Abstract
Multicellular organisms are not created through cell proliferation alone. It is through cell death that an indefinite cellular mass is pared back to reveal its true form. Cells are also lost throughout life as part of homeostasis and through injury. This detritus represents a significant burden to the living organism and must be cleared, most notably through the use of specialized phagocytic cells. Our understanding of these phagocytes and how they engulf cell corpses has been greatly aided by studying the fruit fly, Drosophila melanogaster. Here we review the contribution of Drosophila research to our understanding of how phagocytes respond to cell death. We focus on the best studied phagocytes in the fly: the glia of the central nervous system, the ovarian follicle cells, and the macrophage-like hemocytes. Each is explored in the context of the tissue they maintain as well as how they function during development and in response to injury.
LIFE, DEATH, AND THE FRUIT FLY
Cell death is an unavoidable part of life. During embryogenesis and development, animals generate an excess of cells, which are then selectively culled through programmed cell death to help shape the emerging organism. Even after birth, deliberate killing of defective or even otherwise viable cells continues throughout adult life with the purpose of maintaining tissue homeostasis and good health. Cell death also occurs unpredictably in response to injury and infection. Ultimately, a significant fraction of our cells have to die for us to live. However, clearing all this cell debris presents a further challenge to every living organism. This is achieved through the engulfment of the dead cells by the living through phagocytosis (also known as “efferocytosis”). Many cells within a tissue have some inherent ability to take up cellular debris in this way in what is termed “nonprofessional phagocytosis.” However, as animals have become larger and more complex, their burden of dead cells has increased exponentially. This has necessitated the enlistment of professional phagocytes, which are specifically tasked with removing cell corpses.
Cell death and the clearance thereof has been studied with great success in the nematode Caenorhabditis elegans and many of the seminal findings discussed in this review have their origin in this organism. For example, the highly conserved cell death abnormality (CED) pathways were first discovered in the worm through screens to identify mutants in which cell corpses were not cleared (Hedgecock et al. 1983; Ellis et al. 1991). This led to the characterization of two parallel, partially redundant pathways acting in the phagocyte to drive engulfment (Ellis et al. 1991). Both these CED pathways converge on the Rho-GTPase, CED-10 (Rac1), the activity of which stimulates the actin cytoskeleton to promote phagocytic cup formation (Kinchen et al. 2005). CED-2/CED-5/CED-12 form one arm of the pathway and act downstream of a number of partially redundant corpse-recognition receptors, including the phosphatidylserine receptor and integrins (Ellis et al. 1991; Gumienny et al. 2001; Wu et al. 2001; Wang et al. 2003; Hsu and Wu 2010). CED-2, CED-5, and CED-12 were subsequently identified in mammals as crkII, Dock180, and ELMO, respectively, and together form an unconventional Rho-GEF that activates Rac1 (Wu and Horvitz 1998a; Gumienny et al. 2001; Wu et al. 2001; Brugnera et al. 2002).
Alternatively, CED-1/CED-6/CED-7 can independently promote CED-10 activation and engulfment (Kinchen et al. 2005). CED-1 (Draper in flies, MEGF10 in mammals) is a receptor involved in corpse recognition, which acts upstream of the adaptor protein CED-6 (Zhou et al. 2001; Callebaut et al. 2003; Freeman et al. 2003). CED-6 (GULP in mammals) binds the NPxY motif within the cytoplasmic tail of CED-1 via its PTB domain (Su et al. 2002). CED-7 is an ABC transporter (ABC1 in mammals) and is interestingly required in both the apoptotic corpse and the phagocyte for effective clearance (Wu and Horvitz 1998b). In the dying cell, it acts to help expose the “eat-me” signal, phosphatidylserine, on the outer leaflet of the plasma membrane (Hamon et al. 2000). This, in turn, is detected by CED-1 in the phagocyte during engulfment (Venegas and Zhou 2007). However, the role of CED-7 plays in the phagocyte is less clear. Furthermore, how CED-1/CED-6 connect to Rac activation to aid corpse uptake is also not known.
The nematode has revealed much about the basics underlying the clearance of cell corpses. However, it lacks the professional class of phagocytes required to engulf and clear the sheer volume of cellular debris found in more complex organisms such as ourselves. The invariant nature of programmed cell death in the nematode is also at odds with the more plastic apoptosis found in most animals. Alternatively, the use of the fruit fly, Drosophila melanogaster, offers many advantages for studying the clearance of cell corpses. Like the worm, the fly is highly genetically tractable, aiding the identification and dissection of molecular pathways. Moreover, compared with the nematode, there is an order of magnitude more cell death in the developing fly, which is addressed in part through the possession of professional phagocytes (Abrams et al. 1993). Furthermore, although the patterns of programmed cell death are broadly similar between individuals, the exact cells removed are not precisely delineated as they are in the worm (Abrams et al. 1993; Rogulja-Ortmann et al. 2007). This requires a far greater degree of plasticity from not only the tissues from which cells will be removed, but also from the responding phagocytes themselves. This additional complexity is much closer to the scenario found in mammals. Pioneering studies in Drosophila revealed the transcriptional induction of programmed cell death and the inhibitory control of apoptosis (White et al. 1994; Hay et al. 1995). However, this review focuses on the contribution of Drosophila research to our understanding of how both professional and nonprofessional phagocytes respond to and clear cell death. The phagocytic glia of the central nervous system (CNS), the follicular cells of the ovary, and the macrophage-like hemocytes are the three best studied fly phagocytes, and their mode of engulfment will be compared and contrasted in this review. Drosophila possess conserved homologs of all the CED pathway members and a particular focus will be placed on the findings that have emerged from their study in these three phagocytic cell types. We will also highlight the central role of the fly CED-1 homolog, Draper, as it operates in all three of these phagocytes and in each step of corpse engulfment. Finally, an emphasis will also be placed on the tissue context in which these phagocytes operate as this has profound consequences for how cellular debris is ultimately cleared.
PHAGOCYTIC GLIA OF THE FLY CENTRAL NERVOUS SYSTEM
The developing CNS of the fly embryo contain specialized phagocytic glial cells that act to clear apoptotic neurons generated during development and metamorphosis (Sonnenfeld and Jacobs 1995; Cantera and Technau 1996). Glia are nonneuronal cells that play many different supportive roles within the brain. Unlike vertebrates, flies do not have CNS resident macrophages such as microglia, making glia the sole phagocytes within the fly brain.
