Abstract
Purpose
The seasonal distribution patterns of febrile seizures and of respiratory and enteric viral pathogens are similar. In this study, we analyzed trends in febrile seizures and viral infection in Korean children, using big data from the Korean Health Insurance Review and Assessment Service (HIRA).
Methods
We analyzed children younger than 6 years who visited the hospital and were diagnosed with febrile seizures from 2009 to 2016, using medical records in the HIRA database. A total of 666,136 medical records of children with a main or subdiagnosis of febrile seizure from 2008 to 2016 were included. Of these records, patients younger than 1 month and records before 2009 were excluded. Finally, 558,130 records were extracted.
Results
The medical records included 315,774 male children and 242,356 (43.4%) female children, with a mean age of 2.31 ± 1.31 years. The annual incidence of febrile seizure was 25.4 per 1000 person-years (27.9 for boys and 22.7 for girls). The ratio of male to female children was 1.30: 1, and records of 1-year-old children comprised the highest proportion (n = 210,400, 33.70%). The total monthly number of patients was highest in May (n = 64,969, 11.6%), and peaks were formed from April to July. The fewest patients were seen in October (n = 34,424, 6.17%). The most common viral pathogens were influenza in April and enterovirus during May–July.
Conclusion
The seasonal distribution of febrile seizures was high from late spring to summer, and influenza virus and enterovirus were most frequently associated.
Keywords: Febrile seizure, Seasonal distribution, Virus infection
1. Introduction
Febrile seizure is a benign convulsive disorder that affects 2–8% of children between the ages of 6 months and 5 years [1], [2] This disease is preceded by or accompanied with fever in the absence of other causes, such as central nervous system infection or metabolic disease [3]. The etiology of febrile seizures remains unclear, although fever, age, and genetic predisposition have been identified as major factors. In addition, multifactorial models based on genetic and environmental causes have been proposed recently [4]. Pediatric infectious diseases are the most frequent environmental causes, of which viral infections are the most frequent entities in children. Recent studies have explored the connections between febrile seizures and respiratory and enteric viral infections [5], [6].
Some studies have investigated the seasonal distribution of febrile seizures. In those studies, febrile seizures were reported to be associated with seasonal viral epidemics, and the two events exhibited similar trends [7], [8], [9], [10], [11]. However, those studies were mostly conducted at single institutions with small numbers of patients, and no studies have been based on data from an entire population. In South Korea, the National Health Insurance (NHI) covers approximately 98% of the national population. Moreover, the Korean Health Insurance Review and Assessment Service (HIRA) claims that their data includes 46 million patients each year. [12]. This study aimed to investigate seasonal trends in febrile seizures in a nationwide sample of South Korean children and to analyze the relationship between febrile seizures and commonly associated respiratory and enteral viral infections.
2. Materials and methods
2.1. Big data from the Korean Health Insurance Review and Assessment Service (HIRA) database
The NHI is the only compulsory public medical insurance system in South Korea [13]. This program covers almost 98% of the health insurance administered to South Koreans [12], [14]. Big data from HIRA includes information for most of the population of South Korea and are provided to researchers for academic purposes, using a strict screening process. All data provided to researchers are anonymized. This study was approved by the Institutional Review Board of Chung-Ang University Hospital (IRB no.: 1710-005-16112). Informed consent from each patient was not required because of the anonymized nature of the information extracted from the HIRA database.
2.2. Study population
The study population included patients who were diagnosed with febrile seizure and included in the HIRA database from 2008 to 2016. Our initial request for data did not include children aged ≥6 years, according to the diagnostic criteria of febrile seizures. The diagnoses were coded using the 5th, 6th and 7th Korean Classifications of Diseases (KCD-5 from 2008 to 2010; KCD-6 from 2011 to 2015; and KCD-7 in 2016). KCD-5, KCD-6, and KCD-7 are classification systems modified from the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). We extracted medical records coded with a main or sub-diagnosis of febrile seizure (KCD-5, KCD-6, and KCD-7 code R560). A total of 666,136 records were selected from 2008 to 2016. Medical records in the HIRA database are classified by ages of <1 month, 1 month to 1 year, and ≥1 year. We excluded records with ages of <1 month according to the definition of febrile seizure [15]. We also excluded records from 2008, which was the first year in which the HIRA database was compiled. A total of 108,006 records were excluded. Finally, a total of 558,130 medical records with a diagnosis of febrile seizure were selected for this study.
