Abstract
Infectious diseases that are transmitted from wildlife hosts to humans, such as the Ebola virus and MERS virus, can be difficult to understand because the pathogens emerge from complex multifaceted ecological interactions. We use a wildlife–pathogen system—prairie dogs (Cynomys ludovicianus) and the plague bacterium (Yersinia pestis)—to describe aspects of disease ecology that apply to many cases of emerging infectious disease. We show that the monitoring and surveillance of hosts and vectors during the buildup to disease outbreaks are crucial for understanding pathogen-transmission dynamics and that a community-ecology framework is important to identify reservoir hosts. Incorporating multidisciplinary approaches and frameworks may improve wildlife–pathogen surveillance and our understanding of seemingly sporadic and rare pathogen outbreaks.
Keywords: Yersinia pestis, prairie dog, plague, disease ecology
Outbreaks of infectious zoonotic pathogens
Novel infectious diseases that spread from wildlife species to human populations can have profound impacts ranging from individual morbidity and mortality to global pandemics, with consequences for public health, economies, and culture. Zoonotic diseases (transmitted from animals to humans) result from the “spillover” of the pathogen from wildlife species into humans—with the initial transmission in human populations possibly going undetected or undiagnosed—followed by wider-scale outbreaks that take place before effective therapies are available or developed. Zoonotic spillovers can be difficult to predict because they result from complex multifaceted ecological interactions and often exhibit patterns of sporadic epidemics that affect local host populations, interspersed by periods of “disappearance” into long-term quiescent phases of cryptic persistence in unknown reservoirs. These events do not lend themselves to accurate disease forecasting and prediction.
Recent outbreaks of the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and the Ebola virus illustrate these challenges. MERS-CoV was first discovered in a patient in Saudi Arabia in 2012 and has since infected more than 950 people globally, resulting in more than 350 deaths (Rasmussen et al. 2015). Camels are believed to be the infectious source for humans, and retrospective antibody surveys indicate that the virus circulated in camels for a substantial time—at least 20 years—before MERS was identified in humans (e.g., Azhar et al. 2014, Meyer et al. 2014). But this type of pathogen discovery does not tell the whole story of spillover and zoonosis emergence. The 2014 Ebola virus outbreak in West Africa revealed that virus transmission can change from a local, rural, and rare zoonotic spillover to an urban epidemic with thousands of fatalities. With rapid international transport, several infected travelers died after transit from West Africa to other parts of the word, which demonstrates the potential of a worldwide epidemic. Although fruit bats have been touted as potential Ebola virus reservoirs, no consensus exists on the identity of the Ebola virus reservoir species or even of the natural history of the virus in putative wildlife hosts (Olival and Hayman 2014). The ongoing MERS-CoV and Ebola virus outbreaks underscore the inherent difficulties in predicting the emergence, transmission, and sporadic outbreaks of zoonotic pathogens that persist in multihost systems but lack a clearly identified wildlife reservoir.
This pattern of events—emerging outbreaks of disease, global travel, unidentified reservoir hosts, and variable transmission routes—has often been repeated. MERS-CoV and Ebola virus are simply the latest examples; the list of recently emerged pathogens includes Severe Acute Respiratory Syndrome (SARS) coronavirus, HIV, Chikungunya virus, and tick-borne pathogens (e.g., Borrelia burgdorferi, which causes Lyme disease). Emerging pathogens such as these constitute an important public health issue but also create problems for wildlife conservation and management. For example, the arrival of West Nile virus in the United States reduced native bird populations (LaDeau et al. 2007). Large-scale mortality of wildlife (lowland gorillas) has been attributed to Ebola virus spread (Leroy et al. 2004). Furthermore, control initiatives to protect public health from disease originating in wildlife may involve culling and other disruptions of wildlife (e.g., badger culling and bovine tuberculosis in the United Kingdom), which gives rise to controversies over the value of ecosystem function, protection, and environmental ethics.
Ideally, to control or predict the size and frequency of outbreaks, we should understand how pathogens persist in the cryptic phase, and we need to identify and predict conditions that cause outbreaks. Persistence and outbreaks can be affected by rates of transmission that are influenced by multiple factors, such as rainfall and temperature, host and vector abundance, exposure rates, and host behavior. But not all hosts are equal: Pathogens often display broad host ranges, infecting multiple host species that vary in predisposition to infectiousness, susceptibility to disease, and modes of transmission (e.g., direct contact, vector borne, and environmental exposure). Detection of the pathogen can be difficult if incidence becomes so low that ongoing surveillance efforts cannot detect outbreaks without an increase in sampling efforts. Similarly, efforts to study pathogen ecology may be stymied by environments that present logistic challenges for field studies—for instance, subterranean burrows, marine environments, forest canopies, and cave networks—and therefore affect our ability to detect disease dynamics before a full-scale outbreak is underway.
The prairie dog–plague (Yersinia pestis) disease system
As an example of the patterns and properties of emerging zoonotic pathogens, we describe research on the ecology and epidemiology of Yersinia pestis, the bacterium that causes plague, on grasslands of the Great Plains of North America. Plague is most famous for the Black Death that devastated human populations of medieval Europe beginning in 1347 and persisting with repeated outbreaks for four centuries. During the late nineteenth century, plague spread from its endemic range in Asia to Africa and the Americas, and outbreaks still cause hundreds of human cases annually, especially in Africa (Gage and Kosoy 2005). Introduced into the United States circa 1900, Y. pestis is now established and affects wildlife populations throughout the western United States, with occasional spillover to humans (approximately 10 cases per year) and domestic animals (Antolin et al. 2002, Gage and Kosoy 2005).
On the Great Plains, plague sporadically erupts in outbreaks that decimate populations of black-tailed prairie dogs (Cynomys ludovicianus; hereafter, prairie dogs). Prairie dogs are diurnal, social ground squirrels (figure 1) that occupy spatially distinct colonies that can extend across several hundred hectares of grassland habitat. Within colonies, prairie dogs are organized into smaller social groups called coteries (1–2 adult males, 2–4 adult females, yearlings and juvenile offspring) that inhabit a well-developed burrow system within a territory that they defend from neighboring individuals (Hoogland 1995). During plague outbreaks, it is common for 95%–100% of individuals in colonies to die, making plague the most important nonanthropogenic threat to prairie dog populations (Cully and Williams 2001, Antolin et al. 2002, Pauli et al. 2006), whose abundance was already reduced (estimated as high as 90%) as a result of habitat loss and poisoning programs for agriculture and urban development. Plague's impact in altering the prairie ecosystem can be profound because prairie dogs are ecosystem engineers that modify the environment by their grazing and burrowing activities and thereby can create habitat for a variety of other species (Antolin et al. 2002, Kotliar et al. 2006). Consequently, plague-induced die-offs can have effects that reverberate throughout the prairie ecosystem. For example, the black-footed ferret (Mustela nigripes), one of the most endangered mammals in the world, depends on large complexes of prairie dog colonies as its primary source of prey and is itself highly susceptible to plague (Antolin et al. 2002, Matchett et al. 2010). Here, we describe recent research into the plague–prairie dog system, which employed approaches such as community ecology, population biology, and epidemiology. We use this investigation as a model for illustrating how disease ecology may prove helpful in developing strategies for understanding and predicting the emergence of other pathogens, such as MERS-CoV or Ebola virus.
Figure 1.
