Abstract
There are a number of newly described and emerging disease syndromes affecting the domestic ferret, and the purpose of this article is to make veterinarians aware of these diseases. A recently described systemic coronavirus infection appears to be a variant of the ferret enteric coronavirus and is currently termed “ferret infectious peritonitis.” Disseminated immunopathologic myositis, aplastic anemia/bone marrow aplasia, acute hemorrhagic syndrome, and oral ulcerations are also described, although the exact etiologies for these diseases have yet to be determined. There appears to be at least 2 important amino acid metabolism deficiencies in ferrets: hindlimb weakness in older ferrets (L-carnitine) and cysteine urolithiasis. Ferrets have recently been found to be susceptible to H1N1 influenza, so knowledge regarding this zoonotic disease is essential for veterinarians working with these animals. A novel Mycoplasma spp. has also recently been identified in ferrets with chronic respiratory problems that originated from one breeding colony. Because these diseases are still being investigated, practitioners who treat a ferret patient exhibiting clinical signs consistent with any of the conditions mentioned are encouraged to contact people who are knowledgeable of that particular illness.
Key words: Coronavirus, ferret, hemorrhagic syndrome, influenza, Mycoplasma, myositis
Because the information in this article will attempt to highlight newly identified diseases in ferrets, there are few references. Although proceedings articles may contain preliminary information, definitive investigations are still being performed. Veterinary practitioners who treat cases diagnosed with new disease conditions are encouraged to tabulate their data and submit them to the pathologists and/or clinicians trying to discern the underlying etiology, treatment, and prevention. Table 1 lists the contact information for the veterinarians currently working on the emerging disease conditions mentioned in this article. Networking through online veterinary exotic animal discussion groups is extremely helpful because it establishes a pattern from which an epidemiological assessment can be formulated.
Table 1.
Professional contacts for emerging diseases in ferrets
| Disease | Pathology | Clinical |
|---|---|---|
| Ferret infectious peritonitis | Michael Garner, DVM, Dip. ACVP | Katrina Ramsell, PhD, DVM |
| Northwest Zoopath | Northwest Exotic Pet Vet LLC | |
| 654 W. Main St | 6895 SW 160th Ave | |
| Monroe, WA 98272 | Beaverton, OR 97007 | |
| 360-794-0630 | 503-646-6101 | |
| fax: 360-794-4312 | E-mail: NWExoticPetVet@hotmail.com | |
| E-mail: zoopath@aol.com | ||
| Disseminated immunopathic myositis | Michael Garner, DVM, Dip. ACVP | Katrina Ramsell, PhD, DVM |
| Northwest Zoopath | Northwest Exotic Pet Vet LLC | |
| 654 W. Main St | 6895 SW 160th Ave | |
| Monroe, WA 98272 | Beaverton, OR 97007 | |
| 360-794-0630 | 503-646-6101 | |
| fax: 360-794-4312 | E-mail: NWExoticPetVet@hotmail.com | |
| E-mail: zoopath@aol.com | ||
| Aplastic anemia | Michael Garner, DVM, Dip. ACVP | Angela Lennox, DVM, Dip. ABVP (Avian) |
| Northwest Zoopath | Avian & Exotic Animal Clinic | |
| 654 W. Main St | 9330 Waldemar Rd | |
| Monroe, WA 98272 | Indianapolis, IN 46268 | |
| 360-794-0630 | 317-879-8633 | |
| fax: 360-794-4312 | fax: 317-879-0823 | |
| E-mail: zoopath@aol.com | E-mail: birddr@aol.com | |
| Amino acid metabolism abnormalities, urolithiasis | Michelle Hawkins, VMD, Dip. ABVP (Avian) VM: Medical & Epidemiology University of California-Davis 2108 Tupper Hall Davis, CA 95616 530-752-1393 E-mail: mghawkins@ucdavis.edu |
Michelle Hawkins, VMD, Dip. ABVP (Avian) |
| VM: Medical & Epidemiology | ||
| University of California-Davis | ||
| 2108 Tupper Hall | ||
| Davis, CA 95616 | ||
| 530-752-1393 | ||
| E-mail: mghawkins@ucdavis.edu | ||
| Acute hemorrhaging syndrome | Drury Reavill, DVM, Dip. ABVP (Avian), Dip. ACVP | Cathy Johnson-Delaney, DVM, Dip. ABVP (Avian), Dip. ABVP (ECM) |
| Zoo/Exotic Pathology Service 2825 Kovr Dr West Sacramento, CA 95605 916-725-5100 fax: 916-725-6155 E-mail: Mail@zooexotic.com |
Eastside Avian & Exotic Animal Medical Center 13603 100th Ave NE Kirkland, WA 98034 1-800-821-6165 fax: 425-821-6130 E-mail: cajddvm@hotmail.com |
|
| Oral Ulceration | Matti Kiupel, Dr.Med.Vet., PhD, Dip. ACVP Associate Professor, Section Chief Anatomic Pathology Michigan State University Department of Pathobiology and Diagnostic Investigation Diagnostic Center for Population and Animal Health |
Cathy Johnson-Delaney, DVM, Dip. ABVP (Avian), Dip. ABVP (ECM) Eastside Avian & Exotic Animal Medical Center 13603 100th Ave NE Kirkland, WA 98034 1-800-821-6165 |
