Abstract
Cells are equipped with mechanisms that allow them to rapidly detect and respond to viruses. These defense mechanisms rely partly on receptors that monitor the cytosol for the presence of atypical nucleic acids associated with virus infection. RIG-I-like receptors detect RNA molecules that are absent from the uninfected host. DNA receptors alert the cell to the abnormal presence of that nucleic acid in the cytosol. Signaling by RNA and DNA receptors results in the induction of restriction factors that prevent virus replication and establish cell-intrinsic antiviral immunity. In light of these formidable obstacles, viruses have evolved mechanisms of evasion, masking nucleic acid structures recognized by the host, sequestering themselves away from the cytosol or targeting host sensors, and signaling adaptors for deactivation or degradation. Here, we detail recent advances in the molecular understanding of cytosolic nucleic acid detection and its evasion by viruses.
Main Text
Cell-Intrinsic Antiviral Immunity
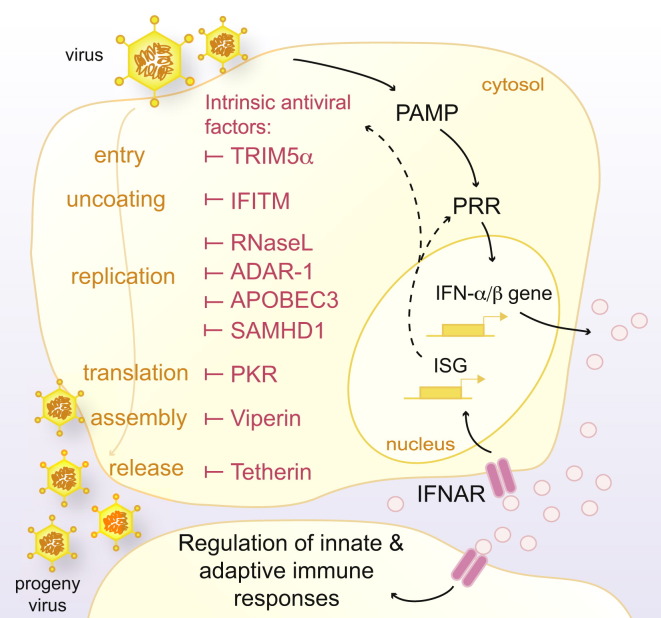
Virus infections can have devastating consequences for the host and must therefore be resisted quickly and effectively. A cell-intrinsic ability to restrict viral infection is found in all domains of life. This form of immunity is mediated by restriction factors that bind viral components and inhibit viral replication. Verterbrate cells have evolved a vast arsenal of viral restriction factors that directly inhibit all steps of viral replication (Duggal and Emerman, 2012; Yan and Chen, 2012; García-Sastre, 2011) (Figure 1 ). For instance, cytoplasmic entry of viruses such as influenza A virus, West Nile virus (WNV), and Dengue virus (DENV) is restricted by IFITM (interferon [IFN]-inducible transmembrane) proteins, whereas incoming retroviruses, such as HIV-1, are blocked by the capsid-binding protein TRIM5α (tripartite motif protein 5α) (Yan and Chen, 2012). When viral entry cannot be prevented, host factors inhibit subsequent steps in the viral life cycle. Mx (myxovirus resistance) GTPases form oligomeric structures that trap and degrade viral nucleocapsids and polymerases after infection with orthomyxoviruses, such as influenza (Yan and Chen, 2012). Host deaminases, including APOBEC3 (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide 3) and ADAR-1 (adenosine deaminase, RNA-specific 1) mutagenize viral genomes. The deoxynucleoside triphosphate triphosphohydrolase SAMHD1 (SAM-domain- and HD-domain-containing protein) degrades deoxynucleoside triphosphates and prevents reverse transcription of retroviruses (Duggal and Emerman, 2012; Yan and Chen, 2012). Viral protein synthesis is inhibited by antiviral proteins such as IFIT (interferon-induced protein with tetratricopeptide repeats) or Schlafen 11, whereas viperin and tetherin prevent assembly and release of progeny virions (Duggal and Emerman, 2012; Yan and Chen, 2012). Thus, cell-intrinsic antiviral immunity acts as a major barrier to infection, which must be circumvented for any virus to become a successful pathogen.
Figure 1.
Cell-Intrinsic Restriction and Recognition of Viruses
Cells express many intrinsic antiviral restriction factors capable of blocking different stages of the virus replication cycle. They are also equiped with PRRs that detect viral PAMPs and trigger the expression of cytokines, including type I IFNs (IFN-α and IFN-β). IFNs signal via the interferon receptor (IFNAR) and upregulate the expression of hundreds of ISGs, including the antiviral factors and PRRs themselves, as well as proteins important in regulating immune responses.
Cell-intrinsic antiviral effector proteins exert their function by targeting different viral components. However, their ability to discriminate virus from host is often imperfect or lacking altogether. For example, PKR (protein kinase R) causes a global shutdown in protein translation, and RNaseL (ribonuclease L) cleaves both viral and host RNA (García-Sastre, 2011). This lack of specificity allows both PKR and RNaseL to act as effective host restriction factors for a vast array of viruses but at a severe cost to the infected cell. So that detrimental effects on the uninfected host can be avoided, the activities of antiviral factors must be tightly regulated. This is typically achieved in two ways: first, antiviral effectors are not constitutively active and require a virus trigger (viral protein, viral genome, etc.) to exert their function; second, the expression of antiviral effectors is maintained at very low levels in the steady state and is upregulated only in response to IFN produced after viral infection. Type III (IFN-λ) and type I (mainly IFN-α and IFN-β) IFNs can be produced by all cell types upon virus infection and, once secreted, communicate a state of antiviral alertness to surrounding cells by inducing the expression of hundreds of IFN-stimulated genes (ISGs). Most cell-intrinsic viral restriction factors are ISGs, thereby ensuring that their expression is restricted to the infected state (Figure 1). ISGs further include proteins, such as costimulatory molecules, cytokines, and chemokines, that favor the initiation of adaptive immune responses. Thus, type I and type III IFNs act as the primary switch for initiating antiviral immunity in vertebrates.
The importance of type I and type III IFNs and the fact that they are produced upon infection have naturally led to interest in the mechanisms that cells use to detect viral presence. Research in this field first uncovered a mechanism utilizing membrane-bound Toll-like receptors (TLRs) for detecting extracellular viruses and virus-infected cells (Takeuchi and Akira, 2010). These pattern-recognition receptors (PRRs) detect viral pathogen-associated molecular patterns (PAMPs), such as viral proteins or viral nucleic acids, that are found in the extracellular milieu or within endosomes and then signal to the nucleus to induce transcription of IFNs and other genes encoding antiviral or proinflammatory mediators. Yet, the topology (extracellular facing) and restricted expression of TLRs (mostly leukocytes) cannot account for the fact that all cells can produce IFNs in response to direct infection. The latter involves a cytosolic mode of virus detection that has been recognized since the discovery of IFNs (Isaacs and Lindenmann, 1957) but that has only recently begun to be understood at the molecular level. The receptors involved include the RIG-I-like receptors (RLRs), which detect viral RNA in the cytosol, as well as cGAS (cyclic GMP-AMP synthase), IFI16 (IFN gamma-inducible protein 16), DAI (DNA-dependent activator of IFN-regulatory factors), and several other cytosolic proteins that detect DNA (see also the accompanying review by Paludan and Bowie, 2013, in this issue of Immunity). Here, we review recent progress in our understanding of cell-intrinsic detection of viruses by RLRs and DNA sensors and its consequences for the control of virus infection.
RLRs Are Superfamily 2 DExD/H-Box RNA Helicases
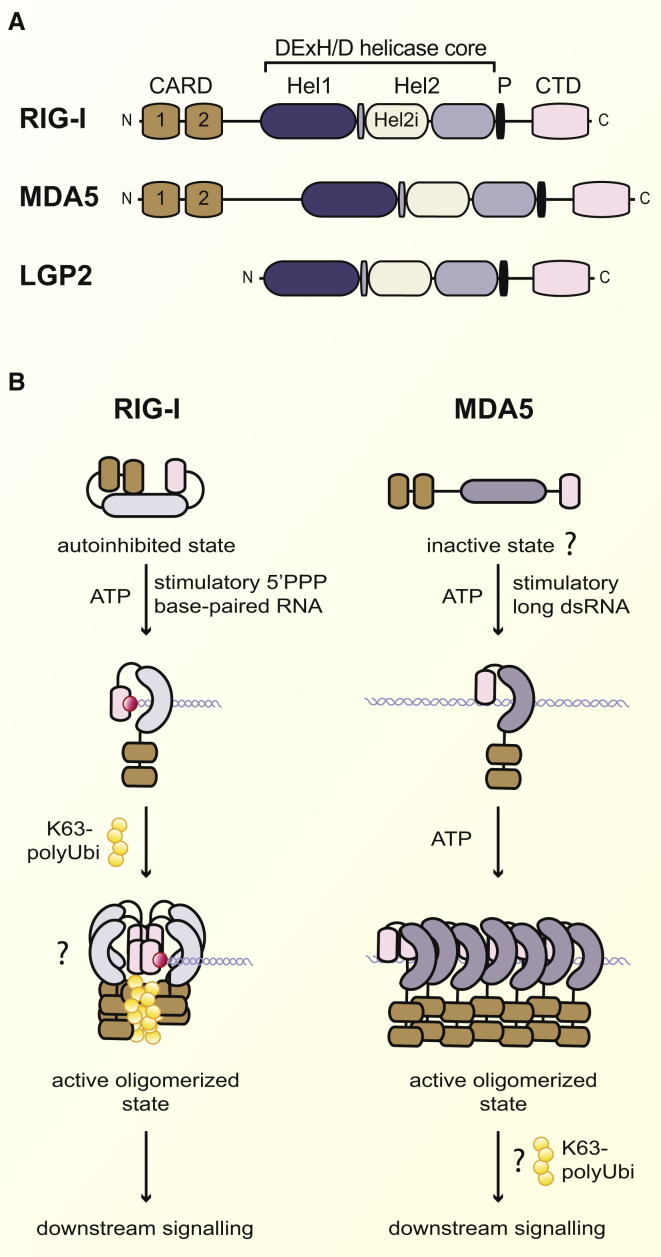
The three central members of the mammalian RLR family, RIG-I (retinoic acid-inducible gene I), MDA5 (melanoma differentiation factor 5), and LGP2 (laboratory of genetics and physiology 2), are found in the cytosol of most cell types and are strongly induced by IFNs in a positive-feedback loop of virus detection (Takeuchi and Akira, 2010) (Figure 1). They belong to the DExD/H-box family of helicases, which in turn is part of helicase superfamily 2 (DExD/H refers to Asp-Glu-x-Asp/His, and “x” can be any amino acid) (Luo et al., 2013). Like other helicases, RLRs possess a conserved helicase core comprising two highly similar tandem helicase domains (Hel1 and Hel2) separated by a unique insertion (known as Hel2i) that is important for RNA-triggered signal integration (Luo et al., 2013) (Figure 2 A). Although RNA helicases were originally named for their ability to unwind double-stranded (ds) RNA, they perform more general functions such as displacing RNA-bound proteins, annealing RNA strands, and promoting RNA conformational changes (Luo et al., 2013). This is true of RIG-I, which has been shown to unwind RNA duplexes and translocate along base-paired RNA (Leung and Amarasinghe, 2012). In addition to having a helicase domain, RIG-I, MDA5, and LGP2 all have a C-terminal domain (CTD), which confers part of their ligand specificity (further detail below). Finally, RIG-I and MDA5, but not LGP2, have at their N terminus two adjacent CARD (caspase activation and recruitment) domains, which are key for coupling to downstream signaling adaptors (Figure 2A).
Figure 2.
Domain Architecture of RLRs and Model of RLR Activation
(A) The three mammalian RLRs (RIG-I, MDA5, and LGP2) are superfamily 2 DExD/H-box RNA helicases. RLRs have a similar helicase core, which comprises two helicase domains termed Hel1 and Hel2, as well as an insertion domain within Hel2 known as Hel2i. At the C terminal of the Hel2 is a pincer (P) domain, also known as the bridging helices. All three RLRs also share a C-terminal domain (CTD), but only RIG-I and MDA5 have two N-terminal tandem CARDs (CARD1 and CARD2).
(B) ATP-dependent conformational changes and activation of RIG-I and MDA5 occur after the recognition of stimulatory RNAs, such as base-paired 5′ PPP RNA (5′ PPP is represented by the red dot) and long dsRNA, respectively. Whereas RIG-I binds to the 5′ PPP end of base-paired RNA, MDA5 binds the stem-loop and cooperatively assembles in a head-to-tail fashion along the length of dsRNA to form a filament-like structure. K63 polyubiquitylation and oligomerization of RLRs are thought to promote downstream signaling.
