Abstract
IKBKB immune deficiency is a rare but life-threatening primary immunodeficiency disorder, involving activation defects in adaptive and innate immunity. We present sixteen cases of a homozygous IKBKB mutation (c.1292dupG) in infants characterized by early-onset bacterial, viral, fungal and Mycobacterial infections. In most cases, T- and B-cells were quantitatively normal, but phenotypically naïve, with severe hypogammaglobulinemia. T-cell receptor excision circles were normal, meaning newborn screening by TREC analysis would miss IKBKB cases. Although IKBKB immune deficiency does not meet traditional laboratory based definitions for SCID, this combined immune deficiency appears to be at least as profound. Urgent HSCT, performed in eight patients, remains the only known curative therapy, although only three patients are survivors. Ongoing infections after transplant remain a concern, and may be due to combinations of poor social determinants of health, secondary graft failure, and failure of HSCT to replace non-hematopoietic cells important in immune function and dependent upon IKK/NF-κB pathways.
Abbreviations: BCG, Bacillus Calmette-Guerin; CID, Combined Immune Deficiency; CMV, Cytomegalovirus; HSCT, Hematopoietic stem cell transplant; HLH, Hemophagocytic Lymphohistiocytosis; IKKβ, Inhibitor of nuclear factor kappa B kinase subunit beta; NEMO, Nuclear factor Kappa-B essential modifier; PHA, Phytohemagglutinin; RSV, Respiratory Syncytial Virus; SCID, Severe combined immune deficiency; TCR, T-cell receptor; TRECs, T-cell receptor excision circles
Keywords: IKBKB, IKKβ (IKK2), IKK/NF-κB, Combined Immune Deficiency, Hematopoietic Stem Cell Transplant
1. Introduction
In the Canadian province of Manitoba, our group has periodically managed young infants of Northern Cree First Nations (Aboriginal) descent presenting with early-onset and life-threatening viral, bacterial, Mycobacterial, and fungal infections, clinically resembling severe combined immune deficiency (SCID). Infants were initially identified in the 1970s from two small northern Manitoba communities. In later years, infants from the neighboring province of Saskatchewan were also identified. In 2013, our group and collaborators identified the genetic defect in four patients as being due to a homozygous duplication mutation (c.1292dupG) in exon 13 of IKBKB, the gene encoding inhibitor of nuclear factor kappa B kinase subunit beta (IKKβ, also known as IKK2) [1].
The homozygous IKBKB duplication mutation seen in our population results in complete loss of IKKβ expression, a critical component of the canonical IKK-nuclear factor kappa B (NF-κB) pathway [1]. Normally, signaling through tumor necrosis family receptors, toll-like receptors, and antigen receptors on T- and B-cells induces activation of the IKK complex, which consists of the kinases IKKα and IKKβ, as well as the regulatory protein nuclear factor Kappa-B essential modifier (NEMO) [2]. The active IKK complex, and specifically IKKβ, phosphorylates the inhibitory protein IκBα, resulting in release of cytoplasmic NF-κB. Free from the influence of IκBα, NF-κB translocates to the nucleus to induce the transcription of a multitude of immune and inflammatory genes.
Given the central role of the IKK/NF-κB pathway in regulating both innate and adaptive immune responses, it is not surprising that patients with mutations in genes from this pathway manifest with primary immunodeficiency disorders [3]. Males with hypomorphic hemizygous mutations in IKBKG, which encodes for the NEMO protein and results in reduced NEMO expression, present with a combined immune deficiency that includes enormous allelic, immunologic and clinical heterogeneity [[4], [5], [6], [7], [8], [9]]. Patients with hypermorphic gain of function mutations in NFKBIA [[10], [11], [12]], resulting in reduced degradation of the IκBα protein, have also been shown to exhibit significant combined immune deficiencies. Furthermore, the IKK/NF-κB pathway operates in both hematopoietic and non-hematopoietic cells, leading to the extra-immune findings seen in patients with defects in NEMO and IκBα, such as ectodermal dysplasia and colitis.
Similar to patients with IKBKG and NFKBIA mutations, the first four patients reported by our group with deficiency of IKKβ due to the c.1292dupG IKBKB mutation also presented with immune deficiency [1]. Since then, six additional patients from various ethnic backgrounds (including Turkey, Qatar, and the Arabian Peninsula) with different nonsense IKBKB mutations have been reported, with all patients presenting with early onset and profound combined immune deficiencies, but typically without findings of ectodermal dysplasia [[13], [14], [15]]. In our initial report, patients had normal to near-normal numbers of T- and B-cells of an almost exclusively naïve phenotype, severe hypogammaglobulinemia, and activation defects to a variety of stimuli in a number of immune cells, both innate and adaptive, more suggestive of a global functional defect as opposed to a quantitative lymphocyte deficiency [1].
Herein we describe the clinical presentation, immunologic features, and HSCT outcomes for the largest cohort of infants with IKBKB immune deficiency resulting from complete loss of IKKβ expression published to date.
2. Materials and methods
2.1. Patients and study design
The study protocol was approved by the Health Research Ethics Board of the University of Manitoba. The authors identified all known (genetically confirmed) or highly suspected infants with the IKBKB mutation managed through the Department of Pediatrics, Winnipeg Children's Hospital, CancerCare Manitoba and the Manitoba Blood and Marrow Transplant Program dating to the 1970s. Data collected by retrospective chart review included clinical presentation, immunologic features, and HSCT approaches and outcomes. Although data was complete for patients diagnosed in a contemporary era (after 2000), data availability was variable for some patients before this (1970–2000). Young infants of Northern Cree descent from the known affected communities presenting with a clinical picture consistent with a severe immune deficiency but without genetic confirmation of an IKBKB mutation were considered highly suspected if they also had a close familial relationship to a genetically confirmed infant with the mutation (e.g. sibling, or second-degree relative where there was known consanguinity within the family). We chose to report the decade of diagnosis (instead of year) to provide the reader with perspective on the immunologic investigations and treatments available at the time, while protecting against the potential for inadvertently identifying individuals, given that patients and their families reside in small communities. Detailed family trees documenting consanguineous relationships within families have not been included for the same reason.
2.2. Immunologic and genetic investigations
Patient laboratory values, including absolute lymphocyte count, CD3, CD4 and CD8 T-cell counts, CD19 B-cell counts, CD56 NK-cell counts, and immunoglobulin levels were compared against published, age-adjusted variables [16,17]. Laboratory parameters are described as low, normal, or high with respect to these ranges. Maternal engraftment of T-cells was assessed by analysis of short tandem repeats using the AmpF STR® Profiler Plus™ PCR Amplification Kit from Life Technologies. Chimerism analysis after HSCT was performed on T-cells, B-cells, and myeloid cells labeled with monoclonal antibodies to CD3, CD19, and CD33/66b and separated using an automated immunomagnetic cell separation method with the RoboSep instrument from StemCell Technologies. Due to a potential paucity of cells with each chimerism test, purity of the various lymphocyte and myeloid subsets was not evaluated with each assay. From validation experiments, however, the purity of separated subsets is >95% with a ±5% error around lineage specific chimerism results.
