Abstract
Background
Few studies have examined long-term neurocognitive outcomes in survivors of childhood soft tissue sarcoma.
Methods
150 survivors (41% female; mean[SD] current age 33[8.9] years; time since diagnosis 24[8.7] years) and 349 community controls (56% female; age 35[10.2] years) completed comprehensive neuropsychological testing, echocardiography, electrocardiography, pulmonary function tests, endocrine evaluation, and physical examination. Patient-reported outcomes of health-related quality of life (HRQOL) and social attainment were collected. Survivors were compared to norms and controls on neurocognitive outcomes using general linear models, and on HRQOL and social attainment using modified Poisson models. The impacts of treatment and chronic health conditions on outcomes were examined using multivariable general linear models (effect size expressed as unstandardized β estimates that reflect unit of change from mean = 0 and standard deviation = 1) and modified Poisson models (effect size expressed as relative risks).
Results
Compared to controls and population norms, survivors demonstrated lower performance on measures of verbal reasoning (mean z-score[SD] −0.45[1.15], p<0.001) mathematics (−0.63[1.07], p<0.001), and long-term memory (−0.37[1.14], p<0.001). Cumulative anthracycline exposure (per 100mg/m2) was associated with poorer verbal reasoning (β=−0.14 Z-scores, p=.04), reading (β=−0.09 Z-scores, p=.04) and patient-reported vitality (relative risk= 1.32, 95% confidence interval 1.09–1.59). Neurologic and neurosensory chronic conditions were associated with poorer mathematics (neurologic conditions β=−0.63 Z-scores, p=0.02; hearing impairment: β=−0.75 Z-scores, p<0.01). Better cognitive performance was associated with higher social attainment.
Conclusion
Long-term survivors of soft tissue sarcoma are at risk for neurocognitive problems and poor HRQOL associated with anthracycline treatment and chronic health conditions.
Keywords: Survivorship, childhood soft tissue sarcoma, neurocognition, health related quality of life, anthracycline and cognition
Precis
Long-term survivors of soft tissue sarcoma are at risk for neurocognitive problems and poor HRQOL associated with anthracycline treatment and chronic health conditions. Survivors should be offered appropriate interventions.
Introduction
Previous investigations have observed 84% of soft tissue sarcoma (STS) survivors report have at least one chronic health condition, with 42% reporting at least one severe, disabling or life-threatening condition.1 Further, 14% report experiencing performance limitations in their everyday life and 10% endorse health-related limitations affecting work/school attendance.
Little is known about neurocognitive functioning in survivors of STS, though reports from 613 STS survivors in the Childhood Cancer Survivor Study, compared to 382 siblings, indicate survivors self-reported more problems with task efficiency (effect size = 0.14).2 A Danish study reported no difference in school grades between survivors of STS and healthy controls,3 whereas U.S. investigators identified school problems (e.g. behavior problems, learning disabilities or intellectual disability) in up to 16% of such survivors.4 In studies on health-related quality of life (HRQOL), survivors of STS more often endorse impaired physical, but not mental health problems compared to siblings.5
As long-term STS survivors experience chronic health conditions, conditions which have recently been associated with neurocognitive impairment in other childhood cancer diagnosis,6, 7 these survivors may also be at risk for impairment. Reports of direct neuropsychological testing in long-term STS survivors are lacking. The aims of the current study were to examine neurocognitive function, HRQOL, and social attainment in adult survivors of STS compared to population norms and community controls, and to identify demographic, treatment exposures, and chronic health conditions associated with impairment.
Methods
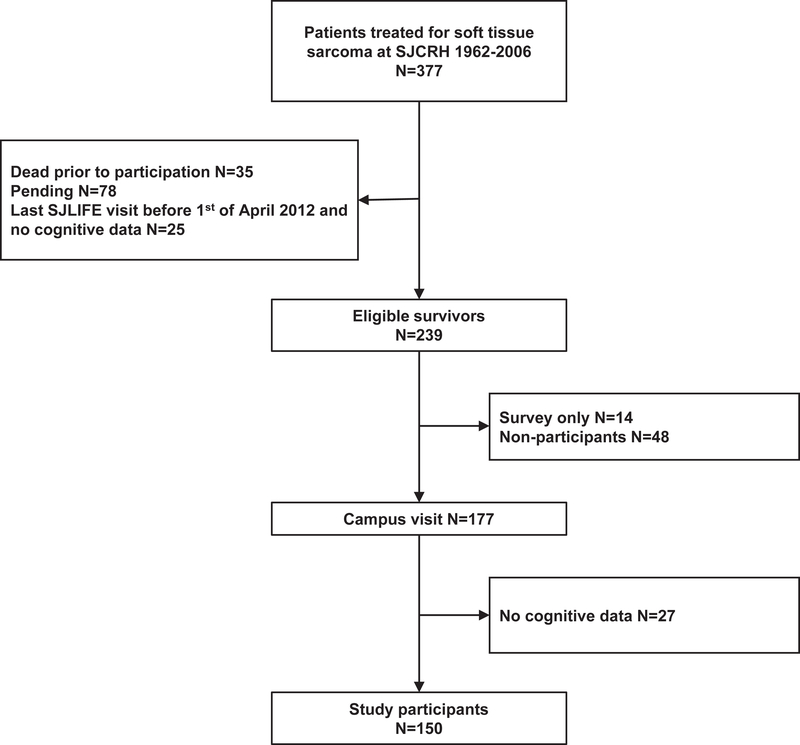
This study was conducted within the St. Jude Lifetime Cohort Study (SJLIFE), an ongoing longitudinal study of ≥5 year survivors treated for childhood cancer at St. Jude Children’s Research Hospital, Memphis, USA.8 Survivors are followed prospectively with physical, psychosocial and neurocognitive evaluations. Prior to April 1, 2012, only survivors who were exposed to neurosurgery, cranial radiation, intrathecal methotrexate, or high-dose intravenous methotrexate or cytarabine were eligible for neurocognitive testing. Beginning on April 1, 2012, eligibility criteria were changed, and all participants were considered eligible for neurocognitive testing. For the present study, survivors of STS who were at least 18 years old and at least 10 years from diagnosis were included in analyses. Exclusion criteria were CNS relapse, pre-existing non-cancer-related genetic or neurodevelopmental disorder associated with neurocognitive impairment, brain injury unrelated to cancer and non-proficiency in English. A total of 239 survivors were eligible for the present study, 62 declined clinical assessment and 27 survivors did not complete neurocognitive testing, leaving 150 (63%) participants (Figure 1). A community control group (n=349) was recruited from non-first-degree relatives and friends of survivors. The study was approved by the IRB and all participants provided informed written consent for participation.
