Abstract
Background
The Striving to be Strong (StbS) study tested the efficacy of a multifaceted, theory based, complex osteoporosis prevention smartphone application (app). We hypothesized use of the app would improve bone mineral density and trabecular bone scores.
Methods
The study was a three-group, prospective, repeated measure, longitudinal randomized trial. Baseline sample consisted of 290 healthy women between 40 and 60 years of age. Participants were randomly assigned to one of three groups: “Striving,” a dynamically tailored, person-centered app; “Boning Up,” standardized osteoporosis-education e-book; and “Wait List,” participant choice of intervention in the final three months of the 12 month study. Participants had or were provided a smart phone. Bone mineral density and trabecular bone scores were measured using dual energy X-ray absorptiometry at baseline and 12 months. To assess engagement in health behavior change processes, ecological momentary assessments were administered via text messaging during the 12-months participants actively used the app.
Results
The final sample reflects an 89.6% retention rate. There were decreases in bone mineral density over time but not among the three groups. The percent of bone density lost over 12 months was lower than expected. Trabecular bone scores were not different over time or by group, but improved across all three groups.
Discussion
Small but positive results were observed across all groups, suggesting one or more aspects of participation might have affected outcomes including dissemination of the intervention across groups, retention without participation, ecological momentary assessments functioning as both an intervention and measure, and selective engagement in research based recommendations.
Keywords: bone mineral density, trabecular bone score, osteoporosis prevention, mobile application, health behavior, intervention
Osteoporosis is a condition that compromises the density and microarchitecture of bone (National Osteoporosis Foundation, 2018). Decreases in the amount and strength of bone can result in fractures, which occur primarily in the trabecular bones of the wrist, spine, and hip and are associated with increased mortality, disability, disfigurement, and acute and chronic pain (National Osteoporosis Foundation, 2018). Osteoporotic fractures occur in 50% of all White women and its prevalence is rapidly increasing among Latina and Black women (Sanchez-Riera et al., 2010). Osteoporosis negatively affects quality of life and functional independence, result in an increased need for family and professional caregiving, and increase the demand on healthcare services and costs (National Osteoporosis Foundation, 2018). While recognized as a condition associated with aging, significant changes in bone mineral density (BMD) can occur during and in the two to three years following menopausal transition (mid-life) (Sowers et al., 2013). While it is recommended that all women should engage in behaviors that promote or maintain healthy bones (nutrition including calcium and vitamin D, balance, leg and core strength, and physical activity (PA) fewer than 20% of healthy middle age women regularly follow these recommendations (Recker, 2011; Ryan, 2009; Ryan, Schlidt, & Ryan, 2013; Wilbur, Vassalo, Chandler, McDevitt, & Miller, 2005).
Interventions that enhance long term maintenance of osteoporosis health promotion behaviors have not been identified. The results of research clearly provide evidence that in addition to one’s general health, genetic background, and life course, engagement in select preventative health behaviors contributes to bone health and the prevention or delay of osteoporosis. However, many women struggle to make and maintain health behavior change (Bouton, 2014; Kelly & Barker, 2016). Over the past fifty decades, health care professionals have developed and tested theories and interventions to enhance health behavior change. These efforts, in general, have resulted in higher rates of initiation of health behavior change but not long-term maintenance of change over time. Together, advances in person-centered approaches, use of new theories focusing on individualized change processes that integrate the complexity of simultaneously engaging in multiple health behaviors, and the availability and affordability of technology provide opportunities to develop and test new types of interventions designed to promote maintenance of health behavior change.
A relatively new mid-range theory, the Individual and Family Self-management Theory (IFSMT) (Ryan, 2009; Ryan & Sawin, 2009; Ryan & Papanek, 2019) postulates that people can improve health outcomes by engaging in processes that enhance self-management of health behaviors by enriching knowledge and beliefs, enhancing self-regulation skills and abilities, and engaging in social facilitation activities supporting health-behavior change. Given the association between osteoporosis-prevention behaviors and outcomes, the IFSMT model predicts that enhancing one’s health beliefs, engaging in self-regulation processes, and social facilitation bolster self-management and improve proximal outcomes (such as calcium intake and strength-training exercises), thereby improving distal outcomes such as BMD and trabecular bone scores (TBS).
Technology is an increasingly popular way to deliver interventions. However, actual use of technology varies widely, and its effects are not well understood (Baysari & Westbrook, 2015; Daly, Horey, Middleton, Boyle, & Flenday, 2017; de Jongh, Gurol-Urganci, Vodopivec-Jamsek, Car, & Atun, 2012; Free et al., 2013). Many commercially available applications (apps) are not based in theory or research, and their efficacy has not been determined (Modave et al., 2015). Traditional electronic media, such as e-books or general alerts, might lack specificity and tailoring to meaningfully affect self-regulation skills, knowledge, and beliefs.
The goal of this health promotion study was to test the efficacy of an intervention designed to enhance knowledge and beliefs, engagement in self-regulation processes, and social facilitation using an app that dynamically and automatically prepared information and activities matched to each individual. We hypothesized that active use of the app over the 12 month study period would result in better distal outcomes (BMD and TBS) than use of a more-traditional e-book app. This study tested the efficacy of the intervention while holding constant the delivery media – a smart phone app.
Methods
Design
Focusing on the prevention of osteoporosis, the StbS study was a three-group, prospective, repeated-measure, longitudinal randomized clinical trial with a 12-month intervention period. Participants were randomly assigned to one of three groups ((Ryan et al., 2018)). The “Striving” group received the newly developed dynamically tailored app. The “Boning Up” group received the National Osteoporosis Foundation’s (NOF) standardized informational intervention converted to an e-book app. The “Wait List” group received their choice of either intervention during the final three months of the study; this group served as the control group for the experimental intervention. Participants in all groups received a second app specifically designed to obtain Ecological Momentary Assessments (EMAs) focusing on the four accepted osteoporosis prevention health behaviors (calcium intake, balance training, strength training, and PA), and components of the self-regulation process (e.g., goal setting, tracking, reflection). We collected data from January 2014 through May 2016 including bone-strength measures of BMD and bone-microarchitecture measures of TBS at baseline and at the end of month twelve via Dual Energy X-Ray Absorptiometry (DXA).
