Abstract
Objective
To test the hypothesis that the inflammatory marker plasma soluble CD14 (sCD14) associates with incident dementia and related endophenotypes in 2 community-based cohorts.
Methods
Our samples included the prospective community-based Framingham Heart Study (FHS) and Cardiovascular Health Study (CHS) cohorts. Plasma sCD14 was measured at baseline and related to the incidence of dementia, domains of cognitive function, and MRI-defined brain volumes. Follow-up for dementia occurred over a mean of 10 years (SD 4) in the FHS and a mean of 6 years (SD 3) in the CHS.
Results
We studied 1,588 participants from the FHS (mean age 69 ± 6 years, 47% male, 131 incident events) and 3,129 participants from the CHS (mean age 72 ± 5 years, 41% male, 724 incident events) for the risk of incident dementia. Meta-analysis across the 2 cohorts showed that each SD unit increase in sCD14 was associated with a 12% increase in the risk of incident dementia (95% confidence interval 1.03–1.23; p = 0.01) following adjustments for age, sex, APOE ε4 status, and vascular risk factors. Higher levels of sCD14 were associated with various cognitive and MRI markers of accelerated brain aging in both cohorts and with a greater progression of brain atrophy and a decline in executive function in the FHS.
Conclusion
sCD14 is an inflammatory marker related to brain atrophy, cognitive decline, and incident dementia.
Cost-effective blood-based biomarkers are greatly needed to detect and track the progression of preclinical brain injury predisposing to dementia. Such biomarkers could also act as endpoints in clinical trials of disease-modifying interventions1 and expand our understanding of disease biology. Biomarkers of neural inflammation could be useful because inflammation may be a common pathway triggered by a variety of injurious mechanisms including vascular ischemia and proteinopathy-linked neurodegenerative processes.
Cluster of differentiation 14 (CD14) is a glycoprotein expressed on monocytes and neutrophils in both membrane-bound (mCD14) and soluble (sCD14) forms.2 sCD14 comprises mCD14 still in microvesicles, mCD14 that has been cleaved off the cell via ectodomain shedding, and an alternate splice form from the liver, which is a weak acute phase reactant. CD14 is a key component of innate immunity and is responsible for facilitating the generation of proinflammatory and anti-inflammatory cytokines in response to numerous potentially harmful molecular changes.3 Emerging evidence from animal models suggests that CD14 regulates the microglial inflammatory response.4,5 However, it is unknown whether peripheral levels of sCD14 levels can predict neurologic conditions with heightened inflammation, such as small vessel disease or neurodegeneration. Accordingly, we examined circulating sCD14 as a predictor of incident dementia and related endophenotypes in 2 large community-based cohorts: The Framingham Heart Study (FHS) and the Cardiovascular Health Study (CHS). Our primary outcome was the incidence of all-cause dementia.
Methods
Study samples
The FHS is a community-based prospective cohort comprising 3 generations of participants from Framingham, Massachusetts. The original cohort was established in 1948. The Offspring cohort began in 1971 with the recruitment of 5,124 individuals who were offspring of the Original cohort or spouses of these offspring.6 Offspring cohort participants have been examined 9 times since inception, with the latest examination cycle concluding in 2014. At the 7th Offspring cohort examination (1998–2001), participants completed a blood draw, which was used to quantify sCD14 levels.
The CHS was established in 1989 as an observational cohort study spanning multiple sites across the United States including Forsyth County, North Carolina; Washington County, Maryland; Sacramento County, California; and Pittsburgh, Pennsylvania. The initial wave of participants comprised 5,201 noninstitutionalized adults aged 65 years or older, recruited from Medicare eligibility lists. From 1992 to 1993, the sample was complemented by the inclusion of 687 African American participants. Details of recruitment can be seen elsewhere.7 Annual clinic visits were conducted from the baseline assessment between 1989–1990 and 1999–1999, with data collection involving demographics, anthropometry, medical history, phlebotomy, vital signs, cognitive function, psychosocial interviews, depression screening, and physical function. sCD14 was measured at the baseline examination.
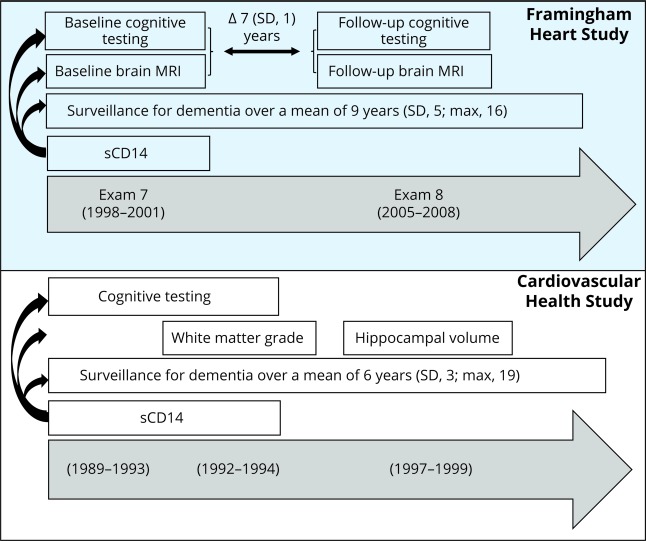
An overview of the study design is depicted in figure 1. The study flow diagram for each analysis sample is presented in figure e-1 (doi.org/10.5061/dryad.7th5ff0).