| 4125 Beaumont Rd, Rm 152A | fax: 425-821-6130 | |
| Lansing, MI 48910 | E-mail: cajddvm@hotmail.com | |
| 517-432-2670 | ||
| fax: 517-432-6557 | ||
| E-mail: kiupel@dcpah.msu.edu | ||
| Mycoplasmosis | Matti Kiupel, Dr.Med.Vet., PhD, Dip. ACVP Associate Professor, Section Chief Anatomic Pathology Michigan State University Department of Pathobiology and Diagnostic Investigation Diagnostic Center for Population and Animal Health 4125 Beaumont Rd, Rm 152A Lansing, MI 48910 517-432-2670 fax: 517-432-6557 E-mail: kiupel@dcpah.msu.edu |
Cathy Johnson-Delaney, DVM, Dip. ABVP (Avian), Dip. ABVP (ECM) Eastside Avian & Exotic Animal Medical Center 13603 100th Ave NE Kirkland, WA 98034 1-800-821-6165 fax: 425-821-6130 E-mail: cajddvm@hotmail.com |
| Michael Garner, DVM, Dip. ACVP | ||
| Northwest Zoopath | ||
| 654 W. Main St | ||
| Monroe, WA 98272 | ||
| 360-794-0630 | ||
| fax: 360-794-4312 | ||
| E-mail: zoopath@aol.com |
The Domestic Ferret: Background
The domestic ferret, Mustela putorius furo, is a highly inbred animal that is an extremely popular pet. In the past 10 years, there has been an increase in the number of ferrets available in pet stores within the United States. Most pet stores obtain their ferret stock from one commercial breeder, although there are other commercial breeders and small, individual breeders who supply the retail market. Most of the diseases presented in this article are being diagnosed in ferrets from different sources, although in the pet trade different shipments may be mixed.
Ferret suppliers have established a trend to neuter/spay and descent their ferrets at 3 to 4 weeks of age. There are no published reports on the implications of surgery at that age on the immune system and/or disease exposure in ferrets; however, performing surgery on these animals at this young age does appear to have an effect on growth and development (e.g., adrenal gland disease). Regardless of any negative health effects early surgery may have on the ferret, this practice will likely continue because of consumer demand.
Ferrets are typically shipped to the retail market at 6 to 8 weeks of age, shortly after their surgery and after being given 1 vaccination against canine distemper virus. These newly shipped animals need to have a complete series of canine distemper vaccinations and a rabies vaccine. Many of these ferrets do not receive a full vaccination series because pet store personnel repeatedly (erroneously) inform new owners “they've had their shots.” Completing a distemper vaccination series and obtaining a rabies vaccine are frequently achieved after the ferret is 6 months to 1 year old, or after it has developed other medical problems.
During their life, most pet ferrets will develop dental disease, neoplasia, cardiomyopathy, or some form of gastrointestinal disorder. The pet ferret has one of the highest tumor rates of any mammal. These serious illnesses are in addition to the usual ferret disease conditions (e.g., ear mites, trauma, and foreign body ingestion). A wise suggestion to new ferret owners is the establishment of a savings account for their ferret's future medical needs.
Ferret Infectious Peritonitis
Ferrets are susceptible to coronavirus infections; experimentally to severe acute respiratory syndrome and ferret enteric coronavirus (FECV). FECV is the cause of epizootic catarrhal enteritis. Lesions associated with FECV are only found in the gastrointestinal tract, whereas the virus may be isolated from the saliva, feces, and enterocytes. Feline coronavirus is responsible for a feline enteric self-limiting disease, and the mutated form is responsible for feline infectious peritonitis (FIP). The agent responsible for the “FIP-like” disease in ferrets appears to be a variant of the FECV virus and is being called ferret systemic coronavirus (FSCV). Gross, histological, and immunohistochemical features of FSCV are identical to FIP.1, 2, 3
Clinical signs of FECV include weight loss (in some cases, the loss of body weight may be severe), anorexia, palpable intraabdominal mass or masses, lethargy, vomiting, splenomegaly, dehydration, bruxism, renomegaly, sneezing/nasal discharge, systolic heart murmur, urine discoloration, dyspnea, peripheral lymphadenomegaly, rectal mucosa erythema, and/or rectal prolapse. Central nervous system (CNS) signs are observed in some ferrets diagnosed with FECV and include acute or progressive hindlimb paresis or paraparesis, ataxia, seizures, abnormal posture, opisthotonus, abnormal gait, and proprioceptive deficits. Some ferret patients may also become pyrexic (body temperature: 103.0-105.4°F, 39.4-40.8°C; normal rectal temperature: 101.0-103.0°F, 38.3-39.4°C).