Study Participants
Eligibility was based on the recommendations from a review of osteoporosis prevention studies (Ryan, Schlidt, et al., 2013). Because of the documented low rate of engagement in osteoporosis prevention behaviors during a period of accelerated bone loss (menopausal transition and menopause) it was determined changes in bone would be most apparent in women between 40 and 60 years of age. All participants were required to speak and read English and to safely engage in PA. Participants had to have not used medications mediating bone turnover nor have chronic or acute illnesses. Exclusion criteria included a self-report of any of the following: pregnancy or lactation; less than five years post active cancer treatment; calcium intake within or greater than recommended levels; or a regimen of vigorous PA of 20 minutes or longer three or more times a week. Women diagnosed with osteoporosis were excluded for two reasons: 1) prevention and self- management behaviors are similar but differ for prevention and treatment; 2) once aware of the diagnosis of osteoporosis women are increasingly likely, with a less intense intervention, to engage in osteoporosis self-management behaviors (Lee, Jong-Duek, Yang, & Yoon, 2012; Sedlak, Doheny, Estok, Zeller, & Winchell, 2007; Wu et al., 2014). Based on previously observed attrition rates of about 33% and effect sizes (Ryan, Maierle, Csuka, Thomson, & Szabo, 2013), our a priori power calculations indicated that a final sample size of 192 (64 in each of the three groups) would be sufficient to detect a moderate effect size (Cohen’s d = 0.4–0.7) at power = 0.8 and alpha = 0.01.
Measures and Procedures
Descriptive measures
We collected participants’ demographic information and physical attributes (Body Mass Index [BMI], Fracture Risk Assessment [FRAX®](World Health Organization Collaborating Centre for Metabolic Bone, 2010), and self-reported Menopausal Status) (Table S1) using a testing battery described previously (Ryan et al., 2018; Ryan, Weiss, & Papanek, 2019b).
EMAs
Self-management of health-behavior change processes were assessed using EMAs (Marszalek, Morgulec-Adamowicz, Rutkowska, & Kosmol, 2014; Shiffman & Rathbun, 2011; Spook, Paulussen, Kok, & VanEmpelen, 2013; Ryan & Papanek, 2019). EMAs are a type of self-report with data collected real-time in natural settings which are reported to minimize bias associated with retrospective recall, maximize ecological validity, and increase both the accuracy and the completeness of the data.
For this study EMA questions gathered data relative to the participants behaviors (calcium intake, balance and strength, and PA), frequency of use, and engagement in specific aspects of the self-regulation processes (goal-setting, planning, tracking, reflecting, decision-making, managing emotions, and miscellaneous). Women both received and could initiate EMAs, hence data were completed immediately or within hours of actually engaging in a behavior. EMA questions remained constant throughout the duration of the study.
BMD and TBS
We used results DXA scans (IDXA General Electric model, Madison, WI, Software 1410.002) to obtain baseline and end of study measures of BMD. Following daily calibration of the DXA scanner total, femoral neck (hip) and L1-L4 (lumbar spine) BMDs were obtained by one of two individuals trained to perform DXA scans by the International Society of Clinical Densitometry; these individuals were masters and PhD prepared professionals who had prior confirmation of high levels of intra-rater reliability. With rare exception, the initial operator performed both baseline and end of study BDM measurement. Together with the principal investigator (PI), the results of all scans were reviewed by an exercise physiologist and a rheumatologist, both certified and experienced in managing osteoporosis clinical care and research. Women with BMD < −2.5 at baseline (World Health Organization Collaborating Centre for Metabolic Bone, 2010) were not eligible for the study.
We collected information required to calculate body composition and FRAX: race, ethnicity, birthdate, height, weight, previous fragile fracture, parental hip fracture, smoking status, use of glucocorticoids, and rheumatic arthritis. Height was measured using a calibrated, wall-mounted stadiometer. Weight in pounds and ounces was measured using an electronic scale (Tanita BWG800A, 3-point weight calibrated staff twice yearly). Consistent with the official positions of the American College of Radiology (American College of Radiology, 2014), we asked all participants about their menstrual status and possible pregnancies. Participants unsure of their pregnancy status completed an over-the-counter pregnancy test.
TBS scores were calculated from each participant’s lumbar spine DXA scan image using commercially available software (TBS iNsight v3.0.2.0, Medimaps, Needham, MA)(Harvey et al., 2016; Romagnoli et al., 2013). We used identical scan protocols for both the baseline and 12-month measurements.
Interventions
“Striving” app
We created a smartphone app to operationalize the IFSM process dimension for osteoporosis-prevention health behaviors (Ryan & Papanek, 2019). The app contained five major content sections. The first section contained information about the study, goals, and participant responsibilities; bone, bone growth, and osteoporosis; and exercise principles including appropriate exercise clothing and shoes, management of exercise-related discomfort, and safety. The other four content sections focused on one of our four operationalized health behaviors (calcium intake, balance training, strength training, and PA). For each of the four behaviors, the app provided behavior specific education, information specific to self-regulation processes, dietary or exercise assessments, progression tracking, and feedback. We selected and progressed training exercises in accordance with research-based protocols and guidelines (Cosman et al., 2014). Information was tailored to match individualized assessments and changed over time to match participants’ progress. This dynamic tailoring process utilized mechanisms of repeated assessments, an extensive message library, computerized decisional algorithms, and normative and ipsative feedback (Ryan & Lauver, 2002; Ryan et al., 2018).
The Striving app coached exercise performance by using multimedia delivery, pictures, voice-over videos, and static images with textual instructions. Progression to more-advanced exercises was predicated on participants’ safe completion of earlier levels (achievement of required reps and sets without symptoms). While the app was able to track information about participants’ engagement in exercises guided by the app (time, frequency, progression) it did not collect information about activities participants engaged in without the use of the app; for example, attending a Zumba class or walking with a friend or neighbor. Real time app data were used to operate the automated functionality of the app and enabled researchers to regularly monitor participant’s use of the app and study participation. All app data were stored in the HIPAA compliant cloud based server.
“Boning Up” app
We obtained permission from the NOF to convert Boning Up, an instructional osteoporosis prevention booklet (National Osteoporosis Foundation, 2008), into an e-book format for use as a smartphone app. We adapted only those chapters relevant for osteoporosis prevention and testing. We added the capacity to bookmark sections and linked all technical terms to definitions provided in the original glossary. For each chapter we created a corresponding quiz located on the StbS website that provided automated feedback based on the accuracy of participant response. NOF was credited with development of the content, and all acknowledgements were included.
