Abstract
Surface antigen one (Sao) is a newly identified protein from the major zoonotic pathogen, Streptococcus suis. In search of functional proteins related to the pathogenesis of Chinese S. suis 2 (SS2), unexpectedly, a variant of Sao protein was obtained. To test its prevalence in S. suis, PCR assay was adopted to address the coding genes systematically. It was found that there are three allelic variants of sao gene, namely sao-S, sao-M, and sao-L based on the different lengths of the genes (∼1.5, ∼1.7, and ∼2.0 kb, respectively). These differences were determined to be caused by heterogeneity within the number of C-terminal repeat sequences (R), which had been seen as a pathogenicity-related domain in the plant pathogen, Xanthomonas oryzae. Two variants (sao-M and sao-L) were only found in SS2. All three variant proteins were prepared in vitro and their biochemical and biophysical properties were characterized. A soluble form of Sao-M protein was then used as a capture antigen to develop an enzyme-linked immunosorbent assay method to detect antibodies against SS2 in convalescent pig sera. Taken together, the results exhibit the properties of Sao proteins and provide an efficient Sao-M-based method for monitoring SS2 infection.
Keywords: Streptococcus suis, Sao protein, allelic variants, antibody, ELISA
Introduction
Streptococcus suis infection of swine is recognized as the leading cause of highly invasive diseases such as meningitis, septicemia, arthritis, and even sudden death, and has spread over 20 countries, resulting in great economic losses in pig-industries each year (Staats et al., 1997; Huang et al., 2005). Streptococcus suis is also a notorious zoonotic agent responsible for more than 200 cases of severe infections in humans worldwide since its first discovery in Denmark in 1968 (Staats et al., 1997). Thirty-five serotypes of S. suis have been determined based on differences among their capsule antigens (Staats et al., 1997). However, Hill (2005) recently suggested that the two serotypes (serotype 32 and serotype 34) may be excluded from S. suis species and redesignated as Streptococcus orisratti. Virulence varies greatly among the serotypes and can be divided into three groups: highly pathogenic, hypovirulent, and avirulent (Staats et al., 1997). Streptococcus suis 2 (SS2) is the most prevalent serotype isolated from both diseased piglets and patients worldwide and is often associated with severe clinical syndrome (Staats et al., 1997). In particular, two large-scale outbreaks of lethal SS2 infections with a hallmark of streptococcal toxic shock syndrome (STSS) emerged in China (one in Jiangsu Province, 1998, and the other in Sichuan Province, 2005), posing a great global concern to the public health (Tang et al., 2006). As it is known that human SS2 infections have been documented for several decades, the molecular mechanism underlying the high pathogenicity of this bacterial agent is still poorly understood (Staats et al., 1997; Gottschalk et al., 1999). Several bacterial components have been previously implicated in S. suis virulence, but the exact role remains unclear (Staats et al., 1997; Gottschalk et al., 1999; Gottschalk & Segura, 2000). So far, the virulence-associated factors identified include components of capsule polysaccharide (CPS) (Smith et al., 2000), extracellular factor (EF) (Smith et al., 1993; Staats et al., 1999), muraminidase-released protein (MRP) (Staats et al., 1999), suilysin (Allen et al., 2001; Lun et al., 2003), adhesions (Tikkanen et al., 1996; Brassard et al., 2004), 38 kDa protein (Okwumabua & Chinnapapakkagari, 2005), glutamate dehydrogenase (GDH) (Okwumabua et al., 2001), fibronectin-binding protein (FBP) (de Greeff et al., 2002; Wang, 2006), hayluronate lyase (King et al., 2004), sortase A (srtA) (Osaki et al., 2002), arginine deiminase (Gruening et al., 2006), etc. In some instances, the virulence determination of some S. suis strains was still ambiguous, indicating that the virulence of S. suis is a complex interplay between the pathogen, its host, and the environment (Gottschalk et al., 1999). Considerable efforts to control severe SS2 infections have still been hampered greatly by the limited knowledge of S. suis pathogenesis (Staats et al., 1997; Gottschalk et al., 1999; Gottschalk & Segura, 2000; Haesebrouck et al., 2004).
