losses to follow‐up to time of primary hospital discharge: 9 fetuses/infants (5 in the repeat antenatal corticosteroid group and 4 in the placebo group) were stillbirths within multiple pregnancies that took place before randomisation and were excluded from the analyses. 5 women (0.3%) with 5 fetuses were lost to follow‐up (2 women and 2 fetuses in the repeat antenatal corticosteroid group and 3 women and 3 fetuses in the placebo group). |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting bias detected. |
| Other bias | Low risk | No obvious risk of other bias detected. |
Peltoniemi 2007.
| Methods | Type of study: placebo‐controlled randomised trial. | |
| Participants | Location: 5 Finnish university and 3 central hospitals. Time frame: May 2001 to March 2005. Included: women at < 34 weeks' gestation who had received a single course of betamethasone > 7 days previously and were to have elective delivery within 48 hours or were at very high risk of spontaneous delivery within 48 hours (cervical opening 3 cm or more, and regular contractions at 5‐ to 10‐minute intervals). Exclusion criteria: long‐term maternal corticosteroid use, clinical chorioamnionitis, or lethal disease of the fetus. Total recruited: 249 women (125 betamethasone, 124 placebo), 326 infants (159 betamethasone, 167 placebo). |
|
| Interventions | Repeat corticosteroid: a single dose of betamethasone 12 mg intramuscularly. Placebo: isotonic saline intramuscularly. |
|
| Outcomes | To time of primary hospital discharge: primary outcome: survival without severe RDS or severe IVH (grade 3 or 4) during the first hospital admission. RDS was defined on the basis of typical chest radiograph findings, requirement for continuous distending airway pressure, supplemental oxygen for 48 hours or more, or requirement for surfactant in cases of established respiratory failure. Severe IVH was defined as IVH with ventricular dilation (grade 3) or parenchymal haemorrhage (grade 4). Cranial ultrasound was performed for all infants at 4 to 8 days of age and at 36 weeks post‐menstrual age or before discharge. The most severe grade of IVH was recorded. Secondary outcomes: cystic PVL; necrotising enterocolitis grade 2 or higher; BPD (defined as a requirement for supplemental oxygen or any form of ventilation with continuous distending pressures at postnatal age of 36 weeks or at postnatal age of 4 weeks for those born after postmenstrual age of 31 weeks); patent ductus arteriosus requiring treatment (defined as a requirement for prostaglandin inhibitor therapy or surgery for closure). For the early childhood follow‐up at 2 years' corrected age: a range of outcomes were assessed. Survival without serious neurological, cognitive or sensory impairment (NDI); respiratory problems; infections, medical history; child's weight, length and head circumference; cerebral palsy; speech; deafness; blindness; child behaviour. |
|
| Funding Support | Funding: foundation for paediatric research in Finland, Alma and KA Snellmann Foundation, Sigrid Juselius foundation, hospital research funds. | |
| Notes | Tocolytics were not used and 79% of mothers gave birth < 24 hours after the intervention. All infants were born < 36 weeks' gestation. Sample‐size calculation: yes. Sample size based on a 25% increase in the primary outcome rate, from an estimated 50% in the control group to 62.5% in the repeat group. The planned sample size was 220 women in each arm (2 tailed alpha 0.05, beta 0.2). Recruitment was terminated early, after 249 women had been enrolled, primarily because of safety concerns due a decrease in intact survival in the repeat corticosteroid group. In addition, recruitment was slower than expected. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method by which the sequence was generated was not described. |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally and was stratified according to centre by using 4 sets of sequentially‐labelled, opaque, sealed envelopes ( for the 4 strata of gestational age (< 28 weeks or between 28 and ≤ 34 weeks and multiple gestation), which were sent to each centre." "Both gestation groups were stratified additionally on the basis of number of fetuses (singleton or multiple pregnancies)." "The sealed envelopes were opened after informed consent was obtained." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The doctors and nurses as well as the study investigators were blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At the follow‐up at 2 years' corrected age: "The examiners and families were unaware of treatment‐group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up to time of primary hospital discharge: none reported. Analysis was by "intention‐to‐treat". Losses to follow‐up to time of 2‐year corrected age assessment: 259 of 312 (83%) survivors completed 2‐year follow‐up. 66 survivors (21%) had no paediatric assessment, 32 (21%) in the repeat corticosteroid group and 34 (21%) in the placebo group; 116 (37%) had no psychological tests, 61 (41%) in the repeat corticosteroid group and 56 (34%) in the placebo group. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting detected. |
| Other bias | High risk | Recruitment was terminated early, after 249 women had been enrolled, primarily because of safety concerns due a decrease in intact survival in the repeat corticosteroid group. There were additional concerns about the long‐term adverse effects of glucocorticoid. Potential imbalance at trial entry for multiple pregnancy and gestational age not addressed. |
Wapner 2006.