T-cell proliferative responses using PHA were performed by 3H-thymidine incorporation assay of peripheral blood mononuclear cells after stimulation with PHA at 1:400–1:800 following a 3-day culture. Percentage of normal PHA response was measured by the stimulation index of the patient relative to the average stimulation index of two concurrently run healthy adult control samples. TREC analysis, when performed at the time of diagnosis (n = 6), was either done locally in our laboratory (n = 3) [18] or at a clinical laboratory (Mayo Clinical Laboratories) (n = 3). Units of measure for TRECs (copies/μL whole blood, copies/million PBMCs, copies/million CD3 cells) changed over time.
Sanger sequencing for the c.1292dupG in exon 13 of IKBKB was performed locally on a 3500 Genetic Analyzer™ as per the method developed by Pannicke [1]. Samples containing homozygous G insertions within the region of interest were classified as affected, and confirmed by Sanger sequencing at an independent laboratory.
3. Results
3.1. Clinical presentation of IKBKB immune deficiency
Sixteen patients with genetically confirmed (n = 11) or highly suspected (n = 5) IKBKB mutations were included (Table 1 ). Genetic confirmation for the five infants with highly suspected IKBKB immune deficiency was not possible since all had died, and DNA was not available (all were born in years before the IKBKB mutation was known). One patient was diagnosed immediately after birth by direct IKBKB mutational analysis of a Guthrie card, as part of a targeted newborn screening project started in November 2013 in two of the heavily affected communities. This patient's younger sibling was later diagnosed in-utero, due to the known family history, with confirmation of the mutation after birth. Both siblings were placed in protective isolation and proceeded to HSCT by 1–2 months of age. The other fourteen patients presented to health care facilities with severe infections at a median of 2.5 months of age (range: 2 weeks - 4 months). Twelve patients came from the province of Manitoba and four from the province of Saskatchewan. Four patients were previously reported in less detail in our initial report of IKBKB immune deficiency [1]. We included these patients in order to describe additional clinical and immunologic characteristics and provide updates on long-term outcomes.
Table 1.
Clinical presentation and outcome of IKBKB immune deficiency.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Decade of Diagnosis | 1970s | 1970s | 1980s | 2000s | 1990s | 2000s |
| IKBKB Mutation Confirmed | No | No | No | Yes | Yes | Yes |
| Direct Family Relationships to Known IKBKB Cases | Affected siblings, and second degree relative with confirmed mutation | Affected siblings, and second degree relative with confirmed mutation | Affected siblings, and second degree relative with confirmed mutation | Affected second degree relatives | Affected half sibling and distant relative | Affected half sibling and distant relative |
| Age at Clinical Presentation | Unknown | 3 months | 2 weeks | 2.5 months | 1 month | 3.5 months |
| Major Infections at Presentation and Before HCT* | Disseminated BCG* | Bacteremia (Klebsiella pneumoniae, E. Coli)* Otitis Media (E. Coli) Pyelonephritis (E.Coli) Pneumonia* (polymicrobial gram negatives) Candida albicans (oropharyngeal, perineum) Tinea capitis |
Bacteremia (E. Coli – recurrent) Thrush Recurrent pneumonias (undocumented pathogens) Disseminated HSV |
Disseminated BCG* Disseminated CMV (lungs, blood, urine)* Disseminated Adenovirus (lungs, urine)* Bacteremia (Streptococcus pneumoniae, Stenotrophomonas maltophilia) |
Disseminated BCG* Disseminated CMV (lungs, brain, blood, adrenal glands, heart)* Candida albicans (oropharyngeal, meningitis)* Bacteremia (E. Coli, Morganella morganni, Staphylococcus aureus) Meningitis (Staphylococcus aureus) Varicella zoster virus |
Disseminated Adenovirus (nasal, stool, urine)* Disseminated CMV (Blood) * Bacteremia (E. Coli, Pseudomonas aeruginosa) Thrush |
| Died Before HCT | Yes | Yes | No | No | Yes | No |
| Received HCT | No. HCT not available at the time | No. HCT not available at the time | Yes | Yes | No. Multi-organ failure present. Not HCT candidate. | Yes. Two maternal HLA 10/10 identical bone marrow transplants. |
| Died After HCT | N/A | N/A | Yes | Yes (Day +9) | N/A | Yes (Day +122) |
| Age at Death | Infant (<4 months) | 14 months | 7 years | 4 months | 5 months | 8 months |
| Patient | 7** | 8** | 9 | 10** | 11 |
|---|---|---|---|---|---|
| Decade of Diagnosis | 2000s | 2000s | 2000s | 2010s | 2010s |
| IKBKB Mutation Confirmed | Yes | Yes | No | Yes | Yes |
| Direct Family Relationships to Known IKBKB Cases | None | None | Affected siblings with confirmed mutations | Affected siblings | Affected siblings |
| Age at Clinical Presentation | 2 months | 1 month | 1 month | 3 months | 2 months |
| Major Infections at Presentation and Before HCT* |
Listeria monocytogenes bacteremia and meningitis * Thrush |
Bacteremia (E. Coli) Pneumonia syndrome ND Thrush |
Pneumonia (RSV)* Bacteremia (Staphylococcus aureus, Enterococcus faecalis, Enterococcus faecium, Leuconostoc lactic) Meningitis (Enterococcus species)* Candida albicans (oropharyngeal, perineum) |
Bacteremia (Staphylococcus hominis, Enterococcus faecium) Thrush Pneumonia (Parainfluenza, Mycoplasma pneumoniae) |
Pneumonia (Rhinovirus)* Klebseilla pneumoniae bacteremia, pneumonia and meningitis* Thrush Scabies |
| Died Before HCT | Yes | No | Yes | No | Yes |
| Received HCT | No. Multi-organ failure present. Not HCT candidate. | Yes | No. SCID was suspected but since IKBKB mutation had not yet been described diagnosis was never confirmed. | Yes | No. Multi-organ failure present. Not HCT candidate. |
| Died After HCT | N/A | Yes (2 years) | N/A | No – Alive 7 years post-HCT | N/A |
| Age at Death | 2 months | 2 years | 15 months | N/A | 2.5 months |
| Patient | 12** | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|
| Decade of Diagnosis | 2010s | 1980s | 1990s | 2010s | 2010s |
| IKBKB Mutation Confirmed | Yes | No | Yes | Yes (NBS) | Yes (in-utero and post-natal) |
| Direct Family Relationships to Known IKBKB Cases | No | Affected sibling with confirmed mutation | Affected sibling | Affected sibling | Affected sibling |
| Age at Clinical Presentation | 3 months | 4 months | 2.5 months | 0 months - Newborn Screening | 0 months – Family History |
| Major Infections at Presentation and Before HCT* | Serratia marcescens bacteremia and intracerebral abscesses. Bacteremia (E. Coli, Klebsiella pneumoniae) Candida albicans (oropharyngeal, urine) Pneumonia (RSV, coronavirus) Hemophagocytic Lymphohistiocytosis |
“Sepsis”* | Disseminated BCG* (including M. bovis meningitis and intracerebral abscesses) Fungemia (Candida albicans)* Bacteremia (Stenotrophomonas maltophilia, Enterobacter cloacae, E. Coli) |
None | None |
| Died Before HCT | No | Yes | Yes | No | No |
| Received HCT | Yes | No. HCT not available at the time. | No. Multi-organ failure present. Not HCT candidate. | Yes | Yes |
| Died After HCT | No – Alive 6 years post-HCT | N/A | N/A | Yes | No – Alive 6-months post-HCT |
| Age at Death | N/A | 4.5 months | 3.5 months | 11 months | N/A |
*Single asterisks refer to infections that ultimately resulted in death due to multi-organ failure and / or severe neurological injury. Involvement of organs was confirmed at the time of autopsy.
