Abstract
Objective:
Increasing physical activity (PA) is regularly cited as a modifiable target to improve health outcomes and quality of life in the aging population, especially postmenopausal women who exhibit low bone mineral density (BMD) and high fracture risk. In this cross-sectional study, we aimed to quantify real-world PA and its association with BMD in postmenopausal women.
Methods:
Seventy postmenopausal women, aged 46 to 79 years, received a dual-energy X-ray absorptiometry scan measuring total hip BMD and wore bilateral triaxial accelerometers on the ankles for 7 days to measure PA in their free-living environment. Custom step detection and peak vertical ground reaction force estimation algorithms, sensitive to both quantity and intensity of PA, were used to calculate a daily bone density index (BDI) for each participant. Multiple regression was used to quantify the relationship between total hip BMD, age, step counts, and mean BDI over the span of 7 days of data collection.
Results:
All participants completed the full 7 days of PA monitoring, totaling more than 7 million detected steps. Participants averaged 14,485 ± 4,334 steps daily with mean peak vertical ground reaction force stepping loads of 675 ± 121 N during daily living. Across the population, total hip BMD was found to be significantly correlated with objective estimates of mean BDI (r = 0.44), as well as participant age (r = .285).
Conclusion:
Despite having higher-than-expected PA, the low stepping loads observed in this cohort, along with half of the participants having low BMD measures, underscores the need for PA intensity to be considered in the management of postmenopausal bone health.
Keywords: Accelerometer, Bone density index, Bone loading, Physical activity, Postmenopause, Wearables
Routinely, physical activity (PA) and exercise are targeted by care providers to improve bone health with the goal of reducing fracture risk in aging adults.1–3 Hip fractures, which increase exponentially with age and occur disproportionally in women,4 account for the majority of the disability and financial burden associated with osteoporosis (OP),5 and often lead to premature death.6 Low bone mass (osteopenia) and the disease of OP, which are key contributors to hip fractures,7 afflict a significant portion of the US population, including 50% of adults older than 50 years, and 30% of all postmenopausal women.8 In clinical practice, dual-energy x-ray absorptiometry (DEXA) is the criterion standard for measuring bone mineral density (BMD), estimating bone mass and diagnosing OP.9 These evaluations are, however, costly,10 rely on specialized equipment, which may not be widely available, and require a clinic visit, limiting its use in preventative care and early identification of hip fracture risk. Furthermore, BMD measures, even if performed with frequency, are unable to provide adequate insights into the association between risk factor(s) related to bone health maintenance.
Evidence suggests that increasing daily PA in postmenopausal women could lead to clinically meaningful increases in hip BMD under appropriate loading conditions.1,11–13 How-ever, a well-defined relationship between daily PA impact loads and hip BMD has not been determined. The assessment of ground reaction force (GRF) impact loads during PA has been traditionally performed in a laboratory setting using force plates and in-shoe pressure measurement systems; however, these applications are limited in their ability to capture real-world PA loading. Furthermore, the seminal work of Whalen et al14 suggests that loading alone cannot be used as an independent variable to correlate with BMD, due to the complex and nonlinear relationship of BMD with loading, anthropomorphics, and exercise. Instead, the concept of an activity-based bone density index (BDI), relating dynamic skeletal loading (based on daily steps and their peak vertical GRFs) with BMD has been developed to capture this interrelationship.14–16 To date, the application of this frame-work to PA measured in the field has been limited, especially in populations at risk for BMD-related injuries, such as postmenopausal women.
