Summary
The intestine is colonized by a considerable community of microorganisms that cohabits within the host and plays a critical role in maintaining host homeostasis. Recently, accumulating evidence has revealed that the gut microbial ecology plays a pivotal role in the occurrence and development of cardiovascular disease (CVD). Moreover, the effects of imbalances in microbe–host interactions on homeostasis can lead to the progression of CVD. Alterations in the composition of gut flora and disruptions in gut microbial metabolism are implicated in the pathogenesis of CVD. Furthermore, the gut microbiota functions like an endocrine organ that produces bioactive metabolites, including trimethylamine/trimethylamine N‐oxide, short‐chain fatty acids and bile acids, which are also involved in host health and disease via numerous pathways. Thus, the gut microbiota and its metabolic pathways have attracted growing attention as a therapeutic target for CVD treatment. The fundamental purpose of this review was to summarize recent studies that have illustrated the complex interactions between the gut microbiota, their metabolites and the development of common CVD, as well as the effects of gut dysbiosis on CVD risk factors. Moreover, we systematically discuss the normal physiology of gut microbiota and potential therapeutic strategies targeting gut microbiota to prevent and treat CVD.
The fundamental purpose of this review is to summarize recent studies on illustrating the complex interactions between the gut microbiota, their metabolites and the development of common CVD, as well as the effects of gut dysbiosis on CVD risk factors. Moreover, we systematically discuss the normal physiology of gut microbiota and potential therapeutic strategies targeting gut microbiota to prevent and treat CVD.
![]()
Introduction
Cardiovascular disease (CVD), including hypertension, atherosclerosis, cardiomyopathy and heart failure, is a leading cause of high morbidity and mortality, producing immense health and economic burdens globally (Mozaffarian, et al., 2016). Inflammation, dyslipidaemia and diabetes mellitus are common pathological processes and risk factors, both of which can affect the initiation and progression of CVD (Zoungas, et al., 2014; Haybar, et al., 2019). Other widely known CVD risk factors include obesity, insulin resistance and unhealthy lifestyle choices, such as smoking, lack of exercise and poor dietary habits (GBD, 2013; Mozaffarian, et al., 2016). However, these risk factors cannot fully explain all of the consequences of CVD.
Recently, there has been growing interest in studies primarily focused on interactions between the gut microbiota and CVD. Genome sequencing and metagenomic analyses show that the presence of the gut microbiota has potential effects on CVD (Karlsson, et al., 2012; Vinje, et al., 2014). Accumulating evidence demonstrates that manipulation of the composition of the gut microbiota affects host cardiovascular phenotypes (Kootte, et al., 2012; Sirisinha, 2016). Furthermore, the gastrointestinal tract can be viewed as a diverse and enormous ecosystem that produces a considerable amount of microbial metabolites (Eckburg, et al., 2005), which can be absorbed into the circulation and serve as mediators of gut microbial effects on the host (Furusawa et al., 2013; Seldin, et al., 2016; Krautkramer, et al., 2017). Thus, the gut microbiota contributes directly or indirectly to our health status. In this review, we describe the normal composition and physiological role of the gut microbiota and highlight emerging discoveries about the gut microbiota and its associated metabolites that are involved in several common CVDs. We focus on experimental studies and potential molecular pathways, as well as clinical evidence. Moreover, we summarize findings on the association of the gut microbiota with inflammation, lipid metabolism disorders and diabetes, which underlie increased cardiovascular risk. Finally, we discuss therapeutic strategies for the improvement of human cardiovascular health via modulation of the gut microbial ecology.
Components and physiological roles of the gut microbiota
The term ‘microbiota’ describes a collection of microorganisms defined as ‘the ecological community of commensal, symbiotic and pathogenic microorganisms that literally share our body space’ (Grice and Segre, 2012). As we are abruptly and continuously exposed to the environment after birth, the gut is rapidly colonized by trillions (1013–1015) of normally non‐pathogenic bacteria (Human Microbiome Project Consortium, 2012; Sender, et al., 2016). Age is the major driver of functional taxa and metabolic performance reconstruction. Although the composition and metabolism functions of gut microbiota are more similar during early life, the divergence of gut microbial succession will increase with time (Cerdo, et al., 2018). In healthy individuals, the composition of the gut microbiota remains relatively stable, dominated mainly by a few phyla, for example Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria and Verrucomicrobia (Human Microbiome Project Consortium, 2012). These organisms inhabit different ecological niches on mucosal surfaces and in the gut lumen, forming complex biochemical interaction networks between themselves and their host (Shkoporov and Hill, 2019). However, there is considerable variation in bacterial diversity between individuals that is caused by differences in the host genome and also by lifestyle factors, such as diet, drug use and environmental exposure (Tamburini, et al., 2016).
The gut microbiota remains in a symbiotic relationship within us, its human host, and it makes crucial contributions that shape our health. Interactions between the gut microbiota and intestinal cells regulate barrier functions, continually stimulating the immune system to defend against pathogens (De Santis et al., 2015). For instance, structural components of the microbiota, such as lipopolysaccharide (LPS) and peptidoglycan, can directly interact with host intestinal cells via Toll‐like receptors (TLRs) (Larsson, et al., 2012). Furthermore, gut microbes also affect the bioavailability and absorption of many oral drugs and can contribute to the production of pharmacologically active metabolites that can enhance toxicities or have other adverse effects on the host (Wallace, et al., 2015; Spanogiannopoulos, et al., 2016). Among the myriad of physiological functions, the most significant function of the gut microbiota is supporting the digestion of food components to allow the metabolites to interact with our human biology (Cani, et al., 2016). For example, vitamin synthesis and metabolic regulation in the intestine or distal organs occur via several metabolic molecules, including bile acids (BAs), short‐chain fatty acids (SCFAs), trimethylamine N‐oxide (TMAO), peptide YY (PYY) and glucagon‐like peptide 1 (GLP‐1) (Tremaroli, et al., 2015), indicating that gut microbiota functions as a pseudo‐organ with unparalleled endocrine potential. Different from host endocrine organs which produce only a few hormones, the gut microbiota performed as a high‐yield endocrine organ to produce hundreds of humoral molecules through metabolism‐dependent or metabolism‐independent pathways. Such gut microbial‐derived hormones are recognized by host receptors to elicit diverse biological effects (Brown and Hazen, 2015). Overall, emerging evidence supports the concept that the composition of the gut microbiota and its fundamental functions alters physiological responses relevant to the cardiometabolic health of the host.
Interactions between the gut microbiota and CVD
Hypertension
Hypertension is the most prevalent risk factor associated with CVD and is the leading cause of disability and death in developed countries, affecting 594 million in 1975 and as many as 1.13 billion in 2015 worldwide (NCD Risk Factor Collaboration, 2017). Gut dysbiosis and microbial functions affect pathological effects beyond the gastrointestinal system. Emerging evidence implicates the gut microbiota in blood pressure (BP) regulation, and abnormal bacterial communities are associated with hypertension (Li et al., 2017a,2017b; Wilck, et al., 2017).
Relatively few studies have examined the roles of the microbiota in hypertensive patients. A small study showed that hypertensive patients exhibited significantly lower diversity in their microbial communities and distinct clustering between normotensive individuals and hypertensive patients (Yang, et al., 2015). Patients with prehypertension and patients with hypertension had not only lower gene richness and α‐diversity than healthy controls, but also a higher percentage of bacteria from the genus Prevotella (Li, et al., 2017a). Although these differences between the disease groups do not prove causation, they do suggest that the metabolic profile associated with hypertension is linked to the composition of the gut microbiota in humans (Table 1). A study conducted in mice reported that maternal gut microbial modulation protected the adult male offspring against hypertension when they were fed a high‐fat diet and that a maternal high‐fat diet had long‐term programming effects on the adult offspring’s gut microbiota (Hsu et al., 2018). It has been postulated that the epigenome and microbiome might be inherited from the parents and that they might have more robust effects than previously believed. If this idea is true, then these factors might play more important roles than the host genome alone in the development of hypertension. Consistent with this idea, the gut microbiomes of monozygotic twins are more similar than those of dizygotic twins, and several taxa were recently shown to be heritable and stable for more than 3 years (Marques, 2018). The collection of faecal samples from families with well‐characterized hypertensive histories could help to address the contribution of the microbiota to essential hypertension related to the contributions of genetic, epigenetic and environmental factors; however, for now, this approach suffers from significant limitations. Horizontal gene transfer (HGT) refers to the genes transfer between organisms in a reproduction‐independent manner. HGT was first described in antibiotic resistance that could be transferred from one microbial strain to another laterally (Ochiai, et al., 1959). Although HGT is common among bacteria, the HGT rate of microbes in human gut is 25‐fold higher than that in other ecosystems (Smillie, et al., 2011), which foster HGT to be a key consideration for translating gut microbial genome into host hypertensive pathogenesis.
Table 1.
The association between gut microbiota and the development and progression of CVD.
| Hypertension | Atherosclerosis | Heart failure | |
|---|---|---|---|
| Participation of gut microbiota | α diversity↓, Prevotella↑, the F/B ratios↑, Erwinia↑, Corynebacteriac‐eae↑, Anaerostipes↓, Lactobacillus murinus↓ | Streptococcus↑, Enterobacteriaceae↑, Lactobacillales↑, Clostridium subcluster XIVa↑, Bacteroides↓ | Campylobacter↑, Candida↑, Shigella↑, Salmonella↑, Yersinia enterocolitica↑, Escherichia/Shigella↑ |
| Participation of gut microbial metabolites |
SCFAs 4‐ethylphenylsulfate |
TMAO↑ BAs, butyrate, NAPEs |
TMAO↑ Plasma primary BAs↓, specific secondary BAs↑ Propionate, PAG, PCS |
| Summary of potential mechanisms | GPCRs (GPR42, Olfr78 and Gper1) related signalling to elicit biological effects |
Intestinal permeability↑ Endothelial cell–cell conjunctions↓ and cell permeability↑ TLR activation↑ Macrophage scavenger receptors and CD36↑ NF‐κB and inflammasome activation↑ Cyp7a1 and Cyp27a1↓ Intracellular Ca2+ release↑ |
Intestinal perfusion↓ and congestion↑ Intestinal permeability↑ Prolong the effect of angiotensin↑ NLRP3 inflammasome‐associated TGF‐β/Smad3 signalling activation↑ |
| Medications |
Captopril: Allobaculum↑ Candesartan and Irbesartan: normalize the F/B ratio, preserve Lactobacillus levels |
Statins: Firmicutes↓, Proteobacteria↑, BAs alteration, butyrate↓ Aspirin:Prevotella, Bacteroides, Ruminococcaceae and Barnesiella alterations, TMAO‐mediated platelet hyper‐responsiveness↓ |
Digoxin: Eggerthella lenta could inactivate digoxin |
A growing body of literature reporting animal experiments supports the presence of interactions between the gut microbiota and hypertension. In a proof‐of‐concept study, ablation of the entire gut microbiota via an antibiotic cocktail significantly reduced the incidence of hypertension‐related aneurysms (Shikata, et al., 2019). Another study reported that germ‐free mice were protected from aortic vascular inflammation and endothelial dysfunction, as well as cardiac fibrosis and systolic dysfunction, and from angiotensin II (Ang II)‐induced hypertension (Karbach, et al., 2016). These experiments were not designed to test whether the gut microbiota had beneficial or harmful roles in the context of hypertension, but rather to reveal the contributions of the gut microbiota to the pathophysiology of hypertension and its related diseases. Evidence for a causative role of gut dysbiosis in the genesis of hypertension came from faecal microbiota transplantation experiments, in which dysbiotic faecal samples transferred from hypertensive patients and rat donors elevated the BP in normotensive mice and rat recipients respectively (Durgan, 2017; Li, et al., 2017a; Toral, et al., 2018; Toral, et al., 2019). These findings point to a strong correlation between hypertension and gut microbiota dysbiosis, revealing a possible contribution or even a cause–effect relationship between elevated BP and altered gut microbiota.
Short‐chain fatty acids are a major class of bacterial metabolites that are mainly generated in the colon via bacterial fermentation of dietary fibres (Cummings, et al., 1987). SCFAs, such as acetate and propionate, are produced mainly by Bacteroidetes, while butyrate is typically produced by Firmicutes (Kasselman, et al., 2018). Therefore, the ratio of the levels of faecal Firmicutes to Bacteroidetes (the F/B ratio) is a useful proxy for classifying the metabolic composition of the microbiome of an individual. Changes in bacteria composition associated with hypertension are followed by alterations in the levels of bacterial metabolic products (Kim, et al., 2018). This concept is corroborated by data from several studies in hypertensive models that demonstrated higher F/B ratio in affected hosts (Yang, et al., 2015; Marques, et al., 2017; Toral, et al., 2018). Furthermore, fibre‐rich diets or magnesium acetate and oral minocycline supplementation could restore gut microbiota homeostasis, reduce the F/B ratio and attenuate BP levels (Yang, et al., 2015; Marques, et al., 2017). In addition, treatment with propionate or butyrate could protect the host from cardiac damage in experimental hypertension models (Wang, et al., 2017; Bartolomaeus, et al., 2019). In summary, solid data clearly demonstrate the involvement and therapeutic potential of SCFAs in hypertension. One way that SCFAs influence host cells is by interacting with host G protein‐coupled receptors (GPCRs), including Gpr41 and Olfr78, among others. Activation of Gpr41 leads to hypotension, whereas Olfr78 stimulation favours elevated BP (Pluznick, 2013). Notably, the SCFA EC50 values (i.e. the concentration required to reach 50% of the maximal effect) for Gpr41 and Olfr78 vary significantly over a wide range, which perhaps at least partly explains why similar SCFAs can target both receptors to promote opposite BP trends. The complex effects of metabolite‐sensing GPCRs in the gut require further exploration (Table 1).
