Abstract
Background. We sought to determine whether infections with human coronaviruses (HCoVs) 229E, OC43, HKU1, and NL63 are associated with pneumonia and to define the epidemiology of HCoV infection in rural Thailand.
Methods. We developed a real-time reverse-transcription polymerase chain reaction (RT-PCR) assay panel for the recognized HCoV types and compared HCoV infections in patients hospitalized with pneumonia, outpatients with influenza-like illness, and asymptomatic control patients between September 2003 and August 2005.
Results. During study year 1, 43 (5.9%) of 734 patients with pneumonia had HCoV infections; 72.1% of the infections were with OC43. During study year 2, when control patients were available, 21 (1.8%) of 1156 patients with pneumonia, 12 (2.3%) of 513 outpatients, and 6 (2.1%) of 281 control patients had HCoV infections. Compared with infection in control patients, infection with any HCoV type or with all types combined was not associated with pneumonia (adjusted odds ratio for all HCoV types, 0.67 [95% confidence interval, 0.26–1.75]; P= .40 ). HCoV infections were detected throughout both study years; 93.6% of OC43 infections in the first year occurred from January through March.
Conclusions. HCoV infections were infrequently detected in rural Thailand by use of sensitive real-time RTPCR assays. We found no association between HCoV infection and illness. However, we noted year-to-year variation in the prevalence of HCoV strains, which likely influenced our results.
Human coronaviruses (HCoVs) are positive-stranded, enveloped RNA viruses classified within the family Coronaviridae (genus, Coronavirus) [1]. First identified in the mid 1960s [2–4], HCoV-229E and HCoV-OC43 are recognized to be an important cause of the common cold [4–6]. Epidemiologic studies have since shown that these viruses are responsible for up to 30% of mild upper respiratory tract infections [7–11]. More recently, 2 new HCoVs, NL63 and HKU1, have been reported, both detected in respiratory tract specimens of patients with lower respiratory tract illness [12–15]. NL63 has since been reported worldwide in 2%–9% of persons with acute respiratory tract illness, including in some hospitalized with pneumonia [16–20]. HKU1 has also been identified in respiratory tract specimens from children and adults with acute respiratory tract illness at a prevalence ranging from 0.3% to 4.4% [21–23].
Much of the early diagnostic work on HCoVs relied on cumbersome and insensitive methods such as serologic analysis, virus culture, and antigen detection. The subsequent development of sensitive reverse-transcription polymerase chain reaction (RT-PCR) assays has permitted a wider study of these viruses [24–27], increasing the number of reports possibly linking HCoV infection to more-severe lower respiratory tract infections. However, these studies often failed to use well-designed epidemiologic or laboratory methods to establish HCoV etiology, including (1) use of sensitive, well-validated assays that detect and distinguish all 4 currently recognized HCoV types; (2) comprehensive testing for other respiratory pathogens that may be present in mixed infections with HCoVs and, thus, may be responsible for the disease; and (3) comparison of HCoV infections between asymptomatic control patients and symptomatic patients, controlling for age and month of illness.
In the present study, we developed a sensitive and specific real-time RT-PCR assay panel for all currently recognized HCoVs (229E, OC43, NL63, and HKU1) that complements our previously described severe acute respiratory syndrome (SARS)—CoV—specific real-time RT-PCR assay [28]. We used this assay panel to test specimens from patients hospitalized with pneumonia, outpatients with influenza-like illness (ILI), and asymptomatic control patients from a rural Thai province. We describe the epidemiology of HCoV infection in rural Thailand and compare infections in control patients versus patients with pneumonia.
Methods
HCoV Assay Panel
Viral nucleic acid. HCoV RNA used for development and validation of the real-time RT-PCR panel was obtained from several sources (see Acknowledgments). HCoV prototype strains 229E (ATCC VR-740) and OC43 (ATCC VR-1558) were available from Centers for Disease Control and Prevention (CDC) archives, whereas the NL63 prototype strain was donated. Thirty-one available HCoV-positive clinical specimens (16 OC43, 4 229E, 10 HKU1 and 1 NL63) were previously identified by RT-PCR and probe hybridization [29] or by RTPCR and sequencing [30, 31]. Other respiratory viruses were available for specificity testing from CDC archives, including laboratory strains of influenza virus A and B, respiratory syncytial virus (RSV), human parainfluenza viruses (HPIV)—1—4, human metapneumovirus (HMPV), adenovirus, rhinovirus, human bocavirus (HBoV), and SARS-CoV.