| Methods | Type of study: randomised controlled trial. | |
| Participants | Location: 18 US hospitals (NICHD MFMU network centres). Timeframe: March 2000 to April 2003. Eligibility criteria: pregnant women with intact membranes between 23 weeks 0 days and 31 weeks and 6 days if they had received a single full course of betamethasone or dexamethasone between 7 and 10 days earlier and were at high risk for spontaneous preterm birth, or had the diagnosis of placenta praevia or chronic abruption Exclusions: preterm premature rupture of the membranes prior to randomisation, confirmed fetal lung maturity, chorioamnionitis, a major fetal anomaly, non‐reassuring fetal status, systemic corticosteroid use during the current pregnancy, or insulin‐dependent diabetes. Gestational age was determined from the last menstrual period provided that ultrasonography confirmed the estimate. When there was discordance, the duration of gestation at randomisation was determined from the first sonogram performed. Gestational age range: 23 weeks 0 days to 31 weeks 6 days gestation. Total recruited: 495 women (planned for 2400) = 591 fetuses/infants. 252 to the repeat corticosteroid arm and 243 to the placebo. | |
| Interventions | Repeat corticosteroid group: each course consisted of 2 injections of betamethasone 12 mg (as 6 mg betamethasone sodium phosphate and 6 mg betamethasone acetate) repeated once in 24 hours. Placebo group: 'matching placebo' ‐ no other details of preparation given. Initially women received courses until birth or 33 weeks 6 days' gestation, whichever was sooner. After 67 women had been enrolled, the number of courses (not including the qualifying course) was limited to 4 because of difficulty in recruitment and published literature suggesting possible harmful effects of multiple courses. 63.4% of women received 4 or more study courses. | |
| Outcomes | To time of primary hospital discharge: the primary outcome was a composite endpoint of 1 of the following: severe RDS (defined as clinical features of RDS with the need for oxygen and respiratory support from 6 to 24 hours or more of age, an abnormal chest x‐ray, and either administration of a full course of surfactant or a fraction of inspired oxygen (FiO2 of at least 60%); grade 3 or 3 IVH; PVL; chronic lung disease (defined as the need for supplemental oxygen at 36 weeks' corrected age in infants born before 34 weeks' gestation); or stillbirth or neonatal death. Secondary outcomes not stated in the paper. For the early childhood follow‐up at 2 years' corrected age: the pre‐specified developmental outcome was the Bayley Mental Developmental Index Score. Other outcomes included Bayley Psychomotor Developmental Index Score; measurements of weight, height and head circumference; and the occurrence of cerebral palsy. |
|
| Funding Support | Funding: National Institute of Child Health and Human Development. | |
| Notes | Sample‐size calculation: yes. Planned sample size was 2400 women. "A primary outcome rate of 11.5% was anticipated for patients assigned to placebo. Detection of a 30% reduction for patients assigned to repeat corticosteroids required a sample of 1200 patients in each group" (80% power and type 1 error rate of 5% (2‐sided). Recruitment was stopped early based on safety concerns (because of a tendency towards decreased birthweight in the repeat corticosteroid group without any reduction in the primary morbidity outcome and also because of difficulties in recruitment). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of treatment allocation: numbered kits were prepared using randomisation sequences created by an independent data co‐ordinating centre. Sequences were generated using the urn design and were stratified by clinical centre, type of qualifying course, and inpatient/outpatient. Woman was assigned to the next sequentially‐numbered kit ‐ betamethasone or identical looking placebo prepared by a centralised research pharmacy. |
| Allocation concealment (selection bias) | Low risk | Assessed as adequate. Women were assigned to the next sequentially‐numbered kit ‐ betamethasone or identical looking placebo prepared by a centralised research pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants are likely to be blinded due to the use of a matching placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | At early childhood follow‐up the assessments were performed by centrally trained and certified study personnel who were unaware of the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analyses: yes. "For categorical infant outcomes the statistical unit was pregnancy rather than infant, because of the correlation between twins." Continuous variables were compared with an adjustment for the correlation between twins, for infants. Losses to follow‐up to time of primary hospital discharge: 3 (< 1%) women lost to follow‐up (2 in the repeat group and 1 in the placebo group). Losses to follow‐up to time of 2‐year corrected age assessment: There were 582 known survivors. Of the survivors, 96 (16.5%) were not able to be seen, 46 in the repeat corticosteroid group and 50 in the placebo group; 117 (20.1%) did not have a Bayley assessment performed, 59 in the repeat corticosteroid group and 58 in the placebo group. Intention‐to‐treat analyses: yes. |
| Selective reporting (reporting bias) | Low risk | No obvious risk of selective reporting bias detected. |
| Other bias | High risk | Recruitment was stopped early based on safety concerns after the recruitment of 495 women. "At the second interim analysis the Data Safety Monitoring Committee recommended that enrolment be halted because of a tendency towards decreased birthweight in the repeat corticosteroid group without evident reduction in the primary morbidity outcome and also because of difficulties with recruitment." |
ACTH: adrenocorticotropic hormone BPD: bronchopulmonary dysplasia IVH: intraventricular haemorrhageMFMU: Maternal Fetal Medicine Units NICHD: National Institute of Child Health and Human Development PVL: periventricular leukomalacia RDS: respiratory distress syndrome SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bontis 2011 | This is a 'non‐randomized' trial. |
| Mercer 2001 | Women recruited to the trial did not have corticosteroids before entry. The objective of the trial was to evaluate the need for and benefits of weekly antenatal corticosteroids in women at risk of preterm birth. 189 women between 23 and 32 weeks at risk of preterm birth were randomised to weekly antenatal corticosteroids or to a control group where corticosteroids were given if indicated before 35 weeks, if the pregnancy was expected to last more than 1 week. The primary outcome was antenatal corticosteroids given within 7 days of preterm birth (< 35 weeks) (optimal exposure). In the control group only one‐third of infants < 35 weeks' gestation received optimal antenatal corticosteroid exposure. Weekly corticosteroids doubled optimal exposure although the vast majority gave birth > 34 weeks. |
| Romejko‐Wolniewicz 2013 | This is a head‐to‐head trial of 2 different regimens and is eligible for the Cochrane review entitled 'Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth' Brownfoot 2013. |
| Thorp 2000 | Women recruited to the trial were not randomised to receive repeat corticosteroids but antenatal phenobarbital. The abstract is a secondary multivariate analysis of this trial assessing if duration of antenatal betamethasone is associated with perinatal outcome. |
Characteristics of studies awaiting assessment [ordered by study ID]
Atarod 2014.