The developing CNS is shaped by waves of neuronal programmed cell death, which begin during mid–late embryogenesis (Abrams et al. 1993; Rogulja-Ortmann et al. 2007). Once ensheathed, the developing CNS of the embryo (and the apoptotic corpses therein) is isolated from the patroling macrophages (hemocytes) that populate the interstitial space above the CNS (Sonnenfeld and Jacobs 1995). It then falls to the astrocyte-like glia embedded within the embryonic CNS to clear the apoptotic neurons (Kurant et al. 2008). Although classed as “nonprofessional phagocytes,” the phagocytic activity of these glia is comparable to that of the macrophages. Despite their exclusion from the CNS, macrophages do engulf some neuronal debris, presumably occurring before ensheathment is completed at the interface between these immune cells and the CNS (Sonnenfeld and Jacobs 1995).
Clearance of embryonic neuronal debris by glia requires the Drosophila CED-1 homolog, Draper (Freeman et al. 2003). However, Draper is not required for the uptake of apoptotic debris by the embryonic glia and is instead required for processing of the engulfed corpses (Kurant et al. 2008). Therefore, the failure of draper mutant glia to effectively clear embryonic neuronal debris is presumably because of an inability to turn over engulfed corpses, preventing further phagocytosis. A related receptor, SIMU, is required for glial phagocytosis in the embryo and acts upstream of Draper during engulfment (Kurant et al. 2008). Although the extracellular domains of SIMU and Draper share similarities, the former lacks the intracellular cytoplasmic signaling domains possessed by Draper. Both are highly expressed in glia as part of their developmental differentiation and are sufficient to promote the phagocytic function of these cells (Shklyar et al. 2014). It is proposed that SIMU acts as a tethering receptor that binds directly to the phosphatidylserine exposed on dying cells (Shklyar et al. 2013). Although Draper also binds phosphatidylserine, this is seemingly not required for the interaction between glia and apoptotic debris and instead potentially triggers downstream signaling required for subsequent corpse processing. Surprisingly, SIMU is not expressed in postembryonic glia and is not necessary for clearing apoptotic debris during the wave of neuronal programmed cell death occurring during metamorphosis (Hilu-Dadia et al. 2018). Instead, it is Draper that is required for glial engulfment of apoptotic neurons (Hilu-Dadia et al. 2018). Glia also play an active role in pruning, whereby superfluous neuronal projections are removed (Fig. 1; Awasaki and Ito 2004). Although pruning is not associated with neuronal death, it uses the same engulfment machinery required by glia to clear apoptotic debris (Awasaki et al. 2006; Williams et al. 2006). Furthermore, localized caspase activity (which usually drives apoptosis) within the degenerating dendrites is necessary for their clearance during pruning (Williams et al. 2006).
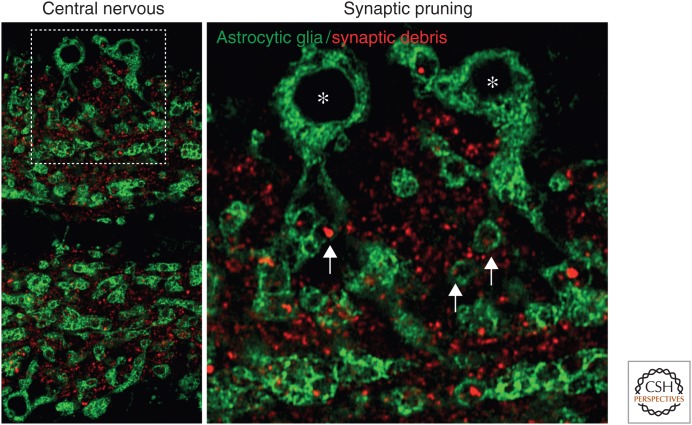
Figure 1.
Phagocytic glia clear synaptic debris within the fly central nervous system. (Left panel) Low-magnification image of pupal ventral nerve cord undergoing synaptic pruning (6 h APF). (Right panel) Magnified image of region outlined by dashed box. Astrocyte-like glia (mCD8-GFP driven by alrm-GAL4, green) and synaptic debris (anti-Brp staining, red) are visualized. Asterisks highlight two astrocyte-like glia in which examples of phagocytic events are seen. Note the extensions of these two cells, highlighted by arrows, in which the anti-Brp red staining is enclosed in the green glial phagocytic cups. (Images provided by Yunsik Kang and Marc Freeman.)
Beyond clearing degenerating neurons as part of development, glia also engulf dying neurons arising from damage to the adult CNS (MacDonald et al. 2006). One powerful model of this response is the glia-mediated clearance of severed olfactory receptor neurons undergoing Wallerian degeneration in the adult fly antennal lobe (MacDonald et al. 2006). In contrast to the embryo, in the adult CNS, it is ensheathing glia and not astrocyte-like glia that clear degenerating axons (Doherty et al. 2009). Whether this difference is because of a dramatic switch in the role of astrocyte-like glia, a further subdivision of this cell type between the embryo and the adult or simply a change in phagocytic glia morphology is not clear. In response to axonal injury, these glia up-regulate their phagocytic machinery, extend infiltrating membrane processes into the antennal lobe and specifically target the cleaved axons for engulfment (MacDonald et al. 2006). Draper is one such up-regulated receptor and in its absence glia fail to infiltrate toward the severed axons, which then persist within the lobe long after injury (MacDonald et al. 2006). Draper possesses a carboxy-terminal immunoreceptor tyrosine-based activation motif (ITAM), a signaling motif commonly found in the cytoplasmic tail of mammalian immunoreceptors including Fc, T-cell, and B-cell receptors (Ziegenfuss et al. 2008). The ITAM of Draper is phosphorylated by the src family kinase, Src42a, leading to association with the Syk family kinase, Shark (Ziegenfuss et al. 2008). Loss of either Src42a or Shark phenocopies loss of Draper, whereby glia fail to clear axonal damage within the antennal lobe (Ziegenfuss et al. 2008). The proposed model emerging from this work is that Draper ligand binding promotes receptor clustering and subsequent ITAM phosphorylation by Src42a. The activated Draper receptor then recruits Shark, which in turn triggers downstream signaling and ultimately engulfment. Interestingly, a second, inhibitory splice variant of Draper (Draper-II) is expressed in glia following glial recruitment to severed axons (Logan et al. 2012). Draper-II lacks an ITAM and instead possesses an immunoreceptor tyrosine-based inhibitory motif (ITIM). Draper-II acts in a negative feedback loop to suppress Draper activity via recruitment of the SHIP phosphatase, corkscrew, to dephosphorylate Shark (Logan et al. 2012). It is proposed that the delayed expression of Draper-II is timed to coincide with the clearance of axonal damage and so prevent excessive, potentially damaging, glial activity.
