Abstract
Background
Onychomycosis is the most common disease of the nails.
Objective
This retrospective study aimed at evaluating the epidemiology of onychomycosis in adult patients in South Greece during the 2015–2017 period.
Material and Methods
A total of 3,226 patients with clinical signs of possible onychomycosis were included. Diagnosis was confirmed by microscopy with KOH 20% and by culture in Sabouraud agar with and without actidione.
Results
Diagnosis of onychomycosis was confirmed in 27.99% of the patients. Men were infected more often (40.04%) than women (23.30%). Toenails (68.77%) were infected more than fingernails (31.23%) in both sexes. Onychomycosis in fingernails was more common among women (39.74%) than men (18.51%). Men were more often diagnosed with onychomycosis in toenails (81.49%) than women (60.26%). Dermatophytes were the most frequently isolated fungi (34.11%), followed by Candida(29.79%) and non-dermatophyte molds (NDM) (7.20%). In fingernails, the most frequently isolated fungus was Candida spp. (84.04%), followed by dermatophytes (3.55%) and NDM (0.71%). In toenails, dermatophytes (47.99%) were more commonly identified, followed by NDM (10.14%) and Candida spp. (5.15%).
Conclusion
Onychomycosis in Greece follows a pattern of higher incidence in males, with toenails more frequently infected with T. rubrum and fingernails more frequently infected with C. albicans in the present era.
Keywords: Dermatophytes, Trichophyton, Candida, Non-dermatophyte molds, Direct microscopy, Culture
Introduction
Onychomycosis is a chronic fungal disease that can affect toenails and fingernails [1]. This infection is usually caused by dermatophytes, yeasts, and non-dermatophyte molds (NDM) [2]. The incidence of onychomycosis is up to 50% of all infections of the nails [3] and 30% of superficial fungal infections of the skin [1, 2, 4]. Onychomycosis appears to be a variable entity that presents in different forms worldwide with varying frequency [5, 6].
Materials and Methods
Study Population
A retrospective study was carried out during the period 01/01/2015 to 31/12/2017 based on files of patients who visited the outpatient clinic of hospital “Andreas Syggros” (Athens, Greece), with clinical signs of possible onychomycosis. The study protocol was approved by the ethics committee of the hospital.
There were 3,548 visits recorded during this period. After excluding patients with multiple visits, there remained 3,226 adult patients (904 men and 2,322 women) who were evaluated for a possible onychomycosis.
Collection
The material was collected from the area of the lesion of the affected nail. Patients were informed that they should not have received any systemic antifungal therapy or topical antifungal therapy, and their nails had to be uncut and not dyed.
Microscopy
Direct microscopy was performed with KOH 20%.
Culture
Τhe culture of the intake material was performed in Sabouraud agar with and without actidione (incubation at 25 ± 2°C for 25 days). Subsequently, the identification of the growth fungus was made in accordance with its macro- and microscopic characteristics. The evaluation of the result was done by taking into account the culture in combination with the microscopy.
Positive diagnosis for onychomycosis was determined (i) in cases of the existence of hyphae and spores in direct microscopy, (ii) in cases that direct microscopy and culture were both positive, and (iii) in cases of positive culture.
NDM were considered significant if they had positive direct microscopy and were isolated 3 times in culture.
Candida spp. were considered as secondary pathogens if they were isolated with dermatophytes or NDM.
Results
Of 3,226 patients with clinical signs of possible onychomycosis, the diagnosis was confirmed in 903 (27.99%). Direct microscopy was positive in 28.68%, culture in 12.62%, and both direct microscopy and culture were positive in 58.69%. Of these patients, 2,322 (71.98%) were females, and the diagnosis was confirmed in 541 (23.30%); 904 (28.02%) were males, and the diagnosis was confirmed in 362 (40.04%). Toenails (621, 68.77%) were infected more often than fingernails (282, 31.23%) in both sexes. Onychomycosis in fingernails was more common among women: 215 women (39.74%) versus 67 men (18.51%). In contrast to the results shown above, a higher percentage of men had onychomycosis in toenails 295 (81.49%) compared with women 326 (60.26%).
