Abstract
Background
Paper-based diaries and self-report of symptom worsening in COPD may lead to underdetection of exacerbations. Epidemiologically, COPD exacerbations exhibit seasonal patterns peaking at year-end. We examined whether the use of a BlackBerry-based daily symptom diary would detect 95% or more of exacerbations and enable characterization of seasonal differences among them.
Methods
Fifty participants with GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage I to IV COPD began a community-based study in December 2007. Another 30 began in December 2008. Participants transmitted daily symptom diaries using a BlackBerry. Alerts were triggered when symptom changes, missed diary transmissions, or medical care for a respiratory problem occurred. Participant encounters were initiated if COPD exacerbations were suspected. Participants used their BlackBerrys to report returns to normal breathing.
Results
Participants transmitted 99.9% of 28,514 possible daily diaries. All 191 (2.5/participant-year) COPD exacerbations meeting Anthonisen criteria were detected. During 148 of the 191 exacerbations (78%, 1.97/participant-year), patients were hospitalized and/or ordered prednisone, an antibiotic, or both. Respiratory viruses were detected in 78 of the 191 exacerbations (41%). Those coinciding with a respiratory viral infection averaged 12.0 days, and those without averaged 8.9 days (P < .04), with no difference in Anthonisen score. Respiratory symptom scores before exacerbations and after normal breathing return showed no differences. Exacerbations were more frequent during the Christmas period than the rest of the year but were not more frequent than in the rest of winter alone.
Conclusions
Smartphone-based collection of COPD symptom diaries enables near-complete identification of exacerbations at inception. Exacerbation rates in the Christmas season do not reach levels that necessitate changes in disease management.
Abbreviations
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- RVI
respiratory viral infection
Studies of COPD that have used self-report of possible COPD exacerbations detect one-third to one-half of all exacerbations that occur.1, 2, 3, 4 Participants using paper-based data recording in studies of respiratory diseases often falsify data,5, 6 and the likelihood of this occurring increases with the length of the study. In one randomized trial of electronic symptom recording vs monitored paper-based recording, actual compliance was 94% in the electronic group, whereas fabricated compliance with paper-based recording was 73%.7
COPD exacerbations requiring hospital treatment are more frequent in winter.8, 9, 10 Peaks in both COPD hospitalization and mortality occur in England at year-end11, 12 and have been observed to be centered on the Christmas period in the United Kingdom, Canada, and Sweden.12 In the Toward a Revolution in COPD Health (TORCH) study, the highest rates of COPD exacerbations for northern hemisphere participants occurred in December, with the next-highest in January.13 Furthermore, children's presentations to general practitioners in England and Wales for acute bronchitis and hospitalization for respiratory diseases increase immediately before the Christmas period,14 preceding peaks in adults.14, 15 Periods of school attendance and high levels of respiratory virus detection in children have been shown to coincide with higher rates of hospitalization for COPD,16 suggesting that school attendance fosters the transmission of respiratory infections between children and adults. Respiratory viral infections (RVIs) coincide with a substantial proportion of COPD exacerbations.17, 18, 19, 20, 21, 22
In a previous study, we used faxed daily symptom diaries to monitor patients with COPD over a single winter period.22 Of 111 exacerbations meeting the Anthonisen criteria,23 only one was not detected during its occurrence. However, the fax-based system requires high levels of patient support by staff and data transmission that could only occur at a single location.
Our primary objective in this study was to determine whether 95% or more of all exacerbations in a cohort of patients with COPD could be detected using secure devices that could be used anywhere, would be well accepted by patients, and would require modest staff support and enable collection of supplementary information when exacerbations occur. Our secondary objective was to confirm our previous observation22 that COPD exacerbations are more frequent during the Christmas period than at other times of the year.
Materials and Methods
Study Design and Period
This was a prospective, observational study of a cohort of patients with COPD of mixed disease severity over a maximum period of 17 months. The study was conducted between December 1, 2007, and April 30, 2009.
Study Participants
Study participants were recruited from the clinics of respiratory specialists or were referred by primary-care physicians in the region of Hamilton, Canada. This study was approved by the Research Ethics Board of St. Joseph's Healthcare, Hamilton (RP07-2864).
