Graphical abstract
Keywords: Copper doping, BET, Light adsorption, Polyurethane, Bioaerosol removal
Highlights
-
•
Cu doping was used to enhance the photocatalytic activity of TiO2 photocatalyst.
-
•
Titania was deposited on porous polyurethane by using the bridging role of silicon.
-
•
Use of the porous polyurethane increased the adsorption ability of the photocatalyst.
-
•
Both PU and TiO2/PU removed C. famata from aerosols under visible light via adsorption.
-
•
Under visible light, Cu–TiO2/PU removed C. famata via both adsorption and photo-oxidization.
Abstract
Polyurethane (PU), a honeycomb structure material, was used as a substrate onto which TiO2 and Cu–TiO2 were deposited in order to integrate the adsorption property to the photocatalysts. TiO2 deposited on PU (TiO2/PU) and Cu-doped TiO2 deposited on PU (Cu–TiO2/PU) were synthesized and applied to the removal of Candida famata (C. famata), a frequently encountered airborne yeast. The removal capacities of C. famata by PU, TiO2/PU and Cu–TiO2/PU were 1.5 × 105, 3.2 × 105 and 6.9 × 105 (CFU/cm3) under dark condition and 1.5 × 105, 3.3 × 105 and 1.8 × 106 (CFU/cm3) under visible light irradiation, respectively. PU and TiO2/PU seemed to exhibit only an adsorption ability for removing C. famata in aerosols under both dark and visible light. The C. famata removal capacity of Cu–TiO2/PU under visible light was increased 2.6-fold compared to that under dark condition. This significant increase was attributed to the Cu dopant, which enhanced the electron–hole separation efficiency and capacity of TiO2, resulting in the high photocatalytic activity of Cu–TiO2/PU under visible light.
1. Introduction
Exposure to a wide class of bioaerosols, containing microorganisms (bacteria, fungi, viruses, pollens), their fragments and the by-products of their metabolism, can result in various adverse effects to human health including infectious diseases, respiratory pathologies and allergic reactions [1], [2]. Such adverse effects have been increasing recently with examples such as severe acute respiratory syndrome (SARS) in 2003 and influenza (H1N1) in 2009 [3]. In addition, the population and resistance of species in bioaerosols or biohazards have also been increasing rapidly, possibly due to increased environmental pollution originated from human activities. Therefore, numerous studies have been conducted to develop powerful technologies and devices for biohazard control [3], [4], [5], [6]. Traditional control methods to eliminate the microorganisms in the aerosol environment include uses of activated charcoal filters and high efficiency particulate air (HEPA) filters with or without UV radiation [4]. Electrostatic field and ozonizers have also been used to inactivate airborne microorganisms [3]. However, organic matter, both dust and microorganism fractions, tends to accumulate in the filters, which in turn become an emission source of microorganisms under certain conditions such as suitable temperature, humidity and presence of nutrients. The disadvantages of using UV irradiation, electrostatic field and ozonizers include high operating costs, high energy consumption, safety issues and the generation of toxic by-products. Therefore, the photocatalytic process combined with adsorption power has been proposed as a potential technology to overcome these limitations. In addition, visible light is progressively replacing UV light in many applications [7], [8]. Thus, the study continuously utilizes visible light as a potential source to initiate for photocatalysis processes.
Photocatalysts can inactivate a wide range of harmful microorganisms such as viruses, bacteria, fungi and algae, as well as cancer cells or protozoa [9]. TiO2 has been widely used as a photocatalyst for the disinfection of aqueous liquid phases due to its superior characteristics such as exceptional optical and electronic properties, chemical stability, non-toxicity and low cost [10], [11], [12], [13], [14], [15], [16], [17], [18]. Recently, the removal of biological airborne pollutants using TiO2 as photocatalyst has also been reported [1], [9]. As the provided energy is higher than the band gap energy of TiO2, electron–hole pairs can be generated on the surface of the photocatalyst and the resulting electrons and holes can react with water and/or oxygen to produce oxidative species, including hydroxyl radical and superoxide radicals [19]. These oxidative species, which are all highly reactive, can then participate in the oxidation reactions to decompose organic compounds contained in microorganisms absorbed on the photocatalyst surface, leading to the mineralization of the microorganism’s cells or their death [9]. However, the practical use of TiO2 has been limited by the following three major issues: (1) low photocatalytic efficiency due to fast recombination of the photogenerated electrons and holes, (2) high energy requirement due to the wide band gap energy of TiO2, which is around 3.2 eV, and (3) recovery issues of TiO2 powder [20], [21].
To overcome these limitations, we have previously used Cu as a doping agent to act as an electron sink to prevent recombination of the photogenerated electrons and holes of TiO2 [22]. Cu doping also decreased the band gap energy of TiO2 and hence the energy requirement for electron–hole pair generation of TiO2. Then, the Cu-doped TiO2 was deposited on glass fiber to overcome the disadvantages of TiO2 powder. The Cu-doped TiO2/glass fiber exhibited high photocatalytic disinfection activity against Escherichia coli in bioaerosol even under visible light irradiation [22]. However, the synthesized Cu-doped TiO2/glass fiber photocatalysts mainly exhibited photocatalytic oxidation properties to inactivate bacteria. The low adsorption properties of the photocatalyst were attributed to the low surface area of the glass fiber substrate. Therefore, in the present study, we investigate a new high surface area substrate onto which the Cu-doped TiO2 is deposited in order to integrate the adsorption properties of the photocatalyst. Polyurethane (PU) foam, a polymer composed of a chain of organic units joined by urethane links with a honeycomb structure and high surface area, could be a suitable substrate for the deposition of Cu-doped TiO2 [23], [24]. The resulting Cu–TiO2/PU photocatalyst is expected to exhibit high bioaerosol removal efficiency by combining both adsorption and photocatalytic oxidation activities. In our previous study, the Cu-doped TiO2/glass fiber exhibited a significant disinfection activity to remove E. coli in bioaerosols. However, the wide diversity of the bioaerosol world is exemplified by the different structure of each strain, and hence different resistance to photocatalytic oxidation and adsorption. The present study, therefore, examines the bioaerosol removal efficiency of Cu–TiO2/PU, which could be fabricated as a dual adsorption/photocatalytic material, against Candida famata, a frequently encountered airborne yeast, as a new microorganism target [25]. It is hoped that the experimental results will lead to the adsorption and photocatalytic purification of bioaerosols under visible light irradiation.