Sensing Viruses by RLRs
Studies in RLR-deficient mice and cells have demonstrated essential and differential requirements for RIG-I, MDA5, and LGP2 in combating viral infection (Table S1, available online). RIG-I is required for type I IFN production in response to Newcastle disease virus, vesicular stomatitis virus, influenza, and Japanese encephalitis virus (Kato et al., 2005; 2008; Loo et al., 2008; Yoneyama et al., 2005). In contrast, IFN production is impaired in MDA5-deficient, but not RIG-I-deficient, cells infected with Picornaviridae (Feng et al., 2012; Gitlin et al., 2006; Kato et al., 2006) (Table S1). Both the murine norovirus 1 and the murine hepatitis virus also trigger an MDA5-dependent IFN response (McCartney et al., 2008; Roth-Cross et al., 2008; Züst et al., 2011). Some viruses such as WNV and DENV are recognized by both MDA5 and RIG-I (Fredericksen et al., 2008; Loo et al., 2008; Schoggins et al., 2011). Finally, the IFN response to some DNA viruses is variably reported to be dependent on RIG-I and/or MDA5 (Table S1). In addition to being important for combating viruses, RIG-I and MDA5 have been implicated in the sensing of bacteria (Table S1).
In contrast to those of MDA5 and RIG-I, the role of LGP2 (encoded by Dhx58) in cytosolic RNA sensing remains unclear. Some reports suggest that LGP2 is required for type I IFN production in response to some RIG-I- and MDA5-dependent viruses (Table S1), whereas others describe LGP2 as a negative regulator of RIG-I-dependent responses (Bruns and Horvath, 2012). Further work will be required for resolving these discrepancies and understanding the function of LGP2 in antiviral immunity.
RNA Structures Recognized by RLRs
The viral specificity of RLRs reflects the remarkable capacity of these helicases to detect RNAs that are present only in infected cells. How RLRs discriminate such RNAs from those present in uninfected conditions remains a topic of intense investigation and has led to the identification of specific primary, secondary, or tertiary structures, as well as modifications in viral or cellular RNAs, that dictate RLR recognition.
The agonist for RIG-I has been defined as an RNA with a triphosphate (PPP) moiety and blunt-ended base-paired region of ∼20 nt at the 5′ end (Hornung et al., 2006; Pichlmair et al., 2006; Schlee et al., 2009; Schmidt et al., 2009). Base pairing at the 5′ end can occur intramolecularly, on individual single-stranded (ss) RNA molecules that possess appropriate secondary structures, or intermolecularly between two complementary RNA molecules that form dsRNA. In either case, the 5′ PPP remains essential. Indeed, thermodynamic analysis revealed that full-length RIG-I recognizes RNA with a 5′ PPP with an affinity that is 126-fold higher than that for RNA with a 5′ hydroxyl group (Vela et al., 2012). This specificity has further been validated in recent structural studies that show a pocket for 5′ PPP within the CTD of RIG-I (Leung and Amarasinghe, 2012). The requirement for 5′ PPP provides a mechanism for virus self-discrimination by RIG-I. Although host RNA transcripts initially contain 5′ PPP, the phosphates are either masked by a 7-methyl-guanosine cap (mRNA) or removed before export from the nucleus (tRNA and rRNA). Thus, the cytosol of uninfected cells is devoid of 5′ PPP RNA, but this is not true of virus-infected cells. Many RNA viruses use primer-independent mechanisms for virus replication and, consequently, possess genomes and replication intermediates that have a PPP-bearing nucleoside at the 5′ end. Notably, many viral genomes, including those of influenza and rabies virus, additionally possess complementary 5′ and 3′ ends that hybridize to form a “panhandle” structure (Schlee et al., 2009). Consistent with these two features, viral genomic RNA was identified as the physiological RIG-I agonist in cells infected with influenza and Sendai virus (Baum et al., 2010; Rehwinkel et al., 2010; Weber et al., 2013).
Some RNAs lacking a 5′ PPP have also been proposed to act as RIG-I agonists. Both short (25 bp) and long (>200 bp) dsRNA with a 3′ or 5′ monophosphate or a 5′ hydroxyl group have been reported to activate RIG-I (Binder et al., 2011; Kato et al., 2008; Takahasi et al., 2008). Furthermore, small structured RNaseL-cleavage-derived RNAs with a 5′ hydroxyl group and a 3′ monophosphate were also reported as RIG-I agonists (Malathi et al., 2007). These types of RNAs can be shown to bind RIG-I, albeit with lower affinities than 5′ PPP base-paired RNAs (Jiang et al., 2011a; Vela et al., 2012). These data raise the possibility that RIG-I might in some instances recognize duplex RNA structures independently of the 5′ end. In vitro reconstitution of RIG-I signaling has confirmed that poly(I:C), a synthetic RNA often used as a dsRNA analog, can act as a direct agonist for RIG-I (Zeng et al., 2010).
In contrast to RIG-I agonists, MDA5 agonists in virally infected cells are not as well understood. Picornaviruses are sensed by MDA5 and produce abundant dsRNA during infection (Pichlmair et al., 2009; Weber et al., 2006) (Table S1). MDA5 could therefore act simply as a dsRNA sensor. Consistent with this notion, MDA5 binds to and is activated by poly(I:C) (Takeuchi and Akira, 2010). Other data suggest that dsRNA recognition by MDA5 is dependent on RNA length and that only relatively long poly(I:C) (0.5–7 kb) efficiently activates MDA5 (Kato et al., 2008). A recent proposal is that MDA5 functions as a “molecular ruler:” whereas relatively short dsRNAs (∼100 nt) can activate MDA5 when present in large quantites, longer dsRNAs (1–2 kb) do so more efficiently as a result of the cooperative assembly of the helicase along dsRNA stems, the complex created by which forms filamentous oligomers that are important for signaling (see below) (Berke and Modis, 2012; Berke et al., 2012; Feng et al., 2012; Peisley et al., 2012; 2011; Wu et al., 2013a). Consistent with such a proposal, long dsRNA generated after encephalomyocarditis virus (EMCV) infection, as well as long segments of the reovirus dsRNA genome, can trigger MDA5-dependent IFN production (Kato et al., 2008). The dependence of MDA5 activation on dsRNA length might have evolved as a means of self-nonself discrimination because short base-paired RNAs, such as regulatory RNAs or transcripts from retrotransposons, are present in uninfected cells (Huang et al., 2012a). The precise nature of the MDA5 agonist in infected cells might actually be more complex than dsRNA and comprise structures that are produced as intermediates during the replication of some RNA viruses (such as EMCV and coxsackievirus) or as a result of convergent transcription of DNA viruses (like vaccinia virus) (Feng et al., 2012; Pichlmair et al., 2009; Triantafilou et al., 2012).
Recent work has pointed to the possibility that MDA5, like RIG-I, could distinguish self- and nonself-RNA by features present in the 5′ end of RNA. Indeed, it was shown that viral mutants lacking the ability to 2′-O-methylate the 5′ cap structure of their mRNAs can induce type I IFN induction via MDA5 (Züst et al., 2011). This suggests that 2′-O-methylation of the 5′ cap structure, a conserved feature of all host mRNAs, could have evolved as a self-marker. Whether MDA5 binds to non-2′-O-methylated mRNA to mediate downstream signaling remains to be determined, especially given that MDA5 has been shown to bind to dsRNA stems rather than to caps (Wu et al., 2013a).
Much less is known of the nature of RNAs that might bind to LGP2, the third RLR family member with no autonomous signaling capacity. Some in vitro studies have shown that LGP2 can bind dsRNA, 5′ PPP RNA, and hepatitis C virus (HCV) genomes (Bruns and Horvath, 2012). A focus on the RNAs that bind to LGP2 might help decipher the true function of this RLR.
RLR Activation
Crystal structures comparing ligand-free and ligand-bound complexes have unveiled the mechanism by which RIG-I is regulated and recognizes RNA to trigger the downstream signaling cascade that leads to type I IFN induction (Leung and Amarasinghe, 2012). At resting state, RIG-I adopts a closed autoinhibited conformation where the CARDs are sterically unavailable for signal transduction (Civril et al., 2011; Ferrage et al., 2012; Kowalinski et al., 2011) (Figure 2B). The rigid autoinhibited state of RIG-I is thought to be achieved via a series of domain interactions: both CARDs are linked to one another in a head-to-tail manner (C terminus of CARD1 with the N terminus of CARD2), and CARD2 also contacts the Hel2i domain (Civril et al., 2011; Ferrage et al., 2012; Kowalinski et al., 2011). Upon infection, RIG-I activation occurs in a dramatic and well-orchestrated sequence of events (Figure 2B). A structural zinc ion and a positively charged cleft-like structure within the CTD specifically recognize the 5′ PPP extremity of blunt-end base-paired RNA (Leung and Amarasinghe, 2012). Binding at the CTD is communicated N-terminally via a long and flexible elbow-like or V-shaped “pincer” (P) domain (also known as the bridging helices) that connects the Hel1 domain to the CTD (Jiang et al., 2011b; Kowalinski et al., 2011; Luo et al., 2011). At the same time, the helicase domain binds to the duplexed-RNA sugar-phosphate backbone in a ring-shaped clamp, which is further compacted upon ATP hydrolysis (Jiang et al., 2011a; Kowalinski et al., 2011; Luo et al., 2012). The interaction between the helicase domain and RNA releases the CARDs, and the P domain functions as a nanomechanical camshaft to “push” them away. Lys172 of the free CARD2 is now available for the addition of polyubiquitin chains generated by the E3 ligase TRIM25 (tripartite-motif-containing 25), although it remains unclear whether those chains are bound covalently or noncovalently (Gack et al., 2007; Zeng et al., 2010). Whichever the case, the ubiquitylation of RIG-I is thought to trigger the formation of a large heterotetrameric complex—consisting of four RIG-I molecules and four ubiquitin chains—that acts as the basic unit for downstream signaling (Jiang et al., 2012) (Figure 2B).
As for RIG-I, the CTDs of LGP2 and MDA5 facilitate RNA binding, and the domain architecture of RIG-I is preserved in MDA5 and LGP2 (Leung and Amarasinghe, 2012). Although information on LGP2 function and activation remains scarce, structural data are beginning to unveil the basis of MDA5 recognition of dsRNA. In contrast to the RIG-I CARDs, the MDA5 CARDs do not form a stable interaction with the helicase domain at steady state, and MDA5 is therefore thought to adopt an open conformation in the absence of ligand (Berke and Modis, 2012). Upon dsRNA binding, the helicase domain of MDA5 forms a ring around the phosphate backbone of the ligand (Wu et al., 2013a). However, rather than being a closed O-ring-like structure, as in RIG-I, the MDA5 ring is C shaped. This more open conformation is due to a distinct orientation of the CTD, which is rotated by 20° compared to that of RIG-I, causing it to be aligned with the dsRNA axis as opposed to forming a cap that closes the helicase ring over the 5′ PPP end of dsRNA (Wu et al., 2013a). Residues on the flat surface of the CTD further permit stem-loop rather than end-mediated recognition of dsRNA. Thus, the CTD provides the basis for recognition of distinct RNA structures by RIG-I and MDA5.
The MDA5 CTD is additionally thought to facilitate the formation of MDA5-dsRNA filaments through cooperative dsRNA recognition (Berke and Modis, 2012; Berke et al., 2012; Peisley et al., 2011; 2012; Wu et al., 2013a). This causes MDA5 monomeric-helicase-CTD rings to become stacked along the RNA in a head-to-tail filament. The CARDs (absent from current crystal structures) are thought to be excluded from the filament core (Berke and Modis, 2012; Wu et al., 2013b). MDA5 signaling therefore differs from that of RIG-I in that it does not involve CARD exposure. Rather, it is proposed that ATP hydrolysis triggered upon ligand binding regulates the conformation of the CARDs of stacked MDA5 monomers and that the CARDs are thus caused to self-assemble into discrete patches that act as the nuclei for downstream signaling (Wu et al., 2013a).
As for RIG-I, MDA5 downstream signaling has been argued to require binding of unanchored K63-linked polyubiquitin to the CARDs, although this observation has been disputed (Jiang et al., 2012; Wu et al., 2013a). RIG-I Lys172 is not conserved in MDA5, yet TRIM25 appears important for MDA5 signaling and is hypothesized to provide specificity for the local delivery of ubiquitin chains (Gack et al., 2007; Jiang et al., 2012; Zeng et al., 2010). In addition to regulating signaling, ATPase activity also regulates MDA5 stability on long versus short dsRNAs by facilitating the dissociation of MDA5 from short RNAs, which, as argued above, contributes to self-nonself RNA discrimination (Berke and Modis, 2012; Peisley et al., 2011; Wu et al., 2013a).