High salt intake promotes the development of hypertension. Since sodium absorption mainly occurs in intestine, the gut microbiota is likely involved in the salt sensitivity associated with hypertension. In high salt diet (HSD)‐induced hypertensive rats, the levels of taxa of the Erwinia genus and the Corynebacteriaceae families are increased. By contrast, taxa of the Anaerostipes genus exhibited decreased abundance versus their levels in the control (Bier, et al., 2018). HSD affects the rodent gut microbiota specifically by decreasing the abundance of Lactobacillus murinus as well as by inducing T helper 17 cells and salt‐sensitive hypertension, which are ameliorated via daily L. murinus supplementation (Wilck, et al., 2017). Moreover, several observations revealed that HSD is also related to changes in the levels of gut microbial metabolites. The level of 4‐ethylphenylsulfate, a gut microbial metabolite linked to benzoate metabolism, is closely related to sodium intake and increases upon sodium restriction (Derkach, et al., 2017) (Table 1). HSD‐fed rats displayed higher faecal levels of acetate and propionate than did the control (Bier, et al., 2018). In the Dahl salt‐sensitive rat hypertensive model, Gper1 deletion via CRISPR/Cas9 gene editing significantly reduced the F/B ratio and BP (Waghulde, et al., 2018). These observations indicate that gut microbiota‐targeted therapies could serve as a novel strategy for salt‐sensitive hypertension prevention and treatment and highlight a specific gene that could have a strong influence on the cross‐talk between the gut microbiota and BP regulation, and the underlying mechanisms are an intriguing target for further investigation.
Several studies have suggested that antihypertensive medications may lower BP via effects on the gut microbiota. Captopril (CAP) is an angiotensin‐converting enzyme inhibitor used clinically in antihypertensive applications, and increased Allobaculum levels can maintain a sustained antihypertensive effect even after CAP withdrawal (Yang, et al., 2019). Ang II receptor blockers, such as candesartan and irbesartan, are also commonly used as antihypertensive medications, and they have been reported to normalize the F/B ratio, preserve Lactobacillus levels and prevent gut microbial disruption (Yisireyili, et al., 2018; Wu et al., 2019a, 2019b) (Table 1).
Collectively, these studies support the concept that the gut microbiota and its metabolites are involved in the development and treatment of hypertension and that gut dysbiosis is not merely a consequence of high BP, but also a direct causal factor. The interactions between the gut microbiota, the environment and the host genome remain to be explored.
Atherosclerosis and thrombosis
Recent studies established a link between the gut microbiota and CVD by describing a case of bacterial translocation from the gut to the heart (Mitra, et al., 2015) and the detection of gut bacterial DNA in the plaques (Jonsson and Backhed, 2017), indicating that the intestine is a potential reservoir of pathogenic microorganisms and that the gut microbiota is involved in the development of atherosclerosis. A metagenomic analysis showed that the gut microbiome of atherosclerotic cardiovascular patients differed from those of healthy individuals as they contained elevated levels of Streptococcus and Enterobacteriaceae species (Jie, et al., 2017). Another study used the terminal restriction fragment length polymorphism method to identify the gut microbiota profiles of patients with coronary artery diseases revealed an increase in the Lactobacillales and Clostridium subcluster XIVa and a reduction in the Bacteroides in faecal samples (Emoto, et al., 2017). The above observations indicate that the gut microbiota is a potential source of plaque bacteria and that its composition is related to the development of atherosclerosis (Table 1).
Gut dysbiosis can also exert pro‐atherosclerotic effects via metabolism‐dependent pathways by altering the generation of various metabolites. Among the various gut microbiota‐derived metabolites, TMAO is a major contributor to the development of atherosclerosis. TMA, a TMAO precursor, is produced by two distinct types of bacterial enzymes, that is carnitine‐specific and choline‐specific lyases, which cleave specific carbon–nitrogen bonds to generate TMA (Koeth, et al., 2013). Once absorbed into the blood, TMA is metabolized into TMAO in the liver by the flavin‐containing monooxygenase family of enzymes (Tang and Hazen, 2014) (Fig. 1). Inhibition of microbial TMA production attenuated intermittent hypoxia (IH)‐induced pulmonary artery atherosclerosis in an obstructive sleep apnoea‐associated mouse model (Xue, et al., 2017). Another study reported that inhibition of the microbial TMA lyases could reduce atherosclerotic lesion formation in ApoE‐/‐ mice (Wang, et al., 2015). Notably, a growing body of clinical evidence related to the prognostic value of TMAO shows that elevated circulating TMAO levels are associated with the presence of coronary plaque vulnerability and that circulating TMAO can directly contribute to enhanced risk for CVD‐related consequences (Li, et al., 2017a; Qi, et al., 2018) (Table 2). Other gut microbiota‐generated metabolites, such as BAs and butyrate, exhibit atheroprotective effects (Jones, et al., 2012; Brown and Hazen, 2015), whereas N‐acyl phosphatidylethanolamines (NAPEs) have no significant effects on aortic lesion size but can markedly decrease the necrotic core area within atherosclerotic lesions (May‐Zhang, et al., 2019). These observations suggest the potential involvement of gut microbiota metabolism in the development of atherosclerosis. Statins, common cholesterol‐lowering drugs used as first‐line agents for coronary disease prevention, have been reported to alter the BA pool and to reduce butyrate production in the intestine (Caparros‐Martin, et al., 2017). HFD‐fed rats treated with atorvastatin showed reduced levels of Firmicutes and relatively increased levels of Proteobacteria, as well as reversion of the levels of some dominant taxa towards their levels in the normal chow diet‐fed control rats (Khan et al., 2018a,2018b). These studies highlight that CVD interventions can exert gut microbiota‐modifying effects and may have implications for the host pathophysiology (Table 1).
Figure 1.
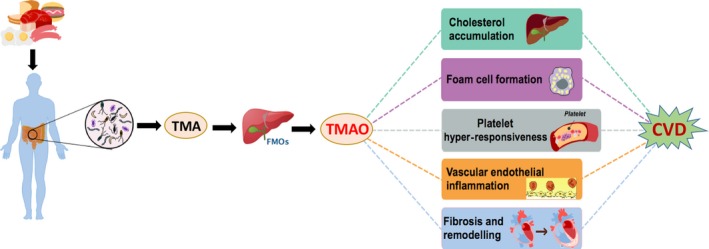
Schematic illustration of the links between the gut microbiota, TMAO and CVD. Dietary trimethylammonium (e.g. phosphatidylcholine, choline and l‐carnitine) can be metabolized into trimethylamine (TMA) by the gut microbiota. In the liver, TMA is converted into trimethylamine N‐oxide (TMAO) by flavin‐containing monooxygenases (FMOs). The effects of TMAO are associated with foam cell formation, alterations in cholesterol metabolism, platelet hyper‐responsiveness, vascular inflammation and adverse cardiac remodelling, all of which can contribute to CVD.
Table 2.
Clinical studies investigated links between TMAO and cardiometabolic diseases.
| Article | Study populations | Measurements | TMAO alterations | Summary of findings |
|---|---|---|---|---|
| (Zhu, et al., 2017) | Eight vegans/vegetarians and 10 omnivores (40% male, age 46 ± 5 years, non‐smokers without diabetes or CVD, no preceding (1‐month) history of antibiotics or probiotics) | Given oral choline supplementation for 2 months with monthly blood examination after overnight fast | TMAO↑ >10‐fold in all subjects at both 1‐ and 2‐month time points (P < 0.01 each) | Striking association between change (compared to baseline) in TMAO level and change (compared to baseline) in platelet aggregation (Spearman rho = 0.38, P = 0.03) |
| (Li et al., 2017a,2017b) | 530 sequential subjects presenting to the emergency department with suspected cardiac origin‐related chest pain within 24 h of onset |
Quantify plasma TMAO levels Reviews of medical records and follow‐up for the 30‐day, 6‐month outcomes Annually followed for all‐cause mortality |
TMAO↑ | Patients in the highest quartile of TMAO levels demonstrated adjusted incident MACE at 30 days (OR 6.30, 95% CI, 1.89–21.00, P < 0.01), 6 months (OR 5.65, 95% CI, 1.91–16.74, P < 0.01) and all‐cause mortality at 7 years (HR 1.81, 95% CI, 1.04–3.15, P < 0.05) |
| (Hayashi, et al., 2018) | 22 HF patients, 11 control subjects |
Gut flora evaluation by 16S rRNA Plasma microbes‐related metabolites evaluation by capillary electrophoresis time‐of‐flight mass spectrometry |
TMAO↑ in both compensated and decompensated HF patients | The genus Escherichial/Shigella is positively correlated with TMAO levels (r = 0.62, P = 0.002) and is more abundant in decompensated than in compensated HF (P = 0.030) |
| (Tang, et al., 2014) | 720 stable cardiac disease patients with a history of HF and undergoing elective coronary angiographic evaluation |
Quantify fasting plasma TMAO levels Tracking for all‐cause mortality for 5 years |
TMAO↑ (median level of HF: 5.0 μM; median level of control: 3.5 μM; P < 0.001) | TMAO is predictive of 5‐year mortality risk (adjusted HR 2.2, 95% CI, 1.42–3.43, P < 0.001) |
| (Winther, et al., 2019) | 1159 individuals with T1DM (58% male, age 46 ± 13 years) |
Quantify plasma TMAO levels Follow‐up for all‐cause and cardiovascular mortality, as well as CVD |
TMAO↑ |
Negative correlation between TMAO and eGFR (R 2 = 0.29, P < 0.0001) Elevated TMAO levels were associated with higher risk of all‐cause mortality (adjusted HR 1.19, 95% CI 1.09–1.29, P < 0.001), cardiovascular mortality (adjusted HR 1.21, 95% CI 1.05–1.39, P = 0.008), and combination CVD (adjusted HR 1.17, 95% CI 1.07–1.27, P < 0.001) in T1DM patients |
| (Tang, et al., 2017a) | 1216 stable T2DM patients undergoing elective coronary angiography |
Quantify fasting plasma TMAO levels Follow‐up for 3‐year MACE risk and 5‐year mortality |
TMAO↑ [4.4 μM (IQR 2.8–7.7 μM) vs 3.6 μM (2.3–5.7 μM), P < 0.001] | Elevated TMAO levels were associated with higher risk of MACE (adjusted HR 2.05, 95% CI 1.31–3.20, P < 0.001), and 5‐year mortality (adjusted HR 2.07, 95% CI .37–3.14, P < 0.001) in T2DM patients |
CI, confidence intervals; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IQR, interquartile range coefficient; MACE, major adverse cardiovascular events; OR, odds ratio; r, correlation of R 2: coefficient of determination.
Currently, some potential mechanisms for the pro‐atherosclerotic effects of gut microbiota have been proposed. Dysbiosis‐associated changes in bacterial composition can lead to increased intestinal permeability, leading to an elevation in circulating LPS levels. Circulating LPS can be sensed by Toll‐like receptor 4 (TLR4) and the downstream signals can be transduced by myeloid differentiation primary response 88 (MYD88) to promote inflammation and foam cell formation (Lau, et al., 2017). Additionally, signals sensed by TLRs cause B2 cell activation in the spleen, thus modifying IgG production to promote the development of atherosclerosis (Chen, et al., 2016). TMAO‐dependent upregulation of macrophage scavenger receptors and CD36 expression impair cholesterol metabolism in macrophages, thus promoting foam cell generation (Wang, et al., 2011). TMAO also inhibits the hepatic bile acid synthetic rate‐limiting enzymes Cyp7a1 and Cyp27a1, which results in decreased cholesterol elimination and reverse cholesterol transport (RCT) (Koeth, et al., 2013). Furthermore, TMAO induces vascular endothelial dysfunction via NF‐κB and inflammasome activation, thus elevating the expression of vascular endothelial inflammation factors (Ma, et al., 2017). Another study reported that TMAO‐induced endothelial dysfunction, including disruption of cell–cell junctions and alterations in cell permeability via activation of high‐mobility group box protein 1, is associated with TLR4 upregulation (Singh, et al., 2019). These data support the view that inflammatory mediators are critical in TMAO‐induced endothelial dysfunction and provide new targets for the treatment or prevention of atherosclerosis (Table 1).
