Abstract
Background
The World Health Organization (WHO) ranks respiratory tract infection (RTI) as the second leading cause of death worldwide for children under 5 years of age. The aim of this work was to evaluate the epidemiology characteristics of respiratory viruses found in children and adults with RTI from July 2009 to June 2012 in southern China.
Methods
In this work, a total of 14 237 nasopharyngeal swabs (14 237 patients from 25 hospitals) were analyzed, and seven respiratory viruses (influenza virus, respiratory syncytial virus, parainfluenza virus, adenovirus, human metapneumovirus, human coronavirus, human bocavirus) were detected using PCR/RT-PCR from nasopharyngeal swabs.
Results
The demographic characteristics, viral prevalence, age distribution, seasonal distribution, and pathogen spectrum of the patients with RTIs were analyzed. Co-infection was observed in 483 specimens, but it was more common in male patients, inpatients, children, and young adults. It varied by season, being more prevalent in the spring and summer and less so in the winter. Human coronavirus and human bocavirus were the most common pathogens, tending to occur in co-infection with other respiratory viruses.
Conclusions
This work adds to our knowledge of the epidemiology characteristics of these seven common respiratory viruses among patients with RTI in southern China. The detection of the specific viral causes of infection provides a useful starting point for an understanding of illness attributable to respiratory infection, and might also provide data relevant to the development of prevention strategies.
Keywords: Respiratory tract infections, Respiratory viruses, Southern China
1. Introduction
Respiratory tract infections (RTI) are a significant health burden for children. The World Health Organization (WHO) estimates that 1.9 million children die each year as a result of the complications of acute RTIs, mainly pneumonia,1 and 70% of RTI deaths occur in Africa and southeast Asia.2 The major viral agents of RTI include influenza A, B, and C viruses (Flu), respiratory syncytial virus (RSV), parainfluenza virus (PIV), adenovirus (ADV), human metapneumovirus (hMPV), human coronavirus (HCoV), and the newly discovered human bocavirus (HBoV). Flu, RSV, and PIV are often associated with pneumonia, especially in children under 5 years of age and those living in developing countries.3, 4, 5, 6, 7, 8 In one study of hospitalized children, RSV was identified in 15–40% of cases, PIV in 6.8–7%, and Flu in 3%.9 ADV, HCoV, and HBoV are associated with a substantial proportion of RTI in infants and young children;7, 10, 11, 12 hMPV has been detected in up to 10% of respiratory specimens.13 RSV and PIV may also contribute substantially to mortality among the elderly, and the annual outbreaks and recurrent infections suggest that RSV and PIV may contribute to the burden of disease throughout life.14, 15
The acquisition and spread of RTIs is known to vary among different study populations in different countries. These variations may be due to cultural and socioeconomic factors, geographical or climatic differences, or variations in health care systems. A clear understanding of the local epidemiology of RTI and the identification of risk factors is critical to the successful implementation of a prevention and control program. Southern China is believed to be the origin of some important respiratory viruses, such as severe acute respiratory syndrome coronavirus (SARS-CoV)16 and influenza virus.17 Southern China has large populations of humans and domestic and wild animals, as well as a large transient population that includes laborers and business people from different provinces of China and from other countries. The mixing of these large regional populations may favor the transmission of respiratory viruses.
The prevalence and clinical presentation of human viral infections in China have been reported previously.8, 10, 18, 19, 20 However, there are no previously published reports describing the etiology of the seven common respiratory tract viruses of inpatient and outpatient RTI across the seasons among the various age and gender categories. Information on RTI from southern China is also sparse. To directly address this situation, nasopharyngeal swabs were collected continuously from children and adults seeking medical attention for RTI from a total of 25 hospitals in southern China between July 2009 and June 2012. Seven respiratory viruses were detected by PCR/RT-PCR and their epidemiological characteristics were analyzed.
2. Materials and methods
2.1. Ethics issues
All research involving human participants was approved by the Institutional Review Board of Zhongshan School of Medicine, Sun Yat-sen University, in accordance with the guidelines for the protection of human subjects. Participants provided written informed consent after being briefed on the purpose of the study and of their right to keep information confidential. Written consent was obtained from all study participants or their guardians.
2.2. Patients and specimens
Study participants had all been admitted to one of the 25 hospitals covering southern China. Selection criteria included having one or more respiratory symptoms, such as headache, cough, expectoration, and pharyngodynia, combined with a body temperature above 37.5 °C. Symptoms, history of illness, results of a clinical examination and laboratory tests, and demographic data were collected for each patient using a standardized form. Nasopharyngeal swabs were collected according to a standard procedure, kept in viral transport medium, and stored at −80 °C prior to analysis (one swab was collected from each patient). For patients with viral infections, some additional clinical information was abstracted retrospectively from the medical treatment records.
