Abstract
Purpose
To report long-term efficacy and treatment outcomes of the combination therapy for treating macular oedema (MO) in retinal vein occlusions (RVOs) from a real-world UK practice.
Methods
The initial reported 66 RVO patients with MO treated with combination therapy (initial Ranibizumab, later optional addition of Ozurdex and laser) were followed up to Year 3: visual acuity (VA) and central retinal thickness (CRT) were analysed against baseline and previous Year 1 results. Safety and adverse events were also recorded.
Results
Baseline LogMAR VA of 0.71 (Snellen 6/30) improved to 0.48 (Snellen 6/18) at Year 3 (p=0.006); 63% experienced VA improvement (40% improved ≥3 lines), 27% had worse vision. Stability of mean VA (6/18) was already achieved at first post-loading phase review and was maintained in each subsequent year. Statistically significant CRT improvement was noted in each year (Year 3 median CRT=264µm) compared to baseline (median CRT=531µm). There was a reduction in the mean number of total injections to 2.5 in Year 3 (vs 5.5 in Year 1). Comparing Year 3 against Year 1, mean Ranibizumab injection frequency was 2.1 vs 4.3; mean Ozurdex injection frequency was 0.2 vs 1.1. In Year 3, 39.6% of patients did not require any form of injections, laser frequency was also reduced to 22.9% (vs 81.8% in Year 1). There was no endophthalmitis in the cohort, one progressed to neovascular glaucoma in Year 2 and mortality rate was recorded as 6%.
Conclusion
Our real-world clinical practice for RVO patients using a combined therapy is associated with good long-term VA and anatomical outcomes with less intravitreal re-treatment rates.
Keywords: RandOL protocol, Ozurdex, ischaemia, laser, mortality, aspirin
Plain Language Summary
Patients suffer from reduced vision due to retinal vein occlusion causing centre retinal swelling, have now available few effective treatment options. However, the efficacy of these drugs when applied separately is short-lived and demands frequent monthly re-treatments, creating undesirable burdens to both patients and hospital service. This article provides real-world clinical outcomes based on a clinical practice using locally adopted combination treatments (of anti-VEGF, steroid and laser), providing guidance in optimal timing when introducing a different agent in this combined regimen, which has been a common dilemma faced by many treating clinicians; and also sub-analysed results of various clinical spectra including difficult challenging cases faced in a real-world clinical practice. Our results show that using a treatment regimen based on combination therapy is associated with good safety, anatomical and visual outcomes with less re-treatment rates at 3-year follow-up.
Introduction
The introduction of intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) has revolutionised the management of macular oedema (MO) secondary to retinal vein occlusions (RVOs) providing promising short-term visual benefits.1–3 Although effective, recurrent MO is a commonly encountered problem with intravitreal monotherapy, leading to the need for multiple and ongoing injections. Patients are inevitably faced with increasing risk of adverse effects and health care services are faced with a significant burden from the increased demand of repetitive intravitreal injections.
Combination therapy using various modalities and intravitreal agents had been explored for the management of MO secondary to RVO, using the concept based on the limited understanding of pathophysiology in RVO eyes where high ocular VEGF levels causing increased vascular permeability which promotes the development of MO and later retinal neovascularization formation.5,6 In our clinical practice, we introduced a combined therapy regimen aimed to reduce the high ocular VEGF levels, by means of both intravitreal anti-VEGF injection and laser therapy. Our rationale is that the anti-VEGF loading dose (for the initial fast effect), with additional target laser therapy on any detectable retinal ischaemia (for longer-term effect), could have better efficacy to lower ocular VEGF levels, hence reducing ischaemic drive (thus preventing neovascularization) and vascular permeability (thus, lessen the number of intravitreal injections). We previously reported the real-world results based on RVO patients treated with this combined treatment regimen which was associated with good Year 1 visual and anatomical outcomes in a wide spectrum of clinical severities of RVO patients with MO.4 The present paper now extends the follow-up of this same cohort to 3 years.
Methods
This auditing report is part of an on-going follow-up of the initial cohort of 66 newly diagnosed RVO patients who received the combined therapy regimen at Birmingham and Midlands Eye Centre (single tertiary-referral centre) between November 2013 and April 2014. Although the care-plan for patients was prospectively arranged, all data were retrospectively collected for further years. Research ethical approval was not applicable as confirmed by our local Research Governance authority, and standard “Good Clinical Practice” applied to all patients when obtaining consent for investigations and treatment procedures.
This report covered all RVO subtypes and severities including neovascular glaucoma (NVG) as a true reflection of real-world clinical practice. Clinical evaluation at every clinic visit included Snellen chart visual acuity (VA) measurement, slit-lamp biomicroscopy, Goldmann tonometry for intraocular pressure (IOP) measurement, and central retinal thickness (CRT) as quantitative measurement of MO using spectral-domain optical coherence tomography (3D-OCT 2000, Topcon, Tokyo).The analyses on VA and CRT, treatment exposure (frequency of injections and laser therapy) were continued at months 24 and 36. Intraocular pressure, adverse events and clinical outcomes for all patients were recorded. Patients’ demography and the study design were already mentioned in our previous publication.4 In our centre, we follow a local protocol of combination therapy regimen, namely RandOL (Ranibizumab and Ozurdex, Laser) protocol for easier understanding of chronological introduction of the treatment modalities.4 The combined therapy (RandOL Protocol flow-chart4) and timing/procedure of choice are summarised as follows.
Guidance on Ranibizumab and Timing
Anti-VEGF injections (Ranibizumab, Novartis, Basel, Switzerland) were the first-line treatment in this cohort. All patients were commenced on an initial loading phase of three Ranibizumab injections at 4-weekly interval. Patients would receive further 1–2 more Ranibizumab after loading dose depending on the persistent severity of retina ischaemic status (plentiful retinal haemorrhages and cotton-wool-spots) even if MO resolved at that initial post-loading assessment. After this stage, in persistent MO or recurrence MO, the decision to continue with Ranibizumab or add dexamethasone implant injection was depending on any co-existing evidence of angiographic retinal ischaemia. Ranibizumab was the choice for recurrent MO with ischaemic retina (especially when the patient was still receiving retinal laser).
