Abstract
The incentive effects of food and related cues are determined by stimulus properties and the internal state of the organism. Enhanced hedonic reactivity and incentive motivation in energy deficient subjects have been demonstrated in animal models and humans. Defining the neurobiological underpinnings of these state-based modulatory effects could illuminate fundamental mechanisms of adaptive behavior, as well as provide insight into maladaptive consequences of weight loss dieting and the relationship between disturbed eating behavior and substance abuse. This article summarizes research of our laboratory aimed at identifying neuroadaptations induced by chronic food restriction (FR) that increase the reward magnitude of drugs and associated cues. The main findings are that FR decreases basal dopamine (DA) transmission, upregulates signaling downstream of the D1 DA receptor (D1R), and triggers synaptic incorporation of calcium-permeable AMPA receptors (CP-AMPARs) in the nucleus accumbens (NAc). Selective antagonism of CP-AMPARs decreases excitatory postsynaptic currents in NAc medium spiny neurons of FR rats and blocks the enhanced rewarding effects of d-amphetamine and a D1R, but not a D2R, agonist. These results suggest that FR drives CP-AMPARs into the synaptic membrane of D1R-expressing MSNs, possibly as a homeostatic response to reward loss. FR subjects also display diminished aversion for contexts associated with LiCl treatment and centrally infused cocaine. An encompassing, though speculative, hypothesis is that NAc synaptic incorporation of CP-AMPARs in response to food scarcity and other forms of sustained reward loss adaptively increases incentive effects of reward stimuli and, at the same time, diminishes responsiveness to aversive stimuli that have potential to interfere with goal pursuit.
Keywords: reward, nucleus accumbens, food restriction, AMPA receptors, dopamine
Introduction
Motivational theory that preceded and influenced recent advances in behavioral neuroscience maintained that incentive properties of environmental stimuli are sufficient to drive behavior and that their potency is determined, in part, by the internal state of the organism [16]. An example of this joint regulation by internal and external factors is positive alliesthesia, in which the hedonic and incentive motivating effects of food and related cues are increased by energy deficit [23]. In animal models, sweet taste reactivity is enhanced by food deprivation [13,73], and the reinforcing potency of food measured in progressive ratio protocols of instrumental responding displays a pattern of increase that parallels body weight loss [60,88,89]. Negative energy balance therefore augments both orosensory reward and the incentive motivation that drives food seeking behavior. Both effects have been confirmed in human studies, where energy deprivation increases the “liking” [26,180,212] and “wanting” of food [54,158]. The neurocircuits that underlie hedonic reactivity and incentive motivation have been differentiated, with the former including a key opioid component, and the latter being largely dopamine mediated [36,99]. These separate but interacting [15,137,169] neurocircuits may be co-regulated when there are shifts in energy balance, though they can be differentially regulated as well, as is the case in addictive disorders where hedonic reactivity declines and incentive motivation escalates [164,165].
The discoveries of endocrine adiposity hormones and feeding-related neuropeptides with receptor populations in both the hypothalamus and mesolimbic pathway point to potential mechanistic bases for regulation of incentive motivation as a fundamental feature of homeostatic behavior [e.g., 50,93,120]. Thus, the presence of receptors for leptin, insulin, ghrelin, orexin, melanocortins, GLP-1 and other metabolic signaling peptides in the ventral tegmental area or nucleus accumbens point to multiple candidate mediators of the link between energy balance and reward. For most of these signaling molecules there is supporting, albeit incomplete and sometimes inconsistent, evidence of involvement in reward modulation [for reviews see: 6,104,120,139,156,213].
Probing reward modulation using drugs as proxies for food
A hypothesis that has been well supported by basic and translational studies maintains that drugs with abuse liability target a final common pathway for the positive reinforcing effects of natural rewards [90,108,209]. Much of the supporting research has focused on the relationship between mechanisms regulating ingestive behavior and drug abuse [28,49,101,204], with the most studied link between the two being the mesoaccumbens dopamine (DA) pathway [7,8,14,59,80,102,141,146,153,157,178,187, 210]. Moreover, just as deprivation increases the rewarding effects of food, food deprivation increases the reward magnitude of abused drugs. In animal models, chronic food restriction (FR) has been shown to increase sensitivity to drug rewarding effects in self-administration [35], conditioned place preference [9,121,186,215], and electrical brain stimulation reward protocols [24,25]. FR also increases the incentive effects of contexts and cues that had been paired with the subjective effects of drugs during prior ad libitum fed states [45,215,217]. The enhancing effect of FR on drug reward magnitude, as assayed in a learning-free curve-shift protocol of intracranial self-stimulation, results from a regimen that leads to a maintained 20% decrease in body weight [24]. The effect is present whether subjects are tested before or after their single daily meal [217], and the return to baseline following restoration of ad libitum feeding occurs gradually over a three week period [25]. These results match the observation that maintenance of a substantially reduced body weight increases drug self-administration, whereas an acute withholding of food does not [33,34]. Most studies conducted to assess metabolic hormone involvement in these effects have not yielded positive results [82,83,127,172,217], though results relating to glucocorticoid involvement suggest differential involvement based on the drug under study and sex of the subjects [27,128,172].
An assumption undergirding this line of research is that just as drugs of abuse exert reinforcing effects by “hijacking” the neural substrate for natural reward, the potentiating effect of FR on drug abuse is based on a “hijacking” of neuroadaptations that normally increase the incentive motivating effects of food and related cues under the condition of negative energy balance. These neuroadaptations may contribute to the increased risk of binge eating and drug abuse among individuals who engage in weight loss dieting [2,38,64,116,131,152,181,203,205]. Relatively few basic research studies have been conducted to model the pathogenic potential of food restriction. However, it has been shown that several weeks of alternating 12-h food deprivation with 12-h sugar access, or combining longer duration food restriction with acute foot shock stress induces binge eating [43]. In the case of drug abuse, food restriction with maintenance of reduced body weight (75–90%) is sufficient to increase self-administration [33–35], reinstatement of extinguished drug-seeking [176], and cue-induced reinstatement of drug-seeking following abstinence [46].
Dopamine-related changes induced by food restriction
The food restriction protocol used in our behavioral studies (i.e, maintenance at 80% of pre-restriction body weight for a minimum of 3–4 weeks), or one very similar to it [154,155], induces a number of changes in the mesoaccumbens DA pathway. Glutamate currents in putative ventral tegmental DA neurons [147], basal DA synthetic activity in NAc, [148], and evoked DA release in NAc slices are all decreased [182]. Basal extracellular NAc DA concentrations in vivo are also decreased [154,155], as are levels of preprodynorphin and preprotachykinin mRNA contained in D1 receptor expressing medium spiny neurons (MSNs) [79]. This set of findings is suggestive of DA conservation in the mesoaccumbens pathway during FR, which is consistent with the adaptive downregulation of energy expenditure [1]. However, in response to challenge with an agonist for D1-like (D1/D5) DA receptors, MAP kinase signaling, CaMKII signaling, gene expression in NAc, and elicited behavioral responses are increased [29–32, 77–79]. This latter combination of findings suggests compensatory upregulation of postsynaptic responsiveness. Intracerebral microinjection studies with d-amphetamine and DA receptor agonists point to the D1R and NAc shell as the key DA receptor type and NAc subregion underlying the enhancing effect of FR [29,30,145,150]. Unlike the D2 receptor, D1 exists in a low affinity state and is not activated until a DA surge increases extracellular concentrations [161]. Thus, low basal DA tone with upregulated signaling downstream of D1R may tune the system to respond selectively and strongly to phasic DA-releasing stimuli (e.g., food, cues, drugs of abuse). When MSNs receive glutamate input, D1R stimulation facilitates the transition from a hyperpolarized downstate to the upstate where membrane potential is near spike threshold [189]. The underlying mechanism is not clear but depends at least in part on protein kinase A (PKA) and glutamate AMPA receptors [196].
AMPA receptor-related changes induced by food restriction
The DA innervation of NAc is convergent with several forebrain glutamate inputs [75] and AMPARs are co-expressed with DA receptors in NAc neurons [12,68]. Most are either GluA1/GluA2 or GluA2/GluA3 heteromers [159]. Changes in AMPA receptor abundance in the synaptic membrane mediate dynamic tuning of synaptic transmission as well as plasticity [40,103]. In cultured MSNs, D1R activation rapidly increases GluA1 surface expression in a PKA-dependent manner [37,126]. It is therefore noteworthy that FR increases phosphorylation of GluA1 at Ser845, the PKA site, in response to administration of a D1R agonist, d-amphetamine, cocaine, sucrose, and a morphine-paired environment [30,98,121,150,151]. This set of findings points to a mechanism, upregulated by FR, that is activated by natural reward, drugs of abuse, and an environment associated with subjective effects of a drug. Phosphorylation at Ser845 stabilizes GluA1 in the membrane and facilitates synaptic insertion [53,55,85,86,125,144,166,177]. Consistent with pSer845 involvement in AMPAR trafficking, it was found that sucrose and d-amphetamine each increased levels of GluA1 and GluA2 in the NAc postsynaptic density (PSD), with a greater effect in FR than in ad libitum fed (AL) rats [150,151,197]. Given that most GluA1 in NAc are associated with GluA2, and most GluA2 not associated with GluA1 are partially assembled receptors [159], it is likely that reward stimuli during FR increase insertion of GluA1/GluA2 heteromers.