Trimethylamine N‐oxide exerts its pro‐atherosclerotic effects not only by increasing macrophage foam cell formation and suppressing RCT, but also by enhancing platelet hyper‐reactivity (Tang and Hazen, 2014), which is associated with platelet aggregation and intra‐arterial thrombi generation. A large prospective cohort of patients undergoing elective coronary angiography showed that TMAO plasma levels were correlated with the incidence of arterial thrombotic events (Zhu, et al., 2017) (Table 2). The caecal microbial taxa are related to TMAO levels and thrombogenesis. TMAO promotes platelet hyper‐responsiveness by stimulating intracellular Ca2+ release (Table 1). In addition, caecal microbial transplantation experiments demonstrated that thrombogenetic potential is a transmissible trait (Zhu, et al., 2016). Consistent with these observations, deletion of the choline utilization C/D (CutC/D) gene, which encodes a rate‐limiting catalyser that converts TMA to TMAO in the host, could reduce thrombosis potential (Skye, et al., 2018). A low dose of aspirin could alter the gut microbial composition (Rogers and Aronoff, 2016) and attenuate the TMAO‐mediated platelet hyper‐responsiveness (Zhu, et al., 2016).
Although the molecular mechanisms of how gut microbiota‐derived TMAO can increase the incidence of atherosclerosis‐ and thrombosis‐related cardiovascular events are not entirely understood, the above observations suggest that TMAO could serve as a rational potential therapeutic target to decrease the risk for the development and progression of atherosclerotic disease and to attenuate pro‐thrombotic tendencies without bleeding complications. Furthermore, the clinical use of TMAO levels to identify subjects potentially suffering from atherosclerosis or thrombosis and who might benefit from CVD therapy is also an interesting area to explore.
Heart failure and cardiomyopathy
Heart failure (HF) includes a large body of complex clinical complications that result in damage to the function or structure of the heart, and it represents the end stage of many CVDs. Our knowledge of the pathogenic mechanisms underlying HF has considerably improved in recent years. The main concept has shifted from haemodynamic changes to neuro‐humoral modulation. Most recently, the link between the gut microbiota and HF has drawn more attention (Dantzer, et al., 2018; Moshkelgosha, et al., 2018).
There is considerable evidence supporting the role of the gut microbiota in the development and progression of HF, and the ‘gut hypothesis’ has been proposed. The gut hypothesis suggests that intestinal hypoperfusion and congestion due to reduced cardiac output could induce bowel wall oedema and impaired barrier function, thus leading to increased bacterial translocation‐related inflammatory responses and alterations in the gut microbiota that could further exacerbate HF (Sandek, et al., 2007; Nagatomo and Tang, 2015; Organ, et al., 2016) (Fig. 2). HF patients with lower intestinal blood flow had higher anti‐LPS IgA serum levels, which were associated with enhanced growth of bacteria obtained from biopsies of the colonic mucosa (Sandek, et al., 2014). Comparison of the faecal bacteria of HF patients with those of healthy individuals revealed that the chronic HF patients were colonized by more pathogenic bacteria, including Campylobacter, Shigella, Salmonella and Yersinia enterocolitica (Lim, 2016), and species as Candida, Campylobacter and Shigella were positively correlated with HF severity (Pasini, et al., 2016). Furthermore, Escherichia/Shigella were more abundant in decompensated HF than in compensated HF over a relatively short period (Yoshihisa, 2018). Digoxin, a cardenolide used for HF treatment, could be inactivated by the human gut actinobacterium Eggerthella lenta (Haiser, et al., 2013; Koppel, et al., 2018) (Table 1). Individual differences in the human gut microbiota lead to metabolic differences that alter the effects of drugs used for HF management (Zhernakova, et al., 2016). These observations emphasize the potential relationships between the pharmacology of CVD drugs and our microbial genomes. Moreover, the development of personalized HF treatment approaches should be considered.
Figure 2.
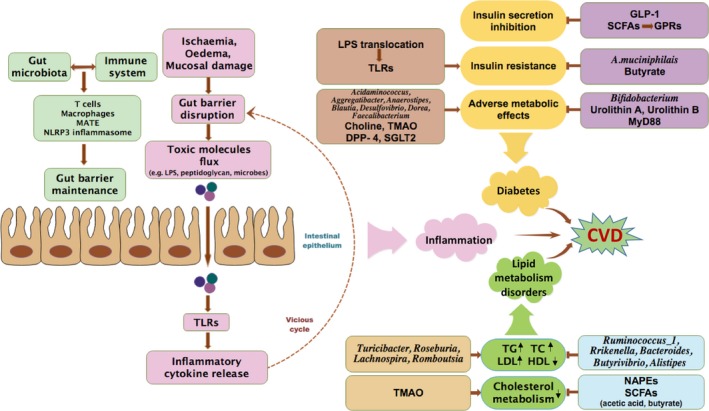
Diagram of the cardiovascular risks associated with inflammation, lipid metabolism disorders and diabetes related to gut microbial ecology. The gut microbiota interacts with the host immune system to maintain gut–barrier functions. Pathological disorders, including ischaemia and oedema, are associated with alterations in gut–barrier functions. A leaky intestinal barrier allows for increased flux of pro‐inflammatory bacterial products into the circulation, causing chronic low‐grade inflammation mainly via TLR activation. Consequently, elevated levels of inflammatory cytokines in the bloodstream are potent inducers of intestinal permeability, thus resulting in the formation of a vicious cycle that promotes toxic molecule translocation and inflammation. Furthermore, TLR activation also contributes to insulin resistance. Changes in the gut microbial composition are related to abnormal serum lipid levels and other adverse metabolic effects, whereas some species shifts result in beneficial effects on metabolism. As for gut microbial metabolites, TMAO induces diabetes‐related metabolic effects and disrupts cholesterol metabolism, whereas urolithin A, urolithin B, NAPEs and SCFAs have protective properties. Molecules such as DPP‐4, SGLT2, GLP‐1 and MyD88 are potential mediators that link the gut microbiota to CVD risk. Diabetes and lipid metabolism disorders, together with inflammation, are risk factors that contribute to CVD development and progression. The brown arrows with single arrowhead indicate the effects of promotion, whereas the horizontal T shape indicates the effects of inhibition; the brown arrows with double arrowhead indicate the interactions with each other; the brown arrow in dash line indicates the feedback; the black arrows indicate the elevation and reduction.
Metabolic derangements resulting from altered gut microbial composition and function may also influence HF development. HF patients had higher TMAO serum levels than control subjects (Table 2). Additionally, decompensated HF patients tended to exhibit a higher CutC/D gene expression level compared with that of compensated HF patients (Hayashi, et al., 2018). In HF patients, higher TMAO levels were associated with 1.18‐ to 1.79‐fold higher mortality and cardiac transplantation rates respectively (Kanitsoraphan, et al., 2018). Elevated plasma TMAO level is a remarkably strong adverse prognostic marker and provides a basis for HF risk stratification. Researchers found that elevated plasma TMAO levels in combination with proBNP were better biomarkers for evaluating the long‐term mortality risk in patients suffering from not only chronic HF, but also from acute HF (Tang, et al., 2014; Suzuki, et al., 2018). Some other gut‐derived metabolites also have effects on HF. Microbial metabolism has a pivotal impact on BA metabolism. BA composition and size were altered in HF patients. In a cohort study, decreased plasma primary BA levels and increased specific secondary BA levels have been observed in patients with chronic HF (Mayerhofer, et al., 2017). The SCFA propionate significantly attenuated cardiac hypertrophy, fibrosis and vascular dysfunction, mainly via regulatory T cells (Bartolomaeus, et al., 2019). Besides the gut metabolites mentioned above, phenyl‐acetyl‐glutamine (PAG) is a strong and independent risk factor for CVD and mortality (Poesen, et al., 2016), and p‐cresyl sulfate (PCS) might function as a predictor of cardiovascular events and all‐cause mortality (Lin, et al., 2013) (Table 1).
In animal experiments, the aforementioned gut microbiota‐derived metabolite TMAO has also been studied. Both choline supplementation (a precursor for TMAO production) and direct dietary TMAO supplementation resulted in higher systemic TMAO levels, increased myocardial fibrosis and worsened functional parameters in transaortic constriction‐induced HF (Organ, et al., 2016). Consumption of a Western diet leads to elevated circulating TMAO levels, subsequently contributing to cardiac fibrosis and dysfunction. Multiple studies indicate that TMAO can affect HF susceptibility and development, partially via increased myocardial fibrosis, ventricular remodelling and the accompanying cardiac dysfunction (Fig. 2). The underlying mechanism by which TMAO aggravates myocardial fibrosis is not fully understood. TMAO prolongs the effect of angiotensin, eventually leading to adverse cardiac remodelling (Ufnal, et al., 2014). Another mechanistic link is that TMAO promotes myocardial fibrosis via activation of NLRP3 inflammasome‐associated TGF‐β/Smad3 signalling; in addition, the finding that NLRP3 silencing can abolish the increases in the levels of IL‐1β, TGF‐β and other profibrotic markers induced by TMAO in cardiac fibroblasts (Li, et al., 2019) suggests that TMAO could be a new potential target for retarding the development of myocardial remodelling and the progression of myocardial functional impairment (Table 1).
These observations imply that an assessment of intestinal barrier function and reliable and reproducible biomarkers may bring greater mechanistic understanding of the impacts of gut‐HF therapy and lead to the development of HF diagnostic strategies. However, the link between increased TMAO levels and HF progression is not fully understood and remains to be determined. Future studies should examine the potential roles of the intestinal microbial composition and metabolism to design novel therapeutic targets in order to tailor treatment strategies to the wide inter‐individual variation for the management of HF.
The link between the gut microbiota and CVD‐related risk factors
Inflammation
Epidemiologic studies show a correlation between the mediators of inflammation and the risk of cardiovascular events (Kaptoge, et al., 2014). Intestinal barrier impairment disrupts host immune homeostasis and is associated with diverse inflammatory diseases (Shreiner, et al., 2015), including CVD. Multiple factors can lead to intestinal barrier function disruption, for example, decreased intestinal perfusion in HF patients particularly affects the structure of the villi (and microvilli), which are susceptible to ischaemia (Jodal and Lundgren, 1986). Bowel wall oedema is associated with the congestion that results from decreased cardiac output (Romano, et al., 2015). Furthermore, increased collagen content in mucosal walls corresponds to HF (Arutyunov, et al., 2008). Due to constant exposure to trillions of microbial antigens, the intestine is constantly at high risk of barrier dysfunction. The gut microbes could maintain and modify the gut barrier directly (Jakobsson, et al., 2015). Commensal bacteria can regulate the differentiation and function of host T cells, thus triggering the participation of T cells in the maintenance of mucosal homeostasis. The MATE (microbial adhesion‐triggered endocytosis) process represents a novel communication pathway that contributes to commensal antigen recognition (Ladinsky, et al., 2019). SCFAs are also involved in the maintenance of the gut barrier by reducing luminal pH and inhibiting pathogenic microorganisms. Furthermore, such gut microbe‐derived molecules are used as a source of energy by intestinal bacteria and host colonocytes (Tang and Hazen, 2017). Interestingly, colonic IL‐10‐producing macrophages are regulated by the gut microbiota and play a role in impaired epithelial recovery in the colon; by contrast, the gut microbiota is dispensable for epithelial recovery in the small intestine (SI), and depletion of the gut microbiota does not alter IL‐10 production by SI macrophages or SI barrier restoration (Morhardt, et al., 2019). Activation of the NLRP3 inflammasome plays a protective role in the maintenance of intestinal epithelium homeostasis. However, once the epithelial barrier is disrupted, NLRP3 inflammasome activation exerts a deleterious effect (Zhen and Zhang, 2019) (Fig. 2).