Figure 1. Overview of the study design.
Framingham Heart Study cohort: soluble cluster of differentiation 14 (sCD14) was measured at examination 7. Baseline brain MRI and cognitive testing were performed within a mean of 0.8 (SD 0.8) years after the blood draw for sCD14. A second round of brain MRI and cognitive testing was performed again after 7 years. Cardiovascular Health Study cohort: sCD14 was measured at the baseline examination (from 1989 to 1990 for the first wave of participants and 1992 to 1993 for the second wave). The first brain MRI (for the quantification of white matter grade) was performed from 1992 to 1994 and the second (for hippocampal volume) from 1997 to 1999.
Standard protocol approvals, registrations, and patient consents
All participants provided written informed consent. The study was approved by the Institutional Review Board and Boston University Medical Center.
sCD14 levels
In the FHS, sCD14 was measured from plasma using a Luminex (Austin, TX) bead-based multiplex assay. Plasma samples were diluted at 1/2,000 and incubated with capture antibody–coated beads at 25°C for 2 hours. Beads were washed to remove unbound proteins and then mixed with 4 biotin-labeled detection antibodies before being reacted with streptavidin-phycoerythrin and read using a BioPlex-200 reader. Each of the plasma samples was tested in duplicate. Intra-assay and interassay coefficients of variation were 3.53%–3.63% and 14.5%–15.4%, respectively. The detectable range for sCD14 levels extended from 58 to 239,000 ng/mL. Values outside these limits were set to the lower or upper detectable limits, respectively. In the CHS, sCD14 was measured at the baseline visit from blood samples drawn in the morning, following an overnight fast. After collection, blood was securely stored at −70°C, before being thawed for analysis. sCD14 levels were measured using a commercial ELISA (CD140; R&D Systems, Minneapolis, MN). The interassay coefficients of variation ranged from 5.32% to 12.36%.
Ascertainment of incident dementia
Incident all-cause dementia was ascertained over a mean follow-up period of 10 years (SD 4; maximum 16) in the FHS and 6 years (SD 3; maximum 19) in the CHS. Both cohorts adjudicated dementia in line with the DSM-IV.8 A diagnosis of Alzheimer disease (AD) dementia was based on the criteria of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association for definite, probable, or possible AD.9 A diagnosis of vascular dementia was determined based on the National Institute of Neurologic Disorders and Stroke–Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria.10 Where appropriate, participants are classified as having multiple contributing pathologies (i.e., AD and vascular dementia). We restricted our analysis samples to participants aged over 60 years since dementia is rare in younger participants and excluded persons with prevalent dementia at baseline. Our analysis samples for incident dementia included 1,588 FHS Offspring study participants and 3,129 participants from the CHS Cognition Study. Details of case ascertainment were as follows.
Dementia case ascertainment in the FHS
FHS participants are under continuous surveillance for incident dementia, the methods for which are detailed elsewhere.11 In brief, cognitive screening is performed at each FHS examination cycle using the Mini-Mental State Examination (MMSE)12 augmented by extensive neuropsychological testing at selected examinations. The MMSE is used to flag suspected cognitive impairment if (1) performance falls below education-based cutoff scores,13 (2) a decline of 3 or more points is observed between consecutive examinations, or (3) a decrease of 5 or more points is observed from the participant's highest past MMSE score. Participants are also flagged for suspected cognitive impairment following referrals from FHS investigators or outside practitioners if concern is expressed by the participant or the family. Once flagged for suspected cognitive impairment, participants complete annual neuropsychological and neurologic evaluations until they develop dementia or are adjudicated to be normal. Assessments suggestive of possible mild cognitive impairment or dementia are followed by referral to our study dementia review committee, comprising a neurologist and neuropsychologist, who adjudicate dementia diagnosis.
Dementia case ascertainment in the CHS
The CHS Cognition Study required participants to have undertaken a brain MRI and Modified Mini-Mental State Examination (3MSE) between 1992 and 1994. There were 3,660 participants who completed brain MRI and, of these, 3,608 also completed the 3MSE and were included in the dementia follow-up cohort. A standardized protocol for dementia surveillance was developed across the 4 sites, the details of which have been described previously.14,15 In brief, the Pittsburgh site endeavored to perform comprehensive neuropsychological testing on all surviving participants. At the 3 remaining sites, participants at high risk of dementia and minority participants were approached for comprehensive neuropsychological testing and study review. High risk of dementia was defined as one of the following: (1) a previous score of less than 80 on the 3MSE, (2) a decrease of 5 or more points on the 3MSE from any previous examination, (3) a previous Telephone Interview for Cognitive Status score less than 28, (4) an Informant Questionnaire on Cognitive Decline in the Elderly score greater than 3.6, (5) incident stroke or medical record review with dementia diagnosis, or (6) residing in a nursing home. Participants with suspected cognitive impairment as identified from follow-up neuropsychological testing underwent a neurologic examination. For persons at high risk of dementia who declined further neuropsychological testing or who were deceased, we performed a medical record review of all hospitalizations, questionnaires sent to the physician, and standardized interviews by phone with living participants or a designated informant. A committee, comprising a neurologist and psychiatrist from each study site, reviewed all available information to determine a consensus dementia diagnosis.