The species most frequently diagnosed in both sexes were dermatophytes (34.11%), followed by Candida(29.79%) and NDM (7.20%). Only 0.22% were diagnosed with mixed infection. Specimens diagnosed by microscopy with failure to identify the species in culture comprised 28.68% of the sample. Of the cases with infected nails by dermatophytes, 270 patients (87.66%) were infected by Trichophyton rubrumand 38 patients (12.34%) by Trichophyton interdigitale. In cases with Candida infections, the most isolated species was C. albicansin 263 patients (97.77%). Regarding NDM, Scopulariopsis brevicaulis (25, 38.46%), Acremonium spp. (22, 33.85%), and Fusariumspp. (9, 13.85%) were the most frequently isolated.
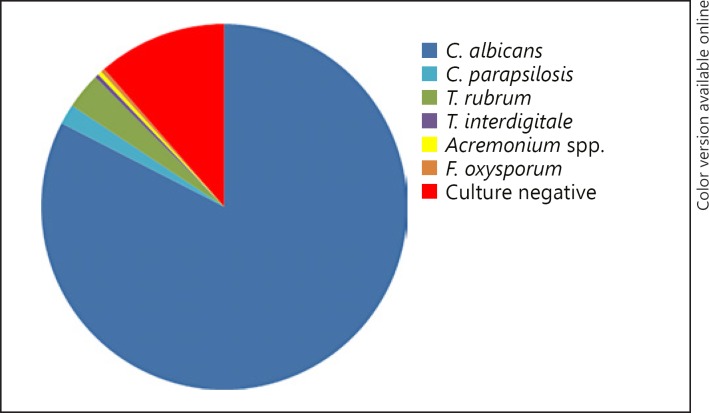
In fingernails, the most frequently isolated fungus was Candidain 237 patients (84.04%), followed by dermatophytes in 10 patients (3.55%) and NDM in 2 patients (0.71%). There was only one case with mixed (0.35%) infection and 32 patients (11.35%) with positive microscopy without the growth of fungus in culture. Of the nail cases infected with Candida, 232 patients were infected by C. albicans(97.89%). Among dermatophytes, the most commonly isolated were T. rubrum in 9 patients (90.00%) and T. interdigitale in only one case (10.00%). For NDM infections, there were only 2 cases, and the infections were caused by Acremonium spp. and Fusarium oxysporum (Fig. 1).
Fig. 1.
Pathogens identified in fingernail onychomycosis: C. albicans 82.6%, C. parapsilosis 1.8%, T. rubrum 3.2%, T. interdigitale 0.3%, Acremonium spp. 0.3%, F. oxysporum 0.3%; culture negative 11.4%.
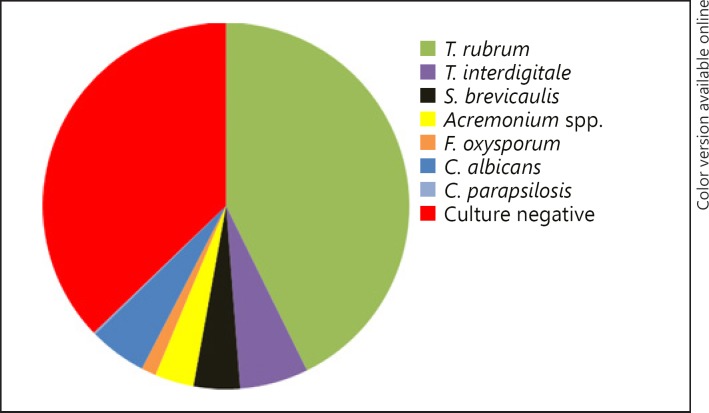
In toenails, dermatophytes had the highest percentage of isolation (298, 47.99%), followed by NDM (63, 10.14%) and Candida spp. (32, 5.15%). Only one case was diagnosed with mixed (0.16%) infection. The percentage of microscopy without a growth of fungus in culture was 36.55% (227 patients). The most frequently isolated dermatophytes were T. rubrum in 261 patients (87.58%) and T. interdigitale in 37 patients (12.42%). Of the nail cases infected with NDM, S. brevicaulis (25, 39.68%), Acremoniumspp. (21, 33.33%), and F. oxysporum (8, 12.70%) were the most commonly isolated. Among Candida, C. albicans was the most prevalent (31, 96.88%) (Fig. 2).