Impaired intestinal barrier function leads to enhanced levels of circulating gut microbiota‐derived toxins and LPS, which is accompanied by increased inflammation and suppressed myocardial function (Romano, et al., 2015). For example, translocation of Weissella spp. from the gut (Kamboj, et al., 2015) and commensal Enterococci‐associated biofilm formation (Ch'ng, et al., 2019) contributed to infective endocarditis. Intestinal Streptococci were implicated in the onset of Kawasaki disease, although the precise aetiology remains unclear (Kinumaki, et al., 2015). Human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) inflict gut mucosal damage, leading to microbial translocation and persistent low‐grade chronic inflammation associated with the contribution of phospholipase to monocyte or macrophage activation, and these effects subsequently result in cardiovascular comorbidities (Kristoff, et al., 2014; Spadaro, et al., 2017) (Fig. 2). Thus, the gut microbiota might be a new target for improving the prognosis of HIV patients and limiting non‐infectious AIDS‐related comorbidities. Elevated concentrations of circulating bacterial DNA in heart disease patients (Dinakaran, et al., 2014), as well as higher LPS levels in the hepatic veins than those detected via direct sampling in the left ventricle, further confirmed that gut bacteria and their related products can be translocated from the gut (Peschel, et al., 2003). LPS induces the expression of a wide array of downstream inflammatory products, mainly via TLR4 signalling, which occurs in multiple cell types, such as dendritic cells, macrophages, cardiac fibroblasts and cardiomyocytes (Frangogiannis, 2014). Increase in LPS plasma concentration over three times the normal threshold is referred to as ‘metabolic endotoxaemia’ because it is prevalent in many metabolic diseases (Kasselman, et al., 2018), which alters CVD risk potentially. Bacteriophages, the most abundant members of the microbiota, activate IFN‐γ‐mediated immune responses via TLR9. Consistently, TLR9/IFN‐γ blockade abrogates phage‐mediated inflammation (Gogokhia, et al., 2019). Elevated levels of circulating inflammatory cytokines, such as TNFα, IL‐6 and CRP (C‐reactive protein), are potent inducers of intestinal permeability (Sandek, et al., 2007; Al‐Sadi, et al., 2013; Al‐Sadi, et al., 2014), which can promote a vicious feed‐forward cycle of heightened endotoxin translocation and inflammatory cytokine accumulation in the circulation (Fig. 2). Furthermore, targeted inhibition of this inflammatory pathway reduces the risk of incident adverse cardiovascular events (Ridker, et al., 2017). SCFAs, such as butyrate, have received increasing interest in relation to hypertension, and their main effects are modulation of the microbial composition and inflammation reduction (Wang, et al., 2017; Kim, et al., 2018). Propionate also exhibits hypotensive effects, possibly via expansion of the splenic Treg cell population (Bartolomaeus, et al., 2019).
As described above, the gut microbiota and intestinal homeostasis affect CVD, in which inflammation plays pivotal roles in determining the severity and progression of the disease. The above studies could generate further interest in the roles of the gut microbiota and endotoxin‐induced inflammation in the pathophysiologic mechanisms of CVD. Indeed, the range of investigations indicates that gut microbiota‐driven inflammatory pathways are potential therapeutic targets for novel pharmacological strategies.
Lipid metabolism disorders
Lipid metabolism disorders, such as dyslipidaemia and hyperlipidaemia, are major risk factors and driving forces for the development of CVD. In recent decades, the term ‘cardiometabolic syndrome’ has been mentioned in the context of various conditions (Yang et al., 2018a,2018b). A study of a population cohort identified metabolic risks related to microbiome functional parameters, such as lipoprotein particle composition, fatty acid saturation, and carbohydrate and sugar derivative metabolism that could predict the probability of CVD consequences (Kurilshikov, et al., 2019). A growing body of studies suggests that the gut microbiota can mechanistically impact host lipid levels. For example, a high‐fat diet alone was not sufficient to restore lipid absorption in germ‐free mice, indicating that the gut microbiota is required for adequate lipid absorption in the gut (Martinez‐Guryn, et al., 2018). Several microbes, including Turicibacter, Roseburia, Lachnospira and Romboutsia, showed positive relationships with abnormal serum triglyceride, total cholesterol and low‐density lipoprotein (LDL) levels, and a negatively correlated relationship with the serum high‐density lipoprotein (HDL) levels. By contrast, the abundance of Ruminococcus_1, Rikenella, Bacteroides, Butyrivibrio and Alistipes was negatively correlated with serum triglyceride, total cholesterol and LDL levels but were positively correlated with HDL levels (Wan, et al., 2018). Gut colonization with bacteria of the phylum Bacteroidetes altered bile salt hydrolase features, leading to changes in the gut bile acid composition that affected host metabolism and gene expression (Yao, et al., 2018) (Fig. 2).
Bioactive products generated by the host or commensal microbes modulate metabolic factors involved in CVD. Urolithin B, which is derived from ellagitannins by components of the gut microbiota, modulates gene expression levels involved in RCT and increases cholesterol efflux from macrophages into HDL particles, thus decreasing lipid deposition in plaques (Zhao, et al., 2019). However, another study reported that the Coriobacteriaceae family, which is involved in urolithin B production, is positively associated with total cholesterol and LDL levels and is a potential CVD risk biomarker (Romo‐Vaquero, et al., 2019). Other molecules, such as indoleamine 2,3‐dioxygenase, serotonin (5‐HT) and NAPEs, are also correlated with gut microbiota changes that alter lipid absorption or lipid metabolism, consequently linking the gut microbiota to cardiometabolic phenotypes (Laurans, et al., 2018; May‐Zhang, et al., 2019; Singhal, et al., 2019). Melatonin improves lipid metabolism, and the potential mechanism is related to gut microbiota reprogramming, especially with respect to Bacteroides‐ and Alistipes‐mediated acetic acid production (Yin, et al., 2018). Furthermore, sodium butyrate administration limits lipid deposition and HFD accumulation in mice (Fang, et al., 2019) (Fig. 2).
These studies demonstrate the roles of the gut microbiota and its associated molecules in regulating lipid metabolism in relation to CVD and show how the effects of host metabolism dysregulation can significantly impact the intestinal microbiome, which in turn likely impacts the overall host metabolic physiology, including cardiometabolic phenotypes. However, we have not yet proven whether gut microbiome perturbation is a driver or a parallel event in lipid metabolism disorders. To date, the underlying biological mechanisms through which the gut microbiota or its metabolites impact host lipid metabolism are poorly understood. Although previous studies provide a basis for cardiometabolic disease prevention and treatment, there is still a long way to go in understanding how the gut microbiota regulates host diseases.
Diabetes
Accumulating data from human and animals suggest that shifts in the gut microbiota play a fundamental role in diabetes, which is an important risk factor for CVD. For instance, Akkermansia muciniphila, a single species of the human gut microbiota, has gained considerable attention since reductions in its abundance are closely linked to insulin resistance, diabetes and other cardiometabolic comorbidities (Hänninen, et al., 2018; Cani, et al., 2019). Other genera found in the human gut microbiota, including Acidaminococcus, Aggregatibacter, Anaerostipes, Bifidobacterium, Blautia, Desulfovibrio, Dorea and Faecalibacterium, are also associated with type 2 diabetes mellitus (T2DM) (Yang, et al., 2018a). In coronary artery disease patients with T2DM, significant decreases in the abundance of commensal or beneficial bacteria and increases in the abundance of opportunistic pathogens were detected (Sanchez‐Alcoholado, et al., 2017).
With regard to the development of diabetes and its cardiovascular comorbidities, changes in the levels of metabolites produced by organisms in the gut microbiota should also be emphasized. Excessive TMAO was detected in T2DM patients with coronary artery disease or chronic kidney disease as well as in type 1 diabetes mellitus (T1DM) patients, and this elevation in TMAO predicts adverse cardiac outcomes and mortality in diabetic patients (Al‐Obaide, et al., 2017; Sanchez‐Alcoholado, et al., 2017; Winther, et al., 2019) (Table 2). Specifically, butyrate has attracted more attention since it is proven to improve insulin sensitivity and to induce beneficial metabolic effects via prevention of metabolic endotoxaemia, enhancement of mitochondrial activity and profound immunometabolic effects (Tilg and Moschen, 2014; Hartstra, et al., 2015; Tang et al., 2017a, 2017b). Urolithin A and urolithin B were reported to reduce diabetes‐induced microenvironmental changes in cardiac tissue and to prevent myocardial dysfunction (Savi, et al., 2017) (Fig. 2). The above‐mentioned gut microbial metabolites linked to common metabolic disorders and CVD are promising targets for reducing diabetes‐associated cardiovascular abnormalities.
Several potential mechanisms have been proposed to link the gut microbiota with diabetes and adverse diabetes‐related cardiovascular events. Gut microbial composition shifts are a signature of low‐grade inflammation (Scheithauer, et al., 2016; Allin, et al., 2018), which interacts with reactive oxygen species to promote atherosclerosis in diabetic individuals (Yuan, et al., 2019). Translocated LPS binds to TLRs to induce insulin resistance and PCSK9 transcription, which in turn limit the clearance of cholesterol and LPS from the blood (Morelli, et al., 2019), thus increasing the risk for CVD. SCFAs could alter the metabolic state via activation of nuclear receptors such as PPARs (Hasan, et al., 2019) or specific GPRs such as GPR41 and GPR43 that are involved in the release of the entero hormone PYY (Samuel, et al., 2008), fat accumulation reduction, energy expenditure (Kimura, et al., 2013) and insulin secretion (Tolhurst, et al., 2012). Several molecules, including GLP‐1, MyD88, dipeptidyl peptidase‐4 (DPP‐4) and sodium glucose cotransporter 2 (SGLT2), interact closely with the gut microbiota to modulate insulin secretion (Grasset, et al., 2017), diabetes‐related metabolic effects (Duparc, et al., 2017; Liao, et al., 2019) and diabetes‐induced vascular dysfunction (Lee, et al., 2018).
In summary, these observations reveal that the gut microbiota and microbial products might alter insulin sensitivity, the immune system and the metabolic ability of host, thus linking them to some disease states, such as diabetes and diabetic CVD. Further study of the casual mechanisms of gut microbial imbalance and diabetic CVD is warranted, as the results of such studies could reveal how specific gut bacteria and their metabolites may be employed as antidiabetic molecules and could suggest potential therapeutic strategies for cardiovascular benefits in diabetic patients with CVD.
Gut microbiota interventions for CVD
The multiple correlations between altered gut microbiota composition and its metabolites and CVD susceptibility have highlighted the gut microbiota as a novel regulator of CVD, and these relationships have become potential targets for novel therapeutics (Fig. 3).
Figure 3.
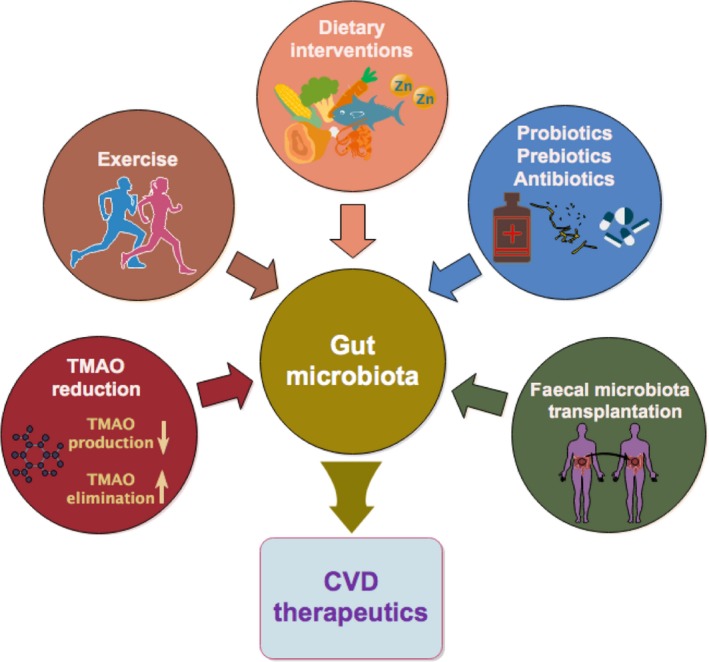
Interventions that target the gut microbiota for improving CVD therapeutics. Increasing evidence has proven the link between the gut microbiota and CVD, indicating that modification of the intestinal microbiota could help to prevent and manage CVD. Current strategies for manipulating gut microbes to elicit positive effects on the cardiovascular system include dietary interventions, use of pre‐ or probiotics and antibiotics, faecal microbiota transplantation, TMAO reduction and exercise.
Dietary interventions
Modulation of the gut microbiota via dietary intervention is a powerful approach for CVD prevention and therapy. A meta‐analysis indicated that higher fibre consumption was associated with lower risk for coronary diseases (Threapleton, et al., 2013) and significantly reduced BP in hypertensive patients (Aljuraiban, et al., 2015). Feeding mice with a high‐fibre diet increased the abundance of acetate‐producing gut bacteria, which significantly decreased myocardial hypertrophy and fibrosis (Marques, et al., 2017). Furthermore, a Mediterranean diet resulted in low TMAO levels, which can consequently prevent cardiovascular consequences and HF (Papadaki, et al., 2017; Estruch, et al., 2018). One study specifically demonstrated that ginger supplementation modulated the gut microbiota community, inducing a significant increase in fatty acid metabolism (Wang, et al., 2019). Furthermore, miRNAs contained by ginger‐derived exosome‐like nanoparticles could alter bacterial gene expression to affect the host (Teng, et al., 2018), representing promising precision tools for dietary therapy to manipulate the gut microbiota for improved consumer health.