In recent work, our group established an accurate method of estimating peak vertical GRFs for individual foot strikes, specific to postmenopausal women, during free-living locomotion using low-cost and widely available ankle-worn accelerometers.17 Using this technique, both the quantity and intensity of PA can be captured in the field to calculate cumulative estimates of dynamic skeletal loading using the BDI framework. The current study’s goal was to explore the cross-sectional relationship between cumulative daily estimates of dynamic skeletal loading in the free-living environment using the BDI15 and measures of total hip BMD. We hypothesized that individuals with higher mean estimates of BDI, representing a greater intensity of PA in the free-living environment, will have higher hip BMD. This study will begin to provide evidence of a modifiable link between in-the-field assessments of PA and total hip BMD, reopening the discussion of PA guidelines often quoted for the management of bone health for postmenopausal women
METHODS
Participant recruitment
A 70-woman sample of convenience was recruited from postmenopausal women residing in a local independent living facility and from community-dwelling adults located in the surrounding area. Participants were required to be postmenopausal (ie, without menses) for at least 1 year by self-report. Exclusion criteria included less than 2 years of self-reported starting, stopping, or modifying OP treatment; currently taking prescription medication which may result in BMD changes; induced menopause; assistive walking device use or any lower body amputations or orthotics; a body mass index of more than 40 kg/m2; prolonged bed rest; musculoskeletal performance deficits history; neuromotor impairment or hip surgery; lower-limb fracture; nondominant hip or knee joint replacement; a current lower extremity injury; moderate to severe hip or knee arthritis; have undergone chemotherapy and long-term or current corticosteroids use; self-reported history within the last 5 years of regular participation in impact or jumping exercises, and self-reported current smoker, or cardiovascular obstructive pulmonary disease including severe allergies or asthma. The Mayo Clinic Institutional Review Board approved the study protocol and informed consent was obtained prior to participation.
Bone mineral density
Upon study enrollment, all participants received DEXA scans (Lunar iDEXA, GE Healthcare Lunar, Madison, WI) to measure total hip BMD on their self-selected nondominant side. These calculations were performed using the manufacturer’s clinical software, which also provided total hip T scores (defined as BMD estimates relative to a young adult reference). Individuals with T scores less than − 1.0 or higher than − 2.5 are considered to have low bone density (osteopenia), whereas − 2.5 or less are considered osteoporotic.
Laboratory data collection and ground reaction force estimation
Under laboratory conditions, described by Madansingh et al,17 participants performed a minimum of five barefoot walking trials at each of three self-selected walking speed categories (slow, normal, and fast) to simultaneously collect bilateral ankle acceleration using ActiGraph GT3X+ (ActiGraph LLC, Pensacola, FL; 100 Hz) activity monitors (AMs) and GRFs using five floor-embedded force plates (3 AMTI, USA and 2 Kistler Instruments, Switzerland; 600 Hz). Mixed models analyses with crossvalidation yielded a model to predict body weight normalized peak vertical GRFs (pVGRFbw; equation (1)) for each individual detected foot strike with low average mean absolute error (6.8% ± 5.5%) and low root-mean-square error (0.087 ± 0.006) suggesting strong predictive qualities.17
| (1) |
where Az is the peak vertical component of ankle acceleration identified from the AMs during foot strike on the laboratory force plates.
Free-living environment activity monitoring and bone density index estimation
Participants were then asked to wear the AMs on both ankles for 7 days in their free-living environments. Participants were instructed to wear the AMs at all times except during sleeping, bathing, or swimming. A valid AM day was defined as 10 or more wear hours per day. ActiGraph GT3X+ devices are cited to have high within-individual validity (ICC = 0.977 vertical axis) when worn on the bilateral ankles during activities of daily living.18 Daily step counts were calculated from ankle acceleration data using our previously developed custom step detection algorithm using MATLAB R2016a (Mathworks, Natick, MA),19 which demonstrated high step count accuracy (92% agreement) with high interrater reliability (ICC = 0.98) using video evaluation as the criterion.19,20 Since our custom algorithm was previously validated for simulated free-living activity in the laboratory19,20 and not in the free-living environment, daily step counts were also calculated using the widely used default (DF) and Low Frequency Extension (LFE) algorithms available in the ActiLife software (ActiGraph LLC), for comparison. These algorithms have been investigated for their validity and show high agreement in the laboratory21–23; however, in the free-living environment, the DF algorithm has been observed to underestimate, whereas the LFE algorithm tends to overestimate when compared with criterion pedometers.21 In the absence of a custom algorithm, the LFE algorithm has been suggested to be most appropriate for an older population.22,23 The custom step detection algorithm used peak anteroposterior acceleration events to detect foot strikes.19 For each detected step, the maximum vertical acceleration (within ± 0.1 s of peak anteroposterior acceleration events) was identified and used to calculate pVGRFbw using Equation (2). A BDI estimate for each day was determined as the sum of the peak vertical GRFs estimated for each detected step16:
| (2) |
where β is the ratio of the individual’s bodyweight (BW) to the cohort’s mean BW and n is the daily step count. The exponent m weights the relative importance of force magnitude and loading cycles and was set to a value of 6.15 Mean BDI was calculated across the 7 days of monitored activity for each participant.
