Abstract
It is well known that the degradation of ecosystems can have serious impacts on human health. There is currently a knowledge gap on what impact restoring ecosystems has on human health. In restoring ecosystems there is a drive to restore the functionality of ecosystems rather than restoring ecosystems to ‘pristine’ condition. Even so, the complete restoration of all ecosystem functions is not necessarily possible. Given the uncertain trajectory of the ecosystem during the ecosystem restoration process the impact of the restoration on human health is also uncertain. Even with this uncertainty, the restoration of ecosystems for human health is still a necessity.
Keywords: Ecosystem restoration, Ecosystem degradation, Public health, Biodiversity, Vector borne disease
Intact, thriving, ecosystems – that is, ecosystems with sufficient biodiversity to maintain functionality – provide humans with a broad range of health–giving ‘services’. The MEA (2005) categorised these ‘ecosystem services’ (Table 1 ) to help highlight their contribution to our wellbeing and make policy recommendations for their sustainable management. Over 1300 experts from 95 countries collaborated to provide a series of landmark publications linking ecosystem functionality directly to the health gains derived from the availability of food, water, timber, fibre, fuel and a sense of place (http://www.unep.org/maweb/en/index.aspx). Unfortunately, the rate of anthropogenic ecosystem change has been greater in the last 50 years than ever before, and rapidly continuing ecosystem degradation poses a barrier to achieving the United Nations Millennium Development Goals of eliminating hunger and disease (MEA, 2005). These adverse impacts on the state of ecological communities have been the focus of detailed investigations by ecologists for decades, generating a rich body of literature that highlights the ecological linkage mechanisms between ecosystem disruption and adverse human health outcomes (Moiseenko et al., 2006, Norris, 2004, O'Hara et al., 2000, Ostfeld and Keesing, 2000, Patz et al., 2008, Zetterstrom, 1998).
Table 1.
Categories of selected ecosystem services that support human health (MEA, 2005).
| Provisioning services | Regulating services | Cultural services |
|---|---|---|
| Food | Climate | Aesthetic |
| Water | Natural hazards | Recreational |
| Fibre | Pests | Educational |
| Fuel | Infectious diseases | Spiritual |
| Medicines |
Human health can be impacted in a number of ways from these environmental changes including increases in exposure to human pathogens, bioaccumulation of toxic substances, reduced crop yields and compromised food supplies, scarcity of potable water and air pollution (Rapport, 2002). Whilst environmental degradation has an impact on ecosystem services, attempts to restore ecosystems do not necessarily result in the full restoration of services. A meta-analysis conducted across a range of ecosystem restoration projects found that biodiversity increased on average 44% and ecosystem services increased 25% when compared to unrestored ecosystems, but restored biodiversity and services were still lower than that of intact reference ecosystems (Rey Benayas et al., 2009). For example, restored wetland ecosystems have, on average, 26% lower biological structure and 23% lower biogeochemical functioning than reference sites (Moreno-Mateos et al., 2012). These findings raise the question of whether restoration reduces the risk of adverse health outcomes, and very little research has been commissioned to specifically answer this question (Weinstein, 2005).
One potential area of research that could provide fruitful ground for addressing this question lies in the area of emerging infectious diseases (EIDs). EIDs are those that have recently appeared de novo, or increased significantly in their incidence, distribution or severity, with obvious examples including HIV (human immunodeficiency virus), DHF (dengue hemorrhagic fever), SARS (Severe acute respiratory syndrome), and Lyme disease (to which we will return). These diseases impose a crippling burden on population health and public health infrastructure, possibly in the order of billions of dollars annually (Fonkwo, 2008). Socio-ecological change is the most significant driver of emergence, with over 60% of emerging pathogens originating in animals, and most of those from developing countries where surveillance and control are least effective (Jones et al., 2008). It is no co-incidence that developing countries generally also have the highest rates of population growth, land clearing for agriculture, and biodiversity loss — a combination of drivers that has in some cases been causally linked to disease emergence. EIDs are not just of concern for developing countries. Lyme disease, for example, has emerged as a public health problem in the north-eastern USA where an increasing number of people spend time in forests that have re-grown following clearing, and that do not contain their original complement of biodiversity. As a result, pathogen-transmitting ticks concentrate their feeding on a Lyme reservoir-competent mouse, creating a higher percentage of infectious ticks than would have been the case if a greater variety of species of host animals had been available for the ticks to feed on. Thus, a higher percentage of ticks that bite humans are infectious, linking biodiversity loss directly to disease emergence (Ostfeld and Keesing, 2000). It is tempting to generalise from this example that biodiversity conservation is protective against infectious disease emergence, and many other examples indeed support that this is the case (Ostfeld, 2009), chiefly for arthropod mediated infections, but also for directly transmitted zoonoses (Derne et al., 2011).