**Double asterisks refer to patients included in the original publication (Pannicke et al, reference 1).
Abbreviations: BCG: bacillus Calmette-Guerin; CMV: Cytomegalovirus; HCT: Hematopoietic Cell Transplantation; HLA: Human Leukocyte Antigen; HSV: Herpes Simplex Virus; HPV: Human Papilloma Virus; NBS: Newborn Screening; N/A: Not Applicable or Available; ND: Not Documented (infectious pathogen); RSV: Respiratory Syncytial Virus.
Details on the number and type of infections present at the time of initial presentation (before HSCT) were complete for twelve and incomplete for two (patient 1 who died of disseminated Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) and patient 13 who died of unknown sepsis). The two siblings identified by newborn screening and family history had no infections at the time of diagnosis of IKBKB immune deficiency and proceeded to urgent HSCT without infections.
For patients not identified by newborn screening, clinical presentation consisted of a multitude of severe bacterial, fungal, Mycobacterial, and viral infections beginning at an early age (<3 months), usually in association with failure to thrive. Mucocutaneous infection of the oropharynx and perineum with Candida was common (n = 11) with evidence of disseminated Candida infection in the blood (n = 1), urine (n = 1), and cerebrospinal fluid (n = 1) in three. Severe, recurrent gram-negative and gram-positive bacterial infections were frequent and included bacteremia / sepsis episodes with Escherichia coli (n = 7), Pseudomonas aeruginosa (n = 3), Klebsiella pneumoniae (n = 2), Enterococcus species (n = 2), Staphylococcus aureus (n = 2), Stenotrophomonas maltophilia (n = 2), Serratia marcescens (n = 1), Streptococcus pneumoniae (n = 1), Staphylococcus hominis (n = 1), Listeria monocytogenes (n = 1), Morganella morganni (n = 1), Leuconostoc lactis (n = 1) and Enterobacter cloacae (n = 1). Intracranial infections were present in six infants and included a combined Staphylococcus aureus and Candida albicans meningitis in one, and Listeria monocytogenes meningitis, Enterococcus meningitis, Klebsiella pneumoniae meningitis, Serratia marcescens intracerebral abscesses, and Mycobacterium bovis meningitis with intracerebral abscesses in the other five. Disseminated viral infections occurred with cytomegalovirus (n = 3), adenovirus (n = 2), varicella (n = 1), and herpes simplex virus (n = 1). Severe pneumonia syndromes were documented in eleven as result of unknown pathogens (n = 3), cytomegalovirus (n = 2), respiratory syncytial virus (n = 2), polymicrobial gram negative bacteria (n = 1), adenovirus (n = 1), rhinovirus (n = 1), and parainfluenza (n = 1).
Consistent with standard practice in the two affected communities at the time, four infants received the BCG vaccination after birth, all of whom developed disseminated BCG and died. Biopsies (n = 2) and autopsies (n = 2) revealed Mycobacterium bovis in multiple organs including the lungs, liver, spleen, lymph nodes, thymus, bone marrow, adrenal glands, kidneys, eyes, skin, brain and gastrointestinal tract. Following these deaths, BCG vaccination in the two affected communities was discontinued. More recently, with the advent of targeted newborn screening for the homozygous IKBKB mutation in the two affected communities, BCG vaccination after birth is held until results of the newborn screen confirm that an infant is not affected.
One patient met clinical and laboratory criteria for hemophagocytic lymphohistiocytosis (HLH), although it is possible that additional patients would have met HLH criteria but were unrecognized. Despite the connection of IKBKB to the IKK/NEMO complex, none of our patients exhibited ectodermal dysplasia. Only one patient had documented organ abnormalities (dysplastic kidney at time of autopsy).
3.2. Patient outcomes
Of the sixteen IKBKB immune deficiency patients, eight died of overwhelming infection before HSCT could be performed, and eight proceeded to HSCT. Detailed autopsy reports were available for three who died prior to HSCT. Aside from evidence of infection, all exhibited severe thymic hypoplasia with scant lymphoid cell populations, minimal to no lymph node and tonsillar tissue, and absence of germinal follicles in the spleen.
3.3. Immunologic presentation of IKBKB immune deficiency
A range of immunologic tests were performed over time, with the extent and type of testing dependent upon the year of presentation and initial suspicion of an immunodeficiency diagnosis. Extensive immunologic investigations were more likely to occur in the contemporary era (after 2000).
Median total lymphocyte count at the time of diagnosis was 6.94 × 109/L (range: 1.52–11.85 × 109/L), with 11/14 patients exhibiting normal (n = 6) to elevated (n = 5) total lymphocyte counts for age. The three lymphopenic infants all had severe infections (disseminated BCG in two and Klebsiella sepsis in one) at the time their initial blood work was performed, along with various lymphocyte subset and hematologic cytopenias (anemia, thrombocytopenia), more consistent with bone marrow suppression from infection. Median CD3 T-cell count (12 patients) was 3298 cells/μL (range: 240–8480 cells/μL), with only one patient having a CD3 T-cell count <1500 cells/μL and one other <300 cells/μL. As above, in both patients with CD3 T-cell lymphopenia, severe infection was present at the time of assessment. Median CD4 T-cell count (11 patients) was 2490 cells/μL (range: 120–6520 cells/μL) and median CD8 T-cell count (11 patients) was 1660 cells/μL (range: 100–2654 cells/μL), with 6/11 patients having at least one of the CD4 and/or CD8 T-cell count above the normal range for age. Maternal engraftment studies were performed at diagnosis in eight patients, with none showing evidence of maternal engraftment. Median CD19 B-cell count (11 patients) was 750 cells/μL (range: 100–3470 cells/μL), being low in two, normal in eight, and elevated in one. Median CD56 NK-cell count (10 patients) was 240 cells/μL (range: undetectable – 1090 cells/μL), being low in three and normal in seven.
Despite generally normal to elevated T- and B-cell numbers in most IKBKB immune deficiency patients, all infants presenting with infections were severely hypogammaglobulinemic (n = 12), with a median IgG level of 0.75 g/L (range: undetectable – 2.06 g/L). The two infants identified as newborns both had normal IgG levels immediately after birth, likely reflecting maternal placental transfer of IgG.