Among the cases diagnosed with a febrile seizure, 472,482 included additional diagnostic codes along with febrile seizure, and 32,337 records were related to specific viral infections.
2.3. Medical records of identified viral infections with febrile seizure
Patients diagnosed with febrile seizure were classified according to virus-related diagnoses. Eleven respiratory and enteric viruses were selected: enterovirus, rotavirus, norovirus, astrovirus, influenza virus, respiratory syncytial virus (RSV), adenovirus, parainfluenza virus, metapneumovirus, coronavirus, and bocavirus. Enterovirus infection records were defined using codes B084 and B085. Rotavirus infection records were defined using code A080. Norovirus infection records were defined using code A081.02. Astrovirus infection records were defined using code A0831. Influenza virus infection records were defined using codes J09, J10, J100, J101, J110, and J111. RSV infection records were defined using codes B974, J121, J205, and J210. Adenovirus infection records were defined using codes A082, B340, B970, and J120. Parainfluenza infection records were defined using codes B978.03, J122, J204, and J2181. Metapneumovirus infection records were defined using codes B978.01, J123 and J211. Coronavirus infection records were defined using codes B342 and B972. Bocavirus infection records were defined using codes J1280, J2080 and J2180.
2.4. Data and statistical analysis
The data analysis was performed using SAS Enterprise version 9.2 (SAS Institute, Cary, NC, USA) and the HIRA database system via remote access. The statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The mean age and mean values in medical records were estimated using the t-test. We used a Pearson correlation analysis to determine the correlations of febrile seizure with viral infection. A two-tailed P value of <0.05 was considered significant.
3. Results
3.1. Characteristics of febrile seizure medical records
A total of 558,130 records with a diagnosis of febrile seizures were included in the HIRA database from 2009 to 2016. These records corresponded to physician billing data associated with hospital visits; accordingly, a single patient could have multiple records. We did not exclude these records because multiple febrile seizure events could occur in a single patient. The records included 315,774 (56.6%) male children and 242,356 (43.4%) female children with a ratio of 1.30: 1, indicating a male predominance. The mean age of the patients was 2.31 ± 1.31 years. The highest number of cases was recorded among patients aged 1 year (210,403, 37.7%), followed by 137,093 (24.5%) among those aged 2 years. Patients older than 2 years exhibited a gradually decreasing trend beginning at 3 years of age, and the lowest frequency was observed at 5 years (25,754, 4.6%) (Table 1 ).
Table 1.
Characteristics of febrile seizure records.
| Variable | Number of patients (%) |
|---|---|
| Boys | 315,744 (56.6%) |
| Age | 2.31 ± 1.31 |
| <1 year | 65,652 (11.8%) |
| 1 year | 210,400 (37.7%) |
| 2 years | 137,093 (24.5%) |
| 3 years | 75,815 (13.6%) |
| 4 years | 43,406 (7.8%) |
| 5 years | 25,764 (4.6%) |
| Number of records per year | |
| 2009 | 68,968 (12.4%) |
| 2010 | 71,980 (12.9%) |
| 2011 | 59,554 (10.7%) |
| 2012 | 80,543 (14.4%) |
| 2013 | 71,185 (12.7%) |
| 2014 | 74,279 (13.3%) |
| 2015 | 63,689 (11.4%) |
| 2016 | 67,932 (12.2%) |
| Seasonal number of records | |
| Spring | 166,701 (29.9%) |
| Summer | 154,535 (27.7%) |
| Autumn | 109,229 (19.5%) |
| Winter | 127,665 (22.9%) |
| Total | 558,130 (100%) |
The average annual number of cases diagnosed with febrile seizures was 69,766 (95% confidence interval [CI]: 64,397–75,135). The highest annual number of febrile seizure records was recorded in 2012 (80,643, 14.4%), while the lowest frequency was recorded in 2011 (59,554, 10.7%) (Table 1). Because the HIRA database covers the entire Korean population, we calculated the annual incidence of febrile seizure according to the average population of Korean children younger than 6 years. This number was 2,749,488 (1,414,696 for boys and 1,334,792 for girls). The annual incidence of febrile seizure was calculated as 25.4 per 1000 person-years (27.9 for boys and 22.7 for girls).