Statistical analyses
To evaluate the custom step detection algorithm’s performance, a one-way analysis of variance was performed to detect differences between its output and that of the two commercial ActiLife step detection algorithms (DF and LFE). Mean daily BDI, age, and mean daily step count for each participant were entered into stepwise multiple regression to identify the significant predictors of BMD and estimate the strength of the relationship among these variables. Assumptions of normality, independence, and multicollinearity among independent variables were evaluated during analyses. Forward and backward stepwise multiple regressions were performed to ensure agreement among variables selected for inclusion, to minimize suppressor effects, and to reduce type II error.24,25 Statistical significance was defined at α = 0.05 for all tests and Bonferroni corrections for multiple comparisons were performed, where necessary. Statistical analyses were performed in SPSS 25.0 (IBM Corp, Armonk, NY).
RESULTS
All 70 participants successfully completed 7 valid days of AM wear, yielding a compliance rate of 100%. Data from one participant were excluded due to a faulty device. Of the 69 participants, the mean (range) demographics were an age of 61 (46–79) years, a body mass of 69.8 (51.1–105.6) kg, a body mass index of 26 (18–40)kg/m2, a BMD of 0.89(0.62–1.39) g/cm2, a T score of −1.0 (−3.1–3.0) and a BDI of 2.21 (1.95–2.66) BW1/2. Thirty-five participants had T scores of less than −1.0, indicating low BMD.
In total, this sample of free-living activity resulted in the capture of more than 7 million steps. Comparing across step detection algorithms, the custom algorithm estimated a significantly larger number of steps than the ActiGraph DF configuration (mean ± SD) (14,485 ± 4,334 vs 10,565 ± 4,313 steps, t204 = 4.94, P < 0.05) but was not significantly different from the ActiGraph LFE configuration (14,485 ± 4,334 vs 15,792 ± 5,284, t204 = 1.65, P = 0.30; Fig. 1A). The ActiGraph DF configuration produced significantly lower step counts when compared with the ActiGraph LFE configuration (10,565 ± 4,313 vs 14,485 ± 4,334, t204 = 6.58, P < 0.05). The peak vertical acceleration of each step was collected, producing a population representative range depicted as a histogram in Figure 1B. The