Beyond Draper, the clearance of severed axons by glia also requires other CED pathway components, including crk (CED-2/crkII), mbc (CED-5/Dock180), and Drosophila CED-12 (ELMO) (Ziegenfuss et al. 2012). Together, these three form an unconventional Rho-GEF, which activates the RhoGTPase, Rac1 (CED-10), which in turn mobilizes the actin cytoskeleton and drives phagocytic cup formation. Whereas Draper and Rac1 are required for the initial infiltration of glial processes toward the damaged axons, crk/mbc/dCED-12 are only required for the subsequent uptake of cellular debris (Ziegenfuss et al. 2012). Because the output of the crk/mbc/dCED-12 complex is activated Rac1, the requirement for Rac activity for glial recruitment implies the presence of a distinct, earlier activator. The sevenless receptor tyrosine kinase (sev RTK) pathway members downstream of receptor kinase (DRK), daughter of sevenless (DOS), and son of sevenless (SOS) are also required for clearance of axonal damage by glia (Lu et al. 2014). Although the sev RTK pathway conventionally activates the Rho GTPase, Ras, glial expression of dominant-negative ras failed to block engulfment. Instead within the glia, DRK/DOS/SOS appear to be working in parallel with crk/mbc/dCED-12 to activate Rac1. Loss of either pathway blocks glial engulfment rather than glial recruitment to axonal injury. However, the combined disruption of both results in a complete failure of the glia to respond to the damaged axons. Confusingly, this implies that these two pathways are functioning redundantly during the initial infiltration of glia toward axonal damage, but nonredundantly during engulfment. No physical interaction between Draper and DRK has been detected and so remains unknown whether Draper is directly responsible for sev RTK pathway activation. However, what is clear is that Draper plays a central role in orchestrating glial engulfment of axonal debris. Importantly, the research discussed in this section has expanded our understanding of the intracellular signaling occurring downstream of Draper and, in all probability, other CED-1 homologs.
The study of phagocytic glia of the fly have taught us much about how the CNS is kept clear of cell corpses arising from development or injury. These glia function within a highly insulated, compact, and complicated tissue where collateral damage from careless or excessive phagocytosis can have severe consequences. One particular advantage offered by Drosophila is the opportunity to study glia within their in vivo environment and so fully capture their complex role within the brain
PHAGOCYTIC FOLLICLE CELLS OF THE DROSOPHILA OVARY
Within the Drosophila ovary, hundreds of egg chambers progress through the well-defined stages of oogenesis. Within each egg chamber, the oocyte is nourished by 15 nurse cells and surrounding these germ line cells are the somatic epithelial follicle cells (Fig. 2). Post “dumping” of their cytoplasmic contents into the oocyte, nurse cells undergo programmed cell death and are cleared by a phagocytic subset of the follicle cells (Timmons et al. 2016). Experimentally, nutrient deprivation can induce premature nurse cell death, which again leads to their engulfment by the follicle cells (Fig. 2). Because the egg chambers of the fly ovary are sealed off from professional phagocytes such as the macrophages, these follicle cells are solely responsible for the clearance of dying nurse cells. Ultimately, they serve as an important model of nonprofessional phagocytosis including Sertoli cells found in the testes.
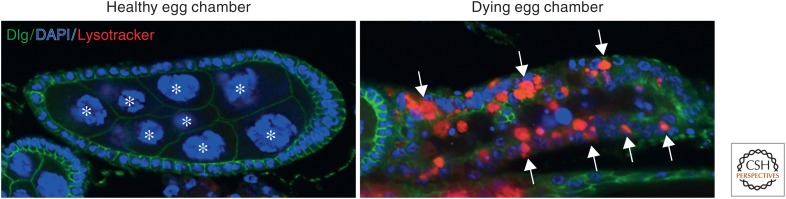
Figure 2.
The follicle cells of the ovary phagocytose dying nurse cells. Egg chambers stained with DAPI (nuclei, blue), anti-Dgl (cell boundaries, green), and Lysotracker (acidified phagosomes, red). (Left panel) Healthy egg chamber containing nurse cells (asterisks) surrounded by follicle cells. (Right panel) Dying egg chamber in which the nurse cells have been engulfed by the follicle cells. The numerous acidified mature phagosomes within the follicle cells (arrows) are indicative of engulfment. (Images provided by Sandy Serizier and Kim McCall.)
As in the glia, efficient nurse cell clearance by follicle cells depends on the fly CED homologs, including Draper (CED-1), dCED-12, Rac1 (CED-10), and a recently identified fly CED-7 homolog, Eato, which act within their canonical parallel pathways (Etchegaray et al. 2012; Timmons et al. 2016; Santoso et al. 2018). Draper, in particular, is enriched at the interface between the engulfing phagocyte and the dying nurse cells (Etchegaray et al. 2012). Integrins (specifically αPS3/βPS heterodimers) are also involved in nurse cell clearance and are actively trafficked to the apical surface of the follicle cells where they accumulate with Draper (Meehan et al. 2015, 2016). The combined loss of Draper and integrins yields a stronger defect in clearance than loss of either one alone, implying that they work independently from one another (Meehan et al. 2016). Nevertheless, together, these receptors likely trigger phagocytosis by allowing follicle cells to physically interact with the nurse cells. Whereas integrins are only involved in the initial uptake of cellular debris, Draper is additionally required for subsequent phagosome maturation/acidification (Meehan et al. 2016). Interestingly, follicle cells lacking both Draper and integrins still retain some residual phagocytic ability, implying there are players still yet to be identified (Meehan et al. 2016).
Overexpression of Draper alone in the phagocytic follicle cells is sufficient to promote the inappropriate engulfment of otherwise healthy nurse cells (Etchegaray et al. 2012). Strikingly, it has emerged that neither apoptosis nor autophagy are necessary for the death of the nurse cells (Peterson and McCall 2013). It appears that the follicle cells induce the death of the nurse cells nonautonomously via their engulfment machinery in what is termed “phagoptosis” (Timmons et al. 2016). The existence of phagoptosis turns what we understand about the interaction between dying cell and phagocyte on its head. Instead of simply responding to and clearing cell corpses, phagocytes can play an active role in inducing cell death raising fascinating questions about selectivity and implementation. Exactly how follicle cells trigger the death of the nurse cells is yet to be addressed, but it appears to require the lysosomal pathway (Peterson and McCall 2013; Timmons et al. 2016). As a self-contained tissue isolated from any other phagocytes and where cell death can be easily induced experimentally, the Drosophila ovary represents an ideal setting to dissect the process of phagoptosis.
PHAGOCYTIC MACROPHAGES OF THE FLY IMMUNE SYSTEM
Macrophage-like blood cells (hemocytes) are the professional phagocytes of the fly and represent the cellular component of the fly's immune system. They are an extremely multifunctional cell type, involved in the inflammatory response to wounds, the engulfment of pathogens, and the deposition of extracellular matrix (Stramer et al. 2005; Vlisidou et al. 2009; Matsubayashi et al. 2017). Like their mammalian counterparts, Drosophila macrophages also have a vital role in clearing cellular debris generated during embryogenesis and metamorphosis, as well as that arising from tissue damage. In contrast to the other two types of phagocytes discussed in this review, macrophages are highly motile during embryogenesis and metamorphosis. Although they show low motility during larval and adult stages, this is because they are instead pumped around the larval/adult body cavity by the fly's open circulatory system. Importantly, this allows macrophages to move toward and clear cellular debris that would be otherwise out of reach to immobile cells.