3.2. Monthly distribution of febrile seizure medical records
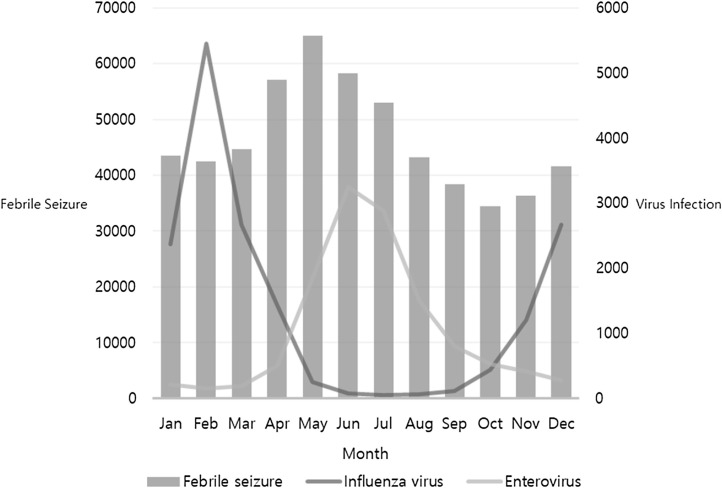
From 2009 to 2016, the monthly average number of febrile seizure records was 46,510 (95% CI: 40,420–52,601). The highest monthly number of febrile seizure records was recorded in May (64,969, 11.64%), followed by June (58,265, 10.44%). A peak in the number of febrile seizure records was observed from April to July. The lowest number of febrile seizure records was recorded in October (34,242, 6.17%) (Fig. 1 ).
Fig. 1.
Monthly distributions of influenza virus, enterovirus and febrile seizures.
3.3. Correlation of febrile seizure with other diagnoses associated with viral infection
Of the 558,130 included medical records, 472,482 (84.7%) included diagnoses of febrile seizures along with additional diagnoses. Of these records, we investigated 32,337 (6.8%) that included virus-related diagnoses. The influenza virus was the most common etiology of infection, according to these records (16,809, 52.0%), followed by enterovirus (12,571, 38.9%), and these two viruses accounted for the majority of cases (29,380, 90.9%). Rotavirus, RSV, and adenovirus cases were also included. However, the frequencies of these viral infections were much lower than those caused by influenza virus and enterovirus. Other viruses accounted for less than 1% of all viral infections (Table 2 ).
Table 2.
Number of viral infections diagnosed with febrile seizure.
| Viruses | Number of records (%) | |
|---|---|---|
| Influenza virus | 16,809 (52.0%) | |
| Enterovirus | 12,571 (38.9%) | |
| Rotavirus | 998 (3.1%) | |
| RSV | 671 (2.1%) | |
| Adenovirus | 639 (2.0%) | |
| Parainfluenza virus | 263 (0.8%) | |
| Metapneumovirus | 203 (0.6%) | |
| Coronavirus | 85 (0.3%) | |
| Norovirus | 59 (0.2%) | |
| Bocavirus | 38 (0.1%) | |
| Astrovirus | 1 (0.0%) | |
| Total | 32,337 (100.0%) | |
During the peak period of febrile seizure records (from April to July), enterovirus was the most frequent causative virus. Influenza virus infection was only most common in April, whereas enterovirus infection was most common from May to July (Fig. 1). Rotavirus was prevalent from January to April, and RSV was prevalent in November and December. Adenovirus exhibited a consistent monthly distributional pattern throughout the year (Table 3 ).
Table 3.