It is widely accepted that the cell-wall-exposed proteins/outer surfaces of pathogenic bacteria are of great importance to understanding their pathogenesis (Navarre & Schneewind, 1999; Cabanes et al., 2002). Not only are surface-associated components implicated in bacterial defense machineries but also they are involved in virulence-related behaviors (e.g., adhesion) (Navarre & Schneewind, 1999; Maione et al., 2005). Thus, for vaccine development against pathogenic bacteria, current interest has shifted from the CPS antigen to surface proteins with robust immunogenicity (Navarre & Schneewind, 1999; Cabanes et al., 2002; Maione et al., 2005; Li et al., 2006). Whole genome-wide screening was carried out to search for a universal Group B Streptococcus (GBS) vaccine (Maione et al., 2005). These surface proteins containing a C-terminal cell wall anchoring motif (LPXTG) have been found in a variety of pathogenic microorganisms, and suggested to execute key steps during the process of infection, which range from colonization to invasion (Navarre & Schneewind, 1999; Cabanes et al., 2002; Osaki et al., 2002; Maione et al., 2005). In S. suis, MRP belongs to this type of surface protein (Smith et al., 1992). More recently, Li (2006) reported a novel surface protein, surface antigen one (Sao), from S735 strain of SS2, and evaluated its potential as a vaccine. Although it failed to protect piglets against SS2 infection completely, it was not determined whether or not this protein played any role in S. suis infection.
The availability of the whole genomes of SS2 Chinese strains allows to mine the functional proteins related to high virulence (Chen et al., 2007). During a genome-wide in silico screening for surface-exposed proteins or cell wall-associated proteins, a mutant of sao was accidentally found, in which 270 bp of repeated sequences at the 3′-terminus was deleted. In light of this unexpected observation, it was attempted to examine the prevalence in various serotypes of S. suis, and test its possible genetic variation or molecular polymorphism. Thus, PCR screening was undertaken to test more than 50 different S. suis strains comprising 34 kinds of serotypes (except for S. suis 12). It was found that Sao proteins exhibit obvious polymorphisms with considerable genetic variation. Furthermore, it was possible to classify Sao into three groups and the proteins were also characterized via a range of biochemical techniques. Immunological data from Western blotting and enzyme-linked immunosorbent assay (ELISA) also demonstrated that Sao-M, the most common type of Sao, has strong immunogenicity. Sao-M has been successfully developed into an effective ELISA method for monitoring S. suis infection in both pigs and humans. Therefore, Sao may serve as a useful marker for clinical surveillance of S. suis infection.
Materials and methods
Strains, plasmids, and culture conditions
The reference strains of S. suis (34 kinds of serotypes) were kindly provided by Prof. Marcelo Gottschalk in Canada and Prof. Astrid de Greeff in Holland. The other SS2 isolates were all kept in the authors' laboratory (listed in Table S1). Streptococcus suis were cultivated in Todd–Hewitt Broth (THB, code CM189; Oxoid) at 37°C for preparing chromosomal DNA as a PCR template (Tang et al., 2006). Escherichia coli strains DH5α and BL21 (DE3) were maintained in Luria–Bertani (LB) broth or agar medium at 37°C for recombinant plasmid amplification and protein expression, respectively (Liu et al., 2006). The commercial pMD18-T vector (Takara) and pET28a (Novagen) were utilized to clone PCR fragments for direct sequencing of sao genes, and construct recombinant expression plasmids, respectively.
Molecular manipulation
Streptococcus suis genomic DNAs were extracted using the routine CTAB method as described by Tang (2006), and their size and quality were evaluated by electrophoresis on a 0.8% agarose gel (Brazil) before they were used as templates of PCR amplification for sao. If necessary, two sets of primers specific for S. suis house-keeping genes, 16S rRNA gene and gyrase, were utilized to further assess the quality of DNA templates.
To amplify sao genes, the specific primers (S-F: 5′-ATGAATACTAAGAAATGGAG-3′; S-R: 5′-TTATAATTTACGTTTACGTGT-3′) were designed, which, according to the available sequence information, cover the entire putative ORF. Subsequently, the standard PCR in a total volume of 50 µL was conducted in a PTC-225 thermocycler (MJ Research) using ExTaq (Takara). All the candidate PCR products were ligated into a pMD18-T vector (Takara) as the recommended protocol to obtain unique clones for direct DNA sequencing by an ABI 3730 DNA sequencer (Perkin-Elmer Applied Biosystems).