RLR Signal Transduction via MAVS
Activated RIG-I and MDA5 induce downstream signaling by binding to the mitochondrial adaptor MAVS (mitochondrial antiviral signaling) (also known as IPS-1 [IFN-β promoter stimulator 1], CARDIF [CARD-adaptor-inducing IFN-β], or VISA [virus-induced signaling adaptor]) via a CARD-CARD-mediated interaction (Takeuchi and Akira, 2010) (Figure 3 ). Although the majority of MAVS is present on the mitochondria, a small proportion is located in the peroxisomes and is also present in the mitochondrial-associated endoplasmic reticulum (ER) membrane (MAM) (Dixit et al., 2010; Horner et al., 2011). This has led to the proposal that these structures act as platforms for antiviral signaling (Dixit et al., 2010; Horner et al., 2011). There is also evidence of recognition of viral RNAs in stress granules, but how this gets translated into signaling at MAMs or peroxisomes is poorly understood (Onomoto et al., 2012).
Figure 3.
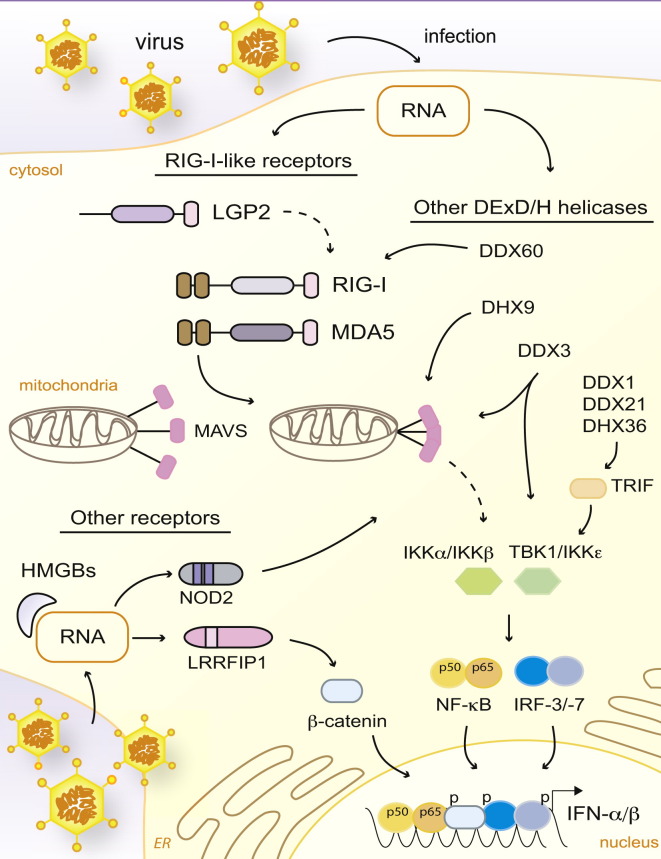
RLR Signaling and Other Receptors Involved in Cytosolic RNA Sensing
RIG-I and MDA5 signaling induces MAVS activation and oligomerization into a prion-like aggregate, which activates the TBK1 and IKK kinases. This culminates in the activation of transcription factors NF-κB, IRF-3, and IRF-7, which translocate to the nucleus and participate in the induction of antiviral genes, including those that encode IFN-α and IFN-β. Other DExD/H-box helicases, including DDX60, DHX9, DDX3, and the DDX1-DDX21-DHX36 complex, are also reported sensors of cytosolic RNA. Most of these helicases are thought to trigger IFN transcription by using RLR-dependent pathways (through RLRs themselves, MAVS, or TBK1), but the DDX1-DDX21-DHX36 complex is thought to signal through TRIF. HMGB proteins and NOD2 have also been described as receptors for cytosolic RNA and inducers of IFN responses. Lastly, it has been argued that after RNA stimulation, LRRFIP1 phosphorylates β-catenin, which translocates to the nucleus and promotes IFN-β expression. Dashed lines indicate indirect or possible signaling, and “p” indicates a phosphorylated protein.
Viral infection appears to induce the formation of large aggregates of MAVS, and it has recently been demonstrated that these result from self-propagation in a prion-like manner (Hou et al., 2011). In other words, the engagement of MAVS by RLRs causes a conformational change that propagates to adjacent unengaged MAVS and thereby results in a large-scale amplification of the signaling cascade. This allows for highly sensitive detection of very small amounts of viral RNA, and it has been calculated that <20 molecules of 5′ PPP viral RNA are sufficient to activate the RIG-I-MAVS pathway (Zeng et al., 2010). Several cytosolic molecules that are also involved in other innate signaling responses are subsequently recruited to MAVS prions (Takeuchi and Akira, 2010). This complex allows for the activation of the kinases TBK1-IKKε (TANK-binding kinase 1-IκB kinase-ε), responsible for the activation of IRF-3 and IRF-7 (IFN regulatory factors 3 and 7, respectively), and IKKα and IKK-β, responsible for NF-κB (nuclear factor kappa-light-chain enhancer of activated B cells) activation (Figure 3). These transcription factors translocate to the nucleus and coordinate the expression of the genes encoding IFN-α and IFN-β, as well as other antiviral genes (Takeuchi and Akira, 2010). In addition to IRF-3 and IRF-7, IRF-5 might also induce type I IFN responses downstream of MAVS in some dendritic cells (Lazear et al., 2013).
Independently of MAVS, RIG-I has also been implicated in activating the intracellular signaling complex termed the inflammasome, the activation of which leads to the proteolytic processing of prointerleukin 1β (proIL-1β) into mature IL-1β, a proinflammatory cytokine (Abdullah et al., 2012; Poeck et al., 2010). Finally, the activation of MDA5 or RIG-I in melanoma cells might couple to the induction of caspase-dependent apoptotic responses via the BH3-only protein Noxa (Besch et al., 2009).
Modulation of RLR Signaling
Many proteins are reported to modulate the RLR signaling pathway (Eisenächer and Krug, 2012). One class is those that regulate the posttranslational status and lifetime of RLRs. Like TRIM25, the E3 ligase Riplet (also known as RNF135 or REUL) can trigger K63-linked polyubiquitylation of RIG-I and positively affect antiviral signaling (Oshiumi et al., 2012). Others, including RNF125 (ring-finger protein 125), Smurf1, AIP4, and c-Cbl, negatively regulate RIG-I, MDA5, and/or MAVS signaling by targeting them for proteasomal degradation (Eisenächer and Krug, 2012; Chen et al., 2013b; Wang et al., 2012a). Deubiquitylating enzymes such as CYLD (cylindromatosis) and USP4 (ubiquitin-specific protease 4) can also regulate RIG-I responses by removing K63-linked or K48-linked polyubiquitin chains, respectively (Friedman et al., 2008; Wang et al., 2013). Phosphorylation and SUMOylation have additionally been reported to regulate RIG-I and MDA5 function (Ferrage et al., 2012; Gack et al., 2010; Mi et al., 2010; Nistal-Villán et al., 2010; Wies et al., 2013).
A different class of RLR regulators is mitochondrial proteins. Two homologous regulators of mitochondrial fusion, MFN1 and MFN2 (mitofusin 1 and 2, respectively), interact with MAVS but have opposing effects. Whereas MFN2 directly inhibits MAVS, MFN1 was suggested to positively regulate RLR-mediated innate antiviral responses by affecting mitochondrial dynamics (Arnoult et al., 2011). Targeted deletion of both MFN1 and MFN2 was shown to result in impaired mitochondrial fusion and decreased mitochondrial-membrane potential, which correlated with a defective antiviral response (Koshiba et al., 2011). In addition, the mitochondrial NLR (Nod-like receptor) protein NLRX1 (also known as NOD5) has been reported to constitutively interact with MAVS and inhibit RLR signaling (Xiao and Ting, 2012). NLRX1 has also been shown to partner with TUFM (mitochondrial Tu translation elongation factor) to promote autophagy, a cellular process that can limit RLR signaling (Lei et al., 2012; 2013). Whether the RNA binding capacity of NLRX1 is important in regulating RLR responses remains to be investigated (Hong et al., 2012). However, the function of NLRX1 is controversial, given that two out of three strains of NLRX1-deficient mice fail to display any alteration of MAVS signaling (Allen et al., 2011; Rebsamen et al., 2011; Soares et al., 2012). Similarly ambiguous results have been reported for a second NLR, NLRC5 (also known as NOD4), which has variably been reported to inhibit RIG-I and/or MDA5 or to have no function in RLR signaling (Cui et al., 2010; Kumar et al., 2011a). The precise role of NLRX1 and NLRC5 in antiviral immunity will need to be clarified in future studies.
The third class of regulators includes miscellaneous proteins such as SHP-1 (Src homology phosphatase 1) and EYA4 (eyes absent 4), as well as Ankrd17 (ankyrin repeat protein 17), the scaffold protein 14-3-3ε, the dsRNA binding protein PACT, and ZAPS (zinc-finger CCCH-type antiviral protein 1), all of which are reported to enhance RLR signaling (An et al., 2008; Hayakawa et al., 2011; Kok et al., 2011; Liu et al., 2012; Okabe et al., 2009; Wang et al., 2012b). The ribonucleoprotein PTB-binding 1 (RAVER1) was also found to specifically regulate MDA5 activation (Chen et al., 2013a). Focal adhesion kinase (FAK) and the complement receptor gC1qR appear to translocate to the mitochondria to interact with MAVS. Whereas FAK acts as a positive regulator, gC1qR has been found to inhibit RLR signaling (Bozym et al., 2012; Xu et al., 2009). Tetraspanin 6 can also negatively affect RLR signaling in that it can associate with MAVS and interfere with the recruitment of TRAF3 (Wang et al., 2012c). In sum, RLR signaling is subject to a complex system of posttranslational regulation, as well as regulation by other cellular proteins. The large number of RLR regulators underlines the importance of these receptors in antiviral responses and the severe consequences that might ensue from their misfiring.
Other Proteins Implicated in Cytosolic Sensing of Viral RNA
Additional DExD/H-box helicases outside the RLR helicase subfamily have been implicated in the IFN-α and IFN-β response to viruses. They include DDX3 (also known as DDX3X or DBX), DHX9 (also known as RHA [RNA helicase A]), DDX60 (also known as DHX60), and the DDX1-DDX21-DHX36 complex. These helicases are thought to mediate IFN-α and IFN-β expression either by directly sensing nucleic acids after viral infection and/or by interacting with components of the IFN-α and IFN-β induction pathway (Figure 3).
DDX3 and DHX9 are constitutively expressed and have been proposed to play a role in the early phases of viral infection when RLR levels have yet to be upregulated by IFNs (Oshiumi et al., 2010; Zhang et al., 2011d). These helicases are thought to sense viral RNA, couple to MAVS, and induce IFN expression (Oshiumi et al., 2010; Zhang et al., 2011d). In addition to sensing RNA, DDX3 is reported to precipitate with RIG-I, MDA5, TBK1, and IKKε, as well as associate with the Ifnb1 promoter (Gu et al., 2013; Oshiumi et al., 2010; Schröder et al., 2008; Soulat et al., 2008). Overexpression and knockdown studies suggest that DDX3 and DHX9 are required for the full induction of IFN responses to a number of RNA viruses and poly(I:C) (Oshiumi et al., 2010; Schröder et al., 2008; Zhang et al., 2011d).
Another helicase, DDX60, has been proposed to promote the antiviral response at the level of RLRs (Miyashita et al., 2011). DDX60 is a member of the Ski2-like subfamily of DExD/H-box helicases, but unlike DDX3 and DHX9 expression, its expression is not constitutive but induced by IFNs, similar to RLRs. Coimmunoprecipitation studies have shown that DDX60 binds to nucleic acids and have identified RIG-I, MDA5, LGP2 as interacting partners (Miyashita et al., 2011). Furthermore, cells in which DDX60 expression was stably knocked down produced lower levels of mRNA for IFN-β and ISGs in response to both RNA and DNA stimuli (Miyashita et al., 2011). Because DDX60 expression also appeared to increase binding of RNA to RIG-I, it was hypothesized that this helicase might bind viral RNA and associate with RLRs during viral infections to enhance signaling (Miyashita et al., 2011). However, we have failed to reproduce such data and have found no role for DDX60 in IFN induction either in vitro or in a DDX60-deficient mouse (data not shown). Rather, DDX60 appears to be an ISG that acts as a virus-specific restriction factor and whose overexpression in cultured cells can restrict the replication of HCV, but not yellow fever virus, WNV, Chikungunya virus, Venezuelan equine encephalitis virus, or HIV-1 (Schoggins et al., 2011).
