Abstract
Infectious bronchitis virus (IBV), the causative agent of infectious bronchitis, results in respiratory disease, nephritis, and poor egg production and quality in chicken. Antimicrobial peptides possess potent antimicrobial activities and are regarded as promising therapeutic alternatives in the fight against microorganisms. To assess the in vitro antiviral activity of swine intestine antimicrobial peptides (SIAMP) against IBV, 45 chick embryos were randomly assigned into 3 groups, 15 for each group. Embryos in group 1 were inoculated with IBV. Group 2 was inoculated with SIAMP-IBV intermixture. Group 3 was used as a control and inoculated with sterile PBS. Allantoic fluid was collected for hemagglutination titer assay. In addition, weight gain, mortality, and pathological changes for each group were recorded. The results showed that no distinct pathological changes were found in chick embryos after they were inoculated with SIAMP-IBV intermixture and the mortality was reduced remarkably compared with the IBV-infected group. Weight gain of embryos in the SIAMP-IBV intermixture group was significantly higher than the IBV-infected embryos (P < 0.01), which was also higher than the control group (P < 0.05). Furthermore, the hemagglutination titer in the SIAMP-IBV group was significantly lower than that in the IBV-infected group (P < 0.01). These results indicated that SIAMP can inhibit virus replication and reduce tissue injury caused by IBV.
Key words: swine intestine peptide, infectious bronchitis virus, chick embryo, antiviral activity
INTRODUCTION
Antimicrobial peptides are an abundant and diverse group of molecules that are produced by many tissues and cell types in a variety of invertebrate, plant, and animal species. Antimicrobial peptides possess potent antimicrobial activities and are regarded as promising therapeutic alternatives in the fight against resistant microorganisms. In addition to their role as endogenous antibiotics, antimicrobial peptides have functions in innate immune response and regulation of the adaptive immune system (Hancock and Chapple, 1999; Wilson et al., 1999; Zasloff, 2002; Ganz, 2003).
Recent studies revealed that some antimicrobial peptides are also involved in host defense against viral infection. It has been demonstrated that melittin, which is discovered in honey bee venom, can inhibit tobacco mosaic virus (Marcos et al., 1995), herpes simplex virus (HSV; Baghian et al., 1997), and human immunodeficiency virus 1 (HIV-1; Wachinger et al., 1998) replication. Cecropins can contribute to an inhibition of HIV-1 (Wachinger et al., 1998). Some of the magainins also showed significant reduction of HSV plaque-forming units. Moreover, it was reported that dermaseptin S4, which was isolated from the skin of rhacophorus, can inhibit cell-free and cell-associated HIV-1 infection of P4-CCR5 indicator cells and human primary T lymphocytes (Lorin et al., 2005). Indolicidin, which was isolated from bovine neutrophil, was shown to reduce virus infectivity remarkably, including HSV-1, HSV-2, and Arenavirus Junin virus (Albiol Matanic and Castilla, 2004). Recently, some studies confirmed that human α-defensin 1, 2, and 3 can contribute to anti-HIV-1 activity after viral entry (Zhang et al., 2002; Chang et al., 2003). More recently, we isolated a swine intestine antimicrobial peptide (SIAMP) from the small intestines of pig and also we have evaluated its antibacterial activity (Wang et al., 2009).
Infectious bronchitis virus (IBV), the causative agent of infectious bronchitis, has been effectively controlled by the extensive use of vaccines but it still remains a major economic problem since it was first reported in 1931. The constant emergence of variant strains has challenged vaccination strategies (Raj and Jones, 1997). Antimicrobial peptides are providing templates for the design and development of both antiviral peptides and peptides that selectively modulate innate immunity to increase protection against IBV infections. A previous report confirmed that SIAMP has antibacterial activity (Wang et al., 2009), and the present study was conducted to evaluate the antiviral activity of SIAMP on IBV.