Guidance on Ozurdex and Timing
Intravitreal dexamethasone implant (Ozurdex, Allergan Inc., Irvine, California) was the additional treatment option in this cohort after anti-VEGF. Ozurdex injection was considered in recurrent MO with no retinal ischaemia (or already treated retinal ischaemia), hence often only considered at a later stage when retina ischemia was sufficiently treated. Patients with recurrent MO who previously received Ozurdex injection could be reconsidered for Ranibizumab if the Ozurdex effect was too short-lasting (3–4 months) or if the patient suffered Ozurdex-related complication such as post-injection IOP spike >30 mmHg. Very often, patients would also specify the re-injection choice based on their experience or preference reflecting a common real-world clinical practice encounters; the combined therapy regimen has the flexibility to adapt for individual patient’s need.
Guidance on Laser and Timing
In practice, the retinal laser was delivered to non-perfused retina in all RVOs depicted by an ultra-widefield fundus fluorescein angiography (FFA) where pre-mapping of the ischaemic retina area was possible, aiding the safety and precision of “target-laser”. Hence, laser was performed as pan-retinal photocoagulation (for CRVO) or just sector retina laser (for HRVO or BRVO), and laser was repeated if retinal ischaemic area was found inadequately covered. In patients with evidence of ischaemia involving part of macula as in some BRVO/HRVO patients, macular grid laser was applied on the angiographically defined ischaemic macula area only but sparing the central fovea area (safety margin of one-disc-diameter wide from centre fovea). Macular grid laser, however, was not recognized as beneficial for ischaemic CRVO patients with established pan-macular ischaemia.7 Hence, when applying needed laser, this group of patients would receive just pan-retinal photocoagulation without macular laser.
Guidance on Fluorescein Angiography
Ultra-widefield FFA (Optos200Tx scanning-laser-Ophthalmoscope, Optos PLC, Dunfermline, United Kingdom) was performed after the first clinic assessment post-Ranibizumab loading phase when retinal haemorrhages were sufficiently cleared allowing better definition of any retinal non-perfusion area. In general, FFA was repeated 6–12 monthly when there were clinical indications: suggestion of a new or recurrence RVO, suspecting residual ischaemia from persistent non-resolving retinal haemorrhages, suspect of retinal neovascularisation development or to confirm resolution of treated retinal neovascularisation. All patients would have a “exit FFA” before being discharged to ensure no untreated residual retinal ischaemia.
Discharge Criteria
In this cohort, we adopted a discharge criterion on “stable patients” when patients were satisfactorily treated and received no further injections nor laser treatment for at least 12 months. Our criteria of a “stable-discharge-period” differs from Royal College RVO guidelines7 (2 years for stable central RVO, 18 months for branch RVO) where laser application was merely an optional intervention, whereas our treatment-protocol encompassed regular reviews of laser adequacy, aiming to minimise and avoid the likelihood of retinal neovascularisation development in future years after discharge.
Statistical Analysis
Wilcoxon Signed Rank Test was used to determine the statistical significance between year 2 and 3 VA and CRT in comparison to baseline. SSPS Statistics (IBM Corp., Armonk, USA) was used for the statistical analysis and p<0.05 was taken as statistically significant. Non-parametric data are presented as median and interquartile range (IQR) and parametric data as mean and standard deviation. Snellen visual acuities were converted to LogMAR for analysis purposes.8
Results
Of the original cohort of 66 patients, a total of 61 (92.4%) and 48 (72.7%) patients completed a full follow-up period of 2 and 3 years, respectively. Reasons for discontinuation of care are summarised in Table 1. As per our discharge criteria on “stable patients”, there were no discharges in Years 2, and 8 (12.1%) were discharged by Year-3; 62.5% were BRVO and 37.5% CRVO. Overall, 5 (7.6%) patients who still needed treatment-monitoring had their care transferred to more convenient local hospitals over Years 2 and 3; one patient did not attend further appointments after Years 1 and 4 (6.1%) patients were deceased.
Table 1.
Discontinuation of Care
| Reasons for Discontinuation of Care | Year 1 n = 66 | Year 2 n = 61 (92%) | Year 3 n = 48 (72%) | |
|---|---|---|---|---|
| Planned discharge | N (%) | 0 | 0 | 8 (12%) |
| RVO subanalysis | 0 | 3 CRVO 5 BRVO |
||
| Transfer of care (to other eye hospitals) |
N (%) | 0 | 2 (3%) | 3 (4.5%) |
| RVO subanalysis | 1 CRVO 1 HRVO |
1 CRVO 1 NVG 1 BRVO |
||
| Persistent non-attendance |
N (%) | 0 | 1 (1.5%) | 0 |
| RVO subanalysis | 1 CRVO | 0 | ||
| Deceased | N (%) | 0 | 2 (3%) | 2 (3%) |
| RVO subanalysis | 1 CRVO 1 HRVO |
1 CRVO 1 BRVO |
||
Notes: The table shows various reasons for discontinuation of care with related subgroup analyses. A total of 92.4% and 72.7% of patients reached a full follow-up period of 2 and 3 years, respectively. Discharge criteria for stable patients in this cohort were “patients received no treatment for at least one year”, the overall planned discharge was 12% recorded only in Year 3. The total number deceased patients over the 3-year follow-up period was 6%. n = total number of patients in the cohort in that year (% = n/66 x100%). N = number of patients left the cohort, Percentage of discontinuation of care was calculated based on original cohort number of 66 patients (% = N/66 x100%).