Zinc is a trace element required by organisms, and it is a cofactor for some commensal bacterial proteins involved in metabolism (Cerasi, et al., 2013). Zinc deficiency can alter the microbial community composition and result in higher β‐diversity (Gaulke, et al., 2018); meanwhile, microbiota shifts can in turn aggravate the zinc‐deficient conditions (Reed, et al., 2015). However, during the early stage of zinc deficiency, gut microbial composition was mildly disrupted, whereas significant functional modifications were indicated by decreased choline demethylation efficiency (Mayneris‐Perxachs, et al., 2016). Zinc transporters, such as ZnT8 and ZnuABC, are involved in the maintenance of the cellular zinc level, deficiencies in which were reported to reshape the gut microbial pattern (Mao, et al., 2019) and impact the infectious ability of gut pathogens (Battistoni, et al., 2017); thus, zinc transporters are new targets for the treatment of metabolic dysfunction or infection‐related comorbidities. Dietary zinc–curcumin supplementation improved the gut dysbiosis associated with zinc dysregulation and the cardiotoxicity induced by doxorubicin in rats (Wu, et al., 2019a), providing a basis for future clinical studies to support the development of novel approaches for cardiotoxicity prevention. However, zinc supplementation affected the gut microbial composition in a dosage‐dependent manner (Watson, et al., 2018), and excess dietary zinc increased intestinal permeability and enriched pathogenic gut microbial species (Podany, et al., 2019), suggesting that zinc levels have critical effects on the susceptibility of individuals to pathogenic effects and disease severity.
Probiotics, prebiotics and antibiotics
Probiotics are live microorganisms administered to re‐establish an intestinal ecological balance. By contrast, prebiotics are non‐microbial and non‐digestible entities involved in microbial community modulation. Some preliminary evidence suggests that such supplements can be cardioprotective. In one study, Lactobacillus rhamnosus GR‐1 administration reduced infarct sizes and improved cardiac functions (Gan, et al., 2014). A randomized controlled pilot study reported that Saccharonyces boulardii had benefits in HF patients as indicated by improved left ventricular ejection fraction (Costanza, et al., 2015). Other common probiotics include Bifidobacterium, Enterococcus and Streptococcus (Markowiak and Slizewska, 2017). However, in very sick patients with weak immunity, clinically applied probiotics might become opportunistic pathogens that can engender endocarditis (Kothari, et al., 2019), indicating that careful consideration is required before the administration of probiotics to vulnerable populations. Inulin (a prebiotic) supplementation significantly decreased atherosclerotic lesions in ApoE−/− mice (Jin, et al., 2019). Furthermore, some studies demonstrated that probiotics and prebiotics can beneficially modify lipid metabolism (Korcz, et al., 2018; Lew, et al., 2018). Several cardiovascular experiments have focused on the elimination of bacterially induced diseases via antibiotics. For example, vancomycin and minocycline interventions decreased systolic BP in spontaneously hypertensive rats (Galla, et al., 2018). Furthermore, ampicillin reduced atherosclerotic risk factors, such as lipoprotein levels (Rune, et al., 2016). However, the general consensus is that non‐specific antimicrobial approaches may lead to a wide range of side‐effects and that even probiotics with excellent safety records could potentially increase the risk of probiotic translocation into the circulation (Liong, 2008). Although these results suggest that prebiotics, probiotics and antibiotics are effective interventions for altering the function of colonizing microbes associated with cardiovascular health and disease, further investigation into how to preserve their beneficial effects while minimizing potential safety concerns is required.
Faecal microbiota transplantation
Faecal microbiota transplantation (FMT) is a possible therapeutic intervention designed to inhibit intestinal pathogens via the transfer of functional bacteria from healthy subjects into the gastrointestinal tract of patients, consequently reconstituting the normal functions of the gut microbiota (Gallo, et al., 2016). Numerous studies demonstrate that FMT is effective in the treatment of refractory and recurrent Clostridium difficile infection (van Nood, et al., 2013; Youngster, et al., 2014; Khan, et al., 2018a), likely via a secondary bile acid‐dependent mechanism (Buffie, et al., 2015). In an experimental autoimmune myocarditis mouse model, FMT could rebalance the gut microbiota community and attenuate myocarditis‐associated myocardial injury (Hu, et al., 2019). However, the obvious limitation of FMT is that endotoxins or infectious agents might also be transferred, thus leading to new complications. The potential of FMT for treating CVD requires further investigation to reduce the risk of adverse effects and to improve the efficiency of its application.
TMAO reduction therapeutics
Due to the numerous clinical links between TMAO and adverse CVD consequences as well as its adverse effects on cardiometabolic phenotypes, strategies for targeted inhibition of TMAO production and removal of TMAO or its precursor (TMA) have attracted attention. Treatment with DMB (a TMA‐lyase inhibitor) reduced TMA production and, consequently, limited the conversion to TMAO, thus significantly improving haemodynamic parameters and reduced ventricular remodelling (Chen, et al., 2017). Furthermore, DMB also plays a role in anti‐atherogenesis (Wang, et al., 2015; Xue, et al., 2017). Oral charcoal adsorbent (AST‐120) has been used clinically to remove uremic toxins to prevent the progression of cardiac hypertrophy and fibrosis in a model of chronic kidney disease plus HF (Fujii, et al., 2009). These results provide evidence to support the future development of interventions against cardiometabolic phenotypes. However, the efficacies of these strategies have not been fully demonstrated.
Exercise
Emerging research indicates that exercise modulates the gut microbiota to influence cardiac function. Several studies suggest that exercise elevates the ratio of Firmicutes to Bacteroidetes (Petriz, et al., 2014; Lambert, et al., 2015) and increases the levels of the bacterial metabolite butyrate (Allen, et al., 2018). Alterations in the gut microbial structure induced by physical exercise are associated with the prevention of cardiac dysfunction in myocardial infarction mice (Liu, et al., 2017). Interestingly, LPS levels are elevated in CVD and some cardiometabolic disorders (Kallio, et al., 2015), and high‐endurance training can decrease plasma LPS levels (Lira, et al., 2010). Notably, the benefits imparted by the gut microbiota disappeared after the suspension of endurance exercise training (Allen, et al., 2018), indicating that the effects of exercise on the gut microbiota were transient and reversible. Ultimately, the aforementioned findings highlight the benefits of gut microbiota modulation via physical exercises for the host beyond the cardiovascular system. However, longer duration and higher intensity aerobic training are required to induce significant and long‐term benefits.
Conclusions and future perspectives
In conclusion, complex correlations exist between the gut microbiota and CVD, as they mutually influence each other via microbiota‐associated products, the circulatory system, immune responses and metabolic changes. Modifications of the gut microbiota via dietary foods, FMT, pre‐ or probiotics, molecular inhibitors or binders and daily exercise can drastically alter the gut microbiota profile, thus driving the host cardiometabolism in a favourable direction. However, current studies do not typically determine how specific constituents of the microbiota or their products interact with each other or with their host nor have they elucidated the relevant underlying molecular players. Future investigations should focus on identifying, at a mechanistic level, whether the interconnected pathways underlying gut dysbiosis that contribute to CVD are causal, correlational or consequential. Unique genetic factors of the gut microbiota and host might predispose individuals towards CVD susceptibility. Microbial technologies related to combination of microbial omics with phenotypes could help to obtain the desired outcome (De Vrieze, et al., 2018), which will be promising strategies to compose and modulate gut microbiome for CVD prevention and therapeutics. Moreover, bacteria also produce membrane vesicles and release their non‐coding RNA into host cells, thus inducing epigenetic changes in the host. Therefore, future genome association studies should be combined with analyses of genetic factors associated with microbiota‐dependent epigenetic modifications. Although TMA/TMAO are potential biomarkers for CVD development, additional gut microbiota components or related metabolites should be explored for use as early CVD markers. Furthermore, clinical studies are required to test whether interventions targeting the pathways associated with these biomarkers could reduce adverse cardiac consequences. Tailored and personalized therapies could offer benefits by modulating microbiota‐cardiovascular cross‐talk.
Conflicts of interest
None declared.
Acknowledgements
The current study was supported in part by grants from the National Natural Science Foundation of China (No. 81770372 to S.Z. and No. 81570338 to Q.L.), and China‐U.S. University of Louisville Pediatric Research Exchange Training Program. We thank BioEdit for the language editing of the manuscript. Personnel expenses and partial research‐related expenses for Hi Xu were provided by Jilin University through a collaborative research agreement between the University of Louisville and Jilin University, Changchun, China.
Microbial Biotechnology (2020) 13(3), 637–656
Funding information
The current study was supported by grants from the National Natural Science Foundation of China (No. 81770372 to S.Z. and No. 81570338 to Q.L.).
Contributor Information
Shanshan Zhou, Email: 36581940@qq.com.
Quan Liu, Email: quanliu888@163.com.
References
- Aljuraiban, G. S. , Griep, L. M. , Chan, Q. , Daviglus, M. L. , Stamler, J. , Van Horn, L. , et al (2015) Total, insoluble and soluble dietary fibre intake in relation to blood pressure: the INTERMAP Study. Br J Nutr 114: 1480–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, J. M. , Mailing, L. J. , Niemiro, G. M. , Moore, R. , Cook, M. D. , White, B. A. , et al (2018) Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 50: 747–757. [DOI] [PubMed] [Google Scholar]
- Allin, K. H. , Tremaroli, V. , Caesar, R. , Jensen, B. A. H. , Damgaard, M. T. F. , Bahl, M. I. , et al (2018) Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 61: 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Obaide, M. A. I. , Singh, R. , Datta, P. , Rewers‐Felkins, K. A. , Salguero, M. V. , Al‐Obaidi, I. , et al (2017) Gut Microbiota‐dependent trimethylamine‐N‐oxide and serum biomarkers in patients with T2DM and advanced CKD. J Clin Med 6, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Sadi, R. , Guo, S. , Ye, D. , and Ma, T. Y. (2013) TNF‐alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk‐1. Am J Pathol 183: 1871–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Sadi, R. , Ye, D. , Boivin, M. , Guo, S. , Hashimi, M. , Ereifej, L. , and Ma, T. Y. (2014) Interleukin‐6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin‐2 gene. PLoS ONE 9: e85345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arutyunov, G. P. , Kostyukevich, O. I. , Serov, R. A. , Rylova, N. V. , and Bylova, N. A. (2008) Collagen accumulation and dysfunctional mucosal barrier of the small intestine in patients with chronic heart failure. Int J Cardiol 125: 240–245. [DOI] [PubMed] [Google Scholar]
- Bartolomaeus, H. , Balogh, A. , Yakoub, M. , Homann, S. , Marko, L. , Hoges, S. , et al (2019) Short‐chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation 139: 1407–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistoni, A. , Ammendola, S. , Chiancone, E. , and Ilari, A. (2017) A novel antimicrobial approach based on the inhibition of zinc uptake in Salmonella enterica. Future Med Chem 9: 899–910. [DOI] [PubMed] [Google Scholar]
- Bier, A. , Braun, T. , Khasbab, R. , Di Segni, A. , Grossman, E. , Haberman, Y. , and Leibowitz, A. (2018) A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt‐sensitive hypertension rat model. Nutrients 10: 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. M. , and Hazen, S. L. (2015) The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med 66: 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffie, C. G. , Bucci, V. , Stein, R. R. , McKenney, P. T. , Ling, L. , Gobourne, A. , et al (2015) Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani, P. D. , Plovier, H. , Van Hul, M. , Geurts, L. , Delzenne, N. M. , Druart, C. , and Everard, A. (2016) Endocannabinoids–at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol 12: 133–143. [DOI] [PubMed] [Google Scholar]
- Cani, P. D. , Van Hul, M. , Lefort, C. , Depommier, C. , Rastelli, M. , and Everard, A. (2019) Microbial regulation of organismal energy homeostasis. Nature metabolism 1: 34. [DOI] [PubMed] [Google Scholar]