Subject Inclusion and Exclusion Criteria
The inclusion criteria were age ≥ 40 years, ability to communicate in English, COPD diagnosis and meeting criteria for GOLD (Global Initiative for Chronic Obstructive Lung Disease) stages I to IV classification, current or ex-smoker with a minimum 10 pack-years of exposure, previous demonstration of < 20% reversibility in FEV1 or an increase in FEV1 of < 200 mL after inhalation of 200 μg of salbutamol, willingness to provide informed consent, and residing in a noninstitutional domicile without current plans to transfer to institutional care. The exclusion criteria were significant comorbidity that would interfere with the participant's ability to complete the study protocol, including use of a BlackBerry; planning to be away for a significant part of the study; or a history of asthma or seasonal allergic rhinitis.
Participants meeting the inclusion criteria underwent a baseline assessment, including clinical history, spirometry, BODE (BMI, airflow obstruction, dyspnea, and exercise capacity) index calculation, completion of the St. George's Respiratory Questionnaire, and collection of samples of sputum and nasal brushings for virologic testing and blood for measurement of C-reactive protein titer. During the study, participants' medical care remained with their own physicians.
Data Collection and Detection of COPD Exacerbations
At enrollment, participants were provided with and trained in the use of model 8700 BlackBerry Smartphones (BlackBerry) controlled and monitored centrally. Functions of the BlackBerrys not needed for the study were disabled. Participants were alerted when a new daily diary (e-Appendix 1) was delivered to their BlackBerry. When all daily questions had been answered, diaries were encrypted and transmitted wirelessly to a data server. Flags were programmed to alert staff when one or more symptoms moved up two levels of severity on a given day; any symptom reached its worst level on a given day; or a participant reported a health-system encounter for a respiratory problem, reported symptoms of a respiratory infection, had not transmitted two sequential daily diaries, or requested contact by staff.
Participants whose diaries met one of the aforementioned criteria were contacted by a study nurse and were asked, using lay terminology, to confirm whether they thought their breathlessness was worse than the usual day-to-day variation; they had an increase in sputum volume and/or sputum purulence; and/or medical attention, if sought, was sought for “breathing problems”; and/or a respiratory infection was probable.
If a new possible exacerbation was confirmed, a meeting with a study nurse in the participant's home was scheduled for as soon as possible. During these, participants underwent postbronchodilator spirometry (SpiroPro, BTL Inc) and had specimens of sputum and nasal brushings (both nostrils) taken for virologic analysis. The participants were notified that three supplementary questions (e-Appendix 1) would appear in their daily diary to determine if prednisone, antibiotic, or both were taken or if breathing had returned to normal on a given day. When this occurred, supplementary questions were removed by remote command from the daily diary.
During hospitalizations, staff met with the participants as soon as medically acceptable. With the approval of attending physicians, participants continued their diary submissions while in hospital.
Assessment of Exacerbations
The severity of COPD exacerbations by the Anthonisen criteria23 was assessed by a COPD nurse. Exacerbation length was defined as the period between the date on which an exacerbation was confirmed and the date on which the participant reported a return to normal breathing.
Data Analysis
As in a prior study,22 we defined the Christmas COPD epidemic period as December 1 to January 17. This includes the period of frequent social activity before, during, and after both Christmas (December 25) and the Orthodox Christmas on January 7 (Ontario has a significant population of Orthodox Christian heritage) and an arbitrary 10-day period following that for related symptom episode development. The “rest of winter” was defined as January 18 to April 30, and the third study period was May 1 to November 30.
Assessment of compliance with the completion of daily diary questionnaires and the detection of COPD exacerbations are descriptive. Secondary analyses compared the annualized rates of COPD exacerbation and the proportion of exacerbations coinciding with RVIs during two Christmas periods, two rest-of-winter periods, and the period from May 1, 2008, to November 30, 2008. Differences in exacerbation rates between periods were tested using a χ2 test, in which the observed rate of exacerbations/participant-year was compared with the number expected across all time periods or between the Christmas and rest-of-winter periods. Exacerbation length and Anthonisen scores with and without the presence of RVIs were examined using a Student t test. To determine whether there was a difference among the symptom levels before, during, and after an exacerbation, daily diary data for questions 1 to 4 (e-Appendix 1) were analyzed with a repeated-measures analysis of variance with two factors: before/during/after and question. The analysis was repeated for the before and after scores only to determine if symptom levels returned to baseline after an exacerbation.