MATERIALS AND METHODS
Preparation of SIAMP
Swine intestine antimicrobial peptides were isolated from swine small intestines as described previously (Ghosh et al., 2002; Wang et al., 2009). The peptides were extracted by 5% acetic acid extracting and were then purified by Sephadex G-100 and Sephadex G-25 gel (Sigma Ltd., Beijing, China) filtration chromatography. Peptide purity was assessed by tricine SDS-PAGE (Evans et al., 1994). Protein concentration was determined by spectrophotometric absorbance at 280 nm. The absorbance was converted to protein concentration (μg/mL) according to a published procedure (Stoscheck, 1990). From 49 to 58 mg of CP, 200 to 250 μg of purified peptides was obtained. The SIAMP extracts were diluted by PBS to 100 μg/mL (SIAMP). The peptide was adjusted to pH 7.0 with sodium hydroxide before it was used in the chick embryos.
Virus
The M41 strain of IBV was obtained from the China Institute of Veterinary Drug Control (Beijing). The virus was propagated in 9-d-old embryonated chicken eggs, After 48 h of incubation at 37.5°C, the allantoic fluid was harvested and reinoculated to a new embryo. This procedure was repeated 6 times. Then the allantoic fluid was diluted by sterilized saline to 200 half-maximal lethal dose/mL of chick embryo. To determine the antiviral activity of SIAMP on IBV in vivo, IBV were incubated with SIAMP extracts for 2 h at 37°C in the SIAMP-pretreated group then inoculated in 9-d-old chick embryos to evaluate the effect of SIAMP on the weight gain, mortality, pathological changes, and the hemagglutination titer.
Birds and Experimental Design
Embryos (n = 45; 9 d old) were randomly separated into 3 different groups of 15 embryos each (Merial Co. Ltd., Beijing, China). These chick embryos were negative for IBV. They were inoculated by the chorioallantoic-sac route with 0.2 mL of 200 half-maximal lethal dose IBV M41 strain (IBV inoculation group), 0.2 mL of sterile PBS (control group), or 0.2 mL of SIAMP extracts-treated IBV M41 strain (0.1 mL of the IBV allantoic fluid was treated with 0.1 mL of activated SIAMP at 37°C for 2 h before inoculation) for each embryo. All chick embryos were incubated in incubator at 37°C and 60% RH until the embryos reached stage 18. This study was approved by the Institutional Animal Care and Use Committee of China Agricultural University.
Effect of SIAMP on Weight Gain, Mortality, and Pathological Changes of Chick Embryos
After 9 d of incubation, all embryos were examined every 12 h. The dead embryos were harvested and pathological changes were recorded. After the allantoic fluid was collected, all of the embryos were weighed. Proventriculus, liver, and brain samples were obtained from all embryos and were then immersed in 10% buffered formalin, pH 7.4. Once fixed, samples were embedded in paraffin following the conventional procedure and were cut with a microtome to obtain 4-μm sections, which were stained with hematoxylin-eosin. The histological changes were observed under an Olympus BH-2 microscope (Olympus Optical Co. Ltd., Beijing, China).
Effect of SIAMP on Replication of IBV
The method used to prepare hemagglutinating virus was conducted as described previously (Bingham et al., 1975). The allantoic fluid was harvested from eggs at the onset of embryo death. The virus in the allantoic fluid concentrated 100-fold by pelleting at 30,000 × g for 45 min and resuspending in 0.01 M Tris-HCl buffer at pH 6.4. An equal volume of phospholipase C type 1 (Sigma Ltd.) containing 1 unit of enzyme per milliliter in phosphate buffer at pH 6.4 was added to the virus suspension and the mixture was incubated for 30 min at 37°C. Hemagglutination titrations were done at 4°C using the microtiter system as described by Bingham et al. (1975).
The hemagglutination titer was defined as the reciprocal of the highest dilution of the virus that caused complete agglutination with an equal volume of appropriately diluted red blood cells (Cotter and Van Eerden, 2006; Dalloul et al., 2006).
Statistical Analysis
Experimental data were analyzed by SPSS 13.0 (SPSS Inc., Chicago, IL) statistical program. The results were expressed as means and SE. Differences were considered significant at P < 0.05 or P < 0.01.
