Abstract
Phospholipase A2 (PLA2) enzymes are involved in various inflammatory pathological conditions including arthritis, cardiovascular and autoimmune diseases. The regulation of their catalytic activity is of high importance and a great effort has been devoted in developing synthetic inhibitors. We summarize the most important small-molecule synthetic PLA2 inhibitors developed to target each one of the four major types of human PLA2 (cytosolic cPLA2, calcium-independent iPLA2, secreted sPLA2, and lipoprotein-associated LpPLA2). We discuss recent applications of inhibitors to understand the role of each PLA2 type and their therapeutic potential. Potent and selective PLA2 inhibitors have been developed. Although some of them have been evaluated in clinical trials, none reached the market yet. Apart from their importance as potential medicinal agents, PLA2 inhibitors are excellent tools to unveil the role that each PLA2 type plays in cells and in vivo. Modern medicinal chemistry approaches are expected to generate improved PLA2 inhibitors as new agents to treat inflammatory diseases.
Abbreviations: 3D-QSAR, three-dimensional quantitative structure-activity relationship; AA, arachidonic acid; AD, Alzheimer's disease; AR, acrosome reaction; BEL, bromoenol lactone; BRB, blood-retina barrier; CLP, cecal ligation and puncture; COX, cyclooxygenase; cPLA2, cytosolic PLA2; DME, diabetic macular edema; DPPC, 1-palmitoyl-2-palmitoyl-sn-glycero-3-phosphocholine; DXMS, hydrogen/deuterium exchange mass spectrometry; ER, endoplasmic reticulum; ERK, extracellular signal-regulated kinase; ESI, electrospray ionization; FAAH, fatty acid amide hydrolase; HDL, high-density lipoprotein; HUVECs, human umbilical vein endothelial cells; IFD, induced fit docking; IFNγ, interferon gamma; iPLA2, calcium-independent PLA2; LDL, low-density lipoprotein; LOX, lipoxygenase; LPC, lysophosphatidylcholine; LpPLA2, lipoprotein-associated PLA2; LPS, lipopolysaccharide; LTB4, leukotriene B4; MD, molecular dynamics; MRM, multiple reaction monitoring; mTOR, mammalian target of rapamycin; NOD, non-obese diabetic; NOX2, NADPH oxidase 2; NTD, neglected tropical diseases; PAF-AHs, platelet-activating factor-acetylhydrolases; PAPC, 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine; PET, positron emission tomography; PGE2, prostaglandin E2; PLPC, 1-palmitoyl-2-lauroyl-sn-glycero-3-phosphocholine; ROS, reactive oxygen species; SAR, structure-activity relationship; SD rats, Sprague-Dawley rats; SFN, sulforaphane; sPLA2, secreted PLA2; TXB2, thromboxane B2; TNFα, tumor necrosis factor alpha; UCB, unconjugated bilirubin; VEGF, vascular endothelial growth factor
Keywords: Clinical trials, Inflammation, Inhibitors, Phospholipase A2
1. Introduction
Enzymes are one of the most important classes of drug targets [1]. Small-molecule enzyme inhibitors have transformed human medicine and it is estimated to comprise roughly one-third to half of all marketed drugs [2]. Since the importance of bioactive lipids has been recognized [3], a great attention has been devoted to enzymes involved in lipid metabolism. Phospholipases A2 (PLA2s) are a superfamily of enzymes [4] characterized by their ability to hydrolyze the ester bond at the sn-2 position of membrane glycerophospholipids releasing free fatty acids, including arachidonic acid (AA), and initiating the eicosanoids cascade [5]. As a matter of fact, PLA2s are the upstream regulators of the eicosanoid cascade supplying free fatty acids to cyclooxygenases, lipoxygenases, and cytochrome P450 enzymes, which thus produce various inflammatory mediators including prostaglandins, leukotrienes and thromboxanes. Because of their involvement in the development and progression of numerous inflammatory diseases [4,6], PLA2s have attracted a great interest as medicinal targets for more than twenty years. As a consequence, many small-molecule synthetic inhibitors have been developed in both academia and pharmaceutical companies [4].
In mammals, the phospholipase A2 superfamily consists of six types of diverse enzymes: GIV PLA2 [cytosolic calcium-dependent PLA2 (cPLA2)], GVI PLA2 [calcium-independent PLA2 (iPLA2)], several groups of secreted PLA2 (sPLA2), two groups of platelet-activating factor-acetylhydrolases PLA2 (PAF-AHs or GVII and GVIII PLA2), GXV PLA2 (lysosomal PLA2), and GXVI PLA2 (adipose PLA2) [4]. However, four types of human PLA2s have been targeted for the development of synthetic inhibitors as new medicinal agents: GIVA cPLA2, sPLA2, cytosolic GVIA iPLA2 and lipoprotein-associated PLA2 (LpPLA2 or GVII PLA2). The various classes of small-molecule synthetic PLA2 inhibitors are summarized in two recent review articles [7,8]. Apart from their importance as new potential therapeutics to treat inflammatory diseases, small-molecule PLA2 inhibitors are excellent tools to unveil the role that each PLA2 type plays in cells and in vivo.
In the present review article, we discuss the inhibitors targeting each one of GIVA cPLA2, GVIA iPLA2, sPLA2, and LpPLA2. We present the most important small-molecule synthetic PLA2 inhibitors developed and studied, focusing on recent research on PLA2 inhibitors, their applications to understand the role of each PLA2 type in cells and in vivo and their therapeutic potential. Computational studies aiming at understanding in depth enzyme-inhibitor interactions as well as recent advances in assaying the activity of PLA2s are discussed. Inhibitors that reached clinical trials are also summarized, although none of them entered the clinical market yet.
2. Inhibitors of cytosolic phospholipase A2
Although multiple subgroups of cytosolic PLA2 have been discovered in different cell types [4], the first purified and sequenced Group IVA cPLA2 (also known as cPLA2α) is the most well studied one. This enzyme is widely distributed in cells throughout most types of human tissue and it exhibits a marked preference for hydrolysis of AA at the sn-2 position of phospholipid substrates. GIVA cPLA2 is an 85 kDa protein that is regulated by intracellular calcium. It contains 749 amino acids and consists of an N-terminal C2 domain and a C-terminal catalytic domain. The crystal structure of GIVA cPLA2 was solved in 1999 by Dessen et al. [9] For the hydrolysis of its substrate phospholipid, the catalytic domain of GIVA cPLA2 utilizes an unusual catalytic dyad, Ser-228/Asp-549, located in the α/β hydrolase domain [10,11]. In a recent review article, Leslie has reviewed the role of cytosolic PLA2 in normal physiological processes and disease [12].
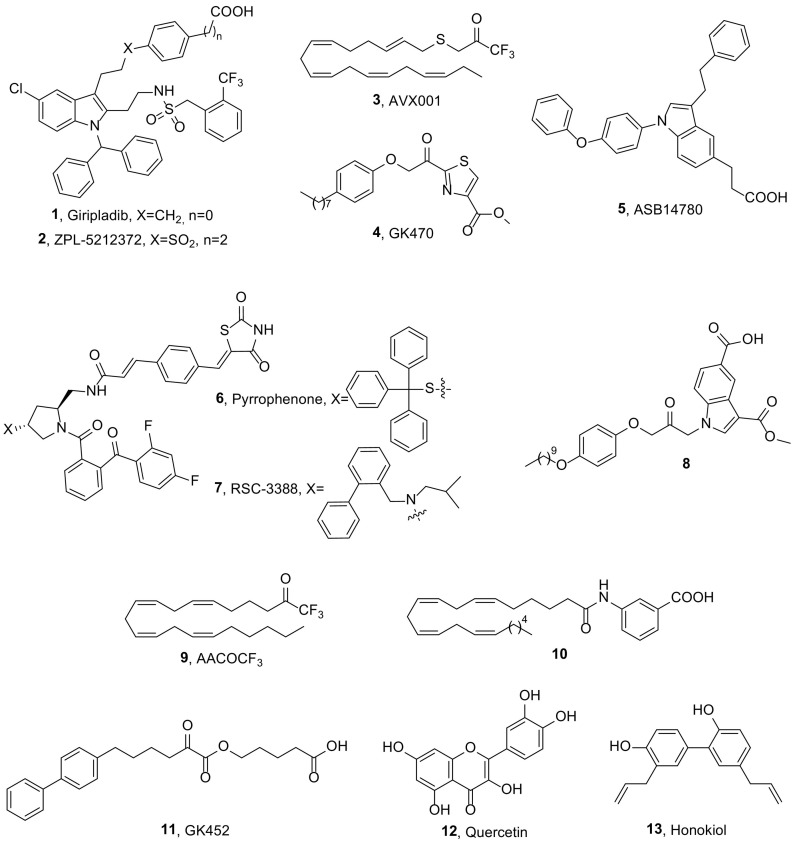
Indole derivatives, initially developed by Wyeth, constitute one of the most well studied classes of GIVA cPLA2 inhibitors [4,13]. Ecopladib, presenting IC50 values 0.15 μM in a GLU assay and 0.11 μM in a rat whole blood assay [14], displayed oral efficacy in the rat carrageenan air pouch and rat carrageenan-induced paw edema models and advanced to phase I clinical trials. Giripladib (1, Fig. 1 ) was the most promising of this indole series and it was advanced into a Phase II clinical trial for osteoarthritis. However, the trial was terminated due to a failure to differentiate from the standard of care with naproxen because of gastroenterologic effects [15].
Fig. 1.
Structures of GIVA cPLA2 inhibitors.
The structurally related compound 2 (ZPL-5212372, Fig. 1, formerly known as PF-5212372), is a highly potent and selective inhibitor of GIVA cPLA2 against both isolated enzyme and in whole cell systems (IC50 value 7 nM in a GLU assay) [16], that exhibits slow-offset inhibitory kinetics affording long duration of action. It demonstrated excellent efficacy in small and large animal models of airway and skin inflammation and Ziarco Pharma conducted a Phase I single ascending dose study via the inhaled route in healthy volunteers. ZPL-5212372 was found to be safe and well tolerated up to high doses. A randomized, adaptive design, double-blind, placebo controlled, sequential group study to determine the safety, tolerability, pharmacokinetics and efficacy of twice daily application of a topical ZPL-5212372 (1.0% w/w) ointment administered for up to 2 weeks in adult healthy volunteers and patients with moderate to severe atopic dermatitis is in progress. Results information has been submitted to ClinicalTrials.gov in February 2018, but is not yet publicly available [17].
Avexxin's ω-3 polyunsaturated fatty acid derivative AVX001 (3, Fig. 1), which has been found to inhibit GIVA with an IC50 value of 120 nM [18], entered clinical trials. A randomized, double-blind, placebo-controlled, dose-escalation first-in-man study to assess the safety and efficacy of topical AVX001 in patients with mild to moderate plaque psoriasis has been carried out demonstrating that treatment with AVX001 is well tolerated in doses up to 5% [19]. Avexxin has also claimed 2-oxothiazoles and related compounds as anti-inflammatory agents acting through the inhibition of GIVA cPLA2 and AA release [20]. AVX235 (4, Fig. 1, known also as GK470) is a potent inhibitor of GIVA cPLA2 (X I(50) value of 0.011 mol fraction in a mixed micelle assay and IC50 of 300 nM in a vesicle assay), which was found to suppress the release of AA with an IC50 value of 0.6 μM, in SW982 fibroblast-like synoviocytes [21]. This inhibitor exhibited in vivo anti-inflammatory effects comparable to the reference drugs methotrexate and Enbrel in a prophylactic and in a therapeutic collagen-induced arthritis model, respectively. More recently, the anti-angiogenic effects of this inhibitor, in a patient-derived triple-negative basal-like breast cancer model was evaluated and significant tumor growth inhibition was observed after 8 days of treatment [22]. Decreased endothelial cell proliferation and fewer immature vessels in treated tumors were shown by histology.
Asubio Pharma developed another indole-based GIVA cPLA2 inhibitor, ASB14780 (5, Fig. 1). This compound was found to be a potent GIVA cPLA2 inhibitor via enzyme assay, cell-based assay, and guinea pig and human whole-blood assays (IC50 value 0.020 μM in human whole blood assay) [23]. The daily administration of ASB14780 markedly ameliorated liver injury and hepatic fibrosis following 6 weeks of treatment with CCl4 indicating that a GIVA cPLA2 inhibitor could be useful for the treatment of nonalcoholic fatty liver diseases, including fatty liver and hepatic fibrosis [24].
Another important class of GIVA cPLA2 inhibitors is the pyrrolidine-based compounds, such as pyrrophenone (6, Fig. 1) and RSC-3388 (7, Fig. 1). This class of inhibitors having 1,2,4-trisubstituted pyrrolidine framework was developed in 2000 by Seno et al. at Shionogi [25]. Both of these inhibitors are commercially available and have been used as tools to understand the role of the enzyme. RSC-3388 exhibited an IC50 value of 1.8 nM in a PC/DOG assay [25], while pyrrophenone was found to be a potent and reversible inhibitor of human GIVA cPLA2 (IC50 4.2 nM), that strongly inhibits AA release, prostaglandin E2 (PGE2), thromboxane B2 (TXB2) and leukotriene B4 (LTB4) formation in human whole blood [26]. A combination of extensive molecular dynamics (MD) simulations and deuterium exchange mass spectrometry experimental results was developed as a tool to define more accurate binding sites. Following such a methodology, it has been demonstrated that pyrrophenone is bound to the enzyme through numerous hydrophobic residues located distal from the active site, more precisely bound in the cap region near the interfacial binding surface of the enzyme [27].
Recently, it was reported that pyrrophenone blocked calcium release from the endoplasmic reticulum (ER) and concomitant increases in mitochondrial calcium in response to stimulation by ATP, serum and A23187 [28]. This off-target effect, implicating a serine hydrolase in regulation of ER calcium release, highlights the importance of careful dose-response studies with pyrrophenone to study GIVA cPLA2 function. The role of GIVA cPLA2 in local and systemic disease during S. pneumonia infection was investigated and it was demonstrated that pharmacological inhibition of GIVA cPLA2 by RSC-3388 blocked Streptococcus pneumoniae-induced polymorphonuclear cells transepithelial migration in vitro [29]. Those results suggest that the enzyme plays a crucial role in eliciting pulmonary inflammation during pneumococcal infection. GIVA cPLA2 has been shown to increase and mediate various activities in several human cancers including colon, prostate and lung cancers [[30], [31], [32]]. Most recently, the expression level of the enzyme in breast cancer cells in the absence and presence of doxorubicin, and in patients before and after chemotherapy was studied. It was found that blockage of GIVA cPLA2 by either RSC-3388 or pyrrophenone sensitized aggressive breast cancer to doxorubicin through suppressing extracellular signal-regulated kinases (ERKs) and mammalian target of rapamycin (mTOR) kinases [33]. Thus, inhibition of GIVA cPLA2 may be of therapeutic value to overcome chemoresistance in breast cancer.
Recent results have shown that GIVA cPLA2 upregulates CD40 protein expression induced by either lipopolysaccharide (LPS) or interferon gamma (IFNγ) and this effect is mediated via the activation of NOX2-NADPH oxidase and NF-κB [34]. Reduction of the enzyme upregulation by a specific antisense or inhibition by pyrrophenone prevented the induction of CD40 protein expression by either LPS or IFNγ. The results suggest that GIVA cPLA2 has a direct role in CD40 upregulation, a feature of the pro-inflammatory M1-phenotype and indicate that GIVA cPLA2 may serve as a pivotal amplifier of the inflammatory response in the CNS.
Another study has provided evidence that GIVA cPLA2 activity is critically involved in the replication of various +RNA virus families and may thus represent a candidate target for broad-spectrum antiviral drug development [35]. The inhibition of GIVA cPLA2 activity by the low-molecular-weight inhibitor pyrrolidine-2 (RSC-3388) has profound effects on viral RNA and protein accumulation in human coronavirus 229E-infected Huh-7 cells.