Monthly records of viral infections diagnosed with febrile seizure.
| January | February | March | April | May | June | July | August | September | October | November | December | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enterovirus | 212 | 158 | 190 | 506 | 1842 | 3246 | 2883 | 1504 | 806 | 534 | 413 | 277 |
| Rotavirus | 115 | 159 | 186 | 169 | 107 | 62 | 33 | 39 | 16 | 23 | 21 | 68 |
| Norovirus | 11 | 3 | 2 | 8 | 1 | 6 | 1 | 3 | 0 | 2 | 6 | 16 |
| Astrovirus | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Influenza virus | 2375 | 5452 | 2673 | 1425 | 259 | 81 | 48 | 64 | 111 | 443 | 1210 | 2668 |
| RSV | 72 | 38 | 28 | 31 | 28 | 8 | 10 | 6 | 26 | 63 | 162 | 199 |
| Adenovirus | 52 | 34 | 39 | 77 | 65 | 52 | 47 | 59 | 68 | 45 | 52 | 49 |
| Parainfluenza virus | 2 | 10 | 11 | 31 | 62 | 39 | 31 | 27 | 15 | 10 | 18 | 7 |
| Metapneumovirus | 8 | 19 | 33 | 56 | 40 | 13 | 7 | 0 | 9 | 7 | 8 | 3 |
| Coronavirus | 11 | 7 | 3 | 10 | 11 | 0 | 5 | 2 | 2 | 2 | 9 | 23 |
| Bocavirus | 9 | 0 | 2 | 10 | 5 | 4 | 1 | 0 | 2 | 3 | 2 | 0 |
| Total | 2867 | 5880 | 3167 | 2324 | 2420 | 3511 | 3066 | 1704 | 1055 | 1132 | 1901 | 3310 |
Bold numbers refer to months in which febrile seizure records were frequent.
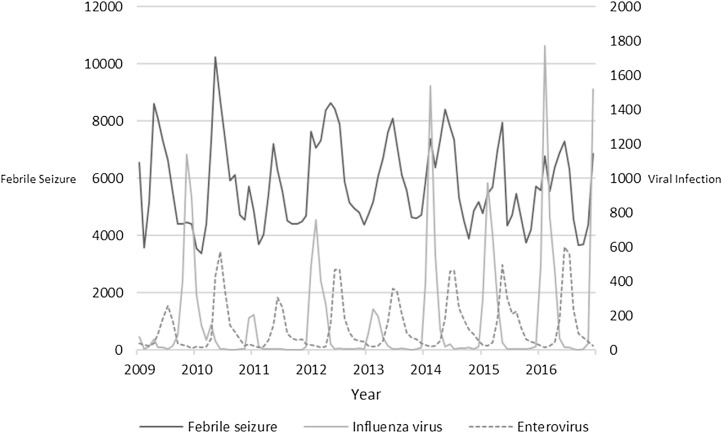
We further made a time series graph of febrile seizure, influenza virus and enterovirus during the study period (Fig. 2 ) and performed a correlation analysis using these data. We identified a significant correlation between febrile seizure and enterovirus infection. However, there was no significant correlation between febrile seizure and influenza virus infection (Table 4 ).
Fig. 2.
Time series of influenza virus, enterovirus and febrile seizures during the study period.
Table 4.
Correlations of the time series of febrile seizure with influenza virus and enterovirus.
| Characteristics | Febrile seizure | Viruses |
|||
|---|---|---|---|---|---|
| Influenza virus | Enterovirus | ||||
| Febrile seizure | r | 1 | |||
| P | – | ||||
| Viruses | Influenza virus | r | 0.96 | 1 | |
| P | 0.351 | – | |||
| Enterovirus | r | 0.504 | −0.329 | 1 | |
| P | <0.001 | 0.001 | – | ||
Significant findings with a P value <0.05 are in bold.
r = correlation coefficient.