largest proportion of peak accelerations (18.6%) was captured in the 1.0 to 1.25 g bin with an average of 1.11 ± 0.71 g. The overall average peak vertical acceleration was 1.96 ± 0.76 g. The range of estimated peak vertical GRFs are depicted in Figure 1C, where the largest proportion of stepping loads (14.5%) was observed in the 650 to 700 N bin, with an average of 673.8 ± 14.6 N. The overall average stepping load was approximately 675 ± 121 N during normal daily activity. Table 1 highlights the relative proportion of observed peak accelerations and estimated GRFs throughout the range of observed values.
FIG. 1.
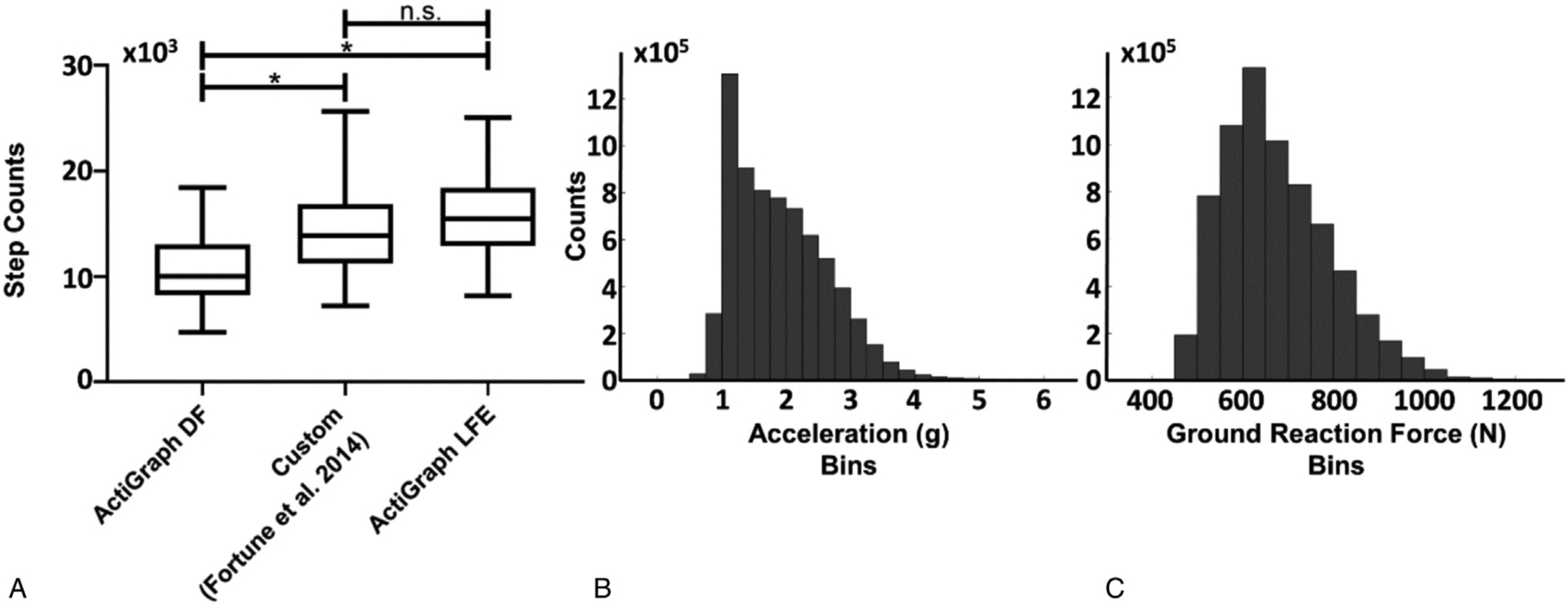
(A) Comparison among step detection algorithms including the ActiLife default (DF) algorithm, the custom algorithm of Fortune et al19 and the ActiLife Low Frequency Extension (LFE) algorithm. Significant differences are denoted with *. The central line represents the median, the edges of the box are the 25th and 75th percentiles, and the whiskers define the 2.5 to 97.5 percentile range of measured values. (B) Histogram of peak acceleration (g) values detected step throughout 7 days of activity monitoring where each bin represents a span of 0.25 g. (C) Histogram of ground reaction forces derived from peak step accelerations and Equation (1), where each bin represents a span of 50 N.
TABLE 1.
Percentage of steps with peak vertical accelerations and peak vertical ground reaction force estimates in the binned ranges
| Peak vertical accelerations | |||||
|---|---|---|---|---|---|
| Range (g) | <1 | 1–2 | 2–3 | 3–4 | >4 |
| Ground reaction force estimates | |||||
| Range (N) | <500 | 500–600 | 600–700 | 700–800 | >800 |
Stepwise regression in the forward (inclusionary) and reverse (exclusionary) directions revealed mean BDI and age to be significant predictors of BMD (F2,66 = 12.60, P < 0.001), see Equation (3).
| (3) |
With a standardized beta of 0.391 (P < 0.05), mean BDI was the strongest predictor of BMD, accounting for 19.4% of the variance in BMD (r = 0.44) (Fig. 2). Age accounted for an additional 8.1% of the variance in BMD (r = 0.285), with a beta of −0.289 (P < 0.05). Overall, the multiple regression model including mean BDI and age explains 27.5% of the variance in BMD (r = 0.525), suggesting a medium-strength relationship.
FIG. 2.