2. Experimental and analysis
2.1. Material preparation and characterization
Before the TiO2 photocatalyst was deposited on the PU substrate, pristine PU was activated to introduce isocyanate groups (NCO) on its surface. Silicon in γ-aminopropyl triethoxysilane (APTES) was used as a bridge element to silanize titanium by creating a Si–O–Ti bond. Then, TiO2 was deposited on the PU surface, based on the urea bond formed from the reaction of the isocyanate group in the activated PU with the amino group (NH2) in the silanized titanium. Scheme 1 shows the four-step material preparation processes: pre-treatment of pristine PU, silanization process, coating TiO2 on activated PU and synthesis of Cu–TiO2/PU.
Scheme 1.
Material preparation processes.
2.1.1. Pre-treatment of pristine PU
Pristine honeycomb PU foams (2 cm × 2 cm × 3 cm) were cleaned under strong ultrasonication condition for 20 min each in toluene and ethanol solutions, dried at 40 °C under vacuum for 2 h and then placed in a mixed solution of toluene (50 mL), toluene-2,4-diisocyanate (TDI) (5 mL), and anhydrous triethylamine (2 mL) in a 150 mL flask. The solution containing PU was stirred continuously and heated to 60 °C for 1 h under nitrogen as a protective gas in order to introduce isocyanate groups (NCO) on the surface of the PU [26]. After this procedure, the PU containing isocyanate groups (activated PU) was washed repeatedly with toluene and dried in nitrogen gas at 60 °C for 4 h.
2.1.2. Silanization process
To prepare for the silanization titanium, 5 mL titanium tetraisopropoxide (TTIP) solution was slowly dropped into a flask containing 100 mL toluene under stirring condition [27]. Then, 100 mL toluene containing 4% APTES was added to the flask. The mixture flask was stirred continuously for 1 h and then kept in an oven at 40 °C for 4 h to silanize the titanium. The final product was a colloid solution of titanosiloxane.
2.1.3. TiO2 coating process
The activated PU was immersed in the titanosiloxane solution at 40 °C for 1 h, after which the TiO2 coated on PU was slowly washed with 100 mL distilled water, dried at 80 °C for 2 h, and calcined in protective nitrogen gas at 200 °C for 2 h to obtain the TiO2/PU photocatalysts.
2.1.4. Synthesis Cu–TiO2/PU
A 0.1 M Cu(NO3)2 solution was slowly dropped onto TiO2/PU after the immersing process to synthesize Cu–TiO2/PU photocatalysts. The addition volume of Cu(NO3)2 was adjusted to synthesize Cu–TiO2/PU materials with 6% weight fractions of Cu/TiO2. Then, the Cu–TiO2/PU was cleaned using 50 mL of 1 M oxalic acid solution. The obtained material was continuously irradiated with an UV-C light (60 W) for 5 h and calcined in protective nitrogen gas at 200 °C for 2 h to afford Cu–TiO2/PU photocatalyst.
2.1.5. Analysis of physicochemical properties
A Nicolet 380 spectrometer was used to obtain Fourier transform infrared spectroscopy (FTIR) spectra of the pristine PU, activated PU, TiO2/PU and Cu–TiO2/PU materials. A Thermo Fisher K-Alpha X-ray Photoelectron Spectrometer system was used to obtain X-ray photoelectron spectroscopy spectra (XPS) of TiO2/PU and Cu–TiO2/PU to determine the chemical composition of the synthesized materials. A Hitachi S-4700 scanning electron microscope (SEM) was used to determine the surface morphology of the PU, TiO2/PU and Cu–TiO2/PU. An energy dispersive X-ray spectrometer was integrated to the Hitachi S-4700 SEM to determine X-ray mapping images of Cu and Ti in the Cu–TiO2/PU. The Brunauer–Emmett–Teller (BET) isotherm was used to calculate the surface area (S BET) of the PU, TiO2/PU and Cu–TiO2/PU materials. An Evolution 300 spectrophotometer (UV-1700 Shimadzu) was used to measure the UV–Vis absorption spectra of the shredded pristine PU, TiO2/PU and Cu–TiO2/PU.
2.2. Yeast removal methods
2.2.1. Yeast cultivation
C. famata (obtained from an environmental bioengineering laboratory at the University of Ulsan, Korea) was cultivated at 30 °C in YPD medium (0.5% yeast extract, 1% peptone and 2% glucose) for 48 h. Then, 400 mL cultivated yeast was centrifuged at 500 rpm for 3 min to obtain 200 mL condensed yeast for use as the yeast source for each removal experiment.
2.2.2. Experimental model design
An experimental model was designed to test the yeast removal efficiency using the synthesized materials (Fig. 1 ). Fresh air (input air) was pumped into the condensed yeast solution to incite the yeast in the condensed yeast solution and was diffused into aerosol to generate an atmospheric yeast stream that was pumped to two similar volume pipes. The atmospheric yeast in pipe 1 was directed to phosphate buffer saline (PBS) solution (5) by pump (4). The atmospheric yeast in pipe 2 was directed to pass through the reaction chamber by pump (10) (Fig. 1). The top and bottom of the reaction chamber were made of quartz so that the experimental light could easily pass through the reaction chamber walls and irradiate the synthesized materials inside. The reaction chamber was isolated in a cask with a dark cover that ensured no light penetration to prevent any interference with the photocatalytic activities. Two 20 W fluorescent bulbs (1200 lm) were placed at the top and bottom of the isolated cask to generate visible light with power density of 0.025 W/cm2 for the photocatalytic activities.
Fig. 1.
Experimental model for C. famata removal tests.