In the course of investigating effects of reward stimuli on AMPAR trafficking, it was discovered that FR, itself, increases NAc synaptic abundance of GluA1. Using a BS3 cross-linking method [18], it was shown that FR increases surface expression of GluA1 but not GluA2 [145]. Subcellular fractionation revealed that FR specifically increases GluA1 protein levels in the NAc PSD [145]. These findings suggested that FR increases synaptic insertion of GluA2-lacking, calcium-permeable AMPA receptors (CP-AMPARs). This conclusion was substantiated by electrophysiological findings in which Naspm, a selective antagonist of CP-AMPARs, decreased the amplitude of evoked excitatory postsynaptic currents (EPSCs) in NAc shell MSNs of FR, but not AL, rats [145]. Further, behavioral experiments indicated that CP-AMPARs mediate the enhanced responses of FR subjects to drug challenge. Microinjection of Naspm into NAc shell reversed the enhanced rewarding effects of SKF-82958 (D1/D5R agonist) [30] and d-amphetamine [150], and reversed the enhanced locomotor-activating effect of SKF-82958 in FR subjects [145]. Naspm had no effect on the rewarding or locomotor-activating effects of the D2-like (D2/D3R) agonist, quinpirole [30,145]. The significance of a synaptic population of CP-AMPARs lies in distinct properties they possess relative to other AMPAR types, including larger single channel conductance, faster kinetics, and triggering of postsynaptic signaling cascades that depend on Ca2+ [115].
FR does not increase density or affinity of D1 binding sites in NAc [77] nor does it increase D1R-stimulated adenylyl cyclase activity [32], which activates canonical signaling leading to phosphorylation of GluA1 at Ser845 via activation of PKA. However, recent findings point to an alternative mechanistic basis for increased DA-dependent pSer845-GluA1 and AMPAR surface expression in the NAc of FR rats. In cultured striatal MSNs, a cooperative relationship was shown between CP-AMPARs and D1Rs in regulating phosphorylation and trafficking of AMPARs [198]. Briefly, D1R stimulation increased phosphorylation of GluA1 at Ser845, via PKA, thereby increasing extrasynaptic accumulation of GluA1-containing AMPARs. Concurrent stimulation of CP-AMPARs triggered intracellular Ca2+ signaling, activating cGMP and cGKII, which augmented the phosphorylation of GluA1 at Ser845, and was followed by PKC-mediated synaptic insertion of GluA1 and GluA2. According to this model, synaptic insertion of CP-AMPARs may be the adaptive response to FR that enables amplification of cellular and behavioral effects downstream of D1R stimulation.
Glutamate inputs to nucleus accumbens
Many distinct components of reward-related behavior are encoded by NAc neurons [170], and strong evidence indicates that orosensory reward and consummatory behavior are initiated and sustained by neuronal inhibition [5,57,100,110,124,160,167,168,183,191,206]. Recently, O’Connor and coworkers identified D1R-expressing MSNs in NAc medial shell that project to lateral hypothalamus as the specific sub-population whose inhibition drives consummatory behavior [142]. In contrast, incentive motivation, as reflected in the initiation and vigor of approach triggered by reward cues, correlates with NAc neuronal firing rate [44,84,91,133], and is dependent on NAc neuronal excitation [42]. Taking a direct approach to assessing functional consequences of NAc neuronal excitation, optogenetic studies have shown that activation of glutamate terminals in NAc is positively reinforcing [19,188], as is direct activation of NAc medial shell neurons. These effects, and the potentiating effect of NAc neuronal activation on cocaine reward, have been attributed to D1R-expressing MSNs [19,111,122,199]. A caveat, however, is in recent findings that, depending on context and task, activation of D2R MSNs can also facilitate positively motivated behavior [179]. Nevertheless, based on the aforementioned pharmacological and biochemical findings, it may be predicted that FR increases synaptic insertion of CP-AMPARs in D1R MSNs of NAc medial shell. This prediction remains to be tested.
An important gap in mechanistic understanding of FR effects is the source(s) of glutamate that acts upon altered AMPAR mechanisms to drive behavior, and the question of whether any glutamate input is differentially involved in FR relative to AL rats. Glutamate inputs to NAc include those from medial prefrontal cortex (mPFC), ventral subiculum (vSub), basolateral amygdala (BLA) and paraventricular thalamus (PVT) [11,19,65,66,175]. Most addiction research has been focused on the former three inputs, which display a high degree of convergence upon individual MSNs [19,62,65,66,75,132], and whose terminals in NAc can be stimulated to increase locomotor activity [4] and reinforce intracranial self-stimulation [19,185,188]. The fact that 95% of MSNs are excited by activation of any one of these three input pathways [19], has led to the suggestion that the amount of glutamate release in NAc may be of greater functional importance than the source [19,199]. Based on such findings, it might be expected that all glutamate inputs to NAc have the potential to interact with a behaviorally significant population of CP-AMPARs. Yet, a source of glutamate input to NAc shell that is well-suited to signal in a manner that is dependent on diet and metabolic status, is PVT [11,20,118]. PVT neurons are excited by food deprivation [195], glucoprivation [51], orexigenic peptide pathways [130], and are activated when FR subjects are anticipating a meal [138]. Importantly, drugs of abuse also activate PVT neurons [48,63,67]. Selective elimination of PVT neurons that innervate NAc decreases acquisition of cocaine self-administration [140], and inactivation of PVT blocks cocaine prime-induced reinstatement [95], CPP expression [21], and meal-anticipatory locomotor activity [138]. However, not unlike the sub-population of NAc shell neurons whose inhibition promotes consummatory behavior, experimental inhibition of PVT neurons increases consumption [184,214]. PVT contains a nearly pure population of glutamate cells [61], most of which innervate NAc shell [52,92,119], where they form synaptic connections with MSNs [119] and converge with DA afferents [10]. There has been a recent test of PVT involvement in drug-seeking behavior of rats on a mild FR regimen in which body weight was maintained at 90% of the pre-FR value. Chemogenetic inhibition of PVT had no effect on heroin seeking or its augmentation by FR in subjects that were withdrawn from heroin [39]. However, chemogenetic excitation preferentially altered the behavior of FR relative to AL rats, but the effect was to inhibit their heroin-seeking. While this result is consistent with the existence of an upregulated response to glutamate of PVT origin, it is not known which PVT target area(s) is involved, nor is the status of NAc AMPAR mechanisms under this protocol known. That is, it is not known whether a mild FR protocol induces the same neuroadaptations as the protocol described above, and opioid withdrawal, itself, induces excitatory plasticity in NAc [123].
Synaptic insertion of CP-AMPARs and motivational valence
In addition to increasing the unconditioned rewarding effects of abused drugs, FR increases the magnitude and persistence of a cocaine conditioned place preference (CPP) acquired during a prior ad libitum (AL) fed state [215–217]. The same effect is seen in morphine CPP [98]. Further, in both cases, CPP expression was associated with increased NAc pSer845-GluA1, which correlated with magnitude of CPP. However, when a LiCl place aversion was conditioned, and subjects were switched to FR for testing, the CPA weakened and extinguished more rapidly than in AL subjects. The opposing effects of FR on cocaine/morphine CPP and LiCl CPA suggest that the effects on CPP expression are not due to a general enhancement of recall or attentiveness but rather, increased incentive effects of the drug-paired context.