Prairie dogs (left) are burrowing, herbivorous, diurnal ground squirrels that inhabit the grasslands and shrublands of the Great Plains of North America (photograph: Austin Allison, Colorado Parks and Wildlife). The most widespread of the five species, the black-tailed prairie dog (Cynomys ludovicianus) is the most social and highly susceptible to plague mortality (Cully and Williams 2001). In suitable habitat, black-tailed prairie dogs can occupy large (hundreds of hectares), densely populated colonies. Within a colony, prairie dogs live in territorially defended social groups called coteries, consisting of 1–2 adult males, 2–4 adult females, yearlings, and juvenile offspring (Hoogland 1995). Prairie dogs are commonly infested with fleas: 88% of prairie dogs in Colorado had fleas, with an average of 14.3 fleas per infested host (Tripp et al. 2009). The vast majority of fleas were one of three species: Oropsylla hirsuta (right, photograph: Dan Tripp, Colorado Parks and Wildlife), O. tuberculata cynomuris, and Pulex simulans. Except for P. simulans, which is also found on carnivores, the fleas associated with prairie dogs are rarely found on other mammals (Brinkerhoff et al. 2006, McGee et al. 2006, Salkeld et al. 2007, Stapp et al. 2009, Tripp et al. 2009).
Variation in modes of transmission
The plague bacterium possesses multiple modes of transmission. Classically, flea-borne transmission was thought to be most efficient after Y. pestis forms a biofilm that blocks the flea's proventriculus and midgut, typically 2–3 weeks postinfection. The blockage causes fleas to starve and escalate feeding attempts, which increases Y. pestis transmission (Bacot and Martin 1914, Eisen et al. 2006, Wilder et al. 2008). Black-tailed prairie dogs are most commonly infested by the prairie dog flea Oropsylla hirsuta (figure 1), and although blockage of the proventriculus had been demonstrated in O. hirsuta, it is an infrequent and inefficient mode of infection in this species (Wilder et al. 2008). An epidemiologic SEI (susceptible–exposed–infected) model suggested that Y. pestis transmission by blocked fleas is incapable of driving plague outbreaks in prairie dog colonies because of the temporal delay until blockage occurs (Webb et al. 2006). Laboratory investigations have since demonstrated that fleas are also capable of early-phase transmission—that is, transmitting plague during the first few days following ingestion of an infectious blood meal and prior to the development of a proventricular blockage (Eisen et al. 2006, Wilder et al. 2008). Both O. hirsuta and the other main prairie dog flea, Oropsylla tuberculata cynomuris, can transmit plague for the 24–48 hours postinfection—and at much higher rates than had been previously measured (Wilder et al. 2008). Models incorporating early-phase transmission have been able to simulate plague dynamics observed during prairie dog die-offs (Eisen et al. 2006, Salkeld et al. 2010, Buhnerkempe et al. 2011).
The plague bacterium can also be spread through direct contact with infected tissue or blood, through trophic or vehicle-borne transmission by consumption of infectious prey or carrion, and through pneumonic transmission via inhalation of respiratory droplets. Prairie dogs are highly social, exhibiting close contact within burrows and while grooming ectoparasites from each other. Like other ground-dwelling squirrels, prairie dogs are known to engage in cannibalism (Hoogland 1995). Therefore, transmission via direct contact may be expected in prairie dogs, and the conditions of subterranean nesting chambers may also be conducive to pneumonic transmission. Laboratory experiments have shown that rodents can be infected with plague by consuming infected prey tissue (Thomas et al. 1989), and pneumonic transmission has been demonstrated between carnivores and between carnivores and humans (Salkeld and Stapp 2006, Wong et al. 2009). Webb and colleagues’ (2006) SEI model, however, suggested that pneumonic transmission is unlikely to be important in driving plague outbreaks, and flea-dusting with insecticides slows or stops die-offs in the field (Seery et al. 2003), implying that vector-borne transmission is likely the predominant mechanism during outbreaks. Although the relative importance of pneumonic, contact, or trophic transmission mechanisms in plague persistence and outbreaks in wild prairie dog colonies remains largely unknown, human exposures are known to have occurred by these routes and by flea-borne transmission (Gage and Kosoy 2005).
Contact rates and transmission dynamics change during the course of a disease outbreak
Until recently, observed patterns of plague outbreaks in prairie dogs suggested that Y. pestis’ presence in a prairie dog colony caused high and rapid levels of mortality, with colony extinction occurring in a matter of 6–8 weeks after first observation of plague activity (Webb et al. 2006). In northern Colorado, the probability of colony extinction was influenced by the size and fate of adjacent colonies (Stapp et al. 2004, Savage et al. 2011), although the timing of die-offs did not reveal neat colony-to-colony waves of plague spread at a timescale consistent with rapid die-offs. Consequently, one interpretation has been that the bacterium was unlikely to persist within prairie dog populations, and instead, it was thought that plague was normally absent from prairie dog colonies between outbreaks and persisted either in disease-resistant alternate hosts that shared the same habitat (e.g., deer mice, Peromyscus maniculatus) or in nearby locations, such as the foothills of the Rocky Mountains, with occasional incursions into prairie dog populations (Cully and Williams 2001, Gage and Kosoy 2005, Brinkerhoff et al. 2009).
A combination of intensive fieldwork, laboratory studies, and modeling efforts has resulted in an improved understanding of the dynamics of plague in hosts and vectors during die-offs in prairie dog colonies. During plague outbreaks, both the prevalence and abundance of fleas (O. hirsuta, O. cynomuris tuberculata, and Pulex simulans—the last a flea found on both carnivores and prairie dogs) on prairie dogs increase (figure 2) as hungry, infected fleas search for living prairie dogs after the death of their hosts (Pauli et al. 2006, Tripp et al. 2009). At the same time, fleas in prairie dog burrows become more abundant, and the prevalence of Y. pestis in the fleas increases (St. Romain et al. 2013). The close-knit nature of the prairie dog coterie suggests that invasion by Y. pestis—in an infectious flea or an infected prairie dog—will almost certainly result in exposure to all of the coterie members. When a coterie perishes or is abandoned, the territory is usually absorbed by an adjacent coterie (Hoogland 1995), which would imply that infected and hungry fleas can infest exploring Y. pestis–naive prairie dogs, which are then carried back to the home coterie, resulting in coterie-to-coterie pathogen transmission. In this scenario, as plague begins to spread, transmission rates snowball because of increased abundance of fleas searching for meals and the consequent increased opportunities for Y. pestis transmission (Tripp et al. 2009).
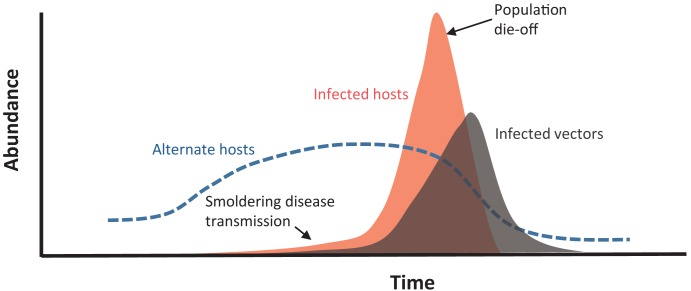
Figure 2.