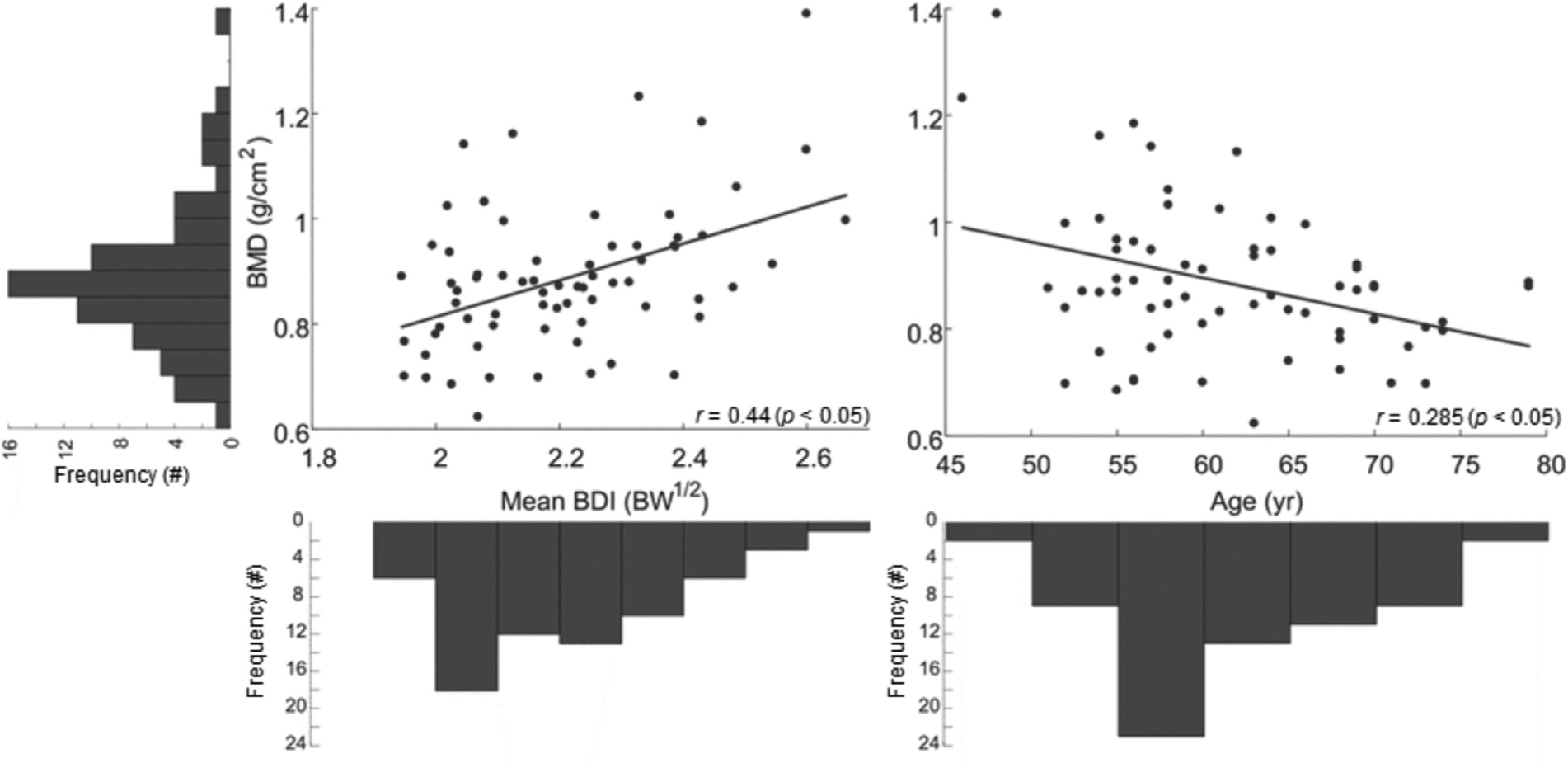
Partial regression plots highlighting the significant linear relationships among mean bone density index (BDI), in units of body weight (BW)1/2 and age with bone mineral density (BMD), accompanied by the frequency distributions of each measure in the free-living environment. Each bin of BMD represents a span of 0.05 g/cm2; each bin of mean BDI represents a span of 0.1 BW1/2; each bin of age represents a span of 5 years.
DISCUSSION
This study presents estimates of the quantity and intensity of PA performed by 70 postmenopausal women during 1 week in their uncontrolled, free-living environment and highlights the potential for PA intensity to be a modifiable factor associated with the maintenance of lower-body bone health. Participant compliance in this study was exceptional (100%) compared with rates of 80% typically reported in the literature for studies on older populations,26–28 with participants expressing little to no challenge with the technology. The PA measured in this cohort suggests a very active population, averaging more than 10,000 steps per day with all three step count algorithms; however, this quantity of steps did not relate to measures of bone density across the population. Custom step detection counts were found to be within the range of estimates provided by ActiGraph’s algorithms, which have previously been reported to underestimate in the DF configuration and overestimate in the LFE configuration21,29 compared to criterion pedometers in free-living environments. There was no detected difference between the custom step detection algorithm and LFE step detection algorithm; therefore, the custom step detection counts continue to be considered a reasonable estimate of quantity of PA.19,20 The predominance of peak vertical acceleration measurements in the 1.0 to 1.25 g range, suggest fairly low-intensity movement and resulted in GRF estimates predominantly in the 600 to 650 N range.17 Despite the high step counts, the vast majority (92.8%) of estimated GRF loads during daily PA were below the suggested threshold for BMD maintenance of 870 N, proposed by Borer et al,30 underscoring the need to better understand the link between modifiable, real-world, cumulative skeletal loading and bone density maintenance.