A cytosolic triple-helicase complex formed by DDX1, DDX21, and DHX36 is also reported to sense RNA in myeloid cells yet function independently of the RLR pathway to induce IFNs (Zhang et al., 2011b). DDX1 is suggested to directly bind poly(I:C), whereas DDX21 and DHX36 are thought to interact with the adaptor TRIF (TIR-domain-containing adaptor-inducing IFN-β) to mediate downstream signaling. Targeted knockdown of any of the helicases negatively affects the IFN-response to poly(I:C), influenza virus, and reovirus. Lastly, two proteins that are not RNA helicases have also been implicated in the sensing of cytosolic RNA and subsequent IFN induction. These include the HMGB (high-mobility-group box) proteins, leucine-rich-repeat protein LRRFIP1 (leucine-rich repeat in flightless interacting protein 1), and the NLR NOD2 (nucleotide-binding oligomerization domain 2) (Sabbah et al., 2009; Yanai et al., 2009; Yang et al., 2010). The functional relevance of all of these proteins in detecting cytosolic RNA requires further investigation for defining whether these molecules work in concert with RLRs in regulating IFN responses and how this might occur.
RLRs in Development and Disease
In addition to playing a role in innate antiviral signaling, RLRs have been implicated in shaping adaptive immunity, including regulating the magnitude and quality of T cells and antibody responses (Negishi et al., 2012; Suthar et al., 2012; Wang et al., 2007). Indeed, a recent study has defined a cell-autonomous role for LGP2 in regulating CD8+ T cell survival and fitness during WNV infection (Suthar et al., 2012). Interestingly, the first RIG-I-deficient mouse strain generated showed liver degeneration and embryonic lethality at day 12.5, suggesting that RIG-I could be involved in development (Kato et al., 2005). Curiously, this phenotype is different from that observed in a second RIG-I-deficient mouse, which is viable, fertile, and born at Mendelian ratios but exhibits a progressive myeloproliferative disorder, as well as increased susceptibility to dextran-sulfate-sodium-induced colitis (Wang et al., 2007; Zhang et al., 2008). The latter pathology has also been seen in MAVS-deficient mice and might be explained by the fact that the RIG-I-MAVS pathway responds to RNA from commensal bacteria to reinforce gut-barrier protection (Li et al., 2011).
RLRs have been linked to inflammatory disorders besides colitis. IFIH1, which encodes MDA5, was implicated in type I diabetes through genome-wide association studies (Lind et al., 2012). Whereas loss-of-function alleles of IFIH1 confer disease protection, susceptibility genotypes are associated with increased IFIH1 expression levels, which possibly cause exacerbation of ongoing immune pathology (Lind et al., 2012). Conversely, MDA5 was recently shown to play a protective role in virus-induced diabetes in mice after infection with EMCV, a virus with a tropism for the insulin-producing β cells (McCartney et al., 2011). MDA5-deficient mice and cells also show increased susceptibility to infections with coxsackie type B virus, a Picornaviridae family enterovirus that has been implicated in the onset and progression of type I diabetes in humans (Lind et al., 2012) (Table S1). Therefore, investigating how human responses to enteroviruses are affected by SNPs associated with type I diabetes might help to elucidate the function of MDA5 in the development of this disease (Lind et al., 2012). Additional genetic associations between IFIH1 and other immune diseases have been reported and include systemic lupus erythematosus (SLE), psoriasis, and immunoglobulin A deficiency (Lind et al., 2012).
The Interferon Response to Cytosolic DNA
As for RNA, there are cytosolic pathways dedicated to the recognition of DNA (reviewed in detail by Paludan and Bowie, 2013, in this issue). Multiple studies have shown that the introduction of dsDNA into the cytosol of cells induces IFN-α and IFN-β in a TLR-independent fashion (Cavlar et al., 2012). This can happen naturally during infection with DNA viruses (such as HSV-1 or vaccinia virus), bacteria (such as Listeria monocytogenes or Legionella pneumophila), or parasites (like Plasmodium falciparum) or can be mimicked experimentally by the transfection of natural or synthetic DNA. Commonly used types of immunostimulatory B-DNA are poly(dA:dT), a homocopolymer, and ISD (immune-stimulatory DNA), a synthetic double-stranded 45 bp oligonucleotide lacking contiguous CpG sequences (Cavlar et al., 2012). In addition to synthetic and microbial DNA, host DNA present in the cell cytosol can also induce the expression of IFNs. Evidence for this stems from the observation that functional loss of certain deoxyribonucleases (DNases) can precipitate interferonopathies, including SLE and Aicardi-Goutières syndrome in humans (Crow, 2011). For example, mice or humans lacking 3′ repair exonuclease TREX1 (three prime repair exonuclease 1), a cytosolic DNase, cannot degrade endogenous-retroelement-derived cDNA, which accumulates in the cytosol and triggers IFN-α and IFN-β and autoimmunity (Crow, 2011). As such, it appears that the mere presence of DNA in the cytoplasm, normally a DNA-free environment, is sufficient to activate innate immune signaling. Although there is also a suggestion that DNA sensing might additionally occur in the nucleus (Kerur et al., 2011; Li et al., 2012), efforts have focused on identifying the pathways that mediate the sensing of DNA in the cell cytosol and link it to inflammation (Figure 4 ).
Figure 4.
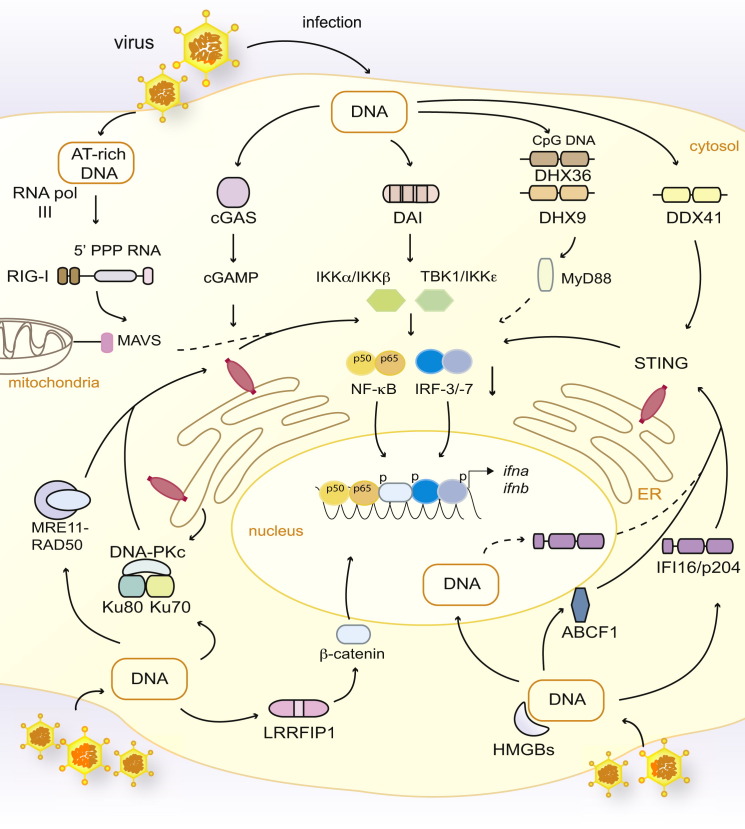
Putative Intracellular DNA Sensors Involved in IFN-α and IFN-β Induction
DNA present in the cytosol after viral infection induces the production of type I IFNs through a central signaling cascade involving STING, which serves as a scaffold for the phosphorylation of IRF-3 by the kinase TBK1. It was recently demonstrated that the cytosolic nucleotidyltransferase cGAS binds DNA and synthesizes the formation of a cyclic-GMP and cyclic-AMP hybrid termed cGAMP, which directly binds to and activates STING. Cytosolic DNA is reported to engage a number of additional receptors, including DAI, human IFI16 (or mouse p204), and the helicases DDX41, DHX36, and DHX9. DHX36 and DHX9 appear to be specific to CpG DNA and are reported to signal via MyD88. HMGB1, HMGB2, and HMGB3 have also been shown to promote cytosolic DNA responses. Data also suggest that ABCF1 binds DNA and interacts with HMGB2 and p204 to stimulate innate immune responses. Moreover, AT-rich DNA can be transcribed by RNA pol III into 5′-PPP-containing RNA (5′ PPP RNA), which serves as a RIG-I agonist. LRRFIP1 senses cytosolic DNA and phosphorylates β-catenin, which translocates to the nucleus and promotes IFN-β transcription. The DNA-PKc-Ku70-Ku80 and MRE11-RAD50 complexes, involved in DNA-damage responses, have additionally been suggested to bind cytosolic DNA and promote STING-dependent type I IFN responses. Ku70 is further reported to trigger the expression of type III IFNs in an IRF-1- or IRF-7-dependent manner in response to cytosolic DNA (not depicted; Zhang et al., 2011a). Proteins involved in DNA-damage responses, as well as IFI16 and RNA pol III, are abundantly present in the nucleus, highlighting the possibility that DNA sensing might also occur in that organelle (only depicted here for IFI16). Dashed lines indicate indirect or possible signaling, and “p” indicates phosphorylated proteins.
Initial conflicting data reporting a role for MAVS as an adaptor in the cytosolic DNA response led to the discovery of RIG-I as an “indirect” sensor of some types of DNA (Ablasser et al., 2009; Chiu et al., 2009) (Figure 4). Two groups demonstrated that cytosolic AT-rich DNA, such as poly(dA:dT), can be transcribed in the cytosol by RNA polymerase (pol) III into uncapped 5′ PPP-RNA, which then functions as a RIG-I agonist (Ablasser et al., 2009; Chiu et al., 2009). The pathway involving RNA pol III and RIG-I was shown to be required for sensing RNA encoded with Epstein-Barr virus and the induction of a full IFN response after infections with HSV-1 and adenovirus, as well as the intracellular bacteria L. pneumophila and L. monocytogenes (Abdullah et al., 2012; Ablasser et al., 2009; Chiu et al., 2009) (Table S1). However, the findings obtained with HSV-1 and L. pneumophila have not been validated in further reports, and additional studies are required for determining the true relevance of the pathway involving RIG-I and RNA pol III for pathogen detection (Melchjorsen et al., 2010; Monroe et al., 2009).
The ER-localized protein STING (stimulator of IFN genes) (also known as TMEM173 [transmembrane protein 173], MITA [mediator of IRF3 activation], MPYS [methionine-proline-tyrosine-serine], and ERIS [ER IFN stimulator]) is essential for the RNA-pol-III-independent IFN response to cytosolic DNA (Cavlar et al., 2012; Ishikawa et al., 2009) (Figure 4). STING-deficient cells show a complete abrogation of IFN-β production in response to a variety of DNA stimuli of bacterial, viral, parasitic, or synthetic origin (Cavlar et al., 2012). Some studies have also reported a decrease in IFN induction in STING-deficient cells infected with RNA viruses (Ishikawa and Barber, 2008; Ishikawa et al., 2009; Jin et al., 2008; Sun et al., 2009; Zhong et al., 2008), whereas others have found no difference (Chen et al., 2011; Sauer et al., 2011). These discrepancies might be attributable to another function of STING, which is independent of RNA or DNA recognition, in inducing IFNs and ISGs upon fusion of viral envelopes with target cells (Holm et al., 2012). Finally, STING further acts as a sensor for the prokaryotic second messenger molecules cyclic-di-GMP and cyclic-di-AMP (Burdette et al., 2011; Huang et al., 2012b; Jin et al., 2011; Ouyang et al., 2012; Sauer et al., 2011; Yin et al., 2012) and the chemotherapeutic agent 5,6-dimethylxanthenone-4-acetic acid (Brunette et al., 2012; Prantner et al., 2012).
STING signaling is thought to be controlled by K63-linked ubiquitylation by both TRIM56 (tripartite motif 56) and TRIM32 (tripartite motif 32) (Tsuchida et al., 2010; Zhang et al., 2012). After dsDNA stimulation, STING rapidly traffics from the ER through the Golgi into perinuclear punctate structures that also contain the kinase TBK1 (Ishikawa et al., 2009; Saitoh et al., 2009; Tanaka and Chen, 2012). STING appears to function as a scaffold for downstream activation by bringing IRF-3 in close proximity to TBK1, which then is able to phosphorylate it (Tanaka and Chen, 2012). Inappropriate activation of STING (encoded by Tmem173) leads to the development of autoimmunity associated with Trex1 or DNaseII mutations, and Trex1 −/− Tmem173 −/− mice are completely protected from mortality and autoimmune tissue damage (Gall et al., 2012). Protection from anemia and polyarthritis has also been reported in animals deficient in both DNaseII and STING (Ahn et al., 2012). These data indicate that the STING pathway is triggered by both foreign DNA and self-DNA.