One determinant of vulnerability to drug abuse is the balance between rewarding and aversive drug effects [163,201,202]. For example, a substantial percentage of psychostimulant users report adverse effects (e.g., depression, anxiety, irritability), and this often leads to moderation of use [208]. Moreover, a hallmark feature of addiction is drug-seeking despite adverse conditions [47,200], which may be homologous with the suppression of aversion that accompanies strong food-motivated behavior [22,107]. Consequently, organismic states that include a negative gating of aversive stimuli may enhance use of drugs with mixed effects and promote addictive behavior. It was therefore considered whether the attenuation of LiCl CPA might be reflective of an aversion-attenuating effect, possibly related to the anxiolytic and analgesic effects that can be induced by FR [81,94,117,134,211]. To assess whether FR may have a moderating effect on the aversive subjective effect of cocaine, we adapted a protocol in which cocaine reinforces a CPA if pairing of drug and context are delayed by 15-min [56]. Using i.c.v. administration, delayed pairing reinforced a modest but significant cocaine CPA. When testing resumed three weeks later, AL subjects continued to display avoidance of the cocaine-paired compartment, but subjects that had been switched to FR displayed preference [Figure 1]. This reversal in motivational valence could result from the known reward-enhancing effect of FR. That is, if cocaine has mixed effects that net out to aversion in the AL fed subject, and those mixed effects are elicited by the paired context as well, FR may selectively enhance the rewarding component and switch valence. Yet, in the LiCl experiment, neither the unconditioned nor conditioned stimulus has a rewarding component available for augmentation.
Figure 1.
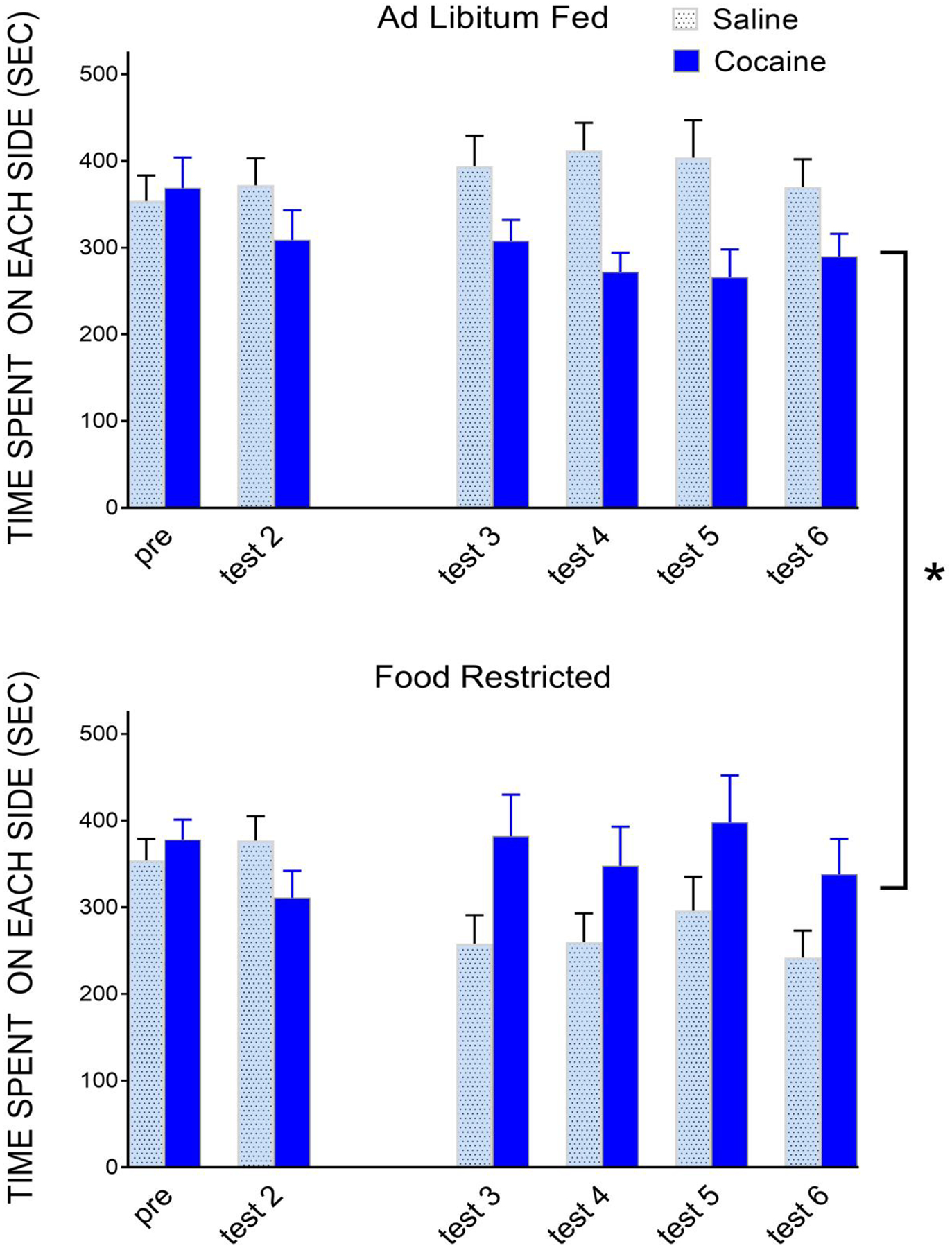
In eight consecutive daily conditioning sessions, rats (male and female) were injected with cocaine (200 μg, i.c.v.) or saline vehicle in alternation. Fifteen minutes after injection, rats were confined (15-min) to corresponding compartments of a place conditioning apparatus. Acquisition of a cocaine conditioned place aversion was confirmed (Test 2; n=25 total; p<.05). Rats were then either maintained AL (top) or switched to FR (bottom) for 3 weeks prior to resumption of testing. Mean time (sec) on the saline- and cocaine-paired sides are displayed. Time not spent on either side was spent in the center compartment. Analysis of difference scores (tests 3–6) indicates an effect of diet (F1,23=7.45, p=.01) [Lee and Carr, unpublished].
An aversion-attenuating mechanism is in line with interest in NAc as a target for modulating pain and negative affect [136], and basic science studies point to a possible mechanistic overlap between these effects and the reward potentiating effect of FR. Specifically, chronic pain increases NAc synaptic abundance of CP-AMPARs, and these receptors mediate a negative feedback mechanism which attenuates the accompanying aversive motivational-affective state [71,114]. Current understanding of the way in which reward and aversion are coded in NAc does not readily lend itself to a model in which separate glutamate inputs acting through CP-AMPARs facilitate reward and inhibit aversion. Optogenetic studies generally support the view that activation of D1R-expressing MSNs has a pro-reward effect and, though somewhat less well supported, activation of D2R-expressing MSNs has an anti-reward effect [179]. In this simple model of valence coding, synaptic insertion of CP-AMPARs in D1R-expressing MSNs might be expected to have the dual effect of enhancing reward and diminishing aversion by shifting the balance of excitability between functionally opponent cell types. It is also possible that reward-enhancing and aversion-attenuating populations of CP-AMPARs are anatomically separable. Optogenetic activation of glutamate terminals from prelimbic cortex in NAc core induces analgesia, inhibits the aversive motivational state, and restores sucrose preference in the chronic pain model [114]. The research on CP-AMPAR involvement in the reward-enhancing effect of FR has, on the other hand, implicated the NAc shell, while the core has not been similarly examined.
The triggering condition for NAc neuroadaptations
The triggering condition for NAc synaptic incorporation of CP-AMPARs during FR has not been identified, though reward deficiency and the neurochemical concomitant of decreased DA transmission merit investigation. There are several other conditions in which loss of reward is followed by synaptic incorporation of CP-AMPARs in NAc. These include withdrawal from chronic cocaine [41], amphetamine [96], morphine [87], ‘junk food’ [143], and ventral tegmental DA neuronal stimulation [149]. Moreover, these CP-AMPARs have been shown to mediate the enhanced cue-triggered motivation and “craving” which characterize the withdrawn state [41,143]. Synaptic insertion of CP-AMPARs may therefore be a cellular response aimed at maintaining reward homeostasis by enhancing the incentive potency of appetitive stimuli and, inadvertently, their proxies (e.g., drugs of abuse). This line of thinking is a speculative extension of findings obtained in cultured neurons, sensory cortices, and brainstem sensory relays following deprivation of input, where homeostatic trafficking of CP-AMPARs to the synapse either restores stable activity or compensates for loss of function [69,70,76,97,105,115,171,190,192,193]. To our knowledge, the only prior evidence bearing on the possibility that deficient DA transmission is a stimulus for CP-AMPAR insertion comes from extreme cases of DA depletion; a genetic mouse model of Parkinson’s Disease increases striatal levels of GluA1 and pSer845-GluA1 [109], and 6-OHDA-induced DA depletion causes dorsal striatal synaptic incorporation of CP-AMPARs [3] and an L-DOPA-induced dyskinesia that is dependent on striatal CP-AMPARs [106]. However, there is also the recent finding that mice withdrawn from chronic optogenetic self-stimulation of ventral tegmental DA neurons show synaptic insertion of CP-AMPARs in D1R-, but not D2R-, expressing MSNs in NAc, coinciding with emergence of vigorous cue-induced relapse to seeking DA stimulation [149].