Microbiologic Testing
Sputum samples were homogenized by shaking for 15 min after the addition of an equal volume of SPUTASOL (Thermo Fisher Scientific Inc). Nucleic acid was extracted from 200 μL of homogenized sputum using the MagNA Pure LC 2.0 system (Hoffman-La Roche Inc) following the manufacturer's instructions. Two hundred microliters of the nasal phosphate-buffered saline were extracted without further manipulation using the MagNA Pure 96 DNA instrument and the Viral NA Small Volume Extraction Kit (Hoffman-La Roche Inc). Elution volume was set at 100 μL. Virologic testing was undertaken using real-time polymerase chain reaction against a panel of viral targets: influenza A and B, respiratory syncytial virus, parainfluenza virus types 1 to 3, adenovirus, human rhinovirus, the four currently circulating human coronavirus types (229E, OC43, NL-63, and HK-1), human metapneumovirus, and bocavirus. Real-time polymerase chain reaction against a cellular gene transcript, RNAseP, was used as a measure of specimen quality, and an extraction control, MS2 RNA phage, was added to each specimen during extraction.
Results
Of 166 potential participants identified through chart review, 65 declined eligibility assessment, 51 were ineligible (see e-Appendix 2 for details), and 50 were recruited. One participant withdrew after assessment but before beginning the study. Forty-nine participants provided data between December 1, 2007, and November 30, 2008, and one died during the period. The study was extended from December 1, 2008, to April 30, 2009. Forty-six of the original participants continued through the extension period. One hundred forty-one new candidates were identified; 66 declined eligibility assessment, and 45 were ineligible (e-Appendix 2). During the second study period, five participants died. Seventy-one people completed the study extension. Demographic and clinical characteristics of the study participants are shown in Table 1 .
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Subject Characteristics | GOLD I (n = 1) and II (n = 39) | GOLD III (n = 28) and IV (n = 11) |
|---|---|---|
| Age, mean (range), y | 66.9 (44–85) | 68.0 (44–89) |
| Male | 17 (43) | 22 (56) |
| Smoking, median (interquartile range), pack-y | 44.1 (39.5) | 53.0 (32.5) |
| Current smokers | 13 (33) | 11 (28) |
| BODE score, mean (SD) | 2.1 (1.5) | 5.5 (2.3) |
| Dyspnea score, median | 2.0 | 3.0 |
| % predicted FEV1 at baseline postbronchodilator, mean (SD) | 60.7 (7.5) | 35.3 (8.0) |
| 6-min walk distance, mean (SD), m | 408 (97) | 324 (124) |
| BMI, mean (SD), kg/m2 | 28.5 (5.4) | 25.9 (6.6) |
| ED visit for breathing difficulties in previous year | 9 (23) | 13 (33) |
| ≥ 1 hospitalization for breathing difficulties in previous year | 5 (13) | 9 (23) |
| C-reactive protein titer, median (interquartile range), mg/L | 3.0 (4.7) | 4.3 (5.8) |
| Subject medication profile at baseline | ||
| Short-acting β2-agonist | 24 (60) | 37 (95) |
| Long-acting β2-agonist | 3 (8) | 3 (8) |
| Inhaled steroid | 3 (8) | 5 (13) |
| Combination inhaled corticosteroid and long-acting β2-agonist | 27 (68) | 29 (74) |
| Anticholinergics including tiotropium and ipratropium/albuterol | 30 (75) | 34 (87) |
| Oral steroid | 0 (0) | 2 (5) |
| Leukotriene receptor antagonist | 1 (3) | 0 (0) |
| Theophylline | 1 (3) | 1 (3) |
| Antibiotics | 2 (5) | 1 (3) |
Data are given as No. (%) unless otherwise indicated. BODE = BMI, airflow obstruction, dyspnea, and exercise capacity; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Daily Diary Data and Characteristics of Exacerbations
Participants transmitted all but 26 of 28,514 possible daily diaries. Participants completed their diaries on 176 of 191 inpatient hospital days (92%).