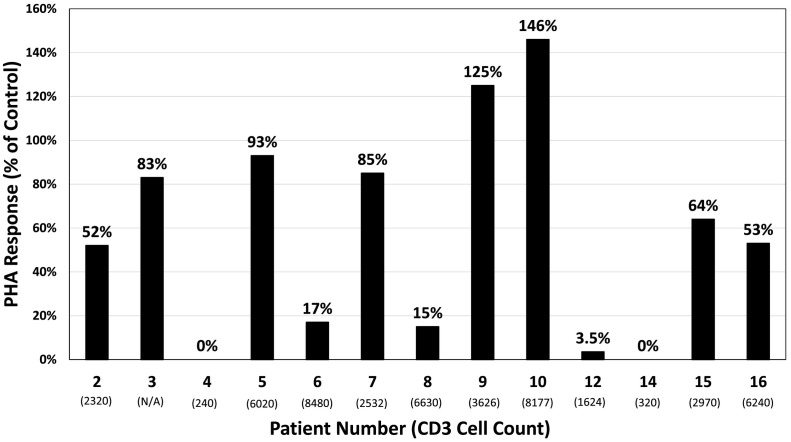
T-cell proliferative responses were available for 13 patients (Fig. 1 ). Relative to controls, three had PHA responses <10%, two had PHA responses between 10 and 30%, and eight had PHA responses above 30% (range: 53% - 146%). No relation was evident between PHA response and total CD3 T-cell count, although two of the patients with severe CD3 T-cell lymphopenia and disseminated BCG had no PHA response. The two infants identified at birth without infections both had normal PHA responses (64% and 53%).
Fig. 1.
Phytohemagglutinin responses at the time of IKBKB diagnosis by patient number. Percent of control is defined as the stimulation index of the patient relative to the average stimulation index of two healthy controls. Corresponding CD3 T-cell count (cells/μL) at the time of diagnosis is under each patient number. Patients 4 and 14 both had disseminated BCG with bone marrow involvement and severe cytopenias, including lymphopenia, which may have impacted PHA response.
TRECs were performed in six infants. All had normal TREC expression levels (9815 copies/106 PBMCs; 19,663 copies/106 CD3; 17,965 copies/106 CD3; 535 copies/μL; 1727 copies/μL; 1462 copies/μL). More extensive T-cell immune phenotyping was performed in three patients. All exhibited a preponderance of naïve CD4 T-cells (CD4 + CD45RA + CD62L + CD27+) between 84%–96% of the CD4 T-cell population, and naïve CD8 T-cells (CD8 + CD45RA + CD62L + CD27+) between 82 and 84% of the CD8 T-cell population. Minimal to no central and effector memory T-cells (0–8%) were present in any of the cases. One patient had TCR v-beta repertoire performed, revealing a normal polyclonal T-cell population.
3.4. Hematopoietic stem cell transplant for IKBKB immune deficiency
3.4.1. Hematopoietic stem cell transplant approaches
Eight patients underwent HSCT, using either bone marrow from a human leukocyte antigen matched parent or sibling (n = 4) or unrelated umbilical cord blood (n = 4) as the graft source (Table 2 ). Two patients not initially receiving a conditioning regimen (patients 4 and 6) due to critical illness from infections both failed to engraft, with a second transplant performed in one using myeloablative conditioning, resulting in subsequent engraftment. Conditioning regimens were initially used in the other six. Two patients (3 and 8), one of whom received a reduced intensity conditioning regimen followed by a cord blood transplant, initially engrafted but experienced secondary graft failure over two years post-HSCT. Four patients received a busulfan-based myeloablative conditioning regimen (with busulfan pharmacokinetic monitoring after 2010) with either full or mixed donor chimerism, and generally more favorable myeloid engraftment.
Table 2.
Hematopoietic stem cell transplant outcomes for IKBKB immune deficiency.
| Patient: |
3 |
4 |
6 |
8 |
10 |
12 |
15 |
16 |
|---|---|---|---|---|---|---|---|---|
| Decade of Diagnosis | 1980s | 2000s | 2000s | 2000s | 2010s | 2010s | 2010s | 2010s |
| Identified by IKBKB NBS | No | No | No | No | No | No | Yes | Yes |
| Major Infections Present at Start of HCT | HSV | Disseminated BCG, Adenovirus, CMV | Disseminated Adenovirus, CMV | Pneumonia Syndrome ND (Likely Viral) | Parainfluenza Pneumonia | RSV Pneumonia and Serratia Marcescens Abscesses | None | None |
| Age at First HCT | 19 months | 4 months | 4.5 months | 5 months | 7 months | 8 months | 2 months | 1 month |
| Donor | Father | Half-Sibling | Mother | Unrelated | Brother | Unrelated | Unrelated | Unrelated |
| HLA Match | 6/6 antigen match (HLA-A, B, HLA—C) and non-reactive in mixed leukocyte culture | 10/10 | 10/10 | 6/6 | 10/10 | 5/6 | 5/6 | 5/6 |
| Graft Source | Marrow | Marrow | Marrow | Cord Blood | Marrow | Cord Blood | Cord Blood | Cord Blood |
| Conditioning | MAC - Bu/Cy/ATG | None | None | RIC - Flu/Mel/Campath | MAC - Bu/Cy/ATG | MAC – Bu/Cy/ATG | MAC – Bu/Flu/ATG | MAC – Bu/Flu/ATG |
| GVHD Prophylaxis | None | None | Tacrolimus / Methotrexate | Tacrolimus / Methotrexate | Tacrolimus / Methotrexate | Tacrolimus / Methotrexate | Tacrolimus / Methotrexate | Tacrolimus / Methotrexate |
| Time to ANC >0.5 × 109/L | N/A | Never Achieved | Primary Graft Failure | +14 | +23 | +15 | +15 | + 14 |
| Time to Platelets >50 × 109/L | N/A | Never Achieved | Primary Graft Failure | N/A | +31 | +54 | +44 | +35 |
| Second Transplant or Boost | No | No | Yes. Mother, Bone Marrow, Myeloablative (Bu/Flu/ATG) with neutrophil engraftment day +12. | No | No | No | No | No |
| Donor Chimerism at Last Follow-Up (Time Post-HCT) | Secondary graft-rejection of paternal marrow determined by karyotype by 3-years. | N/A. Uncertain engraftment of half-sibling marrow | 97% donor at day +12 after second transplant | Mixed Donor:Recipient with declining donor chimerism to <10% over 2-years. No myeloid donor chimerism. | T-cell: 67% | T-cell: 98% | T-cell: 95% | T-cell: 82% |
| B-cell: 96% | B-cell: 98% | B-Cell: 98% | B-Cell: 100% | |||||
| Myeloid: 80% (7-years) | Myeloid: 98% (2-years) | Myeloid: 100% (7-months) | Myeloid: 94% (5-months) | |||||
| CD3 T-Cell Count Post-HCT (x109/L) | N/A | N/A | N/A | 6.55 | 3.51 | 5.78 | 6.55 | 3.04 |
| CD4 T-Cell Count Post-HCT (x109/L) | N/A | N/A | N/A | 3.84 | 1.51 | 4.36 | 5.27 | 1.91 |
| CD8 T-Cell Count Post-HCT (x109/L) | N/A | N/A | N/A | 2.71 | 1.73 | 1.27 | 0.9 | 0.11 |
| CD56 NK cell Count Post-HCT (x109/L) | N/A | N/A | N/A | 0.61 | 0.22 | 0.13 | 0.9 | 0.11 |
| CD19 B-Cell Count Post-HCT (x109/L) | N/A | N/A | N/A | 1.57 | 1.62 | 3.26 | 5.27 | 7.98 |