Assessment of brain aging, brain injury, and cognitive function in the FHS
The initial neuropsychological assessment and brain MRI were performed a mean of 0.8 (SD 0.8) years after the blood draw for sCD14. A repeat neuropsychological test battery and MRI were used to measure annualized change in cognition and total brain and hippocampal volume over a mean of 7 (SD 1) years, up to a maximum of 12 years.
Brain MRI endpoints in our cross-sectional analysis included total brain parenchymal volume, hippocampal volume, white matter hyperintensity volume (WMHV), and the presence of covert brain infarcts. The brain MRI used in our cross-sectional analysis consisted of a Siemens (Munich, Germany) 1T or 1.5T field strength machine with a T2-weighted double spin-echo coronal imaging sequence in contiguous slices of 4 mm. The repeat MRI used to quantify annualized change in brain volume consisted of a 1.5T Siemens Avanto scanner with 3D T1-weighted coronal spoiled gradient-recalled echo (SPGR) acquisition and fluid-attenuated inversion recovery (FLAIR) sequences. Hippocampal volume was calculated using a semiautomatic multi-atlas segmentation algorithm. The presence of covert brain infarcts was determined manually by STRIVE criteria.16 WMHV was first expressed as a percent of total cranial volume, and log-transformed to normalize its distribution. In all cross-sectional analysis, brain volumes were expressed as a percentage of total cranial volume, thus adjusting for differences in head size. Full details of the imaging methodology, including methods for WMHV segmentation, have been published.17 Analysis of MRI images was completed by a neurologist (C.D.), blind to sCD14 levels as well as participant demographics.
Clinical neuropsychologists and trained research assistants administered a well-validated neuropsychological battery that included Similarities (verbal reasoning), Logical Memory delayed recall (verbal memory), Visual Reproductions delayed recall (visual memory), and the Trail-Making Test; we used the difference between the time taken to complete parts B and A (executive function). We also examined a global cognitive score derived from principal component analysis. This variable was created previously using a principal component analysis and forcing a single score solution. The score combines weighted loadings for Trail-Making Test Part B, Hooper Visual Organization Test, Logical Memory, Visual Reproductions, Paired Associate Learning, and Similarities. Higher scores across all cognitive endpoints indicate superior performance, except Trail-Making Test, whereby higher scores indicate slower task completion.
We excluded persons with prevalent dementia and stroke at baseline, providing an analysis sample of 2,331 and 2,068 participants for the cross-sectional cognitive and MRI outcomes, respectively. Of these persons, 1,770 and 1,478 participants returned for a repeat neuropsychological assessment and brain MRI, respectively, allowing for analysis of cognitive decline and brain atrophy. Persons with brain MRI were a subset of those with cognitive testing.
Assessment of brain aging, brain injury, and cognitive function in the CHS
An initial MRI brain scan was completed on all willing participants from 1992 to 1994 to measure white matter abnormality grade, with a repeat scan conducted between 1997 and 1999 to measure hippocampal volume. Details of the scanning procedures have been described previously.18 In short, the study investigators used 1.5T scanners, a sagittal T1-weighted localizer sequence, axial T1-weighted spin-density, and T2-weighted images. Axial images were 5 mm thick without interslice gaps. The repeat MRI scan involved 3D volumetric T1-weighted images with spoiled gradient recall acquisition sequences. Scans were read at a central reading site by board-certified radiologists with neuroradiology subspecialties, in conjunction with MRI technologists. White matter abnormality was estimated from the initial MRI using spin density–weighted axial images along a 10-point grading scale as the total extent of periventricular and subcortical white matter signal abnormality. Grades 0 and 1 categorized no changes or barely detectable white matter changes, respectively, with grade 9 indicating almost complete white matter involvement.19 This scale has been validated against white matter hyperintensity volume.20 Intrareader agreement within 1 grade was 97%, with a κ of 0.96. Hippocampal volumes were calculated from the second brain MRI scan as gray matter voxel counts using an automated segmentation algorithm.
At baseline, participants completed the 3MSE (general cognitive function) and the Digit Symbol Substitution Test (DSST) (processing speed with elements of motor skills, visual tracking, visuospatial ability, and working memory). Higher scores on both tasks indicate superior performance. There were 5,140 participants available for the study of cognitive function, 2,963 for white matter grade, and 766 for hippocampal volume.
For the subclinical outcomes, we did not impose any age restrictions on our analyses but we excluded persons with prevalent dementia or stroke at baseline.
Statistical analysis
We examined the associations between sCD14 and incident dementia using Cox proportional hazards regression models. Results are reported as hazard ratios (HRs) and 95% confidence intervals (CIs). Results from each cohort were first examined separately and then combined using random effects meta-analysis. The associations between sCD14 and the MRI and cognitive outcomes were examined using linear and logistic regression. Brain MRI and cognitive outcomes were related cross-sectionally to sCD14 levels and then examined as annualized changes (in the FHS only). sCD14 was analyzed as both a continuous and categorical variable. For the former, values of sCD14 were log-transformed to achieve normality and then standardized within cohort. For the latter, we examined the top quintile of sCD14 relative to the bottom 4 and the top decile relative to the bottom 9. We implemented these various thresholds a priori to explore the most appropriate cutoffs for predicting each of the outcomes.
