Graphical abstract
The direct chemoselective differential functionalization of the ring-C hydroxyl groups present in the Amaryllidaceae alkaloid lycorine is described allowing for selective manipulation of the 1,2-hydroxyl groups. A mini-library comprised of synthetic and natural lycorane alkaloids was prepared and their apoptosis-inducing activity investigated in human leukemia (Jurkat) cells. Further insights into the nature of this interesting apoptosis-inducing pharmacophore are described, including the requirement of both free hydroxyl groups in ring-C.
Keywords: Narcissus bujei, Amaryllidaceae, Lycorine, Apoptosis-induction, Anticancer agent, Alkaloid
Abstract
The direct chemoselective differential functionalization of the ring-C hydroxyl groups present in the Amaryllidaceae alkaloid lycorine is described allowing for selective manipulation of the 1,2-hydroxyl groups. A mini-library comprised of synthetic and natural lycorane alkaloids was prepared and their apoptosis-inducing activity investigated in human leukemia (Jurkat) cells. Further insights into the nature of this interesting apoptosis-inducing pharmacophore are described, including the requirement of both free hydroxyl groups in ring-C.
1. Introduction
Three major structurally distinct classes of Amaryllidaceae alkaloids are recognized as the lycorane, galanthamine or crinane types (Jin, 2005), while several other less common structural variants are also known (Kornienko and Evidente, 2008). These structural types are related as a consequence of their common biogenesis from the amino acid-derived precursor norbelladine 1 (Scheme 1 ). Representatives from each of the major series include lycorine 2, galanthamine 3 and crinamine 4, respectively, illustrating the remarkable structural diversity that Nature has evolved from 1. The plethora of biological properties exhibited by these compounds is not surprisingly as equally diverse (Bastida et al., 2006). Of the galanthamine class, galanthamine 3 itself is the first of the Amaryllidaceae alkaloids to be approved as a pharmaceutical in the treatment of a human disease. Its remarkable ability to reversibly inhibit acetylcholinesterase, of relevance in Alzheimer’s and other neuro-degenerative diseases, has very recently led to its approval as a prescription drug under the generic name Reminyl (Houghton et al., 2006).
Scheme 1.
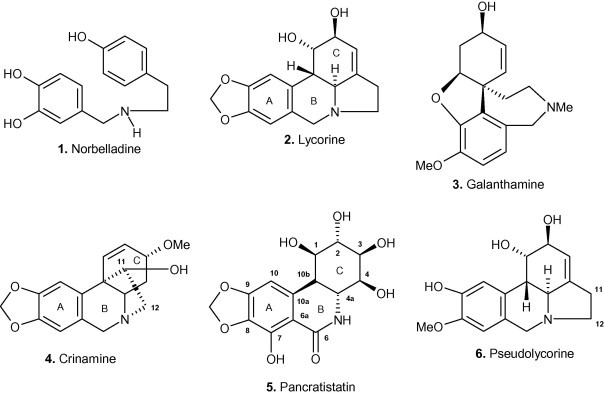
Structurally diverse alkaloid representatives of the Amaryllidaceae.
Alkaloids of the crinane series have been shown to exhibit a range of biological activities (Tram et al., 2002). For example, crinamine 4 is known to be cytotoxic to the malarial parasite and to a series of tumor cell lines (Likhitwitayawuid et al., 1993), while the activity of 6-hydroxycrinamine against mouse melanoma cells has been documented (Nair et al., 1998) and haemanthamine (the C-3 epimer of crinamine) is known to inhibit protein synthesis and have anti-proliferative action (Jimenez et al., 1976, Hohmann et al., 2002). We recently demonstrated the potent apoptosis-inducing ability of the α-ethano bridged crinane alkaloids crinamine 4 and the C-3 epimeric haemanthamine. These compounds were shown to be significantly more active than the β-ethano bridged compounds (McNulty et al., 2007a, McNulty et al., 2009) demonstrating the critical structural requirements in the vicinity of the C-ring. In the lycorane series, pancratistatin 5, and its analogues such as narciclasine (Kornienko and Evidente, 2008), are known for their potent and selective anticancer properties as well as their synthetically challenging structures. Synthetic efforts in this area involving a complementary pair approach and pharmacophore minimization (McNulty and Mo, 1998, McNulty et al., 2001, McNulty et al., 2005, McNulty et al., 2007b, McNulty et al., 2008, Rinner and Hudlicky, 2005) disclosed the strict requirements of the free hydroxyl groups in ring-C of the compounds for potent apoptosis-inducing activity. We have shown that pancratistatin induces apoptosis selectively in cancer cells with minimal effect on normal cells and that mitochondria are the site of action (McLachlan et al., 2005). Evidence for the apoptotic mode of death in cancer cells was related to early activation of caspase-3 followed by flipping of phosphatidyl serine (Kekre et al., 2005).
The interesting structural requirements for apoptosis-inducing activity in the pancratistatin-type of lycorane derivative drew our attention to alkaloids of the lycorine subclass. Lycorine 2 is by far the most common alkaloid within the Amaryllidaceae family. This compound exhibits a vast array of biological properties. It is active against poliovirus (Hwang et al., 2008), vaccinia smallpox virus (Deng et al., 2007) and SARS-associated coronavirus (Li and Tan, 2005). Furthermore, it exhibits antifungal activity against Saccharomyces cerevisiae (Del Giudice et al., 2005), is fatal to the protozoan parasite Trypanosoma brucei (Mackey et al., 2006) and is more potent than indomethacin as an anti-inflammatory agent (Citoglu et al., 1998). In addition, lycorine 2 has been shown to have insect antifeedant activity (Evidente et al., 1986), inhibit ascorbic acid biosynthesis (Arrigoni et al., 1975) and inhibit the enzyme acetylcholinesterase of significance in motor neuron disease (Lopez et al., 2002). The fact that it has an inhibitory effect towards cell division and cell elongation in plants suggests that it may inhibit either (or both) RNA and protein synthesis (De Leo et al., 1973, Jimenez et al., 1976). As a potential chemotherapeutic, it has shown most promise as an antiproliferative agent of a number of cancer cell lines (Likhitwitayawuid et al., 1993). Investigation into the in vitro mode of action in a leukemia (HL-60) cell line model established that lycorine 2 suppressed tumor cell growth and reduced cell survival via cell cycle arrest and induction of apoptosis (Liu et al., 2004). Further investigation of the effect of lycorine 2 on severe combined immuno-deficiency (SCID) mice inoculated with HL-60 cells showed that it decreased tumor cell growth and increased survival rates with no observable adverse effects on treated animals (Liu et al., 2007). Arrest of cell cycle progression and apoptosis induction was also the demonstrated mechanism of action of lycorine 2 on multiple myeloma (KM3) cells (Li et al., 2007).
Motivated by these interesting and diverse biological properties, we decided to investigate structure-activity studies in the lycorine-series in order to further refine some of the pharmacophoric requirements. Herein we describe a concise, chemo- and regioselective three-step synthesis of 1-acetyllycorine 9 from lycorine 2 utilizing the reactivity of the allylic pseudoequatorial C-2 hydroxy group. This selective functionalization allowed us to prepare a mini-library of lycorine derivatives analogues 7–10 (Scheme 2 ). Investigations on the apoptosis-inducing properties of six derivatives showed that only lycorine 2 and pseudolycorine 6 were able to induce apoptosis in human leukemia (Jurkat) cells at the micromolar level.
Scheme 2.
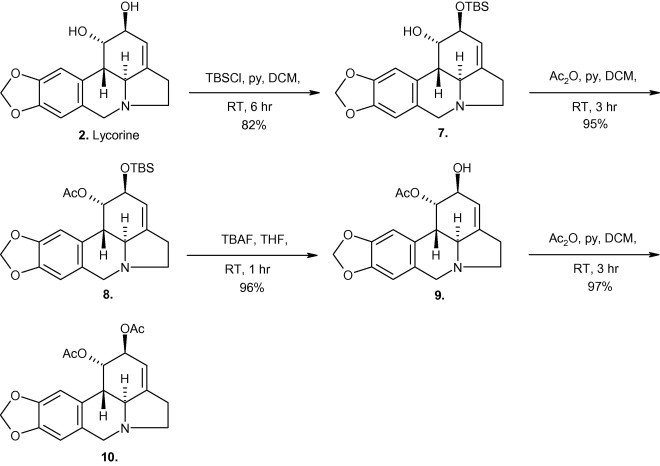
Regio-chemoselective route to 1-acetyllycorine 9.
2. Results and discussion
Previous studies have highlighted the difficulties attending the differential functionalization of the hydroxyl groups present within the lycorine series (Takeda et al., 1958). Previous work in our group has shown that highly selective mono-silylation of 1,2-diols can be achieved under certain conditions (McNulty and Mao, 2002). The reaction of lycorine 2 with tert-butyldimethylsilyl chloride (TBSCl) and pyridine in dichloromethane (DCM) (Scheme 2) was therefore investigated. To our delight, this reaction afforded the novel monosilyl ether 7 (in 82% yield), most likely due to the greater reactivity of the allylic pseudoequatorial 2-hydroxy group over the axially-orientated C-1 hydroxyl. The 1H NMR spectrum of 7 (see Section 3) had the H-2 signal at δ 4.29 (dd), upfield-shifted from δ 5.37 (dd) where it resonates for lycorine (2) while COSY contours were established between H-2 and both H-1 (δ 4.43, dd) and H-3 (δ 5.42, dd). In addition, three-bond HMBC showed H-2 to be correlated to both C-4 (δ 142.01, s) and C-10b (δ 41.20, d).
Direct access to 7 allowed us to prepare the new allylic acetate 8, which was obtained in near quantitative yield from silyl ether 7 upon acetylation with acetic anhydride and pyridine, and its 1H NMR spectrum showed the expected deshielding effect on H-1 (δ 5.56, dd). Desilylation of 8 proceeded smoothly with tetrabutylammonium fluoride (TBAF) in tetrahydrofuran (THF) to give 1-acetyllycorine 9, the proton spectrum of which indicated a slight upfield shift for H-2 (δ 4.15, dd) compared to 8, whose spectroscopic data (mpt, αD, IR, 1H NMR, 13C NMR) was similar to literature values (Kobayashi et al., 1984). Acetylation of 9 then provided in 97% isolated yield 1,2-diacetyllycorine 10 which had the H-2 signal at δ 5.25 (dd) and its spectroscopic data were in close agreement with documented values (Kobayashi et al., 1984, Campbell et al., 1998).
The minipanel of synthetic compounds 7–10 together with natural products lycorine 2 and pseudolycorine 6 were then screened for their ability to induce apoptosis in human leukemia (Jurkat) cells. Fig. 1 depicts the apoptotic index of Jurkat cells treated for 72 h with 1, 5 and 10 μM final concentration of alkaloids 2,6 and 7–10 respectively. The control cells were treated with vehicle (DMSO) so the final concentrations in the culture media was 0.1, 0.5 and 1%, respectively, to match the volume of vehicle added to treated cells. Generally, mammalian cells tolerate 0.1–0.5% DMSO, but 1% DMSO can show toxicity over 72 h. It is not surprising then, that the 10 μM (10 μL/ml of 1 mm stock solution of compounds) equivalent of vehicle control (1% DMSO) caused an increase in apoptotic cells. The index for lycorine 2 and pseudolycorine 6 is striking with over 85% of cells appearing apoptotic after 72 h treatment at 10 μM. Interestingly, 1-acetyllycorine 9 was apoptotic up to ∼45% at 10 μM while the other analogues (7,8,10) exhibited indices at control levels. Nuclear morphological changes characteristic to apoptosis were visualized by Hoechst staining (Fig. 2, Fig. 3 ), by which condensed nuclei of cells affected by the treatment are more fluorescent and fragmented in appearance. In parallel with Hoechst staining, cells were also treated with Trypan Blue, to evaluate the extent of necrotic cells, and subjected to Annexin-V binding assays as a further indication of the induction of programmed cell death (data not shown). The mild activity (∼45%) exhibited by 1-acetyllycorine 9 indicates that a small substituent at C-1 is partly tolerated. Overall, it is clear from these results that a free ring-C 1,2-diol is required in the lycorine series (lycorine and pseudolycorine) for potent apoptosis-inducing activity since both the monosubstituted silyl ether 7 as well as disubstituted analogues (8 and 10) were all inactive.
Fig. 1.
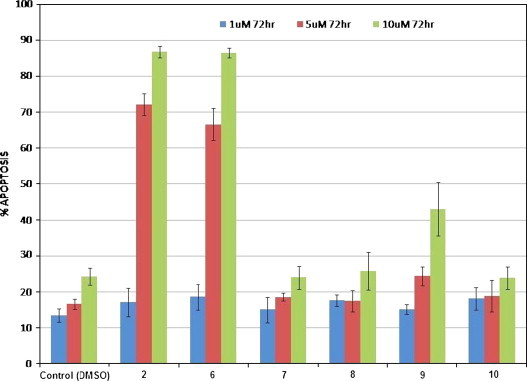
Apoptotic index in Jurkat cells after 72 h treatment with compounds 2, 6, 7, 8, 9 and 10. Data for compounds 2 and 6 at 5 μM concentration, and compounds 2, 6 and 9 at 10 μM concentration are statistically significant from control values (p > 0.05).
Fig. 2.
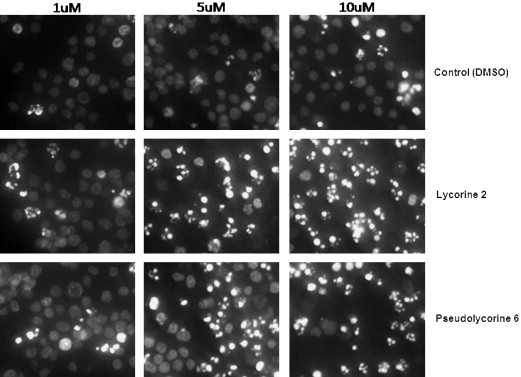
Hoechst staining of Jurkat cells treated over 72 h with lycorine and pseudolycorine.
Fig. 3.
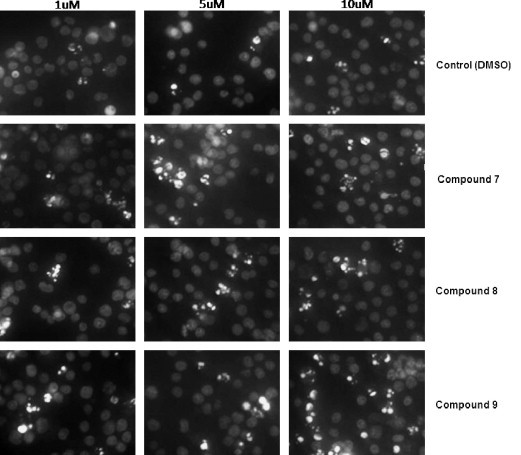
Hoechst staining of Jurkat cells treated over 72 h with compounds 7, 8 and 9.
In the present study, apoptosis initiation was retained upon replacement of the 8,9-methylenedioxy group in lycorine 2 by 8-methoxy-9-hydroxy substituents in pseudolycorine 6. This is in contrast to the observation of Rinner et al. (2004) where substitution of the methylenedioxy group in pancratistatin 5 led to a 10 to 100-fold drop in activity. Intriguingly, the phenanthridine nucleus is common to the crinane, lycorine and pancratistatin series of compounds; with the N to C-10b ethano bridge common to crinane compounds and the N to C-4 ethyl tether characteristic of lycorine compounds.
The present results also draw attention to the subtle, often critical nature of the region around the C-4a, C-10b ring junction. In the crinane alkaloid series, the apoptosis-inducing ability of the α-series far superseded those of the β-series (McNulty et al., 2007a, McNulty et al., 2009) in all of the cases examined. The presence of a C-1,C-10b double bond in the pancratistatin series, such as narciclasine, is known to have no detrimental effect on its anticancer activity profile (Rinner and Hudlicky, 2005). We have also demonstrated the mildly modulating influence of the C-1,C-2 double bond in the α-crinane compounds (McNulty et al., 2007a, McNulty et al., 2009). The introduction of a sp 2 hybridized carbon at the ring junction appears to introduce only a minor conformational effect on the pharmacophore. The C-3, C-4 double bond in lycorine 2 may play a similar conformational role to that in the crinane and pancratistatin series since dihydrolycorine derivatives have also been shown to possess activity (Jimenez et al., 1976).
The overall results (Fig. 1) superficially appear to be reminiscent of the strict requirement for apoptosis induction in pancratistatin 5, in which a minimum of three free hydroxyls in ring C is necessary for activity (McNulty et al., 2005). However the 1,2-dihydroxy groups present in lycorines 2 and 6, which we now show to be required for activity, have the opposite absolute configuration to analogous positions as found in pancratistatin. Furthermore, the C-4a and C-10b ring junction in lycorines 2 and 6 is also opposite in configuration to that found in pancratistatin. Given the strict requirements of the ring-C hydroxyl groups that are known for activity in the pancratistatin series, it is remarkable to note that ent-pancratistatin derivatives have also been shown to have only slightly reduced anticancer activity (Hudlicky et al., 2002). While removing a single hydroxyl group may result in inactive compounds, changing the absolute configuration of the entire molecule can result in retention of activity. These results demonstrate the importance and high sensitivity of the ring-C region in modulating cytotoxicity and heightens the possibility that all of these compounds (pancratistatin, ent-pancratistatin, narciclasine and now also the lycorines), may act upon the same biological receptor.
In conclusion we have demonstrated the selective mono-silylation of the C-2 hydroxyl group in the alkaloid lycorine 2. This work allowed for the synthesis of a mini-library of differentially functionalized 1,2-diol lycorine derivatives. Biological assaying of these compounds demonstrated that the free 1,2-diol is a pharmacophoric requirement for induction of apoptosis in the lycorine series. Comparisons are drawn between this activity and that demonstrated by the pancratistatin/ent-pancratistatin structural types that may indicate a common pharmacophore, drawing attention to the critical nature of the ring-C region (stereochemistry and functionality) and subtleties that are manifest. Further structure-activity studies and biological assays aimed at elucidating the mechanism of action of these derivatives is in progress in our laboratories. Based on the structural similarity of the active compounds to that of pancratistatin, one can hypothesize that these compound could trigger apoptosis by activating Fas-associated Caspase-3, or by destabilizing mitochondria in Jurkat cells as has been previously observed (Griffin et al., 2007, McLachlan et al., 2005). A significant amount of work must be done to delineate the biochemical mechanism of induction of apoptosis by these compounds.
3. Experimental
General: Melting points (uncorrected) were measured on a Gallenkamp melting point apparatus. Optical rotations were determined on a Perkin–Elmer 241 polarimeter installed with a λ589 sodium lamp. IR spectra were measured on a Bio-Rad FTS-40 series spectrometer in dry film. CIMS were run on a Micromass Quattro Ultima spectrometer fitted with a direct injection probe (DIP) with ionization energy set at 70 eV and HRMS (CI) were performed with a Micromass Q-Tof Ultima spectrometer. 1H and 13C NMR spectra were recorded on a Bruker AV700 spectrometer in CDCl3 or CD3OD with TMS as internal standard, chemical shifts are reported in units of δ (ppm) and coupling constants (J) are expressed in Hz. Silica gel Merck (70–230 mesh) was used for CC, silica gel SIL G/UV254 for analyt. and SIL G-25/UV254 for prep. TLC (both Macherey-Nagel). Spots on chromatograms were detected under UV light (254 nm) and by Dragendorff’s reagent stain.
3.1. Extraction and isolation of alkaloids
Lycorine 2 and pseudolycorine 6 were obtained from Narcissus bujei as previously described by us (Campbell et al., 1998, Labrana et al., 2002) and voucher specimens have been deposited (Herbarium, Institut Botanic, Barcelona, Spain, specimen No. 900191). All alkaloids were single components as indicated by NMR and HPLC analyses.
3.2. Cell Culture
Human leukemia (Jurkat) cancer cells were purchased from ATCC, Manassas, VA. Cells were grown and maintained in an incubator set at 37 °C with a 5% CO2 atmosphere and 95% relative humidity. RPMI-1640 media (Sigma–Aldrich, Oakville, ON) supplemented with 10% fetal bovine serum (FBS) and 10 μg/mL gentamycin (Life Technologies, Mississauga, ON) was used for cell culture.
3.3. Cell treatment
To explore the induction of apoptosis by lycorane alkaloids 2,6,7–10, Jurkat cells were grown and treated for 24, 48 and 72 h with each alkaloid. Various concentrations of the alkaloids diluted in culture media, from a 10 mM stock in DMSO, ranging from 1 to 20 μM were used and apoptotic induction by alkaloid treatment was calculated. Controls were treated with DMSO (solvent) at concentrations relative to alkaloid treatment.
3.4. Cellular staining
Nuclear morphological changes associated with apoptosis were visualized using 10 μM final concentration of cell permeable Hoechst 33342 dye (Molecular Probes, Eugene, OR). Following treatment with various alkaloid concentrations over multiple treatment times, Hoechst 33342 dye was added directly to culture media and allowed to incubate for 10 min at 37 °C. Cells were examined using a fluorescent microscope (Leica DM IRB, Germany); pictures were taken at 40X objective. To obtain an apoptotic index, brightly stained condensed nuclei (apoptotic cells) were counted in a minimum of 5 fields, each containing more than 100 cells, so that a minimum of 500 total cells were counted for each data point. The apoptotic index was created such that apoptotic cells were expressed as a percentage of the total number of cells counted. Statistical analysis was performed using STATISTICA from the data of three separate experiments and p < 0.05 was obtained for alkaloids 2,6,7–10.
3.5. Synthesis
3.5.1. Synthesis of 2-tert-butyldimethylsilyloxylycorine 7
Lycorine 2 (30 mg, 0.105 mmol) was dissolved in 0.315 ml of dry CH2Cl2 (3 ml/mmol) to which was added dry pyridine (0.025 ml, 0.314 mmol) followed by tert-butyldimethylsilyl chloride (16 mg, 0.105 mmol) and the solution stirred at room temperature until TLC (30% EtOAc/hexane) indicated consumption of all starting material (∼6 h). The mixture was then quenched with saturated NH4Cl solution (1 ml) and extracted with CH2Cl2 (5 × 1 ml), the combined organic fractions dried (anhydr. Na2SO4) and the solvent removed under reduced pressure. The crude gum obtained was subjected to silica gel CC to give 7 as an off-white powder (34.5 mg, 82%). M.p. 130–132 °C. −78.1 (c 0.1 in CHCl3). IR υ MAX/cm−1 (NaCl): 3610 (OH), 2920, 1605 (Ph), 1506, 1486, 1258 (SiCH3), 1110, 1034 (C–O), 932 (OCH2O). HRMS (CI): calcd. 402.2101 for C22H31NO4Si, found 402.2108. CIMS 70 eV, m/z (rel. int.): 402 [M+1]+ (75), 381 (15), 322 (10), 268 (12), 250 (100), 227 (30), 192 (15), 149 (10), 92 (12). 1H NMR (700 MHz, CDCl3): δ 0.12 (3H, s, Si(CH 3), 0.15 (3H, s, Si(CH 3), 0.90 (9H, s, SiC(CH 3)3, 2.34 (1H, ddd, J = 9.1, 8.4, 1.4 Hz, H–12α), 2.61 (2H, m, 2H-11), 2.71 (1H, d, J = 10.5 Hz, H-4a), 2.81 (1H, dd, J = 10.5, 1.4 Hz, H-10b), 3.35 (1H, ddd, J = 9.0, 7.0, 2.1 Hz, H-12β), 3.49 (1H, d, J = 14.0 Hz, H-6α), 4.15 (1H, d, J = 14.0 Hz, H-6β), 4.29 (1H, dd, J = 3.5, 2.8 Hz, H-2), 4.43 (1H, dd, J = 3.5, 1.4 Hz, H-1), 5.42 (1H, dd, J = 2.8, 1.0 Hz, H-3), 5.93 (2H, s, OCH 2O), 6.60 (1H, s, H-7), 6.84 (1H, s, H-10). 13C NMR (176 MHz, CDCl3): δ 4.58 (q, Si(CH3), −4.30 (q, Si(CH3), 18.29 (s, SiC(CH3)3, 25.99 (3q, SiC(CH3)3), 28.73 (t, C-11), 41.20 (d, C-10b), 54.07 (t, C-12), 57.34 (t, C-6), 61.10 (d, C-4a), 72.35 (d, C-2), 72.69 (d, C-1), 101.14 (t, OCH2O), 104.58 (d, C-10), 107.93 (d, C-7), 118.46 (d, C-3), 127.97 (s, C-6a), 130.66 (s, C-10a), 142.01 (s, C-4), 146.42 (s, C-8), 146.74 (s, C-9).
3.5.2. Synthesis of 1-acetyl-2-tert-butyldimethylsilyloxylycorine 8
Silyl ether 7 (25.0 mg, 0.0621 mmol) was dissolved at room temperature in 0.186 ml CH2Cl2 (3 ml/mmol) to which was added consecutively dry pyridine (0.010 ml, 0.124 mmol) and Ac2O (0.010 ml, 0.093 mmol) and the solution stirred for 3 h when TLC (EtOAc/hexane 3:7) indicated the reaction to be complete. The mixture was then diluted with satd. NaHCO3 solution (1 ml) and extracted with CH2Cl2 (5 × 1 ml), the combined organic fractions dried (anhydr. Na2SO4) and the solvent removed under reduced pressure to yield a gum which was purified on silica gel eluting with (30% EtOAc/hexane) to give compound 8 (26.2 mg, 95%) as a white crystalline powder. M.p. 123–125 °C. −58.2 (c 0.1 in CHCl3). IR υ MAX/cm−1 (NaCl): 2915, 1720 (CO), 1610 (Ph), 1505, 1485, 1255 (SiCH3), 1115, 1032 (C–O), 934 (OCH2O). HRMS (CI): calcd. 444.2206 for C24H33NO5Si, found 444.2216. CIMS 70 eV, m/z (rel. int.): 444 [M+1]+ (60), 384 (43), 322 (15), 269 (10), 250 (40), 227 (100), 192 (10), 117 (12). 1H NMR (700 MHz, CDCl3): δ 0.11 (3H, s, Si(CH 3), 0.19 (3H, s, Si(CH 3), 0.89 (9H, s, SiC(CH 3)3, 1.94 (3H, s, O(CO)CH 3), 2.38 (1H, ddd, J = 9.1, 8.4, 1.4 Hz, H-12α), 2.63 (2H, m, 2H-11), 2.74 (1H, d, J = 9.8 Hz, H-4a), 2.94 (1H, dd, J = 9.8, 1.4 Hz, H-10b), 3.35 (1H, ddd, J = 9.1, 7.7, 2.1 Hz, H-12β), 3.51 (1H, d, J = 14.0 Hz, H-6α), 4.15 (1H, d, J = 14.0 Hz, H-6β), 4.18 (1H, dd, J = 3.5, 2.1 Hz, H-2), 5.39 (1H, dd, J = 2.1, 1.0 Hz, H-3), 5.56 (1H, dd, J = 3.5, 1.4 Hz, H-1), 5.91 (2H, s, OCH 2O), 6.57 (1H, s, H-7), 6.73 (1H, s, H-10). 13C NMR (176 MHz, CDCl3): δ 4.59 (q, Si(CH3), −4.56 (q, Si(CH3), 18.16 (s, SiC(CH3)3, 21.24 (q, O(CO)CH3), 25.91 (3q, SiC(CH3)3), 28.71 (t, C-11), 39.47 (d, C-10b), 53.93 (t, C-12), 57.16 (t, C-6), 61.59 (d, C-4a), 69.84 (d, C-2), 72.65 (d, C-1), 101.05 (t, OCH2O), 105.00 (d, C-10), 107.45 (d, C-7), 118.39 (d, C-3), 127.89 (s, C-6a), 129.66 (s, C-10a), 142.28 (s, C-4), 146.25 (s, C-8), 146.53 (s, C-9), 170.61 (s, O(CO)CH3).
3.5.3. Synthesis of 1-acetyllycorine 9
Silyl acetate 8 (20 mg, 0.045 mmol) was dissolved at room temperature in 0.225 ml of dry THF (5 ml/mmol) to which was added 1 M tetrabutylammonium fluoride (TBAF) solution (0.0675 ml, 0.0675 mmol) and the solution left stirring until TLC (30% EtOAc/hexane) indicated the reaction to be complete (∼1 h). The mixture was then diluted with satd. NH4Cl solution (1 ml) and extracted with EtOAc (5 × 1 ml), the combined organic fractions dried (anhydr. Na2SO4) and the solvent removed under reduced pressure to yield a gum which was purified on a silica gel column eluted with (EtOAc/hexane; 3:7) to give compound 9 as a light yellow crystalline powder (14.2 mg, 96%). M.p. 218–220 °C. −110.5 (c 0.5 in CHCl3). IR υ MAX/cm−1 (NaCl): 3605 (OH), 2918, 1725 (CO), 1608 (Ph), 1503, 1482, 1260, 1114, 1035 (C–O), 933 (OCH2O). HRMS (CI): calcd. 330.1341 for C18H19NO5, found 330.1328. CIMS 70 eV, m/z (rel. int.): 330 [M+1]+ (20), 313 (10), 268 (15), 252 (100), 237 (10), 227 (20), 192 (15), 167 (10), 135 (12), 105 (15). 1H NMR (700 MHz, CD3OD): δ 1.91 (3H, s, O(CO)CH 3), 2.56 (1H, ddd, J = 9.1, 8.4, 1.4 Hz, H-12α), 2.67 (2H, m, 2H-11), 2.91 (1H, d, J = 9.8 Hz, H-4a), 2.94 (1H, dd, J = 9.8, 1.4 Hz, H-10b), 3.36 (1H, ddd, J = 9.1, 7.7, 2.1 Hz, H-12β), 3.62 (1H, d, J = 14.0 Hz, H-6α), 4.14 (1H, d, J = 14.0 Hz, H-6β), 4.15 (1H, dd, J = 3.5, 2.1 Hz, H-2), 5.57 (1H, dd, J = 3.5, 1.4 Hz, H-1), 5.70 (1H, dd, J = 2.1, 1.0 Hz, H-3), 5.91 (2H, s, OCH 2O), 6.66 (1H, s, H-7), 6.73 (1H, s, H-10). 13C NMR (176 MHz, CD3OD): δ 20.73 (q, O(CO)CH3), 29.34 (t, C-11), 40.01 (d, C-10b), 54.64 (t, C-12), 57.42 (t, C-6), 62.89 (d, C-4a), 70.20 (d, C-2), 73.30 (d, C-1), 102.49 (t, OCH2O), 105.69 (d, C-10), 108.38 (d, C-7), 119.41 (d, C-3), 128.40 (s, C-6a), 130.03 (s, C-10a), 143.32 (s, C-4), 148.04 (s, C-8), 148.29 (s, C-9), 172.00 (s, O(CO)CH3).
3.5.4. Synthesis of 1,2-diacetyllycorine 10
Hydroxy acetate 9 (9.0 mg, 0.0273 mmol) was dissolved at room temperature in 0.137 ml CH2Cl2 (5 ml/mmol) to which was added consecutively dry pyridine (0.004 ml, 0.0546 mmol) and Ac2O (0.004 ml, 0.00409 mmol) and the solution stirred for 3 h when TLC (EtOAc/hexane; 3:7) indicated the reaction to be complete. The mixture was then diluted with satd. NaHCO3 solution (1 ml) and extracted with CH2Cl2 (5 × 1 ml), the combined organic fractions dried (anhydr. Na2SO4) and the solvent removed under reduced pressure to yield a gum which was purified on silica gel eluting with EtOAc/hexane (3:7) to give compound 10 (9.8 mg, 97%) as a white crystalline powder. M.p. 210–212 °C. −25.2 (c 0.9 in CHCl3). IR υ MAX/cm−1 (NaCl): 2915, 1720 (CO), 1605 (Ph), 1500, 1485, 1262, 1115, 1034 (C–O), 932 (OCH2O). HRMS (CI): calcd. 372.1447 for C20H21NO6, found 372.1438. CIMS 70 eV, m/z (rel. int.): 372 [M+1]+ (100), 357 (10), 341 (12), 329 (14), 311 (60), 250 (80), 222 (15), 165 (10). 1H NMR (700 MHz, CDCl3): δ 1.95 (3H, s, O(CO)CH 3), 2.08 (3H, s, O(CO)CH 3), 2.40 (1H, ddd, J = 9.1, 8.4, 1.4 Hz, H-12α), 2.65 (2H, m, 2H-11), 2.77 (1H, d, J = 9.8 Hz, H-4a), 2.88 (1H, dd, J = 9.8, 1.4 Hz, H-10b), 3.37 (1H, ddd, J = 9.1, 7.7, 2.1 Hz, H-12β), 3.53 (1H, d, J = 14.0 Hz, H-6α), 4.15 (1H, d, J = 14.0 Hz, H-6β), 5.25 (1H, dd, J = 3.5, 2.1 Hz, H-2), 5.53 (1H, dd, J = 2.1, 1.0 Hz, H-3), 5.73 (1H, dd, J = 3.5, 1.4 Hz, H-1), 5.92 (2H, s, OCH 2O), 6.57 (1H, s, H-7), 6.74 (1H, s, H-10). 13C NMR (176 MHz, CDCl3): 21.09 (q, O(CO)CH3), 21.29 (q, O(CO)CH3), 28.83 (t, C-11), 40.67 (d, C-10b), 53.78 (t, C-12), 57.06 (t, C-6), 61.38 (d, C-4a), 69.41 (d, C-1), 71.05 (d, C-2), 101.12 (t, OCH2O), 105.22 (d, C-10), 107.47 (d, C-7), 113.97 (d, C-3), 126.73 (s, C-6a), 129.58 (s, C-10a), 146.28 (s, C-4), 146.47 (s, C-8), 146.59 (s, C-9), 169.92 (s, O(CO)CH3), 170.15 (s, O(CO)CH3).
Acknowledgments
We thank NSERC (JMcN, SP) and the Lotte & John Hecht Memorial Foundation, Vancouver, B.C. (SP, JMcN) for financial support of this work. We also thank Dr. D. Hughes for obtaining high field NMR data.
References
- Arrigoni O., Arrigoni Liso R., Calabrese G. Lycorine as an inhibitor of ascorbic acid biosynthesis. Nature. 1975;256:513–514. [Google Scholar]
- Bastida J., Lavilla R., Viladomat F. Chemical and biological aspects of Narcissus alkaloids. In: Cordell G.A., editor. vol. 63. Elsevier; Amsterdam: 2006. pp. 87–179. (The Alkaloids). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W.E., Nair J.J., Gammon D.W., Bastida J., Codina C., Viladomat F., Smith P.J., Albrecht C.F. Cyotoxic and antimalarial alkaloids from Brunsvigia littoralis. Planta Medica. 1998;64:91–93. doi: 10.1055/s-2006-957381. [DOI] [PubMed] [Google Scholar]
- Citoglu G., Tanker M., Gamusel B. Antiinflammatory effects of lycorine and haemanthidine. Phytotherapy Research. 1998;12(3):205–206. [Google Scholar]
- De Leo P., Dalessandro G., De Santis A., Arrigoni O. Inhibitory effect of lycorine on cell division and cell elongation. Plant and Cell Physiology. 1973;14(3):481–486. [Google Scholar]
- Del Giudice L., Massardo D.R., Pontieri P., Wolf K. Interaction between yeast mitochondrial and nuclear genomes: null alleles of RTG genes affect resistance to the alkaloid lycorine in rho0 petites of Saccharomyces cerevisiae. Gene. 2005;354:9–14. doi: 10.1016/j.gene.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Deng L., Dai P., Ciro A., Smee D.F., Djaballah H., Shuman S. Identification of novel antipoxviral agents: mitoxantrone inhibits vaccinia virus replication by blocking virion assembly. Journal of Virology. 2007;81(24):13392–13402. doi: 10.1128/JVI.00770-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evidente A., Arrigoni O., Luso R., Calabrese G., Randazzo G. Further experiments on structure-activity relationships among lycorine alkaloids. Phytochemistry. 1986;25:2739–2743. [Google Scholar]
- Griffin, C., McNulty, J., Hamm, C., Pandey, S., 2007. Pancratistatin: a novel highly selective anti-cancer agent that induces apoptosis by the activation of membrane-Fas-receptor associated caspase-3. In: Trends in Cell Apoptosis Research. Nova Science Publishers Inc., Hauppauge, NY, pp. 93–110.
- Hohmann J., Forgo P., Molnar J., Wolfard K., Molnar A., Thalhammer T., Mathe I., Sharples D. Antiproliferative Amaryllidaceae alkaloids isolated from the bulbs of Sprekelia formosissima. Planta Medica. 2002;68:454–457. doi: 10.1055/s-2002-32068. [DOI] [PubMed] [Google Scholar]
- Houghton P.J., Ren Y., Howes M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006;23:181–199. doi: 10.1039/b508966m. [DOI] [PubMed] [Google Scholar]
- Hudlicky T., Rinner U., Gonzales D., Akgun H., Schilling S., Siengalewicz P., Martinot T.A., Pettit G.R. Total synthesis and biological evaluation of Amaryllidaceae alkaloids: narciclasine, ent-7-deoxypancratistatin, regioisomer of 7-deoxypancratistatin, 10b-epi-deoxypancratistatin, and truncated derivatives. J. Org. Chem. 2002;67:8726–8743. doi: 10.1021/jo020129m. [DOI] [PubMed] [Google Scholar]
- Hwang Y.C., Chu J.J.H., Yang P.L., Chen W., Yates M.V. Rapid identification of inhibitors that interfere with poiliovirus replication using a cell-based assay. Antiviral Research. 2008;77:232–236. doi: 10.1016/j.antiviral.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A., Santos A., Alonso G., Vazquez D. Inhibitors of protein synthesis in eukaryotic cells. Comparative effects of some Amaryllidaceae alkaloids. Biochim. Biophys. Acta. 1976;425:342–348. doi: 10.1016/0005-2787(76)90261-6. [DOI] [PubMed] [Google Scholar]
- Jin Z. Amaryllidaceae and sceletium alkaloids. Nat. Prod. Rep. 2005;22:111–126. doi: 10.1039/b316106b. [DOI] [PubMed] [Google Scholar]
- Kekre N., Griffin C., McNulty J., Pandey S. Pancratistatin causes early activation of caspase-3 and the flipping of phosphatidyl serine followed by rapid apoptosis specifically in human lymphoma cells. Cancer Chemother. Pharmacol. 2005;56:29–38. doi: 10.1007/s00280-004-0941-8. [DOI] [PubMed] [Google Scholar]
- Kornienko A., Evidente A. Chemistry, biology and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008;108:1982–2014. doi: 10.1021/cr078198u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrana J., Machocho A.K., Kricsfalusy V., Brun R., Codina C., Viladomat F., Bastida J. Alkaloids from Narcissus angustifolius subsp. transcarpathicus (Amaryllidaceae) Phytochemistry. 2002;60(8):847–852. doi: 10.1016/s0031-9422(02)00154-1. [DOI] [PubMed] [Google Scholar]
- Li R., Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Research. 2005;67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Chen C., Zhang H., Guo H., Wang H., Wang L., Zhang X., Hua S., Yu J., Xiao P., Li Y., Liu J., Tang L., Shi Y., Ren W., Hu W. Apoptosis induced by lycorine in KM3 cells is associated with the G0/G1 cell cycle arrest. Oncology Reports. 2007;17:377–384. [PubMed] [Google Scholar]
- Likhitwitayawuid K., Angerhofer C.K., Chai H., Pezzuto J.M., Cordell G.A., Ruangrungsi N. Cytotoxic and antimalarial alkaloids from the bulbs of Crinum amabile. J. Nat. Prod. 1993;56:1331–1338. doi: 10.1021/np50098a017. [DOI] [PubMed] [Google Scholar]
- Liu J., Hu W., He L., Ye M., Li Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS letters. 2004;578:245–250. doi: 10.1016/j.febslet.2004.10.095. [DOI] [PubMed] [Google Scholar]
- Liu J., Li Y., Tang L.J., Zhang G.P., Hu W.X. Treatment of lycorine on SCID mice model with human APL cells. Biomedicine & Pharmacotherapy. 2007;61:229–234. doi: 10.1016/j.biopha.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S., Bastida J., Viladomat F., Codina C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sciences. 2002;71:2521–2529. doi: 10.1016/s0024-3205(02)02034-9. [DOI] [PubMed] [Google Scholar]
- Mackey Z.B., Baca A.M., Mallari J.P., Apsel B., Shelat A., Hansell E.J., Chiang P.K., Wolff B., Guy K.R., Williams J., McKerrow J.H. Discovery of trypanocidal compounds by whole cell HTS of Trypanosoma brucei. Chem. Biol. Drug Des. 2006;67:355–363. doi: 10.1111/j.1747-0285.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- McLachlan A., Kekre N., McNulty J., Pandey S. Pancratistatin: a natural anticancer compound that targets mitochondria specifically in cancer cells to induce apoptosis. Apoptosis. 2005;10:619–630. doi: 10.1007/s10495-005-1896-x. [DOI] [PubMed] [Google Scholar]
- McNulty J., Mao J. On the direct 2,3-hydroxyl group differentiation of tartaric acid esters. Tetrahedron Lett. 2002;43:3857–3860. [Google Scholar]
- McNulty J., Mo R. Diastereoselective intramolecular nitroaldol entry to lycoricidine alkaloids. J. Chem. Soc. Chem. Comm. 1998;93:3–934. [Google Scholar]
- McNulty J., Mao J., Gibe R., Mo R., Wolf S., Pettit G.R., Herald D.L., Boyd M.R. Studies directed towards the refinement of the pancratistatin cytotoxic pharmacophore. Bioorg. Med. Chem. Lett. 2001;11:169–172. doi: 10.1016/s0960-894x(00)00614-4. [DOI] [PubMed] [Google Scholar]
- McNulty J., Larichev V., Pandey S. A synthesis of 3-deoxydihydrolycoricidine: refinement of a structurally minimum pancratistatin pharmacophore. Bioorg. Med. Chem. Lett. 2005;15:5315–5318. doi: 10.1016/j.bmcl.2005.08.024. [DOI] [PubMed] [Google Scholar]
- McNulty J., Nair J.J., Codina C., Bastida J., Pandey S., Gerasimoff J., Griffin C. Selective apoptosis-inducing activity of crinum-type Amaryllidaceae alkaloids. Phytochemistry. 2007;68:1068–1074. doi: 10.1016/j.phytochem.2007.01.006. [DOI] [PubMed] [Google Scholar]
- McNulty J., Nair J.J., Sliwinski M., Harrington L.E., Pandey S. Unusual magnesium chloride catalyzed non-Evans’ anti-aldol reactions of an enolizable l-threose derivative. Eur. J. Org. Chem. 2007;566:9–5673. [Google Scholar]
- McNulty J., Nair J.J., Griffin C., Pandey S. Synthesis and biological evaluation of fully functionalized seco-pancratistatin analogues. J. Nat. Prod. 2008;71:357–363. doi: 10.1021/np0705460. [DOI] [PubMed] [Google Scholar]
- McNulty J., Nair J.J., Bastida J., Pandey S., Griffin C. Structure activity studies on the crinane alkaloid apoptosis inducing pharmacophore. Natural Product Communications. 2009;4:483–488. [PubMed] [Google Scholar]
- Nair J.J., Campbell W.E., Gammon D.W., Albrecht C.F., Viladomat F., Codina C., Bastida J. Alkaloids from Crinum delagoense. Phytochemistry. 1998;49:2539–2543. [Google Scholar]
- Rinner U., Hudlicky T. Synthesis of Amaryllidaceae constituents-an update. Synlett. 2005;3:365–387. [Google Scholar]
- Rinner U., Hillebrenner H.L., Adams D.R., Hudlicky T., Pettit G.R. Synthesis and biological activity of some structural modifications of pancratistatin. Bioorg. Med. Chem. Lett. 2004;14:2911–2915. doi: 10.1016/j.bmcl.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Takeda K., Kotera K., Mizukami S. A Partial Synthesis of Caranine. J. Am. Chem. Soc. 1958;80:2562–2567. [Google Scholar]
- Tram N.T.M., Titorenkova T.V., Bankova V.S., Handjieva N.V., Popov S.S. Crinum L. (Amaryllidaceae): a review. Fitoterapia. 2002;73:183–208. doi: 10.1016/s0367-326x(02)00068-0. [DOI] [PubMed] [Google Scholar]