Pathology
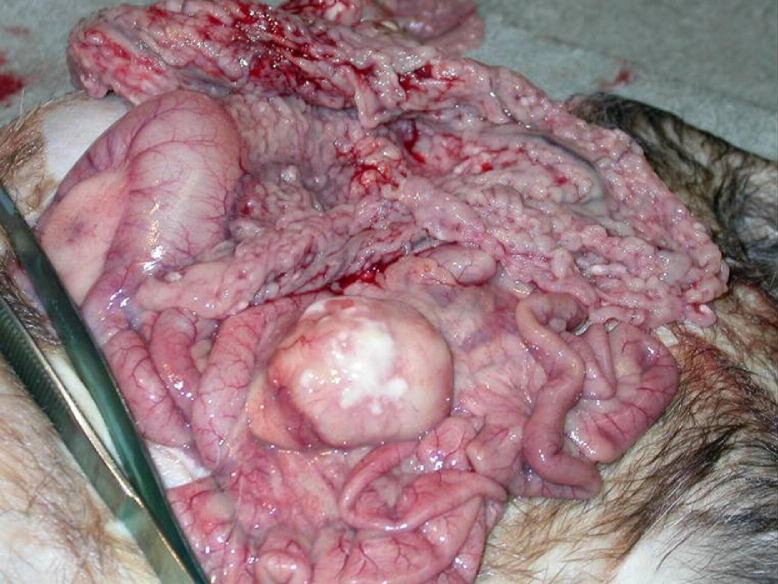
On biopsy or necropsy, the primary gross lesions associated with FSCV are circumscribed to coalescing white, tan, or slightly pink irregular nodules or foci of white discoloration ranging in size from 5 to 30 mm on the surface and within the parenchyma of the spleen, liver, kidneys, lung, mesentery, and lymph nodes.1, 2, 3 Splenomegaly, renomegaly, hepatomegaly, and ascites have also been noted with FSCV (Fig 1).
Figure 1.
Gross findings of ferret diagnosed with FSCV.
Histologic lesions have been detected in the mesentery/peritoneum, lymph nodes, spleen, kidneys, liver, lung, intestine, pancreas, stomach, brain, and adrenal glands. Other notable lesions include a nonsuppurative meningoencephalitis and suppurative or nonsuppurative tubulointerstitial nephritis.1 Coronavirus antigen has been detected in all tissues with lesions; however, the staining reaction was most prominent in lymph nodes, splenic lymphoid follicles, and all foci identified with pyogranulomatous inflammation.1, 3
Current Assessment and Virology
FSCV has similar gross and histologic lesions to those in cats with FIP. FSCV resembles the “dry form” of FIP, with widespread nodular foci on serosal surfaces and within the parenchyma of thoracic and abdominal viscera. There is also nodular enlargement of mesenteric lymph nodes. Clinically, some practitioners have reported an effusion in the body cavity of their patients that resembles the “wet form” of FIP, but this has not been a common condition associated with this disease in ferrets. Designating “wet” or “dry” forms is somewhat arbitrary, because many cats exhibit lesions consistent with both forms during the course of the illness; it is likely that this is also occurring in ferrets. As more cases of FSCV are diagnosed, more effusive “wet” forms of the disease may be documented.
When gene sequencing is used to identify the virus, there is slight cross-reactivity in some samples with the FECV-specific primers, which suggests there is sequence conservation between the nucleocapsid genes of FSCV and FECV. Further genomic sequencing of the virus is required to establish a definitive classification of this virus. In conclusion, the relatively recent recognition of FSCV in pet ferrets suggests a recent mutation or shift in the FECV, which has resulted in the development of a systemic disease. This apparent viral mutation is apparently similar to the changes that occurred in feline coronavirus preceding the development of FIP.1, 2, 3
Disseminated Immunopathic Myositis
Disseminated immunopathic myositis (DIM) was not described in ferrets before 2003 and is characterized by a fatal, inflammatory condition of the muscles.4, 5 Ferrets that have been diagnosed with DIM have originated from various breeding facilities and are typically between 3 and 24 months of age (with the average age being 10 months); there appears to be no sex predilection.
The clinical presentation of a ferret with DIM is one of rapid onset, characterized by high fever (105-108°F, 40.5-42.2°C), anorexia, and a reluctance to move. Lymphadenopathy and splenomegaly may be present. Complete blood count results often indicate a neutrophilic leukocytosis, whereas the serum chemistries may show an elevated aspartate transaminase, hyperglycemia, and/or hypoalbuminemia. Most ferrets are dehydrated, cachexic, and in poor body condition. Some ferrets diagnosed with DIM also have diarrhea, mild serous nasal discharge, tachycardia, and tachypnea. Radiography and ultrasonography are usually unrewarding. Skeletal muscle biopsy samples collected from the hindleg or lumbar region, lymph nodes, and/or any abnormal masses submitted for histopathological evaluation may be helpful with determining an antemortem diagnosis and subsequent prognosis. Tissue samples harvested during a necropsy examination that will provide the best chance for a definitive diagnosis include those collected from the esophagus, heart, skeletal muscle (several sites), diaphragm, lymph nodes, spleen, and bone marrow.