Lehr and coworkers have developed 1-heteroarylpropan-2-ones, like compound 8 (Fig. 1), as potent inhibitors of GIVA cPLA2 [36]. In a recent work, they studied in detail structurally related compounds as dual inhibitors of GIVA cPLA2 and fatty acid amide hydrolase (FAAH). Bioisosteric replacement of the carboxylic acid functionality of 1-(5-carboxyindazol-1-yl)propan-2-ones by inverse amides, sulfonylamides, carbamates and ureas showed that the carboxylic acid functionality of the lead compounds is of special importance for a pronounced inhibition of GIVA cPLA2 and FAAH [37]. Since the serine reactive ketone functionality of such compounds is readily metabolized to inactive alcohol derivatives, this moiety was replaced by α-ketoheterocycle, cyanamide and nitrile serine traps, in an effort to obtain metabolically stable inhibitors. However, studies of the inhibitory activity and metabolic stability of these substances revealed that in all cases an increased metabolic stability was accompanied by a loss of inhibitory potency against GIVA cPLA2 and FAAH [38]. Structure-activity relationship studies on 1-(2-oxopropyl)indole-5-carboxylic acids explored the effect of butanoyl and hexanoyl substituents in position 3 of the indole scaffold bearing terminal groups of varying polarity [39]. The inhibitory potency was not considerably affected in most cases, while metabolic phase I and phase II in vitro stability and aqueous solubility were influenced and modulated by the structural modifications performed.
Since GIVA cPLA2 plays a critical role in neurodegenerative disorders associated with neuroinflammation, an effective positron emission tomography (PET) radioligand for imaging GIVA cPLA2 in living brain might prove a useful tool for biomedical research. Thus, four high-affinity indole-5-carboxylic acid-based inhibitors of GIVA cPLA2 (IC50 2.1 to 12 nM) were selected for labelling in carboxyl position with carbon-11 (t 1/2 = 20.4 min) to provide candidate PET radioligands for imaging brain enzyme [40]. However, all the synthesized and tested [11C]arylcarboxylic acids showed low brain penetration and lack of retention in mouse brain in vivo showing that these compounds are ineffective brain PET radioligands for GIVA cPLA2.
For better efficiency, an ideal GIVA cPLA2 inhibitor should not only be able to inhibit inflammation, but should also reach the site where inflammatory processes are taking place. Drug delivery systems that target the site of inflammation, or protect PLA2 inhibitors from in vivo degradation or detoxification are urgently needed. Arachidonoyl trifluoromethyl ketone (9, Fig. 1, AACOCF3, X I(50) 0.036) is the first GIVA cPLA2 inhibitor reported in 1993 [41], and, although not potent and selective inhibitor, it has been widely used to study the role of this enzyme in cells and in animals. A novel nanoliposomal delivery system of inhibitor AACOCF3 was developed for melanoma treatment [42].This system, called NanoATK, loaded 61.7% of the inhibitor and was stable at 4 °C for 12 weeks. The formulation decreased toxicity-enabling administration of higher doses, which was more effective at killing melanoma cells compared to free AACOCF3.
Recently, a new generation of AA analogues was developed as potential neurological agents targeting GIVA cPLA2 [43]. Among these compounds, inhibitor 10 (Fig. 1) exhibited 5.5-fold stronger GIVA cPLA2 inhibition than AACOCF3. Inhibitor 10 was found to be a GIVA cPLA2 selective inhibitor, non-cytotoxic, cell and brain penetrant and capable of reducing reactive oxygen species (ROS) and nitric oxide (NO) production in stimulated microglial cells.
GIVA cPLA2 inhibitors usually suffer from high lipophilicity. Inhibitors with ClogP values higher than 5 are not expected to present favorable ADME properties, according to Lipinski's rule of five [44]. The ClogP value is a measure of the hydrophobicity, representing the calculated partition coefficient in octanol/water on a logarithmic scale. In addition, the molecular weight of the inhibitor should be lower than 500, again according to Lipinski's rule [44]. The ClogP values, calculated using ChemDraw, together with the molecular weights of inhibitors 1–10 are summarized in Table 1 . It is obvious that none of them presents a ClogP value lower than 5. Furthermore, a number of them (1, 2, 6, 7 and 8) have molecular weights higher than 500. Kokotos and Dennis have developed in the past 2-oxoamides as potent inhibitors of GIVA cPLA2 and demonstrated their in vivo activities [45,46]. In a continuation of that research, they have recently reported new 2-oxoamides with reduced lipophilicity [47]. A reduction in the lipophilicity of the in vivo active 2-oxoamide inhibitor AX048 was achieved by replacing the long aliphatic chain with a chain containing an aromatic ring along with one or two ether oxygens.
Table 1.
ClogP values and molecular weights of some GIVA cPLA2 inhibitors.
| Inhibitor | ClogPa | MW |
|---|---|---|
| Giripladib (1) | 10.50 | 745.25 |
| ZPL-5212372 (2) | 7.85 | 823.34 |
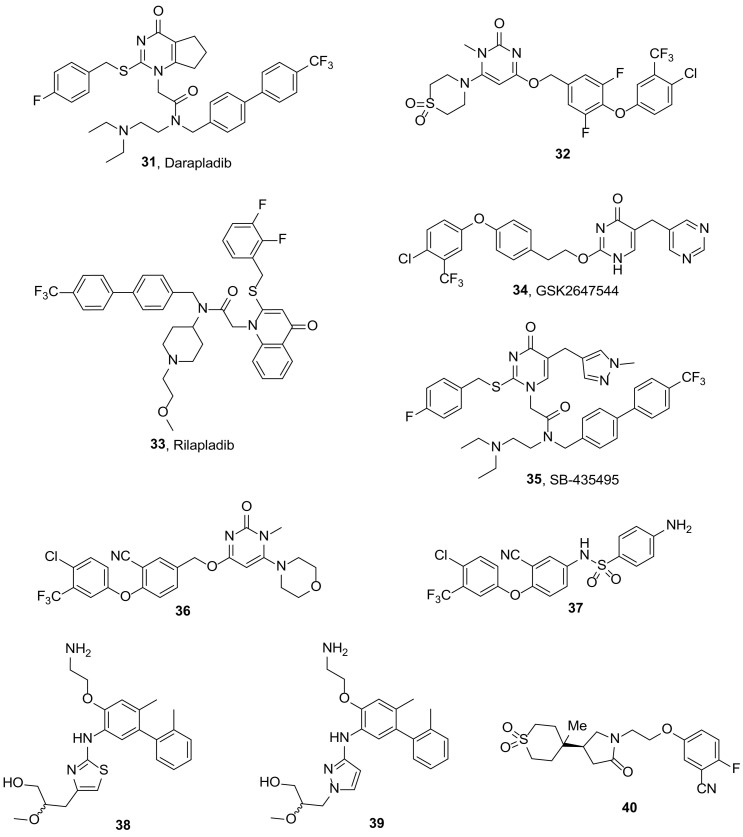
| AVX001 (3) | 7.58 | 386.52 |
| GK470 (4) | 5.96 | 389.51 |
| ASB14780 (5) | 8.98 | 461.56 |
| Pyrrophenone (6) | 9.19 | 876.01 |
| RSC-3388 (7) | 8.92 | 838.97 |
| Inhibitor 8 | 8.50 | 523.63 |
| AACOCF3 (9) | 7.94 | 356.47 |
| Inhibitor 10 | 9.31 | 437.62 |
| GK452 (11) | 4.70 | 382.46 |
Calculated using ChemDraw.
Most recently, Kokotos and Dennis have developed a novel class of highly potent GIVA cPLA2 inhibitors, namely 2-oxoesters [48]. 2-Oxoester GK452 (11, Fig. 1), containing a biphenyl system and a free carboxyl group, led to highly potent and selective GIVA cPLA2 in vitro inhibition exhibiting an X I(50) value of 0.000078. This inhibitor is the first example of a highly potent GIVA cPLA2 inhibitor, which presents a ClogP value lower than 5 (ClogP value 4.70). In RAW264.7 macrophages, treatment with the most potent 2-oxoester inhibitor GK452 resulted in over 50% decrease in KLA-elicited prostaglandin D2 production.
Apart from synthetic small-molecules, some natural products have been found to inhibit GIVA cPLA2. The enzyme has been reported to play an important role in ROS/NO signaling during microglial activation through the lipoxygenase pathway [49]. Botanical polyphenols, such as quercetin (12, Fig. 1) and honokiol (13, Fig. 1), were effective in inhibiting LPS-induced NO production and phosphorylation of GIVA cPLA2 indicating a potential use of botanical polyphenols to ameliorate the neurotoxic effects [50].
3. Inhibitors of calcium-independent phospholipase A2
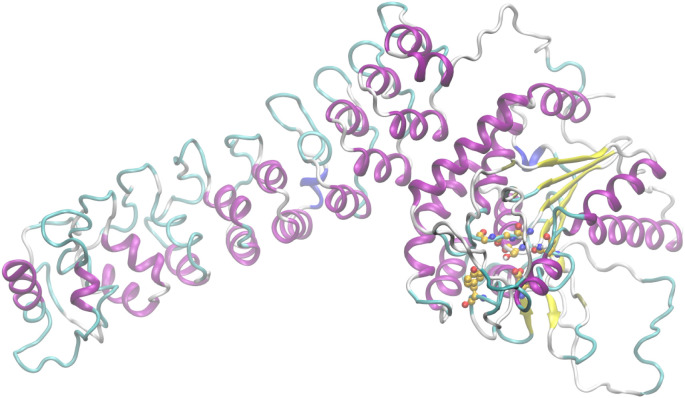
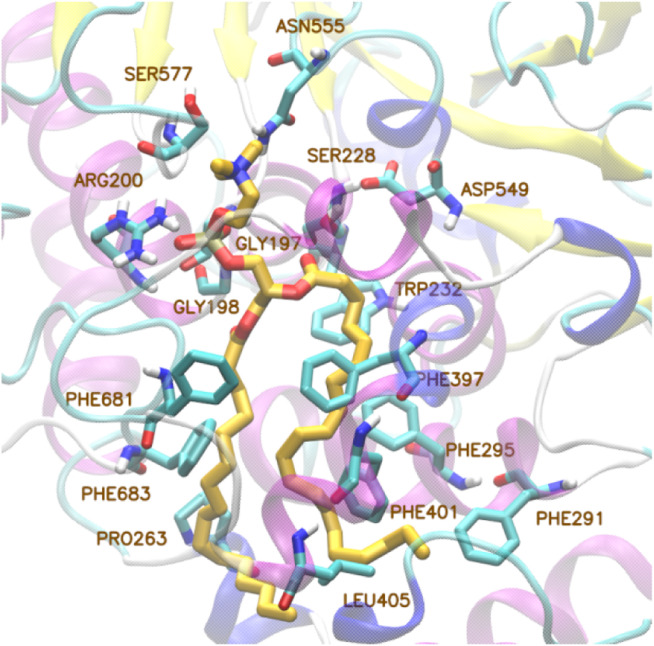
The distinctive features of the many isoforms belonging to group VI iPLA2 are the fact that they are calcium-independent and that they do not show specificity for AA [51]. They are the most widespread PLA2s throughout human tissues. The first and most widely described member of this family, GVIA iPLA2 (also known as iPLA2β), purified from macrophages in 1994 [52], also possesses lysophospholipase, transacylase and acyl-CoA thioesterase activity [53,54]. According to homology models and sequence alignments, the lipid hydrolysis is executed by a Ser/Asp catalytic dyad and the sequence consists of a linker region, seven ankyrin repeats and a catalytic domain [55]. Very recently, the crystal structure of GVIA iPLA2 revealed that the protein forms a stable dimer mediated by catalytic domains with both active sites in close proximity, positioned for cooperative activation and internal transacylation [56]. Both active sites of the dimer are wide open and provide sufficient space for phospholipids to access the catalytic centers. The ankyrin repeat domains are oriented toward the membrane-binding interface, so that they can interact with membrane proteins. The structure of GVIA iPLA2 monomer (PDB ID: 6AUN) is presented in Fig. 2 . The missing loops were filled with Modeller [57] and the residues of the active site are represented with balls (yellow: C atoms, red: O atoms, blue: N atoms, white: H atoms).
Fig. 2.
Structure of GVIA iPLA2 monomer.
In a recent review article, Ramanadham et al. discuss in detail how increased or decreased expression of iPLA2s may affect the metabolic state, CNS function, cardiovascular performance and cell survival [58]. Since dysregulation of iPLA2s may play a critical role in the development of many diseases, such as diabetes, Barth syndrome, ovarian cancer, ischemia, multiple sclerosis, research for the discovery of potent small-molecule inhibitors is therefore considered crucial [4,7,8,13].
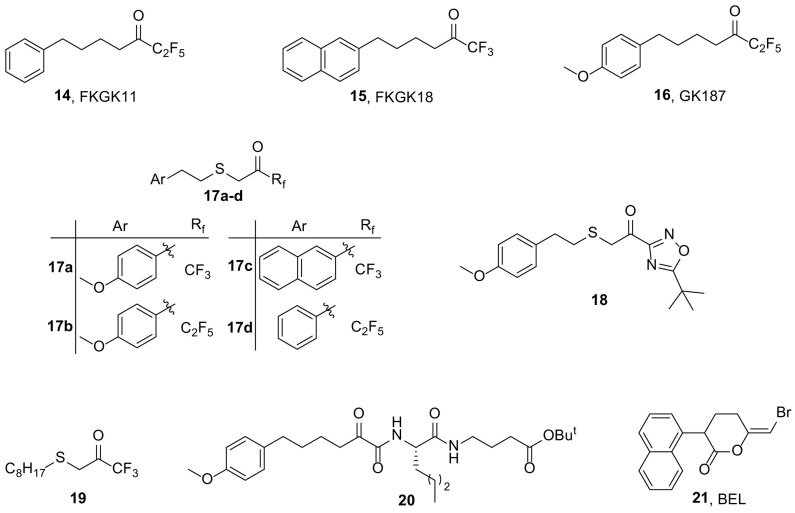
Polyfluoroketones constitute the most important class of potent and selective small-molecule GVIA iPLA2 inhibitors [[59], [60], [61]] and they have been used to study the role of the enzyme ex vivo and in vivo and in particular in autoimmune diseases [[62], [63], [64]]. FKGK11 (14, Fig. 3 ), presenting an X I(50) value of 0.0014 [60], caused a strong reduction in the clinical severity and progression of experimental autoimmune encephalomyelitis, the animal model of multiple sclerosis, showcasing that GVIA iPLA2 plays a key-role in both the onset and the progression of the disease [62]. FKGK18 (15, Fig. 3) was found to be seven times more potent inhibitor of GVIA iPLA2 (X I(50) value of 0.0002) than FKGK11 and 195 and 455 times more potent for GVIA iPLA2 than for GIVA cPLA2 and GV sPLA2, respectively [60]. GK187 (16, Fig. 3) is even more potent than FKGK18 (X I(50) value of 0.0001) [61].
Fig. 3.
Structures of GVIA iPLA2 inhibitors.
FKGK18, a reversible inhibitor which is 100-fold more selective for iPLA2β (GVIA iPLA2) than iPLA2γ (GVIB iPLA2) and does not inhibit other serine proteases, is not cytotoxic and inhibits β-cell apoptosis [65], has been investigated as for its impact on autoimmune diabetes development [66]. When administrated to spontaneous diabetes-prone non-obese diabetic (NOD) mice, FKGK18 was found to significantly decrease incidence of diabetes in association with reduced insulitis, improved glucose homeostasis, higher circulating insulin, and preservation of β-cell area. Moreover, FKGK18 reduced tumor necrosis factor alpha (TNFα) production from CD4+ T-cells and antibodies from B-cells, suggesting modulation of immune cell responses by iPLA2β or GVIA iPLA2-derived products. Further, adoptive transfer of diabetes by CD4+ T-cells to immunodeficient and nondiabetogenic NOD.scid mice was moderated by FKGK18 pretreatment and TNFα production from CD4+ T-cells was reduced by inhibitors of cyclooxygenase (COX) and 12-lipoxygenase (12-LOX). Taken together, the observations suggest that GVIA iPLA2 activation promotes immune responses, while GVIA iPLA2 inhibition may be beneficial in ameliorating diabetes.
