In this systematic review and meta-analysis, we found evidence for a protective effect of facemasks and respirators against clinical respiratory infection among healthcare workers, and limited evidence for superiority of respirators. However, the existing evidence is sparse and findings inconsistent.
Keywords: Facemasks, N95 respirators, respiratory infections, severe acute respiratory syndrome (SARS), influenza
Abstract
This systematic review and meta-analysis quantified the protective effect of facemasks and respirators against respiratory infections among healthcare workers. Relevant articles were retrieved from Pubmed, EMBASE, and Web of Science. Meta-analyses were conducted to calculate pooled estimates. Meta-analysis of randomized controlled trials (RCTs) indicated a protective effect of masks and respirators against clinical respiratory illness (CRI) (risk ratio [RR] = 0.59; 95% confidence interval [CI]:0.46–0.77) and influenza-like illness (ILI) (RR = 0.34; 95% CI:0.14–0.82). Compared to masks, N95 respirators conferred superior protection against CRI (RR = 0.47; 95% CI: 0.36–0.62) and laboratory-confirmed bacterial (RR = 0.46; 95% CI: 0.34–0.62), but not viral infections or ILI. Meta-analysis of observational studies provided evidence of a protective effect of masks (OR = 0.13; 95% CI: 0.03–0.62) and respirators (OR = 0.12; 95% CI: 0.06–0.26) against severe acute respiratory syndrome (SARS). This systematic review and meta-analysis supports the use of respiratory protection. However, the existing evidence is sparse and findings are inconsistent within and across studies. Multicentre RCTs with standardized protocols conducted outside epidemic periods would help to clarify the circumstances under which the use of masks or respirators is most warranted.
The emergence of novel respiratory pathogens, such as severe acute respiratory syndrome (SARS)–Coronavirus (SARS-CoV) [1] and pandemic H1N1 influenza (pH1N1) [2] highlighted the vulnerability of healthcare workers (HCWs) to respiratory infections [3]. Nonpharmaceutical interventions, such as respiratory personal protective equipment (rPPE), are particularly important to decrease the occupational risk of respiratory infection when vaccination or specific anti-infective treatments are unavailable [4].
Medical masks [5] can help to protect users from large respiratory droplets [6, 7]. They vary in thickness and permeability and are not certified to protect users from airborne infection [6]. N95 respirators are specifically designed to protect users from small airborne particles, including aerosols [6, 7]. Strict regulations dictate the filtration efficiency and breathing resistance of N95 respirators, which also require fit-testing to ensure a tight seal around the user’s face [7].
Current guidelines on rPPE use in healthcare settings are based on limited evidence of their effectiveness [4]. Studies to investigate the efficacy of rPPE are challenging, because of difficulties ensuring users’ compliance and limited statistical power to evaluate effectiveness against low-incidence outcomes [8]. Thus, results are often incongruent, leading to inconsistent international guidelines [8, 9] and conflicting practice recommendations [10, 11].
Previous reviews discussed the performance of rPPE in community and healthcare settings, but did not quantify their protective effect [8, 12]. One recent meta-analysis compared the effectiveness of N95 respirators and medical masks but did not compare their effectiveness against a “no mask” control [13]. This information is critical to assess the utility of universal mask use policies relative to targeted rPPE use during high-risk procedures, because universal policies have significant disadvantages in terms of personal discomfort and quality of care.
Additionally, the superiority of N95 respirators over medical masks may have limited practical relevance in low-resource settings, where N95 respirators may be unaffordable and resources for respirator fit-testing, regulation, and certification unavailable [9]. Finally, no reviews have quantified the effectiveness of rPPE against different pathogens, although their effectiveness may differ against viral and bacterial agents or pathogens with potentially different transmission modes [14, 15].
We conducted a systematic review and meta-analysis to quantify the effectiveness of different rPPE in reducing the risk of clinical and laboratory-confirmed respiratory outcomes among HCWs. In addition, we compared the protective effect of masks and respirators against bacterial and viral infections separately and evaluated the frequency of mask use as a potentially contributing factor. The main goal of this review was to develop evidence-based recommendations to reduce the occupational risk of respiratory infection among medical personnel.
METHODS
We conducted this systematic review and meta-analysis using a prespecified protocol (Appendix C).
Search Strategy
We searched Pubmed, Web of Science, and EMBASE databases without language or time restrictions for articles satisfying the following criteria:
Inclusion
Study design: Published, peer-reviewed randomized control trials (RCTs) and observational studies;
Population: HCWs;
Intervention: Any type of rPPE;
Outcome: Effectiveness of rPPE in reducing the risk of clinical or laboratory-confirmed respiratory outcomes;
Settings: Healthcare settings worldwide.
Exclusion
Editorials, reviews, guidelines, public press articles; theoretical models.
A detailed description of the search strategy is provided in Appendix B (Tables S1 and S2). We conducted the literature search on November 3rd, 2015. Two authors independently selected the studies and consulted a third author in case of disagreement.
Data Extraction and Quality Assessment
We extracted the following data from all included studies (Appendix B, Tables S3–S12): author, publication year, journal, and location; details of study population and interventions; study design and methods, including randomization procedures (RCTs) and statistical analysis; results, conclusions, and limitations.
We used the Cochrane Risk of Bias tool [16] and Review Manager 5.3 to assess the risk of bias within and across RCTs. Adapted Newcastle-Ottawa scales were used to assess the risk of bias in case-control, cohort [17], and cross-sectional studies [18]. Two authors (V. O. and M. S. F. L.) independently assessed study quality and consulted a third author (CCT) in case of disagreement.
