Abstract
Platensimycin (PTM) is an excellent natural product drug lead against various gram-positive pathogens, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. In this study, twenty PTM derivatives with varying aminobenzoic acids were semisynthesized. In contrast to all the previous reported inactive aminobenzaote analogues, a few of them showed moderate antibacterial activities against S. aureus. Our study suggested that modification of the conserved aminobenzoic acid remains a viable approach to diversify the PTM scaffold.
Keywords: semisynthesis, platensimycin, antibacterial activities
Graphical Abstract
We have semisynthesized twenty PTM analogues with varying aminobenzoic acids and a few of them showed moderate antibacterial activities against S. aureus. Our results suggested that both electronegative properties and the steric hindrance of the substituents in the 5’ position of the ADHBA moiety in these molecules would affect their binding to the target enzyme, while the former would probably play a major role; And the 4’-OH substituent in the ADHBA moiety of PTM is more important than the 2’-OH substituent. Our study suggested that modification of the conserved aminobenzoic acid remains a viable approach to diversify the PTM scaffold.
Platensimycin (PTM, 1), a potent inhibitor of both bacterial and mammalian fatty acid synthases, was initially discovered from the soil-dwelling bacteria Streptomyces platensis MA7327 from South Africa by Merck scientists using a RNAi-based whole cell screening strategy, and it was later re-isolated from similar strains in China (Figure 1A).[1] It showed potent antibacterial activity against multi-drug resistant gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA) in a mouse peritonitis model through continuously infusion, as well as antidiabetic effects in both diabetic mice and monkeys through oral administration.[1] PTM consists of a 2, 4-dihydroxyaminobenzoate (ADHBA) moiety and a unique tetracyclic terpene cage, connected though a propionylamide linker. The promising biological activities and novel structure of PTM have sparked interest to generate analogues to study the structure activity relationships, to improve its poor pharmacokinetic properties.[2]
Figure 1.
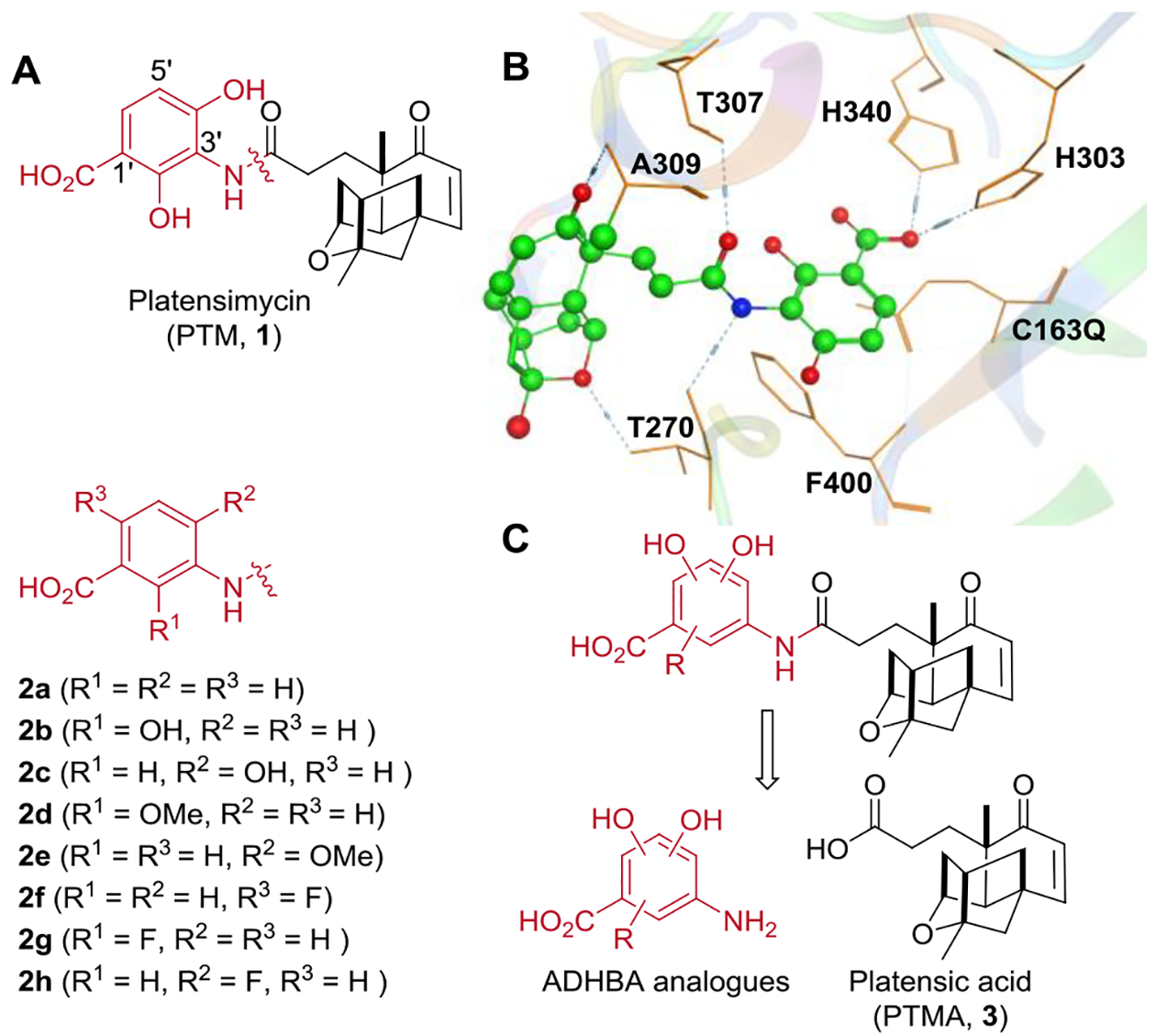
The design of the aminobenzoate analogues of platensimycin. (A) The structures of platensimycin and selected platensimycin aminobenzoate analogues. (B) The co-crystal structure of PTM with E. coli FabF (C163Q). (C) The retrosynthetic analysis for the semisynthesis of most platensimycin analogues in this study.