To prepare the soluble recombinant Sao proteins, it was attempted to construct three plasmids using PCR products generated with the primers (Sao-F: 5′-CG GGATCC CAAGAAGTAAAAAATACCATC-3′; Sao-R: 5′-CCAA GTCGAC TTATTTCTCACCAGTTACTGG-3′). The primers introduced BamHI and SalI sites into the truncated DNA fragments. The truncated DNA fragments from three sao variants (88–1938 bp of sao-L, 88–1668 bp of sao-M, and 88–1395 bp of sao-S) equivalent to mature extra-domains (30–646 aa of Sao-L, 30–556 aa of Sao-M, and 30–465 aa of Sao-S) were introduced into the BamHI and XhoI sites of pET28a (Novagen). Finally, the resulting recombinant plasmids, designated pET28∷Sao-L, pET28∷Sao-M, and pET28∷Sao-S, were all verified to be in frame with the initiation codon of the vector and to have the expected sequence by direct DNA sequencing.
Production of soluble Sao proteins
To obtain soluble versions of the Sao proteins, a general procedure was performed as described earlier in the authors' laboratory with some minor modifications (Liu et al., 2006). First, a single colony from BL21(DE3) transformants was inoculated in LB medium containing 50 mg L−1 kanamycin (Sigma) at 37°C and grown overnight. Then, the culture was diluted 1 : 100 in 2 L of fresh LB medium and incubated at 37°C. When the culture density (OD600 nm) reached 0.8–1.0, the culture was induced with 0.8 mM isopropyl-β-d-thiogalactopyranoside (Sigma) and kept for about 4–5 h at 37°C until the bacterial cells were collected by centrifugation at 2400 g. Third, the harvested bacterial pellet was suspended in chilled phosphate-buffered saline (PBS) and lysed by sonication. The extract was centrifuged at 16 200 g for 20 min at 4°C, and subsequently filtered through a 0.22 µm membrane for clarification. Finally, the supernatant was loaded onto the nickel-ion (Ni+) affinity column (Qiagen), and the N-terminus 6 × histine-tagged Sao proteins were eluted in elution buffer with 100 mM imidazole after removing the contaminant proteins. The proteins of interest were visualized on a 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and stained with Coomassie brilliant blue R250 (Sigma, St Louis, MO).
Biochemical characterization of Sao proteins
To further characterize Sao variants, FPLC or gel filtration, chemical cross-linking, and circular dichroism (CD) were utilized as described earlier (Ma et al., 2005; Liu et al., 2006).
First, SDS-PAGE was performed according to the standard procedure (Liu et al., 2006). Then, for the analysis by gel filtration, the acquired recombinant Sao proteins were loaded on a Superdex 75 column (Pharmacia) with an AKTA Purifier System (Pharmacia) after they were concentrated by ultra-filtration (10 kDa cutoff) and exchanged from 1 × PBS buffer into the exclusion buffer. The peak fractions were collected and analyzed by 12% SDS-PAGE, and the apparent molecular weights (MW) were estimated by comparison with the standard protein marker (Sangon, Shanghai) run on the same gel.
Second, to discriminate the solution structures of the different Sao proteins in vitro, a chemical cross-linking assay was adopted. Briefly, Sao protein purified from gel filtration were dialyzed against a cross-linking buffer (50 mM HEPES, pH8.3; 100 mM NaCl) and concentrated to c. 10 mg L−1 by ultra-filtration (10 kDa cutoff). Then, the resultant protein was subjected to a chemical cross-linking reaction with ethylene glycol bis-succinimidylsuccinate (EGS) (Pierce). The reactions were incubated for 1 h on ice at different concentrations of EGS, respectively (0, 1.0, and 5.0 mM EGS), and quenched with 50 mM glycine. Eventually, the cross-linked samples were analyzed by 12% SDS-PAGE (Ma et al., 2005; Liu et al., 2006).
Lastly, to acquire information on the secondary structures of these proteins, CD spectra were recorded on a Jasco J-715 spectrophotometer in a 0.1 cm path-length cuvette consisting of a 1 × PBS buffer at 25°C (Ma et al., 2005; Liu et al., 2006). The records were the mean of triplicate independent experiments.
Immunogenic evaluation of Sao protein
Western blotting and ELISA were used to test the immunogenicity of Sao. Here, negative swine sera were sampled from SPF-pigs and convalescent-phase swine sera were collected from those piglets that had survived infection by S. suis ZY05.