Abbreviations: RVO, retinal vein occlusion; CRVO, central retinal vein occlusion; HRVO, Hemi retinal vein occlusion; BRVO, branch retinal vein occlusion; NVG, neovascular glaucoma.
Treatment Exposure
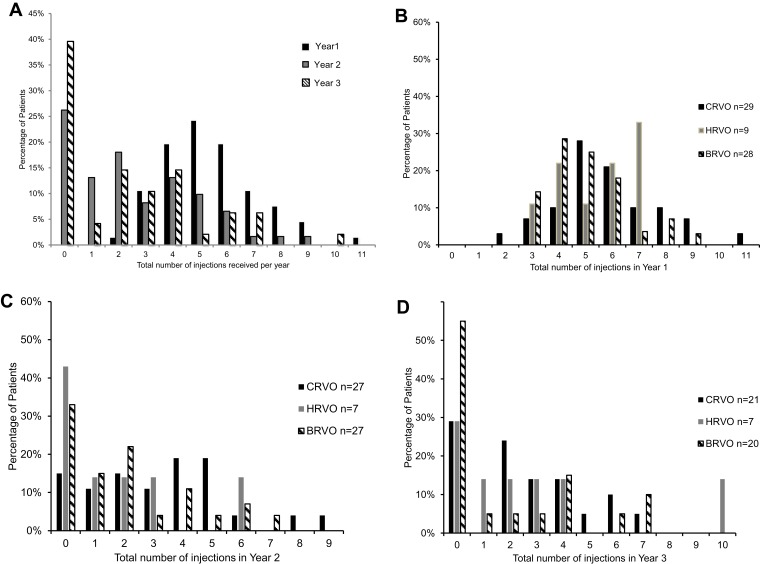
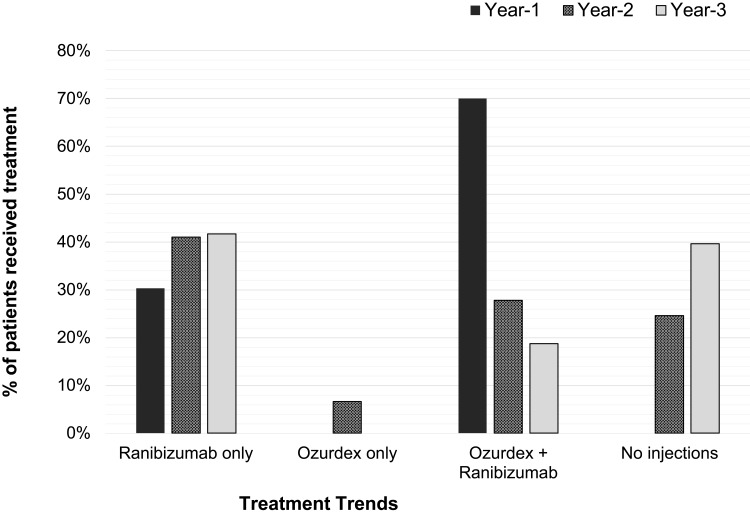
Table 2 shows a reduction in the mean number of total injections to 2.5 per year in Years 2 and 3 compared to 5.5 in Year 1, and the variation in frequency range was small. Figure 1 shows the intravitreal injection frequency received by the whole cohort with further subgroup analyses: 25% and 40% patients (mainly belonged to BRVO and HRVO groups) did not require any injections in Years 2 and 3, respectively, severe disease spectrum such as neovascular glaucoma patients was in CRVO group, hence also accounted for the higher injection frequency demand. Figure 2 shows various treatment modalities received by the whole cohort of each year, confirming a significant reduction in the number of patients requiring additional Ozurdex injections, with 34.4% and 18.8% receiving at least one Ozurdex in Years 2 and 3 compared to 69.7% in Year 1. Table 3 depicts varied combination modes received by the number of patients in each year. All patients who received Ozurdex injection(s) had already received a minimal of 3 Ranibizumab injections. On average, effectiveness of Ozurdex was 3–4 months, re-injection of Ozurdex depends on any post-injection intraocular pressure spike and patient’s preference.
Table 2.
Treatment Exposure Over 3 Years with Subgroup Analyses
| Year 1 n= 66 | Year 2 n= 61 | Year 3 n= 48 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Injections | |||||||||
| Mean frequency (SD) | 5.5 (1.8) | 2.5 (2.3) | 2.5 (2.3) | ||||||
| Mode (Range) | 5 (2–11) | 0 (0–9) | 0 (0–10) | ||||||
| Subgroup Analyses | CRVO | HRVO | BRVO | CRVO | HRVO | BRVO | CRVO | HRVO | BRVO |
| Mean frequency (SD) Mode (Range) |
5.9 (2.0) 5 (0–11) |
5.4 (1.5) 7 (0–7) |
5.0 (1.6) 4 (0–9) |
3.3 (2.4) 4 (0–9) |
1.7 (2.2) 0 (0–6) |
2.0 (2.1) 0 (0–7) |
2.6 (2.2) 0 (0–7) |
2.8 (3.5) 0 (0–1) |
1.9 (2.6) 0 (0–7) |
| Ranibizumab Only Mean frequency (SD) Mode (Range) |
4.3 (1.8) 3 (2–11) |
2.2 (2.1) 0 (0–8) |
2.1 (2.4) 0 (0–10) |
||||||
| Subgroup Analyses | CRVO | HRVO | BRVO | CRVO | HRVO | BRVO | CRVO | HRVO | BRVO |
| Mean frequency (SD) Mode (Range) |
4.7 (2.1) 3 (2–11) |
4.4 (1.2) 5 (3–6) |
4.0 (1.5) 3 (2–8) |
2.8 (2.2) 4 (0–8) | 1.6 (2.2) 0 (0–6) | 1.7 (1.9) 0 (0–6) |
2.3 (2.2) 0 (0–7) |
2.7 (3.5) 2 (0–10) |
1.8 (2.3) 0 (0–7) |
| Ozurdex Only Mean frequency (SD) Mode (Range) |
1.1 (0.8) 1 (0–1) |
0.4 (0.6) 0 (0–2) |
0.2 (0.5) 0 (0–2) |
||||||
| Subgroup Analyses | CRVO | HRVO | BRVO | CRVO | HRVO | BRVO | CRVO | HRVO | BRVO |
| Mean frequency (SD) Mode (Range) |
1.1 (0.9) 2 (0–3) |
1.0 (0.7) 1 (0–2) |
1.0 (0.8) 1 (0–2) |
0.5 (0.6) 0 (0–2) |
0.4 (0.8) 0 (0–2) |
0.3 (0.6) 0 (0–2) |
0.3 (0.4) 0 (0–1) |
0.1 (0.4) 0 (0–1) |
0.2 (0.5) 0 (0–2) |
| Laser Procedures | 54 (81.8%) | 29 (47.5%) | 11 (22.9%) | ||||||
| Subgroup Analyses | CRVO | HRVO | BRVO | CRVO | HRVO | BRVO | CRVO | HRVO | BRVO |
| 24/29 (82.8%) | 7/9 (77.8%) | 23/28 (82.1%) | 14/27 (51.9%) | 5/7 (71.4%) |
10/27 (37.0%) |
6/21 (28.6%) | 3/7 (42.9%) | 2/20 (10.0%) | |
Notes: The average number of total intravitreal injections required per patient in Year 1 was 5.5, reduced to 2.5 in Years 2 and 3, there was no difference for total injections (or Ranibizumab only injections) for each subgroup in Year 1 but CRVO patients did still require more injections in Year 2. All RVO subgroups shared similar Ozurdex frequency pattern and mode. Laser requirement declined in subsequent years but top-up laser request was common in ischaemic eyes due to inadequate laser (confirmed by repeat fluorescein angiography).
Abbreviations: n, number of eyes; SD, standard deviation; CRVO, central retinal vein occlusion; HRVO, hemi-retinal vein occlusion; BRVO, branch retinal vein occlusion.