Analyses were adjusted according to 2 statistical models. Model 1 included adjustments for age and sex, with the addition of race and clinic site for the CHS cohort, age squared for the MRI outcomes (association with age is nonlinear),17 and education for the cognitive outcomes. A second statistical model was designed to reduce confounding using a range of potential mediators and confounders based on the literature. Model 2 included additional adjustments for systolic blood pressure, treatment for hypertension, prevalent diabetes, prevalent atrial fibrillation, prevalent cardiovascular disease (CVD), total cholesterol, high-density lipoprotein cholesterol, smoking status, body mass index, and positivity for an APOE ε4 allele. We also explored whether sCD14 was associated with the risk of incident dementia over and above C-reactive protein (CRP) and interleukin 6 (IL-6), 2 commonly used blood markers of systemic inflammation. For this analysis, CRP and IL-6 were entered together in a single model with sCD14 and model 2 covariates. Missing data were excluded from analysis. All results were considered significant if p < 0.05.
Sensitivity analysis
We examined whether sCD14 was associated with incident dementia following adjustments for nonsteroidal anti-inflammatory drug (NSAID) use and model 1 covariates. Next, we explored whether sCD14 was associated with dementia due to clinical AD or vascular disease as separate outcomes. For the analysis of incident all-cause dementia, we investigated the interaction between sCD14 levels and sex as well as APOE ε4 allele carrier status. This was investigated given evidence that the APOE ε4 allele may interact with chronic low-grade inflammation to increase AD risk21 and given potential sex differences in the susceptibility to inflammation and vulnerability to AD.22–24
Data availability
The FHS and CHS studies make phenotypic and genetic data available through the online repositories BioLINCC and dbGap, respectively.
Results
Cohort demographics
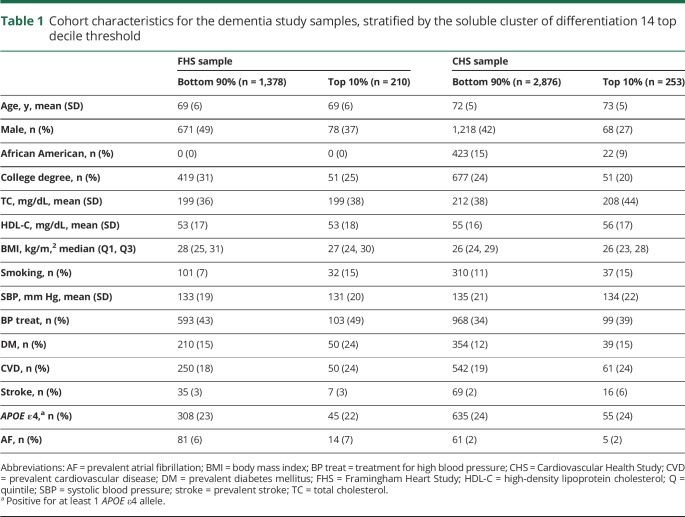
Cohort demographics for the dementia sample at baseline are displayed in table 1. In the FHS, the median sCD14 levels were 16,600 ng/mL (Q1–Q3, 14,500–19,200) and the thresholds for the top quintile and decile were >19,200 and >21,300 ng/mL, respectively. In the CHS, the median sCD14 levels were 1,598 ng/mL (Q1–Q3, 1,402–1,829) and the thresholds for the top quintile and decile were >1,881 and >2,072 ng/mL, respectively. Across both cohorts, persons classified as having high sCD14 levels were more likely to be female, less well-educated, and current smokers, and to have diabetes and CVD (table 1). Cohort demographics for the subclinical outcomes are provided in tables e-1 and e-2 (doi.org/10.5061/dryad.7th5ff0).
Table 1.
Cohort characteristics for the dementia study samples, stratified by the soluble cluster of differentiation 14 top decile threshold
sCD14 and incident dementia
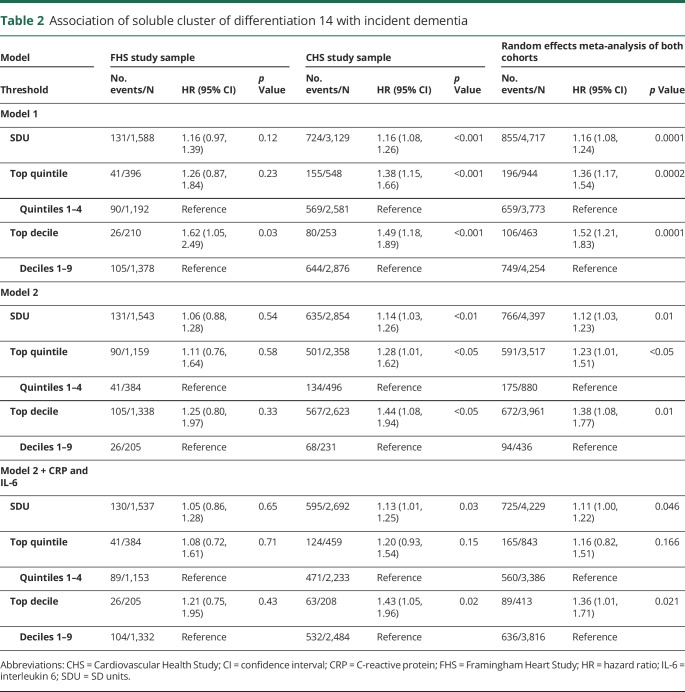
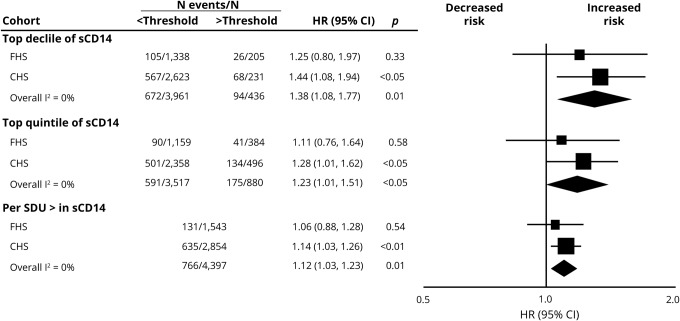
We observed 131 cases of incident dementia in the FHS (8%) and 724 in the CHS cohort (23%). In meta-analysis of both cohorts, higher levels of sCD14 were associated with a higher risk of incident dementia across all thresholds after adjustment for basic demographic variables (table 2) and following full multivariable adjustment (figure 2). Results were strongest at the threshold of the top decile; persons with sCD14 in the top decile (relative to the bottom 9) displayed a 38% increase in the risk of incident dementia. Following adjustments for CRP and IL-6, meta-analysis of both cohorts revealed that persons with sCD14 in the top decile, relative to the bottom 9, displayed a 36% increase in the risk of incident dementia. Persons with sCD14 levels in the top quintile, relative to the bottom 4, displayed a nonsignificant 16% higher risk of incident dementia, whereas each SD unit increase in sCD14 was associated with an 11% increase in the risk of incident dementia.