Meta-Analysis
We performed separate meta-analyses of (i) RCTs and (ii) observational studies conducted during the 2003 SARS pandemic (Appendix B, Table 1). We combined studies in different meta-analyses according to type of rPPE and outcomes assessed (Appendix B, Table 1).
We summarized effect sizes and pooled estimates using forest plots and assessed publication bias using funnel plots and the Harbord test for funnel plot asymmetry.
We used the odds ratio (OR) as the effect measure for observational studies. This was possible because all identified cohort studies had fixed follow-up times. However, ORs may not approximate risk ratios (RRs) in high-incidence settings such as hospital outbreaks and could give misleading information about the actual protective effect of rPPE [19]. We therefore calculated a range of plausible RRs for each summary OR using the formula RR = OR/(1-rb + (rb*OR)), where rb is the baseline risk of infection. We assumed baseline risks of SARS infection between 20% and 60%, estimated from the available cohort studies [20–22]. To allow comparability between studies, we conducted meta-analyses with unadjusted statistics.
We assessed between-study heterogeneity using the I2 statistic and used a random-effects model for I2 > 60% and heterogeneity P-value < .05. Because of the small number of studies available for each meta-analysis, we did not conduct meta-regression to investigate factors affecting heterogeneity. Statistical analyses were performed with Stata version 12 (Stata Corporation).
RESULTS
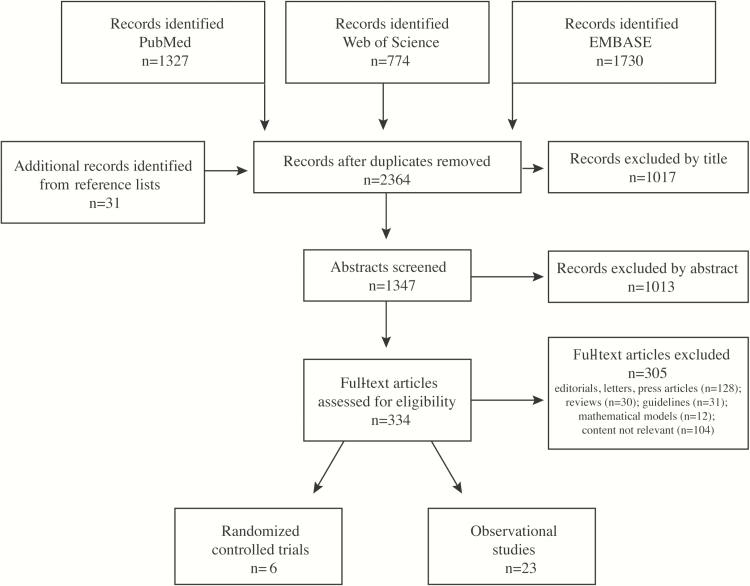
We retrieved 2333 unique articles from the three databases and 31 potentially relevant publications from reference lists (Figure 1). Of 334 full-text articles assessed for eligibility, 305 did not meet our exclusion criteria. Ultimately, we included 6 RCTs (Table S3) and 23 observational studies (Tables S4–S12). We found no evidence of publication bias (Figure S2).
Figure 1.
Summary of the literature search and inclusion process.
Randomized Controlled Trials
We combined 5 RCTs [23–27] in different meta-analyses according to type of rPPE and outcomes assessed (Appendix B, Table 1). We excluded one study [28] with high risk of bias (Figure S1).
Continuous Respiratory Personal Protective Equipment Use vs No Respiratory Personal Protective Equipment
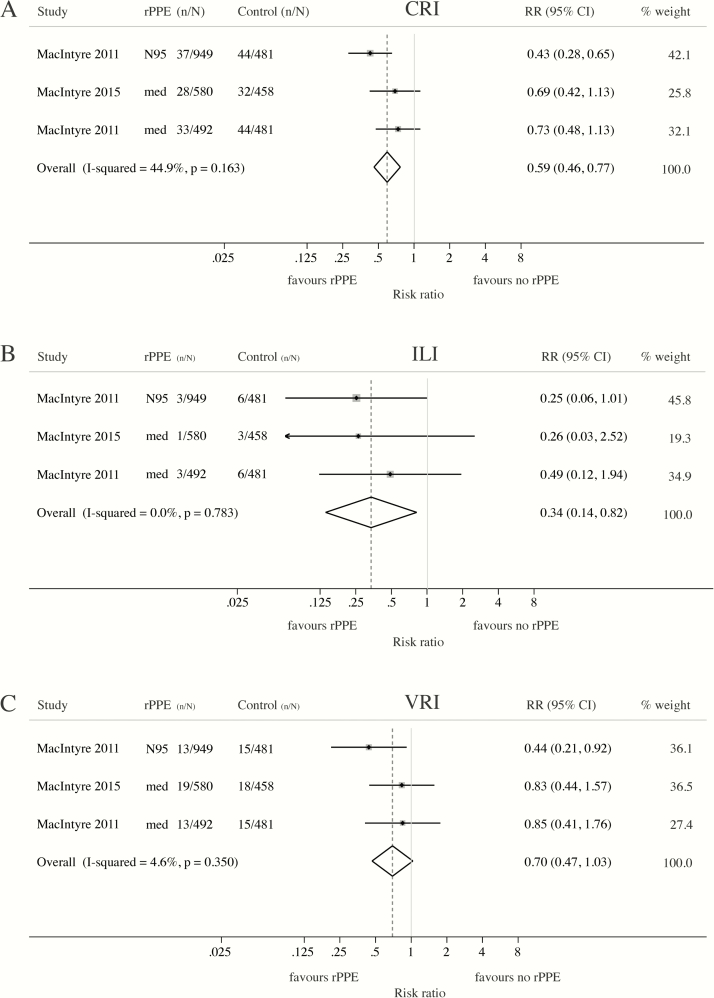
Two RCTs compared respiratory infection risk in HCWs wearing rPPE continuously to convenience-selected controls wearing no rPPE [24] or following routine care [23]. Wearing a medical mask or N95 respirator throughout the work shift conferred significant protection against self-reported clinical respiratory illness (CRI) (RR = 0.59; 95% CI: 0.46–0.77) (Figure 2A) and influenza-like illness (ILI) (RR = 0.34; 95% CI: 0.14–0.82) (Figure 2B). Meta-analysis suggested a protective, but nonstatistically significant, effect against laboratory-confirmed viral infections (VRI) (RR = 0.70; 95% CI: 0.47–1.03) (Figure 2C).