Procedure
Human subject protection was assured through the efforts of a single Institutional Review Board (IRB) for multi-institutional study with the University of Wisconsin-Milwaukee serving as the IRB of record.
Recruitment
Women were recruited within southeastern Wisconsin (Papanek, Csuka, Prigmore, & Ryan, 2019 Under Review; Ryan et al., 2018). We attracted community dwelling women using a number of advertising strategies (publically displayed flyers [e.g., libraries, grocery stores, and beauty shops], work site intranet communication, newspaper articles, radio programs, internet sites, women’s conferences, and nurse managed clinics). These marketing strategies provided women with basic information about the study purpose, eligibility criteria, and study requirements including pre and post in-person appointments and use of smart phone app as the delivery. Volunteers initiated contact with the study via email, text messaging, web site, or phone contact. When volunteers contacted us, details about the study and participation requirements were provided and we performed a two-phase screening process to determine eligibility. Initial eligibility requirements were evaluated via phone interview conducted by experienced and trained professional nurses. Women who met initial eligibility were scheduled for an in-person appointment for a DXA scan to confirm a BMD > −2.5. During the in-person appointment women were consented for the DXA scan and preliminary information were collected. Scan results were immediately reviewed and eligible women were consented to participate in the study and scheduled or preceded to a six-hour baseline appointment. Both the participant and researcher were blinded to group assignment. Participants signed the consent form and then opened a sealed, opaque envelope containing group assignment, un-blinding group assignment. Detailed description of this appointment can be found elsewhere (Ryan & Sawin, 2009).
Preparing participants and apps
We uploaded the appropriate apps to each participant’s smartphone at their baseline appointment. Each participant then received usage instruction in the form of a voice-over PowerPoint presentation. After the presentation, the administrating researchers conducted an assessment of participants’ ability to use the phone and the apps. All participants received printed copies of the PowerPoint tutorials and could ask for additional one-to-one assistance at any time during the duration of the study.
All apps (Striving, Boning Up, and EMAs) ran on Iphone 5, 5s, or 6s. For participants who already owned a compatible smartphone, we loaded the intervention app directly onto their personal phone; these participants received monthly compensation to offset the usage cost. Participants who did not have a compatible smartphone were given an Iphone 5s for use during the duration of the study. For these participants, the study provided a phone plan as long as the woman remained an active participant including completion of online measures five times during the year, and weekly electronic contact with the study. Participants who successfully completed the study were allowed to keep their study smartphone.
The separate EMA app was loaded onto all phones, including members of the Wait List group. Using a computer generated random schedule 276 EMA messages were sent via an automated text-message small message system (SMS) (i.e., text message) to all study participants across the 12-month study period. Participant responses, along with the data from participant-initiated EMAs, were collected and stored in the study’s HIPAA–compliant cloud server. Data from the EMA app identify the specific health behaviors the participant was working on (calcium intake, balance or strength training, or PA), the frequency of the activity, and specific types of health-behavior change processes used.
Intervention period and retention
Women in all three groups actively participated in the study for 12 months. Participants in the Striving group were instructed to work with their Striving app three to five times a week over the course of the year, while participants in the Boning Up group were instructed to read and study the book and then use the e-book as reference for the study year. All participants were required to maintain active participation. Active participation included attendance at baseline and end-of-study appointments; completion of repeated-measures using the app, web site, or the e-book; and engagement in weekly electronic communication with the study through study devices. Participants’ study devices automatically recorded all electronic communication, including usage time, measure response/input, use of study web site, and completion of EMAs. Failure to communicate electronically (e.g., app, EMS, website) on a weekly basis triggered a message to the research team indicating the participant was not actively participating in the study. A designated researcher initiated personal contact with the participant to help them re-establish the required communication.
Retention strategies included allowing participants to keep the assigned study phone or to be compensated for use of personal monthly phone plans for study duration, regularly mailed tokens of appreciation (e.g., recipe cards with study logo, book mark), and progressive financial compensation for electronic data completion every three months. Participants who chose to withdraw from the study or who failed to respond to email or phone contact following a weekly communication failure were removed. Removal or withdrawal from the study triggered a cessation in monthly payment or cancelation of the phone plan and removal of all apps. With assistance from the phone carrier, we remotely locked any unreturned study phones for use or sale.
Analysis
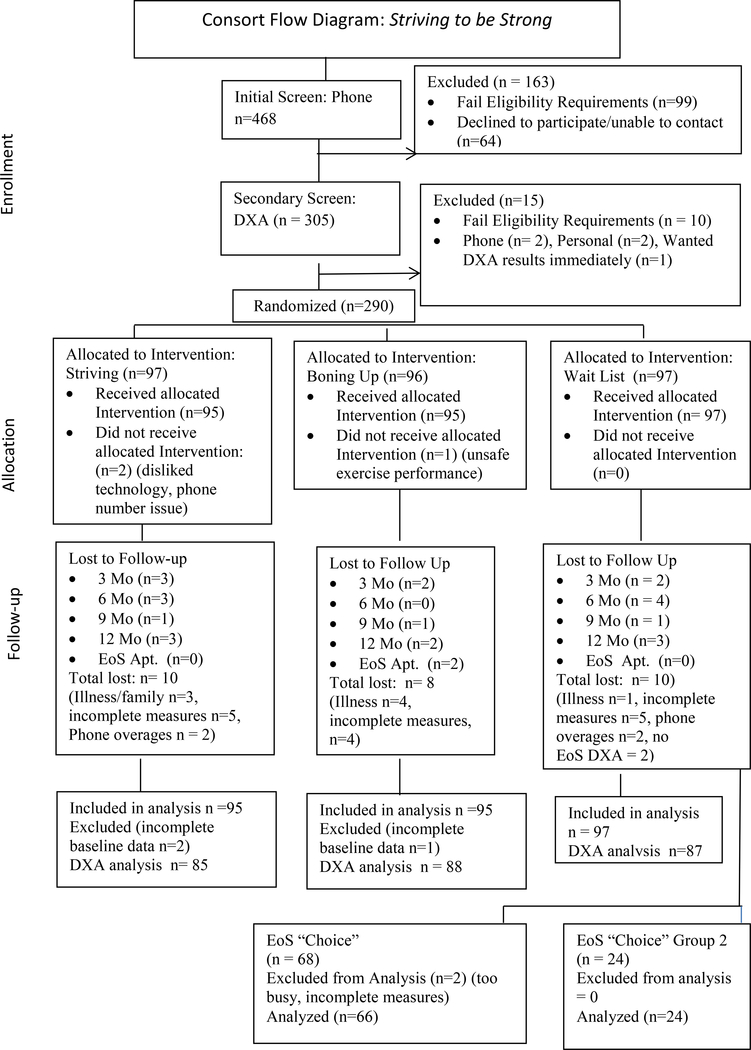
Figure 1 shows screening, enrollment, and retention numbers for total sample and each arm along with details related to drop-out and follow-up. Physical, sociodemographic, and other clinical characteristics for each of the three study groups in frequency and percent for categorical data and means and SD for continuous data can be found in Table S1 (Supplemental Digital Content). We determined the total and percent EMA use for each group and each behavior. Using an intent to treat analysis with general linear mixed modeling (GLMM) (Chakraborty & Gu, 2009; Cnaan, Laird, & Slasor, 1997), we described differences between groups, over time, and group by time interaction. Although numerous techniques exist for analyzing continuous, repeated-measures data, when the design includes only two measurements (pre and post), the use of a repeated-measures GLMM produces the least about of bias in the results when there is moderate to high correlation between the outcome measures (Hyer & Waller, 2014). These data are described in both table and graphic format (Table 1 & Figure 2). We described DXA data, BMD baseline and end-of-study scores, and created a difference score for each study group for total hip, femoral neck, spine, and TBS for those participants for whom baseline and end of study data were available (Table 2).