One hundred ninety-two participant encounters were initiated for possible COPD exacerbations, with 191 events meeting the Anthonisen criteria (101 type 1, 57 type 2, 33 type 3); in the remaining event, the participant experienced worsened dyspnea and an antibiotic was ordered. Physician visits for breathing problems occurred during 122 exacerbations (64%) (Table 2 ). Overall, the rate of exacerbations observed was 2.54/participant-year. During 148 of 191 exacerbations (78%), participants were hospitalized and/or began oral prednisone, an antibiotic, or both (1.97/patient-year).
Table 2.
Encounters Initiated and Characteristics of Exacerbations
| Characteristics of COPD Exacerbations | GOLD I and II (n = 40) | GOLD III and IV (n = 39) |
|---|---|---|
| Participant encounters initiated, No. | 89 | 103 |
| Exacerbations by Anthonisen type | ||
| 3 | 18 (20) | 15 (15) |
| 2 | 30 (34) | 27 (26) |
| 1a | 41 (46) | 60 (58) |
| Total | 89 | 102 |
| Exacerbations per participant, No. | ||
| 0 | 5 | 5 |
| 1 | 13 | 10 |
| 2 | 8 | 8 |
| 3 | 2 | 6 |
| 4 | 8 | 3 |
| ≥ 5 | 4 | 7 |
| Exacerbations requiring help from any health-care professional | 53 (61) | 69 (67) |
| Exacerbations requiring visit to respiratory specialist | 7 (8) | 33 (32) |
| Exacerbations requiring family physician visit | 35 (40) | 54 (22) |
| Exacerbations requiring ED treatment | 10 (11) | 12 (12) |
| Exacerbations requiring hospitalization | 4 (5) | 9 (9) |
| Exacerbation length,b median (range), d | 8 (0–120) | 12 (0–74) |
| Exacerbation length, virus positive, median (range), d | 10.5 (2–73) | 16 (1–44) |
| Exacerbation length, virus negative, median (range), d | 7 (1–120) | 9 (2–74) |
| Unresolved exacerbation (no return to normal breathing) | 2 (2) | 14 (14) |
| Exacerbations with prednisone prescription without hospitalization | 19 (21) | 44 (43) |
| Exacerbations with antibiotic prescription without hospitalization | 60 (67) | 76 (74) |
| Exacerbations with virus detection | 38 (43) | 40 (39) |
| Absolute decline in postbronchodilator % predicted FEV1 at exacerbation from baseline, mean (SD) | 7.2 (7.3) | 0.7 (6.9) |
Data are given as No. (%) unless otherwise indicated. See Table 1 legend for expansion of abbreviations.
Most severe.
Data included for 173 exacerbations; in 16 there was no return to normal breathing by study end, and in two the participant died.
The median length of the 173 exacerbations in which participants reported a return to normal breathing before the end of the study was 10.0 (interquartile range, 14.0 days). Participants in GOLD III and IV categories were twice as likely to be prescribed a course of prednisone during an exacerbation than were those in GOLD I and II. The former participants' exacerbations were also longer on average; however, they experienced minimal reductions from baseline in their percentage of predicted FEV1, whereas those in GOLD I and II had an average drop of 7%.
The mean average symptom score across questions 1 to 4 was 1.97 preexacerbation and 1.99 postexacerbation. The difference was not significant (F = −0.59, P = .44).
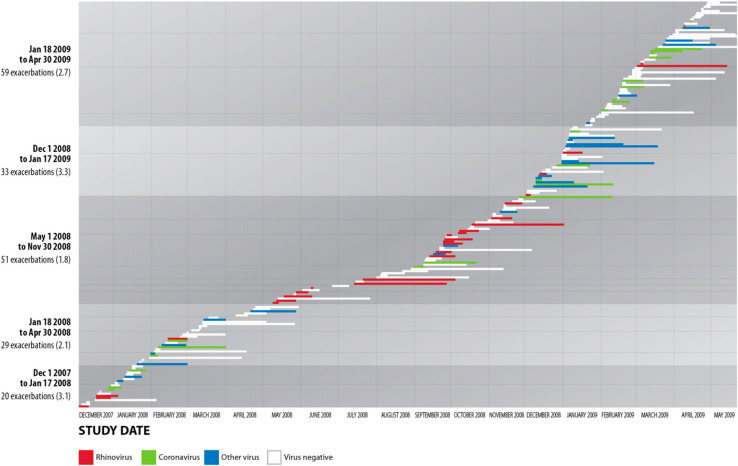
The rate of exacerbations during the Christmas period (3.3/participant-year) was higher than in the other two periods (2.5/participant-year for the rest of winter and 1.8/participant-year for the rest of the year) The difference between exacerbation rates during the Christmas period and the rest of the year was significant (χ2 = 17.2, P < .0001). The difference between exacerbation rates during the Christmas period and the rest of winter alone was not significant (χ2 = 2.26, not signficant). Characteristics of exacerbations in the three study periods are shown in Table 3 .
