Abstract
A focused nucleoside library was constructed around a 3′-C-ethynyl-d-ribofuranose sugar scaffold, which was coupled to variously modified purine nucleobases. The resulting nucleosides were probed for their ability to inhibit tumor cell proliferation, as well as for their activity against a panel of relevant human viruses. While C6-aryl substituted purine nucleosides were found to be weakly active, several C7-substituted 7-deazapurine nucleosides elicited potent antiproliferative activity. Their activity spectrum was evaluated in the NCI-60 tumor cell line panel indicating activity against several solid tumor derived cell lines. Analog 32, equipped with a 7-deaza 7-chloro-6-amino-purin-9-yl base was evaluated in a metastatic breast tumor (MDA-MB-231-LM2) xenograft model. It inhibited both tumor growth and reduced the formation of lung metastases as revealed by BLI analysis. The dideazanucleoside analog 66 showed interesting activity against hCMV. These results highlight the potential advantages of recombining known sugar and nucleobase motifs as a library design strategy to discover novel antiviral or antitumor agents.
Keywords: Antiviral; Antiproliferative; 3′-C-ethynylnucleoside; 7-deazapurine nucleoside; 1,7-dideazapurine nucleoside; Vorbrüggen glycosylation
Graphical abstract
Highlights
-
•
3′-C-ethynylribofuranose deazapurine nucleosides were synthetized and evaluated for antiproliferative and antiviral activity.
-
•
Significant in vitro antiproliferative activity was identified.
-
•
BLI in vivo analysis with analog 32 showed reduced tumor growth and metastasis.
-
•
Interesting starting points for further optimization of antiviral activity were discovered.
1. Introduction
Nucleoside analogs are the cornerstones for antiviral therapy with notable successes in the treatment of HIV and Hepatitis C virus infections [1]. Furthermore, nucleoside analogs have also found widespread use in oncology, with most approved derivatives active against various forms of lymphomas [[1], [2], [3]].
A focused screening library, comprised of nucleoside analogs surrounding a single modified d-ribofuranose moiety could be a viable strategy to discover attractive hits for the aforementioned disease areas, as evidenced by a number of recent publications [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]]. In this paper, a rather underrepresented 3′-C-ethynylribofuranose motif [[15], [16], [17], [18]] was used as the sugar scaffold to construct a small library of nucleoside analogs. Previous reports mainly focused on pyrimidine (-like) nucleobase moieties [17,19], structurally resembling the cytidine analog, ECyd (1, Fig. 1 ), which emerged as the most promising derivative from the initial discovery of 3′-C-ethynyl nucleosides in 1996 [15]. ECyd has been evaluated in clinical trials as a new antitumoral agent [3] and recently attracted renewed interest as a combination therapy, e.g. with carboplatin [3], or as part of a ‘duplex drug’, in which it is linked to 2′-deoxy-5-fluorouridine [20].
Fig. 1.
Overview of previously reported nucleoside analogs featuring a 3′-C-ethynylribofuranose (ECyd and EAdo); known purine-modified nucleoside analogs with interesting biological activity (2 and 3), and their combination to construct a small library.
The purine counterparts on the other hand, exemplified by the 3′-C-ethynyladenosine analog (EAdo, 5), have received little attention, which motivated us to combine this peculiar 3′-C-ethynyl sugar element with different purine nucleobases to build a focused library. Previously we reported a series of C2- and C6-substituted purine analogs (4, Fig. 1) of 3′-C-ethynyladenosine (EAdo, 5) [21]. Considering the interesting biological properties reported for nucleosides comprising a C6 arylpurine [22,23] (e.g. 2, Fig. 1) or a C7-substituted-7-deazapurine base, which was recently coined a ‘privileged scaffold’ [24] and is part of the natural nucleoside antibiotic tubercidin (3) [[24], [25], [26]], in this contribution we investigate the effect(s) of combining these base moieties with the 3′-C-ethynylribofuranose moiety.
2. Results and discussion
2.1. Chemistry
The target nucleoside analogs were prepared by Vorbrüggen glycosylation using either acetate (6, Scheme 1 ) [21] or benzoate (18, Scheme 2 ) [15] protected sugar precursors. Reaction conditions depended on the type of nucleobase (purine [21] vs. 7-deazapurine analogs [27]). Synthesis of the 6-substituted purine analogs 13–17 was accomplished via Suzuki reaction of the 6-chloropurine nucleoside 7 with appropriate phenylboronic acids [22]. Final compounds were obtained after deprotection with NH3/MeOH (Scheme 1).
Scheme 1.
Reagents and conditions: a) (i) 6-chloropurine, HMDS, cat. (NH4)2SO4, reflux; (ii) TMSOTf, 1,2-dichloroethane, reflux, 81%; b) (substituted) phenylboronic acid, K2CO3, Pd(Ph3P)4, toluene, 100 °C, 30–52%; c) 7 N NH3/MeOH, 43–75%.
Scheme 2.
Reagents and conditions: a) 4-chloro-5-halo-7H-pyrrolo[2,3-d]pyrimidine (F [32,33], Cl [34], Br [34], I [34]), TMSOTf, MeCN, 80 °C; b) NaN3, DMF, 65 °C; c) (i) 1.0M PMe3 in THF, THF; (ii) aq. HOAc, MeCN, 65 °C; d) 7N NH3/MeOH, 30–95%; e) (i) iPrMgCl.LiCl (1.3M in THF), toluene, −65 °C; (ii) sat. aq. NH4Cl, 76%; f) furan-2-yl-boronic acid, Na2CO3, Pd(OAc)2, TPPTS, MeCN/water (1/2), 100 °C, 29%.
For the synthesis of the C7-modified 7-deazanucleosides1 (Scheme 2), the use of the aforementioned sugar precursor 6 only provided the desired glycosylation product in low yield after cumbersome purification procedures. Switching to the benzoate protected sugar derivative 18 [15] improved the coupling yields [27], but the isolated glycosylation products were contaminated with residues of glycosyl donor degradation products (tentative assignment based on 1H NMR data; not shown). Treatment of the crude intermediates 19–22 with NH3/MeOH [27] (or NH4OH [28]) at elevated (>100 °C) temperatures caused decomposition. Therefore, an alternative protocol to introduce the 6-amino group was employed [29,30]. Nucleophilic displacement of the 6-chloride with sodium azide efficiently delivered the corresponding azide derivatives (23–26), which generally could be obtained in pure form due to the marked difference in polarity induced by the predominating tetrazolo tautomer. Staudinger reduction furnished the corresponding 6-amino derivatives. Deprotection afforded the final compounds (31, 32, 33, 34). To dehalogenate the 7-iodo intermediate 26, it was subjected to I/Mg exchange using Knochel's iPrMgCl.LiCl [26,31], and the magnesiated intermediate was quenched with aqueous acid to give 35 in good yield. Further conversion to 37 was realized as described above. Introduction of a furan-2-yl moiety was achieved via an aqueous Suzuki reaction on 34 [25]. Remarkably, Suzuki reaction on the 7-iodo-7-deazapurine substrate gave significantly lower yields than reaction with the 6-chloropurine starting material.
A similar glycosylation strategy was followed to synthetize the C7 (C5)2 trifluoromethyl analog 49 from 39 (upper line in Scheme 3 ) [35,36] by treating commercial 4-chloro-7H-pyrrolo[2,3-d]pyrimidine with the Langlois reagent (sodium trifluoromethanesulfinate) [35]. The presence of a 3 J H-1′-C-8 cross peak in the 1H-13C gHMBC spectrum of compound 48 (see Supporting Information) allowed to ascertain glycosylation at N9 (N7). However, a large coupling constant (J = 37.8 Hz) observed for C8 (C6) was inconsistent with the CF3 group being attached to C7 (C5). This led us to assign the structure of the trifluoromethylated compound prepared using Langlois' reagent as the C8 (C6) regio-isomer. The C7 (C5) substituted heterocycle 42 could be obtained from the known C7 (C5) iodide 40 [34] by subsequent N-Boc protection and trifluoromethylation using the Ruppert reagent [37] (the N-Boc protecting group was lost during the reaction). Comparison of the 1H NMR spectra of both regioisomers 39 and 42 (see Supporting Information) led to the confirmation that 39 and thus also 48, are the C8 (C6) substituted isomers. Of note, a recent patent application [38] described both regio-isomers 39 and 42, in which the regiochemical assignment is opposite to our conclusions. The C7 substituted heterocycle 42 was used to synthetize the desired product 49, following the same reaction sequence as above.
Scheme 3.
Reagents and conditions: a) Langlois reagent, t-BuOOH, DCM/water; b) Boc2O, DBU, DMAP, 1,4-dioxane, 96%; c) Ruppert reagent (TMSCF3), B(OMe)3, KF, CuI, 1,10-phenanthroline, DMSO, 60 °C, 23%; d) 39 or 42, BSA, TMSOTf, MeCN, 80 °C; e) NaN3, DMF, 65 °C, 43% (2 steps, 46); f) (i) 1.0M PMe3 in THF, THF; (ii) aq. HOAc, MeCN, 65 °C, 21% (3 steps, 47), 55 % (48); g) 7N NH3/MeOH, 80% (49), 87% (50).
To obtain the C7 ethynyl substituted analog 54 (Scheme 4 ) a Sonogashira reaction was envisioned. To avoid selectivity issues with the 3′-C-ethynyl group of 18, this group was protected with a TMS group (51) and glycosylated (52). After introduction of the C7 ethynyl chain [25], 53 was transformed into 54 employing the same reaction sequence as described above. The synthesis of 3′-C-ethyl analog 59 started with catalytic hydrogenation of 18, giving rise to 55, which was subjected to glycosylation conditions. The glycosylation product 56 was directly used and elaborated as described above.
Scheme 4.
Reagents and conditions: a) 1. iPrMgCl.LiCl (1.3M in THF), toluene, −65 °C; 2. TMSCl, 60%; b) 4-chloro-5-iodo-pyrrolo[2,3-d]pyrimidine (40) [34], BSA, TMSOTf, MeCN, 80 °C, 30%; c) ethynyltrimethylsilane, CuI, Pd(Ph3P)2Cl2, Et3N, DMF, 39%; d) (i) NaN3, DMF, 65 °C; (ii) 1.0M PMe3 in THF, THF; (iii) aq. HOAc, MeCN, 65 °C; (iv) 7N NH3/MeOH, 46%; e) Pd/C, H2 (balloon), ethyl acetate, 92%; f) 4,5-dichloro-7H-pyrrolo[2,3-d]pyrmidine [34], BSA, TMSOTf, MeCN, 80 °C; g) NaN3, DMF, 65 °C, 55% (2 steps); h) (i) 1.0M PMe3 in THF, THF; (ii) aq. HOAc, MeCN, 65 °C, 88%; i) 7N NH3/MeOH, 87%.
For the synthesis of 1,7-dideazapurine (7-azaindole or pyrrolo[2,3-b]pyridine)3 nucleoside analogs (Scheme 5, Scheme 6 ), commercially available 1H-4-chloro-pyrrolo[2,3-b]pyridine was halogenated with the appropriate halosuccinimide [34]. Glycosylation products were obtained using the same conditions as for their C7-deazapurine counterparts. Lewis acid-mediated glycosylation with this type of heterocycle has only been reported once [39]. Generally, nucleobase-anion glycosylation [40] or acid-catalyzed fusion [41] are employed to ensure this transformation. Both regio- and stereochemistry were ascertained by 1H-13C gHMBC and 2D NOESY experiments (see Supporting Information). Deprotection with NH3/MeOH gave final products 66, 67 and 68. De-iodination by I/Mg exchange of 65 gave 69 in good yield, after which deprotection furnished 70. As expected, introduction of the C6 (C4) azido group on e.g. 63 was problematic due to the higher electron density of the pyrrolo[2,3-b]pyridine system with respect to the pyrrolo[2,3-d]pyrimidine system. No desired product could be detected after reaction with NaN3 at 65 °C, while gradual increase of the temperature to 100 °C (and higher) only led to degradation.
Scheme 5.
Reagents and conditions: a) appropriate N-halosuccinimide, DMF, 93% (X = Cl), 96% (X = Br), 92% (X = I); b) 60–62, BSA, TMSOTf, MeCN, 80 °C, 25–39%; c) 7 N NH3/MeOH, 70–80%; d) (i) iPrMgCl.LiCl (1.3M in THF), toluene, −65 °C; (ii) sat. aq. NH4Cl, 75%.
Scheme 6.
Reagents and conditions: a) NaN3, NH4Cl, DMF, 110 °C, 66% (71), 71% (72); b) NIS, DMF, 93%; c) 72 or 73, BSA, TMSOTf, MeCN, 80 °C, 25% (78); d) (i) 1.0M PMe3 in THF, THF; (ii) aq. HOAc, MeCN, 65 °C, 22% (2 steps, 76), 13% (2 steps, 77), 74% (79); e) 7N NH3/MeOH, 73% (80), 74% (81).
These issues led us to introduce the azido group [42] before the glycosylation step (Scheme 6). Glycosylation, employing the same conditions as for the chloride-substituted heterocycles, was first attempted with 72, which afforded two products, 74 and 75. The identity of each isomer was assigned after Staudinger reduction to 76 and 77 (Scheme 6) to facilitate purification. The 1H-13C gHMBC spectrum of 76 and 77 showed a markedly different cross-peak pattern between H-1′ and the heterocyclic moiety (see Supporting Information). The synthesis of iodo-substituted 79 was accomplished using the same conditions (the tentative N3 (N7) isomer could be observed from TLC analysis, but was not isolated nor formally characterized). Both intermediates (76 and 79) afforded, after deprotection with saturated NH3/MeOH, the desired nucleosides 80 and 81.
2.2. Biological evaluation
All final nucleoside analogs were assayed for their ability to inhibit cell proliferation of three different tumor cell lines (L1210, CEM and HeLa; Table 1 ) and for their antiviral activity against a representative panel of human viruses, including herpex simplex virus (HSV) 1 and 2, cytomegalovirus (CMV), varicella zoster virus (VZV), vaccinia virus (VV), adenovirus-2, influenza-A virus (H1N1, H3N2), influenza B virus, feline corona virus, feline herpes virus, para-influenza virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus, vesicular stomatitis virus, respiratory syncytial virus (RSV) (Table 4, Table 5 ).
Table 1.
Effects of different 3′-C-ethynyl purine derivatives on the proliferation of three tumor cell lines. IC50 values represent the concentration of compound able to inhibit proliferation by 50% (Coulter Counter cell count endpoint).
| Cpd. | L1210 IC50 (μM) | CEM IC50 (μM) | HeLa IC50 (μM) | Cpd. | L1210 IC50 (μM) | CEM IC50 (μM) | HeLa IC50 (μM) |
|---|---|---|---|---|---|---|---|
| 5a | 0.73 ± 0.14 | 0.61 ± 0.08 | 0.29 ± 0.11 | 38 | 30 ± 1 | 17 ± 5 | 14 ± 1 |
| 13 | >250 | >250 | >250 | 49 | 0.11 ± 0.03 | 0.36 ± 0.26 | 0.75 ± 0.19 |
| 14 | 205 ± 45 | 154 ± 8 | >250 | 50 | >250 | >250 | >250 |
| 15 | >250 | >250 | >250 | 54 | 159 ± 49 | 63 ± 0 | 114 ± 20 |
| 16 | 225 ± 23 | 124 ± 49 | 170 ± 53 | 59 | 1.2 ± 0.1 | 2.6 ± 1.2 | 4.4 ± 1.5 |
| 17 | >250 | 223 ± 21 | 220 ± 30 | 66 | 75 ± 19 | 1.3 ± 0.1 | 6.3 ± 3.8 |
| 31 | 0.035 ± 0.008 | 0.16 ± 0.02 | 0.15 ± 0.01 | 67 | 30 ± 6 | 0.56 ± 0.07 | 0.89 ± 0.11 |
| 32 | 0.014 ± 0.009 | 0.012 ± 0.001 | 0.051 ± 0.006 | 68 | 20 ± 5 | 0.26 ± 0.03 | 1.1 ± 0.1 |
| 33 | 0.028 ± 0.013 | 0.030 ± 0.007 | 0.093 ± 0.009 | 70 | 197 ± 62 | 86 ± 4 | 135 ± 1 |
| 34 | 0.056 ± 0.012 | 0.12 ± 0.02 | 0.18 ± 0.04 | 80 | 0.98 ± 0.03 | 5.4 ± 0.8 | 1.7 ± 0.4 |
| 37 | 0.38 ± 0.04 | 0.71 ± 0.13 | 0.88 ± 0.09 | 81 | 0.044 ± 0.14 | 0.31 ± 0.27 | 0.18 ± 0.03 |
Results are taken from Ref. [21].
Table 4.
Proliferation inhibition on different endothelial cell types: HMEC-1 (Human Dermal Microvascular Endothelial Cells), HMVEC (Human Microvascular Endothelial Cells) and HUVEC (Human Umbilical Vein Endothelial Cells), as well as Hel (Human embryonic lung fibroblasts). IC50 values represent the concentration of compound able to inhibit proliferation by 50% (Coulter Counter cell count endpoint).
| Cpd. | HMEC-1 IC50 (μM) | HMVEC IC50 (μM) | HUVEC IC50 (μM) | Hel IC50 (μM) |
|---|---|---|---|---|
| 31 | 0.061 ± 0.006 | <0.00128 | 0.067 ± 0.03 | 0.076 ± 0.019 |
| 32 | 0.0035 ± 0.0009 | <0.00128 | 0.0049 ± 0.0022 | 0.0092 ± 0.0066 |
| 33 | 0.0045 ± 0.0003 | 0.0019 | 0.018 ± 0.006 | 0.017 ± 0.006 |
| 34 | 0.018 ± 0.015 | <0.00128 | 0.0099 ± 0.0074 | 0.032 ± 0.023 |
| 37 | 0.061 ± 0.006 | N.D | N.D | N.D |
Table 5.
Antiviral activity against herpes virus-1 (HSV-1), herpes virus-2 (HSV-2), acyclovir-resistant HSV (Thymidine kinase knock-out) and vaccinia virus cultured in Hel (human embryonic lung) cells.
| Cpd | HSV-1 (KOS) EC50a (μM) | HSV-2 (G) EC50a (μM) | HSV-1 (TK−) KOS ACVr EC50a (μM) | vaccinia virus EC50a (μM) | MCCb (μM) | CC50c (μM) |
|---|---|---|---|---|---|---|
| 5 | >4 | >4 | >4 | 0.35 ± 0.05 | 20 | 0.73 ± 0.07 |
| 59 | 2.10 ± 0.97 | 0.63 ± 0.09 | 1.27 ± 0.52 | 0.73 ± 0.33 | ≥20 | N.D. |
| 66 | 16.3 ± 3.7 | >100 | 10.57 ± 4.84 | >100 | ≥100 | 5.25 ± 3.80 |
| 67 | 6.67 ± 2.67 | >100 | 5.83 ± 3.17 | >100 | >100 | 0.35 ± 0.02 |
| 68 | >100 | >100 | >100 | >100 | >100 | 0.16 ± 0.0 |
| 70 | >100 | >100 | >100 | >100 | >100 | N.D. |
| Acyclovir | 0.2 | 0.2 | 10 | >250 | >250 | N.D. |
| Cidofovir | 1.5 | 1.2 | 2.0 | 22 | >250 | N.D. |
| Ganciclovir | 0.03 | 0.03 | 0.5 | >100 | >100 | N.D. |
Antiviral activity is expressed as EC50 values (μM) and represent the concentration of test compound necessary to reduce viral-induced cytopathogenicity by 50%.
MCC or minimal cytotoxic concentration represents the concentration of test compound that is able to cause a microscopically detectable alteration of normal cell morphology.
CC50 represents the concentration (μM) of test compound that reduces cell (Hel) proliferation by 50% as determined by Coulter Counter.
2.2.1. Antiproliferative activity
The results of the inhibition of cell line proliferation are depicted in Table 1.
Introduction of (substituted) phenyl rings in the C6 position of the purine nucleobase, known to confer cytostatic activity in ribofuranosylpurine nucleosides [22,23], failed to display any inhibitory activity on the cell line proliferation. Remarkably, 3′-C-ethynyl-7-deaza-adenosine (3′-C-ethynyltubercidin) 37, as well as its 7-halogenated analogs 31–34 inhibited the proliferation of the different tumor cell lines with nanomolar IC50 values. The chloro (32) and bromo (33) analogs showed the highest antiproliferative activity irrespective of the cell line studied, while unsubstituted derivative 37 showed similar activity as EAdo (5). Interestingly, the observed structure-antiproliferative activity relationship from this small subset significantly differs from that observed for the corresponding ribofuranose derivatives [25]. Furan-2-yl substituted analog 38 was found to only weakly inhibit tumor cell proliferation, which contrasts to the potent activity observed for the corresponding 7-(furan-2-yl)-7-deazaadenosine [25]. Similarly, the 7-ethynyl analog 54 only showed weak antiproliferative activity, which also contrasts with the activity observed for the corresponding ribofuranose analog [25]. Saturation of the ethynyl substituent as in 59, resulted in two orders of magnitude lower IC50's than those for 32.
The 7-trifluoromethyl analog 49 gave submicromolar activity, while the corresponding C8 isomer 50 was completely devoid of activity, possibly due to a preferred anti-orientation of the purine ring for activity.
To investigate the importance of N1, we synthetized the 1,7-dideazapurine or pyrrolo[2,3-b]pyridine analogs 66–68, 70 and 80 and 81. Interestingly, all the analogs elicited antiproliferative effects, most notably for the CEM cell line (C6 chloride analogs), except for 70. In the C6 chloride series, the antiproliferative activity correlated with halogen size (I > Br > Cl > H). While analog 80 was significantly less active than the related 32 (approximately 100-fold); this was not the case for the iodo-substituted analog 81, which displayed potent antiproliferative activity, especially on the L1210 cell line.
To further explore the potential of these new nucleoside analogs, the most potent analogs (31–34 and 37) were selected for testing in the NCI-60 cell line panel [43,44]. Table 2, Table 3 summarize the GI50 values of representative cell lines. Full assay data as well as mean GI50 graphs are provided in the Supporting Information.
Table 2.
Summary of the growth inhibitory potential (expressed as GI50) of selected nucleoside analogs against the NCI-60 tumor cell line panel with Sulforhodamine B (SRB) read-out at 48 h [43,44]. Full details (GI50 values for all cell lines) can be found in the Supporting Information. Values represent mean ± SEM of two independent evaluations.
| Cpd | Leukemia |
Lung |
Colon |
|||
|---|---|---|---|---|---|---|
| CCRF-CEM GI50 (μM) | HL-60 GI50 (μM) | SR GI50 (μM) | A549 GI50 (μM) | NCI-H460 GI50 (μM) | HCT116 GI50 (μM) | |
| 31b | 0.338 | 0.436 | 0.185 | 0.377 | 0.231 | 0.153 |
| 32 | 0.248 ± 0.009 | 0.059 ± 0.025 | 0.074 ± 0.013 | 0.188 ± 0.050 | [<0.01–0.046]a | 0.020 ± 0.002 |
| 33 | 0.213 ± 0.073 | 0.047 ± 0.025 | 0.064 ± 0.02 | 0.019 ± 0.097 | 0.034 ± 0.016 | 0.028 ± 0.002 |
| 34 | 0.272 ± 0.023 | 0.050 ± 0.018 | 0.28 ± 0.098 | 0.443 ± 0.146 | 0.098 ± 0.039 | 0.045 ± 0.01 |
| 37b | 2.3 | 1.57 | 0.817 | 1.31 | 0.473 | 0.595 |
Values in brackets represent the obtained GI50 values from both experiments.
Compounds 31 and 37 were only tested once.
Table 3.
Summary of the growth inhibitory potential (expressed as GI50) of selected nucleoside analogs against the NCI-60 tumor cell line panel with Sulforhodamine B (SRB) read-out at 48 h [43,44]. Full details (GI50 values for all cell lines) can be found in the Supporting Information. Values represent mean ± SEM of two independent evaluations.
| Cpd | CNS |
Melanoma |
Prostate |
Breast |
||
|---|---|---|---|---|---|---|
| U251 GI50 (μM) | Lox IMVI GI50 (μM) | PC-3 GI50 (μM) | DU145 GI50 (μM) | MCF7 GI50 (μM) | MDA-MB-231 GI50 (μM) | |
| 31b | 0.631 | 0.279 | 0.293 | 0.354 | 0.103 | 0.379 |
| 32 | [<0.01–0.02]a | 0.059 ± 0.023 | 0.192 ± 0.019 | 0.134 ± 0.025 | 0.027 ± 0.005 | 0.170 ± 0.02 |
| 33 | 0.022 ± 0.009 | 0.067 ± 0.038 | 0.107 ± 0.060 | 0.107 ± 0.044 | 0.023 ± 0007 | 0.136 ± 0.08 |
| 34 | 0.074 ± 0.006 | 0.060 ± 0.021 | 0.076 ± 0.010 | 0.131 ± 0.033 | [<0.01–0.014]a | 0.186 ± 0.02 |
| 37b | 2.44 | 0.667 | 0.808 | 0.563 | 0.239 | 1.53 |
Values in brackets represent the obtained GI50 values from both experiments.
Compounds 31 and 37 were only tested once.
Potent growth inhibitory activity was observed for 32, 33 and 34, while 31 and 37 were less active. The activity spectrum of the former analogs was found to be broad, and especially pronounced for leukemia cell lines as observed for e.g. clofarabine. GI50 values for several solid tumor cell lines (e.g. MCF-7, HCT-116, U251, NCI-H460) are below 100 nM.
Additionally, these analogs were evaluated for their potential to inhibit the cell proliferation of three different endothelial cell types. Agents capable of modifying the tumor vasculature, either by antiangiogenic or vascular-disrupting action, are of interest for antitumor therapies both as single agents and in combination with other chemotherapeutic drugs. Disruption of vascular networks in solid tumors may induce their collapse by deprivation of oxygen and other nutrients [45]. Nucleoside analogs 31–34, 37 were found to potently inhibit the proliferation of the three endothelial cell types studied, with the halogenated derivatives 32 (Cl) and 33 (Br) being most potent. Unfortunately, these analogs also significantly inhibited the proliferation of Hel-fibroblasts.
2.2.2. In vivo evaluation of compound 32
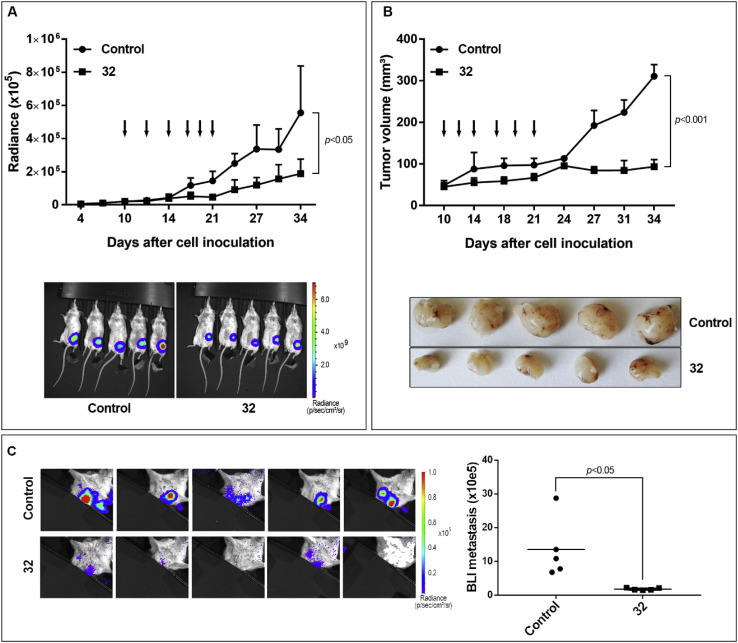
Encouraged by the strong in vitro anti-proliferative activity of analogs 31–34, 37, 49 and 81 against various tumor cell lines (Table 1, Table 2, Table 3), analog 32 was selected for in vivo evaluation of its antitumor activity in a metastatic breast cancer xenograft mouse model employing MDA-MB-231 (LM2) cells expressing firefly luciferase [46]. Antitumor activity was assessed by measurement of both the BLI signal (Fig. 2 , Panel A) and the tumor volume (Panel B). LM2 cells were orthotopically engrafted in SCID mice and treatment commenced once the tumor was palpable (day 10). Compound 32 was injected intratumorally (0.3 mg/kg) 3 times a week for two consecutive weeks. The tumor growth was significantly retarded starting from day 18, i.e. after four i.t. injections, as measured by bioluminescent radiance (Panel A). The reduced tumor growth was also obvious when the tumor size was measured using a digital caliper (Panel B). At day 35, mice were sacrificed and the tumors removed, after which they were macroscopically examined (Panel B). The average control tumor weight was 551 ± 74 mg versus 241 ± 61 mg for the treatment group.
Fig. 2.
In vivo evaluation of 32: MDA-MB-231-LM2 cells were orthotopically engrafted in the mammary fat pad of SCID mice. Compound 32 or vehicle were injected i.t. starting 10 days after inoculation and dosed 3 times a week, for 2 consecutive weeks. Arrows indicate compound administration. Panel A: BLI signal at regular time intervals. Data are mean ± STDEV, n = 5. Representative bioluminescence images of vehicle control and 32-treated mice at day 34 are shown. Panel B: Tumor volumes calculated from caliper measurements. Data are mean ± STDEV, n = 5. At day 35 mice were sacrificed and the corresponding pictures of dissected tumors are shown. Panel C: Lung metastasis at day 34 was quantified after shielding the primary tumor. Data are mean ± STDEV, n = 5. Statistical significance is indicated (multiple t-test).
To analyze the effect of 32 on lung metastasis, mice were covered with a black paper during imaging to shield the primary tumor. Interestingly, the total metastasis burden in the lungs was significantly lower in the treated group at day 34 versus the vehicle control group (Panel C). This indicates that the nucleoside analog not only exhibits antitumor activity but also reduces breast cancer metastasis to secondary organs.
2.2.3. Antiviral evaluation
Most analogs either showed no antiviral activity up to the highest concentration tested (100 μM) when assayed against a panel of relevant viruses (see Experimental section), or the activity was accompanied by significant toxicity for the host cell, making the derivatives non-specific (data not shown). Only a few analogs combined antiviral activities with acceptable selectivity indices. The results are summarized in Table 5 & Table 6 . EAdo (5) showed activity against vaccinia virus (Table 5), although it also potently inhibited Hel cell line proliferation (Table 6). Activity against vaccinia virus and HSV-2 was also found for 59, which, however generally inhibited cell proliferation (see also Table 1).
Table 6.
Antiviral activity against varicella-zoster virus and human cytomegalovirus.
| Cpd. | VZV TK+ (OKA) EC50a (μM) | VZV TK− (07_01) EC50a (μM) | MCCb (μM) | CC50c (μM) | CMV (AD-169) EC50d (μM) | CMV (Davis) EC50d (μM) | MCCe (μM) | CC50f (μM) | |
|---|---|---|---|---|---|---|---|---|---|
| 5 | 1.82 ± 0.24 | 1.64 ± 0.44 | 20 | 0.7 ± 0.1 | ≥4 | ≥4 | 20 | 0.73 ± 0.07 | |
| 59 | 1.71 ± 0.63 | 3.74 ± 2.69 | ≥20 | 3.41 ± 2.25 | 1.79 | >4 | 4 | N.D. | |
| 66 | 16.55 | 3.06 | 20 | N.D. | 0.51 ± 0.0 | 0.53 ± 0.09 | ≥20 | 5.25 ± 3.80 | |
| 67 | 2.76 ± 0.09 | 2.27 ± 1.74 | ≥100 | 0.35 ± 0.02 | 0.36 ± 0.0 | 0.35 ± 0.02 | >100 | 0.35 ± 0.02 | |
| 68 | 9.82 ± 2.21 | 8.26 ± 2.74 | 20 | 0.16 ± 0.0 | 2.31 ± 1.70 | 0.66 ± 0.15 | 20 | 0.16 ± 0.0 | |
| 70 | >100 | >100 | >100 | N.D. | >100 | >100 | 20 | N.D. | |
| 80 | >100 | 17.49 | >100 | N.D. | >100 | 20 | 100 | N.D. | |
| Acyclovir | 1.26 ± 0.73 | 36.74 ± 2.95 | >440 | >440 | N.D. | N.D. | N.D. | N.D. | |
| Cidofovir | N.D. | N.D. | N.D. | N.D. | 1.59 ± 0.35 | 1.45 ± 0.18 | >300 | N.D. | |
| Ganciclovir | N.D. | N.D. | N.D. | N.D. | 11.75 ± 0.32 | 6.52 ± 0.53 | >350 | N.D. | |
aActivity against varicella-zoster virus (VZV) in Hel (human embryonic lung) culture; TK−: thymidylate kinase knock-out strain; EC50 values (μM) represent the concentration of compound that reduces virus-induced cytopathicity by 50%.b,e MCC or minimal cytotoxic concentration represents the concentration of test compound that causes a microscopically detectable alteration of normal cell morphology.c,f CC50 represents the concentration (μM) of test compound that reduces cell (Hel) proliferation by 50% as determined by Coulter Counter.d Antiviral activity against cytomegalovirus (CMV) in Hel culture; EC50 values (μM) represent the concentration of compound that reduce virus-induced cytophathicity by 50%.
The most promising antiviral activity was found for compound 66, which exhibited potent activity against human cytomegalovirus (hCMV), with a reasonable selectivity index (10-fold). Other halogen-substituted derivatives 67 (Br) and 68 (I) displayed elevated cytotoxicity and are therefore non-selective. Apparently, the observed activity is specific for halogen bearing compounds, as the parent compound without halogen (70) was inactive. Furthermore, changing the 6-chloride for an amino group, mimicking a natural adenine, was detrimental for the activity (compare 66 and 80).
3. Conclusion
We have developed a nucleoside library around a 3′-C-ethynylribofuranose moiety, which was combined with several purine nucleobases that bear substituents, which were previously found to confer biological activity when combined with other sugar motifs. While 6-aryl purine nucleoside analogs 13–17 were devoid of antiproliferative or antiviral activity, the 7-halogenated 7-deazapurine analogs (31–34), as well as trifluoromethylated derivative 49 and the C7 unsubstituted analog 37 were found to significantly inhibit the growth of three tumor cell lines. Their spectrum of activity was thoroughly investigated (except for 49) by assaying against the NCI-60 panel, which also revealed nanomolar activity against several solid tumor derived cell lines. Removal of the N7 nitrogen and introduction of substitutions at C7 (particularly halogens, derivatives 31–34) was shown to lead to significantly more potent antiproliferative nucleosides than the C2/C6 modified derivatives we have previously reported. Additionally, analogs 31–34 potently inhibited proliferation of endothelial cell types, which could be particularly interesting for the treatment of solid tumors. As a proof-of-concept, analog 32 was investigated in a metastatic breast cancer xenograft mouse model. This derivative inhibited both tumor growth as well as metastasis as assessed by means of BLI. However, 32 also potently inhibited in vitro proliferation of Hel-fibroblasts, requiring further optimization to improve on selectivity. Several 1,7-dideaza-3-C-ethynyl analogs were prepared and some of them found to be potent inhibitors of human cytomegalovirus (hCMV) in vitro. Particularly, analog 66 requires further evaluation.
In conclusion, the results presented in this paper showcase the utility of screening a focused nucleoside library for both antiviral as well as antiproliferative activity as a means of finding novel hits for further elaboration.
4. Experimental
4.1. General experimental
All reagents and solvents were obtained from standard commercial sources and were of analytical grade. Unless otherwise specified, they were used as received. 6 [21], 18 [15], were prepared according to literature procedures. Halogenated heterocycles 4-chloro-5-halo-pyrrolo[2,3-d]pyrimidine: 5-fluoro [32,33], 5-chloro [34], 5-bromo [34], 5-iodo (40) [34], were prepared from commercially available 4-chloro-7H-pyrrolo[2,3-d]pyrimidine employing literature conditions.
All moisture sensitive reactions were carried out under argon atmosphere. Reactions were carried out at ambient temperature unless otherwise indicated. Analytical TLC was performed on Machery-Nagel® pre-coated F254 aluminum plates and were visualized by UV followed by staining with basic aq. KMnO4 or sulfuric acid-anisaldehyde spray. Column chromatography was performed using Davisil® (40–63 μm) or on a Reveleris X2 (Grace/Büchi) automated Flash unit employing pre-packed silica columns. Exact mass measurements were performed on a Waters LCT Premier XE™ Time of Flight (ToF) mass spectrometer equipped with a standard electrospray (ESI) and modular Lockspray™ interface. Samples were infused in a MeCN/water (1:1) + 0.1% formic acid mixture at 100 μL/min. NMR spectra were recorded on a Varian Mercury 300 MHz spectrometer. Chemical shifts (δ) are given in ppm and spectra are referenced to the residual solvent peak. Coupling constants are given in Hz. In 19F NMR, signals were referenced to CDCl3 or DMSO‑d 6 lock resonance frequency according to IUPAC referencing with CFCl3 set to 0 ppm. After glycosylation, both the correct stereochemistry at C1’ (β) and the correct regiochemistry (N9; purine numbering) of the glycosylation products was ascertained by means of 2D NMR techniques (2D NOESY, 1H-13C gHMBC, respectively), either on the protected or on the deprotected derivative (depending on peak resolution). Melting points were determined on a Büchi-545 apparatus and are uncorrected. Purity was assessed by means of analytical LC-MS employing either:
-
(1)
Waters Alliance 2695 XE separation Module using a Phenomenex Luna® reversed-phase C18 (2) column (3 μm, 100 × 2.00 mm) and a gradient system of HCOOH in H2O (0.1%, v/v)/HCOOH in MeCN (0.1%, v/v) at a flow rate of 0.4 mL/min, 10:90 to 0:100 in 9 min. High-resolution MS spectra were recorded on a Waters LCT Premier XE Mass spectrometer.
-
(2)
Waters AutoPurification system (equipped with ACQUITY QDa (mass; 100–1000 amu)) and 2998 Photodiode Array (220–400 nm)) using a Waters Cortecs® C18 (2.7 μm 100 × 4.6 mm) column and a gradient system of HCOOH in H2O (0.2%, v/v)/MeCN at a flow rate of 1.44 mL/min, 95:05 to 00:100 in 6.5 min.
All obtained final compounds had purity >95%, as assayed by analytical HPLC (UV) unless otherwise indicated.
4.2. Chemistry
4.2.1. General procedure A (Suzuki coupling (6-Cl-purine derivatives))
In a flame-dried 25 mL round-bottom flask, equipped with a stir bar was added under argon, 6-chloro purine nucleoside derivative 6 (0.5 mmol, 1 eq.), the corresponding boronic acid (1.5 eq.), anhydrous K2CO3 (1.5 eq.) and Pd(Ph3P)4 (0.05 eq.). The flask was evacuated and refilled with argon three times. Then, anhydrous degassed toluene (5 mL, 10 mL/mmol SM) was added and the mixture stirred for approximately 5 min before being heated to 100 °C. After TLC monitoring showed full conversion of the starting material (∼2–4h), the mixture was allowed to cool to room temperature, filtered and evaporated till dryness. The residue was purified by column chromatography (0 → 5% acetone/DCM).
4.2.2. General procedure B (nucleoside deprotection (ester hydrolysis))
The ester protected nucleoside (1 eq.) was dissolved in 7N NH3 in MeOH and stirred at ambient temperature until TLC showed full conversion (generally overnight to 36h). Then, the mixture was evaporated to dryness and the residue purified by column chromatography (typically 2 → 10% MeOH/DCM).
4.2.3. General procedure C (Vorbrüggen glycosylation of pyrrolo [2,3-d]pyrimidine and pyrrolo [2,3-b]pyridine derivatives)
In a flame-dried 2-neck round bottom flask, equipped with a stir bar was added the appropriate heterocycle (1.1 eq.) under argon. Then, anhydrous MeCN (7.5 mL/mmol SM) was added, followed by BSA (1.2 eq.). The resulting suspension was stirred at ambient temperature for approximately 10 min; after which a clear solution was obtained. Then, glycosyl donor, 18 [15] (1 eq.) was added in one portion, immediately followed by TMSOTf (1.25 eq.). The resulting solution was stirred at ambient temperature for another 15 min, and then transferred to a pre-heated oil bath at 80 °C. Heating was continued until TLC analysis showed full consumption of the starting material (∼2–3h), after which the mixture was cooled to ambient temperature. EA was added, and the mixture poured into a sat. aq. NaHCO3 solution. The layers were separated and the water layer extracted twice more with EA. The organic layers were combined, dried over Na2SO4, filtered and evaporated till dryness. The residue was purified by column chromatography 15% EA/Hexanes.
4.2.4. General procedure D (nucleophilic displacement with sodium azide)
The appropriate chloro-nucleoside (1 eq.) was dissolved in DMF (10 mL/mmol). Then, NaN3 (2 eq.) was added and the mixture stirred at 65 °C for 30 min. Then, the mixture was cooled to ambient temperature after which it was poured into half-saturated aq. NaHCO3 solution and EA. The layers were separated and the water layer extracted two more times with EA. The organic layers were combined, dried over Na2SO4, filtered and evaporated till dryness. The residue was purified by column chromatography (EA/Hexanes) to yield the protected azidonucleoside.
4.2.5. General procedure E (Staudinger reduction & iminophosphorane hydrolysis)
The appropriate azidonucleoside (1 eq.) was dissolved in THF (10 mL/mmol). Then, PMe3 solution (1 M in THF; 2 eq.) was added and the mixture stirred at ambient temperature until TLC analysis showed full conversion of starting material (generally 30 min to 1 h). Next, the solution was evaporated till dryness, and subsequently re-dissolved in MeCN (10 mL/mmol). To this solution was added a 1 M aq. HOAc solution (3.33 eq.), and the mixture heated in a pre-heated oil bath at 65 °C for 1h. Next, the mixture was cooled to ambient temperature and poured into sat. aq. NaHCO3 solution. DCM was added, layers were separated, and the water layer extracted two more times with DCM. The organic layers were combined, dried over Na2SO4, filtered and evaporated till dryness. Purification by column chromatography (EA/Hexanes) gave rise to the protected nucleoside aminopurine.
4.2.6. N9-β-d-ribofuranosyl-6-phenylpurine (2)22
2 was prepared according to a literature procedure [22]. Spectral data are in accordance with literature values [22]. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.56–3.64 (m, 1H, H-5″), 3.69–3.76 (m, 1H, H-5′), 4.00 (dd, J = 7.8, 3.8 Hz, 1H, H-4′), 4.20–4.24 (m, 1H, H-3′), 4.66 (dd, J = 10.8, 5.7 Hz, 1H, H-2′), 5.13 (t, J = 5.7 Hz, 1H, OH-5′), 5.25 (d, J = 5.1 Hz, 1H, OH-3′), 5.56 (d, J = 6.0 Hz, 1H, OH-2′), 6.10 (d, J = 5.4 Hz, 1H, H-1’), 7.58–7.65 (m, 3H, HPhe), 8.82–8.85 (m, 2H, HPhe), 8.93 (s, 1H, H-8), 9.02 (s, 1H, H-2).
4.2.7. 6-chloro-N9-(2′,3′,5′-tri-O-acetyl-3′-C-ethynyl-β-d-ribofuranosyl)-purine (7)
6-chloropurine (0.46 g, 2.94 mmol, 1.5 eq.) was suspended in HMDS (16 mL, 8 mL/mmol SM) and a catalytic amount of (NH4)2SO4 was added. The mixture was then refluxed overnight. After cooling to ambient temperature, the resulting solution was carefully evaporated till dryness, and the resulting oil further dried at high vacuum (∼1 h). Then, anhydrous 1,2-dichloroethane (16 mL, 8 mL/mmol SM) was added to dissolve the silylated heterocycle, after which 6 [21] (0.67 g, 1.96 mmol, 1 eq.) was added via syringe, immediately followed by TMSOTf (0.71 mL, 3.91 mmol, 2 eq.). The resulting solution was subsequently refluxed for approximately 30 min and cooled to ambient temperature. Then, the mixture was poured into sat. aq. NaHCO3, and DCM was added. The layers were separated, and the water layer extracted twice more with DCM. The organic layers were combined, dried over Na2SO4, filtered and evaporated till dryness. The residue was purified by column chromatography 5 → 7.5% acetone/DCM to give 7 (0.693 g, 1.59 mmol) as a white foam in 81% yield. 1H NMR (300 MHz, CDCl3) δ: 2.11 (s, 3H, acetyl-CH3), 2.14 (s, 3H, acetyl-CH3), 2.20 (s, 3H, acetyl-CH3), 2.86 (s, 1H, ethynyl-H), 4.54 (dd, J = 14.7, 5.7 Hz, 1H, H-5″), 4.61–4.68 (m, 2H, H-5′, H-4′), 6.05 (d, J = 4.5 Hz, 1H, H-2′), 6.30 (d, J = 4.8 Hz, 1H, H-1′), 8.52 (s, 1H, H-8), 8.77 (s, 1H, H-2). 13C NMR (75 MHz, CDCl3) δ: 20.5 (acetyl-CH3), 20.9 (acetyl-CH3), 21.0 (acetyl-CH3), 63.3 (C-5′), 75.3, 76.0, 77.0, 79.9 (C-2′), 81.6 (C-4′), 86.4 (C-1′), 131.9 (C-5), 143.1 (C-8), 151.7, 152.6 (2C), 168.4 (C=O), 168.7 (C=O), 170.4 (C=O). HRMS (ESI): calculated for C18H19Cl1N5O7 ([M+H]+): 452.0968, found: 452.0970. [Remark: correct stereo- and regiochemistry was ascertained by transforming a small amount into 3′-C-ethynyladenosine (5) and comparing NMR data to literature reference; [15] which confirmed the correct structure].
4.2.8. 6-Phenyl-N9-(2′,3′,5′-tri-O-acetyl-3′-C-ethynyl-β-d-ribofuranosyl)-purine (8)
8 was prepared according to General procedure A. 7 (0.22 g, 0.5 mmol) gave rise to 8 (0.101 g, 0.211 mmol) as a white foam in 42% yield. Purification: 0 → 5% acetone/DCM; second column 0 → 35% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.12 (s, 3H, acetyl-CH3), 2.16 (s, 3H, acetyl-CH3), 2.22 (s, 3H, acetyl-CH3), 2.86 (s, 1H, ethynyl-H), 4.52–4.58 (m, 1H, H-5″), 4.62–4.69 (m, 2H, H-4′, H-5′), 6.12 (d, J = 4.8 Hz, 1H, H-2′), 6.40 (d, J = 4.8 Hz, 1H, H-1′), 7.54–7.61 (m, 3H, HPhe), 8.55 (s, 1H, H-8), 8.75–8.79 (m, 2H, HPhe), 9.04 (s, 1H, H-2). HRMS (ESI): calculated for C24H23N4O7 ([M+H]+): 479.1561, found: 479.1562.
4.2.9. 6-(4-Methylphenyl)-N9-(2′,3′,5′-tri-O-acetyl-3′-C-ethynyl-β-d-ribofuranosyl)-purine (9)
9 was prepared according to General procedure A. 7 (0.22 g, 0.5 mmol) gave rise to 9 (0.107 g, 0.217 mmol) as a white foam in 43% yield. Purification: 0 → 5% acetone/DCM; second column 0 → 35 EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.12 (s, 3H, acetyl-CH3), 2.16 (s, 3H, acetyl-CH3), 2.22 (s, 3H, acetyl-CH3), 2.46 (s, 3H, CH3), 2.86 (s, 1H, ethynyl-H), 4.51–4.58 (m, 1H, H-5″), 4.61–4.70 (m, 2H, H-4′, H-5′), 6.12 (d, J = 4.8 Hz, 1H, H-2′), 6.39 (d, J = 5.1 Hz, 1H, H-1′), 7.36–7.39 (m, 2H, H-3Phe, H-5Phe), 8.53 (s, 1H, H-8), 8.67–8.70 (m, 2H, H-2Phe, H-6Phe), 9.01 (s, 1H, H-2). HRMS (ESI): calculated for C25H25N4O7 ([M+H]+): 493.1718, found: 493.1732.
4.2.10. 6-(4-Methoxyphenyl)-N9-(2′,3′,5′-tri-O-acetyl-3′-C-ethynyl-β-d-ribofuranosyl)-purine (10)
10 was prepared according to General procedure A. 7 (0.22 g, 0.5 mmol) gave rise to 10 (0.131 g, 0.258 mmol) as a white foam in 52% yield. Purification: 0 → 5% acetone/DCM; second column 0 → 50 EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.11 (s, 3H, acetyl-CH3), 2.15 (s, 3H, acetyl-CH3), 2.22 (s, 3H, acetyl-CH3), 2.86 (s, 1H, ethynyl-H), 3.91 (s, 3H, OCH3), 4.54 (dd, J = 14.4, 5.4 Hz, 1H, H-5″), 4.62–4.66 (m, 2H, H-4′, H-5′), 6.11 (d, J = 4.8 Hz, 1H, H-2′), 6.39 (d, J = 5.1 Hz, 1H, H-1′), 7.06–7.09 (m, 2H, H-3Phe, H-5Phe), 8.51 (s, 1H, H-8), 8.80–8.83 (m, 2H, H-2Phe, H-6Phe), 8.97 (s, 1H, H-2). HRMS (ESI): calculated for C25H25N4O8 ([M+H]+): 509.1667, found: 509.1654.
4.2.11. 6-(4-Chlorophenyl)-N9-(2′,3′,5′-tri-O-acetyl-3′-C-ethynyl-β-d-ribofuranosyl)-purine (11)
11 was prepared according to General procedure A. 7 (0.22 g, 0.5 mmol) gave rise to 11 (0.09 g, 0.175 mmol) as a slightly yellow foam in 35% yield. Purification: 0 → 37% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.12 (s, 3H, acetyl-CH3), 2.16 (s, 3H, acetyl-CH3), 2.22 (s, 3H, acetyl-CH3), 2.87 (s, 1H, ethynyl-H), 4.55 (dd, J = 14.7 Hz, 5.7 Hz, 1H, H-5″), 4.62–4.69 (m, 2H, H-4′, H-5′), 6.10 (d, J = 4.8 Hz, 1H, H-2′), 6.39 (d, J = 4.8 Hz, 1H, H-1′), 7.52–7.56 (m, 2H, H-3Phe, H-5Phe), 8.55 (s, 1H, H-8), 8.76–8.81 (m, 2H, H-2Phe, H-6Phe), 9.02 (s, 1H, H-2). HRMS (ESI): calculated for C24H22ClN4O7 ([M+H]+): 513.1172, found: 513.1170.
4.2.12. 6-(4-Fluorophenyl)-N9-(2′,3′,5′-tri-O-acetyl-3′-C-ethynyl-β-d-ribofuranosyl)-purine (12)
12 was prepared according to General procedure A. 7 (0.22 g, 0.5 mmol) gave rise to 12 (0.075 g, 0.152 mmol) as a slightly yellow foam in 30% yield. Purification: 0 → 37% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.11 (s, 3H, acetyl-CH3), 2.16 (s, 3H, acetyl-CH3), 2.22 (s, 3H, acetyl-CH3), 2.86 (s, 1H, ethynyl-H), 4.55 (dd, J = 15, 5.7 Hz, 1H, H-5″), 4.62–4.69 (m, 2H, H-4′, H-5′), 6.10 (d, J = 4.8 Hz, 1H, H-2′), 6.38 (d, J = 4.8 Hz, 1H, H-1′), 7.21–7.27 (m, 2H, H-3Phe, H-5Phe), 8.54 (s, 1H, H-8), 8.82–8.87 (m, 2H, H-2Phe, H-6Phe), 9.01 (s, 1H, H-2). 19F NMR (282 MHz, CDCl3) δ: −108.6 to −108.5 (m, 1 F). HRMS (ESI): calculated for C24H22FN4O7 ([M+H]+): 497.1467, found: 497.1469.
4.2.13. 6-Phenyl-N9-(3′-C-ethynyl-β-d-ribofuranosyl)-purine (13)
13 was prepared according to General Procedure B. 8 (0.1 g, 0.209 mmol) gave rise to 13 (0.032 g, 0.091 mmol) as a white solid in 43% yield. Purification: 3% MeOH/DCM. Melting point: 221 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.61 (s, 1H, ethynyl-H), 3.73–3.84 (m, 2H, H-5′, H-5″), 4.06 (dd, J = 4.5, 3.0 Hz, 1H, H-4′), 4.91 (t, J = 7.2 Hz, 1H, H-2′), 5.18 (t, J = 5.1 Hz, 1H, OH-5′), 6.00 (d, J = 6.9 Hz, 1H, OH-2′), 6.09 (d, J = 7.5 Hz, 1H, H-1′), 6.14 (s, 1H, OH-3′), 7.59–7.65 (m, 3H, H-3Phe, H-4Phe, H-5Phe), 8.81–8.87 (m, 2H, H-2Phe, H-6Phe), 8.95 (s, 1H, H-8), 9.03 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.9 (C-5′), 72.8, 77.3, 78.0 (C-2′), 82.6, 86.2 (C-1′), 87.8 (C-4′), 128.7 (2C, C-3Phe, C-5Phe), 129.4 (2C, C-2Phe, C-6Phe), 130.8 (C-5), 131.2 (C-4Phe), 135.2 (C-1Phe), 145.2 (C-8), 152.0 (C-2), 152.5 (C-4), 153.1 (C-6). HRMS (ESI): calculated for C18H17N4O4 ([M+H]+): 353.1244, found: 353.1259. [Remark: The final product contained ∼1.5 eq. of acetamide; which was added to the MW of the product.]
4.2.14. 6-(4-Methylphenyl)-N9-(3′-C-ethynyl-β-d-ribofuranosyl)-purine (14)
14 was prepared according to General Procedure B. 9 (0.1 g, 0.203 mmol) gave rise to 14 (0.040 g, 0.109 mmol) as a white solid in 54% yield. Purification: 3% MeOH/DCM. Melting point: 221 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 2.42 (s, 3H, CH3), 3.61 (s, 1H, ethynyl-H), 3.71–3.87 (m, 2H, H-5′, H-5″), 4.06 (dd, J = 4.5, 3.0 Hz, 1H, H-4′), 4.90 (t, J = 7.2 Hz, 1H, H-2′), 5.19 (dd, J = 5.7, 4.8 Hz, 1H, OH-5′), 5.99 (d, J = 6.9 Hz, 1H, OH-2′), 6.08 (d, J = 7.5 Hz, 1H, H-1′), 6.13 (s, 1H, OH-3′), 7.41–7.44 (m, 2H, H-3Phe, H-5Phe), 8.74–8.77 (m, 2H, H-2Phe, H-6Phe), 8.92 (s, 1H, H-8), 8.99 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 21.1 (CH3), 61.9 (C-5′), 72.8, 77.3, 78.0 (C-2′), 82.6, 86.2 (C-1′), 87.8 (C-4′), 129.4 (4C, CPhe), 130.6 (C-5), 132.5 (C-1Phe), 141.3 (C-4Phe), 144.9 (C-8), 151.9 (C-2), 152.4 (C-4), 153.2 (C-6). HRMS (ESI): calculated for C19H19N4O4 ([M+H]+): 367.1401, found: 367.1387. [Remark: The final product contained ∼2.5 eq. of acetamide; which was added to the MW of the product.]
4.2.15. 6-(4-Methoxyphenyl)-N9-(3′-C-ethynyl-β-d-ribofuranosyl)-purine (15)
15 was prepared according to General Procedure B. 10 (0.131 g, 0.258 mmol) gave rise to 15 (0.060 g, 0.157 mmol) as a white solid in 61% yield. Purification: 0 → 5% MeOH/DCM. Melting point: 219 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.60 (s, 1H, ethynyl-H), 3.71–3.85 (m, 2H, H-5′, H-5″), 3.87 (s, 3H, OCH3), 4.05 (dd, J = 4.5, 3.3 Hz, 1H, H-4′), 4.90 (dd, J = 7.5, 6.9 Hz, 1H, H-2′), 5.20 (dd, J = 5.7, 4.8 Hz, 1H, OH-5′), 5.96 (d, J = 6.9 Hz, 1H, OH-2′), 6.07 (d, J = 7.5 Hz, 1H, H-1′), 6.11 (s, 1H, OH-3′), 7.15–7.18 (m, 2H, H-3Phe, H-5Phe), 8.84–8.87 (m, 2H, H-2Phe, H-6Phe), 8.89 (s, 1H, H-8), 8.95 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 55.3 (OCH3), 61.9 (C-5′), 72.8, 77.3, 77.9 (C-2′), 82.6, 86.2 (C-1′), 87.8 (C-4′), 114.2 (2C, C-3Phe, C-5Phe), 127.6 (C-1Phe), 130.2 (C-5), 131.2 (2C, C-2Phe, C-6Phe), 144.7 (C-8), 151.9, 152.2, 152.9 (C-4), 161.8 (C-4Phe). HRMS (ESI): calculated for C19H19N4O5 ([M+H]+): 383.1350, found: 383.1359. [Remark: The final product contained ∼1.5 eq. of acetamide; which was added to the MW of the product.]
4.2.16. 6-(4-Chlorophenyl)-N9-(3′-C-ethynyl-β-d-ribofuranosyl)-purine (16)
16 was prepared according to General Procedure B. 11 (0.057 g, 0.111 mmol) gave rise to 16 (0.026 g, 0.068 mmol) as a white solid in 61% yield. Purification: 0 → 5% MeOH/DCM. Melting point: 230 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.61 (s, 1H, ethynyl-H), 3.71–3.87 (m, 2H, H-5′, H-5″), 4.06 (dd, J = 4.5, 3.3 Hz, 1H, H-4′), 4.91 (t, J = 7.2 Hz, H-2′), 5.17 (dd, J = 5.7, 4.8 Hz, 1H, OH-5′), 5.99 (d, J = 7.2 Hz, 1H, OH-2′), 6.09 (d, J = 7.5 Hz, 1H, H-1′), 6.14 (s, 1H, OH-3′), 7.68–7.72 (m, 2H, C-3Phe, C-5Phe), 8.85–8.89 (m, 2H, C-2Phe, C-6Phe), 8.97 (s, 1H, H-8), 9.04 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.8 (C-5′), 72.8, 77.3, 78.0 (C-2′), 82.5, 86.2 (C-1′), 87.9 (C-4′), 128.9 (2C, C-3Phe, C-5Phe), 130.7 (C-5), 131.1 (2C, C-2Phe, C-6Phe), 134.0 (C-1Phe), 136.1 (C-4Phe), 145.4 (C-8), 151.7 (C-6), 152.0 (C-2), 152.6 (C-4). HRMS (ESI): calculated for C18H16ClN4O4 ([M+H]+): 387.0855, found: 387.0869.
4.2.17. 6-(4-Fluorophenyl)-N9-(3′-C-ethynyl-β-d-ribofuranosyl)-purine (17)
17 was prepared according to General Procedure B. 12 (0.075 g, 0.151 mmol) gave rise to 17 (0.041 g, 0.112 mmol) as a white solid in 75% yield. Purification: 0 → 5% MeOH/DCM. Melting point: 235 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.61 (s, 1H, ethynyl-H), 3.72–3.87 (m, 2H, H-5′, H-5″), 4.06 (dd, J = 4.8, 3.3 Hz, 1H, H-4′), 4.91 (t, J = 7.2 Hz, 1H, H-2′), 5.18 (t, J = 5.4 Hz, 1H, OH-5′), 6.00 (d, J = 6.9 Hz, 1H, OH-2′), 6.09 (d, J = 7.5 Hz, 1H, H-1′), 6.14 (s, 1H, OH-3′), 7.43–7.49 (m, 2H, H-3Phe, H-5Phe), 8.90–8.94 (m, 2H, H-2Phe, H-6Phe), 8.95 (s, 1H, H-8), 9.02 (s, 1H, H-2). 19F NMR (282 MHz, DMSO‑d 6) δ: −109.01 (tt, J = 11.3, 5.6 Hz). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.9 (C-5′), 72.8, 77.3, 78.0 (C-2′), 82.5, 86.2 (C-1′), 87.9 (C-4′), 115.8 (d, J = 21.8 Hz, 2C, C-3Phe, C-5Phe), 130.6 (C-5), 131.7 (d, J = 2.3 Hz, 1C, C-1Phe), 131.9 (d, J = 8.0 Hz, 2C, C-2Phe, C-6Phe), 145.3 (C-8), 151.9, 152.0, 152.5 (C-4), 164.0 (d, J = 248.4 Hz, 1C, C-4Phe). HRMS (ESI): calculated for C18H16FN4O4 ([M+H]+): 371.1150, found: 371.1163.
4.2.18. 4-chloro-5-fluoro-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (19)
19 was prepared according to General Procedure C. 18 (0.63 g, 1.2 mmol) gave rise to 19 (0.246 g) as a white foam, containing some impurities. Therefore, 19 was immediately used in the next step.
4.2.19. 4,5-dichloro-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (20)
20 was prepared according to General Procedure C. 18 (0.8 g, 1.5 mmol) gave rise to 20 (0.39 g) as a yellow foam, containing some impurities. Therefore, 20 was immediately used in the next step.
4.2.20. 4-chloro-5-iodo-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (22)
22 was prepared according to General Procedure C. 18 (1.69 g, 3.2 mmol) gave rise to 22 (1.25 g) as a yellow foam, containing some impurities. Therefore, 22 was immediately used in the next step.
4.2.21. 4-azido-5-fluoro-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (23)
23 was prepared according to General Procedure D. 19 (0.246 g, 0.385 mmol) gave rise to 23 (0.14 g, 0.217 mmol) as a white solid in 56% yield. Purification: 0 → 30% EA/Hexanes. 1H NMR (300 MHz, DMSO‑d 6) δ: 4.29 (s, 1H, ethynyl-H), 4.86 (dd, J = 12.0, 6.9 Hz, 1H, H-5″), 5.02 (dd, J = 12.0, 4.2 Hz, 1H, H-5′), 5.20 (dd, J = 6.6, 3.9 Hz, 1H, H-4′), 6.37 (d, J = 5.4 Hz, 1H, H-2′), 6.92 (dd, J = 5.4, 1.5 Hz, 1H, H-1′), 7.40–7.45 (m, 2H, OBz), 7.51–7.57 (m, 4H, OBz), 7.61–7.73 (m, 3H, OBz), 7.88–7.91 (m, 2H, OBz), 8.00–8.08 (m, 5H, OBz, H-6), 9.95 (s, 1H, H-2). 19F NMR (282 MHz, DMSO‑d 6) δ: −163.54 (d, J = 2.0 Hz). 13C NMR (75 MHz, DMSO‑d 6) δ: 63.6 (C-5′), 75.7, 76.4, 78.2 (C-2′), 80.4 (C-4′), 82.2, 85.7 (C-1′), 92.8 (d, J = 14.9 Hz, 1C, C-4a), 108.8 (d, J = 26.4 Hz, 1C, C-6), 127.6, 128.4, 128.9, 129.0, 129.2, 129.3, 129.4, 129.5, 133.7, 134.29, 134.34, 135.3 (C-2), 137.2 (d, J = 3.5 Hz, 1C, C-7a), 143.3 (d, J = 143.3 Hz, 1C, C-5), 144.4 (d, J = 3.5 Hz, 1C, C-4), 163.6 (C=O), 163.8 (C=O), 165.4 (C=O). HRMS (ESI): calculated for C34H24FN6O7 ([M+H]+): 647.1685, found: 647.1686.
4.2.22. 4-azido-5-chloro-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (24)
24 was prepared according to General Procedure D. 20 (0.38 g, 0.579 mmol) gave rise to 24 (0.16 g, 0.24 mmol) as a slightly yellow foam in 41% yield. Purification: 0 → 60% EA/Hexanes. 1H NMR (300 MHz, DMSO‑d 6) δ: 4.30 (s, 1H, ethynyl-H), 4.87 (dd, J = 12.0, 6.9 Hz, 1H, H-5″), 5.04 (dd, J = 12.0, 4.2 Hz, 1H, H-5′), 5.22 (dd, J = 6.6, 4.2 Hz, 1H, H-4′), 6.42 (d, J = 5.4 Hz, 1H, H-2′), 6.89 (d, J = 5.4 Hz, 1H, H-1′), 7.41–7.46 (m, 2H, OBz), 7.51–7.58 (m, 4H, OBz), 7.61–7.76 (m, 3H, OBz), 7.89–7.92 (m, 2H, OBz), 8.01–8.08 (m, 4H, OBz), 8.22 (s, 1H, H-6), 9.97 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 63.6 (C-5′), 75.8, 76.3, 78.2 (C-2′), 80.6 (C-4′), 82.2, 85.7 (C-1′), 102.0 (C-4a), 106.5 (C-5), 122.4 (C-6), 127.7, 128.4, 128.9, 129.0, 129.2, 129.3, 129.4, 129.5, 133.7, 134.29, 134.34, 135.5 (C-2), 140.3 (C-7a), 145.1 (C-4), 163.5 (C=O), 163.8 (C=O), 165.4 (C=O). HRMS (ESI): calculated for C34H24ClN6O7: 663.1390 ([M+H]+), found: 663.1398.
4.2.23. 4-azido-5-iodo-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (26)
26 was prepared according to General Procedure D. 22 (0.133 g, 0.178 mmol) gave rise to 26 (0.082 g, 0.109 mmol) as a slightly yellow foam in 61% yield. Purification: 25% EA/Hexanes. 1H NMR (300 MHz, DMSO‑d 6) δ: 4.31 (s, 1H, ethynyl-H), 4.86 (dd, J = 12.0, 6.3 Hz, 1H, H-5″), 5.03 (dd, J = 12.0, 4.2 Hz, 1H, H-5′), 5.21 (dd, J = 6.3, 4.5 Hz, 1H, H-4′), 6.41 (d, J = 5.4 Hz, 1H, H-2′), 6.86 (d, J = 5.4 Hz, 1H, H-1′), 7.41–7.46 (m, 2H, OBz), 7.52–7.58 (m, 4H, OBz), 7.61–7.76 (m, 3H, OBz), 7.88–7.91 (m, 2H, OBz), 8.01–8.08 (m, 4H, OBZ), 8.19 (s, 1H, H-6), 9.93 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 56.6 (C-5), 63.6 (C-5′), 75.8, 76.4, 78.2 (C-2′), 80.6 (C-4′), 82.2, 85.6 (C-1′), 107.0 (C-4a), 127.6, 128.4, 128.8, 129.0, 129.2, 129.3, 129.4, 129.5, 129.6 (C-6), 133.7, 134.27, 134.32, 135.2 (C-2), 141.8 (C-7a), 146.0 (C-4), 163.5 (C=O), 163.8 (C=O), 165.4 (C=O). HRMS (ESI): calculated for C34H24IN6O7: 755.0746 ([M+H]+), found: 755.0697.
4.2.24. 4-amino-5-fluoro-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (27)
27 was prepared according to General Procedure E. 23 (0.13 g, 0.201 mmol) gave rise to 27 (0.111 g, 0.179 mmol) as a slightly yellow foam in 89% yield. Purification: 20 → 65% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.96 (s, 1H, ethynyl-H), 4.84–4.94 (m, 2H, H-4′, H-5″), 5.01 (dd, J = 10.8, 3.0 Hz, 1H, H-5′), 5.49 (br. s, 2H, NH2), 6.30 (d, J = 5.1 Hz, 1H, H-2′), 6.79 (dd, J = 5.1, 2.1 Hz, 1H, H-1′), 7.15 (d, J = 2.4 Hz, 1H, H-6), 7.28–7.31 (m, 2H, OBz), 7.39–7.51 (m, 5H, OBz), 7.57–7.62 (m, 2H, OBz), 7.87–7.90 (m, 2H, OBz), 8.02–8.06 (m, 2H, OBz), 8.14–8.18 (m, 2H, OBz), 8.22 (s, 1H, H-2). 19F NMR (282 MHz, CDCl3) δ: −166.34 (d, J = 2.0 Hz). HRMS (ESI): calculated for C34H26FN4O7 ([M+H]+): 621.1780, found: 621.1788.
4.2.25. 4-amino-5-chloro-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (28)
28 was prepared according to General Procedure E. 24 (0.14 g, 0.211 mmol) gave rise to 28 (0.120 g, 0.188 mmol) as a white foam in 89% yield. Purification: 20 → 65% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.97 (s, 1H, ethynyl-H), 4.88 (dd, J = 11.4, 5.4 Hz, 1H, H-5″), 4.93–4.96 (m, 1H, H-4′), 5.02 (dd, J = 11.1, 3.3 Hz, 1H, H-5′), 5.70 (br. s, 2H, NH2), 6.34 (d, J = 5.1 Hz, 1H, H-2′), 6.72 (d, J = 4.8 Hz, 1H, H-1′), 7.27–7.32 (m, 2H, OBz), 7.35 (s, 1H, H-6), 7.40–7.52 (m, 5H, OBz), 7.57–7.63 (m, 2H, OBz), 7.88–7.91 (m, 2H, OBz), 8.03–8.06 (m, 2H, OBz), 8.15–8.18 (m, 2H, OBz), 8.23 (s, 1H, H-2). HRMS (ESI): calculated for C34H26ClN4O7 ([M+H]+): 637.1485, found: 637.1455.
4.2.26. 4-amino-5-bromo-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (29)
29 was prepared by employing a sequence of General Procedure C, D & E. As such, 18 (0.53 g, 1.0 mmol) gave rise to 29 (0.077 g, 0.113 mmol) as a white foam in 11% yield. 1H NMR (300 MHz, CDCl3) δ: 2.96 (s, 1H, ethynyl-H), 4.89 (dd, J = 11.1, 5.1 Hz, 1H, H-5″), 4.93–4.86 (m, 1H, H-4′), 5.01 (dd, J = 11.1, 3.0 Hz, 1H, H-5′), 5.76 (br. s, 2H, NH2), 6.34 (d, J = 5.1 Hz, 1H, H-2′), 6.71 (d, J = 5.1 Hz, 1H, H-1′), 7.27–7.32 (m, 2H, OBz), 7.40–7.52 (m, 6H, OBz, H-6), 7.57–7.63 (m, 2H, OBz), 7.88–7.91 (m, 2H, OBz), 8.03–8.06 (m, 2H, OBz), 8.15–8.18 (m, 2H, OBz), 8.21 (s, 1H, H-2). 13C NMR (75 MHz, CDCl3) δ: 63.8 (C-5′), 76.4, 76.9, 78.5 (C-2′), 72.2, 80.9 (C-4′), 85.4 (C-1′), 89.9 (C-5), 102.4 (C-4a), 120.9 (C-6), 128.5, 128.6, 128.8, 128.9, 129.7, 130.0, 130.1, 133.6, 133.9, 134.1, 150.9 (C-7a), 153.3 (C-2), 157.0 (C-4), 164.4 (C=O), 164.6 (C=O), 166.4 (C=O). HRMS (ESI): calculated for C34H26BrN4O7 ([M+H]+): 681.0979, found: 681.1010.
4.2.27. 4-amino-5-iodo-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (30)
30 was prepared according to General Procedure E. 26 (0.075 g, 0.099 mmol) gave rise to 30 (0.065 g, 0.089 mmol) as a slightly yellow foam in 90% yield. Purification: 25 → 75% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.96 (s, 1H, ethynyl-H), 4.88 (dd, J = 11.1, 4.8 Hz, 1H, H-5″), 4.94–4.96 (m, 1H, H-4′), 5.02 (dd, J = 11.1, 3.3 Hz, 1H, H-5′), 5.68 (br. s, 2H, NH2), 6.36 (d, J = 5.1 Hz, H-2′), 6.69 (d, J = 5.4 Hz, 1H, H-1′), 7.27–7.32 (m, 2H, OBz), 7.40–7.53 (m, 5H, OBz), 7.50 (s, 1H, H-6), 7.58–7.64 (m, 2H, OBz), 7.88–7.91 (m, 2H, OBz), 8.03–8.06 (m, 2H, OBz), 8.15–8.18 (m, 2H, OBz), 8.22 (s, 1H, H-2). HRMS (ESI): calculated for C34H26IN4O7 ([M+H]+): 729.0841, found: 729.0861.
4.2.28. 4-amino-5-fluoro-N7-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (31)
31 was prepared according to General Procedure B. 27 (0.105 g, 0.169 mmol) gave rise to 31 (0.05 g, 0.16 mmol) as a white solid in 95% yield. Purification: 1 → 15% MeOH/DCM. Melting point: 235 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 3.55 (s, 1H, ethynyl-H), 3.63–3.75 (m, 2H, H-5′, H-5″), 3.91 (t, J = 3.6 Hz, 1H, H-4′), 4.49 (t, J = 7.5 Hz, 1H, H-2′), 5.20 (t, J = 5.10 Hz, 1H, OH-5′), 5.78 (d, J = 7.2 Hz, 1H, OH-2′), 5.91 (s, 1H, OH-3′), 6.04 (dd, J = 7.5, 1.8 Hz, 1H, H-1′), 7.02 (br. s, 2H, NH2), 7.38 (d, J = 2.1 Hz, 1H, H-6), 8.06 (s, 1H, H-2). 19F NMR (282 MHz, DMSO‑d 6) δ: −167.55 to −167.54 (m, 1 F). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.9 (C-5′), 72.8, 76.8, 78.2 (C-2′), 83.1, 85.4 (C-1′), 86.7 (C-4′), 92.6 (d, J = 16.1 Hz, 1C, H-4a), 104.6 (d, J = 26.3 Hz, 1C, C-6), 142.6 (d, J = 243.8 Hz, 1C, C-5), 146.6 (C-7a), 152.8 (C-2), 155.9 (d, J = 3.4 Hz, 1C, C-4). HRMS (ESI): calculated for C13H14FN4O4 ([M+H]+): 309.0994, found: 309.0993.
4.2.29. 4-amino-5-chloro-N7-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (32)
32 was prepared according to General Procedure B. 28 (0.12 g, 0.188 mmol) gave rise to 32 (0.035 g, 0.109 mmol) as a white solid in 58% yield. Purification: 5 → 10% MeOH/DCM. Melting point: 254 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 3.55 (s, 1H, ethynyl-H), 3.62–3.77 (m, 2H, H-5′, H-5″), 3.92 (t, J = 3.6 Hz, 1H, H-4′), 4.55 (t, J = 7.2 Hz, 1H, H-2′), 5.25 (t, J = 4.8 Hz, 1H, OH-5′), 5.80 (d, J = 7.2 Hz, 1H, OH-2′), 5.94 (s, 1H, OH-3′), 6.02 (d, J = 7.8 Hz, 1H, H-1′), 6.90 (br. s, 2H, NH2), 7.62 (s, 1H, H-6), 8.09 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.8 (C-5′), 72.8, 76.9, 78.2 (C-2′), 83.0, 85.6 (C-1′), 86.9 (C-4′), 99.9 (C-4a), 102.8 (C-5), 119.5 (C-6), 149.5 (C-7a), 152.7 (C-2), 156.8 (C-4). HRMS (ESI): calculated for C13H14ClN4O4 ([M+H]+): 325.0698, found: 325.0691.
4.2.30. 4-amino-5-bromo-N7-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (33)
33 was prepared according to General Procedure B. 29 (0.075 g, 0.110 mmol) gave rise to 33 (0.03 g, 0.081 mmol) as a white solid in 95% yield. Purification: 1 → 15% MeOH/DCM. Melting point: 245 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 3.55 (s, 1H, ethynyl-H), 3.63–3.77 (m, 2H, H-5″, H-5′), 3.93 (t, J = 3.6 Hz, 1H, H-5′), 4.56 (t, J = 7.2 Hz, 1H, H-2′), 5.25 (t, J = 5.1 Hz, 1H, OH-5′), 5.81 (d, J = 6.9 Hz, 1H, OH-2′), 5.95 (s, 1H, OH-3′), 6.03 (d, J = 7.5 Hz, 1H, H-1′), 6.82 (br. s, 2H, NH2), 7.68 (s, 1H, H-6), 8.10 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.8 (C-5′), 72.8, 76.9, 78.2 (C-2′), 82.9, 85.6 (C-1′), 86.9 (2C, C-4′, C-5), 101.1 (C-4a), 122.1 (C-6), 149.9 (C-7a), 152.4 (C-2), 157.0 (C-4). HRMS (ESI): calculated for C13H14BrN4O4 ([M+H]+): 369.0193, found: 369.0199.
4.2.31. 4-amino-5-iodo-N7-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (34)
34 was prepared according to General Procedure B. 30 (0.39 g, 0.535 mmol) gave rise to 34 (0.1 g, 0.24 mmol) as a white solid in 45% yield. Purification: 6% MeOH/DCM. Melting point: 230 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 3.55 (s, 1H, ethynyl-H), 3.63–3.77 (m, 2H, H-5′, H-5″), 3.93 (dd, J = 3.9, 3.3 Hz, 1H, H-4′), 4.56 (t, J = 7.5 Hz, 1H, H-2′), 5.25 (dd, J = 5.7, 4.5 Hz, 1H, OH-5′), 5.78 (d, J = 7.2 Hz, 1H, OH-2′), 5.92 (s, 1H, OH-3′), 6.01 (d, J = 7.5 Hz, 1H, H-1′), 6.69 (br. s, 2H, NH2), 7.71 (s, 1H, H-6), 8.10 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 52.1 (C-5), 61.8 (C-5′), 72.8, 76.8, 78.2 (C-2′), 83.0, 85.6 (C-1′), 86.9 (C-4′), 103.3 (C-5), 127.5 (C-6), 150.4 (C-7a), 151.9 (C-2), 157.2 (C-4). HRMS (ESI): calculated for C13H14IN4O4 ([M+H]+): 417.0054, found: 417.0056.
4.2.32. 4-azido-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (35)
26 (0.22 g, 0.292 mmol, 1 eq.) was co-evaporated with anhydrous toluene (10 mL) three times. Then, the resulting foam was dissolved in anhydrous toluene (2.5 mL, 8.5 mL/mmol SM) under argon and cooled to –65 °C. After stirring at this temperature for ∼15 min, iPrMgCl.LiCl (1.3 M in THF, 0.45 mL, 0.583 mmol, 2 eq.) was added dropwise with the help of a syringe pump (70 μL/min). After complete addition, the mixture was stirred at –65 °C for ∼30min, after which the cooling was removed, and aq. sat. NH4Cl was added. Then, the mixture was diluted with EA and additional water was added. The layers were separated, and the water layer extracted twice more with EA. The organic layers were combined, dried over Na2SO4, filtered and evaporated. the residue was purified by column chromatography 0 → 10% Et2O/Toluene to give 35 (0.14 g, 0.222 mmol) as a white foam in 76% yield. 1H NMR (300 MHz, DMSO‑d 6) δ: 4.30 (s, 1H, ethynyl-H), 4.87 (dd, J = 12.0, 6.0 Hz, 1H, H-5″), 5.01 (dd, J = 12.0, 4.2 Hz, 1H, H-5′), 5.21 (dd, J = 6.0, 4.2 Hz, 1H, H-4′), 6.41 (dd, J = 5.1, 0.9 Hz, 1H, H-2′), 6.89 (d, J = 5.1 Hz, 1H, H-1′), 7.32 (d, J = 3.6 Hz, 1H, H-5), 7.82–7.43 (m, 2H, OBz), 7.51–7.75 (m, 7H, OBz), 7.86–7.89 (m, 2H, OBz), 8.00–8.08 (m, 5H, OBz, H-6), 9.91 (s, 1H, H-2). HRMS (ESI): calculated for C34H25N6O7 ([M+H]+): 629.1779, found: 629.1796.
4.2.33. 4-amino-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (36)
36 was prepared according to General Procedure E. 35 (0.193 g, 0.307 mmol) gave rise to 36 (0.160 g, 0.266 mmol) as a white foam in 87% yield. Purification: 50 → 70% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.93 (s, 1H, ethynyl-H), 4.88 (dd, J = 11.1, 5.1 Hz, 1H, H-5″), 4.96 (dd, J = 5.1, 3.3 Hz, 1H, H-4′), 5.02 (dd, J = 11.1, 3.3 Hz, 1H, H-5′), 5.29 (br. s, 2H, NH2), 6.41 (d, J = 5.1 Hz, 1H, H-2′), 6.45 (d, J = 3.9 Hz, 1H, H-5), 6.74 (d, J = 5.1 Hz, 1H, H-1′), 7.13–7.26 (m, 2H, OBz), 7.40–7.52 (m, 6H, OBz, H-6), 7.56–7.63 (m, 2H, OBz), 7.88–7.91 (m, 2H, OBz), 8.04–8.07 (m, 2H, OBz), 8.15–8.18 (m, 2H, OBz), 8.27 (s, 1H, H-2). HRMS (ESI): calculated for C34H27N4O7 ([M+H]+): 603.1874, found: 603.1877.
4.2.34. 4-amino-N7-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (37)
37 was prepared according to General Procedure B. 36 (0.15 g, 0.249 mmol) gave rise to 37 (0.021 g, 0.073 mmol) as a white solid in 30% yield. Purification: 5 → 20% MeOH/DCM. Melting point: 150 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.53 (s, 1H, ethynyl-H), 3.70–3.72 (m, 2H, H-5′, H-5″), 3.92 (t, J = 3.3 Hz, 1H, H-4′), 4.61 (t, J = 7.5 Hz, 1H, H-2′), 5.49 (dd, J = 6.3, 4.5 Hz, 1H, OH-5′), 5.75 (d, J = 7.2 Hz, 1H, OH-2′), 5.87 (s, 1H, OH-3′), 5.94 (d, J = 7.5 Hz, 1H, H-1′), 6.60 (d, J = 3.6 Hz, 1H, H-5), 7.07 (br. s, 2H, NH2), 7.38 (d, J = 3.9 Hz, 1H, H-6), 8.04 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 62.0 (C-5′), 72.8, 76.7, 78.0 (C-2′), 83.3, 86.6 (C-1′), 86.7 (C-4′), 99.7 (C-5), 103.2 (C-4a), 122.7 (C-6), 150.1 (C-7a), 151.5 (C-2), 157.6 (C-4). HRMS (ESI): calculated for C13H15N4O4 ([M+H]+): 291.1088, found: 291.1089.
4.2.35. 4-amino-5-(furan-2-yl)-N7-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (38)
34 (0.086 g, 0.207 mmol, 1 eq.), furan-2-boronic acid (0.035 g, 0.31 mmol, 1.5 eq.), Na2CO3 (0.2 g, 1.86 mmol, 9 eq.), Pd(OAc)2 (0.002 g, 0.01 mmol, 0.05 eq.) and TPPTS (0.018 g, 0.031 mmol, 0.15 eq.) were added to a 10 mL round-bottom flask, equipped with a stir bar. Next, the flask was evacuated and refilled with argon. This procedure was repeated three times in total. Next, degassed MeCN (0.75 mL) and H2O (1.5 mL) were added to the solids under argon. After 5 min of stirring, the mixture was heated to 100 °C in a pre-heated oil bath. When the starting material was fully consumed (30 min), the mixture was cooled to ambient temperature, and neutralized (pH ∼ 7) with 0.5 M aq. HCl. The mixture was evaporated till dryness, resuspended in MeOH and evaporated (three times). Next, the mixture was adsorbed onto Celite® (from MeOH) and eluted over a short silica pad (∼5 cm) with 20% MeOH/DCM. The liquid was evaporated in vacuo and purified by column chromatography 1 → 10% MeOH/DCM, to give 38 (0.022 g, 0.061 mmol) as a white solid in 29% yield. Melting point: 208–210 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.57 (s, 1H, ethynyl-H), 3.66–3.78 (m, 2H, H-5′, H-5″), 3.95 (dd, J = 4.2, 3.3 Hz, 1H, H-4′), 4.63 (t, J = 7.5 Hz, 1H, H-2′), 5.59 (dd, J = 5.7, 4.8 Hz, 1H, OH-5′), 5.81 (d, J = 7.2 Hz, 1H, OH-2′), 5.94 (s, 1H, OH-3′), 6.07 (d, J = 7.8 Hz, 1H, H-1′), 6.62 (dd, J = 3.3, 1.8 Hz, 1H, H-4furan), 6.68 (dd, J = 3.3, 0.6 Hz, 1H, H-3furan), 6.93 (br. s, 2H, NH2), 7.79 (dd, J = 1.8, 0.6 Hz, 1H, H-5furan), 7.88 (s, 1H, H-6), 8.13 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.9 (C-5′), 72.8, 76.9, 78.1 (C-2′), 83.1, 85.8 (C-1′), 86.9 (C-4′), 99.4 (C-4a), 105.3 (C-3furan), 106.3 (C-5), 111.9 (C-4furan), 120.8 (C-6), 142.1 (C-5furan), 148.2 (C-2furan), 151.2 (C-7a), 152.1 (C-2), 157.3 (C-4). HRMS (ESI): calculated for C17H17N4O5 ([M+H]+): 357.1193, found: 357.1196.
4.2.36. 4-chloro-6-trifluoromethyl-7H-pyrrolo[2,3-d]pyrimidine (39)35
A suspension of 6-chloro-7-deazapurine (0.31 g, 2 mmol, 1 eq.) and sodiumtrifluoromethylsulfinate (0.94 g, 6 mmol, 3 eq.) in a mixture of DCM/water (8 mL/3.2 mL; 2.5/1 ratio; 0.18 M concentration in total) was cooled in an ice bath to 0 °C. After stirring at that temperature for ∼10 min, 70% aq. tBuOOH (1.4 mL, 10 mmol, 5 eq.) was added dropwise (0.1 mL/min). When the addition was complete, the ice bath was removed, and vigorous stirring continued for 3 days. Then, the mixture was partitioned between sat. aq. NaHCO3 solution and DCM, and the layers separated. The water layer was extracted twice more with DCM. The combined organic layers were dried over Na2SO4, filtered and evaporated till dryness. Purification by column chromatography 16% EA/Hexanes gave 39 (0.05 g, 0.226 mmol) as a white solid in 11% yield. 1H NMR (300 MHz, CDCl3) δ: 7.10 (s, 1H, H-5), 8.83 (s, 1H, H-2), 13.29 (br. s, 1H, NH). 19F NMR (282 MHz, CDCl3) δ: −61.6.13C NMR (75 MHz, CDCl3) δ: 101.6 (q, J = 3.5 Hz, 1C, C-5), 117.5 (C-4a), 120.5 (q, J = 267.9 Hz, 1H, CF3), 128.5 (q, J = 40.1 Hz, 1C, C-6), 151.6, 152.4 (C-2), 155.7. Spectral data were in accordance with literature values [35].
4.2.37. t-butyl-4-chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine-7-carboxylate (41)
40 [34] (0.84 g, 3 mmol, 1 eq.) was suspended in anhydrous 1,4-dioxane (12 mL, 4 mL/mmol SM). Then, DMAP (0.073 g, 0.6 mmol, 0.2 eq.) was added, followed by DBU (0.9 mL, 6 mmol, 2 eq.). Next, Boc2O (2.07 mL, 9 mmol, 3 eq.) was added dropwise and the resulting mixture was stirred at ambient temperature overnight. Next, the mixture was added to sat. aq. NH4Cl, and EA/water was added. The layers were separated, and the water layer extracted twice more with EA. The organic layers were combined, dried over Na2SO4, filtered and evaporated till dryness. The residue was purified by column chromatography (15% EA/Hexanes) to yield 41 (1.09 g, 2.87 mmol) as a slightly yellow solid in 96% yield. 1H NMR (300 MHz, CDCl3) δ: 1.68 (s, 9H, t-Bu), 7.87 (s, 1H, H-6), 8.83 (s, 1H, H-2). 13C NMR (75 MHz, CDCl3) δ: 28.1 (3C, t-Bu CH3), 56.6 (C-5), 86.8 (t-Bu, C-(CH3)3), 119.0 (C-4a), 133.4 (C-6), 146.0 (C=O), 151.7 (C-7a), 153.2 (C-2), 153.6 (C-4). HRMS (ESI): calculated for C11H12IN3O2 ([M+H]+): 379.9657; found: 379.9667.
4.2.38. 4-chloro-5-trifluoromethyl-7H-pyrrolo[2,3-d]pyrimidine (42)
In a flame dried culture flask, equipped with a stir bar, was added under argon: 41 (0.76 g, 2 mmol, 1 eq.), KF (0.349 g, 6 mmol, 3 eq.), CuI (0.076 g, 0.4 mmol, 0.2 eq.) and 1,10-phenanthroline (0.072 g, 0.4 mmol, 0.2 eq.). The flask was evacuated and refilled with argon three times. Then, anhydrous DMSO (4 mL, 2 mL/mmol SM) was added, followed by B(OMe)3 and TMSCF3. The mixture was submerged in a pre-heated oil bath at 60 °C for 24h. Then, the mixture was allowed to cool to ambient temperature and partitioned between water and Et2O. The layers were separated, and the water layer extracted once more with Et2O. The combined organic layers were then washed with diluted aq. ammonia solution (1×), followed by brine (1×), and dried over Na2SO4, filtered and evaporated till dryness. The residue was purified by column chromatography 3 → 4% acetone/DCM. The resulting product was found to be sufficiently pure for use in the next step (Vorbrüggen glycosylation). [However, the slight amount of remaining iodoheterocycle ( 40 ; thus without Boc protecting group) could be efficiently removed by employing a Sonogashira reaction [Pd(Ph 3 P) 2 Cl 2 (0.05 eq.), CuI (0.1 eq.), Et 3 N (0.2 mL/mmol), DMF (10 mL/mmol)] with butyn-1-ol (1 eq.) (reaction time 3H). As such, 42 was obtained as a slightly yellow solid (0.1 g, 0.45 mmol) in 23% yield.] Melting point: 217–218 °C. 1H NMR (300 MHz, CDCl 3) δ: 7.83 (d, J = 0.9 Hz, 1H, H-6), 8.80 (s, 1H, H-2), 10.47 (br. s, 1H, NH). 19F NMR (282 MHz, CDCl 3) δ: −55.98.1H NMR (300 MHz, DMSO‑d 6) δ: 8.42 (br. s, 1H, H-6), 8.77 (s, 1H, H-2), 13.38 (br. s, 1H, NH). 19F NMR (282 MHz, DMSO‑d 6) δ: −53.62 (d, J = 2.0 Hz). 13C NMR (75 MHz, DMSO‑d 6) δ: 102.7 (q, J = 37.8 Hz, 1C, C-5), 112.1 (q, J = 2.4 Hz, 1C, C-4a), 122.7 (q, J = 264.5 Hz, 1C, CF3), 130.6 (q, J = 5.78 Hz, 1C, C-6), 150.0, 151.9 (C-2), 152.9. HRMS (ESI): calculated for C7H4ClF3N3 ([M+H]+): 222.0040, found: 222.0041.
4.2.39. 4-chloro-5-trifluoromethyl-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (43)
43 was prepared according to General Procedure C. 18 (0.550 g, 1.03 mmol) gave rise to 43 (0.309 g) as a slightly yellow foam, containing some impurities. Therefore, 43 was immediately used in the next steps (General Procedure D & General Procedure E).
4.2.40. 4-chloro-6-trifluoromethyl-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (44)
44 was prepared according to General Procedure C. 18 (0.634 g, 1.2 mmol) gave rise to 44 (0.436 g) as a slightly yellow foam, containing some impurities. Therefore, 44 was immediately used in the next step.
4.2.41. 4-azido-6-trifluoromethyl-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (46)
46 was prepared according to General Procedure D. 44 (0.436 g, 0.632 mmol) gave rise to 46 (0.191 g, 0.274 mmol) as a white foam in 43% yield. Purification: 5 → 25% EA/Hexanes. 1H NMR (300 MHz, DMSO‑d 6) δ: 4.19 (s, 1H, ethynyl-H), 4.85 (dd, J = 12, 6.9 Hz, 1H, H-5″), 5.10 (d, J = 12, 4.2 Hz, 1H, H-5′), 5.32 (dd, J = 6.9, 4.5 Hz, 1H, H-4′), 6.58 (d, J = 6.9 Hz, 1H, H-1′), 6.95 (d, J = 7.2 Hz, 1H, H-2′), 7.46–7.56 (m, 6H, OBz), 7.64–7.75 (m, 3H, OBz), 7.87–7.90 (m, 2H, OBz), 7.96–8.06 (m, 5H, OBz, H-5), 10.06 (1H, s, H-2). 19F NMR (282 MHz, DMSO‑d 6) δ: −56.73. HRMS (ESI): calculated for C35H24F3N6O7 ([M+H]+): 697.1653, found: 697.1677.
4.2.42. 4-amino-5-trifluoromethyl-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (47)
47 was prepared according to General Procedure D & General Procedure E. 43 (0.309 g, 0.448 mmol) was transformed into 47 (0.12 g, 0.179 mmol) as a white foam in 21% yield (over 3 steps). Purification: 25 → 65% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.97 (s, 1H, ethynyl-H), 4.87–4.95 (m, 1H, H-5″), 4.98–5.05 (m, 2H, H-4′, H-5′), 5.54 (br. s, 2H, NH2), 6.37 (d, J = 4.8 Hz, 1H, H-2′), 6.75 (d, J = 4.8 Hz, 1H, H-1′), 7.28–7.33 (m, 2H, OBz), 7.40–7.54 (m, 5H, OBz), 7.58–7.64 (m, 2H, OBz), 7.85–7.92 (m, 3H, OBz, H-6), 8.04–8.07 (m, 2H, OBz), 8.14–8.17 (m, 2H, OBz), 8.30 (s, 1H, H-2). 19F NMR (282 MHz, CDCl3) δ: −55.76 (d, J = 2.0 Hz). 13C NMR (75 MHz, CDCl3) δ: 63.7 (C-5′), 76.4, 77.4, 78.7 (C-2′), 79.4, 81.1 (C-4′), 85.8 (C-1′), 99.3 (d, J = 2.3 Hz, 1C, C-4a), 106.9 (q, J = 37.8 Hz, 1C, C-5), 122.7 (q, J = 5.8 Hz, 1C, C-6), 123.2 (q, J = 265.6 Hz, 1C, CF3), 128.3, 128.6, 128.76, 128.80, 129.5, 129.9, 130.1, 133.6, 134.0, 134.1, 152.3 (C-7a), 153.6 (C-2), 156.2 (C-4), 164.3 (C=O), 164.5 (C=O), 166.4 (C=O). HRMS (ESI): calculated for C35H26F3N4O7 ([M+H]+): 671.1748, found: 671.1736.
4.2.43. 4-amino-6-trifluoromethyl-N7-(3′-C-ethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (48)
48 was prepared according to General Procedure E. 46 (0.13 g, 0.201 mmol) gave rise to 48 (0.144 g, 0.215 mmol) as a white foam in 55% yield. Purification: 40 → 65% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.88 (s, 1H, ethynyl-H), 5.09–5.24 (m, 3H, H-4′, H-5′, H-5″), 5.53 (br. s, 2H, NH2), 6.32 (d, J = 7.8 Hz, 1H, H-1′), 6.78 (s, 1H, H-5), 7.38–7.64 (m, 10H, OBz, H-2′), 7.99–8.02 (m, 2H, OBz), 8.10–8.13 (m, 2H, OBz), 8.14–8.17 (m, 2H, OBz), 8.24 (s, 1H, H-2). 19F NMR (282 MHz, CDCl3) δ: −58.34.13C NMR (75 MHz, CDCl3) δ: 64.5 (C-5′), 75.8 (C-2′), 76.3, 77.4, 79.0, 82.5 (C-4′), 86.4 (C-1′), 102.5 (C-4a), 103.3 (q, J = 3.8 Hz, 1C, C-5), 120.8 (q, J = 266.8 Hz, 1C, CF3), 124.5 (q, J = 37.8 Hz, 1C, C-6), 128.5, 128.7, 128.8, 129.6, 129.98, 130.03, 130.1, 133.1, 133.9, 134.0, 153.0 (C-7a), 154.4 (C-2), 157.9 (C-4), 164.3 (C=O), 164.9 (C=O), 166.5 (C=O). HRMS (ESI): calculated for C35H25F3N4O7 ([M+H]+): 671.1748, found: 671.1807.
4.2.44. 4-amino-5-trifluoromethyl-N7-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (49)
49 was prepared according to General Procedure B. 47 (0.12 g, 0.179 mmol) gave rise to 49 (0.051 g, 0.142 mmol) as a white solid in 80% yield. Purification: 0 → 10% MeOH/DCM. Melting point: 207 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.57 (s, 1H, ethynyl-H), 3.63–3.81 (m, 2H, H-5′, H-5″), 3.98 (t, J = 3.3 Hz, 1H, H-4′), 4.65 (t, J = 7.5 Hz, 1H, H-2′), 5.30 (dd, J = 5.4, 4.5 Hz, 1H, OH-5′), 5.84 (d, J = 6.9 Hz, 1H, OH-2′), 5.99 (s, 1H, OH-3′), 6.10 (d, J = 7.5 Hz, 1H, H-1′), 6.63 (br. s, 2H, NH2), 8.21 (d, J = 0.9 Hz, 1H, H-6), 8.23 (s, 1H, H-2). 19F NMR (282 MHz, DMSO‑d 6) δ: −53.83.13C NMR (75 MHz, DMSO‑d 6) δ: 61.7 (C-5′), 72.9, 77.0, 78.4 (C-2′), 82.8, 85.9 (C-1′), 87.3 (C-4′), 98.0 (d, J = 2.3 Hz, 1C, C-4a), 103.6 (q, J = 36.6 Hz, 1C, C-5), 123.4 (q, J = 264.5 Hz, 1C, CF3), 124.5 (q, J = 5.7 Hz, 1C, C-6), 151.7 (C-7a), 153.1 (C-2), 156.3 (C-4). HRMS (ESI): calculated for C14H14F3N4O4 ([M+H]+): 359.0962, found: 359.0962.
4.2.45. 4-amino-6-trifluoromethyl-N7-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (50)
50 was prepared according to General Procedure B. 48 (0.116 g, 0.173 mmol) gave rise to 50 (0.054 g, 0.151 mmol) as a white solid in 87% yield. Purification: 6 → 10% MeOH/DCM. Melting point: 150–152 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.53 (s, 1H, ethynyl-H), 3.68–3.85 (m, 2H, H-5′, H-5″), 4.01 (t, J = 3.0 Hz, 1H, H-4′), 5.35 (t, J = 7.5 Hz, 1H, H-2′), 5.63 (d, J = 8.4 Hz, 1H, H-1′), 5.87 (d, J = 6.6 Hz, 1H, OH-2′), 5.94 (s, 1H, OH-3′), 5.94 (dd, J = 9.0, 2.7 Hz, 1H, OH-5′), 7.33 (s, 1H, H-5), 7.68 (br. s, 2H, NH2), 8.18 (s, 1H, H-2). 19F NMR (282 MHz, DMSO‑d 6) δ: −56.89.13C NMR (75 MHz, DMSO‑d 6) δ: 62.2 (C-5′), 72.6, 75.0 (C-2′), 76.7, 82.9, 88.17 (C-1′), 88.25 (C-4′), 101.9 (C-4a), 104.3 (q, J = 4.7 Hz, 1C, C-5), 120.8 (q, J = 265.7 Hz, 1C, CF3), 122.5 (q, J = 37.8 Hz, 1C, C-6), 151.0 (C-7a), 154.0 (C-2), 159.0 (C-4). HRMS (ESI): calculated for C14H14F3N4O4: 359.0962, found: 359.0977.
4.2.46. 1-O-acetyl-2,3,5-tri-O-benzoyl-3-C-trimethylsilylethynyl-α,β-d-ribofuranose (51)
18 (1.32 g, 2.5 mmol, 1 eq.) was co-evaporated with anhydrous toluene (15 mL) three times. Next, the residue was dissolved in anhydrous toluene (25 mL, 10 mL/mmol SM) and cooled to –65 °C. After stirring at –65 °C for ∼15 min, iPrMgCl.LiCl solution (1.3 M in THF, 2.11 mL, 2.75 mmol, 1.1 eq.) was added dropwise, and the resulting solution stirred at –65 °C for 30 min. Then, TMSCl (0.48 mL, 3.75 mmol, 1.5 eq.) was added in one portion and the cooling removed. The mixture was stirred another 30 min at ambient temperature and aq. sat. NH4Cl solution was added, followed by EA and water. The layers were separated, and the water layer extracted twice more with EA. The organic layers were combined, dried over Na2SO4, filtered and evaporated till dryness. The residue was purified by column chromatography 0 → 20% EA/Hexanes to give 51 (0.894 g, 1.49 mmol) as a sticky foam in 60% yield. (mixture of isomers in ∼1:1.67 ratio (α/β)) 1H NMR (300 MHz, CDCl3) δ: 0.11 (s, 9H, TMS-CH3,α), 0.18 (s, 9H, TMS-CH3,β), 1.98 (s, 3H, OAcα), 2.14 (s, 3H, OAcβ), 4.74–5.03 (m, 2 × 3H, H-4, H-5, H-5′); 6.05 (d, J = 4.5 Hz, 1H, H-2α), 6.15 (d, J = 1.5 Hz, 1H, H-2β), 6.35 (d, J = 1.2 Hz, 1H, H-1β), 6.74 (d, J = 4.2 Hz, 1H, H-1α), 7.89–7.62 (m, 2 × 9H, OBz), 7.91–8.17 (m, 2 × 6H, OBz). HRMS (ESI): calculated for C31H29O7Si ([M-OAc]+): 541.1677, found: 541.1682.
4.2.47. 4-chloro-5-iodo-N7-(3′-C-trimethylsilylethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (52)
52 was prepared according to General Procedure C. 51 (0.85 g, 1.42 mmol) gave rise to 52 (0.344 g, 0.42 mmol) as a slightly yellow foam, containing minor impurities. Yield: 30%. Purification: 12.5% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 0.27 (s, 9H, TMS-CH3), 4.83 (dd, J = 11.4, 6.3 Hz, 1H, H-5″), 4.88–4.91 (m, 1H, H-4′), 5.03 (dd, J = 11.4, 3.0 Hz, 1H, H-5′), 6.25 (d, J = 3.3 Hz, 1H, H-2′), 6.70 (d, J = 3.9 Hz, 1H, H-1′), 7.22–7.28 (m, 2H, OBz), 7.40–7.51 (m, 5H, OBz), 7.57–7.63 (m, 2H, OBz), 7.80–7.83 (m, 2H, OBz), 8.00 (s, 1H, H-6), 8.01–8.06 (m, 2H, OBz), 8.12–8.17 (m, 2H, OBz), 8.58 (s, 1H, H-2). HRMS (ESI): calculated for C37H32IN4O7Si ([M+H]+): 820.0737, found: 820.0780.
4.2.48. 4-chloro-5-trimethylsilylethynyl-N7-(3′-C-trimethylsilylethynyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (53)
In a flame-dried 10 mL round bottom flask under argon was added: 52 (0.33 g, 0.402 mmol, 1 eq.), CuI (0.008 g, 0.0402 mmol, 0.1 eq.) and Pd(Ph3P)2Cl2 (0.014 g, 0.0201 mmol, 0.05 eq.). The flask was evacuated and refilled with argon, three times. Then, anhydrous degassed DMF (2 mL, 4 mL/mmol SM) was added, followed by degassed Et3N (0.16 mL, 0.4 mL/mmol SM) and ethynyltrimethylsilane (0.57 mL, 4.02 mmol, 10 eq.). Then, the mixture was stirred at ambient temperature overnight and subsequently evaporated till dryness. The residue was purified by column chromatography (0 → 25% EA/Hexanes; three sequences), to give 53 (0.125 g, 0.158 mmol) as a yellowish foam in 39% yield. 1H NMR (300 MHz, CDCl3) δ: 0.26 (s, 9H, TMS-CH3), 0.29 (s, 9H, TMS-CH3), 4.78–4.90 (m, 2H, H-4′, H-5″), 5.04 (dd, J = 11.4, 3.0 Hz, 1H, H-5′), 6.20 (d, J = 3.3 Hz, 1H, H-2′), 6.69 (d, J = 3.3 Hz, 1H, H-1′), 7.19–7.24 (m, 2H, OBz), 7.39–7.51 (m, 5H, OBz), 7.56–7.63 (m, 2H, OBz), 7.78–7.81 (m, 2H, OBz), 8.00–8.17 (m, 6H, OBz), 8.13 (s, 1H, H-6), 8.60 (s, 1H, H-2). HRMS (ESI): calculated for C42H41ClN3O7Si2 ([M+H]+): 790.2166, found: 790.2181.
4.2.49. 4-amino-5-ethynyl-N7-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (54)
54 was prepared by a sequential combination of General Procedure D, E and B. As such, 53 (0.125 g, 0.158 mmol) gave rise to 54 (0.023 g, 0.072 mmol) as a white solid in 46% yield. Purification: 0 → 10% MeOH/DCM. Melting point: 195 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 3.57 (s, 1H, ethynyl-Hpurine), 3.63–3.78 (m, 2H, H-5′, H-5″), 3.94 (t, J = 3.3 Hz, 1H, H-4′), 4.30 (s, 1H, ethynyl-Hribo), 4.59 (t, J = 7.5 Hz, 1H, H-2′), 5.34 (dd, J = 6.0, 4.8 Hz, 1H, OH-5′), 5.83 (d, J = 7.2 Hz, 1H, OH-2′), 5.97 (s, 1H, OH-3′), 5.99 (d, J = 7.8 Hz, 1H, H-1′), 6.74 (br. s, 2H, NH2), 7.85 (s, 1H, H-6), 8.12 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.8 (C-5′), 72.8 (C-3′), 76.9, 77.1, 78.2 (C-2′), 82.9, 83.2, 86.1 (C-1′), 87.1 (C-4′), 94.2 (C-5), 102.4 (C-4a), 127.8 (C-6), 149.8 (C-7a), 152.8 (C-2), 157.6 (C-4). HRMS (ESI): calculated for C15H15N4O4 ([M+H]+): 315.1088, found: 315.1096.
4.2.50. 1-O-acetyl-2,3,5-tri-O-benzoyl-3-C-ethyl-α,β-d-ribofuranose (55)
18 (1.47 g, 2.78 mmol) was dissolved in EA (15 mL, 5 mL/mmol SM) under a nitrogen atmosphere. Then, a catalytic amount of Pd/C was added, and the reaction mixture was stirred under a hydrogen atmosphere (balloon) for approximately 7 h. Then, the mixture was flushed with nitrogen and filtered over a short pad of Celite®. The filtrate was evaporated till dryness and purified by column chromatography 15% EA/Hexanes to give 55 (1.37, 2.57 mmol) as white foam in 92% yield. 1H NMR (300 MHz, CDCl3) δ: 1.04 (t, J = 7.8 Hz, 3H, CH3β), 1.26 (t, J = 7.2 Hz, 3H, CH3α), 1.91–2.06 (m, 1H, CH2α), 1.97 (s, 3H, OAcα), 2.03 (s, 3H, OAcβ), 2.16–2.28 (m, 1H, CH2β), 2.64–2.76 (m, 1H, CH2β), 2.78–2.91 (m, 1H, CH2α), 4.60 (dd, J = 12.3, 4.8 Hz, 1H, H-5′α), 4.63 (dd, J = 12.0, 6.0 Hz, 1H, H-5′β), 4.82 (dd, J = 12.3, 3.6 Hz, 1H, H-5α), 4.99 (dd, J = 12.0, 3.6 Hz, 1H, H-5β), 5.08 (dd, J = 6.3, 3.6 Hz, 1H, H-4β), 5.18 (dd, J = 4.2, 3.6 Hz, 1H, H-4α), 5.64 (d, J = 4.5 Hz, 1H, H-2α), 5.96 (d, J = 2.4 Hz, 1H, H-2β), 6.40 (d, J = 2.4 Hz, 1H, H-1β), 6.69 (d, J = 4.8 Hz, 1H, H-1α), 7.32–7.65 (m, 2 × 9H, OBz), 7.94–8.19 (m, 2 × 6H, OBz). HRMS (ESI): calculated for C28H25O7 ([M-OAc]+): 473.1595, found: 473.1606.
4.2.51. 4,5-dichloro-N7-(3′-C-ethyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (56)
56 was prepared according to General Procedure C. 55 (0.558 g, 1.05 mmol) gave rise to 56 (0.471 g) as a slightly yellow foam, containing minor impurities. Purification: 14% EA/Hexanes.
4.2.52. 4-azido-5-chloro-N7-(3′-C-ethyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (57)
57 was prepared according to General Procedure D. 56 (0.471 g, 0.71 mmol) gave rise to 57 (0.387 g, 0.581 mmol) as a white foam. Yield = 55%. Purification: 20 → 25% EA/Hexanes. 1H NMR (300 MHz, DMSO‑d 6) δ: 0.91 (t, J = 7.2 Hz, 3H, CH3), 2.31–2.43 (m, 1H, CH2), 2.66–2.78 (m, 1H, CH2), 4.79 (dd, J = 12.0, 6.6 Hz, 1H, H-5″), 4.96 (dd, J = 12.3, 4.2 Hz, 1H, H-5′), 5.28 (dd, J = 6.6, 4.2 Hz, 1H, H-4′), 6.38 (d, J = 7.2 Hz, 1H, H-2′), 6.86 (d, J = 7.2 Hz, 1H, H-1′), 7.45–7.79 (m, 9H, OBz), 7.89–7.92 (m, 2H, OBz), 8.01–8.04 (m, 2H, OBz), 8.14–8.17 (m, 2H, OBz), 8.35 (s, 1H, H-6), 9.90 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 7.4 (CH3), 24.3 (CH2), 63.5 (C-5′), 77.8 (C-2′), 81.8 (C-4′), 84.8 (C-1′), 86.5 (C-3′), 101.1 (C-4a), 106.0 (C-5), 122.9 (C-6), 128.1, 128.9, 128.96, 129.04, 129.2, 129.3, 129.4, 129.6, 133.6, 134.0, 134.2, 135.3 (C-2), 140.5 (C-7a), 145.1 (C-4), 164.3 (C=O), 164.6 (C=O), 165.4 (C=O). HRMS (ESI): calculated for C34H27ClN6O7 ([M+H]+): 666.1630, found: 667.1703.
4.2.53. 4-amino-5-chloro-N7-(3′-C-ethyl-2′,3′,5′-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (58)
58 was prepared according to General Procedure E. 57 (0.374 g, 0.561 mmol) gave rise to 58 (0.32 g, 0.497 mmol) as a white foam. Yield = 88%. Purification: 40 → 75% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 0.95 (t, J = 7.5 Hz, 3H, CH3), 2.16–2.28 (m, 1H, CH2), 2.82–2.95 (m, 1H, CH2), 4.80 (dd, J = 12.6, 3.9 Hz, 1H, H-5″), 4.94 (dd, J = 12.3, 3.9 Hz, 1H, H-5′), 5.24 (t, J = 3.6 Hz, 1H, H-4′), 5.64 (br. s, 2H, NH2), 6.29 (d, J = 7.8 Hz, 1H, H-2′), 6.72 (d, J = 7.5 Hz, 1H, H-1′), 7.13 (s, 1H, H-6), 7.40–7.68 (m, 9H, OBz), 8.03–8.06 (m, 2H, OBz), 8.15 (s, 1H, H-2), 8.17–8.21 (m, 4H, OBz). HRMS (ESI): calculated for C34H30ClN4O7 ([M+H]+): 641.1798, found: 641.1795.
4.2.54. 4-amino-5-chloro-N7-(3′-C-ethyl-β-d-ribofuranosyl)-pyrrolo[2,3-d]pyrimidine (59)
59 was prepared according to General Procedure B. 58 (0.3 g, 0.468 mmol) gave rise to 59 (0.126 g, 0.383 mmol) as a white solid in 87% yield. Purification: precipitation from MeOH. Melting point: 272 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 0.96 (t, J = 7.2 Hz, 3H, CH3), 1.57–1.75 (m, 2H, CH2), 3.50–3.62 (m, 2H, H-5′, H-5″), 3.84 (t, J = 2.7 Hz, 1H, H-4′), 4.29 (t, J = 7.4 Hz, 1H, H-2′), 4.51 (s, 1H, OH-3′), 5.26 (d, J = 6.9 Hz, 1H, OH-2′), 5.42 (t, J = 4.8 Hz, 1H, OH-5′), 6.02 (d, J = 7.8 Hz, 1H, H-1′), 6.86 (br. s, 2H, NH2), 7.64 (s, 1H, H-6), 8.08 (s, 1H, H-2). 13C NMR (75 MHz, DMSO‑d 6) δ: 7.8 (CH3), 25.7 (CH2), 61.2 (C-5′), 77.3 (C-2′), 78.2 (C-3′), 86.4 (C-1′), 87.0 (C-4′), 100.0 (C-4a), 102.4 (C-5), 120.0 (C-6), 149.4 (C-7a), 152.5 (C-2), 156.8 (C-4). HRMS (ESI): calculated for C13H18ClN3O4: 329.1011, found: 329.1034.
4.2.55. 3,4-dichloro-1H-pyrrolo[2,3-b]pyridine (60)
1H-4-chloro-pyrrolo[2,3-b]pyridine (0.763 g, 5.0 mmol, 1 eq.) was dissolved in DMF (7.5 mL, 1.5 mL/mmol SM) and NCS (0.701 g, 5.25 mmol, 1.05 eq.) was added. The resulting mixture was stirred at ambient temperature overnight, protected from light. Then, ice-cold water (25 mL, 5 mL/mmol SM) was added and the resulting precipitate filtered. The solids were washed four additional times with ice-cold water (4 × 10 mL, 2 mL/mmol SM). The solid was collected and dried under high vacuum to give 60 (0.861 g, 4.6 mmol) as an off-white solid in 92% yield. Melting point: 236 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 7.21 (d, J = 4.2 Hz, 1H, H-5), 7.77 (s, 1H, H-2), 8.20 (d, J = 4.2 Hz, 1H, H-6), 12.35 (br. s, 1H, NH). 13C NMR (75 MHz, DMSO‑d 6) δ: 101.1 (C-3), 113.7 (C-3a), 117.1 (C-5), 125.1 (C-2), 133.8 (C-4), 144.4 (C-6), 147.6 (7a). HRMS (ESI): calculated for C7H5Cl2N2 ([M+H]+): 186.9824, found: 186.9824.
4.2.56. 3-bromo-4-chloro-1H-pyrrolo[2,3-b]pyridine (61)
61 was prepared as has been described for 60, except for the use of NBS instead of NCS. 1H-4-chloro-pyrrolo[2,3-b]pyridine (0.763 g, 5 mmol) gave rise to 61 (1.12 g, 4.8 mmol) as a yellow solid in 96% yield. Melting point: 210 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 7.23 (d, J = 5.1 Hz, 1H, H-5), 7.81 (d, J = 2.7 Hz, 1H, H-2), 8.21 (d, J = 5.1 Hz, 1H, H-6), 12.44 (br. s, 1H, NH). 13C NMR (75 MHz, DMSO‑d 6) δ: 85.0 (C-3), 114.6 (C-3a), 117.1 (C-5), 127.7 (C-2), 134.2 (C-4), 144.2 (C-6), 148.0 (C-7a). HRMS (ESI): calculated for C7H5BrClN2 ([M+H]+): 230.9319, found: 230.9332.
4.2.57. 3-iodo-4-chloro-1H-pyrrolo[2,3-b]pyridine (62)
62 was prepared as has been described for 60, except for the use of NIS instead of NCS. 1H-4-chloro-pyrrolo[2,3-b]pyridine (0.763 g, 5 mmol, 1 eq.) gave rise to 62 (1.29 g, 4.6 mmol) as a yellow solid in 92% yield. Melting point: 222 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 7.19 (d, J = 5.1 Hz, 1H, H-5), 7.81 (d, J = 2.4 Hz, 1H, H-2), 8.18 (d, J = 5.1 Hz, 1H, H-6), 12.45 (br. s, 1H, NH). 13C NMR (75 MHz, DMSO‑d 6) δ: 49.7 (C-3), 116.3 (C-3a), 116.9 (C-5), 133.1 (C-2), 134.8 (C-5), 143.7 (C-6), 148.4 (C-7a). HRMS (ESI): calculated for C7H5ClIN2 ([M+H]+): 278.9180, found: 278.9197.
4.2.58. 3,4-dichloro-N1-(3′-C-ethynyl-2′,3′-5-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (63)
63 was prepared according to General Procedure C. 18 (1.11 g, 2.1 mmol) gave rise to 63 (0.519 g, 0.792 mmol) as a slight yellow foam in 39% yield. Reaction time: 2.5 h. Purification: 12 → 15% EA/cHex. 1H NMR (300 MHz, CDCl3) δ: 2.98 (s, 1H, ethynyl-H), 4.89 (dd, J = 11.1, 5.4 Hz, 1H, H-5″), 4.96 (dd, J = 5.1, 3.3 Hz, 1H, H-4′), 5.03 (dd, J = 11.1, 3.3 Hz, 1H, H-5′), 6.39 (d, J = 5.1 Hz, H-2′), 6.85 (d, J = 5.1 Hz, 1H, H-1′), 7.11 (d, J = 5.1 Hz, 1H, H-5), 7.27–7.31 (m, 2H, OBz), 7.40–7.51 (m, 5H, OBz), 7.58–7.63 (m, 2H, OBz), 7.66 (s, 1H, H-2), 7.87–7.90 (m, 2H, OBz), 8.03–8.07 (m, 2H, OBz), 8.14 (d, J = 5.1 Hz, 1H, H-6), 8.15–8.18 (m, 2H, OBz). 13C NMR (75 MHz, CDCl3) δ: 63.8 (C-5′), 76.4, 76.9, 78.4 (C-2′), 79.3, 80.8 (C-4′), 85.8 (C-1′), 106.8 (C-3), 116.1 (C-3a), 119.0 (C-5), 123.1 (C-2), 128.5, 128.5, 128.7, 128.9, 129.7, 129.96, 130.06, 130.09, 133.5, 133.9, 134.0, 136.7 (C-4), 144.6 (C-6), 147.6 (C-7a), 164.3 (C=O), 164.5 (C=O), 166.4 (C=O). HRMS (ESI): calculated for C35H25Cl2N2O7 ([M+H]+): 655.1033, found: 655.1045.
4.2.59. 3-bromo-4-chloro-N1-(3′-C-ethynyl-2′,3′-5-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (64)
64 was prepared according to General Procedure C. 18 (0.666 g, 1.26 mmol) gave rise to 64 (0.275 g, 0.392 mmol) as a slight yellow foam in 32% yield. Reaction time: 3h. Purification: 15% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.98 (s, 1H, ethynyl-H), 4.90 (dd, J = 11.1, 4.5 Hz, 1H, H-5″), 4.96 (dd, J = 5.1, 3.3 Hz, 1H, H-4′), 5.03 (dd, J = 11.1, 3.3 Hz, 1H, H-5′), 6.40 (d, J = 5.1 Hz, 1H, H-2′), 6.85 (d, J = 5.1 Hz, 1H, H-1′), 7.12 (d, J = 5.1 Hz, 1H, H-5), 7.27–7.32 (m, 2H, OBz), 7.40–7.52 (m, 5H, OBz), 7.59–7.64 (m, 2H, OBz), 7.74 (s, 1H, H-2), 7.87–7.90 (m, 2H, OBz), 8.03–8.07 (m, 2H, OBz), 8.13 (d, J = 5.1 Hz, 1H, H-6), 8.15–8.18 (m, 2H, OBz). 13C NMR (75 MHz, CDCl3) δ: 63.8 (C-5′), 76.4, 76.9, 78.5 (C-2′), 79.3, 80.8 (C-4′), 85.9 (C-1′), 90.4 (C-3), 117.0 (C-3a), 119.0 (C-5), 125.9 (C-2), 128.47, 128.54, 128.7, 128.9, 129.7, 130.0, 130.1, 133.6, 133.9, 134.0, 137.2 (C-4), 144.3 (C-6), 147.8 (C-7a), 164.3 (C=O), 164.5 (C=O), 166.4 (C=O). HRMS (ESI): calculated for C35H25BrClN2O7 ([M+H]+): 699.0528, found: 699.0550.
4.2.60. 3-iodo-4-chloro-N1-(3′-C-ethynyl-2′,3′-5-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (65)
65 was prepared according to General Procedure C. 18 (0.666 g, 1.26 mmol) gave rise to 65 (0.354 g, 0.474 mmol) as a slight yellow foam in 39% yield. Purification: 15% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.98 (s, 1H, ethynyl-H), 4.89 (dd, J = 11.1, 4.8 Hz, 1H, H-5″), 4.97 (dd, J = 4.8, 3.6 Hz, 1H, H-4′), 5.02 (dd, J = 11.1, 3.3 Hz, 1H, H-5′), 6.42 (d, J = 5.1 Hz, 1H, H-2′), 6.83 (d, J = 5.1 Hz, 1H, H-1′), 7.11 (d, J = 5.1 Hz, 1H, H-5), 7.26–7.52 (m, 2H, OBz), 7.40–7.52 (m, 5H, OBz), 7.58–7.63 (m, 2H, OBz), 7.84 (s, 1H, H-2), 7.87–7.91 (m, 2H, OBz), 8.03–8.07 (m, 2H, OBz), 8.13 (d, J = 5.1 Hz, 1H, H-6), 8.15–8.18 (m, 2H, OBz). 13C NMR (75 MHz, CDCl3) δ: 53.2 (C-3), 63.8 (C-5′), 76.5, 76.8, 78.5 (C-2′), 79.3, 80.9 (C-4′), 85.9 (C-1′), 118.5 (C-3a), 119.0 (C-5), 128.5, 128.6, 128.76, 128.82, 128.9, 129.7, 130.0, 130.1, 131.6 (C-2), 133.6, 133.9, 134.0, 137.8 (C-4), 144.0 (C-6), 147.9 (C-7a), 164.4 (C=O), 164.5 (C=O), 166.4 (C=O). HRMS (ESI): calculated for C35H25IClN2O7 ([M+H]+): 747.0389, found: 747.0412.
4.2.61. 3,4-dichloro-N1-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (66)
66 was prepared according to General Procedure B. 63 (0.32 g, 0.494 mmol) gave rise to 66 (0.130 g, 0.379 mmol) as a white solid in 77% yield. Purification: 0 → 5% MeOH/DCM. Melting point: 86–88 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.58 (s, 1H, ethynyl-H), 3.65–3.80 (m, 2H, H-5′, H-5″), 3.96 (dd, J = 4.2, 3.0 Hz, 1H, H-4′), 4.60 (t, J = 7.2 Hz, 1H, H-2′), 5.13 (t, J = 4.8 Hz, 1H, OH-5′), 5.82 (d, J = 6.9 Hz, 1H, OH-2′), 5.99 (s, 1H, OH-3′), 6.27 (d, J = 7.5 Hz, 1H, H-1′), 7.35 (d, J = 5.1 Hz, 1H, H-5), 8.13 (s, 1H, H-2), 8.29 (d, J = 5.1 Hz, 1H, H-6). 13C NMR (75 MHz, DMSO‑d 6) δ: 63.8 (C-5′), 72.8, 77.0, 78.5 (C-2′), 82.9, 85.4 (C-1′), 87.0 (C-4′), 102.7 (C-3), 114.5 (C-3a), 118.2 (C-5), 125.2 (C-2), 134.4 (C-4), 144.6 (C-6), 147.3 (C-7a). HRMS (ESI): calculated for C14H12Cl2N2O4: 343.0247, found: 343.0259.
4.2.62. 3-bromo-4-chloro-N1-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (67)
67 was prepared according to General Procedure B. 64 (0.09 g, 0.129 mmol) gave rise to 67 (0.038 g, 0.098 mmol) as a white solid in 76% yield. Purification: 0 → 5% MeOH/DCM. Melting point: 102–104 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.58 (s, 1H, ethynyl-H), 3.66–3.80 (m, 2H, H-5′, H-5″), 3.96 (dd, J = 3.9, 3.3 Hz, 1H, H-4′), 4.61 (t, J = 7.5 Hz, 1H, H-2′), 5.20 (t, J = 4.8 Hz, 1H, OH-5′), 5.83 (d, J = 7.2 Hz, 1H, OH-2′), 5.99 (s, 1H, OH-3′), 6.27 (d, J = 7.5 Hz, 1H, H-1′), 7.34 (d, J = 5.4 Hz, 1H, H-5), 8.16 (s, 1H, H-2), 8.27 (d, J = 5.1 Hz, 1H, H-6). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.8 (C-5′), 72.8, 77.0, 78.5 (C-2′), 82.9, 85.5 (C-1′), 86.8 (C-3), 87.0 (C-4′), 115.4 (C-3a), 118.3 (C-5), 127.8 (C-2), 134.8 (C-4), 144.4 (C-6), 147.6 (C-7a). HRMS (ESI): calculated for C14H13BrClN2O4: 386.9742, found: 386.9756.
4.2.63. 3-iodo-4-chloro-N1-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (68)
68 was prepared according to General Procedure B. 65 (0.10 g, 0.134 mmol) gave rise to 68 (0.040 g, 0.092 mmol) as a white solid in 70% yield. Purification: 0 → 5% MeOH/DCM. Melting point: 176–178 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.57 (s, 1H, ethynyl-H), 3.65–3.80 (m, 2H, H-5′, H-5″), 3.96 (dd, J = 3.9, 3.0 Hz, 1H, H-4′), 4.61 (t, J = 7.5 Hz, 1H, 2′-H), 5.14 (t, J = 4.8 Hz, 1H, OH-5′), 5.81 (d, J = 7.2 Hz, 1H, OH-2′), 5.97 (s, 1H, OH-3′), 6.24 (d, J = 7.8 Hz, 1H, H-1′), 7.30 (d, J = 5.1 Hz, 1H, H-5), 8.17 (s, 1H, H-2), 8.24 (d, J = 5.1 Hz, 1H, H-6). 13C NMR (75 MHz, DMSO‑d 6) δ: 51.9 (C-3), 61.8 (C-5′), 72.8, 77.0, 78.5 (C-2′), 82.9, 85.5 (C-1′), 87.0 (C-4′), 117.3 (C-4a), 118.1 (C-5), 133.2 (C-2), 135.5 (C-4), 143.9 (C-6), 148.0 (C-7a). HRMS (ESI): calculated for C14H13IClN2O4: 434.9603, found: 434.9628. Purity: 91%.
4.2.64. 4-chloro-N1-(3′-C-ethynyl-2′,3′-5-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (69)
69 was prepared as described for 35.65 (0.1 g, 0.134 mmol) gave rise to 69 (0.062 g, 0.0998 mmol) as a foam in 75% yield. 1H NMR (300 MHz, CDCl3) δ: 2.95 (s, 1H, ethynyl-H), 4.89 (dd, J = 10.8, 4.8 Hz, 1H, H-5″), 4.94–5.05 (m, 2H, H-4′, H-5′), 6.48 (d, J = 5.1 Hz, 1H, H-2′), 6.68 (d, J = 3.9 Hz, 1H, H-3), 6.87 (d, J = 5.1 Hz, 1H, H-1′), 7.12 (d, J = 5.4 Hz, 1H, H-5), 7.26–7.31 (m, 2H, OBz), 7.40–7.52 (m, 5H, OBz), 7.57–7.64 (m, 2H, OBz), 7.71 (d, J = 3.9 Hz, 1H, H-6), 7.88–7.91 (m, 2H, OBz), 8.05–8.08 (m, 2H, OBz), 8.14–8.18 (m, 2H, OBz). 13C NMR (75 MHz, CDCl3) δ: 64.0 (C-5′), 76.5, 77.0, 78.5 (C-2′), 79.1, 80.72 (C-4′), 85.9 (C-1′), 101.7 (C-3), 117.4 (C-5), 120.8 (C-3a), 125.7 (C-2), 128.5, 128.6, 128.71, 128.74, 129.0, 129.8, 130.0, 130.09, 130.12, 133.5, 133.8, 134.0, 136.9 (C-4), 143.4 (C-6), 148.5 (C-7a), 164.4 (C=O), 164.6 (C=O), 166.4 (C=O). HRMS (ESI): calculated for C35H26ClN2O7 ([M+H]+): 621.1423, found: 621.1402.
4.2.65. 4-chloro-N1-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (70)
70 was prepared according to General Procedure B. 69 (0.06 g, 0.0967 mmol) gave rise to 70 (0.024 g, 0.078 mmol) as a white solid in 80% yield. Purification: 0 → 8% MeOH/DCM. Melting point: 162 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.57 (s, 1H, ethynyl-H), 3.65–3.79 (m, 2H, H-5′, H-5″), 3.96 (t, J = 3.6 Hz, 1H, H-4′), 4.64 (t, J = 7.2 Hz, 1H, H-2′), 5.16 (t, J = 5.1 Hz, 1H, OH-5′), 5.81 (d, J = 6.9 Hz, 1H, OH-2′), 5.96 (s, 1H, OH-3′), 6.22 (d, J = 7.5 Hz, 1H, H-1′), 6.65 (d, J = 3.6 Hz, 1H, H-3), 7.30 (d, J = 5.1 Hz, 1H, H-5), 7.95 (d, J = 3.6 Hz, 1H, H-2), 8.23 (d, J = 5.1 Hz, 1H, H-6). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.9 (C-5′), 72.8, 76.9, 78.4 (C-2′), 83.1, 86.1 (C-1′), 86.7 (C-4′), 98.9 (C-3), 116.4 (C-5), 119.6 (C-3a), 128.1 (C-2), 134.5 (C-4), 143.3 (C-6), 1483.4 (C-7a). HRMS (ESI): calculated for C14H14ClN2O4([M+H]+): 309.0637, found: 309.0644.
4.2.66. 4-azido-1H-pyrrolo[2,3-b]pyridine (71)42
[ Caution: this reaction employs large amounts of sodium azide in combination with mild acid as well as substantial heating, and can therefore be considered explosive (HN 3 )! No accidents have occurred when performing this reaction (>10 runs), however reaction scale has never exceeded 10 mmol of heterocycle SM. Additional protection by means of a blast shield, and closed fume hood is strongly recommended] 4-chloro-1H-pyrrolo[2,3-b]pyridine (0.765 g, 5 mmol, 1 eq.) was dissolved in DMF (15 mL, 3 mL/mmol SM), and NH4Cl (1.34 g, 25 mmol, 5 eq.) was added, followed by NaN3 (1.63 g, 25 mmol, 5 eq.). the mixture was heated to 110 °C behind a blast shield. After 7 h, the mixture was allowed to cool to ambient temperature, diluted with EA, and poured into half-sat. aq. NaHCO3 solution. The layers were separated, and the water layer washed twice with EA. The organic layers were combined, dried over Na2SO4, filtered and evaporated till dryness. The residue was purified by column chromatography (30% EA/PET) to give 71 (0.53 g, 3.32 mmol) as a white solid in 66% yield. Melting point: 180 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 6.46 (dd, J = 3.3, 1.8 Hz, 1H, H-3), 6.88 (d, J = 5.4 Hz, 1H, H-5), 7.46 (dd, J = 3.6, 2.4 Hz, 1H, H-2), 8.18 (d, J = 5.1 Hz, 1H, H-6), 11.86 (br. s, 1H, NH). 13C NMR (75 MHz, DMSO‑d 6) δ: 96.6 (C-3), 105.0 (C-5), 112.0 (C-3a), 125.9 (C-2), 139.5 (C-7a), 143.7 (C-6), 149.9 (C-4). HRMS (ESI): calculated for C7H6N5 ([M+H]+): 160.0618, found: 160.0585.
4.2.67. 3-chloro-4-azido-1H-pyrrolo[2,3-b]pyridine (72)
[ Caution: See note for 71 with regard to additional safety measures when performing this reaction!] 60 (0.53 g, 2.83 mmol, 1 eq.) and NH4Cl (0.62 g, 14.17 mmol, 5 eq.) were suspended in DMF (10 mL, 3 mL/mmol SM). Then, NaN3 (0.92 g, 14.17 mmol, 5 eq.) was added and the resulting mixture heated at 110 °C for 6 h behind a blast shield. After cooling to ambient temperature, the mixture was diluted with EA, and poured in to half-saturated aq. NaHCO3 solution. The layers were separated, and the water layer extracted twice with EA. The organic layers were combined, dried over Na2SO4, filtered and evaporated. The residue was purified by column chromatography 30% EA/Hexanes to give 72 (0.39 g, 2.01 mmol) as a grey powder in 71% yield. Melting point: 205 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 7.05 (d, J = 5.4 Hz, 1H, H-5), 7.60 (d, J = 1.8 Hz, 1H, H-2), 8.24 (d, J = 5.4 Hz, 1H, H-6), 12.10 (br. s, 1H, NH). 13C NMR (75 MHz, DMSO‑d 6) δ: 100.6 (C-3), 106.0 (C-5), 108.1 (C-3a), 123.6 (C-2), 140.3 (C-4), 144.9 (C-6), 148.24 (C-7a). HRMS (ESI): calculated for C7H5ClN5 ([M+H]+): 194.0228, found: 194.0210.
4.2.68. 3-iodo-4-azido-1H-pyrrolo[2,3-b]pyridine (73)
71 (0.32 g, 2 mmol, 1 eq.) was dissolved in DMF (3 mL, 1.5 mL/mmol SM) and NIS (0.472 g, 2.1 mmol, 1.05 eq.) was added. The mixture was stirred in the dark overnight. Then, ice-cold water (10 mL, 5 mL/mmol SM) was added and the resulting precipitate filtered. The solids were washed four additional times with ice-cold water (2 mL, 1 mL/mmol SM). The solid was collected and dried under high vacuum to give 73 (0.527 g, 1.85 mmol) as a yellow solid in 93% yield. Melting point: 192 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 7.03 (d, J = 5.1 Hz, 1H, H-5), 7.64 (d, J = 2.4 Hz, 1H, H-2), 8.22 (d, J = 5.4 Hz, 1H, H-6), 12.20 (br. s, 1H, NH). 13C NMR (75 MHz, DMSO‑d 6) δ: 48.5 (C-3), 105.8 (C-5), 111.4 (C-3a), 131.40 (C-2), 140.4 (C-7a), 144.4 (C-6), 149.3 (C-4). HRMS (ESI): calculated for C7H5IN5 ([M+H]+): 285.9584, found: 285.9573.
4.2.69. 3-chloro-4-amino-N1-(3′-C-ethynyl-2′,3′-5-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (76) & 3-chloro-4-amino-N7-(3′-C-ethynyl-2′,3′-5-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (77)
76 and 77 were prepared by employing General Procedure C (Reaction time: 3H) and General Procedure E. As such, 18 (0.805 g, 1.52 mmol) gave rise to azido nucleosides [General Procedure C] 74 (0.242 g, 0.366 mmol; Rf = 0.27, 25% EA/hexanes) and a lower running isomer 75 (0.213 g, 0.35 mmol; Rf = 0.19, 25% EA/hexanes) in 24% and 23% yield, respectively (both containing some impurities). Purification: 18 → 25% EA/hexanes. Reaction time: 3H. [Remark: The upper-running fraction, containing 74, has the same Rf on TLC in a variety of solvent systems but can be identified via staining with p-anisaldehyde/sulfuric acid spray, which gives a characteristic reddish colour for 74.]
Next, both isomeric fractions were subjected to General Procedure E.
4.2.70. N-1 isomer
74 (0.242 g, 0.366 mmol) gave rise to 76 (0.192 g, 0.302 mmol) as a yellowish foam in 82% yield. Purification: 0 → 40% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.95 (s, 1H, ethynyl-H), 4.86 (dd, J = 11.1, 5.4 Hz, 1H, H-5″), 4.92–5.00 (br. s, 3H, NH2, H-4′), 5.01 (dd, J = 11.1, 3.3 Hz, 1H, H-5′), 6.21 (d, J = 5.7 Hz, 1H, H-5), 6.40 (d, J = 5.4 Hz, 1H, H-2′), 6.83 (d, J = 5.1 Hz, 1H, H-1′), 7.25–7.34 (m, 2H, OBz), 7.35 (s, 1H, H-2), 7.38–7.50 (m, 5H, OBz), 7.56–7.62 (m, 2H, OBz), 7.88–7.92 (m, 3H, OBz, H-6), 8.02–8.05 (m, 2H, OBz), 8.15–8.18 (m, 2H, OBz). 13C NMR (300 MHz, CDCl3) δ: 63.9 (C-5′), 76.4, 77.1, 78.2 (C-2′), 79.0, 80.5 (C-4′), 85.2 (C-1′), 102.8 (C-5), 105.3 (C-3a), 105.8 (C-3), 118.3 (C-2), 128.5, 128.6, 128.7, 129.0, 129.8, 130.0, 130.1, 133.5, 133.7, 133.9, 145.7 (C-6), 148.1 (C-7a), 164.4 (C=O), 164.6 (C=O), 166.4 (C=O). HRMS (ESI): calculated for C35H27ClN3O7 ([M+H]+): 636.1532, found: 636.1587. [Remark: 1C, namely C-4 could not be detected].
4.2.71. N-7 isomer
75 (0.213 g, 0.322 mmol) gave rise to 77 (0.115 g, 0.181 mmol) as a yellow foam in 56% yield. Purification: 25 → 75% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.91 (s, 1H, ethynyl-H), 4.94–5.06 (m, 3H, H-4′, H-5′, H-5″), 5.93 (br. s, 2H, NH2), 6.13 (d, J = 7.2 Hz, 1H, H-5), 6.29 (d, J = 4.8 Hz, 1H, H-2′), 7.17 (s, 1H, H-2), 7.23 (d, J = 4.8 Hz, 1H, H-1′), 7.25–7.31 (m, 2H, OBz), 7.37–7.51 (m, 5H, OBz), 7.55–7.62 (m, 2H, OBz), 7.89–7.93 (m, 3H, OBz, H-6), 8.00–8.03 (m, 2H, OBz), 8.12–8.16 (m, 2H, OBz). 13C NMR (300 MHz, CDCl3) δ: 63.8 (C-5′), 76.2, 76.8, 78.9 (C-2′), 79.6, 81.4 (C-4′), 88.3 (C-1′), 99.0 (C-5), 101.3 (C-3), 106.8 (C-3a), 128.38, 128.42, 128.67, 128.70, 129.1 (C-6), 129.6, 129.9, 130.06, 130.09, 133.5, 133.8, 134.0, 134.3 (C-2), 145.7 (C-7a), 150.9 (C-4), 164.4 (C=O), 164.5 (C=O), 166.4 (C=O). HRMS (ESI): calculated for C35H27ClN3O7 ([M+H]+): 636.1532, found: 636.1541.
4.2.72. 3-iodo-4-azido-N1-(3′-C-ethynyl-2′,3′-5-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (78)
78 was prepared according to General Procedure C. 18 (0.793 g, 1.5 mmol) gave rise to 78 (0.288 g, 0.382 mmol) as a slight yellow foam in 25% yield. Reaction time: 2.5 h. Purification: 15% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.97 (s, 1H, ethynyl-H), 4.89 (dd, J = 11.1, 4.8 Hz, 1H, H-5″), 4.95–4.97 (m, 1H, H-4′), 5.02 (dd, J = 11.1, 3.8 Hz, 1H, H-5′), 6.41 (d, J = 5.1 Hz, 1H, H-2′), 6.80 (d, J = 5.1 Hz, 1H, H-1′), 6.87 (d, J = 5.1 Hz, 1H, H-5), 7.27–7.32 (m, 2H, OBz), 7.40–7.52 (m, 5H, OBz), 7.57–7.64 (m, 2H, OBz), 7.71 (s, 1H, H-2), 7.87–7.91 (m, 2H, OBz), 8.03–8.06 (m, 2H, OBz), 8.15–8.18 (m, 2H, OBz), 8.20 (d, J = 5.4 Hz, 1H, H-6). 13C NMR (75 MHz, CDCl3) δ: 52.0 (C-3), 63.8 (C-5′), 76.4, 76.8, 78.4 (C-2′), 79.3 (C-4′), 80.8, 85.7 (C-1′), 107.0 (C-5), 113.2 (C-3a), 128.47, 128.53, 128.66, 128.73, 128.8, 128.9, 129.6, 130.0, 130.1, 130.2 (C-2), 133.6, 133.8, 134.0, 142.4 (C-4), 145.0 (C-6), 149.1 (C-7a), 164.3 (C=O), 164.5 (C=O), 166.4 (C=O). HRMS (ESI): calculated for C35H25IN5O7 ([M+H]+): 754.0793, found: 754.0775.
4.2.73. 3-iodo-4-amino-N1-(3′-C-ethynyl-2′,3′-5-tri-O-benzoyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (79)
79 was prepared according to General Procedure E. 78 (0.28 g, 0.372 mmol) gave rise to 79 (0.2 g, 0.275 mmol) as a yellow foam in 74% yield. Purification: 5 → 40% EA/Hexanes. 1H NMR (300 MHz, CDCl3) δ: 2.94 (s, 1H, ethynyl-H), 4.86 (dd, J = 11.4, 5.1 Hz, 1H, H-5″), 4.93–5.03 (br. s, 4H, NH2, H-4′, H-5′), 6.23 (d, J = 5.7 Hz, 1H, H-5), 6.43 (d, J = 5.4 Hz, 1H, H-2′), 6.81 (d, J = 5.1 Hz, 1H, H-1′), 7.27–7.33 (m, 2H, OBz), 7.39–7.52 (6H, OBz, H-2), 7.56–7.64 (m, 2H, OBz), 7.90–7.94 (m, 3H, OBz, H-6), 8.03–8.06 (m, 2H, OBz), 8.16–8.20 (m, 2H, OBz). HRMS (ESI): calculated for C35H27IN3O7 ([M+H]+): 728.0888, found: 728.0899.
4.2.74. 3-chloro-4-amino-N1-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (80)
80 was prepared according to General Procedure B. 77 (0.19 g, 0.299 mmol) gave rise to 80 (0.070 g, 0.218 mmol) as a white solid in 73% yield. Purification: 0 → 20% MeOH/DCM. Melting point: 162 °C. 1H NMR (300 MHz, DMSO‑d 6) δ: 3.53 (s, 1H, ethynyl-H), 3.60–3.75 (m, 2H, H-5′, H-5″), 3.92 (t, J = 3.3 Hz, 1H, H-4′), 4.62 (t, J = 7.5 Hz, 1H, H-2′), 5.65 (dd, J = 6.6, 4.5 Hz, 1H, OH-5′), 5.72 (d, J = 7.2 Hz, 1H, OH-2′), 5.85 (s, 1H, OH-3′), 5.99 (d, J = 7.8 Hz, 1H, H-1′), 6.21 (br. s, 2H, NH2), 6.29 (d, J = 5.4 Hz, 1H, H-5), 7.52 (s, 1H, H-2), 7.76 (d, J = 5.7 Hz, 1H, H-6). 13C NMR (75 MHz, DMSO‑d 6) δ: 61.9 (C-5′), 72.8, 76.7, 77.5 (C-2′), 83.2, 86.6 (C-1′), 86.8 (C-4′), 101.5, 102.1 (C-5), 104.3, 120.2, 144.6 (C-6), 147.3, 148.7. HRMS (ESI): calculated for C14H15ClN3O4 ([M+H]+): 324.0746, found: 324.0753.
4.2.75. 3-iodo-4-amino-N1-(3′-C-ethynyl-β-d-ribofuranosyl)-pyrrolo[2,3-b]pyridine (81)
81 was prepared according to General Procedure B. 79 (0.19 g, 0.261 mmol) gave rise to 81 (0.08 g, 0.19 mmol) as a white solid in 74% yield. Purification: 0 → 5% MeOH/DCM. Melting point: 204 °C (decomposed). 1H NMR (300 MHz, DMSO‑d 6) δ: 3.53 (s, 1H, ethynyl-H), 3.62–3.75 (m, 2H, H-5′, H-5″), 3.92 (t, J = 3.3 Hz, 1H, H-4′), 4.63 (t, J = 7.5 Hz, 1H, H-2′), 5.67–5.71 (m, 1H, OH-5′), 5.71 (d, J = 7.2 Hz, 1H, OH-2′), 5.84 (s, 1H, OH-3′), 5.98 (d, J = 7.8 Hz, 1H, H-1′), 6.12 (br. s, 2H, NH2), 6.31 (d, J = 5.7 Hz, 1H, H-5), 7.61 (s, 1H, H-2), 7.76 (d, J = 5.4 Hz, 1H, H-6). 13C NMR (75 MHz, DMSO‑d 6) δ: 50.1 (C-3), 61.9 (C-5′), 72.8, 76.7, 77.5 (C-2′), 83.3, 86.6 (C-1′), 86.9 (C-4′), 101.6 (C-5), 106.8 (C-3a), 128.3 (C-2), 144.0 (C-6), 148.0 (C-7a), 148.9 (C-4). HRMS (ESI): calculated for C14H15IN3O4 ([M+H]+): 416.0102, found: 416.0113.
4.3. Biology
4.3.1. Cell proliferation
L1210, HeLa and CEM cells [21]: All assays were performed in 96-well microtiter plates. To each well were added (5–7.5) × 104 tumor cells and a given amount of the test compound. The cells were allowed to proliferate for 48 h (murine leukemia L1210 cells) or 72 h (human lymphocytic CEM and human cervix carcinoma HeLa cells) at 37 °C in a humidified CO2-controlled atmosphere. At the end of the incubation period, the cells were counted in a Coulter counter (Analis, Belgium). The IC50 (50% inhibitory concentration) was defined as the concentration of the compound that inhibited cell proliferation by 50%.
NCI-60 evaluation: Assay protocols concerning NCI-60 tumor cell panel evaluation can be found in the following references: [43,44].
Endothelial cells (ECs). Primary human umbilical vein endothelial cells (HUVEC) and human dermal microvascular endothelial cells (HMVEC-d) were purchased from Lonza (Verviers, Belgium) and the human microvascular endothelial cell line HMEC-1 was obtained from the Centers for Disease Control and Prevention (CDC, Atalanta, GA, USA). The ECs were seeded in gelatin-coated 48-well plates at 20,000 cells/well in EC growth medium (EGM2, Lonza) and after an overnight incubation, 5-fold dilutions of the compounds were added. The ECs were allowed to proliferate for four days in the presence of the compounds, trypsinized and counted by means of a Coulter counter to determine the IC50 values.
4.3.2. In vivo evaluation of antitumor activity of 32
Female severe combined immunodeficient (SCID) mice were used at the age of 8 weeks. The animals were bred at the animal facility of the Rega Institute for Medical Research (KU Leuven, Belgium). The MDA-MB-231-LM2 lung metastatic cell line (clone 4715) was a kind gift of Prof. Massagué [46]. LM2 cells (106) were suspended in 50% matrigel (BD Bioscience) in PBS and orthotopically engrafted in the exposed left, fourth inguinal mammary fat pad of anesthetized SCID mice. Once the tumor was palpable (day 10), 32 was injected intratumorally (i.t.) at 0.3 mg/kg in PBS containing 1% DMSO, 3 times a week, for 2-consecutive weeks. Control mice received only PBS with 1% DMSO.
The growth of luciferase-positive LM2 cells was quantified with an IVIS Spectrum imaging system (Caliper Life Sciences, Hopkinton, MA, USA). Before imaging, mice were anesthetized and injected subcutaneously with 150 mg/kg d-luciferin (PerkinElmer). Images were acquired every 2 min and plateau radiance values (photons/sec) were retained. Lung metastasis was determined after shielding the primary tumor with a black paper. Tumor size was measured using a digital caliper and calculated with the following formula: tumor volume (mm³) = 0.5 × a x b2, where a is the longest diameter and b is the shortest diameter.
Ethics statement: All studies were done in compliance with the ethical guidelines for animal welfare of the KU Leuven (P277/2015).
4.3.3. Antiviral Evaluation [21]
The compounds were evaluated against the following viruses: herpes simplex virus type 1 (HSV-1) strain KOS, thymidine kinase-deficient (TK−) HSV-1 KOS strain resistant to ACV (ACVr), herpes simplex virus type 2 (HSV-2) strains Lyons and G, varicella-zoster virus (VZV) strain Oka, TK− VZV strain 07−1, human cytomegalovirus (HCMV) strains AD-169 and Davis, vaccinia virus Lederle strain, adenovirus-2, respiratory syncytial virus (RSV) strain Long, vesicular stomatitis virus (VSV), Coxsackie B4, parainfluenza 3, influenza virus A (subtypes H1N1, H3N2), influenza virus B, Sindbis, reovirus-1, Punta Toro. The antiviral assays were based on inhibition of virus-induced cytopathicity or plaque formation in human embryonic lung (Hel) fibroblasts, African green monkey cells (Vero), human epithelial cells (HeLa) or Madin-Darby canine kidney cells (MDCK). Confluent cell cultures in microtiter 96-well plates were inoculated with 100 CCID50 of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) or with 20 plaque forming units (PFU) (VZV) in the presence of varying concentrations of the test compounds. Viral cytopathicity or plaque formation was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds. Antiviral activity was expressed as the EC50 or compound concentration required to reduce virus-induced cytopathogenicity or viral plaque formation by 50%. Cytotoxic concentration was expressed as the MCC or minimal cytotoxic concentration being the compound concentration that was required to afford a microscopically visible alteration of cell morphology.