2.2.3. Yeast removal capacity
PU, TiO2/PU and Cu–TiO2/PU were used as photocatalytic and adsorption materials for the yeast removal experiments. The light bulbs in the isolated cask could be turned off or on to create dark or visible light condition, respectively. The flow rate of the two pumps was maintained almost constant at 2 L/min during each experiment so that almost the same amount of yeast passed through pipes 1 and 2 (Fig. 1). The yeast in pipe 1, for absorption into PBS solution (5), was considered as the input yeast amount of the yeast removal processes. The remaining yeast in pipe 2, which passed through the reaction chamber, was absorbed into the PBS solution (11) to determine the output yeast amount of the yeast removal processes. According to the count method reported by White et al., the amount of the viable yeast (C. famata) in the PBS solution was determined by counting colony-forming unit (CFU) on agar plates [28]. The reaction time, which was similar to the working time of the pumps, was adjusted to control the input yeast amount for each removal experiment. The yeast removal capacities by using pristine PU, TiO2/PU and Cu–TiO2/PU as photocatalytic and adsorption materials (having volume of 12 cm3) under dark and visible light conditions were calculated by Eq. (I):
| (I) |
In the control experiments, the reaction chamber was kept empty but other procedures were similar to the previous yeast removal experiments. The amount of yeast absorbed on the solutions (5) and (11) was compared to verify the hypothesis, assuming that the yeast passing through pipe 1, considered as the input yeast amount, was almost the same as that passing through pipe 2 used for the yeast removal experiments.
3. Results and discussion
3.1. Material characterization
3.1.1. TiO2 coating
Fig. 2 shows the FTIR spectrum results of pristine PU, activated PU and TiO2/PU. The FTIR bands and their assigned vibrations are shown in Table 1 . The FTIR spectra indicate that the urethane group (–NH–CO–O–), which contains N–H, C–N, C O and C–O bonds, in the pristine PU was successfully converted to an isocyanate group (–N C O) in the activated PU. The Si–O–Ti bond recorded in the FTIR spectra of TiO2/PU confirmed the silanization process between TTIP and APTES to create titanosiloxane (Scheme 1). As compared to the FTIR spectrum of the activated PU, the FTIR spectrum of TiO2/PU showed a strong increase in the N–H and C O adsorption peaks, which are typical peaks of the urea group (–NH–CO–NH–). However, the isocyanate peak in TiO2/PU greatly decreased as compared to that of the activated PU. The peak intensity change revealed the successful formation of the urea linkage based on reaction between the isocyanate group of the activated PU and the amine group of the titanosiloxane (Scheme 1). Therefore, titania was successfully coated on the surface of PU by using the bridging role of APTES. Silicon in APTES could link to titania in TTIP via the Si–O–Ti bond. The amine group in the APTES could react with the isocyanate group in the activated PU to create a urea linkage. The FTIR spectra of the TiO2/PU exhibited adsorption peaks at 670 and 570 cm−1, which were assigned to vibrations of the Ti–O–Ti bond. The peaks indicated that TiO2 was coated on the surface of PU based on reaction between titanium alkoxide and H2O.
Fig. 2.
FTIR spectra of pristine PU, activated PU and TiO2/PU.
Table 1.
FTIR bands and assigned vibrations.
| Wavenumber (cm−1) | Materials | Assigned vibrations | References |
|---|---|---|---|
| 3400–3250 | C | N–H | [53], [54] |
| 2950–2850 | A, B, C | C–H | [55] |
| 2250 | B, C | N C O | [56] |
| 1730 | A, C | C O | [53], [54] |
| 1600 | A, B, C | C C | [53], [54] |
| 1510, 1240 | A, C | C–N | [53], [54] |
| 1200 | C | C–Si | [57] |
| 1100, 790 | C | Si–O–Si | [58] |
| 970 | C | Si–O–Ti | [58] |
| 570, 670 | C | Ti–O–Ti | [16] |
Note: A – pristine PU, B – activated PU, C – TiO2/PU.
3.1.2. Cu dopant
Fig. 3 shows the high-resolution XPS spectrum of the Cu 2p3/2 peak of Cu in Cu–TiO2/PU. The Gaussian multipeak shapes were applied to fit the Cu 2p3/2 peak to determine the state of copper in Cu–TiO2/PU. The peak analysis result revealed two components of copper in Cu–TiO2/PU: Cu+ in Cu2O (932.3 eV) and Cu2+ in CuO (933.5 eV) [29]. CuO in Cu–TiO2/PU was produced from thermal decomposition of Cu(NO3)2 during UV irradiation or calcination processes [22]. The UV irradiation (photo-reduction) and oxalic acid, rolled as a cleaner for the cleaning process, could reduce Cu2+ into Cu+ during the decomposition of Cu(NO3)2 to form Cu2O [30], [31], [32], [33]. Lin et al. reported that the Cu2+ cations could penetrate into the TiO2 lattice and substitute the Ti4+ cations, leading to formation of Cu–O–Ti bonds [34]. The substitution of Cu2+ into TiO2 lattice was confirmed by XRD analysis (see Supplementary material). The substitution could also lead to the formation of Cu+ in TiO2 lattice. Therefore, the elemental states of the Cu dopant in Cu–TiO2/PU included Cu+ and Cu2+, most of which existed in Cu2O and CuO forms, respectively, which are physically adsorbed on the TiO2 surface. Another Cu+ existed in the TiO2 lattice in the form of Cu–O–Ti bonds.
Fig. 3.
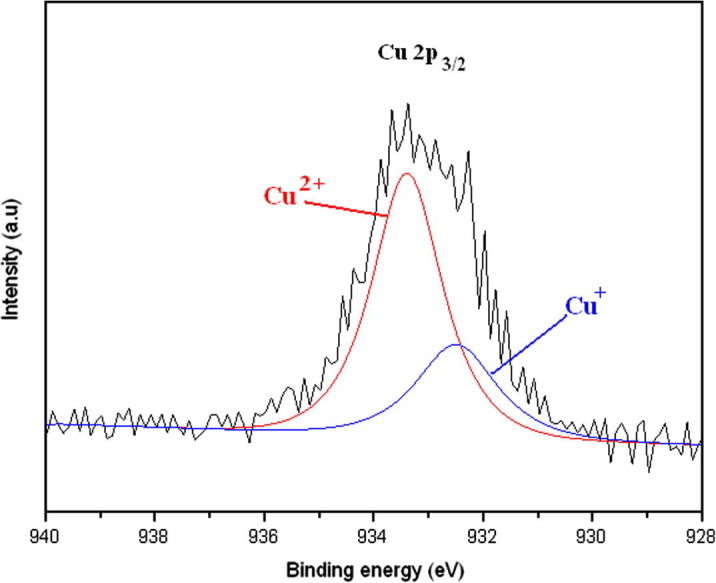
High-resolution XPS spectrum of Cu 2p3/2 in Cu–TiO2/PU.