Table 2.
Association of soluble cluster of differentiation 14 with incident dementia
Figure 2. Association of soluble cluster of differentiation 14 (sCD14) with incident dementia.
Both the top decile and top quintile are compared to the remainder of the sample. All estimates are adjusted for age, sex, systolic blood pressure, treatment for hypertension, prevalent diabetes, prevalent atrial fibrillation, prevalent cardiovascular disease, total cholesterol, high-density lipoprotein cholesterol, smoking status, body mass index, and positivity for an APOE ε4 allele. Estimates for the Cardiovascular Health Study (CHS) are further adjusted for race and clinic site. CI = confidence interval; FHS = Framingham Heart Study; HR = hazard ratio; SDU = SD increase.
sCD14 and cross-sectional brain volume and brain injury
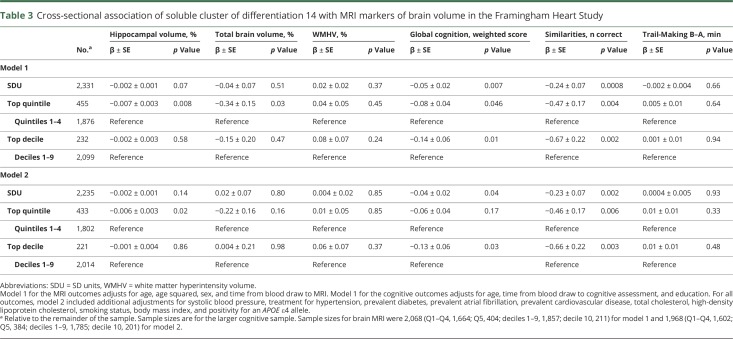
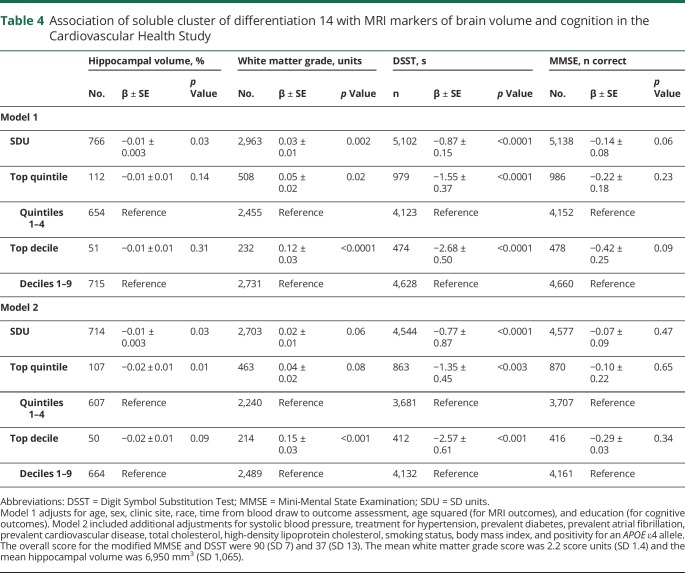
In the FHS, higher sCD14 levels were associated with poorer global cognition and poorer performance on the test of Similarities (verbal reasoning) across both statistical models (table 3). Findings were strongest for the top decile of sCD14 vs the bottom 9. Of the MRI outcomes, the top quintile of sCD14, relative to the bottom 4, was associated with lower hippocampal (models 1 and 2) and total brain volumes (model 1 only). In the CHS, higher sCD14 levels were associated with smaller hippocampal volumes, greater white matter injury, and slower processing speed (DSST scores); as with the risk of incident dementia, associations tended to be strongest at the threshold of the top decile (table 4). Higher levels of sCD14 were not consistently associated with memory or covert brain infarcts in the FHS (table e-3, doi.org/10.5061/dryad.7th5ff0).