Sensors of Intracellular DNA
There has been considerable effort to try to elucidate the very initial signaling events that permit cells to detect the presence of cytosolic DNA and engage STING. This search has led to the identification of multiple DNA sensors (Figure 4). The IFN-inducible protein DAI was the first protein reported as a potential mediator of the IFN response to cytosolic DNA (Takaoka et al., 2007). LRRFIP1, HMGB proteins, and ABCF1 (ATP-binding cassette, subfamily F member 1) have also been shown to bind DNA directly and positively regulate IFN responses (Lee et al., 2013; Yanai et al., 2009; Yang et al., 2010). The AIM2-like proteins, including human IFI16 and its mouse ortholog p204, have also been implicated in STING-dependent IFN responses (Brunette et al., 2012; Unterholzner et al., 2010). Moreover, some DExD/H-box helicases, including DDX41, DHX9 and DDX36, are also postulated to act as sensors of cytosolic DNA (Kim et al., 2010; Zhang et al., 2011c). Lastly, some proteins with known functions in DNA-damage responses have also been reported as mediators of the antiviral response triggered by cytosolic DNA. These include components of the DNA-PK (DNA-dependent protein kinase; composed of Ku70, Ku80, and DNA-PKc) and MRE11 (meiotic recombination 11 homolog A)-RAD50 complexes (Ferguson et al., 2012; Kondo et al., 2013; Zhang et al., 2011a). The lack of redundancy among these receptors, as well as their role in other cellular functions, has led to the hypothesis that they might function as cytosolic DNA sensors only in certain cell types and/or in response to certain pathogens (Paludan and Bowie, 2013, in this issue).
A recent study has suggested that the molecule responsible for binding and activating STING after DNA transfection or infection with a DNA virus might not be DNA itself but rather a novel second messenger termed cyclic-GMP-AMP (cGAMP) (Wu et al., 2013b) (Figure 4). The ability of STING to directly bind eukaryotic cGAMP is reminiscent of its reported aditional function as a sensor of prokaryotic cyclic-di-GMP and cyclic-di-AMP (see above) and suggests that the key DNA sensor is a DNA-dependent cytosolic cyclase. Consistent with that prediction, a newly identified nucleotidyltransferase family member, named cytosolic GAMP synthase (or cGAS), was shown to mediate the production of cGAMP in response to DNA (Sun et al., 2013). The identification of the cGAS-cGAMP-STING pathway is an exciting development that offers a new perspective in DNA sensing, but it remains to be established to what extent it can account for responses to pathogen infection. It will also be important to investigate whether and how other DNA sensors function in concert with cGAS to promote cytosolic DNA responses.
Viral Evasion and Subversion of Cytosolic Detection
Although hosts have developed a complex immune system to fend off invaders, viruses have counteracted with a series of sophisticated mechanisms to successfully replicate within host cells. In this final section, we describe some of the ways in which viruses escape and block cell-intrinsic detection or use it to their advantage.
One strategy viruses employ to evade innate immune detection is by modifying and concealing their genomes and replication intermediates. For example, some negative-strand RNA viruses, such as Hantaan virus, Crimean-Congo haemorrhagic fever virus (Nairovirus), Prospect Hill virus (all Bunyaviridae), and Borna disease virus (BDV; Bornaviridae), avoid RIG-I recognition by using virus-encoded endonucleases or phosphatases to process the 5′ PPP of their genomes to a 5′ monophosphate (Garcin et al., 1995; Habjan et al., 2008; Wang et al., 2011b; Weber et al., 2013). In addition to having a 5′ monophosphate, the majority of genomes and antigenomes of BDV have a 3′ overhang as a result of trimming of the 5′ end, making them refractory to RIG-I detection (Schlee et al., 2009; Schmidt et al., 2009; Schneider et al., 2007). Similarly, Arenaviridae (which include Lassa virus and Tacaribe arenavirus) have PPPs on their genomes but contain a 5′ nucleotide overhang (PPP-G), which has been shown to interfere with RIG-I recognition (Marq et al., 2010; Schlee et al., 2009; Schmidt et al., 2009). More recent data suggest that the genomes of these viruses could also act as RIG-I decoys by binding the receptor but failing to cause its activation (Marq et al., 2011). In contrast, Picornaviridae and Caliciviridae have positive ssRNA genomes that are covalently bound to a viral protein, VPg (viral genome-linked protein), and are therefore not subjected to RIG-I recognition (Knipe and Howley, 2007). Another way in which viruses avoid RLR sensing is by hiding their genomes. Certain RNA and DNA viruses, like Orthmomyxoviridae and Adenoviridae, respectively, replicate in the nucleus, a location that is not subjected to surveillance by cytosolic PRRs (Knipe and Howley, 2007). Most RNA and some DNA viruses that replicate in the cytoplasm do so in specific compartments composed of both viral- and host-encoded proteins, often in association with cytosolic membranes or organelles such as the mitochondria, ER, and Golgi (Knipe and Howley, 2007). These structures act as viral factories to facilitate the production of virions while sequestering viral RNA or DNA away from innate immune sensors. Specific viral proteins can also help sequester viral nucleic acids and outcompete PRRs. For example, the influenza nonstructural protein 1 (NS1), the vaccinia virus protein E3, and Ebola virus protein VP35 allow these viruses to disrupt immunity in multiple ways, including through binding of viral RNA, to avoid PRR detection (Leung et al., 2012). Recent structural studies have demonstrated that VP35 binding to viral RNA mimics that of RIG-I and provides an effective mechanism for excluding the sensor (Leung et al., 2012). In contrast, the C protein of the human parainfluenza virus type 1 functions somewhat differently. Rather than sequester viral RNA, it is thought to suppress the accumulation of dsRNA that would otherwise trigger MDA5 (Boonyaratanakornkit et al., 2011). Finally, HIV manages to evade immune detection by benefiting from the functions of the host protein, TREX1. This exonuclease degrades unintegrated HIV cDNA after cytosolic reverse transcription, allowing its escape from detection by cytosolic DNA sensors (Yan and Chen, 2012).
A second distinct strategy used by viruses to evade cell-intrinsic detection is direct targeting and inhibition of cytosolic PRRs and downstream signaling molecules (Leung et al., 2012; Taylor and Mossman, 2013). For example, NS1 from influenza interacts with RIG-I and efficiently antagonizes downstream signaling, in part by disrupting TRIM25 and Riplet ubiquitin E3 ligases (García-Sastre, 2011; Leung et al., 2012). Other viruses encode deubiquitinating enzymes that target RIG-I or can harness negative-feedback loops of RIG-I signaling (Inn et al., 2011; Sun et al., 2012; van Kasteren et al., 2012; Wang et al., 2011a). Viruses have also been shown to decrease RLR and MAVS levels by lysosomal targeting, sequestration into viral inclusion bodies, and transcriptional downregulation (Taylor and Mossman, 2013). In addition, paramyxoviruses directly block all three RLRs via their V and C proteins (Goodbourn and Randall, 2009). A recent structure of the porcine MDA5 helicase domain in complex with the V protein of parainfluenza virus 5 revealed that the V protein binds and disrupts the MDA5 ATPase domain and thus inhibits the cooperative assembly of MDA5-dsRNA filaments (Motz et al., 2013). Picornaviridae have come up with a more dramatic strategy: they trigger MDA5 and RIG-I cleavage and degradation by using the cell’s caspase and proteasome machinery and the viral protease 3Cpro (Barral et al., 2007; 2009). MAVS is also a target for coxsackievirus-B3-encoded 3Cpro (Mukherjee et al., 2011) and the hepatitis B virus (Hepadnaviridae) viral protein HBx (hepatitis B virus X) (Kumar et al., 2011b; Wei et al., 2010). In addition, MAVS can be cleaved off mitochondria by the serine protease NS3-4A of HCV (Flaviviridae) and the ABC cysteine protease of hepatitis A virus (Picornaviridae) (Li et al., 2005; Meylan et al., 2005). Finally, the three polymerase subunits (PB1, PB2, and PA) and PB1-F2 of influenza virus can also bind MAVS and inhibit the induction of type I IFNs (Graef et al., 2010; Iwai et al., 2010; Varga et al., 2012).
Given the more recent identification of the receptors involved in sensing cytosolic DNA, viral proteins thought to inhibit these PRRs are only just beginning to emerge. There is work suggesting that viral proteins encoded by members of the Herpesviridae family inhibit DAI, DHX9, and IFI16 responses. Recently, it was shown that the HSV-1-encoded protein ICP0 targets the degradation and nuclear relocalization of IFI16 and thereby limits IRF-3 activation (Orzalli et al., 2012). The human CMV matrix protein pUL83 (pp65), which is a known inhibitor of ISG induction and an important virulence factor, has been shown to interact with IFI16 (Cristea et al., 2010). Moreover, the murine CMV viral inhibitor of RIP activation (encoded by M45) and the KSHV viral protein kinase are thought to target DAI and DHX9 responses, respectively (Jong et al., 2010; Rebsamen et al., 2009; Upton et al., 2012; Welz and Pasparakis, 2012). Other viral proteases, including the coronavirus papain-like proteases and the DENV NS2B3 protease, target STING directly (Aguirre et al., 2012; Sun et al., 2012; Yu et al., 2012). The proteolytic activity of NS2B3 appears to be species specific given that it has been shown to cleave human, but not murine, STING (Aguirre et al., 2012; Yu et al., 2012). Nevertheless, targeting of STING by some RNA viruses suggests that it serves as an important antiviral factor for both DNA and RNA viruses.
Conclusion
Cells are equipped with systems that allow them to rapidly detect the presence of viral intruders and coordinate antiviral defense programs. In this review, we have highlighted recent advances in our understanding of how viral nucleic acids are detected inside the cell and activate innate immune defenses. RNA sensors, such as MDA5 and RIG-I, discriminate self-RNA from foreign RNA present in the cytosol by binding specific RNA structures, such as long dsRNA or 5′ PPP base-paired extremities, which are only found upon viral invasion. The molecular basis for this ligand specificity has been revealed by structural studies that further elucidate how ligand binding can result in signal transduction. Analysis of the latter has led to the discovery of a MAVS-mediated self-propagating signal that confers remarkable sensitivity to low levels of viral RNA and results in the induction of an IFN-mediated positive-feedback loop that reinforces antiviral immunity. Further studies have indicated that the system is highly regulated to prevent autoimmune disease and is targeted by viruses to permit their replication. Finally, emerging work has shown that DNA can also be sensed in the cytosol of cells and that this detection involves both novel and previously described proteins, as well as second messengers, such as cGAMP, that had not been known to be produced in eukaryotic cells. Thus, in little under a decade, we have gone from knowing very little about cytosolic detection of viruses to having a rich understanding. However, this understanding is by no means comprehensive. The spatiotemporal aspects of cytosolic viral detection remain mysterious and will need to be elucidated via mapping the viral life cycle with respect to immune activation in infected cells. Further progress is needed for defining the true nature of RLR agonists, particularly in cells infected with viruses recognized by both RIG-I and MDA5 and in which these two helicases might detect distinct RNA species present at different times during virus replication. Little is known about the detection of DNA and whether self-nonself DNA discrimination is simply based on nuclear versus cytoplasmic localization. Even if the latter is true, it will be important to determine how it is regulated in mitotic cells upon disintegration of the nuclear envelope. Finally, understanding how the triggering of multiple PRRs is integrated during viral infection and how this dictates host immunity remains an important issue. Thus, molecular and cellular understanding of cell-intrinsic virus detection and its evasion promises to continue to be a rich area for future study, which will help clarify immune mechanisms of self-nonself discrimination and might lead to novel strategies for intervention in viral disease pathogenesis.
Acknowledgments
We thank members of the Immunobiology laboratory for discussions, as well as Jan Rehwinkel and Cecilia Johansson for critical reading of the manuscript. D.G. is a recipient of Cancer Research UK and Fondation Baxter & Alma Ricard fellowships. S.D. is a recipient of Marie-Curie long-term fellowships. The C.R.S. laboratory is funded by Cancer Research UK, the European Research Council, and Fondation Bettencourt-Schueller.
Footnotes
Supplemental Information includes one table and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2013.05.007.