A conceptual representation of the anatomy of a plague outbreak in a prairie dog colony. Field observations show that plague activity may be ongoing several months prior to a recognized prairie dog die-off (St. Romain et al. 2013). High grasshopper mouse abundance is correlated with a higher likelihood of plague outbreak the following year, although grasshopper-mice populations are also affected by Y. pestis activity (Stapp et al. 2008, 2009). The abundance of fleas infected with Y. pestis also rises during the observed outbreaks (Tripp et al. 2009).
Hypothetically, outbreaks may also occur if conditions on colonies exacerbate prairie dog contacts via territorial disputes between coteries and therefore result in greater flea exchange and pathogen transmission between neighboring coteries. Recent observations that prairie dog dispersal is elevated after the disappearance or death of close kin within a coterie (Hoogland 2013) suggest that dispersal and transmission rates may increase substantially as mortality increases. That is, if one or two individuals within a coterie die from plague, remaining individuals may be motivated to disperse, carrying Y. pestis with them. Although a modeling scenario that incorporated dispersal and breeding seasons did not reveal noticeable changes in plague transmission rates (Salkeld et al. 2010), the relationship between coterie boundary dynamics and prairie dog density requires more investigation, especially under the circumstances of a plague outbreak.
Smoldering disease transmission
Determining the duration of a disease outbreak is not a trivial matter. Despite the sheer numbers of prairie dogs that are killed during plague outbreaks, most mortality occurs belowground (potentially creating a large reservoir of infectious tissue and infectious fleas that may ultimately slow re-establishment of the colony). Carcasses on the surface are removed quickly by scavenging carnivores (on average, in less than 2 days; Boone et al. 2008), and finding a prairie dog carcass is not a common event. Annual monitoring programs that track size and fate of colonies rely on noticeable declines in prairie dog numbers and lack the fine temporal resolution required to accurately describe spatiotemporal patterns of mortality leading up to and during outbreaks. PCR analysis of prairie dog fleas in northern Colorado demonstrates that plague activity can occur for 6–15 months before obvious prairie dog mortality that may signal an outbreak (figure 2; St. Romain et al. 2013). In Montana, Y. pestis-positive fleas have been found in burrows without evidence of ongoing prairie dog die-offs (Hanson et al. 2007), although other studies have struggled to find Y. pestis persisting in fleas (Holmes et al. 2006).
Further evidence for slow, smoldering pathogen transmission prior to large plague outbreaks and die-offs comes from experimental management—vaccinations and flea-dusting (application of insecticides to prairie dog burrows)—which increased survival of prairie dogs and black-footed ferrets even in the absence of conspicuous plague activity (Biggins et al. 2010, Matchett et al. 2010). In the case of flea-dusting, reduced prairie dog mortality may have occurred because of the suppression of other unrecognized vector-borne pathogens. Increased survival of black-footed ferrets after vaccination may be caused by protection when animals are exposed to soil or carcasses that harbor viable Y. pestis. A spatially explicit agent-based model also suggested that outbreaks occur as a culmination of plague activity lasting for several months to multiple years, although disease fade-outs were also likely (Salkeld et al. 2010). Taken together, these observations suggest that Y. pestis transmission can occur at low rates without widespread mortality, making pathogen activity difficult to observe.
Slow, smoldering, cryptic disease transmission in animal populations prior to outbreaks in humans is also a hypothetical explanation for the persistence of pathogens such as MERS-CoV and Ebola virus. For example, MERS-CoV has circulated in camels for at least two decades (Meyer et al. 2014). Presumably, human cases have occurred during that time span but have not been accurately diagnosed. Similarly, the H1N1 “swine-flu” outbreak that was detected initially in Mexico and the United States in 2009—and was thought to have traveled globally within a few weeks of detection—was probably circulating in humans for several months prior to its discovery (Smith et al. 2009). In the case of Ebola virus, the identification of seropositive wildlife hosts normally occurs in the wake of observed human outbreaks, but an early-warning sentinel species has yet to be identified. These observations beg the question of how to develop more effective surveillance for zoonotic pathogens of epidemic and pandemic potential in the early stages of an outbreak.
Pathogen maintenance through metapopulation dynamics
Prairie dog colonies can comprise several thousand individuals and are often separated from one another by several kilometers of uninhabited grasslands (figure 3). Colony fates vary among those that experience outbreaks, recover, and then undergo recurrent die-offs (figure 4); colonies that experience plague and are never repopulated; and colonies that appear to remain unaffected by plague (Stapp et al. 2004, Augustine et al. 2008, Johnson et al. 2011). Landscape context can affect plague activity: A higher degree of geographic isolation from colonies with plague outbreaks reduces the risk of plague at a given colony (Collinge et al. 2005, Savage et al. 2011). The framework of rapidly occurring die-offs makes plague persistence between outbreaks difficult to explain.
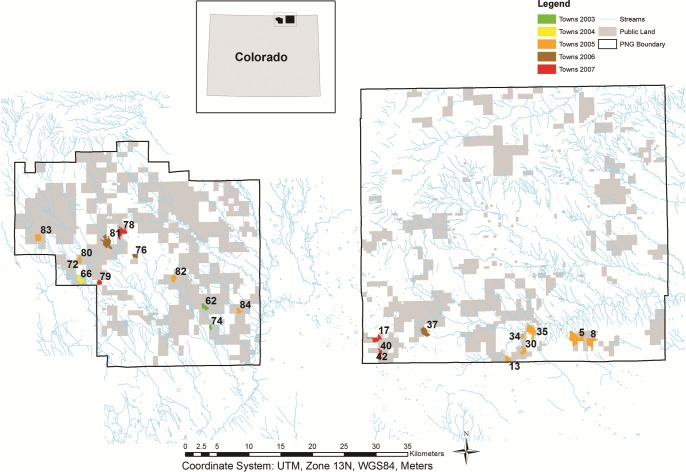
Figure 3.
A map showing black-tailed prairie dog colonies on the Pawnee National Grassland, Colorado, that experienced plague activity during 2003–2008. Colony size is monitored annually in late summer or fall by the US Forest Service, and active prairie dog colony area is determined by the presence of burrows that are cleared of vegetation and cobwebs and/or show signs of fresh digging and/or fresh scat (Savage et al. 2011). Colony area is shown here for the year prior to collapse as a result of Yersinia pestis–induced die-off (confirmed by the screening of fleas or prairie dog carcasses, in most cases). The decline in colony area ranged from 92% to 100% in 20 of 22 colonies and was 35% in colony 81 and 75% in colony 82. Measured declines in colony area were sometimes only observed the year after known plague activity, presumably because burrows may still have been considered active if the die-off had occurred shortly before US Forest Service monitoring.