For Western blotting, the soluble Sao-M protein was subjected to 10% SDS-PAGE, transferred onto a nitrocellulose membrane (Amersham Pharmacia Biotech), and blocked with 5% skim milk in TBST [50 mM Tris (pH 7.3), 150 mM NaCl, 0.1% Tween 20] at room temperature (RT) for 1 h. The membrane was incubated with convalescent-phase swine sera in a 1 : 200 dilution in TBST at RT for 1 h, which was followed by the incubation with HRP-conjugated antiswine antibody (Santa Cruz) in a 1 : 5000 dilution in TBST at RT for 1 h.
To probe whether Sao protein is associated with S. suis infection, an Sao-based ELISA was performed. Ninety-six-microtube ELISA plates (Greiner bio-one, Germany) were coated overnight at 4°C with 100 µL well−1 of purified Sao protein at an appropriate concentration in carbonate buffer. After three washes with TBST, the plates were blocked with 5% skim milk in TBST for 1 h at 37°C. Next, several pig sera diluted in 1 : 1000 in TBST were added to appropriate wells in triplicate at 100 µL well−1, incubated for 1 h at 37°C, and washed three times. Then, the bound antibodies against Sao were detected by incubation for 1 h at 37°C with HRP-conjugated goat antiswine IgG (Sigma). These plates were developed with O-phenylenediamine as a substrate (Amresco) and H2O2 (Sigma) as the oxidation agent; 1 M sulfuric acid was used to stop the reaction. The absorbance score was measured at 490 nm in a micro-plate reader (Model 500; Bio-rad). The results were expressed as means±standard deviations, and statistical significance was determined by Student's t test (Li et al., 2006).
Bioinformatics analysis
All the sequencing results of different sao genes (submitted to GenBank) were assembled with Vector NTI Suite 8.0 (Invitrogen). Also, with the aid of Vector NTI Suite 8.0, multiple sequence alignments of these genes were carried out. The phylogenetic tree of Sao proteins was constructed by the neighbor-joining method. A blastp search was performed in the protein database of Genbank.
Results
Distribution of different sao genes in S. suis
Prompted by the unexpected finding of strains carrying sao gene variants, it was attempted to determine the range of sao variants and correlate variant prevalence with the serotype of S. suis. More than 50 different S. suis strains covering nearly all of the serotypes were cultivated for isolation of genomic DNA. The results of PCR screening yielded three distinct sao amplicons (Fig. 1a). Among those strains tested, the following conclusions could be drawn. First, sao is relatively common in different serotypes and/or strains of S. suis. Although PCR-negative results were obtained in several isolates such as Denmark strains 6704, 4417, 22083, etc. (Table S1, Fig. 2), this was largely in agreement with the Western blot results described by Li (2006). Second, according to various lengths of genes, sao could be referred to as sao-L (∼2.0 kb, L: long), sao-M (∼1.7 kb, M: middle), and sao-S (∼1.5 kb, S: small) (Fig. 1a). These data also suggested that sao-M is the most prevalent variant comprising about 80%, followed by sao-L, and sao-S is detected at no more than 6% (Fig. 2).
1.

Three types of Sao proteins from Streptococcus suis. (a) PCR identification of sao variants. M: 100 bp DNA Ladder (Fermentas, Vilnius, Lithuania). sao-L: sao in ∼2.0 kb; sao-M: sao in ∼1.7 kb; sao-S: sao in ∼1.5 kb. (b) In vitro expression analysis of the three Sao proteins. M: protein MW standard marker (Sangon, Shanghai, China). (c) Multiple-sequence alignment of the repeated regions from different Sao proteins. Three types of Sao proteins are aligned using the software of Vector NTI Suite 8.0. The N-terminal sequences were found to be nearly identical, while the C-terminal regions were discontinuously well matched. Here, the focus is on the variations in the C-terminus and this information is presented as described by Li (2006). R1, R2, R3 … R10: Repeated region1, 2, 3 … 10 in the C-terminus of Sao. S735: Holland isolate of S. suis 2 (Sao-L); SS1: Holland strain 5428 of S. suis 1 (Sao-S); SS3: Holland strain 4961 of S. suis 3 (Sao-L); ZY05: a highly virulent strain of SS2 from Ziyang County of Sichuan Province in China, 2005 (Sao-M). (d) Linear representation of three types of Sao proteins. The vertical arrow indicates the predicted signal peptidase cleavage site. The ellipses in yellow and red correspond to the hydrophobic region at the N-terminus and the charged amino acids of the C-terminal tail. The curved blue boxes refer to the possible cell wall-associated regions. The dashed lines represent the deletion of repeated regions. TM refers to the possible trans-membrane region at the C-terminus.
2.