RNA transcripts. Primer pairs containing T7 and SP6 RNA polymerase promoter sequences were used to amplify regions bracketing the real-time RT-PCR signatures for each HCoV type. A 488-bp fragment of the OC43 nucleoprotein (N) gene was generated using the forward primer 5′-SP6-GAGCTCAACCCAAGCAAACTG-3′ and the reverse primer 5′-T7-TGTGCGCGAAGTAGATCTGG-3′; 476 bp of the 229E N gene was generated using the forward primer 5′-T7-ATGGCTACAGTCAAATGGGCTG-3′ and the reverse primer 5′-SP6-CCGCGACTCTGCGACCT-3′; 401 bp of the NL63 N gene was generated using the forward primer 5′-T7-ATCAGTGCTAACCTCACTATCAAAGAAT-3′ and the reverse primer 5′-SP6-CTTCTCGTAGCACTTCAAGACAACA-3′; and 440 bp of the HKU1 replicase 1b gene was generated using the previously published primers [23] 5′-SP6-GGTTGGGATTATCCTAAATGTGA-3′ (forward) and 5′-T7-CCATCATCACTCAAAACATCATA-3′ (reverse). Amplification products were gel purified using the QIAquick Gel Extraction Kit (Qiagen), and RNA transcripts were prepared using the MEGAshortscript High Yield Transcription Kit (Ambion). RNA transcripts were purified using the MEGAclear Kit (Ambion) and were quantified by UV light spectroscopy. For limit-of-detection tests, serial dilutions of the quantified RNA transcripts were prepared in a diluent containing yeast tRNA (50 ng/mL; Ambion).
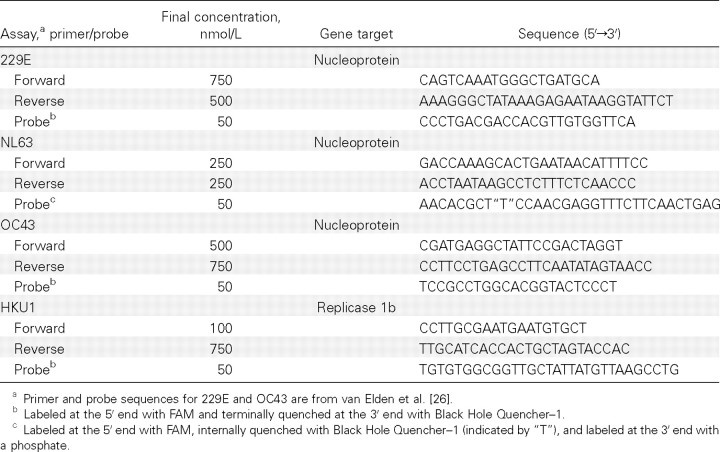
Primers and probes. Multiple primer and probe sequences for HCoV-HKU1 and HCoV-NL63 were designed to identify type-specific conserved regions of the viral replicase 1b and N genes identified from alignments of sequences available in GenBank (AY884001, DQ190472, and DQ206693-9 for HKU1 [31, 32] and NC_005831, AY563107-8, and AY758276-82 for NL63 [13, 14]), using Primer Express (version 3.0; Applied Biosystems) and Beacon Designer (version 5.0; Premier Biosoft International) software (table 1). Primer and probe sequences for HCoV-OC43 and HCoV-229E have been described elsewhere [26]. All probes were labeled at the 5′ end with FAM and quenched at the 3′ end or internally with Black Hole Quencher—1 (Biosearch Technologies). Sequences of different representative HCoVs were aligned to check for potential cross-reactivity of the real-time assays (SARS-CoV [AY864806], OC43 [NC_005147], 229E [NC_002645], NL63 [NC_005831], and HKU1 [NC_006577]) [14, 15, 33–35].
Table 1.
Human coronavirus real-time reverse-transcription polymerase chain reaction (RT-PCR) panel primer and probe sequences.
Real-time RT-PCR assays. The real-time RT-PCR panel consisted of 4 differential HCoV monoplex assays run under identical amplification conditions. Reaction mixtures were prepared using the iScript One-Step RT-PCR Kit for Probes (Bio-Rad). Twenty-five—microliter reactions contained 2× reaction buffer, 50× iScript reverse transcriptase, primers, probe, nuclease-free water, and nucleic acid. Amplification was performed on an iCycler iQ Real Time Detection System (Bio-Rad) with the following cycling conditions: 48°C for 10 min (1 cycle); 95°C for 5 min (1 cycle); and 95°C for 15 s followed by 55°C for 1 min (45 cycles). Each specimen was also tested for the human ribonuclease P gene to measure nucleic acid integrity [28].
Clinical Study
Study subjects and specimens. Specimens were collected from patients of all ages enrolled in an enhanced active surveillance program for pneumonia requiring hospitalization in Sa Kaeo Province, Thailand [36], over a 2-year period from 1 September 2003 to 31 August 2005; we previously tested these specimens for HBoV infections [37, 38]. In general, a patient was enrolled in the study if he or she had at least 1 sign of acute infection (e.g., fever, chills, and/or abnormal white blood cell count), symptoms of lower respiratory tract disease (e.g., abnormal breath sounds, tachypnea, and/or cough), and the physician ordered a chest radiograph (CXR) within 48 h of admission. Final CXR findings were not available for the present analysis. A nasopharyngeal swab, acute and convalescent serum, urine, and clinical and demographic information were collected from each patient with pneumonia.