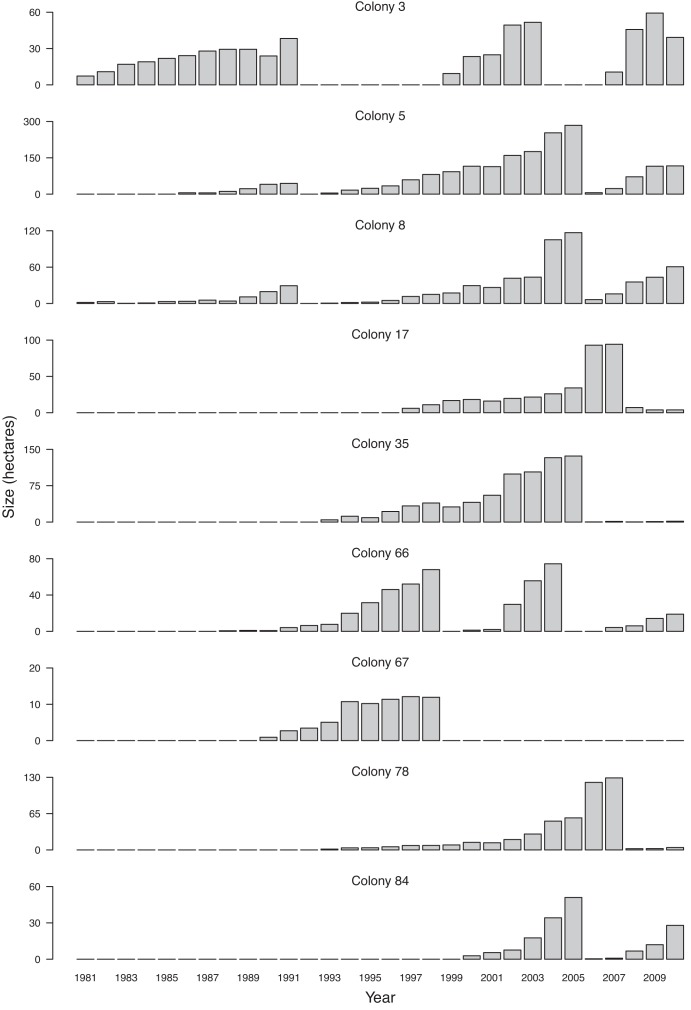
Figure 4.
A time series of the size of nine prairie dog colonies on the Pawnee National Grassland (PNG), north Colorado, from 1981 to 2010. Sudden declines in colony area can be attributed to plague outbreaks, and in 2004–2008, the presence of Y. pestis was confirmed by postmortem examination of dead prairie dogs, or by polymerase chain reaction (PCR) screening of fleas. Note that the scales of the y-axes vary. The patterns shown here are representative of other PNG prairie dog colonies; on average, 14% of prairie dog colonies experienced die-offs each year (n = 70 colonies active for at least 3 consecutive years; data from 1982 to 2009), although several colonies (23%) have shown no evidence of die-offs. Colonies normally show evidence of repopulation within a year of an outbreak (52%), although it may take several years to re-establish a population size comparable to that prior to the plague outbreak. Some colonies (16%) have never recovered even after more than 20 years (see colony 67).
However, if plague activity begins several months before causing a colony's extinction, then a broader window of opportunity exists for prairie dogs to disperse between colonies, thereby spreading Y. pestis. Thus, if prairie dog colonies are viewed as a connected network or metapopulation on the landscape (Stapp et al. 2004, Snall et al. 2008, Savage et al. 2011, George et al. 2013), a snapshot of colonies at a particular time would reveal that plague may be absent, smoldering, fading out, or causing die-offs, with local conditions (e.g., flea populations, prairie dog behavior, alternate host densities) determining the likelihood of each colony's fate. In other words, plague persists at the landscape scale because of host spatial dynamics (figure 5).
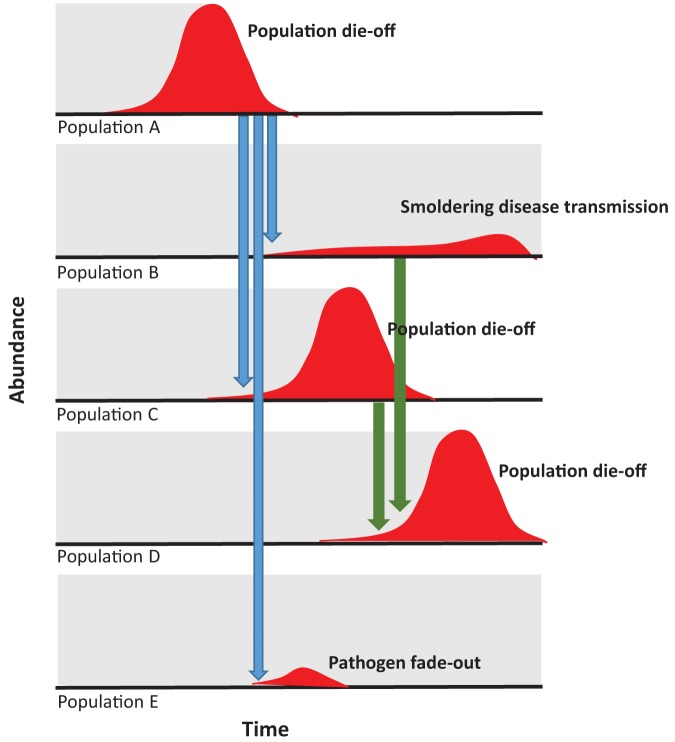
Figure 5.
A conceptual diagram of the metapopulation dynamics of plague and prairie dogs. The perceived abundance of the prairie dog population is shown in gray. The numbers of prairie dogs infected with Yersinia pestis are shown in dark gray. A plague outbreak in population A may cause a die-off in the hosts, but the pathogen is transmitted to nearby populations (light gray arrows). In the new populations, Y. pestis may (a) smolder and transmit slowly (e.g., population B), (b) cause a new outbreak (e.g., population C), or (c) fade out (e.g., population E). Subsequent spread (dark gray arrows) may occur from populations actively experiencing die-offs (e.g., population C to population D) or from populations experiencing smoldering plague transmission (e.g., population B to population C). Across a mosaic of prairie dog colonies on the landscape, plague may therefore be absent, smoldering, fading out, or causing die-offs. Researchers monitoring prairie dog colonies may not notice declines in population density (background gray) in the cases in which fade-out or smoldering transmission occurs; therefore, there is an observer bias in understanding Y. pestis’ presence and spread.
Synchronized outbreaks
If pathogen transmission between relatively isolated and independent host colonies allows for long-term persistence at the landscape level, we must ask these questions: How far and how often does the pathogen spread? The frequency of colony die-offs varies across years, and an epidemiologic challenge is to discover the degree to which different disease clusters are linked. Determining the distance that Y. pestis travels between and during outbreaks will provide insight into complex underlying mechanisms that dictate long-term persistence of Y. pestis in the environment. Molecular genetic analyses of Y. pestis in Colorado (Lowell et al. 2015) revealed locally confined genotypes, suggesting that the clones of bacteria are maintained at local scales representative of rodent dispersal patterns. This supports the idea that distinct Y. pestis genotypes persist in localized plague foci at spatial scales reflecting prairie dog spatial dynamics and that the transmission and persistence of Y. pestis are facilitated by prairie dog colonies recovering before subsequent reinvasion by Y. pestis. Furthermore, genetic evidence does not support the hypothesis that plague outbreaks on the grasslands occur because Y. pestis is transmitted from a distant maintenance reservoir, such as in the Rocky Mountain foothills, about 100 kilometers (km) from the Pawnee National Grassland (PNG). Interpreted in this way, widespread regional plague activity is the result of Y. pestis clones propagating locally and simultaneously rather than the result of sweeping clonal spread across the landscape. More systematic sampling (spatially and geographically) and improved genotyping of Y. pestis clones are required to determine the spatial extent of Y. pestis foci even though favorable climatic conditions spur plague outbreaks at several locales in chorus (Ben Ari et al. 2011, Savage et al. 2011, Lowell et al. 2015).