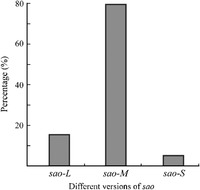
Distribution of different sao genes in Streptococcus suis. The PCR technique is utilized to test the prevalence of the sao gene among various strains of S. suis. About 75% of all the strains tested here can be confirmed to be PCR positive (also seen in Table S1). Three types of sao genes (sao-L, sao-M, and sao-S) have been drawn according to the different sizes of the PCR fragments. The proportion of the above three sao types is measured based on the samples collected in this study.
Molecular characterization of Sao
To further characterize sao variation at the molecular level, numerous representative PCR products were cloned into a pMD-T vector, and subjected to DNA direct sequencing on an ABI sequencer 3730. Multiple sequence alignments of these genes indicated that although point mutations are relatively common (not shown), the major cause of the variation could be attributed to deletions within the 3′ end (Fig. 1c and d). Phylogenetic analysis suggested molecular polymorphism as well (not shown). Various numbers of repeated units (27 aa U−1) (Fig. 1c) were identified in a series of Sao proteins. Furthermore, nearly all the deletion events converged in the repeat regions at the C′-terminus of Sao (Fig. 1c and d). For this reason, the repeated regions were used as a query for a blastp search of the Genbank database and it was found that repeated regions matched well with the avirulence domain, AvaXa7, in the plant pathogen, Xanthomonas oryzae pv. oryzae, a principal causative agent of rice blight (not shown). This validated strongly a similar finding given by Li (2006).
Soluble expression of Sao proteins in vitro
To obtain Sao proteins for further analysis, the pET28a/BL21(DE3) expression system was applied to express the fragment of sao lacking the two hydrophobic domains. Fortunately, soluble forms of the three Sao proteins were successfully expressed (Fig. 1b). However, these proteins behaved abnormally in SDS-PAGE. The apparent MW of the Sao proteins purified by affinity chromatography were considerably larger than those estimated by deduction from the DNA sequences (Fig. 1b and Table 1). Meanwhile, it was observed that the size error of Sao-S was much less than that of Sao-L in the SDS-PAGE profile (Fig. 1b and Table 1).
1.
Biochemical properties of different versions of Sao
| Sao types | Strains | Serotype | Length (aa) | Predicted IP | Estimated MW (kDa) | Apparent MW (kDa) |
| Sao-L | 4961 | 3 | 670 | 4.8 | 74.8 | ∼130 |
| Sao-M | ZY05 | 2 | 580 | 4.8 | 64.6 | ∼110 |
| Sao-S | 5428 | 1 | 489 | 5.0 | 54.5 | ∼60 |
The apparent MW was estimated on the basis of migratory rates of the equivalent Sao proteins in 12% SDS-PAGE.
IP:, isoelectric point; MM, molecular weight.
Biochemical properties of Sao proteins
To better understand the biochemical properties of three Sao proteins, each protein was analyzed by gel filtration, chemical cross-linking, and CD. The three Sao proteins (Sao-L, Sao-M, and Sao-S) were eluted from a Superdex-200 column at ∼11.8 mL (Fig. 3a), ∼12.8 mL (Fig. 3b), and ∼13.1 mL (Fig. 3c), respectively, and were resolved from one another almost completely (95%). Also, the elution volumes of the Sao proteins suggested that they are monomeric in solution, which was supported by the chemical cross-linking experiments (Fig. 3d). CD experiments presented preliminary information on the protein secondary structure for Sao proteins (not shown).
3.

Biochemical characterization of Sao proteins. (a), (b), and (c) FPLC profiles of three types of Sao proteins (Sao-L, Sao-M, and Sao-S). They are run on a Superdex 200 column, and the arrows indicate their respective elution volumes. (d) Chemical cross-linking of Sao proteins. 0, 1.0, and 5.0 correspond to the mM concentration of EGS. M: protein MW standard marker (Sangon, Shanghai, China).
Development of a Sao-M-based ELISA method and its application
Because it had been determined that Sao-M was the most common form of Sao in S. suis, this variant was subjected to immunological evaluation. Western blotting demonstrated that Sao-M reacts strongly with convalescent-phase sera from pigs clinically infected by SS2 (Fig. 4a). As a further confirmation, sera were sampled from SPF-pigs (negative control) and piglets after SS2 infection. Then, ELISA was performed and the specificity of convalescent-phase sera for Sao-M was confirmed (Fig. 4b).