The embryonic macrophages extend large, dynamic, actin-rich protrusions known as “lamellipods” to move toward and engulf pathogenic intruders and cellular debris (Davidson et al. 2019). Macrophages originate from the head mesoderm and migrate along predetermined routes to populate the entire embryo (Tepass et al. 1994; Wood et al. 2006). During this developmental dispersal, these highly phagocytic cells clear apoptotic corpses that have been eliminated through programmed cell death as part of embryogenesis (Tepass et al. 1994). The engulfment of these first apoptotic corpses triggers the up-regulation of phagocytic receptors such as the CED-1 homolog, Draper, and the CD36 homolog, croquemort (Franc et al. 1999; Weavers et al. 2016a). This in turn undoubtedly heightens their phagocytic activity and reinforces their macrophage identity. Remarkably, in the absence of the key blood cell differentiation factor, serpent, expression of either crq, Draper or SIMU is sufficient to partially restore the dispersal and phagocytic ability of macrophages within the embryo (Shlyakhover et al. 2018).
Draper is a promiscuous receptor and has several reported ligands. One such ligand is Pretaporter, which during apoptosis is released from the endoplasmic reticulum, at which point it relocates to the cell surface and is subsequently recognized by phagocytes via Draper (Kuraishi et al. 2009). However, this initial finding has yet to be advanced. Macroglobulin complement-related (mcr), is another proposed Draper ligand (Lin et al. 2017). However, as of yet no physical interaction been Draper and mcr has been detected. By far the best characterized Draper ligand thus far is phosphatidylserine, which is one of the best known “eat me” signals exposed on the outer leaflet of cells undergoing apoptosis (Tung et al. 2013). Furthermore, this receptor/ligand interaction is consistent with that found with CED-1 in the worm (Venegas and Zhou 2007). The overexpression of Draper in hemocyte-derived, Drosophila “S2” cell lines is sufficient to increase apoptotic corpse uptake in vitro (Williamson and Vale 2018). During S2 cell engulfment of apoptotic corpses or phosphatidylserine-coated beads in vitro, Draper is strongly enriched at the phagocytic cup (Williamson and Vale 2018). This promotes phosphorylation of Draper's cytoplasmic tail, including on tyrosine residues within the ITAM motif. As in phagocytic glia, the phosphorylation of Draper's ITAM motif triggers intracellular signaling, including recruitment of Shark, which becomes enriched at S2 cell phagocytic cups during corpse engulfment (Williamson and Vale 2018). Initially it was reported that the knockdown of Draper severely impairs macrophage phagocytosis of cell corpses both in vitro and within the embryo (Manaka et al. 2004). However, surprisingly, Draper is not required for embryonic macrophage uptake of apoptotic cells in vivo, as draper mutant macrophages are full of engulfed corpses (Kurant et al. 2008; Evans et al. 2015). In fact, draper mutant macrophages are excessively vacuolated, containing a higher apoptotic load than their wild-type counterparts, implying a downstream corpse processing defect (Evans et al. 2015). The related receptor, SIMU, has instead been implicated in corpse uptake, acting upstream of Draper (Kurant et al. 2008). Furthermore, the loss of SIMU results in a failure to clear apoptotic debris from the embryo (Roddie et al. 2019). However, even the combined loss of both SIMU and Draper fails to block all engulfment.
Another phagocytic receptor, the fly homolog of the vertebrate scavenger receptor CD36 named croquemort (crq), is first detected in macrophages during their embryonic dispersal and is required for efficient clearance of apoptotic corpses (Franc et al. 1996, 1999). However, the downstream effectors of crq and how they feed into the established CED pathways is poorly understood. A screen for genes required for efficient clearance of corpses by the embryonic macrophages identified Pallbearer, which acts through an E3 ubiquitin ligase complex to target proteins for proteasomal degradation (Silva et al. 2007). Exactly which proteins Pallbearer earmarks for destruction and how this contributes to corpse uptake is not known and this finding has not been further advanced. In the same screen, the junctophilin Undertaker/Retinophilin was also implicated in clearance of apoptotic corpses by embryonic macrophages (Cuttell et al. 2008). Undertaker/Retinophilin was found to work downstream of Draper and dCED-6 and regulate intracellular calcium release from the endoplasmic reticulum. Interestingly, transient increases in macrophage intracellular calcium are observed with every phagocytic event and are required for the up-regulation of Draper via JNK activity (Weavers et al. 2016a). This suggests a feedback loop whereby Draper increases its own expression via Undertaker and intracellular calcium release.
Although clearing apoptotic debris arising from programmed cell death is a major role of the embryonic macrophages, they are also highly responsive to tissue damage induced by wounding. When laser ablation is used to wound the overlying epithelium, macrophages chemotax to the site of injury where they engulf cellular debris (Fig. 3). Given the instantaneous nature of laser-induced damage and the lack of cleaved-caspase staining, it is assumed that the cellular debris within the wound is necrotic (Weavers et al. 2016a). As the presence of macrophages is not required for wound closure itself, the clearance of these necrotic cells is likely to be a primary function of the recruited immune cells (alongside phagocytosis of any invading pathogens) (Stramer et al. 2005). Interestingly, during their developmental dispersal throughout the embryo, macrophages will prioritize the engulfment of apoptotic cells over recruitment to wounds (Moreira et al. 2010). However, this is likely attributable to their immaturity because until these macrophages have engulfed an apoptotic corpse, they are unresponsive to wounds (Weavers et al. 2016a). These early phagocytic events are vital for functionalizing (or “priming”) these macrophages, without which they fail to mount the appropriate inflammatory response (Weavers et al. 2016a). In ΔH99 mutant embryos, which are deficient in programmed cell death, macrophages disperse throughout the embryo without ever encountering an apoptotic corpse. Although their basal migration is normal, these cells are only weakly recruited to wounds (Weavers et al. 2016a). Reintroduction of apoptotic corpses into ΔH99 mutant embryos via UV irradiation, rescues the inflammatory response. The engulfment of apoptotic corpses activates JNK signaling (see above), which up-regulates the expression of Draper (Weavers et al. 2016a). Elevated Draper is required for robust macrophage recruitment to wounds and exogenously expressed Draper alone is sufficient to rescue inflammation in the absence of engulfment (Evans et al. 2015; Weavers et al. 2016a). Interestingly, too much cell death also impairs the ability of macrophages to mount a subsequent inflammatory response (Roddie et al. 2019). Therefore, it appears that the amount of corpse uptake by macrophages plays a critical role in defining their future behavior.