2.3. Nucleic acid extraction and cDNA synthesis
DNA or RNA was extracted from 200 μl of the nasopharyngeal swab specimen using the QIAamp MiniElute Virus Spin kit (Qiagen). Reverse transcription of virus RNA was conducted using SuperScript III RT (Invitrogen, Life Technology) in order to detect RNA viruses (Flu, RSV, PIV, hMPV, and HCoV). DNA samples extracted using the kit were used directly to detect DNA viruses (ADV and HBoV). Both kits were used in accordance with the manufacturer's instructions.
2.4. Pathogen screening
Flu (A, B, C), RSV, PIV, ADV, hMPV, HCoV, and HBoV were detected by standard PCR or reverse transcription PCR (RT-PCR), as described previously, using specific primers listed in the Supplementary Material Table S1,21, 22, 23, 24, 25 and amplified products were detected using agarose gel electrophoresis.
2.5. Statistical analysis
Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Viral prevalences were compared using the Chi-square test for categorical variables, and the cartogram was drawn using Excel software. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Demographic characteristics
A total of 14 237 nasopharyngeal swabs were collected from July 2009 to June 2012. In all, 7323 specimens were from outpatients and 6914 specimens were from inpatients. More specimens were collected from males than from females (ratio 1.48). The median patient age was 21 years (range 0–110 years). The number of children (age ≤14 years) was 6117 and the number of other patients (>14 years) was 8120. The seasonal distribution of patients sampled was 3756 in spring (January to March), 2617 in summer (April to June), 3993 in autumn (July to September), and 3871 in winter (October to December). An estimated 99.9% of eligible patients volunteered to participate. Our data showed a significant difference in inpatient proportion by age, and showed the difference in sex distribution by age. They did not show the 20–30 and 50–55 years groups, and the relative numbers of male participants in other age groups were all over 50%.
3.2. Clinical characteristics of the patients
The clinical characteristics of the patients are listed in the Supplementary Material Table S2. Most patients presented with symptoms of respiratory tract illness (RTI), including fever (≥37.5 °C; 100.0%), cough (84.1%), expectoration (42.1%), runny nose (36.4%), and sore throat (19.8%).
3.3. Viral prevalence
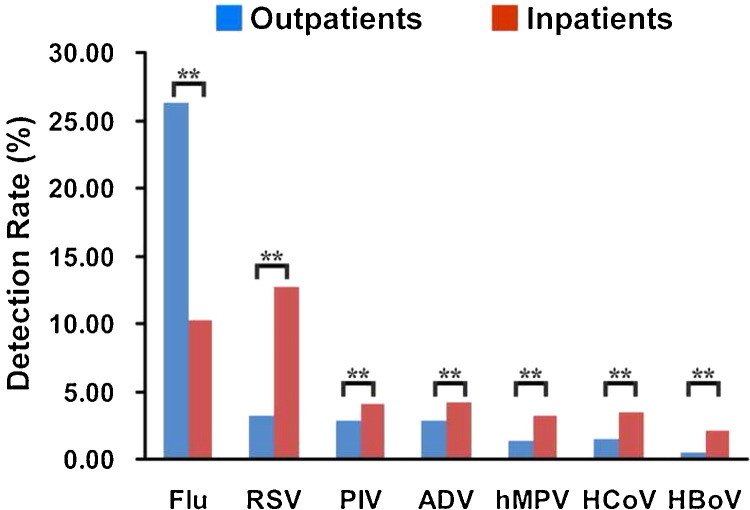
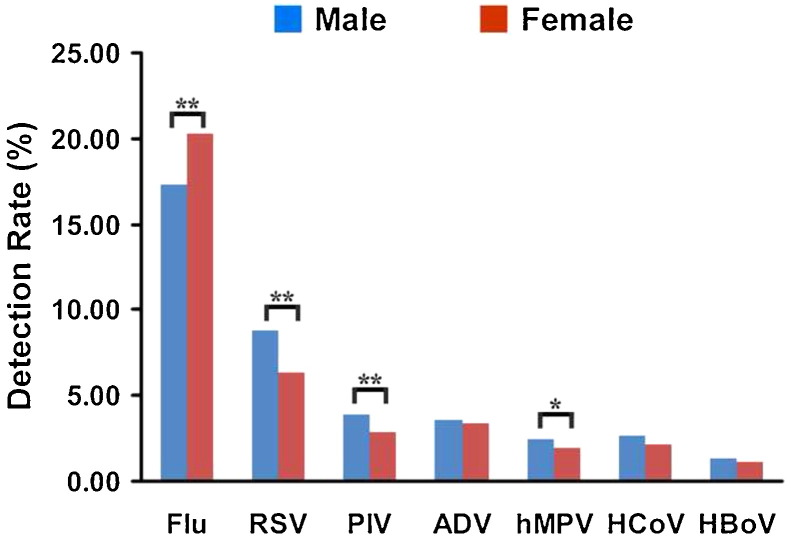
The total rate of detection of all seven viruses for all specimens was 39.24% (5582/14 237). Flu viruses were detected in 2632 specimens (18.50%), RSV in 1120 (7.86%), PIV in 494 (3.47%), ADV in 493 (3.47%), hMPV in 319 (2.24%), HCoV in 351 (2.47%), and HBoV in 180 (1.26%). The total viral detection rate (all seven viruses) for all inpatients was 40.01% (2772/6914), which was higher than that of outpatients (38.47%, 2817/7323) (Chi-square = 3.937, p = 0.047). With the exception of Flu, the viruses were more common in inpatients than in outpatients (Figure 1 ). The total viral detection rate (all seven viruses) was higher in male patients (40.11%, 3403/8485) than in female patients (37.95%, 2179/5742) (Chi-square = 6.687, p = 0.010). The rates of detection of RSV, PIV, and hMPV were higher in male patients than in female patients, and the detection rate of Flu was lower in male patients than in female patients (Chi-square = 19.262, p < 0.0001). There was no difference in the detection rates for ADV, HCoV, and HBoV (Figure 2 ).