The co-crystal structure of PTM with Escherichia coli β-ketoacyl-[acyl carrier protein] synthase II (FabF) (C163Q) revealed that the interaction between the active site residues of FabF and the ADHBA moiety is critical: the two His residues H340 and H303 form salt bridge with the carboxylate, while the C-4’ phenol hydroxyl group is engaged with a hydrogen bond with D265 through H2O (Figure 1B).[1a] The fine-tuned interaction in the binding of ADHBA with the FabF active sites, makes generating ADHBA-modified active PTM analogues extremely difficult. Even minor perturbation of ADHBA leads to the abolishment of the antibacterial activities, despite dozens of aminobenzoate analogues have been obtained from fermentation or synthesis (Figure S1 and Table S1).[3] For example, compounds 2a-2c synthesized by Nicolaou and co-workers displayed minimum inhibitory concentrations (MICs) above 82 μg mL−1 against MRSA, while other aminobenzoate analogues 2d-2h from semisynthetic approaches also showed no antibacterial activities against MRSA. In contrast, the terpene cage was solvent-exposed and several terpene cage modified analogues were reported to have improved or comparable activities with PTM, such as the carbaplatensimycin, adamantaplatensimycin, the 7-phenyl- and 11-methyl-7-phenylplatensimycin, as well as the recent semisynthesized PTM sulfur analogues.[4] Since most of the previously obtained PTM aminobenzoate analogues suffered reduced structure complexity, such as compounds 2a-2h, it appeared to us that the FabF active site could still accommodate more substitutions on the ADHBA moiety of PTM, which might lead to additional interactions with amino acid residues within the active site. Therefore our semisynthetic platform may enable the facile preparation of additional and complicated ADHBA analogues of PTM for biological evaluation, by taking advantage of its decagram-scale production from microbial fermentation using a high-yield mutant, and the gram-scale preparation of the key synthetic substrate platensic acid (PTMA, 3) from PTM (Figure 1C).[3f, 4c, 5]
The synthesis of the 5’-F-substituted aniline derivative 6 started from the commercially available 2, 4, 5- trifluorobenzonitrile 4 (Scheme 1). Substitution of the 2-F and 4- F of 4 by benzyl alcohol, followed by high yield hydrolysis to convert nitrile to carboxylic acid and then methylation, afforded intermediate 5.[6] Catalytic hydrogenation of 5 [H2 (1.1 atm.), 10% Pd/C], followed by nitration with 65% HNO3 and then catalytic hydrogenation again, delivered compound 6.[7] HATU-mediated amide coupling of platensic acid 3 with aniline 6 yielded amide 7a, which could be smoothly hydrolyzed with aqueous KOH in MeOH to furnish 5’-F-platensimycin 7b in 23% overall yield in eight steps. No hydrolyzed product of amide 7a was observed using aqueous LiOH in THF, which was used in the previous basic hydrolysis of the methyl esters of PTM analogues, such as carbaplatensimycin.[3c, 3g] The 5’-F-substituted aniline TMS ester of PTM could also be prepared, but it suffered low yield when coupled to 3 (Scheme S1).
Scheme1. Synthesis of 5’-F-platensimycin 7b.
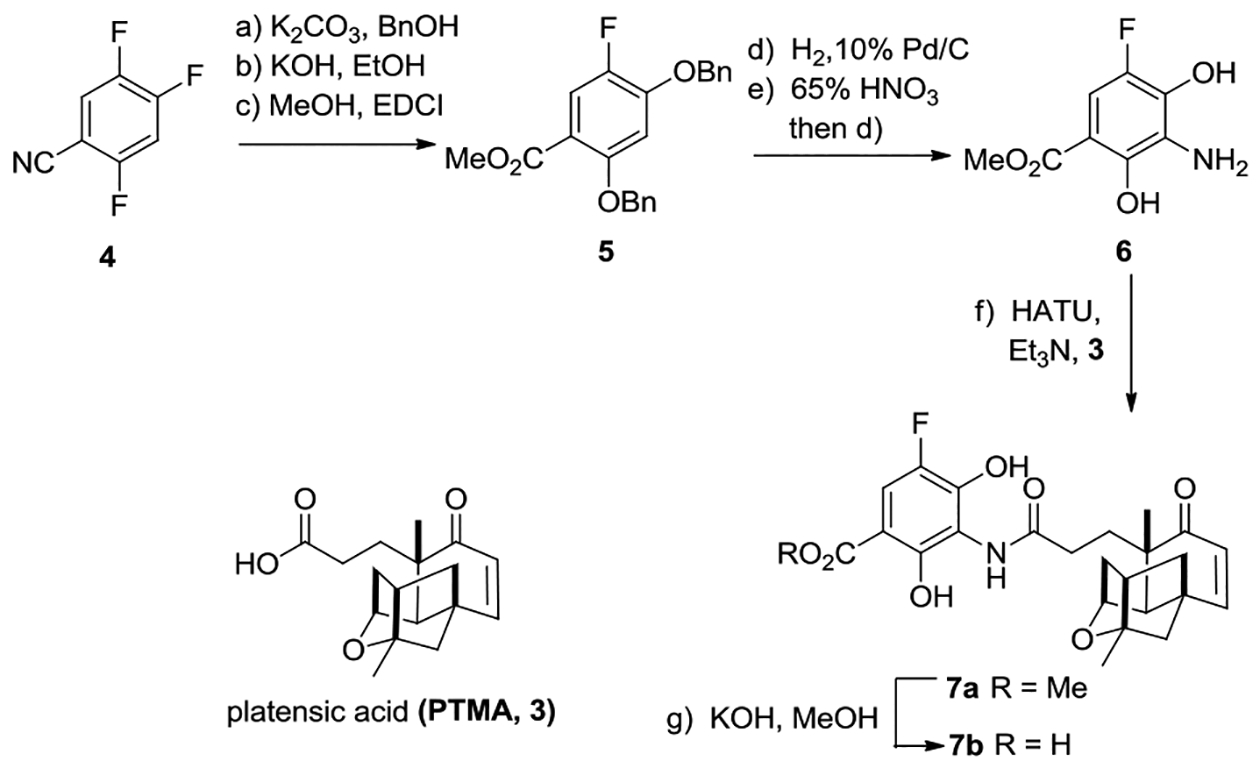
Reagents and conditions: (a) K2CO3 (5 equiv.), BnOH (2.5 equiv.), DMF, 105 °C, 16 h, 85%; (b) 6 N KOH, EtOH, 140 °C, 1.5 h, 95%; (c) MeOH, DMAP (0.1 equiv.), EDCI (1.2 equiv.), NEt3 (2.5 equiv.), 10 h, 65%; (d) H2 (1.1 atm.), 10% Pd/C (0.2 equiv.), MeOH, 25 °C, 3 h, 90%; (e) 65% HNO3, CHCl3, 5 min, 72%; (f) 3 (1.0 equiv.), HATU (2.0 equiv.), NEt3 (3.0 equiv.), DCM, 25 °C, 12 h, 83%; (g) aq. KOH, MeOH, 45 °C, 4 h, 89%. Abbreviations: Bn = benzyl, DMF = N,N-dimethylformamide, DMAP = dimethylaminopyridine, EDCI = 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, HATU = 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate, DCM = dichloromethane.
Four additional aminobenzoate analogues of PTM 8–10 were smoothly synthesized from PTM in a one-step reaction (Scheme 2). Electrophilic iodination of PTM (I2, DMAP, pyridine, CCl4) afforded the 5’-I-platensimycin 8 in a good yield of 88%.[8] Treatment of PTM by 65% HNO3 in 5 min furnished the 5’-nitro substituted PTM analogue 9 in 84% yield. Treatment of PTM by formaldehyde under basic conditions (HCHO, KOH, MeOH, CaCl2), followed by quenching the reaction with NH4OH led to the methoxylmethyl derivative of PTM 10a with an excellent yield of 90%, while efficient synthesis of the similar hydroxymethyl PTM 10b could be achieved in a similar procedure without NH4OH treatment.[9]
Scheme 2. Synthesis of platensimycin analogues 8–10.
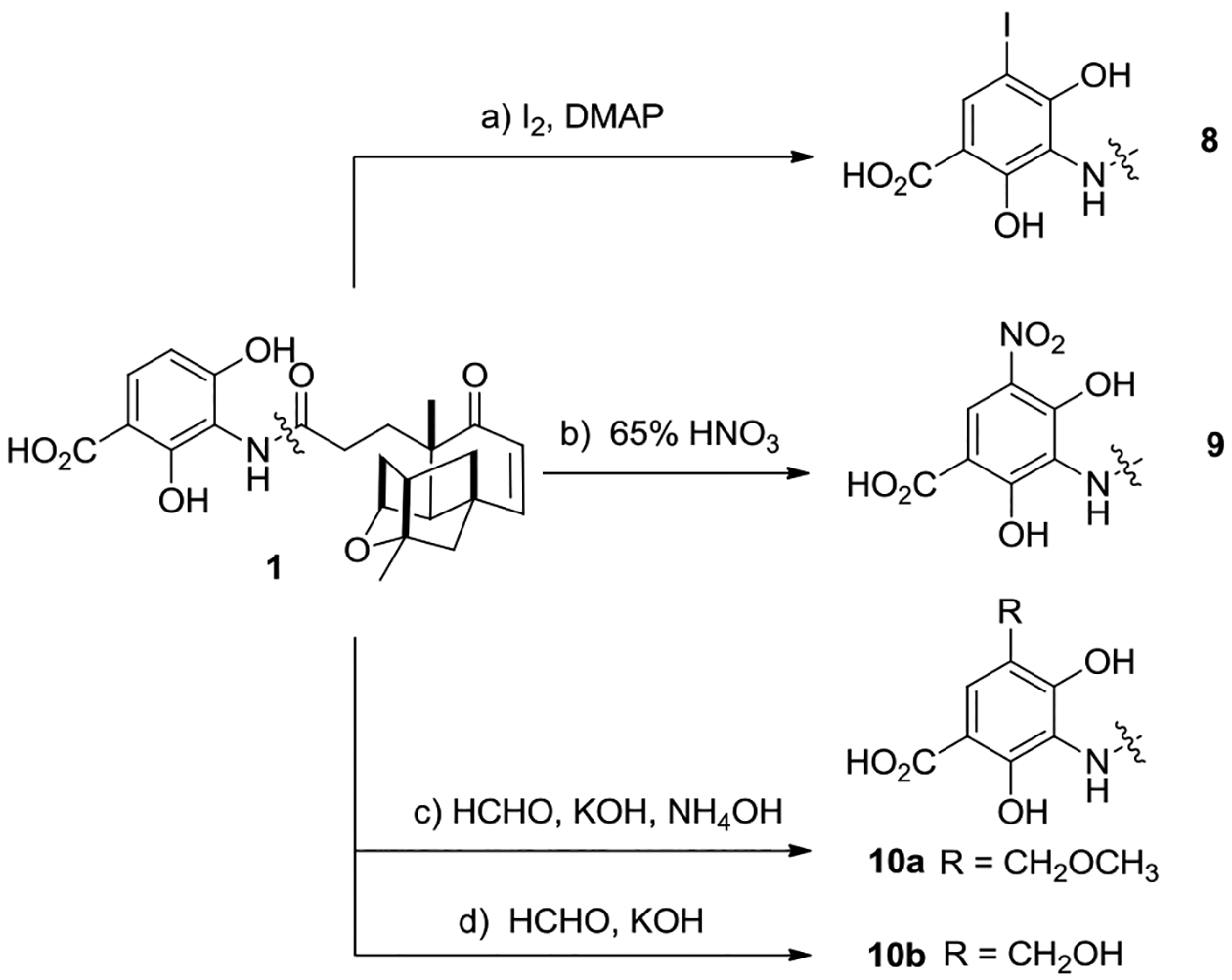
Reagents and conditions: (a) CCl4:pyridine (4:1), I2 (3 equiv.), DMAP (0.2 equiv.), 110 °C, 4 h, 88%; (b) 65% HNO3, CHCl3, r.t. 5 min, 84%; (c) HCHO (3.0 equiv.), KOH (3.0 equiv.), CaCl2 (3.0 equiv.), MeOH (2 ml), NH4OH (10.0 equiv.), r.t. 4 h, 90%; (d) HCHO (3.0 equiv.), KOH (3.0 equiv.), CaCl2 (3.0 equiv.), MeOH (2 ml), r.t. 4 h, 92%.