Table 3.
Characteristics of Exacerbations During the Christmas Period (December 1-January 17), the Rest of Winter (January 18-April 30), and the Rest of the Year
| Characteristics of COPD Exacerbations | Christmas Period (n = 2) | Rest of Winter (n = 2) | Rest of Year (n = 1) |
|---|---|---|---|
| Total participant-y in study period | 16.2 | 34.8 | 28.7 |
| Exacerbations, No. | 53 | 88 | 51 |
| Exacerbations/participant-year, No. | 3.3 | 2.5 | 1.8 |
| Exacerbations by Anthonisen type | |||
| 3 | 9 (17) | 14 (16) | 10 (20) |
| 2 | 14 (26) | 28 (32) | 15 (29) |
| 1a | 30 (57) | 45 (51) | 26 (51) |
| Total | 53 | 87 | 51 |
| Exacerbations by GOLD category | |||
| I and II | 29 (55) | 39 (44) | 21 (41) |
| III and IV | 24 (45) | 49 (56) | 30 (59) |
| Exacerbations requiring help from any health-care professional | 30 (57) | 52 (60) | 38 (78) |
| Exacerbations requiring visit to respiratory specialist | 7 (13) | 19 (22) | 14 (29) |
| Exacerbations requiring physician visit | 17 (32) | 25 (29) | 13 (27) |
| Exacerbations requiring ED treatment | 5 (9) | 8 (9) | 9 (18) |
| Exacerbations requiring hospitalization | 3 (6) | 5 (6) | 5 (10) |
| Exacerbation length,b median (range), d | 10.5 (1–74) | 9 (1–73) | 12.5 (2–120) |
| Exacerbation length, virus positive, median (range), d | 12 (4–63) | 16.5 (1–73) | 13.5 (2–42) |
| Exacerbation length, virus negative, median (range), d | 9 (1–74) | 7 (2–52) | 10.5 (2–120) |
| Exacerbations with prednisone prescription | 16 (30) | 25 (28) | 22 (43) |
| Exacerbations with antibiotic prescription | 31 (58) | 66 (75) | 38 (75) |
| Exacerbations with virus detection | 25 (47) | 28 (32) | 25 (49) |
Data are given as No. (%) unless otherwise indicated. See Table 1 legend for expansion of abbreviations.
Most severe.
Data for 173 exacerbations; in 16 there was no return to normal breathing by study end, and in two the patients died.
Virus Detection
Baseline
Nasal-fluid samples were obtained from all 80 participants, and sputum specimens from 48. One baseline nasal-fluid specimen (2.5%) and seven baseline sputum specimens (15%) were virus positive, all for rhinovirus.
Exacerbations
Nasal-fluid samples were obtained during all 191 confirmed COPD exacerbations and sputum samples during 141 (74%). Respiratory viruses were present in 78 of 191 of the COPD exacerbations (41%) (Table 4 ). Forty-five nasal specimens (24%) and 58 sputum specimens (45%) were positive for one or more viruses.
Table 4.