Figure 1.
CONSORT diagram
Measures
Neurocognitive tests/subtests (Table S1) included: Wechsler Abbreviated Scale of Intelligence (WASI; vocabulary and matrix reasoning),9 Wechsler Adult Intelligence scale (WAIS; coding, symbol search, digit span),10 Woodcock Johnson Tests of Achievement III (WJ-III [letter word identification and calculation]),11 Conners Continuous Performance Test II (CPT-II [variability, omissions, perseverations]),12 California Verbal Learning Test II (CVLT-II; total trial 1–5 and long delay free recall),13 Trail Making Test (TMT; part A & part B),14 Test of Memory and Learning II (TOMAL-II; visual selective reminding),15 and Controlled Oral Word Association Test (COWAT; FAS).16 Testing was conducted by masters-level licensed examiners under the supervision of a board-certified neuropsychologist in a fixed order, arranged to reduce distractions and fatigue. All cognitive measures were entered as continuous variables in the multivariable models, higher numbers representing better outcome.
Patient-reported outcomes were administered to assess HRQOL and social attainment. For HRQOL the Medical Outcomes Survey 36 Short Form survey (SF-36)17 was used, which includes eight subscales (physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitation due to emotional problems, mental health). For the multivariable models, HRQOL outcomes were dichotomized as impaired (> 1 SD below the mean) and non-impaired. The following social attainment variables were used: education (college graduate or higher vs <college graduate), employment (full-time vs < full-time), marriage (never vs ever), household income (<$40,000 vs ≥$40,000), independent living (yes/no) and health insurance (yes/no).
Medical record abstraction was performed for treatment exposure. Cumulative doses of vincristine, dactinomycin, alkylating agents (cyclophosphamide equivalent dose), anthracyclines (doxorubicin and daunorubicin, doxorubicin equivalent dose), and etoposide were used. Radiation dosimetry was calculated from treatment records of radiation fields, source and doses to cranium, abdomen and chest.18 Chronic health conditions were grouped by organ system and graded per a modified version of the Common Terminology Criteria for Adverse Events.8 Conditions at the time of testing were dichotomized as none/mild (grade 0–1) vs moderate to life threatening (grade 2–4). Exploratory analysis was done for specific conditions within each organ system associated with any outcome measure.
Statistical analysis
Measures of neurocognitive function and HRQOL were converted to age-adjusted z-scores (μ=0, σ=1) using national normative data. Chi-square was used to compare categorical variables and Student’s t-test to compare continuous variables between survivors and the community controls. Further, analyses of outcomes of interest were adjusted for variables that differed between survivors and controls.
General linear models were fitted to compare differences between survivors and controls on neurocognitive measures, adjusting for race and sex, with p-values adjusted for false discovery rates. Differences on HRQOL and social attainment outcomes between survivors and controls were compared using modified Poisson regression models and adjusted for current age, with p-values again adjusted for false discovery rates.53 Using one-sample t-tests, the survivor group was compared to the population expected mean of 0, p-values adjusted for false discovery rates. Those outcomes that were significantly below community controls and population norms (p < 0.05) were examined for associations with treatment exposures and chronic health conditions (in separate models) adjusting for age at diagnosis and race. Associations of treatment exposures and chronic health conditions with HRQOL and social attainment outcomes were examined using multivariable modified Poisson regression models. Cranial radiation, chemotherapy agents and chronic conditions to be included as predictors in multivariable models were identified a priori based on known prevalence and previous literature. Analyses were conducted using Statistical Analysis System software version 9.4 (SAS Institute, Cary, NC). Statistical significance level was set to p<0.05.