Nine new aminobenozoic acid derivatives were synthesized from the known methyl esters 11, 14, 16, 20 and 22 (Scheme 3). Basic hydrolysis of the methyl ester 11 (aq. LiOH, THF, 45 °C), followed by esterification with TMSE-OH (TMSE-OH, EDCI, Et3N, DCM, 25°C) and catalytic hydrogenation, led to aniline 13 (Scheme 3A).[6c, 9] Aniline 15b was prepared similarly by LiOH-mediated hydrolysis, followed by catalytic hydrogenation (Scheme 3B). Direct catalytic hydrogenation of methyl esters 14, 16 and 20, afforded the corresponding aniline methyl ester 15a, 17a and 21a[7] in 95, 89 and 89% yield, respectively (Scheme 3B–3D). Starting from 16 and 20, ester exchange under basic conditions (TMSE-OH, Bu2SnO, toluene, 150°C), followed by catalytic hydrogenation, furnished anilines 17b and 21b.[10] Unfortunately, 14 and 23 could only be converted to the corresponding TMS esters in very low yields, when the above ester exchange conditions were used. The aniline derivative 19 was swiftly converted from the methyl ester 16, by basic hydrolysis, demethylation mediated by AlCl3 (AlCl3, 1, 2-DCE, 90 °C), and catalytic hydrogenation.[7] Finally, selective benzylation of 22 (BnBr, K2CO3, acetone), followed by mild nitration (Bi(NO3)3·5H2O, MMT K10, DCM, 25 °C), provided the aniline 24 (Scheme 3E).[9]
Scheme 3. Construction of aromatic fragments.
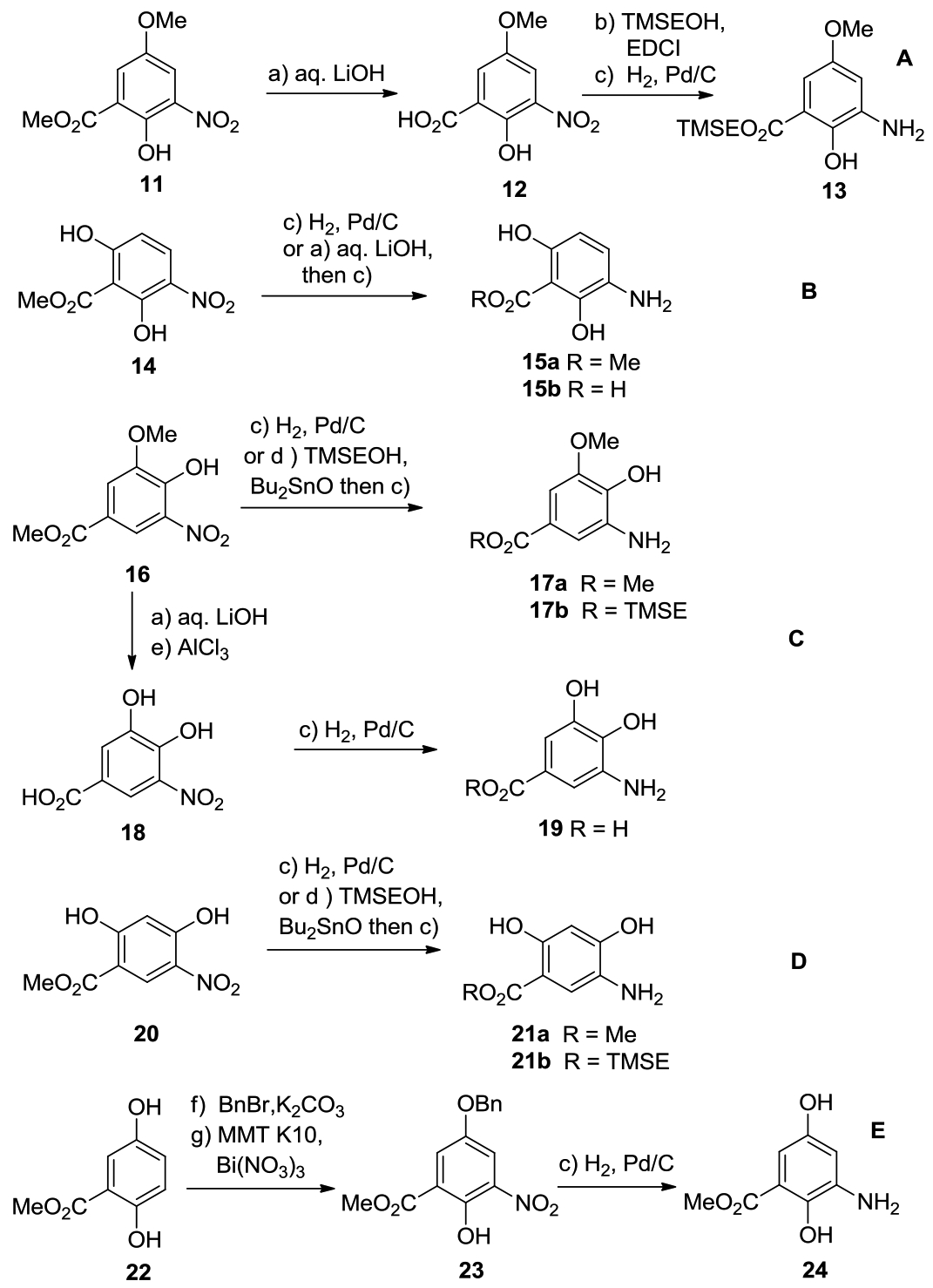
Reagents and conditions: (a) aq. LiOH, THF, 45 °C, 91–95%; (b) TMSEOH (1.2 equiv.), EDCI (1.0 equiv.), NEt3 (2.0 equiv.), DCM, 25 °C, 12 h, 89%; (c) H2 (1.1 atm.), 10% Pd/C (0.2 equiv.), MeOH, 25 °C, 3 h, 95% (for 13), 91% (for 15a), 89% (for 15b), 93% (for 17a), 82% (for 17b), 89% (for 19), 84% (for 21a), 92% (for 21b), 87% (for 24); (d) TMSEOH (1.5 equiv.), toluene, Bu2SnO (1.0 equiv.), 150 °C, 85–95%; (e) AlCl3 (3.0 equiv.), 1,2-DCE, 90 °C, 85%; (f) BnBr, K2CO3, acetone, 3 h, 87%; (g) Bi(NO3)3.5H2O (1.0 equiv.), MMT K10 (1.0 equiv.), DCM, 25 °C, 2 h, 81%. Abbreviations: THF = tetrahydrofuran, TMSE = 2-(trimethylsilyl)ethyl, Bu = butyl, MMT K10 = bentonite clay K-10.