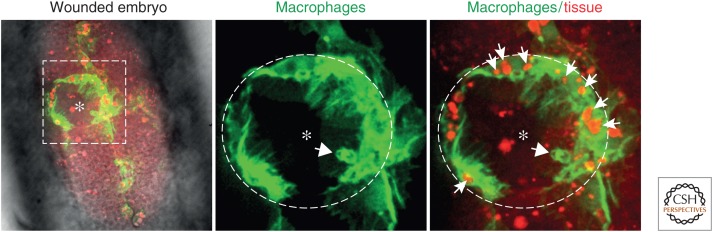
Figure 3.
Inflammatory macrophages engulf debris at a wound. (Left panel) Low-magnification image of the ventral surface of a stage 15 embryo. An epithelial wound (asterisk) has been generated by laser ablation resulting in macrophage recruitment (LifeAct-GFP driven by sn-GAL4, green). (Middle panel) Magnified image of region outlined by the dashed box. Macrophages encircle the wound edge (dashed circle) and phagocytose debris (arrow). (Right panel) Same image with inclusion of whole embryo tissue label (sqh-mCherry-Moesin, red). Internalized labeled tissue is present in macrophages (barbed arrows) originating from wound and earlier apoptotic events.
When the embryo is wounded, one of the earliest damage signals released is hydrogen peroxide (Moreira et al. 2010; Razzell et al. 2013). This is detected within these immune cells by a redox-sensitive cysteine within the src family kinase, Src42a (Evans et al. 2015). Src42a is known to phosphorylate the ITAM motif within the cytoplasmic tail of Draper (Ziegenfuss et al. 2008; Evans et al. 2015). This, in turn, leads to the recruitment of the downstream effector kinase Shark and recruitment to the wound. Although mathematical modeling of macrophage behavior in response to wounding suggests that hydrogen peroxide is not the de facto chemoattractant that is guiding macrophages to the wound, it is important in triggering the inflammatory response (Razzell et al. 2013; Weavers et al. 2016b). Draper's responsiveness to hydrogen peroxide combined with its requirement for the inflammatory recruitment of macrophages to the wound implies it is a chemotactic receptor (Evans et al. 2015). Thus, Draper is necessary for chemotaxis to wounds, is involved in phagocytosis (albeit not strictly required), and needed for efficient corpse processing.
It is increasingly clear from studies in Drosophila that the inflammatory function of macrophages is intricately intertwined with their role in engulfing cell corpses. Combined with its powerful genetics and excellent in vivo imaging, the great advantage offered by Drosophila for macrophage research is the ability to challenge these immune cells with a full array of different stimuli within the context of a living organism. Such an approach will be necessary if we are to fully appreciate the consequences of cell corpse uptake on the inflammatory response and vice versa. Furthermore, the increasing use of the pupae for live imaging is offering the opportunity to visualize macrophage behavior in a whole variety of contexts not possible in the embryo (Thuma et al. 2018). As such, there remains much to learn from the fly about these truly multifunctional phagocytes.
Draper: The Many Headed Hydra
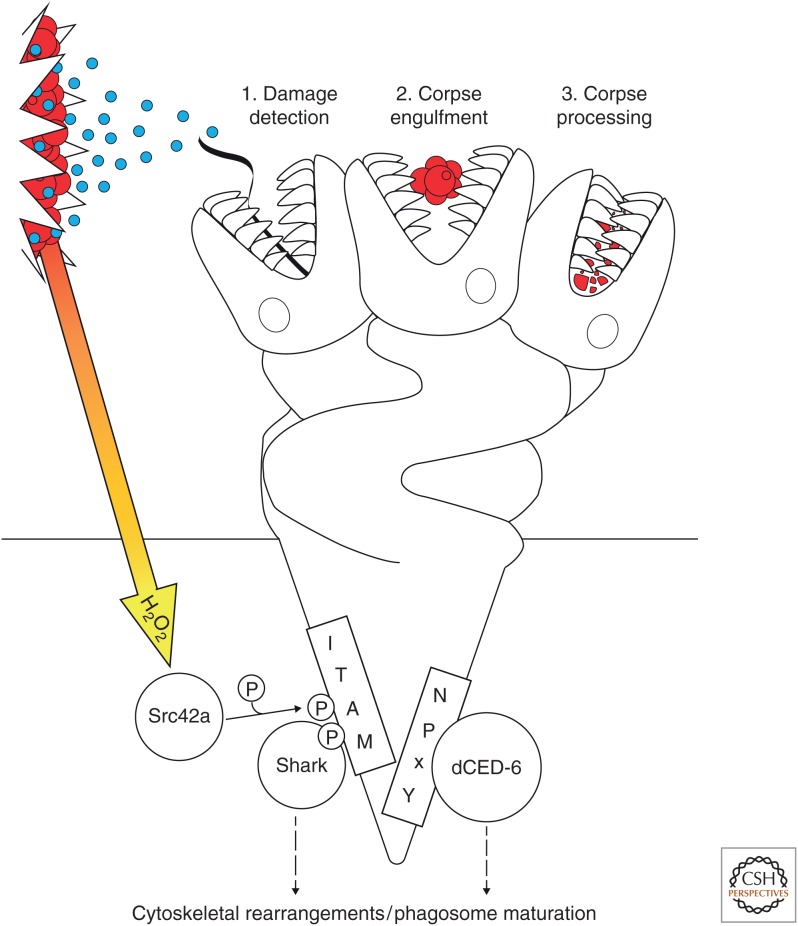
In each of the three Drosophila phagocytes discussed within this review, the CED-1 homolog Draper is a recurring protagonist (Fig. 4). Furthermore, in response to cell death, Draper is acting as a multifunctional “Hydra” playing a central role in all aspects of corpse clearance, including chemotaxis, phagocytosis, and phagosome maturation/acidification (Fig. 5). As discussed throughout this review, Draper has been implicated in corpse recognition, uptake, and processing in each of the phagocytes in the fly, highlighting its key role in shepherding cellular debris through the entire engulfment process (Freeman et al. 2003; Kurant et al. 2008; Evans et al. 2015; Meehan et al. 2016). Quite how this one receptor is capable of contributing to all these functions is intriguing and perplexing. One might think that Draper's promiscuity offers one possible solution, whereby the recognition of different ligands triggers different molecular and, therefore, cellular, responses. Furthermore, hydrogen peroxide release on wounding activates Draper via Src42, bypassing the need for receptor engagement (Evans et al. 2015). Nevertheless, how different stimuli can evoke different responses from the same receptor is difficult to explain. Remarkably, much of Draper's extracellular sequence is dispensable for clearance of neuronal damage by glia in the CNS (Logan et al. 2012). This result awaits further exploration in all of the established roles of Draper and the different phagocytes. It is also possible that tethering receptors such as SIMU relieve Draper of the necessity of interacting with cellular debris directly. However, in the absence of definitive answers, such findings further complicate our understanding of Draper.