Figure 2.
Meta-analysis of RCTs assessing the protective effect of medical masks and N95 respirators against clinical and laboratory-confirmed respiratory outcomes. Meta-analyses comparing the risk of (A) clinical respiratory illness (CRI), (B) influenza-like illness (ILI) or (C) laboratory-confirmed viral respiratory infection (VRI) among HCWs continuously wearing respiratory personal protective equipment (rPPE) during working hours and convenience-selected HCWs wearing no mask (MacIntyre 2011 [42]) or following routine care, which may or may not include mask wearing (MacIntyre 2015 [41]). (A) CRI = 2 or more respiratory symptoms, or one respiratory symptom and a systemic symptom; (B) ILI = fever ≥38°C and 1 respiratory symptom; (C) VRI = detection of adenovirus, metapneumovirus, coronavirus 229E ⁄ NL63, parainfluenza 1- 3, influenza A and B, respiratory syncytial virus A and B, rhinovirus A⁄ B or coronavirus OC43⁄HKU1 by multiplex PCR. Abbreviations: CI, confidence interval; HCW, healthcare worker; med, medical mask; n/N, number of cases/number at risk; PCR, polymerase chain reaction; RCT, randomized controlled trial; RR, risk ratio.
N95 Respirators vs Medical Masks
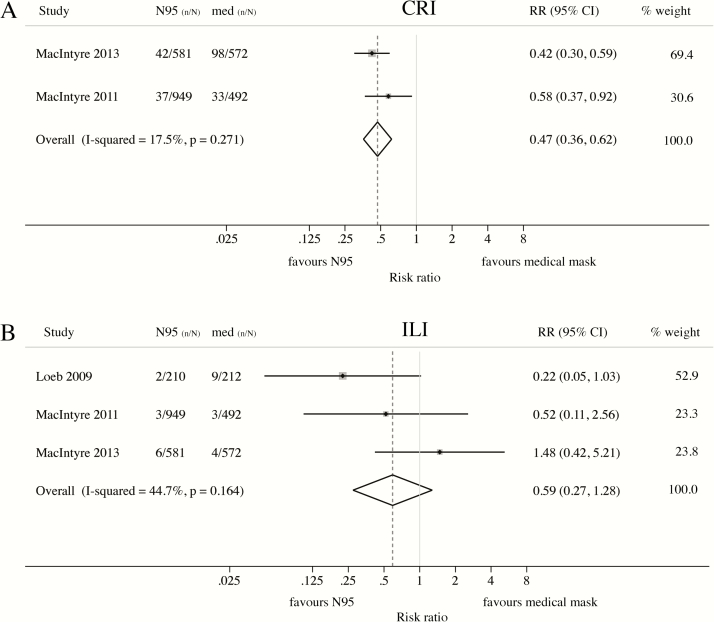
Four RCTs compared protection from N95 and medical masks against different clinical or laboratory-confirmed outcomes [24–27]. Of these, 3 [24–26] specified rPPE use throughout the work shift. Compared to medical masks, N95 respirators conferred significant protection against self-reported CRI (RR = 0.47; 95% CI: 0.36–0.62) (Figure 3A), but evidence of superiority against ILI was limited (RR = 0.59; 95% CI: 0.27–1.28) (Figure 3B).
Figure 3.
Meta-analysis of RCTs comparing the protective effect of N95 respirators and medical masks against clinical respiratory outcomes. Protective effect of N95 respirators compared to medical masks against (A) clinical respiratory illness (CRI) or (B) influenza-like illness (ILI). Masks and respirators were worn at all times during the work shift (MacIntyre 2011 [42] and MacIntyre 2013 [44]) or only when providing care to patients with febrile respiratory illness (Loeb 2009 [45]). (A) CRI = 2 or more respiratory symptoms, or 1 respiratory symptom and a systemic symptom; (B) ILI (MacIntyre 2011 [42] and MacIntyre 2013 [44]) = fever ≥38°C and 1 respiratory symptom; ILI (Loeb 2009 [45]) = fever ≥38°C and cough. Abbreviation: CI, confidence interval; n/N, number of cases/number at risk; RCT, randomized controlled trial; RR, risk ratio.