Results
Demographic, disease, and treatment data are shown in Table 1. Survivors were more likely to be male and non-Hispanic black than controls. Subsequent analyses were adjusted for race and sex, though sex was not associated with outcomes and was dropped from final models. 19% of survivors received cranial, 11% abdominal and 11% chest radiation. Chronic conditions were more common in survivors compared to controls.
Table 1.
Demographics, treatment and disease data, chronic conditions
| Survivors n=150 | Community Controls n=349 | ||
| Variable | M (SD) | M (SD) | |
| Age at evaluation, years | 33.3 (8.9) | 35.1 (10.2) | |
| Age at diagnosis, years | 9.1 (5.5) | ||
| Time since diagnosis, years | 24.3 (8.7) | ||
| Sex | N (%) | N (%) | |
| Female | 61 (40.7) | 194 (55.6) | |
| Male | 89 (59.3) | 155 (44.4) | |
| Race/Ethnicity | |||
| Non-Hispanic White | 111 (74.0) | 297 (85.6) | |
| Non-Hispanic Black | 35 (23.3) | 20 (5.7) | |
| Other | 4 (2.7) | 30 (8.6) | |
| Diagnosis | |||
| Rhabdomyosarcoma | 91 (60.7) | ||
| Non-rhabdomyosarcoma soft tissue sarcoma | 59 (39.3) | ||
| Tumor location | |||
| Chest/abdomen | 21 (14.0) | ||
| Extremities | 30 (20.0) | ||
| Head/neck | 65 (43.3) | ||
| Pelvis/Genitourinary region | 34 (22.7) | ||
| Radiation, cGy | M (SD)§ | ||
| Cranial radiation | 28 (18.7) | 4400 (1377) | |
| Chest radiation | 17 (11.3) | 4124 (1630) | |
| Abdominal radiation | 17 (11.3) | 4253 (1655) | |
| Chest and abdominal radiation dose | 8 (5.3) | 3425 (1601) | |
| Chemotherapy, mg/m2 | M (SD)§ | ||
| Vincristine | 114 (76.0) | 34.97 (16.6) | |
| Dactinomycin | 99 (66.0) | 12.52 (6.1) | |
| Alkylating agents CED† | 104 (69.3) | 16453.93 (9284.9) | |
| Anthracycline DED‡ | 67 (44.7) | 274.11 (104.9) | |
| Etoposide | 15 (10.0) | 2333.24 (1278.1) | |
| Intrathecal methotrexate | 2 (1.3) | 84.95 (99.1) | |
| High dose methotrexate | 10 (6.7) | 43734.96 (30847.3) | |
| Chronic conditions grade 2–4 at assessment | |||
| Cardiovascular | 22 (14.7) | 35 (10.0) | |
| Respiratory | 35 (23.3) | 20 (5.7) | |
| Reproductive | 46 (30.7) | 33 (9.5) | |
| Endocrine | 85 (56.7) | 149 (42.7) | |
| Renal | 1 (0.7) | 4 (1.1) | |
| Musculoskeletal | 21 (14.0) | 15 (4.3) | |
| Neurology | 23 (15.3) | 12 (3.4) | |
| Hearing impairment | 22 (14.7) | 3 (0.9) | |
| Vision impairment | 10 (6.7) | 3 (0.9) | |
Compared to normative data and controls, survivors demonstrated poorer verbal reasoning, reading, mathematics, sustained attention, verbal learning, long-term verbal memory, visual memory, working memory, cognitive flexibility, and initiation (Table 2). Survivors had significantly lower HRQOL on all measures compared to the control group, and nearly all subscales were lower than normative data. Survivors had lower social attainment compared to controls; fewer graduated from college (31% vs. 55%), worked full-time (60% vs. 74%), or were living independently (68% vs. 87%). Survivors were more likely to report a lower household income (51% vs 21% earning less than $40000 per year)
Table 2.