FKGK18 was also used, along with other inhibitors, so that the role of three different PLA2 types in spontaneous and progesterone (P4)-induced acrosome reaction (AR) could be studied. More specifically, pyrrolidine-1 was used as a GIVA cPLA2 inhibitor, LY329722 as a sPLA2 (group X) inhibitor, while BEL and FKGK18 as GVIA iPLA2 inhibitors. According to the results, GVIA iPLA2 is crucial for spontaneous AR, both GVIA iPLA2 and GX sPLA2 are involved in P4-induced AR, while GIVA cPLA2 is dispensable in both types [67]. Moreover, progesterone-induced AR was found to be a long lasting process, spreading over 30 min in the mouse and kinetic analyses suggest the presence of different sperm subpopulations, using distinct PLA2 pathways to achieve AR. At low physiological P4 concentration (2 μM), sperm undergoing early AR (0–5 min post-P4) rely on GVIA iPLA2, whereas sperm undergoing late AR (20–30 min post-P4) rely on GX sPLA2. The role of PLA2s in AR seemed to depend on P4 concentration, with the PLA2s being key actors at low physiological P4 concentrations (≤2 μM), but not at higher ones (~10 μM).
In 2014, GIVA cPLA2 and GVIA iPLA2 were studied for their substrate specificities during inflammatory activation of macrophages with zymosan, using selective inhibitors, more specifically, GIVA cPLA2 inhibitor pyrrophenone (6, Fig. 1) and GVIA iPLA2 inhibitor FKGK18 (15, Fig. 3) [68]. The data showed that the two enzymes act on different phospholipid pools. Unlike GIVA cPLA2, GVIA iPLA2 does not participate in AA release, but shows specificity for choline glycerophospholipid (PC) species containing palmitic acid at the sn-1 position to generate lysoPC (LPC) (16:0), which is a major acceptor for AA incorporation back into phospholipids.
Molecular docking calculations and MD simulations were employed so that a structure-activity relationship (SAR) between GVIA iPLA2 and previously synthesized inhibitors could be established. Based on the SAR model, new compounds were synthesized and tested for the in vitro inhibitory activity against GIVA cPLA2, GV sPLA2 and GVIA iPLA2. Among them, the thioether fluoroketone compounds 17a-d (Fig. 3) and thioether keto-1,2,4-oxadiazole compound 18 (Fig. 3) potently inhibited GVIA iPLA2 and were quite selective relative to GIVA cPLA2 and GV sPLA2 [69].
Linear aliphatic beta-substituted trifluoromethyl ketones were tested against human GVIA iPLA2, GIVA cPLA2 and GV sPLA2. The beta-thiotrifluoromethyl ketone 19 (Fig. 3) was found to be highly potent inhibitor of GVIA iPLA2 with an X I(50) value of 0.0002 mol fraction (IC50110 nM), while it did not show significant inhibition toward GIVA cPLA2 and GV sPLA2 [70]. Additionally, combination of hydrogen/deuterium exchange mass spectrometry (DXMS) with computer-aided design techniques, led to an enzyme-inhibitor complex that disclosed the binding mode of 19 (Fig. 3) in the binding site of the enzyme.
New 2-oxoamides based on dipeptides and pseudodipeptides were synthesized and studied for their in vitro inhibitory activity against human GVIA iPLA2, as well as for their selectivity over the other PLA2 types. Compound 20 (Fig. 3), a 2-oxoamide based on Nle-GABA-OBut, presented significant inhibition of GVIA iPLA2 (X I(50) 0.007), as well as selectivity over GIVA cPLA2 and GV sPLA2 [71].
Bromoenol lactone (21, Fig. 3, BEL) is an irreversible, covalent inhibitor of GVIA iPLA2, exhibiting 1000-fold selectivity for iPLA2 over cPLA2 and sPLA2 [72,73]. Over the years, BEL has been used to discern the involvement of GVIA iPLA2 in biological processes at cellular level as well as in vivo. However, several features of BEL decrease its feasibility for in cells and in vivo use: (a) irreversible inhibition of iPLA2, (b) inactivation of other serine proteases, and (c) high toxicity due to its interaction with cysteines. In 2016, it was reported that iPLA2β and iPLA2γ are expressed and are active in murine cells of the osteoblastic phenotype and that their inhibition with BEL results in an initial increase in PGE2 formation, due to iPLA2-independent AA accumulation, followed by a decrease at higher BEL concentrations [74]. Moreover, BEL seems to react with intracellular cysteine and glutathione leading to glutathione depletion, which is responsible for the decrease in PGE2 production. These findings suggest that BEL must be used with caution in a cellular environment, since all data presented resemble conditions of extreme oxidative stress.
4. Inhibitors of secreted phospholipase A2
Secreted PLA2s were the first type of PLA2 enzymes identified and in mammals constitute the largest family, containing 10 catalytically active isoforms (IB, IIA, IIC, IID, IIE, IIF, III, V, X, and XIIA) and one inactive isoform (XIIB) [4,[75], [76], [77]], that differ in source, structure and function. They are typically small proteins (14–18 kDa), which require Ca2+ in mM concentration for their catalytic activity. They utilize an Asp/His dyad in the active site and they are stabilized by six common disulfide bonds and one or two variable additional ones. Individual members of sPLA2s exhibit unique tissue and cellular distributions and enzymatic properties, suggesting their distinct biological roles. They can act either by catalyzing reactions as enzymes or by binding to receptors and hence, they are involved in the activation of several biological pathways. Maintaining sPLA2 homeostasis appears critical for several physiological functions, as up-regulation or down-regulation of the expression of some sPLA2 isoforms is related to pathological conditions, such as atherosclerosis, immune disorders and cancer, as summarized in several review articles [[78], [79], [80]]. As a result, a variety of synthetic inhibitors targeting sPLA2 have been developed [4,7,8,13].
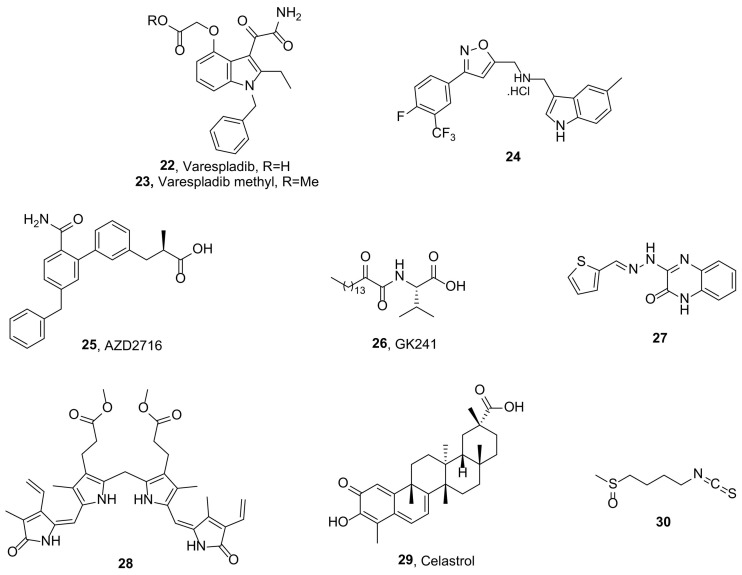
The most known sPLA2 inhibitors are the indole-based varespladib (22, Fig. 4 ) [81] and its orally bioavailable prodrug, varespladib methyl (23, Fig. 4), which entered clinical trials for inflammatory diseases (e.g. sepsis, rheumatoid arthritis, acute coronary syndrome), but failed demonstrating poor efficacy [[82], [83], [84]].
Fig. 4.
Structures of sPLA2 inhibitors.
Recently, the World Health Organization (WHO) has added snakebite envenoming to the priority list of Neglected Tropical Diseases (NTD) [85]. It is estimated that >75% of the fatal snakebites occur before victims can reach the hospital for antivenom treatment. Thus, small molecule therapeutics seem attractive tools for initiating the treatment of snakebite in the pre-hospital environment and as adjuncts to antivenom therapy [86]. Varespladib and varespladib methyl have shown high-level sPLA2 inhibition at nano-and picomolar concentrations against 28 medically important snake venoms using chromogenic assays [87]. In vivo proof-of-concept studies with varespladib had striking survival benefit against lethal doses of Micrurus fulvius and Vipera berus venom and suppressed venom-induced sPLA2 activity in rats challenged with 100% lethal doses of M. fulvius venom.
Inhibitory potential of varespladib for snake envenomation was also evaluated by another research group. Treatment with varespladib showed a significant inhibitory effect to snake venom PLA2, estimated by IC50 in vitro and ED50 in vivo (Deinagkistrodon acutus: IC50 0.0037 μg/μL, ED50 1.14 μg/g, Agkistrodon halys: IC50 0.0016 μg/μL, ED50 0.45 μg/g) [88]. In animal models, the severely hemorrhagic toxicity of D. acutus and A. halys venom was almost fully inhibited after administration. Moreover, signs of edema in gastrocnemius muscle were remarkably attenuated by administration of varespladib, with a reduced loss of myonecrosis and desmin. Serum levels of creatine kinase, lactate dehydrogenase isoenzyme 1, aspartate transaminase and alanine transaminase were down-regulated after treatment with varespladib, which indicated the protection to viscera injury.
In an effort to combine the PLA2 inhibitory activities of indoles and isoxazoles, a series of indole containing isoxazole derivatives were studied as sPLA2 inhibitors. Among them, compound 24 (Fig. 4) showed sPLA2 inhibitory activity with an IC50 value of 10.23 μM in vitro. Tested on a group of rats using carrageenan model and paw volumes, 24 ( Fig. 4) showed 75.67% and 76.54% edema inhibition at 3 h and 4 h, respectively. Further studies demonstrated that 24 (Fig. 4) showed in vitro antiproliferative activity, when tested against MCF-7 breast and DU145 prostate cancer cells in MTT assay [89].
Astra Zeneca developed a series of biphenyl derivatives as sPLA2 inhibitors. AZD2716 (25, Fig. 4) inhibited GIIA, GV and GX sPLA2 with IC50 values of 10, 40, and 400 nM, respectively, and demonstrated high plasma sPLA2 inhibition (ICu,50 0.1 nM) [90]. In vivo, a dose of 30 mg orally administered to cynomolgus monkeys, generated a concentration-dependent inhibition of sPLA2 activity in plasma (ICu,80 13 nM). When incubated with HepG2 cells, 25 (Fig. 4) inhibited sPLA2 activity (IC50 < 14 nM) and suppressed production of GIIA sPLA2 (IC50 176 nM). In addition, 25 (Fig. 4) showed sPLA2 inhibition (IC50 56 nM) in atherosclerotic plaque homogenates, as obtained from carotid endarterectomy of coronary artery disease patients. Exhibiting excellent preclinical pharmacokinetic properties across different animal species and minimized safety risk, inhibitor 25 ( Fig. 4) was selected as a clinical candidate for the treatment of coronary artery disease.
Since 2002, 2-oxoamide inhibitors have been synthesized and evaluated for their inhibitory activity against various PLA2 types [45,46,[91], [92], [93]]. A continuation of this research, guided by molecular docking simulations, led to derivative 26 (Fig. 4, GK241), a long chain 2-oxoamide based on (S)-valine. Compound 26 ( Fig. 4) exhibited high potency for inhibition of GIIA sPLA2 (IC50 143 nM and 68 nM against human and mouse GIIA sPLA2, respectively). The inhibitor was proven ten times more selective for GIIA than GV sPLA2 and did not exhibit any appreciable inhibition against other human and mouse sPLA2 enzymes [94]. Additionally, inhibitor 26 (Fig. 4), as well as other sPLA2 inhibitors, presented a significant suppression of IL-1β-stimulated PGE2 release in rat renal mesangial cells, indicating that sPLA2 plays a predominant role in the production of PGE2 [95].
Quinoxalinone derivatives with dual activities on biochemically unrelated enzymes, but mutually involved in diabetes and its complications, were presented [96]. All of the studied compounds showed low micromolar IC50 values against the different sPLA2 isozymes (IC50s < 20 μM) and satisfactory IC50 values (IC50s < 50 μM) against pancreatic α-glucosidase. For instance, compound 27 (Fig. 4) inhibited human GIIA, human GV, human GX and human GXIIA sPLA2 with IC50 values of 2.81, 6.28, 4.43 and 3.81 μM, respectively, as well as α-glucosidase (IC50 9.99 μM).
Unconjugated bilirubin (UCB), an endogenous antioxidant, has been previously reported as a GIIA sPLA2 inhibitor [97]. Chemical modifications led to more hydrophobic derivatives, which were then evaluated for their inhibitory activity against AA cascade enzymes. Dimethyl ester of bilirubin (28) inhibited GIIA sPLA2 activity in a concentration dependent manner with an IC50 value of 4.0 μM (~3 fold lower compared to UCB) [98]. In vivo, 28 (Fig. 4) reduced GIIA sPLA2 induced edema in a dose dependent manner. Compound 28 (Fig. 4) also inhibited 5-LOX and COX-2 peroxidase activity in a concentration dependent manner with IC50 values of 2.0 μM and 1.0 μM, respectively. Furthermore, 28 (Fig. 4) inhibited AA induced platelet aggregation, as well as dose dependently decreased carrageenan induced mouse paw edema.
Celastrol (29, Fig. 4), a quinine methide triterpene, was found to modulate inflammation through inhibition of the catalytic activity of mediators of AA pathway. Briefly, celastrol inhibited GIIA sPLA2 (IC50 6 μM) and 5-LOX activity (IC50 5 μM), in a concentration-dependent manner, and COX-2 peroxidase activity (IC50 20 μM) in vitro. It, additionally, inhibited carrageenan-induced edema and GIIA sPLA2-induced edema in mice. Co-injection of different concentrations of celastrol with 4 μg of human GIIA sPLA2 into hind paws of mice decreased the edema ratio in a dose-dependent manner, while at 20 μg concentration, the edema was completely inhibited. Celastrol also inhibited LPS-stimulated production of PGE2 in human neutrophils and exhibited strong antioxidant activities [99].
Various natural products have been claimed as sPLA2 inhibitors. Maslinic acid, a natural pentacyclic triterpenoid, was proved to interact directly with human GIIA sPLA2 and inhibit the enzyme activity in a concentration-dependent manner, by binding to the calcium binding and phospholipid interfacial site. Maslinic acid also inhibited human GIIA sPLA2-induced THP-1 cell differentiation and migration [100].
Sulforaphane (30, Fig. 4, SFN), a natural isothiocyanate present in cruciferous vegetables, was examined for its effects on the expression and activity of GIIA sPLA2 in vitro and in vivo. First, the effects of SFN on the expression and activity of GIIA sPLA2 induced by LPS in human umbilical vein endothelial cells (HUVECs) were determined. Post-treatment of SFN (at 10–30 mM) potently inhibited the expression and activity of GIIA sPLA2. In vivo, in a cecal ligation and puncture (CLP) model of sepsis, post-treatment with SFN markedly reduced GIIA sPLA2 expression in both LPS-injected and CLP-induced sepsis mice. SFN also suppressed the activation of GIVA cPLA2 and ERK1/2 by LPS [101].