| Methods | Randomised trial. |
| Participants | Women at risk of preterm birth between 28 and 35 weeks' gestation. |
| Interventions | Repeat betamethasone compared with no repeat. |
| Outcomes | RDS, need for oxygen, surfactant, need for ventilation, duration of hospital stay, mortality to hospital discharge, birthweight, height, head circumference. |
| Notes | It is unclear as to the time point at which randomisation to repeat betamethasone or no repeat occurred. The authors have been contacted for this information in February 2015 and we are awaiting a response. |
Crowther 2011.
| Methods | 8 conference abstracts reporting on follow‐up data and 1 report on the follow‐up of a subgroup of the original trial population of the ACTORDS trial (Crowther 2006). |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | The review authors will add any data eligible for inclusion when the ACTORDS follow‐up data have been published in full. |
Murphy 2012.
| Methods | 4 papers reporting further follow‐up data from the Murphy 2008 trial. |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | The review authors will add any data eligible for inclusion in the next update of this systematic review. |
RDS: respiratory distress syndrome
Characteristics of ongoing studies [ordered by study ID]
Sobhrabvand 2001.
| Trial name or title | Effects of single versus multiple courses of corticosteroid therapy on pregnancy results in women with PPROM. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
TEAMS 1999.
| Trial name or title | Trial of the effects of antenatal multiple courses of steroids versus a single course (TEAMS). |
| Methods | RCT. |
| Participants | Women who had received a course of antenatal corticosteroids. |
| Interventions | 2 intramuscular injections of betamethasone versus placebo. |
| Outcomes | Death within first year after birth; developmental quotient less than 85 at 2 years. |
| Starting date | 1/10/1999. End date (recruitment): 1/05/2001. |
| Contact information | Ms Helen Adams, TEAMS administrator. |
| Notes | ISRCTN46614711 |
PPROM: preterm prelabour rupture of the membranes RCT: randomised controlled trial
Differences between protocol and review
Amendments made to the protocol for the 2011 update that clarify the longer‐term health outcomes: total deaths added under outcomes for the child and the child as an adult; survival free of any disability and survival free of major disability added under primary outcomes for the child and for the child as an adult.
In addition: periventricular haemorrhage changed to intraventricular haemorrhage; small‐for‐gestational age, intraventricular haemorrhage, intraventricular haemorrhage grade 3/4 and periventricular leukomalacia moved to secondary outcomes for the infant; "However defined by authors" has been added to the definitions of the following outcomes: necrotising enterocolitis; patent ductus arteriosus; retinopathy of prematurity; and early systemic neonatal infection.
Methods updated according to the current Cochrane Pregnancy and Childbirth Group standard methods text (2015). In the 2015 update the prespecified groups for subgroup analysis were edited to describe specific populations of interest .
Contributions of authors
CAC and JEH prepared the original protocol, CAC wrote the draft of the original review, and both CAC and JEH commented on subsequent draft and prepared the previous updates. For the 2011 update, CAC prepared the first draft; CAC, CM and PM prepared the 'Risk of bias' tables, CAC and CM assessed identified studies for eligibility and CAC, CM and PM extracted data for the included trials. All authors commented on subsequent drafts and approved the final version.
All authors (CAC, CM, PM, JEH) commented on subsequent drafts and approved the final version.
Sources of support
Internal sources
Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia.
Liggins Institute, University of Auckland, New Zealand.
External sources
Australian Department of Health and Ageing, Australia.
-
National Institute for Health Research, UK.
NIHR Programme of centrally‐managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS:10/4001/02
Declarations of interest
CAC and JEH are investigators in the Australasian Collaborative Trial of Repeat Doses of Corticosteroid for the Prevention of Neonatal Respiratory Disease (Crowther 2006).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aghajafari 2002 {published data only}
- Aghajafari F, Murphy K, Ohlsson A, Amankwah K, Matthews S, Hannah M. Multiple versus single courses of antenatal corticosteroids for preterm birth: a pilot study. Journal of Obstetrics and Gynaecology Canada: JOGC 2002;24(4):321‐9. [DOI] [PubMed] [Google Scholar]
Crowther 2006 {published data only}
- Ashwood PJ, Crowther CA, Willson KJ, Haslam RR, Kennaway DK, Hiller JE, et al. Neonatal adrenal function after repeat dose prenatal corticosteroids: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;194:861–7. [DOI] [PubMed] [Google Scholar]
- Battin M, Bevan C, Harding J. Repeat doses of antenatal steroids and hypothalamic‐pituitary‐adrenal axis (HPA) function. American Journal of Obstetrics and Gynecology 2007;197:40.e1‐40.e6. [DOI] [PubMed] [Google Scholar]
- Battin M, Bevan C, Harding J. Growth in the neonatal period after repeat courses of antenatal corticosteroids: data from the ACTORDS randomised trial. Archives of Disease in Childhood Fetal & Neonatal Edition 2012;97(2):F99‐F105. [DOI] [PubMed] [Google Scholar]
- Battin MR, Bevan C, Morton SM, Harding JE. Repeat courses of antenatal corticosteroids do not alter hypothalamic‐pituitary‐adrenal axis function after birth; results of a randomised controlled trial. Pediatric Academic Societies Annual Meeting; 2004 May 1‐4; San Francisco, USA. 2004.
- Crosby DD, Ashwood PJ, Khong TY, Crowther CA. Effects of repeat dose prenatal corticosteroids on placental pathology: results from the ACTORDS trial. Journal of Paediatrics and Child Health 2011;47(Suppl 1):53. [Google Scholar]
- Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson JS, et al. Outcome at 2 years of age after repeat doses of antenatal corticosteroids. New England Journal of Medicine 2007;357:1179‐89. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Haslam RR, Doyle LW, Harding JE, Hiller JE, Robinson JS, for the ACTORDS Study Group. Repeat doses of prenatal corticosteroids for women at risk of preterm birth: follow‐up of children at 2 years corrected age in the ACTORDS trial. Journal of Paediatrics and Child Health 2007;43(Suppl 1):A76. [Google Scholar]
- Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS, for the Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet 2006;367:1913‐9. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Hiller JE, Haslam RR, Doyle LW, Robinson JS. Repeat doses of prenatal corticosteroids for women at risk of preterm birth: the ACTORDS Trial 12 month follow up. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:179.
- Mildenhall L, Battin M, Bevan C, Kuschel C, Harding J. Repeat prenatal corticosteroid doses do not alter neonatal blood pressure or myocardial thickness: randomized, controlled trial. Pediatrics 2009;123(4):e646‐e652. [DOI] [PubMed] [Google Scholar]
- Mildenhall L, Battin M, Morton S, Bevan C, Kuschel C, Harding J. Exposure to repeat doses of antenatal glucocorticoids is associated with altered cardiovascular status after birth. Archives of Disease in Childhood. Fetal and Neonatal Edition 2006;91:F56‐F60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildenhall LFJ, Battin MR, Bevan C, Kuschel CA, Harding JE. Repeat doses of antenatal corticosteroids do not alter neonatal cardiovascular status after birth; a randomised controlled trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:55.
Garite 2009 {published data only}
- Garite T, Kurtzman J, Maurel K, Clark R, for the Obstetrix Collaborative Research Network. Impact of a 'rescue course' of antenatal corticosteroids: a multicenter randomized placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2009;200:248.e1‐248.e9. [DOI] [PubMed] [Google Scholar]
- Kurtzman J, Garite T, Clark R, Maurel K, The Obstetrix Collaborative Research Network. Impact of a "rescue course" of antenatal corticosteroids (ACS): a multicenter randomized placebo controlled trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S2. [DOI] [PubMed] [Google Scholar]
- Obstetrix Medical Group. A randomized trial comparing the impact of one versus two courses of ACS on neonatal outcome. ClinicalTrials.gov (www.clinicaltrials.gov) (accessed 20 February 2008).
Guinn 2002 {published data only}
- Guinn D, Atkinson M, Sullivan L, Lee M, MacGregor S, Parilla B, et al. Single versus weekly courses of antenatal corticosteroids for women at risk of preterm delivery: a randomized controlled trial. Obstetrics & Gynecology 2003;101(1):195. [Google Scholar]
- Guinn D, BMZ Study Group. Multicenter randomized trial of single versus weekly courses of antenatal corticosteroids (ACS). American Journal of Obstetrics and Gynecology 2001;184(1):S6. [Google Scholar]
- Guinn DA, Atkinson MW, Sullivan L, Lee M, MacGregor S, Parilla B, et al. Single vs weekly courses of antenatal corticosteroids for women at risk of preterm delivery. JAMA 2001;286(13):1581‐7. [DOI] [PubMed] [Google Scholar]
- Guinn DA, BMZ Study Group. Multicenter randomized trial of single versus weekly courses of antenatal corticosteroids (ACS): interim analysis. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S12. [Google Scholar]
- Lee M, Davies J, Atkinson MW, Guinn D, BMZ Study Group. Efficacy of weekly courses of antenatal corticosteroids (ACS) in preterm premature rupture of the membranes. American Journal of Obstetrics and Gynecology 2001;184(1):S8. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Davies J, Guinn D, Sullivan L, Atkinson MW, McGregor S, et al. Single versus weekly courses of antenatal corticosteroids in preterm premature rupture of membranes. Obstetrics & Gynecology 2004;103(2):274‐81. [DOI] [PubMed] [Google Scholar]
Mazumder 2008 {published data only}
- Mazumder P, Dutta S, Kaur J, Narang A. Single versus multiple courses of antenatal betamethasone and neonatal outcome: a randomized controlled trial. Indian Pediatrics 2008;45:650‐2. [PubMed] [Google Scholar]
McEvoy 2002 {published data only}
- McEvoy C. Effects of single versus weekly courses of antenatal steroids (AS) on functional residual capacity in preterm infants: a randomized trial. Pediatric Research 2001;49(4):388A. [DOI] [PubMed] [Google Scholar]
- McEvoy C, Bowling S, Williamson K, Lozano D, Tolaymat L, Collins J, et al. Effects of single versus weekly courses of antenatal steroids (AS) on functional residual capacity in preterm infants: a randomized trial. Pediatric Academic Societies Annual Meeting; 2001 April 28‐May 1; Baltimore Convention Centre, Baltimore, Maryland, USA. 2001:Abstract no: 2228.
- McEvoy C, Bowling S, Williamson R, Lozano D, Tolaymat L, Izquierdo L, et al. The effect of a single remote course versus weekly courses of antenatal corticosteroids on functional residual capacity in preterm infants: a randomized trial. Pediatrics 2002;110:280‐4. [DOI] [PubMed] [Google Scholar]
McEvoy 2010 {published data only}
- McEvoy C, Schilling D, Clay N, Spitale P, Durand M. Neurodevelopmental outcome and growth in infants randomized to a single rescue course of antenatal steroids. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:3829.270.
- McEvoy C, Schilling D, Peters D, Tillotson C, Spitale P, Wallen L, et al. Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010;202:544.e1‐544.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy C, Schilling D, Segel S, Spitale P, Wallen L, Bowling S, et al. Improved respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized trial. American Journal of Obstetrics and Gynecology 2008;199(Suppl 1):S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy C, Schilling D, Spitale P, Gravett M, Durand M. Pulmonary function and respiratory outcomes at 12‐24 months in preterm infants randomized to a single rescue course of antenatal steroids. Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- McEvoy C, Schilling D, Spitale P, Wallen L, Segel S, Bowling S, et al. Improved respiratory compliance after a single rescue course of antenatal steroids: a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2007 May 5‐8; Toronto, Canada. 2007.
- McEvoy C, Schilling D, Spitale P, Wallen L, Segel S, Bowling S, et al. Improved respiratory compliance after a single rescue course of antenatal steroids: a randomized controlled trial. Pediatric Academic Societies Annual Meeting; 2008 May 2‐6; Honolulu, Hawaii. 2008.
- McEvoy C, Schilling D, Spitale P, Wallen P, Segel S, Bowling S, et al. Growth and respiratory outcomes after a single rescue course of antenatal steroids: a randomized trial. Pediatric Academic Societies Annual Meeting; 2009 May 2‐5; Baltimore, USA. 2009.
- McEvoy CT, Schilling D, Segal S, Spitale P, Wallen L, Bowling S, et al. Improved respiratory compliance in preterm infants < 34 weeks after a single rescue course of antenatal steroids. American Journal of Respiratory and Critical Care Medicine 2009;179:A4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2008 {published and unpublished data}
- Asztalos E, Murphy K, Hannah M, Willan A, MACS Collaborative Group. Outcomes of children at two years after multiple courses of antenatal corticosteroids for threatened preterm birth: the multiple antenatal corticosteroids study (MACS). Pediatric Academic Societies' 2010 Annual Meeting; 2010 May 1‐4; Vancouver, Canada. 2010.
- Asztalos EV, Murphy KE, Hannah ME, Willan AR, Matthews SG, Ohlsson A, et al. Multiple courses of antenatal corticosteroids for preterm birth study: 2‐year outcomes. Pediatrics 2010;126:e1045‐e1055. [DOI] [PubMed] [Google Scholar]
- Maternal Infant and Reproductive Health Research Unit. Multiple courses of antenatal steroids for preterm birth (MACS). Center for Mother, Infant, and Child Research (www.utoronto.ca/miru/macs/protocol02.htm) 2002.
- Murphy K, Asztalos E, Hannah M, Willan A, Hewson S, Ohlsson A, et al. Multiple courses of antenatal corticosteroids for preterm birth study (MACS): results of the three month maternal post partum questionnaire. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S173. [Google Scholar]
- Murphy K, Hannah M, Willan A, Hewson S, Ohlsson A, Kelly E, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet 2008;372:2143‐51. [DOI] [PubMed] [Google Scholar]
- Murphy K, Willan A, Hannah M, Ohlsson A, Kelly E, Matthews S, et al. Do antenatal corticosteroids reduce fetal growth or gestational age at birth? A secondary analysis from the multiple courses of antenatal corticosteroids for preterm birth study (MACS). American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S226. [Google Scholar]
- Murphy K, for the MACS Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S2, Abstract no: 2. [Google Scholar]
- Murphy KE, Hannah ME, Willan AR, Ohlsson A, Kelly EN, Matthews SG, et al. Maternal side‐effects after multiple courses of antenatal corticosteroids (MACS): the three‐month follow‐up of women in the randomized controlled trial of MACS for preterm birth study. Journal of Obstetrics and Gynaecology Canada: JOGC 2011;33(9):909‐21. [PubMed] [Google Scholar]
Peltoniemi 2007 {published data only}
- Janer C, Helve O, Pitkänen OM, Kari MA, Peltoniemi OM, Hallman M, et al. Expression of airway epithelial sodium channel in the preterm infant is related to respiratory distress syndrome but unaffected by repeat antenatal beta‐methasone. Neonatology 2010;97(2):132‐8. [DOI] [PubMed] [Google Scholar]
- Koivisto M, Peltoniemi OM, Saarela T, Tammela O, Jouppila P, Hallman M. Blood glucose level in preterm infants after antenatal exposure to glucocorticoid. Acta Paediatrica 2007;96(5):664‐8. [DOI] [PubMed] [Google Scholar]
- Peltoniemi O, Kari, M, Lano A, Yliherva A, Puosi R, Lehtonen L, et al. Two‐year follow‐up of a randomized trial with repeated antenatal betamethasone. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(6):F402‐F406. [DOI] [PubMed] [Google Scholar]
- Peltoniemi OM. 2‐year follow‐up of randomized trial on a single repeat dose of antenatal betamethasone in imminent preterm birth. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2008 May 2‐6; Honolulu, Hawaii. 2008.
- Peltoniemi OM, Kari MA, Halmesmaki E, Raudaskoski T, Tammela O, Uotila J, et al. The effect of second course of antenatal betamethasone (BM) on neonatal morbidity in preterm infants: a randomised trial. Pediatric Research 2005;58(2):404. [Google Scholar]
- Peltoniemi OM, Kari MA, Jouppila P, Hallman M. The second rescue dose of antenatal betamethasone (BM) shortly before birth may increase respiratory morbidity in high risk preterm infants. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco, CA, USA. 2006.
- Peltoniemi OM, Kari MA, Tammela O, Lehtonen L, Marttila R, Halmesmaki E, et al. Randomized trial of a single repeat dose of prenatal betamethasone treatment in imminent preterm birth. Pediatrics 2007;119(2):290‐8. [DOI] [PubMed] [Google Scholar]
- Pesonen A, Raiikonen K, Lano A, Peltoniemi O, Hallman M, Kari MA. Antenatal betamethasone and fetal growth in prematurely born children: Implications for temperament traits at the age of 2 years. Pediatrics 2009;123:e31‐e37. [DOI] [PubMed] [Google Scholar]
Wapner 2006 {published data only}
- Carroll MA, Vidaeff AC, Mele L, Wapner RJ, Mercer B, Peaceman AM, et al. Bone metabolism in pregnant women exposed to single compared with multiple courses of corticosteroids. Obstetrics & Gynecology 2008;111(6):1352‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church M. Auditory brainstem responses (ABRs) in neonates exposed to repeated courses of antenatal corticosteroids. Program of the Twenty Eighth Annual Midwinter Research Meeting of the Association for Research in Otolaryngology; 2005 Feb 19‐24; New Orleans, Louisiana. 2005:Abstract no: 669.
- Church MW, Wapner RJ, Mele LM, Johnson F, Dudley DJ, Spong CY, et al. Repeated courses of antenatal corticosteroids: Are there effects on the infant's auditory brainstem responses?. Neurotoxicology and Teratology 2010;32(6):605‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca L, Ramin SM, Mele L, Wapner RJ, Johnson F, Peaceman AM, et al. Bone metabolism in fetuses of pregnant women exposed to single and multiple courses of corticosteroids. Obstetrics & Gynecology 2009;114(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashima JN, Lai Y, Wapner RJ, Sorokin Y, Dudley DJ, Peaceman A, et al. The effect of maternal body mass index on neonatal outcome in women receiving a single course of antenatal corticosteroids. American Journal of Obstetrics and Gynecology 2010;202(3):263.e1‐263.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawady J, Mercer B, Wapner R, Zhao, Y, Sorokin Y, Johnson F, et al. The National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network beneficial effects of antenatal repeated steroids study: impact of repeated doses of antenatal corticosteroids on placental growth and histologic findings. American Journal of Obstetrics and Gynecology 2007;281:e1‐e8. [DOI] [PubMed] [Google Scholar]
- Sorokin Y for the NICHD MFMU Network. Effect of maternal BMI, number of courses and timing of antenatal corticosteroids: association with neonatal anthropometric measurements. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S106. [Google Scholar]
- Sorokin Y, Romero R, Mele L, Wapner RJ, Iams JD, Dudley DJ, et al. Maternal serum interleukin‐6, C‐reactive protein, and matrix metalloproteinase‐9 concentrations as risk factors for preterm birth <32 weeks and adverse neonatal outcomes. American Journal of Perinatology 2010;27(8):631‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin Y, Romero R, for the NICHD MFMU Network. Elevated maternal serum IL‐6 and CRP are associated with preterm delivery < 32 weeks and subsequent neonatal intraventricular hemorrhage. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S62. [Google Scholar]
- Wapner R. Long term follow‐up of infants receiving single vs repeat courses of antenatal corticosteroids. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S2. [Google Scholar]
- Wapner R, Sorokin Y, Mele L, Johnson F, Dudley D, Spong CY, et al. Long‐term outcomes after repeat doses of antenatal corticosteroids. New England Journal of Medicine 2007;357:1190‐8. [DOI] [PubMed] [Google Scholar]
- Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. American Journal of Obstetrics and Gynecology 2006;195(3):633‐42. [DOI] [PubMed] [Google Scholar]
- Wapner RJ, for the NICHD MFMU Network. A randomized trial of single versus weekly courses of corticosteroids. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S56. [Google Scholar]
- Wapner RJ, for the NICHD MFMU Network. Maternal and fetal adrenal function following single and repeat courses of antenatal corticosteroids (ACS). American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S5. [Google Scholar]
References to studies excluded from this review
Bontis 2011 {published data only}
- Bontis N, Vavilis D, Tsolakidis D, Goulis DG, Tzevelekis P, Kellartzis D, et al. Comparison of single versus multiple courses of antenatal betamethasone in patients with threatened preterm labor. Clinical and Experimental Obstetrics and Gynecology 2011;38(2):165‐7. [PubMed] [Google Scholar]
Mercer 2001 {published data only}
- Mercer B, Egerman R, Beazley D, Sibai B, Carr T, Sepesi J. Steroids reduce fetal growth: analysis of a prospective trial. American Journal of Obstetrics and Gynecology 2001;184(1):S7. [Google Scholar]
- Mercer B, Egerman R, Beazley D, Sibai B, Carr T, Sepesi J. Weekly antenatal steroids trial in women at risk of preterm birth: a randomized trial. American Journal of Obstetrics and Gynecology 2001;184(1):S6. [Google Scholar]
- Sawady J, Mercer B. Impact of repeated doses of antenatal corticosteroids on placental growth and histology. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S73. [DOI] [PubMed] [Google Scholar]
Romejko‐Wolniewicz 2013 {published data only}
- Romejko‐Wolniewicz E, Oleszczuk L, Zareba‐Szczudlik J, Czajkowski K. Dosage regimen of antenatal steroids prior to preterm delivery and effects on maternal and neonatal outcomes. Journal of Maternal‐Fetal and Neonatal Medicine 2013;26(3):237‐41. [DOI] [PubMed] [Google Scholar]
Thorp 2000 {published data only}
- Thorp JA, Yeast JD, Cohen GR, Wickstrom EA, D' Angelo LJ. Repeated antenatal betamethasone and perinatal outcome. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S21. [Google Scholar]
References to studies awaiting assessment
Atarod 2014 {published data only}
- Atarod Z, Taghipour M, Roohanizadeh H, Fadavi S, Taghavipour M. Effects of single course and multicourse betamethasone prior to birth in the prognosis of the preterm neonates: a randomized, double‐blind placebo‐control clinical trial study. Journal of Research in Medical Sciences 2014;19(8):715‐9. [PMC free article] [PubMed] [Google Scholar]
- Ataroud Z. Single versus multiple courses of antenatal betamethasone: evaluation of preterm infant's outcome. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010). IRCT138711261689N1 2010.
Crowther 2011 {published data only}
- Crowther C, Doyle LW, Anderson P, Harding JE, Haslam RR, Hiller JE. Repeat dose(s) of prenatal corticosteroids for women at risk of preterm birth: early school‐age outcomes (6 to 8 years) for the ACTORDS trial. Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:3123.6.
- Crowther CA, Doyle LW, Anderson P, Harding JE, Haslam RR, Hiller JE, et al. Repeat dose(s) of prenatal corticosteroids for women at risk of preterm birth: Early school‐age outcomes (6 to 8 years) for children in the ACTORDS trial. Journal of Paediatrics and Child Health 2011;47(Suppl 1):52‐3. [Google Scholar]
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics 2015;135(2):e405‐e415. [DOI] [PubMed] [Google Scholar]
- McKinlay CJD, Cutfield WS, Battin MR, Dalziel SR, Crowther CA. Cardiovascular risk factors after exposure to repeat antenatal betamethasone: early school‐age follow‐up of a randomised trial (ACTORDS). Journal of Paediatrics and Child Health 2011;47(Suppl 1):20. [Google Scholar]
- McKinlay CJD, Cutfield WS, Battin MR, Dalziel SR, Crowther CA. Repeat antenatal betamethasone does not affect basal salivary cortisol at early school‐age: a randomised controlled trial (ACTORDS). Journal of Paediatrics and Child Health 2011;47(Suppl 1):19. [Google Scholar]
- McKinlay CJD, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE. Cardiovascular risk factors after exposure to repeat antenatal betamethasone: early school‐age follow‐up of a randomised trial (ACTORDS). Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:1660.5.
- McKinlay CJD, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE. Repeat antenatal betamethasone does not affect basal salivary cortisol at early school‐age: a randomised controlled trial (ACTORDS). Pediatric Academic Societies and Asian Society for Pediatric Research Joint Meeting; 2011 April 30‐May 3; Denver, Colorado, USA. 2011:2919.266.
- McKinlay CJD, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE. Repeat antenatal betamethasone does not affect bone mass at early school‐age: A randomised controlled trial (ACTORDS). Journal of Paediatrics and Child Health 2013;49(Suppl 2):49. [Google Scholar]
- McKinlay CJD, Harding JE, Ashwood PJ, Dalziel SR, Doyle LW, Haslam RR, et al. Effect of repeat antenatal betamethasone on childhood lung function: A randomised controlled trial (ACTORDS). Journal of Paediatrics and Child Health 2013;49(Suppl 2):92‐3. [Google Scholar]
Murphy 2012 {published data only}
- Asztalos E, Willan A, Murphy K, Matthews S, Ohlsson A, Saigal S, et al. Association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years: multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS‐5). BMC Pregnancy and Childbirth 2014;14(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztalos E, Willan A, Murphy K, Matthews S, Ohlsson A, Saigal S, et al. Multiple courses of antenatal corticosteroids for preterm birth study at 5 years of age (MACS‐5): association between gestational age at birth, antenatal corticosteroids, and outcomes at 5 years of age (MACS‐5). American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S321‐S322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asztalos EV, Murphy KE, Willan AR, Matthews SG, Ohlsson A, Saigal S, et al. for the MACS‐5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: Outcomes in children at 5 years of age (MACS‐5). Journal of the American Medical Association Pediatrics 2013;167(12):1102‐10. [DOI] [PubMed] [Google Scholar]
- Murphy KE, Willan AR, Hannah ME, Ohlsson A, Kelly EN, Matthews SG, et al. Effect of antenatal corticosteroids on fetal growth and gestational age at birth. Obstetrics & Gynecology 2012;119(5):917‐23. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Sobhrabvand 2001 {published data only}
- Sohrabvand F, Behbahani B, Kazeminejad A. Effects of single versus multiple courses of corticosteroid therapy on pregnancy results in women with PPROM. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):528. [Google Scholar]
TEAMS 1999 {published and unpublished data}
- Adams H. Trial of the effects of antenatal multiple courses of steroids versus a single course (TEAMS): pilot study. Current Controlled Trials (http://www.controlled‐trials.com) (accessed 4 November 2003) 2003.
- Brocklehurst P, Gates S, Johnson A. Effects of multiple courses of antenatal steroids are uncertain [letter]. BMJ 2000;321:47. [PMC free article] [PubMed] [Google Scholar]
Additional references
Ashwood 2006
- Ashwood PJ, Crowther CA, Willson KJ, Haslam RR, Kennaway DJ, Hiller JE, et al. Neonatal adrenal function after repeat dose prenatal corticosteroids: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;194:861‐7. [DOI] [PubMed] [Google Scholar]
Benediktsson 1993
- Benediktsson R, Lindsay RS, Noble J, Secki JR, Edwards CRW. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet 1993;341:339‐41. [DOI] [PubMed] [Google Scholar]
Brownfoot 2013
- Brownfoot FC, Gagliardi DI, Bain E, Middleton P, Crowther CA. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD006764.pub3] [DOI] [PubMed] [Google Scholar]
Crowther 2012
- Crowther C, Aghajafari F, Askie L, Asztalos E, Brocklehurse P, Bubner T, et al. Repeat prenatal corticosteroid prior to preterm birth: a systematic review and individual participant data meta‐analysis for the PRECISE study group (prenatal repeat corticosteroid international IPD study group: assessing the effects using the best level of evidence) ‐ study protocol. Systematic Reviews 2012;1(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalziel 2005a
- Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30‐year follow‐up of a randomised controlled trial. Lancet 2005;365(9474):1856‐62. [DOI] [PubMed] [Google Scholar]
Dalziel 2005b
- Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ 2005;331(7518):665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunlop 1997
- Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. Journal of Maternal‐Fetal Medicine 1997;6:309‐13. [DOI] [PubMed] [Google Scholar]
Esplin 2000
- Esplin M, Fausett M, Smith S, Oshiro B, Porter TF, Branch DW, et al. Multiple courses of antenatal steroids associated with a delay in long‐term psychomotor development in children with birth weight < 1500 grams. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S24. [Google Scholar]
Fowden 1996
- Fowden AL, Szemere J, Hughes RS, Forhead AJ. The effects of cortisol on the growth rate of the sheep fetus during late gestation. Journal of Endocrinology 1996;151:97‐105. [DOI] [PubMed] [Google Scholar]
French 1998
- French N, Hagan R, Evans S, Godfrey M, Newnham J. Repeated antenatal corticosteroids: behaviour outcomes in a regional population of very preterm infants. Pediatric Research 1998;43:214A. [Google Scholar]
French 1999
- French N, Hagan R, Evans S, Godfrey M, Newnham J. Repeated antenatal corticosteroids: size at birth and subsequent development. American Journal of Obstetrics and Gynecology 1999;180:114‐21. [DOI] [PubMed] [Google Scholar]
French 2004
- French N, Hagan R, Evans S, Mullan A, Newnham J. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behaviour. American Journal of Obstetrics and Gynecology 2004;190:588‐95. [DOI] [PubMed] [Google Scholar]
Hasbargen 2001
- Hasbargen U, Reber D, Versmold H, Schulze A. Growth and development of children to 4 years of age after repeated antenatal steroid administration. European Journal of Pediatrics 2001;160:552‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ikegami 1997
- Ikegami M, Jobe AH, Newnham J, Polk DH, Willet KE, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. American Journal of Respiratory and Critical Care Medicine 1997;156:178‐84. [DOI] [PubMed] [Google Scholar]
Jensen 2002
- Jensen EC, Gallaher BW, Breier BH, Harding JE. The effect of a chronic maternal cortisol infusion on the late gestation fetal sheep. Journal of Endocrinology 2002;174:27‐36. [DOI] [PubMed] [Google Scholar]
Jobe 1994
- Jobe AH. National Institutes of Health. Report of the Consensus Development Conference on the effect of corticosteroids for fetal lung maturation on perinatal outcomes. Bethesda, MD: US Department of Health and Human Services, Public Health Service, 1994:39‐41. [Google Scholar]
Johnson 1993
- Johnson A, Townshend P, Yudkin P, Bull D, Wilkinson AR. Functional abilities at age 4 years of children born before 29 weeks gestation. BMJ 1993;306:1715‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laws 2005
- Laws PJ, Sullivan EA. Australia's mothers and babies 2003. Perinatal Statistics Series no. 16. Sydney: Australian Institute of Health and Welfare, National Perinatal Statistics Unit, 2005. [Google Scholar]
Liggins 1972
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515‐25. [PubMed] [Google Scholar]
McKenna 2000
- McKenna DS, Witter GM, Nagaraja HN, Samuels P. The effects of repeat doses of antenatal corticosteroids on maternal adrenal function. American Journal of Obstetrics and Gynecology 2000;183:669‐73. [DOI] [PubMed] [Google Scholar]
McLaughlin 2003
- McLaughlin KJ, Crowther CA, Walker N, Harding JE. Effects of a single course of corticosteroids given more than 7 days before birth: a systematic review. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43:101‐6. [DOI] [PubMed] [Google Scholar]
Mildenhall 2006
- Mildenhall LF, Battin MR, Morton SMB, Bevan C, Kuschel CA, Harding JE. Exposure to repeat doses of antenatal glucocorticoids is associated with altered cardiovascular status after birth. Archives of Disease in Childhood Fetal and Neonatal Edition 2006;91:F56‐F60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moise 1995
- Moise AA, Wearden ME, Kozinetz CA, Gest AL, Welty SE, Hansen TN. Antenatal steroids are associated with less need for blood pressure support in extremely premature infants. Pediatrics 1995;95:845‐50. [PubMed] [Google Scholar]
Newnham 1999
- Newnham JP, Evans S, Godfrey M, Huang W, Ikegami M, Jobe A. Maternal, but not fetal, administration of corticosteroids restricts fetal growth. Journal of Maternal‐Fetal Medicine 1999;8(3):81‐7. [DOI] [PubMed] [Google Scholar]
NIH 1995
- NIH Consensus Development Panel. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 1995;273:413‐8. [DOI] [PubMed] [Google Scholar]
NIH 2000
- NIH Consensus Development Panel. Antenatal corticosteroids revisited: repeat courses. National Institutes of Health Consensus development conference statement. Obstetrics & Gynecology 2000;98:144‐50. [DOI] [PubMed] [Google Scholar]
Padbury 1996
- Padbury JF, Ervin G, Polk D. Extrapulmonary effects of antenatally administered steroids. Journal of Pediatrics 1996;128:167‐72. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2006
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Rojas‐Reyes 2012
- Rojas‐Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD000510.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seckl 2004
- Seckl JR, Meaney MJ. Glucocorticoid programming. Annals of the New York Academy of Sciences 2004;1032:63‐84. [DOI] [PubMed] [Google Scholar]
Thorp 2002
- Thorp JA, Etzenhouser J, O'Connor M, Jones A, Jones P, Belden B, et al. Effects of phenobarbital and multiple‐dose antenatal/postnatal steroid on developmental outcome at age 7 years. American Journal of Obstetrics and Gynecology 2002;185(6):S87. [DOI] [PubMed] [Google Scholar]
Tschanz 1995
- Tschanz SA, Danke BM, Burri PH. Influence of postnatally administered glucocorticoids on rat lung growth. Biology of the Neonate 1995;68:229‐45. [DOI] [PubMed] [Google Scholar]
Utama 2010
- Utama DP, Crowther CA. Transplacental versus direct fetal corticosteroid treatment for accelerating fetal lung maturation where there is a risk of preterm birth. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008981.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Crowther 2000
- Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD003935] [DOI] [PubMed] [Google Scholar]
Crowther 2007
- Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD003935.pub2] [DOI] [PubMed] [Google Scholar]
Crowther 2011
- Crowther CA, McKinlay CJD, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD003935.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