Cognition, HRQOL and social attainment
| Survivors | Controls | FDR-P vs. norms | FDR-P vs. control | |||
|---|---|---|---|---|---|---|
| M (SD) | %Imp | M (SD) | %Imp | |||
| Global Cognition | ||||||
| Verbal reasoning | −0.45(1.15) | 25.5% | 0.19(0.9) | 6.6% | <0.001 | <0.001 |
| Non-verbal reasoning | 0.06(0.88) | 10.1% | 0.27(0.73) | 3.7% | 0.48 | 0.065 |
| Academics | ||||||
| Reading | −0.37(0.66) | 8.8% | 0(0.54) | 1.7% | <0.001 | <0.001 |
| Mathematics | −0.63(1.07) | 25.0% | −0.12(0.81) | 7.8% | <0.001 | <0.001 |
| Attention | ||||||
| Sustained attention: omissions | −0.23(1.52) | 14.5% | 0.14(1.11) | 7.2% | 0.101 | 0.011 |
| Sustained attention: variability | −0.35(1.28) | 19.3% | −0.03(1.1) | 11.3% | 0.0034 | 0.017 |
| Focused attention | 0.01(1.23) | 12.1% | 0.58(0.83) | 2.9% | 0.98 | <0.001 |
| Processing speed | ||||||
| Visuomotor | −0.15(1.03) | 16.8% | 0.45(0.94) | 3.7% | 0.101 | <0.001 |
| Cognitive | 0.22(1.14) | 12.5% | 0.64(0.91) | 1.4% | 0.050 | 0.0011 |
| Memory | ||||||
| Memory span | 0(1) | 5.3% | 0.15(0.94) | 4.6% | 0.98 | 0.15 |
| Verbal learning | −0.18(1.04) | 18.4% | 0.35(1) | 7.5% | 0.048 | <0.001 |
| Long-term verbal memory | −0.37(1.14) | 20.0% | 0.11(1.03) | 9.2% | <0.001 | <0.001 |
| Visual memory | −0.45(1.13) | 30.1% | −0.06(0.97) | 11.8% | <0.001 | 0.0015 |
| Executive function | ||||||
| Working memory | −0.23(0.98) | 7.3% | 0.02(0.86) | 2.3% | 0.0092 | 0.0096 |
| Self-monitoring | −0.28(1.37) | 20.0% | −0.12(1.48) | 12.7% | 0.025 | 0.43 |
| Cognitive flexibility | −0.52(1.73) | 23.0% | 0.3(1.18) | 7.5% | <0.001 | <0.001 |
| Initiation | −0.21(1.04) | 18.8% | 0.08(1.08) | 13.0% | 0.025 | 0.0071 |
| Health Related Quality of Life SF-36 | ||||||
| Physical function | −0.16(1.1) | 20.6% | 0.39(0.7) | 4.7% | 0.097 | <0.001 |
| Role limitations physical problems | −0.26(1.22) | 27.7% | 0.43(0.67) | 6.0% | 0.022 | <0.001 |
| Bodily pain | −0.22(1.22) | 27.0% | 0.41(0.91) | 8.9% | 0.047 | <0.001 |
| General health | −0.56(1.2) | 36.4% | 0.26(0.91) | 7.4% | <0.001 | <0.001 |
| Vitality | −0.2(1.15) | 24.1% | 0.15(0.97) | 14.5% | 0.052 | <0.001 |
| Social functioning | −0.34(1.14) | 22.0% | 0.18(0.86) | 8.6% | 0.0018 | <0.001 |
| Role limitations emotional problems | −0.29(1.31) | 22.1% | 0.22(0.79) | 9.0% | 0.018 | <0.001 |
| Mental health | −0.28(1.24) | 24.8% | 0.07(0.92) | 15.0% | 0.018 | 0.0002 |
| Social attainment | N (%) | N (%) | ||||
| Education: college graduate or more | 46 (30.7) | 190 (54.6) | <0.001 | |||
| Employment: full time | 84 (59.6) | 248 (74.0) | 0.0026 | |||
| Marital status: ever married | 97 (69.3) | 267 (79.5) | 0.091 | |||
| Household income: ≥$ 40 000 | 56 (49.1) | 249 (79.0) | <0.001 | |||
| Independent living | 95 (68.3) | 294 (87.0) | <0.001 | |||
| Health insurance | 108 (75.5) | 294 (87.5) | 0.013 | |||
Z-scores, M=0, SD=1, higher score indicates better outcome. %Imp = percent of the group falling in the impaired range, defined as a score in the bottom 10th percentile of the normal distribution. Comparisons between survivors and controls were adjusted for difference in race, sex and age. Multiple comparisons between survivors and controls and survivors and population norms were adjusted using false discovery rates (FDR).
Neither tumor location nor treatment with cranial radiation was associated with neurocognitive, HRQOL or social attainment outcomes. Higher exposure to anthracyclines was associated with worse performance in verbal reasoning (β =−0.14, p=0.04), reading (β =−0.09, p=0.04), and initiation (β =−0.14, p=0.02; Table 3) [Note: β represents the non-standardized estimate, unit = z-score/ (100mg/m2); e.g., with average anthracycline dose of 274.11 mg/m2, verbal reasoning score was 0.38 standard deviations lower]. Higher doses of anthracyclines were also associated with poorer patient-reported HRQOL (Supplemental Table S2), including role limitation due to physical health (risk ratio (RR)=1.25, 95% confidence interval (CI)=1.06–1.47), vitality (RR=1.32, 95%CI=1.09–1.59), and mental health (RR=1.26, 95%CI=1.06–1.51).
Table 3.