| IgG (g/L) off IVIg | N/A | N/A | N/A | 19.2 | 14.2 | 6.53 | 2.06 | N/A (on IVIg) |
| IgA (g/L) | N/A | N/A | N/A | 1.62 | 2.04 | 0.65 | <0.07 | <0.07 |
| Response to Post HCT Vaccinations | N/A | N/A | N/A | No response to tetanus, diphtheria (3 vaccinations). No pneumococcal titres despite Streptococcus pneumoniae infections and conjugate and PV23 vaccination. | No response to tetanus, diphtheria (4 vaccinations). Negative anti-HBs (6 hepatitis B vaccinations). No pneumococcal titres (2 PV23 vaccinations) | Not tested. | No Post-HCT vaccinations | Too early post-HCT |
| PHA Response Post-HCT | N/A | N/A | N/A | Normal | Normal | Normal | Normal | N/A |
| Major Infections in First 6-Months Post-HCT | HSV (stomatitis, perianal, ocular) Thrush |
Disseminated BCG, Adenovirus, CMV* Streptococcus pneumoniae bacteremia |
Disseminated Adenovirus, CMV* Enterobacter cloacae bacteremia |
Streptococcus pneumoniae bacteremia | Varicella Zoster Recurrent Pneumonias |
Disseminated Adenovirus Pseudomonas aeruginosa bacteremia |
Candida Diaper Dermatitis |
Streptococcus pneumoniae bacteremia Candida Diaper Dermatitis Methicillin-Resistant Staphylococcus aureus bacteremia |
| Major Infections After 6-Months Post-HCT |
Salmonella bacteremia / gastroenteritis Disseminated Mycobacterium avium-intracellulaire* |
N/A | N/A |
Streptococcus pneumoniae bacteremia / osteomyelitis. Disseminated Mycobacterium avium-intracellulaire* |
Mycobacterium tuberculosis pulmonary disease. Staphylococcus aureus bacteremia / osteomyelitis / cellulitis HPV-70 Warts Norwegian Scabies |
Streptococcus pneumoniae bacteremia / septic arthritis Bordetella pertussis pneumonia Scabies Varicella Zoster |
Parainfluenza pneumonia Influenza A pneumonia Candida Diaper Dermatitis Streptococcus pneumoniae bacteremia / meningitis* |
N/A |
| Graft Versus Host Disease | “Mild” Acute GVHD (skin) | None | None | Grade III acute GVHD (gastrointestinal) | None | None | None | None |
| Died After HCT | Yes | Yes | Yes | Yes | No | No | Yes | No |
| Time of Death Post-HCT | 5-years | Day +9 | Day +122 | 2-years | N/A | N/A | 8-months | N/A |
| Time of Last Follow Up Post-HCT if Alive | N/A | N/A | N/A | N/A | 7-years | 6-years | N/A | 6-months |
Abbreviations: ANC: Absolute Neutrophil Count; ATG: Antithymocyte globulin; BCG: bacillus Calmette-Guerin; Bu: Busulfan; CMV: Cytomegalovirus; Cy: Cyclophosphamide; Flu: Fludarabine; GVHD: Graft-versus-host disease; HCT: Hematopoietic Cell Transplant; HPV: Human papilloma virus. HSV: Herpes simplex virus; IVIg: Intravenous immunoglobulin; MAC: Myeloablative conditioning; Mel: Melphalan; N/A: Not available; NBS: Newborn screening; ND: Not determined; RIC: Reduced intensity conditioning; RSV: Respiratory syncytial virus;
*Asterisks refer to infections that ultimately resulted in death due to multi-organ failure and/or severe neurological injury. Involvement of organs was confirmed at the time of autopsy.
3.4.2. Clinical outcomes following hematopoietic stem cell transplantation
Three patients receiving transplant remain alive 6-months, 6-years and 7-years post-HSCT. Two patients died soon after HSCT (days +9 and + 122) from complications related to infections present at the time of transplant. Three others died of new infections acquired post-HSCT, including two (patients 3 and 8) who died of disseminated Mycobacterium avium-intracellulaire infection in association with secondary graft failure at 2- and 5-years post-HSCT. The third (patient 15) died of Streptococcus pneumoniae bacteremia and meningitis despite full donor chimerism at 8-months post-HSCT. New clinically relevant infections, including viral, bacterial, fungal, and Mycobacterial infections, were common in patients who survived at least 6-months after transplant, and were still occurring years after transplant despite full and mixed lymphoid and myeloid donor chimerism and normal neutrophil and lymphocyte counts. Such infections included Streptococcus pneumoniae bacteremia (n = 5), meningitis (n = 1), osteomyelitis (n = 1), and septic arthritis (n = 1); Staphylococcus aureus bacteremia, cellulitis, and osteomyelitis (n = 1); Salmonella bacteremia and enterocolitis (n = 1); and Pseudomonas aeruginosa bacteremia (n = 1). Viral infections (e.g. influenza, parainfluenza, varicella, adenovirus) also occurred, with resolution in all patients. One patient developed Mycobacterial tuberculosis pneumonia at 1-year post-HSCT, was successfully treated with anti-tuberculosis medications, and remains a long-term survivor.
3.4.3. Immune reconstitution following hematopoietic stem cell transplantation
All patients achieved normal numbers of CD3+, CD4+, CD8+, CD56+, and CD19+ cells post-HSCT. Immunoglobulin replacement was variable between patients and generally continued between 3- and 12-months post-HSCT. PHA responses were normal (above 50%) for all patients. Despite these findings, specific antibody production to post-transplant vaccinations once immunoglobulin was discontinued was poor. For instance, patients 8 and 10 did not make anti-pneumococcal antibodies despite both conjugated and unconjugated pneumococcal vaccinations, and in the case of patient 8, a Streptococcus pneumoniae infection. Patient 10 received six combined hepatitis A and B vaccinations and despite this, did not make antibody against hepatitis A virus or hepatitis B surface antigen. Similarly, neither patient made protective titres against tetanus or diphtheria despite being vaccinated after HSCT.