With the nine aromatic fragments in hand, coupling to platensic acid via amidation would afford the corresponding PTM analogues. Analogues 25a, 26a, 27a, 29a, 29b and 30 were efficiently prepared from their corresponding aniline derivatives 12, 14, 16, 20 and 23, with platensic acid 3 through HATU-mediated amide coupling (HATU, Et3N, DMF) (Scheme 4). Deprotection of the resulting PTM TMS esters 25a, 27b and 29b using fluoride (TBAF, DCM) furnished compounds 25b, 27c and 29c.[10b] Alternatively, compounds 27c and 29c could be obtained by the hydrolysis of 27a and 29a using aqueous KOH in MeOH, in 74 and 34% yield, respectively. Inspired by the direct coupling of unprotected ADHBA with platencin core using DCC (DCC, DMAP, Et3N, MeCN/DMF, rt), compounds 26b and 28 were conveniently obtained by coupling of the respective aminobenzoic acids 15b or 19 with 3 (PyBOP, Et3N, DCM, rt, 30 min), in 80 and 75% yield, respectively.[7, 11]
Scheme 4. Synthesis of platensimycin analogues 25–30.
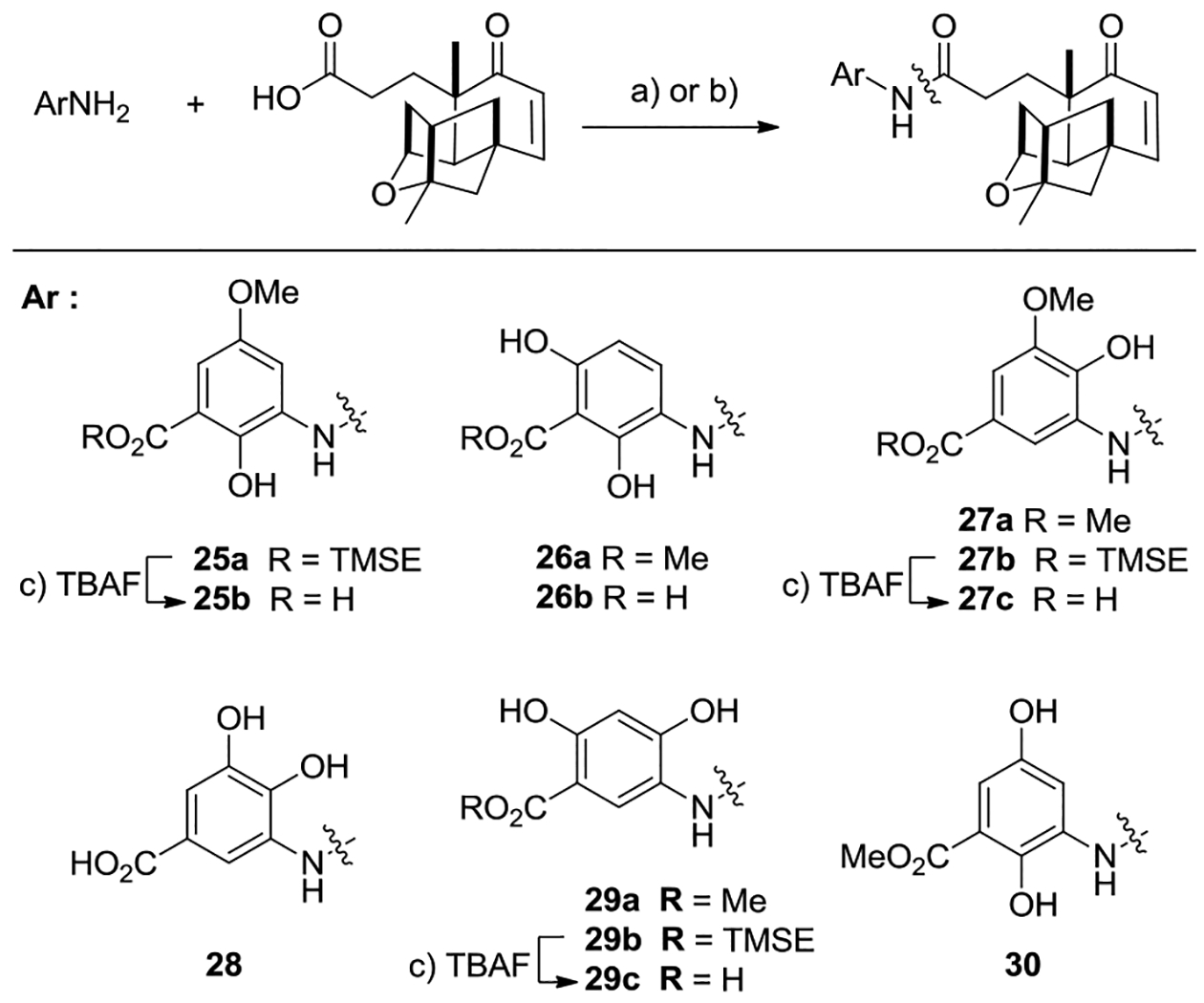
Reagents and conditions: (a) HATU (2.0 equiv.), NEt3 (3.0 equiv.), DMF, r.t, 8 h, 86% (for 25a), 74% (for 26a), 70% (for 27a), 80% (for 27b), 63% (for 29a), 82% (for 29b), 59% (for 30); (b) PyBOP (0.9 equiv.), NEt3 (2.0 equiv.), DCM, r.t, 30 min, 80% (for 26b), 75% (for 28); (c) TBAF, DCM, 12 h, 92% (for 25b), 76% (for 27c), 85% (for 29c). Abbreviations: PyBOP =benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate, TBAF = tetrabutylammonium fluoride.
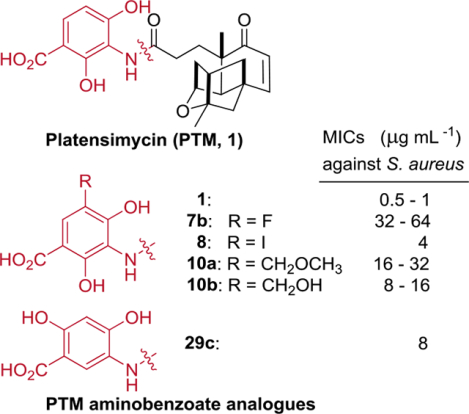
The eighteen synthesized PTM analogues 8–10 and 25–30 were first tested against the gram-positive S. aureus ATCC 29213 and the gram-negative Klebsiella pneumoniae using the paper disc method, with 5 μg or 25 μg per disc, and PTM and linezolid (5 μg/disc) were used as controls (Figure S2). The 5’-Br-platensimycin and 5’-Cl-platensimycin were previously synthesized,[3b] but their antibacterial activities have not been reported (Figure S1). We also synthesized these two analogues according to the previous reported procedure and included them in our assay.