A sample of outpatients with ILI, defined as fever within the previous 3 days and cough or sore throat in the absence of other diagnoses, were recruited from the outpatient departments of 5 hospitals in Sa Kaeo Province [39]. Only outpatient specimens from the second year of the study (1 September 2004 to 31 August 2005) were tested for HCoV infection. A nasopharyngeal swab and demographic information was collected from each outpatient participant. In addition, during the second study year, we enrolled a sample of control patients from the same outpatient clinics; these were patients with other health-related complaints but without a fever and with no history of fever, cough, sore throat, or diarrhea within the previous 3 days. We sought to enroll equal numbers of control patients and patients hospitalized with pneumonia from each age group (⩽2 years, 3–5 years, 6–15 years, 16–54 years, and ⩾55 years) during each month of the year. A nasopharyngeal swab and demographic information was collected from each control patient.
Nasopharyngeal swabs were collected in viral transport medium, and aliquots were processed and transported as described elsewhere [37, 38]. The swabs were tested for RSV, HPIVs, influenza virus A and B, and adenovirus by culture at the Thailand National Institute of Health [38], and total nucleic acid extracts were tested for influenza virus A and B, RSV, HPIV-1–3, HMPV, adenovirus, rhinovirus, and HBoV (year 2 only) by PCR at the CDC [37, 40–42]. Aliquots of the extracts were kept at -70°C for HCoV testing. We did not test for SARS-CoV infection. Acute and convalescent serum samples were tested for RSV, HPIV-1–3, adenovirus, and HMPV by inhouse EIAs and for influenza viruses by hemagglutination-inhibition assays (year 1 only). A specimen was considered to be positive if either culture or PCR results were positive or if there was a 4-fold or greater increase in antibody titer between acute and convalescent serum samples.
All participants were informed of the study objectives, and written consent was obtained. The study protocol was reviewed and approved by the internal review boards of the CDC and the Thailand Ministry of Public Health.
Statistical methods. Admissions for pneumonia were counted individually, including readmissions ⩾14 days after a previous discharge; readmissions occurring within 13 days were considered to be 1 admission. We compared HCoV infections between patients hospitalized with pneumonia and control patients during the study year using the χ2 test and a multivariable unconditional logistic regression model controlling for age group and month of illness. The clinical characteristics of patients with HCoV infection, without coinfections, were compared to patients with infection with other common viruses. Variables were compared with the χ2 test for dichotomous variables. Two-tailed P < .05 was considered to indicate statistical significance. Analyses were performed using SAS software (version 9.1; SAS Institute).
Results
Development and validation of the HCoV real-time RT-PCR assay panel. Different primer and probe combinations were evaluated for the NL63 and HKU1 assays, and those with the most efficient amplification were selected for further studies. All real-time assays were internally specific for the other HCoVs, and no amplification was obtained with other respiratory viral pathogens—including influenza viruses A and B, RSV, HPIV1–4, HMPV, adenovirus, rhinovirus, HBoV, and SARS-CoV— or with human DNA. All assays were successfully validated against 31 clinical specimens previously shown to be positive for HCoVs by alternate methods; 3 specimens originally reported to be positive for OC43 by RT-PCR [30] were identified as being HKU1 by the real-time assay and were confirmed by sequencing. The sensitivity of each assay was determined by testing limiting dilutions of specific viral RNA transcripts, and all 4 HCoV assays could detect as few as 5 copies. Every assay in the panel detected 15 (100%) of 15 and 15 (100%) of 15 replicates of 500 and 50 copies, respectively. The OC43, 229E, HKU1, and NL63 assays detected 5 (33.3%) of 15, 14 (93.3%) of 15, 5 (33.3%) of 15, and 12 (80.0%) of 15 replicates of 5 copies, respectively. All 4 assays achieved linear amplification of respective RNA transcripts ranging from 5×101 to 5×105 copies. Reaction efficiencies, slopes, and R2 values for these assays were 100%, —3.32, and 0.99 for OC43; 99.2%, —3.34, and 0.99 for 229E; 100%, -3.32, and 0.99 for HKU1; and 99.6%, —3.33, and 1.00 for NL63, respectively. Limited attempts to multiplex the reactions in a single tube resulted in reduced assay sensitivity, and these studies were not pursued further (data not shown).
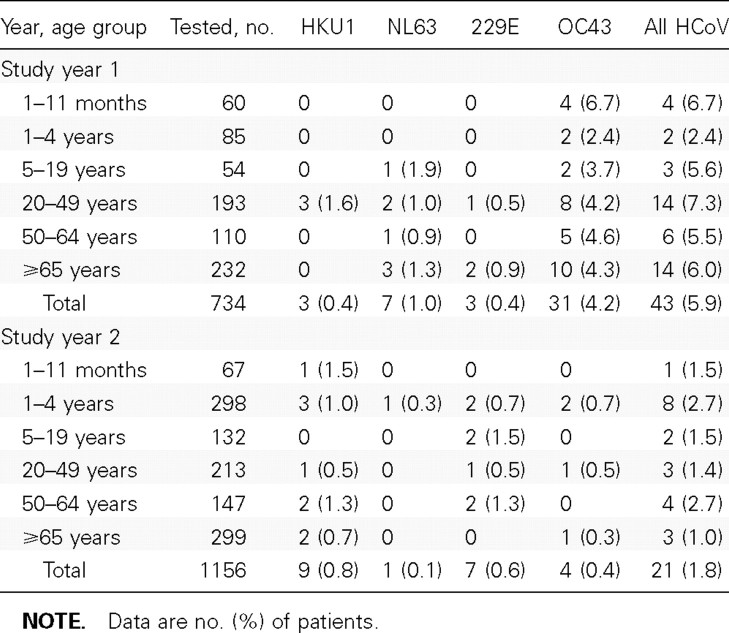
HCoV epidemiology in Sa Kaeo Province. During the first study year (1 September 2003 to 31 August 2004), 3489 patients with signs and symptoms of clinical pneumonia were admitted to a Sa Kaeo Province hospital, and 2059 (59.0%) of these patients had a CXR taken. We enrolled 755 (36.7%) of the patients hospitalized with pneumonia who had a CXR taken; 16 were being readmitted. Respiratory specimens were unavailable for 21 patients with pneumonia, who were excluded from the analysis (n=734). There were 43 (5.9%) HCoV infections during study year 1, and all HCoV types were identified (table 2). OC43 was detected most frequently, representing 72.1% of all HCoV infections, and was present in all age groups. 229E, NL63, and HKU1 infections were uncommon.