Supplemental Information
References
- Abdullah Z., Schlee M., Roth S., Mraheil M.A., Barchet W., Böttcher J., Hain T., Geiger S., Hayakawa Y., Fritz J.H. RIG-I detects infection with live Listeria by sensing secreted bacterial nucleic acids. EMBO J. 2012;31:4153–4164. doi: 10.1038/emboj.2012.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablasser A., Bauernfeind F., Hartmann G., Latz E., Fitzgerald K.A., Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre S., Maestre A.M., Pagni S., Patel J.R., Savage T., Gutman D., Maringer K., Bernal-Rubio D., Shabman R.S., Simon V. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8:e1002934. doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J., Gutman D., Saijo S., Barber G.N. STING manifests self DNA-dependent inflammatory disease. Proc. Natl. Acad. Sci. USA. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen I.C., Moore C.B., Schneider M., Lei Y., Davis B.K., Scull M.A., Gris D., Roney K.E., Zimmermann A.G., Bowzard J.B. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An H., Hou J., Zhou J., Zhao W., Xu H., Zheng Y., Yu Y., Liu S., Cao X. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat. Immunol. 2008;9:542–550. doi: 10.1038/ni.1604. [DOI] [PubMed] [Google Scholar]
- Arnoult D., Soares F., Tattoli I., Girardin S.E. Mitochondria in innate immunity. EMBO Rep. 2011;12:901–910. doi: 10.1038/embor.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral P.M., Morrison J.M., Drahos J., Gupta P., Sarkar D., Fisher P.B., Racaniello V.R. MDA-5 is cleaved in poliovirus-infected cells. J. Virol. 2007;81:3677–3684. doi: 10.1128/JVI.01360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral P.M., Sarkar D., Fisher P.B., Racaniello V.R. RIG-I is cleaved during picornavirus infection. Virology. 2009;391:171–176. doi: 10.1016/j.virol.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A., Sachidanandam R., García-Sastre A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc. Natl. Acad. Sci. USA. 2010;107:16303–16308. doi: 10.1073/pnas.1005077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke I.C., Modis Y. MDA5 cooperatively forms dimers and ATP-sensitive filaments upon binding double-stranded RNA. EMBO J. 2012;31:1714–1726. doi: 10.1038/emboj.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke I.C., Yu X., Modis Y., Egelman E.H. MDA5 assembles into a polar helical filament on dsRNA. Proc. Natl. Acad. Sci. USA. 2012;109:18437–18441. doi: 10.1073/pnas.1212186109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besch R., Poeck H., Hohenauer T., Senft D., Häcker G., Berking C., Hornung V., Endres S., Ruzicka T., Rothenfusser S., Hartmann G. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J. Clin. Invest. 2009;119:2399–2411. doi: 10.1172/JCI37155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M., Eberle F., Seitz S., Mücke N., Hüber C.M., Kiani N., Kaderali L., Lohmann V., Dalpke A., Bartenschlager R. Molecular mechanism of signal perception and integration by the innate immune sensor retinoic acid-inducible gene-I (RIG-I) J. Biol. Chem. 2011;286:27278–27287. doi: 10.1074/jbc.M111.256974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit J., Bartlett E., Schomacker H., Surman S., Akira S., Bae Y.-S., Collins P., Murphy B., Schmidt A. The C proteins of human parainfluenza virus type 1 limit double-stranded RNA accumulation that would otherwise trigger activation of MDA5 and protein kinase R. J. Virol. 2011;85:1495–1506. doi: 10.1128/JVI.01297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozym R.A., Delorme-Axford E., Harris K., Morosky S., Ikizler M., Dermody T.S., Sarkar S.N., Coyne C.B. Focal adhesion kinase is a component of antiviral RIG-I-like receptor signaling. Cell Host Microbe. 2012;11:153–166. doi: 10.1016/j.chom.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette R.L., Young J.M., Whitley D.G., Brodsky I.E., Malik H.S., Stetson D.B. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J. Exp. Med. 2012;209:1969–1983. doi: 10.1084/jem.20121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns A.M., Horvath C.M. Activation of RIG-I-like receptor signal transduction. Crit. Rev. Biochem. Mol. Biol. 2012;47:194–206. doi: 10.3109/10409238.2011.630974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette D.L., Monroe K.M., Sotelo-Troha K., Iwig J.S., Eckert B., Hyodo M., Hayakawa Y., Vance R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavlar T., Ablasser A., Hornung V. Induction of type I IFNs by intracellular DNA-sensing pathways. Immunol. Cell Biol. 2012;90:474–482. doi: 10.1038/icb.2012.11. [DOI] [PubMed] [Google Scholar]
- Chen H., Sun H., You F., Sun W., Zhou X., Chen L., Yang J., Wang Y., Tang H., Guan Y. Activation of STAT6 by STING is critical for antiviral innate immunity. Cell. 2011;147:436–446. doi: 10.1016/j.cell.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Chen H., Li Y., Zhang J., Ran Y., Wei J., Yang Y., Shu H.-B. RAVER1 is a coactivator of MDA5-mediated cellular antiviral response. J. Mol. Cell. Biol. 2013;5:111–119. doi: 10.1093/jmcb/mjt006. [DOI] [PubMed] [Google Scholar]
- Chen W., Han C., Xie B., Hu X., Yu Q., Shi L., Wang Q., Li D., Wang J., Zheng P. Induction of Siglec-G by RNA viruses inhibits the innate immune response by promoting RIG-I degradation. Cell. 2013;152:467–478. doi: 10.1016/j.cell.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Chiu Y.-H., Macmillan J.B., Chen Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civril F., Bennett M., Moldt M., Deimling T., Witte G., Schiesser S., Carell T., Hopfner K.-P. The RIG-I ATPase domain structure reveals insights into ATP-dependent antiviral signalling. EMBO Rep. 2011;12:1127–1134. doi: 10.1038/embor.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea I.M., Moorman N.J., Terhune S.S., Cuevas C.D., O’Keefe E.S., Rout M.P., Chait B.T., Shenk T. Human cytomegalovirus pUL83 stimulates activity of the viral immediate-early promoter through its interaction with the cellular IFI16 protein. J. Virol. 2010;84:7803–7814. doi: 10.1128/JVI.00139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow Y.J. Type I interferonopathies: a novel set of inborn errors of immunity. Ann. N Y Acad. Sci. 2011;1238:91–98. doi: 10.1111/j.1749-6632.2011.06220.x. [DOI] [PubMed] [Google Scholar]
- Cui J., Zhu L., Xia X., Wang H.Y., Legras X., Hong J., Ji J., Shen P., Zheng S., Chen Z.J., Wang R.F. NLRC5 negatively regulates the NF-kappaB and type I interferon signaling pathways. Cell. 2010;141:483–496. doi: 10.1016/j.cell.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit E., Boulant S., Zhang Y., Lee A.S.Y., Odendall C., Shum B., Hacohen N., Chen Z.J., Whelan S.P., Fransen M. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal N.K., Emerman M. Evolutionary conflicts between viruses and restriction factors shape immunity. Nat. Rev. Immunol. 2012;12:687–695. doi: 10.1038/nri3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenächer K., Krug A. Regulation of RLR-mediated innate immune signaling—it is all about keeping the balance. Eur. J. Cell Biol. 2012;91:36–47. doi: 10.1016/j.ejcb.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Feng Q., Hato S.V., Langereis M.A., Zoll J., Virgen-Slane R., Peisley A., Hur S., Semler B.L., van Rij R.P., van Kuppeveld F.J.M. MDA5 Detects the Double-Stranded RNA Replicative Form in Picornavirus-Infected Cells. Cell Rep. 2012;2:1187–1196. doi: 10.1016/j.celrep.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B.J., Mansur D.S., Peters N.E., Ren H., Smith G.L. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. Elife. 2012;1:e00047. doi: 10.7554/eLife.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrage F., Dutta K., Nistal-Villán E., Patel J.R., Sánchez-Aparicio M.T., De Ioannes P., Buku A., Aseguinolaza G.G., García-Sastre A., Aggarwal A.K. Structure and Dynamics of the Second CARD of Human RIG-I Provide Mechanistic Insights into Regulation of RIG-I Activation. Structure. 2012;20:2048–2061. doi: 10.1016/j.str.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen B.L., Keller B.C., Fornek J., Katze M.G., Gale M., Jr. Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J. Virol. 2008;82:609–616. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman C.S., O’Donnell M.A., Legarda-Addison D., Ng A., Cárdenas W.B., Yount J.S., Moran T.M., Basler C.F., Komuro A., Horvath C.M. The tumour suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Rep. 2008;9:930–936. doi: 10.1038/embor.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gack M.U., Shin Y.C., Joo C.-H., Urano T., Liang C., Sun L., Takeuchi O., Akira S., Chen Z., Inoue S., Jung J.U. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- Gack M.U., Nistal-Villán E., Inn K.-S., García-Sastre A., Jung J.U. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J. Virol. 2010;84:3220–3229. doi: 10.1128/JVI.02241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall A., Treuting P., Elkon K.B., Loo Y.-M., Gale M., Jr., Barber G.N., Stetson D.B. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36:120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sastre A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 2011;162:12–18. doi: 10.1016/j.virusres.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin D., Lezzi M., Dobbs M., Elliott R.M., Schmaljohn C., Kang C.Y., Kolakofsky D. The 5′ ends of Hantaan virus (Bunyaviridae) RNAs suggest a prime-and-realign mechanism for the initiation of RNA synthesis. J. Virol. 1995;69:5754–5762. doi: 10.1128/jvi.69.9.5754-5762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin L., Barchet W., Gilfillan S., Cella M., Beutler B., Flavell R.A., Diamond M.S., Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Randall R.E. The regulation of type I interferon production by paramyxoviruses. J. Interferon Cytokine Res. 2009;29:539–547. doi: 10.1089/jir.2009.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef K.M., Vreede F.T., Lau Y.-F., McCall A.W., Carr S.M., Subbarao K., Fodor E. The PB2 subunit of the influenza virus RNA polymerase affects virulence by interacting with the mitochondrial antiviral signaling protein and inhibiting expression of beta interferon. J. Virol. 2010;84:8433–8445. doi: 10.1128/JVI.00879-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L., Fullam A., Brennan R., Schröder M. Human DEAD Box Helicase 3 Couples IκB Kinase {varepsilon} to Interferon Regulatory Factor 3 Activation. Mol. Cell. Biol. 2013;33:2004–2015. doi: 10.1128/MCB.01603-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habjan M., Andersson I., Klingström J., Schümann M., Martin A., Zimmermann P., Wagner V., Pichlmair A., Schneider U., Mühlberger E. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS ONE. 2008;3:e2032. doi: 10.1371/journal.pone.0002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa S., Shiratori S., Yamato H., Kameyama T., Kitatsuji C., Kashigi F., Goto S., Kameoka S., Fujikura D., Yamada T. ZAPS is a potent stimulator of signaling mediated by the RNA helicase RIG-I during antiviral responses. Nat. Immunol. 2011;12:37–44. doi: 10.1038/ni.1963. [DOI] [PubMed] [Google Scholar]
- Holm C.K., Jensen S.B., Jakobsen M.R., Cheshenko N., Horan K.A., Moeller H.B., Gonzalez-Dosal R., Rasmussen S.B., Christensen M.H., Yarovinsky T.O. Virus-cell fusion as a trigger of innate immunity dependent on the adaptor STING. Nat. Immunol. 2012;13:737–743. doi: 10.1038/ni.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M., Yoon S.-I., Wilson I.A. Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. Immunity. 2012;36:337–347. doi: 10.1016/j.immuni.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner S.M., Liu H.M., Park H.S., Briley J., Gale M., Jr. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc. Natl. Acad. Sci. USA. 2011;108:14590–14595. doi: 10.1073/pnas.1110133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V., Ellegast J., Kim S., Brzózka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K.-K., Schlee M. 5′-Triphosphate RNA Is the Ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Hou F., Sun L., Zheng H., Skaug B., Jiang Q.-X., Chen Z.J. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.R.L., Burns K.H., Boeke J.D. Active transposition in genomes. Annu. Rev. Genet. 2012;46:651–675. doi: 10.1146/annurev-genet-110711-155616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-H., Liu X.-Y., Du X.-X., Jiang Z.-F., Su X.-D. The structural basis for the sensing and binding of cyclic di-GMP by STING. Nat. Struct. Mol. Biol. 2012;19:728–730. doi: 10.1038/nsmb.2333. [DOI] [PubMed] [Google Scholar]
- Inn K.-S., Lee S.-H., Rathbun J.Y., Wong L.-Y., Toth Z., Machida K., Ou J.-H.J., Jung J.U. Inhibition of RIG-I-mediated signaling by Kaposi’s sarcoma-associated herpesvirus-encoded deubiquitinase ORF64. J. Virol. 2011;85:10899–10904. doi: 10.1128/JVI.00690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs A., Lindenmann J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai A., Shiozaki T., Kawai T., Akira S., Kawaoka Y., Takada A., Kida H., Miyazaki T. Influenza A virus polymerase inhibits type I interferon induction by binding to interferon β promoter stimulator 1. J. Biol. Chem. 2010;285:32064–32074. doi: 10.1074/jbc.M110.112458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F., Ramanathan A., Miller M.T., Tang G.-Q., Gale M., Jr., Patel S.S., Marcotrigiano J. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011;479:423–427. doi: 10.1038/nature10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Österlund P., Sarin L.P., Poranen M.M., Bamford D.H., Guo D., Julkunen I. Innate immune responses in human monocyte-derived dendritic cells are highly dependent on the size and the 5′ phosphorylation of RNA molecules. J. Immunol. 2011;187:1713–1721. doi: 10.4049/jimmunol.1100361. [DOI] [PubMed] [Google Scholar]