However, a recent review article (Randolph and Dobson, 2012) and meta-analysis (Salkeld et al., 2013) have seriously questioned the generalisability of the statement “biodiversity protects against disease” (Randolph and Dobson, 2012). The review points to the complexity of the relationship, showing that both theoretical and empirical evidence supports the hypothesis only under limited, variable, and case-specific circumstances. Worse still, in some circumstances, pathogen amplification is facilitated by biodiversity, increasing the risk of disease emergence. The meta-analyses came to a similar conclusion, finding only a “weak and highly heterogeneous relationship between host diversity and disease” (Salkeld et al., 2013). It appears that the relationship between biodiversity and disease can be positive or negative and the relationship may not be simple or consistent (Wood et al., 2014).
General consensus exists that anthropogenic environmental changes commonly result in non-random community dis-assembly (loss and/or changes in the abundance of individual species, and alterations in community composition, species interactions and function) (Hobbs et al., 2009). The ecological processes operating in a degraded or altered ecosystem are often different from those operating in a non-degraded ecosystem (Suding et al., 2004), with impacted ecosystems taking on altered ecological states with communities dominated by tolerant or newly colonising species (Pinder et al., 2005). Furthermore, these impacts can extend to processes that govern the transmission of pathogens in particular circumstances (Jardine et al., 2007, Patz et al., 2004). For example, habitat modification can disrupt aquatic invertebrate communities resulting in colonisation and dominance of Anopheles vectors of malaria that would normally be excluded by predating and competing taxa (Patz et al., 2000).
Although there is an understanding of the consequences of ecosystem degradation, community re-assembly following ecological restoration is less well understood (Wilson et al., 2000). Currently, understanding of the ecological processes underlying the recovery of ecosystems is often incomplete and poorly integrated across different ecosystems (Montoya et al., 2012). New ecosystems can arise through abiotic changes, these ecosystems will comprise different species, interactions and functions (Hobbs et al., 2009). Novel ecosystems result when species occur in combinations and relative abundances that have not previously occurred within a given biome (Hobbs et al., 2006). In restoring an ecosystem there are several alternative states that the ecosystem may pass through during restoration and the end point of the restoration may not be the original ecosystem state but an alternative state with differing processes from the original, i.e. the degradation and recovery trajectories are different (Suding et al., 2004). This may be due to changes in landscape connectivity and organisation, loss of native species pools, shifts in species dominance, trophic interactions and/or invasion by exotic species, and contamination effects on biogeochemical processes (Suding et al., 2004). Restored ecosystems do not necessarily return to their original state or functioning (Hobbs et al., 2006). Where ecosystem degradation has resulted in enhanced potential for disease transmission, such as increased abundance and dominance of vector species (Carver et al., 2009a, Carver et al., 2010, Patz et al., 2004), the effects of ecosystem restoration on community re-assembly and disease risk remains a poorly studied area (Dale and Knight, 2008). Given the unknown trajectory and endpoint of restored ecosystems the impacts on human health could also be unknown as the ecosystem functions regulating diseases may be different from the initial ecosystem state (Fig. 1 ).
Fig. 1.