4. Discussion
Our study describes the monthly and seasonal distributions of febrile seizures and the relationship of this disorder with associated respiratory and gastrointestinal viral infections in South Korea, based on an analysis of HIRA data. Many studies have investigated the seasonal distribution of febrile seizure. Most studies reported frequent febrile seizures in winter, but differed with respect to events in summer. According to a study conducted in Japan, febrile seizure was predominant during both the winter (from November to January) and summer (from June to August). This result was attributed to the prevalence of respiratory virus infection in winter and gastrointestinal virus infection in summer [7]. Similar results were reported in Italy and Iran [9], [20]. However, a study conducted in Finland reported that febrile seizure occurred most frequently in winter but was uncommon in summer [16]. The authors reported that this result was associated with febrile episodes, which were also frequent in winter and uncommon in summer [10]. In a study of febrile seizure distribution and associated viral infection in in Bosnia and Herzegovina, the distributions of febrile seizure from spring to winter were similar, and no statistically significant association with virus infection was observed [11]. Our study showed that febrile seizures occurred most frequently from late spring to summer. We believe that this result can be attributed to enterovirus infection, which occurs predominantly in the summer.
Influenza virus, enterovirus, rotavirus, RSV, and adenovirus were the most frequent types of viral infection among cases of febrile seizures in the HIRA database. Of these viruses, the pattern of enterovirus infection was most similar to that of febrile seizure, and these patterns exhibited a significant correlation. Previous studies internationally and in South Korea have reported the high prevalence of enterovirus infection in the summer [21], [22], [23], [24]. As enterovirus infection frequently causes a high fever, this high summer prevalence might be the cause of febrile seizures shown in this study. Enterovirus has an affinity for neuronal cells and is a major cause of central nervous system infections, such as viral meningitis. These central nervous system infections may present with mild symptoms, or with severe infection and consequent encephalitis associated with disability and death [25], [26]. In a study conducted in Japan, 13 of 21 children with febrile seizures in the summer yielded positive results in an enterovirus polymerase chain reaction examination of cerebrospinal fluid [27]. In our study, febrile seizures that occurred during summer might have been mild neurologic manifestations of enterovirus infections.
Influenza virus infection is a major viral infection associated with febrile seizure during the winter season. In Australia, the number of pediatric patients who visited the emergency department for febrile seizures increased in during influenza pandemics [28]. In China, the incidence of febrile seizures associated with influenza A infection was higher than that associated other respiratory viruses in a study of hospitalized children [29]. The rate of recurrent febrile seizure was also associated with influenza A infection [30]. In accordance with previous studies, we identified influenza virus infection as the most common viral infection associated with febrile seizures in winter. This finding suggests that influenza virus infection is the major cause of febrile seizures in the winter season.
After influenza virus and enterovirus, the next most frequent viruses in our study were rotavirus, RSV, and adenovirus. Rotavirus causes gastrointestinal infection and is prevalent in winter [31]. RSV, a respiratory virus, is prevalent in winter and is the most common cause of acute bronchiolitis in children younger than 2 years [32]. The incidence of adenovirus infection remains fairly consistent throughout the year [33]. In our study, the seasonal distribution patterns of rotavirus, RSV, and adenovirus infection were similar to those reported in previous studies. All these viruses appear to be associated with febrile seizures.
Our study had some limitations. First, the HIRA dataset lacked clinical information about febrile seizures. Therefore, we could not assess these events using detailed criteria (e.g., simple febrile seizure, complex febrile seizure and febrile status epilepticus). Second, the diagnoses of febrile seizure and viral infection were based on the codes in medical records, due to a limitation of the HIRA database. In reality, however, discrepancies between diagnostic codes and actual disease might occur in patients with febrile seizure. Third, in Korea, physicians commonly apply viral diagnostic methods such as PCR of stool samples and nasal swabs to hospitalized patients. In Korea, however, influenza virus is prevalent in winter, and a rapid enzyme immunoassay-based influenza diagnostic test was used frequently in local clinics and emergency rooms. Therefore, the high number of influenza virus infections may be due to diagnostic bias. However, our study provides a reliable assessment of the seasonal distribution of febrile seizures and the relationship of febrile seizure with viral infection at the national level, as the analysis was based on a database that covered almost the entire South Korean population.
5. Conclusion
This is the first nationwide HIRA database study in South Korea concerning the seasonal distributions of febrile seizures and associated viral infections. According to our findings, the prevalence of febrile seizure was high from late spring to summer, and enterovirus was the most commonly associated virus.
Authors’ contributions
Do Hoon Han and Soo Ahn Chae: Drs. Han and Chae designed and wrote the manuscript.