Figure 1.
Detection rates of the seven viruses (Flu, RSV, PIV, ADV, hMPV, HCoV, and HBoV) in outpatients and inpatients (%). Analyses were performed using the Chi-square test; statistical significance is shown with asterisks: *p < 0.05, **p < 0.01. Flu (Chi-square = 598.226, p < 0.0001), RSV (Chi-square = 450.854, p < 0.0001), PIV (Chi-square = 17.077, p < 0.0001), ADV (Chi-square = 18.256, p < 0.0001), hMPV (Chi-square = 59.509, p < 0.0001), HCoV (Chi-square = 59.858, p < 0.0001), and HBoV (Chi-square = 64.685, p < 0.0001).
Figure 2.
Detection rates of the seven viruses (Flu, RSV, PIV, ADV, hMPV, HCoV, and HBoV) in male and female patients (%). Analyses were performed using the Chi-square test; statistical significance is shown with asterisks: *p < 0.05, **p < 0.01). Flu (Chi-square = 19.262, p < 0.0001), RSV (Chi-square = 30.076, p < 0.0001), PIV (Chi-square = 11.529, p = 0.001), ADV (Chi-square = 0.312, p = 0.577), hMPV (Chi-square = 5.195, p = 0.023), HCoV (Chi-square = 2.977, p = 0.084), and HBoV (Chi-square = 2.176, p = 0.140).
3.4. Age distribution
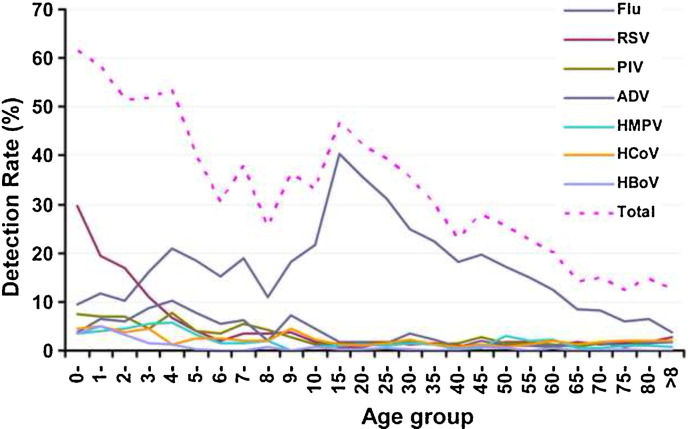
A decline in the incidence of viral infections with age was observed for respiratory viruses, except for Flu. The detection rates of RSV, PIV, ADV, hMPV, HCoV, and HBoV among children (≤14 years) were higher than among adults (>14 years old). The detection rates for RSV and HBoV were highest among children <5 years old. The detection rate for Flu was highest among patients aged 15–35 years (Figure 3 ).
Figure 3.
Age distribution of the incidence of the seven viruses (Flu, RSV, PIV, ADV, hMPV, HCoV, and HBoV).
3.5. Seasonal distribution
A combined graph for all three study years showing the monthly distribution of the seven respiratory viruses was drawn. The total detection rate of the seven respiratory viruses was highest in February (48.03%) and August (47.16%), and lowest in October (23.14%). The highest rate of RSV was detected in February (16.10%); the highest rate of hMPV was detected in March (4.30%); PIV, HCoV, and HBoV showed the highest rates of detection in June: 7.87%, 3.68%, and 3.43%, respectively. Flu showed the highest rate of detection in August (32.03%), and ADV showed the highest rate of detection in December (4.38%).