Treatment related to cognitive outcomes among survivors (n=150)
| Verbal reasoning | Reading | Mathematics | Attention variability | Verbal learning | ||||||
| β | P | β | P | β | P | β | P | β | P | |
| Vincristine, per 10 mg/m2 | 0.10 | 0.15 | 0.07 | −0.11 | 0.02 | 0.74 | −0.11 | −0.19 | 0.02 | 0.74 |
| Dactinomycin, per 10 mg/m2 | −0.33 | 0.07 | −0.20 | 0.06 | −0.16 | 0.37 | 0.05 | 0.80 | −0.16 | 0.37 |
| Alkylating agents, CED†, per 10 000 mg/m2 | 0.06 | 0.63 | 0.01 | 0.92 | −0.03 | 0.77 | 0.10 | 0.47 | −0.03 | 0.77 |
| Anthracycline, per 100 mg/m2, DED‡ | −0.14 | 0.04 | −0.09 | 0.04 | −0.01 | 0.90 | −0.14 | 0.08 | −0.01 | 0.90 |
| Cranial radiation therapy | 0.08 | 0.73 | 0.16 | 0.27 | 0.13 | 0.58 | 0.10 | 0.72 | 0.13 | 0.58 |
| Long-term verbal memory | Visual memory | Working memory | Cognitive flexibility | Initiation | ||||||
| β | P | β | P | β | P | β | P | β | P | |
| Vincristine, per 10 mg/m2 | 0.08 | 0.27 | 0.02 | 0.80 | 0.16 | 0.01 | −0.01 | 0.92 | 0.04 | 0.56 |
| Dactinomycin, per 10 mg/m2 | −0.35 | 0.06 | −0.40 | 0.03 | −0.23 | 0.14 | −0.21 | 0.44 | −0.08 | 0.63 |
| Alkylating agents, CED†, per 10 000 mg/m2 | 0.09 | 0.46 | 0.11 | 0.38 | −0.10 | 0.30 | −0.22 | 0.23 | −0.13 | 0.21 |
| Anthracycline, per 100 mg/m2 DED‡ | 0.05 | 0.46 | 0.04 | 0.53 | −0.06 | 0.27 | −0.20 | 0.05 | −0.14 | 0.02 |
| Cranial radiation therapy | 0.27 | 0.28 | −0.17 | 0.51 | 0.19 | 0.37 | 0.22 | 0.56 | 0.16 | 0.48 |
Separate models for each cognitive outcome and for radiation therapy. Chemotherapeutic agents in one model. Models adjusted for age at diagnosis, race/ethnicity.
CED=cyclophosphamide equivalent dose.
DED=doxorubicin equivalent dose.
Bold text indicates significant models. β represents the non-standardized coefficient; cognitive measures in z-scores, m=0, sd=1.
The presence of at least moderate hearing impairment was associated with lower performance on measures of verbal reasoning (β=−0.68, p<0.01), mathematics (β=−0.75, p<0.01), verbal learning (β=−0.48, p=0.05) and long-term verbal memory (β=−0.66, p=0.01). Having a neurologic condition (grade 2–4) was associated with lower performance on mathematics (β=−0.63, p=0.02), sustained attention (β=−1.17, p<0.01) and initiation (β=−0.56, p=0.02). Survivors had a higher prevalence of peripheral sensory (5.3% vs 1.4%) and motor neuropathy (6.0% vs 0%) compared to community controls (Table S4).
Neurologic conditions were associated with poor patient-reported HRQOL (Supplementary Table S3), including poor physical function (RR = 2.90, 95%CI=1.64–5.14), role limitations due to physical (RR=2.27, 95%CI=1.34–3.86), poor general health (RR=1.99, 95%CI=1.33–2.98), and impaired social functioning (RR=2.09, 95%CI=1.11–3.95). Cardiovascular conditions were associated with poorer general health (RR=1.96, 95%CI=1.30–2.96) and vitality (RR=2.06, 95%CI=1.12–3.79), while respiratory conditions were associated with poorer role limitations due to physical health problems (RR=1.78, 95%CI=1.07–2.98), and bodily pain (RR=1.70, 95%CI=1.02–2.82).
Better neurocognitive performance was associated with higher social attainment, including education, employment, and household income (Table 5). One standard deviation higher performance on any given neurocognitive test was associated with a 4–32% greater chance of having a better social outcome (e.g. verbal reasoning: college graduate or higher RR=1.11, 95%CI=1.07–1.15, full-time employment RR=1.23, 95%CI=1.09–1.40, household income >$40000 RR=1.10, 95%CI=1.05–1.16). Better cognitive outcome was also associated with a lower risk of impairment in most domains of HRQOL (supplemental Table S5), RRs ranging from 0.70 (cognitive processing speed and role limitation due to physical health) to 0.87 (cognitive flexibility and mental health).
Table 5.