Table 3.
Cross-sectional association of soluble cluster of differentiation 14 with MRI markers of brain volume in the Framingham Heart Study
Table 4.
Association of soluble cluster of differentiation 14 with MRI markers of brain volume and cognition in the Cardiovascular Health Study
sCD14 and annualized change in neuropsychological performance and brain volume in the FHS
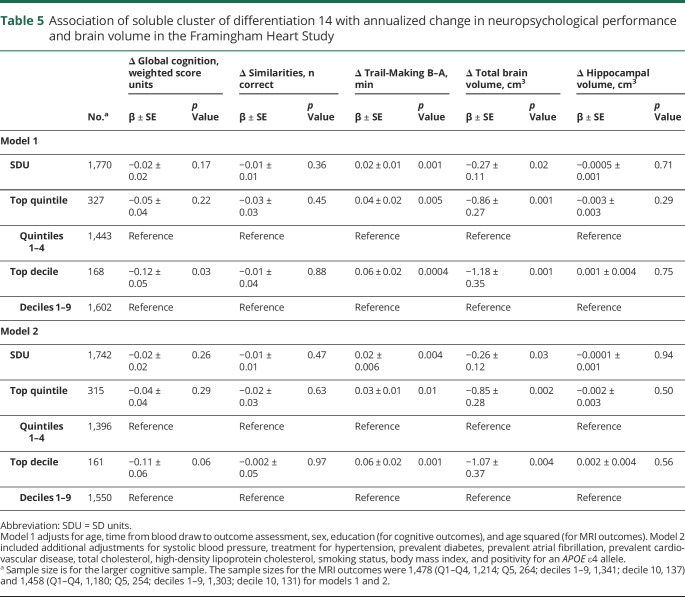
Higher sCD14 levels were associated with greater progression of total brain atrophy and greater cognitive decline in the form of poorer executive functioning (Trail Making B–A scores; table 5). These findings were observed across both statistical models using linear increments, quintiles, and deciles of sCD14. Persons with sCD14 levels in the top decile displayed an approximate 1 cm3 greater decline in brain volume per year, as compared to persons in the bottom 9 deciles. Those with sCD14 levels in the top decile also displayed a greater decline in global cognitive function, but in model 1 only. sCD14 levels were not associated with hippocampal atrophy.
Table 5.
Association of soluble cluster of differentiation 14 with annualized change in neuropsychological performance and brain volume in the Framingham Heart Study
Sensitivity analyses and sex interactions
For the outcome of incident dementia, results were essentially unchanged following adjustment for NSAID use (table e-4, doi.org/10.5061/dryad.7th5ff0). Meta-analysis across both cohorts revealed similar HRs between sCD14 and incident AD dementia as compared with sCD14 and vascular dementia (table e-5, doi.org/10.5061/dryad.7th5ff0). When predicting incident all-cause dementia in the CHS, we observed a significant sex–sCD14 interaction when comparing the top quintile of sCD14 to the bottom 4 (p = 0.04). Stratification of results revealed that the association between the top quintile of CD14 (vs the bottom 4) and incident all-cause dementia was stronger in women (HR 1.53; 95% CI 1.23–1.89) than men (HR 1.03; 95% CI 0.71–1.48). There were no sex–sCD14 interactions observed in the FHS and no sCD14–APOE ε4 allele interactions in either cohort (p > 0.1).
Discussion
We examined the associations of sCD14 with incident dementia and related endophenotypes in 2 large independent prospective cohorts. Results were largely consistent between cohorts despite differing population characteristics and the use of different sCD14 assays yielding vastly different mean sCD14 levels. Across both cohorts, higher levels of sCD14 were associated with a higher risk of incident dementia irrespective of the threshold and independent of vascular risk factors and APOE ε4 status. Higher levels of sCD14 were associated with various markers of brain aging and injury, including total brain atrophy and a decline in executive functioning in the FHS.
To our knowledge, we are among the first to report on the association of sCD14 with incident dementia as well as related subclinical outcomes in the community. We extend an emergent body of literature suggesting that sCD14 is involved in the brain's response to vascular insult and AD pathology. It has been shown that the microglial response to fibrillar forms of β-amyloid (Aβ) requires CD14 and toll-like receptors, which bind fibrillar amyloid at the cell surface.25 Thus, CD14 may play a direct role in microglial activation in response to Aβ. In previous animal models of AD, CD14 appeared to mediate the inflammatory response to Aβ4226 and the destruction of neurons damaged by Aβ.27 Liu et al.5 extended this work and demonstrated that CD14 colocalizes with fibrillar Aβ42 and that CD14+ microglia ingest more fibrillar Aβ42 than microglia from CD14-deficient mice. However, the literature is somewhat inconsistent. Transgenic mice without CD14 display a reduced Aβ plaque burden, a reduction in the number of microglia, and an increase in the expression of genes encoding both proinflammatory and anti-inflammatory cytokines.4 Concerning humans, immunohistochemical staining of the AD brain reveals augmented CD14 expression in microglia throughout various parenchymal regions and around senile plaques.5 Such results suggest that CD14 can alter the neuroinflammatory environment and affect AD pathology.