Figure 1.
Number of intravitreal injections received by the whole cohort and by different RVO subgroups in each year.
(A) Percentage of patients (of the whole cohort) received X number of injections each year. (B) Year 1 RVO subgroup analyses: all subgroups shared similar frequency distribution, one CRVO patient (of neovascular glaucoma) needed 11 injections in Year 1. (C) Year 2 RVO subgroup analyses: BRVO and HRVO had the most numbers of patients not needing re-injections this year; and CRVO patients accounted for higher injection frequency. (D) Year 3 RVO subgroup analyses: 55% of BRVO needed no injections in Year 3, one HRVO patient had a severe recurrence and needed 10 injections.
Abbreviations: RVO, retinal vein occlusion; CRVO, central retinal vein occlusion; HRVO, hemi-retinal vein occlusion; BRVO, branch retinal vein occlusion.
Figure 2.
Trend of combined modalities received by the whole cohort of each year. All patients received injections in Year 1: 30% required only the initial loading dose of three Ranibizumab for disease stability and 69.7% needed combination therapy for disease stability. In Year 2, 41% received Ranibizumab alone, 6.6% needed just Ozurdex for the whole Year 2. Combination therapy was less frequent in subsequent years; 25% and 40% of patients received no injections in Year 2 and Year 3 respectively.
Table 3.
Number of Patients Received Injections with Varied Combination Modes in Each Year
| R=0 | R=1 | R=2 | R=3 | R=4 | R=5 | R=6 | R=7 | R=8 | |
|---|---|---|---|---|---|---|---|---|---|
| Year 1 Received Combined Therapy, n= 46/66 (69.7%) | |||||||||
| Oz =1 | 9 | 2 | 3 | 5 | 3 | 1 | |||
| Oz =2 | 13 | 7 | 1 | 2 | |||||
| Year 2 Received Combined Therapy, n= 21/61 (34.4%) | |||||||||
| Oz =1 | 3 | 4 | 1 | 4 | 3 | 1 | 1 | ||
| Oz =2 | 1 | 1 | 1 | 1 | |||||
| Year 3 Received Combined Therapy, n = 9/48 (18.7%) | |||||||||
| Oz =1 | 2 | 2 | 4 | ||||||
| Oz =2 | 1 | ||||||||
Notes: This table shows detailed breakdown of different combined therapy modes received by number of patients in that year. For example, in Year 1, all patients who received Ozurdex injection(s) had already received a minimal of 3 Ranibizumab injections – in particular, 9 patients received one Ozurdex injection after 3 Ranibizumab injections, 2 patients received 3 initial Ranibizumab and later had additional one Ozurdex and one Ranibizumab for the rest of Year 1. On average, effectiveness of Ozurdex lasted for 3–4 months, continuation with Ranibizumab injections was common after Ozurdex effect had worn off. Re-injection of Ozurdex was decided by any post-injection intraocular pressure spike and other factors.
Abbreviations: R, ranibizumab injection frequency; Oz, Ozurdex injection frequency.
Laser requirement was recorded to decline in later years: from 81.8% in Year 1 to 22.9% in Year 3 (Table 2). Top-up laser treatment was common in ischaemic eyes due to inadequate laser coverage at one laser setting, not because of worsening angiographic retinal ischaemia (confirmed by repeat fluorescein angiography), and no correlation with subgroups analyses.
Visual and Anatomical Outcomes
Visual improvement to 6/18 (LogMAR 0.48) was statistically significant compared to baseline (6/30, LogMAR 0.71) for the whole cohort in each year and remained so only in BRVO subgroup in each subsequent year (Table 4). Subgroup analyses showed BRVO and HRVO patients maintained their improved median VA at 6/12 (LogMAR 0.39) in Year 3 whereas CRVO reduced to 6/60 (LogMAR 0.78). The three neovascular glaucoma patients belonged to CRVO subgroup accounting for the poorer visual outcome: two patients did not recover from “Hand Movement” vision; one patient achieved best 6/24 after cataract surgery when CMO resolved but needed maintaining dose of 6 Ranibizumab injections in Year 3.