RESULTS
Macroscopic Lesions
At 36 h after inoculation, the embryos of group A began to die, whereas the embryos in group B and C were normal. At 18 d of age, the yolk sac of the embryos in group A was contracted. The allantoic fluid increased and the amniotic membrane was thickened and adhered to the embryo proper. Some embryos were observed runting, curling, and misshaping, with compression of feet overhead. No evident macroscopic lesions were recorded in groups B and C (Figure 1 ).
Figure 1.
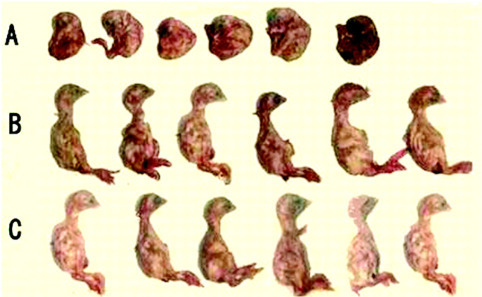
Nine-day-old embryos inoculated in the allantoic cavity with (A) infectious bronchitis virus (IBV), (B) swine intestine antimicrobial peptide-pretreated IBV, and (C) sterile PBS. Color version available in the online PDF.
Fatality Rate of Embryos
As shown in Table 1 , 14 out of 15 chick embryos died after inoculation of IBV. Pretreated with SIAMP, the IBV caused 2 of all 15 chick embryo deaths. In the control group, no embryo was found dead. The death time was focused on 36 to 60 h postinoculation. The fatality rate in the IBV inoculation group was 93.3% and was 13.3% in the SIAMP-pretreated group.
Table 1.
Effect of swine intestine antimicrobial peptides (SIAMP) on fatality rate of chick embryos
| Group1 | Time postinoculation |
Total | Mortality (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | 60 h | 72 h | 80 h | |||
| IBV inoculation group | 0 | 0 | 1 | 11 | 2 | 0 | 0 | 14/15 | 93.3 |
| SIAMP-pretreated group | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2/15 | 13.3 |
| Control group | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0/15 | 0 |
IBV = infectious bronchitis virus.
Embryo Weight Gain
The data in Table 2 show that embryo weight gain in the SIAMP-pretreated group was significantly higher than that in the IBV group (P < 0.01). Compared with the control group, the embryo weight was increased in the SIAMP-pretreated group (P < 0.05).
Table 2.
Effect of swine intestine antimicrobial peptides (SIAMP) on chick embryo weight gain
| Group1 | Embryo weight (g) |
|---|---|
| IBV inoculation group | 13.26 ± 2.64a–c, A,B |
| SIAMP-pretreated group | 19.44 ± 2.95a–c, A,B |
| Control group | 17.20 ± 2.28a–c, A,B |
Means with different superscripts are significantly different at the 0.05 level.
Means with different superscripts are significantly different at the 0.01 level.
IBV = infectious bronchitis virus.
Hemagglutination Titer
The data in Table 3 show that the IBV can be detected in each embryo of the IBV-infected group. The coverage of agglutination titer was present from 25 to 28; the average was 26.5. In all 15 embryos of the SIAMP-pretreated group, 2 embryos were detected with IBV (the 2 embryos in the SIAMP group were the same 2 embryos that died) and the average was 20.7. The agglutination titer in every embryo in control group was 0.
Table 3.
Agglutination titer of chick embryo allantoic fluids
| Group1 | Agglutination titer | Average |
|---|---|---|
| IBV inoculation group | 27, 26, 27, 25, 28, 26, 27, 25, 27, 27, 25, 27, 28, 27, 26 | 26.5 |
| SIAMP-pretreated group | 26, 25, 20, 20, 20, 20, 20, 20, 20, 20, 20, 20, 20, 20, 20 | 20.7 |
| Control group | 20, 20, 20, 20, 20, 20, 20, 20, 20, 20, 20, 20, 20, 20, 20 | 20 |
IBV = infectious bronchitis virus; SIAMP = swine intestine antimicrobial peptides.
Histological Changes
Tissue edema was observed in the proventriculus of IBV-infected chick embryos. The gland base at Figure 2 , panel A1, showed complete denudation of overlying epithelium with only necrotic debris remaining. In addition, the gland papillary branching was lower compared with the SIAMP-pretreated group and the control. In contrast, there was no obvious pathological changes present in the SIAMP-pretreated group and the control (arrows in proventriculus; Figure 2).