3.1.3. Morphology and surface area
Fig. 4A shows that the pristine PU contained pores sized from 100 to 300 μm. Since, TiO2 was smoothly and thinly coated on the PU surface in TiO2/PU, the TiO2 layer did not greatly affect the pore size change of the PU substrate (Fig. 4B). The SEM features of Cu–TiO2/PU exhibited small Cu2O and CuO particles that were deposited with good dispersion on the TiO2 layer (Fig. 4C). Fig. 4D shows the mixed mapping of Ti and Cu elements in the Cu–TiO2/PU material. The elemental mapping clearly shows that the Ti and Cu elements were deposited almost evenly or alternately on the PU frame, which may have caused the distinct photocatalytic activity of Cu–TiO2/PU for bioaerosol removal.
Fig. 4.
SEM of PU (A), TiO2/PU (B), Cu–TiO2/PU (C) and mapping of Cu and Ti in Cu–TiO2/PU (D).
The BET surface area of the synthesized TiO2/PU was 111.4 m2/g, which was much higher than that of PU (31.3 m2/g) or TiO2 commercial powder (AEROXIDE® TiO2 P25) obtained from Evonik Degussa Corporation (60 m2/g) [35]. The high surface area indicates that the immobilization process of TiO2 onto the surface of PU, which could minimize the coagulation of the TiO2 particles, strongly enhanced the surface area of TiO2. In addition, during TiO2/PU preparation process, several silicon of APTES could substitute Ti4+ ions in TiO2 lattice to form Si–O–Ti bond (Table 1) contributing to enhancement in surface area and porosity of TiO2/PU [36]. Thus, the surface area of TiO2/PU could be higher than that of pure TiO2. The BET surface area of the synthesized Cu–TiO2/PU was 166.3 m2/g, which was higher than that of PU or TiO2/PU. This strong improvement in the surface area was attributed to significant deposition of small Cu2O and CuO particles on the TiO2 layer (Fig. 4C). The distribution of the particles increased the degree of surface roughness leading to increase in the surface areas of the Cu–TiO2/PU.
3.1.4. Light adsorption ability
The UV–Vis adsorption spectra of PU, TiO2/PU and Cu–TiO2/PU are shown in Fig. 5 . As compared to TiO2/PU and Cu–TiO2/PU, PU did not exhibit any adsorption ability for light in the wavelength range from 300 to 700 nm. The UV–Vis adsorption spectrum of TiO2/PU exhibited an adsorption edge at 390 nm. However, it did not exhibit any noticeable adsorption in the visible region, which corresponds to the light adsorption properties of TiO2 [37]. As compared to the UV–Vis adsorption spectrum of TiO2/PU, that of Cu–TiO2/PU, however, not only showed a red shift of the absorption edge but also exhibited higher adsorption ability in the visible region. The red shift of the absorption edge for Cu–TiO2/PU was possibly due to the absorption role of CuO in the synthesized material. Pham and Lee reported that a composite material, containing TiO2 and CuO, could create a suitable semiconductor–semiconductor system that decreased the band gap energy of TiO2 leading to a red shift of the absorption edge of the synthesized material [22]. The direct interfacial electron transfer from the valence band of TiO2 to the valence band and conduction band of CuO also led to the red shift of the absorption edge for Cu–TiO2/PU [38]. The strong enhancement of the light adsorption ability of Cu–TiO2/PU was attributed to the Cu dopant, which increases the light-harvesting ability of TiO2 within the visible light region [39]. This enhanced visible light absorbance of Cu–TiO2/PU is expected to considerably increase the photocatalytic activity of the synthesized material in the visible light region.
Fig. 5.
UV–Vis absorption spectra of PU, TiO2/PU and Cu–TiO2/PU.
3.2. Yeast removal
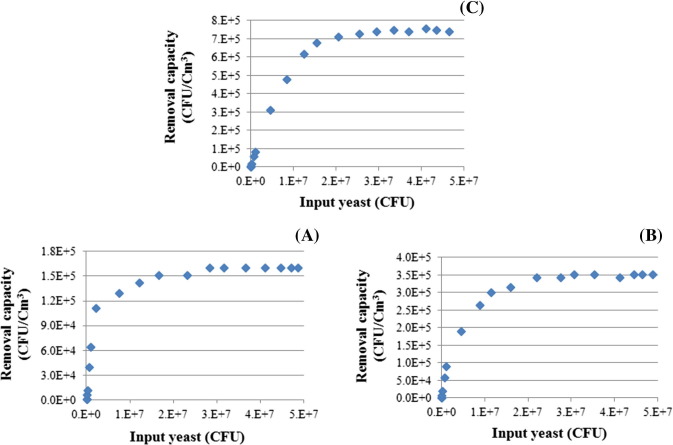
The control experimental results (data not shown) indicated that when the reaction chamber was empty, the yeast amounts absorbed into the PBS solutions (5) and (11) were similar and that the yeast amounts passing through pipes 1 and 2 were similar. Therefore, the hypothesis that the yeast in pipe 1 could be considered as the input yeast amount of the yeast removal experiment was verified. The aerosol yeast removal capacities by PU, TiO2/PU and Cu–TiO2/PU under dark and visible light over the input yeast concentration range of 500–5 × 107 (CFU) are shown in Fig. 6, Fig. 7 . The yeast removal capacities initially increased with increasing input yeast to a maximum but then plateaued with further increase in input yeast. Table 2 shows the stable maximum yeast removal capacities by PU, TiO2/PU and Cu–TiO2/PU under dark and visible light.
Fig. 6.
Yeast removal capacities by PU (A), TiO2/PU (B) and Cu–TiO2/PU (C) under dark condition.