Table 2.
Human coronavirus (HCoV) infections among patients hospitalized with pneumonia during 2 years of surveillance, Sa Kaeo Province, Thailand, 1 September 2003 to 31 August 2005.
During the second study year (1 September 2004 to 31 August 2005), 3960 patients with pneumonia were admitted to a hospital in Sa Kaeo Province, and 2288 (57.8%) of these patients had a CXR taken. We enrolled 1156 (50.5%) of the hospitalized pneumonia patients who had a CXR taken; 32 were being readmitted. Only 21 (1.8%) had any HCoV infection detected (table 2). Each HCoV type was detected in <1% of patients with pneumonia. There were significantly more HCoV infections detected among patients with pneumonia in study year 1 than in study year 2, and this difference remained when we analyzed for OC43 infection alone (for all HCoV infections, 5.9% vs. 1.8% [P<.001]; for OC43 infections, 4.2% vs. 0.4% [P<.001]).
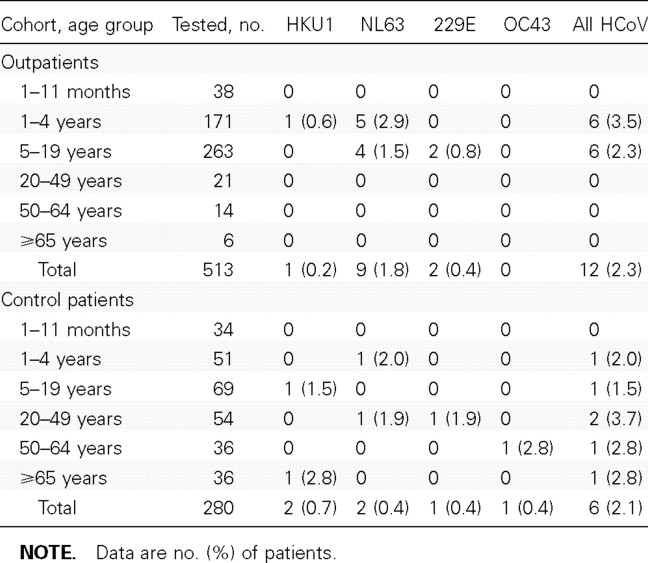
Of the outpatients with ILI, 12 (2.3%) had HCoV infections (table 3). NL63 was the most common type detected among the outpatients (75.0% of all HCoVs). We detected a similar proportion of HCoV infections among asymptomatic control patients; 6 (2.1%) of the 280 control patients had 1 of the HCoV types detected. Among the control patients, HCoV infections were detected in all age groups except for children <1 year of age.
Table 3.
Human coronavirus (HCoV) testing results for outpatients with influenza-like illness and control patients (persons without respiratory tract symptoms, fever, or diarrhea during the previous 3 days) in study year 2 (1 September 2004 to 31 August 2005).
We compared HCoV infections between patients hospitalized with pneumonia and control patients for study year 2. Neither all HCoV infections combined nor infections with individual HCoV types were associated with pneumonia requiring hospitalization, even after controlling for age and month of illness (for all HCoV infections, adjusted odds ratio [aOR] of 0.68 [95% confidence interval {CI}, 0.26–1.75] [P=.4]; for HKU1, aOR of 0.68 [95% CI, 0.14–3.30] [P=.63]; for NL63, aOR of 0.07 [95% CI, 0.01–0.84] [P=.04]; for 229E, aOR of 2.18 [95% CI, 0.25–19.03] [P=.48 ]; for OC43, aOR of 0.75 [95% CI, 0.08–6.78] [P=.79]). Fewer NL63 infections were detected among patients with pneumonia than among control patients, and the association between NL63 and pneumonia appears to be protective. However, the numbers are small, and this result should be interpreted with caution.