Translation and caveats
The basic science findings relating to food restriction, dopamine, AMPA receptors, and drugs of abuse were obtained in male rats. This points to a serious gap in the literature. National surveys in the US indicate that at any given time twice as many women as men are dieting to lose weight [17,162,174,207], and women consistently seek to lose a greater percentage of body weight than men [17,58,112]. Moreover, transition from dieting to an eating disorder occurs predominantly in girls and women [181]. Comorbidity of eating disorders with substance use disorders ranges from 23 to 37%, which is much higher than prevalence in the general population of 2–3% for illicit drugs and 12% for alcohol [135]. Considering the female predominance among those who diet, suffer from eating disorders, and express a comorbid substance use disorder, the use of males in the FR studies cited above provides an incomplete view of the translational relevance of findings obtained.
Should continuing research support a model in which NAc synaptic insertion of CP-AMPARs during FR increases the incentive potency of appetitive reward stimuli and concurrently diminishes aversion, the utility of such a neuroadaptation during periods of food scarcity in the wild would be clear. However, modern human societies offer opportunities for maladaptive consequences. These could include self-medication of negative affective states by abstinence from feeding, breakthrough episodes of bingeing among weight loss dieters, and increased relapse risk among those who diet to combat rebound weight gain which occurs during cessation of smoking and other psychostimulant addictions.
A moderating consideration when judging the above model as a feature of homeostatic control or a basis for the pathogenic potential of weight loss dieting is the impoverished environment occupied by rodent subjects in most of the aforementioned studies. Because subjects in these studies typically have surgical implants, and/or their food access and consumption need to be precisely regulated, they are usually housed individually without social contact or enrichment objects. The incentive value of food is accentuated under these housing conditions, as suggested by the fact that free-feeding rats housed in this manner take in more calories and are more obese than rats housed in an enriched environment [129]. Consequently, “impoverished” housing would increase the severity of reward loss when food is removed. Interestingly, transfer of rats from impoverished housing to social housing with novel objects increases NAc metabolic activity in both the short- and long-term [72,113] and specifically increases basal extracellular [DA] and potassium-induced DA release [173]. These changes are accompanied by decreased potency of other incentive stimuli. For example, just 22 hours of environmental enrichment decreased rats’ consumption and responsiveness to sucrose-paired cues [74], and three weeks of enrichment decreased cue-induced reinstatement of cocaine-seeking [194]. Consequently, CNS and behavioral effects of FR in naturalistic environments may be less extreme than those identified in the studies described, and this additional factor merits systematic investigation.
Supplementary Material
Highlights.
Food restriction increases the reward magnitude of abused drugs and associated cues
Behavioral effects are mediated by Ca2+-permeable AMPA receptors in n. accumbens
Results may provide insight into dieting as a risk factor for binge eating and drug abuse
Acknowledgements
This work was supported by the National Institutes of Health [DA-003956]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Almundarij TI, Gavini CK, Novak CM, Suppressed sympathetic outflow to skeletal muscle, muscle thermogenesis, and activity energy expenditure with calorie restriction. Physiol Reports 5 (2017) e13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin SB, Gortmaker SL, Dieting and smoking initiation in early adolescent girls and boys: A prospective study. Am J Public Health 91 (2001) 446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagetta V, Sgobio C, Pendolino V, Del Papa G, Tozzi A, Ghiglieri V, Giampa C, Zianni E, Gardoni F, Calabresi P, Picconi B, Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson’s Disease. J Neurosci 32 (2012):17921–17931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagot RC, Parise EM, Peña CJ, Zhang H, Maze I, Chaudhury D, Persaud B, Cachope R, Bolaños-Guzman CA, Cheer JF, Deisseroth K, Han M, Nestler EJ, Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nature Commun (2015) 6:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE, Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci 19 (2004) 376–386. [DOI] [PubMed] [Google Scholar]
- 6.Barson JR, Leibowitz SF, Orexin/hypocretin system: Role in food and drug overconsumption. Int Rev Neurobiol. 136 (2017) 199–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassareo V, Di Chiara G, Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neurosci 89 (1999) 637–641. [DOI] [PubMed] [Google Scholar]
- 8.Bassareo V, Di Chiara G, Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci 11 (1999) 4389–4397. [DOI] [PubMed] [Google Scholar]
- 9.Bell SM, Stewart RB, Thompson SC, Meisch RA, Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacol 131 (1997) 1–8. [DOI] [PubMed] [Google Scholar]
- 10.Berendse HW, Galis-de Graaf Y, Groenewegen HJ, Topographical corticostriatal projections in the rat. J Comp Neurol 316 (1992) 314–347. [DOI] [PubMed] [Google Scholar]
- 11.Berendse HW, Groenewegen HJ, Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J Comp Neurol 299 (1990) 187–228. [DOI] [PubMed] [Google Scholar]
- 12.Bernard V, Somogyi P, Bolam JP, Cellular, subcellular, and subsynaptic distribution of AMPA type glutamate receptor subunits in the neostriatum of the rat. J Neurosci 17 (1997) 819–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge KC, Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat, Appetite 16 (1991) 103–120. [DOI] [PubMed] [Google Scholar]
- 14.Berridge KC, The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacol 191(2007) 391–431. [DOI] [PubMed] [Google Scholar]
- 15.Berridge KC, ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav 97 (2009) 537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bindra D, The interrelated mechanisms of reinforcement and motivation, and the nature of their influence on response, in: Arnold WJ & Levine D (Eds.), Nebraska Symposium on Motivation: University of Nebraska Press, Lincoln, 1969, 1–38. [Google Scholar]
- 17.Bish CL, Blanck HM, Serdula MK, Marcus M, Kohl HW 3rd, Khan LK, Diet and physical activity behaviors among Americans trying to lose weight: 2000 Behavioral Risk Factor Surveillance System. Obes Res 13 (2005) 596–607. [DOI] [PubMed] [Google Scholar]
- 18.Boudreau AC, Wolf ME, Behavioral sensitization to cocaine associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci 25 (2005) 9144–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A, Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76 (2012) 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brog JS, Salyapongse A, Deutch AY, Zahm DS, The patterns of afferent innervation of the core and shell in the ‘accumbens’ part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338 (1993) 255–278. [DOI] [PubMed] [Google Scholar]
- 21.Browning JR, Jansen HT, Sorg BA, Inactivation of the paraventricular thalamus abolishes the expression of cocaine conditioned place preference in rats. Drug Alcohol Depend 134 (2014) 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett CJ, Li C, Webber E, Tsaousidou E, Xue SY, Brüning JC, Krashes MJ, Hunger-driven motivational state competition. Neuron 92 (2016) 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabanac M, Physiological role of pleasure, Science 173 (1971) 1103–l107. [DOI] [PubMed] [Google Scholar]
- 24.Cabeza de Vaca S, Carr KD, Food restriction enhances the central rewarding effect of abused drugs. J Neurosci 18 (1998) 7502–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabeza de Vaca S, Krahne L, Carr KD, A progressive ratio schedule of self-stimulation testing reveals profound augmentation of d-amphetamine reward by food restriction but no effect of a “sensitizing” regimen of d-amphetamine. Psychopharmacol 175 (2004) 106–113. [DOI] [PubMed] [Google Scholar]
- 26.Cameron JD, Goldfield GS, Cyr MJ, Doucet E, The effects of prolonged caloric restriction leading to weight-loss on food hedonics and reinforcement. Physiol Behav 94 (2008) 474–480. [DOI] [PubMed] [Google Scholar]
- 27.Campbell UC, Carroll ME, Effects of ketoconazole on the acquisition of intravenous cocaine self-administration under different feeding conditions in rats. Psychopharmacol. 154 (2001) 311–318. [DOI] [PubMed] [Google Scholar]
- 28.Cardinal RN, Everitt BJ, Neural and psychological mechanisms underlying appetitive learning: Links to drug addiction. Curr Opin Neurobiol 14 (2004)156–162. [DOI] [PubMed] [Google Scholar]