Fig. 7.
Yeast removal capacities by PU (A), TiO2/PU (B) and Cu–TiO2/PU (C) under visible light condition.
Table 2.
Maximum yeast removal capacity (CFU/cm3) by different materials under dark and light conditions.
| Materials | PU |
TiO2/PU |
Cu–TiO2/PU |
|||
|---|---|---|---|---|---|---|
| Conditions | Dark | Vis-light | Dark | Vis-light | Dark | Vis-light |
| Removal capacity (CFU/cm3) | 1.5 × 105 | 1.5 × 105 | 3.2 × 105 | 3.3 × 105 | 6.9 × 105 | 1.8 × 106 |
3.2.1. Yeast removal by PU
The maximum removal capacity of the aerosolized yeast by PU was similar under both dark and visible light conditions: 1.5 × 105 (CFU/cm3) (Table 2). This result indicated that PU only acted as an absorbent in removing the aerosolized yeast under both dark and visible light conditions. The reported yeast removal mechanism indicates that a microorganism is adhered into an adsorbent. Kara et al. reported that removal of a microorganism is a complex process affected by many parameters such as the chemical and physical properties of the adsorbent, the microorganism’s characteristics and the environmental conditions [40]. The porous structure and large surface area of the PU are favorable for the adsorption of airborne microorganisms [41]. Yeast can be adhered on the inner surface of the pores of PU by van der Waals force [42]. The yeast adsorption mechanism by PU is also due to its hydrophobicity and non-polarity. Most previous studies reported that microorganisms tend to attach to hydrophobic and non-polar materials [41], [43]. Li et al. reported that hydrophobic interaction between the yeast cell surface and the adsorbent surface can enable the cell to overcome the repulsive forces within a certain distance from the adsorbent and yeast [41].
3.2.2. Yeast removal by TiO2/PU
The maximum removal capacity of the aerosolized yeast by TiO2/PU under visible light condition was 3.3 × 105 (CFU/cm3), which was only slight increased from the capacity of 3.2 × 105 under dark light condition. This was due to the TiO2 photocatalysts wide band gap energy of approximately 3.2 eV, which required excitation light with a wavelength lower than 388 nm to excite for the photocatalytic process. Therefore, visible light could not significantly excite the electron–hole pairs necessary for TiO2/PU to develop any photocatalytic activity to remove the aerosolized yeast. Thus, TiO2/PU only exhibited an adsorption ability to remove the aerosolized yeast under both dark and visible light conditions. The maximum removal capacity of the aerosolized yeast by TiO2/PU was twice that of PU, which was attributed to the much higher surface area of TiO2/PU (111.4 m2/g) compared to that of PU (31.3 m2/g). Li et al. reported that the surface area is one of main factors affecting the increased adsorption ability of microorganisms by an adsorbent [41]. The van der Waals force, which could adhere the yeast into the inner surface of pores of TiO2/PU, contributed strongly to the adsorption process [42]. The SiO2 component in the TiO2/PU could enhance the free energy or charge density on the surface of TiO2/PU [41], [42]. This charge density increase could increase the electrostatic interaction between the surface of the TiO2/PU and the charges on the cell wall of the yeast, thereby increasing the adhesion between the yeast and TiO2/PU [44]. Therefore, the yeast adsorption ability of TiO2/PU was higher than that of PU.
3.2.3. Yeast removal by Cu–TiO2/PU
The maximum removal capacity of the aerosolized yeast by Cu–TiO2/PU under dark condition was 6.9 × 105 (CFU/cm3), which was approximately five- and two-fold higher than that by PU and TiO2/PU, respectively. This significant enhancement was firstly due to the increased surface area of Cu–TiO2/PU (166.3 m2/g), which was much higher than that of PU (31.3 m2/g) and TiO2/PU (111.4 m2/g). Kara et al. reported that the rough surface of an adsorbent could promote adhesion of the microorganisms [40]. Therefore, the higher surface roughness of Cu–TiO2/PU (Fig. 4C), as compared to PU (Fig. 4A) and TiO2/PU (Fig. 4B), may also have enhanced the yeast removal capacity by Cu–TiO2/PU. In addition, the Cu2+ ions existing on the surface of Cu–TiO2/PU may have killed the yeast and thus increased the yeast removal capacity of the synthesized Cu–TiO2/PU [17], [45].
The maximum yeast removal capacity by Cu–TiO2/PU of 1.8 × 106 (CFU/cm3) under visible light was approximately three-fold higher than that under dark condition. This significant enhancement was attributed to the photocatalytic activity of Cu–TiO2/PU under visible light as the excitation sources. The greatly increased photocatalytic activity even under visible light could be attributed to the role of the Cu dopant in Cu–TiO2/PU. The doped Cu could defect into the TiO2 lattice leading to reduction of Ti4+ ions into Ti3+ ions. The formation of Ti3+ in the TiO2 lattice was confirmed by XPS analysis of titanium (see Supplementary material). Thus, in Cu–TiO2/PU, the titanium was found to exist in the forms of Ti3+ and Ti4+, while copper existed in the forms Cu+ and Cu2+. All these different ionic states of titanium and copper created a mixture of different Fermi levels as shown in Fig. 8 [21], [46], [47]. When considering the mixture of Fermi levels, the low energy levels provided by incident visible light could be utilized to transfer electrons from the valence band (VB) to the intermediate Fermi levels of Cu+, Cu2+ and Ti3+, and finally, to the Ti4+ conduction band (CB), in series (Fig. 8). CuO and Cu2O oxides existing on the surface of Cu–TiO2/PU could also act as electron sinks to inhibit the recombination of the photo-generated electrons with holes, thus increasing the lifetime of the photo-generated electrons or electron–hole pair separation efficiency [48]. In addition, Ti3+ could more easily generate excited electrons than Ti4+ because the electron number of Ti3+ (19) is higher than that of Ti4+ (18). Thus, Cu doping, which led to formation of Ti3+ in TiO2 lattice, could increase the electron–hole separation capacity of the synthesized Cu–TiO2/PU photocatalyst [22], [49]. Therefore, as compared to TiO2, Cu–TiO2/PU exhibited an increase in the electron–hole pair separation efficiency and capacity even under visible light irradiation. The lifetime of the photo-generated electrons in Cu–TiO2/PU was also prolonged longer than that in TiO2/PU. Thus, electron–hole pairs were easily generated and could exist for a longer time when Cu–TiO2/PU was irradiated by visible light. The photo-generated electron–hole pairs then reacted with water and molecular oxygen absorbed on the surface of the Cu–TiO2/PU material, leading to the formation of oxy radicals such as hydroxyl radicals (*OH) and superoxide radical anions (*O2 −) [17], [49], [50], [51]. The formed oxy radicals could cause destruction of the outer cell wall of the yeast, oxidation of coenzyme A and hence inhibition of cell respiration, oxidation of unsaturated phospholipids in the yeast cell membrane, leakage of intracellular K+ ions and detrimental effects on DNA and RNA, leading to inactivation or death of the yeast [5], [45], [50]. After destruction of the outer cell wall of the yeast by oxy radicals, the Cu ions could also permeate inside the cell wall, leading to mutation of key biochemical processes inside of the cell and finally resulting in the death of the yeast cell [50]. In addition, the CuO and Cu2O distributed on surface of TiO2 could also disinfect a certain amount of C. famata via a mechanism called “contact killing” [52].