The total detection rates for the seven respiratory viruses in spring, summer, autumn, and winter were 44.31%, 41.15%, 41.66%, and 30.52%, respectively. The rates of detection were different during the four seasons (Chi-square = 177.859, p < 0.0001). However, the detection rates in summer and autumn were similar (Chi-square = 0.171, p = 0.679). The rates of detection of all viruses except ADV (Chi-square = 2.170, p = 0.538) differed across the seasons: Flu (Chi-square = 187.068, p < 0.0001), RSV (Chi-square = 327.662, p < 0.0001), PIV (Chi-square = 32.731, p < 0.0001), hMPV (Chi-square = 103.609, p < 0.0001), HCoV (Chi-square = 27.864, p < 0.0001), and HBoV (Chi-square = 43.512, p < 0.0001).
In all four seasons, the detection rate of Flu was the highest of the seven respiratory viruses, followed by RSV. The detection rate of Flu was highest in autumn and there was no statistically significant difference in rates between spring/summer and summer/winter. However, the detection rate of RSV was highest in spring. The detection rate for PIV was highest in summer. There was no difference in the rate of detection of PIV during spring, autumn, and winter. For hMPV, the detection rates during spring and summer were higher than during autumn and winter. There was no difference between spring/summer and autumn/winter. The detection rate for HCoV was lowest in autumn; there was no difference among spring, summer, and winter. The detection rate for HBoV was lower in spring than in summer, autumn, and winter.
3.6. Pathogen spectrum
The pathogen spectrum differed with patient age. The proportion of patients infected with Flu increased with age, peaking in the group including age 15 years (86.31%), after which it fluctuated slightly but remained stable, and declined significantly after age 60 years. However, the rates of detection of RSV, PIV, ADV, hMPV, and HCoV relative to the total rate of detection decreased after the patients reached adulthood, and then fluctuated only slightly until age 60 years, after which they declined significantly.
RSV was the most common pathogen detected in children, accounting for 31.72% of all viruses detected in children. Flu accounted for 25.91% of all the viruses detected in children. Flu was the most common pathogen detected in adults, accounting for 74.97% of all viruses detected. Flu was the most common pathogen detected in outpatients (68.25%). RSV was the most common pathogen detected in inpatients (31.78%). However, RSV was the most common pathogen detected in inpatient children (34.77%). Flu was the most common pathogen detected in inpatient adults (81.04%), outpatient children (34.46%), and outpatient adults (42.02%).
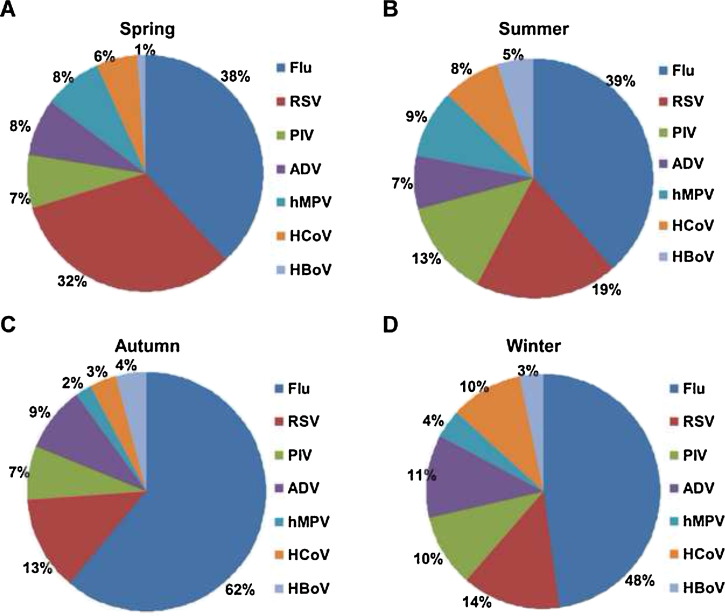
The proportion of Flu infections among female cases (53.33%) was greater than among male cases (43.20%). However, there were fewer RSV, PIV, HCoV, and hMPV among female cases than among male cases. The pathogen spectrum also differed across the seasons. RSV accounted for a larger proportion of the total number of infections during spring (32.27%); PIV, hMPV, and HBoV accounted for a larger proportion during summer (12.92%, 9.01%, and 5.11%, respectively). Flu accounted for a larger proportion during autumn (61.23%). ADV and HCoV accounted for a larger proportion during winter (11.35% and 9.99%, respectively) (Figure 4 ).
Figure 4.
Rates of detection of the seven viruses (Flu, RSV, PIV, ADV, hMPV, HCoV, and HBoV) by season. The percentages were calculated as the total number of virus-positive cases for the individual virus divided by the number of cases positive for one of the seven viruses in the same season.