Cognitive measures related to social attainment
| Educational attainment | Employment | Household income | Independent living | Health Insurance | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI |
| Verbal reasoning | 1.11 | 1.07–1.15 | 1.23 | 1.09–1.40 | 1.10 | 1.05–1.16 | 1.12 | 1.01–1.24 | 1.04 | 0.96–1.14 |
| Reading | 1.19 | 1.11–1.27 | 1.29 | 1.01–1.64 | 1.11 | 1.02–1.21 | 1.32 | 1.09–1.61 | 1.17 | 0.98–1.40 |
| Mathematics | 1.14 | 1.09–1.20 | 1.19 | 1.06–1.35 | 1.11 | 1.05–1.17 | 1.11 | 0.99–1.24 | 1.03 | 0.93–1.14 |
| Sustained attention | 1.04 | 1.01–1.09 | 1.19 | 1.04–1.36 | 1.06 | 1.02–1.11 | 1.02 | 0.92–1.13 | 1.10 | 0.99–1.21 |
| Verbal learning | 1.08 | 1.02–1.15 | 1.10 | 0.96–1.26 | 1.03 | 0.98–1.09 | 1.01 | 0.89–1.14 | 1.00 | 0.92–1.09 |
| Long-term verbal memory | 1.07 | 1.01–1.14 | 1.13 | 0.99–1.28 | 1.03 | 0.97–1.08 | 1.02 | 0.91–1.14 | 1.00 | 0.93–1.08 |
| Visual memory | 1.06 | 1.01–1.12 | 1.09 | 0.96–1.24 | 1.01 | 0.96–1.07 | 1.01 | 0.91–1.13 | 0.97 | 0.89–1.07 |
| Working memory | 1.06 | 1.01–1.12 | 1.08 | 0.94–1.23 | 1.05 | 0.99–1.11 | 1.06 | 0.95–1.19 | 0.94 | 0.84–1.04 |
| Cognitive flexibility | 1.03 | 0.99–1.07 | 1.12 | 1.01–1.24 | 1.05 | 0.99–1.10 | 1.12 | 1.03–1.22 | 1.02 | 0.96–1.09 |
| Initiation | 1.06 | 1.01–1.11 | 1.24 | 1.08–1.42 | 1.05 | 0.99–1.11 | 1.08 | 0.96–1.21 | 1.11 | 1.02–1.20 |
Adjusted for age at diagnosis and race/ethnicity. RR=Risk ratio, reflecting chances for better outcome. Bold text indicates significant models.
Discussion
Survivors of STS have not historically been considered at risk for adverse neurocognitive outcomes. Compared to non-cancer controls and population norms, STS survivors in the current study performed worse on measures of intelligence, academics, attention, memory, and executive functioning. We also observed significant associations between treatment exposures, chronic health conditions and neurocognitive performance. Importantly, neurocognitive deficits were associated with reduced social attainment and quality of life, suggesting that survivors of STS may require neurocognitive surveillance and access to interventions.
Treatment with anthracyclines was associated with worse neurocognitive and HRQOL outcomes. This association has not previously been reported in other studies of survivors of childhood cancer,6, 19 perhaps because of the generally higher doses of anthracyclines used in STS patients who also do not receive central nervous system directed therapy that can overshadow effects from other chemotherapy agents. Anthracyclines are associated with cardiotoxicity,20 which in turn could lead to neurocognitive impairment.21 However, in this study, chronic cardiac conditions were not associated with worse neurocognitive outcomes, which may be due to use of gross chronic condition grading rather than more sensitive cardiac imaging. Anthracyclines have been associated with neurocognitive impairment in adult survivors of breast cancer.22 Research has shown that anthracyclines can penetrate the blood-brain-barrier in mice,23 though such evidence in humans is lacking. Recent studies have suggested CNS toxicity develops due to peripheral inflammatory cytokines and oxidative stress induced in the brain, disturbances in mitochondrial respiration, and depletion of CNS antioxidants.24 In those studies anthracyclines have been associated with temporary neurocognitive impairments, during and immediately after treatment, but results from our study suggest a more long-term effect.
In contrast to earlier studies2, 25 we did not find an association between outcome measures and primary tumor site or CRT. This is likely due to the fact that only 28 (19%) of the survivors received CRT, with tumor and CRT location being highly variable. Studies of survivors of CNS tumors and leukemia have shown whole brain radiation to be more detrimental to cognitive functions than focal radiation,26, 27 though none of the survivors in our study received whole brain radiation. The target of radiation for STS is not brain matter, as it is in leukemia or brain tumors, but rather the soft tissue on the outside of the skull. This may result in less total brain volume being directly treated with radiation than in leukemia or CNS tumors. Regarding focal CRT, studies have found midline and medial temporal structures of the brain, especially the hippocampus and hypothalamus to be more vulnerable to radiation therapy,28, 29 though these regions are not as likely to have been exposed during radiation for STS. Still, neurocognitive deficits have been reported in adult-onset head/neck cancer patients following treatment with chemotherapy and/or radiation.30
Having a moderate to severe neurologic condition was associated with both worse neurocognitive performance and poor HRQOL. The most common neurologic condition was peripheral neuropathy. Peripheral neuropathy is generally considered an acute effect of certain chemotherapies (vincristine), but several recent studies have suggested that the condition might be long-lasting and associated with severe disability and reduction of quality of life.31 Associations between long-term peripheral neuropathy and development of neurocognitive dysfunction should be further investigated.
Hearing impairment was associated with reduced scores on verbal tests and reduced ratings of HRQOL. The former is in line with one previous study, showing hearing impairment to be associated with reading skills and general ability in survivors of childhood medulloblastoma.28 Our findings support the importance of detecting and providing rehabilitation for hearing impairment in survivors of childhood soft tissue sarcoma.
There are several limitations worth noting. Survivors were diagnosed over a long period of time (1969–2004) and cancer treatment protocols have changed over those years. Further, radiation treatment records are inconsistent over this time, which limits precision in calculated dosimetry. This is a single-institution-sample, generalization to other samples may be reduced. This is a cross-sectional study and conclusions cannot be drawn on causality or temporal associations. Further research should include pre-treatment testing and longitudinal follow-up to explore potential causal associations (e.g. neurocognitive impairment primary or secondary to tumor and disease). Finally, specific types of chronic conditions vary across the relatively small sample and limit the ability to examine associations beyond the gross organ level. Future research is needed to clarify such mechanisms.