Table 4.
Comparison of VA and CRT Against Baselines
| Year 1 n = 66 | Year 2 n = 61 | Year 3 n = 48 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparison of Vision in LogMAR (Snellen Equivalent) | |||||||||
| Baseline median VA | 0.71 (6/30) | ||||||||
| [IQR] | [0.43–1.16] | ||||||||
| Median VA | 0.48 (6/18) | 0.48 (6/18) | 0.48 (6/18) | ||||||
| [IQR] | [0.18–0.78] | [0.18–0.89] | [0.18–1.00] | ||||||
| of cohort year | p<0.001 | p<0.001 | p=0.006 | ||||||
| Subgroup Analyses |
CRVO n=29 |
HRVO n=9 |
BRVO n=28 |
CRVO n=27 |
HRVO n=7 |
BRVO n=27 |
CRVO n=21 |
HRVO n=7 |
BRVO n=20 |
| Baseline median VA LogMAR (Snellen) |
1.00 (6/60) |
0.72 (6/30) |
0.61 (6/24) |
||||||
| Median VA each year (Snellen equivalent) p value |
0.78 (6/36) 0.055 |
0.44 (6/15) 0.033 |
0.54 (6/20) <0.001 |
1.00 (6/60) 0.628 |
0.30 (6/12) 0.272 |
0.30 (6/12) <0.001 |
1.00 (6/60) 0.627 |
0.33 (6/12) 0.106 |
0.39 (6/12) <0.001 |
| Visual Changes in Comparison to Baseline per Each Cohort Year | |||||||||
| Worse | 10 (15%) | 12 (21%) | 13 (27%) | ||||||
| Stable | 5 (8%) | 6 (10%) | 5 (10%) | ||||||
| Improved | 51 (77%) | 42 (69%) | 30 (63%) | ||||||
| Improved by ≥ 3-lines | 34 (52%) | 23 (38%) | 19 (40%) | ||||||
| Achieved 6/12 or better | 30 (46%) | 28 (46%) | 19 (40%) | ||||||
| Comparison of Central Retinal Thickness (in μm) | |||||||||
| Baseline median CRT [IQR] |
531 [435–622] |
||||||||
| Median CRT [IQR] |
245 [221–351] p<0.001 |
266 [231–369] p<0.001 |
264 [230–378] p<0.001 |
||||||
| Subgroup Analyses |
CRVO n=29 |
HRVO n=9 |
BRVO n=28 |
CRVO n=27 |
HRVO n=7 |
BRVO n=27 |
CRVO n=21 |
HRVO n=7 |
BRVO n=20 |
| Baseline median CRT |
571 | 513 | 499 | ||||||
| Median CRT each year p value |
236 <0.001 |
285 0.009 |
252 <0.001 |
264 <0.001 |
256 0.022 |
276 <0.001 |
280 0.002 |
271 0.030 |
258 <0.001 |
Notes: Visual improvement was statistically significant compared to baseline for the whole cohort in each year and remained so only in BRVO subgroup in each subsequent year. The 3 neovascular glaucoma patients belonged to CRVO subgroup accounting for the poorer visual outcome. Macular oedema improvement, however, remained statistically significant in each subgroup from year 1 to 3. (Statistical analysis using Wilcoxon signed-rank test; statistical significant: p<0.05).
Abbreviations: VA, visual acuity; CRT, central retinal thickness; IQR, Inter-quartile-range; Crvo, central retinal vein occlusion; Hrvo, hemi-retinal vein occlusion; Brvo, branch retinal vein occlusion.
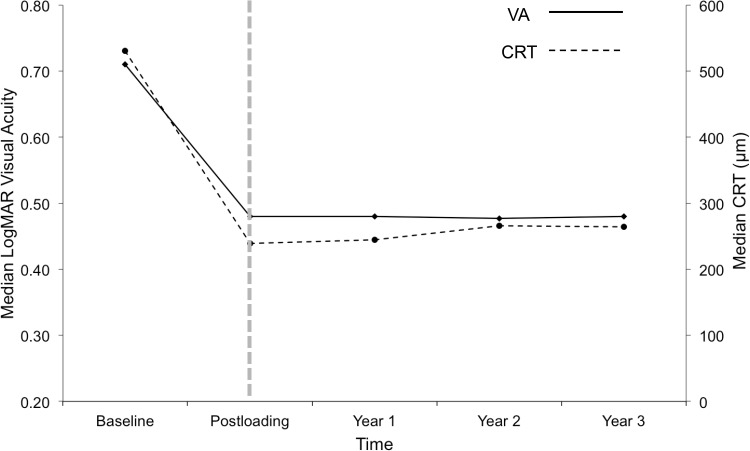
In addition, baseline LogMAR VA in the original cohort of 66 patients was 0.71 (Snellen 6/30), which improved significantly to 0.48 (Snellen 6/18) in Year 1 and was maintained at subsequent years (Figure 3), with 63% experiencing VA improvement in Year 3. Patients achieving VA 6/12 or better vision were similar in Years 1 to 3, approximately 40–46%. VA improvement by ≥3-lines ranging from 38% to 52% over the 3 years, with the slightly lower figure in Year 3 due to the discharge of 8 “stable patients”, most of whom achieved at least 6/9 vision at discharge. There was a low percentage of patients losing vision, but the percentage was higher in Year 3 as the majority of remained patients in later years belonged to the severe-end of RVO clinical entity with gross ischaemic MO requiring repetitive injections.
Figure 3.