Pathogen spread on the landscape by host dispersal
The exact mechanism(s) of Y. pestis movement between colonies are still debated. Given the distances between colonies, some authors have presumed that Y. pestis is transported by dispersing prairie dogs that are infected or carrying infected fleas (figure 3; Girard et al. 2004, Stapp et al. 2004). Field observations of dispersing black-tailed prairie dogs demonstrate that prairie dogs can move easily between nearby colonies, often negotiating distances of 2–7 km (Garrett and Franklin 1988) and occasionally moving up to 10 km (Knowles 1985). These distances readily encompass the estimated mean dispersal distance for plague (6.9 km) generated for a complex of prairie dog colonies in Montana (Snall et al. 2008). Genetic studies suggest that that dispersal and gene flow continues on prairie dog colonies after initial colonization, probably along drainage routes, although there is some isolation by distance (Garrett and Franklin 1988, Roach et al. 2001, Jones and Britten 2010). Studies of prairie dog fleas have failed to show an effect of distance on flea gene flow, suggesting that flea dispersal can be widespread (Jones and Britten 2010, Brinkerhoff et al. 2011). Given that prairie dog dispersal is commonplace, the movement of infected prairie dogs—or the movement of infectious fleas on prairie dogs—can account for a large portion of plague spread and persistence at the landscape level (Stapp et al. 2004) and corroborates recent analyses that show outbreaks can be predicted by connectivity to other towns experiencing plague (as well as current-year climatic conditions). Thus, plague outbreaks cluster in groups of prairie dog colonies with short intercolony distances, whereas isolated colonies experience fewer plague outbreaks (figure 3; Savage et al. 2011).
Pathogen spread on the landscape facilitated by interacting species
However, prairie dog dispersal is not universally accepted as the driver of plague spread (Snall et al. 2008, George et al. 2013). Instead, Y. pestis dispersal may be explained by the wider-ranging behavior of carnivores or other plague-resistant species that carry infected fleas between colonies or through stepwise transmission between individuals of other rodent species (Girard et al. 2004, Snall et al. 2008, George et al. 2013). Coyotes and swift foxes interact closely with prairie dog populations—as predators and as scavengers of prairie dog carcasses, and they also exhibit higher use of prairie dog colony habitat—raising the possibility that predators functionally link colonies (Salkeld et al. 2007, Boone et al. 2009). Furthermore, carnivores are frequently exposed to plague and have been found carrying flea species commonly found on prairie dogs and previously observed as infected with Y. pestis (e.g., Pulex simulans), although Y. pestis-positive fleas have yet to be found on carnivores (McGee et al. 2006, Salkeld and Stapp 2006, Salkeld et al. 2007, Tripp et al. 2009). It is worth noting that prairie dog– and carnivore-mediated dispersal of Y. pestis are not mutually exclusive, and the importance of carnivores in the spread of plague will likely vary, depending on the species involved (Brinkerhoff et al. 2009). But Y. pestis spread on wider-ranging carnivores might explain the higher estimates of plague dispersal estimates in Montana (i.e., 4.5–12.6 km; Snall et al. 2008). Ebola virus transmission dynamics in the context of multiple species movements remains underexplored (Olival and Hayman 2014) but will be complex because of the different scales of movement of bats and primates—and of humans, both domestically and internationally. In the case of MERS-CoV, human-determined camel movements and market economics will also influence pathogen transport.
Surveillance bias and the identification of reservoir hosts
If disease outbreaks are sporadic and difficult to predict, a bias toward studies of the pathogen's ecology during the peaks or aftermaths of the outbreaks will naturally arise. This bias toward postoutbreak studies may lead to overlooking the wildlife species involved in the initial introduction of the disease and an erroneous identification of reservoir hosts. For example, on the PNG, deer mice and ground squirrels are sometimes found seropositive after a prairie dog die-off but do not seem to be involved in Y. pestis transmission dynamics before or during the die-off (Stapp et al. 2008). The inherent difficulty in studying rare pathogens prior to noticeable outbreaks is understated, but greater recognition that outbreaks are the zenith of longer-term, possibly cryptic transmission dynamics may allow improved insights into a pathogen's persistence and dynamics by motivating the monitoring and surveillance of wildlife populations prior to outbreaks.
Traditionally, because of plague's high lethality, prairie dog die-offs were believed to follow Y. pestis’ introduction to a colony from a disease-resistant alternative host species, such as deer mice (Cully and Williams 2001, Gage and Kosoy 2005). However, field research has failed to indubitably identify a nonprairie dog reservoir host on our field sites on the PNG—that is, a host species that is able to maintain the pathogen within its population and also possess transmission routes that allow the pathogen the opportunity to be introduced to the highly susceptible prairie dog population (Salkeld and Stapp 2008). Although deer mice and other rodents occasionally are seropositive on the PNG, their seroconversion normally occurs in the wake of the prairie dog die-off (Salkeld and Stapp 2008). Under the hypothesis that plague may persist solely in prairie dog metapopulations, the requirement for an alternate reservoir host could be moot.
Disease outbreaks and community ecology of alternate hosts
Nonetheless, Y. pestis’ ability to infect multiple species of hosts and vectors means that other animals are still important in Y. pestis transmission dynamics in the prairie dog–plague system. On the PNG, the northern grasshopper mouse (Onychomys leucogaster) has been implicated as an important component in the ecology of plague outbreaks. Grasshopper mice are common residents of prairie dog colonies, have large active ranges that may encompass multiple coterie territories, and regularly visit multiple prairie dog burrows each night, which, in effect, functionally connects prairie dog coteries despite prairie dog territorial behavior (Kraft and Stapp 2013). Plague outbreaks tend to occur on prairie dog colonies with high numbers of grasshopper mice or during periods when these mice are abundant (figure 2; Stapp et al. 2009). Grasshopper mouse populations decline by approximately two-thirds during prairie dog die-offs, but they exhibit some degree of plague resistance because they frequently seroconvert (Stapp et al. 2008). During plague outbreaks grasshopper mice become infested and bitten by O. hirsute—a flea normally host specific to prairie dogs (Stapp et al. 2009, Franklin et al. 2010). Both O. hirsuta and Pleochaetis exilis—the latter a flea specific to grasshopper mice—have been found infected with Y. pestis while infesting grasshopper mice, suggesting that grasshopper mice can be infectious hosts (Stapp et al. 2009). A spatially explicit agent-based model that incorporated grasshopper mouse movements, their ability to harbor prairie dog fleas, prairie dog social structure, and early-phase transmission suggested that higher grasshopper mouse density may increase the rate of spread of plague between coteries that would otherwise be socially isolated (Salkeld et al. 2010).
Although grasshopper mice and prairie dogs closely interact (figure 6) and plague activity occurs simultaneously in both species, it is currently impossible to discern whether plague is introduced from grasshopper mouse populations to prairie dog populations or vice versa—or indeed from some other source. No seropositive grasshopper mice have been found away from prairie dog colonies or on colonies without recent plague, although one grasshopper mouse was found harboring O. hirsuta several kilometers from the nearest active colony (Stapp et al. 2009). In the absence of additional evidence, it seems likely that the role of grasshopper mice is to amplify the rate of Y. pestis transmission within prairie dog populations.
Figure 6.
Prairie dogs and grasshopper mice interact closely on prairie dog colonies. Left: Grasshopper mouse running into prairie dog burrow (photograph: Kim Pollard). Right: Evidence of grasshopper mouse interactions with prairie dog carcasses. The prairie dog carcass (roadkill) had been previously intact and had been placed on a prairie dog colony to estimate rates of removal by carnivores (see Boone et al. 2006). Simultaneously, grasshopper mice on the prairie dog colony were being tracked by marking with fluorescent powder. The ring of pink fluorescent powder around the gaping hole in the prairie dog reveals that a grasshopper mouse scavenged this prairie dog's entrails (photograph: John Kraft).