Figure 1:
Consort Flow Diagram: Striving to be Strong
Table 1.
Results of GLMM Analysis of Distal Outcomes of Bone Mineral Density and Trabecular Bone Scores: An Intention to Treat Analysis
| Model Term | Chi-square | df | p-value |
|---|---|---|---|
| Femoral Neck BMD (N=290) | |||
| Time | 23.89 | 1 | <0.001 |
| Group | 4.62 | 2 | 0.099 |
| Time X Group | 1.38 | 2 | 0.502 |
| Total Hip BMD (N=290) | |||
| Time | 6.75 | 1 | 0.009 |
| Group | 2.01 | 2 | 0.365 |
| Time X Group | 0.54 | 2 | 0.762 |
| Spine BMD L1-L4 (n=284)a | |||
| Time | 15.87 | 1 | <0.001 |
| Group | 2.19 | 2 | 0.335 |
| Time X Group | 0.26 | 2 | 0.876 |
| Trabecular Bone Score (N=290) | |||
| Time | 0.81 | 1 | 0.367 |
| Group | 1.54 | 2 | 0.462 |
| Time X Group | 0.32 | 2 | 0.852 |
Note. GLMM = General Linear Mixed Model; BMD = Bone mineral Density (g/cm2)
BMD = Bone Mineral Density
Spine BMD baseline and end of study for 284 women rather than 290 because 6 women had some form of metal (e.g., surgical repair or bullet) in at least one or more vertebra and as such, a valid measurement of L1-L4 spine BMD could not obtained at baseline or end of study.
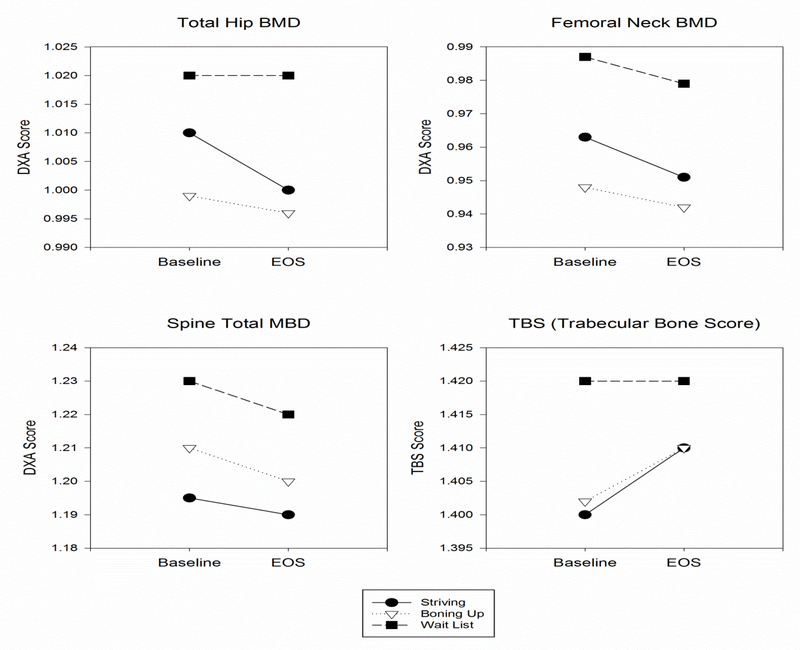
Figure 2:
Graphic Representation of Change in Bone Mineral Density and Trabecular Bone Score over Time and Among Groups
The downward slopes of the lines depict loss of bone mineral density & trabecular bone score between baseline and end of study for total hip, femoral neck, and spine. No difference among the groups occurred as evidenced by none of the lines crossing over time.
Table 2.