We found that DEXA measurements of hip BMD were significantly correlated with cumulative estimates of skeletal loading, as measured by the mean BDI. Mean daily step counts were not correlated with BMD. Individuals who experienced greater mean BDI tended to have higher BMD, suggesting a potentially modifiable dose-response relationship. Participant age was also found to be significantly related to hip BMD, where BMD was observed to decrease with age; a phenomenon well documented in the literature.26–28 Interestingly, the strength of the relationship observed between hip BMD and mean BDI in this study mirrored the results found by Boyer et al,16 with proximal femur BMD, and by Bowley and Whalen15 with calcaneal BMD. Genetic factors are often cited as the primary explanatory mechanism dictating changes in BMD throughout age, with some estimates accounting for 40% to 80% of the difference between individuals.31–33 The ability for PA to account for 20% of the variance in BMD in the current study provides support for and reinforces the conclusion that BDI estimates provide some representation of the mechanical stimulus experienced during real-world PA responsible for lower body bone remodeling and maintenance.16
The age-related results in this study are contrary to those of Boyer et al,16 who did not observe a significant effect of age in their sampled population including 49- to 64-year-old men and women. The inclusion of a larger age range in the current study (46–79 years) serves to provide greater insight to the complex relationship between BMD and mean BDI at ages 65 years or older. In secondary analyses, we remodeled the relationship between age and mean BDI with hip BMD, excluding those participants 65 years or older and found no significant relationship between hip BMD and age, similar to those findings in Boyer et al.16 One interpretation of these findings suggests that the relationship between BMD and age changes after 65 years, where there is an increase in the rate of BMD loss, as observed in the longitudinal study of Berger et al.34 It has been suggested that there exists a complex moderating effect of age upon mean BDI, implying that the natural progression of increased age results in decreased skeletal loading, and this decreased stimulus results in accelerated BMD loss, especially in postmenopausal women.34 This interaction between age and modifiable PA factors is currently an area of significant exploration.35–38
One possible limitation of the current study is the level of activity observed in the sampled population may not be representative of typical postmenopausal women. This is likely due to the extensive list of exclusionary criteria used in this study to reduce BMD confounders. Individual diet and nutritional supplement intake, such as calcium, vitamins, or fish oils supplements, were not controlled for in this study, and are evidenced to influence BMD changes in postmenopausal women.39,40 Future studies will necessitate including these confounding factors systematically, or will require dramatically larger populations to support a causal relationship. In addition, the technique for estimating GRFs was validated in barefoot walking under multiple speeds17; therefore, it may not account for individual variations in footwear or environmental factors, such as varying terrains, which may serve to heighten or dampen AM-measured accelerations during PA. Although these sources of variability may decrease the accuracy of the predicted GRFs, observing a consistent relationship between mean BDI and BMD as previously seen in the literature provides confidence that the impact of this variability was minimal. Finally, it bears mentioning that although the need for increased PA intensity is suggested, low-impact PA has been observed to be beneficial for postmenopausal health outcomes,41,42 and are more strongly advisable in individuals with high fracture risk.
CONCLUSIONS
These results highlight a potentially modifiable link between daily cumulative PA intensity and total hip BMD, and provide a direction for future exploration of a simple and low-cost ankle accelerometer to aid in BMD maintenance. This study has built on previous work understanding the relationship between BMD, step counting, and the BDI,15,16 by incorporating validated and accurate estimates of in-the-field GRFs.17 The current study’s results for postmenopausal women, many of which had low BMD but higher-than-expected levels of PA, underscore the need for considerations of PA intensity when PA is suggested to patients for bone health management. Future research will be needed to investigate the longitudinal effects of PA intensity on BMD, as well as the effectiveness of PA intensity-focused exercise interventions on the maintenance of bone health throughout aging.