Importantly, the roles of alternate hosts and multispecies interactions most likely vary across time and space; therefore, the dynamics of plague outbreaks are dependent on the local species assemblages of fleas and their hosts. Illustrative of this is the absence of grasshopper mice in study sites in Boulder County, Colorado—approximately 200 km from the PNG—but where plague outbreaks also occur in prairie dog colonies (Collinge et al. 2005, Cully et al. 2010). It is therefore advisable for disease ecology studies to investigate the local host community even when the pathogen appears to target a single animal species. This may lead to insights on previously unsuspected species roles—whether as species susceptible to the pathogen, species that affect the abundance of the main reservoir, or species that can inhibit transmission dynamics.
Disease ecologists must wrestle with the interpretation of available field data. Like the prairie dog–plague system, previous arguments about Ebola virus dynamics have suggested that the high lethality of Ebola virus in nonhuman primate populations indicates the need for a nonprimate reservoir host (Olival and Hayman 2014). Also like prairie dog plague, most surveys of possible Ebola virus–reservoir species have occurred in the wake of observed outbreaks, which can lead to false interpretations of seropositive species post-outbreak as being integral in Ebola virus ecology prior to the outbreak. If, as in prairie dog systems, host carcasses can be hard to find, there may also be a bias in determining which species are affected by the Ebola virus. The putative Ebola virus reservoir is a fruit bat species, on the basis of experimental inoculations of the virus into bats, similar geographic distributions between Ebola virus outbreaks and bats, and the identification of nucleotide and antibody presence in wild-caught bats (Olival and Hayman 2014). Some fruit bat species may have reservoir potential, but it is important to remember that this may be only one facet in Ebola virus ecology and that surveys identifying seropositive fruit bats after human outbreaks may have biased subsequent investigations toward bat–Ebola virus ecology, with the consequence that the potential role of nonhuman primates or other ungulates in Ebola virus circulation may have been neglected.
Environmental persistence and vector species
The absence of a smoking gun that implicates a specific vertebrate reservoir host for Y. pestis has shifted attention to other potential reservoir mechanisms. Recent hypotheses have advocated for new frameworks for plague transmission and maintenance and remain controversial. For example, a growing body of work suggests that Y. pestis may persist in soil. Viable plague bacteria have been isolated from natural soil samples collected three weeks after contamination by the death of mountain lion (Felis concolor) from plague (Eisen et al. 2008). Transmission of Y. pestis was demonstrated in lab experiments that allowed mice to burrow through plague-inoculated soil, although very rarely (Boegler et al. 2012). Colonizing prairie dogs often reuse abandoned burrow systems, and the re-excavation of sealed burrows containing infected carcasses may expose prairie dogs to infected soils or tissues. However, the importance of persistence in soil is unclear: Does a demonstrated three-week persistence of Y. pestis in soil in the case of the mountain lion have implications for longer-term Y. pestis persistence?
Infected fleas may also act as long-term (several months) reservoirs if they can persist postdie-off and infect immigrating prairie dogs, which would then enable a new generation of fleas to become infected. Although Y. pestis-positive fleas have been found remaining on sites several months after the prairie dog die-off, their presence can be rare (Holmes et al. 2006, Hanson et al. 2007, St. Romain et al. 2013), and their infectiousness is unknown, because transmission efficiency of O. hirsuta declines rapidly postinfection (Wilder et al. 2008). Transmission efficiency by fleas may be temperature-dependent, with higher transmission efficiency at lower temperatures (Williams et al. 2013), which suggests a re-evaluation of Y. pestis dynamics in the context of the host's burrow environments.
The evidence for nonvertebrate persistence mechanisms for Y. pestis remains equivocal. However, entertaining these developing hypotheses and accruing evidence for and against the role of these phenomena in the wild may allow for breakthroughs in our understanding of plague disease ecology.
Conclusions
Despite its importance to public health and conservation, we still are making only educated guesses as to Y. pestis’ persistence and spread and movement at the landscape scale. However, ecological studies of plague outbreaks in wildlife have resulted in important lessons that can be applied to zoonotic disease dynamics and persistence in general. For instance, we know that the ecological community surrounding the putative reservoir hosts is important; that transmission rates and mechanisms can vary over time and space; that our understanding of the pathogen's ecology may often be biased by post-outbreak surveillance; the local context; and that pathogens may persist in host populations even when they cause near-certain death of their hosts. These insights—sometimes still controversial—have required us to challenge conventional wisdoms and dogmas in Y. pestis ecology and epidemiology.
To be successful, the surveillance and control of zoonotic pathogens must accurately capture the dynamics of disease risk and spread—and do so at a local scale. A misplaced focus on a single animal host species may nullify efforts to create useful early warning monitoring programs, especially if the chosen sentinel species is more indicative of post-outbreak exposure as opposed to preoutbreak transmission dynamics. For example, are fruit bats that are seropositive for Ebola virus evidence of heightened disease risk for human populations because fruit bats can be infectious and demonstrate local Ebola virus activity? Or are seropositive fruit bats simply indicative of recent Ebola virus transmission in an unidentified reservoir host some months prior? Without directly addressing these questions, precious public health resources may be misdirected into inappropriate monitoring and surveillance programs. We recognize that an ecological understanding of rare pathogens that cause sporadic outbreaks in wildlife and human populations is no trivial undertaking. We nonetheless encourage the development of integrated surveillance programs for zoonotic diseases within wildlife populations that recognize ecological context before, during, and after outbreaks that occur in humans or animal species of conservation concern.
Acknowledgments
Long-term prairie dog town size and location data were generously provided by the Ranger District for the Pawnee National Grasslands, with special thanks to Mark Ball, Steve Curry, Richard Hill, Beth Humphrey, and Kristen Philbrook. We are grateful to Robert Flynn for GIS support, Kelly Pierce for R expertise, and Mark Lindquist in general. Many thanks to the field crews for their excellent work. The original research was predominantly supported by Short Grass Steppe Long Term Ecological Research Grants (nos. DEB-0217631, 0823405) and Ecology of Infectious Diseases programs (nos. DEB 0224328, EID 0327052).