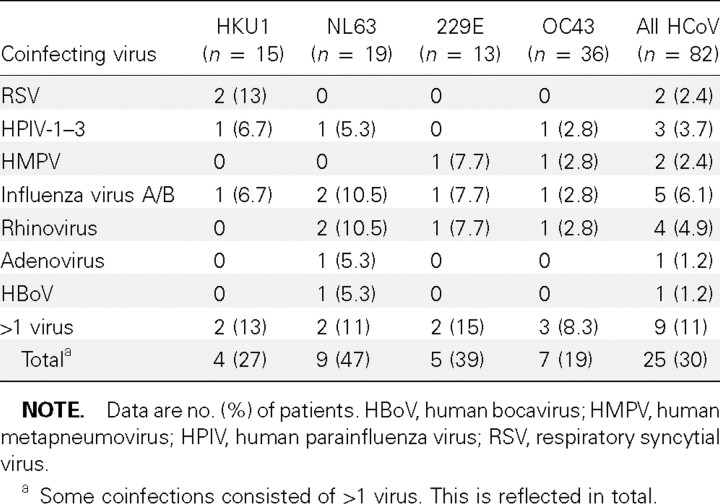
Of all 82 patients with HCoV infections, 30.5% were coinfected with other respiratory viruses, including almost half of those who were infected with NL63 (table 4). Among the patients with pneumonia, 17 (26.6%) of the 64 who were infected with HCoV had a viral coinfection. Among the outpatients and control patients with HCoV infections, 7 (58.3%) of 12 and 1 (16.7%) of 6, respectively, were coinfected with another virus.
Table 4.
Coinfections of respiratory specimens with human coronavirus (HCoV) and another respiratory virus during the 2-year study, 1 September 2003 to 31 August 2005.
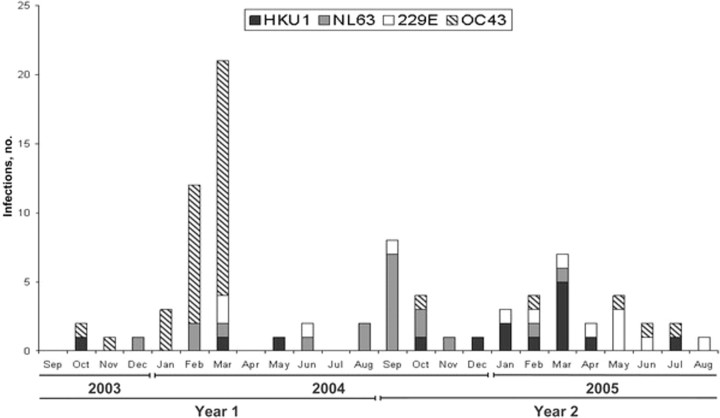
HCoV infections were detected in Sa Kaeo Province throughout the 2-year study (figure 1). OC43 was the most frequently detected HCoV type, representing 36 (43.4%) of all 83 HCoV infections. During study year 1, OC43 was present in 31 (72.1%) of the 43 HCoV-infected patients; these infections clustered in January–March 2004 and made up 93.6% of the OC43 infections that year. The following year, 8 (53.3%) of all 15 HKU1 infections were detected during the same 3 months and only 1 OC43 infection (2.7%) was detected, indicating a shift in circulation of the predominant HCoV type. NL63 infections appeared to peak in September 2004. 229E was detected at low levels throughout the study.
Figure 1.
Seasonality of human coronavirus (HCoV) infections during 2 years of surveillance, Sa Kaeo Province, Thailand, 1 September 2003 to 31 August 2005. The first year includes HCoV infections in hospitalized patients with pneumonia, and the second year includes HCoV infections in pneumonia patients, outpatients with influenza-like illness, and control patients.
We compared the clinical characteristics of OC43-infected patients with pneumonia to those of patients with pneumonia who were infected with influenza virus or RSV (2 common infections) and limited our analysis to study year 1, when the prevalence of OC43 infections was highest. Fifty percent of the 24 patients with OC43 infections had fever, 91.7% had cough, 41.7% had tachypnea, 22.7% received oxygen therapy, and 8.3% required mechanical ventilation; no statistically significant differences were noted between patients infected with OC43 and those infected with influenza virus or RSV (data not shown).
A few differences were noted—3 (12.5%) of 24 patients with OC43 infections had rhinitis versus 0 of 47 patients with influenza virus infections (P=.04), and only 3 persons (12.5%) with OC43 infections had wheezing on admission versus 9 (47.4%) of 19 persons with RSV infections (P=.01 ). Patients with HCoV infections other than with OC43 also had similar proportions of fever (63.2%), cough (89.5%), and tachypnea (21.1%).
Discussion
In the present study, we developed a sensitive and specific real-time RT-PCR assay panel for the 4 HCoV types (229E, OC43, NL63 and HKU1) and used this assay panel to assess the disease burden and epidemiology of HCoV infection in a rural province of Thailand over a 2-year period. Infections were infrequently detected among hospitalized patients with pneumonia and among outpatients with ILI. During study year 2, the asymptomatic control patients and the ill patients had similar proportions of HCoV infections. OC43 infections were detected in significantly higher numbers during the first study year. However, we did not have a control group for comparison during that year. Thus, in rural Thailand from 1 September 2003 to 31 August 2005, infection with HCoVs occurred infrequently. We were unable to show an epidemiologic association between any of the HCoV types and pneumonia requiring hospitalization.