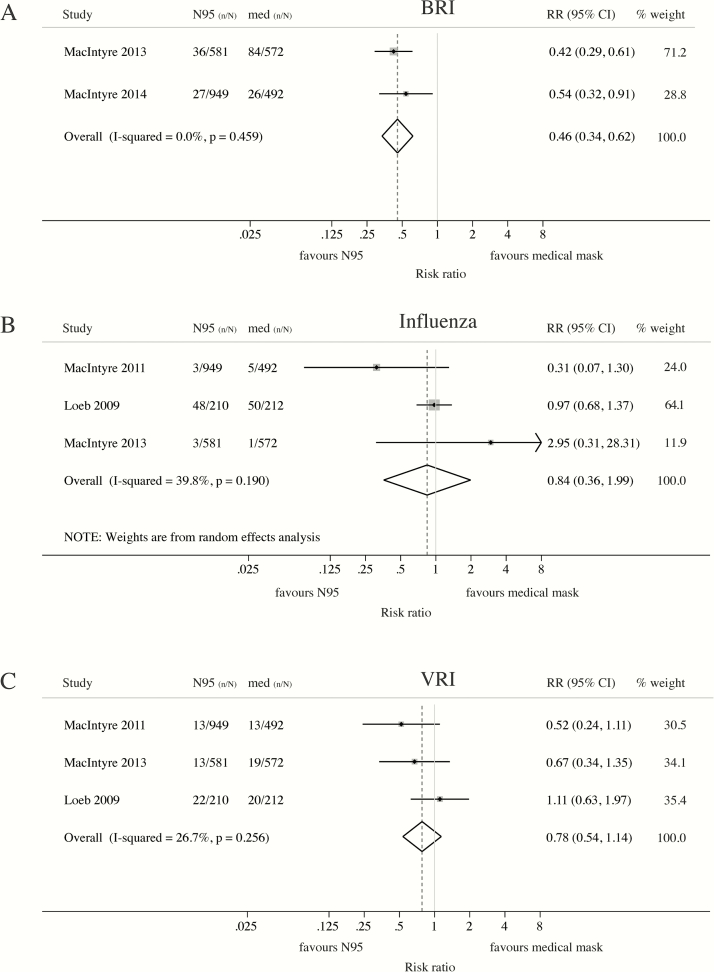
Meta-analysis indicated statistically significant superiority of N95 respirators over medical masks against laboratory-confirmed upper respiratory tract bacterial colonization (BRI) (RR = 0.46; 95% CI: 0.34–0.62) (Figure 4A) but not laboratory-confirmed influenza (RR = 0.84; 95% CI: 0.36–1.99) (Figure 4B) or other viral infections (RR = 0.78; 95% CI: 0.54–1.14) (Figure 4C).
Figure 4.
Meta-analysis of RCTs comparing the protective effect of N95 respirators and medical masks against laboratory-confirmed respiratory outcomes. Protective effect of N95 respirators compared to medical masks against laboratory-confirmed (A) bacterial respiratory infection (BRI), (B) influenza or (C) other viral respiratory infections (VRI). Masks and respirators were worn at all times on shift (MacIntyre 2011 [42], MacIntyre 2013 [44] and MacIntyre 2014 [43]) or only when providing care to patients with febrile respiratory illness (Loeb 2009 [45]). (A) BRI = detection of Streptococcus pneumoniae, Legionella, Bordetella pertussis, Chlamydia, Mycoplasma pneumoniae, or Haemophilus influenzae type B by multiplex PCR. (B) Influenza = laboratory-confirmed influenza A or B in symptomatic subjects. (C) VRI (MacIntyre 2011 [42], MacIntyre 2013 [44]) = detection of adenovirus, metapneumovirus, coronavirus 229E ⁄ NL63, parainfluenza 1–3, influenza viruses A and B, respiratory syncytial virus A and B, rhinovirus A/ B or coronavirus OC43 ⁄HKU1 by multiple PCR; VRI (Loeb 2009 [45]) = detection of respiratory syncytial virus A and B, metapneumovirus, parainfluenza 1–4, rhinovirus, coronavirus OC43, 229E, NL63, and HKU1by multiplex PCR. Abbreviations: CI, confidence interval; n/N = number of cases/number at risk; PCR, polymerase chain reaction; RCT, randomized controlled trial; RR, risk ratio.
Observational Studies
Most observational assessed the effectiveness of rPPE during the care of high-risk patients involved in outbreaks. We did not exclude any articles based on quality assessment (Appendix B, Tables S21–S23) but summarized their limitations in the discussion.
Severe Acute Respiratory Syndrome
Eight case-control [29–36] (Table S4) and 4 cohort studies [20–22, 37] (Table S5) assessed the effectiveness of rPPE in protecting HCWs from SARS infection (Appendix B, Table 2).
With one exception [30], case-control studies consistently reported a protective effect of medical masks against SARS [31, 32, 34] (Appendix B, Table 2). Compared to “no rPPE” controls, N95 respirators conferred protection against confirmed SARS-CoV infection in 2 of 3 case-control studies [32, 33]; no protective effect against SARS was reported for disposable [29, 34], cotton [35], or paper [32] masks (Appendix B, Table 2).
Evidence from the 4 cohort studies was less conclusive. Two studies reported lower risk of pneumonic SARS (RR = 0.24; 95% CI: 0.08–0.71; P < .001) [22] and moderate protection against laboratory-confirmed SARS-CoV infection (RR = 0.23; 95% CI: 0.05–0.93; P < .058) [20] among HCWs wearing a N95 respirator (Appendix B, Table 3). Another study reported reduced risk of SARS-CoV infection among HCWs wearing a medical mask (RR = 0.08; 95% CI: 0.01–0.50; P < .01) [37]. Two studies found no protective effect of either medical masks or N95 respirators against SARS [20, 21], although lower attack rates were reported among nurses consistently wearing either type of rPPE (RR = 0.23; 95% CI: 0.07–0.78; P = .023) (Appendix B, Table 3) [20].