Comparison of and Difference between Baseline and End of Study Bone Mineral Density and Trabecular Bone Scores for Women with Baseline and End of Study Data
| Total N = 260 μ (SD) |
Striving n = 85 μ (SD) |
Boning Up n = 88 μ (SD) |
Wait List n = 87 μ (SD) |
|
|---|---|---|---|---|
| Total Hip BMD | ||||
| Baseline | 1.010 (.135) | 1.018 (.144) | .994 (.122) | 1.020 (.138) |
| EoS | 1.006 (.137) | 1.012 (.147) | .990 (.123) | 1.017 (140) |
| Difference | −.004 (.026 | −.006 (.018) | −.003 (.034) | −.003 (.022) |
| Femoral Neck BMD | ||||
| Baseline | ||||
| EoS | .963 (.128) | .967 (.133) | .942 (.118) | .980 (.130) |
| Difference | .955 (.129) | .956 (.135) | .9364 (.119) | .972 (.132) |
| −.008 (.028) | −.0116 (.025) | −.006 (.026) | −.007 (.032) | |
| Spine Total*BMD | ||||
| Baseline | 1.208 (.163) | 1.195 (.144) | 1.210 (.174) | 1.22 (.170) |
| EoS | 1.200 (.167) | 1.188 (.152) | 1.200 (.176) | 1.212 (.173) |
| Difference | −.008 (.034) | −.007 (.033) | −.009 (.034) | −.009 (.039) |
| TBS | ||||
| Baseline | 1.406 (.120) | 1.400 (.121) | 1.402 (.124) | 1.416 (.117) |
| EoS | 1.412 (.108) | 1.408 (.113) | 1.409 (.109) | 1.419 (.102) |
| Difference | .006 (.082) | .007 (.091) | .007 (.080) | .002 (.077) |
Note. EoS = End of Study; TBS = Trabecular Bone Score; BMD = Bone Mineral Density (g/cm2)
Note: Spine BMD baseline and end of study for 284 women rather than 290 because 6 women had some form of metal (e.g., surgical repair or bullet) in at least one or more vertebra and as such, a valid measurement of L1-L4 spine BMD could not obtained.
Difference (actual and percent) between baseline and end-of-study BMD and TBS scores were calculated and classified as in increase in BMD or a decrease in BMD. A decrease in BMD was further classified into a loss of less than 1% or greater than 1% (Table 3). Because the national norm for loss of BMD in women in this age group is 1-to- 5% annually, we interpreted a loss of BMD of less than 1% as a weak but positive outcome (Looker, Isfahani, Fan, & Shepherd, 2017). Reports of other study data can be found at the following web-site (Ryan & Papanek, 2019)
Table 3.
2-Month Change in DXA and TBS Scoresa
| Difference: Baseline to End of Study | Total N = 260 Mean (SD) % Change |
Striving n = 85 Mean (SD) % Change |
BoningUp n = 88 Mean (SD) % Change |
Wait List n = 87 Mean (SD) % Change |
|---|---|---|---|---|
| Total Hip BMD* | −.004 (. 026) | −.006 (.018) | −.003 (.034) | −.003 (.022) |
| % Change | −0.39% | −0.58% | −0.30% | −0.29% |
| Femoral Neck BMD | −.008 (.028) | −.0116 (.025) | −.006 (.026) | −.007 (.032) |
| % Change | −0.83% | −1.19% | −0.65% | −0.71% |
| Spine Total* MBD | −.008 (.034) | −.007 (.033) | −.009 (.034) | 0.36 |
| % Change | −0.74% | −0.58% | −0.74% | −0.73% |
| TBS (Trabecular Bone Score) | +0.006 (.082) | +0.007 (.091) | +0.007 (.080) | +0.002 (.077) |
| % Change | +.43% | +0.50 | +0.50 | +0.14 |
Note. BMD = Bone Mineral Density; TBS = Trabecular bone score. Cells with no background indicate improvement; cells with light background indicate a decrease of less than 1% per year in scores; single darker cell indicates a decrease of greater than 1 % decrease in scores.
Normal loss = 1 to 5%/year in this age group.
Results
Hypothesis Testing
We hypothesized that a person-centered, dynamically tailored intervention would result in the improvement of BMD and TBS over the use of standardized education or a wait list. When using an intention to treat analysis (GLMM), the null hypothesis was accepted as there were no significant differences in BMD (total hip, femoral neck, or spine) and TBS among intervention groups (Table 1, Figure 2). Although there were no significant between-group differences, the overall BMD loss across all groups (with a single exception) was less than 1%, a level of BMD loss lower than commonly observed (Table 3) (Looker et al., 2017). EMA responses indicated that all groups were actively responding to EMAs throughout the 12-month study period, with some participants working on more than one health behavior at a time.
Participants
All women who were recruited, screened, and met BMD requirements via DXA were accepted into the study for an enrollment sample of 290. A 12-month attrition rate of 10.4% resulted in a final sample size of n = 260 (Figure 1, Follow up). Attrition across study groups was similar as were reason for withdrawing. Participants averaged 50 years of age (minimum-maximum, 40–60) and were predominately White, college educated, and of a moderately high socioeconomic status (Table 1). The majority of participants were overweight based on body mass index and had a 4% 10-year risk for any osteoporotic fracture based on the FRAX. Consistent with national norms for this age group (Gold, 2011), approximately half of the participants were post-menopausal, slightly less than 25% were in menopausal transition, and slightly over 25% were pre-menopausal (Table S1, Supplemental Digital Content).
EMAs
Much to our surprise women responded positively to the EMAs, described them as motivating, with a number of study participants requesting to keep the EMA app following completion of the study. Over the course of the 12-month study, active participants received a total of 78,166 EMA messages with an average of 284 responses per participant. Women in the Striving group responded to approximately 30 EMAs for each 28-day block of time, while women in both the Boning Up and Wait List groups responded to approximately 28 EMAs for each 28-day block of time.
Participants provided data for 94,480 individual behaviors (more responses than requests), indicating participants were actively working on more than one health behavior at a time. All participants responded some of the time and few participants responded regularly. Most participants focused on increasing calcium intake (47.5% in Striving, 52.6% Boning Up, and 48.1% Wait List). The next most-frequent behavior was PA (36.6% in Striving Group, 33.7% in Boning Up, and 42.3% Wait List), followed by strength (15.5%, 13.1%, and 18%). Balance received the fewest number of responses (13.6%, 12.2%, and 13.7%). Example of EMA screens can be found at the study website (Ryan & Papanek, 2019).
Discussion
This article provides the results of a multifaceted health promotion program delivered via an app dynamically tailored to enhance the participants’ health beliefs, self-regulation skills and abilities, and social facilitation. This unique approach to app development extends the current paradigm for m-health delivery media to support and enhance self-management behaviors in its use of person-centered strategies directed at strengthening change processes. Results demonstrate that while distal outcomes changed over time, there were no statistically significant differences among study groups for the distal outcomes of BMD and TBS. Small but positive results were observed across all groups, suggesting one or more aspects of study participation might have affected the outcomes. In addition, the large variance observed within study groups and the time by group interaction differences across groups supports a need for future analysis to identify pattern of usage and sub-group analysis.