The real-time RT-PCR assays for the 4 HCoV types were combined into a single test panel using identical amplification conditions. The assay panel was shown to be sensitive and specific with defined targets and was validated by use of human clinical specimens that were independently confirmed to be positive for the different virus types. Samples previously determined to be OC43 positive by use of conventional methods were found to be HKU1 positive and OC43 negative by use of this panel, exemplifying the panel's specificity in relation to other HCoV diagnostic tools. Although real-time assays have been described for some HCoV types [26, 43], most studies failed to report analytical and clinical data by which assay performance could be evaluated. Although our assay panel could detect and discriminate among the 4 known HCoV types, it could have failed to detect or improperly classify unrecognized HCoVs.
Our study represents the first comprehensive analysis of HCoV infection in a tropical rural population in Southeast Asia. Despite sensitive assays, we found the prevalence of infection with HCoVs to be very low during the study period. Similar to our findings, a recent study from Bangkok, Thailand, conducted during 2002–2003 found 229E and OC43 infections in 4.9% of young children with acute respiratory tract illness; however, the majority were 229E infections rather than OC43 infections [44]. The proportion of HCoV types we detected is similar to those of other international studies reporting HCoVs from both upper and lower acute respiratory tract illness by use of PCR assays [12–16, 18, 20–23, 31, 45]. In the present study, we detected very few NL63 infections. Moreover, a larger proportion of the patients with detected NL63 infections were coinfected with other viruses, compared with patients infected with other HCoV types. Additional years of surveillance will be necessary to clearly establish the prevalence and circulation patterns of HCoVs in Thailand.
We did not find an epidemiologic association between HCoV infection and pneumonia. However, correlation between HCoV and severe pneumonia during the first study year could not be assessed because of the absence of a control group, and the low number of HCoV infections during the second study year made it difficult to accurately assess disease correlation. Given the year-to-year variation in the prevalence of HCoV infections, only a multiyear study may be able to definitively assess whether an association between HCoV infection and severe illness exists.
Only a few studies have tested asymptomatic control subjects for HCoV infection, and most of these studies have tested only for OC43 and 229E. Using serologic assays, McIntosh et al. [46] detected OC43 and 229E infection in 34 (8%) of 417 children hospitalized with acute lower respiratory tract disease and in 1 (8%) of 13 control children. Nokso-Koivisto et al. [47] failed to find 229E or OC43 by PCR among hospitalized children aged 1 month to 16 years with or without a history of respiratory tract illness. In contrast, van Elden et al. [26] found significantly more 229E and OC43 by PCR in patients with respiratory tract illness than in bone-marrow transplant recipients and healthy volunteers without respiratory tract illness. However, they did not control for age and month of illness/admission, and it is unclear how frequently OC43 and 229E were detected among patients with pneumonia versus those with the common cold. Thus, although numerous studies have tentatively linked 229E and OC43 infections to severe respiratory tract illness over many years [48], no study controlling for age and month of illness has demonstrated an epidemiologic association between infection with these HCoVs and any illness other than the common cold. Also, no study has demonstrated an epidemiologic association between NL63 and HKU1 infection and severe respiratory tract illness in relation to asymptomatic control subjects. Two studies did find NL63 and HKU1 in bronchoalveolar lavage specimens from patients with severe illness [49, 50].
In temperate regions, HCoV infections occur most frequently in late winter and early spring [7, 8, 17, 20]. However, there are few reports describing the seasonal circulation patterns of these viruses in the tropics [18, 23]. HCoV infections were detected year-round in the present study. However, during study year 1, OC43 infections appeared to cluster from January through March. In contrast, influenza virus infections clustered from June through October in Sa Kaeo Province [39]. There was a statistically significant difference in the number of OC43 infections between year 1 and year 2, suggesting year-to-year variation in strain circulation. Previous multiyear studies [6, 8–11] in temperate regions have made similar observations.
Our study has some limitations. First, the number of HCoV infections detected among patients with pneumonia was likely underestimated. Only 55%–59% of patients admitted to a Sa Kaeo Province hospital with signs and symptoms of clinical pneumonia had a CXR taken, and only 37%–51% of these were enrolled in the study; children and severely ill patients were less likely to enroll. Second, adults were underrepresented in the outpatient sample. Third, we found a high proportion of patients infected with HCoV to be coinfected with other viruses, especially among those infected with NL63, making it difficult to determine the responsible etiology; however, we tested for more viruses than have other studies [26]. Finally, we cannot exclude the possibility that some control patients may have been shedding HCoV following an earlier symptomatic infection. Using sensitive PCR assays, van Elden et al. [26] reported detecting HCoV 14 days after illness. Future studies that include control subjects should account for this possibility.
Well-planned studies are needed to evaluate whether newly described viruses are epidemiologically associated with severe respiratory tract illness. We sought to determine whether infections with new and previously recognized HCoV types were associated with pneumonia requiring hospitalization in Thailand. We found very low numbers of HCoV infections and failed to find an association with severe respiratory tract disease. However, because year-to-year variation in overall prevalence and type of circulating HCoV can occur, a multiyear study with appropriate control subjects will be necessary to adequately assess whether there is an association between HCoV infection and severe respiratory disease.