Detailed autopsy reports were available for 2 patients after HSCT. Aside from evidence of infection, both exhibited severe thymic hypoplasia with scant lymphoid cell populations, minimal to no lymph node and tonsillar tissue, and absence of germinal follicles in the spleen. Patient 3, who experienced secondary graft failure by 3-years post-HSCT, developed disseminated Mycobacterium avium-intracellulaire and was managed for 1-year with anti-tuberculous medications and splenectomy before death. The spleen was entirely replaced by atypical Mycobacteria. At autopsy, performed 5-years after HSCT, the thymus was small and atrophic, weighing only 2 g, and lymph nodes were not readily apparent.
4. Discussion
This series presents the clinical features, immunologic presentation, and HSCT outcomes for the largest cohort of IKBKB immune deficiency patients published to date. A number of important conclusions can be made.
First, the clinical presentations in our case series indicate that IKBKB immune deficiency, as result of profound defects in innate and adaptive immunity, manifest as exceptional susceptibility to a variety of different but severe infections, including bacteria, viruses, fungi, and Mycobacteria. We believe that IKBKB immune deficiency, although not meeting conventional laboratory-based diagnostic criteria for SCID [19] (due to higher CD3 T-cell counts, moderately decreased to normal PHA responses, normal TRECs, high proportions of naïve CD45RA+ T-cells, and low CD45RO+ T-cells in IKBKB immune deficiency), is as severe an immune deficiency as SCID at a clinical level. We would suggest therefore, that inclusion of IKBKB deficiency under the descriptive category of “combined immune deficiencies generally less profound than SCID” in the most recent 2017 International Union Immunologic Societies classification [20], likely underestimates the severity of the immune deficiency and should be modified in future iterations.
Supporting a more profound immune deficiency are the other six reported patients with IKBKB mutations, who also presented with severe bacterial, fungal and viral infections as young infants. Mycobacterial infection was also prominent, with disseminated BCG noted in four of the six [[13], [14], [15]]. Similar to our cohort, previously reported IKBKB immune deficient patients exhibited normal to elevated absolute T-cell and B-cell numbers, low CD45RO+ memory T-cells, and absent or low class-switched memory B-cells. Immunoglobulins were universally low or undetectable, but proliferative responses to PHA were present and typically normal. Only one patient was noted to have features of ectodermal dysplasia at an older age (18 months) [13], a feature which we have not seen in any of our three post-HSCT survivors. Two of the other reported patients died from overwhelming infection prior to definitive therapy, and four received HSCT, with only one long-term survivor.
Secondly, this data further expands the understanding of primary immune deficiencies involving aberrant IKK/NF-κB signaling. At least 14 patients with germline gain of function mutations in NFKBIA have been reported, with a severe but more variable clinical and immune phenotype that appears dependent upon the genotype (with missense mutations resulting in a more severe clinical phenotype) [21]. Like IKBKB immune deficiency, patients with hypermorphic NFKBIA mutations that result in reduced degradation of IκBα, present with multiple and severe bacterial, fungal and viral infections starting at an early age, typically before 3-months. Infections reported include invasive bacteria such as Klebsiella, Pseudomonas, Haemophilus influenzae, and Staphylococcus aureus, Mycobacteria (including vaccine-strain BCG), as well as chronic mucocutaneous candidiasis, pneumocystis pneumonitis, and severe viral infections (rotavirus, norovirus, parainfluenza, RSV, CMV). Similarly, patients with NFKBIA mutations present with normal to elevated T-cell counts, an excess of naïve CD4 and CD8 cells, and normal mitogen proliferative response to PHA in most. Dysgammaglobulinemia (usually with one or more quantitatively abnormal immunoglobulin isotypes), impaired response to vaccine antigens, and when tested, absent or reduced memory B-cells and class switched memory B-cells have also been reported. Unlike patients with IKBKB immune deficiency, however, almost all patients with IκBα defects show some degree of ectodermal dysplasia.
By comparison, individuals with hemizygous hypomorphic IKBKG mutations with reduced NEMO expression manifest a more variable clinical and immunologic phenotype when compared to patients with IKBKB immune deficiency and hypermorphic NFKBIA mutations [22]. In addition to infectious predisposition, patients with NEMO deficiency may present with autoimmunity (25%) and inflammatory conditions (most notably inflammatory bowel disease in 21%), features not described in either our cohort of IKBKB immune deficient patients nor in those with hypermorphic IκBα defects. As in patients with IKBKB immune deficiency, patients with NEMO deficiency commonly present with infections due to pyogenic bacteria (87%), Mycobacteria (44%), viruses (21%) and fungal and opportunistic pathogens (10%) [22] The median age at HSCT for a large cohort of NEMO deficient patients was 3.3 years old (range: 4.4 months – 18.8 years), an age that is older compared to our cohort, suggesting NEMO deficiency may be a less severe immune deficiency relative to IKBKB immune deficiency [23]. NEMO deficiency also has a more variable immunologic phenotype, with hypogammaglobulinemia in only 24/41 (59%) and defects in specific antibody production in 18/28 (64%). Similar to IKBKB immune deficiency, most patients with NEMO deficiency have normal to elevated CD4 and CD8 counts, and 91% have normal mitogen induced proliferations [22].
A third important finding of this study is that SCID newborn screening by TREC assays will not detect IKBKB immune deficiency. Given the severe clinical phenotype of IKBKB immune deficiency, a pilot project of targeted newborn screening by direct genetic mutation analysis of Guthrie cards for the IKBKB mutation was developed in select Northern Cree communities in Manitoba. This investigation identified a carrier frequency of 1 in 13.1 newborns, with a predicted mutation homozygosity of 1/686 births [24]. Over 5-years (2013–2018), 760 newborns from two communities were screened, with one infant identified at birth (patient 15) and a subsequent sibling (patient 16) identified in-utero. Both were placed in protective isolation after birth and received HSCT at an early age, before the onset of serious infections, important variables associated with excellent outcomes in SCID HSCT [25,26]. In other populations with founder mutations or high rates of consanguinity, a similar genetic-based newborn screening strategy such as ours might be applicable.
Unfortunately, patient 15 later died of invasive Streptococcus pneumoniae infection in their home community, 8-months following an otherwise successful transplant. This illustrates a complex interplay between what we suspect are ongoing defects in immunity post-HSCT, combined with poor social determinants of health. For familial and cultural reasons, our patients often have strong desires to return home soon after HSCT, and the tertiary care HSCT center is not easily accessible from their remote communities. With the death of patient 15, and with the recent transplant of patient 16, we have instituted a program of housing the family close to the tertiary care center for at least 1.5 years post-HSCT, as well as regular immunoglobulin replacement and indefinite daily anti-pneumococcal antibiotic prophylaxis.
Much remains unknown about the immune abnormalities in patients with IKBKB immune deficiency, both before and after HSCT. From our previous investigations, the broad immune defects relate to abnormal IKK/NF-κB signaling in T-cells, B-cells and innate immune cells, resulting in generalized activation defects to a variety of stimuli [1]. With the exception of regulatory T-cells and gamma-delta T-cells, which are absent in IKBKB immune deficiency, other lymphocyte subsets appear to develop normally. Despite thymic hypoplasia in five of our patients who died either prior to or after HSCT, six other patients had normal TREC levels at the time of diagnosis, suggesting no intrinsic defect in T-cell development and maturation within the thymus. Unfortunately, none of the patients who had autopsies performed had detailed TREC analysis, making direct correlation with the underdevelopment of the thymus and TREC levels in a single patient impossible. Detailed T-cell studies were not available for most of our patients pre-HSCT, but when previously studied in one patient (and in one additional patient in this series), the T-cell receptor beta-chain variable region repertoire was polyclonal [1]. We are unable to resolve this apparent contradiction in the autopsy and immunologic findings, and believe it warrants further study. Likewise, when studied in two patients, kappa-deleting recombination excision circles (KRECs) in dried blood spots from newborn Guthrie cards of two patients were found to be normal, suggesting normal B cell development.
In a mouse model involving a hypermorphic mutation of IκBα, NF-κB signaling in non-immune “architectural” cells were shown to be severely disrupted [27]. Although such cells are not derived from hematopoietic stem cells, they are nonetheless integral to lymphoid organogenesis, such as in the development of lymph nodes, Peyer's patches, splenic marginal zones, follicular dendritic cells, and the formation of germinal centers in the spleen, which were absent. Further, the immune defect in this model was not fully corrected by HSCT. It is reasonable to suspect, therefore, that similar architectural cell dysfunction with resultant disruption of the immunologic niche could also play a role in IKBKB immune deficiency. Failure to correct non-immune cells with HSCT could explain the ongoing infectious complications seen years after an otherwise apparently successful, lymphoid engrafted transplant in IKBKB immune deficiency. The absence of splenic germinal centers and lack of tonsils and lymphoid tissues in the autopsies of patients who died post-HSCT, and absent specific vaccine responses despite excellent donor T- and B- cell chimerism, supports that HSCT does not fully correct all aspects of the immune deficiency in IKBKB.
In years past, and in patients who were profoundly ill at presentation, full immune evaluations were not always available to further elucidate the nature of the immune deficiency in this population. At the moment, there are only three survivors in our cohort, for whom detailed T cell studies pre- and post-HSCT (including serial measurements of T cell markers such as recent thymic emigrants, markers of naivety and memory cells and markers of activation or exhaustion) have been limited to date. Moving forward, and with genetic-based newborn screening introduced for this condition in our province, we will now have this opportunity.
Despite these challenges, we believe that IKBKB immune deficiency is still a disorder that requires HSCT. To our knowledge, no individual has been a long-term survivor of IKBKB immune deficiency without HSCT. We are unaware of any reports of hypomorphic IKBKB mutations producing a milder clinical phenotype, allowing clinicians to take a wait and see approach. HSCT for other disorders involving the IKK/NF-κB pathway have been previously described, including for hemizygous hypomorphic IKBKG mutations [23] and hypermorphic NFKBIA mutations [21]. In the cohort of fourteen IκBα gain of function patients reported by Boisson et al., eleven received HSCT, with only five survivors. One patient died without receiving HSCT, and two patients survived without receiving HSCT.
Since most centers will not have genetically based newborn screening for IKBKB immune deficiency, clinicians would likely be faced with an infant presenting with severe life-threatening infections, given that TREC based newborn screening will not detect the disorder. Commercially available next-generation sequencing gene panels for immune deficiency disorders (including many SCID panels) increasingly include IKBKB, which should facilitate diagnosis, although not always in a timely manner before HSCT. In our series, two of the three patients surviving HSCT had serious life-threating infections before transplant, including a case of multiple Serratia marcescens intracerebral abscesses, where the long-term neurocognitive outcome was initially unclear. We have tended to be aggressive with antimicrobials and other supportive care measures, with the goal of reaching HSCT as quickly as possible. This strategy has resulted in long-term survival of 2/6 infants receiving HSCT with active infections. That being said, we suspect that survival will be significantly improved with current newborn screening strategies, in the absence of pre-HSCT infection.
Unfortunately, and based on this limited dataset, we believe myeloablative conditioning may be required for engraftment in IKBKB immune deficiency. The largest and most recent study of HSCT in NEMO patients, however, did not find this to be necessarily true [23]. In their cohort of fifteen patients receiving classical myeloablative conditioning and thirteen patients receiving reduced intensity conditioning, the global engraftment rate was 93%, with similar survival rates and secondary graft failure rates, independent of conditioning regimen intensity. Our center currently uses once daily intravenous dosing of busulfan for 4 days (aiming for an area under the curve approximating 4000 μmol/L*min), fludarabine 1.33 mg/kg/dose for 4 days, and rabbit anti-thymocyte globulin 2.5 mg/kg/dose for 3 days. We acknowledge other conditioning regimens might also be efficacious, although caution that late secondary graft-failure appears to be a concern in IKBKB immune deficiency.
Finally, and importantly, clinicians should be mindful that despite apparently reasonable basic laboratory immune reconstitution data for IKBKB immune deficiency patients post-HSCT (e.g. T-cell counts, chimerism studies), late infections, including bacterial and Mycobacterial infections in particular, appeared to be common. Of interest, one of our long-term survivors who was fully engrafted developed primary Mycobacterium tuberculosis pulmonary disease at 14-months post-HSCT. With appropriate therapy, the infection resolved, suggesting HSCT corrected enough of the immune deficiency. In contrast to our post-HSCT patients with IKBKB immune deficiency, Miot et al. found that bacterial infections only recurred at >6-months post-HSCT for NEMO deficiency in 2/27 patients [23]. We hypothesize that failure of HSCT to improve all aspects of innate and adaptive immune function after transplant, as shown by the number of ongoing serious bacterial and Mycobacterial infections and lack of protective specific antibody responses to vaccinations, could be because HSCT does not correct cells of non-hematopoietic origin important in the immune response that are also dependent upon IKK/NF-κB signaling. A similar pattern of incomplete immune correction and ongoing bacterial and viral infections after HSCT has been reported in a patient with the related autosomal dominant anhidrotic ectodermal dysplasia with immune deficiency due to hypermorphic IκBα defect [28].
In summary, we present a relatively large cohort of a rare primary immunodeficiency disorder, IKBKB immune deficiency, characterized by the presence of naïve T-cells, evidence of a functional activation defect, and amenable to HSCT, although with ongoing long-term infectious challenges.
Acknowledgements
We would like to thank all of the patients and their families; the nursing staff (Debbie Hanson, Jennie Pitura, Shannon Cisneros) who have taken care of the patients; Drs. Ulrich Pannicke, Stephan Ehl, and Klaus Schwarz who were instrumental in the discovery of the IKBKB gene; Drs. Cheryl Greenberg and Paul Van Caeseele for their contribution to the newborn screening project for IKBKB deficiency; and Luvinia Kwan and Agapito Gentile for performing the IKBKB mutational analysis.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Pannicke U., Baumann B., Fuchs S., Henneke P., Rensing-Ehl A., Rizzi M. Deficiency of innate and acquired immunity caused by an IKBKB mutation. N. Engl. J. Med. 2013;369(26):2504–2514. doi: 10.1056/NEJMoa1309199. [DOI] [PubMed] [Google Scholar]
- 2.Vallabhapurapu S., Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 3.Paciolla M., Pescatore A., Conte M.I., Esposito E., Incoronato M., Lioi M.B. Rare mendelian primary immunodeficiency diseases associated with impaired NF-kappaB signaling. Genes Immun. 2015;16(4):239–246. doi: 10.1038/gene.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filipe-Santos O., Bustamante J., Haverkamp M.H., Vinolo E., Ku C.L., Puel A. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J. Exp. Med. 2006;203(7):1745–1759. doi: 10.1084/jem.20060085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ku C.L., Dupuis-Girod S., Dittrich A.M., Bustamante J., Santos OF, Schulze I. NEMO mutations in 2 unrelated boys with severe infections and conical teeth. Pediatrics. 2005;115(5):e615–e619. doi: 10.1542/peds.2004-1754. [DOI] [PubMed] [Google Scholar]
- 6.Ku C.L., Picard C., Erdos M., Jeurissen A., Bustamante J., Puel A. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J. Med. Genet. 2007;44(1):16–23. doi: 10.1136/jmg.2006.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picard C., Casanova J.L., Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clin. Microbiol. Rev. 2011;24(3):490–497. doi: 10.1128/CMR.00001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zonana J., Elder M.E., Schneider L.C., Orlow S.J., Moss C., Golabi M. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma (NEMO) Am. J. Hum. Genet. 2000;67(6):1555–1562. doi: 10.1086/316914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doffinger R., Smahi A., Bessia C., Geissmann F., Feinberg J., Durandy A. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat. Genet. 2001;27(3):277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 10.Courtois G., Smahi A., Reichenbach J., Doffinger R., Cancrini C., Bonnet M. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. J. Clin. Invest. 2003;112(7):1108–1115. doi: 10.1172/JCI18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Granados E., Keenan J.E., Kinney M.C., Leo H., Jain N., Ma C.A. A novel mutation in NFKBIA/IKBA results in a degradation-resistant N-truncated protein and is associated with ectodermal dysplasia with immunodeficiency. Hum. Mutat. 2008;29(6):861–868. doi: 10.1002/humu.20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen R., van Wengen A., Hoeve M.A., ten Dam M., van der Burg M., van Dongen J. The same IkappaBalpha mutation in two related individuals leads to completely different clinical syndromes. J. Exp. Med. 2004;200(5):559–568. doi: 10.1084/jem.20040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns S.O., Plagnol V., Gutierrez B.M., Al Zahrani D., Curtis J., Gaspar M. Immunodeficiency and disseminated mycobacterial infection associated with homozygous nonsense mutation of IKKbeta. J. Allergy Clin. Immunol. 2014;134(1):215–218. doi: 10.1016/j.jaci.2013.12.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen C., Jakobsen M.A., Larsen M.J., Muller A.C., Hansen S., Lillevang S.T. Immunodeficiency associated with a nonsense mutation of IKBKB. J. Clin. Immunol. 2014;34(8):916–921. doi: 10.1007/s10875-014-0097-1. [DOI] [PubMed] [Google Scholar]
- 15.Mousallem T., Yang J., Urban T.J., Wang H., Adeli M., Parrott R.E. A nonsense mutation in IKBKB causes combined immunodeficiency. Blood. 2014;124(13):2046–2050. doi: 10.1182/blood-2014-04-571265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soldin S., Brugnara C., Wong E. American Association for Clinical Chemistry Press; Washington: 2007. Pediatric Reference Intervals. Sixth Edition Ed. [Google Scholar]
- 17.Shearer W.T., Rosenblatt H.M., Gelman R.S., Oyomopito R., Plaeger S., Stiehm E.R. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J. Allergy Clin. Immunol. 2003;112(5):973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Jilkina O., Thompson J.R., Kwan L., Van Caeseele P., Rockman-Greenberg C., Schroeder M.L. Retrospective TREC testing of newborns with Severe combined Immunodeficiency and other primary immunodeficiency diseases. Mol. Genet. Metab. Rep. 2014;1:324–333. doi: 10.1016/j.ymgmr.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shearer W.T., Dunn E., Notarangelo L.D., Dvorak C.C., Puck J.M., Logan B.R. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the primary Immune Deficiency Treatment Consortium experience. J. Allergy Clin. Immunol. 2014;133(4):1092–1098. doi: 10.1016/j.jaci.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picard C., Bobby Gaspar H., Al-Herz W., Bousfiha A., Casanova J.L., Chatila T. International union of immunological societies: 2017 primary immunodeficiency diseases committee report on inborn errors of immunity. J. Clin. Immunol. 2018;38(1):96–128. doi: 10.1007/s10875-017-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boisson B., Puel A., Picard C., Casanova J.L. Human IkappaBalpha gain of Function: a Severe and Syndromic Immunodeficiency. J. Clin. Immunol. 2017;37(5):397–412. doi: 10.1007/s10875-017-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanson E.P., Monaco-Shawver L., Solt L.A., Madge L.A., Banerjee P.P., May M.J. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J. Allergy Clin. Immunol. 2008;122(6):1169–1177. doi: 10.1016/j.jaci.2008.08.018. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miot C., Imai K., Imai C., Mancini A.J., Kucuk Z.Y., Kawai T. Hematopoietic stem cell transplantation in 29 patients hemizygous for hypomorphic IKBKG/NEMO mutations. Blood. 2017;130(12):1456–1467. doi: 10.1182/blood-2017-03-771600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin T.S., Rockman-Greenberg C., Van Caeseele P., Cuvelier G.D.E., Kwan L., Schroeder M.L. Newborn screening for IKBKB deficiency in Manitoba using genetic analysis. J. Clin. Immunol. 2018;38(7):742–744. doi: 10.1007/s10875-018-0555-2. [DOI] [PubMed] [Google Scholar]
- 25.Heimall J., Logan B.R., Cowan M.J., Notarangelo L.D., Griffith L.M., Puck J.M. Immune reconstitution and survival of 100 SCID patients post-hematopoietic cell transplant: a PIDTC natural history study. Blood. 2017;130(25):2718–2727. doi: 10.1182/blood-2017-05-781849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pai S.Y., Logan B.R., Griffith L.M., Buckley R.H., Parrott R.E., Dvorak C.C. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N. Engl. J. Med. 2014;371(5):434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mooster J.L., Le Bras S., Massaad M.J., Jabara H., Yoon J., Galand C. Defective lymphoid organogenesis underlies the immune deficiency caused by a heterozygous S32I mutation in IkappaBalpha. J. Exp. Med. 2015;212(2):185–202. doi: 10.1084/jem.20140979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupuis-Girod S., Cancrini C., Le Deist F., Palma P., Bodemer C., Puel A. Successful allogeneic hemopoietic stem cell transplantation in a child who had anhidrotic ectodermal dysplasia with immunodeficiency. Pediatrics. 2006;118(1):e205–e211. doi: 10.1542/peds.2005-2661. [DOI] [PubMed] [Google Scholar]