References cited
- Antolin MF, Gober P, Luce B, Biggins DE, Van Pelt WE, Seery DB, Lockhart M, Ml Ball. The influence of sylvatic plague on North American wildlife at the landscape level, with special emphasis on black-footed ferret and prairie dog conservation. Transactions of the North American Wildlife and Natural Resources Conference. 2002;67:104–127. [Google Scholar]
- Augustine DJ, Matchett MR, Toombs TP, Cully JF, Jr., Johnson TL, Sidle JG. Spatiotemporal dynamics of black-tailed prairie dog colonies affected by plague. Landscape Ecology. 2008;23:255–267. [Google Scholar]
- Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, Madani TA. Evidence for camel-to-human transmission of MERS coronavirus. New England Journal of Medicine. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- Bacot AW, Martin CJ. Observations on the mechanism of the transmission of plague by fleas. Journal of Hygiene (Plague Supplement III) 1914;13:423–439. [PMC free article] [PubMed] [Google Scholar]
- Ben Ari T, Neerinckx S, Gage KL, Kreppel K, Laudisoit A, Leirs H, Stenseth NC. Plague and climate: Scales matter. PLOS Pathogens. 2011;7 doi: 10.1371/journal.ppat.1002160. (art. e1002160). doi:10.1371/journal.ppat.1002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins DE, Godbey JL, Gage KL, Carter LG, Montenieri JA. Vector control improves survival of three species of prairie dogs (Cynomys) in areas considered enzootic for plague. Vector-Borne and Zoonotic Diseases. 2010;10:17–26. doi: 10.1089/vbz.2009.0049. [DOI] [PubMed] [Google Scholar]
- Boegler KA, Graham CB, Montenieri JA, MacMillan K, Holmes JL, Petersen JM, Gage KL, Eisen RJ. Evaluation of the infectiousness to mice of soil contaminated with Yersinia pestis-infected blood. Vector-Borne and Zoonotic Diseases. 2012;12:948–952. doi: 10.1089/vbz.2012.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone A, Kraft JP, Stapp P. Scavenging by mammalian carnivores on prairie dog colonies: Implications for the spread of plague. Vector-Borne and Zoonotic Diseases. 2009;9:185–189. doi: 10.1089/vbz.2008.0034. [DOI] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Markeson AB, Knouft JH, Gage KL, Montinieri JA. Abundance patterns of two Oropsylla (Ceratophyllidae: Siphonaptera) species on black-tailed prairie dog (Cynomys ludovicianus) hosts. Journal of Vector Ecology. 2006;31:355–363. doi: 10.3376/1081-1710(2006)31[355:apotoc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Collinge SK, Bai Y, Ray C. Are carnivores universally good sentinels of plague? Vector-Borne and Zoonotic Diseases. 2009;9:491–497. doi: 10.1089/vbz.2008.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Martin AP, Jones RT, Collinge SK. Population genetic structure of the prairie dog flea and plague vector, Oropsylla hirsuta. Parasitology. 2011;138:71–79. doi: 10.1017/S0031182010001046. [DOI] [PubMed] [Google Scholar]
- Buhnerkempe MG, Eisen RJ, Goodell B, Gage KL, Antolin MF, Webb CT. Transmission shifts underlie variability in population responses to Yersinia pestis infection. PLOS ONE. 2011;6 doi: 10.1371/journal.pone.0022498. (art. e22498). doi:10.1371/journal.pone.0022498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge SK, Johnson WC, Ray C, Matchett R, Grensten J, Cully JF, Gage KL, Kosoy MY, Loye JE, Martin AP. Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landscape Ecology. 2005;20:941–955. [Google Scholar]
- Cully JF, Jr, Williams ES. Interspecific comparisons of sylvatic plague in prairie dogs. Journal of Mammalogy. 2001;82:894–905. [Google Scholar]
- Cully JF, Jr, Collinge SK, VanNimwegen RE, Ray C, Johnson WC, Thiagarajan B, Conlin DB, Holmes BE. Spatial variation in keystone effects: Small mammal diversity associated with black-tailed prairie dog colonies. Ecography. 2010;33:667–677. [Google Scholar]
- Eisen RJ, Bearden SW, Ap Wilder, Montenieri JA, Antolin MF, Gage KL. Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics. Proceedings of the National Academy of Sciences. 2006;103:15380–15385. doi: 10.1073/pnas.0606831103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Petersen JM, Higgins CL, Wong D, Levy CE, Mead PS, Schreifer ME, Griffith KS, Gage KL, Beard CB. Persistence of Yersinia pestis in soil under natural conditions. Emerging Infectious Diseases. 2008;14:941–943. doi: 10.3201/eid1406.080029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin HA, Stapp P, Cohen A. Polymerase chain reaction (PCR) identification of rodent blood meals confirms host sharing by flea vectors of plague. Journal of Vector Ecology. 2010;35:363–371. doi: 10.1111/j.1948-7134.2010.00095.x. [DOI] [PubMed] [Google Scholar]
- Gage KL, Kosoy MY. Natural history of the plague: Perspectives from more than a century of research. Annual Review of Entomology. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- Garrett MG, Franklin WL. Behavioral ecology of dispersal in the black-tailed prairie dog. Journal of Mammalogy. 1988;69:236–250. [Google Scholar]
- George DB, Webb CT, Pepin KM, Savage LT, Antolin MF. Persistence of black-tailed prairie dog populations affected by plague in northern Colorado. USA. Ecology. 2013;94:1572–1583. doi: 10.1890/12-0719.1. [DOI] [PubMed] [Google Scholar]
- Girard JM, Wagner DM, Vogler AJ, Keys C, Allender CJ, Drickamer LC, Keim P. Differential plague-transmission dynamics determine Yersinia pestis population genetic structure on local, regional, and global scales. Proceedings of the National Sciences. 2004;101:8408–8413. doi: 10.1073/pnas.0401561101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson DA, Britten HB, Restani M, Washburn LR. High prevalence of Yersinia pestis in black-tailed prairie dog colonies during an apparent enzootic phase of sylvatic plague. Conservation Genetics. 2007;8:789–795. [Google Scholar]
- Holmes BE, Foresman KR, Matchett MR. No evidence of persistent Yersinia pestis infection at prairie dog colonies in north–central Montana. Journal of Wildlife Diseases. 2006;42:164–169. doi: 10.7589/0090-3558-42.1.164. [DOI] [PubMed] [Google Scholar]
- Hoogland JL. The Black-tailed Prairie Dog. University of Chicago Press; 1995. [Google Scholar]
- Hoogland JL. Prairie dogs disperse when all close kin have disappeared. Science. 2013;339:1205–1207. doi: 10.1126/science.1231689. [DOI] [PubMed] [Google Scholar]
- Jones PH, Britten HB. The absence of concordant population genetic structure in the black-tailed prairie dog and the flea, Oropsylla hirsuta, with implications for the spread of Yersinia pestis. Molecular Ecology. 2010;19:2038–2049. doi: 10.1111/j.1365-294X.2010.04634.x. [DOI] [PubMed] [Google Scholar]
- Johnson TL, Cully JF, Jr, Collinge SK, Ray C, Frey CM, Sandercock BK. Spread of plague among black-tailed prairie dogs is associated with colony spatial characteristics. Journal of Wildlife Management. 2011;75:357–368. [Google Scholar]
- Knowles CJ. Observations on prairie dog dispersal in Montana. Prairie Naturalist. 1985;17:33–40. [Google Scholar]
- Kotliar NB, Miller BJ, Reading RR, Clark TW. The prairie dog as a keystone species. In: Hoogland JL, editor. Conservation of the Black-tailed Prairie Dog. Island Press; 2006. pp. 53–64. [Google Scholar]