3.7. Co-infections of respiratory virus
Co-infections occurred in 483 specimens, with detection rates of 3.39% (483/14 237) of all specimens and 9.55% (483/5057) of positive specimens. Among the 483 co-infected specimens, 442 were double infections, 40 were triple infections, and one was a quadruple infection (Table 1 ). The one quadruple infection was in a 1-year-old girl (born April 25, 2010), residing in the Liwan region of Guangzhou city. She was admitted to the hospital on April 26, 2011. The symptoms included fever (39.5 °C), cough, runny nose, and expectoration, with a diagnosis of bronchopneumonia. Mycoplasma pneumoniae and Chlamydophila pneumoniae were also detected. Co-infection viruses included Flu, RSV, HCoV, and HBoV, at rates of 52.17% (252/483), 50.52% (244/483), 39.03% (137/351), and 36.67% (66/180), respectively.
Table 1.
Co-infections with seven respiratory viruses
| Co-infection viruses | Number of cases |
|---|---|
| Flu, RSV | 99 |
| Flu, PIV | 33 |
| Flu, ADV | 26 |
| Flu, hMPV | 16 |
| Flu, HCoV | 47 |
| Flu, HBoV | 10 |
| RSV, PIV | 32 |
| RSV, ADV | 27 |
| RSV, hMPV | 10 |
| RSV, HCoV | 30 |
| RSV, HBoV | 19 |
| PIV, ADV | 16 |
| PIV, hMPV | 11 |
| PIV, HCoV | 10 |
| PIV, HBoV | 8 |
| ADV, hMPV | 6 |
| ADV, HCoV | 12 |
| ADV, HBoV | 11 |
| hMPV, HCoV | 11 |
| hMPV, HBoV | 1 |
| HCoV, HBoV | 7 |
| FluA, RSV, PIV | 5 |
| FluA, RSV, ADV | 1 |
| FluA, RSV, hMPV | 1 |
| FluA, RSV, HCoV | 6 |
| FluA, PIV, ADV | 2 |
| FluA, PIV, HCoV | 2 |
| FluA, hMPV, HCoV | 2 |
| FluA, HCoV, HBoV | 1 |
| RSV, PIV, ADV | 2 |
| RSV, PIV, hMPV | 4 |
| RSV, PIV, HCoV | 2 |
| RSV, PIV, HBoV | 2 |
| RSV, ADV, HCoV | 2 |
| RSV, ADV, HBoV | 2 |
| RSV, hMPV, HBoV | 1 |
| RSV, HCoV, HBoV | 2 |
| PIV, ADV, hMPV | 2 |
| PIV, ADV, HCoV | 2 |
| PIV, ADV, HBoV | 1 |
| ADV, hMPV, HCoV | 1 |
| ADV, hMPV, HBoV | 1 |
| Flu, RSV, PIV, HCoV | 1 |
Flu, influenza virus; RSV, respiratory syncytial virus; PIV, parainfluenza virus; ADV, adenovirus; hMPV, human metapneumovirus; HCoV, human coronavirus; HBoV, human bocavirus.
Flu and RSV were frequently detected as co-infection viruses among the 483 co-infection specimens, accounting for 52.17% (252/483) and 50.52% (244/483), respectively. However, HCoV and HBoV were the pathogens preferring to co-infect with other respiratory viruses; 39.03% (137/351) of HCoV-positive samples and 36.67% (66/180) of HBoV-positive samples were found to contain other respiratory viruses.
Most co-infected patients were male (66.05%, 319/483), inpatients (69.15%, 334/483), children <4 years old (67.07%, 324/483), and younger adults aged 20–35 years (12.84%, 62/483). Co-infection was more common in children ≤14 years old, (6.02%) than in adults (>14 years old; 1.42%) (Chi-square = 225.195, p < 0.0001). The rate of co-infection detection was 3.76% (319/8490) in male patients and 2.85% (164/5747) in female patients (Chi-square = 8.540, p = 0.003), and 2.03% (149/7323) in outpatients and 4.83% (334/6914) in inpatients (Chi-square = 84.833, p < 0.0001).
There were 175, 121, 113, and 74 cases of co-infection in spring, summer, autumn, and winter, respectively; the co-infection rates were 4.66%, 4.63%, 2.83%, and 1.91%, respectively (Chi-square = 60.209, p < 0.0001). The co-infection rates in spring and summer were higher than in autumn and winter, and the rate of co-infection in winter was the lowest.
4. Discussion
The WHO ranks RTI as the second leading cause of death worldwide for children younger than 5 years of age.26 However, the influenza viruses are the only viral respiratory pathogens for which vaccines are currently available.27 Ongoing vaccine research and development are focusing on many other leading viral pathogens.27, 28, 29 The detection of the specific viral causes of infection provides a useful starting point for an understanding of illness attributable to respiratory infection. It also provides data relevant to the development of prevention strategies. The objective of the study was to estimate the prevalence of respiratory viruses in people who presented with acute respiratory tract infections in southern China over a 3-year study period.