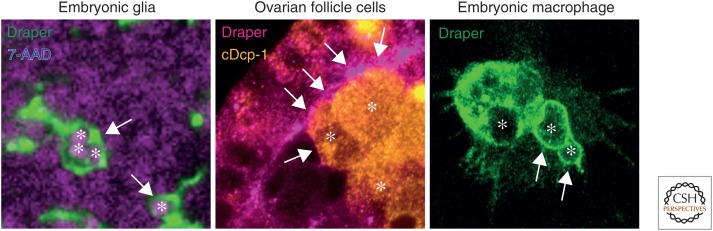
Figure 4.
Draper localization during corpse clearance in multiple fly phagocytes. (Left panel) Draper (anti-Draper, green) localizes to membranes of embryonic glia (arrows) engulfing apoptotic corpses (7-AAD staining, purple) in developing central nervous system. Asterisks highlight engulfed corpses. (Middle panel) Draper (anti-Draper, pink) is enriched (arrows) at the interface between the engulfing follicle cells and the dying nurse cells (anti-cDcp-1, asterisks and orange). (Right panel) Draper (Draper-GFP driven by sn-GAL4) localizes to phagocytic cups (arrows) of embryonic macrophages engulfing apoptotic corpses (negative fluorescence and asterisks). (The glia image was provided by Mary Logan and the ovarian follicle image was provided by Sandy Serizier and Kim McCall.)
Figure 5.
Draper is a multifunctional “Hydra,” participating in all stages of corpse clearance. (1) Draper is required to detect tissue damage (red circles) perhaps via detection of an as-of-yet unidentified ligand released from the wound (blue circles). Hydrogen peroxide emanating from the wound (orange/yellow arrow) bypasses the requirement for Draper ligand binding by directly activating Src42a. Activated Src42a phosphorylates the ITAM domain, which leads to the recruitment of Shark and downstream signaling. This draws the phagocyte toward the debris via cytoskeletal rearrangements. (2) Draper localizes to phagocytic cups during engulfment of cell corpses (red circles) triggering ITAM phosphorylation via Src42a. Shark recruitment and subsequent downstream signaling promotes the cytoskeletal rearrangements required for engulfment. (3) Draper is also required for phagosome maturation and efficient corpse processing via its NPxY motif, which associates with dCED-6/GULP.
Intracellularly, Draper possesses two well-defined signaling motifs: an NPxY motif and an ITAM domain. Each of these motifs interact with different downstream effectors and therefore might drive the different functions of Draper. For instance, although the ITAM motif is necessary for the inflammatory recruitment to wounds, it is not required for the processing of engulfed corpses (Evans et al. 2015). However, whether this holds true for the other phagocytes remains to be confirmed.
Beyond clearance of cellular debris, Draper has also been implicated in mediating cell–cell competition and in the caspase-independent, autophagic cell death of the salivary glands (Li and Baker 2007; McPhee et al. 2010). Cell competition is the process that drives the elimination of cells of one genotype by the cells of a different genotype within the same tissue. The requirement for Draper in this process might prove to be via phagoptosis, whereby the “winner” cells kill the “loser” cells through Draper-mediated engulfment (Li and Baker 2007). How the cells of the salivary glands use Draper to promote their own caspase-independent programmed cell death is less clear. However, to date, no known phagocytes have been implicated in the clearance of these cells, which might otherwise be driving their own removal via autophagy (McPhee et al. 2010). Within this setting, it is possible that Draper is required for the maturation of autolysosomes similar to its requirement for corpse processing during engulfment. However, even if the salivary glands are able to devour themselves, there remains a need for a yet-to-be-identified phagocyte to engulf whatever remains and Draper will undoubtedly have a role in this uptake too.
CONCLUDING REMARKS
In many ways, Drosophila is the ideal model to study the clearance of cellular debris. The fly combines a sophisticated, yet well-defined development and anatomy (and the inevitable associated cell death) with incredible genetic tractability. Furthermore, the fly possesses a variety of phagocytes operating within a range of different tissues, with each presenting unique demands and constraints. The best studied of these phagocytes include the phagocytic glia of the fly CNS, the follicle cells of the Drosophila ovary, and the macrophage-like hemocytes of the fly innate immune system. These have all been intensively studied over the past few decades and so each offer abundant molecular tools as well as a wealth of preexisting knowledge. A general theme emerging from the in vivo study of engulfment within the fly is that not only are phagocytes carefully matched to their quarry, but the mode of cell death is tailored to suit their designated phagocyte. For example, the motility of the macrophages allows them to clear developmental debris from a wide array of different tissues alongside the ability to rapidly concentrate phagocytosis in response to wounding. Tellingly, the voracious macrophages are excluded from particularly delicate tissues such as the CNS and the ovary, where specialized phagocytes operate. Despite the extent to which these various phagocytes differ in their morphology, mode of engulfment, tissue residency and the target cells they ultimately clear, they share clear commonalities in their underlying molecular pathways. More specifically, the CED pathways and, in particular, the CED-1 homolog Draper, are driving clearance of cell corpses in each of these phagocytes. How such phagocytic plasticity is derived from the same set of molecular players is an intriguing open question, but one which Drosophila and its phagocytes are well placed to address.
ACKNOWLEDGMENTS
We would like to thank Yunsik Kang, Marc Freeman, Mary Logan, Sandy Serizier, and Kim McCall for contributing images for the figures presented in this review.