All of the twenty tested PTM analogues showed no antibacterial activities against the gram-negative pathogen K. pneumoniae, suggesting that these PTM ADHBA modified derivatives might share certain drug-efflux mechanisms with PTM against gram-negative pathogens, such as Escherichia coli (Figure S2).[1a] Interestingly, five ADHBA-modified analogues, including 5’-F-platensimycin 7b, 5’-I-platensimycin 8, 5’-methoxymethyl-platensimycin 10a, 5’-hydroxymethylplatensimycin 10b and 4’, 6’- dihydroxyplatensimycin 29c showed clean zone of inhibition, while 5’-Cl-platensimycin only showed a faint inhibition zone when applied against S. aureus ATCC 29213. The other tested PTM derivatives showed no antibacterial activities against the tested S. aureus strains.
The MICs of those active PTM derivatives 7b, 8, 10a, 10b and 29c, were determined against S. aureus ATCC 29213 and the clinical isolated methicillin-sensitive S. aureus (MSSA) and MRSA, using PTM and linezolid as controls (Table 1).[12] Among them, the 5’-I-platensimycin 8 showed a MIC of 4 μg mL−1, only 4–8 fold less potent than PTM, while 5’-F-platensimycin 7b had a MIC of 64 μg mL−1 against the tested S. aureus strains. However, about 10% of 5’-I-platensimycin deiodinated to PTM due to the instability of C-I bond under the assay condition, suggesting that the observed antibacterial activities might be partly due to its parent compound PTM and an interesting pro-drug strategy to deliver PTM in vivo to improve its poor bioavailability (Figure. S3).[13] Both 10a and 10b, bearing slightly larger substituents in ADHBA moiety than 5’-I-PTM, have attenuated antibacterial activities with MICs ranging from 8–32 μg mL−1. In contrast, 5’-Br-platensimycin, 5’-Cl-platensimycin and 5’-nitroplatensimycin did not show any antibacterial activities against S. aureus, while it is surprising that the 5’-F-platensimycin, with potentially enhanced bioavailability, still have weak antibacterial activity.[14] It was consistent with the complete loss of the antibacterial activities of 5’-Cl-platencin and 5’-Cl-iso-platencin, analogues of another potent fatty acid synthase inhibitor platencin, which shares the same ADHBA moiety with PTM.[3b, 15] Taken together, these results suggested that both electronegative properties and the steric hindrance of the substituents in the 5’ position of the ADHBA moiety in these molecules would affect their binding to the target enzyme, while the former would probably play a major role.