Factors Contributing to No Difference among Groups
Four unplanned and unintentional factors might have contributed to the lack of significant differences between-groups on BMD and TBS scores. These factors were: dissemination of the intervention, retention without participation, EMA functioned as both assessment tool and intervention, and personalization of research based recommendations.
Dissemination of the intervention
Random assignment of women to an intervention group was core to the design. Word of mouth or “woman-to-woman” contact occurred as an unplanned recruitment strategy (Ryan et al., 2018)(). Women told other women (friends, family, and co-workers) about the study and encouraged them to participate (Buchholz et al., 2016). Mixing woman-to-woman recruitment with random assignment of individuals to study groups might have resulted in dissemination of the interventions across groups, a threat to the internal validity of the study (Shadish, Cook, & Campbell, 2002). Although end-of-study interviews and surveys indicated a limited amount of actual sharing of the app across members of different study groups, we cannot discount the possibility that the different interventions were blurred across daily activities and conversations through woman-to-woman exchanges.
Retention without participation
The relationship between study retention and intervention use has become an increasingly observable phenomenon with the advent of automated collection of electronic data. Unlike conventional approaches used to evaluate intervention fidelity (e.g., self-report, class attendance, or use of knowledge tests as a proxy measures), electronic media enables researchers to automatically track intervention use. In his review of studies that used electronically collected information, Eyesenbach (Eysenbach, 2005) documented that up to 99% of persons who continued to remain “active” study participants failed to use interventions as intended. It is not known whether the extent of participant inactivity is associated with the use of electronic intervention delivery media or whether high levels of failure to use interventions exist in other types of delivery media but have not been readily observable before the advent of automated data collection. Our study’s initial report of EMA use provides helpful information about intervention use and its differences across participant. Future sub-group analyses may disclose differences in outcomes based on differences in use over time (Zaslavsky et al., 2013).
EMA as assessment and intervention
It is possible that study-generated EMA messages acted as an independent intervention. Because all study participants (regardless of treatment group) received and responded to EMAs throughout the duration of the study, and because participants reported being motivated or reminded by the EMAs, the EMAs might have functioned both as a measurement tool and as an intervention themselves. The results of research published after development of the Striving App suggests that app usage increases when the app contacts the user, either by reminding users to engage in a specific activity or by sending encouraging messages (Birkhoff & Smeltzer, 2017). Our results are consistent with this observation, and they point to a need to further explore the roles that EMAs can serve as both an assessment tool and an intervention.
Personalization of research based recommendations
There is wide-spread agreement about the importance of person-centered interventions (Institute of, 2001), and although the operationalization of such interventions varies, it is generally accepted that professional recommendations should be based on research-based protocols and that individuals need to actively participate in decision making, goal setting, and planning. Although the interventions developed for this study were research based and tailored to match the characteristics of individuals, we know participants altered the recommendations ((Ryan, Brown, & Lynch, 2019). Instead of using protocols as advised, participants selectively chose to use some aspects of the intervention and often did not follow recommendations for amounts of foods or frequency, intensity, or duration of activity and exercises. The process of personalization might have changed the implementation of the intervention such that previously documented research outcomes could no longer be reached.
We believe interventions will have the greatest efficacy when they are both research-based and individualized. The future challenge will be to integrate knowledge and experience of health care professionals with personal preferences of individuals. There is a need to foster shared decision-making, personalized implementation, and evaluation among healthcare providers and individuals to initiate and maintain effective health-behavior change efforts.
Limitations and Implications for Future Studies
The sample has limited generalizability as it was composed primarily of White, educated women. The percentage of African American women enrolled (8.9%) was lower than the percentage living in the community from which recruitment occurred (15%) despite using more vigorous recruitment efforts among communities of color than our prior studies where our minority population was higher (Ryan, Lynch, Schlidt, & Papanek, under review). Recruitment occurred by attracting interested volunteers and led to self-selection by the volunteers. It could be argued that only women with pre-determined interested in preventing osteoporosis or interest and willingness to use electronic technology contacted the study.
The IFSMT is a relatively new theory and its utility to manage complex health behavior change needs to be tested empirically. This study focused on osteoporosis prevention and guided participants through health behavior change processes; clearly focusing on the four health behaviors. The unique contribution of the IFSMT is its focus on process, and while the study incorporated process into the intervention perhaps the intervention would have had greater efficacy with a dominant focus on the dimension of process rather than on behaviors.
Future analysis is needed to identify subgroups and to determine efficacy of intervention across sub groups. For example, future analysis could evaluate the relationship of participants focus on one or more of the four behaviors, actual use of intervention over time, or differences in outcomes across menopausal status. In addition, although BMD and TBS are capable of assessing changes over a 12-months period, additional reassessments (two to three years later) might improve capacity to detect change among groups.
Conclusion
This study used a person-centered intervention to investigate a theory that explains the relationship between processes of health-behavior self-management and health outcomes. We created a smartphone app that contained materials to enhance knowledge, self-management skills, and social facilitation for middle-aged women to prevent osteoporosis. There were few differences across groups. All groups experienced a loss of bone density, but the amount of bone density lost was less than that nationally observed for women in this age range. Study results do not yet support transitioning this osteoporosis prevention app to clinical practice at this time; nevertheless, our findings support additional analyses to extend the scope of this project to identify and evaluate the effect of patterns and the complexities of health-behavior change. Our findings could inform future research related to person-centered interventions and develop and implement interventions that target health-behavior management in under-researched patient populations. New and innovative approaches to healthcare delivery, as well as prevention and management of chronic conditions, are critical to meet increasing healthcare demands, and our study points to a technological media that enhances individuals’ ability to develop skills needed to self-manage health-behavior change.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR013913. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The award was made to the University of Wisconsin-Milwaukee (UWM) with contributions by Board of Regents of the University of Wisconsin System on behalf of the University of Wisconsin-Milwaukee. Drs. Ryan and Papanek served as Primary Investigators for the study reported.
Additional Members of the Striving to be Strong Team:
Co-Investigator: Raymond Hoffmann, PhD†, Medical College of Wisconsin, Milwaukee, WI; Iqbal Ahamad, PhD, Marquette University
Consultant: Roger Brown PhD. University of Wisconsin Milwaukee
Study Team: Sandra Lynn Danduran; Gina Scheidt, MD; Katalyn Skelton Stanaszak, MSN,RN, AGCNS-BC; Jenna Speltz, BS, MS:, Karen Wilson BSN, RN
Information Technology team: Team, Marquette University, College of Mathematics and Computer Science: Mel Bilen, BS; Duc Do, BS; Taskina Fayezeeni, BA; ABM Kowser Patwary, BS
Student Workers: Marquette University College of Health Science: Franceska Wenninger, Zachary Vandenberg, Kelsey Krushinsky, Margaret Smith
Footnotes
The authors have no conficts of interest to report.