Figure 2.
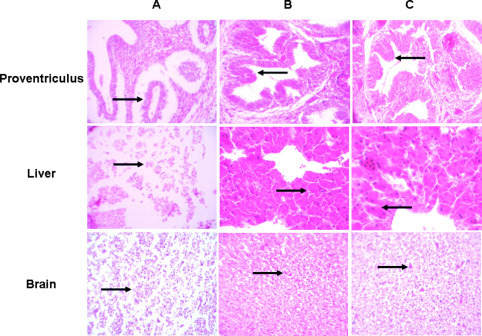
Pathological changes of proventriculus, liver, and brain in an infectious bronchitis virus-infected group (A), swine intestine antimicrobial peptide-pretreated group (B), and control group (C). 200× magnification. Color version available in the online PDF.
After IBV inoculation, necrosis of liver cells was found in hepatic tissue. The structure of bile canaliculus was difficult to identify. There was no evident difference between the control and the SIAMP-treated group (arrows in liver; Figure 2).
The dead embryos due to IBV infection, edema, and necrosis were observed in brain tissue. Also, the number of glial cells decreased. Nerve cells were scarcely observed under a microscope. In the SIAMP-treated group and the control, the brain tissue was relatively normal and nerve cells were observed (arrows in brain; Figure 2).
DISCUSSION
Antimicrobial peptides are widespread in living organisms and constitute an important component of innate immunity to microbial infections. By the early 1980s, more than 800 different antimicrobial peptides had been isolated from mammals, amphibians, fish, insects, plants, and bacterial species (Kamysz, 2005). They are widely regarded as a potential source of future antibiotics owing to a remarkable set of advantageous properties ranging from molecular simplicity to low-resistance swift kill of a broad range of microbial cells(Kamysz, 2005; Agerberth and Gudmundsson, 2006; Hancock et al., 2006; Jenssen et al., 2006).
Previous studies have demonstrated that SIAMP have antibacterial activities. In 1989, Lee and coworkers first reported the isolation and purification of SIAMP cecropin P1. Its molecular weight is 3,339 Da and it is highly sensitive to Escherichia coli, Salmonella, Actinomycete, and pyesis Streptococcus (Lee et al., 1989). Subsequently, another antimicrobial peptide, PR-39, characterized a 31-residue antibacterial peptide with activity against E. coli K12 and several other gram-negative bacteria and was isolated from swine intestine (Agerberth et al., 1991). Recently, a 78-residue antimicrobial, NK-lysin, was purified from pig small intestine; NK-lysin showed high antibacterial activity against E. coli and Bacillus megaterium and moderate activity against Acinetobacter calcoaceticus and Streptococcus pyogenes (Andersson et al., 1995). More recently, we have isolated a novel SIAMP and demonstrated that the molecular weight of this peptide was 5,972 Da (Wang et al., 2009). To our knowledge, little work has been conducted on the antiviral activity of SIAMP. Thus, the present study was designed to investigate the effect of SIAMP on the anti-IBV infection of SPF chick embryos.
The avian coronavirus IBV is a major economic pathogen of domestic poultry that, despite vaccination, causes mortality and significant losses in production (Harrison et al., 2007). Our results confirmed that 36 h postinoculation, the embryo began to die. The dead embryos were observed runting, curling, and misshaping, with compression of feet overhead. This is consistent with a previous report about the pathological changes caused by IBV (Escorcia et al., 2002). Interestingly, we found that the embryos in the SIAMP-treated group were normal, and no evident difference was present compared with the control. Furthermore, the mortality induced by IBV was reduced remarkably after embryos were pretreated with SIAMP. These findings suggested that when embryos were pretreated with SIAMP, the infectivity of IBV was reduced. This may be explained by the fact that preincubation with peptides may prevent viral fusion to target cells and reduce the IBV infectivity. However, the underlying mechanism of the antiviral activity of SIAMP remains unclear.