Fig. 2.
Pathogens identified in toenail onychomycosis: T. rubrum 42.7%, T. interdigitale 6%, S. brevicaulis 4.1%, Acremonium spp. 3.4%, F. oxysporum 1.3%, C. albicans 5.1%, C. parapsilosis 0.2%; culture negative 37.1%.
Discussion
Onychomycosis occurs globally and accounts for 50% of nail diseases [1, 3]. Its prevalence varies across geographical areas and is influenced by the climate of the region, socioeconomic and occupational factors, age, increase in the number of immunocompromised patients, as well as changes in lifestyle and everyday habits [1, 2].
Prevalence of onychomycosis was estimated to be 5.5% in a weighted average of 6 recent studies [7, 8, 9, 10, 11]. Earlier studies report similar prevalence of 2–8% [5, 12, 13, 14]. Based on several studies, the incidence rate in East Asia and Europe is 20 and 23%, respectively, while in North America the incidence rate is estimated at up to 14% [15, 16]. Regarding the incidence of onychomycosis in Greece, it appears that its frequency is around 16% [7, 8]. In a study carried out in southern Greece on the incidence of onychomycosis, the rate was about 15.7% [17], while the rate performed in an earlier study in northern Greece was 20% [18].
The variation in the incidence of onychomycosis can be attributed to the geographic features of each site and the different climatic conditions that sometimes favor the growth of fungi. Fungi of the genus Candida appear more often in warm humid climates, while onychomycosis due to dermatophytes occurs mainly in temperate countries and regions [1, 7]. In the population studied, a predominance of Candida spp. infection in the fingernails and dermatophyte infection in the toenails was observed, suggesting that the epidemiology of onychomycosis in Greece still follows a pattern consistent with the Mediterranean region. Even though there are reports suggesting an increase in the average temperature in the eastern Mediterranean region due to climate change, humidity conditions do not show a similar variation [19]. In addition, the majority of refugees in Greece originate from Mediterranean Middle East countries suggesting a similar epidemiology of onychomycosis in the immigrant population.
Onychomycosis is a condition that can significantly affect patients' quality of life [20, 21]. Feelings of embarrassment and low self-esteem are commonly reported by patients with nail infections. Nail disorders are often visible and long-lasting and may even result in modification of patients' everyday activities such as avoiding public facilities like gyms or swimming pools to minimize the potential of someone seeing their affected nail [17, 20]. Many patients experience pain and difficulty in nail care caused by the dystrophy of onychomycosis [20, 21, 22].
In the population studied, women sought expert opinion for a possible diagnosis of onychomycosis significantly more often than men. This fact may be attributed to a possible increased worry of women regarding both nail appearance and a possible infection of their nails than men. Even though toenails are usually covered by shoes, the long Greek summertime allows women to wear shoes that leave toenails visible for more than 6 months per year, suggesting that a raised concern for toenail appearance might affect women more than men. Among patients with onychomycosis, microscopy and culture were both positive in 58.69%, followed by positive microscopy and negative culture in 28.68% and negative direct microscopy with positive culture in 12.62%. The results of this research seem to be converging with research results carried out by Godoy-Martinez et al. [23] for positive direct microscopy and negative culture and by Satpathi et al. [24] for negative direct microscopy with positive culture, On the other hand, in our study the rates were higher in comparison with the results of the study by Sarma et al. [25]. The combination of direct microscopy and culture is important to revealing both fungal viability and species identification. The sampling process, sample preparation, failure rate of microscopy and culture, and the interpretation of results are related to the different results of global studies [7, 26].
In the sample studied, toenails were more frequently infected (68.77%) than fingernails (31.23%). This may be related to the slower rate of growth in toenails that allows fungi to thrive longer. Predominance of toenail or fingernail infection has been reported in the published literature. Papini et al. [27] reported that toenails had a higher incidence of infection (83%) while fingernails had a lower incidence rate (17%). In contrast, a higher incidence of onychomycosis on the fingernails (66.6%) than on toenails (33.3%) was noticed in the study carried out by Hashemi et al. [26].