Human PIV is a major cause of respiratory tract illness in infants and young children worldwide.30 All children experience at least one PIV infection by the age of 5 years, and re-infection may occur throughout life because of incomplete immunity.31 The virus is associated with a wide variety of RTIs, but most frequently with croup and pneumonia. Annual epidemics of hPIV-3 infection are responsible for considerable economic losses as a result of hospitalization, medication costs, work and school absence, and mortality. However, PIV infections have been less well studied compared to RSV and influenza viral infections. In the present study, PIV was associated with 10.00% of all the RTI hospitalizations and 15.00% of the RTI in outpatient children. A study in the USA found PIV infections to be associated with 11.50% of all pediatric RTI hospitalizations.32
Human bocavirus (HBoV) was first reported by Allander et al. in 2005.33 Subsequently, HBoV was reported in respiratory specimens collected from different countries and regions worldwide,34, 35, 36 and detected in 1.50% to 8.30% of respiratory specimens from individuals with acute RTI, especially young children and infants. In the present study, HBoV was detected in 3.85% of all children <2 years old, who provided 78.33% of all the HBoV-positive specimens. HBoV infection has recently attracted attention worldwide. However, the incidence and clinical presentation of this infection varies widely, often involving co-infection with other potential pathogens.37 Such characteristics have led to debate over the role of HBoV as a true pathogen. In the present study, among the 180 HBoV-positive specimens, there were 66 (36.67%) co-infections with at least one other respiratory virus. Elderly people have also been reported to be susceptible to HBoV. A single lineage of HBoV was detected among a wide age distribution of patients with acute RTI. In this study, a total of 18 adult specimens were positive for HBoV. The virus has also been found in stool specimens from patients with gastrointestinal illness.38, 39 Therefore, additional evidence and studies are needed throughout the world to gain a better understanding of this virus.
This study adds to the knowledge of seasonal variations of respiratory viral infections in southern China. The epidemiology of respiratory viral infection was found to vary tremendously by geographical region. In temperate climates, the prevalence of these viruses is well documented as a cause of yearly winter epidemics of acute lower RTIs.40 This study clearly showed evidence of seven viruses throughout the 3-year study period. The highest rate of infection was in spring, with a peak in February (48.03%), and the lowest rates of infection were in winter, with a nadir in October (23.14%), which may be attributed to the fact that February is the coldest month in southern China. The detection rates for summer and autumn were similar and this is likely due to high humidity and a lack of significant delimitation between summer and autumn.
With recent advances in the detection of respiratory agents, numerous studies have shown that some pediatric patients with acute lower RTIs become infected simultaneously with multiple respiratory viruses.41, 42 Multiple viral infections have been linked in some reports to higher fever, a longer hospital stay, more frequent use of antibiotics, and an increased risk of admission to the ICU.43 However, there is no consensus regarding the effect of co-infection on the severity of disease. The effect may depend upon which viruses co-infect.44 In the present study, 3.39% of specimens had multiple or dual infections, with a predominance of Flu compared to the other co-viruses. However, in the positive specimens, the co-infection rates for HCoV and HBoV were higher than those of other respiratory viruses. The co-infection rates were higher in male patients, inpatients, and patients aged ≤14 years. The rates of co-infection were higher during spring and summer than during autumn and winter. The rates of co-infection were lowest during winter, which corresponded with the overall low detection rates in winter.
Acknowledgements
We owe our special thanks to the four collaborating institutes (the Centre for Disease Control and Prevention of Guangdong Province, Guangzhou Centre for Disease Control and Prevention of the City, the Affiliated Pearl River Hospital of Southern Medical University, and the First Affiliated Hospital of Guangzhou Medical University) and the 25 hospitals (Sun Yat-sen Memorial Hospital, the Third Affiliated Hospital of Sun Yat-sen University, the Chinese Traditional Hospital of Guangdong Province, the people's Hospital of Shijie Town of Dongguan City, the First Affiliated Hospital of Guangzhou Medical University, the Central Hospital of Shantou City, the Central People's Hospital of Zhanjiang City, the Yuebei People's Hospital, the People's Hospital of Zhuhai City, the First People's Hospital of Huizhou City, the People's Hospital of Zhongshan City, Zhaoqing Centre for Disease Control and Prevention of the City, the Donghua Hospital of Dongguan City, the People's Hospital of Qingyuan City, the People's Hospital of Zengcheng, the People's Hospital of Huadu, the Tanzhen Hospital of Zengcheng, the Fourth People's Hospital of Shenzhen City, the Central Hospital of Panyu, the Central Hospital of Changsha City, the Huaqiao Hospital, the Eighth Hospital of Guangzhou City, the Affiliated Pearl River Hospital of Southern Medical University, the Children's Hospital of Guangzhou City, the Red Cross Society of Guangzhou City) involved in the study; we thank the doctors and nurses who participated for their help in collecting the samples. We thank Marissa Valentine and Professor Gregory C. Gray of the University of Florida for their critical review of this manuscript.