Many treatment protocols have been attempted in an effort to reduce the fever and inflammation associated with DIM, but rarely has there been a complete recovery. Antibiotics that have been used unsuccessfully to manage DIM include penicillins, cephalosporins, fluoroquinolones, doxycycline, clarithromycin, azithromycin, trimethoprim-sulfadimethoxine, aminoglycosides, metronidazole, and chloramphenicol. Antifungal and antiviral drugs have likewise been ineffective in controlling the clinical disease conditions associated with DIM. Treatment with steroidal and nonsteroidal antiinflammatory drugs has had little effect on this disease. Cyclophosphamide dosed at 5 mg/kg orally every 12 hours also has been attempted without much success. Interferon-alpha at a dose of 600 IU/day has been found to temporarily reduce the fever in a few ferrets diagnosed with DIM. Because most affected ferret patients require fluid therapy, nutritional supplementation, and analgesia as the disease progresses, many owners elect euthanasia. To date, the author has not successfully treated a patient diagnosed with DIM (100% mortality in confirmed cases).
Pathology
Gross lesions of DIM include skeletal muscle atrophy throughout the body, including the esophagus (described as red and white mottling, dilation). Histologically, there is moderate to severe suppurative to pyogranulomatous inflammation of the skeletal muscles and fascia of the esophagus, heart, limbs, body wall, head, and lumbar regions. Myeloid hyperplasia is commonly observed in the spleen and/or bone marrow. Lesions observed using electron microscopy (EM) include mitochondrial swelling, intracellular edema, disruption of myofibrils, and Z bands.4, 5
At this time, the underlying etiology of DIM is unknown. No pathogen has been isolated in any of the confirmed ferret DIM cases by bacterial or viral cultures, EM, immunohistochemistry, or polymerase chain reaction testing for a wide variety of known pathogens. A vaccine from a sole manufacturer is the only known common connection between animals diagnosed with DIM. This vaccine is no longer available; therefore, it is unknown if this syndrome will continue. However, the author has seen at least one confirmed DIM case (2009) in which the ferret did not receive the aforementioned vaccine; consequently, there may be other disease triggers for DIM. In one clinical investigation using the canine castration vaccine, this myofasciitis syndrome was reproduced in the entire group of ferrets, suggesting that DIM may be an immunological response to an inflammatory stimulation.4 Cytokines in ferrets have recently been studied and found to be similar in structure and function to those of other carnivores.6 The ferret immune system, for the most part, functions in a similar manner to other animals; therefore, future DIM investigations should focus on the immune system rather than trying to identify an infectious etiology, based on similar diseases of dogs and cats.
Aplastic Anemia/Bone Marrow Aplasia
Aplastic anemia/bone marrow aplasia (AAMA) has been diagnosed in ferrets throughout the United States. Case histories from ferrets diagnosed with AAMA are being reviewed, but thus far the only discernible correlation between these cases is that the affected animals are under 18 months of age. Ferrets that present with AAMA present with a history of lethargy and anorexia and, when examined, have pale to white mucus membranes (Fig 2). A few patients have been determined to have moderate splenomegaly, but other physical parameters that can be assessed through external examination appear normal. Packed cell volumes of these animals are generally less than 10%, with bone marrow aspirates being diagnosed as erythrocytic aplasia. AAMA ferrets often have normal white blood cell counts and plasma chemistry parameters. Although transfusions are provided to these patients, erythropoietin therapy is usually ineffective. High doses of corticosteroids have been attempted to rule out an immune-mediated component to this disease process, but do not appear to have a beneficial effect in reducing any of the adverse physical conditions associated with AAMA. The underlying etiology of AAMA in ferrets is currently unknown. Ultimately, compiling case information and full necropsy examinations on patients diagnosed with this disease will be necessary to aid in the pathophysiologic investigation. A few ferrets diagnosed with AAMA have survived with intensive hospital care (e.g., repeated blood transfusions, corticosteroids, and erythropoietin), so there is some potential hope with this disease.
Figure 2.
Pale mucus membranes seen with severe anemia.