In the population studied, onychomycosis was more frequently diagnosed in males than females. These results on the incidence of onychomycosis in men and women appear to be consistent with other global studies [13, 15, 21, 28]. In a study by Gupta et al. [11], a higher frequency was reported in males (65%) compared to females (35%). In a study by Babayani et al. [29], the percentage of men (58.23%) was higher compared to women (41.77%). In contrast, a study by Bedaiwy et al. [1] showed a higher incidence of onychomycosis in women (86%) than men (14%).
Fingernail onychomycosis was more frequent in women (39.74%) compared to men (18.51%). In contrast, toenail onychomycosis was more frequent in males (81.49%) compared to females (60.26%). The results of this research seem to be converging with the research results carried out by Husain et al. [2] since toenail onychomycosis of men (66.6%) was more common, while women were more likely to suffer from fingernail onychomycosis (60%). Similarly, Rana et al. [14] reported that toenail onychomycosis appeared more frequently in men (34.8%), while fingernail onychomycosis was more common in women (71.1%). The more frequent appearance of fingernail fungal disease in women has often been attributed to the increased levels of humidity in women's hands as a result of intense housework, regular care, and manicure treatments and therefore gradual destruction of the eponychium [14, 16, 30, 31]. On the other hand, toenail onychomycosis in men is more common, probably due to vigorous daily activity and more systematically engaging in sports activities, resulting in frequent nail injuries [2, 16].
Regarding the species of fungus most frequently isolated in both genders, dermatophytes (34.11%) were the majority, followed by Candida species (29.79%). Patients with other NDM accounted for 7.20% of the positively diagnosed cases. Similar results were published by Segal et al. [32], where a higher percentage of dermatophytes was measured (61.5%), followed by lower percentages of Candida (34.45%) and other NDM (4.05%). In a study by Cengiz et al. [33], dermatophytes (55.1%) were more frequently isolated than Candida species (40.4%) and other NDM (3.1%). Other authors report different results showing the variations in onychomycoses epidemiology in different regions. In the study of Chadeganipour and Mohammadi [34], Candida species (51.1%) had a higher incidence than dermatophytes (26.8%) or NDM (22%). Raghavendra et al. [35] reported that NDM infections (35.33%) had a higher incidence, while dermatophytes (18.66%) and Candida species (10%) were more rarely isolated. These variations in different regions could be attributed to both genetic and environmental factors including temperature and humidity of the area studied [1, 4, 7, 19].
In patients with fingernail onychomycosis, Candida species (84.04%) were more frequently isolated, followed by dermatophytes (3.55%) and other NDM (0.35%) in both sexes. In patients with toenail onychomycosis, dermatophytes (47.99%) were the most commonly isolated species in both males and females. Other NDM (10.14%) and Candida species (5.15%) were less common. The results of this study are in line with the results of a study conducted in a different part of Greece by Maraki and Mavromanolaki [8], where the most frequently isolated species in fingernails was Candida (97.8%) followed by dermatophytes (2.2%). In the toenails of both sexes, dermatophytes were more common (56.3%), followed by Candida (28.6%) and NDM (16.1%).
Regarding fingernail infections for both men and women, onychomycosis is most commonly caused by Candida, as reported in international studies [1, 36]. In the population studied, C. albicans was identified in 97.89% of Candida infections. In studies that are consistent with the results of this one, C. albicans was found to be the most common cause of fingernail infection, in 33.9% of the patients in the study of Cengiz et al. [33], and 55.6% in the study of Youssef et al. [37]. In contrast, in a study conducted by Feng et al. [38], Candida parapsilosis (52.1%) showed higher isolation rates in the fingernails of both sexes, and C. albicans had a lower percentage (27.8%). Segal et al. [32], also reported C. parapsilosis to be the predominant cause of fingernail infection in men (75%) and women (63%). This variability in results reported demonstrates the importance of various demographic and geographic factors that influence the prevalence and cause of the infection. Candida spp. could often be just a contaminant, particularly in the course of fingernail chronic paronychia and in toenail presence of dermatophyte pathogens [39].
It appears that the spectrum of toenail onychomycosis in our population follows the global pattern of predominance of the anthropophilic fungus T. rubrum in terms of toenail infections [2, 40]. T. rubrum was the species with the highest rate of isolation (87.58%) on toenails of both sexes. This result is consistent with the results of studies by Cengiz et al. [33] and Youssef et al. [37], in which T. rubrum was most frequently isolated from the toenails of both men and women at 35.1% and 96.8%, respectively.