- Kraft J, Stapp P. Movements and burrow use by northern grasshopper mice as a possible mechanism of plague spread in prairie dog colonies. Journal of Mammalogy. 2013;94:1087–1093. [Google Scholar]
- LaDeau SL, Kilpatrick AM, Marra PP. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447:710–713. doi: 10.1038/nature05829. [DOI] [PubMed] [Google Scholar]
- Leroy EM, et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004;303:387–390. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith JO, George D, Pepin KM, Pitzer VE, Pulliam JRC, Dobson AP, Hudosn PJ, Grenfell BT. Epidemic dynamics at the human–animal interface. Science. 2009;326:1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell JL, Antolin MF, Andersen GL, Hu P, Stowkowski RP, Gage KL. Single nucleotide polymorphisms reveal spatial diversity among clones of Yersinia pestis during plague outbreaks in Colorado and the western United States. Vector-Borne Zoonotic Diseases. 2015;15:291–302. doi: 10.1089/vbz.2014.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchett MR, Biggins DE, Carlson V, Powell B, Rocke T. Enzootic plague reduces black-footed ferret (Mustela nigripes) survival in Montana. Vector-Borne and Zoonotic Diseases. 2010;10:27–35. doi: 10.1089/vbz.2009.0053. [DOI] [PubMed] [Google Scholar]
- McGee BK, Butler MJ, Pence DB, Alexander JL, Nissen JB, Ballard WB, Nicholson KL. Possible vector dissemination by swift foxs following a plague epizootic in black-tailed prairie dogs in northwestern Texas. Journal of Wildlife Diseases. 2006;42:415–420. doi: 10.7589/0090-3558-42.2.415. [DOI] [PubMed] [Google Scholar]
- Meyer B, et al. Antibodies against MERS coronavirus in dromedaries, United Arab Emirates, 2003 and 2013. Emerging Infectious Diseases. 2014;20:552–559. doi: 10.3201/eid2004.131746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olival KJ, Hayman DTS. Filoviruses in bats: Current knowledge and future directions. Viruses. 2014;6:1759–1788. doi: 10.3390/v6041759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli JN, Buskirk SW, Williams ES, Edwards WHA. A plague epizootic in the black-tailed prairie dog (Cynomys ludovicianus) Journal of Wildlife Diseases. 2006;42:74–80. doi: 10.7589/0090-3558-42.1.74. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Gerber SI, Swerdlow DL. Middle East respiratory syndrome coronavirus (MERS-CoV): CDC update for clinicians. Clinical Infectious Diseases. 2015;60:1686–1689. doi: 10.1093/cid/civ118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach JL, Stapp P, Van Horne B, Antolin MF. Genetic structure of a metapopulation of black-tailed prairie dogs. Journal of Mammalogy. 2001;82:946–959. [Google Scholar]
- Salkeld DJ, Stapp P. Seroprevalence rates and transmission of plague (Yersinia pestis) in mammalian carnivores. Vector-Borne and Zoonotic Diseases. 2006;6:231–239. doi: 10.1089/vbz.2006.6.231. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Stapp P. No evidence of deer mouse involvement in plague (Yersinia pestis) epizootics of prairie dogs. Vector-Borne and Zoonotic Diseases. 2008;8:331–337. doi: 10.1089/vbz.2007.0199. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Eisen RJ, Stapp P, Wilder AP, Lowell J, Tripp DW, Albertson D, Antolin MF. The potential role of swift foxes (Vulpes velox) and their fleas in plague outbreaks in prairie dogs. Journal of Wildlife Diseases. 2007;43:425–431. doi: 10.7589/0090-3558-43.3.425. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Salathe M, Stapp P, Jones JH. Plague outbreaks in prairie dog populations explained by percolation thresholds of alternate host abundance. Proceedings of the National Academy of Sciences. 2010;107:14247–14250. doi: 10.1073/pnas.1002826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LT, Reich RM, Hartley LM, Stapp P, Antolin MF. Climate, soils, and connectivity predict plague epizootics in black-tailed prairie dogs (Cynomys ludovicianus) Ecological Applications. 2011;21:2933–2943. [Google Scholar]
- Seery DB, Biggins DE, Montenieri JA, Enscore RE, Tanda DT, Gage KL. Treatment of black-tailed prairie dog burrows with deltamethrin to control fleas (Insecta: Siphonaptera) and plague. Journal of Medical Entomology. 2003;40:718–722. doi: 10.1603/0022-2585-40.5.718. [DOI] [PubMed] [Google Scholar]
- Smith GJD, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- Snall T, O'Hara RB, Ray C, Collinge SK. Climate-driven spatial dynamics of plague among prairie dog colonies. American Naturalist. 2008;171:238–248. doi: 10.1086/525051. [DOI] [PubMed] [Google Scholar]
- Romain K, Tripp DW, Salkeld DJ, Antolin MF. Duration of plague (Yersinia pestis) outbreaksin black-tailed prairie dog (Cynomys ludovicianus) colonies of northern Colorado. EcoHealth. 2013;10:241–245. doi: 10.1007/s10393-013-0860-4. [DOI] [PubMed] [Google Scholar]
- Stapp P, Antolin MF, Ball M. Patterns of extinction in prairie dog metapopulations: Plague outbreaks follow El Niño events. Frontiers in Ecology and the Environment. 2004;2:235–240. [Google Scholar]
- Stapp P, Salkeld DJ, Eisen RJ, Pappert R, Young J, Carter LG, Gage KL, Tripp DW, Antolin MF. Exposure of small rodents to plague during epizootics in black-tailed prairie dogs. Journal of Wildlife Diseases. 2008;44:724–730. doi: 10.7589/0090-3558-44.3.724. [DOI] [PubMed] [Google Scholar]
- Stapp P, Salkeld DJ, Franklin HA, Kraft JP, Tripp DW, Antolin MF, Gage KL. Evidence for the involvement of an alternative rodent host in the dynamics of introduced plague in prairie dog colonies. Journal of Animal Ecology. 2009;78:807–817. doi: 10.1111/j.1365-2656.2009.01541.x. [DOI] [PubMed] [Google Scholar]
- Thomas RE, Beard ML, Quan TJ, Carter LG, Barnes AM, Hopla CE. Experimentally induced plague infection in the northern grasshopper mouse (Onychomys leucogaster) acquired by consumption of infected prey. Journal of Wildlife Diseases. 1989;25:477–480. doi: 10.7589/0090-3558-25.4.477. [DOI] [PubMed] [Google Scholar]
- Tripp DW, Gage KL, Montenieri JA, Antolin MF. Flea abundance on black-tailed prairie dogs (Cynomys ludovicianus) increases during plague epizootics. Vector-borne and Zoonotic Diseases. 2009;9:313–321. doi: 10.1089/vbz.2008.0194. [DOI] [PubMed] [Google Scholar]
- Webb CT, Brooks CP, Gage KL, Antolin MF. Classic flea-borne transmission does not drive plague epizootics in prairie dogs. Proceedings of the National Academy of Sciences. 2006;103:6236–6241. doi: 10.1073/pnas.0510090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder AP, Eisen RJ, Bearden SW, Montenieri JA, Tripp DW, Brinkerhoff RJ, Gage KL, Antolin MF. Transmission efficiency of two flea species (Oropsylla tuberculata cynomuris and Oropsylla hirsuta) involved in plague epizootics among prairie dogs. EcoHealth. 2008;5:205–212. doi: 10.1007/s10393-008-0165-1. [DOI] [PubMed] [Google Scholar]
- Williams SK, Schotthoeffer AM, Montenieri JA, Holmes JL, Vetter SM, Gage KL, Bearden SW. Effects of low-temperature flea maintenance on the transmission of Yersinia pestis by Oropsylla montana. Vector-borne and Zoonotic Diseases. 2013;13:468–478. doi: 10.1089/vbz.2012.1017. [DOI] [PubMed] [Google Scholar]
- Wong D, et al. Primary pneumonic plague contracted from a mountain lion carcass. Clinical Infectious Diseases. 2009;49:e33–e38. doi: 10.1086/600818. [DOI] [PubMed] [Google Scholar]