- Caparros‐Martin, J. A. , Lareu, R. R. , Ramsay, J. P. , Peplies, J. , Reen, F. J. , Headlam, H. A. , et al (2017) Statin therapy causes gut dysbiosis in mice through a PXR‐dependent mechanism. Microbiome 5: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasi, M. , Ammendola, S. , and Battistoni, A. (2013) Competition for zinc binding in the host‐pathogen interaction. Front Cell Infect Microbiol 3: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdo, T. , Ruiz, A. , Acuna, I. , Jauregui, R. , Jehmlich, N. , Haange, S. B. , et al (2018) Gut microbial functional maturation and succession during human early life. Environ Microbiol 20: 2160–2177. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Ishigami, T. , Nakashima‐Sasaki, R. , Kino, T. , Doi, H. , Minegishi, S. , and Umemura, S. (2016) Commensal microbe‐specific activation of B2 cell subsets contributes to atherosclerosis development independently of lipid metabolism. EBioMedicine 13: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, K. , Zheng, X. , Feng, M. , Li, D. , and Zhang, H. (2017) Gut microbiota‐dependent metabolite trimethylamine N‐oxide contributes to cardiac dysfunction in western diet‐induced obese mice. Front Physiol 8: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'ng, J. H. , Chong, K. K. L. , Lam, L. N. , Wong, J. J. , and Kline, K. A. (2019) Biofilm‐associated infection by enterococci. Nat Rev Microbiol 17: 82–94. [DOI] [PubMed] [Google Scholar]
- Costanza, A. C. , Moscavitch, S. D. , Faria Neto, H. C. , and Mesquita, E. T. (2015) Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double‐blind, placebo‐controlled pilot trial. Int J Cardiol 179: 348–350. [DOI] [PubMed] [Google Scholar]
- Cummings, J. H. , Pomare, E. W. , Branch, W. J. , Naylor, C. P. , and Macfarlane, G. T. (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28: 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer, R. , Cohen, S. , Russo, S. J. , and Dinan, T. G. (2018) Resilience and immunity. Brain Behav Immun 74: 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach, A. , Sampson, J. , Joseph, J. , Playdon, M. C. , and Stolzenberg‐Solomon, R. Z. (2017) Effects of dietary sodium on metabolites: the Dietary Approaches to Stop Hypertension (DASH)‐Sodium Feeding Study. Am J Clin Nutr 106: 1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakaran, V. , Rathinavel, A. , Pushpanathan, M. , Sivakumar, R. , Gunasekaran, P. , and Rajendhran, J. (2014) Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS ONE 9: e105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duparc, T. , Plovier, H. , Marrachelli, V. G. , Van Hul, M. , Essaghir, A. , Stahlman, M. , et al (2017) Hepatocyte MyD88 affects bile acids, gut microbiota and metabolome contributing to regulate glucose and lipid metabolism. Gut 66: 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan, D. J. (2017) Obstructive sleep apnea‐induced hypertension: role of the gut microbiota. Curr Hypertens Rep 19: 35. [DOI] [PubMed] [Google Scholar]
- Eckburg, P. B. , Bik, E. M. , Bernstein, C. N. , Purdom, E. , Dethlefsen, L. , Sargent, M. , et al (2005) Diversity of the human intestinal microbial flora. Science 308: 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto, T. , Yamashita, T. , Kobayashi, T. , Sasaki, N. , Hirota, Y. , Hayashi, T. , et al (2017) Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels 32: 39–46. [DOI] [PubMed] [Google Scholar]
- Estruch, R. , Ros, E. , Salas‐Salvado, J. , Covas, M. I. , Corella, D. , Aros, F. , et al (2018) Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra‐virgin olive oil or nuts. N Engl J Med 378: e34. [DOI] [PubMed] [Google Scholar]
- Fang, W. , Xue, H. , Chen, X. , Chen, K. , and Ling, W. (2019) Supplementation with sodium butyrate modulates the composition of the gut microbiota and ameliorates high‐fat diet‐induced obesity in mice. J Nutr 149: 747–754. [DOI] [PubMed] [Google Scholar]
- Frangogiannis, N. G. (2014) The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii, H. , Nishijima, F. , Goto, S. , Sugano, M. , Yamato, H. , Kitazawa, R. , et al (2009) Oral charcoal adsorbent (AST‐120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant 24: 2089–2095. [DOI] [PubMed] [Google Scholar]
- Furusawa, Y. , Obata, Y. , Fukuda, S. , Endo, T. A. , Nakato, G. , Takahashi, D. , et al (2013) Commensal microbe‐derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. [DOI] [PubMed] [Google Scholar]
- Galla, S. , Chakraborty, S. , Cheng, X. , Yeo, J. , Mell, B. , Zhang, H. , et al (2018) Disparate effects of antibiotics on hypertension. Physiol Genomics 50: 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, A. , Passaro, G. , Gasbarrini, A. , Landolfi, R. , and Montalto, M. (2016) Modulation of microbiota as treatment for intestinal inflammatory disorders: an uptodate. World J Gastroenterol 22: 7186–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, X. T. , Ettinger, G. , Huang, C. X. , Burton, J. P. , Haist, J. V. , Rajapurohitam, V. , et al (2014) Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail 7: 491–499. [DOI] [PubMed] [Google Scholar]
- Gaulke, C. A. , Rolshoven, J. , Wong, C. P. , Hudson, L. G. , Ho, E. , and Sharpton, T. J. (2018) Marginal zinc deficiency and environmentally relevant concentrations of arsenic elicit combined effects on the gut microbiome. mSphere 3: e00521-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD (2013) Mortality and Causes of Death Collaborators (2015) Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385: 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogokhia, L. , Buhrke, K. , Bell, R. , Hoffman, B. , Brown, D. G. , Hanke‐Gogokhia, C. , et al (2019) Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 25: 285–299.e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasset, E. , Puel, A. , Charpentier, J. , Collet, X. , Christensen, J. E. , Terce, F. , and Burcelin, R. (2017) A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP‐1 resistance through an enteric no‐dependent and gut‐brain axis mechanism. Cell Metab 25: 1075–1090.e1075. [DOI] [PubMed] [Google Scholar]
- Grice, E. A. , and Segre, J. A. (2012) The human microbiome: our second genome. Annu Rev Genomics Hum Genet 13: 151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiser, H. J. , Gootenberg, D. B. , Chatman, K. , Sirasani, G. , Balskus, E. P. , and Turnbaugh, P. J. (2013) Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 341: 295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen, A. , Toivonen, R. , Pöysti, S. , Belzer, C. , Plovier, H. , Ouwerkerk, J. P. , et al (2018) Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 67: 1445–1453. [DOI] [PubMed] [Google Scholar]
- Hartstra, A. V. , Bouter, K. E. , Backhed, F. , and Nieuwdorp, M. (2015) Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 38: 159–165. [DOI] [PubMed] [Google Scholar]
- Hasan, A. , Rahman, A. , and Kobori, H. (2019) Interactions between host PPARs and gut microbiota in health and disease. Int J Mol Sci 20: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, T. , Yamashita, T. , Watanabe, H. , Kami, K. , Yoshida, N. , Tabata, T. , et al (2018) Gut microbiome and plasma microbiome‐related metabolites in patients with decompensated and compensated heart failure. Circ J 83: 182–192. [DOI] [PubMed] [Google Scholar]
- Haybar, H. , Shokuhian, M. , Bagheri, M. , Davari, N. , and Saki, N. (2019) Involvement of circulating inflammatory factors in prognosis and risk of cardiovascular disease. J Mol Cell Cardiol 132: 110–119. [DOI] [PubMed] [Google Scholar]
- Hsu, C.‐N. , Lin, Y.‐J. , Hou, C.‐Y. , and Tain, Y.‐L. (2018) Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients 10: 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, X. F. , Zhang, W. Y. , Wen, Q. , Chen, W. J. , Wang, Z. M. , Chen, J. , et al (2019) Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol Res 139: 412–421. [DOI] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium (2012) Structure, function and diversity of the healthy human microbiome. Nature 486: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson, H. E. , Rodriguez‐Pineiro, A. M. , Schutte, A. , Ermund, A. , Boysen, P. , Bemark, M. , et al (2015) The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep 16: 164–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie, Z. , Xia, H. , Zhong, S. L. , Feng, Q. , Li, S. , Liang, S. , et al (2017) The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun 8: 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, M. , Qian, Z. , Yin, J. , Xu, W. , and Zhou, X. (2019) The role of intestinal microbiota in cardiovascular disease. J Cell Mol Med 23: 2343–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodal, M. , and Lundgren, O. (1986) Countercurrent mechanisms in the mammalian gastrointestinal tract. Gastroenterology 91: 225–241. [DOI] [PubMed] [Google Scholar]
- Jones, M. L. , Martoni, C. J. , Parent, M. , and Prakash, S. (2012) Cholesterol‐lowering efficacy of a microencapsulated bile salt hydrolase‐active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br J Nutr 107: 1505–1513. [DOI] [PubMed] [Google Scholar]
- Jonsson, A. L. , and Backhed, F. (2017) Role of gut microbiota in atherosclerosis. Nat Rev Cardiol 14: 79–87. [DOI] [PubMed] [Google Scholar]
- Kallio, K. A. , Hatonen, K. A. , Lehto, M. , Salomaa, V. , Mannisto, S. , and Pussinen, P. J. (2015) Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol 52: 395–404. [DOI] [PubMed] [Google Scholar]
- Kamboj, K. , Vasquez, A. , and Balada‐Llasat, J. M. (2015) Identification and significance of Weissella species infections. Front Microbiol 6: 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanitsoraphan, C. , Rattanawong, P. , Charoensri, S. , and Senthong, V. (2018) Trimethylamine N‐oxide and risk of cardiovascular disease and mortality. Curr Nutr Rep 7: 207–213. [DOI] [PubMed] [Google Scholar]
- Kaptoge, S. , Seshasai, S. R. , Gao, P. , Freitag, D. F. , Butterworth, A. S. , Borglykke, A. , et al (2014) Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta‐analysis. Eur Heart J 35: 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbach, S. H. , Schonfelder, T. , Brandao, I. , Wilms, E. , Hormann, N. , Jackel, S. , et al (2016) Gut microbiota promote angiotensin II‐induced arterial hypertension and vascular. Dysfunction, J Am Heart Assoc 5: e003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, F. H. , Fak, F. , Nookaew, I. , Tremaroli, V. , Fagerberg, B. , Petranovic, D. , et al (2012) Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3: 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasselman, L. J. , Vernice, N. A. , DeLeon, J. , and Reiss, A. B. (2018) The gut microbiome and elevated cardiovascular risk in obesity and autoimmunity. Atherosclerosis 271: 203–213. [DOI] [PubMed] [Google Scholar]
- Khan, M. Y. , Dirweesh, A. , Khurshid, T. , and Siddiqui, W. J. (2018a) Comparing fecal microbiota transplantation to standard‐of‐care treatment for recurrent Clostridium difficile infection: a systematic review and meta‐analysis. Eur J Gastroenterol Hepatol 30: 1309–1317. [DOI] [PubMed] [Google Scholar]
- Khan, T. J. , Ahmed, Y. M. , Zamzami, M. A. , Mohamed, S. A. , Khan, I. , Baothman, O. A. S. , et al (2018b) Effect of atorvastatin on the gut microbiota of high fat diet‐induced hypercholesterolemic rats. Sci Rep 8: 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Goel, R. , Kumar, A. , Qi, Y. , Lobaton, G. , Hosaka, K. , et al (2018) Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond) 132: 701–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, I. , Ozawa, K. , Inoue, D. , Imamura, T. , Kimura, K. , Maeda, T. , et al (2013) The gut microbiota suppresses insulin‐mediated fat accumulation via the short‐chain fatty acid receptor GPR43. Nat Commun 4: 1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinumaki, A. , Sekizuka, T. , Hamada, H. , Kato, K. , Yamashita, A. , and Kuroda, M. (2015) Characterization of the gut microbiota of Kawasaki disease patients by metagenomic analysis. Front Microbiol 6: 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth, R. A. , Wang, Z. , Levison, B. S. , Buffa, J. A. , Org, E. , Sheehy, B. T. , et al (2013) Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kootte, R. S. , Vrieze, A. , Holleman, F. , Dallinga‐Thie, G. M. , Zoetendal, E. G. , de Vos, W. M. , et al (2012) The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab 14: 112–120. [DOI] [PubMed] [Google Scholar]
- Koppel, N. , Bisanz, J. E. , Pandelia, M. E. , Turnbaugh, P. J. , and Balskus, E. P. (2018) Discovery and characterization of a prevalent human gut bacterial enzyme sufficient for the inactivation of a family of plant toxins eLife 7: e33953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korcz, E. , Kerenyi, Z. , and Varga, L. (2018) Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: potential health benefits with special regard to cholesterol‐lowering effects. Food Funct 9: 3057–3068. [DOI] [PubMed] [Google Scholar]
- Kothari, D. , Patel, S. , and Kim, S. K. (2019) Probiotic supplements might not be universally‐effective and safe: A review. Biomed Pharmacother 111: 537–547. [DOI] [PubMed] [Google Scholar]
- Krautkramer, K. A. , Dhillon, R. S. , Denu, J. M. , and Carey, H. V. (2017) Metabolic programming of the epigenome: host and gut microbial metabolite interactions with host chromatin. Transl Res 189: 30–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoff, J. , Haret‐Richter, G. , Ma, D. , Ribeiro, R. M. , Xu, C. , Cornell, E. , et al (2014) Early microbial translocation blockade reduces SIV‐mediated inflammation and viral replication. J Clin Invest 124: 2802–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilshikov, A. , van den Munckhof, I. C. , Chen, L. , Bonder, M. J. , Schraa, K. , Rutten, J. , et al (2019) Gut microbial associations to plasma metabolites linked to cardiovascular phenotypes and risk: a cross‐sectional study. Circ Res 124: 1808–1820. [DOI] [PubMed] [Google Scholar]