Trend in median visual acuity (VA) and median central retinal thickness (CRT) for each cohort year. Both VA and CRT improved and stabilised after a loading phase of Ranibizumab. In particular, VA stabilisation at LogMAR 0.48 (Snellen 6/18) level in each year was similar as VA first achieved after the loading phase. Hence, it is possible that “vision achieved 4-weeks post-loading phase” could potentially be useful as a prognostic VA predictor in longer term.
Table 4 shows statistically significant CRT improvement from a baseline of 531µm to 266µm and 264µm in Years 2 and 3, respectively. This result was achieved and maintained by all 3 subgroups in each year. Figure 3 shows the stabilisation of median CRT after Year 1, and maintained in subsequent years, with 50.8% and 45.8% achieving a dry fovea at Years 2 and 3, respectively.
Adverse Events and Associated Procedures
Table 5 shows the adverse effects recorded in this cohort. There was no record of any IOP rise post-Ranibizumab (measured at each post-injection clinic visit); however, IOP elevations were noted with post-Ozurdex injections. The number of patients experiencing a spike of IOP >21mmHg (compared to normal IOP in pre-Ozurdex injection clinic) were 8/20 (40%) in Year 2 and 3/5 (60%) in Year 3, with the higher percentage reflected by reducing numbers of patients receiving this treatment. These were all successfully managed topically, except for one patient with uncontrolled steroid-related IOP spike, despite on maximum topical therapy, needed to have an extra Selective Laser Trabeculoplasty procedure 8 months post-Ozurdex injection. Cyclodiode laser therapy recorded were all performed on the three existing NVG patients only. Other additional glaucoma procedures recorded in this cohort (trabeculoplasty, iStent with cataract surgery) were all received by patients with pre-existing advanced glaucoma of suboptimal IOP control, not a result of intravitreal procedures as there was no recorded variation in pre- and post-injection IOPs in these patients. In summary, all patients had post-Ozurdex IOP checked at 4 weeks after the procedure. Mono-topical anti-glaucoma treatment would be started and continued indefinitely when IOP was >21. The majority responded well to topical anti-glaucoma alone and received shared care in a glaucoma clinic. We had not explored in this cohort if anti-glaucoma eyedrops could be stopped when Ozurdex effect wore-off, as patients were organised to have glaucoma-shared care. Repeat Ozurdex was not a contraindication in this group of patients after initial IOP spike. We observed few patients who had no IOP spike after received first Ozurdex, but IOP spike was recorded when received subsequent Ozurdex injection.
Table 5.
Adverse Effects and Other Ocular Procedures
| Year 1 | Year 2 | Year 3 | |
|---|---|---|---|
| Ozurdex-Related Complications Raised IOP >21mmHg Vitreous haemorrhage Cataract from injection procedure Cataract from Ozurdex effect |
19/46 (41%) 2/46 (4%) 0 Unknown |
8/20 (40%) 0 0 Unknown |
3/5 (60%) 0 0 Unknown |
| Concomitant Surgeries / Procedures | |||
| Cataract surgeries | 12/66 (18%) | 10/61 (16%) | 1/48 (2%) |
| Laser iridotomies / trabeculoplasty / SLT | 5 | 2 | 2 |
| Cyclodiode laser (for NVG only) | 3 | 1 | 2 |
| Progression to NVG | 0 | 1 | 0 |
| Endophthalmitis | 0 | 0 | 0 |
Notes: number of patients received Ozurdex is different each year. Data recording is unable to determine if a cataract formation is from repetitive Ozurdex injection or natural progression.
Abbreviations: IOP, Intraocular Pressure; SLT, selective laser trabeculoplasty; NVG, neovascular glaucoma.
Cataract operations were performed in 10 patients in Year 2 and one patient in Year 3. All cataract surgeries (including most glaucoma surgeries) were carefully timed to coincide either after an anti-VEGF injection or planned to receive anti-VEGF at the time of the surgery. Hence, we had no record of worsening RVO nor CMO after surgeries. There were no cases of infective endophthalmitis secondary to intravitreal injections in this cohort. One patient with very severe but stabilized ischaemic central RVO failed to attend follow up after Year 1, re-presented later with vitreous haemorrhage and neovascular glaucoma.
Discussion
In this cohort, we show that staged combination therapy was associated with good anatomical and visual outcomes, as well as safety, at 3-year follow-up. This analysis provides additional long-term data supporting our previous 1-year follow-up but with less intravitreal re-treatment rates.4
Although the VA stability was unchanged in each year, percentage of VA improvement was still maintained at 63% in Year 3 compared to 77% in Year 1. In contrast, the percentage of patients experiencing worse vision increased to 6%, perhaps due to the “more stable patients” discharged from the cohort follow-up, thus leaving the more severe RVO patients whose visual stability was very much dependant on on-going repetitive treatment. Of note, the VA improvement to 6/18 level in each year was the same as VA first achieved after the initial anti-VEGF loading phase (Figure 3); hence it is possible that “vision achieved at 4-weeks post-loading phase” may be useful as a prognostic VA predictor in the longer term.
Although many reports had explored options of various combination therapies, few could conclude on a combination regimen that was more superior or re-producible results in the longer term.9–12 Although study numbers were small and study duration short, the randomized BRVO study also suggested that target laser (on non-perfused retina) as a combined therapy with Bevacizumab reduced MO recurrence, achieved a higher VA stability and required fewer injections in the study duration of 6 months.11 Importantly, we are aware of only one other published RVO real-world series, but this failed to report good primary outcomes and averaging highish 5–7 intravitreal injections per year.12 The latter retrospective audit recorded miscellaneous applications of anti-VEGF agents, intraocular steroids and laser therapy (received by 1 in 5 patients). Although not based on combination therapy, OCEAN real-world study of Ranibizumab treatment reported of 35% VA improvement in Year 1 (and maintained in Year 2), comparatively lower than our 77% in Year 1 and 63% in Year 2.13 However, they concluded similar interesting observation of final VA was the same as VA first achieved at post-loading phase. The injection frequency in OCEAN study was higher in Year 2 (4–5 injections) compared to our cohort of 2.5 injections. The less optimal outcomes reported in OCEAN study were likely attributed to several factors: mixture of treatment-naïve and chronic study patients, non-uniform treatment protocol over 369 German study sites with no common recommended injection-loading frequency and re-injections were at the treating clinicians’ discretion. Indeed, these results were commonly reflected in real-world clinical practice where clinicians have varied management plans.14 This further confirms the dilemma amongst treating clinicians and the desperate need of a workable standardized RVO treatment protocol in real-world clinical practice, a protocol of combination therapy may perhaps help to achieve better clinical outcomes.