- Jiang X., Kinch L.N., Brautigam C.A., Chen X., Du F., Grishin N.V., Chen Z.J. Ubiquitin-Induced Oligomerization of the RNA Sensors RIG-I and MDA5 Activates Antiviral Innate Immune Response. Immunity. 2012;36:959–973. doi: 10.1016/j.immuni.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Waterman P.M., Jonscher K.R., Short C.M., Reisdorph N.A., Cambier J.C. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol. Cell. Biol. 2008;28:5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Hill K.K., Filak H., Mogan J., Knowles H., Zhang B., Perraud A.-L., Cambier J.C., Lenz L.L. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J. Immunol. 2011;187:2595–2601. doi: 10.4049/jimmunol.1100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong J.E., Park J., Kim S., Seo T. Kaposi’s sarcoma-associated herpesvirus viral protein kinase interacts with RNA helicase a and regulates host gene expression. J. Microbiol. 2010;48:206–212. doi: 10.1007/s12275-010-0021-1. [DOI] [PubMed] [Google Scholar]
- Kato H., Sato S., Yoneyama M., Yamamoto M., Uematsu S., Matsui K., Tsujimura T., Takeda K., Fujita T., Takeuchi O., Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K.J. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kato H., Takeuchi O., Mikamo-Satoh E., Hirai R., Kawai T., Matsushita K., Hiiragi A., Dermody T.S., Fujita T., Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerur N., Veettil M.V., Sharma-Walia N., Bottero V., Sadagopan S., Otageri P., Chandran B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Pazhoor S., Bao M., Zhang Z., Hanabuchi S., Facchinetti V., Bover L., Plumas J., Chaperot L., Qin J., Liu Y.J. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA. 2010;107:15181–15186. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D.M., Howley P.M., editors. Vol. 1 and 2. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. (Fields Virology, Fifth Edition). [Google Scholar]
- Kok K.-H., Lui P.-Y., Ng M.-H.J., Siu K.-L., Au S.W.N., Jin D.-Y. The double-stranded RNA-binding protein PACT functions as a cellular activator of RIG-I to facilitate innate antiviral response. Cell Host Microbe. 2011;9:299–309. doi: 10.1016/j.chom.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Kondo T., Kobayashi J., Saitoh T., Maruyama K., Ishii K.J., Barber G.N., Komatsu K., Akira S., Kawai T. DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc. Natl. Acad. Sci. USA. 2013;110:2969–2974. doi: 10.1073/pnas.1222694110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T., Yasukawa K., Yanagi Y., Kawabata S.-I. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci. Signal. 2011;4:ra7. doi: 10.1126/scisignal.2001147. [DOI] [PubMed] [Google Scholar]
- Kowalinski E., Lunardi T., McCarthy A.A., Louber J., Brunel J., Grigorov B., Gerlier D., Cusack S. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Kumar H., Pandey S., Zou J., Kumagai Y., Takahashi K., Akira S., Kawai T. NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. J. Immunol. 2011;186:994–1000. doi: 10.4049/jimmunol.1002094. [DOI] [PubMed] [Google Scholar]
- Kumar M., Jung S.Y., Hodgson A.J., Madden C.R., Qin J., Slagle B.L. Hepatitis B virus regulatory HBx protein binds to adaptor protein IPS-1 and inhibits the activation of beta interferon. J. Virol. 2011;85:987–995. doi: 10.1128/JVI.01825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear H.M., Lancaster A., Wilkins C., Suthar M.S., Huang A., Vick S.C., Clepper L., Thackray L., Brassil M.M., Virgin H.W. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 2013;9:e1003118. doi: 10.1371/journal.ppat.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.N., Roy M., Ong S.-E., Mertins P., Villani A.-C., Li W., Dotiwala F., Sen J., Doench J.G., Orzalli M.H. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat. Immunol. 2013;14:179–185. doi: 10.1038/ni.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Wen H., Yu Y., Taxman D.J., Zhang L., Widman D.G., Swanson K.V., Wen K.-W., Damania B., Moore C.B. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 2012;36:933–946. doi: 10.1016/j.immuni.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Wen H., Ting J.P.-Y. The NLR protein, NLRX1, and its partner, TUFM, reduce type I interferon, and enhance autophagy. Autophagy. 2013;9:432–433. doi: 10.4161/auto.23026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D.W., Amarasinghe G.K. Structural insights into RNA recognition and activation of RIG-I-like receptors. Curr. Opin. Struct. Biol. 2012;22:297–303. doi: 10.1016/j.sbi.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D.W., Basler C.F., Amarasinghe G.K. Molecular mechanisms of viral inhibitors of RIG-I-like receptors. Trends Microbiol. 2012;20:139–146. doi: 10.1016/j.tim.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-D., Sun L., Seth R.B., Pineda G., Chen Z.J. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. USA. 2005;102:17717–17722. doi: 10.1073/pnas.0508531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-D., Chiu Y.-H., Ismail A.S., Behrendt C.L., Wight-Carter M., Hooper L.V., Chen Z.J. Mitochondrial antiviral signaling protein (MAVS) monitors commensal bacteria and induces an immune response that prevents experimental colitis. Proc. Natl. Acad. Sci. USA. 2011;108:17390–17395. doi: 10.1073/pnas.1107114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Diner B.A., Chen J., Cristea I.M. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc. Natl. Acad. Sci. USA. 2012;109:10558–10563. doi: 10.1073/pnas.1203447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind K., Hühn M.H., Flodström-Tullberg M. Immunology in the clinic review series; focus on type 1 diabetes and viruses: the innate immune response to enteroviruses and its possible role in regulating type 1 diabetes. Clin. Exp. Immunol. 2012;168:30–38. doi: 10.1111/j.1365-2249.2011.04557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.M., Loo Y.-M., Horner S.M., Zornetzer G.A., Katze M.G., Gale M., Jr. The mitochondrial targeting chaperone 14-3-3ε regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe. 2012;11:528–537. doi: 10.1016/j.chom.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo Y.-M., Fornek J., Crochet N., Bajwa G., Perwitasari O., Martinez-Sobrido L., Akira S., Gill M.A., García-Sastre A., Katze M.G., Gale M., Jr. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Ding S.C., Vela A., Kohlway A., Lindenbach B.D., Pyle A.M. Structural insights into RNA recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Kohlway A., Vela A., Pyle A.M. Visualizing the Determinants of Viral RNA Recognition by Innate Immune Sensor RIG-I. Structure. 2012;20:1983–1988. doi: 10.1016/j.str.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Kohlway A., Pyle A.M. Duplex RNA Activated ATPases (DRAs): Platforms for RNA sensing, signaling and processing. RNA Biol. 2013;10:111–120. doi: 10.4161/rna.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K., Dong B., Gale M., Jr., Silverman R.H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marq J.-B., Kolakofsky D., Garcin D. Unpaired 5′ ppp-nucleotides, as found in arenavirus double-stranded RNA panhandles, are not recognized by RIG-I. J. Biol. Chem. 2010;285:18208–18216. doi: 10.1074/jbc.M109.089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marq J.-B., Hausmann S., Veillard N., Kolakofsky D., Garcin D. Short double-stranded RNAs with an overhanging 5′ ppp-nucleotide, as found in arenavirus genomes, act as RIG-I decoys. J. Biol. Chem. 2011;286:6108–6116. doi: 10.1074/jbc.M110.186262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney S.A., Thackray L.B., Gitlin L., Gilfillan S., Virgin H.W., Colonna M. MDA-5 recognition of a murine norovirus. PLoS Pathog. 2008;4:e1000108. doi: 10.1371/journal.ppat.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney S.A., Vermi W., Lonardi S., Rossini C., Otero K., Calderon B., Gilfillan S., Diamond M.S., Unanue E.R., Colonna M. RNA sensor-induced type I IFN prevents diabetes caused by a β cell-tropic virus in mice. J. Clin. Invest. 2011;121:1497–1507. doi: 10.1172/JCI44005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchjorsen J., Rintahaka J., Søby S., Horan K.A., Poltajainen A., Østergaard L., Paludan S.R., Matikainen S. Early innate recognition of herpes simplex virus in human primary macrophages is mediated via the MDA5/MAVS-dependent and MDA5/MAVS/RNA polymerase III-independent pathways. J. Virol. 2010;84:11350–11358. doi: 10.1128/JVI.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan E., Curran J., Hofmann K., Moradpour D., Binder M., Bartenschlager R., Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- Mi Z., Fu J., Xiong Y., Tang H. SUMOylation of RIG-I positively regulates the type I interferon signaling. Protein Cell. 2010;1:275–283. doi: 10.1007/s13238-010-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita M., Oshiumi H., Matsumoto M., Seya T. DDX60, a DEXD/H box helicase, is a novel antiviral factor promoting RIG-I-like receptor-mediated signaling. Mol. Cell. Biol. 2011;31:3802–3819. doi: 10.1128/MCB.01368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe K.M., McWhirter S.M., Vance R.E. Identification of host cytosolic sensors and bacterial factors regulating the type I interferon response to Legionella pneumophila. PLoS Pathog. 2009;5:e1000665. doi: 10.1371/journal.ppat.1000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motz C., Schuhmann K.M., Kirchhofer A., Moldt M., Witte G., Conzelmann K.-K., Hopfner K.-P. Paramyxovirus V proteins disrupt the fold of the RNA sensor MDA5 to inhibit antiviral signaling. Science. 2013;339:690–693. doi: 10.1126/science.1230949. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Morosky S.A., Delorme-Axford E., Dybdahl-Sissoko N., Oberste M.S., Wang T., Coyne C.B. The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling. PLoS Pathog. 2011;7:e1001311. doi: 10.1371/journal.ppat.1001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi H., Yanai H., Nakajima A., Koshiba R., Atarashi K., Matsuda A., Matsuki K., Miki S., Doi T., Aderem A. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat. Immunol. 2012;13:659–666. doi: 10.1038/ni.2307. [DOI] [PubMed] [Google Scholar]
- Nistal-Villán E., Gack M.U., Martínez-Delgado G., Maharaj N.P., Inn K.-S., Yang H., Wang R., Aggarwal A.K., Jung J.U., García-Sastre A. Negative role of RIG-I serine 8 phosphorylation in the regulation of interferon-beta production. J. Biol. Chem. 2010;285:20252–20261. doi: 10.1074/jbc.M109.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y., Sano T., Nagata S. Regulation of the innate immune response by threonine-phosphatase of Eyes absent. Nature. 2009;460:520–524. doi: 10.1038/nature08138. [DOI] [PubMed] [Google Scholar]
- Onomoto K., Jogi M., Yoo J.-S., Narita R., Morimoto S., Takemura A., Sambhara S., Kawaguchi A., Osari S., Nagata K. Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity. PLoS ONE. 2012;7:e43031. doi: 10.1371/journal.pone.0043031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzalli M.H., DeLuca N.A., Knipe D.M. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc. Natl. Acad. Sci. USA. 2012;109:E3008–E3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiumi H., Sakai K., Matsumoto M., Seya T. DEAD/H BOX 3 (DDX3) helicase binds the RIG-I adaptor IPS-1 to up-regulate IFN-beta-inducing potential. Eur. J. Immunol. 2010;40:940–948. doi: 10.1002/eji.200940203. [DOI] [PubMed] [Google Scholar]
- Oshiumi H., Matsumoto M., Seya T. Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I. J. Biochem. 2012;151:5–11. doi: 10.1093/jb/mvr111. [DOI] [PubMed] [Google Scholar]
- Ouyang S., Song X., Wang Y., Ru H., Shaw N., Jiang Y., Niu F., Zhu Y., Qiu W., Parvatiyar K. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36:1073–1086. doi: 10.1016/j.immuni.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan S.R., Bowie A.G. Immune Sensing of DNA. Immunity. 2013;38:870–880. doi: 10.1016/j.immuni.2013.05.004. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A., Lin C., Wu B., Orme-Johnson M., Liu M., Walz T., Hur S. Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc. Natl. Acad. Sci. USA. 2011;108:21010–21015. doi: 10.1073/pnas.1113651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisley A., Jo M.H., Lin C., Wu B., Orme-Johnson M., Walz T., Hohng S., Hur S. Kinetic mechanism for viral dsRNA length discrimination by MDA5 filaments. Proc. Natl. Acad. Sci. USA. 2012;109:E3340–E3349. doi: 10.1073/pnas.1208618109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A., Schulz O., Tan C.P., Näslund T.I., Liljeström P., Weber F., Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Pichlmair A., Schulz O., Tan C.P., Rehwinkel J., Kato H., Takeuchi O., Akira S., Way M., Schiavo G., Reis e Sousa C. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J. Virol. 2009;83:10761–10769. doi: 10.1128/JVI.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeck H., Bscheider M., Gross O., Finger K., Roth S., Rebsamen M., Hannesschläger N., Schlee M., Rothenfusser S., Barchet W. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat. Immunol. 2010;11:63–69. doi: 10.1038/ni.1824. [DOI] [PubMed] [Google Scholar]