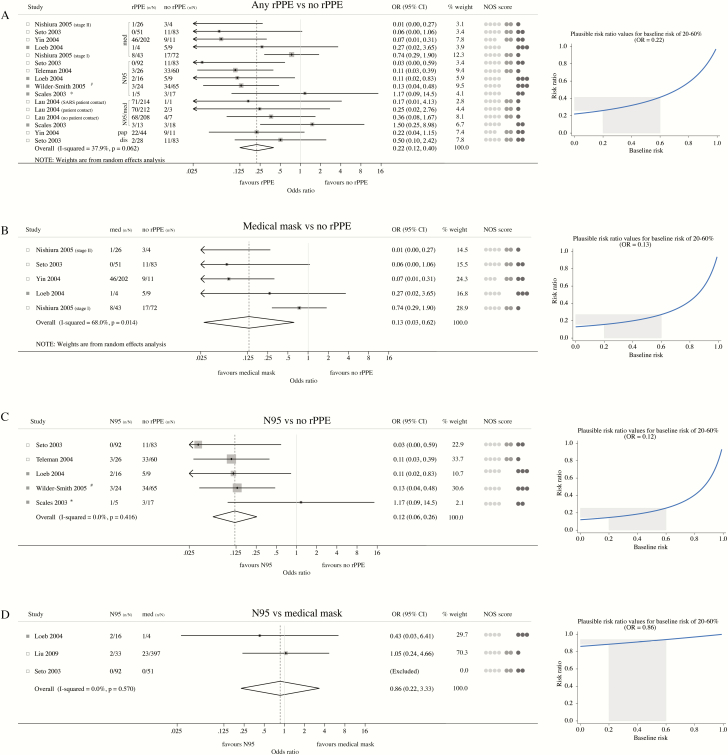
In meta-analyses combining 6 case-control [29, 31–34, 36] and 3 cohort [20–22] studies (Appendix B, Table 1), use of rPPE conferred significant protection against SARS among exposed HCWs (OR = 0.22; 95% CI: 0.12–0.40) (Figure 5A). The corresponding RRs under baseline risks of 20% and 60% were 0.26 (95% CI: 0.15–0.45) and 0.41 (95% CI: 0.25–0.63), respectively. More specifically, wearing medical masks (OR = 0.13; 95% CI: 0.03–0.62) (Figure 5B) or N95 respirators (OR = 0.12; 95% CI: 0.06–0.26) (Figure 5C) both reduced the risk of SARS by approximately 80%. The corresponding RRs under baseline risks of 20% and 60% are 0.16 (95% CI: 0.04–0.67) and 0.27 (95% CI: 0.07–0.80) for medical masks and 0.15 (95% CI: 0.07–0.31) and 0.25 (95% CI: 0.14–0.47) for respirators, respectively. There was no significant difference between N95 respirators and medical masks in protecting HCWs from SARS (OR = 0.86; 95% CI: 0.22–3.33), with corresponding RRs of 0.88 (95% CI: 0.26–2.27) and 0.94 (95% CI: 0.41–1.34) under baseline risks of 20% and 60%, respectively (Figure 5D).
Figure 5.
Meta-analysis of observational studies assessing the protective effect of masks and respirators against SARS infection. (A)–(C) Five case-control (empty squares) and 3 cohort (full squares) studies were combined into different meta-analyses to assess the protective effect of (A) any respiratory personal protective equipment (rPPE), including medical masks, paper masks, disposable masks, and N95 respirators, (B) medical masks or (C) N95 respirators. Controls for studies included in meta-analyses (A)–(C) were HCWs not wearing any rPPE, except for Loeb 2004 [20] and Lau 2004 [36], where the control group consisted HCWs reporting “inconsistent use” of masks or respirators; med = medical mask; pap = paper mask; dis = disposable mask; n/N = number of cases/number at risk. aHCWs wearing N95 during non-invasive positive-pressure ventilation. bOutcome = incidence of pneumonic SARS (excludes asymptomatic SARS cases). (D) Meta-analyses combining observational studies comparing the protective effect of N95 and medical masks against SARS. NOS scores = Newcastle-Ottawa-Scale scores; for each paper, light gray, mid-gray, and dark gray circles represent the score for the “Selection,” “Comparability” and “Exposure” (case-control studies) or “Outcome” (cohort studies) domains of the Newcastle-Ottawa score, respectively. For each meta-analysis (A–D), panels on the right-hand side display a range of plausible risk ratios corresponding to the summary effect estimate for an estimated baseline risk of SARS ranging from 20% to 60%. Abbreviations: CI, confidence interval; HCW, healthcare worker; med, medical mask; n/N, number of cases/number at risk; RR, risk ratio; SARS, severe acute respiratory syndrome.
Pandemic H1N1 Influenza (pH1N1)
Eight observational studies assessed the effectiveness of rPPE in protecting HCWs against pH1N1 infection (Tables S6–S10).
Early in the outbreak, the effectiveness of masks and respirators was assessed in HCWs who had been exposed to pH1N1 cases in California [38]. Seroconversion against pH1N1 was detected in 21% (9/43) of HCWs attending pH1N1-patients without rPPE but none of the HCWs wearing a mask or N95 respirator (Table S14) [38].
In a cohort study from Hong Kong, all HCWs who reported using a medical mask during patient contact remained healthy, whereas 1.5% (4/268) of HCWs not using any rPPE developed laboratory-confirmed pH1N1 infection (Table S14) [39].
Two matched case-control studies in Beijing assessed the protective effect of masks and N95 respirators [40] or “high protection level masks” [41], respectively. In one study, the “high protection level mask” reduced the odds of pH1N1 influenza among HCWs (adjusted OR = 0.05; 95% CI: 0.01–0.35) [41]. In the second study, unadjusted analysis showed no significantly protective effect for N95, medical or cloth masks (Table S13) [40].
In two cross-sectional studies in Thailand [42] and Japan [43], use of medical masks or N95 respirators was not associated with pH1N1 seroprevalence. Two additional studies reported no effectiveness of rPPE in protecting HCWs from laboratory-confirmed pH1N1 infection [44, 45].