Chronic and Recurrent Macular Oedema
Our protocol hypothesis was based on resolving MO by reducing the high level of hypoxic-driven ocular VEGF, by means of direct intravitreal anti-VEGF and indirectly by laser therapy.5 However, the cohort recorded that recurrence MO remains a frequent manifestation in RVO patients with or without retinal ischaemia. Based on angiographic studies of this cohort, we observed two common causes of chronic recurrence MO: ischaemic macula (either patchy or complete) and leakage from retinal collaterals adjacent to fovea. Clearly, laser therapy would not be feasible for these macular “pathologies”, and the only option for stabilization would be continuation with intravitreal injections. In this cohort of our clinical practice, we advised continuation of anti-VEGF injections for chronic recurrence MO with evidence of significant macular ischaemia; and offered Ozurdex implant for MO secondary to leakage from angiographic retinal collaterals, if patients had no contraindication of uncontrolled glaucoma. The observation of retinal collateral formation is interesting, and unfortunately, we have no comprehensive records in this cohort to investigate which RVO subtypes or clinical features are most likely to develop leaky venous re-canalisations.
Neovascular Glaucoma
Although anti-VEGF therapy is superior and remains the mainstay therapy for resolving fovea-involving MO, its limitation is the short-lived effect and the ability to have an effect on retinal ischaemia remains unknown; hence progression to neovascularisation or end-stage NVG complications is a high possibility with anti-VEGF therapy alone.15,16 Despite additional laser therapy in our cohort, there was still one patient (1.5%) who developed NVG when missing clinic attendances and treatment plan for a whole year. We are not alone in believing laser as an important adjunctive therapy to stabilize ischaemic RVO disease and to prevent the development of neovascularisation. The large non-randomised cohort from Tultseva et al compared the efficacy of Ranibizumab and laser (Gp1) versus Ranibizumab alone (Gp2) in RVO patients over 24 months.16 They also reported a high rate of retinal ischaemia in 74.1% of their whole cohort, and 4.6% progressed to NVG in Gp2 compared to 1.1% in Gp1. They also concluded that the combination therapy of anti-VEGF plus retinal laser therapy had added to the stability of VA (achievable by 5 months) in Gp1 but was highly fluctuating in Gp2.16
Mortality and Systemic Risk Factors
Mortality in a RVO cohort is not an uncommon finding. A recent systematic review on published case–control studies reported mortality as high as 34.7%, stroke in 6.8% and myocardial infarction in 5.7% in RVO patients (over a study period of >5 years).17 Unsurprisingly, RVO and cardiovascular complications share common systemic co-morbidities such as hypertension, diabetes, hyperlipidaemia and carotid artery disease. We reported a 6.1% mortality in 3 years, although none were thought to be associated with intravitreal drug and procedures. Perhaps this outcome would be even higher with even longer follow-up duration given that 92.4% of our cohort presented with at least one cardiovascular co-morbidity at diagnosis, with 34.8% already suffering 3 or more co-morbidities and systemic cardiovascular complications. In terms of addressing the common risk factors in a busy eye clinic dealing with RVO patients, blood pressure is a routine measurement in our RVO clinic with an emphasis on an optimal blood pressure control based on NICE diabetic management guidelines of target blood pressure <130/80mmHg.18
With the complexity of co-existing risk factors and cardiovascular complications, the benefits of commencing on low-dose aspirin for RVO patients to prevent future recurrent events remain uncertain.19,20 Sartori et al reported reduced vascular events (including RVO recurrence) in a follow-up period of 5 years for patients started on antithrombotic prophylaxis (10% vs 24% no-aspirin group).20 In our practice, most RVO patients were already on an oral antithrombotic medication, we advise low-dose Aspirin (150mg or less) as prophylaxis to RVO patients with evidence of significant retinal venous congestion and tortuosity in “other non-affected eye”, and with existing cardiovascular disease.18
Strengths and Limitations
The main strength of this report is the availability of 3-year follow-up data on a consecutive cohort of patients (inclusive of a wide spectrum of disease severity) from a clinical practice giving a good representation of the actual “real-world” experience and results. These long-term data also allow understanding of the relatively under-reported late ocular and systemic complications. The limitations of this paper relate to observational and auditing report on the relatively small numbers without a comparator, and the use of non-parametric visual acuity scores derived by conversion from Snellen acuities.
In conclusion, our real-world cohort data show that using a treatment regimen based on combination therapy is associated with good safety, anatomical and visual outcomes at 3-year follow-up. This analysis provides additional long-term data supporting our previous 1-year follow-up and with less intravitreal re-treatment rates.
Acknowledgments
We are sincerely grateful to Sukhdev Sagu and Frances Furhuraire for their professionalism and their total dedication in organising timely appointments for all our patients, to Vinaya Felicida, Hema Kolli, Amoy Ramsay, Eesha Gokhale, Suman Pilli, Akshata Ranganath and Sawasan El Sheikh for their dedications towards patients’ care in laser and RVO clinical services. This work and preparation of manuscript received no commercial sponsorship and assistance.