- 29.Carr KD, Cabeza de Vaca S, Sun Y, Chau LS, Reward-potentiating effects of D-1 dopamine receptor agonist and AMPAR GluR1 antagonist in nucleus accumbens shell and their modulation by food restriction. Psychopharmacol 202 (2009) 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carr KD, Chau LS, Cabeza de Vaca S, Gustafson K, Stouffer M, Tukey DS, Restituito S, Ziff EB, AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neurosci 165 (2010)1074–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr KD, Kim GY, Cabeza de Vaca S, Rewarding and locomotor-activating effects of direct dopamine receptor agonists are augmented by chronic food restriction in rats. Psychopharmacol 154 (2001) 420–428. [DOI] [PubMed] [Google Scholar]
- 32.Carr KD, Tsimberg Y, Berman Y, Yamamoto N, Evidence of increased dopamine receptor signaling in food-restricted rats. Neurosci 119 (2003) 1157–1167. [DOI] [PubMed] [Google Scholar]
- 33.Carroll ME, Meisch RA, The effects of feeding conditions on drug-reinforced behavior: maintenance at reduced body weight versus availability of food. Psychopharmacol 68 (1980) 121–124. [DOI] [PubMed] [Google Scholar]
- 34.Carroll ME, Meisch RA, Determinants of increased drug self-administration due to food deprivation. Psychopharmacol 74 (1981) 197–200. [DOI] [PubMed] [Google Scholar]
- 35.Carroll ME, Meisch RA, Increased drug-reinforced behavior due to food deprivation. Adv Behav Pharmacol 4 (1984) 47–88. [Google Scholar]
- 36.Castro DC, Berridge KC, Opioid hedonic hotspot in nucleus accumbens shell: mu, delta and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci 34 (2014) 4239–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao SZ, Ariano MA, Peterson DA, Wolf ME, D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem 83 (2002) 704–712. [DOI] [PubMed] [Google Scholar]
- 38.Cheskin LJ, Hess JM, Henningfield J, Gorelick DA DA, Calorie restriction increases cigarette use in adult smokers. Psychopharmacol 179 (2005) 430–436. [DOI] [PubMed] [Google Scholar]
- 39.Chisholm A, Iannuzzi J, Rizzo D, Gonzalez N, Fortin É, Bumbu A, Batallán Burrowes AA, Chapman CA, Shalev U, The role of the paraventricular nucleus of the thalamus in the augmentation of heroin seeking induced by chronic food restriction Addiction Biol 2019. doi: 10.1111/adb.12708. [DOI] [PubMed] [Google Scholar]
- 40.Choquet D, Fast AMPAR trafficking for a high-frequency synaptic transmission. Eur J Neurosci 32 (2010) 250–260. [DOI] [PubMed] [Google Scholar]
- 41.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y, Marinelli M, Wolf ME, Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454 (2008)118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbit LH, Balleine BW, The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci 31 (2011)11786–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corwin RL, Avena NM, Boggiano MM, Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav 104 (2011) 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cromwell HC, Schultz W, Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol 89 (2003) 2823–2838. [DOI] [PubMed] [Google Scholar]
- 45.D’Cunha TM, Daoud E, Rizzo D, Bishop AB, Russo M, Mourra G, Hamel L, Sedki F, Shalev U, Augmentation of heroin seeking following chronic food restriction in the rat: Differential role for dopamine transmission in the nucleus accumbens shell and core. Neuropsychopharmacol 42 (2017)1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Cunha TM, Sedki F, Macri J, Casola C, Shalev U, The effects of chronic food restriction on cue-induced heroin seeking in abstinent male rats. Psychopharmacol 225 (2013) 241–250. [DOI] [PubMed] [Google Scholar]
- 47.Deroche-Gamonet V, Belin D, Piazza PV, Evidence for addiction-like behavior in the rat. Science 305 (2004) 1014–1017. [DOI] [PubMed] [Google Scholar]
- 48.Deutch AY, Bubser M, Young CD, Psychostimulant-induced Fos protein expression in the thalamic paraventricular nucleus. J Neurosci 18 (1998) 10680–10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Chiara G, Dopamine in disturbances of food and drug motivated behavior: A case of homology? Physiol Behav 86 (2005) 9–10. [DOI] [PubMed] [Google Scholar]
- 50.DiLeone RJ, Georgescu D, Nestler EJ, Lateral hypothalamic neuropeptides in reward and drug addiction, Life Sci. 73 (2003) 759–768. [DOI] [PubMed] [Google Scholar]
- 51.Dodd GT, Williams SR, Luckman SM, Functional magnetic resonance imaging and c-Fos mapping in rats following a glucoprivic dose of 2-deoxy-D-glucose. J Neurochem 113 (2010) 1123–1132. [DOI] [PubMed] [Google Scholar]
- 52.Dong X, Li S, Kirouac GJ, Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct Funct 222 (2017) 3927–3943. [DOI] [PubMed] [Google Scholar]
- 53.Ehlers MD, Heine M, Groc L, Lee MC, Choquet D, Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron 54 (2007) 447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epstein LH, Truesdale R, Wojcik A, Paluch RA, Raynor HA, Effects of deprivation on hedonics and reinforcing value of food. Physiol Behav 78 (2003) 221–227. [DOI] [PubMed] [Google Scholar]
- 55.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R, PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6 (2003)136–143. [DOI] [PubMed] [Google Scholar]
- 56.Ettenberg A, Raven MA, Danluck DA, Necessary BD, Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav 64 (1999) 507–512. [DOI] [PubMed] [Google Scholar]
- 57.Faure A, Richard JM, Berridge KC, Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS ONE 5 (2010) e11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fayet F, Petocz P, Samman S, Prevalence and correlates of dieting in college women: A cross sectional study. Int J Womens Health 4 (2012) 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feltenstein MW, See RE, The neurocircuitry of addiction: An overview. Br J Pharmacol 154 (2008) 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferguson SA, Paule MG, Progressive ratio performance varies with body weight in rats. Behav Processes 40 (1997) 177–182. [DOI] [PubMed] [Google Scholar]
- 61.Ferrario CR, Labouèbe G, Liu S, Nieh EH, Routh VH, Xu S, O’Connor EC, Homeostasis meets motivation in the battle to control food intake. J Neurosci 36 (2016) 11469–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finch DM, Neurophysiology of converging synaptic inputs from the rat prefrontal cortex, amygdala, midline thalamus, and hippocampal formation onto single neurons of the caudate/putamen and nucleus accumbens. Hippocampus 6 (1996) 495–512. [DOI] [PubMed] [Google Scholar]
- 63.Franklin TR, Druhan JP, Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci 12 (2000) 2097–2106. [DOI] [PubMed] [Google Scholar]
- 64.French SA, Perry CL, Leon GR, Fulkerson JA, Weight concerns, dieting behavior, and smoking initiation among adolescents: A prospective study. Am J Public Health 84 (1994) 1818–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.French SJ, Totterdell S, Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol 446 (2002) 151–165. [DOI] [PubMed] [Google Scholar]
- 66.French SJ, Totterdell S, Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neurosci 119 (2003) 19–31. [DOI] [PubMed] [Google Scholar]
- 67.Garcia MM, Brown HE, Harlan RE, Alterations in immediate-early gene proteins in the rat forebrain induced by acute morphine injection. Brain Res 692 (1995) 23–40. [DOI] [PubMed] [Google Scholar]
- 68.Glass MJ, Lane DA, Colago EEO, Chan J, Schlussman SD, Zhou Y, Kreek MJ, Pickel VM, Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens. Exp Neurol 210 (2008) 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK, Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci 9 (2006) 1001–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goel A, Xu LW, Snyder KP, Song L, Goenaga-Vazquez Y, Megill A, Takamiya K, Huganir RL, Lee HK, Phosphorylation of AMPA receptors is required for sensory deprivation-induced homeostatic synaptic plasticity. PLoSOne 6, (2011) e18264.doi: 10.1371/journal.pone.0018264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goffer Y, Xu D, Eberle SE, D’amour J, Lee M, Tukey D, Froemke RC, Ziff EB, Wang J, Calcium permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J. Neurosci 33 (2013) 19034–19044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzalez-Lima F, Ferchmin PA, Eterovic VA, Gonzalez-Lima EM, Metabolic activation of the brain of young rats after exposure to environmental complexity. Dev Psychobiol 27 (1994) 343–351. [DOI] [PubMed] [Google Scholar]
- 73.Grill HJ, Roitman MF, Kaplan JM, A new taste reactivity Behav Neurosci 108 (1994) 347–352.8037879 [Google Scholar]
- 74.Grimm JW, Hyde J, Glueck E, North K, Ginder D, Jiganti K, Hopkins M, Sauter F, MacDougall D, Hovander D, Examining persistence of acute environmental enrichment-induced anti-sucrose craving effects in rats. Appetite 139 (2019) 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Groenewegen HJ, Wright CI, Beijer AV, Voorn P, Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci 877 (1999) 49–63. [DOI] [PubMed] [Google Scholar]
- 76.Groth RD, Lindskog M, Thiagarajan TC, Li L, Tsien RW, β Ca2+/CaM-dependent kinase type II triggers upregulation of GluA1 to coordinate adaptation to synaptic inactivity in hippocampal neurons. Proc Natl Acad Sci 108 (2011) 828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haberny SL, Berman Y, Meller E, Carr KD, Chronic food restriction increases D-1 dopamine receptor agonist-induced ERK1/2 MAP kinase and CREB phosphorylation in caudate-putamen and nucleus accumbens. Neurosci 125 (2004) 289–298. [DOI] [PubMed] [Google Scholar]