Acknowledgments
We thank the following people who provided HCoV RNA for assay validation: Dr. Lia van der Hoek (University of Amsterdam, Amsterdam, The Netherlands), Dr. Nancy Bellei (University of Sao Paulo, Sao Paulo, Brazil), Dr. Atul Humar (Mount Sinai Hospital, Toronto, Canada), and Dr. Ian Mackay (Royal Children's Hospital, Queensland, Australia). We also thank George Gallucci, Brian Holloway, Xiaoyan Lu, and Karen McCaustland (CDC, Atlanta, Georgia) for technical assistance; Pongpun Sawatwong (Thailand Ministry of Public Health—US CDC Collaboration) for laboratory assistance; and the surveillance officers and research nurses in Sa Kaeo Province, Thailand.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 22nd Annual Clinical Virology Symposium, Clearwater Beach, FL, 30 April–3 May 2006 (abstract T-PM16).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
References
- 1.Holmes KV. Coronaviruses. In: Fields B, Knipe D, Howley P, et al., editors. Fields virology. 4th ed. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1187–203. [Google Scholar]
- 2.Hamre D, Procknow JJ. A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med. 1966;121:190–3. doi: 10.3181/00379727-121-30734. [DOI] [PubMed] [Google Scholar]
- 3.McIntosh K, Becker WB, Chanock RM. Growth in suckling-mouse brain of "IBV-like" viruses from patients with upper respiratory tract disease. Proc Natl Acad Sci USA. 1967;58:2268–73. doi: 10.1073/pnas.58.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyrrell DA, Bynoe ML. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. 1965;1:1467–70. doi: 10.1136/bmj.1.5448.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradburne AF, Bynoe ML, Tyrrell DA. Effects of a "new" human respiratory virus in volunteers. Br Med J. 1967;3:767–9. doi: 10.1136/bmj.3.5568.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradburne AF, Somerset BA. Coronative antibody tires in sera of healthy adults and experimentally infected volunteers. J Hyg (Lond) 1972;70:235–44. doi: 10.1017/s0022172400022294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavallaro JJ, Monto AS. Community-wide outbreak of infection with a 229E-like coronavirus in Tecumseh, Michigan. J Infect Dis. 1970;122:272–9. doi: 10.1093/infdis/122.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaye HS, Dowdle WR. Seroepidemiologic survey of coronavirus (strain 229E) infections in a population of children. Am J Epidemiol. 1975;101:238–44. doi: 10.1093/oxfordjournals.aje.a112091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaye HS, Marsh HB, Dowdle WR. Seroepidemiologic survey of coronavirus (strain OC 43) related infections in a children's population. Am J Epidemiol. 1971;94:43–9. doi: 10.1093/oxfordjournals.aje.a121293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntosh K, Kapikian AZ, Turner HC, Hartley JW, Parrott RH, Chanock RM. Seroepidemiologic studies of coronavirus infection in adults and children. Am J Epidemiol. 1970;91:585–92. doi: 10.1093/oxfordjournals.aje.a121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenzel RP, Hendley JO, Davies JA, Gwaltney JM., Jr Coronavirus infections in military recruits: three-year study with coronavirus strains OC43 and 229E. Am Rev Respir Dis. 1974;109:621–4. doi: 10.1164/arrd.1974.109.6.621. [DOI] [PubMed] [Google Scholar]
- 12.Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191:492–8. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouchier RA, Hartwig NG, Bestebroer TM, et al. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci USA. 2004;101:6212–6. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Hoek L, Pyrc K, Jebbink MF, et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–73. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo PC, Lau SK, Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–95. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arden KE, Nissen MD, Sloots TP, Mackay IM. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J Med Virol. 2005;75:455–62. doi: 10.1002/jmv.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastien N, Robinson JL, Tse A, Lee BE, Hart L, Li Y. Human coronavirus NL-63 infections in children: a 1-year study. J Clin Microbiol. 2005;43:4567–73. doi: 10.1128/JCM.43.9.4567-4573.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu SS, Chan KH, Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. 2005;40:1721–9. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vabret A, Mourez T, Dina J, et al. Human coronavirus NL63, France. Emerg Infect Dis. 2005;11:1225–9. doi: 10.3201/eid1108.050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Hoek L, Sure K, Ihorst G, et al. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005;2:e240–e240. doi: 10.1371/journal.pmed.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Coronavirus HKU1 infection in the United States. Emerg Infect Dis. 2006;12:775–9. doi: 10.3201/eid1205.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau SK, Woo PC, Yip CC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–71. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo PC, Lau SK, Tsoi HW, et al. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J Infect Dis. 2005;192:1898–907. doi: 10.1086/497151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi D, Johnson G, Draker R, et al. Comprehensive detection and identification of human coronaviruses, including the SARS-associated coronavirus, with a single RT-PCR assay. J Virol Methods. 2004;122:29–36. doi: 10.1016/j.jviromet.