One cohort study reported an increased risk of pH1N1 seroconversion among HCWs not wearing rPPE continuously [38]. In 4 other studies, no association was found between compliance with rPPE use and pH1N1 infection [40–43].
DISCUSSION
Randomized Controlled Trials
Compared to non-rPPE wearing HCWs, those wearing medical masks or N95 respirators throughout their work shift were significantly protected against nonspecific respiratory infection. However, assessment of clinical outcomes was self-reported and prone to bias, as the intervention cannot be masked. Evidence of a protective effect of masks or respirators against VRI, a rarer outcome, was not statistically significant, though this may indicate insufficient statistical power in these studies, rather than lack of a protective effect.
Compared to medical masks, N95 respirators provided greater protection against CRI and BRI. These 2 outcomes were common in these trials (average risks of 8.7% and 7.3%, respectively), but the studies may have been underpowered to detect a superior protective effect of N95 respirators against influenza and other lower incidence outcomes.
Several limitations should be considered. First, the source of infection was not ascertained in any of the trials; some HCWs may have acquired infections in the community rather than the workplace. Second, one RCT required HCWs to wear rPPE only when caring for febrile patients [27], whereas others specified continuous rPPE use [23–26]. Third, our meta-analyses included RCTs with different comparison groups, including convenience samples of HCWs not usually wearing masks [24, 25] or following routine infection control policies, which may have included the use of rPPE [23]. Finally, the number of RCTs was small, and 4 of these were conducted in China by the same investigators, limiting generalizability to other settings.
Observational Studies
HCWs wearing N95 respirators were protected against SARS [20, 22, 32, 33], except when exposed to SARS patients during noninvasive positive-pressure ventilation [21]. Evidence of protection through medical masks was available from individual articles [31, 32, 34, 37], although results were inconsistent within [31] and across studies [20, 30]. Differing levels of exposure could explain such discrepancies, but individual studies provided insufficient information for more detailed analysis.
The superiority of N95 respirators over medical masks could reflect the ability of N95 respirators to protect users from infectious aerosols or indicate higher effectiveness against droplet contagion. Nonetheless, our meta-analysis revealed that use of both N95 respirators and medical masks was associated with up to 80% reduction in risk of SARS.
For pH1N1, the evidence was inconsistent. Asymptomatic infection, common in pH1N1 patients [43], could have led to substantial misclassification of infection status in studies without serological confirmation, diluting any protective effect of masks. Compared to SARS cases [46], pH1N1 patients experienced relatively mild symptoms and lower case fatality rates [47], which may have resulted in HCWs being less adherent to rPPE use. Moreover, more drastic infection control measures, such as quarantine and patient isolation [48], may have led to an overestimation of rPPE effectiveness in the SARS studies.
Limitations of Included Studies
Specific brands, models, or even the generic type of mask used, was often omitted.
In most studies, rPPE adherence was self-reported, and definitions of compliance varied across studies [23, 24]. One RCT included external validation, but auditing was irregular and limited to areas outside patients’ rooms [27]. Because individuals overestimate compliance [49], self-reported adherence could result in attenuated effect estimates and potentially biased comparisons of masks and respirators if compliance differs by rPPE type [23, 24]. Continuous adjustments and inappropriate wearing may even reverse the benefits of N95 respirators through the contamination of hands, face, and other PPE [4].
In the included studies, HCWs were usually trained in wearing N95 respirators, but fit-testing was not universal [24–26]. One trial compared fit-testing with no fit-testing and reported no difference in respiratory infection risk between the 2 groups [24].
In some RCTs, influenza vaccine uptake differed between trial arms [24, 26]. Similarly, not all observational studies accounted for differences in influenza vaccination coverage [38, 42, 43] and gown- [31, 32, 36] or hand-washing habits [32, 33, 36]. Several studies adjusted for confounders [23, 25, 26, 29, 31–33, 36, 37, 41, 50, 51], but it was not always clear which factors were accounted for [41, 50, 51]. Finally, most studies lacked statistical power to estimate protective effects, yielding extremely wide confidence intervals. Failure to detect a significant effect may therefore indicate insufficient statistical power, rather than absence of a protective effect, even when the available studies are pooled into a meta-analysis.
Limitations of the Meta-Analysis of Observational Studies
Our meta-analysis of observational studies summarized the protective effect of rPPE on a specific respiratory outcome with an established case definition [52], among reasonably well-defined populations and over a defined time period. However, it was not possible to account for potential between-study differences in exposure, fluctuating compliance with rPPE use [29, 31], potential decreases in infectiousness over the course of the outbreak, or additional confounders affecting the original studies. Nonetheless, relevant confounders were unlikely to be equally distributed across studies in different settings, so that any protective effect of mask use should have become apparent when results of numerous studies were pooled.
Conclusions
In this review and meta-analysis, we analysed the collective evidence from published RCTs and observational studies in order to identify major gaps and methodological shortcomings in the current literature and develop evidence-based recommendations for the use of masks and respirators in healthcare settings. We found evidence to support universal medical mask use in hospital settings as part of infection control measures to reduce the risk of CRI and ILI among HCWs. Overall, N95 respirators may convey greater protection, but universal use throughout a work shift is likely to be less acceptable due to greater discomfort.
Our analysis confirms the effectiveness of medical masks and respirators against SARS. Disposable, cotton, or paper masks are not recommended.
The confirmed effectiveness of medical masks is crucially important for lower-resource and emergency settings lacking access to N95 respirators. In such cases, single-use medical masks are preferable to cloth masks, for which there is no evidence of protection and which might facilitate transmission of pathogens when used repeatedly without adequate sterilization [8].
We found no clear benefit of either medical masks or N95 respirators against pH1N1. However, current policies mandating standard and droplet precautions when performing routine care for influenza patients are reasonable. RCTs conducted in community settings have demonstrated protective effects of medical masks in combination with hand-hygiene and other infection control interventions [53].