- Prantner D., Perkins D.J., Lai W., Williams M.S., Sharma S., Fitzgerald K.A., Vogel S.N. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J. Biol. Chem. 2012;287:39776–39788. doi: 10.1074/jbc.M112.382986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsamen M., Heinz L.X., Meylan E., Michallet M.-C., Schroder K., Hofmann K., Vazquez J., Benedict C.A., Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsamen M., Vazquez J., Tardivel A., Guarda G., Curran J., Tschopp J. NLRX1/NOD5 deficiency does not affect MAVS signalling. Cell Death Differ. 2011;18:1387. doi: 10.1038/cdd.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehwinkel J., Tan C.P., Goubau D., Schulz O., Pichlmair A., Bier K., Robb N., Vreede F., Barclay W., Fodor E., Reis e Sousa C. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Roth-Cross J.K., Bender S.J., Weiss S.R. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J. Virol. 2008;82:9829–9838. doi: 10.1128/JVI.01199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah A., Chang T.H., Harnack R., Frohlich V., Tominaga K., Dube P.H., Xiang Y., Bose S. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Fujita N., Hayashi T., Takahara K., Satoh T., Lee H., Matsunaga K., Kageyama S., Omori H., Noda T. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl. Acad. Sci. USA. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer J.-D., Sotelo-Troha K., von Moltke J., Monroe K.M., Rae C.S., Brubaker S.W., Hyodo M., Hayakawa Y., Woodward J.J., Portnoy D.A., Vance R.E. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 2011;79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M., Roth A., Hornung V., Hagmann C.A., Wimmenauer V., Barchet W., Coch C., Janke M., Mihailovic A., Wardle G. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A., Schwerd T., Hamm W., Hellmuth J.C., Cui S., Wenzel M., Hoffmann F.S., Michallet M.-C., Besch R., Hopfner K.-P. 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc. Natl. Acad. Sci. USA. 2009;106:12067–12072. doi: 10.1073/pnas.0900971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider U., Martin A., Schwemmle M., Staeheli P. Genome trimming by Borna disease viruses: viral replication control or escape from cellular surveillance? Cell. Mol. Life Sci. 2007;64:1038–1042. doi: 10.1007/s00018-007-6545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins J.W., Wilson S.J., Panis M., Murphy M.Y., Jones C.T., Bieniasz P., Rice C.M. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder M., Baran M., Bowie A.G. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 2008;27:2147–2157. doi: 10.1038/emboj.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares F., Tattoli I., Wortzman M.E., Arnoult D., Philpott D.J., Girardin S.E. NLRX1 does not inhibit MAVS-dependent antiviral signaling. Innate Immun. 2012 doi: 10.1177/1753425912467383. Published online December 4, 2012. [DOI] [PubMed] [Google Scholar]
- Soulat D., Bürckstümmer T., Westermayer S., Goncalves A., Bauch A., Stefanovic A., Hantschel O., Bennett K.L., Decker T., Superti-Furga G. The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. EMBO J. 2008;27:2135–2146. doi: 10.1038/emboj.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Li Y., Chen L., Chen H., You F., Zhou X., Zhou Y., Zhai Z., Chen D., Jiang Z. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc. Natl. Acad. Sci. USA. 2009;106:8653–8658. doi: 10.1073/pnas.0900850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Xing Y., Chen X., Zheng Y., Yang Y., Nichols D.B., Clementz M.A., Banach B.S., Li K., Baker S.C., Chen Z. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS ONE. 2012;7:e30802. doi: 10.1371/journal.pone.0030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar M.S., Ramos H.J., Brassil M.M., Netland J., Chappell C.P., Blahnik G., McMillan A., Diamond M.S., Clark E.A., Bevan M.J. The RIG-I-like Receptor LGP2 Controls CD8(+) T Cell Survival and Fitness. Immunity. 2012;37:235–248. doi: 10.1016/j.immuni.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahasi K., Yoneyama M., Nishihori T., Hirai R., Kumeta H., Narita R., Gale M., Jr., Inagaki F., Fujita T. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol. Cell. 2008;29:428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Takaoka A., Wang Z., Choi M.K., Yanai H., Negishi H., Ban T., Lu Y., Miyagishi M., Kodama T., Honda K. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Chen Z.J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 2012;5:ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K.E., Mossman K.L. Recent advances in understanding viral evasion of type I interferon. Immunology. 2013;138:190–197. doi: 10.1111/imm.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou K., Vakakis E., Kar S., Richer E., Evans G.L., Triantafilou M. Visualisation of direct interaction of MDA5 and the dsRNA replicative intermediate form of positive strand RNA viruses. J. Cell Sci. 2012;125:4761–4769. doi: 10.1242/jcs.103887. [DOI] [PubMed] [Google Scholar]
- Tsuchida T., Zou J., Saitoh T., Kumar H., Abe T., Matsuura Y., Kawai T., Akira S. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity. 2010;33:765–776. doi: 10.1016/j.immuni.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Unterholzner L., Keating S.E., Baran M., Horan K.A., Jensen S.B., Sharma S., Sirois C.M., Jin T., Latz E., Xiao T.S. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton J.W., Kaiser W.J., Mocarski E.S. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kasteren P.B., Beugeling C., Ninaber D.K., Frias-Staheli N., van Boheemen S., García-Sastre A., Snijder E.J., Kikkert M. Arterivirus and nairovirus ovarian tumor domain-containing Deubiquitinases target activated RIG-I to control innate immune signaling. J. Virol. 2012;86:773–785. doi: 10.1128/JVI.06277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z.T., Grant A., Manicassamy B., Palese P. Influenza virus protein PB1-F2 inhibits t2he induction of type I interferon by binding to MAVS and decreasing mitochondrial membrane potential. J. Virol. 2012;86:8359–8366. doi: 10.1128/JVI.01122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela A., Fedorova O., Ding S.C., Pyle A.M. The thermodynamic basis for viral RNA detection by the RIG-I innate immune sensor. J. Biol. Chem. 2012;287:42564–42573. doi: 10.1074/jbc.M112.385146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang H.-X., Sun Y.-P., Liu Z.-X., Liu X.-S., Wang L., Lu S.-Y., Kong H., Liu Q.-L., Li X.-H. Rig-I-/- mice develop colitis associated with downregulation of G alpha i2. Cell Res. 2007;17:858–868. doi: 10.1038/cr.2007.81. [DOI] [PubMed] [Google Scholar]
- Wang D., Fang L., Li P., Sun L., Fan J., Zhang Q., Luo R., Liu X., Li K., Chen H. The leader proteinase of foot-and-mouth disease virus negatively regulates the type I interferon pathway by acting as a viral deubiquitinase. J. Virol. 2011;85:3758–3766. doi: 10.1128/JVI.02589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Vaheri A., Weber F., Plyusnin A. Old World hantaviruses do not produce detectable amounts of dsRNA in infected cells and the 5′ termini of their genomic RNAs are monophosphorylated. J. Gen. Virol. 2011;92:1199–1204. doi: 10.1099/vir.0.029405-0. [DOI] [PubMed] [Google Scholar]
- Wang Y., Tong X., Ye X. Ndfip1 negatively regulates RIG-I-dependent immune signaling by enhancing E3 ligase Smurf1-mediated MAVS degradation. J. Immunol. 2012;189:5304–5313. doi: 10.4049/jimmunol.1201445. [DOI] [PubMed] [Google Scholar]
- Wang Y., Tong X., Li G., Li J., Deng M., Ye X. Ankrd17 positively regulates RIG-I-like receptor (RLR)-mediated immune signaling. Eur. J. Immunol. 2012;42:1304–1315. doi: 10.1002/eji.201142125. [DOI] [PubMed] [Google Scholar]
- Wang Y., Tong X., Omoregie E.S., Liu W., Meng S., Ye X. Tetraspanin 6 (TSPAN6) negatively regulates retinoic acid-inducible gene I-like receptor-mediated immune signaling in a ubiquitination-dependent manner. J. Biol. Chem. 2012;287:34626–34634. doi: 10.1074/jbc.M112.390401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhao W., Zhang M., Wang P., Zhao K., Zhao X., Yang S., Gao C. USP4 positively regulates RIG-I-mediated antiviral response through deubiquitination and stabilization of RIG-I. J. Virol. 2013;87:4507–4515. doi: 10.1128/JVI.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Wagner V., Rasmussen S.B., Hartmann R., Paludan S.R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Gawanbacht A., Habjan M., Rang A., Borner C., Schmidt A.M., Veitinger S., Jacob R., Devignot S., Kochs G. Incoming RNA virus nucleocapsids containing a 5′-triphosphorylated genome activate RIG-I and antiviral signaling. Cell Host Microbe. 2013;13:336–346. doi: 10.1016/j.chom.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C., Ni C., Song T., Liu Y., Yang X., Zheng Z., Jia Y., Yuan Y., Guan K., Xu Y. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J. Immunol. 2010;185:1158–1168. doi: 10.4049/jimmunol.0903874. [DOI] [PubMed] [Google Scholar]
- Welz P.-S., Pasparakis M. A way to DAI. Cell Host Microbe. 2012;11:223–225. doi: 10.1016/j.chom.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Wies E., Wang M.K., Maharaj N.P., Chen K., Zhou S., Finberg R.W., Gack M.U. Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity. 2013;38:437–449. doi: 10.1016/j.immuni.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Peisley A., Richards C., Yao H., Zeng X., Lin C., Chu F., Walz T., Hur S. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152:276–289. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T.S., Ting J.P.-Y. NLRX1 has a tail to tell. Immunity. 2012;36:311–312. doi: 10.1016/j.immuni.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Xiao N., Liu F., Ren H., Gu J. Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc. Natl. Acad. Sci. USA. 2009;106:1530–1535. doi: 10.1073/pnas.0811029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N., Chen Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012;13:214–222. doi: 10.1038/ni.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai H., Ban T., Wang Z., Choi M.K., Kawamura T., Negishi H., Nakasato M., Lu Y., Hangai S., Koshiba R. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- Yang P., An H., Liu X., Wen M., Zheng Y., Rui Y., Cao X. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat. Immunol. 2010;11:487–494. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- Yin Q., Tian Y., Kabaleeswaran V., Jiang X., Tu D., Eck M.J., Chen Z.J., Wu H. Cyclic di-GMP sensing via the innate immune signaling protein STING. Mol. Cell. 2012;46:735–745. doi: 10.1016/j.molcel.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y.-M., Gale M., Jr., Akira S. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- Yu C.-Y., Chang T.-H., Liang J.-J., Chiang R.-L., Lee Y.-L., Liao C.-L., Lin Y.-L. Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS Pathog. 2012;8:e1002780. doi: 10.1371/journal.ppat.1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Sun L., Jiang X., Chen X., Hou F., Adhikari A., Xu M., Chen Z.J. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.-N., Shen S.-H., Jiang L.-J., Zhang W., Zhang H.-X., Sun Y.-P., Li X.-Y., Huang Q.-H., Ge B.-X., Chen S.-J. RIG-I plays a critical role in negatively regulating granulocytic proliferation. Proc. Natl. Acad. Sci. USA. 2008;105:10553–10558. doi: 10.1073/pnas.0804895105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Brann T.W., Zhou M., Yang J., Oguariri R.M., Lidie K.B., Imamichi H., Huang D.-W., Lempicki R.A., Baseler M.W. Cutting edge: Ku70 is a novel cytosolic DNA sensor that induces type III rather than type I IFN. J. Immunol. 2011;186:4541–4545. doi: 10.4049/jimmunol.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Kim T., Bao M., Facchinetti V., Jung S.-Y., Ghaffari A.A., Qin J., Cheng G., Liu Y.-J. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011;34:866–878. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yuan B., Bao M., Lu N., Kim T., Liu Y.-J. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yuan B., Lu N., Facchinetti V., Liu Y.-J. DHX9 pairs with IPS-1 to sense double-stranded RNA in myeloid dendritic cells. J. Immunol. 2011;187:4501–4508. doi: 10.4049/jimmunol.1101307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Hu M.-M., Wang Y.-Y., Shu H.-B. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J. Biol. Chem. 2012;287:28646–28655. doi: 10.1074/jbc.M112.362608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B., Yang Y., Li S., Wang Y.-Y., Li Y., Diao F., Lei C., He X., Zhang L., Tien P., Shu H.B. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Züst R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J., Szretter K.J., Baker S.C., Barchet W., Diamond M.S. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.