Ethical Conduct of Research: This study was conducted in compliance with ethical codes for research. Human subjects protection was assured through the efforts of a single Institutional Review Board for multi-institutional study with the University of Wisconsin-Milwaukee serving as the IRB of record (12.402)
Clinical Trial Registration: Clinical Trials.gov, NCT03405103, Registration date Jan. 26, 2018, enrollment January 2014 through May 2016.
Contributor Information
Polly Ryan, School of Nursing University of Wisconsin–Madison, Madison, WI.
Roger Brown, Schools of Nursing and Medicine and Public Health, University of Wisconsin–Madison Madison, WI.
Mary Ellen Csuka, Department of Rheumatology, Medical College of Wisconsin, Milwaukee, WI.
Paula Papanek, Department of Physical Therapy, Marquette University, Milwaukee, WI.
References
- American College of Radiology. (2014). ACR-SPR-SSR practice parameter for the performance of Dual-Energy X-ray Absorptiometry (DXA) resolution 39 Retrieved from http://www.acr.org/guidelines
- Baysari MT, & Westbrook JI (2015). Mobile applications for patient-centered care coordination: A review of human factors methods applied to their design, development and evaluation. Yearbook of Medical Informatics, 10, 47–54. doi: 10.15265/IY-2015-011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhoff SS, & Smeltzer SC (2017). Perceptions of smartphone user-centered mobile health tracking apps across various chronic illness populations: An integrative Review. Journal of Nursing Scholarship, 49(4), 371–378. doi: 10.1111/jnu.12298 [DOI] [PubMed] [Google Scholar]
- Bouton ME (2014). Why behavior change is difficult to sustain. Preventative Medicine, November, 29–36. doi: 10.1016/j.ypmed.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz SW, Wilbur J, Schoeny ME, Fogg L, Ingram DM, Miller A, & Braun L (2016). Retention of African American Women in a Lifestyle Physical Activity Program. Western journal of nursing research, 38(3), 369–385. doi: 10.1177/0193945915609902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty H, & Gu H (2009). A mixed model approach for intent-to-treat analysis in longitudinal clinical trials with missing values. Retrieved from NC: http://www.rti.org/rtipress [PubMed] [Google Scholar]
- Cnaan A, Laird NM, & Slasor P (1997). Using the general linear mixed model to analyze unbalanced repeated measures and longitudinal data. Statistics in Medicine, 16, 2349–2380. [DOI] [PubMed] [Google Scholar]
- Cosman F, de Beur SJ, LeBoff MS, Lewieecki EM, Tanner B, Randall S, & Lindsay R (2014). Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis Int. doi: 10.1007/s00198-014-2794-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly LM, Horey D, Middleton PF, Boyle FM, & Flenday V (2017). The effect of mobile application interventions on influencihg health maternal behavior and imporving preinatal health outcomes: A systematic review protocol. Systematic Reviews, 6, 26. doi: 10.1186/s13643-017-0424-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, Car J, & Atun R (2012). Mobile phone messaging for facilitating self-managment of long-term illness. Cochrane Database of Systematic Reviews, 12. doi:10:1002/14651858.CD007459.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenbach G (2005). The law of attrition. Journal of medical Internet research, 7(1), e11. doi:v7e11 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free GP, Galli L, Watson L, Felix L, Edwards P, Patel V, & Haines A (2013). The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review Caroline. PLOS Medicine, 10(1). doi:e10001362 PMC3548655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB (2011). The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am, 38(3), 425–440. doi: 10.1016/j.ogc.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NC, Gluer CC, Binkley N, McCloskey EV, Brandi M-L, Cooper C, . . . Kanis JA (2016). Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice: A consensus report of a European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Working Group. Bone, 78(216–224). doi: 10.1016/j.bone.2015.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyer JM, & Waller JL (2014). Comparison of five analytic techniques for two-group pre-post repeated measures designs using SAS (1798–2014, SAS Technical Paper). Retrieved from [Google Scholar]
- Institute of, M. (2001). Health and behavior: The interplay of biological, behavioral, and societal influences: Committee on health and behaivor: Research, practice and policy board on neuroscience and behavioral health. Retrieved from Washington, D.C.: [Google Scholar]
- IOM. (2012). Living Well with Chronic Illness: A Call for Public Health Action. Retrieved from Washington D.C.: [DOI] [PubMed] [Google Scholar]
- Kelly MP, & Barker M (2016). Why is changing health-related behaviour so difficult? Public Health, 136, 109–116. doi: 10.1016/j.puhe.2016.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-S, Jong-Duek B, Yang K, & Yoon S (2012). Relationships Between Physical Activity and Awareness and Treatment Status Among Adults With Low Femoral Bone Density in the United States. American Journal of Health Promotion, 27(1), 2–9. doi: 10.4278/ajhp.110107-QUAN-7 [DOI] [PubMed] [Google Scholar]
- Looker AC, Isfahani NS, Fan B, & Shepherd JA (2017). Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporosis International, 28, 1979–1988. doi: 10.1007/s00198-017-3996-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek J, Morgulec-Adamowicz N, Rutkowska I, & Kosmol A (2014). Using ecological momentary assessment to evaluate current physical activity. BioMed Research International, 2014. doi: 10.1155/2014/915172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modave F, Bian J, Leavitt T, Bromwell J, Harris C, & Vincent H (2015). Low quality to free coaching apps with respect to the American College of Sports Medicine Guidelines: A review of current mobile apps. JMIR MHEALTH& UHEALTH, 3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Osteoporosis Foundation. (2008). Boning Up on Osteoporosis: A Guide to Prevention and Treatment. Washington, DC: National 1002Osteoporosis Foundation. [Google Scholar]
- National Osteoporosis Foundation. (2018). What is osteoporosis and what causes it. Retrieved from https://www.NOF.Org/pts/what-is-osteoporosis
- Papanek PE, Csuka ME, Prigmore H, & Ryan P (2019. Under Review). Higher than anticipated prevalence of low bone mass in healthy women 40 to 60 years of age. [Google Scholar]
- Recker R (2011). [NOF President, Dr. Robert Recker, delivered remarks Friday, September 9 at the joint meeting of the FDA Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee]. Web Page.