Footnotes
Editors: Kim Newton, James M. Murphy, and Edward A. Miao
Additional Perspectives on Cell Survival and Cell Death available at www.cshperspectives.org
REFERENCES
- Abrams JM, White K, Fessler LI, Steller H. 1993. Programmed cell death during Drosophila embryogenesis. Development 117: 29–43. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Ito K. 2004. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol 14: 668–677. 10.1016/j.cub.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. 2006. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron 50: 855–867. 10.1016/j.neuron.2006.04.027 [DOI] [PubMed] [Google Scholar]
- Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. 2002. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol 4: 574–582. 10.1038/ncb824 [DOI] [PubMed] [Google Scholar]
- Callebaut I, Mignotte V, Souchet M, Mornon J-P. 2003. EMI domains are widespread and reveal the probable orthologs of the Caenorhabditis elegans CED-1 protein. Biochem Biophys Res Commun 300: 619–623. 10.1016/S0006-291X(02)02904-2 [DOI] [PubMed] [Google Scholar]
- Cantera R, Technau GM. 1996. Glial cells phagocytose neuronal debris during the metamorphosis of the central nervous system in Drosophila melanogaster. Dev Genes Evol 206: 277–280. 10.1007/s004270050052 [DOI] [PubMed] [Google Scholar]
- Cuttell L, Vaughan A, Silva E, Escaron CJ, Lavine M, Van Goethem E, Eid JP, Quirin M, Franc NC. 2008. Undertaker, a Drosophila Junctophilin, links Draper-mediated phagocytosis and calcium homeostasis. Cell 135: 524–534. 10.1016/j.cell.2008.08.033 [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Millard TH, Evans IR, Wood W. 2019. Ena orchestrates remodelling within the actin cytoskeleton to drive robust Drosophila macrophage chemotaxis. J Cell Sci 132: jcs224618 10.1242/jcs.224618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Tasdemir OE, Freeman MR. 2009. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci 29: 4768–4781. 10.1523/jneurosci.5951-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RE, Jacobson DM, Horvitz HR. 1991. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics 129: 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray JI, Timmons AK, Klein AP, Pritchett TL, Welch E, Meehan TL, Li C, McCall K. 2012. Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells. Development 139: 4029–4039. 10.1242/dev.082776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans IR, Rodrigues FS, Armitage EL, Wood W. 2015. Draper/CED-1 mediates an ancient damage response to control inflammatory blood cell migration in vivo. Curr Biol 25: 1606–1612. 10.1016/j.cub.2015.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franc NC, Dimarcq JL, Lagueux M, Hoffmann J, Ezekowitz RA. 1996. Croquemort, a novel Drosophila hemocyte/macrophage receptor that recognizes apoptotic cells. Immunity 4: 431–443. 10.1016/S1074-7613(00)80410-0 [DOI] [PubMed] [Google Scholar]
- Franc NC, Heitzler P, Ezekowitz RA, White K. 1999. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science 284: 1991–1994. 10.1126/science.284.5422.1991 [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. 2003. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron 38: 567–580. 10.1016/S0896-6273(03)00289-7 [DOI] [PubMed] [Google Scholar]
- Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, et al. 2001. CED-12/ELMO, a novel member of the crkII/dock180/rac pathway, is required for phagocytosis and cell migration. Cell 107: 27–41. 10.1016/S0092-8674(01)00520-7 [DOI] [PubMed] [Google Scholar]
- Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, et al. 2000. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol 2: 399–406. 10.1038/35017029 [DOI] [PubMed] [Google Scholar]
- Hay BA, Wassarman DA, Rubin GM. 1995. Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 83: 1253–1262. 10.1016/0092-8674(95)90150-7 [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Sulston JE, Thomson JN. 1983. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science 220: 1277–1279. 10.1126/science.6857247 [DOI] [PubMed] [Google Scholar]
- Hilu-Dadia R, Hakim-Mishnaevski K, Levy-Adam F, Kurant E. 2018. Draper-mediated JNK signaling is required for glial phagocytosis of apoptotic neurons during Drosophila metamorphosis. Glia 66: 1520–1532. 10.1002/glia.23322 [DOI] [PubMed] [Google Scholar]
- Hsu TY, Wu YC. 2010. Engulfment of apoptotic cells in C. elegans is mediated by integrin α/SRC signaling. Curr Biol 20: 477–486. 10.1016/j.cub.2010.01.062 [DOI] [PubMed] [Google Scholar]
- Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H, Schnabel R, Hengartner MO. 2005. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 434: 93–99. 10.1038/nature03263 [DOI] [PubMed] [Google Scholar]
- Kuraishi T, Nakagawa Y, Nagaosa K, Hashimoto Y, Ishimoto T, Moki T, Fujita Y, Nakayama H, Dohmae N, Shiratsuchi A, et al. 2009. Pretaporter, a Drosophila protein serving as a ligand for Draper in the phagocytosis of apoptotic cells. EMBO J 28: 3868–3878. 10.1038/emboj.2009.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurant E, Axelrod S, Leaman D, Gaul U. 2008. Six-microns-under acts upstream of Draper in the glial phagocytosis of apoptotic neurons. Cell 133: 498–509. 10.1016/j.cell.2008.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Baker NE. 2007. Engulfment is required for cell competition. Cell 129: 1215–1225. 10.1016/j.cell.2007.03.054 [DOI] [PubMed] [Google Scholar]
- Lin L, Rodrigues F, Kary C, Contet A, Logan M, Baxter RHG, Wood W, Baehrecke EH. 2017. Complement-related regulates autophagy in neighboring cells. Cell 170: 158–171.e8. 10.1016/j.cell.2017.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan MA, Hackett R, Doherty J, Sheehan A, Speese SD, Freeman MR. 2012. Negative regulation of glial engulfment activity by Draper terminates glial responses to axon injury. Nat Neurosci 15: 722–730. 10.1038/nn.3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TY, Doherty J, Freeman MR. 2014. DRK/DOS/SOS converge with Crk/Mbc/dCed-12 to activate Rac1 during glial engulfment of axonal debris. Proc Natl Acad Sci 111: 12544–12549. 10.1073/pnas.1403450111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. 2006. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 50: 869–881. 10.1016/j.neuron.2006.04.028 [DOI] [PubMed] [Google Scholar]
- Manaka J, Kuraishi T, Shiratsuchi A, Nakai Y, Higashida H, Henson P, Nakanishi Y. 2004. Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages. J Biol Chem 279: 48466–48476. 10.1074/jbc.M408597200 [DOI] [PubMed] [Google Scholar]
- Matsubayashi Y, Louani A, Dragu A, Sanchez-Sanchez BJ, Serna-Morales E, Yolland L, Gyoergy A, Vizcay G, Fleck RA, Heddleston JM, et al. 2017. A moving source of matrix components is essential for de novo basement membrane formation. Curr Biol 27: 3526–3534.e4. 10.1016/j.cub.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee CK, Logan MA, Freeman MR, Baehrecke EH. 2010. Activation of autophagy during cell death requires the engulfment receptor Draper. Nature 465: 1093–1096. 10.1038/nature09127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan TL, Kleinsorge SE, Timmons AK, Taylor JD, McCall K. 2015. Polarization of the epithelial layer and apical localization of integrins are required for engulfment of apoptotic cells in the Drosophila ovary. Dis Model Mech 8: 1603–1614. 10.1242/dmm.021998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan TL, Joudi TF, Timmons AK, Taylor JD, Habib CS, Peterson JS, Emmanuel S, Franc NC, McCall K. 2016. Components of the engulfment machinery have distinct roles in corpse processing. PLoS ONE 11: e0158217 10.1371/journal.pone.0158217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira S, Stramer B, Evans I, Wood W, Martin P. 2010. Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol 20: 464–470. 10.1016/j.cub.2010.01.047 [DOI] [PubMed] [Google Scholar]