Inseok Lim, Sin Weon Yun, Dae Yong Yi, Na Mi Lee and Su Yeong Kim: Drs. Lim, Yun, Yi, Lee, and Kim investigated and analyzed the data (Health Insurance Review and Assessment Service: HIRA).
Dae Yong Yi, Na Mi Lee and Su Yeong Kim: Drs. Yi, Lee, and Kim initially reviewed the manuscript.
Soo Ahn Chae: Dr. Chae critically reviewed the manuscript.
All authors have read and approved the final manuscript.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgements
We thank So Jin Hong of the Big Data Division, Healthcare Data Convergence Department, Korean Health Insurance Review and Assessment Services. We thank Editage Language Editing Services for reviewing the manuscript, tables, and figures.
References
- 1.Depiero A.D., Teach S.J. Febrile seizures. Pediatr Emerg Care. 2001;17(5):384–387. doi: 10.1097/00006565-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Syndi Seinfeld D., Pellock J.M. Recent research on febrile seizures: a review. J Neurol Neurophysiol. 2013;4(165) doi: 10.4172/2155-9562.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadleir L.G., Scheffer I.E. Febrile seizures. BMJ. 2007;334(7588):307–311. doi: 10.1136/bmj.39087.691817.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kjeldsen M.J., Kyvik K.O., Friis M.L., Christensen K. Genetic and environmental factors in febrile seizures: a Danish population-based twin study. Epilepsy Res. 2002;51(1-2):167–177. doi: 10.1016/s0920-1211(02)00121-3. [DOI] [PubMed] [Google Scholar]
- 5.Pokorn M., Jevsnik M., Petrovec M., Steyer A., Mrvic T., Grosek S., et al. Respiratory and enteric virus detection in children. J Child Neurol. 2017;32(1):84–93. doi: 10.1177/0883073816670820. [DOI] [PubMed] [Google Scholar]
- 6.Francis J.R., Richmond P., Robins C., Lindsay K., Levy A., Effler P.V., et al. An observational study of febrile seizures: the importance of viral infection and immunization. BMC Pediatr. 2016;16(1):202. doi: 10.1186/s12887-016-0740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuboi T., Okada S. Seasonal variation of febrile convulsion in Japan. Acta Neurol Scand. 1984;69(5):285–292. doi: 10.1111/j.1600-0404.1984.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 8.Verburgh M.E., Bruijnzeels M.A., van der Wouden J.C., van Suijlekom-Smit L.W., van der Velden J., Hoes A.W., et al. Incidence of febrile seizures in the Netherlands. Neuroepidemiology. 1992;11(4-6):169–172. doi: 10.1159/000110928. [DOI] [PubMed] [Google Scholar]
- 9.Manfredini R., Vergine G., Boari B., Faggioli R., Borgna-Pignatti C. Circadian and seasonal variation of first febrile seizures. J Pediatr. 2004;145(6):838–839. doi: 10.1016/j.jpeds.2004.06.079. [DOI] [PubMed] [Google Scholar]
- 10.Mikkonen K., Uhari M., Pokka T., Rantala H. Diurnal and seasonal occurrence of febrile seizures. Pediatr Neurol. 2015;52(4):424–427. doi: 10.1016/j.pediatrneurol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 11.ZubČEviĆ S., ĐElmo T. The seasonal distribution of febrile seizures does not follow the seasonal distribution of febrile illnesses in infants and toddlers. Pedijatrija Danas: Pediatrics Today. 2015;11(2):40–47. [Google Scholar]
- 12.Kim L., Kim J.-A., Kim S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol Health. 2014;36 doi: 10.4178/epih/e2014008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D.S. Introduction: health of the health care system in Korea. Soc Work Public Health. 2010;25(2):127–141. doi: 10.1080/19371910903070333. [DOI] [PubMed] [Google Scholar]