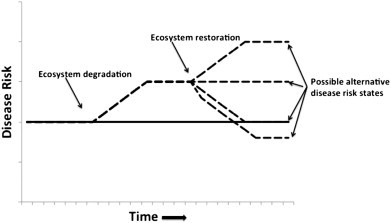
Ecosystem degradation has been shown to increase the risk of some diseases, but, given the unknown trajectory of restored ecosystems the act of restoration may not reduce the disease risk (solid line indicates disease risk in undisturbed ecosystem, broken line indicates disease risk in degraded ecosystem and possible trajectories under different restoration regimes).
A number of studies have shown that increased species diversity can reduce disease risk by regulating the abundance of an important host species, but other studies have shown that increased diversity can increase disease risk (Hough, 2014). This increase can be due to new species providing alternative sources of infection or by increasing vector numbers or providing additional food sources for vectors (Hough, 2014). With the restoration of ecosystems new ecosystems can arise through abiotic changes, and these ecosystems will comprise different species, interactions and functions (Hobbs et al., 2009). Very few studies have examined the impact of ecosystem restoration and its impact on vector-borne disease. The majority of these studies have found a positive effect where the restoration has resulted in a decrease in risk to human health (Table 2 ). A notable exception can be seen in the example of reforestation and Lyme disease (Barbour and Fish, 1993). In this case, reforestation resulted in an increase in biodiversity, but also an increase in Lyme disease cases. The reforestation increased the number of deer and deer ticks and thus increased the incidence of the disease.
Table 2.
Examples of ecosystem restoration and its impact on vector-borne disease.
| Vector | Restoration | Impact |
|---|---|---|
| Mosquito | Habitat modification and biological control | Reduction in mosquito larvae abundance (Rochlin et al., 2009) |
| Ticks (Ixodes scapularis) | Removal of weed species | Reduction in Lyme disease risk by 98% (Morlando et al., 2012) |
| Mosquito | Drainage of salt marsh to more natural state | Reduction in mosquito numbers (Jacups et al., 2012) |
| Ticks (Ixodes ricinus) | Restoration of peatlands | Reduction in abundance of ticks (Gilbert, 2013) |
| Ticks (Ixodes dammini) | Reforestation | Increase in incidence of Lyme disease (Barbour and Fish, 1993) |
Restoring ecosystem function in wetlands whilst benefiting wildlife may have unintended consequences such as providing mosquito habitat (Lawler et al., 2007). A potential example of this can be seen in the case of Ross River Virus (RRV) and dryland salinity. In Western Australia, changes in landuse from perennial native vegetation to annual crops have resulted in an increase in dryland salinity (the removal of deep rooted perennial vegetation results in a rise in the water table bringing dissolved salts to the surface). These changes have resulted in ecosystem disruptions (Jardine et al., 2007), such that the vector for RRV, Aedes camptorhynchus, becomes dominant in saline affected areas — due to other mosquito species which may compete with A. camptorhynchus and macroinvertebrate predators of A. camptorhynchus being unable to tolerate the saline conditions (Carver et al., 2009b, Carver et al., 2010, Jardine et al., 2008). Environmental remediation of saline lands is underway in Western Australia through a variety of means, including revegetation and deep drainage (a process where drains are constructed that pierce the watertable draining away saline water). In restoring an ecosystem there are several alternative states that the ecosystem may pass through during restoration, and the end point may not be the original state but an alternative state with differing ecological processes from the original. It is known that degradation and recovery trajectories are likely to differ (Suding et al., 2004). In the case of restoration of saline lands there are different methods of remediation that reduce salinity in different ways, and therefore directly affect restoration trajectories so that the ecological endpoints are likely to differ. Impacts of these restoration trajectories on A. camptorhynchus are unknown: in the case of revegetation, where waterlogging is reduced and biodiversity increased, there is the potential for a decrease in A. camptorhynchus; with deep drainage, where biodiversity remains low, waterlogging in the surrounding area is decreased but saline water is still in the landscape, and A. camptorhynchus abundance may remain constant or may increase. The two different restoration pathways both have the same aim (reduction of dryland salinity) but have differing ecological, and therefore health, outcomes (Fig. 2 ). Given the potentially unknown trajectories of the ecosystem the actual human health outcomes are not guaranteed.
Fig. 2.
