An outbreak of severe acute respiratory syndrome (SARS) has affected 33 countries on five continents, with 8098 suspected and probable cases and 774 deaths. During the SARS epidemic, more than 1700 infected cases have been reported in Hong Kong. The disease is infectious, particularly within the healthcare setting. Early diagnosis is essential to control the spread of SARS by identifying and isolating infected patients (1)(2). Diagnostic tests have been developed that detect either antibodies to or reverse transcription-PCR (RT-PCR) products of the SARS coronavirus (SARS-CoV). SARS-CoV antibodies can be reliably detected only around 20 days after disease onset (3)(4). Quantitative real-time RT-PCR assays, on the other hand, allow the detection of SARS-CoV within the first week of illness. Our recent study showed that detection sensitivity could approach 80% for plasma and serum samples from SARS patients (5)(6). Aside from improving the analytical and clinical sensitivity of the RT-PCR assays, it is also possible to develop new types of assays on the basis of other biological variables and/or technologies. In the past few years, surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF MS) has been applied to the discovery of diagnostic proteomic signatures for cancers such as prostate cancer, ovarian cancer, breast cancer, and hepatocellular carcinoma (7)(8). Here we report the presence of plasma proteomic signatures in serial blood samples from eight pediatric patients with SARS and their correlation with SARS-CoV RNA concentrations in plasma (6).
The serial plasma samples investigated in this study were those examined for SARS-CoV RNA by real-time RT-PCR in our recently reported study (6). The pediatric patients were admitted to the New Territories East Cluster of Hospital Authority Hospitals in Hong Kong and satisfied the WHO surveillance case definition for SARS (9). These patients were recruited between March 13 and May 17, 2003. Informed consent was obtained from the patients or their parents, and ethics approval was obtained from the Institutional Review Board. The serial blood samples used in this study were collected from the patients during sample collection for routine blood tests, which included the monitoring of lymphocyte counts and biochemical indices and enzymes. The convalescent sera of these patients were tested for IgG antibodies against SARS-CoV with SARS-CoV-infected cells in an indirect immunofluorescence assay (4). All patients were serologically positive for SARS-CoV IgG antibody. As negative controls, blood samples from 15 pediatric patients who suffered from fever and infections other than SARS were collected. The plasma SARS-CoV RNA concentrations in the pediatric patients were compared with the results of adult SARS patients as reported previously (5).
Seven of the eight studied patients had been in close contact with infected adults, whereas one patient had no SARS contact history. All patients had a fever, and the mean duration of the fever was 8 days (range, 4–10 days). During the course of hospitalization, all patients were initially treated with oral ribavirin (40–60 mg/kg daily), which was continued for a mean duration of 10 days (range, 3–14 days). Seven were treated with oral prednisolone starting at a mean of 7 days (range, 6–10 days) after the onset of fever, and the duration of prednisolone treatment was 14 days.
The plasma samples were subjected to SELDI ProteinChip analysis, similar to the method described previously (8). We denatured proteins in 3 μL of the plasma sample by adding 6 μL of U9 solution (9 mol/L urea, 20 g/L CHAPS, 50 mmol/L Tris-HCl, pH 9), and diluted them with 51 μL of binding buffer (50 mmol/L sodium acetate, 1 mL/L Triton X-100, pH 4.0) to give a final dilution of 20-fold. CM10 ProteinChip arrays were preequilibrated twice with 5 μL of binding buffer for 5 min, after which 5 μL of each diluted sample was applied to the ProteinChip array in duplicate and incubated with shaking at room temperature for 90 min. After the incubation, each array was washed five times with the binding buffer and rinsed twice with deionized water. After air-drying, sinapinic acid matrix in 500 mL/L acetonitrile containing 5 mL/L trifluoroacetic acid was added to each array. The ProteinChip Arrays were read on the ProteinChip PBS II reader of a ProteinChip Biomarker System (Ciphergen Biosystems) to measure the masses and intensities of the protein peaks. The common peaks among the SELDI mass spectra were identified and quantified by Biomarker Wizard software (Ciphergen Biosystems). Before data mining, the peak intensities were normalized with the total peak intensities, followed by log2 transformation.
To avoid the identification of proteomic features that might be associated only with the investigated SARS patients but not the SARS disease itself, SARS-specific proteomic features were defined as SELDI proteomic features that fulfilled three criteria: (a) normalized peak intensities that were increased in the SARS patients at the onset of the fever; (b) normalized peak intensities that were decreased at the time of recovery; and (c) normalized peak intensities that were positively correlated with the concentrations of SARS-CoV in plasma.