Overall, the evidence to inform policies on mask use in HCWs is poor, with a small number of studies that is prone to reporting biases and lack of statistical power. Multicenter RCTs with standardized protocols conducted outside periods of unusual epidemic events and including the measurement of compliance and fit-testing would overcome many of the methodological difficulties of current studies, including low statistical power, the use of concurrent epidemic control measures, and unusually high compliance during epidemics. Large, well-designed studies would also enable subanalyses to investigate the role of mask use against different types of infections [54], clarify the circumstances under which rPPE use is most warranted, and yield valuable information about the role of different transmission modes. The inclusion of relevant controls is of paramount importance. Because the source of infection cannot always be ascertained, control groups could include HCWs who do not have any patient contact.
In addition, the protective effect of masks is likely to be related to the baseline risk of infection, because outbreaks with higher attack rates offer more opportunities for infection. We recommend that studies indicate the baseline risk of disease, either from a nonintervention group or occupational health records. This is particularly important for case-control studies, for which the interpretation of the OR as a measure of protective effect is problematic in high-incidence scenarios.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Note
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Peiris JS. Severe acute respiratory syndrome (SARS). J Clin Virol 2003; 28:245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peiris JS, Tu WW, Yen HL. A novel H1N1 virus causes the first pandemic of the 21st century. Eur J Immunol 2009; 39:2946–54. [DOI] [PubMed] [Google Scholar]
- 3. Trajman A, Menzies D. Occupational respiratory infections. Curr Opin Pulm Med 2010; 16:226–34. [DOI] [PubMed] [Google Scholar]
- 4. Jefferson T, Del Mar CB, Dooley L et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev 2011; CD006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care Geneva, Switzerland: 2014; Available at: http://apps.who.int/iris/bitstream/10665/112656/1/9789241507134_eng.pdf. Accessed 27 April 2017. [Google Scholar]
- 6. Occupational Safety and Health Administration. Respiratory infection control: respirators versus surgical mask. OSHA Fact Sheet2009; Available at: https://www.osha.gov/Publications/respirators-vs-surgicalmasks-factsheet.html. Accessed 27 April 2017.
- 7. Centres for Disease Control and Prevention. Respirator Trusted-Source Information The National Personal Protective Technology Laboratory; 2014; Available at: http://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/respsource3healthcare.html - d. Accessed 27 April 2017. [Google Scholar]
- 8. MacIntyre CR, Chughtai AA. Facemasks for the prevention of infection in healthcare and community settings. BMJ 2015; 350:h694. [DOI] [PubMed] [Google Scholar]
- 9. Chughtai AA, Seale H, MacIntyre CR. Availability, consistency and evidence-base of policies and guidelines on the use of mask and respirator to protect hospital health care workers: a global analysis. BMC Res Notes 2013; 6:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. Interim guidance on infection control measures for 2009 H1N1 influenza in healthcare settings, including protection of healthcare personnel 2010; Available at: http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm. Accessed 27 April 2017.
- 11. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Hospitalized Patients with Middle East Respiratory Syndrome Coronavirus (MERS-CoV) 2015; Available at: http://www.cdc.gov/coronavirus/mers/infection-prevention-control.html - infection-prevention. Accessed 27 April 2017.
- 12. Bin-Reza F, Lopez Chavarrias V, Nicoll A, Chamberland ME. The use of masks and respirators to prevent transmission of influenza: a systematic review of the scientific evidence. Influenza Other Respir Viruses 2012; 6:257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith JD, MacDougall CC, Johnstone J, Copes RA, Schwartz B, Garber GE. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ 2016; 188:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu ITS, Li YG, Wong TW et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med 2004; 350:1731–9. [DOI] [PubMed] [Google Scholar]
- 15. Seto WH. Airborne transmission and precautions: facts and myths. J Hosp Infect 2015; 89:225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0) 2011; Available at: http://handbook.cochrane.org/. Accessed 27 April 2017.
- 17. Wells GA, Shea B, O’Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 27 April 2017.