4.

Development of a Sao-M-based ELISA method for monitoring of Streptococcus suis infection. (a) Western blotting of recombinant Sao-M with the convalescent-phase sera clinically infected by S. suis ZY05. (b) ELISA analysis of recombinant Sao-M with negative swine serum and swine serum postinfection. The results are expressed as means of absorbance values with standard errors (SE). P≤0.01. (c) Sao-based ELISA assay of swine serological samples. +: positive serum confirmed in laboratory. −: negative serum confirmed in laboratory. Samples from 1 to 25 are those sera collected from field pig farms or slaughterhouses. The dashed line is used to indicate the background of the sera samples. The results are expressed as means of absorbance values with SEs. *P≤0.01.
Given the robust immunogenicity of Sao-M, next, it was of interest to evaluate the potential of Sao-M as a diagnostic antigen for investigation of S. suis infection. A Sao-M protein-based ELISA method was used successfully to monitor sera from S. suis-infected animals. Based on the results obtained from numerous samples, it was determined that the optimal conditions for the assay consisted of 1 µg mL−1 of Sao-M, sera diluted 1 : 1000, and 1 : 1000 diluted HRP-conjugated goat anti-swine secondary antibody. The ELISA results demonstrated that the assay could unambiguously distinguish between the positive, suspicious, and negative samples (Fig. 4c). Therefore, this method shows promise for application in laboratory, clinical, and even field monitoring of S. suis infection in the swine-industry. This could help establish prevention and alarm systems for dealing with outbreaks of zoonotic diseases (such as meningitis, septicemia, arthritis, etc.) caused by S. suis infections of humans.
Discussion
Streptococcus suis causes a series of severe invasive diseases in piglets, with substantial economic impact upon the swine-industry (Staats et al., 1997; Huang et al., 2005). Recently, S. suis infection garnered great attention worldwide due to public health concerns over its potential to cause serious zoonotic infections with associated clinical syndromes such as septicemia, meningitis, STSS, etc. (Gottschalk & Segura, 2000; Huang et al., 2005; Tang et al., 2006). The conventional strategy to control S. suis infections is overly dependent on prophylactic and therapeutic antibiotics, which has led to the isolation of antibiotic-resistant strains in recent years (Huang et al., 2005; Tang et al., 2006). Also, a lack of data describing the virulence factors contributing to the pathogenesis of S. suis has greatly hampered the development of novel vaccines and pharmaceutics against S. suis infections (Staats et al., 1997; Gottschalk et al., 1999). Thus, there is currently considerable interest in identifying and characterizing surface-exposed virulence factors of S. suis. Recently, a promising candidate called Sao has been described. Sao is a novel C-terminally anchored surface protein identified from S735, a virulent Holland strain (Li et al., 2006).
Sao was found from an in silico search of surface-exposed proteins in the S. suis genome. Based on the search, a deletion mutant of sao could be identified that had 270 bp of repeated sequences missing from its 3′-terminus. Then, 34 serotypes of S. suis were examined and three forms of sao genes were found among the different strains. Although sao genes could not be amplified from several S. suis strains (e.g., 10581, 2726, 865191, etc.), the absence of sao genes might have been caused by the considerable variation during the evolution of these serotypes. This was also validated by the result of a Western blot described by Li (2006). Although the existence of sao genes in a few strains (e.g., 4417) could not be detected despite positive results at the protein level (Li et al., 2006), other approaches will be attempted to determine the reasons for this discrepancy. The most frequently observed form of the sao gene was sao-M, which is the medium-sized version at ∼1.7 kb. The results resemble those reported by Smith (1993). In that study, the authors also found EF, a derivative of EF* lacking the repeat units at its C-terminus, and was correlated with increased virulence (Smith et al., 1993). Therefore, it would be of interest to determine whether or not the present deletion variants in Sao proteins have any correlation with their pathogenicity of the organisms. The aberrantly larger apparent MW of Sao-L and Sao-M proteins, can be surmised to be due to the poor binding of SDS, which gives less charge density to the fragments than to the standard proteins and hence a higher apparent MW.
The homology of the Sao protein to the avirulence domain of a prominent plant pathogen (AvrXa7) (not shown) also hints at a role of the high invasiveness and/or virulence of S. suis (Yang et al., 2000). In the case of avr in plant pathogenesis, this gene is associated with a hypersensitive response of the plant towards the pathogens (Yang et al., 2000). As such, several questions regarding Sao variants require further investigation. These are (1) is there an analogous host R protein binding to the Avr-like repeats of Sao? (2) Is a type III secretion system involved? (3) Does Sao function as a superantigen similar to what occurs with Staphylococcus aureus (Banks et al., 2003) and Streptococcus pyogenes (GAS) (Alouf & Muller-Alouf, 2003)?