- 78.Haberny SL, Carr KD, Food restriction increases NMDA receptor-mediated CaMK II and NMDA receptor/ERK 1/2-mediated CREB phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neurosci 132 (2005) 1035–1043. [DOI] [PubMed] [Google Scholar]
- 79.Haberny SL, Carr KD, Comparison of basal and D-1 dopamine receptor agonist-stimulated neuropeptide gene expression in caudate-putamen and nucleus accumbens of ad libitum fed and food-restricted rats. Molec Brain Res 141 (2005) 121–127. [DOI] [PubMed] [Google Scholar]
- 80.Hajnal A, Smith GP, Norgren R, Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol - Reg Integr Comp Physiol 286 (2004) R31–37. [DOI] [PubMed] [Google Scholar]
- 81.Hamm RJ, Knisely JS, The analgesia produced by food deprivation in 4-month old, 14-month old, and 24-month old rats. Life Sci 39 (1986)1509–1515. [DOI] [PubMed] [Google Scholar]
- 82.Hao J, Cabeza de Vaca S, Carr KD, Effects of chronic ICV leptin infusion on motor-activating effects of D-amphetamine in food-restricted and ad libitum fed rats. Physiol Behav 83 (2004) 377–381. [DOI] [PubMed] [Google Scholar]
- 83.Hao J, Cabeza de Vaca S, Pan Y, Carr KD, Effects of central leptin infusion on the reward-potentiating effect of D-amphetamine. Brain Res. 1087 (2006) 123–133. [DOI] [PubMed] [Google Scholar]
- 84.Hassani OK, Cromwell HC, Schultz W, Influence of expectation of different rewards on behavior related neuronal activity in the striatum. J Neurophysiol 85 (2001) 2477–2489. [DOI] [PubMed] [Google Scholar]
- 85.He K, Goel A, Ciarkowski CE, Song L, Lee HK, Brain area specific regulation of synaptic AMPA receptors by phosphorylation. Commun Integr Biol 4 (2011) 569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK, Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci 106 (2009) 20033–20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, Schmidt C, Larson EB, Thomas MJ, Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci 113 (2016) 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hodos W, Progressive ratio as a measure of reward strength, Science 134 (1961) 943–944. [DOI] [PubMed] [Google Scholar]
- 89.Hodos W, Kalman G, Effects of increment size and reinforcer volume on progressive ratio performance. J Exp Anal Behav 6 (1963) 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoebel BG, Brain neurotransmitters in food and drug reward. Am J Clin Nutr 42 (5 Suppl) (1985) 1133–1150. [DOI] [PubMed] [Google Scholar]
- 91.Hollerman JR, Tremblay L, Schultz W, Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol 80 (1998) 947–963. [DOI] [PubMed] [Google Scholar]
- 92.Hoover WB, Vertes RP, Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 212 (2007) 149–179. [DOI] [PubMed] [Google Scholar]
- 93.Hsu TM, McCutcheon JE, Roitman MF, Parallels and overlap: the integration of homeostatic signals by mesolimbic dopamine neurons. Front Psychiat (2018) 10.3389/fpsyt.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inoue K, Zorrilla EP, Tabarin A, Valdez GR, Iwasaki S, Kiriike N, Koob GF, Reduction of anxiety after restricted feeding in the rat: Implication for eating disorders. Biol Psychiat 55 (2004)1075–1081. [DOI] [PubMed] [Google Scholar]
- 95.James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR, Smith DW, Dayas CV, Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoSONE 5 (2010) 12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jedynak J, Hearing M, Ingebretson A, Ebner S, Kelly M, Fischer RA, Kourrich S, Thomas MJ, Cocaine and amphetamine induce overlapping but distinct patterns of AMPAR plasticity in nucleus accumbens medium spiny neurons. Neuropsychopharmacol 41 (2015) 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB, Kessels HW, Takahashi T, Serotonin mediates cross-modal reorganization of cortical circuits. Neuron 69 (2011) 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jung C, Rabinowitsch A, Lee WT, Zheng D, Cabeza de Vaca S, Carr KD, Effects of food restriction on expression of place conditioning and biochemical correlates in rat nucleus accumbens. Psychopharmacol 233 (2016) 3161–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelley AE, Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27 (2004) 765–776. [DOI] [PubMed] [Google Scholar]
- 100.Kelley AE, Baldo BA, Pratt WE, Will MJ, Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86 (2005) 773–795. [DOI] [PubMed] [Google Scholar]
- 101.Kelley AE, Berridge KC, The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci 22 (2002) 3306–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kenny PJ, Reward mechanisms in obesity: New insights and future directions. Neuron 69 (2011) 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kessels HW, Malinow R, Synaptic AMPA receptor plasticity and behavior. Neuron 61 (2009) 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khanh DV, Choi YH, Moh SH, Kinyua AW, Kim KW, Leptin and insulin signaling in dopaminergic neurons: relationship between energy balance and reward system. Front Psychol 5 (2014) 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim S, Ziff EB, Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+-permeable AMPA receptors. PLoS Biol. 12 (2014) e1001900. doi: 10.1371/journal.pbio.1001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobylecki C, Crossman AR, Ravenscroft P, Alternative splicing of AMPA receptor subunits in the 6-OHDA-lesioned rat model of Parkinson’s disease and L-DOPA-induced dyskinesia. Exper Neurol 247 (2013) 476–484. [DOI] [PubMed] [Google Scholar]
- 107.Konorski J, Integrative Activity of the Brain. University of Chicago Press; (1967) Chicago. [Google Scholar]
- 108.Kornetsky C, Esposito RU, Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 38 (1979) 2473–2476. [PubMed] [Google Scholar]
- 109.Koutsokera M, Kafkalias P, Giompres P, Kouvelas ED, Mitsacos A, Expression and phosphorylation of glutamate receptor subunits and CaMKII in a mouse model of Parkinsonism. Brain Res 1549 (2014) 22–31. [DOI] [PubMed] [Google Scholar]
- 110.Krause M, German PW, Taha SA, Fields HL, A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci 30 (2010) 4746–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kravitz AV, Tye LD, Kreitzer AC, Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci 15 (2012) 816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kruger J, Galuska DA, Serdula MK, Jones DA, Attempting to lose weight: Specific practices among U.S. adults. Am J Prev Med 26 (2004) 402–406. [DOI] [PubMed] [Google Scholar]
- 113.Läck AK, Gill KE, Porrino LJ, Local cerebral glucose utilization in rats exposed to an enriched environment: a comparison to impoverishment. Pharmacol Biochem Behav 96 (2010) 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee M, Manders TR, Eberle SE, Su C, D’amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J, Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci 35 (2015) 5247–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee HK, Ca-permeable AMPA receptors in homeostatic synaptic plasticity. Front Mol Neurosci 5 (2012) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee Y, Lee KS, Relationship between unhealthy weight control behaviors and substance use patterns among Korean adolescents: results from the 2017 national youth risk behavior survey. Public Health 174 (2019) 56–64. [DOI] [PubMed] [Google Scholar]
- 117.Levay EA, Govic A, Penman J, Paolini AG, Kent S, Effects of adult-onset calorie restriction on anxiety-like behavior in rats. Physiol Behav 92 (2007) 889–896. [DOI] [PubMed] [Google Scholar]
- 118.Li S, Kirouac GJ, Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 506 (2008) 263–287. [DOI] [PubMed] [Google Scholar]
- 119.Ligorio M, Descarries L, Warren RA, Cholinergic innervation of thalamic input in rat nucleus accumbens. J Chem Neuroanat 37 (2009) 33–45. [DOI] [PubMed] [Google Scholar]
- 120.Liu S, Borgland SL, Regulation of the mesolimbic dopamine circuit by feeding peptides. Neurosci 289 (2015)19–42. [DOI] [PubMed] [Google Scholar]
- 121.Liu S, Zheng D, Peng XX, Cabeza de Vaca S, Carr KD, Enhanced cocaine-conditioned place preference and associated brain regional levels of BDNF, p-ERK1/2 and p-Ser845-GluA1 in food-restricted rats. Brain Res 1400 (2011) 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ, Cell type specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330 (2010) 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Madayag AC, Gomez D, Anderson EM, Ingebretson AE, Thomas MJ, Hearing MC, Cell-type and region-specific nucleus accumbens AMPAR plasticity associated with morphine reward, reinstatement, and spontaneous withdrawal. Brain Struct Funct 224 (2019) 2311–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maldonado-Irizarry CS, Swanson CJ, Kelley AE, Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15 (1995) 6779–6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Man HY, Sekine-Aizawa Y, Huganir RL, Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci 104 (2007) 3579–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mangiavacchi S, Wolf ME, D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem 88 (2004)1261–1271. [DOI] [PubMed] [Google Scholar]