Histological examination further proved that SIAMP can reduce histopathological changes induced by IBV infection. The virus is primarily epitheliotropic and enters the epithelial cells by viropexis (Patterson and Bingham, 1976). The mechanism behind this may be that SIAMP can interact with IBV and block the binding of IBV to epithelial cells of embryos. Therefore, the virus cannot replicate in host cells. This may be the reason why there was no evident difference between the SIAMP-pretreated and the control group embryos. More interestingly, the embryo weight in the SIAMP-treated group was significantly higher than the IBV group or the control group. It is possible that SIAMP may contribute to the growth of the embryos as reported previously (Liu et al., 2008; Bao et al., 2009; Wang et al., 2009).
To further investigate the effect of SIAMP on the inhibition of IBV, we measured the agglutination titer of allantoic fluid of the chick embryos. Incubated with the enzyme phospholipase C, IBV has the ability to agglutinate red cells (Bingham et al., 1975). The agglutination titer can reflect the content of the virus. The data showed that when embryos are preincubated with SIAMP, the average agglutination titer was decreased significantly compared with the infected group. It is possible that SIAMP inhibited viral replication by binding to virus hemagglutinin, thereby preventing attachment of virus to red blood cells.
In conclusion, SIAMP has an effect on inhibiting virus replication and reducing pathological changes induced by viral infection. Further research is required to reveal the antiviral mechanism of SIAMP.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant no. 30471301).
REFERENCES
- Agerberth B., Gudmundsson G.H. Current Topics in Microbiology and Immunology. Springer Berlin; Heidelberg, Germany: 2006. Host antimicrobial defence peptides in human disease; pp. 67–90. [DOI] [PubMed] [Google Scholar]
- Agerberth B., Lee J.Y., Bergman T., Carlquist M., Boman H.G., Mutt V., Jornvall H. Amino acid sequence of PR-39. Isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur. J. Biochem. 1991;202:849–854. doi: 10.1111/j.1432-1033.1991.tb16442.x. [DOI] [PubMed] [Google Scholar]
- Albiol Matanic V.C., Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents. 2004;23:382–389. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- Andersson M., Gunne H., Agerberth B., Boman A., Bergman T., Sillard R., Jornvall H., Mutt V., Olsson B., Wigzell H., Dagerlind A., Boman H.G., Gudmundsson G.H. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J. 1995;14:1615–1625. doi: 10.1002/j.1460-2075.1995.tb07150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghian A., Jaynes J., Enright F., Kousoulas K.G. An amphipathic α-helical synthetic peptide analogue of melittin inhibits herpes simplex virus-1 (HSV-1)-induced cell fusion and virus spread. Peptides. 1997;18:177–183. doi: 10.1016/s0196-9781(96)00290-2. [DOI] [PubMed] [Google Scholar]
- Bao H., She R., Liu T., Zhang Y., Peng K.S., Luo D., Yue Z., Ding Y., Hu Y., Liu W., Zhai L. Effects of pig antibacterial peptides on growth performance and intestine mucosal immune of broiler chickens. Poult. Sci. 2009;88:291–297. doi: 10.3382/ps.2008-00330. [DOI] [PubMed] [Google Scholar]
- Bingham R.W., Madge M.H., Tyrrell D.A.J. Haemagglutination by avian infectious bronchitis virus: A coronavirus. J. Gen. Virol. 1975;28:381–390. doi: 10.1099/0022-1317-28-3-381. [DOI] [PubMed] [Google Scholar]
- Chang T.L.-Y., Francois F., Mosoian A., Klotman M.E. CAF-mediated human immunodeficiency virus (HIV) type 1 transcriptional inhibition is distinct from α-defensin-1 HIV inhibition. J. Virol. 2003;77:6777–6784. doi: 10.1128/JVI.77.12.6777-6784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P.F., Van Eerden E. Natural anti-Gal and Salmonella-specific antibodies in bile and plasma of hens differing in diet efficiency. Poult. Sci. 2006;85:435–440. doi: 10.1093/ps/85.3.435. [DOI] [PubMed] [Google Scholar]