Sao also shows great promise as a diagnostic antigen. Based on the ELISA results with Sao-M, the protein was found to be very immunogenic and very discriminatory between uninfected and convalescent animals. This should be of great utility for monitoring samples in the laboratory, clinic, and field, which may prevent the further spread of this emerging pathogen.
Authors' contribution
Y.F., F.Z., and X.P. contributed equally to this work.
Acknowledgements
The authors thank Prof. John Cronan Jr at Department of Microbiology, University of Illinois at Urbana-Champaign in USA, Dr Merritt Justin at University of Oklahoma Health Sciences Center in USA, and Prof. Min Wu at University of North Dakota in USA for critically reading the manuscript. The authors are grateful to both Prof. Marcelo Gottschalk in Canada and Prof. Astrid de Greeff in the Netherlands for kindly providing many reference strains of S. suis. This work was supported by national basic research program (973) of Ministry of Science and Technology (MOST) of China (2005CB523001) to Prof. George F. Gao, national project of tackling key problems of the MOST (2003BA712A03-05) and a grant from the National Natural Science Foundation of China (NSFC) (30670105) to Prof. Jiaqi Tang, and a grant from NSFC (30600533), and the Natural Science Fund of Jiangsu Province, China (BK2006014), to Dr Changjun Wang, and National Key Technologies R&D Program (2006BAD06A04) to Prof. Jinghua Yan. George F. Gao is a distinguished young investigator of the NSFC (Grant No. 30525010).
Supplementary material
The following supplementary material is available for this article online:
Table S1. PCR investigation of sao distributed in S. suis.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1574-6968.2007.00859.x (This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Allen A.G., Bolitho S., Lindsay H., et al. (2001) Generation and characterization of a defined mutant of Streptococcus suis lacking suilysin. Infect Immun 69: 2732–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alouf J.E., Muller-Alouf H. (2003) Staphylococcal and streptococcal superantigens: molecular, biological and clinical aspects. Int J Med Microbiol 292: 429–440. [DOI] [PubMed] [Google Scholar]
- Banks M.C., Kamel N.S., Zabriskie J.B., Larone D.H., Ursea D., Posnett D.N. (2003) Staphylococcus aureus express unique superantigens depending on the tissue source. J Infect Dis 187: 77–86. [DOI] [PubMed] [Google Scholar]
- Brassard J., Gottschalk M., Quessy S. (2004) Cloning and purification of the Streptococcus suis serotype 2 glyceraldehyde-3-phosphate dehydrogenase and its involvement as an adhesin. Vet Microbiol 102: 87–94. [DOI] [PubMed] [Google Scholar]
- Cabanes D., Dehoux P., Dussurget O., Frangeul L., Cossart P. (2002) Surface proteins and the pathogenic potential of Listeria monocytogenes. Trends Microbiol 10: 238–245. [DOI] [PubMed] [Google Scholar]
- Chen C., Tang J., Dong W., et al. (2007) A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS ONE 2: e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greeff A., Buys H., Verhaar R., Dijkstra J., Van Alphen L., Smith H.E. (2002) Contribution of fibronectin-binding protein to pathogenesis of Streptococcus suis serotype 2. Infect Immun 70: 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk M., Segura M. (2000) The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet Microbiol 76: 259–272. [DOI] [PubMed] [Google Scholar]
- Gottschalk M., Higgins R., Quessy S. (1999) Dilemma of the virulence of Streptococcus suis strains. J Clin Microbiol 37: 4202–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruening P., Fulde M., Valentin-Weigand P., Goethe R. (2006) Structure, regulation, and putative function of the arginine deiminase system of Streptococcus suis. J Bacteriol 188: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesebrouck F., Pasmans F., Chiers K., Maes D., Ducatelle R., Decostere A. (2004) Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet Microbiol 100: 255–268. [DOI] [PubMed] [Google Scholar]
- Hill J.E., Gottschalk M., Brousseau R., Harel J., Hemmingsen S.M., Goh S.H. (2005) Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet Microbiol 107: 63–69. [DOI] [PubMed] [Google Scholar]
- Huang Y.T., Teng L.J., Ho S.W., Hsueh P.R. (2005) Streptococcus suis infection. J Microbiol Immunol Infect 38: 306–313. [PubMed] [Google Scholar]