5. Inhibitors of lipoprotein-associated phospholipase A2
The phospholipases belonging to Group VII and Group VIII catalyze the hydrolysis of the acetyl group from the sn-2 position of PAF, a potent phospholipid mediator that plays a major role in inflammation [102], therefore were named PAF acetylhydrolases (PAF-AH). GVIIA PLA2 associates with both low- and high-density lipoproteins (LDL and HDL) in human plasma, which led to the name lipoprotein-associated PLA2 (LpPLA2) [103]. LpPLA2 is a calcium independent, secreted extracellularly enzyme of 45 kDa, which uses the catalytic triad Ser/His/Asp [104]. LpPLA2 shows broad substrate specificity; apart from PAF, it can also hydrolyze phosphatidylcholines with short-chain sn-2 residues, as well as oxidized phospholipids [105]. When cloned from human plasma in 1995, it was shown to have anti-inflammatory activity in vivo [106]; however subsequent clinical studies of LpPLA2 levels in patients established this enzyme as a definitive marker of coronary heart disease [107]. Recently, a reagent for measuring LpPLA2 activity received FDA approval [108]. Elevated levels of LpPLA2 are associated with coronary heart disease and stroke [109,110]. Thus, further research is crucial for the development of new inhibitors of LpPLA2.
Darapladib (31, Fig. 5 ) is a potent and selective inhibitor of LpPLA2 (IC50 0.25 nM against recombinant human LpPLA2 in a DNPG assay), developed by GlaxoSmithKline [111], that has undergone two phase 3 trials for cardiovascular disease, but has been discontinued because of failure to reduce the risk of major coronary events [112,113]. Recently, darapladib was found to reduce elevated LpPLA2, Rho kinase activity, and cardiomyocyte apoptosis in atherosclerosis, which can lead to cardiovascular protection [114]. In an in vivo rat type 2 diabetes mellitus model, darapladib was proven to reduce inflammation [115], to decrease vascular cell adhesion molecule-1 and intercellular adhesion molecules-1 aorta expression in early stages of atherosclerosis [116] and to improve insulin resistance occurring in the metabolic disorder [117]. Moreover, investigation of the effects of chronic diabetes mellitus and hypercholesterolaemia on pig retina revealed increased permeability of the blood-retina barrier (BRB) coupled with alterations in retinal architecture, including a leak of plasma components into the retina, selective immunoglobulin G binding to neurons in the ganglion cell layer, thinning of retinal layers due to cell loss and increased glial fibrillary acidic protein expression in Müller cells, which were all curtailed by treatment with darapladib [118].
Fig. 5.
Structures of LpPLA2 inhibitors.
Recently, the crystal structures of human LpPLA2 bound with darapladib and inhibitor 32 (Fig. 5, IC50 1.7 nM against recombinant human LpPLA2) [119] were determined. Briefly, structural investigation into the LpPLA2/darapladib and LpPLA2/32 complexes identified a fairly open, large, relatively hydrophobic and rigid binding pocket. Key interactions between inhibitors and LpPLA2 were revealed. The structure of LpPLA2 proved very rigid and the binding of darapladib and 32 (Fig. 5) into LpPLA2 did not change the overall conformation of the protein. Furthermore, isothermal titration calorimetry experiments revealed that the binding of these two inhibitors to LpPLA2 is driven by enthalpic effects while the influence of entropy on the binding is negative [120].
GlaxoSmithKline has also introduced rilapladib (33, Fig. 5, SB-659032) and GSK2647544 (34, Fig. 5), which have both entered into clinical trials as potential treatment of Alzheimer's disease (AD). Rilapladib has completed a phase ΙΙa study [121] to evaluate its effect in AD, where it demonstrated improved cognitive outcomes and changes to a number of mechanism- and disease-related biomarkers, suggesting that rilapladib and inhibition of LpPLA2 may have the potential to slow the progression of AD and alter the underlying pathology in a subpopulation of AD patients with neuroimaging evidence of cerebrovascular disease [122]. GSK2647544 has been evaluated as for the safety, tolerability, pharmacokinetics and pharmacodynamics in healthy volunteers in phase 1 clinical trials [123]. The compound was generally well tolerated and had a reasonable PK-PD profile [124]. In order to refine therapeutic dose predictions and confirm brain penetration, a radiolabelled form of the inhibitor [18F]GSK2647544 was manufactured for use in a PET biodistribution phase Ι study [125]. According to the results, GSK2647544 is able to cross the blood brain barrier and enter the human brain. An exploratory analysis of the data indicated that a dose of 102 mg, twice daily would be sufficient to inhibit ~80% brain LpPLA2 [126].
Another study has investigated whether LpPLA2 and lysophosphatidylcholine (LPC), are involved in BRB damage during diabetic retinopathy, using an analog of darapladib. Systemic LpPLA2 inhibition using SB-435495 (35, Fig. 5) [127] at 10 mg/kg (i.p.), effectively suppressed BRB breakdown in streptozotocin-diabetic Brown Norway rats. This inhibitory effect was comparable to intravitreal vascular endothelial growth factor (VEGF) neutralization and the protection against BRB dysfunction was additive when both targets were inhibited simultaneously. Mechanistic studies in primary brain and retinal microvascular endothelial cells, as well as occluded rat pial microvessels, showed that luminal but not abluminal LPC potently induced permeability and that this required signaling by the VEGF receptor 2. The results suggested that LpPLA2 could be an efficacious therapeutic target for diabetic macular edema (DME), either alone or in combination with anti-VEGF therapeutics [128].
Another series of pyrimidone derivatives was tested for the LpPLA2 inhibitory activity and the effect on DME. Compound 36 (Fig. 5) demonstrated decent pharmacokinetic profile and robust inhibitory potency against LpPLA2 in male Sprague-Dawley (SD) rats. The derivative presented IC50 values of 1.0 and 2.2 nM against rat plasma and recombinant human LpPLA2, respectively. Moreover, 36 (Fig. 5) significantly inhibited retinal thickening in streptozotocin-induced diabetic SD rats, as a model of DME, after oral dosing for 4 weeks [119].
Combination of fragment screening, crystal structure determination, virtual screening and medicinal chemistry led to the identification of novel sulfonamide LpPLA2 inhibitors. Compound 37 exhibited high inhibitory activity (IC50 14 nM), good stability, as well as good permeability in vitro [129]. Additionally, inhibitor 37 ( Fig. 5) showed favorable oral bioavailability in male SD rats and maintained the inhibitory activity for 24 h after oral administration, which is superior to that of darapladib.
An X-ray fragment screening was conducted in order to identify LpPLA2 inhibitors, which do not make a direct interaction with the catalytic residues of the enzyme. This screening led to the identification of numerous fragment hits that effectively mapped the active site of the enzyme and occupied a binding surface similar to that defined by darapladib. However, a subset of fragment hits were revealed to bind in a novel pocket, approximately 13 Å from the oxyanion hole, formed by rotation of the Phe357 side chain. For instance, compounds 38 (Fig. 5) and 39 (Fig. 5) exhibited low IC50 values, molecular weights <500 Da, and ClogP values of 3.6 and 3.4, respectively. In addition, they have comparable human plasma protein binding and artificial membrane permeability to darapladib. Finally, the work was halted because neither thiazole 38 (Fig. 5) nor pyrazole 39 (Fig. 5) possessed PK properties consistent with once-daily dosing in humans. Nonetheless, the success of fragment based drug discovery in identifying ligand efficient compounds with vastly improved physicochemical properties was demonstrated [130].
Based on the above fragment-based approach, lactam inhibitors of LpPLA2 were developed [131]. Compound 40 (Fig. 5), a γ-lactam that bears a sulfone moiety, presented an IC50 value of 32 nM in whole human plasma assay, an inhibitory potency similar to that of darapladib. However, 40 ( Fig. 5) has significantly lower MW and is less lipophilic than darapladib. Moreover, inhibitor 40 ( Fig. 5) has promising pharmacokinetic properties and was found to bind in the oxyanion hole of the enzyme [131].
6. Computational studies on phospholipase A2 inhibitors
Computational studies not only contribute to better understanding of the enzyme-substrate and enzyme-inhibitor interactions, but also constitute a powerful tool to discover novel inhibitors. The applications of rational drug design on the development of PLA2 inhibitors have been reviewed in the past [132]. The combination of computational and experimental studies have been proven useful in defining inhibitor binding sites, facilitating the generation of improved enzyme inhibitors. Such a combination of MD simulations with DXMS helped to understand how pyrrophenone and 2-oxoamide inhibitors interact with GIVA cPLA2 [27] and how fluoroketones interact with GVIA iPLA2 [133].
Computational studies have been also employed to simulate the interactions of PLA2s with substrates and cell membrane for the membrane-associated members [134]. The binding mode of a phospholipid into the active site of GIVA cPLA2 and GVIA iPLA2 has been explored using MD simulations driven by DXMS data [135]. This study highlighted the importance of the interactions between protein-membrane for the substrate binding and the catalytic mechanism. Since an X-ray structure was not available at that time, a homology model of GVIA iPLA2 was created. The results showed that the polar head of the substrate 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC) occupies the hydrophilic area close to the catalytic center. The phosphate group creates a hydrogen bond with Arg200 in GIVA cPLA2 and Lys489 in GVIA iPLA2 and the carbonyl group at the sn-2 position is located near the catalytic Ser288 in GIVA cPLA2 and Ser519 in GVIA iPLA2. The aliphatic chains of the fatty acids are accommodated in the hydrophobic areas of the channel of both proteins (Fig. 6 ).
Fig. 6.
Binding mode of PAPC substrate in the active site of GIVA cPLA2.
In a continuation of that work, Mouchlis et al. further explored the substrate specificity by lipidomics and MD simulations [136]. They discovered that a unique hydrophobic area in the binding site accommodates the cleaved fatty acid and it thus drives the substrate specificity. Based on structural analysis and MD simulations, they identified an optimal phospholipid binding mode in the case of GIVA cPLA2 and they could explain the substrate specificity differences between GVIA iPLA2 and GV sPLA2.
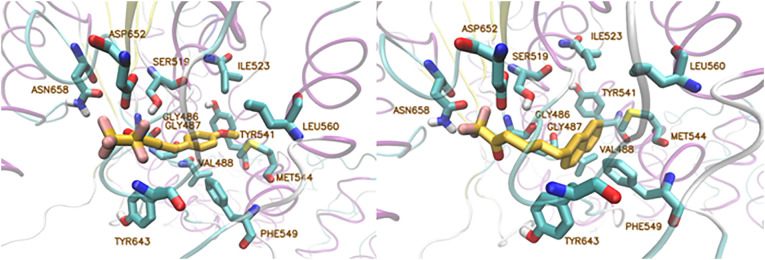
Computational studies, organic synthesis and in vitro assays led to the development of potent and selective GVIA iPLA2 inhibitors. A homology model of GVIA iPLA2 was used and the binding pocket was defined by the Gly486, Gly487, Lys489, Ser519, Val548, Phe549, Leu560, Tyr643, Phe644, Asp652, Lys729, and Leu770 residues [69]. The binding modes of trifluoromethyl ketone GK187 and pentafluoroethyl ketone FKGK18 were generated with IFD (Induced Fit Docking) calculations and the poses were subjected to MD simulations with NAMD [137] for 300 ns. According to the results (Fig. 7 ), although the carbonyl group of each compound interacts with the “oxyanion hole” Gly486/Gly487, it does not create any interaction with the catalytic Ser519. Also, the interactions the fluorine atoms create with Asn658 do not seem to be critical for the binding. Interestingly, the methoxyphenyl group of inhibitor GK187 and the naphthalene group of inhibitor FKGK18, which were located in the entrance of the binding pocket in the initial pose, moved to the hydrophobic region during the simulation.
Fig. 7.
Left: Binding mode of inhibitor GK187 in GVIA iPLA2. Right: Binding mode of inhibitor FKGK18 in GVIA iPLA2.
The binding mode of a fluoroketone inhibitor to GIVA cPLA2 was also studied [69]. The results showed that the same fluoroketone functional group can be used to construct inhibitors exhibiting selectivity for either GVIA iPLA2 or GIVA cPLA2. The size of the hydrophobic chain is very critical for both activity and selectivity. Short-chain compounds tend to selectively inhibit GVIA iPLA2, while long-chain compounds also inhibit GIVA cPLA2. That study, together with studies reported by Mouchlis et al. [70], indicated the importance of the presence of a sulfur atom at the β-position to the activated carbonyl group of fluoroketones.
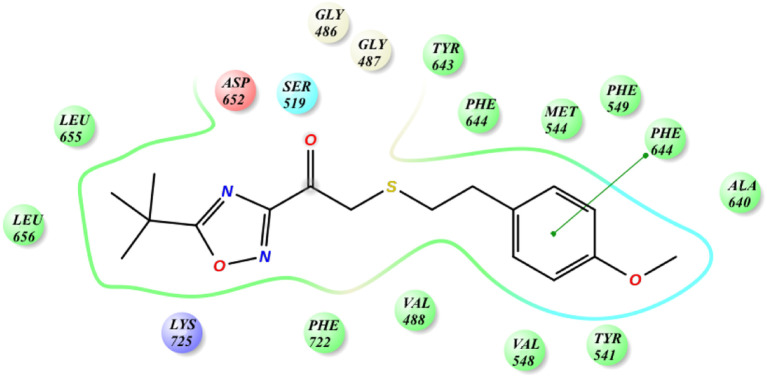
The binding mode of the novel thioether derivative 18 to GVIA iPLA2 was also studied [69]. Again the carbonyl group interacts with the “oxyanion hole” Gly486/Gly487, while Asn658 interacts via a hydrogen bond with the oxadiazole ring. The beneficial insertion of a sulfur atom is depicted by the higher affinity and, according to MD simulations, this is due to the interactions with the residues Tyr643, Phe722 and Leu770 (Fig. 8 ).
Fig. 8.
Interactions of thioether 18 with the residues of GVIA iPLA2.
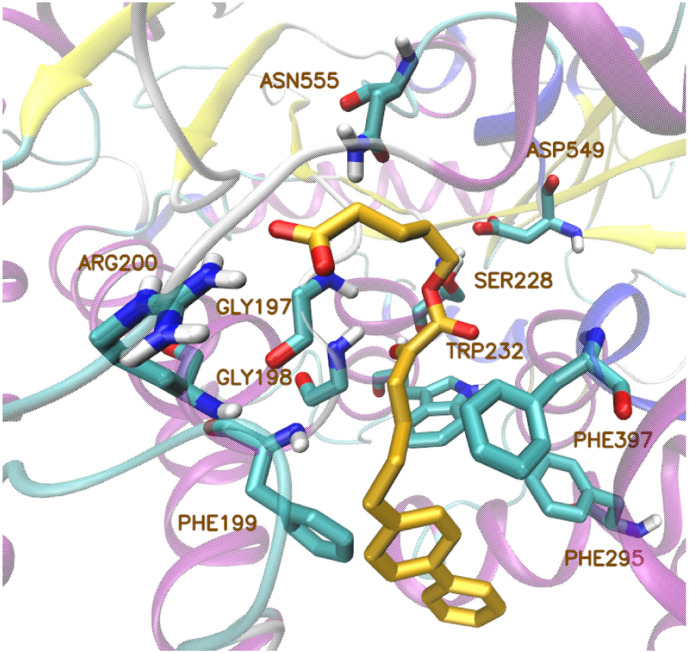
Recently, a library of small molecules, which contain the 2-oxoester functionality, was generated [48]. Inhibitor GK452, a 2-oxoester derivative with a biphenyl group and a free carboxyl group, presented high inhibitory activity against GIVA cPLA2. According to its binding mode described in that paper, the carboxyl group of the molecule interacts with Arg200 and the 2-oxoester moiety interacts with the oxyanion hole (Gly197/Gly198) via a hydrogen bond (Fig. 9 ).
Fig. 9.
Binding mode of inhibitor GK452 in the active site of GIVA cPLA2.
Structural modifications on known sPLA2 inhibitors using computational tools have been reported for the design of new derivatives with improved potency. Docking calculations for the evaluation of derivatives of co-crystallized inhibitor FPL67047XX suggested the synthesis of new derivatives, which reproduced the key interactions with the residues of the binding pocket presenting lower binding energy [138]. In addition, Mouchlis et al. [139] reported the use of a three-dimensional quantitative structure-activity relationship (3D-QSAR) model for the design and evaluation of new indole derivatives against sPLA2. The construction of the 3D-QSAR model was based on a set of 34 indole inhibitors and the generated new indole derivatives reproduced the key interactions.