Acute Hemorrhagic Syndrome
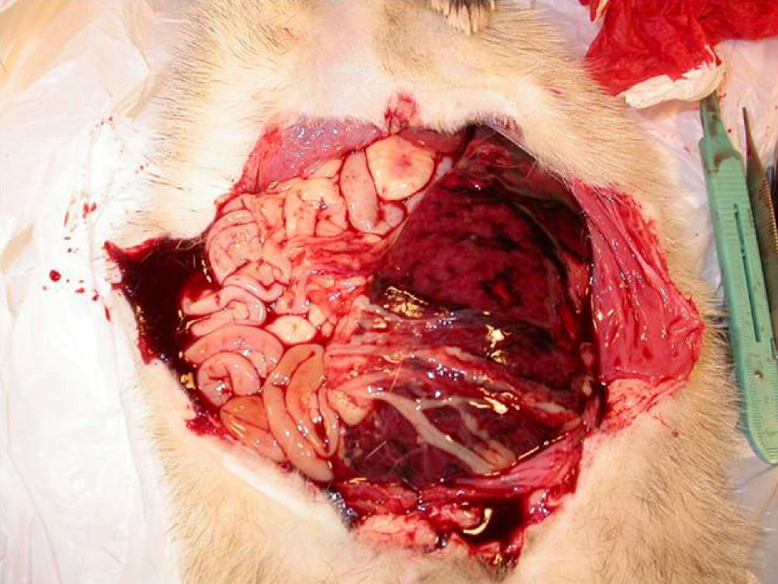
Acute hemorrhagic syndrome (AHS) has been identified in ferrets within the past year and appears to primarily affect recently shipped animals and/or those recently placed in pet stores. Affected kits typically present with acute hemorrhage (e.g., initially as epistaxis, with subsequent development of oral ulceration) (Fig 3 ). Hemorrhage may be observed in the rectal area, as petechial areas on the skin, and/or within the abdominal cavity. Immediate therapy with parenteral vitamin K and basic supportive care appears to control hemorrhaging, if caught early; however, many animals literally “bleed out” no matter what treatment is attempted. If possible, blood should be collected for a coagulation profile to confirm an antemortem diagnosis of a coagulopathy. Currently, AHS is thought to be a hemophilia disorder. Normal coagulation parameters have been collected on ferrets under 1 year of age and are found in Table 2. The affected ferrets had prolonged clotting times, but full coagulation profiles on the 3 animals listed were not available. Normal coagulation values are available for adult ferrets.7 If a ferret presents with clinical signs similar to those described for AHS, the practitioner should attempt to collect blood for a coagulation profile and administer the therapeutic regimen listed above.
Figure 3.
Abdominal hemorrhage seen with acute hemorrhagic syndrome.
Table 2.
Coagulation parameters for ferrets
| Ferret group | aPTT (seconds) | PT (seconds) |
|---|---|---|
| Healthy young ferrets⁎ | 18.25 | 10.25 |
| Affected ferrets† | 99.43 | 76.67 |
| Adult ferrets7 | 18.7 | 12.3 |
Abbreviations: aPTT, Activated partial thromboplastin time; PT, prothrombin time.
Healthy young ferrets (n = 13), average age: 10.2 months (blood collected by the author, processed at Phoenix Central Laboratory, Everett, WA USA).
Affected ferrets (n = 3), all less than 9 months of age.
Amino Acid Metabolism Abnormalities
Two disease conditions have been identified in ferret patients that appear to be genetic metabolism pathway abnormalities. One syndrome is cysteine metabolism, which results in cysteine urolithiasis. Ferrets that form cysteine uroliths have all been fed novel protein diets. Abnormalities with L-carnitine metabolism are being linked to skeletal muscle weakness in older ferrets, particularly in the hindlegs. Supplementation with L-carnitine has ameliorated the most severe clinical signs, but further characterization of this syndrome is necessary. Dr. Michelle Hawkins is working on various genetic disease conditions in ferrets. Ferret urolithiasis analyses are currently being performed at the University of California–Davis, School of Veterinary Medicine (Davis, CA USA).
H1N1 Influenza
The H1N1 (swine) influenza epidemic that has been a worldwide disease concern is transmissible to the ferret. As of this writing, there are a number of reports of the organism being isolated from pet ferrets. Affected ferrets may present with respiratory disease, dehydration, tracheitis, anorexia, weight loss, and pyrexia. Treatment recommendations include bronchodilators, antibiotics for secondary bacterial pneumonias, and meloxicam (0.2 mg/kg, orally, once a day, Metacam; Bohringer–Inglehiem Inc., St. Joseph, MO USA). All patients diagnosed with H1N1 influenza that have been treated by the author using the above therapeutic regimen have recovered. Laboratory confirmation of the disease is possible, and the practitioner should follow guidelines for their public health reporting system and laboratories for sample submission.