Disclosure
BMu: advisory board member and speaker for Alimera Science, Allergan, Bayer, Novartis and ORAYA therapeutics. She also reports travel grants and/or personal fees from Novartis, Bayer, and Alimera, outside the submitted work. RC: advisory board member for Bayer and speaker for Novartis. He also reports personal fees from Bayer as an advisory board member, and personal fees from Novartis as a speaker, outside the submitted work. BMo: received educational travel sponsorship from Bayer, Novartis, and Allergan, outside the submitted work. AM: advisory board member and speaker for Alimera Sciences and Novartis. The authors report no other conflicts of interest in this work.
References
- 1.Braithwaite T, Nanji AA, Lindsley K, Greenberg PB. Anti-vascular endothelial growth factor for macular oedema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2014;5:CD007325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a Phase III study. Ophthalmology. 2011;118(10):2041–2049. doi: 10.1016/j.ophtha.2011.02.038 [DOI] [PubMed] [Google Scholar]
- 3.Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the Phase 3 GALILEO study. Ophthalmology. 2014;121(1):202–208. doi: 10.1016/j.ophtha.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 4.Lip PL, Cikatricis P, Sarmad A, et al. Efficacy and timing of adjunctive therapy in the anti-VEGF treatment regimen for macular oedema in retinal vein occlusion: 12-month real-world result. Eye (Lond). 2018;32(3):537–545. doi: 10.1038/eye.2017.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. [DOI] [PubMed] [Google Scholar]
- 6.Gozawa M, Takamura Y, Miyake S, et al. Photocoagulation of the retinal nonperfusion area prevents the expression of the vascular endothelial growth factor in an animal model. Invest Ophthalmol Vis Sci. 2017;58(13):5946–5953. doi: 10.1167/iovs.17-22739 [DOI] [PubMed] [Google Scholar]
- 7.Retinal Vein Occlusion (RVO). Guidelines 2015 (July). The Royal College of Ophthalmologists Guidelines. Available from: https://www.rcophth.ac.uk/standards-publications-research/clinical-guidelines. Accessed March9, 2020.
- 8.Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina. 2010;30(7):1046–1050. doi: 10.1097/IAE.0b013e3181d87e04 [DOI] [PubMed] [Google Scholar]
- 9.Campochiaro PA, Hafiz G, Mir TA, et al. Scatter photocoagulation does not reduce macular edema or treatment burden in patients with retinal vein occlusion: the RELATE trial. Ophthalmology. 2015;122(7):1426–1437. doi: 10.1016/j.ophtha.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tadayoni R, Waldstein SM, Boscia F, et al. Sustained benefits of ranibizumab with or without laser in branch retinal vein occlusion: 24-month results of the BRIGHTER study. Ophthalmology. 2017;124(12):1778–1787. doi: 10.1016/j.ophtha.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 11.Tomomatsu Y, Tomomatsu T, Takamura Y, et al. Comparative study of combined bevacizumab/targeted photocoagulation vs bevacizumab alone for macular oedema in ischaemic branch retinal vein occlusions. Acta Ophthalmol. 2016;94(3):e225–30. doi: 10.1111/aos.2016.94.issue-3 [DOI] [PubMed] [Google Scholar]
- 12.Jumper JM, Dugel PU, Chen S, Blinder KJ, Walt JG. Anti-VEGF treatment of macular edema associated with retinal vein occlusion: patterns of use and effectiveness in clinical practice (ECHO study report 2). Clin Ophthalmol. 2018;12:621–629. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callizo J, Ziemssen F, Bertelmann T, et al. Real-world data: ranibizumab treatment for retinal vein occlusion in the OCEAN study. Clin Ophthalmol. 2019;7(13):2167–2179. doi: 10.2147/OPTH.S209253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lip PL, Malick H, Damer K, et al. One-year outcome of bevacizumab therapy for chronic macular edema in central and branch retinal vein occlusions in real-world clinical practice in the UK. Clin Ophthalmol. 2015;9:1779–1784. doi: 10.2147/OPTH.S89147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wykoff CC, Brown DM, Croft DE, Major JC Jr, Wong TP. Progressive retinal nonperfusion in ischemic central retinal vein occlusion. Retina. 2015;35(1):43–47. doi: 10.1097/IAE.0000000000000277 [DOI] [PubMed] [Google Scholar]
- 16.Tultseva SN, Astakhov YS, Novikov SA, et al. Alternative ways to optimize treatment for retinal vein occlusion with peripheral capillary non-perfusion: a pilot study. Arq Bras Oftalmol. 2017;80(4):224–228. doi: 10.5935/0004-2749.20170055 [DOI] [PubMed] [Google Scholar]
- 17.Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30(8):1031–1038. doi: 10.1038/eye.2016.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Type 2 diabetes in adults: management NICE guideline (NG28). National Institute for Health and Care Excellence; 2015. Available from: https://www.nice.org.uk/guidance/ng28/chapter/1-recommendations-blood-pressure-management-2. Accessed March9, 2020. [PubMed]
- 19.Squizzato A, Manfredi E, Bozzato S, Dentali F, Ageno W. Antithrombotic and fibrinolytic drugs for retinal vein occlusion: a systematic review and a call for action. Thromb Haemost. 2010;103(2):271–276. doi: 10.1160/TH09-09-0626 [DOI] [PubMed] [Google Scholar]
- 20.Sartori MT, Barbar S, Dona A, et al. Risk factors, antithrombotic treatment and outcome in retinal vein occlusion: an age-related prospective cohort study. Eur J Haematol. 2013;90(5):426–433. doi: 10.1111/ejh.2013.90.issue-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Retinal Vein Occlusion (RVO). Guidelines 2015 (July). The Royal College of Ophthalmologists Guidelines. Available from: https://www.rcophth.ac.uk/standards-publications-research/clinical-guidelines. Accessed March9, 2020.
- Type 2 diabetes in adults: management NICE guideline (NG28). National Institute for Health and Care Excellence; 2015. Available from: https://www.nice.org.uk/guidance/ng28/chapter/1-recommendations-blood-pressure-management-2. Accessed March9, 2020. [PubMed]