Despite these limitations, the current study of STS survivors and community controls demonstrated neurocognitive problems using objective direct neuropsychological testing with well-established test instruments. Survivors demonstrated impairment in several neurocognitive and HRQOL domains. Chemotherapy treatment and chronic health conditions appear to be associated with these problems. Early interventions that mitigate the development of chronic health conditions may reduce risk for poor functional and quality of life outcomes.
Supplementary Material
Table 4.
Chronic conditions related to cognitive measures among survivors (n=150)
|
Chronic conditions Grade 2–4 |
Verbal reasoning | Reading | Mathematics | Attention variability | Verbal learning | |||||
| β | P | β | P | β | P | β | P | β | P | |
| Cardiovascular | −0.06 | 0.83 | 0.18 | 0.27 | 0.15 | 0.56 | 0.24 | 0.44 | 0.13 | 0.59 |
| Respiratory | −0.04 | 0.86 | −0.17 | 0.21 | −0.26 | 0.22 | −0.21 | 0.38 | 0.15 | 0.46 |
| Reproductive | −0.13 | 0.52 | −0.17 | 0.15 | −0.05 | 0.79 | 0.10 | 0.68 | −0.05 | 0.78 |
| Endocrine | 0.10 | 0.61 | −0.04 | 0.75 | −0.02 | 0.93 | −0.20 | 0.34 | −0.05 | 0.76 |
| Musculoskeletal | −0.25 | 0.35 | −0.19 | 0.25 | −0.66 | 0.01 | 0.08 | 0.79 | −0.15 | 0.55 |
| Neurology | −0.36 | 0.16 | −0.05 | 0.77 | −0.63 | 0.02 | −1.17 | <0.01 | −0.10 | 0.68 |
| Hearing impairment | −0.68 | <0.01 | −0.24 | 0.13 | −0.75 | <0.01 | 0.16 | 0.61 | −0.48 | 0.05 |
|
Chronic conditions Grade 2–4 |
Long-term verbal memory | Visual memory | Working memory | Cognitive flexibility | Initiation | |||||
| β | P | β | P | β | P | β | P | β | P | |
| Cardiovascular | 0.13 | 0.62 | 0.09 | 0.76 | 0.01 | 0.97 | −0.29 | 0.48 | −0.29 | 0.23 |
| Respiratory | 0.12 | 0.58 | 0.01 | 0.98 | −0.10 | 0.61 | −0.19 | 0.55 | −0.25 | 0.21 |
| Reproductive | 0.12 | 0.55 | 0.003 | 0.99 | −0.03 | 0.85 | −0.13 | 0.67 | −0.19 | 0.30 |
| Endocrine | 0.08 | 0.69 | 0.15 | 0.44 | −0.05 | 0.74 | 0.06 | 0.83 | 0.00 | 1.00 |
| Musculoskeletal | 0.05 | 0.86 | −0.47 | 0.09 | −0.18 | 0.44 | −1.07 | <0.01 | −0.07 | 0.79 |
| Neurology | −0.05 | 0.86 | −0.38 | 0.16 | −0.01 | 0.95 | −0.63 | 0.10 | −0.56 | 0.02 |
| Hearing impairment | −0.66 | 0.01 | −0.40 | 0.17 | −0.32 | 0.15 | −0.56 | 0.17 | −0.17 | 0.49 |
Separate models for each cognitive outcome and each organ system. All models adjusted for age at diagnosis and race/ethnicity. β represents the non-standardized coefficient; units: Cognitive measures: z-scores, M=0, SD=1, condition grade 2–4 as compared to grade 0–1. Bold text indicates significant models.