- Peterson JS, McCall K. 2013. Combined inhibition of autophagy and caspases fails to prevent developmental nurse cell death in the Drosophila melanogaster ovary. PLoS ONE 8: e76046 10.1371/journal.pone.0076046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzell W, Evans IR, Martin P, Wood W. 2013. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol 23: 424–429. 10.1016/j.cub.2013.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddie HG, Armitage EL, Coates JA, Johnston SA, Evans IR. 2019. Simu-dependent clearance of dying cells regulates macrophage function and inflammation resolution. PLoS Biol 17: e2006741 10.1371/journal.pbio.2006741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogulja-Ortmann A, Luer K, Seibert J, Rickert C, Technau GM. 2007. Programmed cell death in the embryonic central nervous system of Drosophila melanogaster. Development 134: 105–116. 10.1242/dev.02707 [DOI] [PubMed] [Google Scholar]
- Santoso CS, Meehan TL, Peterson JS, Cedano TM, Turlo CV, McCall K. 2018. The ABC transporter Eato promotes cell clearance in the Drosophila melanogaster ovary. G3 (Bethesda) 8: 833–843. 10.1534/g3.117.300427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklyar B, Levy-Adam F, Mishnaevski K, Kurant E. 2013. Caspase activity is required for engulfment of apoptotic cells. Mol Cell Biol 33: 3191–3201. 10.1128/MCB.00233-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklyar B, Sellman Y, Shklover J, Mishnaevski K, Levy-Adam F, Kurant E. 2014. Developmental regulation of glial cell phagocytic function during Drosophila embryogenesis. Dev Biol 393: 255–269. 10.1016/j.ydbio.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Shlyakhover E, Shklyar B, Hakim-Mishnaevski K, Levy-Adam F, Kurant E. 2018. Drosophila GATA factor serpent establishes phagocytic ability of embryonic macrophages. Front Immunol 9: 266 10.3389/fimmu.2018.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Au-Yeung HW, Van Goethem E, Burden J, Franc NC. 2007. Requirement for a Drosophila E3-ubiquitin ligase in phagocytosis of apoptotic cells. Immunity 27: 585–596. 10.1016/j.immuni.2007.08.016 [DOI] [PubMed] [Google Scholar]
- Sonnenfeld MJ, Jacobs JR. 1995. Macrophages and glia participate in the removal of apoptotic neurons from the Drosophila embryonic nervous system. J Comp Neurol 359: 644–652. 10.1002/cne.903590410 [DOI] [PubMed] [Google Scholar]
- Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, Parkhurst SM, Martin P. 2005. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol 168: 567–573. 10.1083/jcb.200405120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HP, Nakada-Tsukui K, Tosello-Trampont AC, Li Y, Bu G, Henson PM, Ravichandran KS. 2002. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP). J Biol Chem 277: 11772–11779. 10.1074/jbc.M109336200 [DOI] [PubMed] [Google Scholar]
- Tepass U, Fessler LI, Aziz A, Hartenstein V. 1994. Embryonic origin of hemocytes and their relationship to cell-death in Drosophila. Development 120: 1829–1837. [DOI] [PubMed] [Google Scholar]
- Thuma L, Carter D, Weavers H, Martin P. 2018. Drosophila immune cells extravasate from vessels to wounds using Tre1 GPCR and Rho signaling. J Cell Biol 217: 3045–3056. 10.1083/jcb.201801013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons AK, Mondragon AA, Schenkel CE, Yalonetskaya A, Taylor JD, Moynihan KE, Etchegaray JI, Meehan TL, McCall K. 2016. Phagocytosis genes nonautonomously promote developmental cell death in the Drosophila ovary. Proc Natl Acad Sci 113: E1246–E1255. 10.1073/pnas.1522830113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung TT, Nagaosa K, Fujita Y, Kita A, Mori H, Okada R, Nonaka S, Nakanishi Y. 2013. Phosphatidylserine recognition and induction of apoptotic cell clearance by Drosophila engulfment receptor Draper. J Biochem 153: 483–491. 10.1093/jb/mvt014 [DOI] [PubMed] [Google Scholar]
- Venegas V, Zhou Z. 2007. Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Mol Biol Cell 18: 3180–3192. 10.1091/mbc.e07-02-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlisidou I, Dowling AJ, Evans IR, Waterfield N, ffrench-Constant RH, Wood W. 2009. Drosophila embryos as model systems for monitoring bacterial infection in real time. PLoS Pathog 5: e1000518 10.1371/journal.ppat.1000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wu YC, Fadok VA, Lee MC, Gengyo-Ando K, Cheng LC, Ledwich D, Hsu PK, Chen JY, Chou BK, et al. 2003. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science 302: 1563–1566. 10.1126/science.1087641 [DOI] [PubMed] [Google Scholar]
- Weavers H, Evans IR, Martin P, Wood W. 2016a. Corpse engulfment generates a molecular memory that primes the macrophage inflammatory response. Cell 165: 1658–1671. 10.1016/j.cell.2016.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavers H, Liepe J, Sim A, Wood W, Martin P, Stumpf MPH. 2016b. Systems analysis of the dynamic inflammatory response to tissue damage reveals spatiotemporal properties of the wound attractant gradient. Curr Biol 26: 1975–1989. 10.1016/j.cub.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. 1994. Genetic control of programmed cell death in Drosophila. Science 264: 677–683. 10.1126/science.8171319 [DOI] [PubMed] [Google Scholar]
- Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. 2006. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci 9: 1234–1236. 10.1038/nn1774 [DOI] [PubMed] [Google Scholar]
- Williamson AP, Vale RD. 2018. Spatial control of Draper receptor signaling initiates apoptotic cell engulfment. J Cell Biol 217: 3977–3992. 10.1083/jcb.201711175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, Faria C, Jacinto A. 2006. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J Cell Biol 173: 405–416. 10.1083/jcb.200508161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Horvitz HR. 1998a. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 392: 501–504. 10.1038/33163 [DOI] [PubMed] [Google Scholar]
- Wu YC, Horvitz HR. 1998b. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 93: 951–960. 10.1016/S0092-8674(00)81201-5 [DOI] [PubMed] [Google Scholar]
- Wu YC, Tsai MC, Cheng LC, Chou CJ, Weng NY. 2001. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev Cell 1: 491–502. 10.1016/S1534-5807(01)00056-9 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hartwieg E, Horvitz HR. 2001. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell 104: 43–56. 10.1016/S0092-8674(01)00190-8 [DOI] [PubMed] [Google Scholar]
- Ziegenfuss JS, Biswas R, Avery MA, Hong K, Sheehan AE, Yeung YG, Stanley ER, Freeman MR. 2008. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature 453: 935–939. 10.1038/nature06901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenfuss JS, Doherty J, Freeman MR. 2012. Distinct molecular pathways mediate glial activation and engulfment of axonal debris after axotomy. Nat Neurosci 15: 979–987. 10.1038/nn.3135 [DOI] [PMC free article] [PubMed] [Google Scholar]