Conclusion
Onychomycosis in Greece follows a pattern of higher incidence in males, with toenails more frequently infected with T. rubrum and fingernails more frequently infected with C. albicans in the present era.
Onychomycosis appears globally, but variations in epidemiology between countries and regions are common [5, 6, 7]. Studies on onychomycosis epidemiology in a specific region are of importance because of the constant change of both environmental and genetic factors due to immigration. Data reported in such studies might offer a better insight into onychomycosis risk factors and help design prevention programs by increasing awareness in the general population.
Statement of Ethics
The study was approved by the ethics committee of the hospital.
Disclosure Statement
None declared by all authors.
Funding Sources
None.
Author Contributions
Stamatios Gregoriou, Nikoletta Mpali, Georgia Vrioni, Stella-Eugenia Chryssou, and Dimitrios Rigopoulos contributed to the design of the work, the acquisition, analysis, or interpretation of data, revised the manuscript critically for important intellectual content, and approved the final version. Eleni Hatzidimitriou contributed to the analysis and interpretation of the data and approved the final version. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgements
This work would not have been possible without the efforts of the laboratory and technical staff of the Microbiology Department of Andreas Sygros Hospital.
References
- 1.Bedaiwy MY, Metwally MA, El Zawawy NA, saba HA. Epidemiology, Causative Agents and Clinical Features of Onychomycosis in El-Gharbia Governorate. Egypt J Bot. 2017;15((0)):187–96. [Google Scholar]
- 2.Husain A, Alam NM, Joarder Y, et al. Correlation between clinical and mycological diagnosis of onychomycosis. J Pak Assoc Dermatol. 2017;27((3)):220–5. [Google Scholar]
- 3.Sigurgeirsson B, Baran R. The prevalence of onychomycosis in the global population: a literature study. J Eur Acad Dermatol Venereol. 2014 Nov;28((11)):1480–91. doi: 10.1111/jdv.12323. [DOI] [PubMed] [Google Scholar]
- 4.Thomas J, Jacobson GA, Narkowicz CK, Peterson GM, Burnet H, Sharpe C. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010 Oct;35((5)):497–519. doi: 10.1111/j.1365-2710.2009.01107.x. [DOI] [PubMed] [Google Scholar]
- 5.Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol. 2010 Mar;28((2)):197–201. doi: 10.1016/j.clindermatol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003 Sep;149((65 Suppl 65)):1–4. doi: 10.1046/j.1365-2133.149.s65.4.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta AK, Versteeg SG, Shear NH. Onychomycosis in the 21st Century: An Update on Diagnosis, Epidemiology, and Treatment. J Cutan Med Surg. 2017 Nov-Dec;21((6)):525–39. doi: 10.1177/1203475417716362. [DOI] [PubMed] [Google Scholar]
- 8.Maraki S, Mavromanolaki VE. Epidemiology of onychomycosis in Crete, Greece: a 12-year study. Mycoses. 2016 Dec;59((12)):798–802. doi: 10.1111/myc.12533. [DOI] [PubMed] [Google Scholar]
- 9.Silva-Rocha WP, de Azevedo MF, Chaves GM. Epidemiology and fungal species distribution of superficial mycoses in Northeast Brazil. J Mycol Med. 2017 Mar;27((1)):57–64. doi: 10.1016/j.mycmed.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Otašević S, Barac A, Pekmezovic M, Tasic S, Ignjatović A, Momčilović S, et al. The prevalence of Candida onychomycosis in Southeastern Serbia from 2011 to 2015. Mycoses. 2016 Mar;59((3)):167–72. doi: 10.1111/myc.12448. [DOI] [PubMed] [Google Scholar]
- 11.Gupta C, Jongman M, Das S, Snehaa K, Bhattacharya SN, Seyedmousavi S, et al. Genotyping and In Vitro Antifungal Susceptibility Testing of Fusarium Isolates from Onychomycosis in India. Mycopathologia. 2016 Aug;181((7-8)):497–504. doi: 10.1007/s11046-016-0014-7. [DOI] [PubMed] [Google Scholar]
- 12.Effendy I, Lecha M, Feuilhade de Chauvin M, Di Chiacchio N, Baran R, European Onychomycosis Observatory Epidemiology and clinical classification of onychomycosis. J Eur Acad Dermatol Venereol. 2005 Sep;19((1 Suppl 1)):8–12. doi: 10.1111/j.1468-3083.2005.01281.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta AK, Gupta G, Jain HC, Lynde CW, Foley KA, Daigle D, et al. The prevalence of unsuspected onychomycosis and its causative organisms in a multicentre Canadian sample of 30 000 patients visiting physicians' offices. J Eur Acad Dermatol Venereol. 2016 Sep;30((9)):1567–72. doi: 10.1111/jdv.13677. [DOI] [PubMed] [Google Scholar]
- 14.Rana M, Altaf F, Bashir B, et al. Frequency of associated factors of onychomycosis. J Pak Assoc Dermatol. 2017;27((3)):226–31. [Google Scholar]
- 15.Ghannoum M, Isham N. Fungal nail infections (onychomycosis): a never-ending story? PLoS Pathog. 2014 Jun;10((6)):e1004105. doi: 10.1371/journal.ppat.1004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoud AG, Salem I, Christensen L. Epidemiology of Onychomycosis. Onychomycosis: Diagnosis and Effective Management. 1st ed. John Wiley & Sons Ltd; 2018. pp. pp. 13–20. [Google Scholar]
- 17.Rigopoulos D, Katsiboulas V, Koumantaki E, Emmanouil P, Papanicolaou A, Katsambas A. Epidemiology of onychomycosis in southern Greece. Int J Dermatol. 1998 Dec;37((12)):925–8. doi: 10.1046/j.1365-4362.1998.00613.x. [DOI] [PubMed] [Google Scholar]
- 18.Koussidou T, Devliotou-Panagiotidou D, Karakatsanis G, Minas A, Mourellou O, Samara K. Onychomycosis in Northern Greece during 1994-1998. Mycoses. 2002 Feb;45((1-2)):29–37. [PubMed] [Google Scholar]
- 19.Cramer W, Guiot J, Fader M, et al. Climate change and interconnected risks to sustainable development in the Mediterranean Nature Clim change. 2018;8:972–980. [Google Scholar]
- 20.Chacon A, Franca K, Fernandez A, Nouri K. Psychosocial impact of onychomycosis: a review. Int J Dermatol. 2013 Nov;52((11)):1300–7. doi: 10.1111/ijd.12122. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen A, Tosti A. Predisposing Factors of Onychomycosis. Onychomycosis. Springer International Publishing; 2017. pp. pp. 11–9. [Google Scholar]
- 22.Adams C, Athanasoula E, Lee W, Mahmudova N, Vlahovic TC. Environmental and Genetic Factors on the Development of Onychomycosis. J Fungi (Basel) 2015 Aug;1((2)):211–6. doi: 10.3390/jof1020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godoy-Martinez P, Nunes FG, Tomimori-Yamashita J, Urrutia M, Zaror L, Silva V, et al. Onychomycosis in São Paulo, Brazil. Mycopathologia. 2009 Sep;168((3)):111–6. doi: 10.1007/s11046-009-9209-5. [DOI] [PubMed] [Google Scholar]
- 24.Satpathi P, Acahr A, Banerjee D, et al. Onychomycosis in Eastern India-study in a peripheral tertiary care centre. J Pak Assoc Dermatol. 2013;23((1)):14–9. [Google Scholar]
- 25.Sarma S, Capoor MR, Deb M, Ramesh V, Aggarwal P. Epidemiologic and clinicomycologic profile of onychomycosis from north India. Int J Dermatol. 2008 Jun;47((6)):584–7. doi: 10.1111/j.1365-4632.2008.03674.x. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi SJ, Gerami M, Zibafar E, Daei M, Moazeni M, Nasrollahi A. Onychomycosis in Tehran: mycological study of 504 patients. Mycoses. 2010 May;53((3)):251–5. doi: 10.1111/j.1439-0507.2009.01703.x. [DOI] [PubMed] [Google Scholar]
- 27.Papini M, Piraccini BM, Difonzo E, Brunoro A. Epidemiology of onychomycosis in Italy: prevalence data and risk factor identification. Mycoses. 2015 Nov;58((11)):659–64. doi: 10.1111/myc.12396. [DOI] [PubMed] [Google Scholar]
- 28.Shemer A, Babaev M. Fungal Infections (Onychomycosis, Tinea Pedis, Tinea Cruris, Tinea Capitis, Tinea Manuum, Tinea Corporis, different Candida Infections, and Pityriasis Versicolor) and Mycological Laboratory Analyses. Gender and Dermatology. Springer International Publishing; 2018. pp. pp. 235–42. [Google Scholar]
- 29.Babayani M, Salari S, Hashemi SJ, Ghasemi Nejad Almani P, Fattahi A. Onychomycosis due to dermatophytes species in Iran: prevalence rates, causative agents, predisposing factors and diagnosis based on microscopic morphometric findings. J Mycol Med. 2018 Mar;28((1)):45–50. doi: 10.1016/j.mycmed.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Shafritz AB, Coppage JM. Acute and chronic paronychia of the hand. J Am Acad Orthop Surg. 2014 Mar;22((3)):165–74. doi: 10.5435/JAAOS-22-03-165. [DOI] [PubMed] [Google Scholar]
- 31.Amartya D, Bala NN, Taher A. Onychomycosis and Its Treatment. Inter J Adv Pharm Biol Chem. 2013;2((1)):123–9. [Google Scholar]
- 32.Segal R, Shemer A, Hochberg M, Keness Y, Shvarzman R, Mandelblat M, et al. Onychomycosis in Israel: epidemiological aspects. Mycoses. 2015 Mar;58((3)):133–9. doi: 10.1111/myc.12287. [DOI] [PubMed] [Google Scholar]
- 33.Cengiz FP, Cemil BC, Emiroglu N, Bahali AG, Ozkaya DB, Su O, et al. Etiology of Onychomycosis in Patients in Turkey. J Am Podiatr Med Assoc. 2018 May;108((3)):253–6. doi: 10.7547/16-139. [DOI] [PubMed] [Google Scholar]
- 34.Chadeganipour M, Mohammadi R. Causative Agents of Onychomycosis: A 7-Year Study. J Clin Lab Anal. 2016 Nov;30((6)):1013–20. doi: 10.1002/jcla.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghavendra KR, Yadav D, Kumar A, Sharma M, Bhuria J, Chand AE. The nondermatophyte molds: emerging as leading cause of onychomycosis in south-east Rajasthan. Indian Dermatol Online J. 2015 Mar-Apr;6((2)):92–7. doi: 10.4103/2229-5178.153010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodman MA, Krishnamurthy K. Onychomycosis. StatPearls Publishing; 2017. [PubMed] [Google Scholar]
- 37.Youssef AB, Kallel A, Azaiz Z, Jemel S, Bada N, Chouchen A, et al. Onychomycosis: which fungal species are involved? Experience of the Laboratory of Parasitology-Mycology of the Rabta Hospital of Tunis. J Mycol Med. 2018 Dec;28((4)):651–4. doi: 10.1016/j.mycmed.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Feng X, Ling B, Yang X, Liao W, Pan W, Yao Z. Molecular Identification of Candida Species Isolated from Onychomycosis in Shanghai, China. Mycopathologia. 2015 Dec;180((5-6)):365–71. doi: 10.1007/s11046-015-9927-9. [DOI] [PubMed] [Google Scholar]
- 39.Tosti A, Piraccini BM, Ghetti E, Colombo MD. Topical steroids versus systemic antifungals in the treatment of chronic paronychia: an open, randomized double-blind and double dummy study. J Am Acad Dermatol. 2002 Jul;47((1)):73–6. doi: 10.1067/mjd.2002.122191. [DOI] [PubMed] [Google Scholar]
- 40.Nenoff P, Krüger C, Schaller J, Ginter-Hanselmayer G, Schulte-Beerbühl R, Tietz HJ. Mycology - an update part 2: dermatomycoses: clinical picture and diagnostics. J Dtsch Dermatol Ges. 2014 Sep;12((9)):749–77. doi: 10.1111/ddg.12420. [DOI] [PubMed] [Google Scholar]