Viruses Detected During Exacerbations
| Virus Type | Nasal Fluid (n = 191), No. (% of Nasal Virus Detections) | Sputum (n = 141), No.(% of Sputum Virus Detections) | Nasal and Sputum, No.(% of Both Sample Virus Detections) | Total Virus Detections,a No. (% of Single Virus Detections) |
|---|---|---|---|---|
| Any virus | 15 (100) | 32 (100) | 26 (100) | 73 (100) |
| Rhinovirus | 3 (20) | 15 (47) | 9 (35) | 27 (37) |
| Coronavirus | 4 (27) | 8 (25) | 11 (42) | 23 (32) |
| Respiratory syncytial virus | 3 (20) | 1 (3) | 4 (15) | 8 (11) |
| Influenza A | 0 | 2 (6) | 0 | 2 (3) |
| Influenza B | 0 | 2 (6) | 0 | 2 (3) |
| Human metapneumovirus | 0 | 2 (6) | 1 (4) | 3 (4) |
| Bocavirus | 3 (20) | 0 | 0 | 3 (4) |
| Parainfluenza virus | 2 (13) | 2 (6) | 0 | 4 (5) |
| Adenovirus | 0 | 0 | 1 (4) | 1 (1) |
In addition, combinations of viruses were detected in five exacerbations, two in which coronavirus was present in nasal fluid and rhinovirus in sputum, one in which respiratory syncytial virus was present in nasal fluid and human metapneumovirus in sputum, one in which adenovirus was present in nasal fluid and human rhinovirus in sputum, and one in which adenovirus and coronavirus were both present in sputum.
Exacerbations coinciding with RVIs, in which a return to normal breathing occurred before the end of the study, lasted an average of 12.0 days, compared with 8.9 days without RVIs (t = 2.06, P = .04) (Fig 1 ). Anthonisen scores for episodes with virus present and absent were 1.52 (SD, 0.71) and 1.73 (SD, 0.78), respectively (t = 1.87, P = .06).
Figure 1.
Incidence and prevalence of COPD exacerbations in all study participants by week of the study. The numbers of COPD exacerbations are shown as horizontal bars over the course of the study (December 1, 2001, to April 30, 2009). The length of the bars represents the length of the exacerbation and the colors represent the categories of virus detected in each. Shading signifies the five periods of the study. Numbers in parentheses represent the rate of exacerbations per participant year. The vertical axis shows the number of exacerbations (number/subject) by study period.
Discussion
We have shown that, over an extended period of time, patients with COPD will provide daily respiratory-health data with near-perfect compliance using BlackBerry devices. In interpreting this finding, one must keep in mind the possibility that this study population was biased toward participants more confident in using electronic devices. The study team was also assiduous in maintaining oversight of participants to a degree that may not be possible in a multicenter study. However, a BlackBerry-based system has been used in other COPD studies, including a large, multicountry clinical trial, with 91% compliance.24 Our participants reported when their breathing returned to normal after an exacerbation using a supplementary BlackBerry diary question. Analysis of diary symptom data established that this self-assessment was accurate, suggesting that assessments of their respiratory health by patients with COPD are perceptive.
The Christmas period, as we defined it, involves an elevated exacerbation risk for patients with COPD, but this was not significant compared with the rest of winter. Since this study was completed, two reports of seasonal patterns of COPD exacerbations in major clinical trials, TORCH15 and Prevention of Exacerbations With Tiotropium in COPD (POET-COPD), have been published.25 In both, in the northern hemisphere exacerbations occurred most frequently in December and January. The original characterization of a Christmas peak in COPD exacerbations12 examined data for 209,274 ED presentations for COPD in Ontario, Canada, over a 4-year period (summary data provided in e-Table 1). In every year, the last week of the year from Christmas Day to New Year's Eve had the highest number of events. That the existence of a specific Christmas effect as opposed to an elevated risk over the broader period can only be demonstrated in large administrative data sets suggests that preparing patients with COPD for the risks of winter as a whole, but not the Christmas season specifically, would be prudent.
We detected one or more respiratory viruses in 41% of exacerbations. Viruses were more likely to be detected in sputum than in nasal fluid, as has been reported previously.21, 26 In a majority of cases, the nasal-fluid and sputum viruses were different, or only found in one type of sample, confirming that the role of RVIs in COPD symptom exacerbations should include examination of both nasal fluid and sputum.
The only virus detected in specimens taken at recruitment was rhinovirus, found in one nasal-fluid sample and seven sputum samples. Recruitment occurred in the fall, a period when rhinovirus is most common,27 and we conclude that the positive participants had colds at the time. Exacerbations coinciding with RVIs took longer to resolve than those without RVIs, consistent with findings in other studies,26, 28 but were no more severe based on Anthonisen scores.
Conclusions
In conclusion, the use of BlackBerry-based technology permitted early and complete detection of COPD exacerbations. Such technologies may foster the adoption of patient-reported outcomes and symptom-based measures in clinical studies and facilitate early detection of deterioration in high-risk patients with COPD in regular clinical practice. However, for this to occur, the effectiveness of the approach must be evaluated in a full spectrum of patients with COPD.