In one documented outbreak of respiratory disease in a ferret colony, tissues were collected from 2 juvenile animals and submitted to the Iowa State University Veterinary Diagnostic Laboratory (Ames, IA USA).8 Microscopic examination of lung samples revealed bronchointerstitial pneumonia with necrotizing bronchiolitis. Influenza A virus was detected in sections of formalin-fixed lung by immunohistochemistry and reverse-transcription polymerase chain reaction assay. A field investigation of the premises and analysis of additional samples led to the confirmation and characterization of an influenza virus with high homology to contemporary reassortant H1N1 swine influenza viruses. Although ferrets have been used extensively to investigate the virulence and transmissibility of avian, human, and swine influenza virus strains and test commercial vaccine effectiveness, naturally occurring outbreaks of swine influenza had not previously been documented in ferrets. As human influenza cases continue, veterinary practitioners will likely diagnose more ferrets with this disease. It is unknown at this time if an infected ferret can transmit H1N1 influenza to humans. People who have routine contact with ferrets should receive influenza vaccinations.8
Oral Ulceration
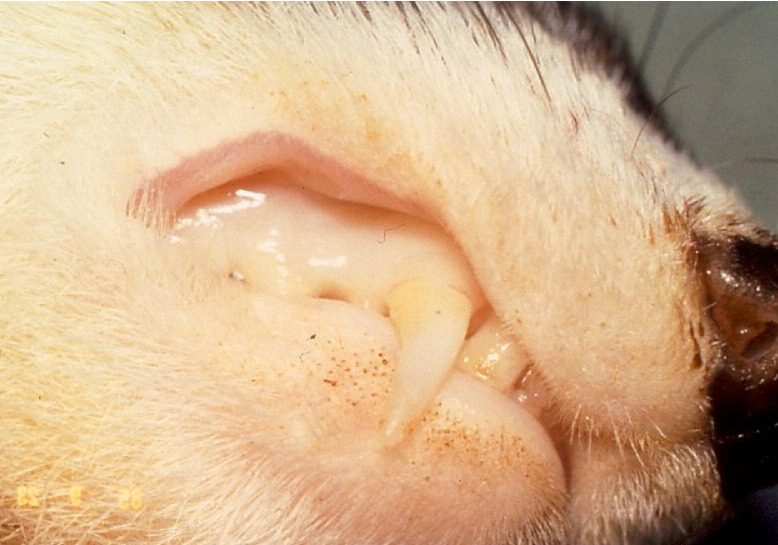
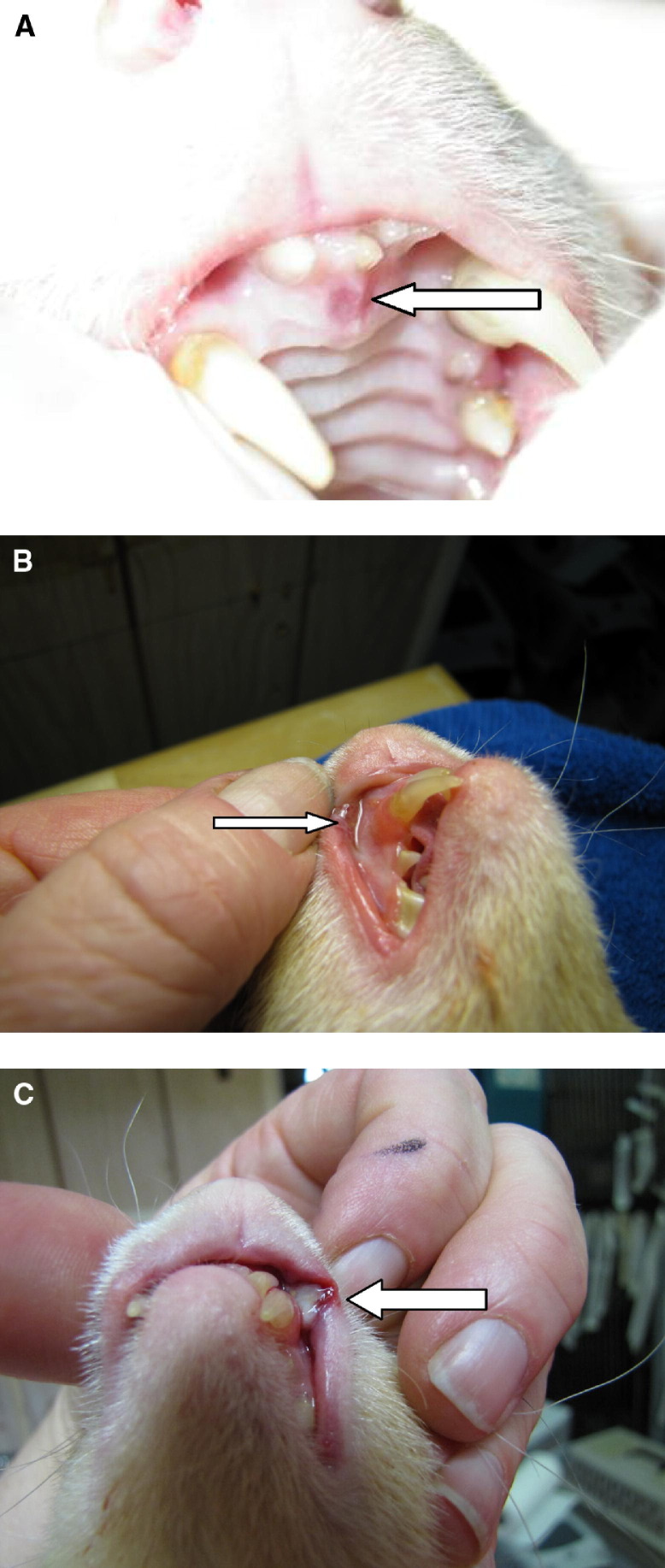
At this time, there is no known etiologic cause associated with the development of oral ulcers in ferret patients. Oral ulceration is often accompanied by cheilitis, pain, and inappetance, and can lead to severe dehydration and weight loss. The abnormal lesions may appear as small ulcers in the oral cavity, particularly on the hard palate and gums (Fig 4A). Larger ulcers are frequently apparent on the mucosa of the cheeks or lips (Fig 4B). Currently, veterinarians are encouraged to take a swab of the affected oral area for viral isolation or collect a biopsy sample to determine a possible etiology. For severe ulcers (Fig 4C), topical lidocaine gel 1% (Xylocain Topical; Astra Zeneca Pharmaceuticals, Wilmington, DE USA), parenteral analgesics, antibiotics (e.g., to control opportunistic bacterial infections), and a ferret “critical care” diet can be used to keep the ferret comfortable while the ulcers heal. Stress and immunosuppression appear to play a significant role in the development of oral ulcers.
Figure 4.