- Romagnoli E, Cipriani C, Nofroni I, Castro C, Angelozzi M, Scarpiello A, . . . Minisola S (2013). “Trabecular Bone Score” (TBS): An indirect measure of bone micro-architecture in postmenopausal patients with primary hyperparathyroidism. Bone, 53(1), 154–159. doi: 10.1016/j.bone.2012.11.041 [DOI] [PubMed] [Google Scholar]
- Ryan P (2009). Integrated Theory of Health Behavior Change: background and intervention development. Clinical nurse specialist CNS, 23(3), 161–170; quiz 171–162. doi: 10.1097/NUR.0b013e3181a42373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P, Brown M, & Lynch SB (2019). Self-management processes used by healthy middle age women to change behavior. Western Journal of Nursing Research. doi: 10.1177/019345919861944 [DOI] [PubMed] [Google Scholar]
- Ryan P, & Lauver DR (2002). The Efficacy of Tailored Interventions. Journal of Nursing Scholarship, 34(4), 331–337. doi: 10.1111/j.1547-5069.2002.00331.x [DOI] [PubMed] [Google Scholar]
- Ryan P, Lynch SB, Schlidt A, & Papanek P (under review). Recruitment and retention strategies supported high retention for health behavior change RCT.
- Ryan P, Maierle D, Csuka M, Thomson A, & Szabo A (2013). Computer-Based intervention to enhance self-management of calcium and vitamin D intake in women. Western Journal of Nursing Research, 35(8), 986–1010. doi: 10.1177/0193945913483369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P, & Papanek P (2019). Striving to be Strong Study. Retrieved from mu.edu/exercise-science/NINR.Osteo [DOI] [PubMed]
- Ryan P, Papanek P, Csuka ME, Brown ME, Hopkins S, Lynch SB, . . . Striving to be Strong Team. (2018). Background and method of the Striving to be Strong Study: a RCT testing the efficacy of a m-health self-management intervention. Contemporary Clinical Trials, 71, 80–87. doi: 10.1016/j.cct.2018,06.006 [DOI] [PubMed] [Google Scholar]
- Ryan P, & Sawin K (2014). Individual and Family Self-Management Theory (Revised Figure). Retrieved from http://www4.uwm.edu/nursing/about/centers-institute/self-management/theory/cfm [DOI] [PMC free article] [PubMed]
- Ryan P, & Sawin KJ (2009). The Individual and Family Self-Management Theory: background and perspectives on context, process, and outcomes. Nursing Outlook, 57(4), 217–225.e216. doi: 10.1016/j.outlook.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P, Schlidt A, & Ryan C (2013). The impact of osteoporosis prevention programs on calcium intake: A systematic review. Osteoporosis International. doi: 10.1007/s00198-012-2259-4 [DOI] [PubMed] [Google Scholar]
- Ryan P, Weiss M, & Papanek P (2019a). A substruction approach to assessing the theoretical validity of measures. Journal of Nursing Measurement, 27(1), 126–145. doi: 10.1891/1061-3749.27.1.126 [DOI] [PubMed] [Google Scholar]
- Ryan P, Weiss M, & Papanek P (2019b). A substruction approach to assessing the theoretical validity of measures. Journal of Nursing Measurement. [DOI] [PubMed] [Google Scholar]
- Sanchez-Riera L, Wilson N, Kamalaraj N, Nolla JM, Kok C, Li Y, . . . March L (2010). Osteoporosis and fragility fractures. Best practice & research.Clinical rheumatology, 24(6), 793–810. doi: 10.1016/j.berh.2010.10.003 [DOI] [PubMed] [Google Scholar]
- Sedlak CA, Doheny MO, Estok PJ, Zeller RA, & Winchell J (2007). DXA, health beliefs, and osteoporosis prevention behaviors. Journal of Aging and Health, 19(5), 742–756. doi: 10.1177/0898264307304303 [DOI] [PubMed] [Google Scholar]
- Shadish WR, Cook TP, & Campbell DT (2002). Experimental and Quasi-Experimental Design for Generalized Causal Inference. Boston: Houghton Mifflin. [Google Scholar]
- Shiffman S, & Rathbun SL (2011). Point process analyses of variations in smoking rate by setting, mood, gender, and dependence. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors, 25(3), 501–510. doi: 10.1037/a0022178; 10.1037/a0022178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers M, Zheng H, Greendale G, Neer R, Cauley J, Ellis J, . . . Finkelstein J (2013). Changes in bone resorption across the menopause transition: Effects of reproductive hormones, body size and ethnicity. Journal of Clinical Endocrine Metabolism, 98(7), 2854–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spook JE, Paulussen T, Kok G, & VanEmpelen P (2013). Monitoring dietary intake and physical activity electronically: feasibility, usability, and ecological validity of a mobile-based ecological momentary assessment tool. Journal of Medical Internet Research, 15(9), e214. doi: 10.2196/jmir.2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur J, Vassalo A, Chandler P, McDevitt J, & Miller AM (2005). Midlife women’s adherence to home-based walking during maintenance. Nursing research, 54(1), 33–40. [DOI] [PubMed] [Google Scholar]
- World Health Organization Collaborating Centre for Metabolic Bone, D. (2010). FRAX WHO Fracture Risk Assessment Tool. Retrieved from http://www.shef.ac.uk/FRAX/tool.jsp?locationValue=9
- Wu F, Laslett LL, Wills K, Oldenburg B, Jones G, & Winzenberg T (2014). Effects of individualized bone density feedback and educational intervention on osteoporosis knowledgs and self efficacy: A 12-yr prospective study. Journal of Clinical Densitometry, 17(4), 466–472. doi: 10.1016/j.jocd.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Zaslavsky O, Cochrane GG, Herting JR, Thompson HJ, Woods NR, & LaCroix A (2013). Application of person-centered analytic methodology in longitudinal research: Exemplars from the Women’s Health Initiative Clinical Trial data. Research in Nursing & Health, 37, 53–64. doi: 10.1002/nurs.21575 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.