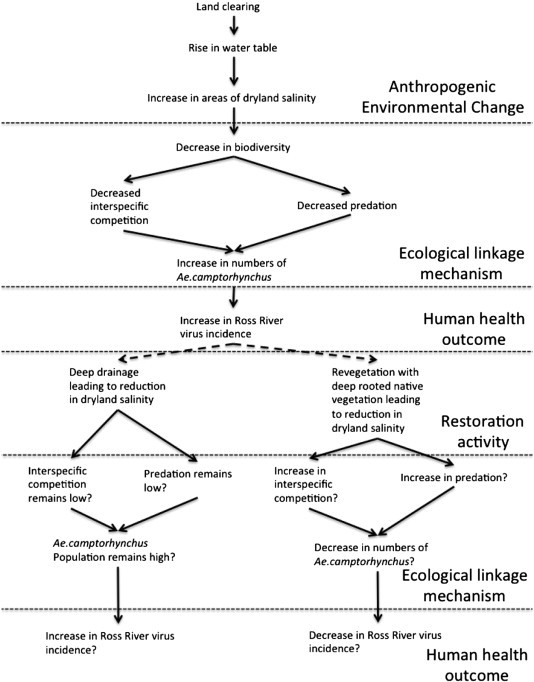
Potential outcomes of two possible processes of ecosystem restoration on incidence of Ross River virus.
With such uncertainty from restoration outcomes, what can land managers conclude about the value of conservation efforts to the health of human populations? One of the great hopes for a positive outcome between infectious disease emergence and biodiversity conservation was that public health practitioners and conservation advocates could work synergistically and concurrently towards healthier sustainable societies and environments. The debate around the mechanistic ecological underpinnings of the relationship – and therefore about the value of restoration – threatens to lessen this opportunity and thereby, as we see it, adversely impact human health. From a public health and clinical perspective, any opportunity to reduce the human disease burden is a good one, and should be grasped in the absence of counterbalancing potential risks. It is important to restore degraded ecosystems, as they can be detrimental to human health, but it is important to realise that there may be adverse health outcomes from poorly planned or implemented restoration projects. The argument is in a way similar to the climate change debate: even if the change is not anthropogenic, can we afford to take the chance of not attempting to reduce the impacts of climate change until we have solid evidence that no adverse outcomes would ensue? From a health perspective, we cannot. The direct and indirect health effects of global warming could be both catastrophic and irreversible (McMichael et al., 2006), and on that basis sound public and environmental health practice dictates that we take the course of action that is most likely to protect the public health, even if that action is later shown to have made no difference (the precautionary principle). In a similar way, the medical and allied health community should be supporting the conservation and restoration of biodiversity both to maintain the health-giving services captured in Table 1, and to reduce the risk of emerging infectious diseases (even if only based on a “weak and heterogeneous” relationship). “The stewards of tomorrow's Earth need to know not only whether interventions work, but which interventions work best, which are most cost-effective, and which are sustainable in terms of protecting the most vulnerable populations” (Weinstein, 2005), which highlights the need to give the restoration ecologists of the future tools and knowledge to tackle the task of restoration (Speldewinde, 2010). Biodiversity loss, like climate change, is irreversible, and both have the potential to seriously and negatively impact population health. Whilst acknowledging uncertainty, sound public health practice would dictate that we restore ecosystems to support biodiversity conservation and work to maintain as much of our planet's ecological integrity as possible (at least until we understand it better) and ensure public health is a priority in the restoration of ecosystems (even those where there is no apparent health risk in the degraded ecosystem).
Editor: C.E.W. Steinberg
References
- Barbour A.G., Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- Carver S., Bestall A., Jardine A., Ostfeld R.S. Influence of hosts on the ecology of arboviral transmission: potential mechanisms influencing dengue, Murray Valley encephalitis, and Ross River virus in Australia. Vector-Borne and Zoonotic Dis. 2009;9:51–64. doi: 10.1089/vbz.2008.0040. [DOI] [PubMed] [Google Scholar]
- Carver S.A., Spafford H., Storey A., Weinstein P. Colonization of ephemeral water bodies in the wheatbelt of Western Australia by assemblages of mosquitoes (Diptera:Culicidae): role of environmental factors, habitat, and disturbance. Environ Entomol. 2009;38:1585–1594. doi: 10.1603/022.038.0609. [DOI] [PubMed] [Google Scholar]
- Carver S., Spafford H., Storey A., Weinstein P. The roles of predators, competitors, and secondary salinization in structuring mosquito (Diptera:Culicidae) assemblages in ephemeral water bodies of the wheatbelt of Western Australia. Environ Entomol. 2010;39:798–810. doi: 10.1603/EN09235. [DOI] [PubMed] [Google Scholar]
- Dale P.E.R., Knight J. Wetlands and mosquitoes: a review. Wetlands Ecol Manage. 2008;16:255–276. [Google Scholar]
- Derne B.T., Fearnley E.J., Lau C.L., Paynter S., Weinstein P. Biodiversity and leptospirosis risk: a case of pathogen regulation? Med Hypotheses. 2011;77:339–344. doi: 10.1016/j.mehy.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Fonkwo P.N. Pricing infectious disease—the economic and health implications of infectious diseases. EMBO Rep. 2008;9:S13–S17. doi: 10.1038/embor.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L. Can restoration of afforested peatland regulate pests and disease? J Appl Ecol. 2013;50:1226–1233. [Google Scholar]
- Hobbs R.J., Arico S., Aronson J., Bridgewater P., Cramer V.A., Epstein P.R. Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob Ecol Biogeogr. 2006;15:1–7. [Google Scholar]
- Hobbs R.J., Higgs E., Harris J.A. Novel ecosystems: implications for conservation and restoration. Trends Ecol Evol. 2009;24:599–605. doi: 10.1016/j.tree.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Hough R.L. Biodiversity and human health: evidence for causality. Biodivers Conserv. 2014;23:267–288. [Google Scholar]
- Jacups S., Warchot A., Whelan P. Anthropogenic ecological change and impacts on mosquito breeding and control strategies in salt-marshes, Northern Territory, Australia. Ecohealth. 2012;19:183–194. doi: 10.1007/s10393-012-0759-5. [DOI] [PubMed] [Google Scholar]
- Jardine A., Speldewinde P., Carver S., P. W Dryland salinity and Ecosystem Distress Syndrome: human health implications. Ecohealth. 2007;4:10–17. [Google Scholar]
- Jardine A., Speldewinde P., Lindsay M.D., Cook A., Johansen C.A., Weinstein P. Is there an association between dryland salinity and Ross River virus disease in southwestern Australia? Ecohealth. 2008;5:58–68. doi: 10.1007/s10393-007-0151-z. [DOI] [PubMed] [Google Scholar]
- Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L. Global trends in emerging infectious disease. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler S.P., Reimer L., Thiemann T., Fritz J., Parise K., Feliz D. Effects of vegetation control on mosquitoes in seasonal freshwater wetlands. J Am Mosq Control Assoc. 2007;23:66–70. doi: 10.2987/8756-971X(2007)23[66:EOVCOM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- McMichael A.J., Woodruff R.E., Hales S. Climate change and human health: present and future risks. Lancet. 2006;367:859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- MEA . Island Press; Washington, D.C.: 2005. Ecosystems and human well-being. Synthesis. [Google Scholar]
- Moiseenko T.I., Voinov A.A., Megorsky V.V., Gashkina N.A., Kudriavtseva L.P., Vandish O.I. Ecosystem and human health assessment to define environmental management strategies: the case of long-term human impacts on an Arctic Lake. Sci Total Environ. 2006;369:1–20. doi: 10.1016/j.scitotenv.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Montoya D., Rogers L., Memmott J. Emerging perspectives in the restoration of biodiversity-based ecosystem services. Trends Ecol Evol. 2012;27:666–672. doi: 10.1016/j.tree.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Moreno-Mateos D., Power M.E., Comin F.A., Yockteng R. Structural and functional loss in restored wetland ecosystems. PLoS Biol. 2012;10:e1001247. doi: 10.1371/journal.pbio.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlando S., Schmidt S.J., LoGiudice K. Reduction in Lyme disease risk as an economic benefit of habitat restoration. Restor Ecol. 2012;20:498–504. [Google Scholar]
- Norris D.E. Mosquito-borne disease as a consequence of land use change. Ecohealth. 2004;1:19–24. [Google Scholar]
- O'Hara S.L., Wiggs G.F.S., Mamedov B., Davidson G., Hubbard R.B. Exposure to airborne dust contaminated with pesticide in the Aral Sea region. Lancet. 2000;355:627–628. doi: 10.1016/S0140-6736(99)04753-4. [DOI] [PubMed] [Google Scholar]
- Ostfeld R.S. Biodiversity loss and the rise of zoonotic pathogens. Clin Microbiol Infect. 2009;15:40–43. doi: 10.1111/j.1469-0691.2008.02691.x. [DOI] [PubMed] [Google Scholar]
- Ostfeld R.S., Keesing F. The function of biodiversity in the ecology of vector-borne zoonotic disease. Can J Zool. 2000;78:2061–2078. [Google Scholar]
- Patz J.A., Graczyk T.K., Geller N., Vittor A.Y. Effects of environmental change on emerging parasitic diseases. Int J Parasitol. 2000;30:1395–1405. doi: 10.1016/s0020-7519(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Patz J.A., Daszak P., Tabor G.M., Aguirre A.A., Pearl M., Epstein J. Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112:1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz J.A., Olson S.H., Uejio C.K., Gibbs H.K. Disease emergence from global climate and landuse change. Med Clin North Am. 2008;92:1473–1491. doi: 10.1016/j.mcna.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Pinder A.M., Halse S.A., McRae J.M., Shiel R.J. Occurrence of aquatic invertebrates of the wheatbelt region of Western Australia in relation to salinity. Hydrobiologia. 2005:543. [Google Scholar]
- Randolph S.E., Dobson A.D.M. Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012;139:847–863. doi: 10.1017/S0031182012000200. [DOI] [PubMed] [Google Scholar]
- Rapport D.J. The health of ecology and the ecology of health. Ecol Risk Assess. 2002;8:205–213. [Google Scholar]
- Rey Benayas J.M., Newton A.C., Diaz A., Bullock J.M. Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science. 2009;325:1121–1124. doi: 10.1126/science.1172460. [DOI] [PubMed] [Google Scholar]
- Rochlin I., Iwanejko T., Dempsey M.E., Ninivaggi D.V. Geostatistical evaluation of integrated marsh management impact on mosquito vectors using before-after-control-impact (BACI) design. Int J Health Geogr. 2009:8. doi: 10.1186/1476-072X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkeld D.J., Padgett K.A., Jones J.H. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol Lett. 2013;16:679–686. doi: 10.1111/ele.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speldewinde P.C. Reinventing the wheel: teaching restoration ecology without the ecology. Biosci Educ. 2010 doi: 10.3108/beej.15.c1. [DOI] [Google Scholar]
- Suding K.N., Gross K.L., Houseman G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol Evol. 2004;19:48–53. doi: 10.1016/j.tree.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Weinstein P. Human health is harmed by ecosystem degradation, but does intervention improve it? A research challenge from the Millennium Ecosystem Assessment. Ecohealth. 2005;2:228–230. [Google Scholar]
- Wilson J.B., Steel J.B., Dodd M.E., Anderson B.J., Ullmann I., Bannister P. A test of community reassembly using the exotic communities of New Zealand roadsides in comparison to British roadsides. J Ecol. 2000;88:757–764. [Google Scholar]
- Wood C.L., Lafferty K.D., DeLeo G., Young H.S., Hudson P.J., Kuris A.M. Does biodiversity protect humans against infectious disease? Ecology. 2014;95:817–832. doi: 10.1890/13-1041.1. [DOI] [PubMed] [Google Scholar]
- Zetterstrom R. Child health and environmental pollution in the Aral Sea region in Kazakhstan. Acta Paediatr Suppl. 1998;88:49–54. doi: 10.1111/j.1651-2227.1999.tb01290.x. [DOI] [PubMed] [Google Scholar]