- 18. Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013; 13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 2014; 348:f7450. [DOI] [PubMed] [Google Scholar]
- 20. Loeb M, McGeer A, Henry B et al. SARS among critical care nurses, Toronto. Emerg Infect Dis 2004; 10:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scales DC, Green K, Chan AK et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerg Infect Dis 2003; 9:1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilder-Smith A, Teleman MD, Heng BH, Earnest A, Ling AE, Leo YS. Asymptomatic SARS coronavirus infection among healthcare workers, Singapore. Emerg Infect Dis 2005; 11:1142–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacIntyre CR, Seale H, Dung TC et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open 2015; 5:e006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacIntyre CR, Wang Q, Cauchemez S et al. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses 2011; 5:170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. MacIntyre CR, Wang Q, Rahman B et al. Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co-infection in hospital healthcare workers - authors’ reply. Prev Med 2014; 65:154. [DOI] [PubMed] [Google Scholar]
- 26. MacIntyre CR, Wang Q, Seale H et al. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med 2013; 187:960–6. [DOI] [PubMed] [Google Scholar]
- 27. Loeb M, Dafoe N, Mahony J et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA 2009; 302:1865–71. [DOI] [PubMed] [Google Scholar]
- 28. Jacobs JL, Ohde S, Takahashi O, Tokuda Y, Omata F, Fukui T. Use of surgical face masks to reduce the incidence of the common cold among health care workers in Japan: a randomized controlled trial. Am J Infect Control 2009; 37:417–9. [DOI] [PubMed] [Google Scholar]
- 29. Liu W, Tang F, Fang LQ et al. Risk factors for SARS infection among hospital healthcare workers in Beijing: a case control study. Trop Med Int Health 2009; 14:52–9. [Google Scholar]
- 30. Ma HJ, Wang HW, Fang LQ et al. A case-control study on the risk factors of severe acute respiratory syndromes among health care workers. Zhonghua Liu Xing Bing Xue Za Zhi 2004; 25:741–4. [PubMed] [Google Scholar]
- 31. Nishiura H, Kuratsuji T, Quy T et al. Rapid awareness and transmission of severe acute respiratory syndrome in Hanoi French Hospital, Vietnam. Am J Trop Med Hyg 2005; 73:17–25. [PubMed] [Google Scholar]
- 32. Seto WH, Tsang D, Yung RW et al. ; Advisors of Expert SARS group of Hospital Authority Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet 2003; 361:1519–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teleman MD, Boudville IC, Heng BH, Zhu D, Leo YS. Factors associated with transmission of severe acute respiratory syndrome among health-care workers in Singapore. Epidemiol Infect 2004; 132:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yin WW, Gao LD, Lin WS et al. Effectiveness of personal protective measures in prevention of nosocomial transmission of severe acute respiratory syndrome. Zhonghua Liu Xing Bing Xue Za Zhi 2004; 25:18–22. [PubMed] [Google Scholar]
- 35. Chen WQ, Ling WH, Lu CY et al. Which preventive measures might protect health care workers from SARS? BMC Public Health 2009; 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lau JT, Fung KS, Wong TW et al. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis 2004; 10:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nishiyama A, Wakasugi N, Kirikae T et al. Risk factors for SARS infection within hospitals in Hanoi, Vietnam. Jpn J Infect Dis 2008; 61:388–90. [PubMed] [Google Scholar]
- 38. Jaeger JL, Patel M, Dharan N et al. Transmission of 2009 pandemic influenza A (H1N1) virus among healthcare personnel-Southern California, 2009. Infect Control Hosp Epidemiol 2011; 32:1149–57. [DOI] [PubMed] [Google Scholar]
- 39. Cheng VC, Tai JW, Wong LM et al. Prevention of nosocomial transmission of swine-origin pandemic influenza virus A/H1N1 by infection control bundle. J Hosp Infect 2010; 74:271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Seale H, Yang P et al. Factors associated with the transmission of pandemic (H1N1) 2009 among hospital healthcare workers in Beijing, China. Influenza Other Respir Viruses 2013; 7:466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deng Y, Zhang Y, Wang XL et al. Pandemic influenza A (H1N1) virus infection factors among healthcare workers—a case-control study. Zhonghua Yu Fang Yi Xue Za Zhi 2010; 44:1075–8. [PubMed] [Google Scholar]
- 42. Chokephaibulkit K, Assanasen S, Apisarnthanarak A et al. Seroprevalence of 2009 H1N1 virus infection and self-reported infection control practices among healthcare professionals following the first outbreak in Bangkok, Thailand. Influenza Other Respir Viruses 2013; 7:359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Toyokawa T, Sunagawa T, Yahata Y et al. Seroprevalence of antibodies to pandemic (H1N1) 2009 influenza virus among health care workers in two general hospitals after first outbreak in Kobe, Japan. J Infect 2011; 63:281–7. [DOI] [PubMed] [Google Scholar]
- 44. Ang B, Poh BF, Win MK, Chow A. Surgical masks for protection of health care personnel against pandemic novel swine-origin influenza A (H1N1)-2009: results from an observational study. Clin Infect Dis 2010; 50:1011–4. [DOI] [PubMed] [Google Scholar]
- 45. Wise ME, De Perio M, Halpin J et al. Transmission of pandemic (H1N1) 2009 influenza to healthcare personnel in the United States. Clin Infect Dis 2011; 52(Suppl 1):S198–204. [DOI] [PubMed] [Google Scholar]
- 46. Fung WK, Yu PL. SARS case-fatality rates. CMAJ 2003; 169:277–8. [PMC free article] [PubMed] [Google Scholar]
- 47. Wong JY, Kelly H, Ip DK, Wu JT, Leung GM, Cowling BJ. Case fatality risk of influenza A (H1N1pdm09): a systematic review. Epidemiology 2013; 24:830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chan-Yeung M, Xu RH. SARS: epidemiology. Respirology 2003; 8 Suppl:S9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. LaFleur J, Oderda GM. Methods to measure patient compliance with medication regimens. J Pain Palliat Care Pharmacother 2004; 18:81–7. [PubMed] [Google Scholar]
- 50. Ng TC, Lee N, Hui SC, Lai R, Ip M. Preventing healthcare workers from acquiring influenza. Infect Control Hosp Epidemiol 2009; 30:292–5. [DOI] [PubMed] [Google Scholar]
- 51. Yang P, Seale H, MacIntyre CR et al. Mask-wearing and respiratory infection in healthcare workers in Beijing, China. Braz J Infect Dis 2011; 15: 102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. World Health Organization. Case Definitions for Surveillance of Severe Acute Respiratory Syndrome (SARS). Emergencies preparedness, response2003, May 1st; Available at: http://www.who.int/csr/sars/casedefinition/en/. Accessed 27 April 2017.
- 53. Wong VW, Cowling BJ, Aiello AE. Hand hygiene and risk of influenza virus infections in the community: a systematic review and meta-analysis. Epidemiol Infect 2014; 142:922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Radonovich LJ Jr, Bessesen MT, Cummings DA et al. The Respiratory Protection Effectiveness Clinical Trial (ResPECT): a cluster-randomized comparison of respirator and medical mask effectiveness against respiratory infections in healthcare personnel. BMC Infect Dis 2016; 16:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.