(A) A mild oral ulcer (arrow pointing to the ulcer) found in an anemic ferret. (B) Moderate oral ulcer (arrow pointing to the ulcer). (C) Severe oral ulcer (arrow pointing to the ulcer).
Ferret Mycoplasmosis
In 2005, a distributor for a major pet store chain began importing ferrets from a single source, a change from the largest US breeder. Many of these ferrets from this new animal source have exhibited chronic conjunctivitis, sneezing, wheezing, and coughing. Conventional diagnostic testing has not provided a definitive diagnosis for this disease condition. Diagnostic tests used to test affected ferrets included bronchiolar lavage (e.g., cytology, aerobic, anaerobic culture), radiographs, endoscopy, heartworm antigen detection, echocardiography, and nasal/tonsil swabs for bacterial/fungal isolation. In addition, a variety of treatment protocols were also tested in these animals, including antihistamines, nonsteroidal antiinflammatory drugs, antibiotics, bronchodilators, and decongestants. However, most of the affected ferrets had no resolution of their clinical signs, despite treatments. The coughing episodes were harsh, paroxysmal, and sometimes productive.
In 2009, one of the severe cases identified by the author died of acute respiratory failure, which was characterized by a sudden increase in congestion and dyspnea that was unresponsive to bronchodilators and oxygen. The 2-year-old female had severe lung pathology consisting of florid lymphoplasmacytic inflammation around the terminal airways, which was associated with luminal attenuation (Figs 5 and 6). Airway epithelial cells appeared attenuated, exfoliated, or eroded. Lumina occasionally contained accumulations of lymphocytes, neutrophils, or necrotic cellular debris. Remaining epithelial cells were denuded of cilia and showed intranuclear edema. There was some mild perivascular lymphoid cuffing. No inclusions or syncytia were observed by the pathologist. Multifocal lipid pneumonia was noted in the parenchyma beneath the capsule or adjacent to the lymphoid nodules. The pulmonary parenchyma was diffusely edematous and congested. All other tissues appeared normal. The diagnosis was severe, nonsuppurative bronchitis, with lesions most closely resembling those of chronic murine pneumonia due to Mycoplasma pulmonis in rats. Immunohistochemistry of the tissues was negative for influenza virus but positive for Mycoplasma spp.
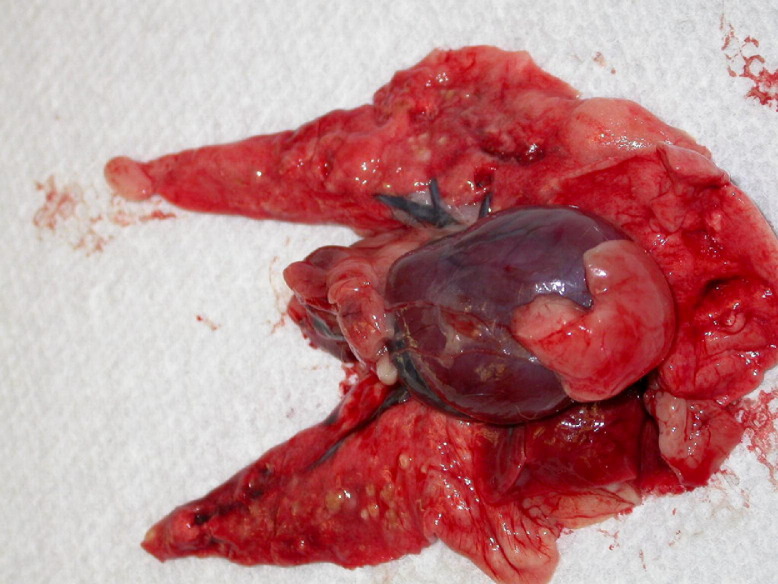
Figure 5.