- Ladinsky, M. S. , Araujo, L. P. , Zhang, X. , Veltri, J. , Galan‐Diez, M. , Soualhi, S. , et al (2019) Endocytosis of commensal antigens by intestinal epithelial cells regulates mucosal T cell homeostasis. Science 363: eaat4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, J. E. , Myslicki, J. P. , Bomhof, M. R. , Belke, D. D. , Shearer, J. , and Reimer, R. A. (2015) Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab 40: 749–752. [DOI] [PubMed] [Google Scholar]
- Larsson, E. , Tremaroli, V. , Lee, Y. S. , Koren, O. , Nookaew, I. , Fricker, A. , et al (2012) Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut 61: 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, K. , Srivatsav, V. , Rizwan, A. , Nashed, A. , Liu, R. , Shen, R. , and Akhtar, M. (2017) Bridging the gap between gut microbial dysbiosis and cardiovascular diseases. Nutrients 9: 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurans, L. , Venteclef, N. , Haddad, Y. , Chajadine, M. , Alzaid, F. , Metghalchi, S. , et al (2018) Genetic deficiency of indoleamine 2,3‐dioxygenase promotes gut microbiota‐mediated metabolic health. Nat Med 24: 1113–1120. [DOI] [PubMed] [Google Scholar]
- Lee, D. M. , Battson, M. L. , Jarrell, D. K. , Hou, S. , Ecton, K. E. , Weir, T. L. , and Gentile, C. L. (2018) SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol 17: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, L. C. , Choi, S. B. , Khoo, B. Y. , Sreenivasan, S. , Ong, K. L. , and Liong, M. T. (2018) Lactobacillus plantarum dr7 reduces cholesterol via phosphorylation of AMPK that down‐regulated the mRNA expression of HMG‐CoA reductase. Korean J Food Sci Anim Resour 38: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Zhao, F. , Wang, Y. , Chen, J. , Tao, J. , Tian, G. , et al (2017a) Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. S. , Obeid, S. , Klingenberg, R. , Gencer, B. , Mach, F. , Raber, L. , et al (2017b) Gut microbiota‐dependent trimethylamine N‐oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 38: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Geng, J. , Zhao, J. , Ni, Q. , Zhao, C. , Zheng, Y. , et al (2019) Trimethylamine N‐oxide exacerbates cardiac fibrosis via activating the NLRP3 inflammasome. Front Physiol 10: 866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, X. , Song, L. , Zeng, B. , Liu, B. , Qiu, Y. , Qu, H. , et al (2019) Alteration of gut microbiota induced by DPP‐4i treatment improves glucose homeostasis. EBioMedicine 44: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, G. B. (2016) Heart failure: gut flora–pathogenic role in chronic heart failure. Nat Rev Cardiol 13: 61. [DOI] [PubMed] [Google Scholar]
- Lin, C. J. , Chuang, C. K. , Jayakumar, T. , Liu, H. L. , Pan, C. F. , Wang, T. J. , et al (2013) Serum p‐cresyl sulfate predicts cardiovascular disease and mortality in elderly hemodialysis patients. Arch Med Sci 9: 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liong, M. T. (2008) Safety of probiotics: translocation and infection. Nutr Rev 66: 192–202. [DOI] [PubMed] [Google Scholar]
- Lira, F. S. , Rosa, J. C. , Pimentel, G. D. , Souza, H. A. , Caperuto, E. C. , Carnevali, L. C. Jr , et al (2010) Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis 9: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Liu, H. Y. , Zhou, H. , Zhan, Q. , Lai, W. , Zeng, Q. , et al (2017) Moderate‐intensity exercise affects gut microbiome composition and influences cardiac function in myocardial infarction mice. Front Microbiol 8: 1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, G. , Pan, B. , Chen, Y. , Guo, C. , Zhao, M. , Zheng, L. , and Chen, B. (2017) Trimethylamine N‐oxide in atherogenesis: impairing endothelial self‐repair capacity and enhancing monocyte adhesion. Biosci Rep 37: BSR20160244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, Z. , Lin, H. , Su, W. , Li, J. , Zhou, M. , Li, Z. , et al (2019) Deficiency of ZnT8 promotes adiposity and metabolic dysfunction by increasing peripheral serotonin production. Diabetes 68: 1197–1209. [DOI] [PubMed] [Google Scholar]
- Markowiak, P. , and Slizewska, K. (2017) Effects of probiotics, prebiotics, and synbiotics on human. Health Nutr 9: 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, F. Z. (2018) Missing heritability of hypertension and our microbiome. Circulation 138: 1381–1383. [DOI] [PubMed] [Google Scholar]
- Marques, F. Z. , Nelson, E. , Chu, P. Y. , Horlock, D. , Fiedler, A. , Ziemann, M. , et al (2017) High‐fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135: 964–977. [DOI] [PubMed] [Google Scholar]
- Martinez‐Guryn, K. , Hubert, N. , Frazier, K. , Urlass, S. , Musch, M. W. , Ojeda, P. , et al (2018) Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe 23: 458–469.e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer, C. C. K. , Ueland, T. , Broch, K. , Vincent, R. P. , Cross, G. F. , Dahl, C. P. , et al (2017) Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail 23: 666–671. [DOI] [PubMed] [Google Scholar]
- Mayneris‐Perxachs, J. , Bolick, D. T. , Leng, J. , Medlock, G. L. , Kolling, G. L. , Papin, J. A. , et al (2016) Protein‐ and zinc‐deficient diets modulate the murine microbiome and metabolic phenotype. Am J Clin Nutr 104: 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May‐Zhang, L. S. , Chen, Z. , Dosoky, N. S. , Yancey, P. G. , Boyd, K. L. , Hasty, A. H. , et al (2019) Administration of N‐acyl‐phosphatidylethanolamine expressing bacteria to low density lipoprotein receptor(‐/‐) mice improves indices of cardiometabolic disease. Sci Rep 9: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra, S. , Drautz‐Moses, D. I. , Alhede, M. , Maw, M. T. , Liu, Y. , Purbojati, R. W. , et al (2015) In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 3: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli, M. B. , Wang, X. , and Santulli, G. (2019) Functional role of gut microbiota and PCSK9 in the pathogenesis of diabetes mellitus and cardiovascular disease. Atherosclerosis 289: 176–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morhardt, T. L. , Hayashi, A. , Ochi, T. , Quiros, M. , Kitamoto, S. , Nagao‐Kitamoto, H. , et al (2019) IL‐10 produced by macrophages regulates epithelial integrity in the small intestine. Sci Rep 9: 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshkelgosha, S. , Masetti, G. , Berchner‐Pfannschmidt, U. , Verhasselt, H. L. , Horstmann, M. , Diaz‐Cano, S. , et al (2018) Gut microbiome in BALB/c and C57BL/6J mice undergoing experimental thyroid autoimmunity associate with differences in immunological responses and thyroid function. Horm Metab Res 50: 932–941. [DOI] [PubMed] [Google Scholar]
- Mozaffarian, D. , Benjamin, E. J. , Go, A. S. , Arnett, D. K. , Blaha, M. J. , Cushman, M. , et al (2016) Heart disease and stroke statistics‐2016 update: a report from the american heart association. Circulation 133: e38–360. [DOI] [PubMed] [Google Scholar]
- Nagatomo, Y. , and Tang, W. H. (2015) Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail 21: 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration (2017) Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19.1 million participants. Lancet 389: 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nood, E. , Vrieze, A. , Nieuwdorp, M. , Fuentes, S. , Zoetendal, E. G. , de Vos, W. M. , et al (2013) Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368: 407–415. [DOI] [PubMed] [Google Scholar]
- Ochiai, K. , Yamanaka, T. , Kimura, K. , and Sawada, O. (1959) Inheritance of drug resistance (and its transfer) between Shigella strains and Between Shigella and E. coli strains. Hihon Iji Shimpor 34: 1861. [Google Scholar]
- Organ, C. L. , Otsuka, H. , Bhushan, S. , Wang, Z. , Bradley, J. , Trivedi, R. , et al (2016) Choline diet and its gut microbe‐derived metabolite, trimethylamine N‐oxide, exacerbate pressure overload‐induced heart failure. Circ Heart Fail 9: e002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadaki, A. , Martinez‐Gonzalez, M. A. , Alonso‐Gomez, A. , Rekondo, J. , Salas‐Salvado, J. , Corella, D. , et al (2017) Mediterranean diet and risk of heart failure: results from the PREDIMED randomized controlled trial. Eur J Heart Fail 19: 1179–1185. [DOI] [PubMed] [Google Scholar]
- Pasini, E. , Aquilani, R. , Testa, C. , Baiardi, P. , Angioletti, S. , Boschi, F. , et al (2016) Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 4: 220–227. [DOI] [PubMed] [Google Scholar]
- Peschel, T. , Schonauer, M. , Thiele, H. , Anker, S. D. , Schuler, G. , and Niebauer, J. (2003) Invasive assessment of bacterial endotoxin and inflammatory cytokines in patients with acute heart failure. Eur J Heart Fail 5: 609–614. [DOI] [PubMed] [Google Scholar]
- Petriz, B. A. , Castro, A. P. , Almeida, J. A. , Gomes, C. P. , Fernandes, G. R. , Kruger, R. H. , et al (2014) Exercise induction of gut microbiota modifications in obese, non‐obese and hypertensive rats. BMC Genom 15: 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznick, J. L. (2013) Renal and cardiovascular sensory receptors and blood pressure regulation. Am J Physiol Renal Physiol 305: F439–F444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podany, A. , Rauchut, J. , Wu, T. , Kawasawa, Y. I. , Wright, J. , Lamendella, R. , et al (2019) Excess dietary zinc intake in neonatal mice causes oxidative stress and alters intestinal host‐microbe interactions. Mol Nutr Food Res 63: e1800947. [DOI] [PubMed] [Google Scholar]
- Poesen, R. , Claes, K. , Evenepoel, P. , de Loor, H. , Augustijns, P. , Kuypers, D. , and Meijers, B. (2016) Microbiota‐derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J Am Soc Nephrol 27: 3479–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, J. , You, T. , Li, J. , Pan, T. , Xiang, L. , Han, Y. , and Zhu, L. (2018) Circulating trimethylamine N‐oxide and the risk of cardiovascular diseases: a systematic review and meta‐analysis of 11 prospective cohort studies. J Cell Mol Med 22: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, S. , Neuman, H. , Moscovich, S. , Glahn, R. P. , Koren, O. , and Tako, E. (2015) Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients 7: 9768–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker, P. M. , Everett, B. M. , Thuren, T. , MacFadyen, J. G. , Chang, W. H. , Ballantyne, C. , et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
- Rogers, M. A. M. , and Aronoff, D. M. (2016) The influence of non‐steroidal anti‐inflammatory drugs on the gut microbiome. Clin Microbiol Infect 22: 178.e171–178.e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano, K. A. , Vivas, E. I. , Amador‐Noguez, D. , and Rey, F. E. (2015) Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine‐N‐oxide. MBio 6: e02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo‐Vaquero, M. , Cortes‐Martin, A. , Loria‐Kohen, V. , Ramirez‐de‐Molina, A. , Garcia‐Mantrana, I. , Collado, M. C. , et al (2019) Deciphering the human gut microbiome of urolithin metabotypes: association with enterotypes and potential cardiometabolic health implications. Mol Nutr Food Res 63: e1800958. [DOI] [PubMed] [Google Scholar]
- Rune, I. , Rolin, B. , Larsen, C. , Nielsen, D. S. , Kanter, J. E. , Bornfeldt, K. E. , et al (2016) Modulating the gut microbiota improves glucose tolerance, lipoprotein profile and atherosclerotic plaque development in ApoE‐deficient mice. PLoS ONE 11: e0146439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, B. S. , Shaito, A. , Motoike, T. , Rey, F. E. , Backhed, F. , Manchester, J. K. , et al (2008) Effects of the gut microbiota on host adiposity are modulated by the short‐chain fatty‐acid binding G protein‐coupled receptor, Gpr41. Proc Natl Acad Sci USA 105: 16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Alcoholado, L. , Castellano‐Castillo, D. , Jordan‐Martinez, L. , Moreno‐Indias, I. , Cardila‐Cruz, P. , Elena, D. , et al (2017) Role of gut microbiota on cardio‐metabolic parameters and immunity in coronary artery disease patients with and without type‐2 diabetes mellitus. Front Microbiol 8: 1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandek, A. , Bauditz, J. , Swidsinski, A. , Buhner, S. , Weber‐Eibel, J. , von Haehling, S. , et al (2007) Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol 50: 1561–1569. [DOI] [PubMed] [Google Scholar]
- Sandek, A. , Swidsinski, A. , Schroedl, W. , Watson, A. , Valentova, M. , Herrmann, R. , et al (2014) Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol 64: 1092–1102. [DOI] [PubMed] [Google Scholar]
- De Santis, S. , Cavalcanti, E. , Mastronardi, M. , Jirillo, E. , and Chieppa, M. (2015) Nutritional keys for intestinal barrier modulation. Front Immunol 6: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vrieze, J. , Boon, N. , and Verstraete, W. (2018) Taking the technical microbiome into the next decade. Environ Microbiol 20: 1991–2000. [DOI] [PubMed] [Google Scholar]