Funding: This work was supported by the Natural Science Foundation of China (81201283, 81102370, 41176128); the National Science and Technique Major Project (201005022-2, 2012ZX09102101-017, 2012ZX10004213, 311030); High-Tech Research (863) Projects (2011AA09070201); Guangdong Provincial Natural Science Foundation (8151008901000210); The Key Science and Technique Research Project of Guangdong Province (2010B030600003, 2009010058).
Ethical approval: All research involving human participants was approved by the Institutional Review Board of Zhongshan School of Medicine, Sun Yat-sen University, in accordance with the guidelines for the protection of human subjects. Participants received ‘written informed consent’ about the purpose of the study and their right to keep information confidential. Written consent was obtained from all participants or their guardians.
Conflict of interest: The authors declare that they have no conflicts of interest.
Corresponding Editor: Eskild Petersen, Aarhus, Denmark
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijid.2014.02.019.
Appendix A. Supplementary data
References
- 1.Mulholland K. Global burden of acute respiratory infections in children: implications for interventions. Pediatr Pulmonol. 2003;36:469–474. doi: 10.1002/ppul.10344. [DOI] [PubMed] [Google Scholar]
- 2.Williams B.G., Gouws E., Boschi-Pinto C., Bryce J., Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 3.Iwane M.K., Edwards K.M., Szilagyi P.G., Walker F.J., Griffin M.R., Weinberg G.A. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 4.Lee M.S., Walker R.E., Mendelman P.M. Medical burden of respiratory syncytial virus and parainfluenza virus type 3 infection among US children. Implications for design of vaccine trials. Hum Vaccin. 2005;1:6–11. doi: 10.4161/hv.1.1.1424. [DOI] [PubMed] [Google Scholar]
- 5.Nair H., Brooks W.A., Katz M., Roca A., Berkley J.A., Madhi S.A. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378:1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 6.Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed J.A., Katz M.A., Auko E., Njenga M.K., Weinberg M., Kapella B.K. Epidemiology of respiratory viral infections in two long-term refugee camps in Kenya, 2007-2010. BMC Infect Dis. 2012;12:7. doi: 10.1186/1471-2334-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu X., Lu R., Wang Z., Zhu N., Wang W., Julian D. Etiology and clinical characterization of respiratory virus infections in adult patients attending an emergency department in Beijing. PLoS One. 2012;7:e32174. doi: 10.1371/journal.pone.0032174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberg G.A., Hall C.B., Iwane M.K., Poehling K.A., Edwards K.M., Griffin M.R. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154:694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Lu R., Yu X., Wang W., Duan X., Zhang L., Zhou W. Characterization of human coronavirus etiology in Chinese adults with acute upper respiratory tract infection by real-time RT-PCR assays. PLoS One. 2012;7:e38638. doi: 10.1371/journal.pone.0038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khamis F.A., Al-Kobaisi M.F., Al-Areimi W.S., Al-Kindi H., Al-Zakwani I. Epidemiology of respiratory virus infections among infants and young children admitted to hospital in Oman. J Med Virol. 2012;84:1323–1329. doi: 10.1002/jmv.23330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J.R., Jin Y., Xie Z.P., Gao H.C., Xiao N.G., Chen W.X. Novel human bocavirus in children with acute respiratory tract infection. Emerg Infect Dis. 2010;16:324–327. doi: 10.3201/eid1602.090553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn J.S. Epidemiology of human metapneumovirus. Clin Microbiol Rev. 2006;19:546–557. doi: 10.1128/CMR.00014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall C.B. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 15.van Asten L., van den Wijngaard C., van Pelt W., van de Kassteele J., Meijer A., van der Hoek W. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206:628–639. doi: 10.1093/infdis/jis415. [DOI] [PubMed] [Google Scholar]
- 16.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 17.Shortridge K.F. The 1918 ‘Spanish’ flu: pearls from swine? Nat Med. 1999;5:384–385. doi: 10.1038/7383. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R.F., Jin Y., Xie Z.P., Liu N., Yan K.L., Gao H.C. Human respiratory syncytial virus in children with acute respiratory tract infections in China. J Clin Microbiol. 2010;48:4193–4199. doi: 10.1128/JCM.00179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z.Y., Du L.N., Chen X., Zhao Y., Liu E.M., Yang X.Q. Genetic variability of respiratory syncytial viruses (RSV) prevalent in southwestern China from 2006 to 2009: emergence of subgroup B and A RSV as dominant strains. J Clin Microbiol. 2010;48:1201–1207. doi: 10.1128/JCM.02258-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W., Cavailler P., Ren P., Zhang J., Dong W., Yan H. Molecular monitoring of causative viruses in child acute respiratory infection in endemo-epidemic situations in Shanghai. J Clin Virol. 2010;49:211–218. doi: 10.1016/j.jcv.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coiras M.T., Perez-Brena P., Garcia M.L., Casas I. Simultaneous detection of influenza A, B, and C viruses, respiratory syncytial virus, and adenoviruses in clinical samples by multiplex reverse transcription nested-PCR assay. J Med Virol. 2003;69:132–144. doi: 10.1002/jmv.10255. [DOI] [PubMed] [Google Scholar]