- King S.J., Allen A.G., Maskell D.J., Dowson C.G., Whatmore A.M. (2004) Distribution, genetic diversity, and variable expression of the gene encoding hyaluronate lyase within the Streptococcus suis population. J Bacteriol 186: 4740–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Martinez G., Gottschalk M., Lacouture S., Willson P., Dubreuil J.D., Jacques M., Harel J. (2006) Identification of a surface protein of Streptococcus suis and evaluation of its immunogenic and protective capacity in pigs. Infect Immun 74: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Feng Y., Gao F., Zhang Q., Wang M. (2006) Characterization of HCoV-229E fusion core: implications for structure basis of coronavirus membrane fusion. Biochem Biophys Res Commun 345: 1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun S., Perez-Casal J., Connor W., Willson P.J. (2003) Role of suilysin in pathogenesis of Streptococcus suis capsular serotype 2. Microb Pathog 34: 27–37. [DOI] [PubMed] [Google Scholar]
- Ma G., Feng Y., Gao F., Wang J., Liu C., Li Y. (2005) Biochemical and biophysical characterization of the transmissible gastroenteritis coronavirus fusion core. Biochem Biophys Res Commun 337: 1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione D., Margarit I., Rinaudo C.D., et al. (2005) Identification of a universal Group B streptococcus vaccine by multiple genome screen. Science 309: 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre W.W., Schneewind O. (1999) Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev 63: 174–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwumabua O., Chinnapapakkagari S. (2005) Identification of the gene encoding a 38-kilodalton immunogenic and protective antigen of Streptococcus suis. Clin Diagn Lab Immunol 12: 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okwumabua O., Persaud J.S., Reddy P.G. (2001) Cloning and characterization of the gene encoding the glutamate dehydrogenase of Streptococcus suis serotype 2. Clin Diagn Lab Immunol 8: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki M., Takamatsu D., Shimoji Y., Sekizaki T. (2002) Characterization of Streptococcus suis genes encoding proteins homologous to sortase of gram-positive bacteria. J Bacteriol 184: 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.E., Vecht U., Gielkens A.L., Smits M.A. (1992) Cloning and nucleotide sequence of the gene encoding the 136-kilodalton surface protein (muramidase-released protein) of Streptococcus suis type 2. Infect Immun 60: 2361–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.E., Reek F.H., Vecht U., Gielkens A.L., Smits M.A. (1993) Repeats in an extracellular protein of weakly pathogenic strains of Streptococcus suis type 2 are absent in pathogenic strains. Infect Immun 61: 3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.E., De Vries R., Van't Slot R., Smits M.A. (2000) The cps locus of Streptococcus suis serotype 2: genetic determinant for the synthesis of sialic acid. Microb Pathog 29: 127–134. [DOI] [PubMed] [Google Scholar]
- Staats J.J., Feder I., Okwumabua O., Chengappa M.M. (1997) Streptococcus suis: past and present. Vet Res Commun 21: 381–407. [DOI] [PubMed] [Google Scholar]
- Staats J.J., Plattner B.L., Stewart G.C., Changappa M.M. (1999) Presence of the Streptococcus suis suilysin gene and expression of MRP and EF correlates with high virulence in Streptococcus suis type 2 isolates. Vet Microbiol 70: 201–211. [DOI] [PubMed] [Google Scholar]
- Tang J., Wang C., Feng Y., et al. (2006) Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med 3: e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen K., Haataja S., Finne J. (1996) The galactosyl-(alpha 1-4)-galactose-binding adhesin of Streptococcus suis: occurrence in strains of different hemagglutination activities and induction of opsonic antibodies. Infect Immun 64: 3659–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. (2006) Development of multiplex PCR for diagnosing the disease caused by Streptococcus suis and cloning and expression the fibrinogen-binding protein of Streptococcus suis type. MSc Degree, Nanjing Agriculture University, pp. 39–41. [Google Scholar]
- Yang B., Zhu W., Johnson L.B., White F.F. (2000) The virulence factor AvrXa7 of Xanthomonas oryzae pv. oryzae is a type III secretion pathway-dependent nuclear-localized double-stranded DNA-binding protein. Proc Natl Acad Sci USA 97: 9807–9812. [DOI] [PMC free article] [PubMed] [Google Scholar]