Acknowledgments
Author contributions: Mr Johnston is responsible for the integrity of the manuscript and the study data.
Mr Johnston: contributed to the leading of the study design team, co-supervision of the conduct of the study, and drafting and review of the manuscript.
Ms Lambert: contributed to the leading of the study operations team, patient assessment, management of patient participation, and writing and review of the manuscript.
Ms Hussack: contributed to the patient assessment, management of patient participation, and writing and review of the manuscript.
Dr Gerhardsson de Verdier: contributed to intellectual guidance throughout the study's inception, design, and conduct and writing and review of the manuscript.
Dr Higenbottam: contributed to the assessment of study events, auditing of patient data, guidance and assistance with study design and result interpretation throughout, and writing and review of the manuscript.
Mr Lewis: contributed to guidance and assistance with the study design and result interpretation throughout and writing and review of the manuscript.
Dr Newbold: contributed to guidance and assistance with study design and result interpretation throughout and writing and review of the manuscript.
Mr Jenkins: contributed to the design and conducting of statistical analyses and writing and review of the manuscript.
Dr Norman: contributed to the design and conducting of statistical analyses and writing and review of the manuscript.
Dr Coyle: contributed to the conducting of virologic testing, interpretation of results, and writing and review of the manuscript.
Dr McIvor: contributed to the clinical assessment of the study patients, leading of patient recruitment, cosupervision of the conduct of the study, and writing and review of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Mr Johnston has received research funding and/or honoraria from Merck Canada Inc, GlaxoSmithKline Canada, and AstraZeneca. He also owns shares in GlaxoSmithKline. Dr McIvor has received honoraria for educational events, advisory boards, and phase 3 clinical trials from pharmaceutical companies including AstraZeneca; Boehringer-Ingelheim GmbH; Takeda Pharmaceutical Company Limited; Pfizer, Inc; Merck & Co, Inc; and GlaxoSmithKline. Dr Higenbottam has been employed by Chiesi Farmaceutici SpA within the past 5 years. Drs Gerhardsson de Verdier and Newbold and Messrs Lewis and Jenkins are employed by AstraZeneca and own shares in the company. Mss Lambert and Hussack and Drs Norman and Coyle have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: Employees of AstraZeneca (Drs Gerhardsson de Verdier, Higenbottam, Newbold, and Jenkins and Mr Lewis) participated in study design, interpretation of results, and review of the manuscript. Dr Jenkins conducted statistical analyses and provided a first draft of Figure 1. Overall conduct of the study, including design, was the responsibility of the academic investigators (Dr McIvor and Mr Johnston).
Additional information: The e-Appendixes and e-Table can be found in the “Supplemental Materials” area of the online article.
Footnotes
Dr McIvor was the coprincipal investigator.
Part of this article has been presented in abstract form at the European Respiratory Society 2008 Annual Meeting, Berlin, Germany (abstract 250496) and American Thoracic Society 2012 Annual Meeting, San Francisco (abstract 28081).