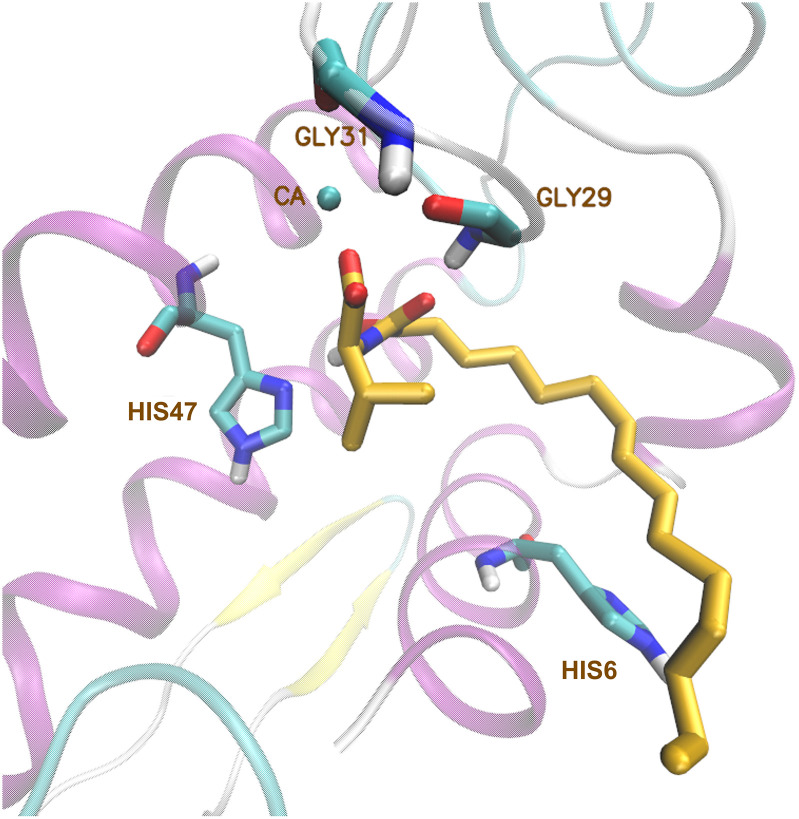
The binding mode of 2-oxoamide inhibitor GK126 to GIIA sPLA2 has been studied using GOLD [93]. Molecular docking calculations and MD simulations with AMBER led to the development of the more potent GIIA sPLA2 inhibitor GK241 (Fig. 10 ) [94,140]. Docking calculations have been also employed to simulate the interaction in GIVA cPLA2 – 2-oxoamide inhibitor complexes [141]. Inhibitor GK241 interacts with the calcium ion of GIIA sPLA2 via its carboxyl group and the 2‑carbonyl group of the amide functionality. During the MD simulation of GK241-enzyme complex, two hydrogen bonds are formed between the amide group and the residues His47 and Gly29, resulting in the stable binding of GK241 to the enzyme.
Fig. 10.
Conformational arrangement of GK241 in the binding pocket of GIIA sPLA2.
7. Assaying the activity of phospholipases A2
Assaying the activity of PLA2s is challenging, because although these enzymes are water-soluble themselves, they act on phospholipid substrates, which aggregate in aqueous solution to form micelles, vesicles, or liposomes [142]. When the inhibition of each PLA2 type is compared, one must note the particular assay conditions and aggregated form of substrate used. Almost twenty years ago, group-specific radiolabel-based assays able to distinguish between the four major types of mammalian PLA2s have been developed [143]. The purpose of this subchapter is to highlight some of the recently reported assays.
Most recently, a novel mass spectrometric-based high-throughput assay (96 well-plate assay) toward both natural and synthetic membrane phospholipids in mixed micelles with a nonionic surfactant has been reported [136]. In this lipidomics-based HPLC/MS assay, a HILIC column and multiple reaction monitoring (MRM) were used for targeted quantification of the assay components including the surfactant (octaethylene glycol monododecyl ether, C12E8), a free fatty acid (AA), a phospholipid (1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine, PAPC), a lysophospholipid (1-palmitoyl-sn-glycero-3-phosphocholine, 16:0 LPC), and an internal standard (1-heptadecanoyl-sn-glycero-3-phosphocholine, 17:0 LPC). AA was detected in negative electrospray ionization (ESI) mode, while lysophospholipids, phospholipids and surfactant (C12E8) in positive ESI mode. Unexpected head group and acyl chain specificity for three major PLA2 types was discovered. These studies allowed a detailed understanding of the preference of GIVA cPLA2 for cleavage of pro-inflammatory AA, and indicated the preference of GIVA iPLA2 for cleavage of linoleic acid and the preference of GV sPLA2 for linoleic acid, saturated fatty acids and phosphatidylglycerol.
Barbour and Ramanadham reported a detailed procedure to measure GVIA iPLA2-specific activity in cell lines or tissue preparations using a simple radiolabel-based assay and to evaluate the impact of small-molecule inhibitors on resting- and disease-state GVIA iPLA2 activity [144]. The assay provides rapid, selective and quantifiable measurement of GVIA iPLA2-specific activity, without requiring purification of the enzyme. Although PAPC is a common choice for the radiolabeled substrate, 1-palmitoyl-2-palmitoyl-sn-glycero-3-phosphocholine (DPPC), 1-palmitoyl-2-lauroyl-sn-glycero-3-phosphocholine (PLPC), and 1-O-(Z)-hexadec-1′-enyl-2-[9,10-3H2]octadec-9′-enoyl-sn-glycero-3-phosphocholine [(16:0p/18:1)-PC] are also suitable. The plasmalogen phospholipid (16:0p/18:1)-PC, containing a vinyl ether linkage in the sn-1 position, is an extremely favored substrate by GVIA iPLA2.
A fluorometric high-throughput screening assay for sPLA2s using phospholipid vesicles was developed and used to identify human GIII sPLA2 inhibitors on a library of 370,276 small molecules [145]. The substrate is present in phospholipid vesicles, because this matrix more closely resembles the natural substrate of human GIII sPLA2, as opposed to phospholipid/detergent mixed micelles. A phospholipid analogue containing BODIPY fluorophores dispersed as a minor component in vesicles of nonfluorescent phospholipids was used as substrate. An increase in fluorescence was observed, when the enzyme acted and liberated a free fatty acid from the phospholipid, because a reduction in quenching of the fluorophore occurred. The assay uses optical detection in a 1536-well plate format.
A colorimetric assay using PAF analog 1-myristoyl-2-(4-nitrophenylsuccinyl) phosphatidylcholine as substrate (chemical LpPLA2 activity assay) is the most commonly used LpPLA2 activity assay in current clinical settings [108,146]. The colorimetric assay proved to be accurate and robust with acceptable linearity through a wide range of activity levels, allowing for adoption of LpPLA2 activity in clinical practice [108]. Most recently, a novel enzymatic method for assaying LpPLA2 in serum has been developed [147]. This LpPLA2 activity assay used 1-O-hexadecyl-2-acetyl-rac-glycero-3 phosphocholine (rac C16 PAF) as a substrate, which was hydrolyzed by LpPLA2 to produce 1-O-hexadecyl-2-hydroxy-sn-glycero-3-phosphocholine (LysoPAF). LysoPAF was then hydrolyzed by lysoplasmalogen-specific phospholipase D (lysophospholipase D) to generate choline, which was detected by choline oxidase. Regression analysis of LpPLA2 activity measured by the enzymatic LpPLA2 activity assay vs. two chemical LpPLA2 activity assays, i.e. LpPLA2 FS and PLAC® test, and ELISA, gave the following correlation coefficients: 0.990, 0.893 and 0.785, respectively (n = 30).
8. Conclusion
Up to now, several small-molecule inhibitors of sPLA2, cPLA2 and LpPLA2 have been studied in clinical trials for their safety and efficacy in humans. The most advanced clinical trials of PLA2 inhibitors, together with the disease indications, are summarized in Table 2 .
Table 2.
Clinical trials of PLA2 inhibitors.
| Inhibitor | Clinical trial | Condition |
|---|---|---|
| Varespladib (sPLA2) |
Phase III clinical trial VISTA-16 clinicaltrials.gov Identifier: NCT01130246 |
Acute coronary syndrome |
| Giripladib (cPLA2) |
Phase II clinical trial clinicaltrials.gov Identifier: NCT00396955 |
Osteoarthritis |
| Phase I clinical trial clinicaltrials.gov Identifier: NCT00440492 |
Rheumatoid arthritis | |
| PF-5212372 (ZPL-5212372) (cPLA2) |
Phase I/II study clinicaltrials.gov Identifier: NCT02795832 |
Atopic dermatitis |
| Darabladib (LpPLA2) |
Phase III clinical trial STABILITY clinicaltrials.gov Identifier: NCT00799903 | Atherosclerosis |
| Phase III clinical trial SOLID-TIMI 52 clinicaltrials.gov Identifier: NCT01000727 | Acute coronary syndrome | |
| Phase III clinical trial clinicaltrials.gov Identifier:NCT01067339 |
Endothelial dysfunction/ Coronary atherosclerosis |
|
| Phase II clinical trial clinicaltrials.gov Identifier:NCT01506895 |
Diabetic retinopathy | |
| Rilapladib (LpPLA2) |
Phase II clinical trial clinicaltrials.gov Identifier: NCT01428453 |
Alzheimer's disease |
| Phase II clinical trial clinicaltrials.gov Identifier: NCT00695305 |
Atherosclerosis | |
| GSK2647544 (LpPLA2) |
Phase I clinical trial clinicaltrials.gov Identifier: NCT01702467, NCT01978327, NCT01924858 |
Alzheimer's disease |
Varespladib (sPLA2 inhibitor) was the first PLA2 inhibitor, which was advanced into clinical trials as an intravenously-administered therapy for sepsis-induced systemic inflammatory response syndrome. Although at the end of the Phase I study it was found to have an acceptable safety profile in patients with severe sepsis, the trial was terminated because the Phase II study showed poor efficacy [82]. A double-blinded placebo-controlled clinical trial of varespladib methyl failed to show efficacy in the treatment of rheumatoid arthritis [83]. Later on, varespladib methyl was evaluated for cardiovascular disease. FRANCIS (Fewer Recurrent Acute Coronary Events with Near-Term Cardiovascular Inflammation Suppression) study demonstrated that treatment with varespladib methyl reduced concentrations of LDL-C, hs-CRP and sPLA2 in ACS patients treated with evidence-based therapies inclusive of high-dose atorvastatin [148]. VISTA-16 (Vascular Inflammation Suppression to Treat Acute Coronary Syndrome for 16 Weeks) clinical trial evaluated the safety and efficacy of 16 weeks of treatment with varespladib methyl on morbidity and mortality when added to atorvastatin and standard of care in subjects with an ACS [149]. However, in 2012 the study was terminated due to lack of efficacy.
Giripladib, an indole-based GIVA cPLA2, was advanced into a Phase II clinical trial for osteoarthritis, but in 2007 the trial was terminated due to gastrointestinal events [15]. A randomized, double-blind, placebo controlled study to determine the safety, tolerability, pharmacokinetics and efficacy of a topical ZPL-5212372 (another indole-based GIVA cPLA2 inhibitor) ointment in adult healthy volunteers and patients with moderate to severe atopic dermatitis is in progress and the results information is not yet publicly available [17].
Darapladib, a potent and selective LpPLA2 inhibitor, has undergone two phase III trials for cardiovascular disease, but it failed to reduce the risk of major coronary events [112,113]. More recently, a clinical trial of rilapladib, another LpPLA2 inhibitor, has completed and demonstrated improved cognitive outcomes and changes to a number of mechanism- and disease-related biomarkers, suggesting that inhibition of LpPLA2 may have the potential to slow the progression of AD [124]. In addition, GSK2647544 (LpPLA2 inhibitor) has been evaluated as for the safety, tolerability, pharmacokinetics and pharmacodynamics in healthy volunteers in phase I clinical trials [123].
Overall, although several small-molecule PLA2 inhibitors have been evaluated in clinical trials, none of them reached the market yet. The trials for sPLA2 inhibitors varespladib and varespladib methyl failed to exhibit the expected in vivo efficacy, while the cPLA2 inhibitor giripladib caused undesired side effects. Recent results showing high-level of sPLA2 inhibition by varespladib and varespladib methyl against 28 medically important snake venoms [87] suggest that these sPLA2 inhibitors may find use in initiating the treatment of snakebite in the pre-hospital environment. In the case of cPLA2 inhibitors, as we previously noticed, the high lipophilicity of the known cPLA2 inhibitors is a serious drawback. Thus, it seems that such inhibitors may be more suitable for topical use, for example for the treatment of dermatitis, rather than for systemic use. Fortunately, potent cPLA2 inhibitors having ClogP values lower than 5, for example 2-oxoesters, have been recently discovered [48]. As for LpPLA2 enzyme, the failure of the potent LpPLA2 inhibitor darapladib to reduce the risk of major coronary events, as demonstrated in both STABILITY and SOLID-TIMI 52 phase III studies, indicates that the enzyme may be a biomarker of vascular inflammation rather than a target for pharmaceutical treatment with beneficial effects.
In conclusion, although both pharmaceutical companies and academic institutions have devoted huge efforts to develop PLA2 inhibitors, it seems that still fundamental issues about the connection of structure with cellular function and the involvement of each PLA2 type in particular diseases have not been clearly understood. For example, most recently a combination of LC/MS lipidomics and MD simulations revealed surprising and previously unrecognized substrate specificity for sPLA2 and iPLA2. It is expected that advanced analytical lipidomic approaches and computational methods may contribute to increase our knowledge on the PLA2 superfamily of enzymes. In addition, the recently solved crystal structure of GVIA iPLA2 may help in designing novel inhibitors for this enzyme. Classical medicinal chemistry approaches, including organic synthesis and structure-activity relationship studies, as well as modern approaches employing functional lipidomics and computer-aided drug design may provide new chemical entities as potential novel pharmaceutical agents.
Conflict of interest
The authors declare no conflict of interest.
Transparency document
Transparency document.
Acknowledgments
The authors would like to thank the “Special Accounts for Research Grants” of the National and Kapodistrian University of Athens, Greece for their support (Grant 13300). M.G.K. would like to thank the National Scholarship Foundation (IKY) for a fellowship.
Footnotes
This article is part of a Special Issue entitled Novel functions of phospholipase A2 Guest Editors: Makoto Murakami and Gerard Lambeau.
The Transparency document associated with this article can be found, in online version.
References
- 1.Imming P., Sinning C., Meyer A. Drugs, their targets and the nature and number of drug targets. Nat. Rev. Drug Discov. 2006;5:821–834. doi: 10.1038/nrd2132. [DOI] [PubMed] [Google Scholar]
- 2.Holdgate G.A., Meek T.D., Grimley R.L. Mechanistic enzymology in drug discovery: a fresh perspective. Nat. Rev. Drug Discov. 2018;17:115–132. doi: 10.1038/nrd.2017.219. [DOI] [PubMed] [Google Scholar]
- 3.Nicolaou A., Kokotos G. The Oily Press; Bridgewater, England: 2004. Bioactive Lipids. [Google Scholar]
- 4.Dennis E.A., Cao J., Hsu Y.H., Magrioti V., Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dennis E.A., Norris P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. Recent progress in phospholipase A2 research: from cells to animals to humans. Prog. Lipid Res. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Kokotou M.G., Limnios D., Nikolaou A., Psarra A., Kokotos G. Inhibitors of phospholipase A2 and their therapeutic potential: an update on patents (2012–2016) Expert Opin. Ther. Pat. 2017;27:217–225. doi: 10.1080/13543776.2017.1246540. [DOI] [PubMed] [Google Scholar]
- 8.Ong W.Y., Farooqui T., Kokotos G., Farooqui A.A. Synthetic and natural inhibitors of phospholipases A2: their importance for understanding and treatment of neurological disorders. ACS Chem. Neurosci. 2015;6:814–831. doi: 10.1021/acschemneuro.5b00073. [DOI] [PubMed] [Google Scholar]
- 9.Dessen A., Tang J., Schmidt H., Stahl M., Clark J.D., Seehra J., Somers W.S. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell. 1999;97:349–360. doi: 10.1016/s0092-8674(00)80744-8. [DOI] [PubMed] [Google Scholar]
- 10.Sharp J.D., Pickard R.T., Chiou X.G., Manetta J.V., Kovacevic S., Miller J.R., Varshavsky A.D., Roberts E.F., Strifler B.A., Brems D.N. Serine 228 is essential for catalytic activities of 85-kDa cytosolic phospholipase A2. J. Biol. Chem. 1994;269:23250–23254. [PubMed] [Google Scholar]
- 11.Pickard R.T., Chiou X.G., Strifler B.A., Defelippis M.R., Hyslop P.A., Tebbe A.L., Yee Y.K., Reynolds L.J., Dennis E.A., Kramer R.M., Sharp J.D. Identification of essential residues for the catalytic function of 85-kDa cytosolic phospholipase A2. J. Biol. Chem. 1996;271:19225–19231. doi: 10.1074/jbc.271.32.19225. [DOI] [PubMed] [Google Scholar]
- 12.Leslie C.C. Cytosolic phospholipase A₂: physiological function and role in disease. J. Lipid Res. 2015;56:1386–1402. doi: 10.1194/jlr.R057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magrioti V., Kokotos G. Phospholipase A2 inhibitors as potential therapeutic agents for the treatment of inflammatory diseases. Expert Opin. Ther. Pat. 2010;20:1–18. doi: 10.1517/13543770903463905. [DOI] [PubMed] [Google Scholar]
- 14.Lee K.L., Foley M.A., Chen L., Behnke M.L., Lovering F.E., Kirincich S.J., Wang W., Shim J., Tam S., Shen M.W., Khor S., Xu X., Goodwin D.G., Ramarao M.K., Nickerson-Nutter C., Donahue F., Ku M.S., Clark J.D., McKew J.C. Discovery of ecopladib, an indole inhibitor of cytosolic phospholipase A2α. J. Med. Chem. 2007;50:1380–1400. doi: 10.1021/jm061131z. [DOI] [PubMed] [Google Scholar]
- 15.http://clinicaltrials.gov (Identifier: NCT00396955)
- 16.Hewson C.A., Patel S., Calzetta L., Campwala H., Havard S., Luscombe E., Clarke P.A., Peachell P.T., Matera M.G., Cazzola M., Page C., Abraham W.M., Williams C.M., Clark J.D., Liu W.L., Clarke N.P., Yeadon M. Preclinical evaluation of an inhibitor of cytosolic phospholipase A2α for the treatment of asthma. J. Pharmacol. Exp. Ther. 2012;340:656–665. doi: 10.1124/jpet.111.186379. [DOI] [PubMed] [Google Scholar]
- 17.http://clinicaltrials.gov (Identifier NCT02795832)
- 18.Huwiler A., Feuerherm A.J., Sakem B., Pastukhov O., Filipenko I., Nguyen T., Johansen B. The ω3-polyunsaturated fatty acid derivatives AVX001 and AVX002 directly inhibit cytosolic phospholipase A2 and suppress PGE2 formation in mesangial cells. Br. J. Pharmacol. 2012;167:1691–1701. doi: 10.1111/j.1476-5381.2012.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omland S.H., Habicht A., Damsbo P., Wilms J., Johansen B., Gniadecki R. A randomized, double-blind, placebo-controlled, dose-escalation first-in-man study (phase 0) to assess the safety and efficacy of topical cytosolic phospholipase A2 inhibitor, AVX001, in patients with mild to moderate plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017;31:1161–1167. doi: 10.1111/jdv.14128. [DOI] [PubMed] [Google Scholar]
- 20.Kokotos G., Johansen B., Magrioti V., Tsakos M. WO2011039365. 2011. Antiinflammatory 2-oxothiazoles and 2-oxooxazoles. [Google Scholar]
- 21.Kokotos G., Feuerherm A.J., Barbayianni E., Shah I., Sæther M., Magrioti V., Nguyen T., Constantinou-Kokotou V., Dennis E.A., Johansen B. Inhibition of group IVA cytosolic phospholipase A2 by thiazolyl ketones in vitro, ex vivo, and in vivo. J. Med. Chem. 2014;57:7523–7535. doi: 10.1021/jm500192s. [DOI] [PubMed] [Google Scholar]
- 22.Kim E., Tunset H.M., Cebulla J., Vettukattil R., Helgesen H., Feuerherm A.J., Engebråten O., Mælandsmo G.M., Johansen B., Moestue S.A. Anti-vascular effects of the cytosolic phospholipase A2 inhibitor AVX235 in a patient-derived basal-like breast cancer model. BMC Cancer. 2016;16:191. doi: 10.1186/s12885-016-2225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomoo T., Nakatsuka T., Katayama T., Hayashi Y., Fujieda Y., Terakawa M., Nagahira K. Design, synthesis, and biological evaluation of 3-(1-aryl-1H-indol-5-yl)propanoic acids as new indole-based cytosolic phospholipase A2α inhibitors. J. Med. Chem. 2014;57:7244–7262. doi: 10.1021/jm500494y. [DOI] [PubMed] [Google Scholar]
- 24.Kanai S., Ishihara K., Kawashita E., Tomoo T., Nagahira K., Hayashi Y., Akiba S. ASB14780, an orally active inhibitor of group IVA phospholipase A2, is a pharmacotherapeutic candidate for non-alcoholic fatty liver disease. J. Pharmacol. Exp. Ther. 2016;356:604–614. doi: 10.1124/jpet.115.229906. [DOI] [PubMed] [Google Scholar]
- 25.Seno K., Okuno T., Nishi K., Murakami Y., Watanabe F., Matsuura T., Wada M., Fujii Y., Yamada M., Ogawa T., Okada T., Hashizume H., Kii M., Hara S., Hagishita S., Nakamoto S., Yamada K., Chikazawa Y., Ueno M., Teshirogi I., Ono T., Ohtani M. Pyrrolidine inhibitors of human cytosolic phospholipase A2. J. Med. Chem. 2000;43:1041–1044. doi: 10.1021/jm9905155. [DOI] [PubMed] [Google Scholar]
- 26.Seno K., Okuno T., Nishi K., Murakami Y., Yamada K., Nakamoto S., Ono T. Pyrrolidine inhibitors of human cytosolic phospholipase A2. Part 2: synthesis of potent and crystallized 4-triphenylmethylthio derivative 'pyrrophenone. Bioorg. Med. Chem. Lett. 2001;11:587–590. doi: 10.1016/s0960-894x(01)00003-8. [DOI] [PubMed] [Google Scholar]
- 27.Burke J.E., Babakhani A., Gorfe A.A., Kokotos G., Li S., Woods V.L., Jr., McCammon J.A., Dennis E.A. Location of inhibitors bound to group IVA phospholipase A2 determined by molecular dynamics and deuterium exchange mass spectrometry. J. Am. Chem. Soc. 2009;131:8083–8091. doi: 10.1021/ja900098y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yun B., Lee H., Ewing H., Gelb M.H., Leslie C.C. Off-target effect of the cPLA2α inhibitor pyrrophenone: inhibition of calcium release from the endoplasmic reticulum. Biochem. Biophys. Res. Commun. 2016;479:61–66. doi: 10.1016/j.bbrc.2016.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhowmick R., Clark S., Bonventre J.V., Leong J.M., McCormick B.A. Cytosolic phospholipase A2α promotes pulmonary inflammation and systemic disease during Streptococcus pneumoniae infection. Infect. Immun. 2017;85 doi: 10.1128/IAI.00280-17. (e00280-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blaine S.A., Wick M., Dessev C., Nemenoff R.A. Induction of cPLA2 in lung epithelial cells and non-small cell lung cancer is mediated by Sp1 and c-Jun. J. Biol. Chem. 2001;276:42737–42743. doi: 10.1074/jbc.M107773200. [DOI] [PubMed] [Google Scholar]
- 31.Parhamifar L., Jeppsson B., Sjölander A. Activation of cPLA2 is required for leukotriene D4-induced proliferation in colon cancer cells. Carcinogenesis. 2005;26:1988–1998. doi: 10.1093/carcin/bgi159. [DOI] [PubMed] [Google Scholar]
- 32.Patel M.I., Singh J., Niknami M., Kurek C., Yao M., Lu S., Maclean F., King N.J., Gelb M.H., Scott K.F., Russell P.J., Boulas J., Dong Q. Cytosolic phospholipase A2-α: a potential therapeutic target for prostate cancer. Clin. Cancer Res. 2008;14:8070–8079. doi: 10.1158/1078-0432.CCR-08-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z., Qu M., Sun Y., Wan H., Chai F., Liu L., Zhang P. Blockage of cytosolic phospholipase A2 alpha sensitizes aggressive breast cancer to doxorubicin through suppressing ERK and mTOR kinases. Biochem. Biophys. Res. Commun. 2018;496:153–158. doi: 10.1016/j.bbrc.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Malada-Edelstein Y.F., Hadad N., Levy R. Regulatory role of cytosolic phospholipase A2 alpha in the induction of CD40 in microglia. J. Neuroinflammation. 2017;14:33. doi: 10.1186/s12974-017-0811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller C., Hardt M., Schwudke D., Neuman B.W., Pleschka S., Ziebuhr J. Inhibition of cytosolic phospholipase A2α impairs an early step of coronavirus replication in cell culture. J. Virol. 2018;92 doi: 10.1128/JVI.01463-17. (e01463-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ludwig J., Bovens S., Brauch C., Elfringhoff A.S., Lehr M. Design and synthesis of 1-indol-1-yl-propan-2-ones as inhibitors of human cytosolic phospholipase A2α. J. Med. Chem. 2006;49:2611–2620. doi: 10.1021/jm051243a. [DOI] [PubMed] [Google Scholar]
- 37.Althaus J., Hake T., Hanekamp W., Lehr M. 1-(5-Carboxyindazol-1-yl)propan-2-ones as dual inhibitors of cytosolic phospholipase A2α and fatty acid amide hydrolase: bioisosteric replacement of the carboxylic acid moiety. J. Enzyme Inhib. Med. Chem. 2016;31:131–140. doi: 10.1080/14756366.2016.1178246. [DOI] [PubMed] [Google Scholar]
- 38.Sundermann T., Hanekamp W., Lehr M. Structure–activity relationship studies on 1-heteroaryl-3-phenoxypropan-2-ones acting as inhibitors of cytosolic phospholipase A2α and fatty acid amide hydrolase: replacement of the activated ketone group by other serine traps. J. Enzyme Inhib. Med. Chem. 2016;31:653–663. doi: 10.3109/14756366.2015.1057721. [DOI] [PubMed] [Google Scholar]
- 39.Arnsmann M., Hanekamp W., Elfringhoff A.S., Lehr M. Structure-activity relationship studies on 1-(2-oxopropyl)indole-5-carboxylic acids acting as inhibitors of cytosolic phospholipase A2α: effect of substituents at the indole 3-position on activity, solubility, and metabolic stability. Eur. J. Med. Chem. 2017;125:1107–1114. doi: 10.1016/j.ejmech.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 40.Fisher M.J., McMurray L., Lu S., Morse C.L., Liow J.S., Zoghbi S.S., Kowalski A., Tye G.L., Innis R.B., Aigbirhio F.I., Pike V.W. [Carboxyl-11C]labeling of four high-affinity cPLA2 inhibitors and their evaluation as radioligands in mice with positron emission tomography. ChemMedChem. 2018;13:138–146. doi: 10.1002/cmdc.201700697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Street I.P., Lin H.K., Laliberté F., Ghomashchi F., Wang Z., Perrier H., Tremblay N.M., Huang Z., Weech P.K., Gelb M.H. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 42.Gowda R., Dinavahi S.S., Iyer S., Banerjee S., Neves R.I., Pameijer C.R., Robertson G.P. Nanoliposomal delivery of cytosolic phospholipase A2 inhibitor arachidonyltrimethyl ketone for melanoma treatment. Nanomedicine. 2018;14:863–873. doi: 10.1016/j.nano.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng C.Y., Kannan S., Chen Y.J., Tan F.C.K., Ong W.Y., Go M.L., Verma C.S., Low C.M., Lam Y. A new generation of arachidonic acid analogues as potential neurological agent targeting cytosolic phospholipase A2. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13996-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 45.Six D.A., Barbayianni E., Loukas V., Constantinou-Kokotou V., Hadjipavlou-Litina D., Stephens D., Wong A.C., Magrioti V., Moutevelis-Minakakis P., Baker S.F., Dennis E.A., Kokotos G. Structure-activity relationship of 2-oxoamide inhibition of group IVA cytosolic phospholipase A2 and group V secreted phospholipase A2. J. Med. Chem. 2007;50:4222–4235. doi: 10.1021/jm0613673. [DOI] [PubMed] [Google Scholar]
- 46.Yaksh T.L., Kokotos G., Svensson C.I., Stephens D., Kokotos C.G., Fitzsimmons B., Hadjipavlou-Litina D., Hua X.Y., Dennis E.A. Systemic and intrathecal effects of a novel series of phospholipase A2 inhibitors on hyperalgesia and spinal prostaglandin E2 release. J. Pharmacol. Exp. Ther. 2006;316:466–475. doi: 10.1124/jpet.105.091686. [DOI] [PubMed] [Google Scholar]
- 47.Antonopoulou G., Magrioti V., Kokotou M.G., Nikolaou A., Barbayianni E., Mouchlis V.D., Dennis E.A., Kokotos G. 2-Oxoamide inhibitors of cytosolic group IVA phospholipase A2 with reduced lipophilicity. Bioorg. Med. Chem. 2016;24:4544–4554. doi: 10.1016/j.bmc.2016.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kokotou M.G., Galiatsatou G., Magrioti V., Koutoulogenis G., Barbayianni E., Limnios D., Mouchlis V.D., Satpathy B., Navratil A., Dennis E.A., Kokotos G. 2-Oxoesters: a novel class of potent and selective inhibitors of cytosolic group IVA phospholipase A2. Sci. Rep. 2017;7:7025. doi: 10.1038/s41598-017-07330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chuang D.Y., Simonyi A., Kotzbauer P.T., Gu Z., Sun G.Y. Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. J. Neuroinflammation. 2015;12:199. doi: 10.1186/s12974-015-0419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chuang D.Y., Simonyi A., Cui J., Lubahn D.B., Gu Z., Sun G.Y. Botanical polyphenols mitigate microglial activation and microglia-induced neurotoxicity: role of cytosolic phospholipase A2. NeuroMolecular Med. 2016;18:415–425. doi: 10.1007/s12017-016-8419-5. [DOI] [PubMed] [Google Scholar]
- 51.Lio Y.C., Dennis E.A. Interfacial activation, lysophospholipase and transacylase activity of group VI Ca2+-independent phospholipase A2. Biochim. Biophys. Acta. 1998;1392:320–332. doi: 10.1016/s0005-2760(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 52.Ackermann E.J., Kempner E.S., Dennis E.A. Ca2+-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J. Biol. Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- 53.Winstead M.V., Balsinde J., Dennis E.A. Calcium independent phospholipase A2: structure and function. Biochim. Biophys. Acta. 2000;1488:28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins C.M., Yan W., Mancuso D.J., Gross R.W. Highly selective hydrolysis of fatty acyl-CoAs by calcium-independent phospholipase A2β. Enzyme autoacylation and acyl-CoA-mediated reversal of calmodulin inhibition of phospholipase A2 activity. J. Biol. Chem. 2006;281:15615–15624. doi: 10.1074/jbc.M511623200. [DOI] [PubMed] [Google Scholar]
- 55.Larsson Forsell P.K., Kennedy B.P., Claesson H.E. The human calcium-independent phospholipase A2 gene multiple enzymes with distinct properties from a single gene. Eur. J. Biochem. 1999;262:575–585. doi: 10.1046/j.1432-1327.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 56.Malley K.R., Koroleva O., Miller I., Sanishvili R., Jenkins C.M., Gross R.W., Korolev S. The structure of iPLA2β reveals dimeric active sites and suggests mechanisms of regulation and localization. Nat. Commun. 2018;9:765. doi: 10.1038/s41467-018-03193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sali A., Blundell T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 58.Ramanadham S., Ali T., Ashley J.W., Bone R.N., Hancock W.D., Lei X. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J. Lipid Res. 2015;56:1643–1668. doi: 10.1194/jlr.R058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baskakis C., Magrioti V., Cotton N., Stephens D., Constantinou-Kokotou V., Dennis E.A., Kokotos G. Synthesis of polyfluoro ketones for selective inhibition of human phospholipase A2 enzymes. J. Med. Chem. 2008;51:8027–8037. doi: 10.1021/jm800649q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kokotos G., Hsu Y.H., Burke J.E., Baskakis C., Kokotos C.G., Magrioti V., Dennis E.A. Potent and selective fluoroketone inhibitors of group VIA calcium-independent phospholipase A2. J. Med. Chem. 2010;53:3602–3610. doi: 10.1021/jm901872v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magrioti V., Nikolaou A., Smyrniotou A., Shah I., Constantinou-Kokotou V., Dennis E.A., Kokotos G. New potent and selective polyfluoroalkyl ketone inhibitors of GVIA calcium-independent phospholipase A2. Bioorg. Med. Chem. 2013;21:5823–5829. doi: 10.1016/j.bmc.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalyvas A., Baskakis C., Magrioti V., Constantinou-Kokotou V., Stephens D., López-Vales R., Lu J.Q., Yong V.W., Dennis E.A., Kokotos G., David S. Differing roles for members of the phospholipase A2 superfamily in experimental autoimmune encephalomyelitis. Brain. 2009;132:1221–1235. doi: 10.1093/brain/awp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.López-Vales R., Navarro X., Shimizu T., Baskakis C., Kokotos G., Constantinou-Kokotou V., Stephens D., Dennis E.A., David S. Intracellular phospholipase A2 group IVA and group VIA play important roles in Wallerian degeneration and axon regeneration after peripheral nerve injury. Brain. 2008;131:2620–2631. doi: 10.1093/brain/awn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.López-Vales R., Ghasemlou N., Redensek A., Kerr B.J., Barbayianni E., Antonopoulou G., Baskakis C., Rathore K.I., Constantinou-Kokotou V., Stephens D., Shimizu T., Dennis E.A., Kokotos G., David S. Phospholipase A2 superfamily members play divergent roles after spinal cord injury. FASEB J. 2011;25:4240–4252. doi: 10.1096/fj.11-183186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ali T., Kokotos G., Magrioti V., Bone R.N., Mobley J.A., Hancock W., Ramanadham S. Characterization of FKGK18 as inhibitor of group VIA Ca2+-independent phospholipase A2 (iPLA2β): candidate drug for preventing beta-cell apoptosis and diabetes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bone R.N., Gai Y., Magrioti V., Kokotou M.G., Ali T., Lei X., Tse H.M., Kokotos G., Ramanadham S. Inhibition of Ca2+-independent phospholipase A2β (iPLA2β) ameliorates islet infiltration and incidence of diabetes in NOD mice. Diabetes. 2015;64:541–554. doi: 10.2337/db14-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abinahed R., Martinez G., Escoffier J., Yassine S., Karaouzène T., Hograindleur J.P., Turk J., Kokotos G., Ray P.F., Bottari S., Lambeau G., Hennebicq S., Arnoult C. Progesterone-induced acrosome exocytosis requires sequential involvement of calcium-independent phospholipase A2β (iPLA2β) and group X secreted phospholipase A2 (sPLA2) J. Biol. Chem. 2016;291:3076–3089. doi: 10.1074/jbc.M115.677799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gil-De-Gómez L., Astudillo A.M., Guijas C., Magrioti V., Kokotos G., Balboa M.A., Balsinde J. Cytosolic group IVA and calcium-independent group VIA phospholipase A2s act on distinct phospholipid pools in zymosan-stimulated mouse peritoneal macrophages. J. Immunol. 2014;192:752–762. doi: 10.4049/jimmunol.1302267. [DOI] [PubMed] [Google Scholar]
- 69.Mouchlis V.D., Limnios D., Kokotou M.G., Barbayianni E., Kokotos G., McCammon J.A., Dennis E.A. Development of potent and selective inhibitors for group VIA calcium-independent phospholipase A2 guided by molecular dynamics and structure-activity relationships. J. Med. Chem. 2016;59:4403–4414. doi: 10.1021/acs.jmedchem.6b00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mouchlis V.D., Morisseau C., Hammock B.D., Li S., McCammon J.A., Dennis E.A. Computer-aided drug design guided by hydrogen/deuterium exchange mass spectrometry: a powerful combination for the development of potent and selective inhibitors of group VIA calcium-independent phospholipase A2. Bioorg. Med. Chem. 2016;24:4801–4811. doi: 10.1016/j.bmc.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smyrniotou A., Kokotou M.G., Mouchlis V.D., Barbayianni E., Kokotos G., Dennis E.A., Constantinou-Kokotou V. 2-Oxoamides based on dipeptides as selective calcium-independent phospholipase A2 inhibitors. Bioorg. Med. Chem. 2017;25:926–940. doi: 10.1016/j.bmc.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ackermann E.J., Conde-Frieboes K., Dennis E.A. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J. Biol. Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- 73.Balsinde J., Bianco I.D., Ackermann E.J., Conde-Frieboes K., Dennis E.A. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in P388D1 macrophages. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8527–8531. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leis H.J., Windischhofer W. Calcium-independent phospholipases A2 in murine osteoblastic cells and their inhibition by bromoenol lactone: impact on arachidonate dynamics and prostaglandin synthesis. J. Enzyme Inhib. Med. Chem. 2016;31:1203–1213. doi: 10.3109/14756366.2015.1114929. [DOI] [PubMed] [Google Scholar]
- 75.Lambeau G., Gelb M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 76.Murakami M., Taketomi Y., Miki Y., Sato H., Yamamoto K., Lambeau G. Emerging roles of secreted phospholipase A2 enzymes: the 3rd edition. Biochimie. 2014;107Pt A:105–113. doi: 10.1016/j.biochi.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Murakami M., Sato H., Miki Y., Yamamoto K., Taketomi Y. A new era of secreted phospholipase A2. J. Lipid Res. 2015;56:1248–1261. doi: 10.1194/jlr.R058123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hui D.Y. Phospholipase A2 enzymes in metabolic and cardiovascular diseases. Curr. Opin. Lipidol. 2012;23:235–240. doi: 10.1097/MOL.0b013e328351b439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quach N.D., Arnold R.D., Cummings B.S. Secretory phospholipase A2 enzymes as pharmacological targets for treatment of disease. Biochem. Pharmacol. 2014;90:338–348. doi: 10.1016/j.bcp.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brglez V., Lambeau G., Petan T. Secreted phospholipases A2 in cancer: diverse mechanisms of action. Biochimie. 2014;107(Pt A):114–123. doi: 10.1016/j.biochi.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 81.Draheim S.E., Bach N.J., Dillard R.D., Berry D.R., Carlson D.G., Chirgadze N.Y., Clawson D.K., Hartley L.W., Johnson L.M., Jones N.D., ER McKinney, Mihelich E.D., Olkowski J.L., Schevitz R.W., Smith A.C., Snyder D.W., Sommers C.D., Wery J.P. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 3. Indole-3-glyoxamides. J. Med. Chem. 1996;39:5159–5175. doi: 10.1021/jm960487f. [DOI] [PubMed] [Google Scholar]
- 82.Abraham E., Naum C., Bandi V., Gervich D., Lowry S.F., Wunderink R., Schein R.M., Macias W., Skerjanec S., Dmitrienko A., Farid N., Forgue S.T., Jiang F. Efficacy and safety of LY315920Na/S-5920, a selective inhibitor of 14-kDa group IIA secretory phospholipase A2, in patients with suspected sepsis and organ failure. Crit. Care Med. 2003;31:718–728. doi: 10.1097/01.CCM.0000053648.42884.89. [DOI] [PubMed] [Google Scholar]
- 83.Bradley J.D., Dmitrienko A.A., Kivitz A.J., Gluck O.S., Weaver A.L., Wiesenhutter C., Myers S.L., Sides G.D. A randomized, double-blinded, placebo-controlled clinical trial of LY333013, a selective inhibitor of group II secretory phospholipase A2, in the treatment of rheumatoid arthritis. J. Rheumatol. 2005;32:417–423. [PubMed] [Google Scholar]
- 84.Nicholls S.J., Kastelein J.J., Schwartz G.G., Bash D., Rosenson R.S., Cavender M.A., Brennan D.M., Koenig W., Jukema J.W., Nambi V., Wright R.S., Menon V., Lincoff A.M., Nissen S.E., for the VISTA-16 Investigators Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- 85.Gutiérrez J.M., Calvete J.J., Habib A.G., Harrison R.A., Williams D.J., Warrell D.A. Snakebite envenoming. Nat. Rev. Dis. Primers. 2017;3 doi: 10.1038/nrdp.2017.79. [DOI] [PubMed] [Google Scholar]
- 86.Laustsen A.H., Engmark M., Milbo C., Johannesen J., Lomonte B., Gutiérrez J.M., Lohse B. From fangs to pharmacology: the future of snakebite envenoming therapy. Curr. Pharm. Des. 2016;22:5270–5293. doi: 10.2174/1381612822666160623073438. [DOI] [PubMed] [Google Scholar]
- 87.Lewin M., Samuel S., Merkel J., Bickler P. Varespladib (LY315920) appears to be a potent, broad-spectrum, inhibitor of snake venom phospholipase A2 and a possible pre-referral treatment for envenomation. Toxins. 2016;8 doi: 10.3390/toxins8090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y., Zhang J., Zhang D., Xiao H., Xiong S., Huang C. Exploration of the inhibitory potential of varespladib for snakebite envenomation. Molecules. 2018;23:391. doi: 10.3390/molecules23020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pedada S.R., Yarla N.S., Tambade P.J., Dhananjaya B.L., Bishayee A., Arunasree K.M., Philip G.H., Dharmapuri G., Aliev G., Putta S., Rangaiah G. Synthesis of new secretory phospholipase A2-inhibitory indole containing isoxazole derivatives as anti-inflammatory and anticancer agents. Eur. J. Med. Chem. 2016;112:289–297. doi: 10.1016/j.ejmech.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 90.Giordanetto F., Pettersen D., Starke I., Nordberg P., Dahlström M., Knerr L., Selmi N., Rosengren B., Larsson L.O., Sandmark J., Castaldo M., Dekker N., Karlsson U., Hurt-Camejo E. Discovery of AZD2716: a novel secreted phospholipase A2 (sPLA2) inhibitor for the treatment of coronary artery disease. ACS Med. Chem. Lett. 2016;7:884–889. doi: 10.1021/acsmedchemlett.6b00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kokotos G., Kotsovolou S., Six D.A., Constantinou-Kokotou V., Beltzner C.C., Dennis E.A. Novel 2-oxoamide inhibitors of human group IVA phospholipase A2. J. Med. Chem. 2002;45:2891–2893. doi: 10.1021/jm025538p. [DOI] [PubMed] [Google Scholar]
- 92.Stephens D., Barbayianni E., Constantinou-Kokotou V., Peristeraki A., Six D.A., Cooper J., Harkewicz R., Deems R.A., Dennis E.A., Kokotos G. Differential inhibition of group IVA and group VIA phospholipases A2 by 2-oxoamides. J. Med. Chem. 2006;49:2821–2828. doi: 10.1021/jm050993h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mouchlis V.D., Magrioti V., Barbayianni E., Cermak N., Oslund R.C., Mavromoustakos T.M., Gelb M.H., Kokotos G. Inhibition of secreted phospholipases A2 by 2-oxoamides based on α-amino acids: synthesis, in vitro evaluation and molecular docking calculations. Bioorg. Med. Chem. 2011;19:735–743. doi: 10.1016/j.bmc.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vasilakaki S., Barbayianni E., Leonis G., Papadopoulos M.G., Mavromoustakos T., Gelb M.H., Kokotos G. Development of a potent 2-oxoamide inhibitor of secreted phospholipase A2 guided by molecular docking calculations and molecular dynamics simulations. Bioorg. Med. Chem. 2016;24:1683–1695. doi: 10.1016/j.bmc.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vasilakaki S., Barbayianni E., Magrioti V., Pastukhov O., Constantinou-Kokotou V., Huwiler A., Kokotos G. Inhibitors of secreted phospholipase A2 suppress the release of PGE2 in renal mesangial cells. Bioorg. Med. Chem. 2016;24:3029–3034. doi: 10.1016/j.bmc.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 96.Alasmary F.A.S., Alnahdi F.S., Ben Bacha A., El-Araby A.M., Moubayed N., Alafeefy A.M., El-Araby M.E. New quinoxalinone inhibitors targeting secreted phospholipase A2 and α-glucosidase. J. Enzyme Inhib. Med. Chem. 2017;32:1143–1151. doi: 10.1080/14756366.2017.1363743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jameel N.M., Frey B.M., Frey F.J., Gowda T.V., Vishwanath B.S. Inhibition of secretory phospholipase A2 enzyme by bilirubin: a new role as endogenous anti-inflammatory molecule. Mol. Cell. Biochem. 2005;276:219–225. doi: 10.1007/s11010-005-4441-x. [DOI] [PubMed] [Google Scholar]
- 98.Joshi V., Umashankara M., Ramakrishnan C., Nanjaraj Urs A.N., Suvilesh K.N., Velmurugan D., Rangappa K.S., Vishwanath B.S. Dimethyl ester of bilirubin exhibits anti-inflammatory activity through inhibition of secretory phospholipase A2, lipoxygenase and cyclooxygenase. Arch. Biochem. Biophys. 2016;598:28–39. doi: 10.1016/j.abb.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 99.Joshi V., Venkatesha S.H., Ramakrishnan C., Nanjaraj Urs A.N., Hiremath V., Kamal Moudgil K.D., Velmurugan D., Vishwanath B.S. Celastrol modulates inflammation through inhibition of the catalytic activity of mediators of arachidonic acid pathway: secretory phospholipase A2 group IIA, 5-lipoxygenase and cyclooxygenase-2. Pharmacol. Res. 2016;113:265–275. doi: 10.1016/j.phrs.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 100.Yap W.H., Ahmed N., Lim Y.M. Inhibition of human group IIA-secreted phospholipase A2 and THP-1 monocyte recruitment by maslinic acid. Lipids. 2016;51:1153–1159. doi: 10.1007/s11745-016-4186-1. [DOI] [PubMed] [Google Scholar]
- 101.Lee Y., Lee W., Kim J., Bae J.S. Inhibitory effect of sulforaphane on secretory group IIA phospholipase A2. Int. J. Pharmacol. 2018;14:187–193. [Google Scholar]
- 102.Prescott S.M., Zimmerman G.A., Stafforini D.M., McIntyre T.M. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 103.Stafforini D.M. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2) Cardiovasc. Drugs Ther. 2009;23:73–83. doi: 10.1007/s10557-008-6133-8. [DOI] [PubMed] [Google Scholar]
- 104.Tjoelker L.W., Eberhardt C., Unger J., Trong H.L., Zimmerman G.A., McIntyre T.M., Stafforini D.M., Prescott S.M., Gray P.W. Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad. J. Biol. Chem. 1995;270:25481–25487. doi: 10.1074/jbc.270.43.25481. [DOI] [PubMed] [Google Scholar]
- 105.Stremler K.E., Stafforini D.M., Prescott S.M., McIntyre T.M. Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J. Biol. Chem. 1991;266:11095–11103. [PubMed] [Google Scholar]
- 106.Tjoelker L.W., Wilder C., Eberhardt C., Stafforini D.M., Dietsch G., Schimpf B., Hooper S., Le Trong H., Cousens L.S., Zimmerman G.A., Yamada Y., McIntyre T.M., Prescott S.M., Gray P.W. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature. 1995;374:549–553. doi: 10.1038/374549a0. [DOI] [PubMed] [Google Scholar]
- 107.Lavi S., Herrmann J., Lavi R., McConnell J.P., Lerman L.O., Lerman A. Role of lipoprotein-associated phospholipase A2 in atherosclerosis. Curr Atheroscler Rep. 2008;10:230–235. doi: 10.1007/s11883-008-0036-9. [DOI] [PubMed] [Google Scholar]
- 108.Donato L.J., Meeusen J.W., Callanan H., Saenger A.K., Jaffe A.S. Advantages of the lipoprotein-associated phospholipase A2 activity assay. Clin. Biochem. 2016;49:172–175. doi: 10.1016/j.clinbiochem.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 109.Oei H.-H.S., van der Meer I.M., Hofman A., Koudstaal P.J., Stijnen T., Breteler M.M.B., Witteman J.C.M. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam study. Circulation. 2005;111:570–575. doi: 10.1161/01.CIR.0000154553.12214.CD. [DOI] [PubMed] [Google Scholar]
- 110.Lavi S., McConnell J.P., Rihal C.S., Prasad A., Mathew V., Lerman L.O., Lerman A. Local production of lipoprotein-associated phospholipase A2 and lyso-phosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 111.Blackie J.A., Bloomer J.C., Brown M.J., Cheng H.Y., Hammond B., Hickey D.M., Ife R.J., Leach C.A., Lewis V.A., Macphee C.H., Milliner K.J., Moores K.E., Pinto I.L., Smith S.A., Stansfield I.G., Stanway S.J., Taylor M.A., Theobald C.J. The identification of clinical candidate SB-480848: a potent inhibitor of lipoprotein-associated phospholipase A2. Bioorg. Med. Chem. Lett. 2003;13:1067–1070. doi: 10.1016/s0960-894x(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 112.White H.D., Held C., Stewart R., Tarka E., Brown R., Davies R.Y., Budaj A., Harrington R.A., Steg P.G., Ardissino D., Armstrong P.W., Avezum A., Aylward P.E., Bryce A., Chen H., Chen M.F., Corbalan R., Dalby A.J., Danchin N., De Winter R.J., Denchev S., Diaz R., Elisaf M., Flather M.D., Goudev A.R., Granger C.B., Grinfeld L., Hochman J.S., Husted S., Kim H.S., Koenig W., Linhart A., Lonn E., López-Sendón J., Manolis A.J., Mohler E.R., 3rd, Nicolau J.C., Pais P., Parkhomenko A., Pedersen T.R., Pella D., Ramos-Corrales M.A., Ruda M., Sereg M., Siddique S., Sinnaeve P., Smith P., Sritara P., Swart H.P., Sy R.G., Teramoto T., Tse H.F., Watson D., Weaver W.D., Weiss R., Viigimaa M., Vinereanu D., Zhu J., Cannon C.P., Wallentin L., STABILITY Investigators Darapladib for preventing ischemic events in stable coronary heart disease. N. Engl. J. Med. 2014;370:1702–1711. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 113.O'Donoghue M.L., Braunwald E., White H.D., Lukas M.A., Tarka E., Steg P.G., Hochman J.S., Bode C., Maggioni A.P., Im K., Shannon J.B., Davies R.Y., Murphy S.A., Crugnale S.E., Wiviott S.D., Bonaca M.P., Watson D.F., Weaver W.D., Serruys P.W., Cannon C.P., SOLID-TIMI 52 Investigators, Steen D.L. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312:1006–1015. doi: 10.1001/jama.2014.11061. [DOI] [PubMed] [Google Scholar]
- 114.Zhang J., Xu D.L., Liu X.B., Bi S.J., Zhao T., Sui S.J., Ji X.P., Lu Q.H. Darapladib, a lipoprotein-associated phospholipase A2 inhibitor, reduces Rho kinase activity in atherosclerosis. Yonsei Med. J. 2016;57:321–327. doi: 10.3349/ymj.2016.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heriansyah T., Wihastuti T.A., Bambang B.S., Anwar S., Renan S., Djanggan S., Imam S., Aulanni'am Nurjati C.S., Saptawati B. Lowering inflammation level by Lp-PLA2 inhibitor (darapladib) in early atherosclerosis development: in vivo rat type 2 diabetes mellitus model. J. Cardiovasc. Disease Res. 2017;8:50–55. [Google Scholar]
- 116.Heriansyah T., Hanifa H., Andarini S., Wihastuti T.A. Darapladib inhibit adhesion molecule expression in aorta at early stages of atherosclerosis using Sprague-Dawley type 2 diabetes mellitus model. Asian J. Pharm. Clin. Res. 2017;10:373–376. [Google Scholar]
- 117.Wihastuti T.A., Andiyani D.Z.P., Andarini S., Heriansyah T. Lp-PLA2 selective inhibitor (darapladib) effect in lowering insulin resistance and aortic tissue inflammation at type 2 diabetes mellitus. J. Appl. Pharm. Sci. 2017;7:110–115. [Google Scholar]
- 118.Acharya N.K., Qi X., Goldwaser E.L., Godsey G.A., Wu H., Kosciuk M.C., Freeman T.A., Macphee C.H., Wilensky R.L., Venkataraman V., Nagele R.G. Retinal pathology is associated with increased blood-retina barrier permeability in a diabetic and hypercholesterolaemic pig model: beneficial effects of the LpPLA2 inhibitor Darapladib. Diab. Vasc. Dis. Res. 2017;14:200–213. doi: 10.1177/1479164116683149. [DOI] [PubMed] [Google Scholar]
- 119.Chen X., Wang K., Xu W., Ma Q., Chen M., Du L., Mo M., Wang Y., Shen J. Discovery of potent and orally active lipoprotein associated phospholipase A2 (Lp-PLA2) inhibitors as a potential therapy for diabetic macular edema. J. Med. Chem. 2016;59:2674–2687. doi: 10.1021/acs.jmedchem.5b01930. [DOI] [PubMed] [Google Scholar]
- 120.Liu Q., Chen X., Chen W., Yuan X., Su H., Shen J., Xu Y. Structural and thermodynamic characterization of protein-ligand interactions formed between lipoprotein-associated phospholipase A2 and inhibitors. J. Med. Chem. 2016;59:5115–5120. doi: 10.1021/acs.jmedchem.6b00282. [DOI] [PubMed] [Google Scholar]
- 121.http://clinicaltrials.gov (Identifier NCT01428453)
- 122.Maher-Edwards G., De'Ath J., Barnett C., Lavrov A., Lockhart A. A 24-week study to evaluate the effect of rilapladib on cognition and CSF biomarkers of Alzheimer's disease. Alzheimers Dementia Transl. Res. Clin. Interv. 2015;1:131–140. doi: 10.1016/j.trci.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.http://clinicaltrials.gov (Identifier NCT01702467)
- 124.Wu K., Xu J., Fong R., Yao X., Xu Y., Guiney W., Gray F., Lockhart A. Evaluation of the safety, pharmacokinetics, pharmacodynamics, and drug-drug interaction potential of a selective Lp-PLA2 inhibitor (GSK2647544) in healthy volunteers. Int. J. Clin. Pharmacol. Ther. 2016;54:935–949. doi: 10.5414/CP202565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.http://clinicaltrials.gov (Identifier NCT01924858)
- 126.Huiban M., Coello C., Wu K., Xu Y., Lewis Y., Brown A.P., Buraglio M., Guan C., Shabbir S., Fong R., Passchier J., Rabiner E.A., Lockhart A. Investigation of the brain biodistribution of the lipoprotein-associated phospholipase A2 (Lp-PLA2) inhibitor [18F]GSK2647544 in healthy male subjects. Mol. Imaging Biol. 2017;19:153–161. doi: 10.1007/s11307-016-0982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Blackie J.A., Bloomer J.C., Brown M.J., Cheng H.Y., Elliott R.L., Hammond B., Hickey D.M., Ife R.J., Leach C.A., Lewis V.A., Macphee C.H., Milliner K.J., Moores K.E., Pinto I.L., Smith S.A., Stansfield I.G., Stanway S.J., Taylor M.A., Theobald C.J., Whittaker C.M. The discovery of SB-435495: a potent, orally active inhibitor of lipoprotein-associated phospholipase A2 for evaluation in man. Bioorg. Med. Chem. Lett. 2002;12:2603–2606. doi: 10.1016/s0960-894x(02)00473-0. [DOI] [PubMed] [Google Scholar]
- 128.Canning P., Kenny B.A., Prise V., Glenn J., Sarker M.H., Hudson N., Brandt M., Lopez F.J., Gale D., Luthert P.J., Adamson P., Turowski P., Stitt A.W. Lipoprotein-associated phospholipase A2 (Lp-PLA2) as a therapeutic target to prevent retinal vasopermeability during diabetes. Proc. Natl. Acad. Sci. U. S. A. 2016;113:7213–7218. doi: 10.1073/pnas.1514213113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Q., Huang F., Yuan X., Wang K., Zou Y., Shen J., Xu Y. Structure-guided discovery of novel, potent, and orally bioavailable inhibitors of lipoprotein-associated phospholipase A2. J. Med. Chem. 2017;60:10231–10244. doi: 10.1021/acs.jmedchem.7b01530. [DOI] [PubMed] [Google Scholar]
- 130.Woolford A.J., Pero J.E., Aravapalli S., Berdini V., Coyle J.E., Day P.J., Dodson A.M., Grondin P., Holding F.P., Lee L.Y., Li P., Manas E.S., Marino J., Jr., Martin A.C., McCleland B.W., McMenamin R.L., Murray C.W., Neipp C.E., Page L.W., Patel V.K., Potvain F., Rich S., Rivero R.A., Smith K., Somers D.O., Trottet L., Velagaleti R., Williams G., Xie R. Exploitation of a novel binding pocket in human lipoprotein-associated phospholipase A2 (Lp-PLA2) discovered through X-ray fragment screening. J. Med. Chem. 2016;59:5356–5367. doi: 10.1021/acs.jmedchem.6b00212. [DOI] [PubMed] [Google Scholar]
- 131.Woolford A.J., Day P.J., Bénéton V., Berdini V., Coyle J.E., Dudit Y., Grondin P., Huet P., Lee L.Y., Manas E.S., McMenamin R.L., Murray C.W., Page L.W., Patel V.K., Potvain F., Rich S.J., Sang Y., Somers D.O., Trottet L., Wan Z., Zhang X. Fragment-based approach to the development of an orally bioavailable lactam inhibitor of lipoprotein-associated phospholipase A2 (Lp-PLA2) J. Med. Chem. 2016;59:10738–10749. doi: 10.1021/acs.jmedchem.6b01427. [DOI] [PubMed] [Google Scholar]
- 132.Mouchlis V.D., Barbayianni E., Mavromoustakos T.M., Kokotos G. The application of rational design on phospholipase A2 inhibitors. Curr. Med. Chem. 2011;18:2566–2582. doi: 10.2174/092986711795933678. [DOI] [PubMed] [Google Scholar]
- 133.Hsu Y.H., Bucher D., Cao J., Li S., Yang S.W., Kokotos G., Woods V.L., Jr., McCammon J.A., Dennis E.A. Fluoroketone inhibition of Ca2+-independent phospholipase A2 through binding pocket association defined by hydrogen/deuterium exchange and molecular dynamics. J. Am. Chem. Soc. 2013;135:1330–1337. doi: 10.1021/ja306490g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vasquez A.M., Mouchlis V.D., Dennis E.A. Review of four major distinct types of human phospholipase A2. Adv. Biol. Regul. 2018;67:212–218. doi: 10.1016/j.jbior.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mouchlis V.D., Bucher D., McCammon J.A., Dennis E.A. Membranes serve as allosteric activators of phospholipase A2, enabling it to extract, bind, and hydrolyze phospholipid substrates. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E516–E525. doi: 10.1073/pnas.1424651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mouchlis V.D., Chen Y., McCammon J.A., Dennis E.A. Membrane allostery and unique hydrophobic sites promote enzyme substrate specificity. J. Am. Chem. Soc. 2018;140:3285–3291. doi: 10.1021/jacs.7b12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Phillips J., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R., Kalé L., Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mouchlis V.D., Mavromoustakos T.M., Kokotos G. Design of new secreted phospholipase A2 inhibitors based on docking calculations by modifying the pharmacophore segments of the FPL67047XX inhibitor. J. Comput. Aided Mol. Des. 2010;24:107–115. doi: 10.1007/s10822-010-9319-7. [DOI] [PubMed] [Google Scholar]
- 139.Mouchlis V.D., Mavromoustakos T.M., Kokotos G. Molecular docking and 3D-QSAR CoMFA studies on indole inhibitors of GIIA secreted phospholipase A2. J. Chem. Inf. Model. 2010;50:1589–1601. doi: 10.1021/ci100217k. [DOI] [PubMed] [Google Scholar]
- 140.Vasilakaki S., Pastukhov O., Mavromoustakos T., Huwiler A., Kokotos G. Small peptides able to suppress prostaglandin E2 generation in renal mesangial cells. Molecules. 2018;23:158. doi: 10.3390/molecules23010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mouchlis V.D., Michopoulou V., Constantinou-Kokotou V., Mavromoustakos T., Dennis E.A., Kokotos G. Binding conformation of 2-oxoamide inhibitors to group IVA cytosolic phospholipase A2 determined by molecular docking combined with molecular dynamics. J. Chem. Inf. Model. 2012;52:243–254. doi: 10.1021/ci2005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reynolds L.J., Washburn W.N., Deems R.A., Dennis E.A. Assay strategies and methods for phospholipases. Methods Enzymol. 1991;197:3–23. doi: 10.1016/0076-6879(91)97129-m. [DOI] [PubMed] [Google Scholar]
- 143.Yang H.C., Mosior M., Johnson C.A., Chen Y., Dennis E.A. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal. Biochem. 1999;269:278–288. doi: 10.1006/abio.1999.4053. [DOI] [PubMed] [Google Scholar]
- 144.Barbour S.E., Ramanadham S. Analyses of calcium-independent phospholipase A2beta (iPLA2β) in biological systems. Methods Enzymol. 2017;583:119–141. doi: 10.1016/bs.mie.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ewing H., Fernández-Vega V., Spicer T.P., Chase P., Brown S., Scampavia L., Roush W.R., Riley S., Rosen H., Hodder P., Lambeau G., Gelb M.H. Fluorometric high-throughput screening assay for secreted phospholipases A2 using phospholipid vesicles. J. Biomol. Screen. 2016;21:713–721. doi: 10.1177/1087057116646742. [DOI] [PubMed] [Google Scholar]
- 146.Kosaka T., Yamaguchi M., Soda Y., Kishimoto T., Tago A., Toyosato M., Mizuno K. Spectrophotometric assay for serum platelet-activating factor acetylhydrolase activity. Clin. Chim. Acta. 2000;296:151–161. doi: 10.1016/s0009-8981(00)00216-3. [DOI] [PubMed] [Google Scholar]
- 147.Yamaura S., Sakasegawa S.I., Koguma E., Ueda S., Kayamori Y., Sugimori D., Karasawa K. Novel enzymatic method for assaying Lp-PLA2 in serum. Clin. Chim. Acta. 2018;481:184–188. doi: 10.1016/j.cca.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 148.http://clinicaltrials.gov (Identifier: NCT00743925)
- 149.http://clinicaltrials.gov (Identifier: NCT01130246)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.