We compared the normalized proteomic data between the earliest available plasma samples collected from the pediatric SARS patients (6 within the first week, 1 on day 8, and 1 on day 11) and the 15 control plasma samples from pediatric patients with influenza and fever, using the two-class unpaired test at a median false discovery rate of zero [Significance Analysis of Microarrays Algorithm; Stanford University (10)]. We identified 41 proteomic features that were positively associated with SARS. Among these 41 proteomic features, normalized peak intensities of 24 proteomic features significantly decreased on recovery (Wilcoxon signed-rank test, P <0.05). The Spearman rank correlation test was used to analyze the correlation between these 24 proteomic features and SARS-CoV RNA in the serial plasma samples from the eight pediatric SARS patients. Fifteen were positively correlated with the plasma SARS-CoV RNA concentrations (all P values <0.05; see Table 1 in the Data Supplement that accompanies the online version of this Technical Brief at http://www.clinchem.org/content/vol50/issue8/). Fig. 1 illustrates the similarities between the changes in the plasma concentrations of SARS-CoV RNA and the peak intensities of three representative SARS-specific proteomic features during the treatment period.
Figure 1.
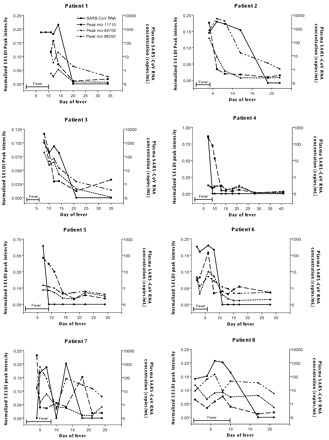
Serial analysis of SARS-CoV RNA concentrations (•) and three representative disease-specific plasma proteomic features at m/z 11 710 (▪), m/z 84 700 (♦), and m/z 98 250 (▴) in pediatric SARS patients.
The proteomic features at m/z 11 710 and m/z 98 250 gave the highest ROC curve areas for differentiating SARS patients from controls, whereas the peak at m/z 84 700 showed the highest correlation with circulating SARS-CoV RNA.
The potential use of the SARS-specific proteomic features in the detection of pediatric SARS patients within the first week of admission was evaluated by ROC curve analysis for the differentiation of 7 SARS samples (6 collected within the first week and 1 collected on the 8th day of admission) from the 15 control samples. The ROC curve areas of the SARS-specific proteomic features were between 0.800 and 0.981 (all P values <0.05; see Table 1 in the online Data Supplement). Our results suggest that all of the identified SARS-specific proteomic features are potential markers for detection of SARS.
Recently, the reliability of the studies using SELDI-TOF MS technology has been questioned. It has been suggested that the findings may be biased by artifacts related to the nature of the clinical samples used, the experimental details, the sample storage conditions, the mass spectrometric instruments, and/or bioinformatic analysis (11)(12)(13). We believe that such problems can be overcome by the use of good experimental design and stringent criteria for defining a proteomic feature as disease specific so that significant proteomic features could be validated at multiple levels. This study illustrates the success of a stringent and systemic approach for analyzing SELDI proteomic data in the identification of SARS-specific proteomic features. With better fulfillment of different data-filtering criteria, it will become less likely that identified proteomic features are identified by chance and that the identified features result from bias in patient selection. In this study, although 3 filtering criteria were set up for the identification of SARS-specific proteomic features, there were 15 significant proteomic features identified. Later ROC curve analyses showed that all of these SARS-specific proteomic features were potential biomarkers for detection of SARS.
Characterization of these circulating SARS-specific proteomic features may help us to understand the disease pathology and patient response. It has been suggested that the SELDI-TOF MS technology has a preference toward detection of high-abundance protein molecules, such as acute-phase reaction proteins. The 11.7-kDa haptoglobin α-subunit (14) and 11.6- and 11.8-kDa isoforms of serum amyloid A (15) have been identified by this technology as cancer-associated proteomic features. It is possible that some of the SARS-specific proteomic features identified in this study are also acute-phase proteins. For example, the SARS-specific proteomic feature at m/z 11 710 may be the haptoglobin α-subunit (11.7 kDa) or the 11.6- or 11.8-kDa serum amyloid A isoforms. The proteomic feature at m/z 78 300 may be transferrin (78 kDa) or clusterin (∼80 kDa). The proteomic feature at m/z 165 600 may be doubly charged (2 H+) fibrinogen (332 kDa). A recent Chinese study has shown that serum concentrations of the acute-phase proteins complement 4 and C-reactive protein were significantly higher in patients with SARS than those in patients with other pneumonias and in healthy individuals (16). In cats, feline coronavirus infection also led to increased concentrations of haptoglobin, serum amyloid A, α1-acid glycoprotein, IgG, and IgM (17).