CD14 expression also appears to be increased in and around the lesion site of deceased patients with traumatic brain injury.28 Therefore, CD14 levels may also be indicative of the brain's inflammatory response to other insults. Elevated CD14 levels have been positively associated with vascular disease in various populations, such as those with vascular disease, HIV, chronic kidney disease, and in surgery.29–32 Occlusion of the middle cerebral artery has been shown to induce expression of CD14 in a mouse model of stroke. Similarly, an autopsy study of 18 human brains with or without focal infarction found CD14 expression to be upregulated both within and around the lesion core in brains affected by stroke.33 Meta-analysis across both cohorts in our study revealed similar effects between sCD14 and incident AD dementia as compared with sCD14 and vascular dementia. In short, the specificity of sCD14 for either vascular or AD-related brain injury is unclear.
sCD14 may be a nonspecific marker of microglial inflammation increasing in response to either vascular or AD-related brain injury. However, we measured sCD14 from plasma and the relationship between peripheral and CNS-derived sCD14 is unknown.
CD14 is a coreceptor for several toll-like receptors. CD14 binds lipopolysaccharides and has numerous roles in the recognition and signaling of microbes.34,35 Thus higher levels of CD14 may relate to dementia through exposure to infection. It has been hypothesized that peripheral infections exacerbate CNS inflammation thereby contributing to AD risk.36 However, CD14 appears to have a myriad of activities, many of which remain elusive.34 Plasma sCD14 may also be elevated in response to systemic inflammation and may relate to dementia through shared risk factors. In both our cohorts, persons with elevated sCD14 levels had a higher prevalence of vascular risk factors and CVD. Earlier analysis of our cohorts has demonstrated that higher levels of circulating sCD14 associate with an increased risk of incident CVD, stroke, and mortality.37,38 Regardless of its source, our findings suggest that sCD14 may be a useful inflammatory biomarker since the highest levels are associated with risk of dementia independent of vascular risk factors and commonly used circulating inflammatory markers (i.e., CRP and IL-6).
Tremendous progress has been made in developing and validating blood biomarkers for dementia in the last 12 months, with biomarkers of Aβ, total tau, and neurofilament light chain all showing promise.39–41 Pending further research, blood biomarkers of inflammation could be investigated in concert with other candidate biomarkers in an attempt to refine risk prediction models and diagnostic screening tools.
Our study was strengthened by the use of 2 large and well-characterized community-based samples and the prospective follow-up for incident dementia. Limitations of our approach include the observational nature of the study, which precludes us from concluding on causality. As optimal thresholds for sCD14 are unknown, we explored different thresholds without making adjustments for multiple comparisons. Consequently, some of the observed results may be attributable to chance. In addition, brain MRI and cognitive testing did not occur on the same day as the blood draw for sCD14. This may have increased the variability of our estimates, meaning that we may underestimate the true associations among sCD14, brain volume, and cognitive function.
We demonstrate that higher levels of plasma sCD14 relate to a higher risk of incident dementia and related endophenotypes in 2 independent community-based cohorts. As sCD14 can be measured by a simple blood test, our findings should encourage further investigation of sCD14 as a potential biomarker for brain injury and dementia. Future studies that measure sCD14 both in blood and CSF combined with CSF or PET biomarkers of AD and microglial activation (i.e., translocator protein) are needed to determine the source of elevated sCD14 in blood and to more completely understand the role of inflammation and immune system function in the development of dementia.
Acknowledgment
The authors thank the Framingham Heart Study and Cardiovascular Health Study participants for their time.
Glossary
- 3MSE
Modified Mini-Mental State Examination
- Aβ
β-amyloid
- AD
Alzheimer disease
- CD14
cluster of differentiation 14
- CHS
Cardiovascular Health Study
- CI
confidence interval
- CRP
C-reactive protein
- CVD
cardiovascular disease
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- DSST
Digit Symbol Substitution Test
- FHS
Framingham Heart Study
- FLAIR
fluid-attenuated inversion recovery
- HR
hazard ratio
- IL-6
interleukin 6
- mCD14
membrane-bound cluster of differentiation 14
- MMSE
Mini-Mental State Examination
- NSAID
nonsteroidal anti-inflammatory drug
- sCD14
soluble cluster of differentiation 14
- SPGR
spoiled gradient-recalled echo
- WMHV
white matter hyperintensity volume
Appendix. Authors
Study funding
M.P.P. is funded by a National Heart Foundation of Australia Future Leader Fellowship (102,052). The Framingham Heart Study is supported by the National Heart, Lung, and Blood Institute (contract no. N01-HC-25195 and no. HHSN268201500001I) and by grants from the National Institute on Aging (R01 AG054076, R01 AG049607, R01 AG058589, R01 AG059421, U01 AG049505, U01 AG052409) and the National Institute of Neurologic Disorders and Stroke (NS017950 and UH2 NS100605 as part of MarkVCID). H.J.A. is supported by a National Institute on Aging Research Supplement to Promote Diversity in Health-Related Research (R01AG054076-02S1) and through support from Boston University School of Medicine's Jack Spivack Neuroscience scholarship and Aram V. Chobanian Assistant Professorship. C.D. directs the UC Davis Alzheimer's Disease Center with funding from the NIH (P30 AG010182). CHS research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and N01HC15103, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurologic Disorders and Stroke. Additional support was provided by R01AG023629, R01AG15928, and R01AG20098 from the National Institute on Aging. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Henriksen K, O'Bryant SE, Hampel H, et al. The future of blood-based biomarkers for Alzheimer's disease. Alzheimers Dement 2014;10:115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan LA, Zheng J, Brester M, et al. Plasma levels of soluble CD14 and tumor necrosis factor-α type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis 2001;184:699–706. [DOI] [PubMed] [Google Scholar]