Acknowledgments
Funding: This study was supported by the National Cancer Institute (CA195547, M. Hudson and L. Robison Principal Investigators). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1.Punyko JA, Gurney JG, Scott Baker K, et al. Physical impairment and social adaptation in adult survivors of childhood and adolescent rhabdomyosarcoma: A report from the Childhood Cancer Survivors Study. Psycho-Oncology. 2007;16: 26–37. [DOI] [PubMed] [Google Scholar]
- 2.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive Functioning in Adult Survivors of Childhood Non-Central Nervous System Cancers. Journal of the National Cancer Institute. 2010;102: 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen KK, Duun-Henriksen AK, Frederiksen MH, Winther JF. Ninth grade school performance in Danish childhood cancer survivors. British Journal of Cancer. 2017;116: 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raney RB, Asmar L, Vassilopoulou-Sellin R, et al. Late complications of therapy in 213 children with localized, nonorbital soft-tissue sarcoma of the head and neck: A descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)-II and - III. IRS Group of the Children’s Cancer Group and the Pediatric Oncology Group. Medical and Pediatric Oncology. 1999;33: 362–371. [DOI] [PubMed] [Google Scholar]
- 5.McDougall J, Tsonis M. Quality of life in survivors of childhood cancer: a systematic review of the literature (2001–2008). Supportive Care in Cancer. 2009;17: 1231. [DOI] [PubMed] [Google Scholar]
- 6.Ehrhardt MJ, Mulrooney DA, Li C, et al. Neurocognitive, psychosocial, and quality-of-life outcomes in adult survivors of childhood non-Hodgkin lymphoma. Cancer. 2018;124: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol. 2012;30: 3618–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiology, Biomarkers and Prevention. 2017;26: 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wechsler D Wechsler Abbreviated Scale of Intelligence, second edition. San Antonio, TX: Pearson, 2011. [Google Scholar]
- 10.Wechsler D Wechsler Adult Intelligence Scale - third edition, technical manual. San Antonio, TX: The Psychological Corporation, 1997. [Google Scholar]
- 11.Woodcock RW, McGrew KS, Johson N. M. Woodcock III Tests of Achievement NU. Rolling Meadows, IL: Riverside Publishing, 2001, 2007. [Google Scholar]
- 12.Conners CK. Conners Continuous Performance Test II. North Tonawanda, NY: Multi-Health Systems Inc., 2001. [Google Scholar]
- 13.Delis DC, Kramer JH, Ober Kaplan E. California Verbal Learning Test - Second Edition. 2 ed. San Antonio, TX, 2000. [Google Scholar]
- 14.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Archives of Clinical Neuropsychology. 2004;19: 203–214. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds CR, Voress JK. Test of Memory and Learning: Second Edition (TOMAL-II). Austin, TX: Pro-Ed, 2007. [Google Scholar]
- 16.Strauss E, Sherman EMS, Spreen OA. A Compendium of Neuropsychological Test: Administration, Norms and Commentary. 3 ed. Oxford: Oxford University Press, 2006. [Google Scholar]
- 17.Ware JE Jr. SF-36 health survey update. Spine. 2000;25: 3130–3139. [DOI] [PubMed] [Google Scholar]
- 18.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiation Research. 2006;166: 141–157. [DOI] [PubMed] [Google Scholar]
- 19.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102: 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raj S, Franco VI, Lipshultz SE. Anthracycline-Induced Cardiotoxicity: A Review of Pathophysiology, Diagnosis, and Treatment. Current Treatment Options in Cardiovascular Medicine. 2014;16: 315. [DOI] [PubMed] [Google Scholar]
- 21.Alagiakrishnan K, Mah D, Ahmed A, Ezekowitz J. Cognitive decline in heart failure. Heart Failure Reviews. 2016;21: 661–673. [DOI] [PubMed] [Google Scholar]
- 22.Jansen CE, Dodd MJ, Miaskowski CA, Dowling GA, Kramer J. Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psycho-Oncology. 2008;17: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 23.Bigotte L, Olsson Y. Cytotoxic effects of adriamycin on the central nervous system of the mouse - Cytofluorescence and electron-microscopic observations after various modes of administration. Acta Neurologica Scandinavica. 1984;70: 55–67. [PubMed] [Google Scholar]
- 24.Aluise CD, Sultana R, Tangpong J, et al. Chemo Brain (Chemo Fog) as a Potential Side Effect of Doxorubicin Administration: Role of Cytokine-Induced, Oxidative/Nitrosative Stress in Cognitive Dysfunction In: Raffa RB, Tallarida RJ, editors. Chemo Fog: Cancer Chemotherapy-Related Cognitive Impairment. New York, NY: Springer New York, 2010:147–156. [DOI] [PubMed] [Google Scholar]
- 25.Paulino AC, Simon JH, Zhen W, Wen BC. Long-term effects in children treated with radiotherapy for head and neck rhabdomyosarcoma. International Journal of Radiation Oncology, Biology, Physics. 2000;48: 1489–1495. [DOI] [PubMed] [Google Scholar]
- 26.Fuss M, Poljanc K, Hug EB. Full Scale IQ (FSIQ) changes in children treated with whole brain and partial brain irradiation. A review and analysis. Strahlentherapie und Onkologie. 2000;176: 573–581. [DOI] [PubMed] [Google Scholar]
- 27.Hoppe-Hirsch E, Brunet L, Laroussinie F, et al. Intellectual outcome in children with malignant tumors of the posterior fossa: influence of the field of irradiation and quality of surgery. Child’s Nervous System. 1995;11: 340–345; discussion 345–346. [DOI] [PubMed] [Google Scholar]
- 28.Merchant TE, Schreiber JE, Wu S, Lukose R, Xiong X, Gajjar A. Critical Combinations of Radiation Dose and Volume Predict Intelligence Quotient and Academic Achievement Scores After Craniospinal Irradiation in Children With Medulloblastoma. International Journal of Radiation Oncology, Biology, Physics. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong GT, Jain N, Robison LL, et al. Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro-Oncology. 2010;12: 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zer A, Pond GR, Razak ARA, et al. Association of Neurocognitive Deficits With Radiotherapy or Chemoradiotherapy for Patients With Head and Neck Cancer. JAMA Otolaryngol Head Neck Surg. 2018;144: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markman M Chemotherapy-associated neurotoxicity: an important side effect-impacting on quality, rather than quantity, of life. Journal of Cancer Research and Clinical Oncology. 1996;122: 511–512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.