- 127.Maric T, Sedki F, Ronfard B, Chafetz D, Shalev U, A limited role for ghrelin in heroin self-administration and food deprivation-induced reinstatement of heroin seeking in rats. Addict Biol 17 (2012) 613–622. [DOI] [PubMed] [Google Scholar]
- 128.Marinelli M, Le Moal M, Piazza PV, Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Res 724 (1996) 251–255. [DOI] [PubMed] [Google Scholar]
- 129.Martin B, Ji S, Maudsley S, Mattson MP, “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci 107 (2010) 6127–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin-Fardon R, Boutrel B, Orexin/hypocretin (orx/hcrt) transmission and drug-seeking behavior: is the paraventricular nucleus of the thalamus (PVT) part of the drug seeking circuitry? Front Behav Neurosci 6 (2012) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Masheb RM, Kutz AM, Marsh AG, Min KM, Ruser CB, Dorflinger LM, “Making weight” during military service is related to binge eating and eating pathology for veterans later in life. Eat Weight Disord 24 (2019) 1063–1070. [DOI] [PubMed] [Google Scholar]
- 132.McGinty VB, Grace AA, Timing-dependent regulation of evoked spiking in nucleus accumbens neurons by integration of limbic and prefrontal cortical inputs. J Neurophysiol 101 (2009)1823–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM, Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron 78 (2013) 910–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McGivern R, Berka C, Berntson GG, Walker JM, Sandman CA, Effect of naloxone on analgesia induced by food deprivation. Life Sci 25 (1979) 885–888. [DOI] [PubMed] [Google Scholar]
- 135.Merikangas KR, McClair VL, Epidemiology of substance use disorders. Hum Genet 131 (2012) 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mitsi V, Zachariou V, Modulation of pain, nociception, and analgesia by the brain reward center Neurosci 338 (2016) 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Münzberg H H, Qualls-Creekmore E, Yu S S, Morrison CD, Berthoud HR, Hedonics act in unison with the homeostatic system to unconsciously control body weight. Front Nutr 3 (2016) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nakahara K, Fukui K, Murakami N, Involvement of thalamic paraventricular nucleus in the anticipatory reaction under food restriction in the rat. J Vet Med Sci 66 (2004) 1297–1300. [DOI] [PubMed] [Google Scholar]
- 139.Navarro M, The role of the melanocortin system in drug and alcohol abuse. Int Rev Neurobiol 136 (2017) 121–150. [DOI] [PubMed] [Google Scholar]
- 140.Neumann PA, Wang Y, Yan Y, Wang Y, Ishikawa M, Cui R, Huang YH, Sesack SR, Schlüter OM, Dong Y, Cocaine-induced synaptic alterations in thalamus to nucleus accumbens projection. Neuropsychopharmacol 41 (2016) 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Norgren R, Hajnal A, Mungarndee SS, Gustatory reward and the nucleus accumbens. Physiol Behav 89 (2006) 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O’Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, Luscher C, Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88 (2015) 553–564. [DOI] [PubMed] [Google Scholar]
- 143.Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR, Eating ‘junk food’ produces rapid and long-lasting increases in. NAc CP-AMPA receptors: implications for enhanced cue-induced motivation and food addiction. Neuropsychopharmacol (2016) doi: 10.1038/npp.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Oh MC, Derkach VA, Guire ES, Soderling TR, Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem 281 (2006) 752–758. [DOI] [PubMed] [Google Scholar]
- 145.Ouyang J, Carcea I, Schiavo JK, Jones KT, Rabinowitsch A, Kolaric R, Cabeza de Vaca S, Froemke RC, Carr KD, Food restriction induces synaptic incorporation of calcium-permeable AMPA receptors in nucleus accumbens. Eur J Neurosci 45 (2017) 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Palmiter RD, Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci 30 (2007) 375–381. [DOI] [PubMed] [Google Scholar]
- 147.Pan Y, Chau L, Liu S, Avshalumov MV, Rice ME, Carr KD, A food restriction protocol that increases drug reward decreases tropomyosin receptor kinase B in the ventral tegmental area, with no effect on brain-derived neurotrophic factor or tropomyosin receptor kinase B protein levels in dopaminergic forebrain regions. Neurosci 197 (2011) 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pan Y, Haberny LY, Berman Y, Meller E, Carr KD, Synthesis, protein levels and phosphorylation state of tyrosine hydroxylase in mesoaccumbens and nigrostriatal dopamine pathways of chronically food-restricted rats. Brain Res 1122 (2006) 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pascoli V, Terrier J, Hiver A, Lüscher C, Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 88 (2015) 1054–1066. [DOI] [PubMed] [Google Scholar]
- 150.Peng XX, Cabeza de Vaca S, Ziff EB, Carr KD, GluA1-containing AMPA receptors are trafficked to the nucleus accumbens postsynaptic density in response to food restriction and d-amphetamine and potentiate reward. Psychopharmacol 231 (2014) 3055–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Peng XX, Ziff EB, Carr KD, Effects of food restriction and sucrose intake on synaptic delivery of AMPA receptors in nucleus accumbens. Synapse 65 (2011) 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pisetsky EM, Chao YM, Dierker LC, May AM, Striegel-Moore RH, Disordered eating and substance use in high-school students: Results from the Youth Risk Behavior Surveillance System. Int J Eat Disord 41 (2008) 464–470. [DOI] [PubMed] [Google Scholar]
- 153.Pontieri FE, Tanda G, Di Chiara G, Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci 92 (1995)12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pothos EN, Creese I, Hoebel BG, Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci 15 (1995) 6640–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pothos EN, Hernandez L, Hoebel BG, Chronic food deprivation decreases extracellular dopamine in the nucleus accumbens: implications for a possible neurochemical link between weight loss and drug abuse. Obes Res Suppl 4 (1995) 525S–529S. [DOI] [PubMed] [Google Scholar]
- 156.Quarta D, Smolders I, Rewarding, reinforcing and incentive salient events involve orexigenic hypothalamic neuropeptides regulating mesolimbic dopaminergic neurotransmission. Eur J Pharmaceut Sci 57 (2014) 2–10. [DOI] [PubMed] [Google Scholar]
- 157.Ranaldi R, Kest K, Zellner MR, Lubelski D, Muller J, Cruz Y, Saliba M, The effects of VTA NMDA receptor antagonism on reward-related learning and associated c-fos expression in forebrain. Behav Brain Res 216 (2011) 424–432. [DOI] [PubMed] [Google Scholar]
- 158.Raynor HA, Epstein LH, The relative-reinforcing value of food under differing levels of food deprivation and restriction, Appetite 40 (2003) 15–24. [DOI] [PubMed] [Google Scholar]
- 159.Reimers JM, Milovanovic M, Wolf ME, Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 1367 (2011) 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reynolds SM, Berridge KC, Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci 21 (2001) 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Richfield EK, Penney JB, Young AB, Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neurosci 30 (1989) 767–777. [DOI] [PubMed] [Google Scholar]
- 162.Rideout CA, Barr SI, “Restrained eating” vs “trying to lose weight”: How are they associated with body weight and tendency to overeat among postmenopausal women? J Am Diet Assoc 109 (2009) 890–893. [DOI] [PubMed] [Google Scholar]
- 163.Riley AL, The paradox of drug taking: The role of the aversive effects of drugs. Physiol Behav 103 (2011) 69–78. [DOI] [PubMed] [Google Scholar]
- 164.Robinson MJ, Fischer AM, Ahuja A A, Lesser EN, Maniates H, Roles of “wanting” and “liking” in motivating behavior: Gambling, food, and drug addictions. Curr Top Behav Neurosci 27 (2016)105–136. [DOI] [PubMed] [Google Scholar]
- 165.Robinson TE, Berridge KC, The incentive sensitization theory of addiction: some current issues, Phil Trans R. Soc 363 (2008) 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL, Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16 (1996) 1179–1188. [DOI] [PubMed] [Google Scholar]
- 167.Roitman MF, Wheeler RA, Carelli RM, Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli encode their predictors, and are linked to motor output. Neuron 45 (2005) 587–597. [DOI] [PubMed] [Google Scholar]