Table 1.
The minimum inhibitory concentrations (MIC μg mL−1) of platensimycin analogues against S. aureus ATCC 29213, and clinical MSSA and MRSA strains
| Compounds |
S. aureus ATCC 29213 |
MRSA | MSSA |
|---|---|---|---|
| PTM | 1 | 0.5 | 1 |
| Linezolid | 1 | 1 | 1 |
| 7b | 64 | 64 | 32 |
| 8 | 4 | 4 | 4 |
| 10a | 32 | 16 | 16 |
| 10b | 16 | 16 | 8 |
| 29c | 8 | 8 | 8 |
The 4’, 6’-dihydroxyplatensimycin 29c had a MIC of 8 μg mL−1, while 2’, 6’-dihydroxyplatensimycin 26b had no antibacterial activity under the tested conditions, suggesting that the 4’-OH substituent in the ADHBA moiety of PTM is more important than the 2’-OH substituent. It is consistent with the reported co-crystal structure of PTM and ecFabF (C163Q), in which the 4’-OH mediated a hydrogen bond with D265.[1a] It seemed that the 2’-OH in PTM and 6’-OH in 29c, both ortho to the carboxylate of PTM or 29c, are also needed for their respective antibacterial activities, because both the previous reported 4’-hydroxyplatensimycin by Nicolaou group and 4’, 5’-dihydroxyplatensimycin 28 showed no antibacterial activity.[3c] Since the ortho phenolic hydroxy group in either PTM or 29c could form a hydrogen bond with the carboxylate, as observed in their proton NMR spectroscopy, it remained to be determined what the role of the intramolecular hydrogen bond plays in the binding of PTM or 29c to FabF.
In summary, we have semisynthesized twenty PTM analogues with varying aminobenzoic acids and a few of them showed moderate antibacterial activities against S. aureus. In contrast to all the previous reported PTM ADHBA analogues, including compounds 2a-2h, as well as other analogues from PTM wild-type producer or the mutant strain through precursor-directed biosynthesis approaches (Figure S1), our study has resulted five active PTM ADHBA derivatives 7b, 8, 10a, 10b and 29c, with diverse functionality. However, they might still suffer the similar metabolic fate of rapid clearance or amide hydrolysis as PTM, challenges that have prevent PTM drug development to date. Our facile preparation of these active analogues would allow the study of their pharmacokinetics and pharmacodynamics in the future. Since PTM analogues could be prepared by convergent synthesis or biosynthetic routes, our discovery of several active ADHBA-modified PTM analogues would suggest the design of next generation of PTM analogues, by varying both ADHBA moiety and its terpene cage. It is further supported by our recent discovery of the active thioplatensimycin, the natural congener of PTM.20 The similar design principle might also be applicable to platencin. Since there are only a few effective inhibitors against type II PKSs, our generation of these active analogues may inspire the discovery of potent antibiotics targeting this essential pathway in major pathogens.
Supplementary Material
Acknowledgements
This work was supported by NSFC grants 81473124 (to Y.H.), the Chinese Ministry of Education 111 Project B0803420 (to Y.D.) and NIH Grant GM114353 (to B.S.). We thank the Center for Advanced Research in Central South University for the HRMS and NMR experiments.
Footnotes
Supporting Information Summary
Detailed information about the experimental procedure, antibacterial activities, spectral characterization data such as 1H-NMR and 13C-NMR along with HRMS spectra of all synthesized products are provided
References
- [1].a) Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio A. n., Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett, Schmatz D, Becker JW, Cully D, Singh SB, Nature 2006, 441, 358–361; [DOI] [PubMed] [Google Scholar]; b) Wu M, Singh SB, Wang J, Chung CC, Salituro G, Karanam BV, Lee SH, Powles M, Ellsworth KP, Lassman ME, Miller C, Myers RW, Tota MR, Zhang BB, Li C, Proc. Natl. Acad. Sci. U.S.A 2011, 108, 5378–5383; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Singh SB, Kang L, Nawrocki AR, Zhou D, Wu M, Previs S, Miller C, Liu H, Hines CD, Madeira M, Cao J, Herath K, Spears LD, Semenkovich CF, Wang L, Kelley DE, Li C, Guan HP, PLoS One 2016, 11, e0164133; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hindra T Huang D Yang JD Rudolf P Xie G Xie Q Teng JR Lohman X Zhu Y Huang L-X Zhao Y Jiang Y Duan B Shen, J. Nat. Prod 2014, 77, 2296–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Nicolaou KC, Chen JS, Edmonds DJ, Estrada AA, Angew. Chem. Int. Ed 2009, 48, 660–719; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Martens E, Demain AL, J. Antibiot. 