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myint S, Johnston S, Sanderson G, Simpson H. Evaluation of nested polymerase chain methods for the detection of human coronaviruses 229E and OC43. Mol Cell Probes. 1994;8:357–64. doi: 10.1006/mcpr.1994.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Elden LJ, van Loon AM, van Alphen F, et al. Frequent detection of human coronaviruses in clinical specimens from patients with respiratory tract infection by use of a novel real-time reverse-transcriptase polymerase chain reaction. J Infect Dis. 2004;189:652–7. doi: 10.1086/381207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vijgen L, Keyaerts E, Moes E, Maes P, Duson G, Van Ranst M. Development of one-step, real-time, quantitative reverse transcriptase PCR assays for absolute quantitation of human coronaviruses OC43 and 229E. J Clin Microbiol. 2005;43:5452–6. doi: 10.1128/JCM.43.11.5452-5456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emery SL, Erdman DD, Bowen MD, et al. Real-time reverse transcription-polymerase chain reaction assay for SARS-associated coronavirus. Emerg Infect Dis. 2004;10:311–6. doi: 10.3201/eid1002.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitkaranta A, Virolainen A, Jero J, Arruda E, Hayden FG. Detection of rhinovirus, respiratory syncytial virus, and coronavirus infections in acute otitis media by reverse transcriptase polymerase chain reaction. Pediatrics. 1998;102:291–5. doi: 10.1542/peds.102.2.291. [DOI] [PubMed] [Google Scholar]
- 30.Kumar D, Erdman D, Keshavjee S, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5:2031–6. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, Mackay IM. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol. 2006;35:99–102. doi: 10.1016/j.jcv.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo PC, Lau SK, Yip CC, et al. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J Virol. 2006;80:7136–45. doi: 10.1128/JVI.00509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shang L, Qi Y, Bao QY, et al. Polymorphism of SARS-CoV genomes. Yi Chuan Xue Bao. 2006;33:354–64. doi: 10.1016/S0379-4172(06)60061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thiel V, Herold J, Schelle B, Siddell SG. Infectious RNA transcribed in vitro from a cDNA copy of the human coronavirus genome cloned in vaccinia virus. J Gen Virol. 2001;82:1273–81. doi: 10.1099/0022-1317-82-6-1273. [DOI] [PubMed] [Google Scholar]
- 35.Vijgen L, Keyaerts E, Moes E, et al. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J Virol. 2005;79:1595–604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen SJ, Laosiritaworn Y, Siasiriwattana S, Chunsuttiwat S, Dowell SF. The incidence of pneumonia in rural Thailand. Int J Infect Dis. 2006;10:439–45. doi: 10.1016/j.ijid.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu X, Chittaganpitch M, Olsen SJ, et al. Real-time PCR assays for detection of bocavirus in human specimens. J Clin Microbiol. 2006;44:3231–5. doi: 10.1128/JCM.00889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fry AM, Lu X, Chittaganpitch M, et al. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–45. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simmerman JM, Lertiendumrong J, Dowell SF, et al. The cost of influenza in Thailand. Vaccine. 2006;24:4417–26. doi: 10.1016/j.vaccine.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 40.Erdman DD, Weinberg GA, Edwards KM, et al. GeneScan reverse transcription-PCR assay for detection of six common respiratory viruses in young children hospitalized with acute respiratory illness. J Clin Microbiol. 2003;41:4298–303. doi: 10.1128/JCM.41.9.4298-4303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 42.Miller EK, Lu X, Erdman DD, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–81. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuypers J, Martin ET, Heugel J, Wright N, Morrow R, Englund JA. Clinical disease in children associated with newly described coronavirus subtypes. Pediatrics. 2007;119:e70–6. doi: 10.1542/peds.2006-1406. [DOI] [PubMed] [Google Scholar]
- 44.Theamboonlers A, Samransamruajkit R, Thongme C, Amonsin A, Chongsrisawat V, Poovorawan Y. Human coronavirus infection among children with acute lower respiratory tract infection in Thailand. Intervirology. 2006;50:71–7. doi: 10.1159/000097392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bastien N, Anderson K, Hart L, et al. Human coronavirus NL63 infection in Canada. J Infect Dis. 2005;191:503–6. doi: 10.1086/426869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIntosh K, Chao RK, Krause HE, Wasil R, Mocega HE, Mufson MA. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis. 1974;130:502–7. doi: 10.1093/infdis/130.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nokso-Koivisto J, Kinnari TJ, Lindahl P, Hovi T, Pitkäranta A. Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J Med Virol. 2002;66:417–20. doi: 10.1002/jmv.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McIntosh K. Commentary: McIntosh K, Chao RK, Krause HE, Wasil R, Mocega HE, Mufson MA. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis 1974; 130:502–7. J Infect Dis. 2004;190:1033–41. doi: 10.1086/422851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garbino J, Crespo S, Aubert JD, et al. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (non-SARS)-related human coronavirus infection. Clin Infect Dis. 2006;43:1009–15. doi: 10.1086/507898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerna G, Percivalle E, Sarasini A, et al. Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients. J Clin Virol. 2007;38:244–50. doi: 10.1016/j.jcv.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]