- 3.Sahay B, Patsey RL, Eggers CH, Salazar JC, Radolf JD, Sellati TJ. CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance. PLoS Pathog 2009;5:e1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed-Geaghan EG, Reed QW, Cramer PE, Landreth GE. Deletion of CD14 attenuates AD pathology by influencing the brain's inflammatory milieu. J Neurosci 2010;30:15369–15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Walter S, Stagi M, et al. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer's amyloid peptide. Brain 2005;128:1778–1789. [DOI] [PubMed] [Google Scholar]
- 6.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med 1975;4:518–525. [DOI] [PubMed] [Google Scholar]
- 7.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 1993;3:358–366. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Ed, Text Revision. Arlington: American Psychiatric Association; 2000. [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 10.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 11.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 2016;374:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 13.Bachman DL, Wolf PA, Linn R, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology 1992;42:115–119. [DOI] [PubMed] [Google Scholar]
- 14.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology 2003;22:1–12. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 2004;52:195–204. [DOI] [PubMed] [Google Scholar]
- 16.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 18.Kuller LH, Arnold AM, Longstreth WT Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the Cardiovascular Health Study. Neurobiol Aging 2007;28:1307–1315. [DOI] [PubMed] [Google Scholar]
- 19.Yue NC, Arnold AM, Longstreth WT Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the Cardiovascular Health Study. Radiology 1997;202:33–39. [DOI] [PubMed] [Google Scholar]
- 20.Kuller LH, Longstreth WT Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ Jr. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke 2004;35:1821–1825. [DOI] [PubMed] [Google Scholar]
- 21.Tao Q, Ang TFA, DeCarli C, et al. Association of chronic low-grade inflammation with risk of Alzheimer disease in ApoE4 carriers. JAMA Netw Open 2018;1:e183597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574. [DOI] [PubMed] [Google Scholar]
- 23.Uchoa MF, Moser VA, Pike CJ. Interactions between inflammation, sex steroids, and Alzheimer's disease risk factors. Front Neuroendocrinol 2016;43:60–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults: association of global amyloid and regional tau deposition measured by positron emission tomography. JAMA Neurol 2019;76:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci 2009;29:11982–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fassbender K, Walter S, Kuhl S, et al. The LPS receptor (CD14) links innate immunity with Alzheimer's disease. FASEB J 2004;18:203–205. [DOI] [PubMed] [Google Scholar]
- 27.Bate C, Veerhuis R, Eikelenboom P, Williams A. Microglia kill amyloid-beta1-42 damaged neurons by a CD14-dependent process. Neuroreport 2004;15:1427–1430. [DOI] [PubMed] [Google Scholar]
- 28.Beschorner R, Nguyen TD, Gozalan F, et al. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol 2002;103:541–549. [DOI] [PubMed] [Google Scholar]
- 29.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014;28:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JW, Cho E, Kim MG, Jo SK, Cho WY, Kim HK. Proinflammatory CD14+CD16+ monocytes are associated with vascular stiffness in predialysis patients with chronic kidney disease. Kidney Res Clin Pract 2013;32:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrijenhoek JE, Pasterkamp G, Moll FL, et al. Extracellular vesicle-derived CD14 is independently associated with the extent of cardiovascular disease burden in patients with manifest vascular disease. Eur J Prev Cardiol 2015;22:451–457. [DOI] [PubMed] [Google Scholar]
- 32.Trøseid M, Nestvold TK, Nielsen EW, Thoresen H, Seljeflot I, Lappegård KT. Soluble CD14 is associated with markers of vascular dysfunction in bariatric surgery patients. Metab Syndr Relat Disord 2015;13:119–124. [DOI] [PubMed] [Google Scholar]
- 33.Beschorner R, Schluesener HJ, Gözalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J Neuroimmunology 2002;126:107–115. [DOI] [PubMed] [Google Scholar]
- 34.Granucci F, Zanoni I. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol 2013;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol 2002;23:301–304. [DOI] [PubMed] [Google Scholar]
- 36.Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Bry M, de Leon MJ. Alzheimer's disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis. J Alzheimer's Dis 2008;13:437–449. [DOI] [PubMed] [Google Scholar]
- 37.Reiner AP, Lange EM, Jenny NS, et al. Soluble CD14: genome-wide association analysis and relationship to cardiovascular risk and mortality in the older adults. Arterioscler Thromb Vasc Biol 2013;33;158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho JE, Lyass A, Courchesne P, et al. Protein biomarkers of cardiovascular disease and mortality in the community. J Am Heart Assoc 2018;7:e008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pase MP, Beiser AS, Himali JJ, et al. Assessment of plasma total tau level as a predictive biomarker for dementia and related endophenotypes. JAMA Neurol 2019;76:598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature 2018;554:249–254. [DOI] [PubMed] [Google Scholar]
- 41.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease: longitudinal plasma neurofilament light and neurodegeneration in Alzheimer disease. JAMA Neurol 2019;76:791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The FHS and CHS studies make phenotypic and genetic data available through the online repositories BioLINCC and dbGap, respectively.