Lungs and heart of the index pathology case.
Figure 6.
Cut surface of the lung from Fig 5.
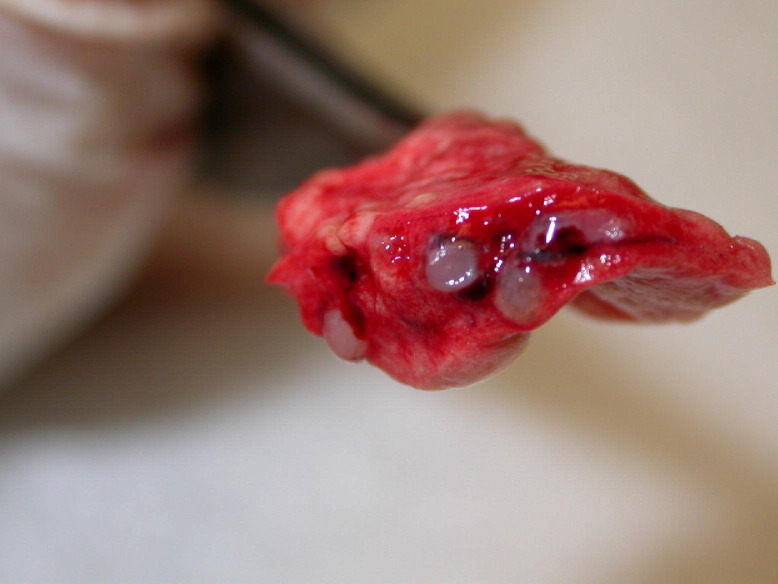
Bronchiolar lavage samples and conjunctival swabs were submitted to Dr. Matti Kiupel at Michigan State University for Mycoplasma spp. identification (Fig 7). A novel Mycoplasma spp. has been identified, and further investigations into this disease condition are ongoing.
Figure 7.
Bronchiolar lavage samples from Mycoplasma sp. suspect ferrets.
Conclusion
A number of emerging diseases in ferrets require further investigation before effective treatments and/or preventive measures can be established. Veterinary practitioners need to share case information of ferrets that present with unusual disease conditions with knowledgeable pathologists and research scientists to determine the etiology and pathophysiologic basis of these currently unknown disease syndromes.
References
- 1.Garner M.M., Ramsell K., Morera N., et al. Clinico-pathologic features of a systemic coronavirus-associated disease resembling feline infectious peritonitis in the domestic ferret (Mustela putorius) Vet Pathol. 2008;45:236–246. doi: 10.1354/vp.45-2-236. [DOI] [PubMed] [Google Scholar]
- 2.Martinez J., Ramis A.J., Reinacher M., et al. Detection of feline infectious peritonitis virus-like antigen in ferrets. Vet Rec. 2006;158:523. doi: 10.1136/vr.158.15.523-b. [DOI] [PubMed] [Google Scholar]
- 3.Garner MM: Systemic corona virus infection in domestic ferrets, Mustela putorius. Proceedings of the American Association of Zoo Veterinarians, Los Angeles, CA, p 39, 2008
- 4.Garner M.M., Ramsell K., Schoemaker N.J., et al. Myofaciitis in the domestic ferret. Vet Pathol. 2007;44:25–38. doi: 10.1354/vp.44-1-25. [DOI] [PubMed] [Google Scholar]
- 5.Garner M.M., Ramsell K. Myofasciitis: an emerging fatal disease of the domestic ferret. Exotic DVM. 2006;8:23. [Google Scholar]
- 6.Nakata M., Itou T., Sakai T. Molecular cloning and phylogenetic analysis of inflammatory cytokines of the ferret (Mustela putorius furo) J Vet Med Sci. 2008;70:543–550. doi: 10.1292/jvms.70.543. [DOI] [PubMed] [Google Scholar]
- 7.Benson K.G., Paul-Murphy J., Hart A.P., et al. Coagulation values in normal ferrets (Mustela putorius furo) using selected methods and reagents. Vet Clin Pathol. 2008;37:286–288. doi: 10.1111/j.1939-165X.2008.00047.x. [DOI] [PubMed] [Google Scholar]
- 8.Patterson A.R., Cooper V.L., Yoon K.J., et al. Naturally occurring influenza infection in a ferret (Mustela putorius furo) colony. J Vet Diagn Invest. 2009;21:527–530. doi: 10.1177/104063870902100417. [DOI] [PubMed] [Google Scholar]