- 168.Roitman MF, Wheeler RA, Tiesinga PHE, Roitman JD, Carelli RM, Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Mem 17 (2010) 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rossi MA, Stuber GD, Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab 27 (2018) 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM, Rapid dopamine dynamics in the accumbens core and shell: Learning and action. Front Biosci 5 (2014) 273–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sarin RM, Mowery TM, Garraghty PE, AMPA receptor subunit expression in the cuneate nucleus of adult squirrel monkeys after peripheral nerve injury. Neurosci Lett 516 (2012) 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sedki F, Abbas Z, Angelis S, Martin J, D’Cunha T, Shalev U, Is it stress? The role of stress related systems in chronic food restriction-induced augmentation of heroin seeking in the rat. Front Neurosci 7 (2013) 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Segovia G, Del Arco A, De Blas M, Garrido P, Mora F, Environmental enrichment increases the in vivo extracellular concentration of dopamine in the nucleus accumbens: a microdialysis study. J Neural Transm 117 (2010) 1123–1130. [DOI] [PubMed] [Google Scholar]
- 174.Serdula MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW, Prevalence of attempting weight loss and strategies for controlling weight. J Am Med Assoc 282 (1999)1353–1358. [DOI] [PubMed] [Google Scholar]
- 175.Sesack SR, Grace AA, Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacol 35 (2010) 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shalev U, Chronic food restriction augments the reinstatement of extinguished heroin-seeking behavior in rats. Addict Biol 17 (2012) 691–693 [DOI] [PubMed] [Google Scholar]
- 177.Shi S, Hayashi Y, Esteban JA, Malinow R, Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105 (2001) 331–343. [DOI] [PubMed] [Google Scholar]
- 178.Smith GP, Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite 43 (2004) 11–13. [DOI] [PubMed] [Google Scholar]
- 179.Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, Sousa N, Rodrigues AJ, Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Comm 7 (2016) 11829 DOI: 10.1038/ncomms11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sorensen LB, Moller P, Flint A, Martens M, Raben A, Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int J Obes Relat Metab Disord 27 (2003) 1152–1166. [DOI] [PubMed] [Google Scholar]
- 181.Stice E, Davis K, Miller NP, Marti NC, Fasting increases risk for onset of binge eating and bulimic pathology: A 5-year prospective study. J Abnorm Psychol 117 (2008) 941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stouffer MA, Lee CR, Patel JC, Witkovsky P, Bao L, Machold RP, Carr KD, Rice ME, Insulin enhances striatal dopamine release via activation of cholinergic interneurons and thereby signals reward. Nat Commun 6 (2015) 8543, doi: 10.1038/ncomms9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stratford TR, Kelley AE, Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci 19 (1999)11040–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stratford TR, Wirtshafter D, Injections of muscimol into the paraventricular thalamic nucleus, but not mediodorsal thalamic nuclei, induce feeding in rats. Brain Res 1490 (2013) 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Stuber GD, Britt JP, Bonci A, Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry 71 (2012) 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stuber GD, Evans SB SB, Higgins MS, Pu Y, Figlewicz DP, Food restriction modulates amphetamine conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse 46 (2002) 83–90. [DOI] [PubMed] [Google Scholar]
- 187.Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A, Reward predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science 321 (2008) 1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stuber GD, Sparta DR, Stamatakis AM, Wieke AL, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A, Amygdala to nucleus accumbens excitatory transmission facilitates reward seeking. Nature 475 (2011) 377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Surmeier J, Ding J, Day M, Wang Z, Shen W, D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30 (2007) 228–235. [DOI] [PubMed] [Google Scholar]
- 190.Teichert M, Liebmann L, Hubner CA, Bolz J, Homeostatic plasticity and synaptic scaling in the adult mouse auditory cortex. Scientific Reports 7 (2017) 17423 | [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tellez LA, Perez IO, Simon SA, Gutierrez R, Transitions between sleep and feeding states in rat ventral striatum neurons. J Neurophysiol 108 (2012)1739–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW, LTP and adaptation to inactivity: Overlapping mechanisms and implications for metaplasticity. Neuropharmacol 52 (2007)156–175. [DOI] [PubMed] [Google Scholar]
- 193.Thiagarajan TC, Lindskog M, Tsien RW, Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47 (2005) 725–737. [DOI] [PubMed] [Google Scholar]
- 194.Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL, Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol 12 (2009) 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Timofeeva E, Richard D, Activation of the central nervous system in obese zucker rats during food deprivation. J Comp Neurol 441 (2001) 71–89. [DOI] [PubMed] [Google Scholar]
- 196.Tseng KY, Snyder-Keller A, O’Donnell P, Dopaminergic modulation of striatal plateau depolarizations in corticostriatal organotypic cocultures. Psychopharmacol 191 (2007) 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tukey D, Ferreira J, Antoine S, D’Amour J, Ninan I, Cabeza de Vaca S, Incontro S, Horwitz J, Hartner D, Guarini C, Khatri L, Goffer Y, Xu D, Titcombe R, Khatri M, Marzan D, Mahajan S, Wang J, Froemke R, Carr KD, Aoki C, Ziff EB, Sucrose ingestion induces rapid AMPA receptor trafficking, J Neurosci 33 (2013) 6123–6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tukey DS, Ziff EB, Ca2+-permeable AMPA receptors and dopamine D1 receptors regulate GluA1 trafficking in striatal neurons. J Biol Chem 288 (2013) 35297–35306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tye KM, Glutamate inputs to the nucleus accumbens: does source matter? Neuron 76 (2012) 671–673. [DOI] [PubMed] [Google Scholar]
- 200.Vanderschuren LJ, Everitt BJ, Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305 (2004)1017–1019. [DOI] [PubMed] [Google Scholar]
- 201.Verendeev A, Riley AL, Relationship between the rewarding and aversive effects of morphine and amphetamine in individual subjects. Learn Behav 39 (2011) 399–408. [DOI] [PubMed] [Google Scholar]
- 202.Verendeev A, Riley AL, The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behav Pharmacol 24 (2013) 363–374. [DOI] [PubMed] [Google Scholar]
- 203.Vidot DC, Messiah SE, Prado G, Hlaing WM, Relationship between current substance use and unhealthy weight loss practices among adolescents Matern Child Health J, 20 (2016), pp. 870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Volkow ND, Wang G-J, Fowler JS, Telang F, Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Phil Trans Royal Soc Brit 363 (2008) 3191–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Warren M, Frost-Pineda K, Gold M M, Body mass index and marijuana use. J Addict Dis 24 (2005) 95–100. [DOI] [PubMed] [Google Scholar]
- 206.Wheeler RA, Carelli RM, Dissecting motivational circuitry to understand substance abuse. Neuropharmacol 56(Suppl 1) (2009)149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Williamson DF, Serdula MK, Anda RF, Levy A, Byers T, Weight loss attempts in adults: Goals, duration, and rate of weight loss. J Public Health 82 (1992) 1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J, Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend 44 (1997) 87–94. [DOI] [PubMed] [Google Scholar]
- 209.Wise RA, The role of reward pathways in the development of drug dependence. Pharmacol Ther 35 (1987) 227–263. [DOI] [PubMed] [Google Scholar]
- 210.Wise RA, Bozarth MA, Brain mechanisms of drug reward and euphoria. Psychiatr Med 3 (1985) 445–460. [PubMed] [Google Scholar]
- 211.Yamamoto Y, Tanahashi T, Kawai T, Chikahisa S, Katsuura S, Nishida K, Teshima-Kondo S, Sei H, Rokutan K, Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol Genomics 39 (2009) 227–235. [DOI] [PubMed] [Google Scholar]
- 212.Yeomans MR, Palatability and the micro-structure of feeding in humans: the appetizer effect. Appetite 27 (1966) 119–133. [DOI] [PubMed] [Google Scholar]
- 213.Zallar LJ, Farokhnia M, Tunstall BJ, Vendruscolo LF, Leggio L, The role of the ghrelin system in drug addiction. Int Rev Neurobiol. 136 (2017) 89–119. [DOI] [PubMed] [Google Scholar]
- 214.Zhang X, van den Pol AN, Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science 356 (2017) 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zheng D, Cabeza de Vaca S, Carr KD, Food restriction increases acquisition, persistence and drug prime-induced expression of a cocaine-conditioned place preference in rats. Pharmacol Biochem Behav 100 (2012) 538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zheng D, Cabeza de Vaca S, Jurkowski Z, Carr KD, Nucleus accumbens AMPA receptor involvement in cocaine-conditioned place preference under different dietary conditions in rats. Psychopharmacol 232 (2015) 2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zheng D, Liu S, Cabeza de Vaca S, Carr KD, Effects of time of feeding on psychostimulant reward, conditioned place preference, metabolic hormone levels, and nucleus accumbens biochemical measures in food-restricted rats. Psychopharmacol 227 (2013) 307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