Funding/support:
Funding for this study was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Numbers R21 AR066643 and UL1 TR002377, in addition to the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery
Footnotes
Financial disclosure/conflicts of interest: None of the authors has a conflict of interest to declare, and all authors were involved in the study design, data collection and interpretation, and contributed to the writing of the manuscript. This manuscript is not currently being considered for publication by another journal.
REFERENCES
- 1.Hannam K, Deere KC, Hartley A, et al. Habitual levels of higher, but not medium or low, impact physical activity are positively related to lower limb bone strength in older women: findings from a population-based study using accelerometers to classify impact magnitude. Osteoporos Int 2017;28:2813–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gába A, Kapuš O, Pelclová J, Riegerová J. The relationship between accelerometer-determined physical activity (PA) and body composition and bone mineral density (BMD) in postmenopausal women. Arch Gerontol Geriatr 2012;54:e315–e321. [DOI] [PubMed] [Google Scholar]
- 3.Krumm EM, Dessieux OL, Andrews P, Thompson DL. The relationship between daily steps and body composition in postmenopausal women. J Womens Health (Larchmt) 2006;15:202–210. [DOI] [PubMed] [Google Scholar]
- 4.Khosla S, Melton LJ. Osteopenia. N Engl J Med 2007;356:2293–2300. [DOI] [PubMed] [Google Scholar]
- 5.Johnell O The socioeconomic burden of fractures: today and in the 21st century. Am J Med 1997;103:S20–S26. [DOI] [PubMed] [Google Scholar]
- 6.Bentler SE, Liu L, Obrizan M, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol 2009;170:1290–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuit SC, Van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 2004;34:195–202. [DOI] [PubMed] [Google Scholar]
- 8.Lim LS, Hoeksema LJ, Sherin K; ACPM Prevention Practice Committee. Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Am J Prev Med 2009;36:366–375. [DOI] [PubMed] [Google Scholar]
- 9.Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J 2007;83:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheldon TA, Raffle A, Watt I. Department of Health shoots itself in the hip. Why the report of the Advisory Group on Osteoporosis undermines evidence based purchasing. BMJ 1996;312:296–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jämsä T, Vainionpää A, Korpelainen R, Vihriälä E, Leppäluoto J. Effect of daily physical activity on proximal femur. Clin Biomech (Bristol, Avon) 2006;21:1–7. [DOI] [PubMed] [Google Scholar]
- 12.Daly RM, Dalla Via J, Duckham RL, Fraser SF, Helge EW. Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther 2019;23:170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain RK, Vokes T. Physical activity as measured by accelerometer in NHANES 2005–2006 is associated with better bone density and trabecular bone score in older adults. Arch Osteoporos 2019;14:29. [DOI] [PubMed] [Google Scholar]
- 14.Whalen RT, Carter DR, Steele CR. Influence of physical activity on the regulation of bone density. J Biomech 1988;21:825–837. [DOI] [PubMed] [Google Scholar]
- 15.Bowley SM, Whalen RT. Physical activity and bone density in women. Bone 2000;1:3. [Google Scholar]
- 16.Boyer KA, Kiratli BJ, Andriacchi TP, Beaupre GS. Maintaining femoral bone density in adults: how many steps per day are enough? Osteoporos Int 2011;22:2981–2988. [DOI] [PubMed] [Google Scholar]
- 17.Madansingh SI, Murphree DH, Kaufman KR, Fortune E. Assessment of gait kinetics in post-menopausal women using tri-axial ankle accelerometers during barefoot walking. Gait Posture 2019;69:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozemek C, Kirschner MM, Wilkerson BS, Byun W, Kaminsky LA. Intermonitor reliability of the GT3X+ accelerometer at hip, wrist and ankle sites during activities of daily living. Physiol Meas 2014;35:129–138. [DOI] [PubMed] [Google Scholar]