- Dalloul R.A., Lillehoj H.S., Lee J.S., Lee S.H., Chung K.S. Immunopotentiating effect of a Fomitella fraxinea-derived lectin on chicken immunity and resistance to coccidiosis. Poult. Sci. 2006;85:446–451. doi: 10.1093/ps/85.3.446. [DOI] [PubMed] [Google Scholar]
- Escorcia M., Fortoul T.I., Petrone V.M., Galindo F., López C., Téllez G. Gastric gross and microscopic lesions caused by the UNAM-97 variant strain of infectious bronchitis virus after the eighth passage in specific pathogen-free chicken embryos. Poult. Sci. 2002;81:1647–1652. doi: 10.1093/ps/81.11.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E.W., Beach G.G., Wunderlich J., Harmon B.G. Isolation of antimicrobial peptides from avian heterophils. J. Leukoc. Biol. 1994;56:661–665. doi: 10.1002/jlb.56.5.661. [DOI] [PubMed] [Google Scholar]
- Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Porter E., Shen B., Lee S.K., Wilk D., Drazba J., Yadav S.P., Crabb J.W., Ganz T., Bevins C.L. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat. Immunol. 2002;3:583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- Hancock R.E.W., Brown K.L., Mookherjee N. Host defence peptides from invertebrates—Emerging antimicrobial strategies. Immunobiology. 2006;211:315–322. doi: 10.1016/j.imbio.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Hancock R.E.W., Chapple D.S. Peptide antibiotics. Antimicrob. Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S.M., Tarpey I., Rothwell L., Kaiser P., Hiscox J.A. Lithium chloride inhibits the coronavirus infectious bronchitis virus in cell culture. Avian Pathol. 2007;36:109–114. doi: 10.1080/03079450601156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen H., Hamill P., Hancock R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamysz W. Are antimicrobial peptides an alternative for conventional antibiotics? Nucl. Med. Rev. Cent. East. Eur. 2005;8:78–86. [PubMed] [Google Scholar]
- Lee J.Y., Boman A., Chuanxin S., Andersson M., Jornvall H., Mutt V., Boman H.G. Antibacterial peptides from pig intestine: Isolation of a mammalian cecropin. Proc. Natl. Acad. Sci. USA. 1989;86:9159–9162. doi: 10.1073/pnas.86.23.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., She R., Wang K., Bao H., Zhang Y., Luo D., Hu Y., Ding Y., Wang D., Peng K. Effects of rabbit sacculus rotundus antimicrobial peptides on the intestinal mucosal immunity in chickens. Poult. Sci. 2008;87:250–254. doi: 10.3382/ps.2007-00353. [DOI] [PubMed] [Google Scholar]
- Lorin C., Saidi H., Belaid A., Zairi A., Baleux F., Hocini H., Bélec L., Hani K., Tangy F. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology. 2005;334:264–275. doi: 10.1016/j.virol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Marcos J.F., Beachy R.N., Houghten R.A., Blondelle S.E., Pérez-Payá E. Inhibition of a plant virus infection by analogs of melittin. Proc. Natl. Acad. Sci. USA. 1995;92:12466–12469. doi: 10.1073/pnas.92.26.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S., Bingham R.W. Electron microscope observations on the entry of avian infectious bronchitis virus into susceptible cells. Arch. Virol. 1976;52:191–200. doi: 10.1007/BF01348016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj G.D., Jones R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoscheck C.M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- Wachinger M., Kleinschmidt A., Winder D., Von Pechmann N., Ludvigsen A., Neumann M., Holle R., Salmons B., Erfle V., Brack-Werner R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 1998;79:731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- Wang D., Ma W., She R., Sun Q., Liu Y., Hu Y., Liu L., Yang Y., Peng K. Effects of swine gut antimicrobial peptides on the intestinal mucosal immunity in specific-pathogen-free chickens. Poult. Sci. 2009;88:967–974. doi: 10.3382/ps.2008-00533. [DOI] [PubMed] [Google Scholar]
- Wilson C.L., Ouellette A.J., Satchell D.P., Ayabe T., Ópez-Boado Y.S.L., Stratman J.L., Hultgren S.J., Matrisian L.M., Parks W.C. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yu W., He T., Yu J., Caffrey R.E., Dalmasso E.A., Fu S., Pham T., Mei J., Ho J.J., Zhang W., Lopez P., Ho D.D. Contribution of human α-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]