Funding/Support: This study was supported by a grant from AstraZeneca.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
Web Extra Material
Online Supplement
References
- 1.Miravitlles M, Ferrer M, Pont A, IMPAC Study Group Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59(5):387–395. doi: 10.1136/thx.2003.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177(4):396–401. doi: 10.1164/rccm.200708-1290OC. [DOI] [PubMed] [Google Scholar]
- 3.Vijayasaratha K, Stockley RA. Reported and unreported exacerbations of COPD: analysis by diary cards. Chest. 2008;133(1):34–41. doi: 10.1378/chest.07-1692. [DOI] [PubMed] [Google Scholar]
- 4.Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl. 2003;41(suppl):46s–53s. doi: 10.1183/09031936.03.00078002. [DOI] [PubMed] [Google Scholar]
- 5.Côté J, Cartier A, Malo JL, Rouleau M, Boulet LP. Compliance with peak expiratory flow monitoring in home management of asthma. Chest. 1998;113(4):968–972. doi: 10.1378/chest.113.4.968. [DOI] [PubMed] [Google Scholar]
- 6.Chowienczyk PJ, Parkin DH, Lawson CP, Cochrane GM. Do asthmatic patients correctly record home spirometry measurements? BMJ. 1994;309(6969):1618–1620. doi: 10.1136/bmj.309.6969.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24(2):182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 8.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959-1999. Am J Epidemiol. 2004;160(5):492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 9.Vilkman S, Keistinen T, Tuuponen T, Kivelä SL. Seasonal variation in hospital admissions for chronic obstructive pulmonary disease in Finland. Arctic Med Res. 1996;55(4):182–186. [PubMed] [Google Scholar]
- 10.Donaldson GC, Wedzicha JA. COPD exacerbations. 1: Epidemiology. Thorax. 2006;61(2):164–168. doi: 10.1136/thx.2005.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansell A, Hollowell J, McNiece R, Nichols T, Strachan D. Validity and interpretation of mortality, health service and survey data on COPD and asthma in England. Eur Respir J. 2003;21(2):279–286. doi: 10.1183/09031936.03.00006102. [DOI] [PubMed] [Google Scholar]
- 12.Johnston NW. The similarities and differences of epidemic cycles of chronic obstructive pulmonary disease and asthma exacerbations. Proc Am Thorac Soc. 2007;4(8):591–596. doi: 10.1513/pats.200706-064TH. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins CR, Celli B, Anderson JA. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39(1):38–45. doi: 10.1183/09031936.00194610. [DOI] [PubMed] [Google Scholar]
- 14.Elliot AJ, Cross KW, Fleming DM. Acute respiratory infections and winter pressures on hospital admissions in England and Wales 1990-2005. J Public Health (Oxf) 2008;30(1):91–98. doi: 10.1093/pubmed/fdn003. [DOI] [PubMed] [Google Scholar]
- 15.Fleming DM, Elliott AJ, Nguyen-Van-Tam JS. A Winter's Tale: Coming to Terms With Winter Respiratory Diseases. Health Protection Agency; London, England: 2005. [Google Scholar]
- 16.McManus TE, Coyle PV, Kidney JC. Childhood respiratory infections and hospital admissions for COPD. Respir Med. 2006;100(3):512–518. doi: 10.1016/j.rmed.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston SL. Overview of virus-induced airway disease. Proc Am Thorac Soc. 2005;2(2):150–156. doi: 10.1513/pats.200502-018AW. [DOI] [PubMed] [Google Scholar]
- 18.Upshur RE, Knight K, Goel V. Time-series analysis of the relation between influenza virus and hospital admissions of the elderly in Ontario, Canada, for pneumonia, chronic lung disease, and congestive heart failure. Am J Epidemiol. 1999;149(1):85–92. doi: 10.1093/oxfordjournals.aje.a009731. [DOI] [PubMed] [Google Scholar]
- 19.Rohde G, Wiethege A, Borg I. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seemungal TA, Harper-Owen R, Bhowmik A. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson AF, Ghimire AK, Thompson MA. A community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPD. Respir Med. 2007;101(12):2472–2481. doi: 10.1016/j.rmed.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Johnston NW, McIvor A, Lambert K. The Christmas season as a risk factor for chronic obstructive pulmonary disease exacerbations. Can Respir J. 2010;17(6):275–281. doi: 10.1155/2010/460532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 24.Wedzicha JA, Agusti A, Vestbo J, et al. Novel detection of exacerbations of COPD with patient reported outcome (EXACT-PRO) and Blackberry (BB). In: Proceedings from the European Respiratory Society Annual Congress; September 18-22, 2010;Barcelona, Spain. Abstract 395.
- 25.Rabe KF, Fabbri LM, Vogelmeier C. Seasonal distribution of COPD exacerbations in the POET-COPD trial. Chest. 2013;143(3):711–719. doi: 10.1378/chest.12-1277. [DOI] [PubMed] [Google Scholar]
- 26.Seemungal TA, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;16(4):677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olenec JP, Kim WK, Lee WM. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125(5):1001–1006. doi: 10.1016/j.jaci.2010.01.059. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurst JR, Donaldson GC, Wilkinson TM, Perera WR, Wedzicha JA. Epidemiological relationships between the common cold and exacerbation frequency in COPD. Eur Respir J. 2005;26(5):846–852. doi: 10.1183/09031936.05.00043405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement