In this prospective registry study of >480 000 children, we propose an association between perioperative RBC transfusions and postoperative VTE in children, infants, and neonates.
Abstract
BACKGROUND AND OBJECTIVES:
Annual incidence of venous thromboembolism (VTE) including postoperative VTE in hospitalized children is rising significantly. A growing body of evidence supports the role of red blood cells (RBCs) in pathologic thrombosis. In this study, we examined the association of perioperative RBC transfusion with postoperative VTE in pediatric patients.
METHODS:
The pediatric databases of the American College of Surgeons’ National Surgical Quality Improvement Project from 2012 to 2017 were used. Multivariable logistic regression was used to examine the association between perioperative RBC transfusion status and the development of new or progressive VTE within 30 days of surgery. The analyses were age stratified, as follows: neonates (≤28 days), infants (>28 days and <1 year), and children (≥1 year).
RESULTS:
In this study, we included 20 492 neonates, 79 744 infants, and 382 862 children. Postoperative development of VTE was reported in 99 (0.48%) neonates, 147 (0.2%) infants, and 374 (0.1%) children. In all age groups, development of VTE was significantly more common among patients with a perioperative RBC transfusion than patients without a perioperative RBC transfusion (neonates: adjusted odds ratio [aOR] = 4.1, 95% confidence interval [CI] = 2.5–6.7; infants: aOR = 2.4, 95% CI = 1.7–3.6; children: aOR = 2.2, 95% CI = 1.7–2.9). Among children who received an intra- or postoperative transfusion, the weight-based volume of RBCs (mL/kg) transfused was associated with postoperative VTE in a dose-dependent manner: second tertile (odds ratio = 2.3, 95% CI = 1.3–4.1) and third tertile (odds ratio = 4.1, 95% CI = 2.3–7.4) versus first tertile.
CONCLUSIONS:
Perioperative RBC transfusions are independently associated with development of new or progressive postoperative VTE in children, infants, and neonates. These findings need further validation in prospective studies and emphasize the need for evidence-based perioperative pediatric blood transfusion decisions.
What’s Known on This Subject:
Annual incidence of venous thromboembolism (VTE) including postoperative VTE in hospitalized children is rising significantly. A growing body of evidence supports the role of red blood cells in physiologic hemostasis as well as pathologic thrombosis.
What This Study Adds:
In this prospective registry study of >480 000 children, perioperative red blood cell transfusions were associated with higher odds of VTE within 30 days of a surgery in neonates, infants, and children, with a potential dose-response relationship among older children.
Annual incidence of venous thromboembolism (VTE) comprising deep venous thrombosis (DVT) and pulmonary embolism (PE) is rising significantly in hospitalized children and neonates.1 VTE in children has been associated with ∼$400 million of excess costs per year in the United States.2,3 Hospital-associated venous thromboembolism (HA-VTE) is now considered the second largest contributor to harm in hospitalized pediatric patients.4 Pediatric VTE management guidelines from the American Society of Hematology note lack of high-quality data regarding putative risk factors for HA-VTE in children.5
There is an evolving body of scientific evidence at both molecular and clinical level that supports the role of red blood cells (RBCs) in physiologic hemostasis and bleeding control but also their association with pathologic thrombosis.2,6,7 In multiple recent studies, researchers have outlined mechanistic pathways linking RBCs to VTE. Both quantitative and qualitative changes in RBCs affect hemostasis and thrombosis and can include RBC counts or hematocrit levels (modulating blood rheology through viscosity) and changes such as deformability, aggregation, expression of adhesive proteins, and release of microparticles or extracellular microvesicles.
RBCs are commonly transfused in relation to a surgical procedure and can further induce a proinflammatory state that may contribute further to development of a thrombosis in a postsurgical patient.8 Perioperative RBC transfusions have been significantly associated with the development of new or progressive VTE, independent of several putative confounders in adults.9,10 However, this association has not been specifically explored in pediatric and neonatal populations. We sought to use a large prospective multicenter registry of hospitalized children undergoing elective and nonelective surgical procedures to examine the association between perioperative RBC transfusion and subsequent development of postoperative VTE in neonates, infants, and children.
Study Design and Methods
Data Source
The American College of Surgeons’ National Surgical Quality Improvement Project (ACS-NSQIP) database is a multicenter prospective registry of surgical patients across North America. ACS-NSQIP is well recognized as the leading nationally validated outcomes-based program to improve quality of surgical care; the accuracy and reproducibility of data have been previously demonstrated.11–13 The American College of Surgeons has collaborated with the American Pediatric Surgical Association to develop a pediatric version called the American College of Surgeons’ National Surgical Quality Improvement Project Pediatric (NSQIP-PEDS) database. Participating teaching and nonteaching hospitals in NSQIP-PEDS include freestanding children’s hospitals, children’s hospitals within larger hospitals, specialty children’s hospitals, and general acute-care hospitals with a dedicated pediatric wing. The number of participating hospitals has increased over time with 109 sites in the 2017 NSQIP-PEDS database. Data were prospectively collected in a standardized fashion following strict definitions by dedicated surgical clinical reviewers. Patients are managed throughout their hospital course and after discharge from the hospital for up to 30 days postoperatively. Data from the 2012 to 2017 NSQIP-PEDS were merged for this study.
The study involved secondary analysis of completely deidentified data and did not meet definition of research with human subjects in accordance with human research regulations at Code of Federal Regulations Title 45, Part 46, and was deemed to be exempt from review by the Johns Hopkins University Institutional Review Board.
Outcomes and Risk Factors
The primary outcome was development of new or progressive VTE within 30 days following surgery. VTE events (PE or DVT) were ascertained initially with the International Classification of Diseases, Ninth Revision or 10th Revision diagnosis code for VTE and confirmed by definitive imaging modality (eg, duplex, venogram, computed tomography, other) or direct pathology examination from autopsy. Patients with known or chronic DVT were included only if they had documented postoperative progression. In addition, cases were required to have therapeutic intervention with anticoagulation therapy and/or placement of a vena cava filter. Arterial thrombosis was not documented as an outcome and thus could not be included in this analysis. PE or DVT cases were not analyzed separately because of a low number of PE events.
The primary exposure variable was perioperative RBC transfusion (≥1 RBC transfusion event from 72 hours before surgery to 72 hours after surgery). The type and timing of perioperative RBC transfusion were examined as (1) no RBC transfusion, (2) only preoperative RBC transfusion (up to 72 hours before surgery), (3) only intraoperative or postoperative RBC transfusion (start of surgery until 72 hours postoperative), and (4) both preoperative and intra- or postoperative RBC transfusions. Weight-based transfusion dose (mL/kg) was calculated from data on total volume of RBCs transfused intra- or postoperatively. The volume of RBC transfused preoperatively was unknown.
Statistical Methods
All analyses were stratified by age groups defined as neonates (<28 days), infants (29 days to 1 year), and older children (1–18 years). Characteristics of the study population were examined by perioperative RBC transfusion status using descriptive statistics.
The primary analysis examined the association of perioperative RBC transfusion and postoperative VTE. Univariate logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of postoperative VTE. Multivariable logistic regression was performed to estimate adjusted odds ratios (aORs). The multivariable models included adjustment for all clinically relevant variables hypothesized to be potential confounders a priori in our conceptual framework and for which data were adequately available: age, sex, race, American Society of Anesthesiology (ASA) class, hospital length of stay, preoperative sepsis status, preoperative ventilation status, disseminated cancer status, central line status, and the work-related relative value units (RVUs). The model for neonates was additionally adjusted for preterm birth status. Age was modeled continuously for neonates (days) and infants (weeks) and as a categorical variable for older children (1–2, 2–5, 6–11, and 12–18 years). Central line status was defined as having either a central line–associated bloodstream infection or a Current Procedural Terminology (CPT) code that indicated the participant having a central line (central line [tunneled or nontunneled] insertion [CPT 36555–36573], repair [CPT 36575 and 36576], partial replacement [CPT 36578], complete replacement [CPT 36580–36585], removal [CPT 36589–36590], or other [CPT 36591–36598]). Similar methods were used to examine the association between the type and timing of perioperative blood transfusion and postoperative VTE.
An ancillary dose response analysis was conducted among children who only received an intra- or postoperative RBC transfusion because data on preoperative transfusion volumes were unavailable. In this group, the association between the intra- and postoperative RBC transfusion volume (mL/kg) and postoperative VTE was examined by using univariable logistic regression. Intra- and postoperative RBC transfusion volume (mL/kg) was categorized into age-specific tertiles. Multivariable dose-response analyses were not performed because of a limited number of events.
Missingness among variables was common in the observed data set (eg, race in neonates missing at 17.7%, see Supplemental Table 3) and was significantly associated with variables in the model (Supplemental Table 4), suggesting that the data were not missing completely at random. Assuming data were more plausibly missing at random, multiple imputation using chained equations was performed to handle missing data and create 5 new data sets.14 Predictive mean matching was used to impute continuous variables, logistic regression was used for binary variables, and polytomous logistic regression was used for unordered categorical variables. The variables for which values were imputed included premature birth status (among neonates), race, ASA class, hospital length of stay, and intra- or postoperative RBC volume transfused (among those who received RBC transfusion). Imputation was performed separately for each age subgroup by using the variables included in the multivariable model, sensitivity analyses, as well as additional auxiliary variables to inform the imputation. All analyses were conducted by using Rubin’s rules, unless specified otherwise.15
Two additional analyses were performed. Using the main analytic approach, a subgroup analysis was performed among those who were reported under the general surgical subtype because the distribution of both RBC transfusion and confounding variables varied by surgical subspecialty. Preoperative hematocrit levels, platelet levels, and international normalized ratio (INR) value were considered potential confounders in our conceptual framework but were not included in the main analysis because of a high percentage of missing data in the database (for example: 88% for children). Because the missingness was high, we did not impute these values. However, a sensitivity analysis was conducted restricted to patients who had complete data on preoperative hematocrit levels, platelet levels, and INR value, in which these variables were added to the main analytic models. For this analysis, we used a complete-case analytic approach.
Two-tailed P values <0.05 were considered statistically significant. Data analysis was performed using Stata/MP version 15.1 (Statacorp, College Station, TX) and R version 3.6.1.
Results
A total of 483 098 pediatric patients were included in this study, of which 20 492 (4.2%) were neonates, 79 744 (16.5%) were infants, and 382 862 (79.3%) were children. Most patients underwent a general surgical procedure, and this was consistent across age groups (Table 1).
TABLE 1.
Characteristics of Study Population Stratified by Perioperative RBC Transfusion Status, ACS-NSQIP Database, 2012–2017
| Characteristic | No. (%) Neonate Patients | No. (%) Infant Patients | No. (%) Children Patients | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 20 492) | Transfusion Group (n = 3179) | Nontransfusion Group (n = 17 313) | Overall (n = 79 744) | Transfusion Group (n = 7023) | Nontransfusion Group (n = 72 721) | Overall (n = 382 862) | Transfusion Group (n = 20 710) | Nontransfusion Group (n = 362 152) | |
| Age in da | 6 (2–18) | 7 (3–16) | 5 (1–19) | — | — | — | — | — | — |
| Premature birth | 6707(32.7) | 2080 (65.4) | 4627 (26.7) | — | — | — | — | — | — |
| Age in wka | — | — | — | 23 (11–35) | 16 (9–30) | 24 (12–36) | — | — | — |
| Age group | |||||||||
| Toddler, 1–2 y | — | — | — | — | — | — | 36 749 (9.6) | 1476 (7.1) | 35 273 (9.7) |
| Early childhood, 2–5 y | — | — | — | — | — | — | 87 115 (22.7) | 2616 (12.6) | 84 499 (23.3) |
| Middle childhood, 6–11 y | — | — | — | — | — | — | 126 548 (33.1) | 4188 (20.2) | 122 360 (33.8) |
| Adolescent, 12–18 y | — | — | — | — | — | — | 132 450 (34.6) | 12 430 (60.0) | 120 020 (33.1) |
| Female | 7779 (38.0) | 1354 (42.6) | 6425 (37.1) | 26 944 (33.8) | 2727 (38.8) | 24 217 (33.3) | 174 440 (45.6) | 12 345 (59.6) | 162 095 (44.8) |
| Race | |||||||||
| White | 16 276 (79.4) | 2201 (69.2) | 14 075 (81.3) | 63 136 (79.2) | 5349 (76.2) | 57 786 (79.5) | 312 363 (81.6) | 15 647 (75.6) | 296 716 (81.9) |
| Black or African American | 3380 (16.5) | 823 (25.9) | 2557 (14.8) | 13 312 (16.7) | 1408 (20.0) | 11 904 (16.4) | 53 310 (13.9) | 4282 (20.7) | 49 028 (13.5) |
| Other | 837 (4.1) | 155 (4.9) | 682 (3.9) | 3297 (4.1) | 266 (3.8) | 3031 (4.2) | 17 189 (4.5) | 781 (3.8) | 16 408 (4.5) |
| ASA class | |||||||||
| 1, no disturbance | 1429 (7.0) | 12 (0.4) | 1418 (8.2) | 19 466 (24.4) | 489 (7.0) | 18 977 (26.1) | 129 270 (33.8) | 2387 (11.5) | 126 883 (35.0) |
| 2, mild disturbance | 5175 (25.3) | 126 (4.0) | 5049 (29.2) | 31 443 (39.4) | 1873 (26.7) | 29 570 (40.7) | 168 921 (44.1) | 8183 (39.5) | 160 737 (44.4) |
| 3, severe disturbance | 8878 (43.3) | 941 (29.6) | 7937 (45.8) | 23 311 (29.2) | 3013 (42.9) | 20 298 (27.9) | 78 996 (20.6) | 8828 (42.6) | 70 169 (19.4) |
| 4, life-threatening | 4624 (22.6) | 1807 (56.8) | 2817 (16.3) | 5343 (6.7) | 1526 (21.7) | 3817 (5.2) | 5497 (1.4) | 1233 (6.0) | 4264 (1.2) |
| 5, moribund | 386 (1.9) | 294 (9.2) | 92 (0.5) | 181 (0.2) | 122 (1.7) | 59 (0.1) | 177 (0.0) | 79 (0.4) | 99 (0.0) |
| Hospital length of stay, da | 16 (4–32) | 33 (17–61) | 14 (3–28) | 2 (1–5) | 11 (4–60) | 1 (0–4) | 1 (0–3) | 5 (4–7) | 1 (0–3) |
| Preoperative sepsis | 1171 (5.7) | 711 (22.4) | 460 (2.7) | 1956 (2.5) | 586 (8.3) | 1370 (1.9) | 37 729 (9.9) | 1252 (6) | 36 477 (10.1) |
| Preoperative ventilation | 5246 (25.6) | 2263 (71.2) | 2983 (17.2) | 4877 (6.1) | 1508 (21.5) | 3369 (4.6) | 5328 (1.4) | 1122 (5.4) | 4206 (1.2) |
| Disseminated cancer | 88 (0.4) | 34 (1.1) | 54 (0.3) | 742 (0.9) | 286 (4.1) | 456 (0.6) | 11 354 (3) | 1892 (9.1) | 9462 (2.6) |
| Central line | 121 (0.6) | 43 (1.4) | 78 (0.5) | 248 (0.3) | 88 (1.3) | 160 (0.2) | 543 (0.1) | 97 (0.5) | 446 (0.1) |
| Work-related RVUsa | 19.5 (11.3–27.8) | 20.9 (18.1–36.8) | 19.0 (11.3–26.4) | 14.0 (8.6–17.7) | 22.6 (15.6–33.6) | 14.0 (8.5–17.6) | 10.0 (8.5–17.0) | 32.1 (20.8–37.5) | 9.8 (8.0–15.0) |
| Surgical subspecialty | |||||||||
| Cardiothoracic | 41 (0.2) | 17 (0.5) | 24 (0.1) | 65 (0.1) | 17 (0.2) | 48 (0.1) | 435 (0.1) | 23 (0.1) | 412 (0.1) |
| General | 16 619 (81.1) | 2857 (89.9) | 13 762 (79.5) | 35 307 (44.3) | 3710 (52.8) | 31 597 (43.4) | 135 050 (35.3) | 3586 (17.3) | 131 464 (36.3) |
| Gynecologic | 2 (0.0) | 0 (0.0) | 2 (0.0) | 4 (0.0) | 1 (0.0) | 3 (0.0) | 1026 (0.3) | 21 (0.1) | 1005 (0.3) |
| Neurosurgical | 2407 (11.7) | 212 (6.7) | 2195 (12.7) | 8937 (11.2) | 1666 (23.7) | 7271 (10) | 34 962 (9.1) | 1710 (8.3) | 33 252 (9.2) |
| Orthopedic | 191 (0.9) | 29 (0.9) | 162 (0.9) | 1979 (2.5) | 33 (0.5) | 1946 (2.7) | 88 530 (23.1) | 14 144 (68.3) | 74 386 (20.5) |
| Otolaryngological | 611 (3.0) | 23 (0.7) | 588 (3.4) | 6653 (8.3) | 217 (3.1) | 6436 (8.9) | 54 785 (14.3) | 241 (1.2) | 54 544 (15.1) |
| Plastic | 166 (0.8) | 7 (0.2) | 159 (0.9) | 11 708 (14.7) | 1288 (18.3) | 10 420 (14.3) | 30 894 (8.1) | 772 (3.7) | 30 122 (8.3) |
| Urologic | 455 (2.2) | 34 (1.1) | 421 (2.4) | 15 091 (18.9) | 91 (1.3) | 15 000 (20.6) | 37 180 (9.7) | 213 (1.0) | 36 967 (10.2) |
—, not applicable.
Data are median (IQR).
Any perioperative RBC transfusion was observed in 3179 (15.5%) neonates, of which, 466 (14.6%) received only preoperative RBC transfusions, 1925 (60.6%) received only intra- or postoperative RBC transfusions, and 788 (24.8%) received both preoperative RBC transfusion and intra- or postoperative RBC transfusions. In infants, 7023 (8.8%) received any perioperative RBC transfusion, of which, 1066 (15.2%) received only preoperative RBC transfusions, 5321 (75.8%) received only intra- or postoperative RBC transfusions, and 636 (9.1%) received both preoperative RBC transfusion and intra- or postoperative RBC transfusions. In children, 20 710 (5.4%) received any perioperative RBC transfusion, of which, 1594 (7.7%) received only preoperative RBC transfusions, 18 682 (90.2%) received only intra- or postoperative RBC transfusions, and 434 (2.1%) received both preoperative RBC transfusion and intra- or postoperative RBC transfusions. Age-specific characteristics of the study population are shown by perioperative RBC transfusion status in Table 1.
Development of VTE within 30 days of surgery was reported in 99 (0.5%) neonates, 147 (0.2%) infants, and 374 (0.1%) children. Table 2 shows the association of perioperative RBC transfusion and the development of VTE within 30 days of surgery. In all age groups, development of VTE was significantly more common among patients with a perioperative RBC transfusion than patients without a perioperative RBC transfusion (neonates: aOR = 4.1, 95% CI = 2.5–6.7; infants: aOR = 2.4, 95% CI = 1.7–3.6; children: aOR = 2.2, 95% CI = 1.7–2.9). Results for each covariate in the multivariable models are shown in Supplemental Tables 5 through 7. For each age group, the type and timing of perioperative RBC transfusion was also significantly associated with the development of VTE (Table 2).
TABLE 2.
Association of Perioperative RBC Transfusion and Development of Postoperative VTE Within 30 d in All Children Aged 0 to 18 y
| Exposure Variable | No. Patients | 30-d Postoperative VTE | ||
|---|---|---|---|---|
| No. (%) | OR (95% CI) | aOR (95% CI)a | ||
| Neonates | ||||
| Perioperative RBC transfusion | ||||
| No | 17 313 | 43 (0.2) | Ref. | Ref. |
| Yes | 3179 | 56 (1.8) | 7.2 (4.8–10.7) | 4.1 (2.5–6.7) |
| Time of perioperative RBC transfusion | ||||
| None | 17 313 | 43 (0.2) | Ref. | Ref. |
| Preoperative only | 466 | 7 (1.5) | 6.1 (2.7–13.7) | 3.7 (1.5–8.7) |
| Intraoperative or postoperative only | 1925 | 29 (1.5) | 6.1 (3.8–9.9) | 3.7 (2.2–6.4) |
| Preoperative and intraoperative | 788 | 20 (2.5) | 10.5 (6.1–17.9) | 6.4 (3.2–12.7) |
| Infants | ||||
| Perioperative RBC transfusion | ||||
| No | 72 721 | 78 (0.1) | Ref. | Ref. |
| Yes | 7023 | 69 (1.0) | 9.2 (6.7–12.8) | 2.4 (1.7–3.6) |
| Time of perioperative RBC transfusion | ||||
| None | 72 721 | 78 (0.1) | Ref. | Ref. |
| Preoperative only | 1066 | 8 (0.8) | 7.0 (3.4–14.6) | 1.5 (0.7–3.2) |
| Intraoperative or postoperative only | 5321 | 47 (0.9) | 8.3 (5.8–11.9) | 2.7 (1.8–4.1) |
| Preoperative and intraoperative | 636 | 14 (2.2) | 21.0 (11.8–37.2) | 2.3 (1.2–4.6) |
| Children | ||||
| Perioperative RBC transfusion | ||||
| No | 362 152 | 252 (0.1) | Ref. | Ref. |
| Yes | 20 710 | 122 (0.6) | 8.5 (6.9–10.6) | 2.2 (1.7–2.9) |
| Time of perioperative RBC transfusion | ||||
| None | 362 152 | 252 (0.1) | Ref. | Ref. |
| Preoperative only | 1594 | 12 (0.8) | 10.9 (6.1–19.5) | 1.8 (1.0–3.3) |
| Intraoperative or postoperative only | 18 682 | 93 (0.5) | 7.2 (5.7–9.1) | 2.2 (1.6–2.8) |
| Preoperative and intraoperative | 434 | 17 (3.9) | 58.5 (35.5–96.6) | 3.7 (2.1–6.5) |
The multivariable models included adjustment for sex, race, age, ASA class, central line, preoperative sepsis status, preoperative ventilation status, disseminated cancer status, and the work-related RVU, hospital length of stay. Model for neonates also was adjusted for preterm birth status. RVUs were modeled continuously using a square root transformation.
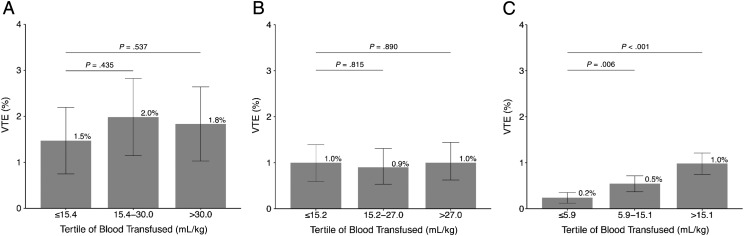
Among patients who only received an intra- or postoperative RBC transfusion, the median volume of blood transfused was 20.3 mL/kg (interquartile range [IQR] = 14.5–37.8) for neonates, 20.1 mL/kg (IQR = 13.9–32.7) for infants, and 10.0 (IQR = 4.4–18.5) for children. The volume of blood transfused was not associated with the development of VTE among neonates or infants (Fig 1A and B). Weight-based volume of RBCs transfused was associated with postoperative VTE among older children in a dose-dependent manner: second tertile (OR = 2.3, 95% CI = 1.3–4.1) and third tertile (OR = 4.1, 95% CI = 2.3–7.4) versus first tertile of mL/kg volume of RBCs (Fig 1C).
FIGURE 1.
Association between the volume of RBC transfusion per kilogram and the development of postoperative VTE within 30 days in (A) neonates, (B) infants, and (C) children, who only received an intraoperative or postoperative transfusion. Given that the analysis used 5 imputed data sets, the actual tertile amounts varied by data set among each age group. The numbers given represent an average of the tertile cut points for each age group.
Among those who received general surgery, the association between perioperative RBC transfusion and VTE remained statistically significant (Supplemental Table 8). Other surgical subspecialties were separately examined, and some had had sparse data on the exposure and/or outcome (Supplemental Table 9). Additionally, the association of perioperative RBC transfusion with development of VTE was robust in the sensitivity analysis that additionally adjusted for preoperative hematocrit levels, preoperative platelet levels, and INR values among neonates (aOR = 5.5, 95% CI = 2.6–11.5) and children (aOR = 1.6, 95% CI = 1.2–2.3) (Supplemental Table 10). In this sensitivity analysis, however, the association was attenuated among infants (aOR 1.4, 95% CI = 0.8–2.4).
Discussion
In data from the largest validated pediatric surgical outcomes registry in North America, it is suggested that perioperative RBC transfusions may be associated with higher odds of postoperative VTE in neonates, infants, and children. Notably, neonates had ∼4 times higher odds of developing VTE with perioperative RBC transfusions in a risk-adjusted model. This association warrants further study in these younger age groups, especially given the higher risk of VTE, as the actual transfusion dose can be a potentially modifiable risk factor.
The initial studies in which researchers hypothesize a possible role of RBCs in thrombosis date back to the early 20th century.16–18 There has been evolving molecular and clinical evidence supporting the role of RBCs in physiologic hemostasis and pathologic thrombosis. There are various proposed mechanistic pathways linking RBCs to venous as well as arterial thrombosis. RBCs are hypothesized to increase the blood viscosity and have platelet-mediated effects in promoting thrombus formation, including increasing platelet margination, increasing platelet adhesion and activation, and enhancing the platelet–thrombus interactions.2 RBCs can also directly or indirectly adhere to the vessel wall and may contribute to thrombin generation within thrombi.19,20 RBCs are not just passively entrapped into a thrombus, but once incorporated into the venous thrombi, they are hypothesized to subsequently reduce the permeability of the thrombus and susceptibility to being lysed, making the clot more resistant.2,21,22 In addition, stored RBCs, especially those stored for longer duration, can undergo various mechanical or physiologic changes and have been shown to have decreased cell membrane deformability,23 increased endothelial adherence,24 and increased aggregability.25 Each of these storage lesions, or all 3 in combination, are plausible mechanisms for increased thrombotic risk after transfusion of RBCs.26
HA-VTE is a major public health concern and is recognized as a preventable27 cause of morbidity and mortality.28–31 Although less common in comparison with adults, HA-VTE is now recognized with increasing frequency in children.32–34 There has been a dramatic increase in VTE incidence in hospitalized children in the United States, particularly in the tertiary care setting, and the rate in hospitalized children is supposed to be increased 100 to 1000 times when compared with the population level.1,2,35 Among children of all age ranges, neonates are recognized as being at the highest risk of VTE. The etiology of this is presumed to be multifactorial, with neonates having an overall procoagulant predisposition with higher hematocrit levels, high von Willebrand factor levels (including higher levels of ultra-large von Willebrand factor polymers), and lower protein C and S levels and hyporeactive platelets. The data from this study revealed that the highest overall incidence of postoperative VTE is in neonates. Additionally, neonates are the most highly transfused age group among children, which is consistent with the literature.36,37 Notably, neonates had high effect size odds for VTE. The degree of prematurity is a known risk factor for VTE in this age group. However, after adjustment for the prematurity status in addition to other previously recognized risk factors for VTE,38 RBC transfusions remained associated with VTE.
In a previous analysis of neonates in the same NSQIP-PEDS database, in initial years (2012–2013) of our study, Goobie et al39 reported on noncardiac surgeries to assess the role of anemia in perioperative mortality. They excluded analysis of infants who received preoperative transfusions and found that neonates with untreated preoperative anemia had ∼2.5 times higher odds of postoperative mortality.39 In light of our study findings, a need for adequate evidence-based guidance to optimize transfusion support decisions perioperatively and properly balance the risks of mortality from severe anemia versus adverse effects from RBC transfusion, such as VTE, is underscored. Their study further begs the question of whether preoperative correction of anemia before surgery would have decreased the likelihood of mortality.38 Similarly, there still remains a need for adequately powered and methodologically robust studies to interrogate the optimal timing and hemoglobin threshold for transfusion versus use of nontransfusion alternatives to treat perioperative anemia in children, infants, and neonates while balancing the risk/benefit profile of transfusion.
Despite the NSQIP-PEDS being the largest prospective, validated surgical outcomes-based database and the use of standardized collection methodologies, this study has inherent limitations. Primarily, findings from this observational study may be subject to unmeasured and residual confounding. For instance, use of concurrent plasma, platelet, and cryoprecipitate transfusions; antifibrinolytics; and procoagulant factors were not available. On adjustment for preoperative INR, hematocrit levels, and platelet counts in an independent supplementary analysis, results remain significant, but details of other blood products, blood derivatives, and medications would have made the analysis stronger. Some clinical markers like posttransfusion hematocrit levels (likely a better marker of actual “red cell mass”) and a composite validated index of severity of illness, like the Case Mix Index, were not available. The ASA classification was used as a surrogate marker of underlying severity of disease, which is a widely accepted grading system for assessing the preoperative health of surgical patients. Likewise, surgical RVUs may not be an ideal predictor of complexity of surgery but have been used and validated previously in various settings as a surrogate marker for surgical complexity.
There are additional limitations of the current investigation to be considered. Although the intra- and postoperative transfusions were in response to intraoperative bleeding, the exact hemoglobin or hematocrit threshold or details of blood loss and hemodynamic changes prompting the transfusion decision were unknown. However, there was a dose-response relationship, with higher volumes of RBCs transfused intra- or postoperatively being associated to a higher odds of postoperative VTE among older children. It is noteworthy that the weight-based transfusion volumes in neonates and infants were much higher than for the older children, which may explain the inability to observe a dose response in these age groups. A few additional clinical details like use of VTE prophylaxis, family history of VTE or history of known thrombophilia, or genetic analyses showing predisposition to VTE would have also made the analysis more robust. In addition, the National Surgical Quality Improvement Project is a selective sampling of disproportionately “high-risk” procedures, and the analysis did not account for competing risks. Finally, the VTE estimates from the registry prospectively capture the VTE events as postoperative complications only and are not accurate for predicting actual incidence or population-level estimates of VTE in pediatric and neonatal populations. Ultimately, these data are hypothesis generating, and the study findings will require rigorous validation in prospectively designed and adequately powered studies.
With this study, we propose a previously underappreciated association of perioperative RBC transfusions with HA-VTE in a postoperative setting in children; a risk factor that can be modifiable at least in some scenarios, especially in elective surgical settings. It is important to highlight that this is a proposed association, and the study results do not imply a causal relationship between perioperative RBC transfusion and risk of postoperative VTE. Given that other blood components frequently accompany red cell transfusions (eg, concurrent platelet, plasma, or cryoprecipitate transfusion), these findings should be interpreted with caution. Nonetheless, these data also underscore need for collaborative inputs from experts and an evidence base to propose guidelines and thresholds for transfusion in children, infants and neonates in the perioperative setting. Validation of these associations in prospective well-designed studies, adequately powered to capture a sufficient number of events, suggest a 2-pronged interventional approach. First, there is a need for rigorous perioperative patient blood management practices in children and exploring nontransfusion alternatives when feasible. Second, early screening may be warranted in this population that is highly transfused and potentially at-risk for VTE to facilitate timely therapeutic interventions in case of thrombotic outcomes.
Glossary
- ACS-NSQIP
American College of Surgeons’ National Surgical Quality Improvement Project
- aOR
adjusted odds ratio
- ASA
American Society of Anesthesiology
- CI
confidence interval
- CPT
Current Procedural Terminology
- DVT
deep venous thrombosis
- HA-VTE
hospital-associated venous thromboembolism
- IQR
interquartile range
- INR
international normalized ratio
- NSQIP-PEDS
American College of Surgeons’ National Surgical Quality Improvement Project Pediatric
- OR
odds ratio
- PE
pulmonary embolism
- RBC
red blood cell
- RVU
relative value unit
- VTE
venous thromboembolism
Footnotes
Drs Goel, Tobian, and Josephson and Mr Patel, Ms Petersen, and Ms Makhani conceptualized and designed the study, conducted the initial analyses, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Frank, Ness, Bloch, Gehrie, Lokhandwala, Nellis, Karam, Shaz, and Patel helped draft the manuscript, critically reviewed the manuscript for important intellectual content, and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Drs Goel and Josephson share the first authorship.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by the National Institutes of Health (NIH) (R01AI120938 and R01AI128779 to Dr Tobian and T32AI102623 to Mr Patel)
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2019-3955.
References
- 1.Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–1008 [DOI] [PubMed] [Google Scholar]
- 2.Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 2019;17(2):271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goudie A, Dynan L, Brady PW, Fieldston E, Brilli RJ, Walsh KE. Costs of venous thromboembolism, catheter-associated urinary tract infection, and pressure ulcer. Pediatrics. 2015;136(3):432–439 [DOI] [PubMed] [Google Scholar]
- 4.Witmer CM, Takemoto CM. Pediatric hospital acquired venous thromboembolism. Front Pediatr. 2017;5:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monagle P, Cuello CA, Augustine C, et al. . American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsey G, Lindholm PF. Thrombosis risk in cancer patients receiving red blood cell transfusions. Semin Thromb Hemost. 2019;45(6):648–656 [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Le Ray I, Lee B, Wikman A, Reilly M. Association of blood group and red blood cell transfusion with the incidence of antepartum, peripartum and postpartum venous thromboembolism. Sci Rep. 2019;9(1):13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zallen G, Moore EE, Ciesla DJ, Brown M, Biffl WL, Silliman CC. Stored red blood cells selectively activate human neutrophils to release IL-8 and secretory PLA2. Shock. 2000;13(1):29–33 [DOI] [PubMed] [Google Scholar]
- 9.Goel R, Patel EU, Cushing MM, et al. . Association of perioperative red blood cell transfusions with venous thromboembolism in a North American registry. JAMA Surg. 2018;153(9):826–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res. 2012;129(5):568–572 [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Cohen ME, Hall BL, Ko CY, Bilimoria KY. Evaluation and enhancement of calibration in the American College of Surgeons NSQIP surgical risk calculator. J Am Coll Surg. 2016;223(2):231–239 [DOI] [PubMed] [Google Scholar]
- 12.Mansmann U, Rieger A, Strahwald B, Crispin A. Risk calculators-methods, development, implementation, and validation. Int J Colorectal Dis. 2016;31(6):1111–1116 [DOI] [PubMed] [Google Scholar]
- 13.Kaafarani HM, Mavros MN, Hwabejire J, et al. . Derivation and validation of a novel severity classification for intraoperative adverse events. J Am Coll Surg. 2014;218(6):1120–1128 [DOI] [PubMed] [Google Scholar]
- 14.Pedersen AB, Mikkelsen EM, Cronin-Fenton D, et al. . Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. 2017;9:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews DA, Low PS. Role of red blood cells in thrombosis. Curr Opin Hematol. 1999;6(2):76–82 [DOI] [PubMed] [Google Scholar]
- 17.Chabanel A, Horellou MH, Conard J, Samama MM. Red blood cell aggregability in patients with a history of leg vein thrombosis: influence of post-thrombotic treatment. Br J Haematol. 1994;88(1):174–179 [DOI] [PubMed] [Google Scholar]
- 18.Smith JR, White AM. Fibrin, red cell and platelet interactions in an experimental model of thrombosis. Br J Pharmacol. 1982;77(1):29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horne MK III, Cullinane AM, Merryman PK, Hoddeson EK. The effect of red blood cells on thrombin generation. Br J Haematol. 2006;133(4):403–408 [DOI] [PubMed] [Google Scholar]
- 20.Mackman N. The red blood cell death receptor and thrombosis. J Clin Invest. 2018;128(9):3747–3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litvinov RI, Weisel JW. Role of red blood cells in haemostasis and thrombosis. ISBT Sci Ser. 2017;12(1):176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walton BL, Byrnes JR, Wolberg AS. Fibrinogen, red blood cells, and factor XIII in venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S208–S215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank SM, Abazyan B, Ono M, et al. . Decreased erythrocyte deformability after transfusion and the effects of erythrocyte storage duration. Anesth Analg. 2013;116(5):975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48(1):136–146 [DOI] [PubMed] [Google Scholar]
- 25.Hovav T, Yedgar S, Manny N, Barshtein G. Alteration of red cell aggregability and shape during blood storage. Transfusion. 1999;39(3):277–281 [DOI] [PubMed] [Google Scholar]
- 26.Ghazi L, Schwann TA, Engoren MC, Habib RH. Role of blood transfusion product type and amount in deep vein thrombosis after cardiac surgery. Thromb Res. 2015;136(6):1204–1210 [DOI] [PubMed] [Google Scholar]
- 27.O’Leary JD, Goobie SM. Improving venous thromboembolism management in children undergoing surgery. Paediatr Anaesth. 2018;28(5):378–379 [DOI] [PubMed] [Google Scholar]
- 28.Monagle P, Adams M, Mahoney M, et al. . Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47(6):763–766 [DOI] [PubMed] [Google Scholar]
- 29.Biss TT, Brandão LR, Kahr WH, Chan AK, Williams S. Clinical features and outcome of pulmonary embolism in children. Br J Haematol. 2008;142(5):808–818 [DOI] [PubMed] [Google Scholar]
- 30.Streiff MB, Brady JP, Grant AM, Grosse SD, Wong B, Popovic T; Centers for Disease Control and Prevention (CDC) . CDC Grand Rounds: preventing hospital-associated venous thromboembolism. MMWR Morb Mortal Wkly Rep. 2014;63(9):190–193 [PMC free article] [PubMed] [Google Scholar]
- 31.Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 suppl):S495–S501 [DOI] [PubMed] [Google Scholar]
- 32.Goldenberg NA, Bernard TJ. Venous thromboembolism in children. Hematol Oncol Clin North Am. 2010;24(1):151–166 [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg NA, Bernard TJ. Venous thromboembolism in children. Pediatr Clin North Am. 2008;55(2):305–322, vii [DOI] [PubMed] [Google Scholar]
- 34.Raffini L. Thrombolysis for intravascular thrombosis in neonates and children. Curr Opin Pediatr. 2009;21(1):9–14 [DOI] [PubMed] [Google Scholar]
- 35.Setty BA, O’Brien SH, Kerlin BA. Pediatric venous thromboembolism in the United States: a tertiary care complication of chronic diseases. Pediatr Blood Cancer. 2012;59(2):258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goel R, Josephson CD. Recent advances in transfusions in neonates/infants. F1000 Res. 2018;7:F1000 Faculty Rev-609 [Google Scholar]
- 37.Keir AK, Yang J, Harrison A, Pelausa E, Shah PS; Canadian Neonatal Network . Temporal changes in blood product usage in preterm neonates born at less than 30 weeks’ gestation in Canada. Transfusion. 2015;55(6):1340–1346 [DOI] [PubMed] [Google Scholar]
- 38.Higgins RD, Patel RM, Josephson CD. Preoperative anemia and neonates. JAMA Pediatr. 2016;170(9):835–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goobie SM, Faraoni D, Zurakowski D, DiNardo JA. Association of preoperative anemia with postoperative mortality in neonates. JAMA Pediatr. 2016;170(9):855–862 [DOI] [PubMed] [Google Scholar]