Fig. 8.
Comparison of disinfection mechanisms between TiO2/PU and Cu–TiO2/PU.
4. Conclusion
The photocatalytic activity of TiO2 was successfully enhanced by the addition of Cu dopant, which acted as both a defecting and an intermediate agent to increase the electron–hole separation efficiency and capacity of TiO2. The adsorption ability was also successfully integrated into the metal-doped TiO2 by using PU as a substrate for the coating process. The yeast removal capacity of the photocatalysts decreased in the order of Cu–TiO2/PU > TiO2/PU > PU under both dark and visible light conditions. The removal of the aerosolized yeast under dark condition was mainly attributed to adsorption ability, which depended on the surface area, surface roughness, polarity, and hydrophobicity of the synthesized materials. The aerosolized yeast removal capacity was highest for Cu–TiO2/PU under visible light condition, at a level 2.6-fold higher than that under dark condition. The greatly enhanced removal capacity of the aerosolized yeast under visible light by Cu–TiO2/PU was mainly due to a combination of the photocatalytic activity and the adsorption ability of the synthesized photocatalysts. Under visible light, the Cu–TiO2/PU photocatalyst could produce oxy radicals, which participated in many oxidation processes to decompose important organic components of yeast and thereby kill the yeast.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (2013R1A2A2A03013138).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cej.2015.10.100.
Appendix A. Supplementary data
This file contains supplementary material.
References
- 1.Pigeot-Remy S., Lazzaroni J.C., Simonet F., Petinga P., Vallet C., Petit P., Vialle P.J., Guillard C. Survival of bioaerosols in HVAC system photocatalytic filters. Appl. Catal. B. 2014;144:654–664. [Google Scholar]
- 2.Pigeot-Remy S., Real P., Simonet F., Hernandez C., Vallet C., Lazzaroni J.C., Vacher S., Guillard C. Inactivation of Aspergillus niger spores from indoor air by photocatalytic filter. Appl. Catal. B. 2013;134–135:167–173. [Google Scholar]
- 3.Xu Z., Yao M. Effects of single-walled carbon nanotube filter on culturability and diversity of environmental bioaerosols. J. Aerosol. Sci. 2011;42:387–396. [Google Scholar]
- 4.Saucedo-Lucero J.O., Quijano G., Arriaga S., Munoz R. Hexane abatement and spore emission control in a fungal biofilter-photoreactor hybrid unit. J. Hazard. Mater. 2014;276:287–294. doi: 10.1016/j.jhazmat.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 5.Grinshpun S.A., Adhikari A., Honda T., Kim K.Y., Toivola M., Rao K.S.R. Control of aerosol contaminants in indoor air: combining the particle concentration reduction with microbial inactivation. Environ. Sci. Technol. 2007;41:606–612. doi: 10.1021/es061373o. [DOI] [PubMed] [Google Scholar]
- 6.Gui X., Wei J., Wang K., Cao A., Zhu H., Yi J. Carbon nanotube sponges. Adv. Mater. 2009;22:617–621. doi: 10.1002/adma.200902986. [DOI] [PubMed] [Google Scholar]
- 7.Bella F., Sacco A., Salvador G.P., Bianco S., Tresso E., Pirri C.F., Bongiovanni R. First pseudohalogen polymer electrolyte for dye-sensitized solar cells promising for in situ photopolymerization. J. Phys. Chem. C. 2013;117:20421–20430. [Google Scholar]
- 8.Zhang S., Li L., Zhao S., Sun Z., Hong M., Luo J. Hierarchical metal–organic framework nanoflowers for effective CO2 transformation driven by visible light. J. Mater. Chem. A. 2015;30:15764–15768. [Google Scholar]
- 9.Chen F., Yang X., Mak H.K.C., Chan D.W.T. Photocatalytic oxidation for antimicrobial control in built environment: a brief literature overview. Build. Environ. 2010;45:1747–1754. [Google Scholar]
- 10.Gaya U.I., Abdullah A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol. C. 2008;9:1–12. [Google Scholar]
- 11.Hu X., Li G., Yu J.C. Design, fabrication, and modification of nanostructured semiconductor materials for environmental and energy applications. Langmuir. 2010;26:3031–3039. doi: 10.1021/la902142b. [DOI] [PubMed] [Google Scholar]
- 12.Guo J.F., Ma B., Yin A., Fan K., Dai W.L. Highly stable and efficient Ag/AgCl@TiO2 photocatalyst: preparation, characterization, and application in the treatment of aqueous hazardous pollutants. J. Hazard. Mater. 2012;211–212:77–82. doi: 10.1016/j.jhazmat.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 13.Amaniampong P.N., Jia X., Wang B., Mushrif S.H., Borgna A., Yang Y. Catalytic oxidation of cellobiose over TiO2 supported gold-based bimetallic nanoparticles. Catal. Sci. Technol. 2015;20:234–239. [Google Scholar]
- 14.Wang J., Wang X., Liu X., Zeng J., Guo Y., Zhu T. Kinetics and mechanism study on catalytic oxidation of chlorobenzene over V2O5/TiO2 catalysts. J. Mol. Catal. A. 2015;402:1–9. [Google Scholar]
- 15.Zhang P., Mo Z., Han L., Wang Y., Zhao G., Zhang C., Li Z. Magnetic recyclable TiO2/multi-walled carbon nanotube nanocomposite: synthesis, characterization and enhanced photocatalytic activity. J. Mol. Catal. A. 2015;402:17–22. [Google Scholar]