Assays involving hCMV and VZV were performed as described in literature [47]. In short; the antiviral assays were based on inhibition of virus-induced cytopathicity or plaque formation in human embryonic lung (Hel) fibroblasts. Confluent cell cultures in microtiter 96-well plates were inoculated with 100 CCID50 of HCMV (1 CCID50 being the virus dose to infect 50% of the cell cultures) or with 20 plaque forming units (PFU) (VZV). After a 1–2 h, the residual virus was removed, and the cell cultures were incubated in the presence of varying concentrations of the test compounds. Viral cytopathicity (hCMV) or plaque formation (VZV) was recorded as soon as it reached completion in the control virus-infected cell cultures (untreated controls). Antiviral activity was expressed as the EC50 or concentration required for reducing virus-induced cytopathicity or viral plaque formation by 50%.
Declaration of interests
Declaration of interests: none.
Acknowledgements
F.H. is indebted to the Flanders Research Fund (FWO) for a PhD-scholarship. The authors would like to acknowledge Melissa Van de L'isle for assistance in the synthesis part of this work.
Footnotes
In the body of the text, purine numbering will be used for nucleoside analogs; however in the Experimental section, IUPAC nomenclature and pyrrolo[2,3-d]pyrimidine numbering will be applied.
In order to allow for a more facile comparison with the assigned NMR data provided in the Experimental section; pyrrolo[2,3-d]pyrimidine numbering is indicated for selected derivates in italic and between brackets.
As for the 7-deazapurine analogs, in the body of the text, purine numbering will be used. In the Experimental section the corresponding pyrrolo[2,3-b]pyridine nomenclature is employed. In order to allow for a more facile comparison with the assigned NMR data (Experimental section), pyrrolo[2,3-b]pyridine numbering is indicated for selected compounds in italic and between brackets.
Copies of 1H, 13C and 19F NMR spectra of compounds 13–17, 23, 24, 26, 31–34, 37, 37–39, 42, 47–50, 54, 57, 59, 63–68, 70, 76–78, 80, 81; as well as 1H— 13C gHSQC & gHMBC, and 2D NOESY spectra of compounds 23, 24, 26, 33, 47–49, 54, 57, 63–65, 76–78 can be found in the Supporting Information. Full NCI-60 assay data for compounds 31–34 and 37 can be found in the Supporting Information.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejmech.2018.07.062.
Abbreviations
- BLI
Bioluminescence imaging
- CMV
cytomegalovirus
- EAdo
3′-C-ethynyladenosine;
- ECyd
3′-C-ethynylcytidine
- HMDS
hexamethyldisilazane
- HMEC-1
human dermal microvascular endothelial cell line;
- Hel
human embryonic lung fibroblasts
- HMVEC
human microvascular endothelial cells
- HUVEC
human umbilical vein endothelial cells
- HSV-1
herpes simplex virus-1
- HSV-2
herpes simplex virus-2
- iPrMgCl.LiCl
isopropylmagnesium chloride.lithiumchloride complex
- TMSOTf
trimethylsilyltriflate
- TPPTS
trisodium 3-bis(3-sulfonatophenyl)phosphanyl benzenesulfonate
- VZV
varicella zoster virus
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Copies of 1H, 13C and 19F NMR spectra of compounds 13–17, 23, 24, 26, 31–34, 37, 37–39, 42, 47–50, 54, 57, 59, 63–68, 70, 76–78, 80, 81; as well as 1H-13C gHSQC & gHMBC, and 2D NOESY spectra of compounds 23, 24, 26, 33, 47–49, 54, 57, 63–65, 76–78 can be found in the Supporting Information. Full NCI-60 assay data for compounds 31–34 and 37 can be found in the Supporting Information.
References
- 1.Jordheim L.P., Durantel D., Zoulim F., Dumontet C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013;12:447–464. doi: 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- 2.Jordheim L.P., Dumontet C. Developments of new compounds and new strategies in the field of cytotoxic nucleoside analogues. Front. Anti-Cancer Drug Discovery. 2010;1:525–540. doi: 10.2174/157489206777442205. [DOI] [PubMed] [Google Scholar]
- 3.Shelton J., Lu X., Hollenbaugh J.A., Cho J.H., Amblard F., Schinazi R.F. Metabolism, biochemical actions, and chemical synthesis of anticancer nucleosides, nucleotides, and base analogs. Chem. Rev. 2016;116:14379–14455. doi: 10.1021/acs.chemrev.6b00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez D., Chakraborty S., Warnick E., Crane S., Gao Z.G., O'Connor R., Jacobson K.A., Carlsson J. Structure-based screening of uncharted chemical space for atypical adenosine receptor agonists. ACS Chem. Biol. 2016;11:2763–2772. doi: 10.1021/acschembio.6b00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou L., Zhang H., Tao S., Ehteshami M., Cho J.H., McBrayer T.R., Tharnish P., Whitaker T., Amblard F., Coats S.J., Schinazi R.F. Synthesis and evaluation of 2,6-modified purine 2′-C-methyl ribonucleosides as inhibitors of HCV replication. ACS Med. Chem. Lett. 2016;7:17–22. doi: 10.1021/acsmedchemlett.5b00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin Z., Chen Y.-L., Schul W., Wang Q.-Y., Gu F., Duraiswamy J., Kondreddi R.R., Niyomrattanakit P., Lakshminarayana S.B., Goh A., Xu H.Y., Liu W., Liu B., Lim J.Y.H., Ng C.Y., Qing M., Lim C.C., Yip A., Wang G., Chan W.L., Tan H.P., Lin K., Zhang B., Zou G., Bernard K.A., Garrett C., Beltz K., Dong M., Weaver M., He H., Pichota A., Dartois V., Keller T.H., Shi P.-Y. An adenosine nucleoside inhibitor of dengue virus. Proc. Natl. Acad. Sci. U.S.A. 2009;106:20435–20439. doi: 10.1073/pnas.0907010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q., Lescrinier E., Groaz E., Persoons L., Daelemans D., Herdewijn P., De Jonghe S. Synthesis and biological evaluation of pyrrolo[2,1-f][1,2,4]triazine C-Nucleosides with a ribose, 2'-deoxyribose, and 2',3'-dideoxyribose sugar moiety. ChemMedChem. 2018;13:97–104. doi: 10.1002/cmdc.201700657. [DOI] [PubMed] [Google Scholar]
- 8.Plebanek E., Lescrinier E., Andrei G., Snoeck R., Herdewijn P., De Jonghe S. Emimycin and its nucleoside derivatives: synthesis and antiviral activity. Eur. J. Med. Chem. 2018;144:93–103. doi: 10.1016/j.ejmech.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 9.McGuigan C., Serpi M., Slusarczyk M., Ferrari V., Pertusati F., Meneghesso S., Derudas M., Farleigh L., Zanetta P., Bugert J. Anti-flavivirus activity of different tritylated pyrimidine and purine nucleoside analogues. Chemistry. 2016;5:227–235. doi: 10.1002/open.201500216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C., Sun C., Liu X., Zhou Y., Hussain M., Wan J., Li M., Li X., Jin R., Tu Z., Zhang J. Design, synthesis, and in vitro biological evaluation of novel 6-methyl-7-substituted-7-deaza purine nucleoside analogs as anti-influenza A agents. Antivir. Res. 2016;129:13–20. doi: 10.1016/j.antiviral.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Yates M.K., Raje M.R., Chatterjee P., Spiropoulou C.F., Bavari S., Flint M., Soloveva V., Seley-Radtke K.L. Flex-nucleoside analogues - novel therapeutics against filoviruses. Bioorg. Med. Chem. Lett. 2017;27:2800–2802. doi: 10.1016/j.bmcl.2017.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhuma N., Burade S.S., Bagade A.V., Kumbhar N.M., Kodam K.M., Dhavale D.D. Synthesis and anti-proliferative activity of 3′-deoxy-3′-fluoro-3′-C-hydroxymethyl-pyrimidine and purine nucleosides. Tetrahedron. 2017;73:6157–6163. [Google Scholar]
- 13.Smith D.B., Martin J.A., Klumpp K., Baker S.J., Blomgren P.A., Devos R., Granycome C., Hang J., Hobbs C.J., Jiang W.-R., Laxton C., Pogam S.L., Leveque V., Ma H., Maile G., Merrett J.H., Pichota A., Sarma K., Smith M., Swallow S., Symons J., Vesey D., Najera I., Cammack N. Design, synthesis, and antiviral properties of 4′-substituted ribonucleosides as inhibitors of hepatitis C virus replication: the discovery of R1479. Bioorg. Med. Chem. Lett. 2007;17:2570–2576. doi: 10.1016/j.bmcl.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Perlíková P., Eberlin L., Ménová P., Raindlová V., Slavětínská L., Tloušťová E., Bahador G., Lee Y.-J., Hocek M. Synthesis and cytostatic and antiviral activities of 2′-deoxy-2′,2′-difluororibo- and 2′-deoxy-2′-fluororibonucleosides derived from 7-(Het)aryl-7-deazaadenines. ChemMedChem. 2013;8:832–846. doi: 10.1002/cmdc.201300047. [DOI] [PubMed] [Google Scholar]
- 15.Hattori H., Tanaka M., Fukushima M., Sasaki T., Matsuda A. Nucleosides and nucleotides. 158. 1-(3-C-Ethynyl-β-d-ribo-pentofuranosyl)-cytosine, 1-(3-C-Ethynyl-β-d-ribo-pentofuranosyl)uracil, and their nucleobase analogues as new potential multifunctional antitumor nucleosides with a broad spectrum of activity. J. Med. Chem. 1996;39:5005–5011. doi: 10.1021/jm960537g. [DOI] [PubMed] [Google Scholar]
- 16.Hrdlicka P.J., Andersen N.K., Jepsen J.S., Hansen F.G., Haselmann K.F., Nielsen C., Wengel J. Synthesis and biological evaluation of branched and conformationally restricted analogs of the anticancer compounds 3′-C-ethynyluridine (EUrd) and 3′-C-ethynylcytidine (ECyd) Bioorg. Med. Chem. 2005;13:2597–2621. doi: 10.1016/j.bmc.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Hrdlicka P.J., Jepsen J.S., Nielsen C., Wengel J. Synthesis and biological evaluation of nucleobase-modified analogs of the anticancer compounds 3′-C-ethynyluridine (EUrd) and 3′-C-ethynylcytidine (ECyd) Bioorg. Med. Chem. 2005;13:1249–1260. doi: 10.1016/j.bmc.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 18.Naik S.D., Chandra G., Sahu P.K., Kim H.-R., Qu S., Yoon J.-s., Jeong L.S. Stereo- and regio-selective synthesis of 3'-C-substituted-(N)-methanocarba adenosines as potential anticancer agents. Org. Chem. Front. 2016;3:1472–1480. [Google Scholar]
- 19.Hrdlicka P.J., Jepsen J.S., Wengel J. Synthesis and biological evaluation of conformationally restricted and nucleobase modified analogs of the anticancer compound 3'-C-ethynylcytidine (ECyd) Nucleosides, Nucleotides Nucleic Acids. 2005;24:397–400. doi: 10.1081/ncn-200059821. [DOI] [PubMed] [Google Scholar]
- 20.Schott S., Wallwiener M., Kootz B., Seeger H., Fehm T., Neubauer H. ATP chemosensitivity testing of new antitumor duplex drugs linking 3`-C-ethynylycytidine (ECyd) and 2′-deoxy-5-fluorouridine (5-FdU) in comparison to standard cytostatica and combinations thereof. Invest. N. Drugs. 2011;29:506–513. doi: 10.1007/s10637-009-9355-0. [DOI] [PubMed] [Google Scholar]
- 21.Hulpia F., Balzarini J., Schols D., Andrei G., Snoeck R., Van Calenbergh S. Exploring the purine core of 3'-C-ethynyladenosine (EAdo) in search of novel nucleoside therapeutics. Bioorg. Med. Chem. Lett. 2016;26:1970–1972. doi: 10.1016/j.bmcl.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hocek M., Holý A., Votruba I., Dvořáková H. Synthesis and cytostatic activity of substituted 6-phenylpurine bases and Nucleosides: application of the Suzuki−Miyaura cross-coupling reactions of 6-chloropurine derivatives with phenylboronic acids. J. Med. Chem. 2000;43:1817–1825. doi: 10.1021/jm991167+. [DOI] [PubMed] [Google Scholar]
- 23.Hocek M., Nauš P., Pohl R., Votruba I., Furman P.A., Tharnish P.M., Otto M.J. Cytostatic 6-arylpurine nucleosides. 6. SAR in anti-HCV and cytostatic activity of extended series of 6-hetarylpurine ribonucleosides. J. Med. Chem. 2005;48:5869–5873. doi: 10.1021/jm050335x. [DOI] [PubMed] [Google Scholar]
- 24.Perlikova P., Hocek M. Pyrrolo[2,3-d]pyrimidine (7-deazapurine) as a privileged scaffold in design of antitumor and antiviral nucleosides. Med. Res. Rev. 2017;37:1429–1460. doi: 10.1002/med.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourderioux A., Naus P., Perlikova P., Pohl R., Pichova I., Votruba I., Dzubak P., Konecny P., Hajduch M., Stray K.M., Wang T., Ray A.S., Feng J.Y., Birkus G., Cihlar T., Hocek M. Synthesis and significant cytostatic activity of 7-hetaryl-7-deazaadenosines. J. Med. Chem. 2011;54:5498–5507. doi: 10.1021/jm2005173. [DOI] [PubMed] [Google Scholar]
- 26.Nauš P., Caletková O., Konečný P., Džubák P., Bogdanová K., Kolář M., Vrbková J., Slavětínská L., Tloušt’ová E., Perlíková P., Hajdúch M., Hocek M. Synthesis, cytostatic, antimicrobial, and anti-HCV activity of 6-substituted 7-(Het)aryl-7-deazapurine ribonucleosides. J. Med. Chem. 2014;57:1097–1110. doi: 10.1021/jm4018948. [DOI] [PubMed] [Google Scholar]
- 27.Seela F., Ming X. 7-Functionalized 7-deazapurine β-d and β-l-ribonucleosides related to tubercidin and 7-deazainosine: glycosylation of pyrrolo[2,3-d]pyrimidines with 1-O-acetyl-2,3,5-tri-O-benzoyl-β-d or β-l-ribofuranose. Tetrahedron. 2007;63:9850–9861. [Google Scholar]
- 28.Peng X., Seela F. An efficient synthesis of 7-functionalized 7-deazapurine β-d- or β-l-Ribonucleosides: glycosylation of pyrrolo[2,3-d]pyrimidines with 1-O-Acetyl-2,3,5-Tri-O-Benzoyl-d-or l-ribofuranose. Nucleosides, Nucleotides Nucleic Acids. 2007;26:603–606. doi: 10.1080/15257770701490332. [DOI] [PubMed] [Google Scholar]
- 29.Yu W., Chory E.J., Wernimont A.K., Tempel W., Scopton A., Federation A., Marineau J.J., Qi J., Barsyte-Lovejoy D., Yi J., Marcellus R., Iacob R.E., Engen J.R., Griffin C., Aman A., Wienholds E., Li F., Pineda J., Estiu G., Shatseva T., Hajian T., Al-awar R., Dick J.E., Vedadi M., Brown P.J., Arrowsmith C.H., Bradner J.E., Schapira M. Catalytic site remodelling of the DOT1L methyltransferase by selective inhibitors. Nat. Commun. 2012;3:1288. doi: 10.1038/ncomms2304. [DOI] [PubMed] [Google Scholar]
- 30.Campeau L.-C., O'Shea P.D. Chemoselective staudinger strategy in the practical, fit for purpose, gram-scale synthesis of an HCV RNA polymerase inhibitor. Synlett. 2011;2011:57–60. [Google Scholar]
- 31.Brückl T., Thoma I., Wagner A.J., Knochel P., Carell T. Efficient synthesis of deazaguanosine-derived tRNA nucleosides PreQ0, PreQ1, and archaeosine using the turbo-grignard method. Eur. J. Org Chem. 2010;2010:6517–6519. [Google Scholar]
- 32.Suydam I.T., Strobel S.A. Fluorine substituted adenosines as probes of nucleobase protonation in functional RNAs. J. Am. Chem. Soc. 2008;130:13639–13648. doi: 10.1021/ja803336y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X., Seth P.P., Ranken R., Swayze E.E., Migawa M.T. Synthesis and biological activity of 5-fluorotubercidin. Nucleos Nucleot. Nucleic Acids. 2004;23:161–170. doi: 10.1081/ncn-120027825. [DOI] [PubMed] [Google Scholar]
- 34.Ju Y., Xiao Q., Song Y., Ding H., Dou Y., Yang R., Sun Q. Efficient and practical synthesis of 5′-deoxytubercidin and its analogues via vorbrüggen glycosylation. Synthesis. 2011;2011:1442–1446. [Google Scholar]
- 35.Ji Y., Brueckl T., Baxter R.D., Fujiwara Y., Seiple I.B., Su S., Blackmond D.G., Baran P.S. Innate C-H trifluoromethylation of heterocycles. Proc. Natl. Acad. Sci. U.S.A. 2011;108:14411–14415. doi: 10.1073/pnas.1109059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fennewald J.C., Lipshutz B.H. Trifluoromethylation of heterocycles in water at room temperature. Green Chem. 2014;16:1097–1100. doi: 10.1039/C3GC42119H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonda Z., Kovács S., Wéber C., Gáti T., Mészáros A., Kotschy A., Novák Z. Efficient copper-catalyzed trifluoromethylation of aromatic and heteroaromatic iodides: the beneficial anchoring effect of borates. Org. Lett. 2014;16:4268–4271. doi: 10.1021/ol501967c. [DOI] [PubMed] [Google Scholar]
- 38.Schiltz G.E., Scheidt K.A., Rosen S.T., Krett N.L. 2016. Preparation of Substituted Pyrrolo[2,3-d]pyrimidines for the Treatment of Cancer and Proliferative Disorders. US20160002252A1. [Google Scholar]
- 39.Iaroshenko V., Wang Y., Sevenard D., Volochnyuk D. Synthesis of fluorinated pyrrolo[2,3-b]pyridine and pyrrolo[2,3-d]pyrimidine nucleosides. Synthesis. 2009;2009:1851–1857. [Google Scholar]
- 40.Seela F., Gumbiowski R. Synthesis of 1,7-dideaza-2'-deoxyadenosine and related pyrrolo[2,3-b]pyridine 2'-deoxy-β-d-ribonucleosides: stereoselective phase-transfer glycosylation via the nucleobase anion. Heterocycles. 1989;29:795–805. [Google Scholar]
- 41.Antonini I., Claudi F., Cristalli G., Franchetti P., Grifantini M., Martelli S. Synthesis of 4-amino-1-β-d-ribofuranosyl-1H-pyrrolo[2,3-b]pyridine (1-deazatubercidin) as a potential antitumor agent. J. Med. Chem. 1982;25:1258–1261. doi: 10.1021/jm00352a035. [DOI] [PubMed] [Google Scholar]
- 42.Leysen D.C.M., Defert O.R., De K.J.O.A., Fourmaintraux E.P.P.R., Arzel P., De W.G.J.H. 2005. Preparation of N-(nitrogen-heterocyclyl)carboxamides as Protein Kinase C Inhibitors. WO2005082367A1. [Google Scholar]
- 43.Holbeck S.L., Collins J.M., Doroshow J.H. Analysis of Food and Drug Administration-approved anticancer agents in the NCI60 panel of human tumor cell lines. Mol. Canc. Therapeut. 2010;9:1451–1460. doi: 10.1158/1535-7163.MCT-10-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shoemaker R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Canc. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 45.Pérez-Pérez M.-J., Priego E.-M., Bueno O., Martins M.S., Canela M.-D., Liekens S. Blocking blood flow to solid tumors by destabilizing tubulin: an approach to targeting tumor growth. J. Med. Chem. 2016;59:8685–8711. doi: 10.1021/acs.jmedchem.6b00463. [DOI] [PubMed] [Google Scholar]
- 46.Minn A.J., Gupta G.P., Siegel P.M., Bos P.D., Shu W., Giri D.D., Viale A., Olshen A.B., Gerald W.L., Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stipković Babić M., Makuc D., Plavec J., Martinović T., Kraljević Pavelić S., Pavelić K., Snoeck R., Andrei G., Schols D., Wittine K., Mintas M. Novel halogenated 3-deazapurine, 7-deazapurine and alkylated 9-deazapurine derivatives of l-ascorbic or imino-l-ascorbic acid: synthesis, antitumour and antiviral activity evaluations. Eur. J. Med. Chem. 2015;102:288–302. doi: 10.1016/j.ejmech.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.