- 19.Fortune E, Lugade VA, Amin S, Kaufman KR. Step detection using multi-versus single tri-axial accelerometer-based systems. Physiol Meas 2015;36:2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortune E, Morrow MMB, Kaufman KR. Assessment of gait kinetics using triaxial accelerometers. J Appl Biomech 2014;30:668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feito Y, Garner HR, Bassett DR. Evaluation of ActiGraph’s low-frequency filter in laboratory and free-living environments. Med Sci Sports Exerc 2015;47:211–217. [DOI] [PubMed] [Google Scholar]
- 22.Korpan SM, Schafer JL, Wilson KC, Webber SC. Effect of ActiGraph GT3X+ position and algorithm choice on step count accuracy in older adults. J Aging Phys Act 2015;23:377–382. [DOI] [PubMed] [Google Scholar]
- 23.Webber SC, St John PD. Comparison of ActiGraph GT3X+ and Step-Watch Step Count Accuracy in geriatric rehabilitation patients. J Aging Phys Act 2016;24:451–458. [DOI] [PubMed] [Google Scholar]
- 24.Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. New York: Routledge; 2013. [Google Scholar]
- 25.Field AP. Discovering Statistics Using SPSS: (And Sex and Drugs and Rock “n” Roll), 3rd ed Los Angeles (Thousand Oaks, CA); London: SAGE Publications; 2009. [Google Scholar]
- 26.Burr DB. Muscle strength, bone mass, and age-related bone loss. J Bone Miner Res 1997;12:1547–1551. [DOI] [PubMed] [Google Scholar]
- 27.Steiger P, Cummings SR, Black DM, Spencer NE, Genant HK. Age-related decrements in bone mineral density in women over 65. J Bone Miner Res 1992;7:625–632. [DOI] [PubMed] [Google Scholar]
- 28.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int 2002;13:105–112. [DOI] [PubMed] [Google Scholar]
- 29.Barreira TV, Brouillette RM, Foil HC, Keller JN, Tudor-Locke C. Comparison of older adults’ steps per day using NL-1000 pedometer and two GT3X+ accelerometer filters. J Aging Phys Act 2013;21:402–416. [DOI] [PubMed] [Google Scholar]
- 30.Borer KT, Fogleman K, Gross M, La New JM, Dengel D. Walking intensity for postmenopausal bone mineral preservation and accrual. Bone 2007;41:713–721. [DOI] [PubMed] [Google Scholar]
- 31.Pocock NA, Eisman JA, Hopper JL, Yeates MG, Sambrook PN, Eberl S. Genetic determinants of bone mass in adults. A twin study. J Clin Invest 1987;80:706–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makovey J, Nguyen TV, Naganathan V, Wark JD, Sambrook PN. Genetic effects on bone loss in peri- and postmenopausal women: a longitudinal twin study. J Bone Miner Res 2007;22:1773–1780. [DOI] [PubMed] [Google Scholar]
- 33.Block JE, Friedlander AL, Brooks GA, Steiger P, Stubbs HA, Genant HK. Determinants of bone density among athletes engaged in weight-bearing and non-weight-bearing activity. J Appl Physiol 1989;67:1100–1105. [DOI] [PubMed] [Google Scholar]
- 34.Berger C, Langsetmo L, Joseph L, et al. Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. CMAJ 2008;178:1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherk VD, Rosen CJ. Senescent and apoptotic osteocytes and aging: exercise to the rescue? Bone 2019;121:255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Saito T, Kobayashi R, Oshiki R, Kitamura K, Watanabe Y. Physical activity modifies the effect of calcium supplements on bone loss in perimenopausal and postmenopausal women: subgroup analysis of a randomized controlled trial. Arch Osteoporos 2019;14:17. [DOI] [PubMed] [Google Scholar]
- 37.Curtis E, Litwic A, Cooper C, Dennison E. Determinants of muscle and bone aging. J Cell Physiol 2015;230:2618–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos L, Elliott-Sale KJ, Sale C. Exercise and bone health across the lifespan. Biogerontology 2017;18:931–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macdonald HM, New SA, Golden MHN, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr 2004;79:155–165. [DOI] [PubMed] [Google Scholar]
- 40.Ilesanmi-Oyelere BL, Brough L, Coad J, Roy N, Kruger MC. The relationship between nutrient patterns and bone mineral density in postmenopausal women. Nutrients 2019;11:pii: E1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev 2011;CD000333. [DOI] [PubMed] [Google Scholar]
- 42.Grove KA, Londeree BR. Bone density in postmenopausal women: high impact vs low impact exercise. Med Sci Sports Exerc 1992;24:1190–1194. [PubMed] [Google Scholar]