In conclusion, this study has demonstrated the presence of unique proteomic signatures in the sera of SARS patients, which were increased within the first week of infection, decreased on recovery, and were positively correlated with SARS-CoV viral load. A similar study on adult samples is being undertaken to determine whether there are similar changes in the plasma/serum proteomic patterns. SELDI TOF-MS, which can be performed in a 96-well microplate format, is a high-throughput technology. It may be possible to develop a quick SELDI ProteinChip assay for detection and monitor of potential SARS patients.
Supplementary Material
Acknowledgments
The authors are supported by the Research Fund for the Control of Infectious Diseases (RFCID) from the Health, Welfare and Food Bureau of the Hong Kong SAR Government.
References
- 1.Tomlinson B, Cockram C. SARS: experience at Prince of Wales Hospital, Hong Kong. Lancet 2003;361:1486-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly C, Ghani AC, Leung GM, Hedley AJ, Fraser C, Riley S, et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 2003;361:1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization Multicentre Collaborative Network for Severe Acute Respiratory Syndrome (SARS) Diagnosis. A multicentre collaboration to investigate the cause of severe acute respiratory syndrome. Lancet 2003;361:1730-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peiris JSM, Chu CM, Cheng VCC, Chan KS, Hung IFN, Poon LLM, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng EKO, Hui DS, Chan KCA, Hung ECW, Chiu RWK, Lee N, et al. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem 2003;49:1976-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng EK, Ng PC, Hon KLE, Cheng WTF, Hung ECW, Chan KCA, et al. Serial analysis of the plasma concentration of SARS coronavirus RNA in pediatric patients with severe acute respiratory syndrome. Clin Chem 2003;49:2085-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petricoin EF, III, Ardekani AM, Hitt AB, Levine PJ, Fusaro VA, Steinberg SM, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet 2002;359:572-577. [DOI] [PubMed] [Google Scholar]
- 8.Poon TCW, Yip TT, Chan ATC, Yip C, Yip V, Mok TSK, et al. Comprehensive proteomic profiling identifies serum proteomic signatures for detection of hepatocellular carcinoma and its subtypes. Clin Chem 2003;49:752-760. [DOI] [PubMed] [Google Scholar]
- 9.Hon KLE, Leung CW, Cheng WTF, Chan PK, Chu WC, Kwan YW, et al. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet 2003;361:1701-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 2001;98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamandis EP. Proteomic patterns in biological fluids: do they represent the future of diagnostics?. Clin Chem 2003;49:1272-1275. [DOI] [PubMed] [Google Scholar]
- 12.Diamandis EP. Analysis of serum proteomic patterns for early cancer diagnosis: drawing attention to potential problems. J Natl Cancer Inst 2004;96:353-356. [DOI] [PubMed] [Google Scholar]
- 13.Diamandis EP. Mass spectrometry as a diagnostic and a cancer biomarker discovery tool: opportunities and potential limitations. Mol Cell Proteomics 2004;3:367-368. [DOI] [PubMed] [Google Scholar]
- 14.Ye B, Cramer DW, Skates SJ, Gygi SP, Pratomo V, Fu L, et al. Haptoglobin-α subunit as potential serum biomarker in ovarian cancer: identification and characterization using proteomic profiling and mass spectrometry. Clin Cancer Res 2003;9:2904-2911. [PubMed] [Google Scholar]
- 15.Cho WC, Yip TT, Yip C, Yip V, Thulasiraman V, Ngan RK, et al. Identification of serum amyloid a protein as a potentially useful biomarker to monitor relapse of nasopharyngeal cancer by serum proteomic profiling. Clin Cancer Res 2004;10:43-52. [DOI] [PubMed] [Google Scholar]
- 16.Liao WJ, Li YM, Chen T, He WQ, Lin YP, Li N. Determination of serum acute phase reaction protein in patients with severe acute respiratory syndrome. Zhonghua Yu Fang Yi Xue Za Zhi 2004;38:92-93. [PubMed] [Google Scholar]
- 17.Giordano A, Spagnolo V, Colombo A, Paltrinieri S. Changes in some acute phase protein and immunoglobulin concentrations in cats affected by feline infectious peritonitis or exposed to feline coronavirus infection. Vet J 2004;167:38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