2011, 64, 705–710 [DOI] [PubMed] [Google Scholar]; c) Saleem M, Hussain H, Ahmed I, van Ree T, Krohn K, Nat. Prod. Rep 2011, 28, 1534–1579 [DOI] [PubMed] [Google Scholar]; d) Singh S, in Expert. Opin. Ther. Pat, Vol. 20, 2010, pp. 1359–1371; [DOI] [PubMed] [Google Scholar]; e) Rudolf JD, Dong L-B, Shen B, Biochem. Pharmacol 2017, 133, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Dong L-B, Rudolf JD, Shen B, Bioorg. Med. Chem 2016, 24, 6348–6353; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Singh SB, Herath KB, Wang J, Tsou N, Ball RG, Tetrahedron. Lett 2007, 48, 5429–5433; [Google Scholar]; c) Nicolaou KC, Stepan AF, Lister T, Li A, Montero A, Tria GS, Turner CI, Tang Y, Wang J, Denton RM, Edmonds DJ, J. Am. Chem. Soc 2008, 130, 13110–13119; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Zhang C, Ondeyka J, Herath K, Jayasuriya H, Guan Z, Zink DL, Dietrich L, Burgess B, Ha SN, Wang J, Singh SB, J. Nat. Prod 2011, 74, 329–340 [DOI] [PubMed] [Google Scholar]; e) Dong L-B, Rudolf JD, Shen B, Org. Lett 2016, 18, 4606–4609; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Qiu L, Tian K, Pan J, Jiang L, Yang H, Zhu X, Shen B, Duan Y, Huang Y, Tetrahedron 2017, 73, 771–775; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Nicolaou KC, Lister T, Denton RM, Montero A, Edmonds DJ, Angew. Chem. Int. Ed 2007, 46, 4712–4714. [DOI] [PubMed] [Google Scholar]
- [4].a) Nicolaou KC, Tang Y, Wang J, Stepan AF, Li A, Montero A, J. Am. Chem. Soc 2007, 129, 14850–14851; [DOI] [PubMed] [Google Scholar]; b) Jang KP, Kim CH, Na SW, Jang DS, Kim H, Kang H, Lee E, Bioorg. Med. Chem. Lett 2010, 20, 2156–2158; [DOI] [PubMed] [Google Scholar]; c) Qiu L, Tian K, Wen Z, Deng Y, Kang D, Liang H, Zhu X, Shen B, Duan Y, Huang Y, J. Nat. Prod 2018, 81, 316–322; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wang J, Sintim HO, Chem. Eur. J 2011, 17, 3352–3357. [DOI] [PubMed] [Google Scholar]
- [5].a) Shi J, Pan J, Liu L, Yang D, Lu S, Zhu X, Shen B, Duan Y, Huang Y, J. Ind. Microbiol. Biot 2016, 43, 1027–1035; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Deng Y, Kang D, Shi J, Zhou W, Sun A, Ju J, Zhu X, Shen B, Duan Y, Huang Y, Med. Chem. Commun 2018, 9, 789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Lee MM, Peterson BR, ACS Omega 2016, 1, 1266–1276; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lignier P, Estager J, Kardos N, Gravouil L, Gazza J, Naffrechoux E, Draye M, Ultrason. Sonochem 2011, 18, 28–31; [DOI] [PubMed] [Google Scholar]; c) Vaidyanathan G, McDougald D, Choi J, Pruszynski M, Koumarianou E, Zhou Z, Zalutsky MR, Org. Biomol. Chem 2016, 14, 1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tiefenbacher K, Gollner A, Mulzer J, Chem. -Eur. J 2010, 16, 9616–9622. [DOI] [PubMed] [Google Scholar]
- [8].Liu H, Yan P, Li Y, Liu J, Sun Q, Wang X, Wang C, Monatsh. Chem 2012, 143, 1055–1059. [Google Scholar]
- [9].Yan Y, Qin B, Ren C, Chen X, Yip YK, Ye R, Zhang D, Su H, Zeng H, J. Am. Chem. Soc 2010, 132, 5869–5879. [DOI] [PubMed] [Google Scholar]
- [10].a) Nicolaou KC, Natarajan S, Li H, Jain NF, Hughes R, Solomon ME, Ramanjulu JM, Boddy CNC, Takayanagi M, Angew. Chem. Int. Ed 1998, 37, 2708–2714; [DOI] [PubMed] [Google Scholar]; b) Ghosh AK, Xi K, J. Org. Chem 2009, 74, 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hayashida J, Rawal VH, Angew. Chem. Int. Ed 2008, 47, 4373–4376. [DOI] [PubMed] [Google Scholar]
- [12].Wiegand I, Hilpert K, Hancock RE, Nat. Protoc 2008, 3, 163–175. [DOI] [PubMed] [Google Scholar]
- [13].Cavina L, van der Born D, Klaren PHM, Feiters MC, Boerman OC, Rutjes F, Euro. J. Org. Chem 2017, 2017, 3387–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Müller K, Faeh C, Diederich F, Science 2007, 317, 1881–1886. [DOI] [PubMed] [Google Scholar]
- [15].Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, González I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB, Proc. Natl. Acad. Sci. U. S. A 2007, 104, 7612–7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sonar VN, Venkatraj M, Parkin S, Crooks PA, Acta Crystallogr., Sect. E: Struct. Rep. Online 2007, 63, o3227–o3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].a) Wang L, Gong J, Deng L, Xiang Z, Chen Z, Wang Y, Chen J, Yang Z, Org. Lett 2009, 11, 1809–1812; [DOI] [PubMed] [Google Scholar]; b) Kiss LE, Ferreira HS, Torrao L, Bonifacio MJ, Palma PN, Soares-da-Silva P, Learmonth DA, J. Med. Chem 2010, 53, 3396–3411. [DOI] [PubMed] [Google Scholar]
- [18].Giannis A, Heretsch P, Synthesis. 2007, 2007, 2614–2616. [Google Scholar]
- [19].Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, González I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully DF, Singh SB. Proc. Natl. Acad. Sci. U.S.A 2007, 104, 7612–7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dong LB, Rudolf JD, Kang D, Wang N, He CQ, Deng Y, Huang Y, Houk KN, Dua Y, Shen B, Nat. Commun 2018, 9, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