- 16.Attarchia N., Montazer M., Toliyat T. Ag/TiO2/β-CD nano composite: preparation and photo catalytic properties for methylene blue degradation. Appl. Catal. A. 2013;467:107–116. [Google Scholar]
- 17.Fisher M.B., Keane D.A., Fernández-Ibanez P., Colreavy J., Hinder S.J., McGuigan K.G., Pillai S.C. Nitrogen and copper doped solar light active TiO2 photocatalysts for water decontamination. Appl. Catal. B. 2013;130–131:8–13. [Google Scholar]
- 18.Chen Q., Liu H., Xin Y., Cheng X. Coupling immobilized TiO2 nanobelts and Au nanoparticles for enhanced photocatalytic and photoelectrocatalytic activity and mechanism insights. Chem. Eng. J. 2014;241:145–154. [Google Scholar]
- 19.Li J., Wu N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: a review. Catal. Sci. Technol. 2015;5:1360–1384. [Google Scholar]
- 20.Ramos-Delgadoa N.A., Gracia-Pinillab M.A., Maya-Trevinoa L., Hinojosa-Reyes L., Guzman-Mara J.L., Hernández-Ramíreza A. Solar photocatalytic activity of TiO2 modified with WO3 on the degradation of an organophosphorus pesticide. J. Hazard. Mater. 2013;263P:36–44. doi: 10.1016/j.jhazmat.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 21.Gurulakshmi M., Selvaraj M., Selvamani A., Vijayan P., Rekha N.R.S., Shanthi K. Enhanced visible-light photocatalytic activity of V2O5/S-TiO2 nanocomposites. Appl. Catal. A. 2012;449:31–46. [Google Scholar]
- 22.Pham T.D., Lee B.K. Cu doped TiO2/GF for photocatalytic disinfection of Escherichia coli in bioaerosols under visible light irradiation: application and mechanism. Appl. Surf. Sci. 2014;296:15–23. [Google Scholar]
- 23.Josset S., Jiesmaili S., Begin D., Edouard D., Huu C.P., Lett M.C., Keller N., Keller V. UV-A photocatalytic treatment of Legionella pneumophila bacteria contaminated airflows through three-dimensional solid foam structured photocatalytic reactors. J. Hazard. Mater. 2010;175:372–381. doi: 10.1016/j.jhazmat.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Liu P., Liu H., Liu G., Yao K. Preparation of TiO2 nanotubes coated on polyurethane and study of their photocatalytic activity. Appl. Surf. Sci. 2012;258:9593–9598. [Google Scholar]
- 25.Yu K.P., Lee G.W.-M., Lin S.Y., Huang C.P. Removal of bioaerosols by the combination of a photocatalytic filter and negative air ions. J. Aerosol Sci. 2008;39:377–392. doi: 10.1016/j.jaerosci.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chattopadhyay D.K., Raju K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007;32:352–418. [Google Scholar]
- 27.Song H.J., Zhang Z.Z., Men X.H. Tribological behavior of polyurethane-based composite coating reinforced with TiO2 nanotubes. Eur. Polym. J. 2008;44:1012–1022. [Google Scholar]
- 28.White P.N., Williams D.W., Kuriyama T., Samad S.A., Lewis M.A.O., Barnes R.A. Detection of Candida in concentrated oral rinse cultures by real-time PCR. J. Clin. Microbiol. 2004;42:2010–2107. doi: 10.1128/JCM.42.5.2101-2107.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jongnavakit P., Amornpitoksuk P., Suwanboon S., Ndiege N. Preparation and photocatalytic activity of Cu-doped ZnO thin films prepared by the sol–gel method. Appl. Surf. Sci. 2012;258:8192–8198. [Google Scholar]
- 30.Zhang Z., Yi J.B., Ding J., Wang L.M., Seng H.L., Wang S.J., Tao J.G., Li G.P., Xing G.Z., Sum T.C., Huan C.H.A., Wu T. Cu doped ZnO nanoneedles and nanonails: morphological evolution and physical properties. J. Phys. Chem. C. 2008;112:9579–9585. [Google Scholar]
- 31.Truong Q.D., Kakihana M. Hydrothermal growth of cross-linked hyperbranched copper dendrites using copper oxalate complex. J. Cryst. Growth. 2012;348:65–70. [Google Scholar]
- 32.Morrison E., Gutiérrez-Tauste D., Domingo C., Vigil E., Ayllón J.A. One step room temperature photodeposition of Cu/TiO2 composite films and its conversion to CuO/TiO2. Thin Solid Films. 2009;517:5621–5624. [Google Scholar]
- 33.Kubacka A., Munoz-Batista M.J., Fernández-García M., Obregón S., Colón G. Evolution of H2 photoproduction with Cu content on CuOx–TiO2 composite catalysts prepared by a microemulsion method. Appl. Catal. B. 2015;163:214–222. [Google Scholar]
- 34.Lin C.J., Yang W.T. Ordered mesostructured Cu-doped TiO2 spheres as active visible-light-driven photocatalysts for degradation of paracetamol. Chem. Eng. J. 2014;237:131–137. [Google Scholar]
- 35.Mahesh K.P.O., Kuo D.H., Huang B.R., Ujihara M., Imae T. Chemically modified polyurethane–SiO2/TiO2 hybrid composite film and its reusability for photocatalytic degradation of Acid Black 1 (AB 1) under UV light. Appl. Catal. A. 2014;475:235–241. [Google Scholar]
- 36.Chen Q., Shi H., Shi W., Xu Y., Wu D. Enhanced visible photocatalytic activity of titania–silica photocatalysts: effect of carbon and silver doping. Catal. Sci. Technol. 2012;2:1213–1220. [Google Scholar]
- 37.Khalid N.R., Ahmed E., Hong Z., Ahmad K.S.M., Zhang Y. Cu-doped TiO2 nanoparticles/graphene composites for efficient visible-light photocatalysis. Ceram. Int. 2013;39:7107–7113. [Google Scholar]