- Savi, M. , Bocchi, L. , Mena, P. , Dall'Asta, M. , Crozier, A. , Brighenti, F. , et al (2017) In vivo administration of urolithin A and B prevents the occurrence of cardiac dysfunction in streptozotocin‐induced diabetic rats. Cardiovasc Diabetol 16: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheithauer, T. P. , Dallinga‐Thie, G. M. , de Vos, W. M. , Nieuwdorp, M. , and van Raalte, D. H. (2016) Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab 5: 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin, M. M. , Meng, Y. , Qi, H. , Zhu, W. , Wang, Z. , Hazen, S. L. , et al (2016) Trimethylamine N‐oxide promotes vascular inflammation through signaling of mitogen‐activated protein kinase and. nuclear factor‐kappaB. J Am Heart Assoc 5: e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender, R. , Fuchs, S. , and Milo, R. (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14: e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata, F. , Shimada, K. , Sato, H. , Ikedo, T. , Kuwabara, A. , Furukawa, H. , et al (2019) Potential influences of gut microbiota on the formation of intracranial aneurysm. Hypertension 73: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkoporov, A. N. , and Hill, C. (2019) Bacteriophages of the human gut: the "Known Unknown" of the microbiome. Cell Host Microbe 25: 195–209. [DOI] [PubMed] [Google Scholar]
- Shreiner, A. B. , Kao, J. Y. , and Young, V. B. (2015) The gut microbiome in health and in disease. Curr Opin Gastroenterol 31: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, G. B. , Zhang, Y. , Boini, K. M. , and Koka, S. (2019) High mobility group box 1 mediates TMAO‐induced endothelial dysfunction. Int J Mol Sci 20: 3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal, M. , Turturice, B. A. , Manzella, C. R. , Ranjan, R. , Metwally, A. A. , Theorell, J. , et al (2019) Serotonin transporter deficiency is associated with dysbiosis and changes in metabolic function of the mouse intestinal microbiome. Sci Rep 9: 2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisinha, S. (2016) The potential impact of gut microbiota on your health: current status and future challenges. Asian Pac J Allergy Immunol 34: 249–264. [DOI] [PubMed] [Google Scholar]
- Skye, S. M. , Zhu, W. , Romano, K. A. , Guo, C. J. , Wang, Z. , Jia, X. , et al (2018) Microbial transplantation with human gut commensals containing CutC is sufficient to transmit enhanced platelet reactivity and thrombosis potential. Circ Res 123: 1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie, C. S. , Smith, M. B. , Friedman, J. , Cordero, O. X. , David, L. A. , and Alm, E. J. (2011) Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480: 241. [DOI] [PubMed] [Google Scholar]
- Spadaro, F. , Cecchetti, S. , and Fantuzzi, L. (2017) Macrophages and phospholipases at the intersection between inflammation and the pathogenesis of HIV‐1 infection. Int J Mol Sci 18: 1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanogiannopoulos, P. , Bess, E. N. , Carmody, R. N. , and Turnbaugh, P. J. (2016) The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol 14: 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T. , Yazaki, Y. , Voors, A. A. , Jones, D. J. L. , Chan, D. C. S. , Anker, S. D. , et al (2018) Association with outcomes and response to treatment of trimethylamine N‐oxide in heart failure (from BIOSTAT‐CHF). Eur J Heart Fail 21, 1291–1294. [DOI] [PubMed] [Google Scholar]
- Tamburini, S. , Shen, N. , Wu, H. C. , and Clemente, J. C. (2016) The microbiome in early life: implications for health outcomes. Nat Med 22: 713–722. [DOI] [PubMed] [Google Scholar]
- Tang, W. H. , and Hazen, S. L. (2014) The contributory role of gut microbiota in cardiovascular disease. J Clin Invest 124: 4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. H. , and Hazen, S. L. (2017) The gut microbiome and its role in cardiovascular diseases. Circulation 135: 1008–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. H. , Wang, Z. , Fan, Y. , Levison, B. , Hazen, J. E. , Donahue, L. M. , et al (2014) Prognostic value of elevated levels of intestinal microbe‐generated metabolite trimethylamine‐N‐oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 64: 1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. H. , Kitai, T. , and Hazen, S. L. (2017a) Gut microbiota in cardiovascular health and disease. Circ Res 120: 1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. H. , Wang, Z. , Li, X. S. , Fan, Y. , Li, D. S. , Wu, Y. , and Hazen, S. L. (2017b) Increased trimethylamine N‐oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem 63: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, Y. , Ren, Y. , Sayed, M. , Hu, X. , Lei, C. , Kumar, A. , et al (2018) Plant‐derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe 24: 637–652.e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threapleton, D. E. , Greenwood, D. C. , Evans, C. E. , Cleghorn, C. L. , Nykjaer, C. , Woodhead, C. , et al (2013) Dietary fibre intake and risk of cardiovascular disease: systematic review and meta‐analysis. BMJ 347: f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg, H. , and Moschen, A. R. (2014) Microbiota and diabetes: an evolving relationship. Gut 63: 1513–1521. [DOI] [PubMed] [Google Scholar]
- Tolhurst, G. , Heffron, H. , Lam, Y. S. , Parker, H. E. , Habib, A. M. , Diakogiannaki, E. , et al (2012) Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein–coupled receptor FFAR2. Diabetes 61: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toral, M. , Romero, M. , Rodriguez‐Nogales, A. , Jimenez, R. , Robles‐Vera, I. , Algieri, F. , et al (2018) Lactobacillus fermentum improves tacrolimus‐induced hypertension by restoring vascular redox state and improving eNOS coupling. Mol Nutr Food Res 62, e1800033. [DOI] [PubMed] [Google Scholar]
- Toral, M. , Robles‐Vera, I. , de la Visitacion, N. , Romero, M. , Yang, T. , Sanchez, M. , et al (2019) Critical role of the interaction gut microbiota – sympathetic nervous system in the regulation of blood pressure. Front Physiol 10: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli, V. , Karlsson, F. , Werling, M. , Stahlman, M. , Kovatcheva‐Datchary, P. , Olbers, T. , et al (2015) Roux‐en‐Y gastric bypass and vertical banded gastroplasty induce long‐term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab 22: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufnal, M. , Jazwiec, R. , Dadlez, M. , Drapala, A. , Sikora, M. , and Skrzypecki, J. (2014) Trimethylamine‐N‐oxide: a carnitine‐derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol 30: 1700–1705. [DOI] [PubMed] [Google Scholar]
- Vinje, S. , Stroes, E. , Nieuwdorp, M. , and Hazen, S. L. (2014) The gut microbiome as novel cardio‐metabolic target: the time has come!. Eur Heart J 35: 883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghulde, H. , Cheng, X. , Galla, S. , Mell, B. , Cai, J. , Pruett‐Miller, S. M. , et al (2018) Attenuation of microbiotal dysbiosis and hypertension in a CRISPR/Cas9 gene ablation rat model of GPER1. Hypertension 72: 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, B. D. , Roberts, A. B. , Pollet, R. M. , Ingle, J. D. , Biernat, K. A. , Pellock, S. J. , et al (2015) Structure and inhibition of microbiome beta‐glucuronidases essential to the alleviation of cancer drug toxicity. Chem Biol 22: 1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, X. , Li, T. , Liu, D. , Chen, Y. , Liu, Y. , Liu, B. , et al (2018). Effect of marine microalga chlorella pyrenoidosa ethanol extract on lipid metabolism and gut microbiota composition in high‐fat diet‐fed rats. Mar Drugs 16: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Klipfell, E. , Bennett, B. J. , Koeth, R. , Levison, B. S. , Dugar, B. , et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Roberts, A. B. , Buffa, J. A. , Levison, B. S. , Zhu, W. , Org, E. , et al (2015) Non‐lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163: 1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Zhu, Q. , Lu, A. , Liu, X. , Zhang, L. , Xu, C. , et al (2017) Sodium butyrate suppresses angiotensin II‐induced hypertension by inhibition of renal (pro)renin receptor and intrarenal renin‐angiotensin system. J Hypertens 35: 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Wang, P. , Li, D. , Hu, X. , and Chen, F. (2019) Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur J Nutr. 10.1007/s00394-019-01938-1 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Watson, H. , Mitra, S. , Croden, F. C. , Taylor, M. , Wood, H. M. , Perry, S. L. , et al (2018) A randomised trial of the effect of omega‐3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 67: 1974–1983. [DOI] [PubMed] [Google Scholar]
- Wilck, N. , Matus, M. G. , Kearney, S. M. , Olesen, S. W. , Forslund, K. , Bartolomaeus, H. , et al (2017) Salt‐responsive gut commensal modulates TH17 axis and disease. Nature 551: 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther, S. A. , Ollgaard, J. C. , Tofte, N. , Tarnow, L. , Wang, Z. , Ahluwalia, T. S. , et al (2019) Utility of plasma concentration of trimethylamine N‐oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care 42: 1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, D. , Tang, X. , Ding, L. , Cui, J. , Wang, P. , Du, X. , et al (2019a) Candesartan attenuates hypertension‐associated pathophysiological alterations in the gut. Biomed Pharmacother 116: 109040. [DOI] [PubMed] [Google Scholar]
- Wu, R. , Mei, X. , Wang, J. , Sun, W. , Xue, T. , Lin, C. , and Xu, D. (2019b) Zn(ii)‐Curcumin supplementation alleviates gut dysbiosis and zinc dyshomeostasis during doxorubicin‐induced cardiotoxicity in rats. Food Funct 10: 5587–5604. [DOI] [PubMed] [Google Scholar]
- Xue, J. , Zhou, D. , Poulsen, O. , Imamura, T. , Hsiao, Y. H. , Smith, T. H. , et al (2017) Intermittent hypoxia and hypercapnia accelerate atherosclerosis, partially via trimethylamine‐oxide. Am J Respir Cell Mol Biol 57: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, T. , Santisteban, M. M. , Rodriguez, V. , Li, E. , Ahmari, N. , Carvajal, J. M. , et al (2015) Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Zhang, Y. , and Ren, J. (2018a) Autophagic regulation of lipid homeostasis in cardiometabolic syndrome. Front Cardiovasc Med 5: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q. , Lin, S. L. , Kwok, M. K. , Leung, G. M. , and Schooling, C. M. (2018b) The roles of 27 genera of human gut microbiota in ischemic heart disease, type 2 diabetes mellitus, and their risk factors: a mendelian randomization study. Am J Epidemiol 187: 1916–1922. [DOI] [PubMed] [Google Scholar]
- Yang, T. , Aquino, V. , Lobaton, G. O. , Li, H. , Colon‐Perez, L. , Goel, R. , et al (2019) Sustained captopril‐induced reduction in blood pressure is associated with alterations in gut‐brain axis in the spontaneously hypertensive rat. J Am Heart Assoc 8: e010721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, L. , Seaton, S. C. , Ndousse‐Fetter, S. , Adhikari, A. A. , DiBenedetto, N. , Mina, A. I. , et al (2018) A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 7: e37182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, J. , Li, Y. , Han, H. , Chen, S. , Gao, J. , Liu, G. , et al (2018) Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high‐fat diet‐fed mice. J Pineal Res 65: e12524. [DOI] [PubMed] [Google Scholar]
- Yisireyili, M. , Uchida, Y. , Yamamoto, K. , Nakayama, T. , Cheng, X. W. , Matsushita, T. , et al (2018) Angiotensin receptor blocker irbesartan reduces stress‐induced intestinal inflammation via AT1a signaling and ACE2‐dependent mechanism in mice. Brain Behav Immun 69: 167–179. [DOI] [PubMed] [Google Scholar]
- Yoshihisa, A. (2018) Altered gut flora and gut microbiome‐derived metabolites in heart failure patients in the compensated and decompensated phases. Circ J 83: 30–31. [DOI] [PubMed] [Google Scholar]
- Youngster, I. , Sauk, J. , Pindar, C. , Wilson, R. G. , Kaplan, J. L. , Smith, M. B. , et al (2014) Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open‐label, controlled pilot study. Clin Infect Dis 58: 1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, T. , Yang, T. , Chen, H. , Fu, D. , Hu, Y. , Wang, J. , et al (2019) New insights into oxidative stress and inflammation during diabetes mellitus‐accelerated atherosclerosis. Redox Biol 20: 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, W. , Wang, L. , Haller, V. , and Ritsch, A. (2019) A novel candidate for prevention and treatment of atherosclerosis: Urolithin B decreases lipid plaque deposition in apoE(‐/‐) mice and increases early stages of reverse cholesterol transport in ox‐LDL treated macrophages Cells. Mol Nutr Food Res 63: e1800887. [DOI] [PubMed] [Google Scholar]
- Zhen, Y. , and Zhang, H. (2019) NLRP3 inflammasome and inflammatory bowel disease. Front Immunol 10: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhernakova, A. , Kurilshikov, A. , Bonder, M. J. , Tigchelaar, E. F. , Schirmer, M. , Vatanen, T. , et al (2016) Population‐based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352: 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. , Gregory, J. C. , Org, E. , Buffa, J. A. , Gupta, N. , Wang, Z. , et al (2016) Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. , Wang, Z. , Tang, W. H. W. , and Hazen, S. L. (2017) Gut microbe‐generated trimethylamine N‐oxide from dietary choline is prothrombotic in subjects. Circulation 135: 1671–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoungas, S. , Curtis, A. J. , McNeil, J. J. , and Tonkin, A. M. (2014) Treatment of dyslipidemia and cardiovascular outcomes: the journey so far–is this the end for statins? Clin Pharmacol Ther 96: 192–205. [DOI] [PubMed] [Google Scholar]