- 14.Park Y.-T., Yoon J.-S., Speedie S.M., Yoon H., Lee J. Health insurance claim review using information technologies. Healthc Inform Res. 2012;18(3):215–224. doi: 10.4258/hir.2012.18.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auvin S., Vallee L. [Febrile seizures: current understanding of pathophysiological mechanisms] Arch Pediatr. 2009;16(5):450–456. doi: 10.1016/j.arcped.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Sillanpaa M., Camfield P., Camfield C., Haataja L., Aromaa M., Helenius H., et al. Incidence of febrile seizures in Finland: prospective population-based study. Pediatr Neurol. 2008;38(6):391–394. doi: 10.1016/j.pediatrneurol.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Sharafi R., Hassanzadeh Rad A., Aminzadeh V. Circadian rhythm and the seasonal variation in childhood febrile seizure. Iran J Child Neurol. 2017;11(3):27–30. [PMC free article] [PubMed] [Google Scholar]
- 21.Durey A., Je Y.S., Kwon H.Y., Im J.H., Baek J.H., Lee S.M., et al. Enterovirus infection in adults presenting with nonspecific febrile illness during summer. Infect Chemother. 2017;49(2):140–141. doi: 10.3947/ic.2017.49.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pichichero M.E., McLinn S., Rotbart H.A., Menegus M.A., Cascino M., Reidenberg B.E. Clinical and economic impact of enterovirus illness in private pediatric practice. Pediatrics. 1998;102(5):1126–1134. doi: 10.1542/peds.102.5.1126. [DOI] [PubMed] [Google Scholar]
- 23.Strikas R.A., Anderson L.J., Parker R.A. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970-1983. J Infect Dis. 1986;153(2):346–351. doi: 10.1093/infdis/153.2.346. [DOI] [PubMed] [Google Scholar]
- 24.Cha S.H. Recently prevalent infectious diseases among children: meningitis due to enteroviral infection. J Korean Med Assoc. 2008;51(10):935–941. [Google Scholar]
- 25.Huang H.I., Shih S.R. Neurotropic enterovirus infections in the central nervous system. Viruses. 2015;7(11):6051–6066. doi: 10.3390/v7112920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudolph H., Schroten H., Tenenbaum T. Enterovirus infections of the central nervous system in children: an update. Pediatr Infect Dis J. 2016;35(5):567–569. doi: 10.1097/INF.0000000000001090. [DOI] [PubMed] [Google Scholar]
- 27.Hosoya M., Sato M., Honzumi K., Katayose M., Kawasaki Y., Sakuma H., et al. Association of nonpolio enteroviral infection in the central nervous system of children with febrile seizures. Pediatrics. 2001;107(1):E12. doi: 10.1542/peds.107.1.e12. [DOI] [PubMed] [Google Scholar]
- 28.Polkinghorne B.G., Muscatello D.J., Macintyre C.R., Lawrence G.L., Middleton P.M., Torvaldsen S. Relationship between the population incidence of febrile convulsions in young children in Sydney, Australia and seasonal epidemics of influenza and respiratory syncytial virus, 2003-2010: a time series analysis. BMC Infect Dis. 2011;11:291. doi: 10.1186/1471-2334-11-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiu S.S., Tse C.Y., Lau Y.L., Peiris M. Influenza A infection is an important cause of febrile seizures. Pediatrics. 2001;108(4):E63. doi: 10.1542/peds.108.4.e63. [DOI] [PubMed] [Google Scholar]
- 30.van Zeijl J.H., Mullaart R.A., Borm G.F., Galama J.M. Recurrence of febrile seizures in the respiratory season is associated with influenza A. J Pediatr. 2004;145(6):800–805. doi: 10.1016/j.jpeds.2004.08.075. [DOI] [PubMed] [Google Scholar]
- 31.Gray J., Vesikari T., Van Damme P., Giaquinto C., Mrukowicz J., Guarino A., et al. Rotavirus. J Pediatr Gastroenterol Nutr. 2008;46(Suppl 2):S24–31. doi: 10.1097/MPG.0b013e31816f78ee. [DOI] [PubMed] [Google Scholar]
- 32.Borchers A.T., Chang C., Gershwin M.E., Gershwin L.J. Respiratory syncytial virus - a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–379. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch J.P., 3rd, Kajon A.E. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin Respir Crit Care Med. 2016;37(4):586–602. doi: 10.1055/s-0036-1584923. [DOI] [PMC free article] [PubMed] [Google Scholar]