- 38.Chiang L.F., Doong R. Cu–TiO2 nanorods with enhanced ultraviolet- and visible-light photoactivity for bisphenol A degradation. J. Hazard. Mater. 2014;277:84–92. doi: 10.1016/j.jhazmat.2014.01.047. [DOI] [PubMed] [Google Scholar]
- 39.Onsuratoom S., Puangpetch T., Chavadej S. Comparative investigation of hydrogen production over Ag-, Ni-, and Cu-loaded mesoporous-assembled TiO2–ZrO2 mixed oxide nanocrystal photocatalysts. Chem. Eng. J. 2011;173:667–675. [Google Scholar]
- 40.Kara F., Aksoy E.A., Yuksekdag Z., Hasirci N., Aksoy S. Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties. Carbohydr. Polym. 2014;112:39–47. doi: 10.1016/j.carbpol.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Li L., Gao M., Liu J., Guo X. Removal of airborne microorganisms emitted from a wastewater treatment oxidation ditch by adsorption on activated carbon. J. Environ. Sci. 2011;23:711–717. doi: 10.1016/s1001-0742(10)60466-4. [DOI] [PubMed] [Google Scholar]
- 42.Erdural B., Bolukbasi U., Karakas G. Photocatalytic antibacterial activity of TiO2–SiO2 thin films: the effect of composition on cell adhesion and antibacterial activity. J. Photochem. Photobiol. A. 2014;283:29–37. [Google Scholar]
- 43.Donlan R.M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li J.F., Huang Y.F., Ding Y., Yang Z.L., Li S.B., Zhou X.S., Fan F.R., Zhang W., Zhou Z.Y., Wu D.Y., Ren B., Wang Z.L., Tian Z.Q. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature. 2010;464:392–395. doi: 10.1038/nature08907. [DOI] [PubMed] [Google Scholar]
- 45.Wei Y., Chen S., Kowalczyk B., Huda S., Gray T.P., Grzybowski B.A. Synthesis of stable, low-dispersity copper nanoparticles and nanorods and their antifungal and catalytic properties. J. Phys. Chem. C. 2010;114:15612–15616. [Google Scholar]
- 46.Yang X., Ma F., Li K., Guo Y., Hu J., Li W., Huo M., Guo Y. Mixed phase titania nanocomposite codoped with metallic silver and vanadium oxide: new efficient photocatalyst for dye degradation. J. Hazard. Mater. 2010;175:429–438. doi: 10.1016/j.jhazmat.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Inturi S.N.R., Boningari T., Suidan M., Smirniotis P.G. Visible-light-induced photodegradation of gas phase acetonitrile using aerosol-made transition metal (V, Cr, Fe Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl. Catal. B. 2014;144:333–342. [Google Scholar]
- 48.Balamurugan B., Mehta B.R., Shivaprasad S.M. Surface-modified CuO layer in size-stabilized single-phase Cu2O nanoparticles. Appl. Phys. Lett. 2001;79:3175–3178. [Google Scholar]
- 49.Carvalho H.W.P., Batista A.P.L., Hammer P., Ramalho T.C. Photocatalytic degradation of methylene blue by TiO2–Cu thin films: theoretical and experimental study. J. Hazard. Mater. 2010;184:273–280. doi: 10.1016/j.jhazmat.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 50.Baghriche O., Rtimi S., Pulgarin C., Sanjines R., Kiwi J. Effect of the spectral properties of TiO2, Cu, TiO2/Cu sputtered films on the bacterial inactivation under low intensity actinic light. J. Photochem. Photobiol. A. 2013;251:50–56. [Google Scholar]
- 51.Yousef A., Barakat N.A.M., Amna T., Al-Deyab S.S., Hassan M.S., Abdel-hay A., Kim H.Y. Inactivation of pathogenic Klebsiella pneumoniae by CuO/TiO2 nanofibers: a multifunctional nanomaterial via one-step electrospinning. Ceram. Int. 2012;38:4525–4532. [Google Scholar]
- 52.Hans M., Erbe A., Mathews S., Chen Y., Solioz M., Mücklich F. Role of copper oxides in contact killing of bacteria. Langmuir. 2013;29:16160–16166. doi: 10.1021/la404091z. [DOI] [PubMed] [Google Scholar]
- 53.Trovati G., Sanches E.A., Neto S.C., Mascarenhas Y.P., Chierice G.O. Characterization of polyurethane resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010;115:263–268. [Google Scholar]
- 54.Hodlur R.M., Rabina M.K. Self assembled graphene layers on polyurethane foam as a highly pressure sensitive conducting composite. Compos. Sci. Technol. 2014;90:160–165. [Google Scholar]
- 55.Pulgarina C., Kiwi J., Nadtochenko V. Mechanism of photocatalytic bacterial inactivation on TiO2 films involving cell-wall damage and lysis. Appl. Catal. B. 2012;128:179–183. [Google Scholar]
- 56.Sebenik U., Krajnc M. Influence of the soft segment length and content on the synthesis and properties of isocyanate-terminated urethane prepolymers. Int. J. Adhes. Adhes. 2007;27:527–535. [Google Scholar]
- 57.Wang J., Lua C.H., Xiong J.R. Self-cleaning and depollution of fiber reinforced cement materials modified by neutral TiO2/SiO2 hydrosol photoactive coatings. Appl. Surf. Sci. 2014;298:19–25. [Google Scholar]
- 58.Cui B., Peng H., Xia H., Guo X., Guo H. Magnetically recoverable core–shell nanocomposites Fe2O3@SiO2@TiO2–Ag with enhanced photocatalytic activity and antibacterial activity. Sep. Purif. Technol. 2013;103:251–257. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains supplementary material.