- 22.Coiras M.T., Aguilar J.C., Garcia M.L., Casas I., Perez-Brena P. Simultaneous detection of fourteen respiratory viruses in clinical specimens by two multiplex reverse transcription nested-PCR assays. J Med Virol. 2004;72:484–495. doi: 10.1002/jmv.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo P.C., Lau S.K., Chu C.M., Chan K.H., Tsoi H.W., Huang Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allard A., Albinsson B., Wadell G. Detection of adenoviruses in stools from healthy persons and patients with diarrhea by two-step polymerase chain reaction. J Med Virol. 1992;37:149–157. doi: 10.1002/jmv.1890370214. [DOI] [PubMed] [Google Scholar]
- 25.Chung J.Y., Han T.H., Kim C.K., Kim S.W. Bocavirus infection in hospitalized children, South Korea. Emerg Infect Dis. 2006;12:1254–1256. doi: 10.3201/eid1208.060261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryce J., Boschi-Pinto C., Shibuya K., Black R.E. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 27.Fiore A.E., Bridges C.B., Cox N.J. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333:43–82. doi: 10.1007/978-3-540-92165-3_3. [DOI] [PubMed] [Google Scholar]
- 28.Wright P.F., Karron R.A., Belshe R.B., Shi J.R., Randolph V.B., Collins P.L. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25:7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato M., Wright P.F. Current status of vaccines for parainfluenza virus infections. Pediatr Infect Dis J. 2008;27:S123–S125. doi: 10.1097/INF.0b013e318168b76f. [DOI] [PubMed] [Google Scholar]
- 30.Henrickson K.J. Parainfluenza viruses. Clin Microbiol Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glezen W.P., Frank A.L., Taber L.H., Kasel J.A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984;150:851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- 32.Almajhdi F.N., Alshaman M.S., Amer H.M. Molecular characterization and phylogenetic analysis of human parainfluenza virus type 3 isolated from Saudi Arabia. J Med Virol. 2012;84:1304–1311. doi: 10.1002/jmv.23326. [DOI] [PubMed] [Google Scholar]
- 33.Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kesebir D., Vazquez M., Weibel C., Shapiro E.D., Ferguson D., Landry M.L. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194:1276–1282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fry A.M., Lu X., Chittaganpitch M., Peret T., Fischer J., Dowell S.F. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maggi F., Andreoli E., Pifferi M., Meschi S., Rocchi J., Bendinelli M. Human bocavirus in Italian patients with respiratory diseases. J Clin Virol. 2007;38:321–325. doi: 10.1016/j.jcv.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Arden K.E., McErlean P., Nissen M.D., Sloots T.P., Mackay I.M. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78:1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pham N.T., Trinh Q.D., Chan-It W., Khamrin P., Nishimura S., Sugita K. Human bocavirus infection in children with acute gastroenteritis in Japan and Thailand. J Med Virol. 2011;83:286–290. doi: 10.1002/jmv.21876. [DOI] [PubMed] [Google Scholar]
- 39.de Sousa T.T., Souza M., Fiaccadori F.S., Borges A.M., da Costa P.S., Cardoso D. Human bocavirus 1 and 3 infection in children with acute gastroenteritis in Brazil. Mem Inst Oswaldo Cruz. 2012;107:800–804. doi: 10.1590/s0074-02762012000600015. [DOI] [PubMed] [Google Scholar]
- 40.Weber M.W., Mulholland E.K., Greenwood B.M. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 41.Kouni S., Karakitsos P., Chranioti A., Theodoridou M., Chrousos G., Michos A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin Microbiol Infect. 2013;19:772–777. doi: 10.1111/1469-0691.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng D., Zhao D., Liu J., Wang X., Yang K., Xicheng H. Multipathogen infections in hospitalized children with acute respiratory infections. Virol J. 2009;6:155. doi: 10.1186/1743-422X-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semple M.G., Cowell A., Dove W., Greensill J., McNamara P.S., Halfhide C. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382–386. doi: 10.1086/426457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin E.T., Kuypers J., Wald A., Englund J.A. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respi Viruses. 2012;6:71–77. doi: 10.1111/j.1750-2659.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.